Abstract
Objective:
Disruptions in the hypothalamic-pituitary-adrenal (HPA) axis are thought to be key neuroendocrine mechanisms involved in psychopathology and may have intergenerational impacts. Hair-derived HPA hormones offer a measure of long-term HPA axis activity that may be useful in assessing maternal and infant health. Building on a community-based randomized control trial of a perinatal depression intervention in Pakistan, we examine intervention effects on HPA axis activity in a subsample of mothers and infants.
Methods:
HPA axis activity was assessed using hair-derived cortisol, cortisone, and dehydroepiandosterone (DHEA). Hair samples were collected from mother-child dyads at one year postpartum from prenatally depressed women randomized to a cognitive-behavioral intervention (n = 35 dyads) or to enhanced usual care (n = 37 dyads), and from a comparison sample of women who screened negative for depression in pregnancy (n = 35 dyads).
Results:
The intervention group had 38 percent (p=0.01) lower maternal cortisol levels and 45 percent (p < 0.01) lower maternal cortisone compared to the EUC group. Maternal DHEA levels were higher among women in the intervention group compared to the EUC group by 29 percent (p = 0.02). Intergenerational intervention effects show higher DHEA levels in infants by 43% (p = 0.03). Infant cortisol and cortisone did not differ across groups.
Conclusions:
Results suggest that the perinatal depression intervention has effects on HPA axis activity in both mothers and children, providing evidence that treating maternal depression may impact physiological stress system functioning intergenerationally. In addition, utilizing hair-derived biomarkers of HPA-axis activity is a potentially useful clinical indicator of intervention impacts on the neuroendocrine system.
Keywords: HPA axis, Cortisol, DHEA, Intervention, Maternal depression, RCT, Infancy, Low and middle income countries
1. Introduction
Dysregulation of the hypothalamic pituitary adrenal (HPA) axis as a result of chronic stress is associated with increased risk for psychopathology and physical health difficulties across the lifespan (McEwen & Seeman, 1999). Improving HPA axis functioning in both parents and children may be a promising avenue for mitigating the impact of chronic stress on families. Insights are especially needed from low resource settings, such as low and middle income countries (LMICs), where families are exposed to persistently stressful environments including high rates of poverty, increased violence exposure, and increased disease risk (Almond, 2009; Mendenhall et al., 2017). In the current study, we added measures of HPA axis activity to an existing randomized control of a community based perinatal psychosocial intervention in rural Pakistan. This intervention was previously shown to improve depressive symptoms in mothers (Maselko et al., 2020a; Rahman et al., 2008; Vanobberghen et al., 2020). The goal of this research is to gain additional insight into the impact of this program by examining intervention effects on HPA axis activity in both mothers and their infants. Objective measures of HPA axis functioning offer another approach to assess intervention impacts, to complement self-report measures.
HPA axis activity can be measured non-invasively through the hormones cortisol, cortisone, and dehydroepiandosterone (DHEA). Cortisol and DHEA act as biological antagonists to one another. DHEA has been shown to offset the toxic effects of prolonged cortisol exposure on neurodevelopment and immune functioning (Kamin & Kertes, 2017). In addition, DHEA contributes to neurodevelopment in infants (Farooqi et al., 2018). This has prompted researchers to examine the cortisol/DHEA ratio as a marker of HPA axis functioning, with the hypothesis that a high ratio (i.e., high cortisol levels and low DHEA levels) reflects dysregulation in the system and places individuals at increased risk for adverse outcomes, such as psychopathology (Chen et al., 2015; Kamin & Kertes, 2017; Maninger et al., 2009). Indeed, studies have shown that decreased DHEA and an increased cortisol/DHEA ratio are both associated with depressive symptoms and life stress (Apter-Levy et al., 2020; Markopoulou et al., 2009; Walther et al., 2019). Similarly, some evidence shows that there are intergenerational effects of parental stress and psychological symptoms on child HPA axis functioning. Studies have demonstrated that maternal histories of depression and trauma are associated with increased salivary cortisol, decreased DHEA, and increased cortisol/DHEA ratios in their children (Goodman et al., 2020; Hagaman et al., 2020; Halligan et al., 2004; Slopen et al., 2018).
Research examining psychosocial interventions has shown that HPA axis activity is a valuable adjunct to self-report measures of intervention effectiveness. Indeed, studies of psychosocial interventions targeting parents and children have shown promising effects on HPA axis activity. For instance, interventions targeting perinatal depression have decreased hair and salivary cortisol, the magnitude of the cortisol awakening response, and increased DHEA in adults (Markopoulou et al., 2009; Richter et al., 2012; Romero-Gonzalez et al., 2020; Urizar & Muñoz, 2011). Some of these interventions show effects on HPA hormones, but not self-reported psychological symptoms (Richter et al., 2012), indicating that HPA axis measures provide additional information beyond what is captured in symptom reports (Rothenberger et al., 2011). Interventions targeting parent-child relationships have shown mixed effects on HPA axis activity in parents and children, with some studies showing improved HPA axis regulation among infants whose parents receive the intervention (Martins et al., 2020). Few studies have examined the effects of psychosocial interventions on both parents and children. One randomized control trial by Urizar and Muñoz found that a cognitive behavioral stress management program delivered prenatally to mothers at risk for depression led to decreased salivary cortisol levels in both mothers and their infants (Urizar & Muñoz, 2011). A small number of studies conducted in LMICs have examined HPA axis activity as an intervention outcome and found emerging evidence that interventions aimed at reducing stress, either by removing stressors (Fernald & Gunnar, 2009; Haushofer et al., 2020; Haushofer & Shapiro, 2016) (e.g. financial stress) or teaching stress management (Dajani et al., 2018), and providing probiotic food supplementation (Brett et al., 2021) can have detectible impacts on individuals’ HPA axis activity in low-resource contexts. To our knowledge, only one study, Dajani et al., 2018, has investigated intervention impacts on hair-derived cortisol levels in LMICs, finding a reduction in HCC in response to a stress management intervention among war-affected adolescents in Jordan.
Most of the previous research investigating impacts of psychological interventions on HPA axis hormones have relied on salivary measures of HPA axis hormones, primarily cortisol (Cristea et al., 2019; Koncz et al., 2021). Although useful for measuring stress reactivity, salivary measures of cortisol have methodological disadvantages due to their high sensitivity to the circadian rhythm of cortisol production and other short-term confounds (Iglesias et al., 2015; Michopoulos et al., 2015; Russell et al., 2012a; Steudte-Schmiedgen et al., 2016). Hair-derived biomarker measurement indicates HPA axis activity over the course of months (analytes in each centimeter of hair approximate hormone secretion over one month), and thus may more accurately reflect chronic hormone secretion (Greff et al., 2019; Russell et al., 2012a). Indeed, a recent study measuring both hair and salivary cortisol in a large sample of children from India found strong positive associations between adversity and HCC, but no relationship between adversity and salivary measures (Bhopal et al., 2019). In addition to Dajani et al., 2018 described above, several studies have evaluated the impact of psychosocial interventions on HCC in high-income countries using randomized design. Romero-Gonzalez et al. (2020) find that a CBT-based intervention reduced HCC in pregnant women. Puhlmann et al. (2021) find that a mindfulness-based intervention reduced HCC and hair cortisone, with no effect on hair DHEA, though three previous studies (Goldberg et al., 2014; Gotink et al., 2017; Younge et al., 2015) evaluating mindfulness-based interventions found no effect on HCC. Analyzing cortisone may give insight into the cumulative amount of active and inactive corticosteroids in the body. In addition, the inclusion of DHEA and the cortisol/DHEA ratio may allow for measurement of multiple facets of the HPA axis and a broader understanding of HPA axis function. Finally, studies examining intervention effects on biomarkers may benefit from including a low-risk comparison group to help contextualize the magnitude and direction of intervention effects (Slopen et al., 2014).
In the current paper we examine whether a psychosocial maternal depression intervention, which has shown effects on maternal mental health (Maselko et al., 2015; Rahman et al., 2008, 2009), may also alter indicators of HPA-axis function in mothers and children. We use data from a subsample of women and their 12-month-old infants who participated in an RCT of the Thinking Healthy Programme Peer-delivered (THPP), and analyze hair-derived cortisol, cortisone, DHEA, and the cortisol/DHEA ratio. The THPP is a psychosocial treatment aimed at improving mothers’ personal health, improving the mother-child relationship, and fostering supportive relationships among mothers’ family and friends (Atif et al., 2017; Sikander et al., 2015, 2019a). This intervention is delivered by lay peers in the community. In addition to examining treatment effects, we also compare HPA axis activity between the two intervention arms and a group of mother-child dyads in which mothers who did not show depressive symptoms during pregnancy.
2. Methods
2.1. Sample and experimental design
The sample for this study comes from a single-blind, stratified cluster-randomized controlled trial that was conducted in Kallar Syedan, a rural subdistrict of Rawalpindi, Pakistan. The sub-district is a socioeconomically deprived area with poverty rates around 50%, female literacy of 40–45%, and a high fertility rate (3.8 births per woman) (National Institute of Pop, 2013). It is largely agrarian with close knit communities co-residing in large households (average 6.2 people per household).
Forty village clusters were randomly allocated in a 1:1 ratio to either the intervention or enhanced usual care (EUC) condition. Cluster randomization was used to avoid contamination within women who lived in the same community. The trial evaluated THPP and the extended version of the intervention, called the Thinking Healthy Programme Peer-delivered Plus (THPP+) (Atif et al., 2019a; Maselko et al., 2020b; Turner et al., 2016). (Clinical trial registration: NCT01826903; Ethics Approval from University of North Carolina at Chapel Hill IRB #19–2706). Eligible participants for the trial were women (aged ≥18 years) in their 3rd trimester of pregnancy (Bhopal et al., 2019). All eligible women were approached by trained research staff and invited to be screened for depression using the Urdu version of the Patient Health Questionnaire-9 (PHQ-9), which has been extensively used as a screening tool in the study setting and has an acceptable criterion validity and reliability for this population (Chronbach’s alpha = 0.844) (Gallis et al., 2018). Women who screened positive for depression (ie, had a PHQ-9 score ≥10) were eligible for enrolment into the trial and follow-up as part of the Bachpan cohort (Sikander et al., 2019b). One of every three women who had a PHQ-9 score of <10 was enrolled to participate in the Bachpan cohort only, resulting in approximately equal numbers of women with and without prenatal depression at the beginning of the cohort (Sikander et al., 2019b). HPA axis measures were not included as outcomes in the pre-registered trial of this intervention, as the main target was maternal depression (NCT01826903).
At the 12-month postnatal follow-up of the trial and cohort study, a random subsample of 177 mother-child dyads was approached for hair collection (forming the sample for our embedded HPA study). Of these, 158 consented, but 54 of the infants did not have at least 1 cm of hair, resulting in a final sample of 104 dyads plus 3 additional hair samples from just mothers. See Fig. S1.
2.2. Intervention
The intervention group received THPP from the third trimester until 6 months postpartum. THPP consisted of individual and group sessions. Individual sessions were delivered weekly during the prenatal period and biweekly between birth and 6 months postpartum. Group sessions were delivered monthly (Atif et al., 2017, 2019a; Maselko et al., 2020b). The intervention group also received THPP+ , a longer-duration continuation of the THPP program. THPP + consisted of 18 group-based “booster” sessions (from 7 months to 36 months postnatal). The first six sessions were delivered monthly, then every 2 months until 36 months postnatal. These sessions were built on the shorter duration THPP intervention and were delivered by the same peers (Atif et al., 2019b; Turner et al., 2016). These sessions aimed for maternal wellbeing and promotion of child development by encouraging mother–infant interaction and play. A detailed list of session timing and content is presented in Table S2. The intervention provided examples of age-appropriate activities, derived from the UNICEF and WHO Care for Development Package, and encouraged demonstration of these activities during the sessions. The peers were lay married women who lived in the same community as that of the women with depression and volunteered their time. The key features of THPP and THPP+ were peer-support, behavioral activation, and problem solving in a culturally sensitive, non-medicalized format, and developmental activities for children up to the 36th month. Peers were trained to use culturally grounded vignettes that served as tools to deliver health and wellbeing messages.
Both the intervention and control groups received enhanced usual care (EUC), which consisted of informing participants about their depression status and ways to seek help for it, informing their respective Lady Health Workers (LHWs) about each woman’s depression status at enrolment, and training all the 11 primary care facility-based physicians in the subdistrict on the mental health Gap Action Programme treatment guidelines for maternal depression (World Health Organization, 2010).
2.3. Patient and public involvement
Our Patient and Public Involvement (PPI) had two important categories a) Lady Health Workers (i.e., government employed community health workers who reside in the same community as the study participants and provide preventive, promotive Maternal and Child Health, and family planning services) and b) the mother-child dyads (i.e., study participants). Both categories were approached prior to the current study. Acceptability and feasibility of collecting hair samples from mothers and their 12-month-old infants was explored from the participants and the LHWs. Based on the feedback and concerns, the study information sheet was developed. This prior engagement through PPI helped streamline the study procedures for collecting hair samples from both mothers and their 12-month-old children. Since the Bachpan cohort study is ongoing, we intend to expand collection of hair samples to all the participants in the subsequent follow ups.
2.4. Measures
2.4.1. Hair derived measures of cortisol, cortisone, and DHEA
Hair samples were collected from women and their infants at 12 months postpartum. A standard protocol for hair sample collection was followed (Russell et al., 2012b). From mothers, approximately 200 strands of hair at least 6 cm long were tied off and then clipped from the posterior vertex. Samples were assayed in 1 cm segments, to 6 cm, representing 6 potential ‘observations. Our analysis allows for the examination of up to 6 months of hormone secretion (corresponding to the period between 6 and 12 months postpartum). No women reported hair treatment, hair product, oral or topical steroid use. The hair samples were collected between April and August 2016, and the laboratory analysis was performed February 2017, at most 10 months following sample collection. Using standardized procedures (Gao et al., 2013a), hair samples were processed and analytes (cortisol, DHEA, and cortisone) were extracted and measured using liquid chromatography mass spectrometry (LC-MS) by Dresden LabService (Gao et al., 2013b; Kirschbaum et al., 2009). The resulting values are presented in picograms per milligram (pg/mg). One woman provided only 5 segments of hair, one woman was excluded because of cortisol levels over 300 pg/mg, and 2 segments from one woman were excluded from DHEA estimation because values were identified as extreme outliers (greater than 6 SDs above the mean). We use the first 3 segments of mother’s hair in the analysis, approximating the past 3 months’ of HPA axis function, as there is degradation of hormone levels in more distal hair segments. Additionally, these correspond more closely to the hair lengths obtained from infants. In total, we utilize 318 segments of cortisol from 106 women, 321 segments from 107 women for cortisone, and 319 segments of DHEA from 107 women. Infants provided only one segment of hair (varying between 1 and 3 cm in length) and no outliers were detected. In total, we have all measurements of infant cortisol, cortisone, and DHEA from 104 infants.
2.4.2. Survey based measures
Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-9), a robust instrument that is validated in our maternal population (Gallis et al., 2018). The PHQ-9 is a nine-item measure that captures depression symptoms within the previous two weeks, with resulting scores ranging from 0 to 27. The PHQ-9 at baseline was used to determine whether the woman was eligible for the clinical trial (“prenatally depressed” if her PHQ-9 score was greater than 9) or not (the “prenatally non-depressed sample”). The Perceived Stress Scale (PSS) was used to measure baseline stress. This 10-item measure assesses the extent to which individuals find their circumstances overwhelming and/or stressful. Each item is rated on a scale from 0 to 4, with a final score ranging from 0 to 40. In our sample, the internal reliability for the PHQ-9 was 0.825 and the PSS was 0.886 (Cronbach’s alpha).
2.5. Statistical analysis
For regression-adjusted estimates of the impact of the intervention, dependent variables (DHEA, cortisol, cortisone, and the cortisol/DHEA ratio) were log-transformed to account for skewed distributions. Because the dependent variables are log-transformed, effect coefficients refer to changes in log points, which approximate percentage changes. Some DHEA levels were below the detectible limit, a total of 17 instances, with 4 women with DHEA levels below the detectible amount across all 3 segments. Five infants also had DHEA levels below detectible amounts. For the regression-adjusted analysis, DHEA levels below detectible amounts were imputed using half of the minimum value (Walther et al., 2019). We conducted power calculations to determine the minimum detectable effect size when examining the difference in hair cortisol concentration between the intervention group (n = 34) and the EUC group (n = 36). With 80% power, a test size of 5%, and accounting for the observed inter-class correlation (0.03) between clusters, we are able to detect a 19% change from the control mean in log-transformed hair cortisol, corresponding to a 0.54 SD difference in means between groups.
We estimated a linear regression of hormone output on an indicator for being randomized into the intervention and calculated randomization inference p-values accounting for clustering at the village level. Due to the randomized study design, results are interpreted as causal effects (Rubin, 1974). Covariates included group indicators (prenatally non-depressed in EUC village, prenatally non-depressed in treatment village), hair segment (indicator variable 1–3), weight of hair sample, and assessor (the researcher who obtained the hair sample). The model was also adjusted for person level variables of child age (days) and sex of the child. Additionally, we included the number of children at baseline and socioeconomic status (through an asset-based measure(Rutstein & Johnson, 2004)) as these characteristics were determined to be imbalanced at baseline. However, results are not sensitive to including these controls. In order to benchmark both the direction and the magnitude of the intervention effects on HPA-axis activity, we examined whether mothers who were screened as depressed at baseline (“prenatally depressed in the EUC arm”) differed from mothers who did not screen positive for depression (“prenatally non-depressed”).
3. Results
The 107 women in our subsample were on average 26.81 years old, with 27 (25%) in their first pregnancy. Comparisons of demographic characteristics within the biomarker sample across treatment arms (see Table 1) revealed only a small difference in parity and socio-economic status in that women in the intervention arm had more children and lower socio-economic status (SES) than those in the EUC arm. All models thus include these two variables as covariates. The joint test across all baseline characteristics cannot reject the null hypothesis that intervention and EUC arms have similar characteristics (p = 0.15).
Table 1.
Sample characteristics by study group.
| (1) |
(2) |
(3) |
|
|---|---|---|---|
| Enhanced Usual Care N = 37 | Intervention N = 35 | Non-depressed N = 35 | |
|
| |||
| mean (sd) | mean (sd) | mean (sd) | |
| Woman’s height (cm) | 156.70 (5.6) | 157.95 (5.7) | 156.34 (6.7) |
| Woman’s weight (kg) | 58.82 (11.0) | 60.62 (10.9) | 60.32 (9.7) |
| Woman’s age | 26.73 (5.2) | 28.03 (4.9) | 25.69 (4.4) |
| Parity | 1.81 (1.6) | 2.60** (1.4) | 1.31 (1.5) |
| Woman’s education (yrs) | 6.38 (4.0) | 6.23 (3.7) | 8.77 (4.0) |
| Husband’s education (yrs) | 8.43 (2.8) | 7.31 (4.0) | 8.80 (3.3) |
| Low SES (poorest 3 quintiles) | 0.62 (0.5) | 0.86** (0.4) | 0.51 (0.5) |
| Baseline depression (PHQ) | 14.76 (3.1) | 16.09 (3.9) | 2.60 (2.3) |
| Baseline stress (PSS) | 23.70 (6.1) | 25.51 (5.5) | 10.60 (4.7) |
| Index child female | 0.59 (0.5) | 0.57 (0.5) | 0.46 (0.5) |
| Age of child (days) | 372.51 (20.4) | 369.69 (14.9) | 374.63 (13.2) |
| Joint test p-value | 0.15 | ||
p < 0.10
p < 0.05
p < 0.01
Significance stars indicate t-tests in the difference in means between intervention and EUC arms (columns 1 and 2).
Comparisons of demographic characteristics between the biomarker subsample and the full sample (see Table S1) also revealed only small differences, with those in the biomarker subsample statistically indistinguishable across the full set of characteristics to the full sample (joint test p = 0.13) and with just one significant difference in that women in the biomarker sample had more children.
Bivariate correlations among study variables are presented in Table 2. In mothers, cortisol and cortisone levels were both significantly positively correlated with baseline depression severity and perceived stress (measured by the PHQ-9 and PSS, respectively), while DHEA levels were significantly negatively correlated. Additionally, women’s weight was significantly positively correlated with cortisol and cortisone but not DHEA. Women’s years of education were positively correlated with maternal DHEA. Child age was negatively correlated with maternal cortisol and cortisone, but not DHEA. In children, maternal depression was not significantly correlated with cortisol or cortisone. There was a significant negative correlation between maternal depression symptoms and child DHEA. In addition, child age was positively correlated with DHEA.
Table 2.
Bivariate correlations between demographic variables and HPA-axis indicators at 12 months postpartum.
| (1) |
(2) |
(3) |
(4) |
(5) |
(6) |
|
|---|---|---|---|---|---|---|
| Maternal Cortisol ln(pg/mg) | Maternal Cortisone ln(pg/mg) | Maternal DHEA ln(pg/mg) | Infant Cortisol ln(pg/mg) | Infant Cortisone ln(pg/mg) | Infant DHEA ln(pg/mg) | |
| Woman’s height (cm) | −0.01 | 0.04 | −0.06 | 0.07 | 0.02 | 0.00 |
| Woman’s weight (kg) | 0.25** | 0.24** | −0.10 | 0.02 | 0.04 | −0.13 |
| Woman’s age | −0.03 | −0.09 | −0.06 | −0.00 | 0.16* | −0.09 |
| Parity | 0.01 | −0.08 | −0.12 | −0.06 | 0.12 | −0.04 |
| Woman’s education (yrs) | 0.07 | 0.08 | 0.17* | 0.10 | 0.04 | 0.00 |
| Husband’s education (yrs) | 0.03 | 0.08 | 0.07 | 0.05 | −0.04 | −0.12 |
| Low SES (poorest 3 quintiles) | 0.02 | −0.04 | 0.01 | −0.15 | 0.05 | −0.02 |
| Baseline depression (PHQ-9) | 0.25** | 0.20** | −0.23** | 0.11 | 0.01 | −0.20** |
| Baseline stress (PSS) | 0.28*** | 0.21** | −0.16* | 0.08 | 0.04 | −0.15 |
| Index child female | 0.05 | −0.00 | −0.04 | 0.09 | 0.02 | 0.04 |
| Age of child (days) | −0.25*** | −0.25*** | 0.11 | 0.06 | −0.03 | 0.20** |
p < 0.10
p < 0.05
p < 0.01
Pairwise correlations between HPA axis hormones and baseline characteristics.
3.1. Intervention effects on mothers
Fig. 1 presents mean levels of cortisol, cortisone, and DHEA across the EUC, intervention, and prenatally non-depressed groups. Fig. 2 shows mother and child biomarkers plotted by group against maternal baseline depression scores. Cortisol and cortisone levels were significantly lower among prenatally depressed women who were randomized into the intervention compared to the EUC group. Intervention effects, based on the adjusted regression models (Table 3), show that the intervention led to a reduction in cortisol levels in the intervention group compared to the EUC group by approximately 38 percent (p = 0.01) and cortisone levels by 45 percent (p < 0.01). DHEA levels were significantly higher in the intervention group compared to the EUC group by approximately 29 percent (p = 0.02). The cortisol/DHEA ratio was 61 percent lower in the intervention group, although this intervention effect did not reach statistical significance (p = 0.09).
Fig. 1.
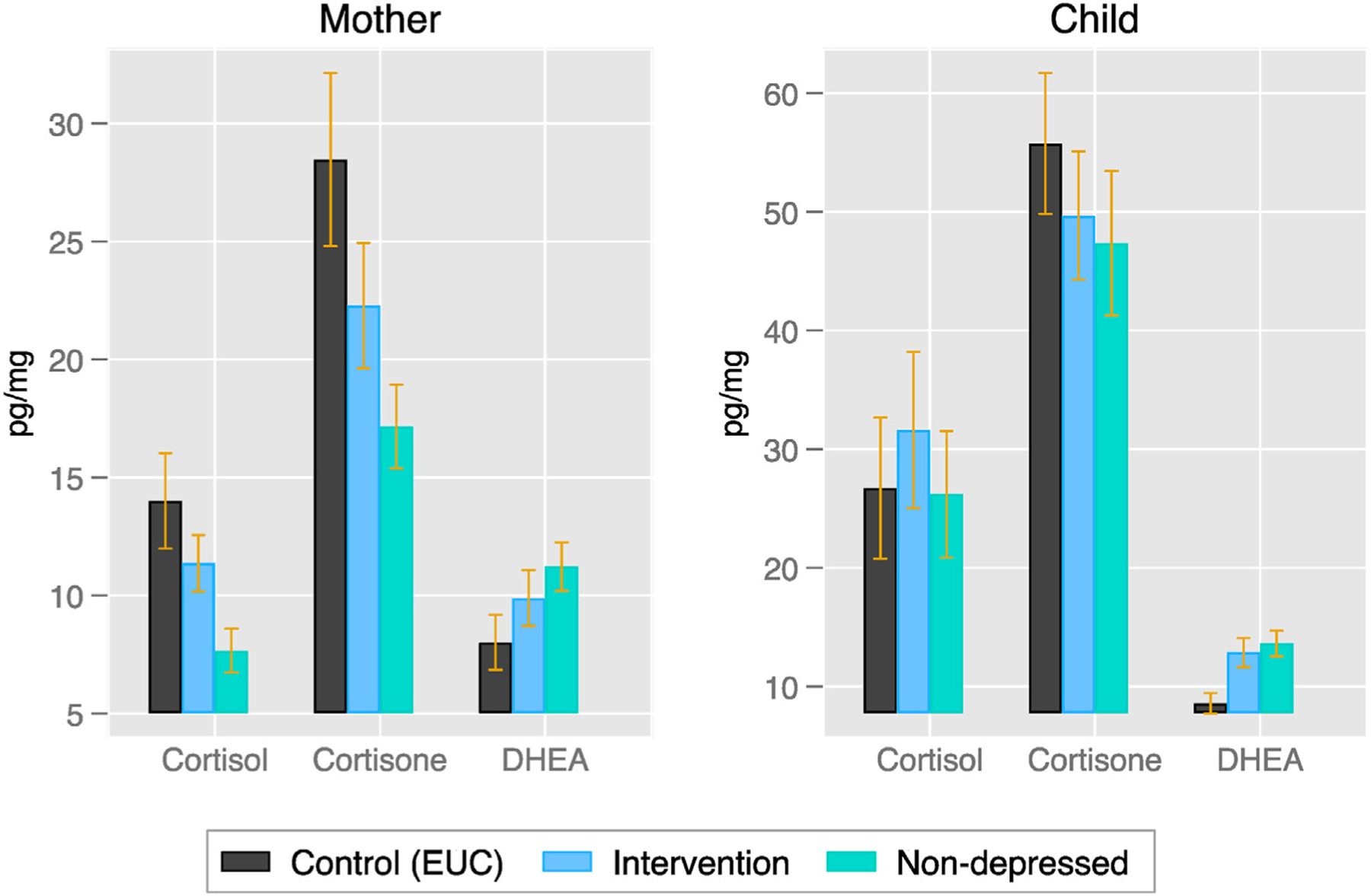
Mother and child biomarkers by study group 12 months postnatally.
Notes: Sample sizes for mothers are 37, 35, and 35 for control (EUC), intervention, and prenatally non-depressed groups respectively. Sample sizes for infants are 36, 34, and 34 for control (EUC), intervention, and non-depressed groups respectively. Error bars indicate 1 SEM. The non-depressed group represents the non-experimental comparison group of women who screened negative (PHQ-9<10) for depression prenatally.
Fig. 2.
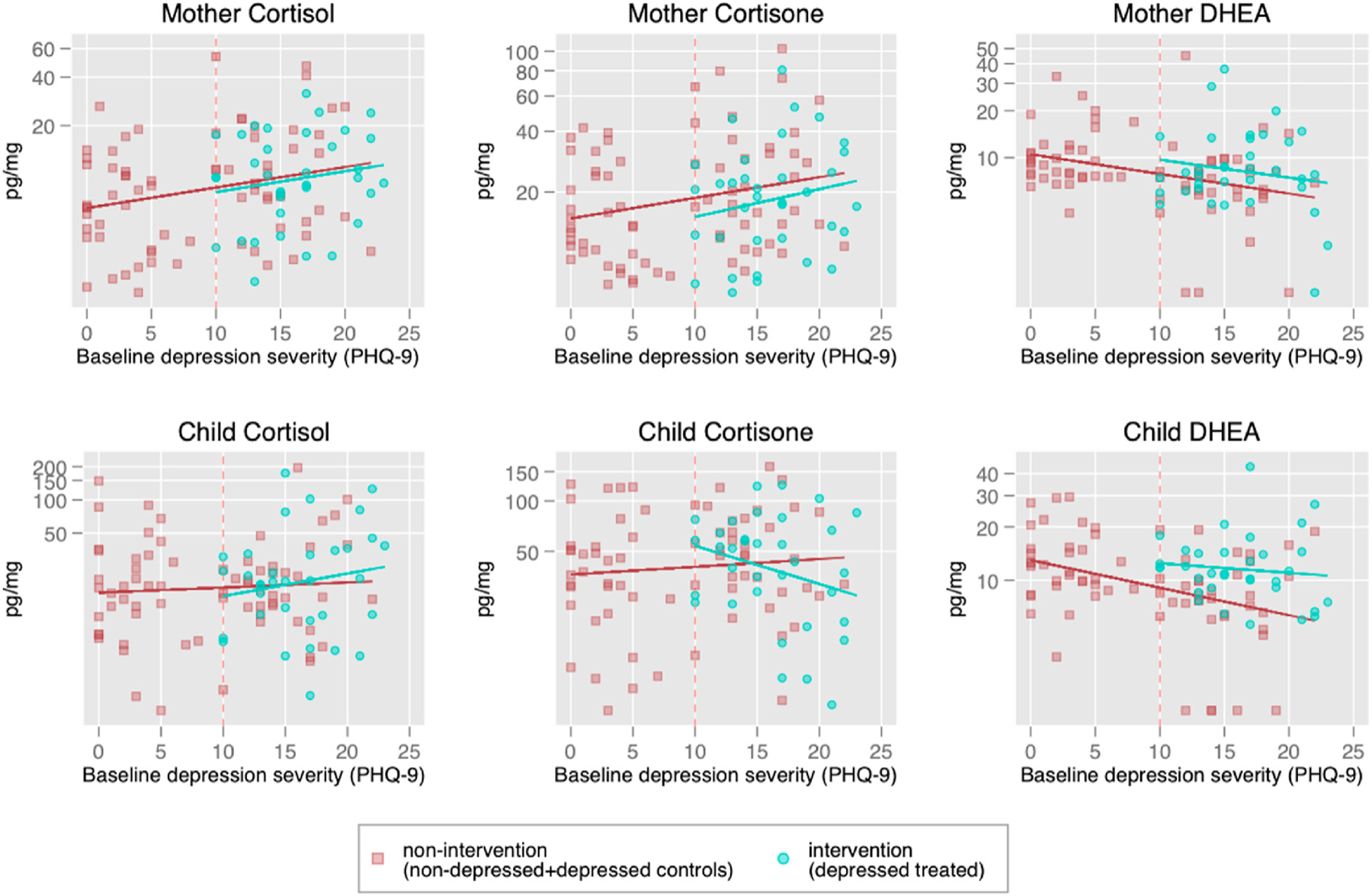
Scatterplots depicting mother and child biomarkers by baseline maternal depression symptom score on the PHQ-9.
Notes: The figures plot the raw data scatterplots of hormone levels (in log-scale) on the y-axis and baseline depression severity (PHQ-9) on the x-axis. Red dashed vertical line represents the PHQ-9 cutoff score of 10. Women who scored 10 or above on the PHQ-9 screened positive for prenatal depression and were included in the trial. Women who scored 9 or lower on the PHQ-9 screened negative for prenatal depression and served as the prenatally non-depressed comparison group. The red solid line represents the regression line of log-hormone on baseline depression severity for women not receiving the intervention (including the baseline non-depressed and baseline depressed randomized into the control (EUC) arm). The green solid line represents the regression line for baseline depression women randomized into the intervention arm.
Table 3.
Intervention effects on mother and infant cortisol, cortisone, and DHEA.
| Intervention (n = 36) |
Enhanced Usual Care (n = 34) |
Treatment effect | p | 95% CI |
||
|---|---|---|---|---|---|---|
| M | M | Lower | Upper | |||
| Maternal cortisol | 11.37 | 14.01 | −0.38 | 0.012 | −0.086 | −0.684 |
| Maternal cortisone | 22.28 | 28.47 | −0.45 | 0.001 | −0.185 | −0.719 |
| Maternal DHEA | 9.88 | 8.01 | 0.29 | 0.024 | 0.040 | 0.544 |
| Maternal cortisol/DHEA ratio | 1.52 | 2.47 | −0.61 | 0.087 | 0.091 | −1.32 |
| Infant cortisol | 31.62 | 26.73 | 0.09 | 0.845 | −0.849 | 1.035 |
| Infant cortisone | 49.69 | 55.77 | −0.27 | 0.420 | 0.396 | −0.939 |
| Infant DHEA | 12.87 | 8.59 | 0.43 | 0.034 | 0.034 | 0.836 |
| Infant cortisol/DHEA ratio | 2.65 | 3.32 | −0.29 | 0.569 | 0.728 | −1.312 |
Notes: Mean cortisol, cortisone, and DHEA values are untransformed and presented in pg/mg. Hormone values were log-transformed for analysis. Treatment effect estimates are based on linear regression, with randomization inference p-values clustered at the level of randomization. All estimates control for segment, assessor, weight of hair sample, age of mother in years (for maternal samples) and child days since birth (for infant samples), gender of the child in the womb at the start of the intervention, maternal parity at baseline and SES quintile.
Prenatally non-depressed mothers exhibited significantly lower levels of cortisol and cortisone, significantly higher levels of DHEA, and a significantly higher cortisol/DHEA ratio compared to the prenatally depressed women in the EUC group (p < 0.01 for all four indices, Fig. 1 and Table S3). The intervention shifted all levels among women in the treatment arm away from the levels of women in the EUC arm and closer to the levels of the prenatally non-depressed women (Fig. 1).
3.2. Intervention effects on infants
Infant cortisol and cortisone did not differ between the intervention and EUC groups (see Fig. 1). Infants whose mothers participated in the intervention showed higher DHEA than infants whose mothers were in the EUC group. Intervention effects (Table 3) show that the DHEA levels in infants in the intervention group were approximately 43% higher (p=0.03). There was no intervention effect on the cortisol/DHEA ratio in infants. There were no differences between the prenatally non-depressed group and the prenatally depressed groups on infant cortisol and cortisone (see Fig. 1). Infants in the EUC group showed lower DHEA compared to infants in the prenatally non-depressed group (p = 0.02) However those in the intervention group were statistically indistinguishable from infants in the prenatally non-depressed group on DHEA (p = 0.79). In addition, there were no differences between the depressed and non-depressed groups on the cortisol/DHEA ratio.
4. Discussion
The goal of this study was to examine the effects of a maternal psychosocial intervention on hair derived HPA axis biomarkers in both mothers and children living in rural Pakistan. At 12 months postpartum, mothers randomized to the intervention had lower cortisol, lower cortisone, and higher DHEA compared to mothers who were randomized into enhanced usual care. Women who received the intervention had hormone levels approximating levels in the prenatally non-depressed comparison group. These findings align with previous work suggesting that psychosocial interventions can result in lower circulating cortisol and higher DHEA in saliva(Cristea et al., 2019; McKay & Zakzanis, 2010) and hair (Dajani et al., 2018; Panter-Brick et al., 2020).
The intervention may affect the maternal HPA axis through reduction of depressive symptoms. In addition, mothers who receive the intervention may utilize effective coping skills, leverage support resources, and experience less stress on a daily basis. Both depressive symptoms and perceived stress are related to dysregulation in the HPA axis (Staufenbiel et al., 2013) and are modifiable following intervention. In addition, depression is often comorbid with other psychological symptoms (e.g., anxiety) that my cause additional stress and HPA axis dysregulation. Future research could explore the relative impacts of these factors and specify the mechanisms by which depression interventions may impact cortisol, cortisone, and DHEA.
Past research has implicated both high and low HPA axis activity in the pathogenesis of psychopathology following stressful experiences (Koss & Gunnar, 2018). The results of this study suggest that a maternal psychosocial intervention leads to lower cortisol and cortisone. This may suggest that high HPA axis activity is especially relevant for depression, the main target of this intervention. Indeed, past research has shown that high cortisol is consistently related to depression, whereas the relation between other forms of psychopathology (e.g., PTSD, bipolar disorder) and cortisol is less consistent (Staufenbiel et al., 2013). However, it is important to note that the relationship between HPA axis activity and psychopathology may be altered based on the timing and severity of previous stress exposure (Cristea et al., 2019). Characterizing the effects of stress exposure, in addition to intervention effects, on HPA axis activity will be an important avenue for future research.
In infants, there were no intervention effects on cortisol or cortisone, although there was also no difference in these hormones between infants in the depressed EUC and the non-depressed group. There was an intervention effect on DHEA such that infants whose mothers received the intervention showed higher DHEA compared to infants whose mothers received enhanced usual care. In addition, infants in the intervention group showed DHEA levels that were similar to DHEA in infants of prenatally non-depressed mothers. This is important as higher DHEA may support neurodevelopment in children. Studies have shown that DHEA has neuroprotective properties and may contribute to neural plasticity and adaptation following stress (Greaves et al., 2019). Further work supports the protective effects of DHEA against psychopathology throughout childhood, indicating that increased DHEA levels are associated with lower rates of internalizing and externalizing symptoms (Dorn et al., 2009; Kimonis et al., 2019; Mulligan et al., 2020). Our findings suggest that infant hair DHEA is sensitive to maternal depressive symptoms and modifiable with maternal psychosocial intervention, but cortisol and cortisone are not. The lack of treatment effects on cortisol and cortisone aligns with some previous work showing that infant cortisol is becomes increasingly sensitive to maternal stress as children age (Laurent, 2017; Slopen et al., 2018).
This intervention may impact infant DHEA through modifying maternal HPA axis activity. Mothers who receive the intervention may show lower cortisol output during pregnancy, thus limiting infant exposure to glucocorticoids in utero and decreasing HPA axis alteration during this period (Glover et al., 2010). Decreased maternal depression symptoms may also be a mechanism linking this intervention to altered HPA axis activity in infants. Decreased depression symptoms in mothers may lead to decreased conflict in the home, improved mother-infant interactions, and an improved mother-child attachment, all of which may impact infant HPA axis regulation (Koss & Gunnar, 2018).
This study has several limitations, including the lack of a baseline measure of HPA axis activity in mothers. Without a baseline hormone measure, we cannot test whether cortisol, cortisone, and DHEA change over time in this sample. We also cannot rule out the possibility that the hormone differences we observe across groups at 12 months postpartum actually reflect baseline differences, and not intervention effects. However, we randomly assigned participants to the EUC and intervention groups and found that there were few differences between those groups on baseline demographic characteristics (see Table 1). This increases our confidence in the assumption that baseline HPA axis activity was similar between the EUC and intervention groups. An additional limitation is that HPA axis measures were not included as an outcome in the pre-registered trial of this intervention (NCT01826903). In addition, the main target of the intervention was maternal depression, not HPA axis functioning, therefore the intervention was not explicitly designed to manipulate HPA axis activity. Finally, our sample size was somewhat small, which limited additional analyses such as those between different subgroups or tests of mediation.
In this study, we utilize hair-derived hormones to measure HPA axis activity, meaning we measure average hormone secretion over the course of months. This allows us to gain a better understanding of chronic HPA axis activity. However, this measure does not capture short-term changes in HPA axis hormones that may be apparent with other sampling methodologies (e.g., high frequency saliva measures). This is especially important to consider in infants, who may show increased variability in hormone secretion during the newborn period (Parker, 1999). Additionally, this study was not powered to examine potential moderators (e.g., family history of psychopathology) or mediators (e.g., mother-child interactions, relationship satisfaction between the father and mother, breastfeeding) of treatment effects. Future studies would benefit by considering these factors.
There is some evidence that shorter duration stressors increase HPA axis activity, while chronic and longer-duration stress may lead to cortisol hyposecretion or blunting in some cases (Miller et al., 2007; Steudte-Schmiedgen et al., 2016). Few studies have incorporated the potential for hyper- and hyposecretion in the analysis, an exception being Dajani et al., 2018. The authors first analyzed trajectories of cortisol production using longitudinal data, identifying 3 latent classes of trajectories: hypersecretion, medium, and hyposecretion. They then explored how the impacts of the stress management intervention varied by which latent class an individual belonged to. They found that for adolescents with medium or hypersecretion, cortisol levels declined with intervention, while adolescents with hypo-secretion, cortisol levels increased. Due to our limited sample size, and that we collected hair samples at one point in time, we are unable to conduct a trajectory analysis to identify study participants that might be experiencing blunting. Thus, the average treatment effect we estimate may mask important heterogeneity due to blunting or hypo-secretion. While it is impossible to identify if individuals in our sample had levels of cortisol that were already “too low” (ie, blunted), we can use the low-risk comparison group as a benchmark to try to understand if the reduction cortisol due to intervention constitutes a positive result, on average. Since the low-risk comparison group also had lower cortisol than depressed controls, the intervention helped to bring the depressed women closer to the low-risk comparison, on average. Further research is needed to learn the prevalence of blunting or hyposecretion and how intervention effects differ by different patterns of HPA axis dysfunction.
Our study has several key strengths. First, the experimental design allows us to identify causal effects of a depression intervention on HPA axis activity. Second, the additional comparison group of prenatally non-depressed mothers provides a benchmark for the magnitude and direction of intervention effects. Third, we capture multiple HPA axis hormones, analyzing three steroid hormones simultaneously. Finally, the study was conducted in a high-risk population from a developing country, which provides much needed evidence outside economically developed Western populations that have been the focus of many previous studies (e.g., Urizar & Muñoz, 2011). As a research study conducted within a low resource, non-western setting, these findings add to the literature relevant to underserved populations in many parts of the world (Bhopal et al., 2019; Dajani et al., 2018; Etwel et al., 2014; Panter-Brick et al., 2020; Schindler et al., 2019; Shaheen et al., 2020; Steudte et al., 2011).
The findings of this study suggest that hair-derived HPA axis hormones are a promising avenue for future global health research. HPA axis activity is potentially an important mechanism shaping mental and physical health, and thus may be a target for intervention efforts or mechanistic studies. Hair-derived hormones are simple to collect and store, relatively cost-effective, and offer a measure of health that is comparable across cultures, unlike questionnaire or interview measures. Finally, as shown in the current study, HPA axis hormones may be modifiable following psychosocial intervention and thus could be valuable markers of intervention effectiveness.
This study advances our understanding of how a maternal psychosocial intervention affects HPA axis activity in both mothers and children living in rural Pakistan. Findings suggest that treating maternal depression may result in meaningful changes in HPA axis biomarkers across generations, and thus may be an avenue for preventing the intergenerational transmission of psychopathology. Further work is needed to clarify the mechanisms by which maternal psychosocial intervention may lead to altered hormonal output in mothers and children, as well as the impact of intervention and HPA axis functioning on child development trajectories over time.
Supplementary Material
Acknowledgements
We would like to thank the team at the Human Development Research Foundation (HDRF), including Rakshanda Liaqat, Tayyiba Abbasi, Maria Sharif, Samina Bilal, Anum Nisar, Amina Bibi, Shaffaq Zufiqar, Ahmed Zaidi, Ikhlaq Ahmad, and Najia Atif. We are also grateful for the assistance and inputs of Prof. Fareed Aslam Minhas from the Institute of Psychiatry, Rawalpindi Medical University. Finally, we are very grateful to the families who participated in the study.
Funding sources
This work was supported by the University of Melbourne, ARC Centre of Excellence - The Life Course Centre, MiSoC ISER at University of Essex, NIH R01HD075875, NIMH U19MH95687, NICHD R03HD097434 and NICHD T32HD007168.
Footnotes
Statement of ethics
This study was approved by institutional review boards at the University of North Carolina at Chapel Hill (#19–2706), Duke University, and Rawalpindi Medical University. Written informed consent (or witnessed consent, if the participant was illiterate) was obtained from mothers before study participation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmmh.2022.100082.
References
- Almond P (2009). Postnatal depression: A global public health perspective. Perspect. Publ. Health, 129(5), 221–227. [DOI] [PubMed] [Google Scholar]
- Apter-Levy Y, Zagoory-Sharon O, & Feldman R (2020). Chronic depression alters mothers’ DHEA and DEHA-to-cortisol ratio: Implications for maternal behavior and child outcomes. Frontiers in Psychiatry, 11, 728, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif N, Bibi A, Nisar A, Zulfiqar S, Ahmed I, LeMasters K, et al. (2019a). Delivering maternal mental health through peer volunteers: A 5-year report. International Journal of Mental Health Systems, 13(1), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif N, Krishna RN, Sikander S, Lazarus A, Nisar A, Ahmad I, et al. (2017). Mother-to-mother therapy in India and Pakistan: Adaptation and feasibility evaluation of the peer-delivered thinking Healthy Programme. BMC Psychiatry, 17(1), 79, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif N, Nisar A, Bibi A, Khan S, Zulfiqar S, Ahmad I, et al. (2019b). Scaling-up psychological interventions in resource-poor settings: Training and supervising peer volunteers to deliver the ‘thinking Healthy Programme’ for perinatal depression in rural Pakistan. Glob. Mental Health, 6, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal S, Verma D, Roy R, Soremekun S, Kumar D, Bristow M, et al. (2019). The contribution of childhood adversity to cortisol measures of early life stress amongst infants in rural India: Findings from the early life stress sub-study of the SPRING cluster randomised controlled trial (SPRING-ELS). Psychoneuroendocrinology, 107, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett BE, Koko BK, Doumbia HO, Koffi FK, Assa SE, Zahé KY, et al. (2021). Salivary biomarkers of stress and inflammation in first graders in Côte d΄ Ivoire: Effects of a probiotic food intervention. Psychoneuroendocrinology, 129, 105255. [DOI] [PubMed] [Google Scholar]
- Chen FR, Raine A, & Granger DA (2015). Tactics for modeling multiple salivary analyte data in relation to behavior problems: Additive, ratio, and interaction effects. Psychoneuroendocrinology, 51, 188–200. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Karyotaki E, Hollon SD, Cuijpers P, & Gentili C (2019). Biological markers evaluated in randomized trials of psychological treatments for depression: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 101, 32–44. [DOI] [PubMed] [Google Scholar]
- Dajani R, Hadfield K, van Uum S, Greff M, & Panter-Brick C (2018). Hair cortisol concentrations in war-affected adolescents: A prospective intervention trial. Psychoneuroendocrinology, 89, 138–146. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Kolko DJ, Susman EJ, Huang B, Stein H, Music E, et al. (2009). Salivary gonadal and adrenal hormone differences in boys and girls with and without disruptive behavior disorders: Contextual variants. Biological Psychology, 81(1), 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etwel F, Russell E, Rieder MJ, Van Uum SH, & Koren G (2014). Hair cortisol as a biomarker of stress in the 2011 Libyan war. Clinical and Investigative Medicine, E403–E408. [DOI] [PubMed] [Google Scholar]
- Farooqi NA, Scotti M, Lew JM, Botteron KN, Karama S, McCracken JT, et al. (2018). Role of DHEA and cortisol in prefrontal-amygdalar development and working memory. Psychoneuroendocrinology, 98, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, & Gunnar MR (2009). Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Sci. Med, 68(12), 2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis J, Maselko J, O’Donnell K, Song K, Saqib K, Turner EL, et al. (2018). Criterion-related validity and reliability of the Urdu version of the patient health questionnaire in community-based pregnant women in Pakistan PeerJ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, & Kirschbaum C (2013a). Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. Journal of Chromatography B, 928, 1–8. [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, & Kirschbaum C (2013. Jun 01). Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J. Chromatogr. B, Anal. Technol. Biomed. Life Sci, 928, 1–8. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, & O’Donnell K (2010). Prenatal stress and the programming of the HPA axis. Neuroscience & Biobehavioral Reviews, 35(1), 17–22. [DOI] [PubMed] [Google Scholar]
- Goldberg SB, Manley AR, Smith SS, Greeson JM, Russell E, Van Uum S, et al. (2014). Hair cortisol as a biomarker of stress in mindfulness training for smokers. Journal of Alternative & Complementary Medicine, 20(8), 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, & Halperin MS (2020). Perinatal depression as an early stres: Risk for the development of psychopathology in children. In Harnkness K, & Hayden EP (Eds.), The oxford handbook of stress and mental health New York, NY: Oxford University Press. [Google Scholar]
- Gotink RA, Younge JO, Wery MF, Utens EM, Michels M, Rizopoulos D, et al. (2017). Online mindfulness as a promising method to improve exercise capacity in heart disease: 12-month follow-up of a randomized controlled trial. PLoS One, 12(5), Article e0175923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves RF, Wudy SA, Badoer E, Zacharin M, Hirst JJ, Quinn T, et al. (2019). A tale of two steroids: The importance of the androgens DHEA and DHEAS for early neurodevelopment. The Journal of Steroid Biochemistry and Molecular Biology, 188, 77–85. [DOI] [PubMed] [Google Scholar]
- Greff MJ, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, & van Uum SH (2019). Hair cortisol analysis: An update on methodological considerations and clinical applications. Clinical Biochemistry, 63, 1–9. [DOI] [PubMed] [Google Scholar]
- Hagaman AK, Baranov V, Chung E, LeMasters K, Andrabi N, Bates LM, et al. (2020). Association of maternal depression and home adversities with infant hypothalamic-pituitary-adrenal (HPA) axis biomarkers in rural Pakistan. Journal of Affective Disorders, 276, 592–599, 2020/11/01/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, & Murray L (2004). Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry, 55(4), 376–381. [DOI] [PubMed] [Google Scholar]
- Haushofer J, Chemin M, Jang C, & Abraham J (2020). Economic and psychological effects of health insurance and cash transfers: Evidence from a randomized experiment in Kenya. Journal of Development Economics, 144, 102416. [Google Scholar]
- Haushofer J, & Shapiro J (2016). The short-term impact of unconditional cash transfers to the poor: Experimental evidence from Kenya. Quarterly Journal of Economics, 131(4), 1973–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias S, Jacobsen D, Gonzalez D, Azzara S, Repetto EM, Jamardo J, et al. (2015). Hair cortisol: A new tool for evaluating stress in programs of stress management. Life Sciences, 141, 188–192. [DOI] [PubMed] [Google Scholar]
- Kamin HS, & Kertes DA (2017). Cortisol and DHEA in development and psychopathology. Hormones and Behavior, 89, 69–85, 2017/03/01/. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Fleming GE, Wilbur RR, Groer MW, & Granger DA (2019). Dehydroepiandrosterone (DHEA) and its ratio to cortisol moderate associations between maltreatment and psychopathology in male juvenile offenders. Psychoneuroendocrinology, 101, 263–271. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, & Dettenborn L (2009). Hair as a retrospective calendar of cortisol production—increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34(1), 32–37. [DOI] [PubMed] [Google Scholar]
- Koncz A, Demetrovics Z, & Takacs ZK (2021). Meditation interventions efficiently reduce cortisol levels of at-risk samples: A meta-analysis. Health Psychology Review, 15(1), 56–84. [DOI] [PubMed] [Google Scholar]
- Koss KJ, & Gunnar MR (2018). Annual Research Review: Early adversity, the hypothalamic-pituitary-adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry, 59(4), 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H (2017). Early calibration of the HPA axis by maternal psychopathology. Psychoneuroendocrinology, 78, 177–184. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, & Mellon SH (2009). Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in Neuroendocrinology, 30(1), 65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markopoulou K, Papadopoulos A, Juruena MF, Poon L, Pariante CM, & Cleare AJ (2009). The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology, 34(1), 19–26. [DOI] [PubMed] [Google Scholar]
- Martins RC, Blumenberg C, Tovo-Rodrigues L, Gonzalez A, & Murray J (2020). Effects of parenting interventions on child and caregiver cortisol levels: Systematic review and meta-analysis. BMC Psychiatry, 20(1), 370, 2020/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselko J, Sikander S, Bhalotra S, Bangash O, Ganga N, Mukherjee S, et al. (2015). Effect of an early perinatal depression intervention on long-term child development outcomes: Follow-up of the thinking Healthy Programme randomised controlled trial. The Lancet Psychiatry, 2(7), 609–617. [DOI] [PubMed] [Google Scholar]
- Maselko J, Sikander S, Turner EL, Bates LM, Ahmad I, Atif N, et al. (2020a). Effectiveness of a peer-delivered, psychosocial intervention on maternal depression and child development at 3 years postnatal: A cluster randomised trial in Pakistan. The Lancet Psychiatry, 7(9), 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselko J, Sikander S, Turner EL, Bates LM, Ahmad I, Atif N, et al. (2020. Sepb). Effectiveness of a peer-delivered, psychosocial intervention on maternal depression and child development at 3 years postnatal: A cluster randomised trial in Pakistan. The Lancet Psychiatry, 7(9), 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Seeman T (1999). Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences, 896(1), 30–47. [DOI] [PubMed] [Google Scholar]
- McKay MS, & Zakzanis KK (2010). The impact of treatment on HPA axis activity in unipolar major depression. Journal of Psychiatric Research, 44(3), 183–192. [DOI] [PubMed] [Google Scholar]
- Mendenhall E, Kohrt BA, Norris SA, Ndetei D, & Prabhakaran D (2017). Non-communicable disease syndemics: Poverty, depression, and diabetes among low-income populations. Lancet (London, England), 389(10072), 951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Norrholm SD, & Jovanovic T (2015). Diagnostic biomarkers for posttraumatic stress disorder: Promising horizons from translational neuroscience research. Biological Psychiatry, 78(5), 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, & Zhou E (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25. [DOI] [PubMed] [Google Scholar]
- Mulligan EM, Hajcak G, Crisler S, & Meyer A (2020). Increased dehydroepiandrosterone (DHEA) is associated with anxiety in adolescent girls. Psychoneuroendocrinology, 119, 104751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Population Studies Islamabad. (2013). Pakistan demographic and health survey Islamabad, Pakistan: National Institute of Population Studies. [Google Scholar]
- Panter-Brick C, Wiley K, Sancilio A, Dajani R, & Hadfield K (2020). C-reactive protein, Epstein-Barr virus, and cortisol trajectories in refugee and non-refugee youth: Links with stress, mental health, and cognitive function during a randomized controlled trial. Brain, Behavior, and Immunity, 87, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CR (1999). Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids, 64(9), 640–647. [DOI] [PubMed] [Google Scholar]
- Puhlmann LM, Vrtička P, Linz R, Stalder T, Kirschbaum C, Engert V, et al. (2021). Contemplative mental training reduces hair glucocorticoid levels in a randomized clinical trial. Psychosomatic Medicine, 83(8), 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Iqbal Z, Roberts C, & Husain N (2009). Cluster randomized trial of a parent-based intervention to support early development of children in a low-income country. Child: Care, Health and Development, 35(1), 56–62. [DOI] [PubMed] [Google Scholar]
- Rahman A, Malik A, Sikander S, Roberts C, & Creed F (2008). Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: A cluster-randomised controlled trial. The Lancet, 372(9642), 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Bittner A, Petrowski K, Junge-Hoffmeister J, Bergmann S, Joraschky P, et al. (2012). Effects of an early intervention on perceived stress and diurnal cortisol in pregnant women with elevated stress, anxiety, and depressive symptomatology. Journal of Psychosomatic Obstetrics and Gynecology, 33(4), 162–170. [DOI] [PubMed] [Google Scholar]
- Romero-Gonzalez B, Puertas-Gonzalez JA, Strivens-Vilchez H, Gonzalez-Perez R, & Peralta-Ramirez MI (2020. 2020/08/01/). Effects of cognitive-behavioural therapy for stress management on stress and hair cortisol levels in pregnant women: A randomised controlled trial. Journal of Psychosomatic Research, 135, 110162. [DOI] [PubMed] [Google Scholar]
- Rothenberger SE, Moehler E, Reck C, & Resch F (2011). Prenatal stress: Course and interrelation of emotional and physiological stress measures. Psychopathology, 44(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Rubin DB (1974). Estimating causal effects of treatments in randomized and nonrandomized studies. Journal of Educational Psychology, 66(5), 688. [Google Scholar]
- Russell E, Koren G, Rieder M, & Uum SV (2012b). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, & Van Uum S (2012a). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. [DOI] [PubMed] [Google Scholar]
- Rutstein SO, & Johnson K (2004). The DHS wealth index. DHS comparative reports no. 6 Calverton: ORC Macro. [Google Scholar]
- Schindler L, Shaheen M, Saar-Ashkenazy R, Bani Odeh K, Sass S-H, Friedman A, et al. (2019). Victims of war: Dehydroepiandrosterone concentrations in hair and their associations with trauma sequelae in palestinian adolescents living in the west bank. Brain Sciences, 9(2), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen M, Schindler L, Saar-Ashkenazy R, Bani Odeh K, Soreq H, Friedman A, et al. (2020). Victims of war—psychoendocrine evidence for the impact of traumatic stress on psychological well-being of adolescents growing up during the Israeli–Palestinian conflict. Psychophysiology, 57(1), Article e13271. [DOI] [PubMed] [Google Scholar]
- Sikander S, Ahmad I, Atif N, Zaidi A, Vanobberghen F, Weiss HA, et al. (2019a). Delivering the thinking Healthy Programme for perinatal depression through volunteer peers: A cluster randomised controlled trial in Pakistan. The Lancet Psychiatry, 6(2), 128–139. [DOI] [PubMed] [Google Scholar]
- Sikander S, Ahmad I, Bates LM, Gallis J, Hagaman A, O’Donnell K, et al. (2019b). Cohort Profile: Perinatal depression and child socioemotional development ; the Bachpan cohort study from rural Pakistan. BMJ Open, 9(5), Article e025644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikander S, Lazarus A, Bangash O, Fuhr DC, Weobong B, Krishna RN, et al. (2015). The effectiveness and cost-effectiveness of the peer-delivered thinking Healthy Programme for perinatal depression in Pakistan and India: The SHARE study protocol for randomised controlled trials. Trials, 16(1), 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, McLaughlin KA, & Shonkoff JP (2014). Interventions to improve cortisol regulation in children: A systematic review. Pediatrics, 133(2), 312–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Roberts AL, LeWinn KZ, Bush NR, Rovnaghi CR, Tylavsky F, et al. (2018). Maternal experiences of trauma and hair cortisol in early childhood in a prospective cohort. Psychoneuroendocrinology, 98, 168–176, 2018/12/01/. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, & van Rossum EF (2013). Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology, 38(8), 1220–1235. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Kirschbaum C, Alexander N, & Stalder T (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience & Biobehavioral Reviews, 69, 124–135. [DOI] [PubMed] [Google Scholar]
- Steudte S, Kolassa I-T, Stalder T, Pfeiffer A, Kirschbaum C, & Elbert T (2011). Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology, 36(8), 1193–1200. [DOI] [PubMed] [Google Scholar]
- Turner EL, Sikander S, Bangash O, Zaidi A, Bates L, Gallis J, et al. (2016. 2016). The effectiveness of the peer-delivered thinking Healthy PLUS (THPP+) program for maternal depression and child socioemotional development in Pakistan: Study protocol for a randomized controlled trial. BioMed Central Trials, 17, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar GG Jr., & Muñoz RF (2011). Impact of a prenatal cognitive-behavioral stress management intervention on salivary cortisol levels in low-income mothers and their infants. Psychoneuroendocrinology, 36(10), 1480–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanobberghen F, Weiss HA, Fuhr DC, Sikander S, Afonso E, Ahmad I, et al. (2020). Effectiveness of the Thinking Healthy Programme for perinatal depression delivered through peers: Pooled analysis of two randomized controlled trials in India and Pakistan. Journal of Affective Disorders, 265, 660–668, 2020/03/15/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Tsao C, Pande R, Kirschbaum C, Field E, & Berkman L (2019). Do dehydroepiandrosterone, progesterone, and testosterone influence women’s depression and anxiety levels? Evidence from hair-based hormonal measures of 2105 rural Indian women. Psychoneuroendocrinology, 109, 104382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2010). mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings: mental health Gap Action Programme (mhGAP) Geneva: World Health Organization. [PubMed] [Google Scholar]
- Younge J, Wester V, Van Rossum E, Gotink R, Wery MF, Utens E, et al. (2015). Cortisol levels in scalp hair of patients with structural heart disease. International Journal of Cardiology, 184, 71–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


