Abstract
Objective:
To estimate the likelihood of an mpox outbreak and the potential impact of mitigation measures in a residential college setting.
Design:
We developed a stochastic dynamic SEIR model of mpox transmission in a study population composed of: a high-risk group representative of the population of men who have sex with men (MSM) with a basic reproductive number (R0) of 2.4; a low-risk group with an R0=0.8. Base input assumptions included: incubation time (days) = 7.6; and time to recovery (days) = 21.
Setting:
U.S. residential college campus.
Participants:
Hypothetical cohort of 6500 students.
Interventions:
Isolation, quarantine, and vaccination of close contacts.
Measurements:
Proportion of 1,000 simulations producing sustained transmission; mean cases given sustained transmission; maximum students isolated, quarantined, and vaccinated. All projections are estimated over a planning horizon of 100 days.
Results:
Without mitigation measures, the model estimated an 83% likelihood of sustained transmission, leading to an average of 183 cases. With detection and isolation of 20%, 50%, and 80% of cases, the average infections would fall to 117, 37, and 8, respectively. Reactive vaccination of contacts of detected cases (assuming 50% detection and isolation) reduced mean cases from 37 to 17, assuming 20 vaccinated contacts per detected case. Pre-emptive vaccination of 50% of the high-risk population prior to outbreak reduced cases from 37 to 14, assuming 50% detection and isolation.
Limitations:
A model is a stylized portrayal of behavior and transmission on a university campus.
Conclusion:
Based on our current understanding of mpox epidemiology among MSM in the US, this model-based analysis suggests that future outbreaks of mpox on college campuses may be controlled with timely detection and isolation of symptomatic cases.
Funding Source:
Research reported in this publication was supported by grants from the National Institute on Drug Abuse (DP2 DA049282 to GSG; R37 DA15612 to AS, GG, and ADP) and from the National Institute of Allergy and Infectious Diseases (R01 AI137093 to VEP) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Introduction
In the spring of 2022, two years after the start of the COVID-19 pandemic, a new infectious disease threat emerged on the world stage: the human mpox virus, an agent which had previously been confined to endemic regions in West and Central Africa. The first American cases of mpox cases were detected in May 2022. By November 2022, cases had been confirmed in every state in the country, and the nationwide case count had reached nearly 30,000. Globally, the outbreak to date has affected over 80,000 people in 110 countries, 103 of which did not historically report mpox infections (1). While the number of cases around the world has declined in recent weeks, the US and other countries in the Americas remain the epicenter, and the World Health Organization still considers the global risk of the outbreak to be moderate among men who have sex with men (MSM) (2).
University and college leaders took note and made plans to address potential mpox outbreaks on campus (3–5). This reflected their concern that mpox could break swiftly out of the dense networks of MSM to which it had originally been confined and into other congregate settings and populations with high levels of regular, frequent physical contact. As of December 2022, only a few cases of mpox have been noted in educational settings (6). However, the national and global outbreaks are still not fully contained, and college students remain a vulnerable population. Persons of college age represent close to half of all sexually transmitted infections (STI) in the US. Chlamydia, gonorrhea, and syphilis alone account for $1.1 billion in direct medical costs, with 60% of this care directed at those from 15-24 years of age (7). Thus, mpox may still emerge, if even sporadically, among young MSM and other sexually active individuals, including on college campuses, with ongoing public health and economic implications for these institutions.
It is difficult to predict how a novel infection like mpox might spread in different settings, especially ones that have unique characteristics like college campuses. Mathematical modeling of disease transmission was widely used during the COVID-19 pandemic to help guide decision-makers and was critical in helping university leaders to design efforts to mitigate spread of the virus on campuses nationwide (8–11). In this analysis, we attempted to employ those same strategies to model the spread of mpox on a residential college campus, to estimate the likelihood of an outbreak, and to evaluate the potential impact of interventions including case detection and isolation, quarantine, and/or vaccination of close contacts. We also developed a web-based tool which college and university officials may use to tailor the analysis presented here to their particular campus settings.
Methods
Disease transmission model
We developed a stochastic dynamic SEIR model of mpox transmission on a college campus (Figure 1). The model simulates transitions between susceptible [S], pre-symptomatic (exposed but not infectious) [E], symptomatic (infectious) [I], and recovered [R] states. We considered a student cohort divided into low- and high-risk groups. We assumed a fixed population size (N) and no movement between the two risk groups. At the start of the semester (time 0), there are I0 infected individuals, all of whom are assumed to be in the high-risk group, and N-I0 susceptible individuals. For example, assuming 6,500 undergraduate students (N=6,500) and 1 infected individual at the start of the semester (I0=1), the remaining 6,499 individuals (N- I0) were assumed to be susceptible. Susceptible individuals can become infected if they are exposed to an infectious, symptomatic, undetected case.
Figure 1.
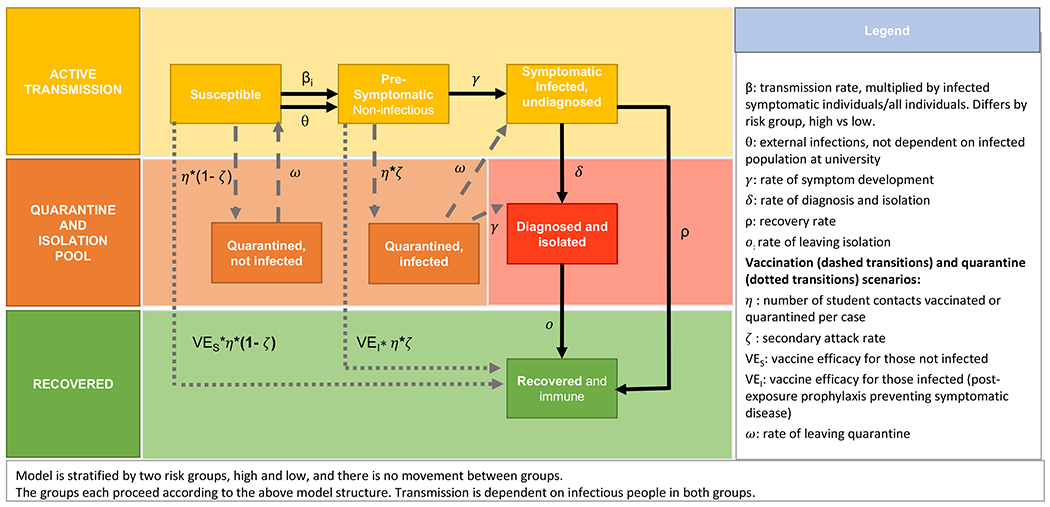
Model diagram.
We assumed frequency-dependent transmission, such that new infections occur at rate (βH*IH/NH + βL*(IH+IL)/N) for the high-risk group and rate βL*(IH+IL)/N for the low-risk group. This assumes two possible forms of transmission: one from casual contact between all students (assuming proportional mixing), with transmission parameter βL; and a second mode of transmission exclusively between high-risk students to denote possible sexual transmission within MSM (with transmission parameter βH). Transmission rates βH and βL are obtained by multiplying the risk-group-level basic reproductive number (R0,H, R0,L) by the rate of departure from the symptomatic, undetected state (i.e. βH = R0,H*ρ, βL = R0,L*ρ). For example, if we assume a symptomatic infectious period of 21 days (ρ=1/21) and R0,H of 1.4 (assuming each high-risk infectious person infects 1.4 other high-risk people on average in a fully susceptible population) and R0,L of 0.8 (assuming each high- and low-risk case infects 0.8 other people on average in a fully susceptible population) then the transmission parameters will be βH=1.4*(1/21) = 0.07 and βL= 0.8*(1/21) = 0.04. The model also permits external introductions of mpox (at a rate θ), allowing for students to be infected by members of the broader community; these external infections are assumed to occur exclusively in members of the high-risk group. Assuming 1 external infection over 100 days, this would give a θ of 1/100 per day.
Interventions
Detection and Isolation
Symptomatic students are assumed to be removed from the active, infectious population at diagnosis rate δ. We assume that diagnosed cases remain isolated until they have recovered and are no longer infectious, at which time they move to the recovered and immune compartment. Students may also recover (at rate ) without ever having been detected and isolated. We assumed that the same rates of detection and recovery applied to both the high- and low-risk groups.
We report detection and isolation as the percent of cases detected (). For example, if we assume the rate of recovery from infectiousness is approximately 0.05 per day, 80% of cases detected and isolated corresponds to an isolation rate (δ) of approximately 0.2 per day. We explored the impact of varying levels of detection and isolation from 0% to 80% of cases.
Vaccination
We also considered the impact of two vaccination strategies—reactive and pre-emptive—on transmission dynamics. For reactive vaccination, when a symptomatic case is detected and isolated, students from the susceptible and pre-symptomatic populations can be vaccinated and thereby move to the recovered population. The number of students removed from the susceptible vs the pre-symptomatic compartments is based on the secondary attack rate of mpox (ζ). We also explored a scenario where 50% of the high-risk group was pre-emptively vaccinated for mpox prior to the start of the simulation. Successfully vaccinated students, as well as recovered students, are considered immune and return to the general population, but can no longer be infected or contribute to transmission.
Quarantine
We model quarantine of students as a function of the number of detected cases. Case contacts move from the susceptible or pre-symptomatic compartments to the quarantine compartment based on the assumed secondary attack rate of mpox (ζ). Those who are not infected (number of students quarantined * (1-ζ)) will return to the susceptible group after completing quarantine. Those who are infected (number of students quarantined * ζ) will either move on to be detected (at a rate = 1/duration of time from infection to symptom onset) or move to the infected compartment, remaining undetected (at a rate ω = 1/duration of quarantine).
Key data inputs
The population size (6,500) was roughly based on the Yale undergraduate population (12). Based on estimates of the MSM population among those aged 18-24 years (13), we assigned 10% of this population to the “high-risk” group (650 people). The duration of time spent in the pre-symptomatic (pre-infectious) state (1/γ) averaged 7.6 days (14–18), and the duration of time in the symptomatic (infectious) state (1/ρ) averaged 21 days (16, 17, 19). We evaluated two base-case assumptions for R0,H in the high-risk group, 2.4 and 1.4, commensurate with estimates of R0 among MSM in the most recent mpox outbreak (15, 20–23). We assumed R0,L was 0.8 in the low-risk group and for casual contact between the high-risk and low-risk groups, commensurate with R0 estimates from prior outbreaks of mpox (24).
For susceptible students who are vaccinated, the vaccine efficacy is assumed to be 80%, which meant that on average 80% of vaccinated people would move to a protected compartment from which progression to symptomatic disease was not possible, and 20% would remain in the susceptible compartment and receive no benefit from vaccination. For pre-symptomatic students who are vaccinated, vaccine efficacy is assumed to be cut in half (40%) to account for students who are not vaccinated early enough in the infection (since vaccination is considered most effective within 4 days of infection) (25). Thus, vaccination as post-exposure prophylaxis is modeled as preventing symptomatic (and therefore infectious) disease in 40% of vaccinated and infected individuals and does not modify disease progression (i.e., shorten length of symptoms or decrease infectiousness and severity of symptoms) in the remaining 60% who are not protected.
For those detected and isolated, we assumed isolation would last 28 days (19). For those quarantined, we assumed an average quarantine period (1/ω ) of 14 days (18).
All model parameter estimates and sources can be found in Table 1.
Table 1.
Summary of model parameter estimates and sources.
| Parameter | Estimate | In model | Source |
|---|---|---|---|
| Total population | 6,500 | N | Yale University undergraduate population, 2021 (12) |
| High-risk population | 650 | NH | Jones (13) |
| Infected students at start of semester | 1 | I 0 | Assumed |
| Number of external infections, monthly | 1 | Assumed | |
| Basic reproductive number, high-risk group (R0,H) | 2.4 | Kaler et al. (23) Endo et al. (20) Guzzetta et al. (15) Branda et al. (22) |
|
| Basic reproductive number, low-risk group (R0,L) | 0.8 | US Department of Homeland Security (24) | |
| Mpox secondary attack rate | 0.2 | Estimated from R0 and estimated number of contacts | |
| Time from infection to symptom onset | 7.6 days | 1/ | Thornhill et al. (14) Guzzetta et al. (15) CDC (16) WHO (17) Miura et al.(18) |
| Time from symptom onset to recovery | 21 days | Adler et al. (19) CDC (16) WHO (17) |
|
| Isolation duration | 28 days | 1/ | Adler et al. (19) |
| Quarantine duration | 14 days | 1/ | Miura et al. (18) |
| Vaccine efficacy, susceptible population | 0.8 | VE1 | Rimoin et al. (34) Fine et al. (35) Reynolds et al. (36) |
| Vaccine efficacy, infected pre-symptomatic population (effectiveness in preventing symptomatic infection) | 0.4 | VE2 | Rimoin et al. (34) Fine et al. (35) Reynolds et al. (36) CDC (25) |
| Percent of cases detected | 0 - 80% | Adler et al. (19) |
Base case analysis
The base case of the model was parameterized with one symptomatic infection in the high-risk group, and on average one additional external infection in the high-risk group over a time horizon of 100 days, which can be considered a typical planning period for universities given the semester system.
Outcomes of interest were the proportion of runs that produced outbreaks (i.e., additional cases beyond the initial cases), average (and range) and maximum number of cases in the event of an outbreak, and estimated number of students isolated, quarantined, and vaccinated. In addition, we recorded the total number of cases and infectious students over 100 days.
Sensitivity analyses
We performed a two-way sensitivity analysis to understand the robustness of our results in the face of variation in both the proportion of the population at high risk for mpox transmission and R0,H for the high-risk group. We varied the proportion of the population at high risk for mpox from 5-50% and R0,H from 0.8 (equal to that of the low-risk population) to 3.5, assuming 20% detection and isolation of symptomatic cases.
Model implementation and online tool
Stochasticity in the model was implemented through use of the Gillespie algorithm (26) in order to allow for the likelihood of small outbreaks given an R0 below 1. For each analysis, the model was run 1,000 times.
In addition, we developed a web-based, interactive implementation of the model to permit college decision-makers to reproduce our findings and to customize the analysis to the particular features of their institutions. The online tool allows decision-makers to vary R0 in the low-risk and high-risk populations, proportion of population at high risk, population size, and presence and availability of intervention measures (detection/isolation and vaccination). For the online tool, 100 model simulations are run for each new parameter combination.
Results
Base case and detection and isolation results
Without detection and isolation of students and assuming an R0,H of 2.4, the model predicted an 82% chance of an outbreak in the high-risk group and 83% chance of cases in the low-risk group (Figure 2, Table 2), resulting in a mean of 124 (95% range: 3-326) additional cases in the high-risk group and 59 (1-184) cases in the low-risk group. Detection and isolation of symptomatic cases reduced likelihood of an outbreak to 51% in the high-risk group and to 29% in the low-risk group when 80% of cases were detected. The mean number of cases fell to below 10 in both groups with 80% detection. Maximum isolation capacity needed was 47 when 50% of students were detected and isolated.
Figure 2. Number of infectious mpox cases over time for different isolation rates.
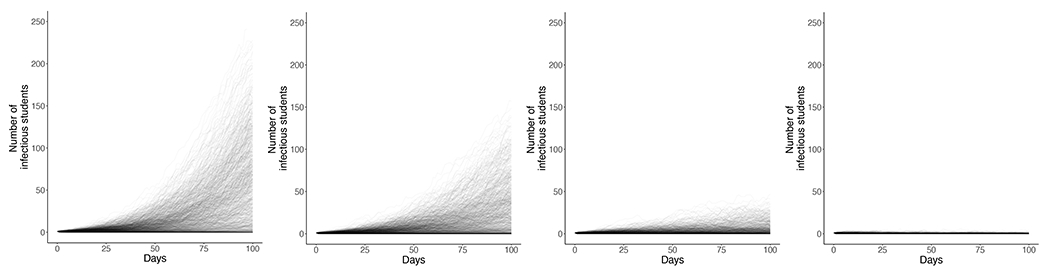
Each line represents one of 1000 stochastic model runs, representing possible outbreak pathways for a population of 6,500 students with 1 infectious student at the start of semester over 100 days and an average of 1 additional external infection in that time period. R0,H is at 2.4, and the R0,L is 0.8. This scenario assumes no vaccination or quarantine. The proportion of cases that are detected and isolated (=δ/(δ+ρ)) varies by panel, from left to right: a. No diagnosis/isolation, b. 20% of students isolated, c. 50% of students isolated, d. 80% of students isolated.
Table 2. Likelihood and size of campus outbreaks with varying diagnosis and isolation rates given one mpox introduction over 100 days, R0,H of 2.4.
Likelihood of more than one additional case and mean number of additional cases given one mpox case introduction on day 0 onto a campus of 6,500 students over 100 days, by percent cases detected and isolated, for 1,000 model runs. R0,H is 2.4, and the R0,L is 0.8.
| High-risk group | Low-risk group | Total population | ||||
|---|---|---|---|---|---|---|
| Detection and Isolation | Likelihood of additional cases beyond initial case | Mean number of additional cases, 95% range | Likelihood of cases | Mean number of cases, 95% range | Mean number of isolated cases, given ongoing outbreak | Maximum isolation capacity needed |
| None | 82% | 124 (3-326) | 83% | 59 (1-184) | - | - |
| 20% of cases | 79% | 80 (3-249) | 76% | 37 (1-121) | 9 | 40 |
| 50% of cases | 75% | 26 (3-109) | 66% | 11 (1-43) | 8 | 47 |
| 80% of cases | 51% | 6 (3-14) | 29% | 2 (1-5) | 3 | 13 |
Without detection and isolation of students and assuming an R0,H of 1.4, the model predicted a 75% chance of an outbreak in the high-risk group and 77% chance of cases in the low-risk group (Appendix Figure 1, Appendix Table 1), resulting in a mean of 24 (3-85) additional cases in the high-risk group and 17 (1-61) cases in the low-risk group. Detection and isolation of symptomatic cases reduced likelihood of an outbreak to 45% in the high-risk group and to 27% in the low-risk group when 80% of cases were detected. The mean number of cases fell to below 10 in both groups with 50% detection. Maximum isolation capacity needed was 17 isolation spaces when 50% of students were detected and isolated.
Vaccination and quarantine results
Assuming 50% of cases were detected and isolated, reactive vaccination did not reduce the likelihood of an outbreak (Table 3, Appendix Table 2); however, with an R0,H of 2.4, reactive vaccination reduced the average number of cases per outbreak. When 20 contacts were vaccinated per identified case, the mean number of cases per outbreak was reduced by half. The same pattern was not observed for an R0,H of 1.4.
Table 3. Likelihood and size of campus outbreaks with varying reactive vaccination rates.
Likelihood of more than one additional case and mean number of additional cases given one initial mpox case on a campus of 6,500 students, by level of vaccination with 50% detection and isolation of cases, for 1,000 model runs. R0,H is 2.4, and the R0,L is 0.8.
| High-risk group | Low-risk group | Total Population | |||||
|---|---|---|---|---|---|---|---|
| Vaccination of identified contacts | Likelihood of additional cases beyond initial case | Mean number of additional cases, 95% range | Likelihood of cases | Mean number of cases, 95% range | Maximum isolation capacity needed | Mean number of students vaccinated | Maximum number of students vaccinated |
| None | 75% | 26 (3-109) | 66% | 11 (1-43) | 57 | ||
| 2 contacts per case | 71% | 23 (3-89) | 66% | 9 (1-36) | 33 | 17 | 164 |
| 6 contacts per case | 72% | 17 (3-65) | 63% | 7 (1-24) | 29 | 43 | 344 |
| 20 contacts per case | 71% | 12 (3-34) | 65% | 5 (1-17) | 17 | 104 | 623 |
Similarly, quarantine was not shown to reduce likelihood of outbreak, but did reduce average number of cases per outbreak when R0,H was 2.4 (Appendix Tables 3–4).
When assuming 50% of those in the high-risk group were pre-emptively vaccinated for mpox prior to start of simulation, the likelihood of an outbreak beyond the initial cases was reduced (Table 4). Assuming no detection and isolation, likelihood of outbreak was 76% in the high-risk and 78% in the low-risk groups. The mean number of cases per outbreak also declined by 33-82% in the high-risk group, and 50-72% in the low-risk group when detection and isolation was below 80%.
Table 4. Likelihood and size of campus outbreaks with varying diagnosis and isolation rates given one mpox introduction over 100 days, assuming pre-emptive vaccination of 50% of high-risk group.
Likelihood of more than one additional case and mean number of additional cases given one mpox case introduction on day 0 onto a campus of 6,500 students over 100 days, by percent cases detected and isolated, for 1,000 model runs. R0,H is 2.4, and the R0,L is 0.8. Sensitivity analysis assumes 50% of high-risk group is vaccinated for mpox before time 0 (assuming 80% vaccine effectiveness).
| High-risk group | Low-risk group | Total Population | ||||
|---|---|---|---|---|---|---|
| Detection and Isolation | Likelihood of additional cases beyond initial case | Mean number of additional cases, 95% range | Likelihood of cases | Mean number of cases, 95% range | Mean number of isolated cases, given ongoing outbreak | Maximum isolation capacity needed |
| None | 76% | 22 (3-73) | 78% | 16 (1-58) | - | - |
| 20% of cases | 71% | 16 (3-57) | 73% | 10 (1-39) | 3 | 13 |
| 50% of cases | 60% | 9 (3-27) | 56% | 5 (1-16) | 4 | 18 |
| 80% of cases | 44% | 4 (3-8) | 27% | 2 (1-4) | 3 | 8 |
Sensitivity analysis results
When we varied the proportion of the population in the high-risk group from 5% to 50% and the R0,H for the high-risk group from 0.8 to 3.5, the probability of additional cases beyond the initial cases ranged from 70% to 90%, and mean additional cases ranged from 11 to 1000 (Appendix Figures 2–3).
Discussion
The future trajectory of mpox in the US is unknown. While the last seven-day average number of cases was under 10 and the Department of Health and Human Services will not renew the Public Health Emergency when it expires at the end of January 2023, HHS Secretary Xavier Becerra said” “we won’t take our foot off the gas — we will continue to monitor the case trends closely” (27). Indeed, while cases are low, there have been small clusters noted in Texas and Southern California according to reports in the popular press (28), which are cause for concern for public health officials. While the progress of the outbreak thus far seems to have spared college campuses, occasional outbreaks of STIs and HIV have occurred among university students, particularly MSM, such as those in 2004 and 2007 (26, 27). Thus, the potential for mpox outbreaks on college campuses remains unless the disease is eradicated in the US and there are no further introductions from abroad.
Our model provides the basis of a contingency plan for college administrators should mpox be introduced to a residential college campus. Our study clearly shows that without any intervention to address the introduction of mpox on a campus, an outbreak is likely to ensue; thus, preparing for such an eventuality is a prudent course. Since our model also showed that simple interventions—detection and isolation of cases in particular--could be highly effective in reducing both the likelihood and the magnitude of potential outbreaks, planning for mpox offers few downsides for administrators. Furthermore, this study and the accompanying online tool are meant to make it easier for university and college leaders to make decisions about containing any nascent outbreaks that may occur in the future.
There are limitations to our modeling strategy. This is not a network model, and greatly simplifies mixing patterns between students. While this reduces the complexity of the analysis, it also means that we may have over- or underestimated the potential downstream influence of the high-risk population. It also limits our ability to consider very targeted vaccination or quarantine strategies or to evaluate changes in behavior that may affect mpox spread. In addition, we were limited in the data we had to parameterize the model. The novelty of the 2022 mpox outbreak undermined our confidence in key values, notably historical R0 estimates and assumptions about mpox epidemiology (29). We attempted to address this limitation using sensitivity analyses and by demonstrating the robustness of our findings across broad parameter value ranges
To date, there has been little modeling of mpox outside of endemic areas in which it is common. While several mpox studies from the last few years have looked at the increasing emergence of human-to-human transmission in endemic settings prior to the current global outbreak (30–32), ours is the first model to examine human-to-human mpox transmission and control on college campuses in an outbreak setting, and one of a few new studies to look at mpox outside of endemic regions. In addition, our work on mpox control in this study is supported by additional work we have done on mpox control in the US (33). We hope these efforts spur additional work to investigate and model mpox emergence outside of west and central Africa among MSM and in congregate settings, such as the college campuses we have described here.
Supplementary Material
References
- 1.CDC 2022;Pages https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html on 11/9 2022.
- 2.World Health Organization. Multi-country outbreak of monkeypox, External situation report #10 - 16 November 2022. Emergency Situational Updates; 2022. [Google Scholar]
- 3.Taylor S Monkeypox Infections Reported by Colleges Raise Concerns of Campus Spread. Bloomberg; 2022. [Google Scholar]
- 4.Dreher A Concern about monkeypox spread shifts to college campuses. AXIOS; 2022. [Google Scholar]
- 5.CDC 2022;Pages https://www.cdc.gov/poxvirus/monkeypox/community/higher-education.html on 11/9 2022.
- 6.Soucheray S US schools, colleges see monkeypox cases. CIDRAP News. Center for Infectious Disease Research and Policy; 2022. [Google Scholar]
- 7.CDC 2022;Pages https://www.cdc.gov/nchhstp/newsroom/fact-sheets/std/STI-Incidence-Prevalence-Cost-Factsheet.html on 11/19 2022.
- 8.Gonsalves GS, Salomon JA, Thornhill T, Paltiel AD. Adventures in COVID-19 Policy Modeling: Education Edition. Curr HIV/AIDS Rep. 2022;19(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paltiel AD, Schwartz JL. Assessing COVID-19 Prevention Strategies to Permit the Safe Opening of Residential Colleges in Fall 2021. Ann Intern Med. 2021;174(11):1563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paltiel AD, Zheng A, Walensky RP. Assessment of SARS-CoV-2 Screening Strategies to Permit the Safe Reopening of College Campuses in the United States. JAMA Netw Open. 2020;3(7):e2016818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultes O, Clarke V, Paltiel AD, Cartter M, Sosa L, Crawford FW. COVID-19 Testing and Case Rates and Social Contact Among Residential College Students in Connecticut During the 2020-2021 Academic Year. JAMA Netw Open. 2021;4(12):e2140602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yale 2022;Pages https://www.yale.edu/about-yale/yale-facts on 9/1 2022.
- 13.Jones JM. LGBT Identification in U.S. Ticks Up to 7.1%. GALLUP; 2022. [Google Scholar]
- 14.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med. 2022;387(8):679–91. [DOI] [PubMed] [Google Scholar]
- 15.Guzzetta G, Mammone A, Ferraro F, Caraglia A, Rapiti A, Marziano V, et al. Early Estimates of Monkeypox Incubation Period, Generation Time, and Reproduction Number, Italy, May-June 2022. Emerg Infect Dis. 2022;28(10):2078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC 2022; Pages https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html on 10/9/2022 2022.
- 17.WHO 2022; Pages https://www.who.int/news-room/fact-sheets/detail/monkeypox on 10/9/2022 2022.
- 18.Miura F, van Ewijk CE, Backer JA, Xiridou M, Franz E, Op de Coul E, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. The Lancet Infectious Diseases. 2022;22(8):1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo A, Murayama H, Abbott S, Ratnayake R, Pearson CAB, Edmunds WJ, et al. Heavy-tailed sexual contact networks and monkeypox epidemiology in the global outbreak, 2022. Science. 2022;378(6615):90–4. [DOI] [PubMed] [Google Scholar]
- 21.Du Z, Shao Z, Bai Y, Wang L, Herrera-Diestra JL, Fox SJ, et al. Reproduction number of monkeypox in the early stage of the 2022 multi-country outbreak. Journal of Travel Medicine. 2022. [DOI] [PubMed] [Google Scholar]
- 22.Branda F, Pierini M, Mazzoli S. Monkeypox: Early estimation of basic reproduction number R(0) in Europe. J Med Virol. 2022:e28270. [DOI] [PubMed] [Google Scholar]
- 23.Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus. 2022;14(7):e26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Homeland Security Science and Technology Directorate. Master Question List for Monkeypox Virus (MPXV). In: Security UDoH, ed; 2022. [Google Scholar]
- 25.CDC 2022; Pages https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html#anchor_1545415186164 on 8/30 2022.
- 26.Johnson P adaptivetau: Tau-Leaping Stochastic Simulation. R package version 2.2-3; 2019. [Google Scholar]
- 27.King R Becerra decides to not extend mpox public health emergency set to expire next month. Fierce Healthcare; 2022. [Google Scholar]
- 28.Waechter P How We Stopped Mpox This Year And What’s Next For The Disease Formerly Known As Monkeypox. Buzzfeed News; 2022. [Google Scholar]
- 29.Gellad WF. Commentary on Daubresse et al. (2017): An epidemic of outdated data. Addiction. 2017;112(6):1054–5. [DOI] [PubMed] [Google Scholar]
- 30.Grant R, Nguyen LL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98(9):638–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peter OJ, Kumar S, Kumari N, Oguntolu FA, Oshinubi K, Musa R. Transmission dynamics of Monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2022;8(3):3423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen PY, Ajisegiri WS, Costantino V, Chughtai AA, MacIntyre CR. Reemergence of Human Monkeypox and Declining Population Immunity in the Context of Urbanization, Nigeria, 2017-2020. Emerg Infect Dis. 2021;27(4):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chitwood MH, Kwon J, Savinkina A, Walker J, Bilinski A, Gonsalves G. Testing, Tracing, and Vaccination Targets for Containment of the US Monkeypox Outbreak: A Modeling Study. medRxiv. 2022:2022.08.01.22278199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107(37):16262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643–50. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds MG, Davidson WB, Curns AT, Conover CS, Huhn G, Davis JP, et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007;13(9):1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


