Abstract
Background
A novel treatment has been developed to reconstruct large skin defects caused by the excision of giant congenital melanocytic nevi. It involves the reimplantation of high-hydrostatic pressurized nevus tissue as a cell-inactivated autologous scaffold for dermal regeneration, followed by the implantation of cultured epithelial autografts on the regenerated dermis. Because this treatment has shown promise in a first-in-human clinical trial which used a prototype pressure machine, a novel pressure device was specifically designed for clinical use.
Methods
In a prospective investigator-initiated clinical trial involving three patients, we evaluated the safety and efficacy of the skin regeneration treatment using a pressure device. All three patients underwent surgical excision of the nevus tissue, primary reimplantation of the inactivated nevus tissue, and secondary implantation of cultured epithelial autografts.
Results
Engraftment of inactivated nevus tissue and cultured epithelial autografts was successful in all three cases, with over 90% epithelialization at 8 weeks post-surgery. No serious adverse events or device malfunction were observed during the trial.
Conclusion
The novel pressure device safely and effectively enabled dermal regeneration using the nevus tissue as an autologous scaffold. This innovative approach offers several advantages, including reduced invasiveness due to minimal sacrifice of normal skin for skin grafting and high curative potential resulting from full-thickness removal of the nevus tissue.
Keywords: Giant congenital melanocytic nevi, High-hydrostatic pressure, Dermal regeneration, Autologous scaffold, Cultured epithelial autografts
Highlights
-
•
A high-pressure device was developed to treat giant congenital melanocytic nevi.
-
•
The device can completely inactivate all cells in nevus tissue.
-
•
The nevus tissue serves as a cell-inactivated autologous dermal scaffold.
-
•
This scaffold helps reconstruct skin defects, resulting from excision of nevi.
1. Introduction
A giant congenital melanocytic nevus is a type of large melanocytic nevus present at birth and measures ≥6 cm on the body or ≥9 cm on the head of neonates, and >20 cm in diameter in adults [1]. It carries a risk of malignant melanoma [2,3], and early prophylactic surgical excision is recommended to reduce this risk [4]. However, complete removal of these skin lesions results in large full-thickness skin defects because nevus cells are histologically present throughout the dermal layer which usually requires harvesting of large amounts of normal skin for reconstruction.
To address this issue, a novel treatment has recently been developed to reconstruct full-thickness skin defects after excision of giant congenital melanocytic nevi [5,6]. This involves the primary reimplantation of high-hydrostatic pressurized nevus tissue as a cell-inactivated autologous scaffold for the dermis regeneration, followed by secondary implantation of cultured epithelial autografts on the regenerated dermis. Nevus tissue is inactivated by applying high-hydrostatic pressurization at 200 MPa for 10 min, which effectively kills all cells in human skin and nevus tissue [[7], [8], [9], [10], [11]].
In a first-in-human clinical trial conducted from 2016 to 2018 [6], full-thickness nevi were inactivated using a portable high-hydrostatic pressure machine (Kitaoka Iron Works Co. Ltd., Osaka, Japan) at 200 MPa for 10 min [[7], [8], [9], [10], [11]], and subsequently reimplanted onto the original site. Four weeks post-surgery, the cultured epithelial autografts were implanted onto the regenerated dermis. The reconstructed skin was epithelized by more than 96% in each graft at 12 weeks, and no recurrence was observed at 52 weeks. This suggests that inactivated nevus tissue can provide a suitable graft bed for cultured epidermal autografts. However, the pressure machine used in the trial was a prototype, and the required manual pressure adjustment posed the risk of inaccuracy and lack of reproducibility. Therefore, to improve the safety and reproducibility of the treatment and facilitate insurance approval, we developed a new medical device, a high-hydrostatic pressure device, and a skin container specifically to inactivate nevus tissue for clinical use. A clinical assessment of the treatment outcomes is necessary to evaluate the performance of these devices. Hence, we conducted this prospective investigator-initiated clinical trial to evaluate the safety and efficacy of skin regeneration treatments using inactivated nevus tissues created using this new device and cultured epithelial autografts.
2. Patients and methods
2.1. Study objectives, design, and participants
This was a prospective, open-label, nonrandomized, noncomparative, single-arm clinical trial to confirm the safety and efficacy of the following treatment methods: surgical excision of nevus tissue, primary reimplantation of inactivated nevus tissue created with the investigational devices, and secondary implantation of cultured epithelial autografts. The eligibility criteria included patients with giant congenital melanocytic nevi deemed unsuitable for resection using standard treatments, including primary closure, tissue expansion, and skin grafting. The exclusion criteria included extensive scarring from previous therapies, where the successful engraftment of the inactivated nevus was deemed unlikely (Table 1). We selected the target nevus to be within 10% or less of the body surface area of each trial patient who met the eligibility and exclusion criteria. A target sample size of three cases was set, considering the rarity of the disease and the feasibility of accumulating cases in this study. The study was registered in the Japan Registry of Clinical Trials (ID: jRCT2052210044) and was conducted between July 2021 and March 2022 at Kyoto University Hospital, Japan.
Table 1.
Study eligibility and exclusion criteria.
| Eligibility Criteria: |
|
|
|
|
| Exclusion Criteria: |
|
|
|
|
|
|
|
|
This study was conducted in accordance with the Declaration of Helsinki and the Pharmaceutical Affairs Law. Following the review process of the Pharmaceuticals and Medical Devices Agency, the study design and amendments received Institutional Review Board approval from Kyoto University Hospital (approval no. K077). Written informed consent was obtained from all the patients. The study was conducted in accordance with the principles of good clinical practice.
2.2. Investigational devices
2.2.1. High-hydrostatic pressure device
The pressure device (Fig. 1A) was manufactured by Sugino Machine Co., Ltd. (Toyama, Japan) according to the Japanese safety requirements for electrical equipment (JIS C1010-1). It was delivered to Kyoto University Hospital by Japan Tissue Engineering Co., Ltd. (J-TEC, Aichi, Japan). The main components of the device include a pressure container, pressure sensor, servomotor, and water supply tank with dimensions of 950 × 850 × 1440 mm (width × depth × height). It was designed to apply hydrostatic pressures of up to 300 MPa on the samples inside a pressure container.
Fig. 1.
(A) High-hydrostatic pressure device and (B) skin container.
2.2.2. Skin container
The skin container (Fig. 1B) was specifically designed to hold the excised nevus tissue during pressurization. It was manufactured by Hanshin Kasei Kogyo Co., Ltd. (Toyama, Japan) using polyethylene and was 45.0 mm in diameter and 89.9 mm in height. The container was sterilized by Radia Industry Co., Ltd. (Gunma, Japan) and delivered to Kyoto University Hospital by J-TEC.
2.3. Intervention
2.3.1. Preparation of cultured epithelial autografts
Two weeks prior to the initial surgical treatment of the target nevus, a normal skin sample of approximately 1 × 2 cm was harvested under either general or local anesthesia and transported to J-TEC. It took three weeks on average to prepare cultured epithelial autografts (JACE®) by J-TEC.
2.3.2. Staged surgical interventions
During the initial surgery, the target nevus was excised to full thickness. A punch biopsy specimen was obtained to evaluate the deep margins. The excised nevus tissue was adjusted to a thickness of 0.5 mm using a Padgett dermatome, and in some cases, it was also meshed. The nevus tissue was placed in a skin container filled with saline solution and sealed. The skin container was set into the pressure container of the device, and pressurized at 210 MPa for 10 min. The inactivated nevus tissue thus obtained was sutured to the original site, fixed, and stabilized using a tie-over dressing and/or splint. The second surgery was performed at 2 (±1) weeks after the initial surgery, during which cultured epithelial autografts were grafted onto the reimplanted nevus tissue. If an area was observed where the inactivated nevus did not survive and the subcutaneous tissue was exposed, it was covered with split-thickness skin grafts and cultured epithelial autografts. Moreover, if needed, additional application of split-thickness skin grafts and/or cultured epithelial autografts was allowed once during 2–6 weeks after the second surgery.
Digital photographs of the target nevus and the reconstructed skin were taken before excision, after reimplantation of the inactivated nevus, 8 weeks after the second surgery, and 24 weeks after the initial surgery. Digital photographs of the donors were obtained when additional skin grafting was required. Blood examinations were performed before the initial surgery and 2 (±1) and 24 (±4) weeks after the initial surgery. After 24 (±4) weeks, a punch biopsy specimen of the reconstructed skin was obtained to diagnose nevus recurrence.
2.4. Outcomes and statistical analysis
The primary outcome was successful engraftment of both the inactivated nevus tissue and cultured epithelial autografts, both of which were evaluated 8 weeks after the second surgery. This evaluation timeframe was set to align with the assessment conducted in a previous clinical trial of JACE® for expanding insurance coverage to giant congenital melanocytic nevi. Successful engraftment was defined as an epithelialization rate (ratio of the epithelialized area to the surface area of the target nevus) exceeding 90%. An independent committee comprising three plastic surgeons assessed the epithelial area of the reconstructed skin at 8 weeks. The point estimate of the proportion of cases judged to have engraftment, along with the two-sided 95% confidence interval (CI) was calculated using the Clopper-Pearson method. The secondary endpoints involved safety assessment, including but not limited to any instance of device malfunction, and adverse events such as nevus recurrence, within 24 weeks after the initial surgery. Efficacy was also assessed by calculating the skin grafting ratio (the ratio of the donor area of the skin grafts required to reconstruct the area where the inactivated autologous nevus did not survive to the area of the reimplanted inactivated autologous nevus). Additionally, the color change between the target nevus and the reconstructed skin was evaluated using Commission Internationale de I'Eclairage (CIE) L × a∗b∗ [12]. Continuous variables were expressed as means (± standard deviations).
3. Results
3.1. Patient demographics and summary
A participant flowchart is shown in Fig. 2. Three patients (one man and two women) with giant congenital melanocytic nevi covering 21.7% (±15.3%) of their body surface area and no history of other disorders were enrolled in the study. All were younger than 1 year, with a mean of 8.7 (±2.1) months. Their height was 67.8 (±2.2) cm and their weight was 7.4 (±0.4) kg. The target nevi were located on the upper back (case 1), lower back (case 2), and left lower leg (case 3), respectively, and their sizes were 4.7 (±2.5) % of the body surface area. A patient summary is shown in Table 2, and the gross appearance of the target nevus at each evaluation point in each patient is shown in Fig. 3.
Fig. 2.
Participant flow chart.
Table 2.
Patient summary.
| Case | Age (months) | Sex | Target site | Target area (cm2) | Additional SG during the second surgery | Additional SG or CEA after the second surgery | Engraftment of inactivated nevus and CEA at 8 weeks after the second surgery (epithelialization rate, %) | Skin grafting ratio (%) | L∗ value |
Recurrence | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| before | 24 weeks | ||||||||||
| 1 | 8 | M | Upper back | 109.4 | No | No | Successful (97.86) | 0 | 92.19 | 100.68 | No |
| 2 | 7 | F | Lower back | 99.57 | No | SG + CEA | Successful (98.71) | 1.37 | 93.47 | 145.21 | Yes |
| 3 | 11 | F | Lower leg | 69.93 | Yes | CEA | Successful (99.50) | 13.37 | 70.93 | 122.02 | No |
M, male; F, female; SG, skin graft; CEA, cultured epithelial autograft.
Fig. 3.
Gross appearances of target lesions at each timepoint.
3.2. Efficacy
3.2.1. Engraftment of inactivated nevus tissue and cultured epithelial autografts
In all three cases, the engraftment of inactivated nevus tissue and cultured epithelial autografts was successful. Eight weeks after the second surgery, in all patients, epithelialization was achieved in over 90% of the target area in all patients (97.9%, 98.7%, and 99.5%, respectively) (Fig. 3). The 95% CI for the successful engraftment rate, calculated using the Clopper-Pearson method, was 29.2–100%.
3.2.2. Skin grafting ratio
In case 1, epithelialization was achieved with only cultured epithelial autografts on the inactivated nevus tissue without the need for additional skin grafting after inactivated nevus reimplantation (skin grafting ratio:0%). In case 2, an additional skin grafting of 1.33 cm2 (skin grafting ratio:1.37%), obtained from normal skin excised simultaneously as small nevi outside the target area was applied four weeks after the second surgery. In case 3, an additional skin grafting of 13.23 cm2 (skin grafting ratio:13.37%) obtained from the scalp was applied during the second surgery.
3.2.3. Color of the reconstructed skin
The color of the inactivated and reimplanted nevus tissue initially remained black, but improved over time. Moreover, the L∗ values of the reconstructed skin were significantly higher than those of the target nevi.
3.3. Safety
3.3.1. Recurrence
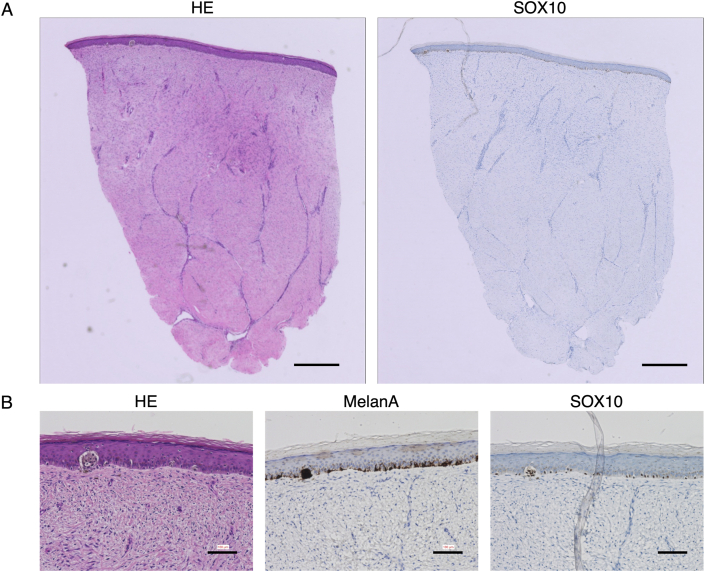
The biopsy results of the excised nevi were negative for deep margins in cases 1 and 3 while they were positive in case 2. Biopsy of the reconstructed skin 24 weeks after the initial surgery showed no presence of nevus cells in cases 1 and 3, but the presence of nevus cells only in the epidermal layer in case 2, indicating recurrence (Fig. 4).
Fig. 4.
Histological sections of the reconstructed skin at 24 weeks in case 2 revealed the presence of nevus cells forming nests only in epidermal layer. (A) Hematoxylin-eosin and SOX10-stained sections. Scale bar: 500 μm. (B) Magnified views of sections stained using hematoxylin-eosin, MelanA, and SO×10. Scale bar: 100 μm.
3.3.2. Adverse events and device malfunction
No serious adverse events were observed in the study population (n = 3). The adverse events that could not be ruled out as having a causal relationship with the investigational devices and treatments included anemia (n = 1), fever (n = 2), pain during postoperative dressing changes (n = 3), wound infection (n = 1), elevation of inflammatory markers (n = 1), blister formation (n = 1), erosion formation (n = 3), hypertrophic scarring (n = 3), and pruritus (n = 1). No device malfunctions were observed during the trials.
4. Discussion
Cultured epithelial autografts prepared using Green's technique [13] have played a major role in advancing the development of regenerative medicine. In Japan, JACE® cultured epithelial autograft has been used for the treatment of severe burns since 2007 and of giant congenital melanocytic nevi since 2016. However, the take rate of cultured epithelial autografts on full-thickness skin defects is low at less than 20%, whereas the rate for partial-thickness skin defects is high at 80% [14]. A 6-year multicenter surveillance in Japan also revealed that the take rate of JACE® on the artificial dermis was only 43% at four weeks and improved to 74% when combined with a wide-mesh autograft [15]. An effective method for regenerating the dermis to provide a suitable wound bed for cultured epithelial autografts has not been established.
This study enrolled three patients who underwent surgical excision of the nevus tissue, primary reimplantation of inactivated nevus tissue created with the investigational devices, and secondary implantation of cultured epithelial autografts. Our previous study showed that full-thickness, inactivated nevus tissue did not survive successfully, while reimplantation of split-thickness tissue resulted in good epithelialization [6]. This suggests a limitation to the thickness at which inactivated nevus tissue can be recellularized and revascularized. Therefore, in this study, the excised nevus tissue was adjusted to a thickness of 0.5 mm. Engraftment of inactivated nevus tissue and cultured epithelial autografts was successful in all three cases, with over 90% epithelialization achieved 8 weeks post-surgery. These results are consistent with those of a first-in-human clinical trial, which also supports the idea that inactivated nevi can provide a suitable graft bed for cultured epidermal autografts [6]. In general, skin grafting for a large full-thickness skin defect requires harvesting of normal skin, which is 17% (1:6 Meek graft) to 60% (1:3 mesh graft) of the defect [[16], [17], [18], [19]]. However, in this clinical trial, the maximum skin grafting ratio was 13.4%, suggesting that our treatment method reduces the sacrifice of normal skin for skin grafting.
No serious adverse events were reported, and no device malfunction was observed during the trial. In case 2, nevus cells were confirmed to be present in the epidermal layer of the reconstructed skin, indicating nevus recurrence. However, the Data and Safety Monitoring Committee determined that this recurrence was not due to device performance issues. Rather, nevus cells likely migrated from the remaining nevus tissue in the surrounding area or deep tissues, as no nevus cells were found in the dermis of the reconstructed skin, and non-clinical studies have shown that nevus cells are completely killed by high-pressure treatment [[7], [8], [9], [10], [11]].
Consequently, the present clinical trial suggests that the investigational devices safely and effectively enable dermal regeneration using the nevus tissue. This method offers several advantages, including reduced invasiveness due to minimal sacrifice of normal skin for reconstruction and high curative potential resulting from full-thickness removal of the nevus tissue. One disadvantage is the requirement for ex vivo removal of the nevus tissue and subsequent inactivation. However, treatments such as laser and cryotherapy without nevus removal are insufficient to kill nevus cells completely and uniformly [20,21], and no alternative treatments have been developed. This innovative approach has the potential to yield favorable outcomes in the treatment of giant congenital melanocytic nevi.
5. Conclusions
A high-hydrostatic pressure device was developed for the reconstruction of full-thickness skin defects resulting from the excision of giant congenital melanocytic nevi. The pressure device safely and effectively enabled dermal regeneration using the excised nevus tissue itself.
Declaration of competing interest
The authors (MS and NM) were financially supported by Japan Tissue Engineering Co., Ltd. (J-TEC) for their collaboration. J-TEC supplied cultured epithelial autografts for this study; however, the company did not play any additional role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Acknowledgements
This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant number 22ck0106583h0003. The authors would like to thank Dr. Hiroshi Naito, Dr. Kazuya Kataoka, Dr. Fumito Ito (Blinded Independent Central Review), and Dr. Jun Arata, Dr. Natsuko Kakudo, and Dr. Toshihiro Ishiko (Data and Safety Monitoring Committee).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Hiroki Yamanaka, Email: ymnkahrk@kuhp.kyoto-u.ac.jp.
Eiichi Sawaragi, Email: sawaragi@kuhp.kyoto-u.ac.jp.
Takashi Nakano, Email: evolve@kuhp.kyoto-u.ac.jp.
Yasuhiro Katayama, Email: hemim@kuhp.kyoto-u.ac.jp.
Tatsuya Ito, Email: taito@wakayama-med.ac.jp.
Harue Tada, Email: haru.ta@kuhp.kyoto-u.ac.jp.
Yu Hidaka, Email: yhidaka@kuhp.kyoto-u.ac.jp.
Satoshi Morita, Email: smorita@kuhp.kyoto-u.ac.jp.
Chihiro Funakoshi, Email: funakosi@kuhp.kyoto-u.ac.jp.
Akemi Kinoshita, Email: kinopiii@kuhp.kyoto-u.ac.jp.
Mieko Watanabe, Email: wamieko@kuhp.kyoto-u.ac.jp.
Itaru Tsuge, Email: itsuge@kuhp.kyoto-u.ac.jp.
Motoki Katsube, Email: katsube@kuhp.kyoto-u.ac.jp.
Michiharu Sakamoto, Email: dojis@kuhp.kyoto-u.ac.jp.
Tetsuji Yamaoka, Email: yamtet@ncvc.go.jp.
Naoki Morimoto, Email: mnaoki22@kuhp.kyoto-u.ac.jp.
References
- 1.Kopf A.W., Bart R.S., Hennessey P. Congenital nevocytic nevi and malignant melanomas. J Am Acad Dermatol. 1979;1:123–130. doi: 10.1016/s0190-9622(79)70009-0. [DOI] [PubMed] [Google Scholar]
- 2.Arad E., Zuker R.M. The shifting paradigm in the management of giant congenital melanocytic nevi: review and clinical applications. Plast Reconstr Surg. 2014;133:367–376. doi: 10.1097/01.prs.0000436852.32527.8a. [DOI] [PubMed] [Google Scholar]
- 3.Watt A.J., Kotsis S.V., Chung K.C. Risk of melanoma arising in large congenital melanocytic nevi: a systematic review. Plast Reconstr Surg. 2004;113:1968–1974. doi: 10.1097/01.prs.0000122209.10277.2a. [DOI] [PubMed] [Google Scholar]
- 4.Marghoob A.A., Agero A.L., Benvenuto-Andrade C., Dusza S.W. Large congenital melanocytic nevi, risk of cutaneous melanoma, and prophylactic surgery. J Am Acad Dermatol. 2006;54:868–870. doi: 10.1016/j.jaad.2006.03.008. discussion 871-3. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto N., Jinno C., Sakamoto M., Kakudo N., Yamaoka T., Kusumoto K. An exploratory clinical trial of a novel treatment for giant congenital melanocytic nevi combining inactivated autologous nevus tissue by high hydrostatic pressure and a cultured epidermal autograft: study protocol. JMIR Res Protoc. 2016;5:e162. doi: 10.2196/resprot.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morimoto N., Mitsui T., Sakamoto M., Mahara A., Yoshimura K., Arata J., et al. A novel treatment for giant congenital melanocytic nevi combining inactivated autologous nevus tissue by high hydrostatic pressure and a cultured epidermal autograft: first-in-human, open, prospective clinical trial. Plast Reconstr Surg. 2021;148:71e–76e. doi: 10.1097/PRS.0000000000008084. [DOI] [PubMed] [Google Scholar]
- 7.Jinno C., Morimoto N., Mahara A., Liem P.H., Sakamoto M., Ogino S., et al. Inactivation of human nevus tissue using high hydrostatic pressure for autologous skin reconstruction: a novel treatment for giant congenital melanocytic nevi. Tissue Eng C Methods. 2015;21:1178–1187. doi: 10.1089/ten.TEC.2015.0054. [DOI] [PubMed] [Google Scholar]
- 8.Liem P.H., Morimoto N., Mahara A., Jinno C., Shima K., Ogino S., et al. Preparation of inactivated human skin using high hydrostatic pressurization for full-thickness skin reconstruction. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto N., Mahara A., Shima K., Ogawa M., Jinno C., Kakudo N., et al. The rapid inactivation of porcine skin by applying high hydrostatic pressure without damaging the extracellular matrix. BioMed Res Int. 2015;2015 doi: 10.1155/2015/587247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto N., Jinno C., Mahara A., Kakudo N., Fujisato T., Kusumoto K., et al. Verification of the inactivation of melanocytic nevus in vitro using a newly developed portable high hydrostatic pressure device. Cells Tissues Organs. 2016;201:170–179. doi: 10.1159/000444048. [DOI] [PubMed] [Google Scholar]
- 11.Mitsui T., Morimoto N., Mahara A., Notodihardjo S.C., Le T.M., Munisso M.C., et al. Exploration of the pressurization condition for killing human skin cells and skin tumor cells by high hydrostatic pressure. BioMed Res Int. 2020;2020 doi: 10.1155/2020/9478789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partl R., Lehner J., Winkler P., Kapp K.S. Testing the feasibility of augmented digital skin imaging to objectively compare the efficacy of topical treatments for radiodermatitis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rheinwald J.G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 14.Auxenfans C., Menet V., Catherine Z., Shipkov H., Lacroix P., Bertin-Maghit M., et al. Cultured autologous keratinocytes in the treatment of large and deep burns: a retrospective study over 15 years. Burns. 2015;41:71–79. doi: 10.1016/j.burns.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura H., Matsushima A., Ueyama M., Kumagai N. Application of the cultured epidermal autograft "JACE((R)") for treatment of severe burns: results of a 6-year multicenter surveillance in Japan. Burns. 2016;42:769–776. doi: 10.1016/j.burns.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Lumenta D.B., Kamolz L.P., Keck M., Frey M. Comparison of meshed versus MEEK micrografted skin expansion rate: claimed, achieved, and polled results. Plast Reconstr Surg. 2011;128:40e–41e. doi: 10.1097/PRS.0b013e318217463a. [DOI] [PubMed] [Google Scholar]
- 17.Rijpma D., Claes K., Hoeksema H., de Decker I., Verbelen J., Monstrey S., et al. The Meek micrograft technique for burns; review on its outcomes: searching for the superior skin grafting technique. Burns. 2022;48:1287–1300. doi: 10.1016/j.burns.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Peeters R., Hubens A. The mesh skin graft--true expansion rate. Burns Incl Therm Inj. 1988;14:239–240. doi: 10.1016/0305-4179(88)90047-2. [DOI] [PubMed] [Google Scholar]
- 19.Henderson J., Arya R., Gillespie P. Skin graft meshing, over-meshing and cross-meshing. Int J Surg. 2012;10:547–550. doi: 10.1016/j.ijsu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Bray F.N., Shah V., Nouri K. Laser treatment of congenital melanocytic nevi: a review of the literature. Laser Med Sci. 2016;31:197–204. doi: 10.1007/s10103-015-1833-3. [DOI] [PubMed] [Google Scholar]
- 21.Elmelegy N., Elghamry S. Carbon dioxide cryotherapy for treatment of nasal and perinasal congenital melanocytic nevi. Ann Plast Surg. 2020;85:107–109. doi: 10.1097/SAP.0000000000002145. [DOI] [PubMed] [Google Scholar]