Abstract
Adaptive immunity is didactically partitioned into humoral and cell-mediated effector mechanisms, which may imply that each arm is separate and does not function together. Here, we report that the activation of CD8+ resident memory T cells (TRM) in nonlymphoid tissues triggers vascular permeability, which facilitates rapid distribution of serum antibodies into local tissues. TRM reactivation was associated with transcriptional upregulation of antiviral signaling pathways as well as Fc receptors and components of the complement cascade. Effects were local, but evidence is presented that TRM in brain and reproductive mucosa are both competent to induce rapid antibody exudation. TRM reactivation in the mouse female genital tract increased local concentrations of virus-specific neutralizing antibodies, including anti-vesicular stomatitis virus, and passively transferred anti-HIV antibodies. We showed that this response was sufficient to increase the efficacy of ex vivo vesicular stomatitis virus neutralization. These results indicate that CD8+ TRM antigen recognition can enhance local humoral immunity.
INTRODUCTION
Cell-mediated immunity, comprised principally of CD8+ and CD4+ T cells, controls intracellular infections and tumor cells via mechanisms requiring cell-to-cell contact. Humoral immunity, comprised of B cells and antibody, controls infectious agents within the extracellular environment. Cell-mediated and humoral immunity are generally conceptualized as two separate arms of the immune system; however, there are examples of crosstalk. For example, some evidence indicates that CD8+ T cells may be able to execute antibody-dependent cell-mediated cytotoxicity1. Moreover, recent vaccine studies indicate that cell-mediated immunity may enhance protective immunity against vaginal simian immunodeficiency virus (SIV) or simian-HIV (SHIV) challenge in the presence of systemic humoral immunity2,3. Whether enhanced protection resulted from two completely independent mechanisms of protection or synergy between antibody and T cells was not investigated.
Tissue-resident memory T cells (TRM) stably patrol regional compartments without recirculating through blood or other organs and are abundant in mice and humans4–9. When established CD8+ TRM re-encounter cognate antigen, they not only kill target cells but also communicate antigen recognition to other local parenchymal and hematopoietic cells, inducing a local anti-pathogen and immunostimulatory state10–13. This response can protect against pathogen re-encounter, which has been studied extensively at barrier sites such as the skin13–16, and mucosal tissues including the lung17–25, and female reproductive tract10,26–29. The ability of TRM to orchestrate immune responses that span innate and adaptive immunity is one of many examples by which the immune system shares information between cell types. Antibody, complement proteins, and other immune effectors patrol vascular compartments and are largely dependent on transudation from serum to accumulate within tissues30,31. Neutralization of many pathogens, SIV or HIV, for example, at mucosal barrier sites, requires high local antibody concentrations that are difficult to durably establish through vaccination2,32–35. Whether tissue-resident T cells can trigger the local exudation of serum immune effector molecules is unknown.
Here, we describe a new function for CD8+ TRM in mediating local increases in vascular permeability within hours of reactivation, resulting in extravasation of circulating antibody. This increase in antibody afforded heightened neutralization of virus ex vivo. These data indicate a form of synergy between CD8+ T cells and humoral immunity and provide one rationale for including both arms of the immune system in vaccine design against certain intracellular pathogens in cases where antibody alone may not be locally available and durably protective.
RESULTS
To study the impact of TRM reactivation in the female reproductive tract (FRT), which, in this study, includes both the uterus and vagina unless noted, and gain insights into potential mechanisms of humoral crosstalk, we established a trackable population of virus-specific memory T cells, taking advantage of transgenic P14 T cells specific for the gp33 epitope of lymphocytic choriomeningitis virus (LCMV). We transferred a small number of naïve Thy1.1 or CD45.1 P14 cells into CD45.2 congenically distinct wild-type C57BL/6 host mice. One day after T cell transfer, we infected mice with LCMV via the intraperitoneal route, which results in the durable establishment of broadly distributed memory T cells, including TRM in the FRT (Fig. 1A)10,11,36. These mice will be referred to as P14 immune chimeras. To first understand the transcriptional changes that occur locally in the FRT when TRM re-encounter antigen, we took a reductionist approach to trigger TRM reactivation in the absence of viral reinfection by delivering gp33 peptide or an irrelevant control peptide (SIINFEKL) transcervically. This was performed 120 days after infection. To gain an understanding of the early transcriptional changes that occur in the FRT following TRM reactivation, we performed a microarray on whole FRT tissue 9 hours following gp33 peptide or control SIINFEKL peptide delivery. We observed significant upregulation of pro-inflammatory transcripts, including cytokines, genes important for T cell chemotaxis and tissue migration, and antiviral responses, including interferon (IFN)-stimulated genes (Fig. 1B and 1C). This is consistent with what has been reported for TRM responses in the skin12,13 and demonstrates durability of TRM sensing and alarm functions. These data also validated our previously published studies in the FRT, which found an upregulation of VCAM-1 and CXCL9 on endothelial cells, CCL2 and CXCL9 on DCs, and CCL3/CCL4 and IFN-γ on T cells following TRM reactivation10,11. In probing this data set, we noted upregulation of genes associated with antibody effector functions, including components of the complement cascade, Fc gamma receptors, as well as the polymeric immunoglobulin (Ig) receptor (PIGR) (Fig. 1D). Levels of PIGR have been reported to change with estrus cycle37. As we did not note the stage of estrus cycle for microarray experiments, we wanted to confirm the upregulation of PIGR was independent of cycle and thus treated mice with medroxyprogesterone acetate (Depo-Provera) to synchronize mice in diestrus. Six hours following TRM reactivation with transcervically delivered peptide, we found an increase in PIGR expression in the uterus by immunofluorescence, consistent with the microarray results (Fig. 1E and F).
Fig. 1.

TRM reactivation induces immune-activating transcriptional changes. (A) Schematic of experimental set-up. P14 immune chimeras 120 days post-infection were challenged transcervically with gp33 or control SIINFEKL peptide and microarray was performed 9 hours later; (B) Volcano plot of differentially expressed genes in FRT where TRM were recalled versus control; (C) Top 100 differentially expressed genes categorized by function; (D) Fc receptor and complement gene expression in FRT tissue where TRM were recalled versus control. Data from n = 3 mice in each group; experiment performed once; (E) Representative PIGR staining in the uterine horns of Depo-Provera treated P14 immune chimera mice 35 days post-infection transcervically challenged with gp33 or control SIINFEKL peptide. Tissue taken 6 hours after peptide challenge. PIGR in magenta, DAPI in blue. < scale bar = 500 μm>; (F) quantification of E (n = 5 mice for TRM recall and n = 6 for control). Data are pooled from two independent experiments. Statistical significance was determined by unpaired two-tailed Mann–Whitney test where *p < 0.05. Error bars indicate standard error of mean. DAPI = 4′,6-diamidino-2-phenylindole dihydrochloride; LCMV = lymphocytic choriomeningitis virus; PIGR = polymeric immunoglobulin receptor; TRM = Tissue-resident memory T cells; WT = wild-type.
Given this increase in antibody effector and transcytosis pathways, we next tested if TRM can trigger an increase in antibody into tissue. To do this, we repurposed a well-established technique to distinguish between T cells in the vasculature and tissue parenchyma by using an intravenous (IV)-injected anti-CD8α antibody prior to animal euthanasia (Fig. 2A). This method is routinely used to selectively stain T cells in the vasculature while sparing cells in the tissue due to the short time (antibody is injected only a few minutes before cell isolation) and restrictive nature of vascular barriers38–40. We hypothesized that TRM reactivation would promote access of IV-injected antibody into tissue, which would manifest as intermediate staining of tissue-restricted CD8+ T cells. To assess this, we quantified the mean fluorescent intensity (MFI) of IV-injected, labeled anti-CD8α antibody on CD8β+ T cells from the FRT following TRM reactivation. Consistent with our hypothesis, we found that there was a significantly higher MFI of a CD8α IV-negative fraction in tissues when TRM were reactivated (Fig. 2A). This suggested that TRM reactivation promoted infiltration of IV-transferred antibody.
Fig. 2.

TRM reactivation induces changes in IV antibody staining in non-lymphoid tissue. (A) P14 immune chimeras were challenged transcervically with gp33 peptide (n = 6 mice) or control peptide (SIINFEKL; n = 6 mice) then 6 hours later biotinylated anti-CD8α antibody was transferred intravenously and mice were euthanized after 3 minutes. Representative flow cytometry plots from FRT tissue. Cells gated on CD8β +. Quantification of mean fluorescent intensity of IV-negative stained cells (gate indicated in histogram); (B) Levels of IgG2c, IgA, and IgM in the vaginal lumen of P14 immune chimera mice, stratified by estrus cycle (Di; n = 34 mice), (Pro; n = 13 mice), (Est; n = 16 mice), (Met; n = 6 mice); (C) Experiment was designed as in (A) except CD8α antibody was injected intravenously 12 hours after intracranial delivered peptides. Representative flow cytometry plots from brain tissue. Cells gated on CD8β +. Quantification of mean fluorescent intensity of IV-negative stained cells (gate indicated in histogram) with n = 4 for control and n = 4 for TRM recall. All data are pooled from at least two independent experiments. Statistical significance was determined by unpaired two-tailed t test in all instances except the IgM panel in (B) where an unpaired two-tailed Mann–Whitney was performed (due to non-normal distribution of the data) where * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Error bars indicate standard error of mean. Di = diestrus; Est = estrus; FRT = female reproductive tract; LCMV = lymphocytic choriomeningitis virus; Ig = immunoglobulin; i.p. = intraperitoneal; IV = intravenous; Met = metestrus; Pro = proestrus; TRM = Tissue-resident memory T cells; WT = wild-type.
To test if endogenous antibody levels were increased upon TRM reactivation in the FRT, we first performed enzyme-linked immunosorbent assays (ELISAs) for antibody on washes from the vaginal lumen of P14 immune chimeras. Steady-state, endogenous antibody levels in the FRT were widely variable and closely associated with estrus cycle (Fig. 2B), consistent with other reports41. Because of this, we wanted to confirm in an alternative tissue site that TRM reactivation leads to increased IV antibody access. We chose to assess this question in the brain because it is surrounded by a highly restrictive blood–brain barrier. We established a population of brain TRM by transferring P14 cells and infecting mice with LCMV intranasally instead of intraperitoneally because the intranasal infection route establishes a higher magnitude of brain TRM (data not shown). At a memory timepoint, we delivered gp33 or control peptide intracranially and 12 hours later, injected IV anti-CD8α antibody, and euthanized mice 3 minutes later, as before. Like the FRT, we found that the MFI of tissue-restricted IV-negative cells was significantly higher, confirming that antibody gains access to tissue when TRM are reactivated (Fig. 2C).
Given the variability in antibody levels with estrus cycle, we synchronized the cycles by treating mice with Depo-Provera to test if endogenous antibody increased upon TRM reactivation in the FRT. Six to 12 days later, when mice were in diestrus, we reactivated P14 TRM with gp33 or control peptide delivered transcervically. Six hours after TRM recall, we performed ELISAs on vaginal washes (Fig. 3B) and whole FRT tissue lysate (Fig. 3C) for different Ig isotypes. While the amount of antibody varied, we found that TRM reactivation led to a significant increase in IgA in the vaginal lumen and IgG1, IgG2b, and IgG2c in FRT tissue. Moreover, although differences were undetectable by ELISA, we found a significant increase in IgM staining in uterine tissue by immunofluorescence (Fig. 3D and 3E). These data also suggest that TRM reactivation in the uterus can have distal effects on IgA in the vagina.
Fig. 3.
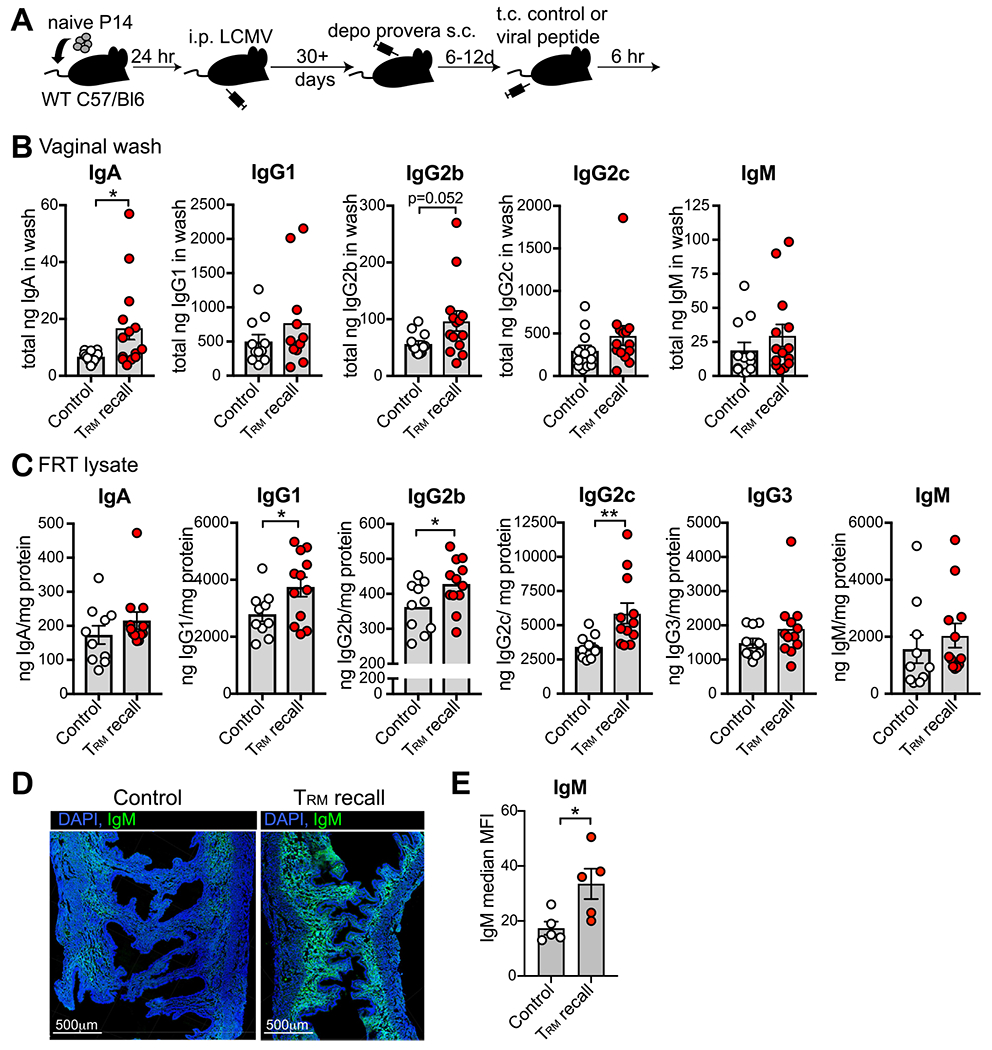
TRM reactivation triggers rapid increase in antibody in the FRT. (A) Depo-Provera treated P14 chimeras were challenged with gp33 or control peptide transcervically and then 6 hours later, vaginal washes and FRT tissues were assayed for antibody by enzyme-linked immunosorbent assay; (B) Levels of antibody in vaginal washes 6 hours following TRM reactivation; n = 12 mice for control and n = 14 mice for TRM recall; (C) levels of antibody in FRT tissue 6 hours following TRM reactivation; n = 10 mice for control and n = 12 mice for TRM recall; (D) Representative IgM staining of FRT tissue where green = IgM and blue = DAPI stained nuclei. Scale bar = 500 μm; E: Quantification of D; n = 5 mice. All data are pooled from at least two independent experiments. Statistical significance was determined by unpaired two-tailed Mann–Whitney in (B) and (C) panel IgG2c, or by unpaired two-tailed t test in (E) and (C) panel IgG1 and IgG2b, where * p < 0.05, ** p < 0.01. Error bars indicate standard error of mean. DAPI = 4′,6-diamidino-2-phenylindole dihydrochloride; FRT = female reproductive tract; LCMV = lymphocytic choriomeningitis virus; Ig = immunoglobulin; TRM = Tissue-resident memory T cells.
Due to the observed rapid changes in immunoglobulin levels within 6 hours, we hypothesized that the observed increase in antibody was at least partly due to TRM-triggered vascular permeability. To test this, we performed an ELISA for albumin, which is a protein that is normally found in circulation, in mice treated with Depo-Provera to synchronize estrus cycles in diestrus. Consistent with our hypothesis, the amount of albumin was significantly increased, by approximately 40%, in FRT tissue following TRM reactivation (Fig. 4A). This was validated through IV injection of Evans blue dye (EBD), which binds to serum albumin and is normally excluded from tissue. Six hours following TRM reactivation, as with albumin, we found a significant increase in EBD in FRT tissue (Fig. 4B). To test if this increase in vasodilation permitted an increase in antibody redistribution from the vasculature into the tissue, we injected anti-chicken ovalbumin (OVA) antibody IV into P14 immune chimeras. Two hours following antibody transfer, we reactivated TRM and then measured the amount of anti-OVA antibody in the FRT 6 hours later. We found that TRM reactivation triggered anti-OVA antibody infiltration into the tissue, which was three times the amount of control.
Fig. 4.
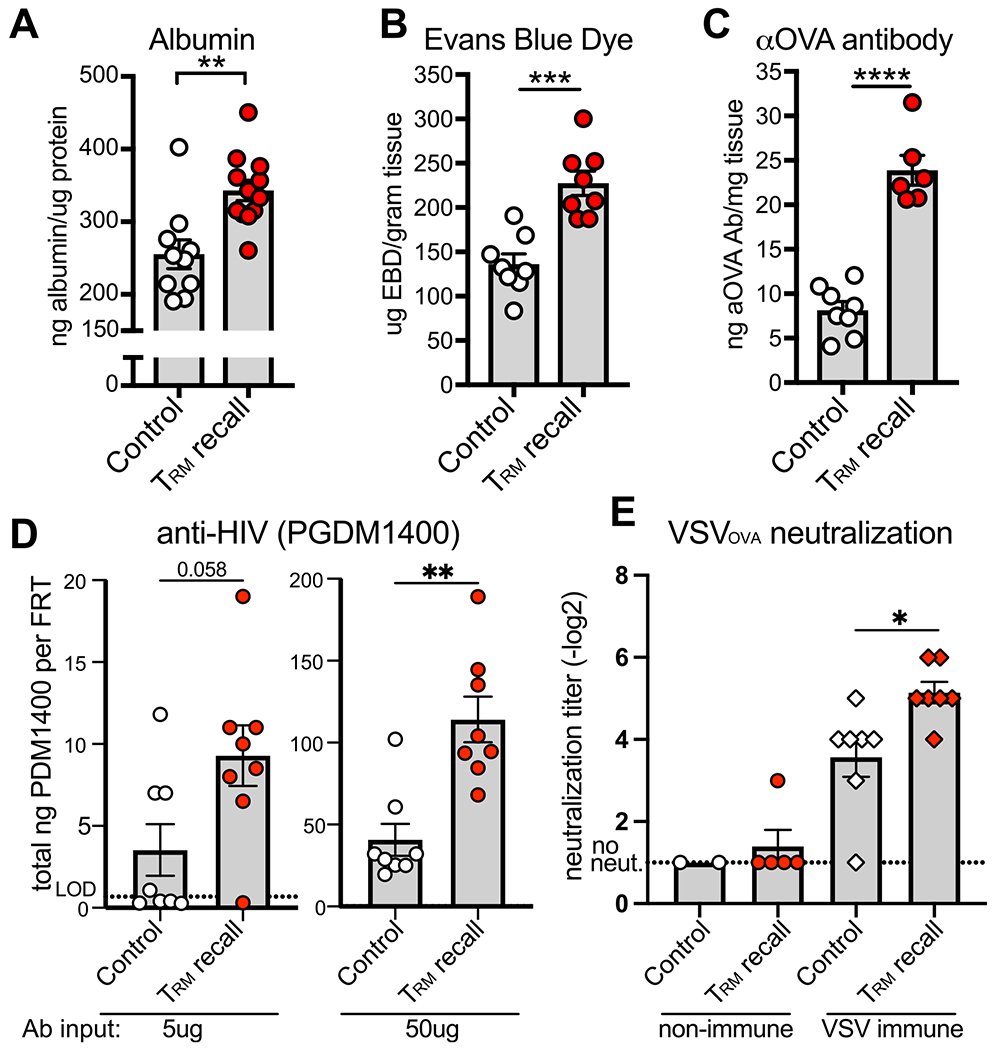
TRM reactivation triggers vascular permeability and neutralizing ab infiltration into the FRT. (A-C) Levels of albumin (n = 10 mice for control and n = 12 for TRM recall) (A), intravenous transferred EBD (n = 8 mice) (B), and IV-transferred anti-OVA ab (n = 8 mice for control and n = 6 for TRM recall) (C) in FRT tissue 6 hours following transcervical delivery of gp33 or control peptide to Depo-Provera treated P14 immune chimera mice. (D) Levels of PGDM1400 HIV neutralizing ab in the FRT after transferring 5 μm (left; n = 8 mice) or 50 μm (right; n = 8 mice) ab intravenous 2.5–3 hours prior to transcervical delivery of gp33 or control peptide; (E) Plaque reduction neutralization assay against VSVOVA using FRT lysate from Depo-Provera treated P14 immune chimeras that were (right) or were not (left) previously infected with VSVOVA. Data are 6 hours following transcervical delivery of gp33 or control peptide. Non-immune; n = 2 mice for control, experiment performed once, and n = 5 for TRM recall. VSV immune; n = 7 mice for both groups. All data are pooled from at least two independent experiments unless noted. Statistical significance was determined by unpaired two-tailed t test in (A–C), unpaired two-tailed Mann–Whitney in (D), and a two-way analysis of variance with Sidak’s multiple comparisons test (E) where * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Error bars indicate standard error of mean. ab = antibody; aOVA = anti-chicken ovalbumin; EBD = evans blue dye; FRT = female reproductive tract; TRM = Tissue-resident memory T cells; VSV = virus expressing ova.
These data raise the possibility that TRM activation may enhance antibody-mediated protection from infection. We recently reported that non-human primates that had low antibody titers following vaccination against SHIV were vulnerable to SHIV challenge compared to animals with high antibody titers2. However, if animals with lower titers had also received a T cell vaccine that established SHIV-specific TRM in the FRT, they were protected against SHIV challenge2. Given these data, we wished to evaluate if TRM could synergize with antibody by triggering rapid re-localization of protective antiviral antibody into the FRT. As a proof-of-concept test, we transferred the HIV broadly neutralizing antibody, PGDM140042, IV into Depo-Provera treated P14 immune chimera mice and delivered gp33 or control peptide transcervically to reactivate TRM. Six hours following TRM reactivation, we tested for levels of PGDM1400 in FRT tissue by ELISA and saw a significant twofold increase in antibody titers (Fig. 4D). To test if increases in local antibody triggered by TRM reactivation could contribute to viral neutralization, we infected P14 immune chimeras with vesicular stomatitis virus expressing ova (VSVOVA) to establish VSVOVA-specific humoral immunity. After viral clearance (30+ days), we reactivated LCMV-specific TRM through transcervical gp33 peptide delivery to Depo-Provera treated mice and 6 hours later, purified protein from the FRT tissue lysate. With this, we performed a plaque reduction neutralization test with VSVOVA and found that tissue extract from FRT where LCMV-specific TRM were reactivated could neutralize VSVOVA to a significantly greater extent (p = 0.014) than tissues that received control peptide. Since VSV is rapidly inhibited by type-I IFN, we performed the same assay with FRTs from P14 immune chimeras that were naïve to VSVOVA to confirm that TRM-triggered IFN responses were not responsible for ex vivo viral neutralization. Consistent with a role for antibody neutralization rather than a generalized antiviral response in vivo, we observed minimal ex vivo neutralization in mice with no VSVOVA-specific immunity regardless of prior TRM reactivation (Fig. 4E).
DISCUSSION
Although vaccine approaches inducing humoral immunity have a history of success against many pathogens, attempts have fallen short for several infections such as malaria, tuberculosis, and those infecting reproductive mucosa such as HIV and herpes simplex virus (HSV), indicating novel approaches are needed43–46. Here, we show that CD8+ resident memory T cells can trigger rapid changes in FRT tissue following reactivation, including pathways that have been observed in other tissues (IFN-stimulated genes, cytokines/chemokines)12,13, and those involving antibody effector functions. Furthermore, we showed that CD8+ TRM-triggered exudation of neutralizing antibody into the tissue through increased vascular permeability. We found this increase in vascular antibody transudation to be independent of B cells, as levels of IV-delivered antibody increased in the FRT following TRM recall. These data have implications for vaccine design and suggest targeting both cellular and humoral immunity may be optimal for protective vaccines. Indeed, studies examining immune correlates of protection against COVID-19 indicate an important role for both T and B cells47–49.
A previous report demonstrated that IFN-γ produced by circulating memory CD4+ T cells triggered increases in vascular permeability in the central nervous system resulting in antibody infiltration 6 days following viral rechallenge50. Here, we show that this vasodilation function extends to resident memory CD8+ T cells, which respond rapidly (within hours rather than days), allowing immediate infiltration of circulating antibody locally into the FRT. This correlated with ex vivo viral neutralization. We explored the requirement for IFN-γ; however, blocking IFN-γ with an antibody had no effect on vascular permeability as measured by EBD (unpublished). We also did not observe an effect when we blocked tumor necrosis factor-α, suggesting there may alternative or synergistic mechanisms of TRM-induced vascular permeability such as IFN-γ and tumor necrosis factor-α combined, or recruitment of histamine or bradykinin-producing basophils or mast cells. This is an area of great interest that warrants future studies. These findings suggest vaccines aimed at establishing both antibody responses and TRM may heighten protective immunity in the FRT.
Although we took a reductionist approach to interrogate TRM functions using viral peptide, it will be important in future studies to understand how this heightened protection is achieved with in vivo viral challenges. By performing passive antibody transfers, we have demonstrated that CD8+ TRM reactivation rapidly increases bloodborne antibody to local tissue. Experimentally, it will be challenging to isolate the in vivo effects of increased antibody transudation from myriad other outcomes of local TRM reactivation that could contribute to protection, including the induction of innate antiviral pathways (Fig. 1)12,13. Moreover, TRM have been shown to recruit B cells, which may eventually contribute to increases in local antibody titers as well10,51. Indeed, this has been demonstrated for CD4 + TRM in the context of HSV infection in the vagina, where antibody increases were seen as early as 24 hours, which were attributed to B cell recruitment into the tissue upon HSV rechallenge of immunized mice51. As we see increases in complement effectors and Fc receptors transcriptionally (Fig. 1) and natural killer cell activation10,11, it will be interesting to see how TRM can affect additional humoral effector mechanisms, including complement activation, antibody-dependent cellular cytotoxicity, and opsonization. Moreover, we noted that levels of IgM were seen to increase by immunofluorescence but not by ELISA. Although we did not observe co-localization of IgM with the B cell marker B220, it may be that increases in IgM+ B cells contribute to this increase or that the ELISA assay was not sensitive enough to detect differences seen by immunofluorescence.
In summary, this study describes a novel function for CD8+ TRM in mediating vascular permeability and antibody extravasation into tissue. These data suggest that antibody-based vaccines may synergize with T-cell vaccines and that targeting both arms of adaptive immunity may provide superior protection against mucosal pathogens.
METHODS
Mice
C57BL/6J (B6) female mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained in specific-pathogen-free conditions at the University of Minnesota or Dartmouth College. Thy1.1 + P14 and CD45.1 OT-I mice were fully backcrossed to C57BL/6J mice and maintained in our animal colony. Sample size was chosen based on previous experience. No sample exclusion criteria were applied. No method of randomization was used during group allocation, and investigators were not blinded. All mice used in experiments were 6–25 weeks of age. All mice were used in accordance with the Institutional Animal Care and Use Committees guidelines at the University of Minnesota and Dartmouth College.
Adoptive transfers and infections
Immune memory mice were generated by transferring 5 × 104 Thy1.1 + P14 CD8+ T cells from female mice into naïve 6–8 week-old C57BL/6J female mice. One day following transfer, mice were infected with 5 × 105 plaque-forming units LCMV Armstrong in 200μl intraperitoneally. For experiments with VSVOVA, 30 days post LCMV infection, mice received 5 × 104 CD45.1 OT-I IV and then were infected the next day with VSVOVA strain Indiana at 105 plaque-forming units intravenously. For intranasal infections, mice were anesthetized with a ketamine/xylazine cocktail and inoculated with 2 x 105 PFU LCMV Armstrong in 30μl PBS intranasally.
Lymphocyte isolation for flow cytometry
We repurposed an intravascular cell labeling technique, as described previously39, to assess availability of antibody to vascular and parenchymal T cells. Briefly, mice were injected intravenously with biotin-conjugated anti-CD8α. Three minutes after the injection, mice were euthanized and tissues were harvested as described10,36. In brief, FRT tissue was removed, digested in Collagenase IV (Sigma) with DNAse for 1 hour, then dissociated via gentleMACS Dissociator (Miltenyi Biotec), and lymphocytes were purified on a 44/67% Percoll (GE Healthcare) gradient. Isolated cells were stained with Steptavidin-BV650 (Biolegend) to label biotin-CD8α bound cells, and antibodies to CD44 (clone IM7, BioLegend), CD45.1 (clone A20, Biolegend), Thy1.1 (Clone OX7, Biolegend), and CD8β (clone Ly-3, Biolegend). All cells were stained at antibody dilutions of 1:100 except Thy1.1, which was 1:1000. Cell viability was determined with Ghost Dye 780 (Tonbo Biosciences). The stained samples were acquired with LSRII or LSR Fortessa flow cytometers (BD Biosciences) and analyzed with FlowJo software (Treestar).
RNA isolation and microarray
FRT tissue was suspended in 1 ml TRIZOL (Invitrogen) and then homogenized with a glass Dounce homogenizer. RNA was then isolated following the TRIZOL recommended protocol. Resulting RNA was then further purified using Qiagen RNA Cleanup Kit. Total RNA samples were processed using the MouseWG-6 v2.0 Expression BeadChip (Illumina). Background correction, normalization, and identification of differentially expressed genes (false discovery rate < 0.05) were performed in R using the limma package.
Immunofluorescence microscopy and quantification
FRT tissue was harvested, then fixed in 1% BD cytofix for 2 hours before being treated with 30% sucrose overnight for cryoprotection. The sucrose-treated tissue was embedded in OCT tissue-freezing medium and frozen in an isopentane liquid bath. Frozen blocks were sectioned into 7 μm sections and stained with antibodies to IgM-FITC (clone II/41, BD Biosciences), and PIGR (clone 7C1, Abcam) conjugated in-house to CF647 (Biotium), and counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride to detect nuclei. Images were captured by confocal microscopy using the Leica Stellaris 8 at 40x final magnification, using a 20x objective (NA = 0.75). Image analysis, cell counting, and calculation of MFI were performed using the spots function in Imaris version 9.8.
Peptide injections
Intracranial:
Intracerebroventricular injections were performed as described previously52. Briefly, 3 μm of control (SIINFEKL), or gp33 peptide (KAVYNFATM) suspended in phosphate-buffered saline (PBS) was injected into the right cerebral ventricle using a Hamilton Syringe, left for 10 seconds, then slowly removed.
Transcervical:
Transcervical injections were done as previously described11. Briefly, 50 μg of gp33 or SIINFEKL control peptide suspended in 30 μl PBS was delivered past the cervix with a gel loading pipette tip and then dispensed. The tip was left for 10 seconds, then slowly removed.
Depo-Provera treatment and estrus cycle determination
Medroxyprogesterone acetate (Depo-Provera) was administered subcutaneously at 100 mg/kg.
Diestrus was confirmed by vaginal cytology 6–12 days later. Estrus cycle staging was also determined by cytology on vaginal washes as described previously53. Briefly, vaginal washes were taken by pipetting 30 μl PBS in and out of the vagina five times. Then, 3 μl of vaginal wash was mounted on a microscope slide with coverslip, and estrus cycle (proestrus, estrus, metestrus, or diestrus) was determined based on the proportion of leukocytes, cornified epithelial, and nucleated epithelial cells.
EBD assay
200 μl of a 1% solution of sterile filtered EBD was injected into the tail vein of mice. Twenty minutes later, mice were thoroughly perfused with 20 ml ice-cold PBS. FRT tissue was removed and incubated in 500 μl formamide overnight at 56 ° C. Tubes were then spun at 2000 × g for 5 minutes, and supernatant was read on a plate reader at 620 nm.
Protein isolation from tissue
FRTs were homogenized in 1.5 ml NP-40 cell lysis buffer (Invitrogen) with protease inhibitor cocktail (Sigma). Detergents were removed via columns (Pierce) then sample was desalted via 7MWCO column (Zeba). The resulting lysate was used for downstream ELISA assays, BCA total protein assay (Pierce), and VSV neutralization assays.
Enzyme-linked immunosorbent assays
Mouse Ig:
Ready-SET-Go mouse Ig ELISA kits (eBioscience) were used per manufacturer’s instruction for all antibody isotypes in vaginal washes and FRT lysate.
Mouse Albumin:
Mouse Albumin ELISA was performed on FRT lysates per manufacturer’s instruction (Immunology Consultants Laboratory, Inc).
PGDM1400:
ELISA to detect PGDM1400 was performed on FRT lysate as previously described42. Briefly, goat-anti-human IgG Fc (Jackson ImmunoResearch) was used to coat 96-well plates overnight. Following washing and blocking with 3% BSA, sample diluted in 1%BSA/0.02% Tween was added and incubated for 1 hour at room temperature. Following washing, detection antibody goat-anti-human IgG Fab’2-Alkaline phosphatase (Jackson ImmunoResearch) was added and incubated for 1 hour. Alkaline phosphatase stain buffer and substrate (Sigma-Aldrich) were added, and plates were read at 10–20 minutes.
Anti-OVA antibody:
96-well plates were coated with OVA protein at a concentration of 10 μg/ml overnight at 4 °C. Plates were washed with 0.05% Tween and blocked with the blocking solution from Ready-SET-Go ELISA kits (eBiosciences). Following washing, sample was incubated with detection antibody goat-anti-mouse IgG HRP (Invitrogen) for 3 hours at room temperature. Following washes, TMB substrate was added and plates read 15 minutes later at 450 nm on a plate reader.
Plaque reduction neutralization assay
Purified FRT lysate free of detergent was heat inactivated for 30 minutes at 56 °C. Samples were diluted in Dulbecco’s Modified Eagle Medium with 1% FBS and twofold serial dilutions were made. Samples were incubated with VSVOVA for 90 minutes at 37 °C, then 200 ul was plated on Vero cell monolayers in 6-well plates and incubated for 1 hour, shaking every 15 minutes. Sample was then removed, and 2 ml of a 0.3% SeaKem Agarose (Lonza) overlay was added. After 24 hours, agarose was removed, and cells stained with 0.1% Crystal Violet. Plaques were counted, and neutralization was determined as the −log2 of the dilution at which the sample can neutralize 50% of infectious virus.
Statistics
Data were subjected to the Shapiro–Wilk normality test to determine whether they were sampled from a Gaussian distribution. If a Gaussian model of sampling was satisfied, parametric tests (unpaired two-tailed Student’s t test for two groups and one-way analysis of variance or two-way analysis of variance with Sidak’s multiple comparison test for more than two groups) were used. If the samples deviated from a Gaussian distribution, non-parametric tests were used (Mann–Whitney U test for two groups, Kruskal–Wallis with Dunn’s multiple comparison test for more than two groups). All statistical analysis was done in GraphPad Prism (GraphPad Software Inc.). A p-value < 0.05 was considered significant.
Cell definitions
TRM:
In this study, we have defined TRM as P14 T cells in the FRT. This is based on our prior data in the same mouse model demonstrating through parabiosis studies > 95% residency36.
Supplementary Material
FUNDING
This work was supported by 1R01AI146032 (DM), 3R01AI084913 (DM), 2P20GM113132-06 (PR), and K22AI148508-02 (PR).
Footnotes
DECLARATION OF COMPETING INTEREST
The authors have no competing interests to declare.
APPENDIX A. SUPPLEMENTARY MATERIAL
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mucimm.2022.11.004.
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request. Microarray data is available through the GEO database under the accession code GSE219002.
REFERENCES
- 1.Naluyima P et al. Terminal effector CD8 T cells defined by an IKZF2+IL-7R–transcriptional signature express FcγRIIIA, expand in HIV infection, and mediate potent HIV-specific antibody-dependent cellular cytotoxicity. J. Immunol 203, 2210–2221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arunachalam PS et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med 26, 932–940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petitdemange C et al. Vaccine induction of antibodies and tissue-resident CD8 + T cells enhances protection against mucosal SHIV-infection in young macaques. JCI Insight 4, e126047 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masopust D & Soerens AG Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol 37, 521–546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo PA, Miron M & Farber DL Location, location, location: tissue-resident memory T cells in mice and humans. Sci. Immunol 4, eaas9673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebhardt T, Palendira U, Tscharke DC & Bedoui S Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev 283, 54–76 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Iijima N & Iwasaki A Tissue instruction for migration and retention of TRM cells. Trends Immunol. 36, 556–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark RA Resident memory T cells in human health and disease. Sci. Transl. Med 7, 269rv1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CO & Kupper TS The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med 21, 688–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenkel JM et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346, 98–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenkel JM, Fraser KA, Vezys V & Masopust D Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol 14, 509–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariotti S et al. T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346, 101–105 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Glennie ND et al. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med 212, 1405–1414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhardt T et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol 10, 524–530 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Jiang X et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483, 227–231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay LK et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. U. S. A 109, 7037–7042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teijaro JR et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol 187, 5510–5514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol 95, 215–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen SG et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med 24, 130–143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Hara JM et al. Generation of protective pneumococcal-specific nasal resident memory CD4+ T cells via parenteral immunization. Mucosal Immunol. 13, 172–182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braverman J et al. Staphylococcus aureus specific lung resident memory CD4+ Th1 cells attenuate the severity of influenza virus induced secondary bacterial pneumonia. Mucosal Immunol. 15, 783–796 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omokanye A et al. Clonotypic analysis of protective influenza M2e-specific lung resident Th17 memory cells reveals extensive functional diversity. Mucosal Immunol. 15, 717–729 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luangrath MA, Schmidt ME, Hartwig SM & Varga SM Tissue-resident memory T cells in the lungs protect against acute respiratory syncytial virus infection. Immunohorizons 5, 59–69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith NM et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 11, 220–235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinnear E et al. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 11 , 249–256 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Shin H & Iwasaki A A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iijima N & Iwasaki A T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346, 93–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stary G et al. Vaccines. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348, aaa8205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng T et al. Tissue-resident-memory CD8+ T cells bridge innate immune responses in neighboring epithelial cells to control human Genital Herpes. Front. Immunol 12, 735643 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gary EN & Kutzler MA Defensive driving: directing HIV-1 vaccine-induced humoral immunity to the mucosa with chemokine adjuvants. J. Immunol. Res 2018, 3734207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasturi SP et al. Adjuvanting a simian immunodeficiency virus vaccine with toll-like receptor ligands encapsulated in nanoparticles induces persistent antibody responses and enhanced protection in TRIM5α restrictive macaques. J. Virol 91, e01844–e10916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauthner MG et al. Vaccine-induced protection from homologous Tier 2 SHIV challenge in nonhuman primates depends on serum-neutralizing antibody titers. Immunity 50, 241–252.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKinley SA et al. Modeling neutralization kinetics of HIV by broadly neutralizing monoclonal antibodies in genital secretions coating the cervicovaginal mucosa. PLoS One 9, e100598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pegu A et al. A meta-analysis of passive immunization studies shows an association of serum neutralizing antibody titer with protection against SHIV challenge. Cell Host Microbe 26, 336–346.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb NE, Montefiori DC & Lee B Dose–response curve slope helps predict therapeutic potency and breadth of HIV broadly neutralizing antibodies. Nat. Commun 6, 8443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinert EM et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaushic C, Frauendorf E & Wira CR Polymeric immunoglobulin A receptor in the rodent female reproductive tract: influence of estradiol in the vagina and differential expression of messenger ribonucleic acid during estrous Cycle 1. Biol. Reprod 57, 958–966 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Potter EL et al. Measurement of leukocyte trafficking kinetics in macaques by serial intravascular staining. Sci. Transl. Med 13, eabb4582 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Anderson KG et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc 9, 209–222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galkina E et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Invest 115, 3473–3483 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallichan WS & Rosenthal KL Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus Type 2 infection in the Genital Tract. Virology 224, 487–497 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Sok D et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl. Acad. Sci. U. S. A 17624–17629 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beura LK, Jameson SC & Masopust D Is a human CD8 T-cell vaccine possible, and if so, what would it take?: CD8 T-cell vaccines: to B or not to B? Cold Spring Harb. Perspect. Biol 10, a028910 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koff WC et al. Accelerating next-generation vaccine development for global disease prevention. Science 340, 1232910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray GE, Laher F, Lazarus E, Ensoli B & Corey L Approaches to preventative and therapeutic HIV vaccines. Curr. Opin. Virol 17,104–109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng’uni T, Chasara C & Ndhlovu ZM Major scientific hurdles in HIV vaccine development: historical perspective and future directions. Front. Immunol 11, 590780 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox RJ & Brokstad KA Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat. Rev. Immunol 20, 581–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sette A & Crotty S Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184, 861–880 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadarangani M, Marchant A & Kollmann TR Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol 21 , 475–484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iijima N & Iwasaki A Access of protective antiviral antibody to neuronal tissues requires CD4 T-cell help. Nature 533, 552–556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh JE et al. Migrant memory B cells secrete luminal antibody in the vagina. Nature 571, 122–126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glascock JJ et al. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J. Vis. Exp 56, 2968 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byers SL, Wiles MV, Dunn SL & Taft RA Mouse estrous cycle identification tool and images. PLoS One 7, e35538 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request. Microarray data is available through the GEO database under the accession code GSE219002.


