Abstract
Introduction
hiPSC-VSMCs have been suggested as therapeutic agents for wound healing and revascularization through the secretion of proangiogenic factors. However, methods of increasing cell paracrine secretion and survivability have thus far yielded inconsistent results. This study investigates the effect of pre-conditioning of hiPSC-VSMCs with TNF-α and their integration into 3D collagen scaffolds on cellular viability and secretome.
Methods
hiPSC-VSMCs were dual-plated in a 2D environment. TNF-α was introduced to one plate. Following incubation, cells from each plate were divided and added to type-I collagen scaffolds. TNF-α was introduced to two sets of scaffolds, one from each 2D plate. Following incubation, scaffolds were harvested for their media, tested for cell survivability, cytotoxicity, and imaged. Intra-media VEGF and bFGF levels were evaluated using ELISA testing.
Results
hiPSC-VSMCs exposed to TNF-α during collagen scaffold proliferation and preconditioning showed an increase in cell viability and less cytotoxicity compared to non-exposed cells and solely-preconditioned cells. Significant increases in bFGF expression were found in pre-conditioned cell groups with further increases found in cells subsequently exposed during intra-scaffold conditioning. A significant increase in VEGF expression was found in cell groups exposed during both pre-conditioning and intra-scaffold conditioning. Fibroblasts treated with any conditioned media demonstrated increased migration potential.
Conclusions
Conditioning hiPSC-VSMCs embedded in scaffolds with TNF-α improves cellular viability and increases the secretion of paracrine factors necessary for wound healing mechanisms such as migration.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12195-023-00764-0.
Keywords: Induced pluripotent stem cells, Vascular smooth muscle cells, TNF-α, Biomaterials, Paracrine growth factor
Introduction
Wound healing is a complex, concerted process involving inflammation, epithelialization, angiogenesis, proliferation, and collagen matrix formation [49]. Disruptions to this process may prevent tissue repair and regeneration with devastating health outcomes. As such, there has been much effort to improve our understanding and ability to manipulate the underlying mechanisms of wound healing.
Stem cells, especially human induced pluripotent stem cells (hiPSCs), are of particular importance owing to their regenerative potential. Their therapeutic efficacy is attributable to secreted trophic factors which regulate fibrosis, apoptosis, angiogenesis, and more [5, 29, 34, 41]. The use of hiPSCs derivatives in the treatment of a wide variety of conditions including myocardial infarction, diabetes, stroke, multiple sclerosis, and liver cirrhosis is under current investigation. [6, 7, 11, 26, 44]
Previous studies have shown that transplantation of hiPSC-derived vascular smooth muscle cells (hiPSC-VSMC) improves tissue regeneration and vascularization in wound healing [17, 25, 38]. Though hiPSC-VSMCs exhibit strong therapeutic potential for wound healing, there are still several limitations associated with their use. Following direct transplantation, these cells encounter a hostile environment causing reduced therapeutic efficacy and rapid, widespread cell death [12, 16]. These limitations make the clinical translation more challenging.
Numerous studies including ones from this laboratory have suggested that a component of the observed therapeutic benefits of hiPSC-VSMCs in wound healing is mediated via paracrine signaling pathways as indicated by altered bioactivity upon exposure to conditioned medium [8, 16, 17]. Utilizing strategies to augment the proportions and forms of secreted trophic factors is crucial to enhance the potency of these cells. Preconditioning of stem cells has emerged as another strategy aimed at increasing paracrine secretion and preparing cells for survival in potentially hostile conditions [3, 43, 56, 57, 60]. Many different methods of preconditioning have been explored including through mechanical forces, H2O2, and hypoxia. Pharmacologic agents have been employed as well, including IFN-γ and N-Methyl-D-aspartic acid, and have been shown to promote their therapeutic benefits. In response to pro-inflammatory agents, studies have suggested that hiPSCs increase their release of cytokines and growth factors with strong effects on endothelial cell proliferation, migration, and tubulogenesis [2, 14].
The creation of extracellular matrix-based biomaterials, such as collagen scaffolds, have been used to assist in delivery of hiPSCs and their secretome [10, 52]. These biomaterials mimic the native cellular environment through biomechanical cues that, in turn, enhance the secretion of paracrine factors. In our previous work, we were able to demonstrate that hydrated, type-I collagen-based fibrillar scaffolds secreted more proangiogenic factors, such as VEGF, bFGF, and MMP-2, with increasing collagen density [10]. We have also demonstrated increases in hiPSC-VSMC viability and paracrine secretion after functionalizing collagen scaffolds with fibronectin and hyaluronic acid [8, 12].
Similar to IFN-γ, TNF-α plays a significant role in regulating the immune, inflammatory, and angiogenic response [46, 47]. However, unlike IFN-γ, no study has explored the effects of this cytokine on hiPSC-VSMCs. The aim of this study was to investigate the effects of conditioning hiPSC-VSMCs with TNF-α before and after integration into type-I collagen scaffolds on cellular viability and proangiogenic paracrine secretion. We used a previously established method for creation of our collagen scaffolds and encapsulating hiPSC-VSMCs [8, 10, 12, 17]. Our results demonstrate that conditioning hiPSC-VSMCs embedded in scaffolds with TNF-α improves cellular viability and increases the secretion of paracrine factors necessary for wound healing mechanisms such as migration.
Methods
hiPSC-VSMC Differentiation and Characterization
A pure population of human hiPSC-VSMCs was obtained through a validated, previously established protocol [8, 10, 12, 17]. Following culture in feeder-free conditions for four days, adhered hiPSC colonies were gently digested and collected following dispase exposure for 15 min at 37 °C. Collected colonies were moved to a low attachment plate with mTeSR™1 media (STEMCELL Technologies, Vancouver, Canada) to develop embryoid bodies. The media was changed to a mixture of mTeSR™1 and a customized differentiation media (DMEM high glucose, 10% FBS, 1% non-essential amino acid (v/v), 2 mM L-glutamine, and 0.012 mM 2-mercaptoethanol) at a 1:3 ratio after 24 h. Following 72 h of mixed media exposure, the media was changed to differentiation media only. Embryoid bodies remained for 48 h in this condition and were then transferred to a gelatin-coated plate for further expansion over five days. A final transfer of the cells to a Matrigel-coated dish (Corning Life Sciences, Corning, NY) was performed and cells remained for one week. Matrigel serves as the basal extracellular matrix for further cell growth. Immunofluorescence staining of SM22α and calponin was performed to verify differentiation to hiPSC-VSMCs, and the resulting cells were utilized for further experimentation. A passage of no more than 10 was used for all experiments.
Determination of Optimal TNF-α Dose
200 µL of hiPSC-VSMCs from a single cell line grown in smooth muscle cell media (SmGM-2) (concentration 1 million cells/mL) were plated onto a 6-well, cell culture-treated, flat-bottom microplate. Following a 24-h settling period, TNF-α was differentially diluted and added to each well. The viability of the cells was measured 48 h later using harvested conditioned media and AlamarBlue reagent according to the previously described methods.
2D TNF-α Pre-conditioning and Expansion
200 µL of hiPSC-VSMCs from a single cell line grown in SmGM-2 (concentration 4 million cells/mL) was cultured in gelatin-treated 6-well petri dishes. After a 24-h settling period, plates were treated with either TNF-α (intra-plate concentration 5 ng/mL) or received no treatment. Following a subsequent 48-h incubation period, cells were digested using TrypLE™ (Thermo Fisher Scientific, Waltham, MA and harvested for scaffold production and analysis.
3D TNF-α Intra-scaffold Conditioning and Expansion
Rat tail-derived type-1 collagen scaffolds at an overall density of 4 mg/mL were produced through a previously established protocol [8, 10, 12, 17]. To prevent premature cross-linking and gelation of collagen, the process was performed on ice. In the following order, 400 µL of type-I collagen (concentration 5 mg/mL), 50 µL of MEM, and 8.4 µL of NaOH (concentration 1 mol/L) were added to a 1.5 mL tube. Following gentle manual homogenization and an observed color change from yellow to light purple, 50 µL of cells (concentration 4 million cells/mL) were added to the fluid. Mixtures contained cells from either the 2D TNF-α treatment group or the 2D no treatment group. The fluid was gently mixed to ensure adequate cell dispersion and distributed at a volume of 100 µL per scaffold into a 96-well, flat-bottom microplate. The plate was incubated at 37 °C for a minimum of 30 min to allow for sufficient gelation. Then, 200 µl of SmGM-2 was added on top of the scaffolds.
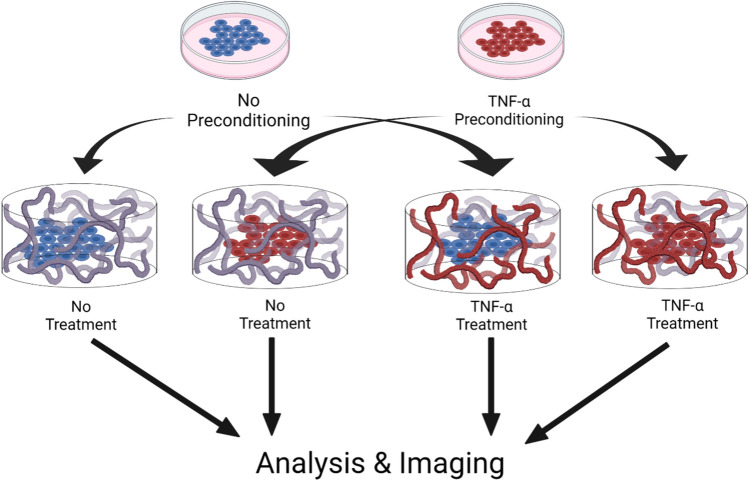
After a 24-h settling period, scaffolds were treated with either TNF-α (intra-well concentration 5 ng/mL) or received no treatment (Fig. 1). This generated four different experimental groups: (1) cells without any TNF-α treatment (negative control), (2) cells pre-conditioned with TNF-α during 2D expansion only (pre-conditioned), (3) cells without TNF-α treatment in 2D culture but received TNF-α treatment during 3D expansion (TNF-α group), and cells treated with TNF-α during 2D and 3D expansion (pre-conditioned TNF-α group). Following a subsequent 72-h incubation period, all scaffolds and conditioned media were collected for future experimental assays.
Fig. 1.
Overview of the experimental process of TNF-α pre-conditioning and treatment regimen for hiPSC-VSMCs in a type-I collagen scaffold. Schematic demonstrating the experimental process (made with BioRender). hiPSC-VSMCs received either no TNF-α pre-conditioning or TNF-α pre-conditioning. Next, the hiPSC-VSMCs were embedded into a collagen scaffold and received either no additional treatments or another treatment with TNF-α. The final 4 groups for further analysis and imaging included no TNF-α pre-conditioning (control), TNF-α pre-conditioning (pre-conditioned), no pre-conditioning and TNF-α treatment (TNF-α), and both TNF-α pre-conditioning and TNF-α treatment (pre-conditioned TNF-α)
Alamar Blue Assay
200 µL of conditioned media was gently aspirated from each scaffold and stored for future experiments. AlamarBlue reagent (ThermoFisher) was thoroughly mixed with SmGM-2 at a dilution of 1:10 and 100 µL was added to each scaffold. The scaffolds were incubated for two hours at 37 °C. After incubation, the plate was analyzed for fluorescence intensity at 540 nm excitation and 590 nm emission wavelengths. Data were normalized relative to negative control measurements per individual round of experimentation.
Lactate Dehydrogenase Assay
The lactate dehydrogenase (LDH) assay was performed according to the manufacturer’s protocol [8, 10, 12, 17]. LDH levels were measured using previously stored conditioned media using the Pierce LDH Cytotoxicity Assay Kit (Thermo Fisher Scientific, Waltham, MA). The LDH levels in each sample were quantified using a plate reader set to a reference wavelength of 680 nm and a measurement at wavelength of 490 nm absorbance for the normalization. Data were further normalized relative to negative control measurements per individual round of experimentation.
Immunofluorescence Staining
Following viability determination, scaffolds were washed with PBS and fixed in 4% paraformaldehyde (PFA) overnight at 4 °C. Scaffolds were then bisected, washed with PBS three times, and blocked with 5% bovine serum albumin (BSA) in PBST (PBS + 0.05% Tween-20) for one hour at room temperature. An overnight, 4 °C incubation with calponin primary antibody (1:200 dilution) followed. The scaffolds were washed with PBST three times and incubated for 1 h at room temperature with secondary antibodies (1:250) tagged with Alexa Fluor 488. Counterstain was then performed with DAPI (1:1000 dilution) for 10 min. Lastly, the scaffolds were washed with PBS an additional three times, mounted on slides, and imaged using a confocal microscope. Images from five different frames of reference were obtained and averaged for each scaffold. Using the DAPI-stained nucleus, the relative level of calponin expression was quantified.
ELISA
Previously collected conditioned media from the scaffolds was used to perform a qualitative ELISA assessing for pro-angiogenic growth factors bFGF, VEGF, TGF-β, ANG-1, IL-10, SDF-1α, and MMP-2. 75 µL of media from each scaffold was placed in a Nunc™ MaxiSorp™ flat-bottom 96 well plate (Thermo Fisher Scientific, Waltham, MA) with replicates and incubated overnight at 4 °C. The plates were washed with PBST three times and blocked using 5% BSA at room temperature for 1 h. The plates were again washed three times with PBST and incubated overnight with primary antibodies (concentration 1:2500 in PBST) at 4 °C. Subsequent steps were conducted in a dark environment. The plates were washed three times with PBST and incubated for 2 h with secondary antibodies (concentration 1:2500 with PBST) at room temperature while avoiding direct lighting. The plates were washed once with PBST, and then 100 µL of TMB substrate solution (Cell Signaling Technology, Danvers, MA) was added. The TMB substrate was incubated on a plate shaker at room temperature for 25 min. Then, 100 µL of 2N HCl stop solution was added to each microwell. Results were measured on a plate reader set to an absorbance of 450 nm. Data were normalized relative to negative control measurements per individual round of experimentation (Supplementary Table 1, Supplementary Table 2).
Migration Assay
For the migration assay, 50,000 primary human dermal fibroblasts were seeded into each well of a 24-well plate. After day 1 of culture, a 1000 µL pipette tip was used to scratch one line down the center of each well. Bright field images of each sample were taken as a pre-migration reference. Next, fibroblasts were treated with conditioned media from our four different experimental groups: DMEM, fibroblast conditioned media, hiPSC-VSMC conditioned media, and conditioned media from hiPSC-VSMC pre-conditioned with TNF-α. The plate was then allowed to incubate for 24 h at 37℃. The following day, bright field images were taken to capture the cell migration of each treatment group. Relative migration was quantified by the absolute increase in cell counts that entered the scratch zone as compared to before incubation with treatment media.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism (Version 9.1.0, San Diego, CA). Significance was set at p < 0.05 for all analyses and was determined using one-way ANOVA and student t-test where appropriate. A varying n of 3–42 was achieved per experiment.
Results
The Optimal Dose of TNF-α Treatment for hiPSC-VSMC is 5 ng/mL
AlamarBlue and LDH cytotoxicity assays were used to determine the ideal dose of TNF-α for cell treatment. Relative cell viability increased significantly with increasing TNF-α dose until 5 ng/mL (Fig. 2A). At 10 ng/mL, the relative cell viability started to significantly decrease. Additionally, none of the tested doses from 0.5 to 10 ng/mL of TNF-α were shown to increase relative cell cytotoxicity more than the baseline of no treatment (Fig. 2B). Thus, we opted to use 5 ng/mL which had improved cell viability and equivalent cell cytotoxicity for further experiments.
Fig. 2.
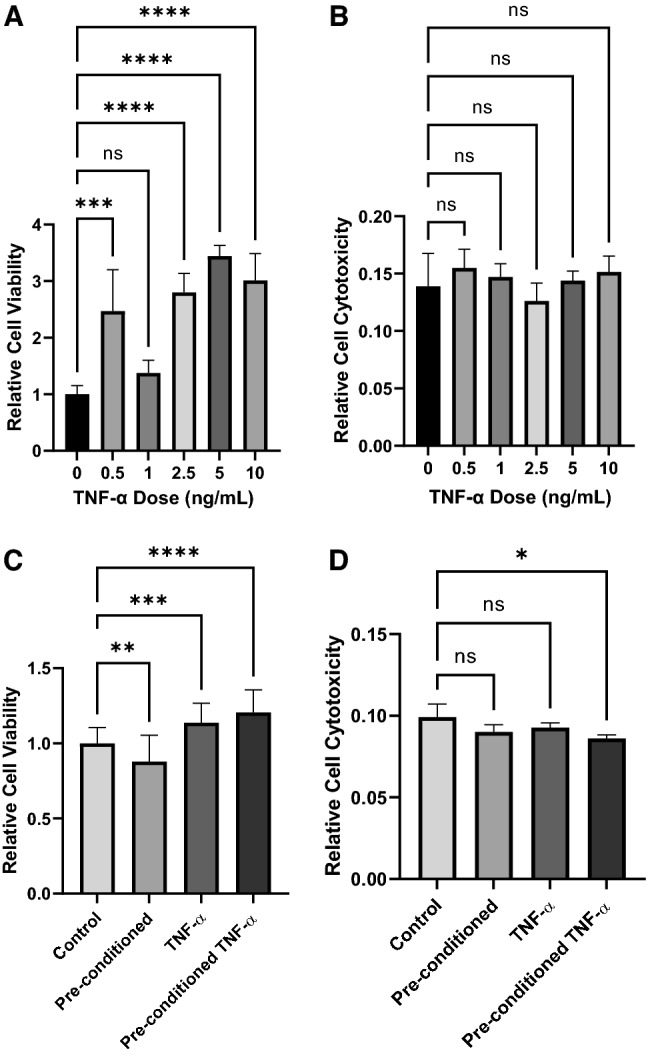
Characterization of the hiPSC-VSMCs for cell viability and cytotoxicity. Treatment with varying doses of TNF-α was assessed (0, 0.5, 1, 2.5, 5, and 10 ng/ml). A AlamarBlue cell viability assay demonstrating the relative viability of varying doses of TNF-α. B LDH assay using conditioned medium of varying treatment doses of TNF-α to demonstrate cellular cytotoxicity. A treatment dose of 5 ng/ml was used for all subsequent experiments. C AlamarBlue cell viability assay demonstrating the relative viability of hiPSC-VSMCs that received no TNF-α pre-conditioning (control), TNF-α pre-conditioning only (pre-conditioned), no pre-conditioning and TNF-α treatment (TNF-α), and both TNF-α pre-conditioning and TNF-α treatment (pre-conditioned TNF-α). D LDH assay using conditioned medium of hiPSC-VSMCs that received no TNF-α pre-conditioning (control), TNF-α pre-conditioning only (pre-conditioned), no pre-conditioning and TNF-α treatment (TNF-α), and both TNF-α pre-conditioning and TNF-α treatment (pre-conditioned TNF-α). *Denotes a statistically significant difference between groups (n = 3–42, one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001)
TNF-α Exposure Increases Cell Viability Without Increasing Cell Cytotoxicity
After the optimal dose of TNF-α was established, the effects of different treatment modalities of TNF-α were assessed. hiPSC-VSMC received either no conditioning, pre-conditioning with TNF-α, TNF-α treatment in the 3D scaffold, or pre-conditioning and 3D scaffold treatment with TNF-α. Relative cell viability, determined via AlamarBlue, significantly increased in groups exposed to any TNF-α (Fig. 2C). Cells that received intra-scaffold treatment with TNF-α had significantly higher viability compared to both 2D culture groups with the TNF-α pre-conditioning and scaffold treatment group having the highest relative cell viability. Complementarily, a decreasing trend in cell cytotoxicity was found with TNF-α exposure (Fig. 2D). The hiPSC-VSMC exposed to TNF-α during both preconditioning and intra-scaffold were found to have significantly less cytotoxicity than all other groups.
TNF-α Exposure Does Not Significantly Alter hiPSC-VSMC Cell Phenotype of Morphology
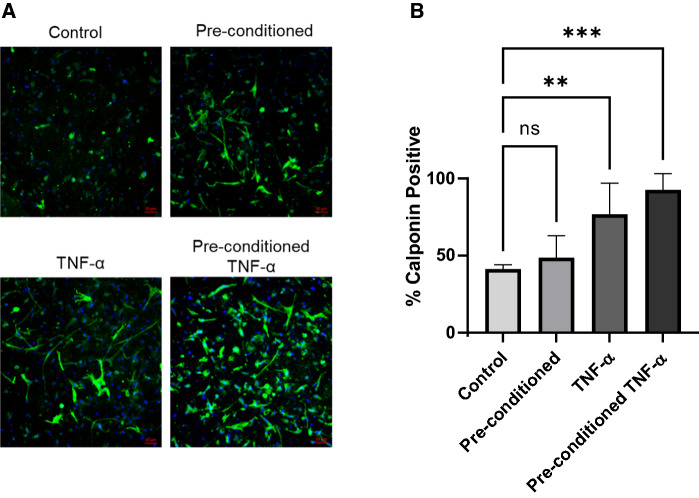
To characterize the phenotype and morphology of hiPSC-VSMC after TNF-α exposure, cells were stained with the smooth muscle cell marker calponin. Representative confocal images revealed abundant staining of all four experimental groups with calponin (Fig. 3A). Analysis of confocal images reveal an enhancement in the level of calponin-positive process formation in scaffolds with greater TNF-α exposure (Fig. 3B). There was no difference in the amount of calponin staining between control and cells pre-conditioned with TNF-α. Cells that received TNF-α treatment only in the 3D scaffolds had significantly more calponin staining than the control and the solely pre-conditioned group. However, cells pre-conditioned with TNF-α and then treated again in the 3D scaffolds had the most abundant staining with calponin.
Fig. 3.
Phenotypic characterization of hiPSC-VSMCs in a collagen scaffold from the four different experimental groups: no TNF-α pre-conditioning (control), TNF-α pre-conditioning only (pre-conditioned), no pre-conditioning and TNF-α treatment (TNF-α), and both TNF-α pre-conditioning and TNF-α treatment (pre-conditioned TNF-α). A Immunofluorescence images of hiPSC-VSMCs after respective treatments, stained with major smooth muscle cell marker calponin in green and nuclei with DAPI in blue. Scale bar measures 50 μm. B Quantification of the number of cells in each experimental group exhibiting positive staining with calponin versus the number of total cells as determined by DAPI staining. *Denotes a statistically significant difference between groups (n = 3–6, one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001)
TNF-α Exposure Increases Paracrine Secretion of bFGF and VEGF
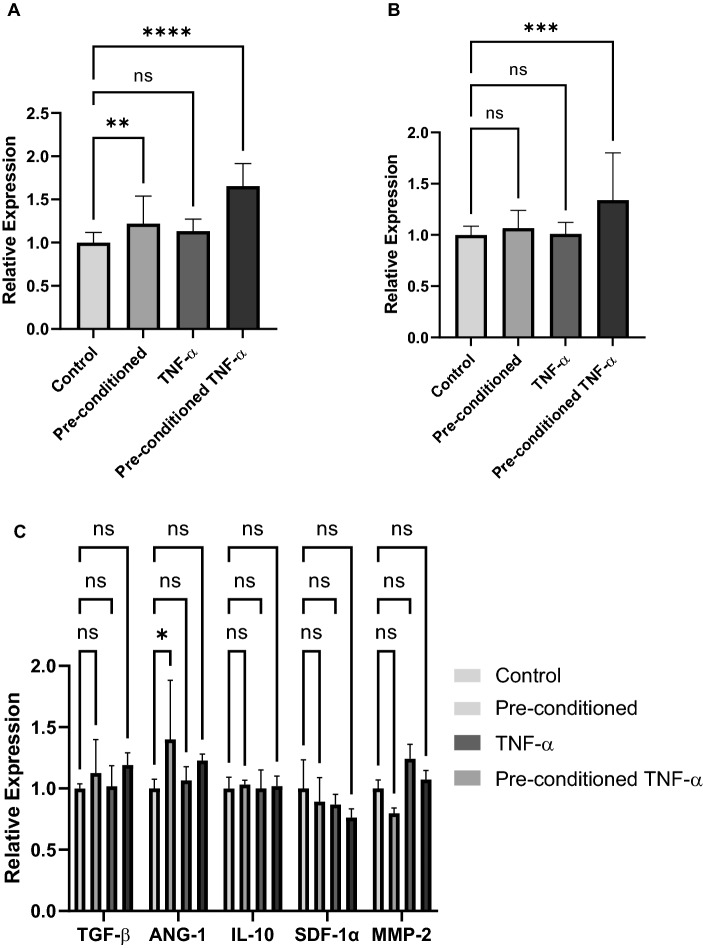
Qualitative ELISA was performed to determine the relative levels of paracrine secretion between the four experimental groups (Fig. 4). Significant increases in relative bFGF expression were found in pre-conditioned cell groups with further increases found in cells subsequently exposed during intra-scaffold conditioning (Fig. 4A). A significant increase in relative VEGF expression was found in cell groups exposed during both pre-conditioning and intra-scaffold conditioning (Fig. 4B). ELISA screening for additional pro-angiogenic growth factors including TGF-β, ANG-1, IL-10, SDF-1α, and MMP-2 did not yield trends suggestive of a true effect from either 2D TNF-α exposure or intra-scaffold TNF-α exposure (Fig. 4C).
Fig. 4.
Characterization of paracrine secretion from hiPSC-VSMCs in a collagen scaffold. ELISA was performed using conditioned medium collected experimental groups: no TNF-α pre-conditioning (control), TNF-α pre-conditioning only (pre-conditioned), no pre-conditioning and TNF-α treatment (TNF-α), and both TNF-α pre-conditioning and TNF-α treatment (pre-conditioned TNF-α). A bFGF, B VEGF C TGF-β, Ang-1, IL-10, SDF-1α, and MMP-2. * denotes a statistically significant difference between groups (n = 3–23, one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001)
Conditioned Media from hiPSC-VSMC Treated with TNF-α Increases Migration of Fibroblasts
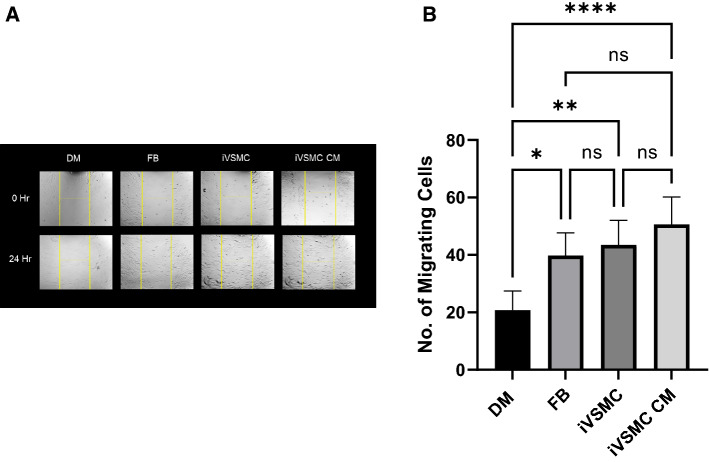
To assess the therapeutic benefits of treatment with TNF-α, a scratch migration assay was conducted. Primary human dermal fibroblasts on a 24-well plate were treated with four different types of media including plain DMEM as a control, fibroblast conditioned media, hiPSC-VSMC conditioned media, and conditioned media from hiPSC-VSMC that had been preconditioned and treated with TNF-α. Migration of fibroblasts was assessed via bright field images (Fig. 5A) and quantified with manual counting (Fig. 5B). Fibroblasts treated with any conditioned media demonstrated increased migration potential when compared to the DMEM group. Although not significant, results trended towards increased migration with the fibroblasts exposed to conditioned media from hiPSC-VSMCs treated with TNF-α.
Fig. 5.
Characterization of conditioned medium from hiPSC-VSMCs pre-conditioned with TNF-α and then treated again with TNF-α in the collagen scaffold (pre-conditioned TNF-α). Human dermal fibroblast cells treated with DMEM (DM), fibroblast conditioned medium (FB), hiPSC-VSMC conditioned medium (iVSMC) without TNF-α treatment, and hiPSC-VSMC pre-conditioned and treated with TNF-α (iVSMC CM). A Bright field images of a migration assay of treatment groups at 0 h and after 24 h. B Quantification of the migration assay after 24 h with images taken from at least 4 different frames of view. * denotes a statistically significant difference between groups (n = 4–11, one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001)
Discussion
hiPSC derivatives have revolutionized the field of tissue engineering and regenerative medicine. To take full advantage of the therapeutic benefits of hiPSCs, cell culture conditions must be optimized and standard protocols maximizing paracrine secretion must be developed. In our previous work, we established that culturing hiPSC-VSMCs in functionalized collagen scaffolds increased cellular viability, secretion of proangiogenic factors, and formation of vascularized networks [8–10, 12, 16, 17]. In vitro preconditioning has been explored to enhance the therapeutic potential of stem cells and includes techniques such as 3D culture, pharmacological compounds, inflammatory cytokines, and hypoxia [4, 19, 21, 33, 39].
There are several suggested benefits to secretome utilization over direct cell transplantation or direct TNF-α exposure. It is unclear what long-term effects, if any, are generated by the reprogramming of pluripotency genes in somatic cells. Poor engraftment and risks of immunogenicity and tumorigenicity are present [1, 22, 51]. Additionally, the direct application of live, human gene-containing cellular material in a clinical environment can generate both logistic and legal difficulties. Cell-free methods allow for timely harvesting of the paracrine factors likely responsible for the majority of published therapeutic benefits while avoiding significant unknowns [8, 16, 17].
TNF-α is a major inflammatory cytokine. Under normal physiologic conditions, TNF-α paradoxically plays a role in both the innate inflammatory process and tissue repair [15, 24, 28, 36, 37]. After injury, the inflammatory phase of wound healing begins and macrophages, monocytes, and mast cells produce TNF-α [36, 45]. It works to stimulate endothelial cell activation and cell adhesion molecule expression [50]. Other molecular events occur after TNF-α stimulates stem cells to produce proangiogenic cytokines such as IL-6 and IL-8 which further work to activate neutrophils and fibroblasts [28, 32, 48, 50]. When tissue is injured, TNF-α release can increase and persist for days [13]. Chronic disease ensues when this proinflammatory phase fails to resolve causing further damage as opposed to priming the wound bed for repair [28]. However, natural cellular reaction to the inflammatory phase can be utilized. By recreating a proinflammatory environment using TNF-α treatment, hiPSC-VSMC can be stimulated to secrete growth factors and cytokines necessary for healing. Increasing the levels of TNF-α treatment could potentially increase the secretion of these factors and in turn accelerate the body’s physiological mechanisms of wound healing. Given the pro-inflammatory profile of TNF-α, there is a theoretical concern that direct impregnation of the cytokine into a wound environment may have non-therapeutic or pathologic effects such as inflammation induced hyperplasia and fibrosis. Though this is a potential limitation of its use, the benefits to wound healing produced by TNF-α at an appropriate dosage cannot be ignored.
Aside from healing, TNF-α has been shown to play a role in osteogenic differentiation, neural cell growth, recovery after myocardial ischemia, and bone repair [13, 15, 23, 27]. Gerstenfeld et al. found that TNF-α is important to attenuating the inflammatory response after injury and in their model, mice deficient of TNF-α receptors experienced delayed bone healing [15]. Another mouse knockout study conducted by Goukassian et al. demonstrated that knocking out TNF-α resulted in decreased neovascularization and hindlimb ischemia [18]. These results are compelling evidence of the ability of TNF-α to promote healing. Other studies have shown adipose-derived MSCs that were treated with TNF-α secreted increased cytokines, extracellular matrix, proteases, and protease inhibitors [35]. Therefore, we attempted to increase hiPSC-VSMC paracrine secretion in the context of wound healing also using a TNF-α conditioning regimen.
Here, we describe the effects of preconditioning with TNF-α on the viability and paracrine secretion of hiPSC-VSMCs captured in type-I collagen scaffolds. The natural mechanisms of healing can potentially be harnessed through conditioning stem cells and then using their secretome to assist in wound healing. We postulated that conditioning hiPSC-VSMCs with TNF-α could stimulate cells to release increased levels of proangiogenic cytokines in response. The effects of TNF-α have been found to be highly variable and dose-dependent between cell types [42, 45]. After testing the effects of TNF-α dose on hiPSC-VSMCs, we elected to use 5 ng/ml as it proved to have the best cellular viability with no risk of added cytotoxicity. Additionally, pre- and post-conditioning with TNF-α proved to increase the abundance of calponin expression from hiPSC-VSMC, as did encapsulation of the cells in the collagen scaffold. Taken together, these results indicate that the hiPSC-VSMC molecular identity is affected by the experimental design.
When the paracrine secretion profile of hiPSC-VSMCs after TNF-α conditioning was examined, increased levels of VEGF and bFGF were found within the secretomes of conditioned cells. TNF-α stimulates release of numerous cytokines including VEGF, hepatocyte growth factor, insulin-like growth factor-1, and TSG6 [53, 55]. VEGF and bFGF specifically contribute to angiogenesis, proliferation, migration, and extracellular matrix reconstruction [10, 54]. Our data showed that the levels of VEGF and bFGF increased with greater exposure to TNF-α indicating that conditioning results in higher levels of cytokines beneficial to wound healing.
Conditioned media from hiPSC-VSMC increased migration of fibroblasts in all experimental groups with a trend favoring the most migration in groups exposed to TNF-α. Existing literature has demonstrated cell migration’s importance in successful wound healing [30, 59]. TNF-α’s effect on cell migration has been experimentally demonstrated to work via chemokine and matrix metalloproteinase expression, specifically IL-6 and IL-8 which promote blood perfusion and regeneration of injured tissues [20, 31, 32, 40, 42, 50]. Other studies have proven that TNF-α conditioned media can stimulate migration of endothelial cells and subsequent re-epithelialization, proliferation, and angiogenesis [20, 58]. With increasing TNF-α exposure, the factors responsible for migration can also be expected to increase.
Conclusions
Our work has demonstrated that conditioning hiPSC-VSMCs with TNF-α can augment cellular viability and increase production of extracellular factors essential to stimulating wound healing. A conditioning regimen can be developed and applied to stimulate cells to produce a specific paracrine profile which can then be used for other applications. Future studies should focus on a multi-step approach for regulating paracrine secretion including hypoxia and biomechanical cues.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 6 kb). Supplementary Table 1: Information related to primary antibodies.
Supplementary file2 (DOCX 6 kb). Supplementary Table 2: Information related to secondary antibodies.
Acknowledgements
The authors would like to thank the research facility faculty at the Yale Department of Surgery.
Author Contributions
BCD and HCH conceived the study and procured funding. DCS, KD, BCD and HCH designed the experiments. BCD, DCS, and KD. performed the experiments. SI and DCS wrote the manuscript. All the authors participated in data analysis, discussed the results, and reviewed the manuscript.
Funding
This work was funded by the Plastic Surgery Foundation Grant 21-003388 (BCD) and 18-003032 (HCH and BCD).
Declarations
Conflict of interest
The authors have no financial interest to disclose in relation to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniel C. Sasson and Sara Islam have contributed equally to this work.
References
- 1.Abad M, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502:340–345. doi: 10.1038/nature12586. [DOI] [PubMed] [Google Scholar]
- 2.Adamiak M, et al. Induced pluripotent stem cell (iPSC)-Derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 2018;122:296–309. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauersachs HG, et al. N-methyl-d-aspartate receptor-mediated preconditioning mitigates excitotoxicity in human induced pluripotent stem cell-derived brain organoids. Neuroscience. 2022;484:83–97. doi: 10.1016/j.neuroscience.2021.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Berniakovich I, Giorgio M. Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int. J. Mol. Sci. 2013;14:2119–2134. doi: 10.3390/ijms14012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braund R, Hook S, Medlicott NJ. The role of topical growth factors in chronic wounds. Curr. Drug Deliv. 2007;4:195–204. doi: 10.2174/156720107781023857. [DOI] [PubMed] [Google Scholar]
- 6.Chau MJ, Deveau TC, Song M, Gu X, Chen D, Wei L. iPSC transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells. 2014;32:3075–3087. doi: 10.1002/stem.1802. [DOI] [PubMed] [Google Scholar]
- 7.Chun YS, Chaudhari P, Jang Y-Y. Applications of patient-specific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int. J. Biol. Sci. 2010;6:796–805. doi: 10.7150/ijbs.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dash BC, Duan K, Xing H, Kyriakides TR, Hsia HC. An in situ collagen-HA hydrogel system promotes survival and preserves the proangiogenic secretion of hiPSC-derived vascular smooth muscle cells. Biotechnol. Bioeng. 2020;117:3912–3923. doi: 10.1002/bit.27530. [DOI] [PubMed] [Google Scholar]
- 9.Dash BC, et al. Tissue-engineered vascular rings from human iPSC-Derived smooth muscle cells. Stem Cell Rep. 2016;7:19–28. doi: 10.1016/j.stemcr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dash BC, et al. A dense fibrillar collagen scaffold differentially modulates secretory function of iPSC-derived vascular smooth muscle cells to promote wound healing. Cells. 2020;9:966. doi: 10.3390/cells9040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douvaras P, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 2014;3:250–259. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan, K., B.C. Dash, D.C. Sasson, S. Islam, J. Parker, and H.C. Hsia. Human iPSC-derived vascular smooth muscle cells in a fibronectin functionalized collagen hydrogel augment endothelial cell morphogenesis. Bioengineering (Basel) 8, 2021. [DOI] [PMC free article] [PubMed]
- 13.Egea V, et al. TNF-α respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell Death Differ. 2011;18:853–863. doi: 10.1038/cdd.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Harane N, et al. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur. Heart J. 2018;39:1835–1847. doi: 10.1093/eurheartj/ehy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerstenfeld LC, et al. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs (Print) 2001;169:285–294. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- 16.Gorecka J, et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019;10:87. doi: 10.1186/s13287-019-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorecka J, et al. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen. Med. 2020;15:1277–1293. doi: 10.2217/rme-2019-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goukassian DA, et al. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins KE, Sharp TV, McKay TR. The role of hypoxia in stem cell potency and differentiation. Regen. Med. 2013;8:771–782. doi: 10.2217/rme.13.71. [DOI] [PubMed] [Google Scholar]
- 20.Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J. Invest. Dermatol. 2011;131:1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 22.Hogan BLM, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, et al. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44:420–427. doi: 10.1111/j.1365-2184.2011.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang B, Liao R. The paradoxical role of inflammation in cardiac repair and regeneration. J. Cardiovasc. Transl. Res. 2010;3:410–416. doi: 10.1007/s12265-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim KL, Song S-H, Choi K-S, Suh W. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng. A. 2013;19:2478–2485. doi: 10.1089/ten.tea.2012.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MJ, Lee EY, You Y-H, Yang HK, Yoon K-H, Kim J-W. Generation of iPSC-derived insulin-producing cells from patients with type 1 and type 2 diabetes compared with healthy control. Stem Cell Res. 2020;48:101958. doi: 10.1016/j.scr.2020.101958. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, et al. TNF-alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front. Biosci. (Landmark Ed) 2009;14:2845–2856. doi: 10.2741/3417. [DOI] [PubMed] [Google Scholar]
- 28.Kizil C, Kyritsis N, Brand M. Effects of inflammation on stem cells: together they strive? EMBO Rep. 2015;16:416–426. doi: 10.15252/embr.201439702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi Y, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krawczyk WS. A pattern of epidermal cell migration during wound healing. J. Cell Biol. 1971;49:247–263. doi: 10.1083/jcb.49.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon MY, Wang C, Galarraga JH, Puré E, Han L, Burdick JA. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials. 2019;222:119451. doi: 10.1016/j.biomaterials.2019.119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon YW, et al. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim. Biophys. Acta. 1832;2136–2144:2013. doi: 10.1016/j.bbadis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Lan Y-W, et al. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res. Ther. 2015;6:97. doi: 10.1186/s13287-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laterza C, et al. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat. Commun. 2013;4:2597. doi: 10.1038/ncomms3597. [DOI] [PubMed] [Google Scholar]
- 35.Lee MJ, et al. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res. 2010;9:1754–1762. doi: 10.1021/pr900898n. [DOI] [PubMed] [Google Scholar]
- 36.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/S0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 37.Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng. A. 2017;23:1212–1220. doi: 10.1089/ten.tea.2016.0548. [DOI] [PubMed] [Google Scholar]
- 38.Park JJ, et al. Coadministration of endothelial and smooth muscle cells derived from human induced pluripotent stem cells as a therapy for critical limb ischemia. Stem Cells Transl. Med. 2021;10:414–426. doi: 10.1002/sctm.20-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc. Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 40.Ponte AL, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 41.Rettinger CL, Kaini RR, Burke TA, Wang H-C. Neurotrophic factors secreted by induced pluripotent stem cell-derived retinal progenitors promote retinal survival and preservation in an adult porcine neuroretina model. J. Ocul. Pharmacol. Ther. 2021;37:301–312. doi: 10.1089/jop.2020.0088. [DOI] [PubMed] [Google Scholar]
- 42.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 43.Rogers AJ, Fast VG, Sethu P. Biomimetic cardiac tissue model enables the adaption of human induced pluripotent stem cell cardiomyocytes to physiological hemodynamic loads. Anal. Chem. 2016;88:9862–9868. doi: 10.1021/acs.analchem.6b03105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas SV, et al. Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction. PLoS ONE. 2017;12:e0173222. doi: 10.1371/journal.pone.0173222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saika S. Yin and yang in cytokine regulation of corneal wound healing: roles of TNF-alpha. Cornea. 2007;26:S70–S74. doi: 10.1097/ICO.0b013e31812f6d14. [DOI] [PubMed] [Google Scholar]
- 46.Saraf A, et al. Functional and molecular effects of TNF-α on human iPSC-derived cardiomyocytes. Stem Cell Res. 2021;52:102218. doi: 10.1016/j.scr.2021.102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sethi JK, Hotamisligil GS. Metabolic messengers: tumour necrosis factor. Nat. Metab. 2021;3:1302–1312. doi: 10.1038/s42255-021-00470-z. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer AJ, Clark RA. Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 50.Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE. Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiology. 1994;62:134–139. doi: 10.1159/000163891. [DOI] [PubMed] [Google Scholar]
- 51.Tan Y, Ooi S, Wang L. Immunogenicity and tumorigenicity of pluripotent stem cells and their derivatives: genetic and epigenetic perspectives. Curr. Stem Cell Res. Ther. 2014;9:63–72. doi: 10.2174/1574888X113086660068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas D, et al. Temporal changes guided by mesenchymal stem cells on a 3D microgel platform enhance angiogenesis in vivo at a low-cell dose. Proc. Natl. Acad. Sci. USA. 2020;117:19033–19044. doi: 10.1073/pnas.2008245117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torihashi S, et al. Acute and temporal expression of tumor necrosis factor (TNF)-α-stimulated gene 6 product, TSG6, in mesenchymal stem cells creates microenvironments required for their successful transplantation into muscle tissue. J. Biol. Chem. 2015;290:22771–22781. doi: 10.1074/jbc.M114.629774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Touani FK, et al. Pharmacological preconditioning improves the viability and proangiogenic paracrine function of hydrogel-encapsulated mesenchymal stromal cells. Stem Cells Int. 2021;2021:6663467. doi: 10.1155/2021/6663467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R880–R884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Umrath F, Cen W, Salgado AJ, Reinert S, Alexander D. Pre-conditioning with IFN-γ and hypoxia enhances the angiogenic potential of iPSC-derived MSC secretome. Cells. 2022;11:988. doi: 10.3390/cells11060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei ZZ, et al. Intracranial transplantation of hypoxia-preconditioned iPSC-derived neural progenitor cells alleviates neuropsychiatric defects after traumatic brain injury in juvenile rats. Cell Transplant. 2016;25:797–809. doi: 10.3727/096368916X690403. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 59.Yue PYK, Leung EPY, Mak NK, Wong RNS. A simplified method for quantifying cell migration/wound healing in 96-well plates. J. Biomol. Screen. 2010;15:427–433. doi: 10.1177/1087057110361772. [DOI] [PubMed] [Google Scholar]
- 60.Zhao L, Hu C, Zhang P, Jiang H, Chen J. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. J. Cell. Mol. Med. 2019;23:720–730. doi: 10.1111/jcmm.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (DOCX 6 kb). Supplementary Table 1: Information related to primary antibodies.
Supplementary file2 (DOCX 6 kb). Supplementary Table 2: Information related to secondary antibodies.