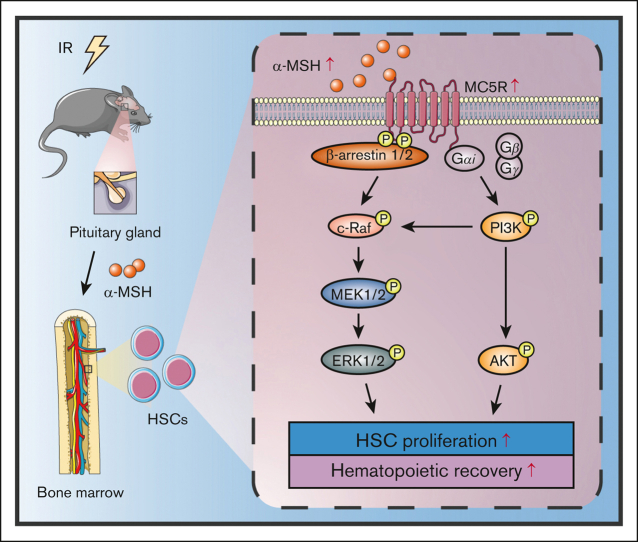
Key Points
-
•
MC5R deficiency impairs HSC proliferation after stress.
-
•
Melanocortin/MC5R axis promotes hematopoietic recovery in irradiated mice.
Visual Abstract
Abstract
Hematopoietic stem cells (HSCs) possess great self-renewal and multidirectional differentiation abilities, which contribute to the continuous generation of various blood cells. Although many intrinsic and extrinsic factors have been found to maintain HSC homeostasis, the precise regulation of hematopoiesis under stress conditions is poorly understood. In this study, we show that melanocortin receptor 5 (MC5R) is abundantly expressed in hematopoietic stem progenitor cells (HSPCs). Using an MC5R knockout mouse model, we observed that it is not essential for steady-state hematopoiesis. Interestingly, the levels of α-melanocyte stimulating hormone (α-MSH), an important subtype of melanocortin, were elevated in the serum and bone marrow, and the expression of MC5R was upregulated in HSPCs from mice after irradiation. MC5R deficiency aggravates irradiation-induced myelosuppression because of impaired proliferation and reconstitution of HSCs. Further investigation revealed that the melanocortin/MC5R axis regulates the proliferation of HSCs by activating the PI3K/AKT and MAPK pathways. More importantly, α-MSH treatment can significantly accelerate hematopoietic recovery in irradiated mice. In conclusion, our data demonstrate that the melanocortin/MC5R axis plays a crucial role in regulating HSC proliferation under stress, thus providing a promising strategy to promote hematopoietic regeneration when suffering from injury.
Introduction
Hematopoietic stem cells (HSCs) are located at the top of the hematopoietic hierarchy, exhibiting the great potential of self-renewal and differentiation into all blood cells.1 Under normal circumstances, adult HSCs primarily settle in the bone marrow (BM) niche and remain in a quiescent state.2,3 After injury induced by various stresses, such as ionizing radiation and chemotherapy drugs, BM residual HSCs undergo rapid proliferation and differentiation to replenish the damaged hematopoietic system.4,5 Studies have identified many intrinsic and extrinsic factors that corporately regulate HSC maintenance under steady-state conditions.6 However, the relevant pathways and mechanisms that regulate HSC behavior and function during stress have not been fully revealed.
It is known that many hormones play a key role in the specific stages of hematopoiesis.7,8 Melanocortins are a class of peptide hormones produced by proopiomelanocortin, including adrenocorticotropic hormone and melanocortin stimulating hormones (α-, β-, and γ-MSH).9 In addition to promoting melanogenesis, melanocortins are involved in the regulation of various physiological processes, such as immunomodulation, inflammatory response, fat metabolism, and exocrine gland secretion.10,11 Achievement of those regulatory functions relies on adrenocorticotropic hormone and melanocortin stimulating hormones to bind and activate melanocortin receptors. To date, 5 subtypes of melanocortin receptors (MC1R to MC5R) have been identified, with distinct physiological functions depending on the differences in tissue distribution and affinity for hormones.12 However, the exact role of melanocortin signaling in regulating hematopoiesis is still obscure.
MC5R, the last discovered type of MCRs, belongs to the family of guanine nucleotide-protein coupled receptors, similar to the other MCRs.13 MC5R is most effectively activated by α-MSH and then causes the activation of downstream signaling pathways, such as cyclic adenosine monophosphate-protein kinase A (cAMP-PKA), phosphoinositide-3-kinase/protein kinase B (PI3K/AKT), β-arrestin1/2/c-Raf, and JAK2/STAT1, in the skin, adrenal glands, or skeletal muscle.14 Notably, recent studies have revealed that MC5R is also expressed in hematopoietic and immune systems, including lymph nodes, the spleen (SP), and BM.15,16 As reported, MC5R inhibits proinflammatory signaling in macrophages and dendritic cells.17 In addition, MC5R participates in the regulation of T-cell immune response during experimental autoimmune uveitis.15
In this study, we showed that MC5R is highly expressed in hematopoietic stem progenitor cells (HSPCs) but is dispensable for normal hematopoiesis. Notably, α-MSH level was increased in the serum and BM of mice after irradiation, accompanied by the upregulation of MC5R expression in HSPCs. Mice with MC5R deficiency displayed more severe myelosuppression after irradiation because of impaired HSC proliferation and reconstitution. Mechanistically, the melanocortin/MC5R axis regulates the activation of AKT and ERK1/2 signaling pathways, which are required to promote the proliferation of HSCs after exposure to irradiation. Collectively, our findings present a new perspective on the modulation of HSC proliferation under stress conditions.
Materials and methods
Mice
Healthy C57BL/6J mice were purchased from Laipite Biotechnology Company (Chongqing, China). MC5R–/– mice were purchased from Cyagen Biosciences Inc (Suzhou, China) and wild-type (WT) littermate mice were served as the control. B6.SJL mice (CD45.1) were kindly gifted by Jinyong Wang (Institutes of Biomedicine and Health, Chinese Academy of Science, Guangzhou, China). All the mice were 8 or 10 weeks old. Animal experiments were performed in accordance with experimental procedures approved by the animal care aommittee of the Third Military Medical University (Chongqing, China).
Irradiation
Mice were subjected to total body irradiation in a 60Co γ-irradiator (Third Military Medical University Irradiation Center), as we previously described.18
Blood routine test
Peripheral blood (PB) was obtained from the tail vein of mice and diluted in a 1% EDTA solution. The counts of white blood cell, red blood cell, and platelet were analyzed automatically on the XT-1800i/2000IV hematology analyzer (Sysmex, Kobe, Japan), as we previously described.19
Flow cytometry
Single-cell suspensions of mouse BM, SP, and PB samples were prepared as we previously described.20 For hematopoietic cell phenotype analysis or sorting, the following antibodies obtained from eBioscience (San Diego, CA) or BioLegend (San Diego, CA) were applied to identify the surface markers: anti–c-Kit (2B8), anti–Sca-1 (D7), anti-Flk2 (A2F10), anti-CD34 (RAM34), anti-CD48 (HM48-1), anti-CD150 (mShad150), anti-CD16/32 (FcγRII/III), anti-CD127 (A7R34), anti-CD3e (145-2C11), anti-B220 (RA3-6B2), anti–Mac-1 (M1/70), anti–Gr-1 (RB6-8C5), anti-CD45.1 (A20), anti-CD45.2 (104), and the anti-lineage cocktail (CD3e, Mac-1, Gr-1, B220, and Ter-119).
After staining with HSPC markers, cells were used for further analysis of the cell cycle, in vivo bromodeoxyuridine (BrdU) incorporation, apoptosis, intracellular protein expression, and mitochondrial properties, as previously reported.19,20 The following antibodies were used in this study: anti-AnnexinV (BioLegend), anti-BrdU (BD Biosciences, San Jose, CA), anti-Ki67 (eBioscience), anti–p-STAT1 (eBioscience), anti–p-STAT3 (eBioscience), anti–p-STAT5 (eBioscience), anti–p-AKT (Cell Signaling Technology, Danvers, MA), anti–p-ERK1/2 (Cell Signaling Technology), anti–p-p65 (Cell Signaling Technology), and anti–p-Smad2/3 (BD Phosflow, San Diego, CA). Flow cytometric data were acquired using a FACSVerse flow cytometer (BD Biosciences) and analyzed using the FlowJo version 10.0 software (BD Biosciences). Cell sorting was performed using the FacsAria III sorter (BD Biosciences).
Protein synthesis assay
The in vivo protein synthesis rate of HSPCs was analyzed using the Click-iT Plus OPP Alexa Fluor 488 Protein Synthesis Assay Kit (Molecular Probes, Carlsbad, CA), as we previously reported.21
HSPC culture
A total of 1 × 103 Lineage– Sca1+ c-Kit+ cells (LSKs) sorted from the BM of WT and MC5R–/– mice were cultured in StemSpan SFEM medium (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with different concentrations of α-MSH (0, 5, 50, or 500 nM; MedChemExpress, Princeton, NJ) for 7 days. The total number of cells in the medium was then counted.
Transplantation assays
For competitive BM transplantation (BMT), 5 × 103 LSKs freshly sorted from the BM of WT and MC5R–/– mice (CD45.2) along with 5 × 105 CD45.1 BM cells were transplanted into lethally irradiated (9.5 Gy) CD45.1 recipient mice. Donor chimerism levels were analyzed via flow cytometry 16 weeks after transplantation. For reciprocal BMT, 1 × 106 CD45.1 BM cells were transplanted into lethally irradiated (9.5 Gy) WT or MC5R–/– recipient mice (CD45.2). Similarly, donor chimerism levels were analyzed using flow cytometry 16 weeks after transplantation. For the homing assay, 2 × 105 LSKs freshly sorted from the BM of WT and MC5R–/– mice were labeled with 5(6)-carboxyfluorescein diacetate succinimidyl ester (Thermo Fisher Scientific) and transplanted into lethally irradiated (9.5 Gy) WT recipient mice. The homing efficiency was analyzed via flow cytometry 16 hours after transplantation, as we previously described.20
Enzyme-linked immunosorbent assay
The serum and BM supernatants of mice were collected at the indicated times after irradiation. Then, α-MSH level was detected using the Alpha-Melanocetin Stimulating Hormone ELISA Kit (Yaji Biological Technology Co, Ltd, Shanghai, China), per the manufacturer's instructions.
In vivo α-MSH, AKT, or ERK1/2 inhibitor treatment
Mice were intraperitoneally injected with saline or α-MSH (1 mg/kg), along with vehicle, AKT inhibitor MK-2206 (30 mg/kg; MedChemExpress, Princeton, NJ), or ERK1/2 inhibitor PD325901 (5 mg/kg; MedChemExpress, Princeton, NJ), once a day for a total of 7 days. For radiation protection experiments, mice were exposed to 5.0 or 7.5 Gy total body irradiation. Three or 9 days after irradiation, mice were administered saline or α-MSH (1 mg/kg) once a day for a total of 7 days and then used for further analysis.
qRT-PCR
Total RNA was extracted from freshly sorted cells using the RNAqueous-Micro Kit (Thermo Fisher Scientific, Waltham, MA). After conversion to complementary DNA using the PrimeScript RT Reagent Kit (Takara, Kyoto, Japan), gene expression level was measured using SYBR Premix Ex Taq II (Takara), following the manufacturer's instructions. All the primers used are provided in supplemental Table 1.
RNA-sequencing (RNA-seq)
Total RNA was isolated from the LSKs of irradiated WT and MC5R–/– mice as described earlier. After quality testing, the RNA samples were used to create a complementary DNA library and then sequenced on an Illumina NovaSeq 6000. The library construction and sequencing were performed at Sinotech Genomics Co, Ltd (Shanghai, China). Gene abundance was expressed as fragments per kilobase of exon per million reads mapped. Stringtie software was used to count the fragments within each gene, and the TMM algorithm was used for normalization. Differential expression analysis of mRNA was performed using the R package edgeR. Genes with fold change >1 and P-value < .05 were considered as significantly changed. Gene set enrichment analysis (GSEA) was performed using GSEA_4.2.1 software (http://www.gsea-msigdb.org/gsea), and gene sets were obtained from the Molecular Signatures Database (MSigDB) database. All raw data were deposited in the NCBI Gene Expression Omnibus (GEO) database.
Immunofluorescence microscopy
Freshly sorted cells were placed on poly-L-lysine–coated slides. After fixation, permeabilization, and blocking, cells were stained with anti-MC5R (Thermo Fisher Scientific), anti–p-AKT (Cell Signaling Technology), or anti–p-ERK1/2 (Cell Signaling Technology) antibodies. The samples were then incubated with FITC-conjugated secondary antibody (Thermo Fisher Scientific) and 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; Sigma, St Louis, MO), followed by imaging using a Zeiss LSM800 confocal microscope (Carl Zeiss, Jena, Germany).
Statistical analysis
Data analysis was performed using the GraphPad Prism 8.0 software (La Jolla, CA). Each experiment was independently performed at least 3 times. Unpaired t test (two-tailed) was used for 2 groups comparisons, and one-way analysis of variance, followed by Tukey test, was used for multiple group comparisons. The survival rates of the mice were analyzed using the log-rank test. All data are presented as the mean ± standard deviation. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 are depicted as statistically significant differences.
Results
MC5R is abundantly enriched in HSPCs but is dispensable for steady-state hematopoiesis
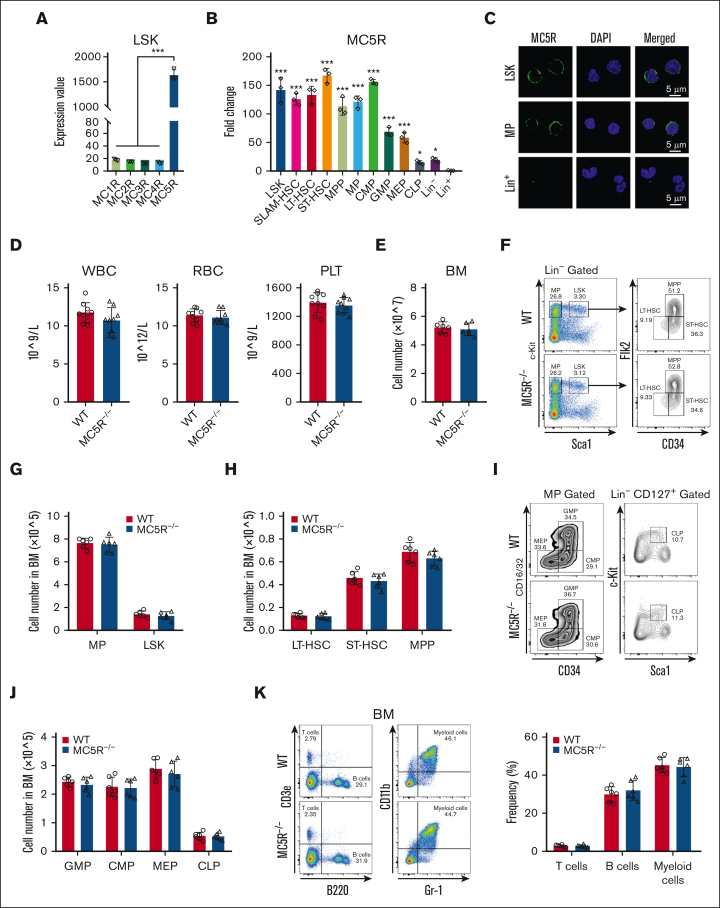
To explore the role of melanocortin signaling in hematopoiesis, we first analyzed the expression of melanocortin receptors, including from MC1R to MC5R, in HSPCs. Interestingly, the microarray data from our previous study showed that MC5R, rather than MC1R to MC4R, was abundantly expressed in LSKs (HSCs enriched compartment) from the BM of healthy mice (Figure 1A). We then searched the Gene Expression Commons, a public database, and found that MC5R was enriched in HSPCs, whereas MC1R to MC4R did not display relative high expression level (supplemental Figure 1A). Consistent with these findings, quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis of purified BM subpopulations revealed that MC5R expression was higher in HSPCs, including HSCs, pluripotent progenitors, and lineage-committed progenitors than in mature hematopoietic cells, which was confirmed at the protein level via immunofluorescence (Figure 1B,C; supplemental Figure 1B). These results suggest that MC5R may be involved in the regulation of HSPC biology.
Figure 1.
MC5R is abundantly enriched in HSPCs but dispensable for steady-state hematopoiesis. (A) Expression value of MC1R, MC2R, MC3R, MC4R, and MC5R in LSKs sorted from healthy WT mice (n = 3). The data were obtained from our previous study (GSE173291). (B) qRT-PCR analysis of MC5R expression in LSKs, SLAM-HSCs, long-term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), multipotent progenitors (MPPs), myeloid progenitors (MPs), common myeloid progenitors (CMPs), granulocyte monocyte progenitors (GMPs), megakaryocyte erythroid progenitors (MEPs), common lymphoid progenitors (CLPs), Lineage– (Lin–) cells, and Lin+ cells sorted from the BM of healthy WT mice (n = 3). The relative expression of MC5R was compared with that in Lin+ cells. (C) Representative immunofluorescence plots showing MC5R expression in LSKs, MPs, and Lin+ cells sorted from the BM of helathy WT mice. (D) WBC, RBC, and PLT counts in the PB of WT and MC5R–/– mice (n = 9). (E) The total number of BM cells in WT and MC5R–/– mice (n = 6). (F) Representative flow cytometric plots showing the percentages of MPs, LSKs, LT-HSCs, ST-HSCs, and MPPs in the BM of WT and MC5R–/– mice. (G) The number of MPs and LSKs in the BM of WT and MC5R–/– mice (n = 6). (H) The number of LT-HSCs, ST-HSCs, and MPPs in the BM of WT and MC5R–/– mice (n = 6). (I) Representative flow cytometric plots showing the percentage of MEPs, CMPs, GMPs, and CLPs in the BM of WT and MC5R–/– mice. (J) The number of GMPs, CMPs, MEPs, and CLPs in the BM of WT and MC5R–/– mice (n = 6). (K) Flow cytometric analysis of the percentage of T, B, and myeloid cells in the BM of WT and MC5R–/–v mice (n = 6). Representative flow cytometric plots (left). (A,B) One-way ANOVA with Tukey multiple comparisons test; (D,E,G,H,J,K) unpaired t test (two-tailed). ∗P < .05; ∗∗∗P < .001. ANOVA, analysis of variance; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
Next, we generated an MC5R–/– mouse model, and the knockout efficiency in LSKs was confirmed via qRT-PCR (supplemental Figure 1C,D). It was observed that PB counts and the total cell numbers of BM and SP were comparable between WT and MC5R–/– mice (Figure 1D,E; supplemental Figure 1E). Flow cytometry analysis showed that MC5R deficiency did not alter the percentage and number of various HSPC populations in the BM (Figure 1F-J, supplemental Figure 1F-K). In addition, there were no significant differences in the percentages of T, B, and myeloid cells in the BM after MC5R knockout (Figure 1K). These results indicate that MC5R is not essential for maintaining normal hematopoiesis.
MC5R deficiency aggravates myelosuppression after radiation injury
Given the fact that the levels of many hormones were markedly changed in response to various stresses,22 we assessed melanocortin levels in mice after irradiation. Notably, α-MSH, a subtype of melanocortin with the highest affinity for MC5R, was significantly elevated in the serum and BM of mice after irradiation, along with the evident upregulation of MC5R in LSKs (Figure 2A,B; supplemental Figure 2A). To investigate whether MC5R regulates hematopoiesis under stress, WT and MC5R–/– mice were subjected to the same dose of ionizing radiation. Compared with WT mice, lower blood counts were observed in MC5R–/– mice from days 12 to 21 after irradiation (Figure 2C-E). Meanwhile, the total number of BM cells in mice decreased after irradiation, when MC5R was knocked out (Figure 2F). In view of these findings that the levels of melanocortin and MC5R as well as conventional hematopoietic parameters were more significantly changed at 15 days than at earlier time points following irradiation exposure, we analyzed HSPC phenotypes at this time point using flow cytometry. It was found that the percentage and number of HSPCs, including MPs, LSKs, long-term HSCs, short-term HSCs, multipotent progenitors, SLAM-HSCs, granulocyte monocyte progenitorss, common myeloid progenitorss, megakaryocyte erythroid progenitorss, and common lymphoid progenitors, were significantly reduced in the BM of MC5R–/– mice experiencing irradiation exposure (Figure 2G-J; supplemental Figure 2B-G). Specifically, these reductions were not because of the increased HSPC apoptosis induced by radiation injury in MC5R–/– mice (supplemental Figure 2H). In contrast, irradiated MC5R–/– mice displayed a reduced percentage of myeloid cells and increased percentages of B and T cells in the BM, suggesting that MC5R may regulate HSPC differentiation (Figure 2K). Taken together, MC5R plays a critical role in facilitating the recovery of HSPC pools in mice with radiation injury.
Figure 2.
MC5R deficiency aggravates myelosuppression after radiation injury. (A) Enzyme-linked immunosorbent assay of α-MSH level in the BM supernatant of WT mice at the indicated time after 5.0 Gy total body irradiation (TBI) (n = 5). Unirradiated WT mice were used as controls (n = 5). (B) qRT-PCR analysis of MC5R expression in LSKs sorted from the BM of WT mice at the indicated time after 5.0 Gy TBI (n = 3). The relative expression of MC5R was compared with that at 0 hour. (C-E) The counts of (C) WBC, (D) RBC, and (E) PLT in the PB of WT and MC5R–/– mice at the indicated time after 5.0 Gy TBI (n = 9). (F) Total number of BM cells in WT and MC5R–/– mice at the indicated time after 5.0 Gy TBI (n = 6). (G) Representative flow cytometric plots showing the percentages of MPs, LSKs, LT-HSCs, ST-HSCs, and MPPs in the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI. (H) The numbers of MPs and LSKs in the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI (n = 6). (I) The number of LT-HSCs, ST-HSCs, and MPPs in the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI (n = 6). (J) The number of MEPs, CMPs, GMPs, and CLPs in the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI (n = 6). (K) Flow cytometric analysis of the percentages of T cells, B cells, and myeloid cells in the BM of WT and MC5R-/- mice 15 days after 5.0 Gy TBI (n = 6). (A,B) one-way ANOVA with Tukey multiple comparisons test; (C-F,H-K) unpaired t test (two-tailed). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Con, control; IR, irradiation.
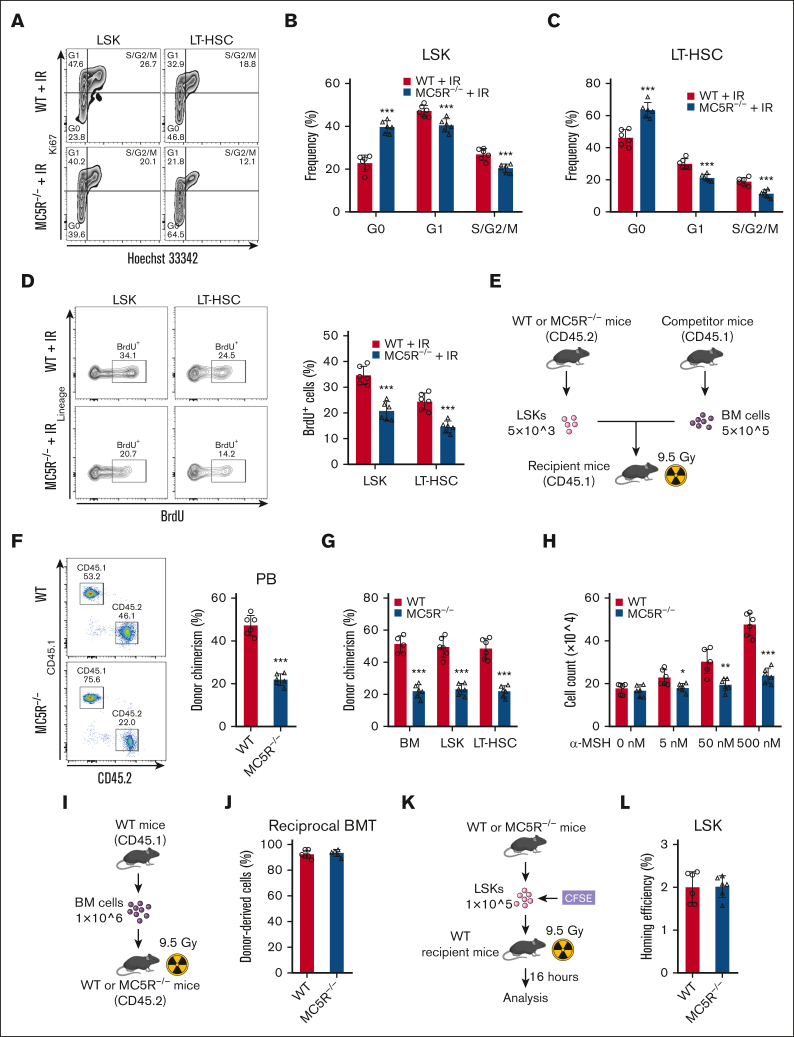
Loss of MC5R compromises the proliferation and reconstitution ability of HSCs
These observations prompted us to investigate whether MC5R deficiency affects the proliferation ability of HSCs. As anticipated, cell cycle analysis of HSCs via Ki67 and Hoechst 33342 staining showed that MC5R deletion led to an increase in the proportion of cells in the G0 phase and a decrease in the proportion of cells in the G1 and S/G2/M phases (Figure 3A-C; supplemental Figure 3A) after irradiation, which was further verified by an in vivo BrdU incorporation assay (Figure 3D). However, these findings were not observed under steady-state conditions, which is consistent with the corresponding phenotypic data (supplemental Figure 3B-E).
Figure 3.
Loss of MC5R compromises proliferation and reconstitution ability of HSCs. (A-C) Flow cytometric analysis of the cell cycle distribution of (B) LSKs and (C) LT-HSCs in the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI (n = 6). Representative flow cytometric plots are shown (A). (D) Flow cytometric analysis of the percentage of BrdU+ cells in LSKs and LT-HSCs from the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI (n = 6). Representative flow cytometric plots (left). (E-G) A total of 5 × 103 LSKs obtained from the BM of WT and MC5R–/– mice (CD45.2) were mixed with 5 × 105 CD45.1 BM cells and transplanted into lethally irradiated CD45.1 recipient mice. (E) The scheme of competitive BMT. (F) Flow cytometric analysis of the percentage of donor-derived cells in the PB of recipient mice 16 weeks after transplantation (n = 6). Representative flow cytometric plots (left). (G) Chimerism levels of BM, LSKs, and LT-HSCs in recipient mice at 16 weeks after transplantation (n = 6). (H) LSKs sorted from the BM of WT and MC5R–/– mice were cultured in the presence of different concentrations of α-MSH (0, 5, 50, or 500 nM). The total cell counts in the medium were counted 7 days after culture (n = 6). (I,J) A total of 1 × 106 CD45.1 BM cells were transplanted into lethally irradiated WT or MC5R–/– recipient mice (CD45.2). (I) The scheme of reciprocal BMT. (J) Flow cytometric analysis of the percentage of donor-derived cells in the PB of recipient mice at 16 weeks after transplantation (n = 6). (K,L) A total of 2 × 105 LSKs obtained from the BM of WT and MC5R–/– mice were labeled with CFSE and transplanted into lethally irradiated WT recipient mice. The percentage of CFSE+ cells in the BM of recipient mice 16 hours after transplantation was detected using flow cytometry. (K) The scheme of homing assay. (L) The homing efficiency of WT and MC5R–/– LSKs (n = 6). (B-D,F-H, J,L) unpaired t test (two-tailed). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. CFSE, 5(6)-carboxyfluorescein diacetate succinimidyl ester.
To assess whether the impairment in HSC proliferation ability induced by MC5R deficiency affected their function in hematopoietic reconstitution, we performed a competitive BMT assay (Figure 3E). It was noticed that the total number of donor-derived cells, especially in the myeloid compartment, was decreased in the PB of recipient mice transplanted with MC5R–/– HSPCs (Figure 3F; supplemental Figure 3F). Similarly, BM chimerism levels were significantly reduced when MC5R was deleted (Figure 3G). In addition, considering that MC5R was not conditionally knocked out in the hematopoietic system, we isolated HSPCs from WT and MC5R–/– mice and cultured them in vitro. Actually, treatment with α-MSH stimulated the proliferation of HSPCs from WT but not MC5R–/– mice in culture in a dose-dependent manner (Figure 3H). In accordance with these data, the reciprocal transplantation assay showed that healthy CD45.1 BM cells comparably reconstructed the BM of WT or MC5R–/– hosts, further validating that MC5R regulates HSPC function in a cell-intrinsic manner (Figure 3I,J). However, the attenuation of hematopoietic reconstitution capacity was not because of defective HSC homing after MC5R knockout (Figure 3K,L). Overall, MC5R intrinsically maintained the proliferation and reconstitution capacity of HSCs.
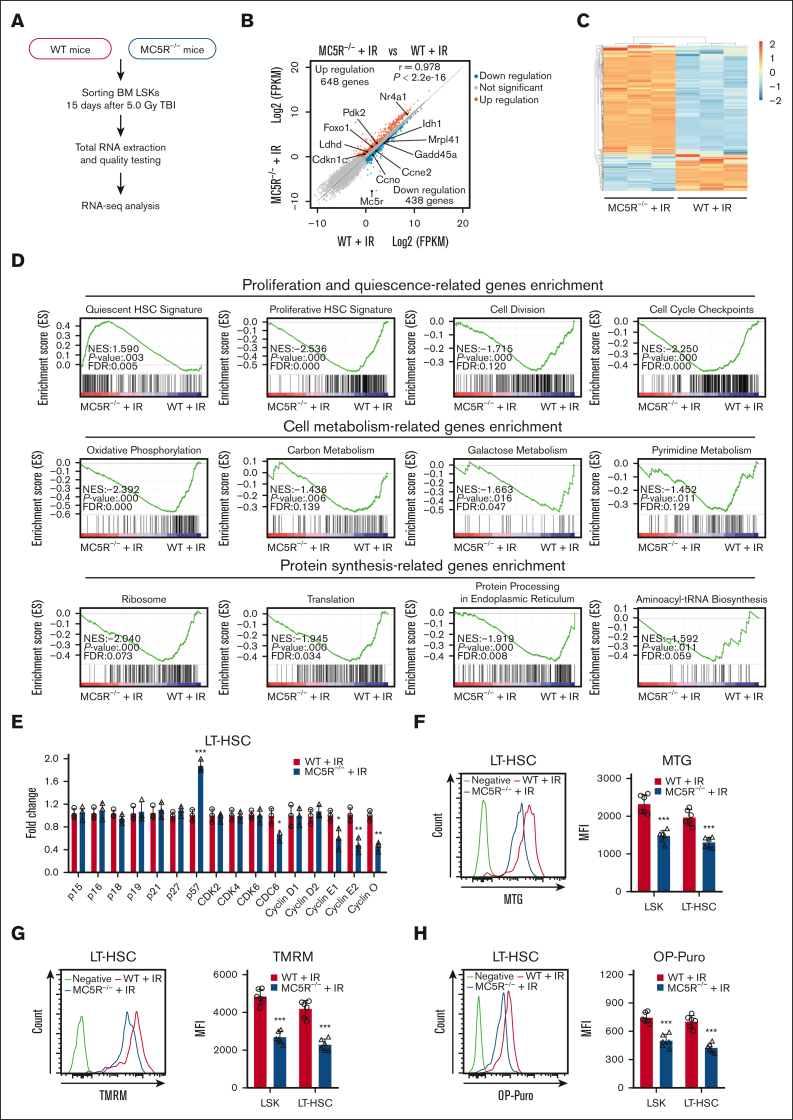
MC5R deletion significantly alters the cell cycle-associated transcriptional profile of HSPCs after irradiation exposure
To explore how MC5R regulates HSPC function, we performed RNA-seq of LSKs from irradiated WT and MC5R–/– mice (Figure 4A). The results showed that the gene expression profile of HSPCs was significantly changed after MC5R deletion, among which 648 genes were upregulated, and 438 genes were downregulated based on the criteria of fold change >1.5 and P < .05 (Figure 4B,C). Then, we conducted a GSEA and found that the gene sets associated with HSC quiescence were enriched in MC5R–/– LSKs, whereas the gene sets associated with HSC proliferation, cell division, and cell cycle checkpoint were enriched in WT LSKs (Figure 4D). In line with these data, qRT-PCR analysis confirmed that the expression of the cell cycle inhibitor p57 had increased, whereas the expression of cyclins (including cyclin E1, cyclin E2, and cyclin O) and CDC6 had decreased in MC5R-deficient HSCs under stress (Figure 4E; supplemental Figure 4A). It is well established that quiescent HSCs usually exhibit a lower metabolic status.21,23 As expected, a significant downregulation of cell metabolic signatures was observed in MC5R–/– LSKs, including oxidative phosphorylation, carbon metabolism, galactose metabolism, and pyrimidine metabolism, along with decreased protein synthesis (Figure 4D). These findings were further confirmed via flow cytometric analysis of mitochondrial activity and protein synthesis rate (Figure 4F-H). However, no significant differences in the transcription profile of cell cycle-associated genes and the levels of metabolism were observed in WT and MC5R–/– HSPCs at steady-state (supplemental Figure 4B-E). These data illustrate that MC5R deficiency inhibits the cell cycle progression and metabolic activation of HSPCs after irradiation.
Figure 4.
MC5R deletion significantly alters cell cycle-associated transcriptional profile in HSPCs following irradiation exposure. (A-C) LSKs sorted from the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI were subjected to RNA-seq analysis (n = 3). Those with a fold change >1.5 and P-value < .05 were defined as differentially expressed genes (DEGs). (A) Experimental scheme. (B) Scatter plot showing DEGs in the LSKs from the BM of irradiated WT and MC5R–/– mice. Representative DEGs are indicated. (C) Heatmap of significantly changed genes in LSKs from the BM of irradiated WT and MC5R–/– mice. (D) GSEA of the RNA-seq data showing proliferation- and quiescence-associated enrichment, cell metabolism-associated enrichment, and protein synthesis-associated enrichment in LSKs from irradiated WT and MC5R–/– mice. (E) qRT-PCR analysis of the relative expression of cell cycle-associated genes in LT-HSCs sorted from the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI (n = 3). (F) Flow cytometric analysis of mitochondrial mass in LSKs and LT-HSCs from the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI using MitoTracker Green staining (n = 6). Representative flow cytometric plots (left). (G) Flow cytometric analysis of mitochondrial membrane potential in LSKs and LT-HSCs from the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI by tetramethylrhodamine methyl ester (TMRM) staining (n = 6). (H) Flow cytometric analysis of the protein synthesis rate in LSKs and LT-HSCs from the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI by O-propargyl-puromycin (OP-Puro) incorporation analysis (n = 6). Representative flow cytometric plots are shown in the left. (E-H) Unpaired t test (two-tailed). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, mean fluorescence intensity.
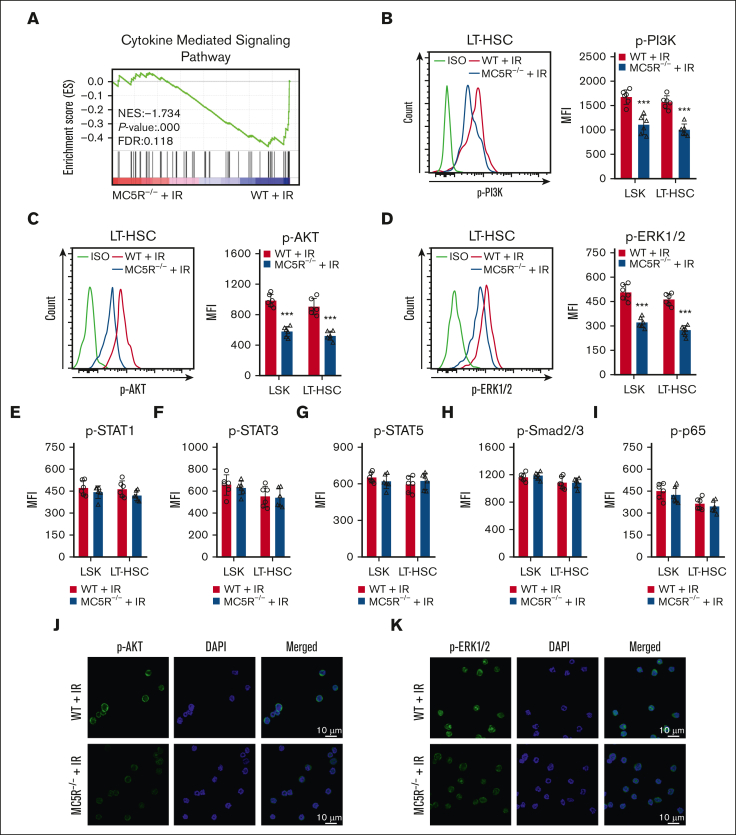
MC5R regulates HSPC proliferation via the PI3K/AKT and MAPK pathways
To further understand the exact molecular mechanism, we analyzed proliferation-associated pathways in HSPCs. Notably, the GSEA results showed that the cytokine-mediated signaling pathway was significantly suppressed in irradiated LSKs lacking MC5R (Figure 5A). Studies have shown that PI3K/AKT, JAK/STAT, MAPK, TGF-β/Smad, and nuclear factor-κB are key pathways that mediate the role of many cytokines in regulating the proliferation of HSPCs.6,24,25 Importantly, MC5R-null HSPCs showed significant decreases in the phosphorylation levels of PI3K, AKT, ERK1/2, but not STAT1, STST3, STAT5, Smad2/3, and p65, especially on day 15 after irradiation (Figure 5B-I; supplemental Figure 5A,B). The attenuated phosphorylation of AKT and ERK1/2 was further confirmed by immunofluorescence (Figure 5J,K). Therefore, these results suggest that MC5R regulates HSPC proliferation probably through the PI3K/AKT and MAPK pathways.
Figure 5.
MC5R regulates HSPC proliferation via the PI3K/AKT and MAPK pathways. (A) GSEA of RNA-seq data showing cytokine signaling-associated enrichment in LSKs from irradiated WT and MC5R–/– mice. (B-D) Flow cytometric analysis of the expression of (B) p-PI3K, (C) p-AKT, and (D) p-ERK1/2 in LSKs and LT-HSCs from the BM of WT and MC5R-/- mice 15 days after 5.0 Gy TBI (n = 6). Representative flow cytometric plots (left). (E-I) Flow cytometric analysis of the expression of (E) p-STAT1, (F) p-STAT3, (G) p-STAT5, (H) p-Smad2/3, and (I) p-p65 in LSKs and LT-HSCs in the BM of WT and MC5R–/– mice 15 days after 5.0 Gy TBI (n = 6). (J,K) Representative immunofluorescence plots showing (J) p-AKT and (K) p-ERK1/2 expression in LSKs sorted from the BM of WT and MC5R–/– mice at 15 days after 5.0 Gy TBI. (B-I) unpaired t test (two-tailed). ∗∗∗P < .001.
Melanocortin/MC5R axis promotes hematopoietic regeneration in mice after irradiation
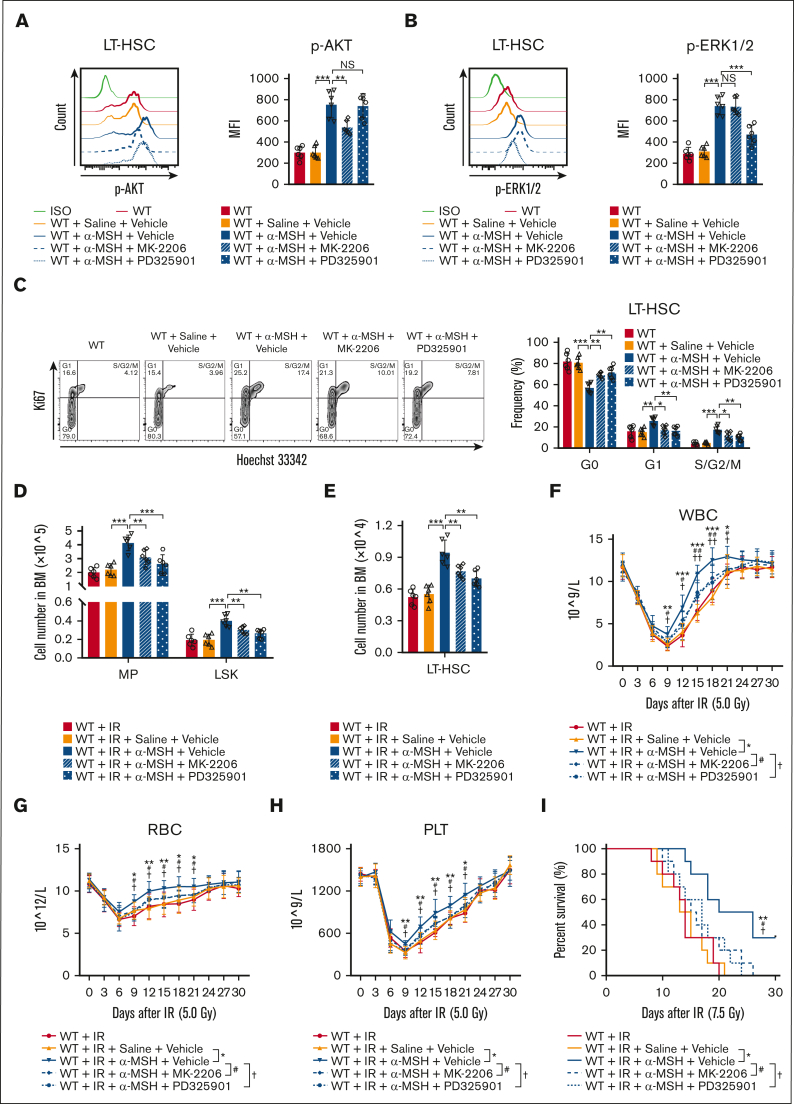
Finally, to determine whether the PI3K/AKT and MAPK pathways mediate the effects of the melanocortin/MC5R axis on HSC proliferation, mice were intraperitoneally injected with α-MSH. Indeed, the phosphorylation levels of AKT and ERK1/2, as well as their proliferation, were remarkably increased in HSCs after α-MSH treatment, which could be largely abrogated by AKT inhibitor (MK-2206) or ERK1/2 inhibitor (PD325901), respectively (Figure 6A-C). More importantly, α-MSH treatment increased the number of HSPCs in the BM, eventually accelerating the recovery of PB in mice after radiation injury (Figure 6D-H). Moreover, α-MSH treatment significantly increased the survival rate of the mice subjected to lethal radiation (Figure 6I). These effects were also significantly eliminated after AKT or ERK1/2 inhibitor treatment (Figure 6D-I). However, these effects of α-MSH were not observed in MC5R–/– mice (supplemental Figure 6A-H). Collectively, our data demonstrated that the melanocortin/MC5R axis is required to facilitate hematopoietic regeneration in mice after irradiation.
Figure 6.
Melanocortin/MC5R axis promotes hematopoietic regeneration in irradiated mice. (A,B) Flow cytometric analysis of the expression of (A) p-AKT and (B) p-ERK1/2 in LT-HSCs from the BM of WT mice treated with saline or α-MSH, along with vehicle, AKT inhibitor MK-2206, or ERK1/2 inhibitor PD325901 (n = 6). (C) Cell cycle analysis of LT-HSCs from the BM of WT mice treated with saline or α-MSH along with vehicle, MK-2206, or PD325901 (n = 6). (D,E) The number of (D) MPs, LSKs, and (E) LT-HSCs from the BM of WT mice treated with saline or α-MSH, along with vehicle, MK-2206, or PD325901, 15 days after 5.0 Gy TBI (n = 6). (F-H) The counts of (F) WBC, (G) RBC, and (H) PLT in the PB of WT mice treated with saline or α-MSH, along with vehicle, MK-2206, or PD325901, at the indicated time after 5.0 Gy TBI (n = 10). (I) The survival rates of WT mice treated with saline or α-MSH, along with vehicle, MK-2206, or PD325901, after 7.5 Gy TBI (n = 10). (A-H) One-way ANOVA with Tukey multiple comparisons test; (I) Log-rank test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; #P < .05; ##P < .01; †P < .05; ††P < .01. NS, not significant.
Discussion
HSCs are extremely rare in adult BM, but they are responsible for maintaining hematopoietic homeostasis throughout life.26,27 After intensive research for decades, the normal hematopoietic process has been well understood. It is known that the hematopoietic system is vulnerable to various damage factors, but the promotion of the rapid proliferation of HSPCs following stress-induced myelosuppression is not fully understood. In recent years, studies have shown that several hormones, such as estrogen, luteinizing, glucocorticoid, and melatonin, play a crucial role in the regulation of HSC behavior.28, 29, 30, 31 Here, we report that melanocortin acts on HSCs via MC5R, upregulating the phosphorylation levels of AKT and ERK1/2, which stimulate hematopoiesis after radiation injury.
Melanocortins, a class of peptide hormones produced in the pituitary gland, can be cleaved into different subtypes to match the melanocortin receptors with tissue specificity and participate in the regulation of relevant physiological and pathological processes.32 The early discovered MCRs subtypes, MC1R to MC4R, have been well known in detail for their major functions. For example, MC1R is mainly distributed in the epidermis and melanocytes and is involved in pigment formation33; MC2R is located in the adrenal cortex and mediates adrenocorticotropic hormone to stimulate glucocorticoid production34; Both MC3R and MC4R are present in the central nervous system and principally act in metabolic regulation–related functions.35,36 However, the physiological function of the last discovered melanocortin receptor, MC5R, has not been well investigated. At present, the public database shows that MC5R is enriched in murine HSPCs from BM, which was confirmed via qRT-PCR and immunofluorescence in our study. In particular, a recent study reported that MC5R was also highly expressed in human HSCs (Lin– CD34+ CD38– CD90+ CD45RA–) purified from PB mononuclear cells.37 These findings suggest that MC5R may have an important regulatory role in HSPCs. Unfortunately, subsequent experiments using MC5R–/– mice proved that the absence of MC5R does not cause significant changes in homeostatic hematopoiesis, which is consistent with the results of a recent study.37
As we know, hormone levels in the body are regulated by several factors. In fact, it has been reported that the levels of melanocortins are significantly elevated after various stresses, such as UV radiation, tumorigenesis, and inflammatory stimuli, etc.33,38,39 However, the involvement of melanocortins in stress hematopoiesis has not been explored. In this study, we observed that α-MSH level was substantially upregulated in the serum and BM of mice after irradiation, together with an increased expression of MC5R in HSPCs. As a result, MC5R deficiency causes more severe myelosuppression in mice after radiation injury. Further studies demonstrated that this delay may be mainly due to the arrest of cell cycle progression rather than increased apoptosis in irradiated HSPCs with MC5R deficiency, which was further confirmed via RNA-seq analysis. It has been well established that most HSCs, particularly LT-HSCs, are retained in a quiescent state under healthy physiological conditions.6,40 Based on this property, primitive HSCs exhibit relatively stronger resistance to ionizing radiation than various hematopoietic progenitor cells with actively cycling.41 When subjected to injuries, residual HSCs will experience rapid proliferation period in order to replenish the blood cells.42 Hence, excessive inhibition of the cell cycle of HSCs may impair their capacity to effectively produce offspring cells, ultimately leading to delayed recovery of the injured hematopoietic system.43 Similarly, repressing HSC proliferation ability will result in failed rebuilding of myeloablative hosts after transplantation, as reported by recent studies, including our own, using some gene-deficient mice, such as CDK19, Gabbr1, Ptbp1, Igf2bp2, Ptp4a, etc.44, 45, 46, 47, 48 Therefore, it can be observed that melanocortin/MC5R axis participates in the regulation of HSC proliferation in response to stress injury.
There is overwhelming evidence that cytokine-mediated pathways play a vital role in regulating various cell behaviors.49 Among these, the PI3K/AKT, JAK/STAT, and MAPK are well recognized pathways to promote the proliferation and differentiation of HSPCs.50, 51, 52 Indeed, many hematopoietic growth factors, including thrombopoietin, granulocyte-macrophage–colony-stimulating factor, and stem cell factor, function predominantly by activating the aforementioned signaling molecules.53, 54, 55 Besides, nuclear factor κB is downstream of Toll-like receptors and mediates the radiation protection effect of several cytokines or small molecule drugs.56,57 On the contrary, the TGF-β/Smad pathway has been shown to sustain the quiescence of HSCs.21,58 In our study, GSEA results revealed that the cytokine-mediated pathway was significantly compromised in HSPCs in the absence of MC5R after irradiation. Further experiments confirmed that the PI3K/AKT and MAPK pathways, but not the other mentioned pathways, were significantly impaired in irradiated HSPCs with MC5R deficiency. According to previous reports, MC5R, as a typical class A guanine nucleotide-protein coupled receptor, can receive upstream melanocortin signals, leading to the dissociation of Gβγ dimers from activated Gαi dimers, thereby causing PI3K phosphorylation as well as downstream AKT activation.59,60 Meanwhile, MC5R activates the ERK1/2 pathway, probably via the PI3K/c-Raf/MEK1/2 or β-arrestin1/2/c-Raf/MEK1/2 signaling cascades.59,60 Consistently, α-MSH administration activates the PI3K/AKT and MAPK pathways, eventually accelerating the expansion of HSPC numbers in the BM. Specifically, these effects could be largely abrogated by AKT or ERK1/2 inhibitor treatment, which means that the melanocortin/MC5R axis exerts regulatory functions, at least in part, through these 2 pathways. Nevertheless, we cannot completely rule out the possibility that other mechanisms are also involved in this process.
In conclusion, our study revealed that melanocortin promoted HSC proliferation, thereby accelerating hematopoietic recovery after irradiation. The generation of the aforementioned effects is closely related to the activation of PI3K/AKT and MAPK pathways via MC5R. These findings not only reveal a previously unrecognized role of melanocortin in HSC biology but also provide a new strategy for hematopoietic regeneration after injury.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors thank Jinyong Wang for gifting the CD45.1 mice, Yang Liu for technical support in flow cytometry, and Liting Wang for technical support in immunofluorescence microscopy. This work was supported by grants from the National Natural Science Foundation of China (81930090 [J.W.], 82203974 [M.H.], and 81725019 [J.W.]), Post-doctoral Innovative Talent Support Program of China (BX20220398 [M.H.]), Post-doctoral Innovative Talent Support Program of Chongqing (CQBX2021017 [M.H.]), and a project funded by the China Postdoctoral Science Foundation (2022M723867 [M.H.]).
Authorship
Contribution: N.C. and Y.Q. performed the experiments, analyzed the data, and wrote the manuscript; M.C., Y.L., L.Y., and S.W. contributed to the animal experiments; F.C. and Y.X. contributed to the in vitro experiments; M.S. and H.Z. contributed to the data analysis; S.C. and F.W. contributed to the initial experimental design and discussed the manuscript; J.W. and M.H. conceived and supervised the study, analyzed the data, and wrote and revised the manuscript; and all authors read and approved the final manuscript.
Footnotes
∗N.C. and Y.Q. contributed equally to this work.
Gene set enrichment analysis was performed using GSEA_4.2.1 software (http://www.gsea-msigdb.org/gsea).
Gene sets were obtained from the Molecular Signatures Database (MSigDB) database.
All raw data were deposited in NCBI Gene Expression Omnibus (GEO) database (accession number GSE210204).
Data are available on request from the corresponding authors, Mengjia Hu (humengjia3260@163.com) or Junping Wang (wangjunping@tmmu.edu.cn).
The full-text version of this article contains a data supplement.
Contributor Information
Junping Wang, Email: wangjunping@tmmu.edu.cn.
Mengjia Hu, Email: humengjia3260@163.com.
Supplementary Material
References
- 1.Cheng H, Zheng Z, Cheng T. New paradigms on hematopoietic stem cell differentiation. Protein Cell. 2020;11(1):34–44. doi: 10.1007/s13238-019-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125(17):2621–2629. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal. 2014;20(9):1447–1462. doi: 10.1089/ars.2013.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forristal CE, Winkler IG, Nowlan B, Barbier V, Walkinshaw G, Levesque JP. Pharmacologic stabilization of HIF-1alpha increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood. 2013;121(5):759–769. doi: 10.1182/blood-2012-02-408419. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Guo Q, Song G, Hou Y. Molecular regulation of hematopoietic stem cell quiescence. Cell Mol Life Sci. 2022;79(4):218. doi: 10.1007/s00018-022-04200-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Hu M, Shen M, et al. IGF-1 facilitates thrombopoiesis primarily through Akt activation. Blood. 2018;132(2):210–222. doi: 10.1182/blood-2018-01-825927. [DOI] [PubMed] [Google Scholar]
- 8.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieudonne M, Ramesh KV. Modeling the interactions between MC2R and ACTH models from human. J Biomol Struct Dyn. 2015;33(4):770–788. doi: 10.1080/07391102.2014.910475. [DOI] [PubMed] [Google Scholar]
- 10.Gong R. Leveraging melanocortin pathways to treat glomerular diseases. Adv Chronic Kidney Dis. 2014;21(2):134–151. doi: 10.1053/j.ackd.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copperi F, Kim JD, Diano S. Melanocortin signaling connecting systemic metabolism with mood disorders. Biol Psychiatry. 2022;91(10):879–887. doi: 10.1016/j.biopsych.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dores RM, Londraville RL, Prokop J, Davis P, Dewey N, Lesinski N. Molecular evolution of GPCRs: melanocortin/melanocortin receptors. J Mol Endocrinol. 2014;52(3):T29–T42. doi: 10.1530/JME-14-0050. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues AR, Sousa D, Almeida H, Gouveia AM. Cell surface targeting of the melanocortin 5 receptor (MC5R) requires serine-rich terminal motifs. Biochim Biophys Acta Mol Cell Res. 2017;1864(7):1217–1226. doi: 10.1016/j.bbamcr.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Guan X, Zhou R, Gong R. Melanocortin 5 receptor signaling pathway in health and disease. Cell Mol Life Sci. 2020;77(19):3831–3840. doi: 10.1007/s00018-020-03511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DJ, Preble J, Lee S, Foster CS, Taylor AW. MC5r and A2Ar deficiencies during experimental autoimmune uveitis identifies distinct T cell polarization programs and a biphasic regulatory response. Sci Rep. 2016;6:37790. doi: 10.1038/srep37790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DJ, Taylor AW. Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. J Immunol. 2013;191(8):4103–4111. doi: 10.4049/jimmunol.1300182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng TF, Manhapra A, Cluckey D, Choe Y, Vajram S, Taylor AW. Melanocortin 5 receptor expression and recovery of ocular immune privilege after uveitis. Ocul Immunol Inflamm. 2022;30(4):876–886. doi: 10.1080/09273948.2020.1849735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng H, Hu M, Lu Y, et al. MicroRNA 34a promotes ionizing radiation-induced DNA damage repair in murine hematopoietic stem cells. FASEB J. 2019;33(7):8138–8147. doi: 10.1096/fj.201802639R. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Zhang Z, Wang S, et al. Srebf1c preserves hematopoietic stem cell function and survival as a switch of mitochondrial metabolism. Stem Cell Rep. 2022;17(3):599–615. doi: 10.1016/j.stemcr.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu M, Zeng H, Chen S, et al. SRC-3 is involved in maintaining hematopoietic stem cell quiescence by regulation of mitochondrial metabolism in mice. Blood. 2018;132(9):911–923. doi: 10.1182/blood-2018-02-831669. [DOI] [PubMed] [Google Scholar]
- 21.Hu M, Lu Y, Wang S, et al. CD63 acts as a functional marker in maintaining hematopoietic stem cell quiescence through supporting TGFbeta signaling in mice. Cell Death Differ. 2022;29(1):178–191. doi: 10.1038/s41418-021-00848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15(9):525–534. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- 23.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Ya F, Li Q, Wang D, et al. Cyanidin-3-o-β-glucoside induces megakaryocyte apoptosis via PI3K/Akt- and MAPKs-mediated inhibition of NF-κB signalling. Thromb Haemost. 2018;118(7):1215–1229. doi: 10.1055/s-0038-1656551. [DOI] [PubMed] [Google Scholar]
- 25.Tothova Z, Šemeláková M, Solarova Z, Tomc J, Debeljak N, Solar P. The role of PI3K/AKT and MAPK signaling pathways in erythropoietin signalization. Int J Mol Sci. 2021;22(14):7682. doi: 10.3390/ijms22147682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Wu B, Li Y, et al. Loss of sphingosine kinase 2 promotes the expansion of hematopoietic stem cells by improving their metabolic fitness. Blood. 2022;140(15):1686–1701. doi: 10.1182/blood.2022016112. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Li X, Wang W, et al. Tracing haematopoietic stem cell formation at single-cell resolution. Nature. 2016;533(7604):487–492. doi: 10.1038/nature17997. [DOI] [PubMed] [Google Scholar]
- 28.Golan K, Kollet O, Markus RP, Lapidot T. Daily light and darkness onset and circadian rhythms metabolically synchronize hematopoietic stem cell differentiation and maintenance: The role of bone marrow norepinephrine, tumor necrosis factor, and melatonin cycles. Exp Hematol. 2019;78:1–10. doi: 10.1016/j.exphem.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Guo B, Huang X, Cooper S, Broxmeyer HE. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat Med. 2017;23(4):424–428. doi: 10.1038/nm.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velardi E, Tsai JJ, Radtke S, et al. Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat Med. 2018;24(2):239–246. doi: 10.1038/nm.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montero-Melendez T, Boesen T, Jonassen TEN. Translational advances of melanocortin drugs: integrating biology, chemistry and genetics. Semin Immunol. 2022;59:101603. doi: 10.1016/j.smim.2022.101603. [DOI] [PubMed] [Google Scholar]
- 33.Swope V, Alexander C, Starner R, Schwemberger S, Babcock G, Abdel-Malek ZA. Significance of the melanocortin 1 receptor in the DNA damage response of human melanocytes to ultraviolet radiation. Pigment Cell Melanoma Res. 2014;27(4):601–610. doi: 10.1111/pcmr.12252. [DOI] [PubMed] [Google Scholar]
- 34.Benjamins JA, Nedelkoska L, Lisak RP. Melanocortin receptor subtypes are expressed on cells in the oligodendroglial lineage and signal ACTH protection. J Neurosci Res. 2018;96(3):427–435. doi: 10.1002/jnr.24141. [DOI] [PubMed] [Google Scholar]
- 35.Bruschetta G, Jin S, Liu ZW, Kim JD, Diano S. MC4R signaling in dorsal raphe nucleus controls feeding, anxiety, and depression. Cell Rep. 2020;33(2):108267. doi: 10.1016/j.celrep.2020.108267. [DOI] [PubMed] [Google Scholar]
- 36.Joseph CG, Yao H, Scott JW, et al. γ2-Melanocyte stimulation hormone (γ2-MSH) truncation studies results in the cautionary note that γ2-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides. 2010;31(12):2304–2313. doi: 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Yan J, Tao Y, et al. Pituitary hormone alpha-MSH promotes tumor-induced myelopoiesis and immunosuppression. Science. 2022;377(6610):1085–1091. doi: 10.1126/science.abj2674. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Guo DY, Lin YJ, Tao YX. Melanocortin regulation of inflammation. Front Endocrinol. 2019;10:683. doi: 10.3389/fendo.2019.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albani A, Perez-Rivas LG, Reincke M, Theodoropoulou M. Pathogenesis of cushing disease: an update on the genetics of corticotropinomas. Endocr Pract. 2018;24(10):907–914. doi: 10.4158/EP-2018-0111. [DOI] [PubMed] [Google Scholar]
- 40.Takihara Y, Nakamura-Ishizu A, Tan DQ, et al. High mitochondrial mass is associated with reconstitution capacity and quiescence of hematopoietic stem cells. Blood Adv. 2019;3(15):2323–2327. doi: 10.1182/bloodadvances.2019032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y, Hu M, Zhang Z, Qi Y, Wang J. The regulation of hematopoietic stem cell fate in the context of radiation. Radiation Medicine and Protection. 2020;1(1):31–34. [Google Scholar]
- 42.Acharya SS, Fendler W, Watson J, et al. Serum microRNAs are early indicators of survival after radiation-induced hematopoietic injury. Sci Transl Med. 2015;7(287):287ra69. doi: 10.1126/scitranslmed.aaa6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patwardhan RS, Sharma D, Checker R, Sandur SK. Mitigation of radiation-induced hematopoietic injury via regulation of cellular MAPK/phosphatase levels and increasing hematopoietic stem cells. Free Radic Biol Med. 2014;68:52–64. doi: 10.1016/j.freeradbiomed.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Lu Y, Qi Y, et al. CDK19 regulates the proliferation of hematopoietic stem cells and acute myeloid leukemia cells by suppressing p53-mediated transcription of p21. Leukemia. 2022;36(4):956–969. doi: 10.1038/s41375-022-01512-5. [DOI] [PubMed] [Google Scholar]
- 45.Shao L, Elujoba-Bridenstine A, Zink KE, et al. The neurotransmitter receptor Gabbr1 regulates proliferation and function of hematopoietic stem and progenitor cells. Blood. 2021;137(6):775–787. doi: 10.1182/blood.2019004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suo M, Rommelfanger MK, Chen Y, et al. Age-dependent effects of Igf2bp2 on gene regulation, function, and aging of hematopoietic stem cells in mice. Blood. 2022;139(17):2653–2665. doi: 10.1182/blood.2021012197. [DOI] [PubMed] [Google Scholar]
- 47.Rehn M, Wenzel A, Frank AK, et al. PTBP1 promotes hematopoietic stem cell maintenance and red blood cell development by ensuring sufficient availability of ribosomal constituents. Cell Rep. 2022;39(6):110793. doi: 10.1016/j.celrep.2022.110793. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi M, Bai Y, Dong Y, et al. PRL2/PTP4A2 phosphatase is important for hematopoietic stem cell self-renewal. Stem Cells. 2014;32(7):1956–1967. doi: 10.1002/stem.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleppe M, Spitzer MH, Li S, et al. Jak1 integrates cytokine sensing to regulate hematopoietic stem cell function and stress hematopoiesis. Cell Stem Cell. 2017;21(4):489–501.e7. doi: 10.1016/j.stem.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgartner C, Toifl S, Farlik M, et al. An ERK-dependent feedback mechanism prevents hematopoietic stem cell exhaustion. Cell Stem Cell. 2018;22(6):879–892.e6. doi: 10.1016/j.stem.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry JM, He XC, Sugimura R, et al. Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 2011;25(18):1928–1942. doi: 10.1101/gad.17421911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kollmann S, Grausenburger R, Klampfl T, et al. A STAT5B-CD9 axis determines self-renewal in hematopoietic and leukemic stem cells. Blood. 2021;138(23):2347–2359. doi: 10.1182/blood.2021010980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao N, Laha S, Das SP, Morlock K, Jesneck JL, Raffel GD. Ott1 (Rbm15) regulates thrombopoietin response in hematopoietic stem cells through alternative splicing of c-Mpl. Blood. 2015;125(6):941–948. doi: 10.1182/blood-2014-08-593392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clayton NP, Khan-Malek RC, Dangler CA, et al. Sargramostim (rhu GM-CSF) improves survival of non-Human primates with severe bone marrow suppression after acute, high-dose, whole-body irradiation. Radiat Res. 2021;195(2):191–199. doi: 10.1667/RADE-20-00131.1. [DOI] [PubMed] [Google Scholar]
- 56.Zhang B, Oyewole-Said D, Zou J, Willliams IR, Gewirtz AT. TLR5 signaling in murine bone marrow induces hematopoietic progenitor cell proliferation and aids survival from radiation. Blood Adv. 2017;1(21):1796–1806. doi: 10.1182/bloodadvances.2017006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu M, Lu Y, Zeng H, et al. MicroRNA-21 maintains hematopoietic stem cell homeostasis through sustaining the NF-kappaB signaling pathway in mice. Haematologica. 2021;106(2):412–423. doi: 10.3324/haematol.2019.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao M, Perry JM, Marshall H, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigues AR, Almeida H, Gouveia AM. Melanocortin 5 receptor signaling and internalization: role of MAPK/ERK pathway and beta-arrestins 1/2. Mol Cell Endocrinol. 2012;361(1-2):69–79. doi: 10.1016/j.mce.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues AR, Pignatelli D, Almeida H, Gouveia AM. Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism. Mol Cell Endocrinol. 2009;303(1-2):74–81. doi: 10.1016/j.mce.2009.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.