Abstract
Introduction
Semaglutide shows significant performance on weight reduction in several clinical trials. However, it is not clear what kind of administration frequency or dosage will achieve better effects. This study aims to explore the different therapeutic effect of semaglutide on weight control under the diverse administration circumstances.
Methods
The PubMed, Embase, Web of Science, Cochrane Library, and the Clinical Trials.gov were searched from inception until 6 June, 2022 to include randomized controlled trials evaluating the Efficacy and safety of subcutaneous semaglutide in overweight or obese adults. Random effects or fixed effects model was conducted based on the heterogeneity among trials. Subgroup analysis was performed to identify the detailed effects under different intervention situations.
Results and discussion
Our study included 13 RCTs involving 5,838 participants with 3,794 ones in semaglutide group and 2,044 in placebo group. Semaglutide was associated with a significant reduction on weight loss related outcomes, including the absolute value of weight loss (WMD -8·97, 95% CI -10·73 to -7·21), percentage of weight loss (WMD -10·00, 95% CI -11·99 to -8·00), body mass index (WMD-3·19, 95% CI -4·02 to -2·37) and waist circumference (WMD -7·21,95% CI -8·87 to -5·56). Subgroup analyses illustrated participants with high weekly dosage, long-term treatment duration and severe baseline BMI (Class II obesity) had a more remarkably decreasing on the main outcomes of weight loss (P for interaction<0·05). Total adverse reactions occurred more frequently in the daily administration group than that in the weekly group (P for interaction =0·01). During the treatment, the incidence rate of hypoglycemia was higher in the group without lifestyle intervention compared with that with lifestyle intervention (P for interaction =0·04). Interpretation Subcutaneous semaglutide had significant benefits on weight loss with reasonable safety in overweight or obese adults. Moreover, additional benefits on cardiometabolic profiles were also seen. We recommended semaglutide treatment to be coupled with lifestyle interventions, and target dose of 2·0 mg or more subcutaneously once weekly. Clinicians can choose suitable treatment schemes based on diverse individual situations.
Systematic review registration
https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=337099, identifier PROSPERO (CRD42022337099).
Keywords: semaglutide, overweight, obese, meta-analysis, subgroup
Graphical Abstract
Summary
This systematic review and meta-analysis aims to explore the different therapeutic effect of semaglutide on weight control under the diverse administration circumstances. Semaglutide was associated with a significant reduction on weight loss related outcomes, including the absolute value of weight loss (WMD -8·97, 95% CI -10·73 to -7·21), percentage of weight loss (WMD -10·00, 95% CI -11·99 to -8·00), body mass index (WMD -3·19, 95% CI -4·02 to -2·37) and waist circumference (WMD -7·21,95% CI -8·87 to -5·56). Subgroup analyses illustrated participants with high weekly dosage, long-term treatment duration and severe baseline BMI (Class II obesity) had a more remarkably decreasing on the main outcomes of weight loss (P for interaction <0·05). Total adverse reactions occurred more frequently in the daily administration group than that in the weekly group (P for interaction =0·01). During the treatment, the incidence rate of hypoglycemia was higher in the group without lifestyle intervention compared with that with lifestyle intervention (P for interaction =0·04). Subcutaneous semaglutide had significant benefits on weight loss with reasonable safety in adults with overweight or obese. Moreover, additional benefits on cardiometabolic profiles were also seen. We recommended semaglutide treatment to be coupled with lifestyle interventions, and target dose of 2·0 mg or more subcutaneously once weekly. Clinicians can choose suitable treatment schemes based on diverse individual situations.
1. Introduction
Obesity, as an identified chronic disease, has become a severe public health challenge worldwide. There are more than 1·9 billion overweight adults and 650 million obese ones globally, according to the World Health Organization (1). Multiple studies have shown that more than 200 co-existing diseases are associated with obesity. Consequently, obesity will lead to a higher risk of early mortality and increasing of all-cause mortality, which gives heavy health burden and financial cost to the population of the whole world. Keeping the weight loss to 5% to 15% can improve situations of the majority of diseases associated with overweight or obesity (2–4).
Maintaining long-term weight control is challenging due to the metabolism variation (5) and non-adherence to lifestyle modification (6, 7). Traditional diet restriction and exercise interventions for weight reduction are sometimes difficult to adhere to, and likely to rebound (8–10). Therefore, it comes increasingly urgent to seek a more safe, effective, and long-term way of weight control. Currently, many guidelines and recommendations encourage the use of anti-obese medications to achieve this goal (11, 12).
Several anti-obesity drugs, such as orlistat, naltrexone-bupropion, phentermine-topiramate and liraglutide, have provided sufficient evidence on weight loss for adults (13). A comprehensive network meta-analysis of pharmacotherapy compared with lifestyle modification only, demonstrated phentermine-topiramate and GLP-1 RAs achieved the best effects (14). GLP-1 is an incretin hormone secreted mainly by intestinal L cells, which can increase the insulin release and inhibit the glucagon release in a manner of glucose-dependent way (15). The mechanisms are mainly focused on appetite restriction and food intake reduction by turning up the satiation signals and turning down the hunger signals in the arcuate nucleus of the hypothalamus (16, 17).
Semaglutide, a novel, long-acting GLP-1 RAs, shows significant performance on weight reduction in several clinical trials (18–20). Besides the good performance on weight loss and hypoglycemic effect, it also demonstrates cardio-protective potential (21, 22), greatly broadening its applications on type 2 diabetes treatment only. Compared with exenatide, dulaglutide and liraglutide, semaglutide shows a superior capability of weight-lowering with similar low risk of adverse events (23–25). Therefore, FDA has officially approved it used for adults with overweight or obese in June 2021 (26). In view of the good performance on weight management and maintenance, many trials with different administration ways (oral or subcutaneous, high or low dosages, long or short treatment durations) has been implemented across the North America, Europe and Asian (27–36). Up to now, it is known that the effect of subcutaneous injection of semaglutide is far better than that of oral medication. However, there is no clear conclusion on the therapeutic effect of semaglutide under the diverse application circumstances (dosages, administration frequencies, trial durations, etc.).
Here, in this study, we conducted a comprehensive systematic review and meta-analysis to provide an up-to-date evaluation of semaglutide on efficacy and safety of weight loss, especially under the different administration situations, such as the weekly dosages, administration frequencies, trial durations, baseline obesity classifications and whether accompanied with lifestyle interventions.
2. Methods
2.1. Protocol registration
This study was implemented according to a priori-written protocol registered in PROSPERO (CRD42022337099, registered 13 June 2022) and the reporting was followed by the PRISMA ( Table S1 ). GRADE criteria for meta-analysis were used to assess the quality of evidence.
2.2. Eligibility criteria and relevant outcomes
Studies which answered the most important question (regarding the efficacy and safety of subcutaneous semaglutide treatment on weight loss) and report the experiences of adults (age ≥18 years) with overweight or obese (BMI ≥25kg/m2) who were prescribed semaglutide treatment for weight management in randomized controlled trials were included.
Outcomes for the first key question were the weight-related indicators [the absolute change and percentage change of body weight, change in BMI and waist circumference, weight loss targets (5%, 10% and 15%) from the baseline to the end of intervention]. Outcomes for the second key question were cardiometabolic parameters, such as change in SBP and DBP, HbA1c, FPG, TC, TG, CRP, and patient-reported outcomes of quality of life, using physical functioning scores by the SF-36v2 and physical function scores by the IWQOL-Lite-CT. Outcomes for the third key question were safety outcome measures mainly referred to adverse events occurring with treatment, including constipation, nausea, vomiting, diarrhea, decreased appetite, hypoglycaemia, and severe side-effects.
To capture the broad field of literature, participants with obesity-related complications were included in this study. For instance, we included overweight or obese people with type 2 diabetes, non-alcoholic fatty liver disease, polycystic ovary syndrome, et al. Studies were excluded if they included patients using semaglutide dosage less than 1.0 mg once weekly, or less than 12 weeks. Non-randomized studies, animal trials, protocols, conference abstracts, case reports and post-hoc analyses were excluded.
2.3. Search strategy
The literature was systematically searched for English articles published up to June 6, 2022 from the following databases which were PubMed, Embase, Web of Science and Cochrane Library. The international or national clinical trial registries, including the ClinicalTrials.gov, European Union Clinical Trials Register, World Health Organization-International Clinical Trials Registry Platform and 9 national clinical trial registries were also searched. The search strategy involved using the medical subject heading terms and the terms of “semaglutide”, “wegovy”, “ozempic”, “overweight”, “obesity” and “randomized controlled trial”. The reference lists from published articles and trials were manually retrieved and screened to identify relevant additional studies. Detailed online search strategies were seen in Table S2 and Text S1 .
2.4. Study selection and data extraction
Two authors (RZ, QH) independently reviewed and extracted the data and information through a pre-planned standardized form in line with the Cochrane handbook for systematic reviews of intervention. Disagreement was resolved by group discussion or by consulting with the other two authors (XL, YL) until consensus was reached. The following information was derived from each of the trials, including the publication details (clinical trial number and trial phase, source of funding, author, year of publication, country), participant profiles (age and BMI at baseline, percentage of female, ethnicity, comorbidity), treatment characteristics (sample size, placebo controlled, trial duration, dosage, with or without lifestyle intervention), and outcome measures. Treatments of all the included trials were based on the full-analysis set and ITT principle. If both exist, ITT was preferred.
2.5. Risk of bias assessment
The risk of bias was assessed by two reviewers (LY, YY) respectively using the Cochrane Collaboration risk of bias tool for RCTs. It involved six aspects, which were the bias generated by the randomization process, the bias generated by the randomization sequence, whether the blind method was implemented between participants and researchers, the bias of result evaluation, the bias caused by incomplete result data, and the bias of selective reporting results. We classified the risk of bias into three levels which was high, low and unclear. If one aspect of the study was identified as low risk, we would judge the overall risk of bias as low. The discordance between two reviewers was resolved through discussion with another reviewer (ZW) until all the researchers reached a consensus.
2.6. Data synthesis and statistical analysis
The results of the systematic evaluation were summarized from quantitative aspects. For the continuous variables, the mean difference and standard deviation were extracted from the intervention and placebo group respectively, using the WMD to estimate the treatment effect of the randomized controlled trials. For the dichotomous variables, we counted the number of cases, using the average RR to estimate the risk ratio.The WMD and RR with 95% CI were calculated for the continuous and dichotomous outcomes respectively, to represent the pooled effects and safety conditions of semaglutide on outcomes of interest. The heterogeneity among individual studies was estimated by the Cochran Q test and quantified by I2 statistic. We used a random effect model based on the DerSimonian-Laird method for those variables with significant heterogeneity (P value <0·1 by Q-test or I2 value ≥50%). Otherwise, a fixed effect model based on the inverse-variance method was applied for trials with no heterogeneity.
Subgroup analysis was performed to identify the detailed effects under different intervention situations [(Class I obesity: 30 kg/m2 ≤ BMI <35 kg/m2, Class II obesity: 35 kg/m2 ≤ BMI <40 kg/m2), administration frequency (weekly or daily), weekly cumulative dose (low dose: 1·0-2·1 mg, high dose: 2·4-2·8 mg), trial duration (short-term: <52 weeks, long-term: ≥52 weeks) and lifestyle interventions (with or without)]. Meta-regression was carried out to explore the possible sources of heterogeneity, such as the baseline degree of obesity involved above (37). Potential publication bias of the included trials was tested by the Egger’s test and inspection of funnel plots. Sensitivity analysis was carried out by excluding studies with high-risk bias each time to explore whether the results were significantly deviated by a specific study.
Statistical analyses were performed using Stata version 16·0 (Stata Corp, College Station, TX, USA) and R software (version 4·1·3). The threshold for significance was set as P <0·05 with a two-sided test.
2.7. Grading of evidence GRADE
The GRADE framework was used to estimate the certainty of evidence on relevant outcomes of interest. Three reviewers (RZ, QH, PS) graded the strength of evidence as high, moderate, low and very low degree, following five assessment criteria which were risk of bias, inconsistency, indirectness, publication bias, and imprecision. Each reviewer was required to describe in detail the reasons that downgrading the classification of evidence based on the principle of evaluation. GRADE pro 3·6 software was used to do the record and rank for the quality of evidence on all outcome measures. Missing data information was requested by email from the corresponding authors. If no response was obtained, we derived the data followed by the rules of the Cochrane handbook of systematic reviews of intervention.
3. Results
3.1. Identification and study selection
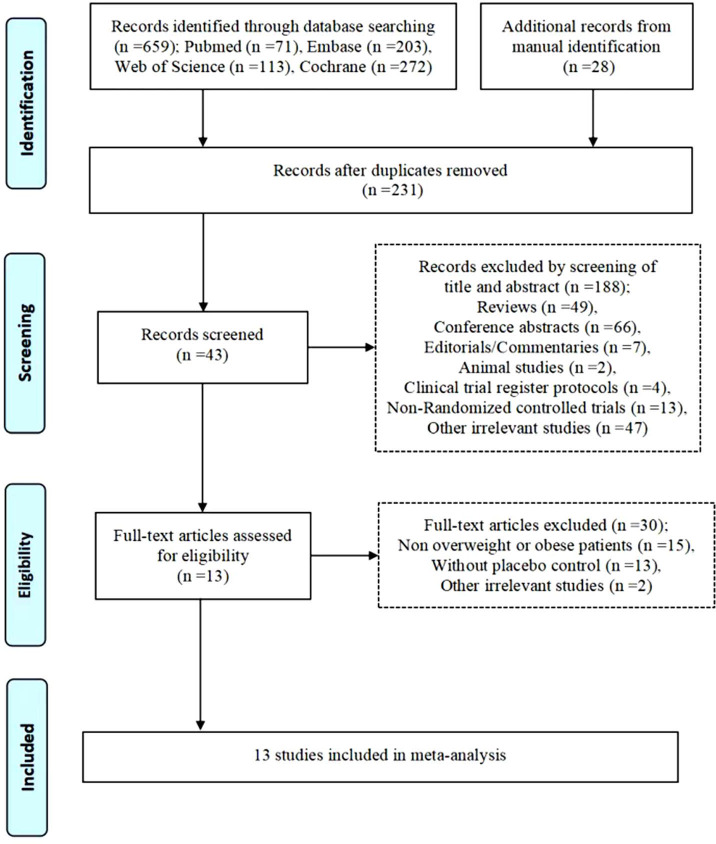
Of 659 potential eligible records were identified according to the search strategy. Among them, 231 duplicate studies and 188 articles which did not meet the inclusion criteria were excluded. Finally, a total of 13 randomized controlled trials involving 5838 participants were included in the final meta-analysis ( Figure 1 ) (18–20, 27–36). All the articles were randomized controlled trials focused on adults with overweight or obese with or without comorbidity that were published from September 2017 to March 2022.
Figure 1.
Flow diagram of study selection process.
The baseline obesity classification fluctuated greatly from 31·9 to 39·3 kg/m2. The comorbidity of 8 studies was not clear, 5 studies had definite specifications (1 with polycystic ovary syndrome, 2 with diabetes, and 2 with nonalcoholic fatty liver disease). Among the 13 studies, 10 were multi-center studies and 3 were single-center ones, 10 were multi-ethnic studies and 3 were single ethnic ones (Slovenians, Caucasians and Asians). In general, most of the studies were implemented in the U.S and the majority of participants were Caucasians. The sample size of the 13 studies varied from a minimum number of 30 to a maximum of 1,961. The trial duration of the trials was from 16 to 104 weeks. Twelve of them were funded by a pharmaceutical company entirely with only one was partly funded by a non-profit organization. The characteristics of the 13 studied were described in Table 1 .
Table 1.
Individual participant characteristics of included studies.
| Trial name | Clinical Trial No | Blinding | Study duration | Trial Phase | Study arms | Sample Size, n | Baseline age (years) | Baseline BMI(kg/m2) | Female, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Blundell 2017 |
NCT02079870 | Double-blind | 12 weeks | Phase 1 | Semaglutide 1·0 mg | 30 | 42 | 33·8 | 10 (33·3) |
| Placebo | 28 | ||||||||
| Lingvay 2018 |
NCT02461589 | Double-blind | 26 weeks | Phase 2 | Semaglutide 1·4 mg | 65 | 58·4 ± 9·6 | 32·8 ± 4·5 | 22 (33·9) |
| Semaglutide 2·1 mg | 63 | 54·8 ± 9·7 | 33·1 ± 4·7 | 31 (49·2) | |||||
| Placebo | 129 | 57·1 ± 9·2 | 32·8 ± 4·2 | 57 (44·2) | |||||
| ONeil 2018 |
NCT02453711 | Double-blind | 52 weeks | Phase 2 | Semaglutide 1·4 mg | 103 | 44 ± 11 | 40·1 ± 6·9 | 66 (64·1) |
| Semaglutide 2·1 mg | 103 | 47 ± 12 | 39·6 ± 7·1 | 66 (64·1) | |||||
| Semaglutide 2·8 mg | 102 | 48 ± 13 | 39·9 ± 8·8 | 66 (64·7) | |||||
| Placebo | 136 | 46 ± 13 | 40·1 ± 7·2 | 88 (64·7) | |||||
| Newsome 2020 |
NCT02970942 | Double-blind | 72 weeks | Phase 2 | Semaglutide 1·4 mg | 78 | 58·1 ± 9·9 | 35·6 ± 6·1 | 52 (67.0) |
| Semaglutide 2·8 mg | 82 | 54·3 ± 10·2 | 35·2 ± 6·6 | 47 (57.0) | |||||
| Placebo | 80 | 52·4 ± 10·8 | 36·1 ± 6·6 | 44 (55.0) | |||||
| Jensterle 2021 |
NCT04263415 | Single-blind | 16 weeks | Phase 4 | Semaglutide 1·0 mg | 15 | 33·7 ± 5·3 | 36·8 ± 3·9 | 13 (100·0) |
| Placebo | 15 | 35·4 ± 3·8 | 12 (100·0) | ||||||
| Flint 2021 |
NCT03357380 | Double-blind | 72 weeks | Phase 2 | Semaglutide 2·8 mg | 34 | 59·5 ± 10·1 | NA | 11 (32·4) |
| Placebo | 33 | 60·5 ± 8·5 | NA | 9 (27·3) | |||||
| Friedrichsen 2021 |
NCT03842202 | Double-blind | 20 weeks | Phase 1 | Semaglutide 2·4 mg | 36 | 40·7 ± 12·2 | 34·2 ± 3·0 | 12 (33·3) |
| Placebo | 36 | 45·0 ± 9·5 | 34·6 ± 3·1 | 16 (44·4) | |||||
| Wadden 2021 |
NCT03611582 | Double-blind | 68 weeks | Phase 3 | Semaglutide 2·4 mg | 407 | 46 ± 13 | 38·1 ± 6·7 | 315 (77·4) |
| Placebo | 204 | 46 ± 13 | 37·8 ± 6·9 | 180 (88·2) | |||||
| Wilding 2021 |
NCT03548935 | Double-blind | 68 weeks | Phase 2 | Semaglutide 2·4 mg | 1306 | 46 ± 13 | 37·8 ± 6·7 | 955 (73·1) |
| Placebo | 655 | 47 ± 12 | 38·0 ± 6·5 | 498 (76·0) | |||||
| Davies 2021 |
NCT03552757 | Double-blind | 68 weeks | Phase 3 | Semaglutide 1·0 mg | 403 | 56 ± 10 | 35·3 ± 5·9 | 203 (50·4) |
| Semaglutide 2·4 mg | 404 | 55 ± 11 | 35·9 ± 6·4 | 223 (55·2) | |||||
| Placebo | 403 | 55 ± 11 | 35·9 ± 6·5 | 190 (47·1) | |||||
| Kadowaki 2022 |
NCT03811574 | Double-blind | 68 weeks | Phase 1 | Semaglutide 1·7 mg | 101 | 51 ± 10 | 31·6 ± 3·7 | 37 (37.0) |
| Semaglutide 2·4 mg | 199 | 52 ± 12 | 32·0 ± 4·6 | 85 (43.0) | |||||
| Placebo | 101 | 50 ± 9 | 31·9 ± 4·2 | 26 (26.0) | |||||
| Rubino 2022 |
NCT04074161 | Double-blind | 68 weeks | Phase 3 | Semaglutide 2·4 mg | 126 | 48 ± 14 | 37·0 ± 7·4 | 102 (81·0) |
| Placebo | 85 | 51 ± 12 | 38·8 ± 6·5 | 66 (77·6) | |||||
|
NCT03693430 2022 |
NCT03693430 | Double-blind | 104 weeks | Phase 3 | Semaglutide 2·4 mg | 152 | 47 ± 12 | NA | 123 (80·9) |
| Placebo | 152 | 47 ± 10 | NA | 113 (74·3) |
NCT, national clinical trial; NA, not available.
3.2. Weight loss efficacy outcomes
All the trials displayed a significant effect on body weight loss compared with placebo ( Table 2 ). There was a significant correlation between semaglutide treatment and a reduction in absolute weight change (WMD -8·97, 95% CI -10·73 to -7·21; P <0·001, I2= 99·15%), percentage of weight change (WMD -10·00, 95% CI -11·99 to -8·00; P <0·001, I2= 96·92%), change in BMI from baseline to the end (WMD -3·19, 95% CI -4·02 to -2·37; P <0·001, I2= 94·89%), as well as waist circumference (WMD -3·19, 95% CI -4·02 to -2·37; P <0·001, I2 = 94·89%). Compared with placebo, semaglutide treatment obtained a higher achievement of the goal of 5% (RR 3·00, 95% CI 2·53 to 3·56; P <0·001, I2= 83·29%), 10% (RR 5·04, 95% CI 3·99 to 6·36; P <0·001, I2= 70·76%) and 15% (RR 6·95, 95% CI 5·39 to 8·96; P =0·07, I2= 42·28%). Details were seen in Figures S1–7 .
Table 2.
Effect of Weight loss, cardiovascular metabolic and quality of life in semaglutide compared with placebo control.
| No of trials | n | WMD | 95% CI | I2 (%) | Pheterogeneity | Tau-squared | ||
|---|---|---|---|---|---|---|---|---|
| body weight (kg) | 18 | 5989 | -8·97 | -10·73 | -7·21 | 99·19 | <0·001 | 13·71 |
| body weight (%) | 11 | 5365 | -10·00 | -11·99 | -8·00 | 96·92 | <0·001 | 10·72 |
| body mass index (kg/m2) | 9 | 3024 | -3·19 | -4·02 | -2·37 | 94·89 | <0·001 | 1·43 |
| waist circumference (cm) | 13 | 5400 | -7·21 | -8·87 | -5·56 | 96·03 | <0·001 | 8·19 |
| weight loss goal (5%) | 13 | 6152 | 3·00 | 2·53 | 3·56 | 83·29 | <0·001 | 0·07 |
| weight loss goal (10%) | 11 | 5766 | 5·04 | 3·99 | 6·36 | 70·76 | <0·001 | 0·10 |
| weight loss goal (15%) | 11 | 5766 | 6·95 | 5·39 | 8·96 | 42·28 | 0·07 | 0·07 |
| HbA1c (%) | 15 | 3947 | -0·82 | -1·03 | -0·60 | 99·06 | <0·001 | 0·18 |
| FPG (mmol/L) | 12 | 3580 | -1·18 | -1·67 | -0·69 | 98·67 | <0·001 | 0·73 |
| TC (mmol/L) | 5 | 952 | -0·30 | -0·52 | -0·08 | 0 | 0·85 | – |
| TG (mmol/L) | 5 | 952 | -0·63 | -1·02 | -0·25 | 8·05 | 0·36 | – |
| DBP (mmHg) | 12 | 5373 | -2·09 | -2·83 | -1·34 | 69·94 | <0·001 | 0·92 |
| SBP(mmHg) | 12 | 5373 | -4·82 | -5·87 | -3·77 | 62·43 | <0·001 | 1·79 |
| CRP (mg/L) | 4 | 927 | -1·37 | -1·82 | -0·92 | 0 | 0·88 | – |
| SF-36v2 | 6 | 4300 | 1·22 | 0·69 | 1·74 | 63·85 | 0·02 | 0·24 |
| IWQOL-Lite-CT | 5 | 3756 | 4·81 | 1·28 | 8·34 | 89·13 | <0·001 | 13·84 |
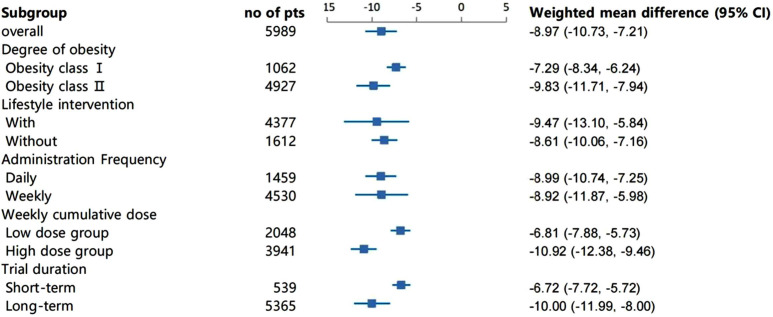
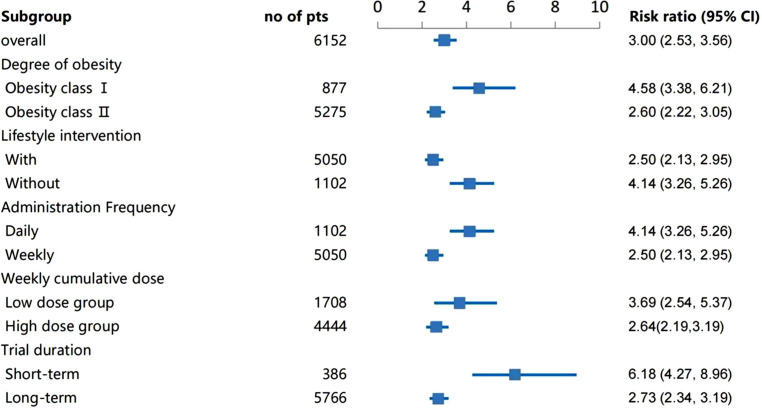
For absolute value of weight change, subgroup analyses revealed the absolute value of weight change decreased more significantly in the Class II obesity group than that in the Class I obesity group (P for interaction =0·02). Compared with the low cumulative weekly dose group, high weekly dose group had a greater improvement on the weight change (P for interaction <0·01). In addition, the absolute value of weight change decreased more obviously in the long-term treatment group than that in the short-term group (P for interaction <0·01), see Figure 2 . For waist circumference, subgroup analyses showed that the high weekly semaglutide dosage group had a greater reduction on waist circumference compared with the low dose group (P for interaction =0·01). Participants in ClassIobesity group, whose baseline BMI were 30 to 34·99 kg/m2, achieved a greater proportion of weight loss goal of 5% and goal of 10% than those in Class II obesity group (P for interaction <0·01). For weight loss goals of 5%, subgroup analysis demonstrated that higher achievement rate of weight loss goal of 5% was found in group of short-term treatment with duration less than 52 weeks, group of once daily administration and that of without lifestyle intervention (P for interaction <0·01), see Figure 3 . No obvious difference on percentage of weight change, BMI reduction,weight loss goals of 10% and 15% was found under the different subgroup. Details were seen in Tables S3–9 .
Figure 2.
Subgroup analysis of the effect of absolute value of body weight change(kg).
Figure 3.
Subgroup analysis of the effect of weight loss goal of 5%.
3.3. Cardiovascular metabolic outcomes
Nine studies had sufficient data of HbA1c. Random effects meta-analyses were conducted and revealed semaglutide treatment had a significant effect on lowering HbA1c level compared with placebo (WMD -0·81, 95% CI -1·05 to -0·57; P < 0·001, I2= 98·87%). For 7 studies with FPG data, semaglutide also showed obvious hypoglycemic effects in contrast with placebo (WMD -1·19, 95% CI -1·64 to -0·74, P <0·001, I2 = 98·36%). Among 7 studies, obvious reduction was seen on blood pressures both with the DBP (WMD -2·09, 95% CI -2·83 to -1·34; P <0·001, I2= 69·94% and SBP (WMD -4·82, 95% CI -5·87 to -3·77; P <0·001, I2 = 62·43%). For the serum lipid, although only 5 trials provided sufficient data, the improvements in semaglutide group were still observed both on total cholesterol (WMD -0·30, 95% CI -0·52 to -0·08; P =0·85, I2= 0%) and triglyceride (WMD -0·63, 95% CI -1·02 to -0·25; P =0·36, I2= 8·05%. 4 trials illustrated that CRP had a distinct reduction after treatment by semaglutide (WMD -1·37, 95% CI -1·82 to -0·92; P =0·88, I2 = 0%), see Table 2 (Details were seen in Figure S8-14 ).
Subgroup analysis proved that participants grouped in Class I obesity at baseline had a greater reduction of HbA1c than those in Class II obesity (P for interaction =0·01) ( Table S10 ). Adults with diet control and exercise enhancement had a greater decrease on triglyceride level than those without lifestyle intervention(P for interaction =0·04) ( Table S11 ). But for other cardiovascular metabolic indicators, like FPG, TC, blood pressure and CRP, no absolute effect was detected under different subgroups. Details were seen in Tables S10–16 .
3.4. Quality of life outcomes
Four studies reported the SF-36v2, and 3 reported the IWQOL-Lite-CT. The quality of life improved more from the scores of SF-36v2 in the semaglutide group than placebo (WMD 1·22, 95% CI 0·69 to 1·74; P =0·02, I2 = 63·85%). Moreover, greater progress in IWQOL-Lite-CT (WMD 4·81, 95% CI 1·28 to 8·34; P <0·001, I2= 89·13%) was seen in the treatment group ( Figures S15–16 ). No direct association was found between quality of life and medication dosage, intervention duration, treatment frequency and whether accompanied with lifestyle intervention based on the meta-regression results, see Table 2 ( Tables S17, 18 ).
3.5. Safety outcomes
11 studies reported the total adverse events and 12 studies reported severe adverse events. The safety evaluation demonstrated that semaglutide treatment had a slightly higher incidence rate of total adverse events compared with placebo (RR 1·08, 95% CI 1·04 to 1·12; P <0·001, I2= 69·23%), as well as the rate of discontinuation due to adverse events (RR 1·64, 95% CI 1·30 to 2·06; P =0·41, I2 = 3·89%). However, for the severe adverse reactions, no significant difference was noted between two groups (RR 1·16, 95% CI 0·97 to 1·38; P =0·35, I2 = 8·38%) ( Figures S17–19 and Table 3 ).
Table 3.
Risk ratio in safety indicators between the semaglutide and placebo control.
| No of trials | n | RR | 95% CI | I2 (%) | Pheterogeneity | Tau-squared | ||
|---|---|---|---|---|---|---|---|---|
| total adverse reactions | 17 | 6756 | 1·08 | 1·04 | 1·12 | 69·23 | <0·001 | 0 |
| serious adverse reactions | 18 | 6814 | 1·16 | 0·97 | 1·38 | 8·38 | 0·35 | – |
| adverse events leading to discontinuation | 18 | 6814 | 1·64 | 1·30 | 2·06 | 3·89 | 0·41 | – |
| hypoglycemia | 11 | 5667 | 212 | 1·50 | 2·98 | 0 | 0·69 | – |
| vomiting | 17 | 6756 | 3·79 | 2·77 | 5·19 | 57·01 | <0·001 | 0·20 |
| nausea | 17 | 6756 | 2·82 | 2·56 | 3·11 | 0 | 0·68 | – |
| constipation | 16 | 6684 | 2·66 | 2·11 | 3·36 | 55·60 | <0·001 | 0·10 |
| loss of appetite | 9 | 1688 | 2·25 | 1·46 | 3·46 | 43·18 | 0·08 | 0·18 |
| diarrhea | 17 | 6756 | 1·90 | 1·65 | 2·19 | 33 | 0·09 | 0·03 |
| cardiovascular disorders | 7 | 4893 | 0·82 | 0·66 | 1·02 | 0 | 0·80 | – |
| psychic disorders | 9 | 5212 | 1·06 | 0·77 | 1·46 | 44·28 | 0·07 | 0·09 |
| allergic reactions | 10 | 5609 | 0·97 | 0·80 | 1·18 | 0 | 0·73 | – |
| injection-site reactions | 10 | 5609 | 0·81 | 0·63 | 1·04 | 0 | 0·99 | – |
The most common adverse events were hypoglycemia and gastrointestinal adverse events. Indeed, the increased risk on the occurrence of hypoglycemia was seen in the semaglutide group (RR 2·13, 95% CI 1·51 to 3·01; P =0·61, I2 = 0%) ( Figure S20 ). In the meanwhile, the incidence rates of gastrointestinal disorders, such as vomiting, nausea, constipation, loss of appetite and diarrhea were comparatively higher than those in the placebo, with the risk ratios of 3.79 (95% CI 2·77 to 5·19), 2·82 (2·56 to 3·11), 2·66 (2·11 to 3·36), 2·25 (1·46 to 3·46), and 1·90 (1·65 to 2·19), respectively ( Figures S21–25 ). No significant difference was found in the followings when compared with placebo group, which were cardiovascular disorders, psychic disorders, allergic reactions, injection-site reactions ( Figures S26–29 ).
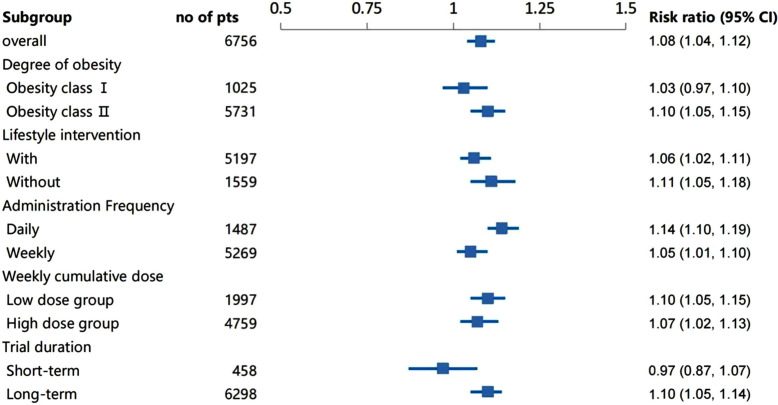
Subgroup analysis was done for further exploration. Results showed the incidence rate of hypoglycemia was higher in the group without lifestyle intervention, compared with that with lifestyle intervention (P for interaction =0·04). Hypoglycemia (RR 3·53, 95% CI 1·99 to 6·27) and total adverse reactions (RR 1·14, 95% CI 1·10 to 1·19) occurred more frequently in the daily group than that in the weekly group ( Table S19 ). Contrasted with the short-term treatment duration group, the long-term treatment group had an increased risk of total adverse reactions (RR 1·10, 95% CI 1·05 to 1·14), vomiting (RR 4·30, 95% CI 3·45 to 5·37) and discontinuation (RR 1·82, 95% CI 1·42 to 2·33), see Figure 4 ( Tables S20–22 ). Generally, heterogeneities were seen for most of the adverse events, except the severe adverse event, nausea and hypoglycemia ( Tables S23–31 ).
Figure 4.
Subgroup analysis of total adverse reactions under different situations.
3.6. Grade of evidence
The GRADE evidence grading system showed all of our primary outcome indicators were rated as moderate to high. Of these, BMI, waist circumference and weight loss of at least 15% were considered to have high quality levels ( Tables S32, 33 ). Due to the inconsistency and publication bias, the quality of evidence on absolute and percentage of weight loss, weight loss targets of at least 5% and 10% were degraded to moderate level.
3.7. Sensitivity analysis
A sensitivity analysis was conducted for the weight related indicators (e.g., the absolute and percentage change of body weight, weight loss target of at least 5%, BMI and waist circumference). High risk studies were sequentially removed. The cumulative statistics for all comparisons of all subjects illustrated the summary results did not change dramatically, suggesting our analysis was generally robust ( Figures S30–34 ).
3.8. Risk and bias assessment
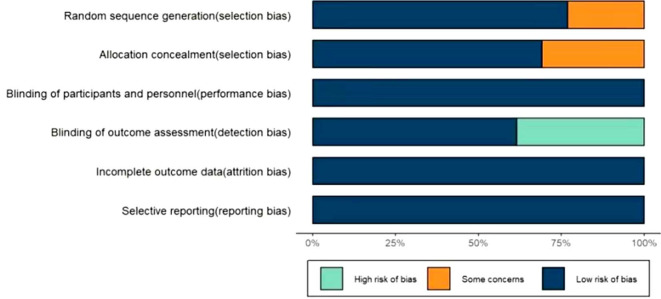
Based on the Cochrane risk assessment tool, 3 (23%) studies did not mention the random sequence generation method and 4 (31%) did not mention the allocation concealment. All the studies were blinded to both participants and personnel. But 5 (38%) of them had a high risk of detection bias for lack of blinding on outcome assessment. The attrition bias and reporting bias were both low as no study was selectively reporting or with incomplete outcome data. Overall, the risks of bias were relatively low for the whole included trials (See Figure 5 ). No evidence of the funnel plot asymmetry was found ( Figures S35–41 ). The Egger’s tests demonstrated no significant publication bias was revealed for the primary outcomes and most of the secondary outcomes (P values were all over 0·05).
Figure 5.
Risk of bias assessment of included studies.
4. Discussion
Several subcutaneous semaglutide randomized controlled trials have shown a great reduction on weight loss, blood glucose and blood pressure, as well as a low rate of adverse events, under different medication treatments. However, which treatment scheme is to be superior, safer, and more durable, especially under diverse and complex personal situations, rare research is reported. Two previous meta-analysis, including 4 and 7 studies respectively, evaluated the efficacy and safety of semaglutide focused on once-weekly treatment only (38, 39). None of them evaluated the quality of evidence for outcomes by the GRADE assessment. Therefore, in this meta-analysis, we explored the efficacy and safety of subcutaneous semaglutide systematically under the comprehensive different treatment conditions, to provide suggestions on medication interventions for adults with overweight or obese.
There were four main findings in our analysis as followed. Firstly, subcutaneous semaglutide showed significant weight loss and cardiometabolic benefits, as well as the improvements in the quality of life in the adults with overweight or obese. Secondly, subcutaneous semaglutide was relatively safe with the consideration of overall adverse events. Thirdly, consistent with the occurrence of side effects in the GLP-1 RAs, gastrointestinal disorders and hypoglycemia were the most common adverse events. Finally, the efficacy and safety of subcutaneous semaglutide varied depending on treatment dosage, administration frequency, trial duration, baseline obesity level and whether accompanied with lifestyle intervention.
Some sources of heterogeneity in this study remained unable to be detected. The likely causes might due to the followings. First of all, the pathogenesis of obesity was multi-factorial and not completely understood, like the genetic factors, different diet and exercise habits, induced by other diseases such as polycystic, et al. (40, 41) Since the mechanism of semaglutide in weight control was primarily appetite restriction and reduction of food intake, there might be some variations on its effectiveness among people with different causes of obesity (42, 43). On the other hand, the medications were administered in a variety of ways, such as the different kinds of starting dosage and methods of dose-escalation. Besides, some of our included trials involved participants with different comorbidities, like diabetes and non-alcoholic fatty liver disease, which might have an impact on treatment effect. Finally, the different sample sizes also had influence on the results.
One pharmacodynamic study reported that semaglutide demonstrated a dose-dependent effect on weight loss (44). In our study, subgroup analysis showed that the absolute weight change and waist circumference values decreased dramatically in the high-dose group than those in the low-dose group. However, the improvements in cardiovascular metabolic indexes, quality of life, and incidence rates of adverse events were identical in the two dosage groups.
Analysis with different treatment frequencies showed no obvious advantages on the reduction of most weight-related outcomes, except that patients in the once-daily group were more likely to achieve the goal of at least 5% weight loss. But in the meanwhile, the incidence rate of overall adverse reactions and hypoglycemia were higher in the once-daily group compared with once-weekly group. Research suggested the weekly administration frequency could enhance patients’ adherence. Indeed, for clinical convenience, frequency of once-weekly was supported by the pharmacokinetic models and drug tolerability trials (28, 30). For safety consideration, as well as for convenience and compliance in the practice, we recommend semaglutide treatment once a week for weight control.
For the analysis of trial duration, the absolute value of weight change was significantly higher in the long-term group than that the short-term group. But participants in the short-term group were more likely to achieve the goal of weight loss at least 5%. Notably, the incidence of total adverse events, withdrawal and vomiting were higher in the long-term group. The reasons to withdraw might be a quick and desirable achievement of weight loss goal after a short period of medication administration. On the other hand, the effect of weight loss in late stage might be not as satisfied as that in the early stage. Besides, adverse reactions were developed intolerable gradually in the late period of treatment and eventually, people ended up the intervention ahead of time. Under these circumstances, it is suggested that patients choose the appropriate length of treatment based on their weight loss results and tolerance of adverse effects.
Lifestyle interventions were the foundation of all obesity treatments according to most of the recommendations and guidelines (45). Although those participants without lifestyle intervention achieved more on weight loss goal of 5%, they had a higher incidence of hypoglycemia actually, compared with those with lifestyle modification. In terms of cardiovascular benefit, greater improvement in TG was seen in the group along with lifestyle intervention. So, we believe that diet and exercise modification can be helpful for stabilization of the blood glucose and serum lipids, especially accompanied with anti-obesity drug usage. Maintaining healthy lifestyle was not only beneficial for weight management, but also good for reducing hypoglycemia, optimizing lipid metabolism when applied with obesity treatment simultaneously. Therefore, we recommend lifestyle interventions in parallel with semaglutide administration.
Our review provided a systematic assessment of efficacy and safety of subcutaneous semaglutide in the treatment of adults with overweight or obese, especially compared the effects under various situations, like different baseline BMI, administration dosages, delivery frequencies, trial duration, with or without lifestyle interventions, hoping to give useful information to clinical practice on pharmacological purpose. The results showed that subcutaneous semaglutide treatment had great beneficial effect on body weight control, with additional improvements on cardiovascular and metabolic profiles. Based on subgroup analysis, we recommend semaglutide to be coupled with lifestyle interventions, and be administered subcutaneously with target dosage of 2·0 mg or more, once weekly. There were some limitations in this study. Firstly, we only compared semaglutide with placebo, no pairwise comparison with other approved anti-obesity drugs. On the other hand, although subgroup analysis and meta-regressions were performed, part of the sources of heterogeneity could not yet be fully identified.
For better medication application on anti-obesity, we suggest in the future, large sample size, more varieties of ethnic, multi-center collaboration with high-quality randomized controlled trials to be conducted. Prolonged follow-up observations after treatment are also recommended to assess the long-term effect and safety on weight loss, as well as the evaluation of cardiometabolic benefits.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
RZ and Q-CH are joint first authors. PS, JT, and Z-WW conceived the study idea and designed the search strategy. RZ, Q-CH, X-LL, and YiL screened studies and collected the data. L-LY, Y-MY, and Z-WW assessed the risk of bias. RZ, Q-CH, and B-HL analyzed the data. RZ, Q-CH, and PS wrote the first drafts of the figures, tables, and appendices. RZ, Q-CH, and PS wrote the first draft of the manuscript and interpreted the data analysis. YuL, LW, T-XL, LD, JT, Z-WW, and X-QY critically revised the manuscript. RZ, Q-CH, and PS are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors contributed to the article and approved the submitted version.
Acknowledgments
Everyone who contributed significantly to the work has been listed. This work was supported by the National Key Technology R&D Program of China (2017YFC0113901); the Key R&D Program of Department of Science and Technology of Sichuan Province (2022YFS0600); Medical and Engineer Cross Joint Fund of University of Electronic Science and Technology of China (ZYGX2021YGLH208). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
GLP-1, glucagon-like peptide-1; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; GRADE, grading of recommendations assessment, development and evaluation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated haemoglobin; FPG, fasting plasma glucose;TC, total cholesterol; TG, triglyceride; CRP, C-reaction protein; SF-36v2, Short Form 36 version 2 healthy survey acute version; IWQOL-Lite-CT, Impact of Weight on Quality of Life-Lite for Clinical Trials Version; ITT, intention-to-treat; WMD, weighted mean difference; RR, relative risk; CI, confidence interval.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1132004/full#supplementary-material
References
- 1. World Health Organization . Obesity and overweight information (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed September 2022).
- 2. Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. CMAJ (2020) 192(31):E875–91. doi: 10.1503/cmaj.191707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne) (2021) 12:706978. doi: 10.3389/fendo.2021.706978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kivimäki M, Kuosma E, Ferrie JE, Luukkonen R, Nyberg ST, Alfredsson L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health (2017) 2(6):e277–85. doi: 10.1016/S2468-2667(17)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leibel RL, Seeley RJ, Darsow T, Berg EG, Smith SR, Ratner R. Biologic responses to weight loss andweight regain: report from an American diabetes association research symposium. Diabetes (2015) 64(7):2299–309. doi: 10.2337/db15-0004 [DOI] [PubMed] [Google Scholar]
- 6. Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence (2016) 10:1547–59. doi: 10.2147/PPA.S103649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kroeger CM, Hoddy KK, Varady KA. Impact of weight regain on metabolic disease risk: a review of human trials. J Obes (2014) 2014:614519. doi: 10.1155/2014/614519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Semlitsch T, Stigler FL, Jeitler K, Horvath K, Siebenhofer A. Management of overweight and obesity in primary care-a systematic overview of international evidence-based guidelines. Obes Rev (2019) 20(9):1218–30. doi: 10.1111/obr.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am (2018) 102(1):183–97. doi: 10.1016/j.mcna.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol (2011) 301(3):R581–600. doi: 10.1152/ajpregu.00755.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract (2016) 22 Suppl 3:1–203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 12. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European Guidelines for obesity management in adults. Obes Facts (2015) 8(6):402–24. doi: 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tak YJ, Lee SY. Long-term efficacy and safety of anti-obesity treatment: where do we stand? Curr Obes Rep (2021) 10(1):14–30. doi: 10.1007/s13679-020-00422-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi Q, Wang Y, Hao Q, Vandvik PO, Guyatt G, Li J, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet (2022) 399(10321):259–69. doi: 10.1016/S0140-6736(21)01640-8 [DOI] [PubMed] [Google Scholar]
- 15. Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol (2018) 14(7):390–403. doi: 10.1038/s41574-018-0016-2 [DOI] [PubMed] [Google Scholar]
- 16. Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor acti- vation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest (2012) 122(1):388–402. doi: 10.1172/JCI42497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP-1 r me- diates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest (2014) 124(6):2456–63. doi: 10.1172/JCI72434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol (2022) 10(3):193–206. doi: 10.1016/S2213-8587(22)00008-0 [DOI] [PubMed] [Google Scholar]
- 19. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA (2021) 325(14):1403–13. doi: 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med (2021) 384(11):989–1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 21. Dagenais GR, Rydén L, Leiter LA, Lakshmanan M, Dyal L, Probstfield JL, et al. Total cardiovascular or fatal events in people with type 2 diabetes and cardiovascular risk factors treated with dulaglutide in the REWIND trail: a post hoc analysis. Cardiovasc Diabetol (2020) 19(1):199. doi: 10.1186/s12933-020-01179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol (2019) 7(10):776–85. doi: 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- 23. Pilitsi E, Farr OM, Polyzos SA, Perakakis N, Nolen-Doerr E, Papathanasiou AE, et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism (2019) 92:170–92. doi: 10.1016/j.metabol.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 24. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med (2019) 381(9):841–51. doi: 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 25. Vosoughi K, Atieh J, Khanna L, Khoshbin K, Prokop LJ, Davitkov P, et al. Association of glucagon-like peptide 1 analogs and agonists administered for obesity with weight loss and adverse events: a systematic review and network meta-analysis. EClinicalMedicine (2021) 42:101213. doi: 10.1016/j.eclinm.2021.101213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. US Food and Drug Administration . Wegovy prescribing information. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215256 (Accessed June 11, 2021).
- 27. Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther (2021) 54(9):1150–61. doi: 10.1111/apt.16608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lingvay I, Desouza CV, Lalic KS, Rose L, Hansen T, Zacho J, et al. A 26-week randomized controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care (2018) 41(9):1926–37. doi: 10.2337/dc17-2381 [DOI] [PubMed] [Google Scholar]
- 29. Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA (2022) 327(2):138–50. doi: 10.1001/jama.2021.23619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obes (Silver Spring) (2020) 28(6):1050–61. doi: 10.1002/oby.22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab (2017) 19(9):1242–51. doi: 10.1111/dom.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab (2021) 23(3):754–62. doi: 10.1111/dom.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet (2021) 397(10278):971–84. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 34. Jensterle M, Ferjan S, Vovk A, Battelino T, Rizzo M, Janež A. Semaglutide reduces fat accumulation in the tongue: a randomized single-blind, pilot study. Diabetes Res Clin Pract (2021) 178:108935. doi: 10.1016/j.diabres.2021.108935 [DOI] [PubMed] [Google Scholar]
- 35. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med (2021) 384(12):1113–24. doi: 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 36. O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet (2018) 392(10148):637–49. doi: 10.1016/S0140-6736(18)31773-2 [DOI] [PubMed] [Google Scholar]
- 37. Djordjevic I, Ivanov B, Sabashnikov A, Gaisendrees C, Gerfer S, Suhr L, et al. Impact of obesity on in-hospital outcomes in veno-arterial ECMO patients. Heart Lung Circ (2022), 31 (10):1393–8. doi: 10.1016/j.hlc.2022.03.014 [DOI] [PubMed] [Google Scholar]
- 38. He K, Guo Q, Zhang H, Xi W, Li J, Jing Z. Once-weekly semaglutide for obesity or overweight: a systematic review and meta-analysis. Diabetes Obes Metab (2022) 24(4):722–6. doi: 10.1111/dom.14612 [DOI] [PubMed] [Google Scholar]
- 39. Zhong P, Zeng H, Huang M, Fu W, Chen Z. Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: a meta-analysis. Endocrine (2022) 75(3):718–24. doi: 10.1007/s12020-021-02945-1 [DOI] [PubMed] [Google Scholar]
- 40. Lindgren CM, Heid IM, Randall JC, Lamina C, Steinthorsdottir V, Qi L, et al. Genome-wide association scan meta-analysis identifies three loci influencing adiposity and fat distribution. PloS Genet (2009) 5(6):e1000508. doi: 10.1371/journal.pgen.1000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Powell LM, Han E, Chaloupka FJ. Economic contextual factors, food consumption, and obesity among U.S. adolescents. J Nutr (2010) 140(6):1175–80. doi: 10.3945/jn.109.111526 [DOI] [PubMed] [Google Scholar]
- 42. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology (2007) 132(6):2131–57. doi: 10.1053/j.gastro.2007.03.054 [DOI] [PubMed] [Google Scholar]
- 43. Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes (2017) 10:123–39. doi: 10.2147/DMSO.S130834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ikushima I, Jensen L, Flint A, Nishida T, Zacho J, Irie S. A randomized trial investigating the pharmacokinetics, pharmacodynamics, and safety of subcutaneous semaglutide once-weekly in healthy Male Japanese and Caucasian subjects. Adv Ther (2018) 35(4):531–44. doi: 10.1007/s12325-018-0677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mehta M, Istfan NW, Apovian CM. Obesity: overview of weight management. Endocr Pract (2021) 27(6):626–35. doi: 10.1016/j.eprac.2021.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.