Abstract
In wheat, TaMYC8 is a negative regulator of cadmium (Cd)-responsive ethylene signaling. In this study, we functionally characterized TabHLH094, a basic helix–loop–helix (bHLH) transcription factor (TF) that inhibits the transcriptional activity of TaMYC8. The TabHLH094 protein was found in the nucleus of tobacco epidermal cells and exhibited transcriptional activation activity. Real-time quantitative PCR (RT–qPCR) indicated that TabHLH094 exhibited root-specific, Cd-responsive expression in wheat seedlings. Overexpression of TabHLH094 enhanced the tolerance of wheat seedlings to Cd exposure. The protein–protein interaction between TabHLH094 and TaMYC8 was verified by glutathione S-transferase (GST) pulldown, coimmunoprecipitation (Co–IP), yeast two-hybrid (Y2H), and bimolecular fluorescence complementation (BiFC) analyses. TabHLH094 was found to reduce the ability of TaMYC8 to bind to the TaERF6 promoter. Furthermore, TabHLH094 could also reduce aminocyclopropanecarboxylate oxidase (ACO) and ACC synthase (ACS) activities, both of which are necessary for ethylene biosynthesis. Taken together, these results indicate that TabHLH094 mediates Cd tolerance by regulating the transcriptional activity of TaMYC8 and decreasing ethylene production.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-023-01404-1.
Keywords: Wheat, TabHLH094, TaMYC8, Complex, Cadmium
Introduction
One highly significant threat to global soil quality is heavy metal contamination. Although heavy metals are present naturally in many soils, human activities are responsible for increasing their concentration in many contexts (Ma et al. 2020a). In particular, industrial activities, fossil fuel combustion, inadequate sewage sludge treatment, and the overuse of chemical fertilizers and pesticides have been linked to increased heavy metal contamination (Leškovï et al. 2020). Because plants are capable of bioaccumulating heavy metals, these pollutants can enter the food web and endanger both animal and human health (Zhao et al. 2018).
Exposure to the toxic and nonessential heavy metal cadmium (Cd) is severely harmful to living cells (Brunetti et al. 2015), even at miniscule concentrations (Elobeid et al. 2012). In plants, Cd exposure can lead to destruction of the photosynthetic system, a reduction in leaf photosynthetic capacity, inhibition of root elongation, and even death (Ma et al. 2020b). Because Cd contamination of agricultural soils poses such a high risk to plants, developing Cd stress-resistant crop cultivars is critical for food safety and security.
In plants, the extensive basic helix–loop–helix (bHLH) transcription factor (TF) superfamily regulates an array of physiological operations (Mao et al. 2017), including the physiological and biochemical responses to environmental stress exposure (Xu et al. 2017). For instance, the transgenic expression of maize-derived ZmbHLH124 results in significantly increased tolerance to water scarcity (Wei et al. 2021). Salt-inducible OsbHLH035 is expressed primarily in germinated rice seeds and seedlings (Chen et al. 2018). In Arabidopsis, AtbHLH39 overexpression confers enhanced tolerance to Cd exposure (Wu et al. 2012). Recently, we found that the wheat-derived bHLH-type TF TaMYC8 negatively regulates the Cd stress response (Wang et al. 2022a, b).
Mounting evidence suggests that interactions between proteins may be crucial for the regulation of gene expression. Specifically, many TFs interact with other TFs to form homo- and heterodimeric complexes. For example, jasmonic acid-mediated anthocyanin accumulation is regulated by the WD–Repeat/bHLH/MYB complex in Arabidopsis (Qi et al. 2011). In Brassica napus, resistance to Sclerotinia infection is regulated by the interaction between BnWRKY15 and BnWRKY33 (Liu et al. 2018). In Medicago truncatula, nitrogenase activity is maintained during drought stress by the interaction between MtCAS31 and MtLb120–1 (Li et al. 2018).
Here, we sought to functionally characterize the wheat-derived bHLH TF TabHLH094 in regulating the uptake of and tolerance to Cd. We found that overexpression of TabHLH094 resulted in decreased root-to-shoot translocation of Cd. Additionally, we found that TabHLH094 interacts with the bHLH-type TF TaMYC8, thereby inhibiting the ability of this TF to interact with the promoter of TaERF6. The resultant induction of TaERF6 expression affected ethylene biosynthesis and conferred tolerance to Cd exposure in wheat. The results of this study help clarify the mechanism by which bHLH TFs regulate the wheat Cd stress response.
Materials and methods
Plant materials
We germinated seeds of “Fielder” wheat (Triticum aestivum L.) on sterile filter paper. After 5 days, seedlings exhibiting uniform growth were transplanted into sand. Seedlings were watered daily with half-strength Hoagland medium. Three-leaf-stage wheat seedlings were watered with Hoagland medium mixed with either 0 (control) or 2 mM (experimental) CdCl2 for either 3 h or 5 days, depending on the experiment.
Soil pot experiments were carried out at the greenhouse of Guizhou Normal University, Guiyang, China. The potting soil consisted of clay and humus and was prepared at a ratio of 1:1. The treatment group was supplemented with Cd at a dose of 5 mg/kg. Plant seeds were first sterilized and sown in ddH2O to germinate and then transferred to soil. After complete maturation, the seeds were harvested for further analysis.
TabHLH094 expression
Total RNA was sampled from both the aerial and root tissues of wheat seedlings and reverse‐transcribed into cDNA using kits purchased from CwBio (Beijing, China). Taβ–actin was used as the internal reference. Real-time quantitative PCR (RT–qPCR) was accomplished utilizing SYBR Green Super Mix (CwBio, Beijing, China) with a QuantStudio 3 RT–PCR System (Thermo Fisher Scientific, MA, USA).
Determination of subcellular localization
The TabHLH094 coding DNA sequence (CDS) was inserted into the pBI121–GFP vector after digestion with EcoR V. Subsequently, the 35S::TabHLH094–GFP construct was introduced into Agrobacterium tumefaciens “GV3101,” and these cells were used to transform tobacco epidermal cells. Detection of TabHLH094–GFP fluorescence was performed using an FV500 confocal laser-scanning microscope (Olympus, Tokyo, Japan).
Transcriptional activation assay
The TabHLH094 CDS was digested with EcoR V and BamH I and integrated into the pGBKT7 vector. The recombinant construct was then introduced into Saccharomyces cerevisiae “AH109.” The method of Song et al. (2020) was used for all transcriptional activation assays.
Vector construction and genetic transformation
To produce a plasmid for TabHLH094 overexpression lines, the TabHLH094 CDS was cloned into the pCambia1300 vector (Supplementary Fig. S1). Immature “Fielder” wheat embryos were transformed through Agrobacterium-mediated gene transfer (Jin et al. 2021). Three independent T2 generation TabHLH094 overexpression lines were selected for further analysis.
Cd content analysis
Leadmium Green AM Dye (Thermo Fisher Scientific, MA, USA) was utilized to visualize Cd accumulation in root tissues. After exposing WT and transgenic wheat lines to 2 mM Cd for 5 days, the roots were washed with ddH2O and subsequently soaked in Leadmium Green AM Dye (5 μL/mL) for 6 h. Fluorescence (480 nm) was detected with an FV500 confocal laser-scanning microscope (Olympus, Tokyo, Japan). Fluorescence intensity was quantified by measuring fluorescence pixel intensity with ImageJ software (Liu et al. 2018). To quantify the Cd concentration in root tissues, WT and transgenic wheat seedlings were exposed to 2 mM Cd for 5 days, after which the roots were collected and rinsed with ddH2O and subsequently dried at 60 °C. The content of Cd in wheat tissues was quantified using inductively coupled plasma–mass spectrometry (ICP–MS) (Thermo Fisher Scientific, MA, USA).
Electrophoretic mobility shift assay (EMSA)
Utilizing our previously published method (Wang et al. 2022a, b), the 5′–GGCAAACTCACGTTCGTCCATTGCA–3′ sequence was labeled at the 3′ end with biotin as the hot probe. The TaMYC8 and TabHLH094 CDSs were cloned into the pET32a vector to produce the TaMYC8–His and TabHLH094–His fusion proteins, respectively. LightShift Chemiluminescent EMSA Kits (Thermo Fisher Scientific, MA, USA) were utilized for all EMSA experiments.
Yeast two-hybrid (Y2H) experiment
The Y2H experiment was performed utilizing the Matchmaker Gold Y2H System (Clontech, CA, USA) using the manufacturer’s standard directions. Briefly, the TabHLH094 and TaMYC8 CDSs were cloned into the pGBKT7 and pGADT7 vectors, respectively. The recombinant constructs were then cotransformed into Saccharomyces cerevisiae “AH109.”
Coimmunoprecipitation (Co–IP) experiment
The TabHLH094 and TaMYC8 open reading frames (ORFs) were inserted into the pTCK303–Flag and pCAMBIA2300–MYC binary vectors, respectively. To study the interaction between TabHLH094 and TaMYC8, both 35S::TabHLH094–Flag and 35S::TaMYC8–MYC were transiently coexpressed in tobacco leaves. Anti‐Flag affinity gel (Abmart, Shanghai, China) was used to precipitate Flag‐fused protein. Anti-MYC and anti-Flag antibodies (Abmart, Shanghai, China) were utilized for immunoblotting.
GST pulldown experiment
To study the interaction between TabHLH094 and TaMYC8, the TabHLH094–His and TaMYC8–GST fusion proteins were expressed in pET32a–TabHLH094 or pGEX–4 T–1–TaMYC8 construct-carrying Escherichia coli “BL21(DE3).” Purification of fusion proteins was performed with either a GST–Tag Protein Purification Kit (Beyotime, Shanghai, China) or a His–Tagged Protein Purification Kit (CwBio, Beijing, China). The pulldown assay was then performed using a GST pulldown kit (FitGene, Guangzhou, China).
Bimolecular fluorescence complementation (BiFC)
The TabHLH094 and TaMYC8 ORFs were inserted into the EcoR V-digested pXY106 vector and the BamH I-digested pXY104 vector to construct the TabHLH094–nYFP and TaMYC8–cYFP fusion proteins, respectively. The plasmid combinations were infiltrated into tobacco leaves using A. tumefaciens. Yellow fluorescent protein (YFP) in tobacco leaves was visualized with an FV500 confocal laser-scanning microscope (Olympus, Tokyo, Japan).
Luciferase complementation imaging (LCI) experiment
The reporter construct was produced by inserting the TaERF6 promoter into pGreenII 0800–LUC. The effectors were constructed by inserting the ORFs of TabHLH094 and TaMYC8 into pGreenII 62–SK. Subsequently, four A. tumefaciens combinations were infiltrated into four distinct portions of the same individual tobacco leaves. After culturing the tobacco leaves for 36 h, each leaf was treated with 100 mM luciferin and kept for 5 min in darkness prior to luminescence detection. A NightOWL II LB 983 low-light cooled CCD imaging apparatus (Berthold Technologies, Bad Wildbad, Germany) was utilized to measure luciferase activity.
Aminocyclopropanecarboxylate oxidase (ACO) and ACC synthase (ACS) activities
To each sample of plant material (1 g), 10 μM pyrrolyl phosphate (PLP), 3% crosslinked polyvinylpyrrolidone (PVPP), 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 M potassium phosphate buffer (pH 8.0), 1 mM acetylphenylenediamine tetraacetic acid (EDTA), and 4 mM dithiothreitol (DTT) were added. The mixture was centrifuged at 12,000 g and 4 °C for 15 min. A 500 µL sample of the supernatant was incubated in 1.5 mL of a mixture containing 10 µM PLP, 0.1 M potassium phosphate buffer (pH 8.0), and 250 µM s–adenosylmethionine (SAM). Incubation of the reaction mixture was carried out at 30 °C for 1 h, after which we added 700 μL of 0.1% HgCl2 to arrest the reaction. The arrested reaction mixture was subsequently incubated for 10 min at 4 °C, after which 200 μL of a precooled NaOH solution saturated with 5% NaClO (NaClO:NaOH = 2:1, v/v) was added. The method of Zhang et al. (2018) was utilized for the measurement of ACO and ACS enzymatic activities.
Statistical analyses
All of the assays were performed under controlled environmental conditions utilizing three biological replicates. Statistically significant differences among treatments were analyzed with either Student’s t-tests or one-way analysis of variance (ANOVA).
Primers
Each primer utilized in the present work can be found in Supplementary Table S1.
Results
TabHLH094 is positively regulated by Cd exposure
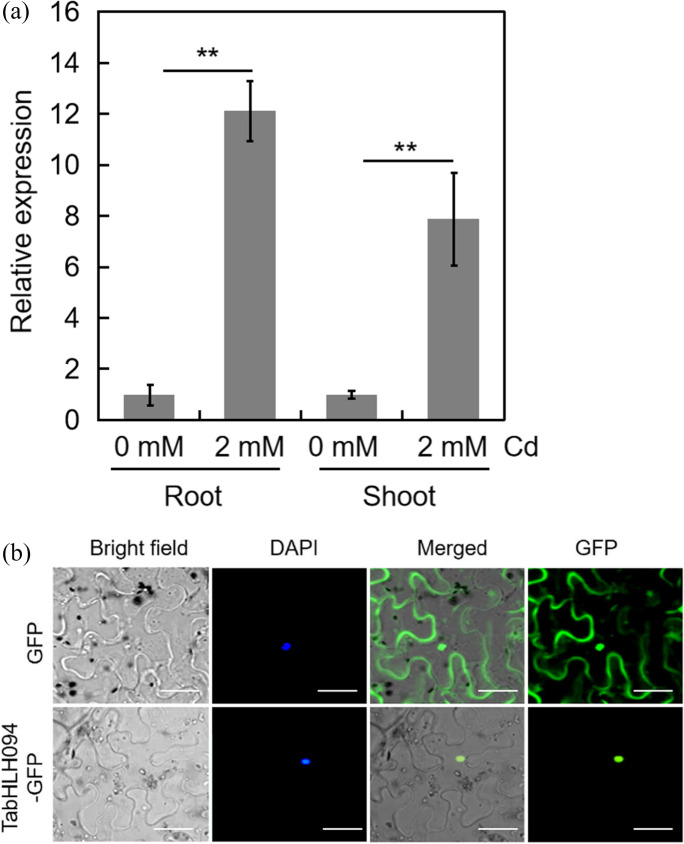
To determine the Cd responsiveness of TabHLH094, wheat seedlings were exposed to Cd, and the transcript level of TabHLH094 was analyzed and compared to that of untreated (control) seedlings. Exposure to Cd resulted in a significant upregulation of TabHLH094 expression in both roots and shoots (Fig. 1a), indicating that TabHLH094 is involved in the reaction of wheat seedlings to Cd exposure.
Fig. 1.
Expression of TabHLH094 and subcellular localization of TabHLH094. a TabHLH094 expression in Cd-treated wheat, as determined by RT‒qPCR. Each value is the mean ± SE (n = 3). Significant differences were analyzed by Student’s t-test. **P < 0.01. b Subcellular localization of TabHLH094:GFP in tobacco epidermal cells. Scale bars = 50 μm
TabHLH094 is located in the nucleus
The subcellular localization of the TabHLH094 protein was examined in tobacco epidermal cells. TabHLH094 is located in the nucleus (Fig. 1b), which is consistent with its TF functionality.
TabHLH094 overexpression increased tolerance to Cd exposure
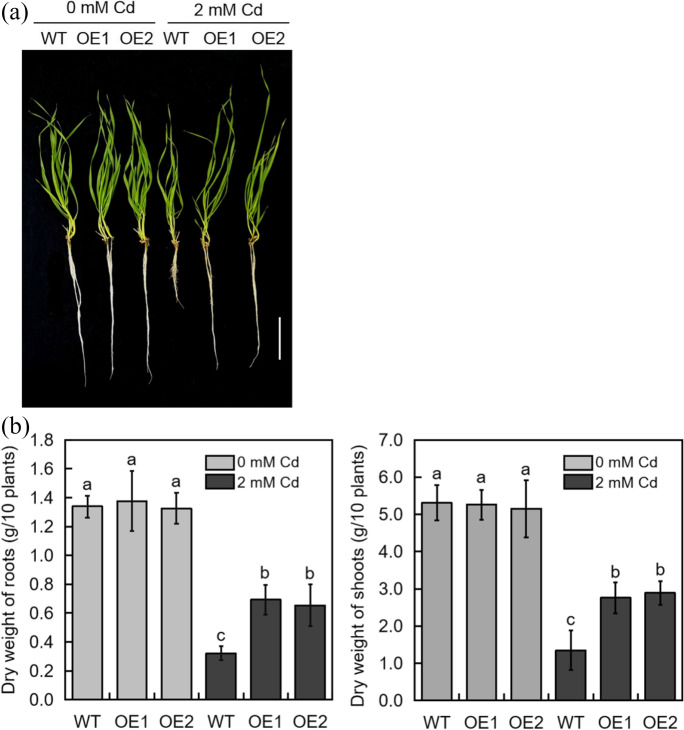
To functionally characterize the TabHLH094 gene, transgenic TabHLH094-overexpressing wheat plants were generated. Five independent transgenic lines were obtained and identified through PCR (Supplementary Fig. S2). RT–qPCR was further used to determine the expression of TabHLH094 in different transgenic lines, and two lines with the highest expression (OE1 and OE2, Supplementary Fig. S3) were selected for the follow-up experiment. Both WT and transgenic wheat exhibited similar phenotypes under control conditions. However, under Cd treatment, WT wheat exhibited significantly weaker growth and shorter roots than transgenic wheat (Fig. 2a). Furthermore, under Cd conditions, the root and shoot dry weights of transgenic wheat were significantly higher than those of WT (Fig. 2b). Our results suggest that TabHLH094 overexpression enhances plant tolerance to Cd exposure.
Fig. 2.
TabHLH094 positively regulates Cd tolerance in wheat. a Phenotypes of Cd-treated WT and transgenic wheat. Three-leaf-stage wheat seedlings were watered with Hoagland medium mixed with 2 mM (experimental) CdCl2 for 5 days. Scale bar = 5 cm. b Dry weight of control and Cd-treated WT and transgenic wheat. Each value is the mean ± SE (n = 3). Different lowercase letters signify significant (P < 0.05) differences
Overexpression of TabHLH094 inhibits uptake of Cd
To visualize Cd accumulation in root tissue, wheat seedlings were exposed for 5 days to 2 mM Cd, after which the roots were soaked in Leadmium Green AM Dye for 6 h. Overall, WT wheat accumulated significantly more Cd in root tissues than transgenic wheat (Fig. 3a, b). ICP–MS was then used to quantify the content of Cd in the roots. Consistent with the fluorescence results, both the roots and shoots of WT wheat were found to contain a higher concentration of Cd than transgenic wheat (Fig. 3c). Furthermore, the average transfer coefficients of Cd in the WT and transgenic lines were 0.86 and 0.54, respectively. Our results suggest that TabHLH094 overexpression reduces the uptake of Cd into root tissues and reduces the transport of Cd to shoots.
Fig. 3.
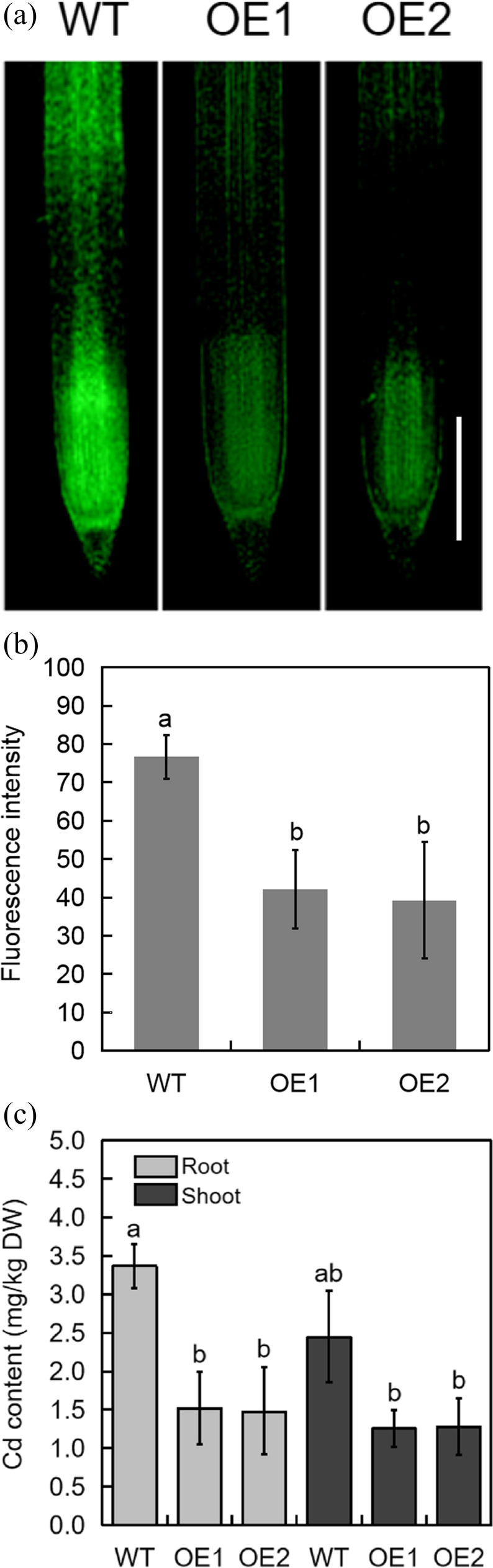
Overexpression of TabHLH094 limited Cd accumulation in root tissues. a Comparison of fluorescence intensities (reflective of Cd content) in the roots of WT and transgenic wheat. b Fluorescence intensities in root tissues were quantified using ImageJ software. The data are expressed as the means ± SDs of three independent experiments. Different lowercase letters signify significant (P < 0.05) differences. c Concentration of Cd in the roots of WT and transgenic wheat. Three-leaf-stage wheat seedlings were watered with Hoagland medium mixed with 2 mM (experimental) CdCl2 for 5 days. Each value is the mean ± SE (n = 3). Different lowercase letters signify significant (P < 0.05) differences
Overexpression of TabHLH094 promotes growth and decreases the grain Cd content of wheat
The effect of TabHLH094 overexpression on plant growth and grain Cd content in wheat under pot conditions was assessed. The growth of transgenic plants under Cd stress treatment was better than that of WT plants (Fig. 4a), and the grain scales of transgenic wheat lines were significantly larger than those of the WT (Fig. 4b). Furthermore, the thousand-grain weight was measured, and Cd stress decreased the thousand-grain weight of WT plants compared with transgenic lines (Fig. 4c). Under Cd conditions, the average thousand-grain weight was 27.6 g for the WT lines and 34.7 g for the transgenic plants. We next analyzed the grain Cd content. As shown in Fig. 4d, the Cd content in the WT was significantly higher than that in the transgenic lines. The average Cd content in the WT wheat grains was 0.32 mg/kg, while in the transgenic plant grains, the average Cd content was 0.18 mg/kg. Thus, overexpression of TabHLH094 promoted the growth of transgenic wheat under Cd stress and decreased Cd accumulation in wheat grains.
Fig. 4.
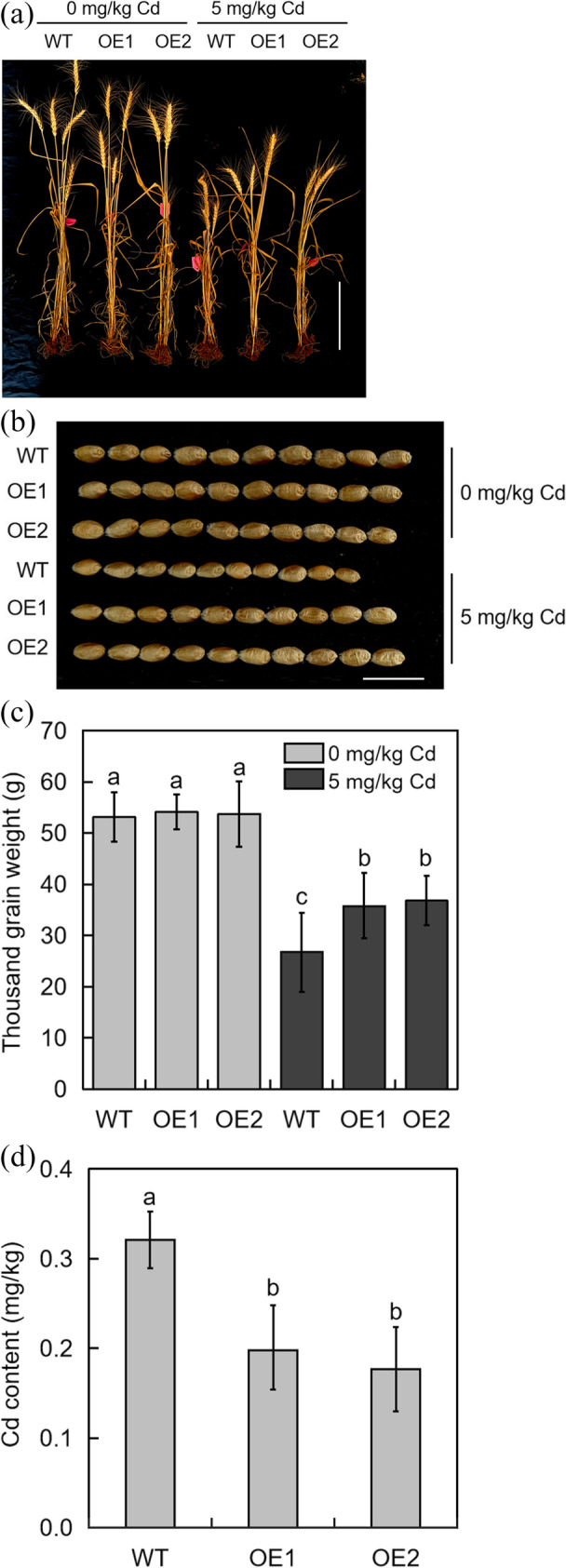
a Plant phenotypes of WT and TabHLH094 overexpression lines were observed with or without Cd. Scale = 30 cm. b Grain phenotypes of WT and TabHLH094 overexpression lines were observed with or without Cd. Scale = 2 cm. c Thousand-grain weight of WT and TabHLH094 overexpression lines under control and Cd stress conditions. Each value is the mean ± SE (n = 3). Different lowercase letters signify significant (P < 0.05) differences. d Cd content in the grains of WT and TabHLH094 overexpression lines under 5 mg/kg Cd conditions. Each value is the mean ± SE (n = 3). Different lowercase letters signify significant (P < 0.05) differences
TabHLH094 interacts with TaMYC8
RNA was extracted from the leaves of Cd-exposed wheat and utilized to construct a cDNA library. TabHLH094 was used as bait in a Y2H screening assay to identify potential protein interaction partners. Sequence analysis of candidate proteins indicated that the bHLH-type TF TaMYC8 can directly interact with TabHLH094. We validated the physical interaction between TabHLH094 and TaMYC8 using a Y2H assay. We found that cells coexpressing both TabHLH094–AD and TaMYC8–BD successfully grew on –Leu/–Trp/–Ade/–His dropout selection medium (Fig. 5a). To further validate whether TabHLH094 can physically interact with TaMYC8–BD, a BiFC assay was performed in tobacco epidermal cells. Pronounced YFP fluorescence was observed in cells cotransformed with both TabHLH094–nYFP and TaMYC8–cYFP (Fig. 5b). GST pulldown assays were performed on the purified recombinant TaMYC8–GST and TabHLH094–HIS proteins, and the results revealed that TaMYC8–GST could pull down TabHLH094–HIS (Fig. 5c). Finally, a Co–IP experiment was carried out utilizing tobacco leaves transiently expressing either TaMYC8–MYC with Flag or TabHLH094–Flag. We found that TaMYC8–MYC could be coimmunoprecipitated only with the TabHLH094–Flag fusion protein (Fig. 5d). Our results indicate that TabHLH094 can physically interact with TaMYC8 in vitro and in vivo.
Fig. 5.
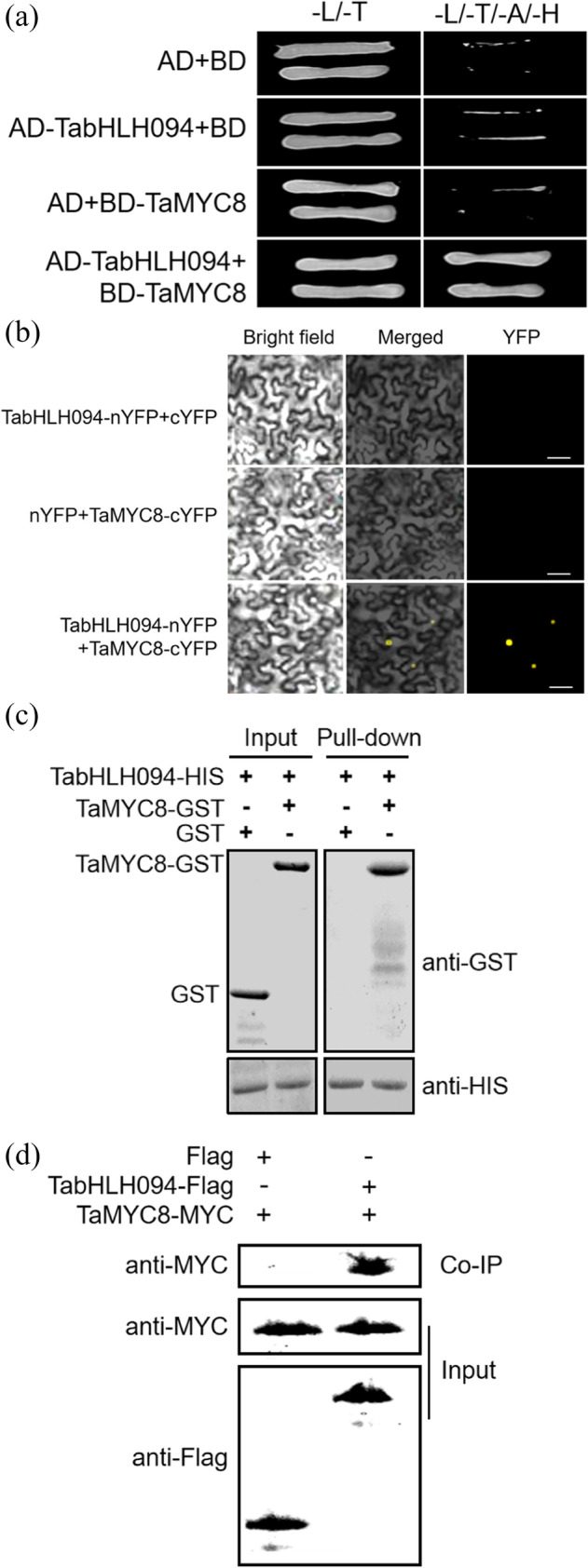
TabHLH094 interacts with TaMYC8 to improve tolerance to Cd exposure. a Y2H analysis of the TabHLH094–TaMYC8 interaction. L: leucine, T: tryptophan, A: alanine, H: histidine. b BiFC analysis of TabHLH094–nYFP and TaMYC8–cYFP in tobacco leaf cells. Scale bars = 30 μm. c Pulldown assays indicating that TabHLH094 physically interacts with TaMYC8 in vitro. d Co–IP assays indicating that TabHLH094 physically interacts with TaMYC8 in vivo
A TabHLH094–TaMYC8 binary complex blocks the transcriptional activity of TaMYC8
Our previous study found that TaMYC8 regulated the expression of TaERF6, thereby altering the Cd exposure response of wheat (Wang et al. 2022a, b). Here, we evaluated whether the TabHLH094–TaMYC8 binary complex affects the ability of TaMYC8 to bind to the TaERF6 promoter. EMSA indicated that the band signal of the TaMYC8–HIS protein and TaERF6 promoter complex was significantly reduced after the addition of the TabHLH094–HIS protein (Fig. 6a). LCI assays in tobacco leaves revealed a strong luminescence signal localized to the TaMYC8 and TaERF6 promoter coinjection area, with minimal signals observed in the TabHLH094, TaMYC8, and TaERF6 promoter coinjection areas (Fig. 6b). Together, these results indicate that the TabHLH094–TaMYC8 binary complex inhibits the ability of TaMYC8 to bind to the promoter of TaERF6 both in vivo and in vitro.
Fig. 6.
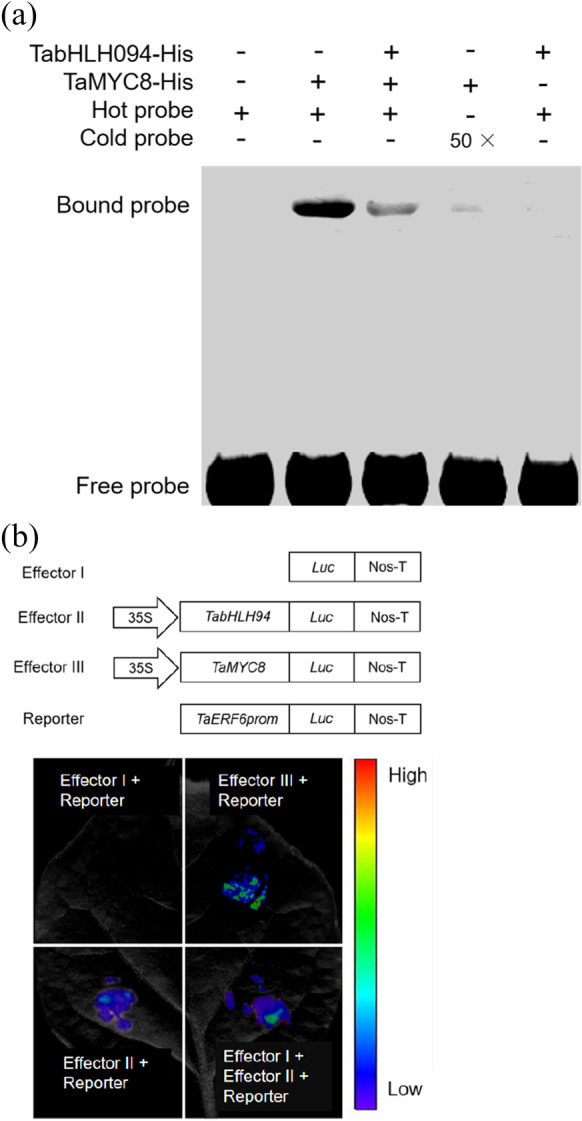
The TabHLH094–TaMYC8 binary complex blocks the transcriptional activation activity of TaMYC8. a In vitro EMSA indicating that the band signal of the TaMYC8-TaERF6 promoter was significantly reduced after the addition of the TabHLH094–HIS protein. b LCI assays indicating that the TabHLH094–TaMYC8 binary complex blocks the transcriptional activity of TaMYC8
Overexpression of TabHLH094 inhibits the activities of ACS and ACO
The ERF gene encodes a TF that regulates ethylene synthesis, and both ACO and ACS are rate-limiting enzymes involved in ethylene synthesis (Yin et al. 2018). Because our results indicated that the TabHLH094–TaMYC8 binary complex inhibits the ability of TaMYC8 to bind to the promoter of TaERF6 and thereby inhibits TaERF6 expression (Fig. 6a, b), we sought to determine whether the enzymatic activities of ACO and ACS were correspondingly inhibited in TabHLH094-overexpressing wheat lines. Under Cd stress, TabHLH094-overexpressing wheat lines exhibited significantly lower ACS and ACO activities than WT plants (Fig. 7a, b). Therefore, it appears that the overexpression of TabHLH094 can inhibit the enzymatic activity of ACO and ACS, thereby reducing ethylene biosynthesis and improving Cd stress tolerance.
Fig. 7.
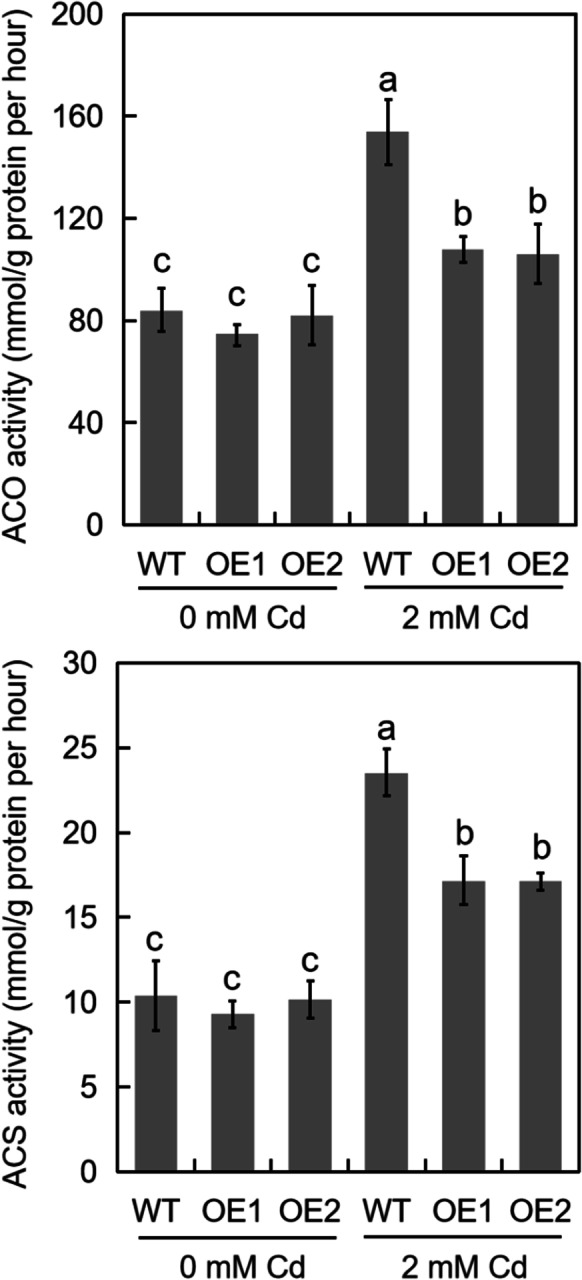
The effect of Cd exposure on the activities of (a) ACO and (b) ACS in TabHLH094-overexpressing wheat. Three-leaf-stage wheat seedlings were watered with Hoagland medium mixed with 2 mM (experimental) CdCl2 for 5 days. Each value is the mean ± SE (n = 3). Different lowercase letters signify significant (P < 0.05) differences
Discussion
The excessive accumulation of the phytohormone ethylene promotes maturation and reduces tolerance to heavy metal exposure (An et al. 2017). Our previous study revealed that TaMYC8 negatively regulates tolerance to Cd exposure in wheat (Wang et al. 2022a, b). Furthermore, we revealed that Cd exposure promoted the expression of TaEFR6, an ethylene-responsive TF, as well as the activities of ACO and ACS (Wang et al. 2022a, b). Here, we discovered that TabHLH094 directly interacts with TaMYC8 to form a binary complex that inhibits the ability of TaMYC8 to transcriptionally regulate TaERF6, thereby conferring enhanced tolerance to Cd exposure.
The primary route of Cd exposure in humans is the ingestion of crops produced on Cd-polluted soils (Luo et al. 2012). Therefore, reducing the Cd content of crop plants may be an effective way to limit the exposure of animals and humans to toxic levels of Cd. Several genes have been discovered to decrease the accumulation of Cd in plant tissues. For example, several ERF TFs, including ERF34 and ERF35, have been reported to be central to the Cd exposure response in plants (Xie et al. 2021). In soybean, GmWRKY142 limits the accumulation of Cd by upregulating the Cd tolerance type 1 genes GmCDT1–2 and GmCDT1–1 (Cai et al. 2020). The purpose of creating a Cd-tolerant wheat line is not only to promote plant growth under Cd stress conditions but also to minimize Cd accumulation in the edible parts. Here, we found that TabHLH094 overexpression enhanced the growth and development of wheat under Cd conditions (Fig. 2a and Fig. 4a–c). Furthermore, in both seedlings and grains, TabHLH094 overexpression lines accumulated significantly less Cd than WT plants (Fig. 3 and 4d).
The plant response to abiotic stress involves an array of TFs, particularly bHLH TFs (Dong et al. 2021), which regulate the abiotic stress response in two ways. First, bHLH TFs can directly modulate downstream gene expression. For example, in apple, the bHLH-type TF MdMYC2 curtails tolerance to aluminum (Al) exposure through direct regulation of MdERF3 expression (An et al. 2017). Our previous study revealed that the bHLH-type TF TaMYC8 regulates TaERF6 expression, thereby weakening the Cd exposure tolerance of wheat plants (Wang et al. 2022a, b). Second, bHLH TFs can form complexes with other TFs to modulate downstream gene expression. In Phalaenopsis, anthocyanin biosynthesis is regulated by a MYB–bHLH complex involving PeMYC4 and PeMYB4L (Wang et al. 2022a, b). In Eriobotrya japonica, the EjbHLH1–EjMYB2–EjAP2–1 ternary complex acts to delay cold-induced lignification (Xu et al. 2019). The IbPYL8–IbbHLH66–IbbHLH118 ternary complex in sweet potato regulates the drought stress response by mediating the expression of tonoplast intrinsic protein 1 (IbTIP1), ABA-responsive element-binding factor 2 (IbABF2), and ABA-insensitive 5 (IbABI5) (Xue et al. 2022). In this study, TabHLH094 enhanced Cd tolerance in wheat by forming a binary complex with TaMYC8 and repressing the expression of the downstream TaERF6 gene (Figs. 5 and 6).
Several phytohormones act to regulate the plant response to stress as well as other physiological functions (Wu et al. 2020). In particular, the phytohormone ethylene negatively regulates the reaction of plants to exposure to Cd (Schellingen et al. 2014). In Petunia hybrida, acdS overexpression limits ethylene biosynthesis in both floral and vegetative tissues, thereby enhancing the lifespan of the flowers and the tolerance to Cd stress (Naing et al. 2022). The process of Cd-induced ethylene production relies on ACO and ACS gene expression (Schellingen et al. 2014). Reducing ACS and ACO gene expression, which results in reduced ethylene biosynthesis, can improve plant tolerance to environmental stress (Li et al. 2019; Espley et al. 2019). Here, we found that the overexpression of TabHLH094 resulted in reduced ACO and ACS activities (Fig. 7), which would likely result in reduced ethylene biosynthesis.
Conclusion
Here, we report the identification of the bHLH-type TF TabHLH094, which positively regulates Cd stress tolerance in wheat. TabHLH094 can interact with TaMYC8, inhibit the ability of TaMYC8 to bind to the promoter of TaERF6, and downregulate TaERF6 expression. These actions should result in a reduction in ethylene biosynthesis, further improving Cd stress tolerance in wheat. The results of this study help clarify the mechanism by which the bHLH TF TabHLH094 regulates the wheat Cd stress response. This TF may prove to be a fruitful target for the breeding of wheat with reduced Cd accumulation.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by the Precursor Projects of Guizhou Province for Biological Breeding Supporting by Science and Technology in 2022 (QKHZC2022ZD026), National Natural Science Foundation of China (grant no. 32260506), and the Science and Technology Department of Guizhou Province, China (grant no. ZK2022YB315).
Data availability
All data generated during the study are given in this published article.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuye Du and Lihe Fang contributed equally to this work.
References
- An JP, Wang XN, Yao JF, Ren YR, You CX, Wang XF, Hao YJ. Apple MdMYC2 reduces aluminum stress tolerance by directly regulating MdERF3 gene. Plant Soil. 2017;418:255–266. doi: 10.1007/s11104-017-3297-7. [DOI] [Google Scholar]
- Brunetti P, Zanella L, De Paolis A, Di Litta D, Cecchetti V, Falasca G, Barbieri M, Altamura MM, Costantino P, Cardarelli M. Cadmium–inducible expression of the ABC–type transporter AtABCC3 increases phytochelatin–mediated cadmium tolerance in Arabidopsis. J Exp Bot. 2015;66:3815–3829. doi: 10.1093/jxb/erv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Cheng WH, Hong CY, Chang YS, Chang MC. The transcription factor OsbHLH035 mediates seed germination and enables seedling recovery from salt stress through ABA–dependent and ABA–independent pathways, respectively. Rice. 2018;11:50. doi: 10.1186/s12284-018-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Xian P, Wang H, Lin R, Lian T, Cheng Y, Ma Q, Nian H. Transcription factor GmWRKY142 confers cadmium resistance by up–regulating the Cadmium Tolerance 1–Like genes. Front Plant Sci. 2020;11:724. doi: 10.3389/fpls.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Chen Q, Dai Y, Hu W, Zhang S, Huang X. Genome–wide identification of PbrbHLH family genes, and expression analysis in response to drought and cold stresses in pear (Pyrus bretschneideri) BMC Plant Biol. 2021;21:86. doi: 10.1186/s12870-021-02862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elobeid M, Göbel C, Feussner I, Polle A. Cadmium interferes with auxin physiology and lignification in poplar. J Exp Bot. 2012;63:1413–1421. doi: 10.1093/jxb/err384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley RV, Leif D, Plunkett B, McGhie T, Henry-Kirk R, Hall M, Johnston JW, Punter MP, Boldingh H, Nardozza S, Volz RK, O’Donnell S, Allan AC. Red to brown: an elevated anthocyanic response in apple drives ethylene to advance maturity and fruit flesh browning. Front Plant Sci. 2019;10:1248. doi: 10.3389/fpls.2019.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Chao K, Li J, Wang Z, Cheng P, Li Q, Wang B. Functional verification of two genes related to stripe rust resistance in the wheat–Leymus mollis introgression line M8664–3. Front Plant Sci. 2021;12:754823. doi: 10.3389/fpls.2021.754823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BF, Du ST, Lu KX, Liu WJ, Lin XY, Jin CW. Iron uptake system mediates nitrate–facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J Exp Bot. 2012;63:3127–3136. doi: 10.1093/jxb/ers036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li XX, Wang MR, Wen J, Yi B, Shen JX, Ma CZ, Fu TD, Tu JX. Interactions of WRKY15 and WRKY33 transcription factors and their roles in the resistance of oilseed rape to Sclerotinia infection. Plant Biotech J. 2018;16:911–925. doi: 10.1111/pbi.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Feng H, Wen J, Dong J, Wang T. MtCAS31 aids symbiotic nitrogen fixation by protecting the leghemoglobin MtLb120–1 under drought stress in Medicago truncatula. Front Plant Sci. 2018;9:633. doi: 10.3389/fpls.2018.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang L, Wang M, Di D, Kronzucker HJ, Shi W. The Arabidopsis AMOT1/EIN3 gene plays an important role in the amelioration of ammonium toxicity. J Exp Bot. 2019;70:1375–1388. doi: 10.1093/jxb/ery457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leškovï A, Zvarï KM, Araya T, Giehl RFH. Nickel toxicity targets cell wall–related processes and PIN2–mediated auxin transport to inhibit root elongation and gravitropic responses in Arabidopsis. Plant Cell Physiol. 2020;61:519–535. doi: 10.1093/pcp/pcz217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K, Dong Q, Li C, Liu C, Ma F. Genome wide identification and characterization of apple bHLH transcription factors and expression analysis in response to drought and salt stress. Front Plant Sci. 2017;8:480. doi: 10.3389/fpls.2017.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QJ, Sun MH, Lu J, Hu DG, Kang H, You CX, Hao YJ. Phosphorylation of a malate transporter promotes malate excretion and reduces cadmium uptake in apple. J Exp Bot. 2020;71:3437–3449. doi: 10.1093/jxb/eraa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wei M, Wang Z, Hou S, Li X, Xu H. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J Hazard Mater. 2020;388:122065. doi: 10.1016/j.jhazmat.2020.122065. [DOI] [PubMed] [Google Scholar]
- Naing AH, Campol JR, Chung MY, Kim CK. Overexpression of acdS in Petunia hybrida improved flower longevity and cadmium–stress tolerance by reducing ethylene production in floral and vegetative tissues. Cells. 2022;11:3197. doi: 10.3390/cells11203197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Song S, Ren Q, Wu bD, Huang H, Chen Y, Fan M, Peng W, Ren C. Xie D (2011) The Jasmonate–ZIM–domain proteins interact with the WD–Repeat/bHLH/MYB complexes to regulate Jasmonate–mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell. 2011;23:1795–814. doi: 10.1105/tpc.111.083261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellingen K, Van Der Straeten D, Vandenbussche F, Vandenbussche F, Prinsen E, Remans T, Vangronsveld J, Cuypers A. Cadmium–induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 2014;14:214. doi: 10.1186/s12870-014-0214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YS, Yang WJ, Fan H, Zhang XS, Sui N. TaMYB86B encodes a R2R3–type MYB transcription factor and enhances salt tolerance in wheat. Plant Sci. 2020;300:110624. doi: 10.1016/j.plantsci.2020.110624. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen C, Du J, Liu H, Cui Y, Zhang Y, He Y, Wang Y, Chu C, Feng Z, Li J, Ling HQ. Co–overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis–enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 2012;158:790–800. doi: 10.1104/pp.111.190983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Li J, Li Z, Zhang F, Tan X. Transcriptomic analyses of Camellia oleifera ‘Huaxin’ leaf reveal candidate genes related to long–term cold stress. Int J Mol Sci. 2020;21:846. doi: 10.3390/ijms21030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Xia R, Chen C, Shang X, Ge F, Wei H, Chen H, Wu Y, Xie Q. ZmbHLH124 identified in maize recombinant inbred lines contributes to drought tolerance in crops. Plant Biotechnol J. 2021;19:2069–2081. doi: 10.1111/pbi.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Zuo D, Zhu B, Du XY, Gu L. TaMYC8 regulates TaERF6 and inhibits ethylene synthesis to confer Cd tolerance in wheat. Environ Exp Bot. 2022;198:104854. doi: 10.1016/j.envexpbot.2022.104854. [DOI] [Google Scholar]
- Wang R, Mao CJ, Ming F. PeMYB4L interacts with PeMYC4 to regulate anthocyanin biosynthesis in Phalaenopsis orchid. Plant Sci. 2022;324:111423. doi: 10.1016/j.plantsci.2022.111423. [DOI] [PubMed] [Google Scholar]
- Xu Z, Liu X, He X, Xu L, Huang Y, Shao H, Zhang D, Tang B, Ma H. The soybean basic helix–loop–helix transcription factor ORG3–like enhances cadmium tolerance via increased iron and reduced cadmium uptake and transport from roots to shoots. Front Plant Sci. 2017;8:1098. doi: 10.3389/fpls.2017.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li SJ, Liu XF, Yin XR, Grierson D, Chen KS. Ternary complex EjbHLH1–EjMYB2–EjAP2–1 retards low temperature–induced flesh lignification in loquat fruit. Plant Physiol Bioch. 2019;139:731–737. doi: 10.1016/j.plaphy.2019.04.032. [DOI] [PubMed] [Google Scholar]
- Xie Q, Yu Q, Jobe TO, Pham A, Ge C, Guo Q, Liu J, Liu H, Zhang H, Zhao Y, Xue S, Hauser F, Schroeder JI. An amiRNA screen uncovers redundant CBF and ERF34/35 transcription factors that differentially regulate arsenite and cadmium responses. Plant Cell Environ. 2021;44:1692–1706. doi: 10.1111/pce.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LY, Wei ZW, Zhai H, Xing SH, Wang YX, He SZ, Gao SP, Zhao N, Zhang H, Liu QC. The IbPYL8–IbbHLH66–IbbHLH118 complex mediates the abscisic acid–dependent drought response in sweet potato. New Phytol. 2022;236:2151–2171. doi: 10.1111/nph.18502. [DOI] [PubMed] [Google Scholar]
- Yin W, Yu X, Chen G, Tang B, Wang Y, Liao C, Zhang Y, Hu Z. Suppression of SlMBP15 inhibits plant vegetative growth and delays fruit ripening in tomato. Front Plant Sci. 2018;9:938. doi: 10.3389/fpls.2018.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang H, Fu Z, Chen H, Lin Y, Yan P, Li W, Xie H, Guo Z, Zhang X, Tang J. Genetic–based dissection of arsenic accumulation in maize using a genome–wide association analysis method. Plant Biotechnol J. 2018;16:1085–1093. doi: 10.1111/pbi.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AD, Wang WQ, Tong Y, Li MJ, Grierson D, Ferguson I, Chen KS, Yin XR. Transcriptome analysis identifies a zinc finger protein regulating starch degradation in kiwifruit. Plant Physiol. 2018;178:850–863. doi: 10.1104/pp.18.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during the study are given in this published article.