Abstract
Purpose
To evaluate cumulative incidence of metastasis at specific timepoints after treatment of uveal melanoma in a large cohort of patients and to provide comparison of conditional outcomes in the youngest and oldest cohorts (extremes of age).
Methods
Retrospective analysis of 8091 consecutive patients with uveal melanoma at a single center over a 51-year period. The patients were categorized by age at presentation (0–29 years [n = 348, 4%], 30–59 years [n = 3859, 48%], 60–79 years [n = 3425, 42%], 80 to 99 years [n = 459, 6%]) and evaluated for nonconditional (from presentation date) and conditional (from specific timepoints after presentation) cumulative incidence of metastasis at five, 10, 20, and 30 years.
Results
For the entire population of 8091 patients, five-year/10-year/20-year/30-year nonconditional cumulative incidence of metastasis was 15%/23%/32%/36%, and the conditional incidence improved to 6%/15%/25%/30% for patients who did not develop metastasis in the first three years. For the extremes of age (0–29 years and 80–99 years), the nonconditional cumulative incidence of metastasis revealed the younger cohort with superior outcomes at 8%/15%/19%/27% and 21%/29%/29%/29%, respectively (P < 0.001). The conditional incidence (at one-year and two-year timepoints with metastasis-free survival) showed persistent superior younger cohort survival (P < 0.001, P = 0.001), but no further benefit for patients with three-year metastasis-free survival at 4%/12%/16%/24% and 7%/18%/18%/18%, respectively (P = 0.09).
Conclusions
Non-conditional metastasis-free survival analysis for patients with uveal melanoma revealed the youngest cohort to have significantly better survival than the oldest cohort, and this persisted into one-year and two-year conditional metastasis-free survival but diminished at the three-year conditional timepoint.
Keywords: conditional outcomes, uveal melanoma, non-conditional outcomes
The management of uveal melanoma is complex and involves treatment of the primary tumor with methods of radiotherapy, enucleation, local resection, or nanoparticle therapy, identification of genetic alterations within the tumor, post-radiotherapy techniques to minimize radiation complications to the eye, and prognostication of uveal melanoma-related risk for metastasis and death.1–3 Recent data has indicated that prognostication of uveal melanoma depends heavily on the tumor genetic profile4–23 and tumor size,8,10,15,22 more so than the American Joint Committee on Cancer classification.9,20,21
Prognostication of uveal melanoma has typically relied on nonconditional (static) analysis of outcomes from date of diagnosis.15 More recently, conditional (dynamic) analysis of outcomes has been reported as a more pragmatic approach for estimating cancer risks because this method incorporates the accrued years of patient survival from the date of diagnosis into the ultimate survival calculation. This methodology has been applied for several cancers, including high-risk malignancies such as pancreatic adenocarcinoma, colon carcinoma, cutaneous melanoma, retinoblastoma, and more recently uveal melanoma.24–35 Swords et al.24 explored conditional survival with pancreatic ductal adenocarcinoma, acknowledging that most affected patients die within 5 years, but also elaborated on long-term conditional survival for those who endure beyond the first few years. Of nearly 11,000 patients with pancreatic adenocarcinoma in the Surveillance, Epidemiology, and End Results (SEER) database, they found that those who survived three years, six years, or nine years demonstrated cancer-specific survival at 12 years of 46%, 75%, or 90%, respectively. Thomas25 commented that conditional survival is often more sensible than nonconditional survival because it addresses patients’ concerns for future risks at specific timepoints in the disease course.
Conditional analysis has been explored for uveal melanoma using the SEER database and in relation to The Cancer Genome Atlas (TCGA) classification.34,35 Herein, we investigate conditional outcomes for uveal melanoma in a large, single center database of 8091 affected patients over a 37-year treatment period and a 51-year follow up period, stratifying conditional outcomes by age.
Methods
The medical records on the Ocular Oncology Service at Wills Eye Hospital, Thomas Jefferson University, Philadelphia, Pennsylvania, USA, were retrospectively reviewed for patients with the clinical diagnosis of uveal melanoma between August 25, 1970, and August 27, 2007, and with a follow-up period extending through September 14, 2021. This study was approved by the Institutional Review Board of Wills Eye Hospital, adhered to the tenets of the Declaration of Helsinki, and complied with the Health Insurance Portability and Accountability Act. Informed consent was obtained from each patient.
All patients were examined by an ocular oncology team for clinical confirmation of the diagnosis of uveal melanoma based on indirect ophthalmoscopy with detailed fundus drawings and multimodal imaging. Ophthalmic imaging included fundus photography with wide-angle or montage imaging, fundus autofluorescence, ultrasonography (A- and B-scan techniques), optical coherence tomography, fluorescein angiography, and occasionally indocyanine green angiography and optical coherence tomography angiography, as needed for documentation at the initial and subsequent examinations. Patients were then stratified by age: 0 to 29 years, 30 to 59 years, 60 to 79 years, and 80 to 99 years, so trends in the extremes of age could be assessed.
Data were recorded at each examination and documented on the patient's medical record. Demographic data included sex (male, female), race (White, non-White), and affected eye (right, left). The tumor features at presentation included largest tumor basal diameter (mm), tumor thickness (mm), tumor location with distance to the optic disc (mm) and distance to the foveola (mm), tumor epicenter (macula, macula to equator, equator to ora, ciliary body, iris), anterior margin of the tumor, posterior margin of the tumor, tumor pigmentation (amelanotic, mixed, melanotic), and tumor shape (dome-shaped, mushroom-shaped, diffuse, tapioca). Presence or absence of other clinical features, such as subretinal fluid, rupture in Bruch's membrane, and vitreous or subretinal hemorrhage were noted. The primary treatment was recorded for each patient (observation, argon laser photocoagulation, cryotherapy, transpupillary thermotherapy, plaque radiotherapy, external beam radiotherapy, proton beam radiotherapy, partial lamellar sclerouvectomy (PLSU), enucleation, or exenteration). Metastasis was documented based on the date of histopathologic confirmation via biopsy or on the date of appropriate imaging, such as magnetic resonance imaging or computerized tomography. Death, both from melanoma-related and non-melanoma-related causes, were documented from correspondence with other physicians or the patients’ families.
Primary outcomes included cumulative incidence of metastasis (CIM) from uveal melanoma. Non-conditional cumulative incidence of metastasis (ncCIM) was assessed at presentation. Conditional cumulative incidence of metastasis (cCIM) was assessed after each year for the first 10 years. Cox regression analysis for competing risks was performed for ncCIM, as well as cCIM for the entire group and then stratified by age group (0–29 years vs. 30–59 years vs. 60–79 years vs. 80–99 years). The cCIM was assessed primarily for five-year, 10-year, 20-year, and 30-year endpoints. Annual likelihood of metastasis for each age group was obtained through Cox regression analysis for competing risks. All analysis for CIM were run both with and without patients with iris melanoma (n = 321), demonstrating no significant difference in any statistical trends in any of the primary outcomes; therefore, iris melanoma was included in the total cohort. Additionally, Kaplan-Meier analyses were compared with Cox regression analyses for competing risks and were found to give overly pessimistic outcome estimates because of the significant number of patients with non-melanoma-related death; therefore, the latter statistical approach was used to determine CIM.
Statistical analysis was performed using SAS Software Suite (version 9.4; SAS Institute). Continuous variables were expressed as mean (median, range). The one-sample Shapiro-Wilk test was used to assess normality of distribution. Comparison between groups was performed using the one-way ANOVA test for continuous variables with normal distribution and the Kruskal-Wallis test for continuous variables without normal distribution. Comparison of categorical variables was performed using the likelihood ratio χ2 test and Fisher's exact test when indicated. Bonferroni correction for multiple testing was used in the assessment of demographics data. For Tables 12–3, the abovementioned tests were used, but the two P values correspond to how the youngest (age 0–29 years) and oldest (age 80–99 years) age groups relate to the remaining population. Multivariate Cox cause-specific hazard regression was used to establish hazard ratios for development of uveal melanoma metastasis in a nonconditional fashion for two-year, five-year, and 10-year outcomes, as well as five-year and 10-year outcomes in a conditional fashion for patients who survived three years without developing metastasis. Variables incorporated into the multivariate model included sex, race, tumor basal diameter, tumor thickness, tumor location, presence of subretinal fluid, rupture in Bruch's membrane, vitreous or subretinal hemorrhage, and death from non-melanoma-related causes. Competing risks analysis was performed for CIM for uveal melanoma predicting cumulative incidence function, which produced survival curves and also determined an annual likelihood of metastasis. In this model, the cause-specific hazard is metastasis resulting from uveal melanoma confirmed on histopathology via biopsy or appropriate imaging, such magnetic resonance imaging or computerized tomography, and the variable of competing risk was death from causes unrelated to uveal melanoma that occurred prior to development of metastatic disease. It was assumed that all melanoma-related deaths were a result of metastatic disease. Gray's test was used to analyze the difference in CIM by age. Assessment of the Kendall Tau correlation coefficient was performed to determine the significance of trends for the annual risk of metastasis. All analyses were run with and without the inclusion of iris melanoma, which demonstrated no notable statistically different trends between age groups; therefore the entire cohort was used for the purpose of this study. A P value <0.05 was considered statistically significant for results of all analyses.
Table 1.
Conditional Metastasis of Uveal Melanoma in 8091 Patients over Half-Century by Age Group: Assessing the Entire Population and the Extremes of Age—Patient Demographics
| Patient Age at Presentation | |||||||
|---|---|---|---|---|---|---|---|
| Patient Demographics | Youngest p-Values | 0–29 Years (n = 348) [n (%)] | 30–59 Years (n = 3859) [n (%)] | 60–79 Years (n = 3425) [n (%)] | 80–99 Years (n = 459) [n (%)] | Oldest P Values | Total Population (n = 8091) [n (%)] |
| Sex | 0.001 | <0.001 | |||||
| Male | 147 (42) | 1992 (52) | 1760 (51) | 187 (41) | 4086 (51) | ||
| Female | 201 (58) | 1867 (48) | 1665 (48) | 272 (59) | 4005 (49) | ||
| Race | 0.008 | 0.006 | |||||
| White | 332 (95) | 3731 (97) | 3389 (99) | 456 (99) | 7908 (98) | ||
| Non-White | 16 (5) | 128 (3) | 36 (1) | 3 (1) | 183 (2) | ||
| Affected eye | 0.751 | 0.271 | |||||
| Right | 173 (50) | 1896 (51) | 1679 (49) | 207 (45) | 3955 (49) | ||
| Left | 175 (50) | 1963 (49) | 1746 (51) | 252 (55) | 4136 (51) | ||
Bold values indicate significant P value.
Youngest P value compares patients of the youngest age group with the remaining population.
Oldest P value compares patients of the oldest age group with the remaining population.
Table 2.
Conditional Metastasis of Uveal Melanoma in 8091 Patients over Half-Century by Age Group: Assessing the Entire Population and the Extremes of Age—Tumor Features
| Patient Age at Presentation | |||||||
|---|---|---|---|---|---|---|---|
| Youngest | 0–29 Years | 30–59 Years | 60–79 Years | 80–99 Years | Oldest | ||
| Tumor Features | P Values | (n = 348) | (n = 3859) | (n = 3425) | (n = 459) | P Values | Total Population (n = 8091) |
| Largest basal diameter (mm), mean (median, range) | <0.001 | 10.2 (10.0, 2.0 – 24.0) | 10.8 (11.0, 1.5 – 24.0) | 11.4 (11.0, 1.3 – 24.0) | 12.3 (12.0, 2.0 – 24.0) | <0.001 | 11.1 (11.0, 1.3 – 24.0) |
| Thickness (mm), mean (median, range) | 0.054 | 5.3 (4.2, 0.5 – 19.0) | 5.3 (4.2, 0.3 – 20.0) | 5.6 (4.5, 0.5 – 24.0) | 6.5 (5.7, 0.5 – 23.0) | <0.001 | 5.5 (4.5, 0.3 – 24.0) |
| Distance to optic disc (mm), mean (median, range) | 0.582 | 6.4 (3.5, 0.0 – 25.0) | 5.0 (3.5, 0.0 – 25.0) | 5.3 (4.0, 0.0 – 25.0) | 5.2 (4.0, 0.0 – 25.0) | 0.441 | 5.2 (3.8, 0.0 – 25.0) |
| Distance to foveola (mm), mean (median, range) | 0.337 | 6.3 (3.0, 0.0 – 25.0) | 4.8 (3.0, 0.0 – 25.0) | 5.1 (3.5, 0.0 – 25.0) | 5.1 (4.0, 0.0 – 22.0) | 0.263 | 5.0 (3.0, 0.0 – 25.0) |
| Tumor epicenter | <0.001 | n = 348 | n = 3852 | n = 3420 | n = 457 | <0.001 | n = 8077 |
| Macula | 21 (6) | 240 (6) | 145 (4) | 11 (2) | 417 (5) | ||
| Macula to equator | 216 (62) | 2731 (71) | 2377 (70) | 311 (68) | 5635 (70) | ||
| Equator to ora | 41 (12) | 511 (13) | 565 (17) | 90 (20) | 1207 (15) | ||
| Ciliary body | 21 (6) | 213 (6) | 227 (7) | 36 (8) | 497 (6) | ||
| Iris | 49 (14) | 157 (4) | 106 (3) | 9 (2) | 321 (4) | ||
| Anterior margin | <0.001 | n = 348 | n = 3852 | n = 3420 | n = 457 | <0.001 | n = 8077 |
| Macula | 6 (2) | 58 (2) | 30 (1) | 0 (0) | 94 (1) | ||
| Macula to equator | 175 (42) | 1737 (45) | 1340 (39) | 144 (32) | 3366 (42) | ||
| Equator to ora | 74 (21) | 1015 (26) | 1005 (29) | 151 (33) | 2245 (28) | ||
| Ciliary body | 45 (13) | 645 (17) | 699 (20) | 109 (24) | 1498 (19) | ||
| Iris | 78 (22) | 397 (10) | 346 (10) | 53 (12) | 874 (11) | ||
| Posterior margin | <0.001 | n = 348 | n = 3852 | n = 3420 | n = 457 | 0.051 | n = 8077 |
| Macula | 152 (44) | 1682 (44) | 1375 (40) | 176 (39) | 3385 (42) | ||
| Macula to equator | 126 (36) | 1857 (48) | 1757 (51) | 244 (53) | 3984 (49) | ||
| Equator to ora | 13 (4) | 111 (3) | 137 (4) | 21 (5) | 282 (3) | ||
| Ciliary body | 18 (5) | 84 (2) | 61 (2) | 9 (2) | 172 (2) | ||
| Iris | 39 (11) | 118 (3) | 90 (3) | 7 (2) | 254 (3) | ||
| Tumor Pigmentation | n = 347 | n = 3849 | n = 3415 | n = 457 | n = 8068 | ||
| Amelanotic | 0.355 | 59 (17) | 623 (16) | 504 (15) | 43 (9) | <0.001 | 1229 (15) |
| Mixed | 0.317 | 96 (28) | 1217 (32) | 990 (29) | 122 (27) | 0.103 | 2425 (30) |
| Melanotic | 0.812 | 192 (55) | 2009 (52) | 1921 (56) | 292 (64) | <0.001 | 4414 (55) |
| Tumor Shape | n = 346 | n = 3845 | n = 3415 | n = 458 | n = 8065 | ||
| Dome-shaped | 0.168 | 250 (72) | 2947 (77) | 2556 (75) | 329 (72) | 0.071 | 6082 (75) |
| Mushroom-shaped | 0.147 | 54 (16) | 683 (18) | 649 (19) | 107 (23) | 0.007 | 1493 (19) |
| Diffuse | 0.001 | 35 (10) | 212 (6) | 202 (6) | 21 (5) | 0.227 | 470 (6) |
| Tapioca | <0.001 | 7 (2) | 3 (<1) | 4 (<1) | 1 (<1) | 0.812 | 15 (<1) |
| Other features | |||||||
| Subretinal fluid | 0.006 | 219 (64) | 2824 (73) | 2338 (68) | 299 (63) | 0.015 | 5680 (70) |
| Rupture in Bruch's membrane | 0.148 | 61 (18) | 770 (20) | 721 (21) | 120 (26) | 0.004 | 1672 (21) |
| Vitreous or subretinal hemorrhage | 0.001 | 19 (5) | 330 (9) | 402 (12) | 71 (16) | <0.001 | 822 (10) |
Bold values indicate significant P value.
Youngest P value compares patients of the youngest age group with the remaining population.
Oldest P value compares patients of the oldest age group with the remaining population.
Table 3.
Conditional Metastasis of Uveal Melanoma in 8091 Patients over Half-Century by Age Group: Assessing the Entire Population and the Extremes of Age—Primary Treatment
| Patient Age at Presentation | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Youngest P Values | 0–29 Years (N = 348), No. (%) | 30–59 Years (N = 3859), No. (%) | 60–79 Years (N = 3425), No. (%) | 80–99 Years (N = 459), No. (%) | Oldest P Values | Total Population (n = 8091), No. (%) |
| Observation | 0.191 | 20 (6) | 134 (3) | 149 (4) | 49 (11) | <0.001 | 352 (4) |
| Argon laser photocoagulation | 0.302 | 4 (1) | 23 (1) | 25 (1) | 2 (<1) | 0.504 | 54 (1) |
| Cryotherapy | 0.298 | 1 (<1) | 2 (<1) | 4 (<1) | 0 (0) | 0.366 | 7 (<1) |
| Transpupillary thermotherapy | 0.376 | 11 (3) | 194 (5) | 117 (3) | 6 (1) | <0.001 | 328 (4) |
| Plaque radiotherapy | <0.001 | 128 (37) | 2364 (61) | 2221 (65) | 291 (63) | 0.482 | 5004 (62) |
| External beam radiation therapy | 0.706 | 1 (<1) | 10 (<1) | 18 (1) | 4 (1) | 0.160 | 33 (<1) |
| Proton beam radiation therapy | 0.099 | 0 (0) | 21 (1) | 10 (<1) | 0 (0) | 0.057 | 31 (<1) |
| PLSU | <0.001 | 48 (14) | 217 (6) | 130 (4) | 6 (1) | <0.001 | 401 (5) |
| Enucleation | <0.001 | 135 (39) | 889 (23) | 747 (22) | 102 (22) | 0.625 | 1873 (23) |
| Exenteration | 0.402 | 0 (0) | 2 (<1) | 4 (<1) | 2 (1) | 0.072 | 8 (<1) |
Bold values indicate significant P value.
Youngest P value compares patients of the youngest age group with the remaining population.
Oldest P value compares patients of the oldest age group with the remaining population.
Results
There were 8091 consecutive patients with uveal melanoma managed over a 51-year period (August 25, 1970–August 27, 2007) at the Ocular Oncology Service at Wills Eye Hospital of Thomas Jefferson University, Philadelphia, Pennsylvania USA. In this analysis, we explored cCIM in 8091 patients with uveal melanoma, subdivided by select age groups (0–29 years [n = 348, 4%], 30–59 years [n = 3859, 48%], 60–79 years [n = 3425, 42%], 80–99 years [n = 459, 6%]). Of 8091 initial patients, 6679 (83%), 4100 (51%), 2010 (25%), 459 (6%), and 99 (1%) patients were followed up at one year, five years, 10 years, 20 years, and 30 years, respectively. Mean follow-up time was 6.9 years, and longest follow-up duration was 46.9 years.
Demographic features are listed in Table 1. The patient sex was male (n = 4086, 51%) or female (n = 4005, 49%), race was White (n = 7908, 98%) or non-White (n = 183, 2%), and affected eye was right (n = 3955, 49%) or left (n = 4136, 51%). Compared to the remaining population the youngest and oldest patients were more likely to be female (P = 0.001 and P < 0.001). Although the youngest patients were more likely to be non-White (P = 0.008), the oldest patients were more likely to be White (P = 0.006). There was no difference per age category regarding affected eye.
Tumor features are listed in Table 2. The tumor showed largest basal diameter (mean 11.1 mm, median 11.0 mm) and thickness (mean 5.5 mm, median 4.5 mm) with location to optic disc (mean 5.2 mm, median 3.8 mm) and foveola (mean 5.0 mm, median 3.0 mm). Increasing age category was associated with larger tumor basal diameter (10.2 vs. 10.8 vs. 11.4 vs. 12.3 mm, P < 0.001) and thickness (5.3 vs. 5.3 vs. 5.6 vs. 6.5 mm, P < 0.001), and more peripheral tumor location (P < 0.001). The oldest patients were more likely to have greater tumor pigmentation (P < 0.001), greater mushroom configuration (P = 0.007), greater Bruch's membrane rupture (P = 0.004), greater vitreous or subretinal hemorrhage (P < 0.001), and less subretinal fluid (P = 0.015). The youngest patients were more likely to have diffuse (P = 0.001) or tapioca (P < 0.001) tumor configuration and less likely to have subretinal fluid (P = 0.006) or vitreous or subretinal hemorrhage (P = 0.001).
Tumor primary treatment is listed in Table 3. Overall, treatment included plaque radiotherapy (n = 5004, 62%), enucleation (n = 1873, 23%), transpupillary thermotherapy (n = 328, 4%), partial lamellar sclerouvectomy (resection) (n = 401, 5%), and others (n = 485, 6%). The youngest patients were more likely to undergo PLSU (P < 0.001) or primary enucleation (P < 0.001) and less likely to have plaque radiotherapy (P < 0.001). The oldest patients were less likely to undergo transpupillary thermotherapy (P < 0.001) and PLSU (P < 0.001).
The ncCIM and cCIM are listed in Table 4. In the entire population, the ncCIM revealed five-year/10-year/20-year/30-year metastatic rate at 15%/23%/32%/36%. The cCIM for the entire population revealed five-year/10-year/20-year/30-year metastatic rate (for those without metastasis at two years) at 10%/19%/28%/33% and the conditional 10-year/20-year/30-year metastatic rate (for those without metastasis at five years) at 10%/21%/26%. Furthermore, the cCIM for the entire population revealed 20-year/30-year metastatic rate (for those without metastasis at 10 years) at 13%/18%. For the total population, 330 patients (4%) were documented to have died of non-melanoma-related causes before the development of metastasis, including one patient (<1%) 0 to 29 years, 77 patients (2%) 30 to 59 years, 210 patients (6%) 60 to 79 years, and 42 patients (9%) 80 to 99 years.
Table 4.
Cumulative Incidence of Metastasis in Uveal Melanoma in 8091 Patients over 30 Years by Age Group. Analysis at Initial Presentation (Non-conditional Survival) and Specific Metastasis-Free Timepoints (Conditional Survival)
| Patient Age at Presentation | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–29 Years | 30–59 Years | 60–79 Years | 80–99 Years | |||||||||||||||||||
| Duration of Achieved Metastasis-Free Survival | (n = 348 Patients) | (n = 3859 Patients) | (n = 3425 Patients) | (n = 459 Patients) | Total Population (n = 8091 Patients) | |||||||||||||||||
| Type of Survival | Metastasis From Uveal Melanoma | 5 Years | 10 Years | 20 Years | 30 Years | 5 Years | 10 Years | 20 Years | 30 Years | 5 Years | 10 Years | 20 Years | 30 Years | 5 Years | 10 Years | 20 Years | 30 Years | p-Value | 5 Years | 10 Years | 20 Years | 30 Years |
| ncCIM [n (%)] | At presentation (n = 8091 patients) | 19 (8) | 31 (15) | 34 (19) | 36 (27) | 347 (12) | 520 (21) | 615 (30) | 625 (35) | 466 (18) | 575 (26) | 624 (36) | 627 (39) | 53 (21) | 60 (29) | 60 (29) | 60 (29) | <0.001 | 885 (15) | 1186 (23) | 1333 (32) | 1348 (36) |
|
cCIM [n (%)] |
1 year (n = 6635 patients) | 16 (7) | 28 (14) | 31 (18) | 33 (26) | 303 (11) | 476 (20) | 571 (29) | 581 (34) | 370 (16) | 479 (24) | 528 (34) | 531 (38) | 46 (20) | 53 (29) | 53 (29) | 53 (29) | <0.001 | 735 (13) | 1036 (21) | 1183 (31) | 1198 (35) |
| 2 years (n = 5895 patients) | 14 (6) | 26 (14) | 29 (17) | 31 (25) | 218 (8) | 391 (17) | 486 (28) | 496 (32) | 248 (12) | 357 (20) | 406 (31) | 409 (35) | 27 (14) | 34 (24) | 34 (24) | 34 (24) | 0.001 | 507 (10) | 808 (19) | 955 (28) | 970 (33) | |
| 3 years (n = 5062 patients) | 9 (4) | 21 (12) | 24 (16) | 26 (24) | 125 (5) | 298 (15) | 393 (25) | 403 (30) | 139 (8) | 248 (16) | 297 (28) | 300 (32) | 11 (7) | 18 (18) | 18 (18) | 18 (18) | 0.087 | 284 (6) | 585 (15) | 732 (25) | 747 (30) | |
| 4 years (n = 4485 patients) | 4 (2) | 16 (10) | 19 (14) | 21 (22) | 52 (2) | 225 (12) | 320 (23) | 330 (28) | 66 (4) | 175 (13) | 224 (26) | 227 (30) | 2 (6) | 9 (14) | 9 (14) | 9 (14) | 0.185 | 124 (3) | 425 (12) | 572 (23) | 587 (28) | |
| 5 years (n = 3931 patients) | 12 (8) | 15 (12) | 17 (21) | 173 (10) | 268 (21) | 278 (26) | 109 (10) | 158 (23) | 161 (27) | 7 (13) | 7 (13) | 7 (13) | 0.519 | 301 (10) | 448 (21) | 463 (26) | ||||||
| 6 years (n = 3412 patients) | 10 (7) | 13 (11) | 15 (20) | 118 (8) | 213 (19) | 223 (24) | 79 (8) | 128 (21) | 131 (26) | 5 (11) | 5 (11) | 5 (11) | 0.692 | 212 (8) | 359 (19) | 374 (24) | ||||||
| 7 years (n = 2960 patients) | 9 (7) | 12 (11) | 14 (19) | 78 (6) | 173 (17) | 183 (23) | 51 (6) | 100 (20) | 103 (25) | 4 (10) | 4 (10) | 4 (10) | 0.868 | 142 (6) | 289 (17) | 304 (23) | ||||||
| 8 years (n = 2589 patients) | 7 (5) | 10 (9) | 12 (18) | 57 (4) | 152 (16) | 162 (22) | 32 (4) | 81 (18) | 84 (23) | 2 (7) | 2 (7) | 2 (7) | 0.885 | 98 (4) | 245 (16) | 260 (21) | ||||||
| 9 years (n = 2262 patients) | 4 (3) | 7 (7) | 9 (16) | 28 (2) | 123 (14) | 133 (20) | 14 (2) | 63 (17) | 66 (22) | 0 (0) | 0 (0) | 0 (0) | 0.437 | 46 (2) | 193 (14) | 208 (20) | ||||||
| 10 years (n = 1981 patients) | 3 (4) | 5 (13) | 95 (12) | 105 (18) | 49 (15) | 52 (20) | 0 (0) | 0 (0) | 0.312 | 147 (13) | 162 (18) | |||||||||||
Bold values indicate statistical significance.
Hazard ratios for development of uveal melanoma metastasis at specific time points are listed in Table 5. Patients presenting under the age of 30 years were less likely to develop metastasis at two years (HR = 0.30, P = 0.01), five years (HR = 0.52, P = 0.01), and 10 years (HR = 0.66, P = 0.04) compared to the remaining population, while patient presenting age 80 years or older were more likely to develop metastasis at two years (HR = 1.68, P = 0.02), five years (HR = 1.75, P = 0.001), and 10 years (HR = 1.78, P = 0.03) compared to the remaining population. However, for patients who maintain metastasis-free survival through the first three years, there is no difference in likelihood to develop metastasis in the remaining five-year and 10-year periods based on age at presentation.
Table 5.
Conditional Metastasis of Uveal Melanoma in 8091 Patients over Half-Century by Age Group: Assessing the Entire Population and the Extremes of Age
| Age 0–29 (n = 348) | Age 80–99 (n = 459) | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Nonconditional multivariate analysis | ||||
| Metastasis in the first two years | 0.30 (0.12-0.75) | 0.010 | 1.68 (1.10-2.58) | 0.017 |
| Metastasis in the first five years | 0.52 (0.32-0.86) | 0.011 | 1.75 (1.29-2.39) | 0.001 |
| Metastasis in the first 10 years | 0.66 (0.44-0.97) | 0.037 | 1.78 (1.04-2.50) | 0.034 |
| Conditional multivariate analysis for patients with three years metastasis free survival | ||||
| Metastasis in the first five years | 1.15 (0.53-2.52) | 0.726 | 1.12 (0.60-2.08) | 0.731 |
| Metastasis in the first 10 years | 1.07 (0.65-1.75) | 0.802 | 0.86 (0.52-1.41) | 0.547 |
Multivariate Cox regression, hazard ratios for metastasis at specific time points.
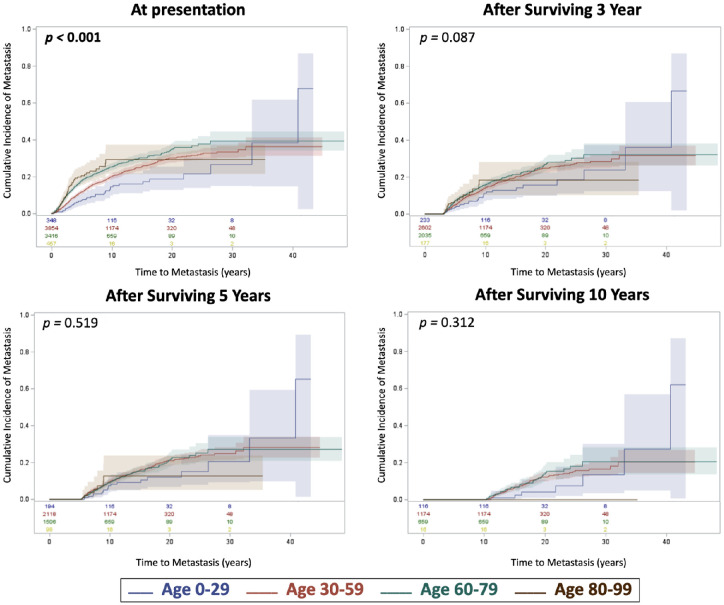
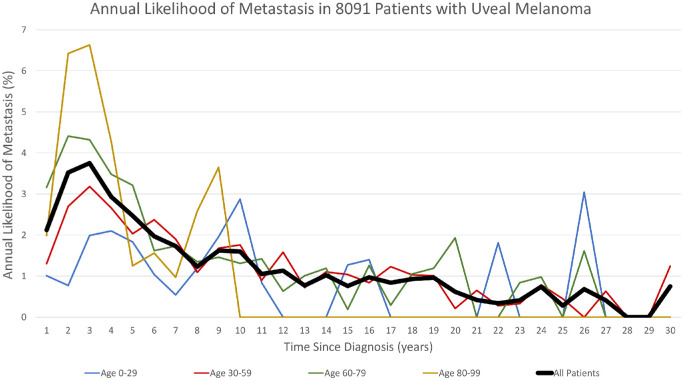
Risk regression analyses for cumulative incidence of metastasis per age group are illustrated in Figure 1. By comparison, increasing age (0–29 vs. 30–50 vs. 60–79 vs. 80–99) was associated with greater incidence of metastasis at initial presentation (ncCIM) (P < 0.001). The conditional outcomes (cCIM) after surviving three years, five years, and 10 years were no different per age group (Fig. 1). The annual rate of metastasis for all patients decreased over time since diagnosis (P < 0.001) and peaked at approximately three to four years after diagnosis (Fig. 2). The average annual rate of metastasis for the total population within the first five years was 2.96%, for years five to 10 (1.62%), for years 10 to 20 (1.00%), and for years 20 to 30 (0.40%). After more than 30 years’ follow-up, three surviving patients developed extremely late metastasis at 31.2, 32.1, and 39.5 years after diagnosis.
Figure 1.
Competing risk regression for cumulative incidence of metastasis over time with comparison of age stratification (0–29 vs. 30–59 vs. 60–79 vs. 80–99 years) at presentation, and after 3-year, 5-year, and 10-year metastasis-free survival. Number at risk at each time point were included at the bottom of each graph and are color coordinate with its respective age group.
Figure 2.
The annual likelihood of metastasis over time since diagnosis (years) of all 8091 patients with uveal melanoma and by age stratification (0–29 vs. 30–59 vs. 60–79 vs. 80–99 years).
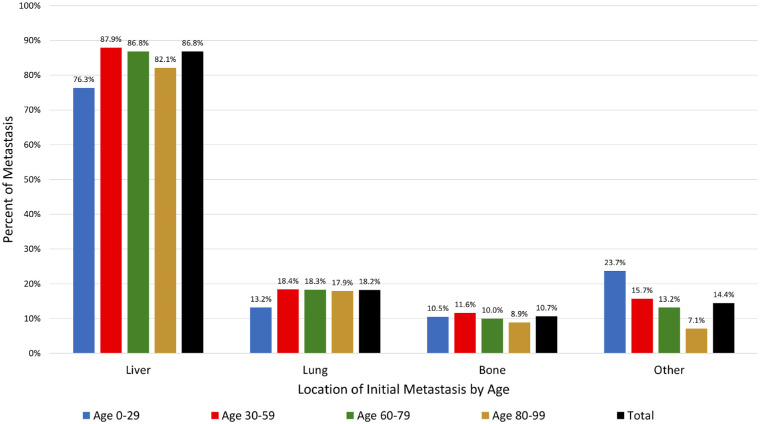
The location of the initial metastasis is shown in Figure 3. Among all age groups, the initial metastasis involved the liver (86.8%), lung (18.2%) or bone (10.7%). There was no difference in metastatic location by age group (P > 0.05 for all locations).
Figure 3.
The percent of metastasis by location (liver, lung, bones, other) of initial metastasis with comparison of age (0–29 vs. 30–59 vs. 60–79 vs. 80–99 years).
Discussion
In 2016, Merrill and Bateman summarized the importance of conditional survival rates, emphasizing that these rates are tailored to a patient's accrued survival time and provide outcomes that are more relative to the single patient as compared to non-conditional survival.27 They investigated cutaneous melanoma from the SEER database registry and noted that five- and 10-year relative survival decreased with age and stage at diagnosis and were lower in males, Blacks, and Hispanics; however, the conditional survival rates improved with each accrued year. Van der Leest et al.30 studied 40,050 affected patients with cutaneous melanoma in the Netherlands between 1994 and 2008 and noted that those with thick (>4 mm) melanoma showed five-year non-conditional survival at 60% compared to conditional survival (for those with 7 accrued years) at 90%. They additionally noted that conditional survival improved for all melanoma survivors year-by-year with the exception of those with the smallest malignancies (≤1 mm thickness) which showed no excessive mortality. Kim et al.26 explored 5145 cases of anal squamous cell carcinoma in the SEER database from 1988 to 2012 and found that the most advanced cases demonstrated the greatest improvement in conditional survival.
In 2020, Zabor et al.34 evaluated conditional survival in 6863 patients with uveal melanoma in the SEER database and quoted the five-year and 10-year nonconditional survival (no melanoma-related death) at 80% and 69%, respectively. After surviving one, two, three, or four years after diagnosis, the five-year conditional survival improved to 82%, 87%, 92%, and 96%, respectively. After survival at five, six, seven, eight, or nine years after diagnosis, the 10-year conditional survival improved to 87%, 90%, 93%, 96%, and 98%, respectively. In 2021, Shields et al.35 further studied conditional survival of uveal melanoma based on genetic testing in 1001 consecutive cases, classified according to TCGA, with the population distributed as follows: Group A (49%), B (14%), C (26%), and D (11%). The nonconditional five-year/10-year metastatic rate showed the following: Group A (4%/6%), Group B (12%/20%), Group C (23%/49%), and Group D (60%/68%). This improved with conditional evaluation for metastasis, and those who survived five years with no metastasis demonstrated 10-year metastasis in Group A (2%), Group B (10%), Group C (33%), and Group D (20%).
In the current analysis, we studied a large, single-center cohort of 8091 eyes with uveal melanoma and found the five-year/10-year/20-year/30-year nonconditional cumulative incidence of metastasis was 15%/23%/32%/36%. For those who accrued three years with no metastasis, the conditional metastasis improved to 6%/15%/25%/30%, and for those who accrued 10 years with no metastasis, the 20-year/30-year metastatic rate was 13%/18%, respectively. Additionally, we studied conditional risk for metastasis by age and noted that the youngest cohort showed better metastasis-free survival than the oldest cohort in the first two years, but this diminished to no difference at the three-year timepoint. The cCIM, after surviving three years, five years, and 10 years was not different per age group. The annual rate of metastasis for all patients decreased over time since the time of diagnosis (P < 0.001) and peaked at three to four years after diagnosis for all age groups. The average annual rate of metastasis decreased over time for the total population including those followed up for years 0 to five (2.96%), for years five to 10 (1.62%), for years 10 to 20 (1.00%), and for years 20 to 30 (0.40%). There was no difference in location of metastasis by age group (P > 0.05).
Regarding patients at the extremes of age with uveal melanoma, there have been no previous analyses evaluating cCIM of youngest compared to oldest adults with uveal melanoma, and our report represents the first publication on this topic. In 2012, Shields et al. evaluated uveal melanoma based on age in a ncCIM analysis of 8033 patients and found that younger patients (≤20 years, n = 106 [1%]) demonstrated more frequent iris melanoma, smaller tumors, and less extraocular extension, leading to less ncCIM at three, five, 10, and 20 years (2%, 9%, 9%, 20%) compared to older adults (>60 years, n = 3640, 45%) (11%, 19%, 28%, 39%).36 In 2016, Al-Jamal et al.37 published on pediatric choroidal and ciliary body melanoma from 24 ocular oncology centers in Europe involving 299 pediatric patients (age 0–18 years). Melanoma-related nonconditional survival (pediatric [0–18 years] vs. young adults [18–25 years]) was 97% and 90% at 5 years and 92% and 80% at 10 years (P = 0.013). This study suggested that children with choroidal and ciliary body melanoma had a more favorable survival than young adults, adjusting for TNM stage and gender. More recently, Dogrusoz et al analyzed prognostic factors five years after enucleation for uveal melanoma in 583 cases and found that older age was not a significant factor in the multivariable analysis.38 Our current results in 8091 patients suggest that young patients (≤29 years) had lower cCIM than both the middle age groups (30–59 and 60–79 years) and the oldest age group (≥80 years); however, this difference in long-term outcome by age was nullified for patients who survived at least three years without developing metastasis. Interestingly, as demonstrated by Shields et al.11 in 1001 eyes with uveal melanoma who underwent cytogenetic analysis for TCGA classification, patients with higher-genetic-risk Group D tumors were older than patients with Group A tumors (64 vs. 57 years). Moreover, the peak incidence of metastasis was earlier for patients with Group D tumors compared to tumors with less cytogenetic atypia.35 Similarly, the oldest age group in our study demonstrated an early peak incidence of metastasis that precipitously dropped more rapidly than younger groups (Fig. 2). Although cytogenetic analysis was not performed in most eyes in our current cohort because many were treated before the development of such testing, it stands to reason that the oldest group of patients may be more likely to possess tumors with a higher degree of cytogenetic atypia compared to their younger counterparts based on their trends of metastasis over time.
There are limitations to this study, including the unique rarity of uveal melanoma and the retrospective data collection and analysis in such a large cohort over 51 years. This comprehensive cohort of patients, managed at a single center of excellence for uveal melanoma, provides important perspective regarding patient conditional outcomes. We realize that this study, conducted over 5 decades, might have experienced different philosophies regarding management of this intraocular malignancy over time, but the data reports important conditional outcomes that may contribute to our understanding of patient prognosis at specific timepoints following therapy. In addition, this “real world” large cohort data included patients treated by our team and followed with us for a few years then preferred follow-up closer to home. For such patients, although information pertaining to the development of metastasis was highly reliable through the re-referral or the correspondence of local physicians, information related to deaths not related to melanoma may be underreported due to limited correspondence following the patients’ deaths. This could potentially lead to overly pessimistic estimations of CIM.
Because of its generally more favorable survival, inclusion of iris melanoma may alternatively lead to an overly optimistic estimation of CIM, despite no statistically significant change in relation to survival between the age groups. However, iris melanoma was included to offer a “real world” analysis for all patient with uveal melanoma, not just a specific cohort. Furthermore, in the oldest age cohort, patient follow-up after four years was low and could be unreliable. These data allow us the opportunity to counsel patients with a greater understanding of uveal melanoma and its disease course, specifically considering patient age and other prognostic factors at diagnosis and on subsequent follow-up.
In conclusion, our analysis of 8091 patients with uveal melanoma revealed the entire population with five-year/10-year/20-year/30-year cumulative incidence of metastasis improved with accrued years. The ncCIM risk improved from 15%/23%/32%/36% to the cCIM risk (with patient survival of three years with no metastasis) of 6%/15%/25%/30%. A separate analysis on the impact of conditional analysis on the extremes of age (youngest age [0–29 years, n = 348] vs. oldest age [80–99 years, n = 459]) showed no difference in cCIM at 5-year, 10-year, 20-year, and 30-year outcomes. Although age seemed to be a prognosticator of metastasis within the first two years of diagnosis (P < 0.001), there was no difference after three years when the cumulative incidence of metastasis was similar among all age groups (P = 0.09).
Acknowledgments
Support was provided in part by the Eye Tumor Research Foundation, Philadelphia, PA (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, and in the preparation, review or approval of the manuscript. Carol L. Shields, M.D. has had full access to all the data in the study and takes responsibility for the integrity of the data.
Presented in part as the 2023 Andrew Schachat Distinguished Lecture, Cleveland OH (September 2023).
Disclosure: C.L. Shields, None; A.G. Samuelson, None; G.J. Oh, None; J.D. DeSimone, None; Z.L. Sajjadi, None; Z. Bas, None; N.E. Kalafatis, None; S.E. Lally, None; J.A. Shields, None; P.W. Dockery, None
References
- 1. Shields JA, Shields CL.. Management of posterior uveal melanoma. Past, present and future. The 2014 Charles L. Schepens Lecture. Ophthalmology. 2015; 122: 414–428. [DOI] [PubMed] [Google Scholar]
- 2. Jager M, Shields CL, Cebulla CM, et al.. Uveal melanoma. Nat Rev Dis Primers. 2020; 6: 24–49. [DOI] [PubMed] [Google Scholar]
- 3. Shields CL, Lim LAS, Dalvin LA, Shields JA.. Small choroidal melanoma: detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy. Curr Opin Ophthalmol. 2019; 30: 206–214. [DOI] [PubMed] [Google Scholar]
- 4. Cancer Genome Atlas Research Network, Weinstein JN, Collisson EA, et al.. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013; 45: 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robertson AG, Shih J, Yau C, et al.. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017; 32: 204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jager MJ, Brouwer NJ, Esmaeli B.. The Cancer Genome Atlas Project: an integrated molecular view of uveal melanoma. Ophthalmology. 2018; 125: 1139–1142. [DOI] [PubMed] [Google Scholar]
- 7. Tomczak K, Czerwińska P, Wiznerowicz M.. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol. 2015; 19(1A): A68–A77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vichitvejpaisal P, Dalvin LA, Mazloumi M, et al.. Genetic analysis of uveal melanoma in 658 patients using the Cancer Genome Atlas Classification of uveal melanoma as A, B, C, and D. Ophthalmology. 2019; 126: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 9. Mazloumi M, Vichitvejpaisal P, Dalvin LA, et al.. Accuracy of The Cancer Genome Atlas Classification vs American Joint Committee on Cancer Classification for prediction of metastasis in patients with uveal melanoma. JAMA Ophthalmol. 2020; 138: 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shields CL, Dalvin LA, Vichitvejpaisal P, et al.. Prognostication of uveal melanoma is simple and highly predictive using The Cancer Genome Atlas (TCGA) classification: a review. Indian J Ophthalmol. 2019; 67: 1959–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shields CL, Mayro EL, Bas Z, et al.. Ten-year outcomes of uveal melanoma based on The Cancer Genome Atlas (TCGA) classification in 1001 cases. Indian J Ophthalmol. 2021; 69: 1839–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sen M, Card KR, Caudill GB, et al.. Relationship between Fitzpatrick skin type and The Cancer Genome Atlas classification with uveal melanoma outcomes in 854 patients at a single ocular oncology center. Ophthalmic Genet. 2022; 43: 742–755. [DOI] [PubMed] [Google Scholar]
- 13. Bas Z, Shields CL.. Uveal melanoma classified by the cancer genome atlas (TCGA). Retina Today. 2021; July/August(4): 50–53. [Google Scholar]
- 14. Card KR, Konstantinou EK, Shields CL.. A classification system for uveal melanoma. Rev Ophthalmol . 2022; October: 64–68, 76. [Google Scholar]
- 15. Shields CL, Furuta M, Thangappan A, et al.. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009; 127: 989–998. [DOI] [PubMed] [Google Scholar]
- 16. Mellen P, Morton S, Shields CL.. American Joint Committee on Cancer (AJCC) Staging of Uveal Melanoma. Practical application to clinical practice. Oman J Ophthalmol . 2013; 6: 116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baron ED, DiNicola M, Shields CL.. American Joint Committee on Cancer (AJCC) Classification (updated 8thedition) for posterior uveal melanoma. Retina Today . 2018; May/June: 30–34. [Google Scholar]
- 18. Shields CL, Kaliki S, Furuta M, et al.. American Joint Committee on Cancer classification of uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology . 2013; 120: 2066–2071. [DOI] [PubMed] [Google Scholar]
- 19. Shields CL, Kaliki S, Furuta M, et al.. American Joint Committee on Cancer classification of uveal melanoma (anatomic stage) predicts prognosis in 7731 patients. The 2013 Zimmerman Lecture. Ophthalmology . 2015; 122: 1180–1186. [DOI] [PubMed] [Google Scholar]
- 20. Gelmi MC, Bas Z, Malkani K, et al.. Adding The Cancer Genome Atlas chromosome classes to American Joint Committee on Cancer system offers more precise prognostication in uveal melanoma patients. Ophthalmology . 2022; 129: 431–437. [DOI] [PubMed] [Google Scholar]
- 21. Gill VT, Sabazade S, Herrspiegel C, et al.. A prognostic classification system for uveal melanoma based on a combination of patient age and sex, the American Joint Committee on Cancer and The Cancer Genome Atlas models. Acta Ophthalmol . 2023; 101: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walter SD, Chao DL, Feuer W, et al.. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol . 2016; 134: 734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai L, Paez-Escamilla M, Walter SD, et al.. Gene expression profiling and PRAME status versus tumor-node-metastasis staging for prognostication in uveal melanoma. Am J Ophthalmol. 2018; 195: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swords DS, Mulvihill SJ, Firpo MA, Scaife CL.. Causes of death and conditional survival estimates of medium- and long-term survivors of pancreatic adenocarcinoma. JAMA Oncol. 2018; 4: 1129–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas CR Jr. Conditional survival as a pragmatic resource for cancer survivors and health care professionals. JAMA Oncol. 2018; 4: 1130–1131. [DOI] [PubMed] [Google Scholar]
- 26. Kim E, Kim JS, Choi M, Thomas CR Jr.. Conditional survival in anal carcinoma using the national population-based Survey of Epidemiology and End Results Database (1988-2012). Dis Colon Rectum. 2016; 59: 291–298. [DOI] [PubMed] [Google Scholar]
- 27. Merrill RM, Bateman S.. Conditional melanoma cancer survival in the United States. Cancers. 2016; 8(2): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rueth NM, Groth SS, Tuttle TM, et al.. Conditional survival after surgical treatment of melanoma: an analysis of the Surveillance, Epidemiology, and End Results database. Ann Surg Oncol. 2010; 17: 1662–1668. [DOI] [PubMed] [Google Scholar]
- 29. Xing Y, Chang GJ, Hu CY, et al.. Conditional survival estimates improve over time for patients with advanced melanoma: results from a population-based analysis. Cancer. 2010; 116: 2234–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Leest RJ, van Steenbergen LN, Hollestein LM, et al.. Conditional survival of malignant melanoma in The Netherlands: 1994-2008. Eur J Cancer. 2014; 50: 602–610. [DOI] [PubMed] [Google Scholar]
- 31. Banerjee M, Lao CD, Wancata LM, et al.. Implications of age and conditional survival estimates for patients with melanoma. Melanoma Res. 2016; 26: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shields CL, Dockery PW, Yaghy A, et al.. Conditional analysis on new tumor formation with solitary unilateral retinoblastoma in 482 consecutive patients. Saudi J Ophthalmol. 2021; 35: 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zabor EC, Gonen M, Chapman PB, Panageas KS.. Dynamic prognostication using conditional survival estimates. Cancer. 2013; 119: 3589–3592. [DOI] [PubMed] [Google Scholar]
- 34. Zabor EC, Radivoyevitch T, Singh AD, et al.. Conditional survival in uveal melanoma. Ophthalmol Retina. 2021; 5: 536–542. [DOI] [PubMed] [Google Scholar]
- 35. Shields CL, Dockery PW, Mayro EL, et al.. Conditional survival of uveal melanoma using The Cancer Genome Atlas (TCGA) classification (simplified version) in 1001 cases. Saudi J Ophthalmol . 2022; 36: 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shields CL, Kaliki S, Furuta M, et al.. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina . 2012; 32: 1363–72. [DOI] [PubMed] [Google Scholar]
- 37. Al-Jamal RT, Cassoux N, Desjardins L, et al.. The pediatric choroidal and ciliary body melanoma study: a survey by the European Ophthalmic Oncology Group. Ophthalmology . 2016; 123: 898–907. [DOI] [PubMed] [Google Scholar]
- 38. Dogrusoz M, Brouwer NJ, de Geus SJR, et al.. Prognostic factors five years after enucleation for uveal melanoma. Invest Ophthalmol Vis Sci. 2020; 61: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]