Abstract
Objective
Breast cancer patients and survivors are increasing in the last years such as their mean age. A feasible and useful complementary intervention to improve physical and psychological health, and decrease some disease symptoms seems to be physical activity. Consequently, this umbrella review wanted to analyze the protocols of different physical activity interventions and to eventually propose a standard operating procedure for possible exercise training in breast cancer patients.
Design, Data sources, Eligibility criteria. The electronic databases PubMed, Scopus, and Web of Science were searched till 25 March 2022 to detect all systematic review and meta-analysis of randomized controlled trials on this topic. The studies were analyzed narratively and evaluated with a scale to assess their quality.
Results
The studies presented heterogeneity in their population included in terms of disease stage and treatments, intervention protocols and outcomes evaluated. This made difficult to synthesize the findings.
Conclusion
It was not possible to propose a standard operating procedure but some indications were proposed to provide feedback for future studies. Ideally, an intervention should be composed of combined training (aerobic and resistance training) with a component of a mindfulness intervention, with an intensity from moderate to high, and 3 times a week. The intervention should be supervised in the first period and then it could be home-based. Exercise training should be personalized to the patients treated.
Keywords: Tumors, Breast cancer, Exercise, Movement, Exercise training
Highlights
-
•
The studies on physical activity intervention in breast cancer patients are heterogeneous.
-
•
Indications for a physical activity intervention for breast cancer patients are proposed.
-
•
The recommendations of this study can be adopted in the clinical setting.
1. Introduction
In the last years, the number of cancer survivors has risen, they often reach older ages presenting other pathologies such as diabetes, hypertension, sarcopenia, or osteoporosis [1]. It is important to reduce the comorbidities and physical activity (PA) practice is functional for the physical, psychological, and social well-being among breast cancer (BC) patients and survivors [2,3]. Physical activity improves the physical sphere including mobility, muscle strength, cardiorespiratory fitness, body composition, bone health, and lymphedema [[4], [5], [6]]. It has also positive outcomes on the levels of proliferation and apoptotic biomarkers in the tumoral microenvironment [7]. From a psychological point of view, PA alleviates the symptoms of depression and anxiety and improves quality of life (QoL) [6], reduces pain and fatigue [8]. Furthermore, exercise training (ET) during an adjunct therapy helps to reduce vascular toxicity, by improving or maintaining the endothelial function, reducing inflammation and hyperlipidemia, as well as promoting endothelial repair [9]. Furthermore, PA is a safe, feasible, and efficacious intervention in BC patients who are undergoing different types of treatments [10].
Although different typologies of PA at different doses appear to reduce the risk caused by BC, there is a stronger risk reduction for specific ET typologies and doses [11]. Some studies suggest that aerobic training is better than resistance training for some symptoms [12], while other studies propose that interventions such as flexibility, Tai Chi, Yoga, Pilates, and Qigong [13] or ET in general [14] have limited evidence.
There is a potential deficiency in exercise programs designed for patients with BC [15]. The differences in the PA interventions with consequent differences in the outcomes on the BC symptoms make necessary a deeper investigation of the training protocols [16], especially during established BC treatments [10]. Furthermore, other important aspects to consider to uniform the treatments and facilitate comparisons of results from different studies are the intensity, duration and frequency of ET [7,17]. The guidelines should have to consider the patients (during medical treatment and in survivorship) the therapy stage, medication typology, fitness level, and the exercise mode and dosage [18]. Guidelines proposed by the American College of Sports Medicine exist [19] but a more specific protocol for people with BC is strongly suggested. The quality of research that adopted randomized controlled trials relate to PA if compared to pharmacological treatments, is inferior making necessary the creation of guidelines to facilitate the generation of high-quality evidence to improve the safety, the efficacy and the implementation of ET [20].
The protocols in other disciplines are often proposed as standard operating procedures, to make the intervention safe, comparable and of good quality [21]. Considering the importance of having a standardized PA intervention, also for the topic of oncology and exercise [22] the objective of the present study was to analyze systematic reviews and meta-analyses with similar questions [23] and to eventually extrapolate a standard operating procedure for an ET to reduce BC related symptoms and increase the QoL.
2. Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24] structure is followed in this umbrella review. This review was not registered but the protocol was written before the collection of the articles and it was followed step by step for the entire study.
2.1. Search strategy
The electronic databases PubMed, Scopus, and Web of Science were searched and articles published between the 1st of January 2012 to the 25th of March 2022 were included. The keywords breast neoplasm and breast cancer, physical activity and exercise, review and meta-analysis were adopted and they were matched through the Boolean operators AND or OR. A string was adopted (“breast cancer” OR “breast neoplasm”) AND (exercise OR “physical activity”) AND (review OR meta-analysis).
2.2. Eligibility criteria
Different eligibility criteria were adopted for population, intervention, comparison, outcomes, and study design (PICO-S). BC patients or survivors were only considered. No limitations were adopted for their current physical activity level, or their background. Reviews were excluded if the sample had other types of cancer patients. ET intervention had to be structured and described. The term of comparison was the control group (pre-and post-intervention, and sedentary or active). Outcomes had no eligibility criteria. The study designs of the included studies were English written systematic reviews and meta-analysis of randomized controlled trials. Other study typologies were excluded.
2.3. Data sources, studies sections and data extraction
The manuscripts were collected in EndNote X8 (EndNote version X8; Thompson Reuters, New York, USA) to eliminate, automatically, the duplicates. In the next phase the collected studies were screened against eligibility criteria by two investigators, who worked independently. The screening was performed by title, abstract, and full text. If there was disagreement between the two investigators, the principal investigator took the final decision. A flow diagram that summarizes the selection process is reported. A table was created with information related to the first investigator and publication year, methodology, electronic databases screened, number of studies included in the review, objective, risk of bias assessment and score, conclusion, population and ET characteristics. The information extracted from the manuscripts was descriptively summarized. The information related to the training methodology extracted from the articles were compared to try to find common aspects, furthermore, the training methodology was also compared with the current literature to try to understand its feasibility and reliability in BC population. Finally, to create a proper standard operating procedure, the population characteristics were considered to make the training specific to the stage or the condition of the patient.
2.4. Study quality assessment
The scale “Assessment of Multiple Systematic Reviews” (AMSTAR) [25] was adopted to study the quality of the included studies. It is a reliable and valid 11-item scale to assess the methodologic quality of systematic reviews [26]. Zero was adopted if “no sufficient information was available”, and 1 if “enough information” were collected. A final score between zero and four corresponds to poor quality; from five to seven was of moderate quality, and above eight the quality was high. The included studies were evaluated, independently, by two authors and disagreements were resolved by the principal investigator.
3. Results
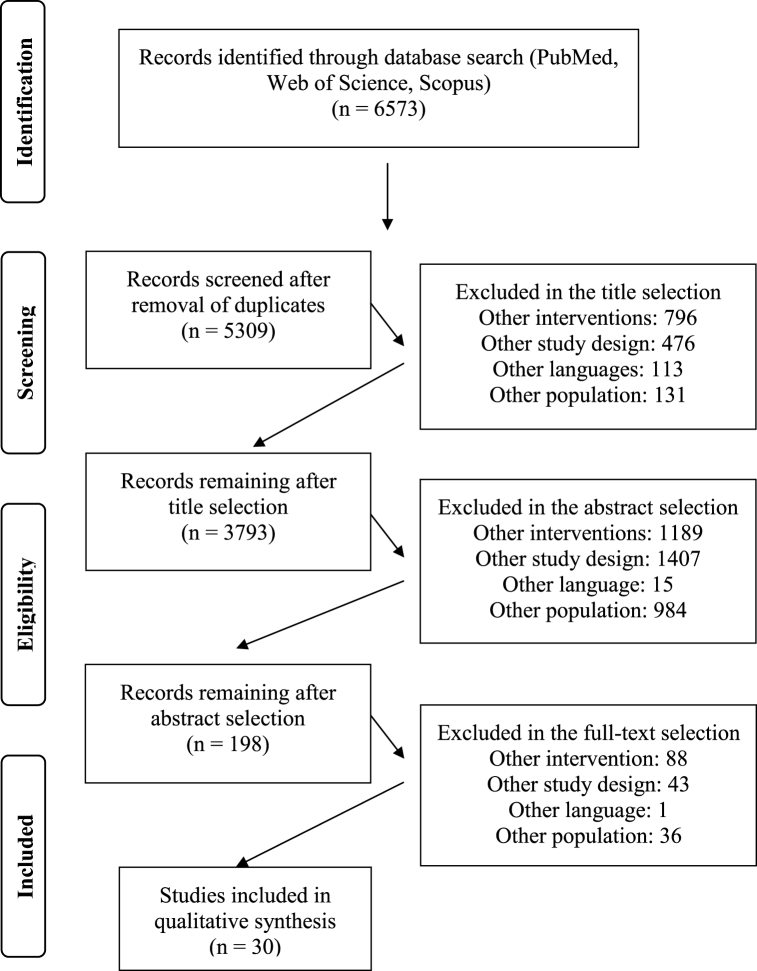
A total of 6573 articles (PubMed 1266; Web of Science 2623; Scopus 2684) were initially included. After duplicate removal, title and abstract screening a total of 282 studies were screened in their full text. After the screening, 30 studies were included. The screening process is summarized in Fig. 1.
Fig. 1.
Flow diagram of the manuscript screening process.
3.1. Characteristics of the included studies
Twenty-one studies used PRISMA guidelines, two studies Cochrane Handbook, three adopted both guidelines while of four studies information were not provided. Pubmed, Web of Science, Scopus, PsycINFO, EBSCO, and CENTRAL were mainly adopted by the included studies to search the articles. The number of included reviews in the studies ranged from four to sixty. Seventeen studies adopted Cochrane Handbook to detect the quality of the included studies while the Physiotherapy Evidence Database (PEDro) scale was adopted in eight studies. Other scales were also adopted and three studies hadn't provided this information. More information about the studies are provided in Table 1.
Table 1.
Characteristics if the studies included in the manuscript.
| 1st Author, year | Guideline | Databases screaned | Objective | N of study | Risk of bias | Main conclusions |
|---|---|---|---|---|---|---|
| Abbasi, 2022 [27] | PRISMA | MEDLINE, Scopus, EMBASE, Google Scholar | ET on inflammatory biomarkers | 18 | Cochrane tool; poor-fair | A consistent reduction in c-reactive protein after concurrent AT and RT and in long-term intervention |
| Antunes, 2021 [28] | PRISMA | MEDLINE, EMBASE, CENTRAL, Web of Science, Scopus | ET on conventional cardiac function and circulating biomarkers | 4 | Cochrane tool: low | ET seems to have a beneficial effect in mitigating left ventricular ejection fraction decline, especially for long intervention |
| Bluethmann, 2015 [29] | PRISMA | Medline; PsycINFO; Ebsco … | ET on behavior change and characteristics of interventions | 14 | NI | Effectiveness in short-term behavior changes of ET, but varied greatly relative to intervention strategies and intensity of supervision/monitoring |
| Carayol, 2015 [30] | PRISMA | MEDLINE, PsycINFO, Francis, PSYarticles CENTRAL | ET on CRF, QoL, anxiety and depression | 33 | PEDro and Cochrane tool: moderate-high | ET may improve CRF, QoL, anxiety and depression |
| Coutino-Escamilla, 2019 [31] | Cochrane | CENTRAL, MEDLINE, SCIELO, EMBASE … | Non- pharmacological therapies on depressive symptoms | 41 | NI | Psychotherapy and mind-body therapies may reduce depressive symptoms. |
| Cramer, 2012 [32] | PRISMA | MEDLINE, Cochrane Library, PsycInfo, EMBASE … | Yoga on health-related QoL and psychological health | 12 | Cochrane tool: unclear | Evidence for short-term effects of yoga in improving psychological health |
| Dong, 2019 [33] | PRISMA | Cochrane Library, MEDLINE, Web of Science … | Yoga on CRF | 17 | Cochrane tool: moderate | Yoga had a small but statistically significant beneficial effect for relieving CRF |
| El-Hashimi, 2019 [34] | NI | CINAHL, CENTRAL, MEDLINE, PsycINFO, Scopus, SPORTDiscus … | Yoga on QoL compared with other physical intervention | 8 | NI | Interventions in general (no yoga specific) diminished CRF, anxiety and depression, and so with an overall enhancement of QoL |
| Espindula, 2017 [35] | Cochrane | MEDLINE, Ovid, EBSCO, Cochrane Library … | Efficacy of pilates | 5 | Cochrane tool: low | Pilates (better) or home-based exercise are effective on CRF, range of motion, mood and it does not bring risks |
| Falcetta, 2018 [36] | PRISMA and Cochrane | Pubmed, Embase, Cochrane Library | ET on anthropometric measurements, QoL, and survival | 60 | Cochrane tool: poor | Significant effects on anthropometric measures and QoL |
| Han, 2021 [37] | PRISMA | MEDLINE, CENTRAL, Embase, SPORTDiscus | ET in modulating the IGF | 12 | Cochrane tool: unclear-high | ET had a positive effect on the IGF system |
| Hong, 2019 [38] | PRISMA | MEDLINE, Web of Science, SPORTDiscus, EMBASE | ET (also modalities) on QoL, social and physical functioning | 26 | Cochrane tool: medium | ET improves QoL, social and physical functioning. The “time of session” appeared to be crucial for an effective improvement in the QoL |
| Juvet, 2017 [39] | PRISMA. | Cochrane Library, MEDLINE, EMBASE, PsycINFO, PEDro … | ET on QoL. Focus on self-reported physical functioning and CRF | 25 | Norwegian Knowledge Centre for the Health Services: moderate |
ET program can produce short-term improvements in physical functioning and can reduce CRF |
| Kang, 2017 [40] | PRISMA and Cochrane | PubMed, EMBASE, CENTRAL, CINAHL, SportDiscuss … | ET on insulin markers | 18 | Cochrane tool: low | Exercise reduces fasting insulin levels. This may be due to exercise-induced reductions in body weight |
| Lee, 2020 [41] | NI | MEDLINE and EMBASE | ET different protocols on physical fitness, QoL, CRF, depression, anxiety, and body compositions | 29 | Quality Assessment of Controlled Intervention Studies | Interventions that involve moderate to vigorous exercise 150 min for 3 times per week and in any modality may provide a better outcome |
| Lin, 2021 [42] | PRISMA | Cochrane Library, EMBASE, Medline, CINAHL, PsycINFO … | ET type, duration and intensity on CRF | 9 | JBI-MASTARI: high | ET can reduce CRF |
| Lipsett, 2017) [43] | NI | MEDLINE, EMBASE, Google Scholar, CINAHL … |
ET during adjuvant radiotherapy on CRF and QoL | 9 | PEDro scale: moderate to high | ET are more effective in alleviating CRF than home-based interventions. Combined AT-RT seems to be a promising in regulation of CRF |
| Liu, 2020 [44] | PRISMA | OVID MEDLINE, AMED, EMBASE, CINAHL, CENTRAL … | Effectiveness of tai chi of CRF | 16 | PEDro score: moderate to high | Tai chi not improves CRF. It significantly relieves CRF symptom when used with conventional supportive care interventions |
| Liu, 2021 [45] | PRISMA | MEDLINE, Cochrane Library, EMBASE, Web of Science … | Mind-body exercise on CRF | 16 | Cochrane tool: fair, medium | Mind-body exercise can effectively improve CRF |
| Luo, 2020 [46] | PRISMA | MEDLINE, EMBASE, CENTRAL … | Effects of Tai Chi Chuan | 15 | Cochrane tool: fair, medium | Tai Chi Chuan appears to be effective on some physical and psychological symptoms and QoL |
| Maginador, 2020 [47] | PRISMA | PubMed, Scopus, and Web of Science | AT (also intensity and mode) on cardio-respiratory fitness | 9 | Cochrane tool: fair-medium | AT improves cardiorespiratory fitness in. Training intensity (vigorous) significantly impacts the VO2max response |
| Medeiros Torres, 2022 [48] | PRISMA | MEDLINE, Web of Science, CENTRAL, Embase … | ET on CRF and which is the most effective type in reducing adverse effect | 20 | PEDro scale: medium-high | ET can be considered beneficial in reducing CRF, especially for supervised training RT or combined AT-RT |
| Meneses-Echavez, 2015 [49] | PRISMA | PubMed, Embase, Scopus, CENTRAL … |
ET on mediators of inflammation | 8 | PEDro scale: fair-medium | ET positively modulates chronic low-grade inflammation |
| Meneses-Echavez, 2016 [50] | PRISMA | EMBASE, CENTRAL, Scopus, SPORTDiscus … | ET in modulating IGF's system | 5 | PEDro score: medium | ET improved IGF. molecular effects of exercise on tumoral microenvironment, apoptosis and survival |
| Meneses-Echavez, 2016 [51] | PRISMA | MEDLINE, EMBASE, Scopus, CENTRAL, CINAHL | Supervised ET on CRF | 9 | PEDro score: medium | Supervised ET reduces CRF and must be implemented in rehabilitation settings |
| O'Neil, 2020 [52] | PRISMA | MEDLINE, Embase, CENTRAL … | Yoga on CRF and QoL | 24 | Cochrane tool: low-moderate | Yoga has small-medium effects on CRF and QoL compared to non-PA. No difference with other ET |
| Ramírez‐Vélez, 2021 [53] | PRISMA | MEDLINE, Embase, Web of Science | ET (type, intensity, volume and frequency) on mental wellbeing | 57 | PEDro: medium | ET significant reduce anxiety, depression, and CRF, as well as increases in body image and QoL, emotional function |
| Wang, 2021 [54] | PRISMA. | MEDLINE, CINAHL, Cochrane Library, Web of Science and Scopus … | ET on cardiovascular system during the convalescence | 11 | Cochrane tool: low | ET could improve the cardiovascular system function associated |
| Yan, 2014 [55] | NI | MEDLINE, EMBASE, CINAHL … | Tai Chi on QoL and other clinical outcomes | 5 | Cochrane tool: low | Lack of sufficient evidence to support Tai Chi to improve QoL/other clinical endpoints. |
| Yi, 2021 [56] | PRISMA and Cochrane | MEDLINE, EMBASE, CENTRAL … | Yoga on health-related quality, physical and psychological health. | 7 | Cochrane tool: medium | Yoga may benefit to reduce cardiorespiratory fitness, depression and anxiety, improve sleep disturbance, and improve QoL in the short-term |
Note: aerobic training: AT; cancer-related fatigue: CRF; Cochrane Central Register of Controlled Trials: CENTRAL; exercise training: ET; No information: NI; Preferred Reporting Items for Systematic Reviews and Meta-Analyses: PRISMA; quality of life: QoL; resistance training: RT; Scientific Electronic Library On-Line: SCIELO.
3.2. Characteristics of the intervention
Participants in the included studies were BC patients at stages zero to four, early diagnosis, and survivors. Participants were under multiple treatment modalities and conditions; neoadjuvant or adjuvant anthracycline-containing and trastuzumab-containing therapy; surgery; radiation therapy; chemotherapy; antihormonal therapy.
The majority of the interventions adopted aerobic ET (n = 17) followed by resistance ET (n = 15) with different intensities and typologies methodologies. Eight studies adopted also a combination of aerobic and resistance ET. A total of 13 studies adopted Yoga with different yoga typologies: Iyengar Yoga, Yoga of awareness, Vin yoga, Restorative Yoga, Yoga based on Patanjali's Yoga tradition, Anusara Yoga, Dru Yoga, Tibetan Yoga, Hatha Yoga, integrated Yoga, Vivekananda Yoga Anusandhana Samsthana (VYASA), Satyanada, Baba Yoga, Bali, and personalized Yoga [[32], [33], [34],52,56]. Mindfulness and Yoga are feasible and efficient interventions [31]. Tai-chi was also widely adopted (n = 8) with different typologies proposal: simplified Tai-chi Chuan, Yang-style Tai-chi Chuan, Tai-chi Chih and Qigong/Tai-chi easy Chen, Tai-chi Yunshou, 18, 24, and 8-form Tai-chi Chuan [44,46]. Qigong was adopted in 4 studies, flexibility in 2 studies and Pilates in 1 study [35]. Water exercise and horseback riding were adopted in 1 study [42].
The duration of the interventions ranged from 1 to 96 weeks, the frequency ranged from 1 to 7 times a week and the duration of the sessions ranged from 115 to 150 min. Six studies were proposed with the intervention supervised, and 5 other studies were with the intervention mixed: supervised and home-based.
Aerobic ET reduces CRF (n = 2) and improves cardiorespiratory fitness (n = 1). Positive outcomes are on CRF after supervised resistance ET (n = 1). Best results if aerobic ET is combined with resistance ET in terms of physical functioning (n = 1), CRF (n = 2), and reduction of the c-reactive protein (n = 1). Yoga, Tai-chi or Qigong are better than aerobic or resistance ET on depression (n = 1), psychological health (n = 1), and CRF (n = 4). Another study that adopted mind-body therapies noted a reduction in depressive symptoms. Yoga may benefit to reduce depression and anxiety, sleep disturbance, and improve cardiorespiratory fitness and QoL (n = 1). Tai-chi Chuan appears to be effective on some physical and psychological symptoms and QoL (n = 1). A study found a lack of sufficient evidence to support Tai-chi to improve QoL and other important clinical endpoints (n = 1). Tai-chi shows no improvement in CRF compared with conventional supportive ET, but it significantly relieves CRF symptoms when used with conventional supportive care interventions (n = 1). There is also evidence for Pilates to improve CRF, range of motion, and mood (n = 1).
High intensity ET had the largest effect (n = 1) on cardiac function (n = 1), and cardiorespiratory response (n = 1). Moderate to vigorous ET may provide a better outcome on physical fitness, QoL, CRF, depression, anxiety, and body composition (n = 1). Low-intensity mind-body ET, reduce CRF (n = 1). High-volume ET is safe and effective in improving CRF and overall QoL (n = 1).
Long-term ET seems to reduce the c-reactive protein (n = 1) and mitigate the left ventricular ejection fraction decline (≥36 exercise sessions) (n = 1). Three weeks improve shoulder function, 12 weeks improve pain, the strength of the arm, and anxiety [46] while 10 weeks are enough to maximize CRF reduction [30]. No long-term improvements were for mental and physical well-being, anxiety, depression, and psychological distress (n = 1). Also, the time of the session seems important: sessions conducted for medium-(>45 to ≤60 min) and longer-time (>60–90 min) considerably improve QoL [38]. Supervised ET seems more productive than home-based ET (n = 2). Highly structured ET produces larger behavior change effects overall, but important effects come from ET supported by phone counseling or e-mail (n = 1). More details of the studies are included in Table 2.
Table 2.
Interventions description.
| 1st Author, year | Participants | Length (D) Session a week (S/w) Session in min (S) |
Intervention |
|---|---|---|---|
| Abbasi, 2022 [27] | BC | D: 4–48 weeks | AT, RT, and yoga. Combination (AT-RT; stretching; tai chi) |
| Antunes, 2021 [28] | Undergoing therapy | D: 9–16 weeks; S/w: 3–5 days; S: 15–45 min | AT-RT Supervised |
| Bluethmann, 2015 [29] | BC survivors; stage I, II, IV, undergoing therapy and surgery | D: 12–26 weeks; S/w: 1–3 days; S: 45 min or less | Walking, group exercise, HB (mix) |
| Carayol, 2015 [30]. | Any tumor stage, undergoing therapy | D: 5–34 weeks | AT, RT, yoga, tai chi or Qigong |
| Coutino-Escamilla, 2019 [31] | BC | D: 2–8 weeks | Mindfulness and yoga |
| Cramer, 2012 [32] | Stage 0–4; survivor; under treatment | D: over 24 weeks; S/w: 1–7 days | Different yoga type |
| Dong, 2019 [33] | BC stages 1–3; post-treatment | D: 4–24 weeks; S/w: 1–5 days; S: 40–90 min | Different yoga type. Supervised, home-based, self-practice |
| El-Hashimi, 2019 [34] | Non-metastasized; first course of cancer treatment; survivors | D: 4–12 weeks; S/w: 1–3 days; S: 60–90 min | Different yoga type. Personalized, small group, DVDs, leaflets |
| Espindula, 2017 [35] | BC | D: 8–12 weeks; S/w: 3 days; S: 45–60 min | Pilates |
| Falcetta, 2018 [36] | BC stage 1–3 | D: 1–96 weeks; F/w: 2–5 days; S: 30–90 min | AR, RT, AT-RT Supervised and HB |
| Han, 2021 [37] | BC | D: 8–24 weeks | AT, RT, AT-RT, tai chi, ET for bring active life |
| Hong, 2019 [38] | BC stage 0–4 | D: 6–26 weeks; S/w: 2–7 days; S: 25–90 min | AT, RT, AT-RT, yoga, and Qigong |
| Juvet, 2017 [39] | Early stage; surgical followed by therapy | No information | AT. RT, AT-RT |
| Lee, 2020 [41] | BC, during adjuvant therapy | D: 17 weeks; S/w: 3 days; S: 150 min | AT, RT. HB; supervised |
| Lin, 2021 [42] | Survivors, stages 0–3, completed treatments, no hormonal therapy | D: 12 weeks; S/w: 3 days; S: 20 min | yoga, mixed AT, water exercise, horseback riding, cycle ergometers |
| Lipsett, 2017 [43] | Stage 0–3, under adjuvant radiotherapy | No information | AT, RT, AT-RT, mind-body, qigong, yoga. Supervised, HB |
| Liu, 2020 [44] | Active BC treatment | D: 10–24 weeks; S/w: 1–4 days; S: 20–120 min | Tai chi |
| Liu, 2021 [45] | BC | D: 6 weeks; S/w: >3 days; S: 40–90 min | Yoga, tai chi, and Qigong |
| Luo, 2020 [46] | BC | D: 12–25 weeks; S/w: 3 days; S: 20–60 min | Tai chi |
| Maginador, 2020 [47] | Undergoing chemotherapy | D: 8–18 weeks | AT |
| Medeiros Torres, 2022 [48] | BC stages 1–4, adjuvant treatment | D: 6–24 weeks | AT, RT, AT-RT, mind–body. Supervised |
| Meneses-Echavez, 2015 [49] | BC type 0–3b, survivors | D: mean 19 weeks; S/w: 2–4 days; S: mean 69 min | AT, AT-RT, yoga. The majority of ET were supervised |
| Meneses-Echavez, 2016 [50] | Survivors, BC stages 1–3A, after anti-cancer treatment | D: mean 22.2 weeks; S/w: 2–3 days; S: mean 73 min | AT, RT and Tai Chi Chuan |
| Meneses-Echavez, 2016 [51] | Survivors, therapy | D: mean 21 weeks; S/w: mean 2.5 days; S:mean44min | AT, RT, stretching exercises. Supervised |
| O'Neill, 2020 [52] | BC at any stage | D: 6–26 weeks; S: 30–90 min | Yoga different programs. Supervised |
| Ramirez-Velez, 2021 [53] | Survivors; all stages, undergoing therapy | D: 6–52 weeks; S/w: 1–6 days | AT, RT, AT-RT, combining AT, strength training, and flexibility. Supervised, HB, mix |
| Wang, 2021 [54] | Completed the primary treatment | D: 8–24 weeks; S/w: 1–7 days; S: 15–90 min | AT, RT, functional training |
| Yan, 2014 [55] | Diagnosed BC | S/w: 1–3days; S: 40–90min | Tai-chi |
| Yi, 2021 [56] | undergoing chemotherapy | D: 8–16 weeks; S/w: 1–4 days; S: 50–90 min | Yoga |
Note: aerobic training: AT; breast cancer: BC; days: D; exercise training: ET; home-based: HB; resistance training: RT; session length: S; sessions/week: S/w.
3.3. Risk of bias assessment
A range from four to ten with a mean of 7/11 indicated a moderate quality of the included studies. Within the studies, the overall quality is mainly moderate and risk of bias is medium. Four studies had no scores. The results of two reviews are unclear. Table 1, Table 3 provide a summary of these information.
Table 3.
Quality assessment with the “assessment of multiple systematic reviews” of the included studies.
| 1st Author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | tot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbasi, 2022 [27] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Antunes, 2021 [28] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Bluethmann, 2015 [29] | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 4 |
| Carayol, 2015 [30] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Coutino-Escamilla, 2019 [31] | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Cramer, 2012 [32] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 5 |
| Dong, 2019 [33] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| El-Hashimi, 2019 [34] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Espindula, 2017 [35] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Falcetta, 2018 [36] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Han, 2021 [37] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Hong, 2019 [38] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 7 |
| Juvet, 2017 [39] | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Kang, 2017 [40] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Lee, 2020 [41] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 9 |
| Lin, 2021 [42] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Lipsett, 2017 [43] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Liu, 2020 [44] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Liu, 2021 [45] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Luo, 2020 [46] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Maginador, 2020 [47] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 6 |
| Medeiros Torres, 2022 [48] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Meneses-Echavez, 2015 [49] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Meneses-Echavez, 2016 [50] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Meneses-Echavez, 2016 [51] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| O'Neil, 2020 [52] | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
| Ramírez‐Vélez, 2021 [53] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Wang, 2021 [54] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Yan, 2014 [55] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Yi, 2021 [56] | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
Note: 1. Priori design; 2. Duplicate article selection/data extraction; 3. Comprehensive literature search (two electronic sources); 4. Status of publication used as an inclusion criteria; 5. List of articles included and excluded; 6. Characteristics of the articles; 7. Scientific quality of the included articles; 8. Scientific quality used appropriately for conclusions; 9. Appropriate methods adopted; 10. Assess of the likelihood of publication bias; 11. Conflicts of interest.
4. Discussion
There is high heterogeneity in the ET protocols designed for BC population. Furthermore, the included studies have important data missing that made it impossible to create and propose a standard operating procedure for an intervention adapted to the cancer stage. Consequently the main goal of this umbrella review was not satisfied; the findings, despite only systematic review and meta-analysis of randomized controlled trials were investigated, is the miss of important information in the included manuscripts. Too often the population included in the same study presented different cancer stages and there wasn't information about the sample background (i.e., habit, physical activity routine, body mass index, working situation). This highlights the necessity of further patients' adapted ET protocol studies. Considering all these limitations it was only possible to propose ET recommendations for future studies that can be adopted only in a second stage, after a personalized intervention planned to reach the minimum level of physical activity. Our ET recommendations for BC patients was based on the main results and the training protocols adopted of the included studies considering the benefits of each intervention proposal. It consists of combined training (aerobic and resistance training) with a moderate-to high-intensity, three times a week and with a component of mindfulness ET (Table 4). Furthermore, a study [22] highlighted the importance of the periodic assessment of patient's physical activity level, their supervision and motivation but also the necessity to have appropriate spaces for the interventions. In this regard, new technologies and systems can help the healthcare system and the metaverse is an example [57]. This new tool could bring the medical doctors but also trainers in the houses of the patients, supervising them 24/24 h with the new wearable devices such as smartwatches and monitoring the training with interactive treadmills or bikes [57].
Table 4.
Recommendations for an intervention in breast cancer patients.
| Main recommendation | Other indications | |
|---|---|---|
| Session duration | >45 min | Better if > 60 min |
| Weekly frequency | 3 times a week | Better if 5 times a week |
| Intensity | Moderate to vigorous exercises | Gradual increment of the intensity Person adapted |
| Training type | Combined (aerobic + resistance training) Mindfulness (yoga) |
Supervised and then home-based Groups activity are suggested |
| Consideration | Guidelines indicated only after personalized training period | The personal doctor has to approve the practice of an exercise training |
Our findings are in line with other studies’ interventions in which different ET modalities were proposed during different treatment phases, both supervised or home-based [58]. The decision to include aerobic exercise, resistance training, and mindfulness activities is also because it is a safe and effective intervention in the management of symptoms of BC-related lymphedema that negatively affect many areas of daily living [59]. Related to ET type, aerobic ET mainly improves cardiorespiratory fitness [47], and reduces CRF [39,43], also during chemotherapy treatment [60]. Consequently, an aerobic ET proposal could be a Nordic Walking training that has positive outcomes on lymphedema, physical fitness and disability with no adverse effects [61]. Resistance ET is obviously suggested to increase, safety, the level of strength, not increasing the risk of lymphedema [[62], [63], [64]]. This ET modality, decreasing muscle mass loss, has positive outcomes also on CRF [48]. Generally, low-volume doses are suitable and suggested because the effects are superior if compared to higher-volume training [65]. Resistance ET should be adapted for those patients at risk of or with bone metastases and possible precautions should be the minimization loading to the lesion area, or also adopting resistance bands instead of machines [66]. Aerobic and resistance ET alone have a good effect on physical and psychological health but the best results are obtained if there is a combination of the two ET modalities [39]. Positive effects are detected also in CRF reduction [43,48] and on c-reactive protein [27]. The literature confirms the effectiveness of this ET strategy [[67], [68], [69]]. Furthermore aerobic, resistance and other ET modalities could be proposed in the aquatic environment because this is feasible, safe, well-tolerated and with positive outcomes on different BC symptoms, improve QoL, cardiorespiratory fitness and muscle strength and achieve high percentages of adherence [70]. One aspect that must be considered in the case of water therapy is the intervention of those that are at risk of suffering or presenting BC-related lymphedema. They must be constantly supervised to control possible adverse events such as inflammations, severe cardiac disease or vascular disorders [71]. Even if water therapy seems feasible and with few side effects, the positive effects on patients that present lymphedema, is limited [71] making this intervention typology not ideal.
Even if it has poor methodology standardization [[32], [33], [34],52,56], similarly to other studies [72,73] Yoga training has promising results on cardiorespiratory fitness, depression and anxiety, sleep quality, and QoL [56,74]. Furthermore, Yoga seems also a safe and feasible training for BC-related lymphedema patients which is a common complication among BC patients having positive outcomes especially on range of motion and QoL [75]. Yoga is an ET with no restriction in equipment, age and gender [73] and despite the Yoga style, there is a similar efficacy [76], it is an ET that should have to be included in a training routine. Tai-chi, other mindfulness ET widely adopted (see Table 2) with different training modalities, it reduces anxiety and depression [44,46]. No long term improvements were found for mental and physical well-being, anxiety, depression, and psychological distress [32], and QoL [55]. The findings on Tai-chi are in line with the literature on this topic [77]. Tai-chi Chuan is effective on some physical and psychological symptoms and QoL [46]. Qigong was adopted different times, the literature suggests positive outcomes on inflammation, QoL, mood disturbance, and the increase in survival rate, depression and anxiety [78,79]. One last ET detected in our review was Pilates and emerged that it improves range of motion and mood [35]. This is confirmed by a study in which they detected also positive outcomes on QoL, pain, and self-reported functional status, mood, fitness and upper extremity circumference [80]. Generally, mindfulness and Yoga are feasible and efficient ET [31] and they must be included in all ET. To work on mental health, dance or movement therapy could be successfully considered as a complementary therapy [81].
High-intensity ET had the largest effect [30] on cardiac function [29], cardio-respiratory response [47] and the literature suggest also positive outcomes on fatigue reduction, improvement of sleep quality and bone mineral density [82]. Positive outcomes on physical and psychological health, CRF and QoL are also from moderate to vigorous exercises [41,47] suggesting that this training modality is suitable for all BC patients. The intensity has to be adapted to the person, the possible medical restriction and the progression of the ET are fundamental aspects to consider: ideally is to start with low intensity (it depends on the level of the patient) and arrive at high-intensity (75–85% of 1RM and 70–80% of VO2max) [83]. Related to the duration, according to Luo and colleagues, 3 weeks of training improve shoulder function while 12 weeks improved pain, the strength of the arm, and anxiety [46]. For Carayol and colleagues [30] 10 weeks are enough to maximize CRF reduction. Generally, long-term ET seems to reduce the c-reactive protein [27] and mitigate the left ventricular ejection fraction decline (≥36 exercise sessions) [28]. According to one study, type, duration, frequency, and total time were not associated with QoL, the only important aspect was the length of the session [38]: medium-time (>45 to ≤60 min) and longer-time (>60–90 min) considerably improved the QoL [38]. Other aspects to consider for an ET to improve the QoL are the intensity and volume: moderate to vigorous exercises [41,82] and high volume [51] is suggested.
Supervised ET is more productive than home-based ET [43,48]. Highly structured ET produces larger behavior change effects, but ET supported by phone counseling or e-mail are feasible [29]. It is fundamental in supervised or home-based ET to have a knowledgeable instructor, tailored information, and a supportive environment [84]. Even if there is no restriction for exercise practice [85] precautions are required when BC patients are under therapies such as surgery, radiation, chemotherapy, hormones, and immunotherapy because this could damage tissues limiting the ability to attend an ET [19]. Furthermore, an aspect to consider is supervising bone metastases [66]. CRF is prevalent during chemotherapy and radiation, this could restrict the ability to exercise [19]. Possible further barriers are pain, and working and caring responsibilities [84].
The main limitation is the heterogeneity of the included reviews in the methodology adopted (inclusion and exclusion criteria, and outcomes) and in the sample (age of the participants and cancer trajectory). The inclusion of BC patients in different stages and treatment in the same manuscript made impossible the analysis according to the participants’ characteristics. Another important limitation was the impossibility to stratify the population based on their training level and routine, this aspect makes our guidelines general. In our manuscript, we tried to include only moderate- or high-quality systematic reviews and meta-analyses, but the included original articles of the reviews presented different results in the quality assessment. Furthermore, the heterogeneity was between the reviews but also within the articles included in the reviews making even harder to synthesize the findings. Another limitation is the inclusion of studies that could be included in more than one systematic review, consequently, a meta-analysis was not performed due to the possible redundant findings, such as other studies did [86].
Future studies should focalize the attention, with original researches, on the level of PA adapted to the BC patients. Information should be collected before, during and after the treatment to present data that can be personalized according to the patient's necessities. Furthermore, a second aspect to consider is the possibility to adopt wearable technologies in BC patients: it seems that positive outcomes are the reduction of sedentary behaviors and an increase in moderate-to-vigorous intensity PA but also to improve the attitude, and cognitive functions [87]. Similar is for the eHealth interventions [88] and for the internet-based approach to delivery ET during rehabilitation [89].
5. Conclusion
The studies presented heterogeneity in the ET protocols and the type of training, frequency, intensity, and time of the session. Furthermore, it was detected a lack of information related to the background of the patients. This highlights the necessity of specific future original studies on this topic. Despite these limitations recommendations are proposed, this want to provide suggestions to experts in the fields proposing an idea of a general interventions that can be followed.
Funding
This work was funded by the University Research Project Grant (PIACERI Found – NATURE-OA – 2020–2022), Department of Biomedical and Biotechnological Sciences (BIOMETEC), University of Catania, Italy.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Luca Petrigna, Email: luca.petrigna@unict.it.
Marta Zanghì, Email: marta.zanghi@phd.unict.it.
Grazia Maugeri, Email: grazia.maugeri1@unict.it.
Velia D'Agata, Email: vdagata@unict.it.
Giuseppe Musumeci, Email: g.musumeci@unict.it.
References
- 1.Casla S., et al. Running away from side effects: physical exercise as a complementary intervention for breast cancer patients. Clin. Transl. Oncol. 2015;17(3):180–196. doi: 10.1007/s12094-014-1184-8. [DOI] [PubMed] [Google Scholar]
- 2.Lu G.L., Zheng J., Zhang L. The effect of exercise on aromatase inhibitor-induced musculoskeletal symptoms in breast cancer survivors :a systematic review and meta-analysis. Support. Care Cancer. 2020;28(4):1587–1596. doi: 10.1007/s00520-019-05186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanghì M., et al. The practice of physical activity on psychological, mental, physical, and social wellbeing for breast-cancer survivors: an umbrella review. Int. J. Environ. Res. Publ. Health. 2022;19(16) doi: 10.3390/ijerph191610391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loh S.Y., Musa A.N. vol. 7. Dove Med Press); 2015. pp. 81–98. (Methods to Improve Rehabilitation of Patients Following Breast Cancer Surgery: a Review of Systematic Reviews. Breast Cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkham A.A., et al. Clinically relevant physical benefits of exercise interventions in breast cancer survivors. Curr. Oncol. Rep. 2016;18(2):12. doi: 10.1007/s11912-015-0496-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhu G.Q., et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails. OncoTargets Ther. 2016;9:2153–2168. doi: 10.2147/OTT.S97864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueira A.C.C., et al. Efficacy of exercise on breast cancer outcomes: a systematic review and meta-analysis of preclinical data. Int. J. Sports Med. 2018;39(5):327–342. doi: 10.1055/s-0044-101149. [DOI] [PubMed] [Google Scholar]
- 8.Moller U.O., et al. A comprehensive approach to rehabilitation interventions following breast cancer treatment - a systematic review of systematic reviews. BMC Cancer. 2019;19 doi: 10.1186/s12885-019-5648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin M., Florida-James G., Ross M. Breast cancer chemotherapy vascular toxicity: a review of mediating mechanisms and exercise as a potential therapeutic. Vasc Biol. 2021;3(1):R106–r120. doi: 10.1530/VB-21-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairman C.M., et al. Effects of exercise interventions during different treatments in breast cancer. J Community Support Oncol. 2016;14(5):200–209. doi: 10.12788/jcso.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer M.C., et al. The mechanisms and effects of physical activity on breast cancer. Clin. Breast Cancer. 2017;17(4):272–278. doi: 10.1016/j.clbc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Bekhet A.H., et al. Benefits of aerobic exercise for breast cancer survivors: a systematic review of randomized controlled trials. Asian Pac. J. Cancer Prev. APJCP. 2019;20(11):3197–3209. doi: 10.31557/APJCP.2019.20.11.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang M.Y., et al. Exercise for fatigue in breast cancer patients: an umbrella review of systematic reviews. Int. J. Nurs. Sci. 2020;7(2):248–254. doi: 10.1016/j.ijnss.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley G.A., Kelley K.S. Exercise and cancer-related fatigue in adults: a systematic review of previous systematic reviews with meta-analyses. BMC Cancer. 2017;17 doi: 10.1186/s12885-017-3687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg J., et al. Quantity of resistance exercise for breast cancer patients: does the dose match the objective? J. Strength Condit Res. 2021;35(5):1467–1476. doi: 10.1519/JSC.0000000000003996. [DOI] [PubMed] [Google Scholar]
- 16.Abdin S., et al. A systematic review of the effectiveness of physical activity interventions in adults with breast cancer by physical activity type and mode of participation. Psycho Oncol. 2019;28(7):1381–1393. doi: 10.1002/pon.5101. [DOI] [PubMed] [Google Scholar]
- 17.Lee J. A meta-analysis of the association between physical activity and breast cancer mortality. Cancer Nurs. 2019;42(4):271–285. doi: 10.1097/NCC.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 18.Gerland L., Baumann F.T., Niels T. Resistance exercise for breast cancer patients? Evidence from the last decade. Breast Care. 2021;16(6):657–663. doi: 10.1159/000513129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liguori G., Medicine A.C.o.S. Lippincott Williams & Wilkins; 2020. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 20.Adams S.C., et al. Comparing the reporting and conduct quality of exercise and pharmacological randomised controlled trials: a systematic review. BMJ Open. 2021;11(8) doi: 10.1136/bmjopen-2020-048218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrigna L., et al. Sport Sciences for Health; 2021. The Importance of Standard Operating Procedures in Physical Fitness Assessment: a Brief Review. [Google Scholar]
- 22.Schmitz K.H., et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. Ca - Cancer J. Clin. 2019;69(6):468–484. doi: 10.3322/caac.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant M.J., Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009;26(2):91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 25.Shea B.J., et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea B.J., et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Abbasi F., et al. Cytokine; 2022. The Effects of Exercise Training on Inflammatory Biomarkers in Patients with Breast Cancer: A Systematic Review and Meta-Analysis; p. 149. [DOI] [PubMed] [Google Scholar]
- 28.Antunes P., et al. Effects of exercise on cardiac function outcomes in women receiving anthracycline or trastuzumab treatment for breast cancer: a systematic review and meta-analysis. Applied Sciences-Basel. 2021;11(18) [Google Scholar]
- 29.Bluethmann S.M., et al. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res. Treat. 2015;149(2):331–342. doi: 10.1007/s10549-014-3255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carayol M., et al. Population-, intervention- and methodology-related characteristics of clinical trials impact exercise efficacy during adjuvant therapy for breast cancer: a meta-regression analysis. Psycho Oncol. 2015;24(7):737–747. doi: 10.1002/pon.3727. [DOI] [PubMed] [Google Scholar]
- 31.Coutino-Escamilla L., et al. Non-pharmacological therapies for depressive symptoms in breast cancer patients: systematic review and meta-analysis of randomized clinical trials. Breast. 2019;44:135–143. doi: 10.1016/j.breast.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Cramer H., et al. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong B., et al. Yoga has a solid effect on cancer-related fatigue in patients with breast cancer: a meta-analysis. Breast Cancer Res. Treat. 2019;177(1):5–16. doi: 10.1007/s10549-019-05278-w. [DOI] [PubMed] [Google Scholar]
- 34.El-Hashimi D., Gorey K.M. Yoga-specific enhancement of quality of life among women with breast cancer: systematic review and exploratory meta-analysis of randomized controlled trials. J Evid Based Integr Med. 2019;24 doi: 10.1177/2515690X19828325. 2515690x19828325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espíndula R.C., et al. Pilates for breast cancer: a systematic review and meta-analysis. Revista da Associacao Medica Brasileira. 2017;63(11):1006–1011. doi: 10.1590/1806-9282.63.11.1006. [DOI] [PubMed] [Google Scholar]
- 36.Falcetta F.S., et al. Effects of physical exercise after treatment of early breast cancer: systematic review and meta-analysis. Breast Cancer Res. Treat. 2018;170(3):455–476. doi: 10.1007/s10549-018-4786-y. [DOI] [PubMed] [Google Scholar]
- 37.Han J.K., Kim G. Role of physical exercise in modulating the insulin-like growth factor system for improving breast cancer outcomes: a meta-analysis. Exp. Gerontol. 2021;152 doi: 10.1016/j.exger.2021.111435. [DOI] [PubMed] [Google Scholar]
- 38.Hong F., et al. Exercise intervention improves clinical outcomes, but the "time of session" is crucial for better quality of life in breast cancer survivors: a systematic review and meta-analysis. Cancers. 2019;11(5) doi: 10.3390/cancers11050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juvet L.K., et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–177. doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Kang D.W., et al. Effects of exercise on insulin, IGF Axis, adipocytokines, and inflammatory markers in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2017;26(3):355–365. doi: 10.1158/1055-9965.EPI-16-0602. [DOI] [PubMed] [Google Scholar]
- 41.Lee J., Lee M.G. Effects of exercise interventions on breast cancer patients during adjuvant therapy: a systematic review and meta-analysis of randomized controlled trials. Cancer Nurs. 2020;43(2):115–125. doi: 10.1097/NCC.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 42.Lin H.P., et al. Exercise effects on fatigue in breast cancer survivors after treatments: a systematic review and meta-analysis. Int. J. Nurs. Pract. 2021 doi: 10.1111/ijn.12989. [DOI] [PubMed] [Google Scholar]
- 43.Lipsett A., et al. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast. 2017;32:144–155. doi: 10.1016/j.breast.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Liu L.Z., et al. The effectiveness of tai chi in breast cancer patients: a systematic review and meta-analysis. Compl. Ther. Clin. Pract. 2020;38 doi: 10.1016/j.ctcp.2019.101078. [DOI] [PubMed] [Google Scholar]
- 45.Liu C., et al. A meta-analysis: intervention effect of mind-body exercise on relieving cancer-related fatigue in breast cancer patients. Evid. base Compl. Alternative Med. 2021:2021. doi: 10.1155/2021/9980940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo X.C., et al. Effect of tai chi chuan in breast cancer patients: a systematic review and meta-analysis. Front. Oncol. 2020;10:607. doi: 10.3389/fonc.2020.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maginador G., et al. Aerobic exercise-induced changes in cardiorespiratory fitness in breast cancer patients receiving chemotherapy: a systematic review and meta-analysis. Cancers. 2020;12(8) doi: 10.3390/cancers12082240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medeiros Torres D., Jorge Koifman R., da Silva Santos S. Support Care Cancer; 2022. Impact on Fatigue of Different Types of Physical Exercise during Adjuvant Chemotherapy and Radiotherapy in Breast Cancer: Systematic Review and Meta-Analysis. [DOI] [PubMed] [Google Scholar]
- 49.Meneses-Echavez J.F., Gonzalez-Jimenez E., Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15 doi: 10.1186/s12885-015-1069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meneses-Echavez J.F., et al. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016;25(7):1009–1017. doi: 10.1158/1055-9965.EPI-15-1061. [DOI] [PubMed] [Google Scholar]
- 51.Meneses-Echavez J.F., et al. The insulin-like growth factor system is modulated by exercise in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2016;16 doi: 10.1186/s12885-016-2733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Neill M., et al. The effect of yoga interventions on cancer-related fatigue and quality of life for women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Integr. Cancer Ther. 2020;19 doi: 10.1177/1534735420959882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramírez‐vélez R., et al. Evidence‐based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: a systematic review and meta‐analysis. Cancers. 2021;13(2):1–27. doi: 10.3390/cancers13020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S.R., et al. Effectiveness of physical exercise on the cardiovascular system in breast cancer patients: a systematic review and meta-analysis of randomized controlled trials. Compl. Ther. Clin. Pract. 2021:44. doi: 10.1016/j.ctcp.2021.101426. [DOI] [PubMed] [Google Scholar]
- 55.Yan J.H., et al. Lack of efficacy of tai chi in improving quality of life in breast cancer survivors: a systematic review and meta-analysis. Asian Pac. J. Cancer Prev. APJCP. 2014;15(8):3715–3720. doi: 10.7314/apjcp.2014.15.8.3715. [DOI] [PubMed] [Google Scholar]
- 56.Yi L.J., et al. Effects of yoga on health-related quality, physical health and psychological health in women with breast cancer receiving chemotherapy: a systematic review and meta-analysis. Ann. Palliat. Med. 2021;10(2):1961–1975. doi: 10.21037/apm-20-1484. [DOI] [PubMed] [Google Scholar]
- 57.Petrigna L., Musumeci G. The metaverse: a new challenge for the healthcare system: a scoping review. Journal of functional morphology and kinesiology. 2022;7(3):63. doi: 10.3390/jfmk7030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gebruers N., et al. The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: a systematic review. Support. Care Cancer. 2019;27(1):109–122. doi: 10.1007/s00520-018-4490-9. [DOI] [PubMed] [Google Scholar]
- 59.Panchik D., et al. Effect of exercise on breast cancer–related lymphedema: what the lymphatic surgeon needs to know. J. Reconstr. Microsurg. 2019;35(1):37–45. doi: 10.1055/s-0038-1660832. [DOI] [PubMed] [Google Scholar]
- 60.Zou L.Y., et al. Effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy: a meta-analysis. Tumour Biol. 2014;35(6):5659–5667. doi: 10.1007/s13277-014-1749-8. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Lastra M.A., et al. Nordic walking for women with breast cancer: a systematic review. Eur. J. Cancer Care. 2019;28(6) doi: 10.1111/ecc.13130. [DOI] [PubMed] [Google Scholar]
- 62.dos Santos W.D.N., et al. Chronic effects of resistance training in breast cancer survivors. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/8367803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasenoehrl T., et al. Resistance exercise and breast cancer-related lymphedema-a systematic review update and meta-analysis. Support. Care Cancer. 2020;28(8):3593–3603. doi: 10.1007/s00520-020-05521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasenoehrl T., et al. Resistance exercise and breast cancer related lymphedema–a systematic review update. Disabil. Rehabil. 2020;42(1):26–35. doi: 10.1080/09638288.2018.1514663. [DOI] [PubMed] [Google Scholar]
- 65.Lopez P., et al. Resistance training in breast cancer patients undergoing primary treatment: a systematic review and meta-regression of exercise dosage. Breast Cancer. 2021;28(1):16–24. doi: 10.1007/s12282-020-01147-3. [DOI] [PubMed] [Google Scholar]
- 66.Weller S., et al. Exercise for individuals with bone metastases: a systematic review. Crit. Rev. Oncol.-Hematol. 2021;166 doi: 10.1016/j.critrevonc.2021.103433. [DOI] [PubMed] [Google Scholar]
- 67.Mok J., et al. The lasting effects of resistance and endurance exercise interventions on breast cancer patient mental wellbeing and physical fitness. Sci. Rep. 2022;12(1):3504. doi: 10.1038/s41598-022-07446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ficarra S., et al. Breast Cancer; 2022. Impact of Exercise Interventions on Physical Fitness in Breast Cancer Patients and Survivors: a Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boing L., et al. Effects of exercise on physical outcomes of breast cancer survivors receiving hormone therapy - a systematic review and meta-analysis. Maturitas. 2020;141:71–81. doi: 10.1016/j.maturitas.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 70.Mur-Gimeno E., et al. Systematic review of the effect of aquatic therapeutic exercise in breast cancer survivors. Eur. J. Cancer Care. 2022;31(1) doi: 10.1111/ecc.13535. [DOI] [PubMed] [Google Scholar]
- 71.Reger M., et al. Water therapies (hydrotherapy, balneotherapy or aqua therapy) for patients with cancer: a systematic review. J. Cancer Res. Clin. Oncol. 2022;148(6):1277–1297. doi: 10.1007/s00432-022-03947-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J., et al. Effects of yoga on psychologic function and quality of life in women with breast cancer: a meta-analysis of randomized controlled trials. J. Alternative Compl. Med. 2012;18(11):994–1002. doi: 10.1089/acm.2011.0514. [DOI] [PubMed] [Google Scholar]
- 73.Wei C.W., et al. Effectiveness of yoga interventions in breast cancer-related lymphedema: a systematic review. Compl. Ther. Clin. Pract. 2019;36:49–55. doi: 10.1016/j.ctcp.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Sharma M., Lingam V.C., Nahar V.K. A systematic review of yoga interventions as integrative treatment in breast cancer. J. Cancer Res. Clin. Oncol. 2016;142(12):2523–2540. doi: 10.1007/s00432-016-2269-2. [DOI] [PubMed] [Google Scholar]
- 75.Saraswathi V., et al. Managing lymphedema, increasing range of motion, and quality of life through yoga therapy among breast cancer survivors: a systematic review. Int. J. Yoga. 2021;14(1):3. doi: 10.4103/ijoy.IJOY_73_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cramer H., et al. Is one yoga style better than another? A systematic review of associations of yoga style and conclusions in randomized yoga trials. Compl. Ther. Med. 2016;25:178–187. doi: 10.1016/j.ctim.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 77.Husebo A.M.L., Husebo T.L. Quality of life and breast cancer: how can mind-body exercise therapies help? An overview study. Sports. 2017;5(4) doi: 10.3390/sports5040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan C.L.W., et al. A systematic review of the effectiveness of qigong exercise in supportive cancer care. Support. Care Cancer. 2012;20(6):1121–1133. doi: 10.1007/s00520-011-1378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng T., et al. Qigong for women with breast cancer: an updated systematic review and meta-analysis. Compl. Ther. Med. 2021:60. doi: 10.1016/j.ctim.2021.102743. [DOI] [PubMed] [Google Scholar]
- 80.Pinto-Carral A., et al. Pilates for women with breast cancer: a systematic review and meta-analysis. Compl. Ther. Med. 2018;41:130–140. doi: 10.1016/j.ctim.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 81.Fatkulina N., et al. Dance/movement therapy as an intervention in breast cancer patients: a systematic review. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/4989282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schutz S., et al. Different methods of physical training applied to women breast cancer survivors: a systematic review. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.639406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Jesus Leite M.A.F., et al. Effects of combined and resistance training on the inflammatory profile in breast cancer survivors: a systematic review. Compl. Ther. Med. 2018;36:73–81. doi: 10.1016/j.ctim.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 84.Lavallée J.F., et al. Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: a qualitative metasynthesis. Psycho Oncol. 2019;28(3):468–476. doi: 10.1002/pon.4980. [DOI] [PubMed] [Google Scholar]
- 85.Baumann F.T., et al. Effects of physical exercise on breast cancer-related secondary lymphedema: a systematic review. Breast Cancer Res. Treat. 2018;170(1):1–13. doi: 10.1007/s10549-018-4725-y. [DOI] [PubMed] [Google Scholar]
- 86.Smith V., et al. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blount D.S., McDonough D.J., Gao Z. Effect of wearable technology-based physical activity interventions on breast cancer survivors' physiological, cognitive, and emotional outcomes: a systematic review. J. Clin. Med. 2021;10(9) doi: 10.3390/jcm10092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dorri S., et al. A Systematic Review of Electronic Health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer. 2020;27(1):25–46. doi: 10.1007/s12282-019-00982-3. [DOI] [PubMed] [Google Scholar]
- 89.Sotirova M.B., et al. Acceptability of online exercise-based interventions after breast cancer surgery: systematic review and narrative synthesis. J Cancer Surviv. 2021;15(2):281–310. doi: 10.1007/s11764-020-00931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.