Abstract
Diabetic macular edema (DME) is a common complication of diabetic retinopathy and is the leading cause of vision loss in diabetic patients. Various factors, such as metabolic disorders and inflammation caused by hyperglycemia, are involved in the occurrence and development of DME, but the specific mechanism is still unclear. Müller cells are a type of macroglial cell unique to the fundus, distributed throughout the retina, and they play a unique role in retinal homeostasis. This article reviews the role of Müller cells in the pathological process of DME and the research progress in the treatment of DME by targeting Müller cells through gene therapy.
Keywords: DME, Müller cells, inflammation, VEGF, gene therapy
Diabetic retinopathy (DR) is a neurovascular complication of diabetes, and it is also one of the most common fundus diseases that seriously threaten people's vision. Currently, DR is the fifth leading cause of blindness or moderate to severe visual impairment in the global working-age population.1 Approximately 463 million people were reported to have type 2 diabetes worldwide in 2019, and this number is expected to increase to 700 million by 2045, with 160 million DR patients.2 DR mainly consists of two stages: nonproliferative diabetic retinopathy, characterized by microaneurysms and bleeding points, and proliferative diabetic retinopathy (PDR), characterized by retinal neovascularization.
Diabetic macular edema (DME) is a complication of DR that can occur at any stage of DR and is mainly characterized by exudative fluid accumulation in the macula. Extracellular fluid can accumulate into a cavity-like structure, known as a “cyst”, or in the subretinal area, known as “subretinal fluid”. In addition, fluid can also accumulate inside cells, causing cell swelling. Because DME mainly occurs in the macula where photoreceptor cells are most densely distributed, most patients will have varying degrees of visual impairment. In most cases, the vision loss is reversible as the fluid in the macula is absorbed, but if the edema persists and the physiological structure of the macula is destroyed, irreversible vision loss can occur.3 Over the past decade, PDR has been controlled by the application of anti-VEGF agents, and DME has replaced PDR as the leading cause of vision loss in diabetic patients.4 Currently, the clinical treatment of DME mainly consists of retinal photocoagulation, vitrectomy, and anti-VEGF therapy, but there are still a considerable number of patients who do not respond to conventional treatment or relapse, and safer and more effective treatments are urgently needed.
Müller cells are a type of macroglia unique to the fundus and they play an important role in the stability of retinal homeostasis. At present, DR is considered to be a neurovascular disease based on neurovascular units (composed of neurons, glial cells, vascular endothelial cells, vascular smooth muscle cells, and pericytes) rather than just a microvascular complication of diabetes.5 Degenerative changes in the neurons precede the microvascular changes, and apoptosis of neurons is observed within a few weeks of the onset of diabetes.6,7 Because of the unique structure, Müller cells become the core of the neurovascular unit where exchanges of nutrients and oxygen occur, and they also play an important role in the neurons and blood vessels interaction.
Intracytoplasmic swelling of the Müller cells is regarded as an important anatomical basis for the macular edema,8 and prolonged edema promotes necrosis of Müller cells and adjacent neural cells resulting in the cystoid cavity in DME.8,9 In addition, numerous studies have shown that Müller cells also play important roles in macular drainage and the integrity of the blood retinal barrier (BRB). These results indicate the importance of Müller cells in fundus diseases, and the role of Müller cells in the pathogenesis of DME has received increasing attention. This article reviews the role of Müller cells in the pathological mechanism of DME and the progress in the development of DME treatments based on Müller cell regulation, which provides a promising avenue for the treatment of DME.
Overview of DME
DME is now the leading cause of vision loss among people with diabetes. The prevalence of DME has been reported in several population-based studies, ranging from 4.2% to 7.9% in type 1 diabetes mellitus and 1.4% to 12.8% in type 2 diabetes mellitus.4 In a latest meta-analysis, investigators estimated the global prevalence of DME in diabetic patients was 5.47% overall, 5.81% in low-to-middle-income countries, and 5.14% in high-income countries.10
DME is traditionally assessed and classified according to the presence of characteristic clinical signs. The advent of optical coherence tomography (OCT) in 1991 revolutionized the clinical evaluation of the DME,11 which provided a non-contact, non-invasive optical biopsy of the retina. The first OCT systems used time-domain (TD) technology, but originally deployed TD-OCT was limited by low axial resolution (10–15 µm) and restricted scan times.12,13 The advancement of spectral-domain OCT (SD-OCT) offered improvements in axial resolution (3–5 µm) and scan times, but the major disadvantage is the loss of resolution with increasing depths.14 Although the enhanced depth imaging protocol can be used by SD-OCT to improve visualization of the choroid, optimal and simultaneous visualization of the vitreous, retina, and choroid is not feasible.13 In the future, swept-source OCT may be widely used in clinic, which can achieve larger scan areas, faster scan times, deeper penetration, and simultaneous high-resolution imaging of the vitreous, retina, and choroid.15
Generally, OCT shows three patterns of structural changes in DME, namely, sponge-like diffuse retinal thickening, cystoid macular edema, and serous retinal detachment, among which sponge-like diffuse retinal thickening and cystoid macular edema are the most common.16 Different structural changes of DME or OCT features, as biomarkers, are associated with different visual outcomes in response to treatment.17–20 DME patients with submacular fluid, no hyper-reflective foci, and a continuous inner segment/outer segment layer responded better to dexamethasone implants than those without these features.19 Disorganization of retinal inner layers, as one more predictive biomarker on the SD-OCT for a functional and anatomical response, can help to predict response to dexamethasone implant in eyes with DME.20 And based on AI technology, deep learning (DL) systems can detect referable diabetic retinopathy and vision-threatening DR from images obtained on ultra-widefield scanning laser ophthalmoscope.21
General Knowledge of Müller in Human Retina
Spatial Distribution of Müller Cells
In 1851, German anatomist Heinrich Müller discovered a new cell type in the retina, the Müller cell.22 Müller cells are the main glial cells in the retina, accounting for 90% of retinal glial cells, and their nucleus is located in the inner nuclear layer (INL) and it is radial in shape.23 The glial processes of Müller cells extend upward to the internal limiting membrane (ILM) to form the apex and down to the outer limiting membrane (OLM) to form microvilli, almost crossing the entire thickness of the retina, coming into contact with most retinal neurons, such as cones, rods, bipolar cells, and ganglion cells.24 In the outer nuclear layer (ONL), the glial processes of Müller cells form a membrane sheath that envelops the outer nuclei of rods and cones.25 Each Müller cell is coupled to a cone cell, approximately ten rods and a variable number of intraretinal neurons, called a columnar microunit of retinal neurons, which is the smallest functional unit required for visual “forward information processing.”26 In addition, the proximity of Müller cells to the vitreous, blood vessels, and subretinal spaces illustrates the anatomical and functional connections that exist between these compartments and retinal neurons.
General Functions of Müller Cells
Maintaining the Homeostasis of Ions and Water
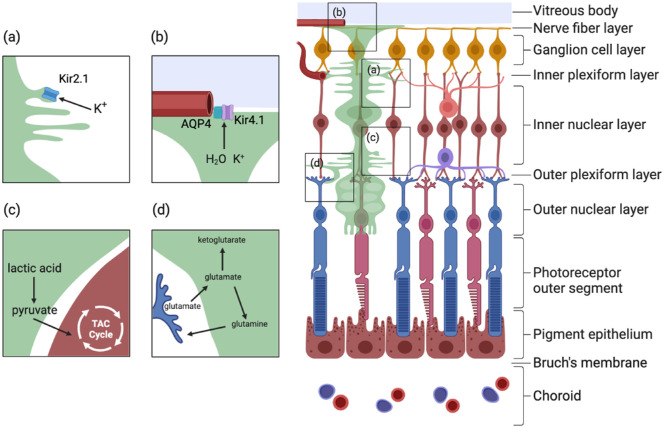
Many ion and water channels in the Müller cell membrane regulate the balance of ions and water inside and outside the cell membrane (Fig. 1). Light-induced neuronal activity causes changes in extracellular K+ concentrations, which can be buffered in three ways: Na+-K+ ATPase, cotransport of K+ with other ions such as Cl-, and inward rectification by the K+ channel (Kir).27 Among them, Kir is generally believed to play a leading role in the "potassium siphon." The Kir subtypes expressed on the cell membrane of Müller cells mainly include Kir2.1 and Kir4.1. Their localization on the membrane of Müller cells is different: the Kir2.1 channel is mainly expressed at membrane sites where glial cells and neurons can interact directly, whereas the Kir4.1 channel subunit is mainly expressed in the podium of cells facing the basement membrane (such as the soleplate membrane adjacent to the vitreous and the floor membrane surrounding the retinal blood vessels).28,29 The coordination of their functions is consistent with their distribution. In response to an increase in K+ in the intercellular space, Müller cells form a very negative membrane potential, the strongly rectified Kir channel Kir2.1 located at the site of direct contact with these neurons opens, the K+ in the intercellular space flows into the Müller cell, and the Müller cell is depolarized. After this, the weakly rectified Kir4.1 channel mediates the flow of K+ out of the Müller cell into the K+ buffer region outside the cell, and the Müller cell is renegatively polarized.28,30,31 In addition, tandem pore domain (2P domain) K+ channels can also regulate neuronal excitability, possibly playing a role in maintaining the negative polarized membrane potential of Müller cells, especially if the Kir4.1 channel is blocked or downregulated.32
Figure 1.
General functions of Müller cells. (a, b) Müller cells maintain the homeostasis of ions and water. Water and ions are transported into Müller cells mainly through Kir2.1 and aquaporin on the Müller cell membrane, which forms synapses with retinal neurons. Then, the water and ions are transported out of the Müller cells through Kir2.1 and AQP4 on the cell membrane adjacent to the vascular plexuses and vitreous cavity. (c) Lactic acid produced from glycolysis in Müller cells can be converted to pyruvate, which can be transported out of Müller cells through the transporter monocarboxylate transporter 2 (MCT2) and then into neurons as a metabolic substrate for the tricarboxylic acid cycle (TAC). (d) Retinal neurons, such as photoreceptors, release glutamate while transmitting visual signals. Müller cells can absorb these neurotransmitters and direct them into two different metabolic pathways: glutamate can be converted into glutamine through glutamate synthetase (GS) and then released out of Müller cells through sodium-coupled neutral amino acid transporter (SNAT) and finally transported back into neurons.
Aquaporin (AQP) is a membrane protein that promotes water transport across the plasma membrane along osmotic gradients, contributing to the regulation of water ions inside and outside the cell, maintaining cellular homeostasis, and regulating signal transduction in the retina.33 All 13 known mammalian aquaporins have been detected in the eye, among which AQP4, AQP9, and AQP11 were found to be expressed on Müller cells. AQP4 and the inwardly rectifying potassium channel Kir4.1 were colocalized in the ILM, and AQP6, AQP7 and AQP11 were localized to the Müller cell terminal of the ILM, where AQP6 colocalized in the ILM with Kir4.1, similar to AQP4.34,35
At present, it is mostly believed that AQP4 and Kir4.1 are colocalized and functionally coupled at the expression location, and AQP4 may be necessary for effective K+ buffering. Transient receptor potential cation channel subfamily V member 4 (TRPV4) and AQP4 also have colocalization relationships, and water inflow through AQP4 can drive calcium inflow through TRPV4 in the glial terminal foot, thereby regulating the gene expression of AQP4 and Kir4.1.36–38 Both Kir4.1 and AQP4 are associated with dystrophin–glycoprotein complex proteins in rat retinas, which may further confirm the coupling relationship between the two.31 However, it has also been reported that Kir4.1 and AQP4 only exhibit physical colocalization and are not functionally related, because the loss of AQP4 did not affect Kir4.1 function or distribution.39 One possible explanation of this finding is that the absence of AQP4 resulted in other compensatory mechanisms, resulting in no significant changes in Kir4.1.
Metabolic Coupling
Retinal cells exhibit special metabolic characteristics, and glial cells and neurons in the retina coordinate complex metabolic relationships and adjust their metabolic activities to meet their needs. Photoreceptor cells have the highest oxidative metabolic rate of all cells in the retina, which is related to their high oxygen and glucose requirements. In the physiological state, Müller cells use glycolysis as their main mechanism of glucose metabolism, that is, the Warburg effect, producing lactic acid and consuming very little oxygen.40 This metabolic mode saves oxygen for consumption by retinal neurons, especially the INL and ganglion cell layer, under normal physiological conditions. It also helps Müller cells resist hypoxic and low glucose environments.26,40 Lactic acid produced by anaerobic glycolysis in Müller cells can be converted into pyruvate and released from Müller cells by the transporter monocarboxylate transporter 2 to the surrounding neuronal cells, which use it as a metabolic substrate for the tricarboxylic acid cycle (Fig. 1).41,42 However, anaerobic glycolysis in Müller cells is not always dominant, and when subjected to persistent glucose deprivation, glycolysis in Müller cells is reduced, and mitochondrial respiration dominates43; therefore their mitochondrial function becomes particularly important when Müller cells are energy limited.
Neurotransmitter Recovery
Müller cells absorb and clear the neurotransmitters produced by nearby neurons, including glutamic acid, γ-aminobutyric acid (GABA), and glycine.26 Glutamate is the main excitatory neurotransmitter in the retina, and it is used to transmit visual signals by photoreceptors, bipolar cells, and ganglion cells (Fig. 1). The Müller cell is mainly responsible for removing excess glutamate released by retinal ganglion cells. Its primary glutamate uptake carrier is the electrogenic glutamate-aspartate transporter (GLAST), which is the main transporter for glutamate removal in the retina, and its efficient operation requires a very negative membrane potential on the membrane of the Müller cell.25,26 Glutamate clearance is also inhibited by blockage of Kir4.1 or by depolarization of Müller cells induced by opening cation channels.44 After being absorbed by Müller cells, glutamate enters different metabolic pathways: Müller cells convert glutamate into glutamine through glutamate synthetase, release it into the extracellular space through sodium-coupled neutral amino acid transporter, allowing it to be transported back to neurons as a precursor for the synthesis of glutamine and GABA.26,43 In addition, glutamic acid can be converted into ketoglutarate through glutamate dehydrogenase or transaminase, which acts as an alternative energy substrate in the case of glucose shortage.45
The γ-GABA is a major inhibitory neurotransmitter in the vertebrate retina and is found in horizontal cells, amacrine cells, ganglion cells, bipolar cells, and plexiform cells. In the outer retina, Müller cells are responsible for the removal of all GABAs, whereas GABA in the inner retina is absorbed by both Müller cells and amacrine cells.46 The uptake of GABA by Müller cells is mainly mediated by sodium- and chlorine-dependent high-affinity GABA transporters.47 GABA is then converted to succinic acid semialdehyde by GABA aminotransferase in Müller cells.46
Other Functions
It is reported that Müller cells also play important roles in many other functions. They can direct light to photoreceptors and mediate image transmission with minimal distortion and loss,48,49 help retina withstand mechanical stress as soft embedded parts and trigger intracellular molecular reactions in Müller cells as mechanical sensors.23,25,50–52 These functions have been reviewed in detail in the literature in recent years, and we will not repeat them here because of the limitation of space.23,25,48–52
Peculiarities of Müller Cells in the Macular Area
Anatomical Features of Müller Cells in the Macular Region
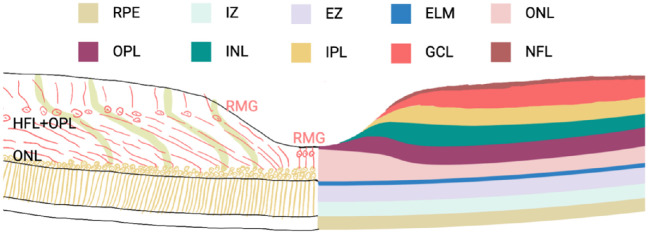
The macula, or fovea, is a special region in primates located at the posterior pole of the retina with the highest density of cones, and the Müller cells in the macula exhibit unique morphological characteristics. There are two different kinds of Müller cells in the fovea, which have different morphological and functional roles (Fig. 2). The most central part contains the “Müller cell cone” formed in the fovea by 25-35 specialized Müller cells, an inverted tapered structure that covers the outside of the fovea.26 The nuclei of these Müller cells are oval in shape, and they extend straight from the OLM to the ILM.53 The extracellular processes of these Müller cells do not leave the fovea and do not join the Henle fiber layer (HFL). Except for cone cells, they do not come into contact with neurons like the typical Muller cell to form functional columns with photoreceptors and neurons.53,54 In fact, these Müller cells do not have any photoreceptor cell processes except when their tip is close to the ILM.55 The other type of Müller cells are located in the foveal wall and parafovea, and their extracellular processes surround the foveal cones. The nuclei of these Müller cells are polygonal, and their overall shape has a characteristic “Z” shape: they extend vertically from the end of the ILM to the outer plexiform layer (OPL), then inward along the HFL in an oblique or horizontal direction toward the foveal ONL, and then vertically to the OLM.54 The HFL is also formed by these cellular processes and the axons of photoreceptor cells. In the HFL, these cell processes are tightly bound to photosensitive axons through connexins such as ZO-1.56
Figure 2.
Two types of Müller cells in the fovea. There are two types of Müller cells in the fovea: specialized Müller cells in the foveola and Müller cells in the foveal walls, which have a characteristic “Z” shape. Müller cells in the foveola are also called Müller cell cones, which run almost straight from the OLM to the ILM. Müller cells in the foveal walls are highly elongated compared to peripheral Müller cells. They run vertically from their foot process in the ILM to the OPL through the Henle fiber layer inward toward the center of the fovea of the retina, where they reach the fovea of the retina almost horizontally and then extend again vertically toward the OLM, forming a characteristic “Z” shape. In this process, obliqued Müller cells and photoreceptor axons constitute the HFL, which is the foveal portion of the OPL. IZ, interdigitation zone; EZ, ellipsoid zone; ELM, external limiting membrane; GCL, ganglion cell layer; NFL, nerve fiber layer.
Functional Characteristics of Müller Cells in the Macular Area
Foveal Müller cells also have different functional characteristics than the typical Müller cells of the peripheral retina. Because the lining of the retina in the fovea is very thin, special structural stabilization support may be needed. In general, the retina is mechanically stabilized by a network of large glia, consisting of processes of Müller cells and astrocytes.57 Near the ILM, the Müller cell cone has many thin internal processes, forming a complex network of lamellar and tubular processes that extend horizontally below the basal lamina and cover the vitreous surface of the fovea.54 This structure, which extends all the way to the transition zone between the fovea and foveal slopes, can compensate for the thinness of the ILM basal layer, increase resistance to mechanical stretching caused by horizontal and vertical traction forces, and may also act as a barrier against additional diffusion of the vitreous cavity and cell channels.53 It has also been reported that the protruding structure of this thin layer of Müller cells smooths the inner surface of the fovea, thereby minimizing image distortion in areas of highly sensitive vision.54
The structural stability of the outer foveal layer (HFL, ONL) is also thought to be provided mainly by the outer processes of the foveal wall and parafoveal Miller cells, which surround the fibers and soma of photoreceptor cells, together with photoreceptor cells constituting the OLM and cobending centripetally with oblique or curved photoreceptor cell processes. In addition, the columnar direction of the cell soma in the ganglion cell layer and INL is roughly parallel to the main Müller cell trend in the inner plexiform layer (IPL), indicating that these cell columns are arranged and mechanically stabilized by Müller cells.53,58
In addition, unlike the typical peripheral Müller cell, which is responsible for all functional and metabolic interactions with the photoreceptors and neurons in the cell column, the foveal Müller cell cone may not be involved in photosensitive functions.54 Müller cells in the macular area also contain a high density of macular pigment.59 Macular and peripheral Müller cells express some proteins differently: both macular and peripheral Müller cells express CD117, but only peripheral Müller cells express CD44. Macular Müller cells also express more AQP4 than peripheral Müller cells, which may help the macula better regulate the balance of water.56,60
In an in vitro experiment, researchers found that macular Müller cells expressed higher levels of key enzymes involved in multiple metabolic pathways, such as phosphoglycerate dehydrogenase, than peripheral Müller cells. They also have significantly higher basal mitochondrial respiration and ATP production capacity, more active serine synthesis levels, and higher glycolytic capacity and reserves.61
Pathological Mechanism of DME—Why Müller Cells Need Our Attention
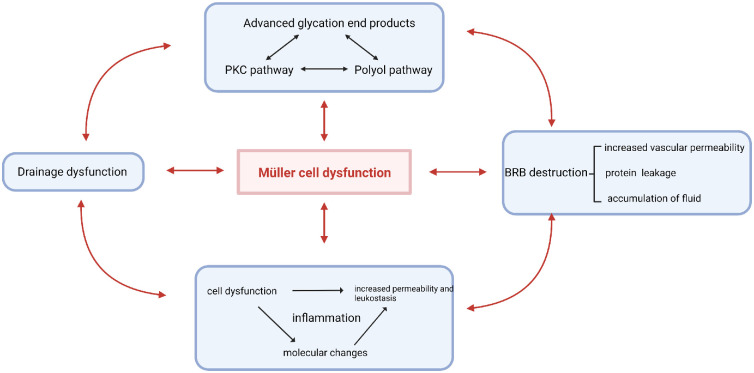
Müller cells play an important role in the progression of DME. The initial metabolic abnormalities caused by hyperglycemia occur in a variety of retinal cells, especially in Müller cells. Subsequently, because of their structural and functional particularities, which we discuss above, Müller cells exacerbate metabolic abnormalities in turn and then participate in other pathological mechanisms, such as drainage dysfunction, BRB destruction and inflammation, ultimately contributing to the formation of the complex and interactive pathological mechanistic network of DME (Fig. 3).
Figure 3.
Pathological mechanisms of DME Diabetic metabolic abnormalities, drainage dysfunction, BRB disruption and inflammation are all associated with Müller cell dysfunction and are vital pathological mechanisms of DME.
Metabolic Abnormalities in DME
AGE Pathway.
Advanced glycation end products (AGEs) accumulate at chronically high glycemic levels in diabetic patients and play an important role in the pathogenesis of DME. Additional oxidation and dehydration reactions in the process of generating AGEs, also known as the Maillard reaction, lead to the formation of irreversible protein-bound AGEs.62,63 An increase in AGE levels has been observed on Spectral Domain Optical Coherence Tomography (SD-OCT) to be significantly associated with disruption of the subfoveal ellipsoid region in DME.64 AGEs can activate white blood cells and increase the production of inflammatory cytokines and chemokines, which in turn leads to the recruitment and adhesion of more inflammatory cells.65 In addition, RAGE is expressed more on cells such as pericytes, microglia, Müller cells and retinal pigment epithelium (RPE) cells in diabetics, and when AGEs bind to these RAGEs, they can activate intracellular signaling pathways such as Ras, MAPK and NF-κB, triggering inflammatory responses.66 AGEs can induce the expression of the VEGF gene, which causes disruption of the blood retinal barrier.67 In addition, the accumulation of AGEs can also lead to the loss of peripheral cells within retinal blood vessels,68 impairment of endothelial cell function69 and the generation of new blood vessels.70
Polyol Pathway.
In diabetics, the hexokinase pathway becomes saturated, and excess glucose enters the polyol pathway. The polyol pathway leads to a decrease in the concentration of NADPH and NAD+, both of which are required for glutathione regeneration, and therefore leads to a decrease in glutathione, which in turn leads to oxidative damage.71 In addition, the decrease in glutathione increases the activity of aldose reductase, which induces the polyol pathway, and aldose reductase also reduces the expression of certain glutathione regulatory genes, ultimately leading to an interaction between the polyol pathway and oxidative damage.72 Sorbitol produced by the polyol pathway also has a damaging effect on the retina. It is highly hydrophilic and does not easily penetrate the cell membrane, so it accumulates in the cells, drawing in excessive intracellular water and causing damage. It also saccharifies nitrogen in protein molecules, thereby promoting the production of AGEs.73,74
PKC Pathway.
The protein kinase C (PKC) isoenzyme family translates multiple signals produced by receptor-mediated phospholipid hydrolysis and plays a key role in a variety of cellular functions. Activation of certain PKC subtypes (mainly α, β1, β2 and δ subtypes) is involved in microangiopathy in diabetic animals.75 Both hyperglycemia and oxidative pathways can lead to the formation of diacylglycerol (DAG) and subsequent activation of PKC subtypes, and increased activation of the DAG-PKC pathway has also been observed in retinal tissue in diabetic patients.76,77 Activated PKC pathways cause retinal vascular dysfunction, decreased blood flow, neoangiogenesis, leukocyte adhesion, and pericyte loss.78 Activation of PKC pathways can also directly or indirectly affect vascular permeability; for example, activated PKC can phosphorylate cytoskeletal proteins (caldesmon, vimentin, talin and vinculin), directly affecting cell permeability, and it can also indirectly affect it by regulating the activation and expression of various growth factors (VEGF, VPF).79
Müller Cells and Metabolic Abnormalities.
The abnormal metabolic pathways induced by diabetes can cause Müller cell dysfunction, resulting in pathological reactions such as oxidative stress, inflammatory responses, and disruption of the water-fluid balance. AGEs may be an important factor in retinal Müller cell dysfunction.80 Diabetes leads to an increase in intraretinal AGEs and the upregulation of RAGEs and S100B (S100 calcium-binding protein B) by Müller cells, which is also accompanied by an increase in glial fibrillary acidic protein (GFAP) within Müller cells.81 Müller cells are one of the main sources of S100B, and S100B may bind to RAGE and cause downstream reactions such as glial hyperplasia and inflammatory factor production.82–84 AGE modification of lamin α-dystroglycan, expressed on Müller cells, can also directly and negatively affect the function of Kir4.1 in Müller cells, leading to disturbances in the ion- and water-fluid balance within the retina.85 Studies have shown that an increase in the formation of AGEs in the vitreous may promote the production of bFGF by Müller cells, which in association with VEGF synergistically promotes the formation and development of new blood vessels in the retina.86 In addition, AGEs also cause Müller cells to produce various inflammatory factors, such as VEGF and IL-6.87,88
Other metabolic pathways are also closely related to Müller cells. Aldose reductase expression by Müller cells, apoptosis of nerve cells in diabetic retinas and changes in GFAP expression may be mediated by increased activity of polyol pathways in Müller cells, leading to the accumulation of damaging products such as sorbate.89,90 PKC activation can reduce the expression of GLAST (glutamate/aspartate transporter) on the surface of Müller cells and affect the transport activity of GLAST.91 It can induce Müller cells to secrete phosphorylated acyl coenzyme A-binding protein, which in turn affects GABA transmission in nearby retinal neurons.92 Müller cells can express TIMP (MMP inhibitors), which are also regulated by PKC.93
Drainage Dysfunction
Structural Susceptibility in the Macular Area.
Structural characteristics of the macular area contribute to its susceptibility to edema. To obtain a more sensitive photoreceptor function, the ganglion cells in the macular region highly express anti-angiogenic pigment epithelial derivatives, resulting in no large-diameter blood vessels in the macula region and almost no vessels in the foveola.94 This structure prevents water from the macular region from being drained through the blood vessels. Most likely as compensation for this pathway loss, the density of Müller cells in the fovea is approximately 5 times higher than that in the rest of the retina, and more water and solutes flow out through the glymphatic pathway of Müller cells.95 If macular Müller cells are dysfunctional, drainage of the macular area will be impaired. In addition, because the Müller cells in the macular region have the structural particularity mentioned in Anatomical Features of Müller Cells in the Macular Region (the Müller cells in the macular region consist of two parts: The inverted tapered Müller cell cone in the fovea and the other type in the foveal wall and parafovea), when connexins (such as ZO-1) and the stability of this structure are destroyed, proteins accumulate between the photoreceptor cell axon and the Z-shaped Müller cell and subsequently absorb fluid, leading to the formation of cysts.56
Dysfunction of Ion and Water Channels in Müller Cells
Physiologically, water is mainly drained by conjugated inward rectification of K+ channel 4.1 (Kir4.1) and aquaporin 4 (AQP4) on the Müller cell cellular membrane, which is mediated by β-1 synthesis protein and dystrophin 71 (DP71).96 Dysfunction of these channels and proteins is involved in the pathogenesis of DME. Decreased expression of Kir4.1 and AQP4 in the retinas of diabetic rats may be a sign of dysfunction of drainage, resulting in swelling of the apical process of Müller cells and the formation of intracellular edema.56,97 The loss of DP71 leads to the downregulation of Kir4.1 and AQP4, which impairs the volume regulatory ability of Müller cells and leads to impaired BRB function.98
There are changes in AQP1 and AQP4 expression in diabetic mice, and the study of AQP4-knockout mice also showed that the channel plays a role in retinal signaling and retinal swelling after injury.99 In fact, other types of water ion channels, such as AQP5 and AQP9, have altered expression in diabetic patients, suggesting complex patterns of AQP expression in response to diabetes, which may involve the adaptation of retinal cells to hyperglycemic conditions and the development of retinal edema.100 As diabetes progresses, the expression of AQP11, mainly located at the end of the Müller cell, is also downregulated by miRNA (HIF-1α-dependent or nondependent), resulting in edema, and overexpression of AQP11 can partially reverse Müller cell edema.101 However, some studies have found that the expression of AQP4 in the ILM in diabetic retinal patients is significantly higher than that in patients without diabetic retinopathy, and there is a positive correlation between AQP4 and VEGF expression in the ILM in patients with diabetic retinopathy.102 This increase in AQP4 and VEGF may be due to oxidative damage and large amounts of ROS in the retinas of diabetic patients, along with an increase in retinal thickness and GFAP expression.103
Intracellular edema and liquefaction necrosis of Müller cells play an important role in cavernous retinal swelling and macular cystic edema.104,105 Dysfunction of ion and water channels first leads to the Müller cell's intracellular toxic edema, which in turn leads to extracellular edema and neuronal toxicity, manifested by the formation of a space of hypo-reflective cystoid edema within the retina that can be observed by optical coherence tomography angiography.56,106 The process of extracellular water penetration might begin with swelling of the INL layer located between two "fluid conductivity barriers" (OPL and IPL) and then penetrate into the loose parts of the fibers of the OPL and IPL, such as the Henle layer of the OPL, which may affect the structural stability of the macular area.107 Whether Müller cells play a major role in the initial swelling of the INL layer needs to be investigated further. In particular, the pyramid structure of the Müller cells in the fovea is also destroyed during this process, with the participation of VEGF.108
Müller Cell Dysfunction and BRB Destruction
BRB Disruption in DME
The BRB is a barrier between the peripheral circulation and the ocular microenvironment, including the inner BRB (iBRB) and the outer blood retinal barrier. The iBRB is mainly composed of endothelial cells and pericytes. In addition, the specialized feet of Müller cells attach to the retinal capillary wall, participating in the formation of the iBRB. The outer BRB is mainly composed of RPE cells and the tight connections between them. In the physiological state, the BRB can prevent proteins and cells in the peripheral circulation from entering the retina, maintaining the "dryness" of the retina and the stability of its structure and function. In diabetic patients, hyperglycemia leads to the rupture of cell‒cell junctions, pericyte loss, and thickening of the basement membrane of BRB,109 which contribute to increased vascular permeability, allowing intravascular fluid and protein component leakage into tissues. Some proteins enter the vitreous cavity and subretinal space, but most proteins accumulate in the retina, resulting in a hypertonic state of the retina, and the accumulation of fluid eventually leads to macular edema.110
Müller Cells’ Role in BRB Formation and Maintenance
As important glial cells in the retina, Müller cells play a vital role in BRB formation and maintenance. Injection of Müller cells from the rabbit retina into the anterior chamber of the rat eye can induce vascular endothelial cell formation, indicating that Müller cells are an important factor in inducing BRB formation.111 The retinal vessels in the superficial vascular plexus are surrounded by Müller cells and astrocytes, while the retinal vessels of the inner plexus and deep plexus are only wrapped by Müller cells.112 Müller cells can induce barrier properties in the deeper plexus.113 In addition, ablation of Müller cells in mice has been found to lead to BRB rupture and deep-retina neovascularization.112
Müller cells can also directly or indirectly maintain the permeability of the iBRB. First, endothelial cell permeability is affected by Müller cells. Under normoxic conditions, the barrier function of endothelial cells is enhanced by cocultured Müller cells. However, under hypoxic conditions, after coculture with Müller cells, endothelial cell barrier function is significantly impaired, which indicates that pathological changes in early diabetes can lead to Müller cell dysfunction, thereby affecting the barrier function of endothelial cells.114 In addition, cytokines produced by Müller cells indirectly mediate the maintenance of BRB permeability, for example, the pigment epithelium–derived factor (PEDF). PEDF can inhibit VEGF-induced retinal vascular permeability through multiple mechanisms. PEDF prevents the dissociation of adherens junction and tight junction proteins and regulates the binding of VEGF receptors to adherens junction proteins. PEDF also prevents phosphorylation of the adherens junction proteins VE-cadherin and β-catenin, thereby inhibiting VEGF-mediated increased permeability.115
Hyperglycemia Mediated Müller Cells’ Dysfunction in BRB Destruction
Müller cells play an important role in the destruction of the BRB. In one study, a Müller-specific adeno-associated virus (AAV) variant was used to ablate Müller cells specifically, which induced retinal remodeling and vascular leakage.116 In another study, upregulation of VEGF and downregulation of claudin-5 expression were observed after intraocular injection of DL-alpha-aminoadipic in rats, which has a toxic effect on Müller cells. These changes increase the BRB permeability, indicating that Müller depletion leads to BRB rupture, which is a significant pathological change in DME.117
Abnormal expression of cytokines produced by Müller cells also mediates the destruction of the BRB. Normally, glial fibrillary acidic protein (GFAP) is mainly expressed by retinal astrocytes, not Müller cells. In diabetic patients, Müller cells have high GFAP expression. GFAP in the aqueous humor of DR patients is higher than that in healthy people. GFAP is a characteristic molecular marker of Müller injury and it can directly cause neuronal and vascular damage.118 Müller cell–derived VEGF can reduce the expression of occludin and ZO-1,119 and anti-VEGF therapy prevents BRB breakdown in diabetic animals.112 MEK/ERK/RUNX2/IL-11 pathway was activated in Müller cells under high glucose condition. IL-11 derived from hyperglycemia-exposed Müller cells bound to IL-11RA on Müller cells can promote Müller cells’ activation and proliferation and the breakdown of BRB in mouse STZ-induced diabetic model.120
Müller cells can also mediate pericyte and its own apoptosis through Connexin43 (Cx43) destruction. Cx43 is widely present on the protrusions at the apex of Müller cells, and Cx43 expression is reduced in Müller cells when they are exposed to high glucose conditions, inhibiting gap junction intercellular communication and leading to inactivation of protein kinase B, inducing apoptosis of Müller cells. High glucose also induces downregulation of Cx43 in Müller cells cultured with pericytes, destruction of the gap junction intercellular communication function, and ultimately apoptosis of Müller cells and pericytes. Müller cell specialized feet and pericytes are important components of the iBRB, whose apoptosis may be associated with BRB destruction.121
The Role of Inflammation in DME
Müller Cell-Derived Inflammation in DME.
Inflammation plays a key role in the pathogenesis of DME. A meta-analysis suggests that increased VEGF, IL-6, IL-8, and MCP-1 levels in aqueous humor are associated with the risk of DME.122 This cytokine imbalance begins with subclinical DR and increases as retinopathy progresses.78 Müller cells are an important source of VEGF and damaging cytokines such as IL-6 and TNF-α, which may be an important cause of the chronic inflammatory state in the diabetic retina.119
Inflammation-mediated leukostasis and increased vascular permeability are important pathological changes in DME. Under high glucose conditions, VEGF expression is upregulated, and by binding to leukocyte surface receptors, leukocyte recruitment to the site of inflammation occurs. At the same time, with the upregulation of ICAM-1, leukostasis increases, and adherent leukocytes directly induce capillary endothelial cell death, causing vascular leakage,123 which leads to protein accumulation in retinal tissue, creating a hyperosmolar state. Increased vascular permeability is another important inflammation-mediated pathological change in DME. Upregulation of VEGF leads to an increase in vascular permeability. TNF-α and IL-6 can disrupt cellular tight junctions via the protein kinase C or STAT3 pathways and increase retinal endothelial cell permeability.124 As a result of the formation of intraretinal hyperosmolar states and increased vascular permeability, fluid accumulates in large quantities, eventually leading to macular edema.123
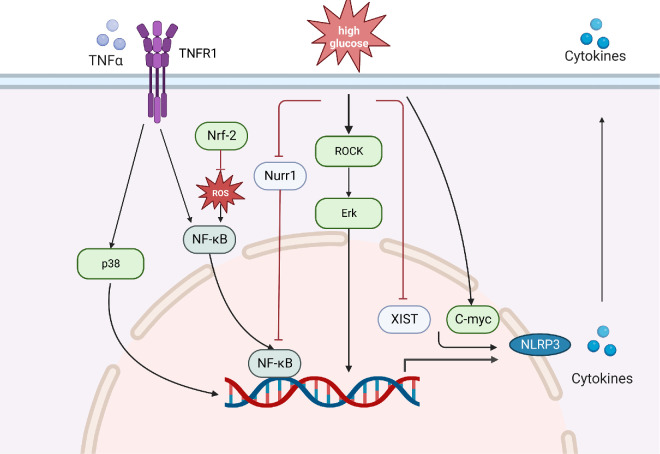
Upstream mechanisms that trigger Müller cell activation and cytokine release include oxidative stress, the NF-κB/NLRP3 pathway, TNFR1/NF-κB, TNFR1/MAPK, ROCK/Erk and other proinflammatory pathways (Fig. 4). NF-κB is a well-known modulator of inflammation that upregulates early inflammation in Müller cells when exposed to high glucose. An increase in ROS can activate the NF-κB pathway, while nuclear factor erythroid 2-related factor 2, as a regulator of intracellular antioxidant defense, can increase the antioxidant capacity of Müller cells to block the NF-κB pathway.119 The transcription factor nuclear receptor subfamily 4 Group A member 2 (Nurr1) inhibits the inflammatory response by inhibiting NF-κB and NLRP3. Nurr1 expression and nuclear translocation are decreased in Müller cells exposed to high glucose in vitro, which leads to activation of the p65 subunit of NF-κB. The interaction of p65 with the NLRP3 inflammasome promoter promotes its upregulation, indicating that the loss of Nurr1 function in Müller cells during DR regulates the inflammatory response through the NF-κB/NLRP3 pathway.125
Figure 4.
Role of Müller cells in inflammation. Müller cells upregulate cytokines during inflammation through oxidative stress, NF-κB/NLRP3, TNFR1/NF-κB, TNFR1/MAPK, ROCK/Erk and other proinflammatory pathways. When they are exposed to high glucose, the expression and nuclear translocation of Nurr1 decreases, leading to the activation of NF-κB, thus contributing to the upregulation of proinflammatory cytokines and NLRP3. Nrf-2 can reduce the role ROS plays in activating the NF-κB pathway. TNF-α binds to TNFR and activates the NF-κB and MAPK pathways. Additionally, high glucose activates the Erk pathway through the activation of ROCK. Downregulation of XIST and upregulation of C-myc are observed in DR, and they contribute to the production of pro-inflammatory factors by Müller cells. Nrf-2, nuclear factor erythroid 2-related factor 2; ROCK, Rho-associated kinase.
In addition, microglia-derived TNF-α increases the expression of proinflammatory factors such as GFAP, TNF-α, IL-1β and other proinflammatory factors derived from Müller cells by activating the TNFR1/NF-κB pathway. Anti-TNFR1 therapy inhibits the upregulation of proinflammatory factors by reducing NF-κB p65 and MAPK p38 phosphorylation.126 Furthermore, hyperglycemia induces activation of Rho-associated kinase to increase Erk pathway signaling and upregulate the inflammatory response.119
The expression of some key genes in Müller cells also affects the occurrence of the inflammatory response. For example, high expression of C-myc in Müller cells is a risk factor for inflammation caused by high glucose, while high expression of X-inactive specific transcript (XIST), a long noncoding RNA (lncRNA), is a protective factor. Physiologically, XIST promotes SIRT1 expression by interacting with SIRT1 and by inhibiting the ubiquitination of SIRT1, ultimately inhibiting the production of proinflammatory factors by Müller cells. However, downregulation of XIST is observed in DR mouse retinal tissue and high glucose-induced human retinal Müller cell lines, indicating that XIST is a protective factor that inhibits retinal inflammation.127 Conversely, C-myc expression in Müller cells in a high-glucose environment is upregulated much earlier than IL-1β, IL-6, and TNF-α. Silencing C-myc in Müller cells inhibits cytokine upregulation, suggesting that this transcription factor initiates the Müller cell inflammatory response in diabetes.119
Targeting these upstream mechanisms and the downstream pathways activated by Müller cells may provide new therapeutic opportunities for the treatment of DME. Later, we will discuss the role Müller cell-derived inflammatory factors play in leukostasis and increased vascular permeability.
Müller Cells’ Contribution to Vascular Permeability.
VEGF, IL-1β, TNF-α and IL-6 are among the most studied Müller cell–derived inflammatory factors in increasing vascular permeability. Müller cells are the main cells producing VEGF, and Müller cell-derived VEGF is involved in important pathological processes in the early stages of DR. Conditional knockout of VEGF in Müller cells reduces the total VEGF in the retina of diabetic animal models by nearly 50%,112 and very low levels of inflammation were observed in the retina of these conditional VEGF knockout rats with diabetes. VEGF production cannot be compensated by other retinal VEGF-producing cells, indicating that Müller cells are a vital source of VEGF in DR.128 As a well-studied regulator in promoting vascular permeability, when VEGF upregulates in DME patients, it promotes the proliferation of vascular endothelial cells, increases capillary permeability, and promotes leukostasis. VEGF can induce the phosphorylation of connexins such as occludin and VE-cadherin by promoting PKC activation, prompting these proteins to translocate from cell‒cell barriers to intracellular compartments.129
TNF-α released from Müller cells under high-glucose conditions increases mitophagy and apoptosis in RPE cells.130 TNF-α also influences RPE permeability. In rodent RPE cells, TNF-α directly affects the distribution of ZO-1. TNF-α-induced permeability in human embryonic stem cell–derived RPE cells is associated with the upregulation of MMP1, MMP2, and MMP9 expression.115 As a factor that can be produced by Müller cells, TNF-α disrupts cellular tight junctions through the PKCζ/NF-κB pathway, reduces the expression of ZO-1 and claudin-5, and increases retinal endothelial cell permeability.124 Diabetes-induced increases in TNF-α can mediate apoptosis in retinal microvascular cells in DR,131 and TNF-α also mediates endothelial cell necrosis.115
It was reported that the mRNA level of IL-6 in Müller cells was increased under high glucose conditions.132 As a factor that can be produced by Müller cells, IL-6 can alter vascular permeability by altering junction integrity and rearranging actin filaments to create gap junctions between cells.133 IL-6 also induces STAT3 activation to reduce the expression of the tight junction proteins ZO-1 and occludin.129 In clinical studies, the IL-6 antibody EBI-031 can inhibit all downstream signaling pathways of IL-6,134 indicating that targeting IL-6 has a certain therapeutic significance in clinical practice.
IL-1β is also important in the pathogenesis of increased vascular permeability. It was demonstrated that high glucose does not significantly induce IL-1β expression in Müller cells. However, IL-1β produced by other cells was reported to induce its own synthesis in Müller cells by paracrine.135 High glucose–induced GAPDH nuclear accumulation in Müller cells was mediated by IL-1β, which is a reliable indicator for early cell death and inhibition of GAPDH nuclear accumulation may be necessary to restore proper retinal function. Interestingly, IL-6 plays a protective role in high glucose and IL-1β-induced GAPDH accumulation. Those cytokines balance might contribute to cellular damage under hyperglycemia.136 Also, both IL-6 and IL-1β can induce the production of other cytokines and growth factors to increase vascular permeability.137 In the eyes of lenses-overexpressing IL-1β mice, VEGF upregulation occurs, and early spikes of its expression are accompanied by BRB decomposition.115
Müller Cells’ Contribution in Leukostasis
ICAM-1 is a key factor that mediates retinal leukostasis. High glucose leads to up-regulation of ICAM-1 in Müller cells, which is proved to be regulated by NF-κB activation.112 The administration of CD-18 or ICAM-1 antibodies inhibits retinal leukostasis and BRB breakdown.138 In a diabetic rat model, blocking the ICAM-1/lymphocyte function-associated antigen 1 axis prevents retinal leukostasis and protects the BRB integrity.133
IL-8 is recognized as an important chemoattractant for neutrophils and monocytes. IL-8 turned out to upregulate in high glucose challenged Müller cells through NF-κB signaling which is regulated by a CamKII-proteasome axis.139 The upregulation of IL-8 in the aqueous humor and vitreous in DME patients may indicate that IL-8 recruits neutrophils and monocytes into the vitreous to promote intraocular neovascularization.129 Targeting signaling pathways like NF-kB may reduce Müller cell-derived ICAM-1 and IL-8 production and leukostasis, thus improving retinal function in DME.
Research Progress in Regulating Müller Cells for the Treatment of DME
Over the past 40 years, we have witnessed the rapid development of DME treatment. From the inevitable vision loss to the ongoing pursuit of long-term vision improvement, large-scale clinical trials have laid the foundation for today's DME treatments, with laser, vitrectomy, and anti-VEGF agents as the primary means. Although the prognosis of DME has improved significantly, nonresponse to conventional therapies, recurrence and long-term vision loss are still troubling us, and the exploration of new disease management methods and the discovery of new therapeutic targets are necessary. Müller cells are unique retinal glial cells, and their role in the pathogenesis of DME has received increasing attention. The rapid development of gene therapy, especially the emergence of gene editing technology, has led to the development of treatments targeting Müller cells. Here, we review the development history and current status of DME therapy and the research progress in treating DME by targeting Müller cells.
History and Current Status of Treatment of DME
Before the 1980s, there was no standardized treatment for DME, and the control of DME mainly depended on blood glucose management. In the mid-1980s, the Early Treatment Diabetic Retinopathy Study demonstrated the effectiveness of retinal photocoagulation for the treatment of DME,140 which greatly improved the success of treatment. However, although retinal photocoagulation could reduce the incidence of vision loss and improve visual acuity in the short term, its five-year outcomes did not meet people's expectations, and adverse effects on visual acuity and the visual field were also observed,141 which forced researchers to find safer and more effective treatments.
One such method was vitrectomy. Studies have found that the vitreous plays an important role in the formation and deterioration of DME,142 and vitrectomy helps slow the progression of the disease and the recovery of visual acuity.143-145 However, vitrectomy is only indicated for patients with severe DME, and it is associated with complications such as cataracts and retinal detachment, and more importantly, recurrence of DME is common.146
At the beginning of the twenty-first century, pharmacologic therapy attracted attention. Considering the role of inflammatory mechanisms in the pathogenesis of DME, large-scale clinical trials of corticosteroids and anti-VEGF agents have been launched.147 However, corticosteroids such as triamcinolone, which were highly anticipated, did not meet people's expectations. In the DRCR.net protocol I study, although the triamcinolone group had a significant effect with reduced central macular thickness (CMT) compared to the sham group, the improvement in visual acuity was not significant. In addition, the incidence of complications such as elevated intraocular pressure and cataracts was high,148 which further limited the clinical use of corticosteroids.
In contrast, anti-VEGF agents such as ranibizumab showed obvious effects in reducing CMT and improving best-corrected visual acuity, and no obvious adverse events were observed.148 The subsequent encouraging results from RISE/RIDE and VIVID/VISTA led to the approval of ranibizumab and aflibercept for the treatment of DME by the U.S. Food and Drug Administration in 2012 and 2014, respectively.149-151 Since then, anti-VEGF agents have become the first-line drugs for the treatment of DME. The advent of anti-VEGF agents revolutionized the treatment of DME, and to date, anti-VEGF agents continue to play an irreplaceable role in clinical practice.
However, the latest report from DRCR.net shows that the five-year outcomes of anti-VEGF agents are not ideal.152 With the intravitreal injection of ranibizumab, bevacizumab, and aflibercept, the mean VA of DME patients improved from baseline by 7.4 letters at two years but decreased by 4.7 letters between two and five years.152 This result forces us to rethink the treatment of DME. In addition to exploring the different management approaches of anti-VEGF agents, searching for other possible therapeutic modalities is essential.
In recent years, intravitreal dexamethasone implants have been shown to be effective in the treatment of naïve DME as well as in eyes nonresponding to anti-VEGF agents.153-155 A retrospective study of intravitreal dexamethasone implants (Ozurdex) by The European DME Registrar Study showed that with a mean follow-up of 1.7 ± 0.8 years, 22.7% achieved ≥15 letters and 37.8% ≥10 letters.156 Another real-world study assessed the effectiveness and safety of intravitreal dexamethasone implants for the treatment of DME conducted in Latin America, and the median best-corrected visual acuity was 50 letters at baseline and 70 letters after treatment.157 At the same time, it is also necessary to consider the effect of corticosteroid-induced increases in intraocular pressure and cataracts on long-term visual acuity, so the effect of corticosteroids is worth considering if the associated side effects can be effectively controlled. However, large-scale clinical studies on the treatment of DME with corticosteroids are relatively few, and comparisons between anti-VEGF drugs and corticosteroids are lacking, so the application of corticosteroids in DME still needs to be investigated.158
Müller Cell–Related Potential Therapeutic Targets for DME
The important role of Müller cells in the production of VEGF and other fundus inflammatory factors has been widely confirmed. In recent years, many new mechanisms of Müller cells involved in DME have been revealed (Fig. 5), and these findings may provide new targets and ideas for the treatment of DME.
Figure 5.
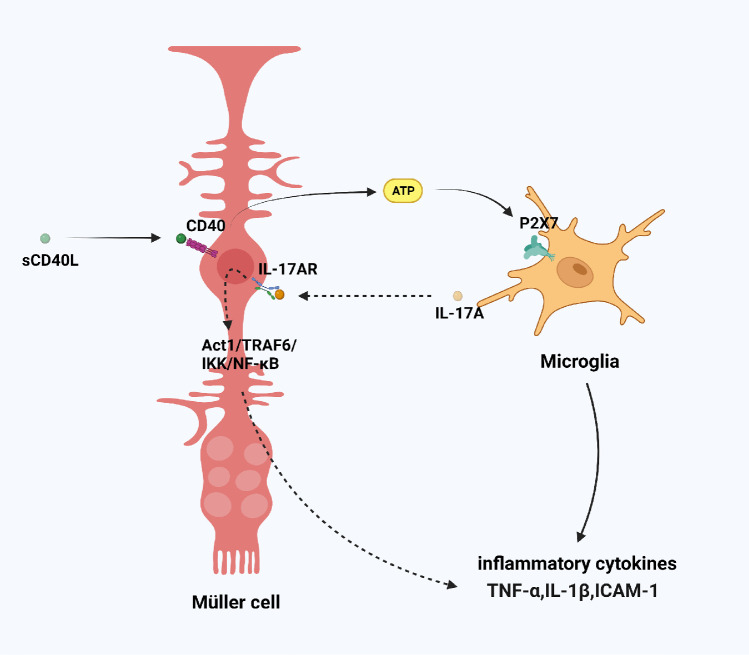
Müller cells and microglia interaction in DME. CD40L could combine with CD40 on the surface of Müller cells to promote ATP release, leading to the activation of P2X7 purinergic receptors on retinal microglia and their subsequent expression of inflammatory cytokines. Microglia produced IL-17A and IL-17A bound to IL-17AR on Müller cells, which activated the Act1/TRAF6/IKK/NF-κB signaling pathway in Müller cells and amplified the inflammatory response in the fundus. sCD40L, circulating CD40 ligand; IL-17AR, IL-17A receptor; Act1, NF-κB activator 1; IKK, NF-κB kinase.
CD40 is an immune-related protein that plays an important role in immune regulation by binding to its ligand CD40L. A significant association was found between circulating CD40 ligand levels in plasma and the severity of DR.159 Portillo et al.160 found that CD40 was expressed in retinal endothelial cells, microglia, ganglion cells, and Müller cells, and immunohistochemistry and flow cytometry studies to assess protein expression revealed that CD40 is upregulated in these cells of diabetic mice.161 In addition, germline deletion of the CD40 gene blocked ICAM1 expression, leukostasis, and acellular capillaries in the retinas of diabetic mice.161 These findings indicate that the CD40-CD40L pathway is activated in diabetes. In subsequent studies, they found that CD40L could combine with CD40 on the surface of Müller cells to promote ATP release, leading to the activation of P2X7 purinergic receptors on retinal microglia and their subsequent expression of inflammatory cytokines.162 Contrary to the conventional view, they believe that Müller cells are the first responders to trigger retinal neurovascular disease in diabetic patients. Targeting signaling pathways downstream of CD40 may represent an alternative approach to inhibit CD40-driven Müller cells activation in diabetic patients. CD40 functions by recruiting TNF Receptor Associated Factors (TRAF) to its TRAF2,3 or TRAF6 binding sites.163 Considering the important role of TRAF6 in dendritic cell maturation and development.164 their recent study applied a peptide that disrupts CD40–TRAF2,3 signaling, effectively reduced retinal expression of inflammatory molecules and reduced leukostasis in diabetic mice.165
IL-17A is a proinflammatory cytokine and has been reported to be involved in many inflammatory diseases. Studies have found that IL-17A levels in peripheral blood and vitreous in DR patients are significantly higher than that in healthy people, and the DR-mediated IL-17A receptor is mainly expressed in Müller cells.166,167 Further studies found that IL-17A activated the NF-κB activator 1/TRAF6/NF-κB kinase/NF-κB signaling pathway in Müller cells and amplified the inflammatory response in the fundus, which in turn led to the destruction of the blood retinal barrier and neuronal necrosis,166 and diabetes-mediated retinal inflammation, oxidative stress, and vascular leakage were all significantly lower in IL-17A−/− mice.168 Another study found that IL-17A aggravated high glucose–induced retinal ganglion cells death in the presence of intact Müller cells but not in the presence of Müller cells in which IL-17RA gene had been knocked down, which indicated that IL-17A injury to retinal ganglion cells is also mediated by retinal Müller cells.169 Notably, in the OIR model, Müller cells did not express IL-17A; in contrast, microglia produced IL-17A via retinoic acid receptor-related orphan nuclear receptor γ in a hypoxic environment, and IL-17A bound to 17A receptor on Müller cells to upregulate VEGF expression. Blocking IL-17A and its upstream retinoic acid-related orphan nuclear receptor γ alleviates diabetic retinopathy in rodents.170-172 These results further confirmed the role of the interaction between Müller cells and microglia in DR and DME and provided a new target for their treatment (Fig. 5).
DME Gene Therapy Targeting Müller Cells
As mentioned above, DME is the most common blinding eye disease in diabetic patients, but the current treatment effect for DME is quite limited. The main problems include insufficient response to first-line treatment, high treatment burden due to the short duration of action of existing drugs and the need for frequent injections.173 In recent years, along with the emergence of gene editing and constant exploration of gene delivery vectors, gene therapy has achieved rapid development. In addition, due to the special location, structure and function of the eyeball, ocular diseases have become the frontier of gene therapy exploration.174 In 2017, the first gene therapy product, Luxturna for RPE65-associated Leber's congenital amaurosis, was approved by the Food and Drug Administration.175 Subsequently, AAV-mediated gene therapy for other monogenic inherited eye diseases, including choroideremia,176,177 X-linked retinitis pigmentosa,178 Leber hereditary optic neuropathy,179 has also produced encouraging results. As to DME, compared with traditional anti-VEGF agents, gene therapy can prolong the duration of the effect, avoid frequent intravitreal injections, and improve patient compliance. The rise of gene therapy may open up a new way to treat DME.
In addition, gene therapy has an unrivaled advantage over conventional therapy when proteins need to be expressed within the cytoplasm of the cells to have a therapeutic effect.180 Gene therapy can more accurately and effectively locate the target cells, using a smaller dose to achieve an ideal effect while controlling the side effects within a certain range. We have previously elaborated on the important role of Müller cells in the pathological process of DME, especially in fundus inflammation. In addition, due to their anatomical structure and functional characteristics, Müller cells are a suitable therapeutic target for DME. First, Müller cells are distributed in the whole layer of the retina and are closely connected with the vitreous body and the subretinal region, which is conducive to drug entry and delivery. Second, Müller cells are resistant to pathological stimuli, allowing them to survive and remain a relevant target in advanced stages of retinal degenerative diseases.181
A number of different AAVs have been shown to have tropism for Müller cells.182 One novel variant named ShH10 demonstrated a striking increase in both transduction efficiency and specificity for Müller cells.183 In addition, the application of Müller cell-specific promoters also makes it possible to target Müller cells. For example, GFAP is a protein mainly expressed in Müller cells in diabetics. Janet Blanks et al. effectively reduced neovascularization in an oxygen-induced retinopathy model using an AAV vector construct expressing endostatin with a GFAP cell-specific promoter.184 Another study targeted Müller cell–derived VEGF164 with CD44 promoters that specifically target Müller cells to reduce intravitreal neovascularization in a rat model of retinopathy of prematurity.185 Recently, Juttner et al.186 developed a library of 230 AAVs, each with a different synthetic promoter and tested their transduction in the retina of mice, nonhuman primates and humans. This screen found a number of synthetic promoters that targeted Müller cells with 100% specificity and one in particular (labeled ProB2) transduced 45% of Müller cells. These results demonstrate the reliability of the both transduction efficiency and specificity of targeting Müller cells.
Another significant advantage of gene therapy over traditional pharmacological approaches is the direct repair of or compensation for defective genes.174 Different types of gene therapy for DME, including gene augmentation, gene-specific targeting, and most recently, genome editing, have been preliminarily verified in cell and animal experiments. Dystrophin-DP71 is an important membrane cytoskeletal protein mainly expressed in Müller cells. One study found that Dystrophin-Dp71 gene knockout mediated fundus VEGF upregulation and capillary degeneration in mice.187 When the BRB is destroyed, the expression of Dystrophin-Dp71 is downregulated, and the loss of Dystrophin-Dp71 further leads to increased permeability of the BRB through delocalization and downregulation of the AQP4 and Kir4.1 channels,188 leading to retinal edema, and these changes can be blocked by dexamethasone.189 An AAV capsid variant ShH10 was used to restore Dp71 in Muller glial cells of Dp71-deficient mice, and the resumption of Dp71 expression led to total reabsorption of the edema and normalization of the BRB permeability,190 providing a new approach to the treatment of diseases involving a permeable BRB. Thioredoxin-interacting protein is a pro-oxidative stress, pro-inflammatory and pro-apoptotic protein that is highly induced by diabetes and high glucose in retinal cells. Thioredoxin-interacting protein knockout via CRISPR/Cas9 prevents mitochondrial damage and mitophagy in Müller cells.191 CRISPR-mediated SOX9 knockout inhibits GFAP expression in Müller cells and attenuates their cell migration ability, which reduces glial cell activity.192
In addition, Müller cells are a major cellular source of survival signals for retinal neurons in physiological situations and diabetes,193 and gene therapy targeting Müller cells is also promising in fundus neuroprotection.181 Dorrell et al. used GFAP-driven gene delivery of NT-4 targeted to activated Müller cells to protect photoreceptors from oxidative stress in a mouse model of neovascularization.194 Dalkara et al.195 slowed retinal degeneration in a rat model of retinitis pigmentosa through AAV-mediated GDNF secretion from Müller cells, and retinal degeneration was postponed for a longer period compared with previous reports using GDNF delivery without Müller cell targeting.
Future Outlook
Over the past several decades, we have gained a deep understanding of the role of Müller cells in DME, but many questions need to be explored further. First, we do not know the specific molecular mechanism by which diabetes leads to the activation of Müller cells or the specific pathological changes in Müller cells in diabetic states. Second, Müller cells are directly and indirectly involved in the destruction of the BRB in diabetic patients. On the one hand, Müller cells are directly involved in the composition of the iBRB. On the other hand, Müller cells release a large number of inflammatory factors under diabetic conditions, but we do not know the specific mechanism of BRB destruction caused by these Müller cell-derived inflammatory factors and whether one or several proteins play a key role. Third, the role of Müller cells in the drainage function of the macular region has received much attention, but the specific mechanism and the important role of these water and ion channels in fluid accumulation in the macular region are still unclear.
In addition, because of the absence of macula in the retinas of rodents, lagomorphs and other laboratory animals commonly used in our studies, we still lack a suitable animal model of DME, which greatly limits the exploration of the pathological mechanism of DME.56 Recently, research on organoids has made great progress, and the culture of retinal organoids has been realized,196 which may provide a feasible way to overcome the problems of current disease models.
Gene therapy has made some achievements in ophthalmology, but currently, it is mainly aimed at single-gene hereditary ocular diseases, and in complex nonhereditary ocular diseases such as DME, the exploration of gene therapy still has a relatively large gap.197 In addition, CRISPR technology still faces the problem of off-target effects and low knockout efficiency, and the resulting safety risks need our attention. However, new technologies such as base editing and primer editing have a relatively short time frame, but they have not been demonstrated by clinical trials. In the future, in addition to the development and advances in gene editing technology itself, more exploration and practice are needed in the field of ophthalmology, especially in nonhereditary eye diseases such as DME and AMD. It is believed that one day, gene therapy based on gene editing will become a routine treatment for ocular diseases.
Conclusion
As a common, recurring, blinding eye disease, DME seriously endangers the vision of diabetic patients. Müller cells, as a unique glial cell of the fundus, play a unique role in fundus inflammation, the blood retinal barrier and macular water balance, and further exploration of the role of Müller cells in DME would promote the development of treatments for DME. In addition, with advances in gene therapy based on gene editing in recent years, gene therapy targeting Müller cells may provide a new direction for the treatment of DME and lay a more solid foundation for the application of gene therapy in ocular diseases.
Acknowledgments
Supported by the National Natural Science Foundation of China (81970811) and the Domestic Science and Technology Cooperation Project of Shanghai Municipal Science and Technology Commission (21015800700). The graphical abstracts were created with BioRender software.
Disclosure: D. Lai, None; Y. Wu, None; C. Shao, None; Q. Qiu, None
References
- 1. Burton MJ, Ramke J, Marques AP, et al.. The lancet global health commission on global eye health: vision beyond 2020. Lancet Global Health. 2021; 9(4): e489–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teo ZL, Tham YC, Yu M, et al.. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021; 128(11): 1580–1591. [DOI] [PubMed] [Google Scholar]
- 3. Daruich A, Matet A, Moulin A, et al.. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018; 63: 20–68. [DOI] [PubMed] [Google Scholar]
- 4. Tan GS, Cheung N, Simo R, Cheung GC, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017; 5: 143–155. [DOI] [PubMed] [Google Scholar]
- 5. Gardner TW, Davila JR. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med. 1995; 1: 527–534. [PMC free article] [PubMed] [Google Scholar]
- 7. Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998; 102: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sebag J, Balazs EA. Pathogenesis of cystoid macular edema: an anatomic consideration of vitreoretinal adhesions. Surv Ophthalmol. 1984; 28(Suppl): 493–498. [DOI] [PubMed] [Google Scholar]
- 9. Hui VWK, Szeto SK, Tang F, et al.. Optical coherence tomography classification systems for diabetic macular edema and their associations with visual outcome and treatment responses—an updated review. Asia Pac J Ophthalmol. 2022; 11: 247–257. [DOI] [PubMed] [Google Scholar]
- 10. Im JHB, Jin YP, Chow R, Yan P. Prevalence of diabetic macular edema based on optical coherence tomography in people with diabetes: a systematic review and meta-analysis. Surv Ophthalmol. 2022; 67: 1244–1251. [DOI] [PubMed] [Google Scholar]
- 11. Huang D, Swanson EA, Lin CP, et al.. Optical coherence tomography. Science. 1991; 254(5035): 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puliafito CA, Hee MR, Lin CP, et al.. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995; 102(2): 217–229. [DOI] [PubMed] [Google Scholar]
- 13. Lains I, Wang JC, Cui Y, et al.. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog Retin Eye Res. 2021; 84: 100951. [DOI] [PubMed] [Google Scholar]
- 14. Drexler W, Morgner U, Ghanta RK, Kärtner FX, Schuman JS, Fujimoto JG. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001; 7: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adhi M, Liu JJ, Qavi AH, et al.. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am J Ophthalmol. 2014; 157: 1272–1281. [DOI] [PubMed] [Google Scholar]
- 16. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999; 127: 688–693. [DOI] [PubMed] [Google Scholar]
- 17. Iglicki M, Lavaque A, Ozimek M, et al.. Biomarkers and predictors for functional and anatomic outcomes for small gauge pars plana vitrectomy and peeling of the internal limiting membrane in naïve diabetic macular edema: The VITAL study. PloS One. 2018; 13(7): e0200365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iglicki M, Loewenstein A, Barak A, Schwartz S, Zur D. Outer retinal hyperreflective deposits (ORYD): a new OCT feature in naïve diabetic macular oedema after PPV with ILM peeling. Br J Ophthalmol. 2020; 104: 666–671. [DOI] [PubMed] [Google Scholar]
- 19. Zur D, Iglicki M, Busch C, et al.. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology. 2018; 125: 267–275. [DOI] [PubMed] [Google Scholar]
- 20. Zur D, Iglicki M, Sala‐Puigdollers A, et al.. Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2020; 98(2): e217–e223. [DOI] [PubMed] [Google Scholar]
- 21. Tang F, Luenam P, Ran AR, et al.. Detection of diabetic retinopathy from ultra-widefield scanning laser ophthalmoscope images: a multicenter deep learning analysis. Ophthalmol Retina. 2021; 5: 1097–1106. [DOI] [PubMed] [Google Scholar]
- 22. Müller H. Zur histologie der netzhaut. Z Wiss Zool. 1851; 3: 234–237. [Google Scholar]
- 23. Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia–neuron interactions in the mammalian retina. Progr Retin Eye Res. 2016; 51: 1–40. [DOI] [PubMed] [Google Scholar]
- 24. Reichenbach A, Bringmann A. Glia of the human retina. Glia. 2020; 68: 768–796. [DOI] [PubMed] [Google Scholar]
- 25. Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013; 61: 651–678. [DOI] [PubMed] [Google Scholar]
- 26. Bringmann A, Pannicke T, Grosche J, et al.. Müller cells in the healthy and diseased retina. Progr Retin Eye Res. 2006; 25: 397–424. [DOI] [PubMed] [Google Scholar]
- 27. Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000; 36(4–5): 291–300. [DOI] [PubMed] [Google Scholar]
- 28. Kofuji P, Biedermann B, Siddharthan V, et al.. Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia. 2002; 39: 292–303. [DOI] [PubMed] [Google Scholar]
- 29. Bringmann A, Francke M, Pannicke T, et al.. Role of glial K+ channels in ontogeny and gliosis: a hypothesis based upon studies on Müller cells. Glia. 2000; 29: 35–44. [DOI] [PubMed] [Google Scholar]
- 30. Gao F, Xu LJ, Zhao Y, Sun XH, Wang Z. K+ channels of Müller glial cells in retinal disorders. CNS Neurol Disord Drug Targets. 2018; 17: 255–260. [DOI] [PubMed] [Google Scholar]
- 31. Connors NC, Kofuji P. Potassium channel Kir4. 1 macromolecular complex in retinal glial cells. Glia. 2006; 53: 124–131. [DOI] [PubMed] [Google Scholar]
- 32. Skatchkov S, Eaton MJ, Shuba YM, et al.. Tandem-pore domain potassium channels are functionally expressed in retinal (Müller) glial cells. Glia. 2006; 53: 266–276. [DOI] [PubMed] [Google Scholar]
- 33. Schey KL, Wang Z, Wenke JL, Qi Y. Aquaporins in the eye: expression, function, and roles in ocular disease. Biochim Biophys Acta. 2014; 1840: 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tran TL, Bek T, Holm L, et al.. Aquaporins 6–12 in the human eye. Acta Ophthalmol. 2013; 91: 557–563. [DOI] [PubMed] [Google Scholar]
- 35. Iandiev I, Dukic-Stefanovic S, Hollborn M, et al.. Immunolocalization of aquaporin-6 in the rat retina. Neurosci Lett. 2011; 490: 130–134. [DOI] [PubMed] [Google Scholar]
- 36. Nagelhus EA, Horio Y, Inanobe A, et al.. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4. 1 and AQP4 in specific membrane domains. Glia. 1999; 26: 47–54. [DOI] [PubMed] [Google Scholar]
- 37. Goodyear MJ, Crewther SG, Junghans BM. A role for aquaporin-4 in fluid regulation in the inner retina. Vis Neurosci. 2009; 26: 159–165. [DOI] [PubMed] [Google Scholar]
- 38. Jo AO, Ryskamp DA, Phuong TT, et al.. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. J Neurosci. 2015; 35: 13525–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruiz-Ederra J, Zhang H, Verkman A. Evidence against functional interaction between aquaporin-4 water channels and Kir4. 1 potassium channels in retinal Müller cells. J Biol Chem. 2007; 282: 21866–21872. [DOI] [PubMed] [Google Scholar]
- 40. Winkler BS, Arnold MJ, Brassell MA, Puro DG. Energy metabolism in human retinal Muller cells. Invest Ophthalmol Vis Sci. 2000; 41: 3183–3190. [PMC free article] [PubMed] [Google Scholar]
- 41. Tsacopoulos M, Poitry-Yamate CL, MacLeish PR, Poitry S. Trafficking of molecules and metabolic signals in the retina. Progr Retin Eye Res. 1998; 17: 429–442. [DOI] [PubMed] [Google Scholar]
- 42. Lin RY, Vera JC, Chaganti RS, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. J Biol Chem. 1998; 273: 28959–28965. [DOI] [PubMed] [Google Scholar]
- 43. Toft-Kehler A, Skytt D, Kolko M. A perspective on the Müller cell-neuron metabolic partnership in the inner retina. Mol Neurobiol. 2018; 55: 5353–5361. [DOI] [PubMed] [Google Scholar]
- 44. Pannicke T, Fischer W, Biedermann B, et al.. P2X7 receptors in Müller glial cells from the human retina. J Neurosci. 2000; 20: 5965–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toft-Kehler AK, Skytt DM, Svare A, et al.. Mitochondrial function in Müller cells-does it matter? Mitochondrion. 2017; 36: 43–51. [DOI] [PubMed] [Google Scholar]
- 46. Marc RE. Structural organization of GABAergic circuitry in ectotherm retinas. Progr Brain Res. 1992; 90: 61–92. [DOI] [PubMed] [Google Scholar]
- 47. Bringmann A, Grosche A, Pannicke T, Reichenbach A. GABA and glutamate uptake and metabolism in retinal glial (Müller) cells. Front Endocrinol. 2013; 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Franze K, Grosche J, Skatchkov SN, et al.. Müller cells are living optical fibers in the vertebrate retina. Proc National Acad Sci. 2007; 104: 8287–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agte S, Junek S, Matthias S, et al.. Müller glial cell-provided cellular light guidance through the vital guinea-pig retina. Biophys J. 2011; 101: 2611–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ryskamp DA, Witkovsky P, Barabas P, et al.. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011; 31: 7089–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schubert H. Cystoid macular edema: the apparent role of mechanical factors. Progr Clin Biol Res. 1989; 312: 277–291. [PubMed] [Google Scholar]
- 52. Lindqvist N, Liu Q, Zajadacz J, Franze K, Reichenbach A. Retinal glial (Müller) cells: sensing and responding to tissue stretch. Invest Ophthalmol Vis Sci. 2010; 51: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 53. Bringmann A, Syrbe S, Görner K, et al.. The primate fovea: structure, function and development. Progr Retin Eye Res. 2018; 66: 49–84. [DOI] [PubMed] [Google Scholar]
- 54. Syrbe S, Kuhrt H, Gärtner U, et al.. Müller glial cells of the primate foveola: an electron microscopical study. Exp Eye Res. 2018; 167: 110–117. [DOI] [PubMed] [Google Scholar]
- 55. Rudich DS, Curcio CA, Wasserstein M, Brodie SE. Inner macular hyperreflectivity demonstrated by optical coherence tomography in Niemann-Pick disease. JAMA Ophthalmol. 2013; 131: 1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Daruich A, Matet A, Moulin A, et al.. Mechanisms of macular edema: beyond the surface. Progr Retin Eye Res. 2018; 63: 20–68. [DOI] [PubMed] [Google Scholar]
- 57. Bringmann A, Karol M, Unterlauft JD, et al.. Foveal regeneration after resolution of cystoid macular edema without and with internal limiting membrane detachment: presumed role of glial cells for foveal structure stabilization. Int J Ophthalmol. 2021; 14: 818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bringmann A, Unterlauft JD, Wiedemann R, Barth T, Rehak M, Wiedemann P. Two different populations of Müller cells stabilize the structure of the fovea: an optical coherence tomography study. Int Ophthalmol. 2020; 40: 2931–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Elsner AE, Burns SA, Beausencourt E, Weiter JJ. Foveal cone photopigment distribution: small alterations associated with macular pigment distribution. Invest Ophthalmol Vis Sci. 1998; 39: 2394–2404. [PubMed] [Google Scholar]
- 60. Too LK, Gracie G, Hasic E, Iwakura JH, Cherepanoff S. Adult human retinal Müller glia display distinct peripheral and macular expression of CD117 and CD44 stem cell-associated proteins. Acta Histochem. 2017; 119: 142–149. [DOI] [PubMed] [Google Scholar]
- 61. Zhang T, Zhu L, Madigan MC, et al.. Human macular Müller cells rely more on serine biosynthesis to combat oxidative stress than those from the periphery. Elife. 2019; 8: e43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006; 114: 597–605. [DOI] [PubMed] [Google Scholar]
- 63. Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Progr Retin Eye Res. 2014; 42: 85–102. [DOI] [PubMed] [Google Scholar]
- 64. Mishra N, Singh M, Singh RK, Saxena S. Elevated advanced glycation end products are associated with subfoveal ellipsoid zone disruption in diabetic macular edema. Ind J Ophthalmol. 2021; 69(11): 3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moore TC, Moore JE, Kaji Y, et al.. The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthalmol Vis Sci. 2003; 44: 4457–4464. [DOI] [PubMed] [Google Scholar]
- 66. Chen M, Curtis T, Stitt A. Advanced glycation end products and diabetic retinopathy. Curr Med Chem. 2013; 20: 3234–3240. [DOI] [PubMed] [Google Scholar]
- 67. Caldwell RB, Bartoli M, Behzadian MA, et al.. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003; 19: 442–455. [DOI] [PubMed] [Google Scholar]
- 68. Motiejūnaitė R, Kazlauskas A. Pericytes and ocular diseases. Exp Eye Res. 2008; 86: 171–177. [DOI] [PubMed] [Google Scholar]
- 69. Guo X, Wang L, Chen B, et al.. ERM protein moesin is phosphorylated by advanced glycation end products and modulates endothelial permeability. Am J Physiol Heart Circ Physiol. 2009; 297(1): H238–H246. [DOI] [PubMed] [Google Scholar]
- 70. Xu J, Wang L, Chen B, et al.. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem. 2018; 48: 705–717. [DOI] [PubMed] [Google Scholar]
- 71. Barnett PA, González RG, Chylack LT Jr, Cheng HM. The effect of oxidation on sorbitol pathway kinetics. Diabetes. 1986; 35: 426–432. [DOI] [PubMed] [Google Scholar]
- 72. Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016; 61: 187–196. [DOI] [PubMed] [Google Scholar]
- 73. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 74. Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973; 288: 831–836. [DOI] [PubMed] [Google Scholar]
- 75. Ishii M, Wen H, Corsa CA, et al.. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009; 114: 3244–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shiba T, Inoguchi TO, Sportsman JR, Heath WF, Bursell SV, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol Endocrinol Metab. 1993; 265(5): E783–E793. [DOI] [PubMed] [Google Scholar]
- 77. Konishi H, Tanaka M, Takemura Y, et al.. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci. 1997; 94: 11233–11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Starace V, Battista M, Brambati M, et al.. The role of inflammation and neurodegeneration in diabetic macular edema. Ther Adv Ophthalmol. 2021; 13: 25158414211055963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Evcimen ND, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007; 55: 498–510. [DOI] [PubMed] [Google Scholar]
- 80. Curtis TM, Hamilton R, Yong PH, et al.. Müller glial dysfunction during diabetic retinopathy in rats is linked to accumulation of advanced glycation end-products and advanced lipoxidation end-products. Diabetologia. 2011; 54: 690–698. [DOI] [PubMed] [Google Scholar]
- 81. Zong H, Ward M, Madden A, et al.. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia. 2010; 53: 2656–2666. [DOI] [PubMed] [Google Scholar]
- 82. Brozzi F, Arcuri C, Giambanco I, Donato R. S100B protein regulates astrocyte shape and migration via interaction with Src kinase. J Biol Chem. 2009; 284: 8797–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ramasamy R, Yan SF, Schmidt AM. Arguing for the motion: yes, RAGE is a receptor for advanced glycation endproducts. Mol Nutr Food Res. 2007; 51: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 84. Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009; 221: 262–272. [DOI] [PubMed] [Google Scholar]
- 85. Thompson K, Chen J, Luo Q, Xiao Y, Cummins TR, Bhatwadekar AD. Advanced glycation end (AGE) product modification of laminin downregulates Kir4. 1 in retinal Müller cells. PLoS One. 2018; 13(2): e0193280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ai J, Liu Y, Sun J-H. Advanced glycation end-products stimulate basic fibroblast growth factor expression in cultured Müller cells. Mol Med Rep. 2013; 7: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hirata C, Nakano K, Nakamura N, et al.. Advanced glycation end products induce expression of vascular endothelial growth factor by retinal Müller cells. Biochem Biophys Res Comm. 1997; 236: 712–715. [DOI] [PubMed] [Google Scholar]
- 88. Nakamura N, Hasegawa G, Obayashi H, et al.. Increased concentration of pentosidine, an advanced glycation end product, and interleukin-6 in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2003; 61: 93–101. [DOI] [PubMed] [Google Scholar]
- 89. Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003; 52: 506–511. [DOI] [PubMed] [Google Scholar]
- 90. Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004; 53: 2404–2411. [DOI] [PubMed] [Google Scholar]
- 91. Wang Z, Li W, Mitchell CK, Carter-Dawson L. Activation of protein kinase C reduces GLAST in the plasma membrane of rat Müller cells in primary culture. Vis Neurosci. 2003; 20: 611–619. [DOI] [PubMed] [Google Scholar]
- 92. Qian Z, Bilderback TR, Barmack NH. Acyl coenzyme A-binding protein (ACBP) is phosphorylated and secreted by retinal Müller astrocytes following protein kinase C activation. J Neurochem. 2008; 105: 1287–1299. [DOI] [PubMed] [Google Scholar]
- 93. Miyata Y, Kase M, Sugita Y, et al.. Protein kinase C-mediated regulation of matrix metalloproteinase and tissue inhibitor of metalloproteinase production in a human retinal Müller cells. Curr Eye Res. 2012; 37: 842–849. [DOI] [PubMed] [Google Scholar]
- 94. Hollborn M, Vogler S, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Regulation of the hyperosmotic induction of aquaporin 5 and VEGF in retinal pigment epithelial cells: involvement of NFAT5. Mol Vision. 2015; 21: 360. [PMC free article] [PubMed] [Google Scholar]
- 95. Distler C, Dreher Z. Glia cells of the monkey retina—II. Müller cells. Vision Res. 1996; 36: 2381–2394. [DOI] [PubMed] [Google Scholar]
- 96. Beverley KM, Pattnaik BR. Inward rectifier potassium (Kir) channels in the retina: living our vision. Am J Physiol Cell Physiol. 2022; 323(3): C772–C782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang T, Zhang C, Xie H, et al.. Anti-VEGF therapy prevents Müller intracellular edema by decreasing VEGF-A in diabetic retinopathy. Eye Vision. 2021; 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sene A, Tadayoni R, Pannicke T, et al.. Functional implication of Dp71 in osmoregulation and vascular permeability of the retina. PLoS One. 2009; 4(10): e7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fukuda M, Nakanishi Y, Fuse M, et al.. Altered expression of aquaporins 1 and 4 coincides with neurodegenerative events in retinas of spontaneously diabetic Torii rats. Exp Eye Res. 2010; 90: 17–25. [DOI] [PubMed] [Google Scholar]
- 100. Hollborn M, Dukic-Stefanovic S, Pannicke T, et al.. Expression of aquaporins in the retina of diabetic rats. Curr Eye Res. 2011; 36: 850–856. [DOI] [PubMed] [Google Scholar]
- 101. Zhang C, Luo D, Xie H, et al.. Aquaporin 11 alleviates retinal Müller intracellular edema through water efflux in diabetic retinopathy. Pharmacol Res. 2022; 187: 106559. [DOI] [PubMed] [Google Scholar]
- 102. Chen Y, Chen H, Wang C, et al.. The expressions of vascular endothelial growth factor and aquaporin 4 in the inner limiting membrane from eyes with diabetic macular edema. Chin J Ocul Fundus Dis. 2021;617–622. [Google Scholar]
- 103. Oosuka S, Kida T, Oku H, et al.. Effects of an aquaporin 4 inhibitor, TGN-020, on murine diabetic retina. Int J Mol Sci. 2020; 21: 2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yanoff M, Fine BS, Brucker AJ, Eagle RC Jr. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984; 28: 505–511. [DOI] [PubMed] [Google Scholar]
- 105. Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981; 92: 466–481. [DOI] [PubMed] [Google Scholar]
- 106. Qin S, Zhang C, Qin H, et al.. Hyperreflective foci and subretinal fluid are potential imaging biomarkers to evaluate anti-VEGF effect in diabetic macular edema. Front Physiol. 2021;2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Byeon SH, Chu YK, Hong YT, Kim M, Kang HM, Kwon OW. New insights into the pathoanatomy of diabetic macular edema: angiographic patterns and optical coherence tomography. Retina. 2012; 32: 1087–1099. [DOI] [PubMed] [Google Scholar]
- 108. Choi M, Yun C, Oh JH, Kim SW. Foveal Müller cell cone as a prognostic optical coherence tomography biomarker for initial response to antivascular endothelial growth factor treatment in cystoid diabetic macular edema. Retina. 2022; 42: 129–137. [DOI] [PubMed] [Google Scholar]
- 109. Frank RN. Diabetic retinopathy. N Engl J Med. 2004; 350: 48–58. [DOI] [PubMed] [Google Scholar]
- 110. Cunha-Vaz J. Mechanisms of retinal fluid accumulation and blood-retinal barrier breakdown. Dev Ophthalmol. 2017; 58: 11–20. [DOI] [PubMed] [Google Scholar]
- 111. Tout S, Chan-Ling T, Holländer H, Stone J. The role of Muller cells in the formation of the blood-retinal barrier. Neuroscience. 1993; 55: 291–301. [DOI] [PubMed] [Google Scholar]
- 112. Coorey NJ, Shen W, Chung SH, Zhu L, Gillies MC. The role of glia in retinal vascular disease. Clin Exp Optom. 2012; 95: 266–281. [DOI] [PubMed] [Google Scholar]
- 113. Kugler EC, Greenwood J, MacDonald RB. The “neuro-glial-vascular” unit: the role of glia in neurovascular unit formation and dysfunction. Front Cell Dev Biol. 2021; 9: 732820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tretiach M, Madigan MC, Wen L, Gillies MC. Effect of Müller cell co-culture on in vitro permeability of bovine retinal vascular endothelium in normoxic and hypoxic conditions. Neurosci Lett. 2005; 378: 160–165. [DOI] [PubMed] [Google Scholar]
- 115. Daruich A, Matet A, Moulin A, et al.. Mechanisms of macular edema: beyond the surface. Progr Retinal Eye Res. 2018; 63: 20–68. [DOI] [PubMed] [Google Scholar]
- 116. Byrne LC, Khalid F, Lee T, et al.. AAV-mediated, optogenetic ablation of muller glia leads to structural and functional changes in the mouse retina. Plos One. 2013; 8(9): e76075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shen W, Li S, Chung SH, Gillies MC. Retinal vascular changes after glial disruption in rats. J Neurosci Res. 2010; 88: 1485–1499. [DOI] [PubMed] [Google Scholar]
- 118. Yang S, Qi SA, Wang CG. The role of retinal Muller cells in diabetic retinopathy and related therapeutic advances. Front Cell Dev Biol. 2022; 10: 1047487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Carpi-Santos R, de Melo Reis RA, Gomes FC, Calaza KC. Contribution of Muller cells in the diabetic retinopathy development: focus on oxidative stress and inflammation. Antioxidants. 2022; 11: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ji N, Guo Y, Liu S, et al.. MEK/ERK/RUNX2 pathway-mediated IL-11 autocrine promotes the activation of Müller glial cells during diabetic retinopathy. Curr Eye Res. 2022; 47: 1622–1630. [DOI] [PubMed] [Google Scholar]
- 121. Muto T, Tien T, Kim D, Sarthy VP, Roy S. High glucose alters Cx43 expression and gap junction intercellular communication in retinal Müller cells: promotes Müller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci. 2014; 55: 4327–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wu JY, Zhong Y, Yue S, et al.. Aqueous humor mediator and cytokine aberrations in diabetic retinopathy and diabetic macular edema: a systematic review and meta-analysis. Disease Markers, 2019; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Scholl S, Kirchhof J, Augustin AJ. Pathophysiology of Macular Edema. Ophthalmologica. 2010; 224(Suppl. 1): 8–15. [DOI] [PubMed] [Google Scholar]
- 124. Aveleira CA, Lin CM, Abcouwer SF, Ambrósio AF, Antonetti DA. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010; 59: 2872–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Li W, Liu X, Tu Y, et al.. Dysfunctional Nurr1 promotes high glucose-induced Müller cell activation by up-regulating the NF-κB/NLRP3 inflammasome axis. Neuropeptides. 2020; 82: 102057. [DOI] [PubMed] [Google Scholar]
- 126. Ji M, Sun Q, Zhang G, et al.. Microglia-derived TNF-α mediates Müller cell activation by activating the TNFR1-NF-κB pathway. Exp Eye Res. 2022; 214: 108852. [DOI] [PubMed] [Google Scholar]
- 127. Zhang J, Chen C, Zhang S, Chen J, Wu L, Chen Z. LncRNA XIST restrains the activation of Müller cells and inflammation in diabetic retinopathy via stabilizing SIRT1. Autoimmunity. 2021; 54: 504–513. [DOI] [PubMed] [Google Scholar]
- 128. Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010; 59: 2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mesquida M, Drawnel F, Fauser S. The role of inflammation in diabetic eye disease. Semin Immunopathol. 2019; 41: 427–445. [DOI] [PubMed] [Google Scholar]
- 130. Liu Y, Li L, Pan N, et al.. TNF-α released from retinal Müller cells aggravates retinal pigment epithelium cell apoptosis by upregulating mitophagy during diabetic retinopathy. Biochem Biophys Res Commun. 2021; 561: 143–150. [DOI] [PubMed] [Google Scholar]
- 131. Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-α production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008; 172: 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ma M, Zhao S, Zhang J, Sun T, Fan Y, Zheng Z. High glucose-induced TRPC6 channel activation decreases glutamate uptake in rat retinal Müller cells. Front Pharmacol. 2019; 10: 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Noma H, Yasuda K, Shimura M. Involvement of Cytokines in the Pathogenesis of Diabetic Macular Edema. Int J Mol Sci. 2021; 22: 3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Urias EA, Urias GA, Monickaraj F, McGuire P, Das A. Novel therapeutic targets in diabetic macular edema: beyond VEGF. Vis Res. 2017; 139: 221–227. [DOI] [PubMed] [Google Scholar]
- 135. Liu Y, Biarnés Costa M, Gerhardinger C. IL-1β is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1β autostimulation. PLoS One. 2012; 7(5): e36949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yego EC, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Müller cells by IL-1beta and IL-6. Invest Ophthalmol Vis Sci. 2009; 50: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 137. Yun J-H, Park SW, Kim KJ, et al.. Endothelial STAT3 activation increases vascular leakage through downregulating tight junction proteins: implications for diabetic retinopathy. J Cell Physiol. 2017; 232: 1123–1134. [DOI] [PubMed] [Google Scholar]
- 138. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015; 122: 1375–1394. [DOI] [PubMed] [Google Scholar]
- 139. Sbardella D, Tundo GR, Mecchia A, et al.. A novel and atypical NF-KB pro-inflammatory program regulated by a CamKII-proteasome axis is involved in the early activation of Muller glia by high glucose. Cell Biosci. 2022; 12: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985; 103: 1796–1806. [PubMed] [Google Scholar]
- 141. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991; 98(5 Suppl): 766–785. [PubMed] [Google Scholar]
- 142. Nasrallah FP, Jalkh AE, Van Coppenolle F, et al.. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988; 95: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 143. Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992; 99: 753–759. [DOI] [PubMed] [Google Scholar]
- 144. Harbour JW, Smiddy WE, Flynn HW Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996; 121: 405–413. [DOI] [PubMed] [Google Scholar]
- 145. Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996; 122: 258–260. [DOI] [PubMed] [Google Scholar]
- 146. Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003; 26: 2653–2664. [DOI] [PubMed] [Google Scholar]
- 147. Ip MS. Intravitreal injection of triamcinolone: an emerging treatment for diabetic macular edema. Diabetes Care. 2004; 27: 1794–1797. [DOI] [PubMed] [Google Scholar]
- 148. Elman MJ, Aiello LP, Beck RW, et al.. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010; 117: 1064–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Brown DM, Nguyen QD, Marcus DM, et al.. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013; 120: 2013–2022. [DOI] [PubMed] [Google Scholar]
- 150. Heier JS, Korobelnik JF, Brown DM, et al.. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID Studies. Ophthalmology. 2016; 123: 2376–2385. [DOI] [PubMed] [Google Scholar]
- 151. Sharma T. Evolving role of anti-VEGF for diabetic macular oedema: from clinical trials to real life. Eye (Lond). 2020; 34(3): 415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Glassman AR, Wells JA III, Josic K, et al.. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T Extension Study). Ophthalmology. 2020; 127: 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Iglicki M, Busch C, Zur D, et al.. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: the International Retina Group Real-Life 24-Month Multicenter Study. The IRGREL-DEX Study. Retina. 2019; 39: 44–51. [DOI] [PubMed] [Google Scholar]
- 154. Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019; 62: 231–236. [DOI] [PubMed] [Google Scholar]
- 155. Iglicki M, Zur D, Busch C, Okada M, Loewenstein A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the “DR-Pro-DEX Study.” Acta Diabetol. 2018; 55: 541–547. [DOI] [PubMed] [Google Scholar]
- 156. Rosenblatt A, Udaondo P, Cunha-Vaz J, et al.. A collaborative retrospective study on the efficacy and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: The European DME Registry Study. Ophthalmology. 2020; 127: 377–393. [DOI] [PubMed] [Google Scholar]
- 157. Mello Filho P, Andrade G, Maia A, et al.. Effectiveness and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: a real-world experience. Ophthalmologica. 2019; 241: 9–16. [DOI] [PubMed] [Google Scholar]
- 158. Ehlers JP, Yeh S, Maguire MG, et al.. Intravitreal pharmacotherapies for diabetic macular edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2022; 129: 88–99. [DOI] [PubMed] [Google Scholar]
- 159. Lamine LB, Turki A, Al-Khateeb G, et al.. Elevation in circulating soluble CD40 ligand concentrations in type 2 diabetic retinopathy and association with its severity. Exp Clin Endocrinol Diabetes. 2020; 128: 319–324. [DOI] [PubMed] [Google Scholar]
- 160. Portillo J-AC, Okenka G, Kern TS, Subauste CS. Identification of primary retinal cells and ex vivo detection of proinflammatory molecules using flow cytometry. Mol Vision. 2009; 15: 1383–1389. [PMC free article] [PubMed] [Google Scholar]
- 161. Portillo J-AC, Greene JA, Okenka G, et al.. CD40 promotes the development of early diabetic retinopathy in mice. Diabetologia. 2014; 57: 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Portillo J-AC, Lopez Corcino Y, Miao Y, et al.. CD40 in retinal Müller cells induces P2X7-dependent cytokine expression in macrophages/microglia in diabetic mice and development of early experimental diabetic retinopathy. Diabetes. 2017; 66: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bishop GA, Hostager BS, Brown KD. Mechanisms of TNF receptor-associated factor (TRAF) regulation in B lymphocytes. J Leukocyte Biol. 2002; 72: 19–23. [PubMed] [Google Scholar]
- 164. Portillo J-AC, Greene JA, Schwartz I, Subauste MC, Subauste CS. Blockade of CD40-TRAF2,3 or CD40-TRAF6 is sufficient to inhibit pro-inflammatory responses in non-haematopoietic cells. Immunology. 2015; 144: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Portillo JC, Yu JS, Vos S, et al.. Disruption of retinal inflammation and the development of diabetic retinopathy in mice by a CD40-derived peptide or mutation of CD40 in Muller cells. Diabetologia. 2022; 65: 2157–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Qiu AW, Bian Z, Mao PA, Liu QH. IL-17A exacerbates diabetic retinopathy by impairing Muller cell function via Act1 signaling. Exp Mol Med. 2016; 48(12): e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Chen H, Ren X, Liao N, Wen F. Th17 cell frequency and IL-17A concentrations in peripheral blood mononuclear cells and vitreous fluid from patients with diabetic retinopathy. J Int Med Res. 2016; 44: 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sigurdardottir S, Zapadka TE, Lindstrom SI, et al.. Diabetes-mediated IL-17A enhances retinal inflammation, oxidative stress, and vascular permeability. Cell Immunol. 2019; 341: 103921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Qiu AW, Huang DR, Li B, Fang Y, Zhang WW, Liu QH. IL-17A injury to retinal ganglion cells is mediated by retinal Muller cells in diabetic retinopathy. Cell Death Dis. 2021; 12: 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Talia DM, Deliyanti D, Agrotis A, Wilkinson-Berka JL. Inhibition of the nuclear receptor RORgamma and interleukin-17A suppresses neovascular retinopathy: involvement of immunocompetent microglia. Arterioscler Thromb Vasc Biol. 2016; 36: 1186–1196. [DOI] [PubMed] [Google Scholar]
- 171. Qiu AW, Liu QH, Wang JL. Blocking IL-17A alleviates diabetic retinopathy in rodents. Cell Physiol Biochem. 2017; 41: 960–972. [DOI] [PubMed] [Google Scholar]
- 172. Zapadka TE, Lindstrom SI, Taylor BE, et al.. RORgammat inhibitor-SR1001 halts retinal inflammation, capillary degeneration, and the progression of diabetic retinopathy. Int J Mol Sci. 2020; 21(10): 3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Iglicki M, González DP, Loewenstein A, Zur D. Next-generation anti-VEGF agents for diabetic macular oedema. Eye. 2022; 36: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lee JH, Wang JH, Chen J, et al.. Gene therapy for visual loss: opportunities and concerns. Prog Retin Eye Res. 2019; 68: 31–53. [DOI] [PubMed] [Google Scholar]
- 175. Russell S, Bennett J, Wellman JA, et al.. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017; 390(10097): 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Xue K, Jolly JK, Barnard AR, et al.. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat Med. 2018; 24: 1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dimopoulos IS, Hoang SC, Radziwon A, et al.. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta Experience. Am J Ophthalmol. 2018; 193: 130–142. [DOI] [PubMed] [Google Scholar]
- 178. Cehajic-Kapetanovic J, Xue K, Martinez-Fernandez de la Camara C, et al.. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020; 26: 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Newman NJ, Yu-Wai-Man P, Carelli V, et al.. Efficacy and safety of intravitreal gene therapy for leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology. 2021; 128: 649–660. [DOI] [PubMed] [Google Scholar]
- 180. MacLaren RE. Gene therapy for age-related macular degeneration. Lancet. 2015; 386(10011): 2369–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Devoldere J, Peynshaert K, De Smedt SC, Remaut K. Muller cells as a target for retinal therapy. Drug Discov Today. 2019; 24: 1483–1498. [DOI] [PubMed] [Google Scholar]
- 182. O'Carroll SJ, Cook WH, Young D. AAV Targeting of glial cell types in the central and peripheral nervous system and relevance to human gene therapy. Front Mol Neurosci. 2020; 13: 618020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Klimczak RR, Koerber JT, Dalkara D, Flannery JG, Schaffer DV. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Muller cells. PLoS One. 2009; 4(10): e7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Biswal MR, Prentice HM, Dorey CK, Blanks JC. A hypoxia-responsive glial cell-specific gene therapy vector for targeting retinal neovascularization. Invest Ophthalmol Vis Sci. 2014; 55: 8044–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Jiang Y, Wang H, Culp D, et al.. Targeting Müller cell-derived VEGF164 to reduce intravitreal neovascularization in the rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2014; 55: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Juttner J, Szabo A, Gross-Scherf B, et al.. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat Neurosci. 2019; 22: 1345–1356. [DOI] [PubMed] [Google Scholar]
- 187. El Mathari B, Sene A, Charles-Messance H, et al.. Dystrophin Dp71 gene deletion induces retinal vascular inflammation and capillary degeneration. Hum Mol Genet. 2015; 24: 3939–3947. [DOI] [PubMed] [Google Scholar]
- 188. Sene A, Tadayoni R, Pannicke T, et al.. Functional implication of Dp71 in osmoregulation and vascular permeability of the retina. PLoS One. 2009; 4(10): e7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Siqueiros-Marquez L, Bénard R, Vacca O, et al.. Protection of glial muller cells by dexamethasone in a mouse model of surgically induced blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci. 2017; 58: 876–886. [DOI] [PubMed] [Google Scholar]
- 190. Vacca O, Charles-Messance H, El Mathari B, et al.. AAV-mediated gene therapy in Dystrophin-Dp71 deficient mouse leads to blood-retinal barrier restoration and oedema reabsorption. Hum Mol Genet. 2016; 25: 3070–3079. [DOI] [PubMed] [Google Scholar]
- 191. Devi TS, Somayajulu M, Kowluru RA, Singh LP. TXNIP regulates mitophagy in retinal Muller cells under high-glucose conditions: implications for diabetic retinopathy. Cell Death Dis. 2017; 8(5): e2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wang X, Shu Q, Ni Y, Xu G. CRISPR-mediated SOX9 knockout inhibits GFAP expression in retinal glial (Muller) cells. Neuroreport. 2018; 29: 1504–1508. [DOI] [PubMed] [Google Scholar]
- 193. Fu S, Dong S, Zhu M, et al.. Müller glia are a major cellular source of survival signals for retinal neurons in diabetes. Diabetes. 2015; 64: 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Dorrell MI, Dong S, Zhu M, et al.. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009; 119: 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Dalkara D, Kolstad KD, Guerin KI, et al.. AAV mediated GDNF secretion from retinal glia slows down retinal degeneration in a rat model of retinitis pigmentosa. Mol Ther. 2011; 19: 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Bellapianta A, Cetkovic A, Bolz M, Salti A. Retinal organoids and retinal prostheses: an overview. Int J Mol Sci. 2022; 23(6): 2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Botto C, Rucli M, Tekinsoy MD, Pulman J, Sahel JA, Dalkara D. Early and late stage gene therapy interventions for inherited retinal degenerations. Prog Retin Eye Res. 2022; 86: 100975. [DOI] [PubMed] [Google Scholar]