Abstract
Purpose of this review
Referred pain is a common but less understood symptom that originates from somatic tissues. A comprehensive recognition of referred pain is important for clinicians when dealing with it. The purpose of this study is to summarize the current understanding of referred pain, including its pathogenesis, characteristics, diagnosis, and treatment.
Recent findings
Referred pain arises not only from pathologies primarily involving local tissue but also from lesions in distant structures. Central sensitization of convergent neurons and peripheral reflexes of dichotomizing afferent fibers are two theories proposed to explain the pathological mechanism of referred pain. Because syndromes related to referred pain of different origins overlap each other, it is challenging to define referred pain and identify its originating lesions. Although various approaches have been used in the diagnosis and treatment of referred pain, including conservative treatment, blockade, radiofrequency, and surgery, management of referred pain remains a clinical challenge.
Summary
Unlike radicular pain and neuropathic pain, referred pain is a less studied area, despite being common in clinics. Referred pain can derive from various spinal structures, and blockage helps identify the primary pathology. Due to the heterogeneity of referred pain, treatment outcomes remain uncertain. Further studies are needed to improve our understanding of referred pain.
Keywords: referred pain, somatic, spinal, neuropathic pain, central sensitization
Introduction
In the field of somatic pain, most clinicians are familiar with radicular pain and neuropathic pain, but little is known about referred pain. Referred pain occurs in an area far from the primary lesion (1, 2) and is sometimes associated with secondary hyperalgesia and trophic changes in the referred areas (3). Although clinically common, the nature of referred pain remains an enigma (4–6). In most cases, pain in the dermatome regions, which are innervated by specific peripheral nerves, is simply regarded as radicular pain or neuropathic pain (1, 7). Referring to this phenomenon, the International Association for the Study of Pain (IASP), citing the classic treatise on pain, stated long ago in 2011 that “Pain in the lower limb should be described specifically as either referred pain or radicular pain” [sic] (2). In addition to the lower limbs, the diagnosis of pain in other somatic areas should also follow this guideline. Some researchers have even suggested that if the nature of pain is not clear, the diagnosis should not be jumped to Vulfsons et al. (7).
A better understanding of referred pain may help physicians in clinical diagnosis and decision-making, thus improving clinical outcomes. To this end, this review characterizes referred pain in various conditions and discusses possible pathological mechanisms and therapeutic measures based on the contributions of previous studies.
Definition and epidemiology of referred pain
Somatic pain arises not only from pathologies primarily involving local tissues, but also from dysfunction in distant tissues, including spinal, neuromuscular, and other somatic structures (8, 9). The term “referred pain” has been documented to describe pain spreading to the somatic regions far from the site of noxious stimulation (10), which is not caused by nerve root stimulation.
Referred pain can be caused by autogenous dysfunction or triggered by external stimuli. For example, referred pain can even be stimulated by hypertonic saline in healthy adults (11, 12). However, referred pain is not directly caused by mechanical or inflammatory stimulation of the nerves or nerve roots (1), nor is it caused by neuromas or lesions of the peripheral nerves or central nervous system (13). On the contrary, referred pain has even been induced by stimulating adjacent structures in patients with denervated or missing limbs in special cases of spinal cord injury (14), plexus avulsion (15, 16), and amputation (13, 17). In another typical example, referred pain has been triggered in areas of neuropathic pain by scraping the earlobe in patients with postherpetic neuralgia (18).
It is clear that referred pain is common and variable. Spinal referred pain, for example, reportedly occurs in 17%–84% of patients with low back pain (19, 20). While referred pain has been a focus of research for a long time, multicenter studies are lacking, and the incidence of referred pain in the general population largely remains unknown.
Possible pathophysiology of referred pain
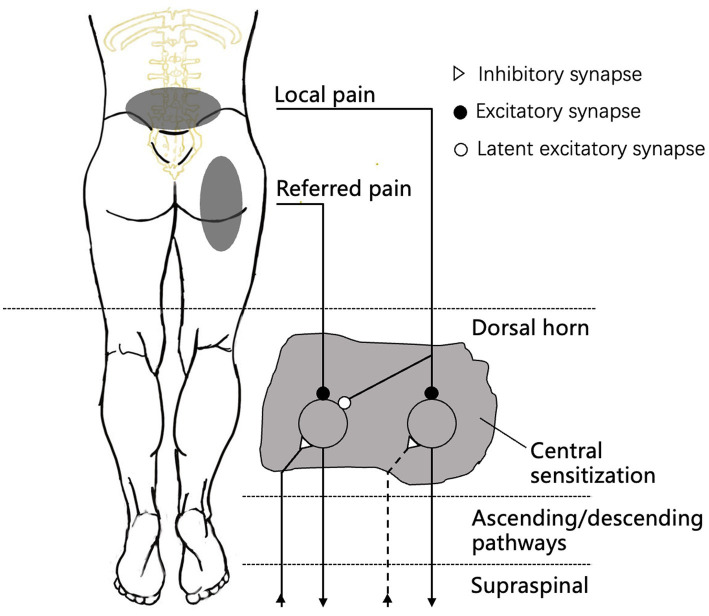
The cortical reorganization theory has long been proposed to explain the pathophysiology of referred pain (21). Contrary to the cortical reorganization theory, however, referred pain has also been found in areas with segregated cortical activation (22). In general, most researchers accept convergence projection theory. In this theory, the convergence of nociceptive afferents on second-order neurons in the spinal cord leads to the occurrence of pain in different somatic areas, like “crossed telephone lines” (9, 10, 23, 24) (Figure 1). While the convergence of sensory afferents at the subcortical level has been confirmed in an animal study (25), referred pain can be considered the central sensitization of convergent neurons. A classic example is the convergence of C1–C3 spinal nerves, where trigeminal input results in migraine and cervicogenic headache (26).
Figure 1.
The neurophysiological schematic model of “central sensitization” in humans. Local pain will excite the pathway mediating pain upward to the dorsal horn, while a sensitization process is initiated at the same time. The release of sensitizing substances will cause the opening of latent synaptic connections to the neurons mediating pain from the referred area. Both the direct excitation of the involved neurons and the facilitation of afferent input in the referred area will result in the perception of referred pain (5).
In another theory, it has been proposed that there are dichotomizing afferent fibers that ramify and distribute to regions of primary dysfunction and referred areas (27–30). While primary lesions stimulate afferent fibers in deep regions, these afferent fibers trigger the activation of a reflex arc toward the muscle via somatic efferent fibers. This theory can be summarized as the pathomechanism underlying physiological reflexes (8). Using double-labeling with fluorescent tracers, dichotomizing axons between the lumbar intervertebral disc and the groin region have been reported (30), supporting this theory (Figure 2). Another example is visceral referred pain, which is regarded as a pathological combination of nociceptive processing pathways for visceral and somatic sensory afferent neurons. As such, visceral referred pain is also called a “viscerosensory reflex” (9, 31).
Figure 2.
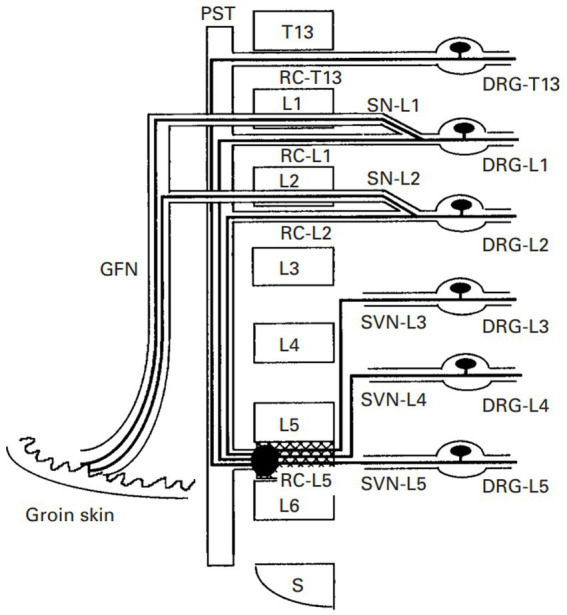
The neurophysiological schematic model of “dichotomizing axons” in rats. There are dichotomizing axons that project to the ventral portion of the L5–L6 disc and the groin skin during the sensory pathways from the ventral portion of the L5–L6 disc and neurons in the L1 and L2 DRG. GFN, genitofemoral nerve; PST, paravertebral sympathetic trunk; RC, ramus communicans; SN, spinal nerve; SVN, sinuvertebral nerve (30).
Referred areas for lesions in various spinal structures
Reported referred pain can originate from almost all spinal structures (5, 31). Among them, the intervertebral disc (31), facet joint (32, 33), and sacroiliac joint (34) are the most common structures involved in spinal referred pain. Referred pain is usually felt in some designated dermatome areas in patients with various spinal disorders, including spinal injuries, disc degeneration, spinal canal stenosis, and lumbar spondylolisthesis (34–36). For example, the pain usually occurs in the distant lumbosacral regions in patients with traumatic or osteoporotic vertebral fractures (37, 38), suggesting that pain may be referred outward and downward from the fracture site according to some predictable patterns. Kellgren et al. first reported that spinal referred pain may be distributed in a segmental pattern (39, 40). However, it is difficult to “map” referred areas because referred pain of different origins often overlaps (12). Moreover, referred pain does not follow dermatomes or patterns of nerve root distribution (11, 41).
While referred pain in the chest, shoulder, and upper limb usually originates from the cervical and thoracic spine (11, 42, 43), referred pain related to lumbar spine diseases tends to spread to the back, abdomen (24), buttocks (12, 31, 44, 45), groin (12, 46, 47), thigh (12, 34), and even the distal limb below the knee (48). Among them, the buttocks and leg are the most common sites of lumbar referred pain (12, 28, 31, 34, 49). Moreover, the distribution patterns for lumbar referred pain vary according to anatomical structures. For example, patients with disc degeneration may feel pain at the dermatome region innervated by the corresponding dorsal root ganglion (DRG) neurons (1, 19, 31, 48, 50), with greater intensity of referred pain being associated with more severe disc degeneration (31, 48).
Referred pain originating from the facet joints is also segmentally distributed, as it may be related to segmental DRGs. For cervical facet joints, the neck and shoulder are the most commonly involved sites of referred pain (42, 51). Referred pain in the back and iliac crest usually originates from the thoracic facet joints (43). For lumbar facet joints, pain may be referred to as the region between the hip and thigh. It has been reported that pathology of the superior facet joints typically induces referred pain to the flank, hip, and lateral thigh regions, while the inferior facet joints usually refer to the posterior thigh (12, 33, 52).
Referred pain from the sacroiliac joint mainly distributes to the lower back and buttocks, in addition to the thigh, groin, leg, and even foot (31, 53, 54). Nevertheless, there is considerable overlap in referral areas, although the pain has been irradiated from various spinal elements (54). A clear map specifying the relationship between anatomical structures and referred regions is currently lacking.
Differentiation of referred pain from radicular pain
Referred pain usually occurs after local pain has persisted for a certain period (5, 55). Typically, referred pain is described as dull, aching, gnawing, annoying, drilling, or pressing (1, 55). Sometimes, referred pain is associated with secondary hyperalgesia and trophic changes (3). Once present, referred pain tends to become fixed in a particular region, depending on the referral pattern (1, 55). Unlike the explicit pathophysiology of radicular pain, it is difficult to locate the exact original site or boundaries of the affected area for referred pain as the convergence of afferent neurons is rather complicated (24, 34, 40, 44). However, referred pain can be identified in a wide area with an identified center or core, although its boundary is difficult to define (11). Although a number of studies have described the segmental patterns of spinal referred pain, these patterns are not consistent among patients and dysfunctions (11, 31, 34, 43, 44). Importantly, referred pain is not dermatomally distributed, which is markedly different from radicular pain (11, 41).
Neuropathic pain is a symptom and also a consequence of various diseases affecting the somatosensory nervous system (13, 56). Typically, neuropathic pain is described as allodynia and hyperalgesia and is usually distributed along the dermatome of peripheral nerve-innervated areas (13, 24). Mechanistically, radicular pain is evoked by ectopic discharges from affected DRGs or nerves. For example, herniated disc tissues compress the spinal root and induce pain traveling along the lower limb, which may be largely resolved after the removal of the herniated disc tissue (57, 58). In many cases, radicular pain is associated with neurological deficits such as decreased muscle strength, weakened reflexes, and specific numbness in the related dermatome (59, 60). The neurophysiological relationship between radicular pain and the affected nerves has been clarified in preclinical animal studies (61–63). The distinguishing features of referred pain and radicular pain are listed in Table 1. However, how to precisely identify referred pain and distinguish it from radicular pain needs further investigation.
Table 1.
Differential features of referred pain and radicular pain.
| Feature | Referred pain | Radicular pain |
|---|---|---|
| Quality | Dull, aching, gnawing, annoying, drilling, pressing | Shooting, lancinating, shocking, electric |
| Concomitant symptom | Secondary hyperalgesia trophic changes | Numbness, decreased muscle strength, weakened reflexes |
| Distribution pattern | A wide area with a boundary that is difficult to define, but with an identified center or core | Travels along the length of the lower limb in a band no more than 2–3 inches in width |
Diagnostic measures for referred pain
As mentioned above, referred pain can originate from various spinal elements, and there is significant overlap in referred areas and distribution patterns. It is thus difficult to locate the exact source of referred pain (24). There are two methods that can help diagnose referred pain: (1) to induce a similar pain pattern using different types of stimuli; (2) to relieve the pain with a local block (5, 31, 55, 64).
In many cases, radiographic imaging, such as CT, MR, and myelography, is not able to identify the exact source of referred pain. As early as 1939, Kellgren et al. first developed an experimental model and found that referred pain could be triggered by injection of hypertonic saline solution (39, 40, 65), as verified in investigating cases of spontaneous pain of unclear etiology (6). Extending this theory, various percutaneous procedures, namely provocative lumbar discography (66–70), facet joint injections (32), blocks (33, 52, 70), intra-articular sacroiliac joint injections (31, 53, 54), and epidural injections (70) have been developed to reproduce or block referred pain and to identify its exact source. In an inflammatory pain model, capsaicin has been used for functional spinal and supraspinal MR imaging, which has been reported to identify the origin of referred pain (18).
A multidisciplinary approach to the treatment of referred pain
Multimodal treatments for referred pain include pharmacotherapy, physical therapy, regular exercise, and psychotherapy (52). Invasive procedures are reserved for those with a confirmed source of somatic referred pain. Invasive therapies, such as local blockage and radiofrequency, are commonly used in treating spinal referred pain. In an early Lancet report, referred pain triggered by injecting hypertonic saline solution could be successfully treated with a local block (6). Later, Hockaday et al. reported that referred pain could be abolished by a local block but was less consistently reduced by blocking the referred areas (11). For instance, intra-articular injections of corticosteroids have been used to treat facetogenic (52, 71) and sacroiliac-referred pain (72). Furthermore, disco block, inspired by discography, was once regarded as a useful approach to relieving discogenic referred pain (50, 73). Shealy et al. first described the method of applying radiofrequency to the facet joints in 1975 (74). Radiofrequency has since been acknowledged as the “gold standard” for the treatment of facetogenic and sacroiliac-referred pain. Thermal intradiscal procedures, such as intradiscal electrothermal annuloplasty and biacuplasty, have also been used to effectively treat discogenic low back pain and referred leg pain (20, 31, 75).
When treating referred pain, non-invasive treatments are usually optional (76). There are some exceptions. For example, discogenic groin pain can be significantly improved after lumbar disc surgery (50). Percutaneous vertebroplasty, or kyphoplasty, is effective in relieving lumbosacral referred pain following osteoporotic vertebral compression fractures (38). It has also been reported that patients with back pain associated with referred inguinal or leg pain had better clinical outcomes after spine surgery than those without (77), suggesting that surgical therapy for the primary conditions may be an effective approach to controlling the secondary referred pain.
Although various interventions have been reported to be effective in the treatment of referred pain, randomized controlled trials are needed to clarify the efficacy of conservative treatments, blockage, radiofrequency, and surgery. In addition, clinical guidelines and expert consensus on the management of referred pain are currently lacking.
Conclusion
In this mini-review, patterns, and characteristics of referred pain from somatic structures are discussed. The similarity between referred pain and peripheral neuropathic pain makes it difficult to approach the diagnosis of referred pain. The main hypotheses for the pathophysiological mechanism of referred pain include central sensitization and peripheral reflex, which may jointly explain the development of referred pain. Convergence of sensory afferents at the subcortical level and dichotomizing afferents have also been proposed in scientific research. The overlap of referred areas from different somatic structures makes it difficult to locate the primary origins of referred pain. In clinical practice, local blockage and radiofrequency are commonly used interventions in the diagnosis and treatment of referred pain, although there is a considerably high false-positive rate for both. Given the complex pathologies, changeable manifestations, and inconsistent distribution patterns, the treatment of referred pain remains a clinical challenge.
Author contributions
QJ and YW carried out the project design and literature summary. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82201358) and the Medicine and Health Science and Technology Plan in Zhejiang Province (grant no. 2023RC019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the colleagues in our spine laboratory for their assistance in this work.
References
- 1.Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain. (2009) 147:17–9. doi: 10.1016/j.pain.2009.08.020, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Merskey H, Bogdduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Pain Suppl. (1986) 3:S1–S226. [PubMed] [Google Scholar]
- 3.Vecchiet L, Vecchiet J, Giamberardino MA. Referred muscle pain: clinical and pathophysiologic aspects. Curr Rev Pain. (1999) 3:489–98. doi: 10.1007/s11916-999-0077-y, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Affaitati G, Costantini R, Tana C, Cipollone F, Giamberardino MA. Co-occurrence of pain syndromes. J Neural Transm. (2020) 127:625–46. doi: 10.1007/s00702-019-02107-8 [DOI] [PubMed] [Google Scholar]
- 5.Graven-Nielsen T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl. (2006) 122:1–43. doi: 10.1080/03009740600865980, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Whitty C, Willison RGJL. Some aspects of referred pain. Lancet. (1958) 272:226–31. doi: 10.1016/S0140-6736(58)90058-8 [DOI] [PubMed] [Google Scholar]
- 7.Vulfsons S, Bar N, Eisenberg E. Back pain with leg pain. Curr Pain Headache Rep. (2017) 21:32. doi: 10.1007/s11916-017-0632-x [DOI] [PubMed] [Google Scholar]
- 8.Giamberardino MA. Referred muscle pain/hyperalgesia and central sensitisation. J Rehabil Med. (2003) 35:85–8. doi: 10.1080/16501960310010205, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Luz LL, Fernandes EC, Sivado M, Kokai E, Szucs P, Safronov BV. Monosynaptic convergence of somatic and visceral C-fiber afferents on projection and local circuit neurons in lamina I: a substrate for referred pain. Pain. (2015) 156:2042–51. doi: 10.1097/j.pain.0000000000000267, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definition of pain terms. Seattle: IASP Press; (1994) 1–28. [Google Scholar]
- 11.Hockaday JM, Whitty CW. Patterns of referred pain in the normal subject. Brain. (1967) 90:481–96. doi: 10.1093/brain/90.3.481 [DOI] [PubMed] [Google Scholar]
- 12.McCall IW, Park WM, O’Brien JP. Induced pain referral from posterior lumbar elements in Normal subjects. Spine (Phila Pa 1976). (1979) 4:441–6. [DOI] [PubMed] [Google Scholar]
- 13.Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. (2021) 101:259–301. doi: 10.1152/physrev.00045.2019 [DOI] [PubMed] [Google Scholar]
- 14.Moore CI, Stern CE, Dunbar C, Kostyk SK, Gehi A, Corkin S. Referred phantom sensations and cortical reorganization after spinal cord injury in humans. Proc Natl Acad Sci U S A. (2000) 97:14703–8. doi: 10.1073/pnas.250348997, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnerup NB, Norrbrink C, Fuglsang-Frederiksen A, Terkelsen AJ, Hojlund AP, Jensen TS. Pain, referred sensations, and involuntary muscle movements in brachial plexus injury. Acta Neurol Scand. (2010) 121:320–7. doi: 10.1111/j.1600-0404.2009.01248.x, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Htut M, Misra P, Anand P, Birch R, Carlstedt T. Pain phenomena and sensory recovery following brachial plexus avulsion injury and surgical repairs. J Hand Surg. (2006) 31:596–605. doi: 10.1016/J.JHSB.2006.04.027, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. (2013) 154:1274–80. doi: 10.1016/j.pain.2013.03.040, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Forstenpointner J, Wolff S, Stroman PW, Jansen O, Rehm S, Baron R, et al. "from ear to trunk"-magnetic resonance imaging reveals referral of pain. Pain. (2018) 159:1900–3. doi: 10.1097/j.pain.0000000000001279, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Mellin G, Hurri H. Referred limb symptoms in chronic low back pain. J Spinal Disord. (1990) 3:52–8. doi: 10.1097/00002517-199003000-00009, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Derby R, Lee SH, Seo KS, Kazala K, Kim BJ, Mi JK. Efficacy of IDET for relief of leg pain associated with Discogenic low Back pain. Pain Pract. (2004) 4:281–5. doi: 10.1111/j.1533-2500.2004.04401.x, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Grüsser SM, Winter C, Mühlnickel W, Denke C, Karl A, Villringer K, et al. The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience. (2001) 102:263–72. doi: 10.1016/S0306-4522(00)00491-7, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Soler MD, Kumru H, Vidal J, Pelayo R, Tormos JM, Fregni F, et al. Referred sensations and neuropathic pain following spinal cord injury. Pain. (2010) 150:192–8. doi: 10.1016/j.pain.2010.04.027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Calcitonin gene-related peptide immunoreactive neurons with dichotomizing axons projecting to the lumbar muscle and knee in rats. Eur Spine J. (2003) 12:576–80. doi: 10.1007/s00586-003-0573-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harding G, Yelland M. Back, chest and abdominal pain—is it spinal referred pain? Australian family physician. Aust Fam Physician. (2007) 36:422–3. [PubMed] [Google Scholar]
- 25.Chen L, Lu X, Jin Q, Gao Z, Wang Y. Sensory innervation of the lumbar 5/6 intervertebral disk in mice. Front Neurol. (2023) 14:1084209. doi: 10.3389/fneur.2023.1084209, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmeads J. The cervical spine and headache. Neurology. (1988) 38:1874–8. doi: 10.1212/WNL.38.12.1874 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Nakajima Y, Sakamoto T, Moriya H, Takahashi K. Capsaicin applied to rat lumbar intervertebral disc causes extravasation in the groin skin: a possible mechanism of referred pain of the intervertebral disc. Neurosci Lett. (1993) 161:1–3. doi: 10.1016/0304-3940(93)90125-5, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Fujii T, Sakuma Y, Orita S, Inoue G, Ochiai N, Kuniyoshi K, et al. Dichotomizing sensory nerve fibers innervating both the lumbar vertebral body and the area surrounding the iliac crest: a possible mechanism of referred lateral back pain from lumbar vertebral body. Spine. (2013) 38:E1571–4. doi: 10.1097/BRS.0b013e3182a879cd [DOI] [PubMed] [Google Scholar]
- 29.Wakai K, Ohtori S, Yamashita M, Yamauchi K, Inoue G, Suzuki M, et al. Primary sensory neurons with dichotomizing axons projecting to the facet joint and the low back muscle in rats. J Orthop Sci. (2010) 15:402–6. doi: 10.1007/s00776-010-1465-1, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Sameda H, Takahashi Y, Takahashi K, Chiba T, Moriya H. Dorsal root ganglion neurones with dichotomising afferent fibres to both the lumbar disc and the groin skin. J Bone Joint Surg Br. (2003) 85:600–3. doi: 10.1302/0301-620X.85B4.13306 [DOI] [PubMed] [Google Scholar]
- 31.Kurosawa D, Murakami E, Aizawa T. Referred pain location depends on the affected section of the sacroiliac joint. Eur Spine J. (2015) 24:521–7. doi: 10.1007/s00586-014-3604-4 [DOI] [PubMed] [Google Scholar]
- 32.Ohtori S, Miyagi M, Inoue G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: a review. Spine Surg Relat Res. (2018) 2:11–7. doi: 10.22603/ssrr.2016-0018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks PRJ. Distribution of pain provoked from lumbar facet joints and related structures during diagnostic spinal infiltration. Pain. (1989) 39:37–40. doi: 10.1016/0304-3959(89)90173-5 [DOI] [PubMed] [Google Scholar]
- 34.Laplante BL, Ketchum JM, Saullo TR, Depalma MJ. Multivariable analysis of the relationship between pain referral patterns and the source of chronic low Back pain. Pain Physician. (2012) 2:171–8. doi: 10.36076/ppj.2012/15/171 [DOI] [PubMed] [Google Scholar]
- 35.Ko S. Correlations between sedimentation sign, dural sac cross-sectional area, and clinical symptoms of degenerative lumbar spinal stenosis. Eur Spine J. (2018) 27:1623–8. doi: 10.1007/s00586-017-5374-2, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Mannion AF, Mutter UM, Fekete TF, Porchet F, Jeszenszky D, Kleinstuck FS. Validity of a single-item measure to assess leg or back pain as the predominant symptom in patients with degenerative disorders of the lumbar spine. Eur Spine J. (2014) 23:882–7. doi: 10.1007/s00586-014-3193-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark EM, Hutchinson AP, Mccloskey EV, Stone MD, Martin JC, Bhalla AK, et al. Lateral back pain identifies prevalent vertebral fractures in post-menopausal women: cross-sectional analysis of a primary care-based cohort. Rheumatology. (2010) 49:505–12. doi: 10.1093/rheumatology/kep414, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YFX, Pan J, Yang M, Li L, Su Q, Tan J. Percutaneous vertebroplasty versus kyphoplasty for thoracolumbar osteoporotic vertebral compression fractures in patients with distant lumbosacral pain. Pain Physician. (2021) 24:E349–56. doi: 10.36076/ppj.2021/24/E349, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Kellgren JH. Observations on referred pain arising from muscle. Clin Sci. (1938) 3:175–90. [Google Scholar]
- 40.Kellgren JH. On the distribution of pain arising from deep somatic structures with charts of segmental pain areas. Clin Sci. (1939) 4:36. [Google Scholar]
- 41.Elliott FA. Tender muscles in sciatica: electromyographic studies. Lancet. (1944) 243:47–9. doi: 10.1016/S0140-6736(00)42562-6 [DOI] [Google Scholar]
- 42.Fukui S, Ohseto K, Shiotani M, Ohno K, Karasawa H, Naganuma Y, et al. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain. (1996) 68:79–83. doi: 10.1016/S0304-3959(96)03173-9 [DOI] [PubMed] [Google Scholar]
- 43.Fukui S, Ohseto K, Shiotani M. Patterns of pain induced by distending the thoracic zygapophyseal joints. Reg Anesth. (1997) 22:332–6. doi: 10.1016/S1098-7339(97)80007-7, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Furman MB, Johnson SC. Induced lumbosacral radicular symptom referral patterns: a descriptive study. Spine J. (2019) 19:163–70. doi: 10.1016/j.spinee.2018.05.029, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Ohnmeiss DD, Vanharanta H, Ekholm J. Relation between pain location and disc pathology: a study of pain drawings and CT/discography. Clin J Pain. (1999) 15:210–7. doi: 10.1097/00002508-199909000-00008, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Yukawa Y, Kato F, Kajino G, Nakamura S, Nitta H. Groin pain associated with lower lumbar disc herniation. Spine. (1997) 22:1736–9. [DOI] [PubMed] [Google Scholar]
- 47.Murphey F. Sources and patterns of pain in disc disease. Clin Neurosurg. (1968) 15:343–51. doi: 10.1093/neurosurgery/15.CN_suppl_1.343 [DOI] [PubMed] [Google Scholar]
- 48.Ohnmeiss DD, Vanharanta H, Ekholm J. Degree of disc disruption and lower extremity pain. Spine. (1997) 22:1600–5. [DOI] [PubMed] [Google Scholar]
- 49.DePalma MJ, Ketchum JM, Thomas S. What is the source of chronic low back pain and does age play a role? Pain Med. (2011) 12:224–33. doi: 10.1111/j.1526-4637.2010.01045.x, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Oikawa Y, Ohtori S, Koshi T, Takaso M, Inoue G, Orita S, et al. Lumbar disc degeneration induces persistent groin pain. Spine. (2012) 37:114–8. doi: 10.1097/BRS.0b013e318210e6b5 [DOI] [PubMed] [Google Scholar]
- 51.Manchikanti L, Kosanovic R, Cash KA, Pampati V, Hirsch JA. Assessment of prevalence of cervical facet joint pain with diagnostic cervical medial branch blocks: analysis based on chronic pain model. Pain Physician. (2020) 23:531–40. doi: 10.36076/ppj.2020.23.531, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Van Kleef M, Vanelderen P, Cohen SP, Lataster A, Van Zundert J, Mekhail N. 12. Pain originating from the lumbar facet joints. Pain Pract. (2010) 10:459–69. doi: 10.1111/j.1533-2500.2010.00393.x, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Slipman CW, Jackson HB, Lipetz JS, Chan KT, Vresilovic EJ. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. (2000) 81:334–8. doi: 10.1016/S0003-9993(00)90080-7, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Van der Wurff P, Buijs EJ, Groen GJ. Intensity mapping of pain referral areas in sacroiliac joint pain patients. J Manip Physiol Ther. (2006) 29:190–5. doi: 10.1016/j.jmpt.2006.01.007, PMID: [DOI] [PubMed] [Google Scholar]
- 55.Domenech-Garcia V, Palsson TS, Herrero P, Graven-Nielsen T. Pressure-induced referred pain is expanded by persistent soreness. Pain. (2016) 157:1164–72. doi: 10.1097/j.pain.0000000000000497, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. (2010) 9:807–19. doi: 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- 57.Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. (2014) 14:180–91. doi: 10.1016/j.spinee.2013.08.003, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Amin RM, Andrade NS, Neuman BJ. Lumbar disc herniation. Curr Rev Musculoskelet Med. (2017) 10:507–16. doi: 10.1007/s12178-017-9441-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Diagnostic value of history and physical examination in patients suspected of lumbosacral nerve root compression. J Neurol Neurosurg Psychiatry. (2002) 72:630–4. doi: 10.1136/jnnp.72.5.630, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vucetic N, Svensson O. Physical signs in lumbar disc hernia. Clin Orthop Relat Res. (1996) 333:192–201. doi: 10.1097/00003086-199612000-00020 [DOI] [PubMed] [Google Scholar]
- 61.Kim SJ, Kim WR, Kim HS, Park HW, Cho YW, Jang SH, et al. Abnormal spontaneous activities on needle electromyography and their relation with pain behavior and nerve fiber pathology in a rat model of lumbar disc herniation. Spine. (2011) 36:E1562–7. doi: 10.1097/BRS.0b013e318210aa10, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Obata K, Tsujino H, Yamanaka H, Yi D, Fukuoka T, Hashimoto N, et al. Expression of neurotrophic factors in the dorsal root ganglion in a rat model of lumbar disc herniation. Pain. (2002) 99:121–32. doi: 10.1016/S0304-3959(02)00068-4 [DOI] [PubMed] [Google Scholar]
- 63.Seki S, Sekiguchi M, Konno SI. Association between neurotrophic factor expression and pain-related behavior induced by nucleus pulposus applied to rat nerve root. Spine. (2018) 43:E7–E15. doi: 10.1097/BRS.0000000000002223 [DOI] [PubMed] [Google Scholar]
- 64.O'Neill S, Graven-Nielsen T, Manniche C, Arendt-Nielsen L. Ultrasound guided, painful electrical stimulation of lumbar facet joint structures: an experimental model of acute low back pain. Pain. (2009) 144:76–83. doi: 10.1016/j.pain.2009.03.014, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Lewis T, Kellgren JH. Observations relating to referred pain, visceromotor reflexes and other associated phenomena. Clin Sci. (1939) 4:47–71. [Google Scholar]
- 66.Moneta GB, Videman T, Kaivanto K, Aprill C, Spivey M, Vanharanta H, et al. Reported pain during lumbar discography as a function of annular ruptures and disc degeneration: a re-analysis of 833 discograms. Spine. (1994) 19:1968–74. [DOI] [PubMed] [Google Scholar]
- 67.Kim HG, Shin DA, Kim HI, Yoo EA, Shin DG, Lee JO. Clinical and radiological findings of Discogenic low Back pain confirmed by automated pressure-controlled discography. J Korean Neurosurg Soc. (2009) 46:333–9. doi: 10.3340/jkns.2009.46.4.333, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindblom K. Technique and results of diagnostic disc puncture and injection (discography) in the lumbar region. Acta Orthop Scand. (1951) 20:315–26. doi: 10.3109/17453675108991178, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Derby R, Kim BJ, Lee SH, Chen Y, Seo KS, Aprill C. Comparison of discographic findings in asymptomatic subject discs and the negative discs of chronic LBP patients: can discography distinguish asymptomatic discs among morphologically abnormal discs? Spine J. (2005) 5:389–94. doi: 10.1016/j.spinee.2005.01.007, PMID: [DOI] [PubMed] [Google Scholar]
- 70.El-Khoury GY, Renfrew DL. Percutaneous procedures for the diagnosis and treatment of lower back pain: diskography, facet-joint injection, and epidural injection. AJR Am J Roentgenol. (1991) 157:685–91. doi: 10.2214/ajr.157.4.1832511, PMID: [DOI] [PubMed] [Google Scholar]
- 71.O'Leary SA, Paschos NK, Link JM, Klineberg EO, Hu JC, Athanasiou KA. Facet joints of the spine: structure-function relationships, problems and treatments, and the potential for regeneration. Annu Rev Biomed Eng. (2018) 20:145–70. doi: 10.1146/annurev-bioeng-062117-120924 [DOI] [PubMed] [Google Scholar]
- 72.Vanelderen P, Szadek K, Cohen SP, De Witte J, Lataster A, Patijn J, et al. 13. Sacroiliac Joint Pain. Pain Pract. (2010) 10:470–8. doi: 10.1111/j.1533-2500.2010.00394.x [DOI] [PubMed] [Google Scholar]
- 73.Ohtori S, Kinoshita T, Yamashita M, Inoue G, Takahashi K. Results of surgery for discogenic low back pain: a randomized study using discography versus discoblock for diagnosis. Spine. (2009) 34:1345–8. doi: 10.1097/BRS.0b013e3181a401bf [DOI] [PubMed] [Google Scholar]
- 74.Shealy CN. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J Neurosurg. (1975) 43:448–51. doi: 10.3171/jns.1975.43.4.0448 [DOI] [PubMed] [Google Scholar]
- 75.Helm Ii S, Simopoulos TT, Stojanovic M, Abdi S, El Terany MA. Effectiveness of thermal annular procedures in treating Discogenic low Back pain. Pain Physician. (2017) 20:447–70. doi: 10.36076/ppj/447, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Brügger A. Über vertebrale, radikuläre und pseudoradikuläre Syndrome II. Acta Rheumatol. (1962) 19:S9–S111. [Google Scholar]
- 77.Ohtori S, Orita S, Yamauchi K, Eguchi Y, Aoki Y, Nakamura J, et al. Do physical symptoms predict the outcome of surgical fusion in patients with Discogenic low Back pain? Asian Spine J. (2016) 10:509–15. doi: 10.4184/asj.2016.10.3.509, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]