Abstract
Evidence indicates that the microbiome plays a significant role in HIV immunopathogenesis and associated complications. This study aimed to characterize the oral and anal microbiome of Men who have Sex with Men (MSM) and Transgender Women (TGW), with and without HIV. One hundred and thirty oral and anal DNA-derived samples were obtained from 78 participants and subjected to shotgun metagenomics sequencing for further microbiome analysis. Significant differences in the microbiome composition were found among subjects associated with HIV infection, gender, sex behavior, CD4+ T-cell counts, antiretroviral therapy (ART), and the presence of HPV-associated precancerous anal lesions. Results confirm the occurrence of oncogenic viromes in this high HIV-risk population. The oral microbiome in HIV-associated cases exhibited an enrichment of bacteria associated with periodontal disease pathogenesis. Conversely, anal bacteria showed a significant decrease in HIV-infected subjects (Coprococcus comes, Finegoldia magna, Blautia obeum, Catenibacterium mitsuokai). TGW showed enrichment in species related to sexual transmission, which concurs that most recruited TGW are or have been sex workers. Prevotella bivia and Fusobacterium gonidiaformans were positively associated with anal precancerous lesions among HIV-infected subjects. The enrichment of Holdemanella biformis and C. comes was associated with detectable viral load and ART-untreated patients. Metabolic pathways were distinctly affected by predominant factors linked to sexual behavior or HIV pathogenesis. Gene family analysis identified bacterial gene signatures as potential prognostic and predictive biomarkers for HIV/AIDS-associated malignancies. Conclusions: Identified microbial features at accessible sites are potential biomarkers for predicting precancerous anal lesions and therapeutic targets for HIV immunopathogenesis.
Subject terms: Infectious-disease diagnostics, Microbial genetics
Introduction
The human body harbors complex microbial communities that ensure vital functions for the host and may affect health and disease susceptibility by modulating physiological homeostasis, energy metabolism, and immune-related bioprocess1,2. The gut and oral microbiomes constitute the first and the second largest microbial communities in humans, and their habitat—the mucosal immune system—is devastated after acute HIV infection3,4. Emergent evidence in recent years has indicated that the microbiome plays a significant role in human immunodeficiency virus (HIV-1) immunopathogenesis and associated complications5–10. A relevant subset of such complications includes virally induced squamous intraepithelial lesions (SILs) and HIV/AIDS-associated malignancies8–11.
Latin America has a high prevalence of HIV/AIDS-associated malignancies caused by viruses (including HPV, EBV, and KSHV) which tend to disproportionally affect underprivileged and socially vulnerable populations, such as men who have sex with men (MSM), transgender women (TGW), commercial sex workers (CSW) and people who inject drugs12,13. Worldwide, TGW have an extremely high HIV prevalence compared to other adults, with almost 50 times higher odds of infection14. In Argentina, the burden of HIV infection among MSM is 14%, while in TGW has been reported as high as 34% compared to 0.4% in the general population15. In addition to biological factors, stigma, discrimination and structural determinants increase the risks for HIV infection and other sexually transmitted infections and represent barriers to care. In particular, TGW suffer social exclusion at an early age, limiting their access to education and jobs, leading many TGW to engage in sex work16,17.
MSM and TGW populations have the highest risk for infection with KSHV and HPV, potentially developing Kaposi sarcoma (KS) and/or SILs and anal cancer. For AIDS-associated malignancies such as KS, the effect of antiretroviral therapy (ART) has been dramatic, with significant decreases in incidence and outcomes improvement. However, for other AIDS malignancies such as HPV-associated anal cancer, ART appears not to affect the natural history of the malignancy.
Beyond the oncogenic viruses, increasing evidence reveals that the microbiota may play a key role in carcinogenesis by affecting the balance of host cell proliferation and apoptosis, hindering anti-tumoral immunity, and influencing the metabolism of host-produced factors, ingested food components, and drugs18. However, limited HIV microbiome studies have been conducted using whole metagenome sequencing to define the compositional and functional consequences of HIV infection on the oral and anal microbiota at the species level19. Importantly, no study to date has comprehensively delineated the oral and anal microbiome portrait of TGW with and without HIV, which is the primary aim of the present study20.
Here we performed a metagenomics-based characterization of the oral and anal microbiome in a cohort of MSM and TGW patients exposed to HIV infection to investigate their relationship with associated factors such as gender, sexual behavior, ART, viral load, CD4+ T-cell counts and the presence of precancerous anal intraepithelial lesions.
Results
Richness and diversity
One hundred and thirty oral and anal DNA-derived samples were obtained from 78 participants (47 MSM and 31 TGW), 50 were HIV-positive and 28 were HIV-negative. When recruited, most of the participants with HIV were on antiretroviral therapy (Table 1 and Supplementary Table 1). We first tested whether microbial richness and diversity differed between anal and oral groups of samples across the different variables. For alpha diversity, there was a significant difference among oral and anal samples, with a greater richness in the distribution of the abundance of species in the oral ones, as indicated by Chao1, Shannon and Simpson indices (p < 0.01; Fig. 1a). As expected, beta diversity shows two main clusters based on sample origin, which determine two locations completely different regarding microbial composition (Fig. 1b). We further evaluated whether the samples cluster beyond that expected by sampling variability using PERMANOVA. There was a significantly greater dispersion of samples from the anal site, with higher inter-sample distance, compared with the oral site (p < 0.001, Supplementary Fig. 1).
Table 1.
Participants, samples, and clinical variables included in the study.
| Participants | MSM | TGW | TOTAL |
|---|---|---|---|
| N | 47 | 31 | 78 |
| AGE (Mean-Median-SD) | (34.8-33-9.5) | (34.0-32.3-8.2) | (34.5-33-8.9) |
| Samples | |||
| Oral swabs | 41 | 19 | 60 |
| Anal swabs | 42 | 28 | 70 |
| Total | 83 | 47 | 130 |
| HIV status | |||
| Positive | 34 | 16 | 50 |
| Negative | 13 | 15 | 28 |
| Current viral load | |||
| Detectable (>50 copies/ml) | 7 | 5 | 12 |
| Undetectable (<50 copies/ml) | 23 | 7 | 30 |
| ND | 4 | 4 | 8 |
| ART | |||
| Yes | 29 | 15 | 44 |
| No | 5 | 1 | 6 |
| Current CD4+ T-cell counts | |||
| <500 (cells/mm3) | 12 | 5 | 17 |
| >500 (cells/mm3) | 20 | 5 | 26 |
| ND | 2 | 5 | 7 |
| Cytology | |||
| Lesions | 34 | 17 | 51 |
| ASCUS | 1 | 2 | 3 |
| LSILs | 28 | 12 | 40 |
| HSILs | 5 | 3 | 8 |
| Normal | 13 | 14 | 27 |
ND no data.
Fig. 1. Richness, diversity, and taxonomy profile of oral and anal samples obtained from TGW and MSM.
a Alpha diversity indices show a greater richness in oral samples compared with anal samples. The p-values displayed on the box plots represent the results of the Wilcoxon test. b Beta diversity shows two main clusters based on sample origin, which determine two locations completely different regarding microbial composition, with a higher inter-sample variability among anal samples assessed by Aitchison distance and PERMANOVA test (p < 0.001, Supplementary Fig. 2) c Box plots of alpha diversity indices of the variables age, HIV status and lesions in anal samples. A decrease in alpha diversity is observed in the group of older subjects, HIV-positive patients and subjects with lesions (p < 0.05). The p-values displayed on the box plots represent the results of the Wilcoxon test. d, e Heatmap representations of the most prevalent virus (d) and bacteria (e) identified in oral and anal samples. In both heatmaps taxa are ordered according to prevalence and abundance; samples were clustered by an unsupervised method. Dominant and mutually exclusive taxa were identified for both locations.
Richness estimation across the variables gender, age, CSW, HIV, ART, CD4+ T-cell counts, and the presence of lesions, was analyzed considering both sites separately. In anal samples, there were no differences in richness between both genders (MSM and TGW) nor in the CSW, CD4+ T-cell counts and ART groups (Supplementary Table 2). However, we found significant differences in aging (under33yo vs. over33yo), HIV status (positive vs. negative), and the presence of lesions (yes vs. no), with lower indices in the group of older subjects (over33yo; Observed, Shannon and Simpson indices, p < 0.05), HIV-positive patients (Shannon and Simpson, p < 0.05) and in the group of patients with lesions (Shannon and Simpson, p < 0.05) (Fig. 1c; Supplementary Table 2). We observed no differences in the analyzed variables when considering oral samples (p > 0.05; Supplementary Table 2).
These results suggest that aging, HIV, and the presence of lesions are associated with changes in the anal microbiome composition. However, further analysis is required to determine any potential interconnectedness between these variables contributing to microbial richness and diversity.
Taxonomic composition and abundance
To define the taxonomic composition of the 130 samples, we used MetaPhlAn 3.0 software. The analysis identified 666 species detected at least once, of which 314 were considered “prevalent” when defined as present in >5% of samples at >0.1% relative abundance (Supplementary Table 3). Of the 666 species, 548 belong to the kingdom Bacteria, 113 to viruses, 4 to eukaryotes and 1 to Archaea. Of the prevalent species, 284 are bacteria, 28 viruses, 1 eukaryote and 1 archaea. Of all detected species, we found a similar number of taxa significantly associated with oral (n = 200) or anal sites (n = 203), according to the MaAsLin 2.0 default significance levels (q value < 0.25; Supplementary Table 3).
To visualize the relative abundance of taxa, we first evaluated the abundances at phylum level for site, gender, aging, CSW, HIV status and the presence of lesions (Supplementary Fig. 2). Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Fusobacteria were the most prevalent phyla (Supplementary Fig. 2). We conducted a multivariate test for differences in the overall composition between groups of samples using the HMP package. Significant differences were observed among sites, with a higher prevalence of Actinobacteria (p < 0.0001), Firmicutes (p < 0.0001), and Proteobacteria (p < 0.0001) in oral samples. In contrast, Bacteroidetes (p < 0.0001), Spirochaetes (p < 0.01), the unusual group Tenericutes (p < 0.01) and Viruses (p < 0.001) were most abundant in anal samples (Supplementary Fig. 2). Interestingly, there was an enrichment of Actinobacteria in oral samples of older subjects (p = 0.009), while Proteobacteria (oral samples, p = 0.01) and Fusobacteria (anal samples, p = 0.001) were more abundant in the youngers (Supplementary Fig. 2). For HIV group, only Viruses yielded a positive association with anal samples of the infected patients (p = 0.038, Additional file 5). The presence of lesions was positively associated with Viruses in anal samples and Proteobacteria in oral samples (Supplementary Fig. 2). Conversely, a decrease of Firmicutes (p = 0.0069) and Actinobacteria (p = 0.0001) was observed in anal samples (Supplementary Fig. 2). Finally, no differences were observed between groups of gender or CSW condition (data not shown).
Next, we defined the most abundant taxa at the species level for viruses and bacteria in oral and anal sites (Pr > 5%, abundance >0.15). A few dominant and mutually exclusive taxa were identified in both locations. For viruses, species of Alphapapillomavirus were prevalent in anal samples, while Human gammaherpesvirus 8 (KSHV) and Human betaherpesvirus 7 (HHV-7) along with bacteriophages of Actynomyces and Streptococcus, among others, were dominant in oral samples (Fig. 1d). In Bacteria, oral samples were enriched by species of Haemophilus (H. influenzae, H. parainfluenzae, H. haemolyticus, H. parahaemolyticus), Neisseria (N. flavescens, N. mucosa, N. sicca), Rothia (R. mucilaginosa, R. dentocariosa) and Streptococcus (S oralis, S mitis) along with other relevant species such as Lautropia mirabilis, Granullicatella elegans, Gemella hemolysans or Corynebacterium matruchotii. In anal samples, species of Prevotella (P. copri, P. corporis, P. bivia, P. disiens, etc.) and Bacteroides (B. vulgatus, B. fragilis, B. uniformis, etc.) were among the most abundant species. Moreover, other frequent bacteria in the anal samples were Eubacterium rectale, Faecalybacteriun prausnitzii or Fusobacterium gonidiaformans, among others (Fig. 1e).
Overall, distinctive microbial compositions were identified in oral and anal mucosa of a cohort of MSM and TGW HIV-positive and negative cases, with a greater richness at the species level in the oral samples. According to the alpha indices, the dysbiosis observed in anal samples due to the HIV infection, aging and the presence of lesions could partly explain the more inter-sample variability of this location compared with oral mucosa.
Taxonomic profile in a multivariable association between phenotypes and microbial species
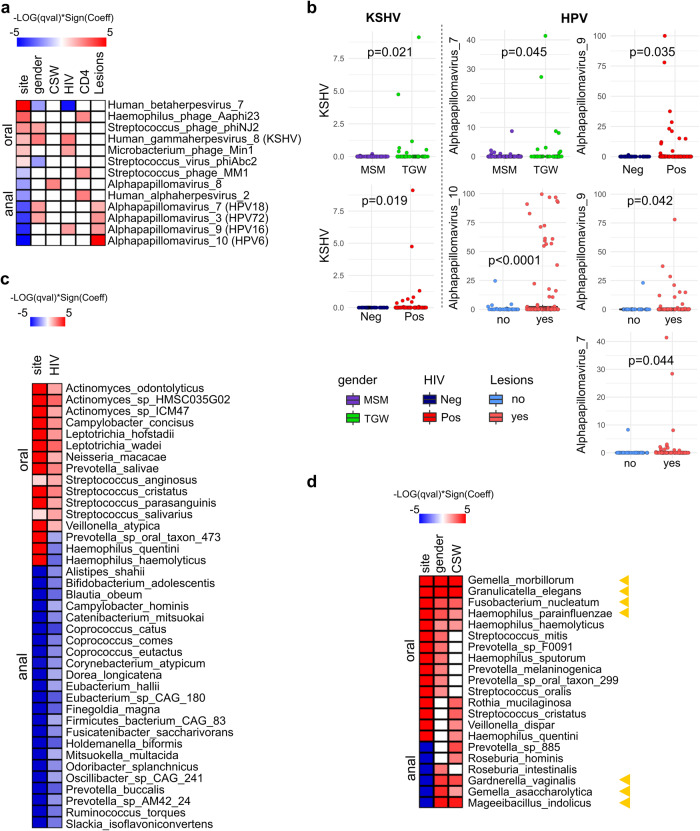
To find associations between the microbiome and patients´ metadata, we used MaAsLin2 multivariate linear model. Results of all significant associations are provided in Supplementary Table 4. When pertinent variables such as alcohol use, tobacco use, or substance use (Table 2) were included in the model as additional adjusting factors, these variables did not show significant differences between MSM and TGW (Supplementary Table 5). Since some relevant species were detected in less than 10% of samples for virus analysis, we set the prevalence threshold at 0.05. Regarding gender, TGW showed enrichment of KSHV and Streptoccuss phage phiNJ2 in oral samples, and Alphapapillomavirus 7 (HPV18) and 3 (HPV72) in the anal ones when compared with MSM (Fig. 2a). For its part, MSM showed a greater abundance of the Human betaherpesvirus 7 (HHV-7) than TGW (Fig. 2a). Just one virus taxon (Alphapapillomavirus 8) was positively associated with CSW (Fig. 2a). In addition, HIV-infected patients were positively associated with KSHV and negatively associated with HHV-7 in coincidence with the TGW group (Fig. 2a, b). Importantly, HIV-positive cases also showed more abundance of Alphapapillomavirus 9 which contains the high-risk genital cancer HPV16, independently of gender condition (Fig. 2a, b). We further evaluated the HIV-related variables, presence of lesions, CD4+ T-cell counts and VL. As expected, the presence of lesions was positively associated with Alphapapillomavirus, which contains HPV types previously related to the development of SILs, such as the HPV16 and HPV18 (Alphapapillomavirus 7) and the low-risk HPV6 (Alphapapillomavirus 10) and HPV72 (Alphapapillomavirus 3) (Fig. 2b)21,22. Thus, since these HPV types would be related to SILs, HPV16 would also be favored by HIV-positive conditions, constituting a potential determinant of anal lesions in HIV-infected patients23.
Table 2.
Sexual behavior and consumption habits of MSM and TGW: age distribution and variable frequencies in the study cohort.
| MSM | TGW | TOTAL | |
|---|---|---|---|
| Sexual behavior | |||
| AFSI (Mean-Median-SD) | (16.5-16-2.9) | (14.5-15-2.7) | (15.7-16-3.0) |
| AASI (Mean-Median-SD) | (18.6-18-3.6) | (14.6-15-2.8) | (17.0-17-3.8) |
| AOSI (Mean-Median-SD) | (18.1-18-3.4) | (14.7-15-3.1) | (16.7-17-3.7) |
| Commercial sex worker | |||
| Yes | 6 | 30 | 36 |
| No | 38 | 1 | 39 |
| ND | 3 | 0 | 3 |
| NSPLM | |||
| None | 12 | 2 | 14 |
| 1–10 | 35 | 13 | 48 |
| 11–50 | 0 | 8 | 8 |
| >50 | 0 | 8 | 8 |
| NSPL | |||
| 1–10 | 5 | 1 | 6 |
| 11–50 | 20 | 2 | 22 |
| 51–100 | 12 | 1 | 13 |
| >100 | 8 | 25 | 33 |
| ND | 2 | 2 | 4 |
| CUAS | |||
| Yes | 20 | 21 | 41 |
| No | 22 | 9 | 30 |
| ND | 5 | 1 | 6 |
| CUOS | |||
| Yes | 31/42 | 20/30 | 41 |
| No | 11/42 | 10/30 | 31 |
| ND | 5 | 1 | 6 |
| Consumption habits | |||
| Alcohol | |||
| Yes | 38/45 | 24/31 | 62 |
| No | 7/45 | 7/31 | 14 |
| ND | 2 | – | 2 |
| Tobacco | |||
| Yes | 28/47 | 20/31 | 48 |
| No | 19/47 | 11/31 | 30 |
| Non-IV drug use | |||
| Yes | 27/47 | 21/31 | 48 |
| No | 20/47 | 10/31 | 30 |
| IV drug use | |||
| Yes | 1/47 | 0/31 | 1 |
| No | 46/47 | 31/31 | 77 |
AFSI age at first sexual intercourse, AASI age of anal sex initiation, AOSI age of oral sex initiation, NPLSM number of sexual partners in last month, NSPL number of sexual partners in life, CUAS condom use in anal sex, CUOS condom use in oral sex, non-IV drug use non-intravenous drug use, IV drug use intravenous drug use.
Fig. 2. Top significant taxa associations (p < 0.05) detected by MaAsLin 2’s linear model.
a Virus species associated with site, gender, CSW, HIV status, CD4+ T-cell counts and presence of lesions. b Box plots showing the significant associations of the relevant viruses KSHV (Human gammaherpesvirus 8), HPV6 (Alphapapillomavirus 10), HPV16 (Alphapapillomavirus 9) and HPV18 (Alphapapillomavirus 7) with the variables gender, HIV and lesions. The p-values displayed on the box plots represent the results of the Wilcoxon test, which was used to compare the dichotomous groups being depicted. c Bacteria species associated with site and HIV status. d Bacteria species associated to site, subject´s gender and CSW condition. Yellow arrowheads point to the most significant taxa shared by TGW and CSW. Heatmap color coding: The red color indicates a positive association to the reference value, referred to as the numerator of the ratio of both terms of each dichotomous variable: for site (oral/anal); gender (TGW/MSM); CSW (yes/no); HIV (positive/negative); CD4+ T-cell counts ( < 500cells/mm3/>500cells/mm3); the presence of lesions (yes/no). Blue color indicates a negative association with the reference value, or a positive association with the opposite term of the ratio (denominator).
Three viral species were found positively associated with the low-CD4+ T-cell counts group: Haemophilus phage Aaphi23, Streptococcus phage MM1, and the Human alphaherpesvirus 2 (HSV-2) (Fig. 2a). The latter is a common pathogen co-infecting more than half of HIV-positive adults and has been postulated to accelerate HIV disease24. Moreover, our results are in agreement with another study from Tan et al. (2013), who found an association of HSV-2 with CD4+ count <350 cells/mm325. Finally, there were no significant associations between viral taxa and VL (data not shown).
Overall, the main results reveal that KSHV, HHV-7, and high-risk HPV16 and HPV18 were associated with gender and/or HIV status. While the high-risk HPV16 and HPV18 and the low-risk HPV6 and HPV72 were positively associated with the presence of lesions. Furthermore, HSV-2 correlated with a decrease in CD4+ T-cell counts.
For bacteria, we found 39 species associated with HIV status (p < 0.05; Pr > 0.1) (Fig. 2c). Most of them (26/39) showed a significant decrease in their relative abundances in HIV-positive compared with HIV-negative (p < 0.05) and belong mostly to the anal site. Notably, the species of higher abundance in HIV-positive (p < 0.05) are more typical of the oral mucosa (13/13) (Fig. 2c). Among those that showed enrichment in HIV-positive, there are species of Streptococcus (S. parasanguinis, S. cristatus, S. anginosus and S. salivarius) and Leptotrichia (L. wadei and L. hofstadii), together with Prevotella salivae, Actynomyces odontolyticus, Neisseria macacae, Veillonella atypica and Campylobacter concisus (Fig. 2c). Many of them share several characteristics, such as being common residents in the oral cavity, being colonizers in the formation of oral biofilms affecting the development of oral plaques (S. parasanguinis, S. salivarius, V. atypica, A. odontolyticus), or being related to the formation of caries and periodontitis processes (V. atypica, S. parasanguinis, S. salivarius, L. wadei, L. hofstadii, C. concisus)26–30. Remarkably, at least at the species level, information about these taxa and HIV status is scarce and scattered. Our results indicate that the enrichment of taxa in the oral site of HIV-positive could be associated with the pathogenesis of periodontal disease in HIV-infected patients on ART31.
On the other side, most taxa that showed a significant decrease in HIV-positive were associated with anal location (Fig. 2c), which would be linked to the lower diversity indices values observed in the anal samples from HIV-positive patients (Fig. 1b). Species of the gender Coprococcus (C. catus, C. comes, C. euctatus), Ruminococcus torques, Blautia obeum, Finegoldia magna, Holdemanella biformis, Catenibacterium mitsuokai, Bifidobacterium adolescentis and Alistipes shahii, have been previously found significantly associated to gut microbiome dysbiosis of HIV-positive individuals in different studies, consistent with our results32–35.
To further identify bacteria species related to HIV variables, we applied MaAsLin2 considering gender, CSW status, presence of lesions, current CD4+ T-cell counts, and current VL. We found fifteen species significantly associated with gender (p < 0.05; Fig. 2d; Supplementary Table 4). Of note, all were increased in TGW compared with MSM. The top relevant (q-value < 0.05) were Gemella morbillorum, Granulicatella elegans, Mageeibacillus indolicus, Gemella asaccharolytica, Gardnerella vaginalis, Fusobacterium nucleatum, and Haemophilus parainfluenzae (Fig. 2d).
As the majority of TGW included in this study are or have been CSW (30/31) while MSM have this condition in a significantly lower proportion (6/38), we hypothesized that TGW-related taxa could be linked to sexual behavior. To support this reasoning we compared the sexual behavior variables between TGW and MSM (Table 2). The statistical analysis showed that TGW in our cohort have a history of engaging in oral and receptive anal sex since a younger age and with multiple partners compared to MSM (Supplementary Table 5), which is consistent with their activity as CSW. This suggests that this population may be at greater risk of acquiring sexually transmitted infections. Therefore, we further analyzed the taxa related to CSW condition and found fourteen species positively associated, of which seven were also associated with TGW (p < 0.05; Fig. 2d). These results led us to speculate that microbiome differences among gender in this cohort of participants could be partly attributable to sexual behavior independently of the HIV condition.
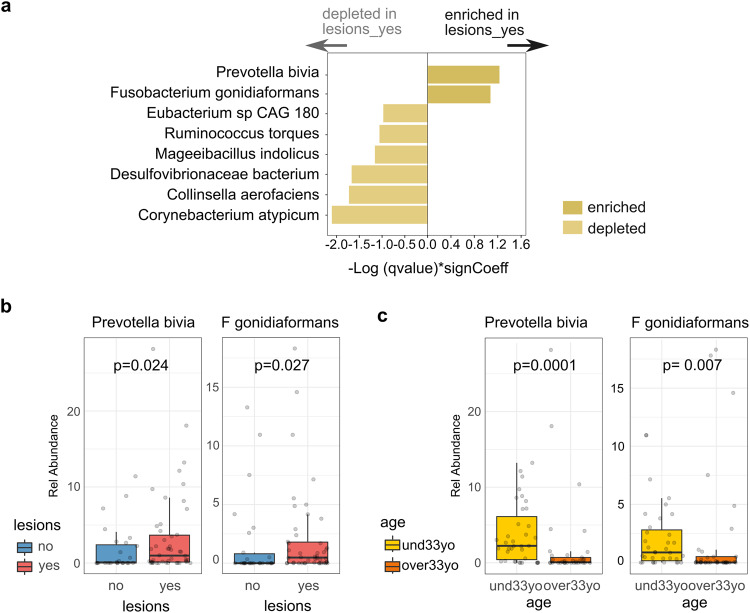
Conversely, the taxonomic profile of groups related to CD4+ T-cell counts, presence of lesions, and VL, showed a distinctive set of associated bacteria with almost no co-occurrence between variables. For the presence of lesions, eight anal bacterial species were significantly associated: Prevotella bivia and Fusobacterium gonidiaformans significantly enriched in patients with lesions; while Mageeibacillus indolicus, Collinsella aerofaciens, Ruminococcus torques, Eubacterium sp CAG 180, Desulfovibrionaceae bacterium and Corynebacterium atypicum were decreased in this group of subjects (Fig. 3a). P. bivia and F. gonidiaformans, in addition to being the two enriched species associated with lesions, highlight for their high abundance and prevalence among subjects (Pr > 0.25; max rel. abundance >0.2), which favors their identification and positions them as interesting markers of the presence of lesions in HIV-positive patients (Fig. 3b).
Fig. 3. Bacteria species associated to the presence of lesions in MSM and TGW HIV related subjects.
a Eight anal species were significantly associated with the presence of lesions (lesions_yes). b P. bivia and F. gonidiaformans, were the only two taxa significantly enriched in the group of subjects with lesions. c P. bivia and F. gonidiaformans were also negatively associated with aging. The p-values displayed on the boxp lots were calculated using the Wilcoxon test.
Remarkable, P. bivia was previously found in penile foreskin microbiome, increasing cytokine production, recruiting HIV-susceptible CD4+ T-cells, and contributing to HIV acquisition36. Moreover, it has recently observed to be significantly higher in samples with cervical intraepithelial neoplasia (CIN), creating a plausible environment for subsequent HPV infection, recurrence of CIN and cancer development37. Regarding F. gonidiaformans, although there is currently no link with premalignant epithelial lesions, it has been associated with other Fusobacterium species with higher abundance in patients with colorectal cancer tumors, meaning a potential association with epithelial malignancy38. We also found that F. gonidiaformans and P. bivia occurred preferentially in younger subjects (Fig. 3c), which would be in accordance with the lower diversity index observed in anal samples from older participants and the higher abundance of the phylum Fusobacteria in the younger group (Fig. 1c; Supplementary Fig. 2).
Furthermore, we estimated the relative risk (RR) associated with the presence of lesions in subjects with P. bivia and F. gonidiaformans in anal samples compared with individuals without detection of these taxa. Results demonstrated that those who had P. bivia or F. gonidiaformans had 2.5 (RR = 2.5; 95%CI = 1.1772−5.3172; p = 0.01) and 2.0 (RR = 2.0; 95%CI = 1.2292−3.2542; p = 0.005) higher risk have anal intraepithelial lesions, respectively, compared with those who did not have the infections (Table 3). Remarkably, when we considered those co-infected with the two species the RR increased to 9.5 (RR = 9.5; 95%CI = 1.4478–62.7231; p = 0.019) compared with those without none of both bacteria (Table 3).
Table 3.
Relative risk (RR) estimation of the presence of P. bivia and F. gonidiaformans in anal samples as predisposing factors to the presence of SILs.
| Lesions | Results | ||||
|---|---|---|---|---|---|
| Groups | Yes | No | RR | 95% CI | p value |
| With P. bivia | 39 | 14 | 2.5 | 1.1772 −5.3172 | 0.017 |
| Without P. bivia | 5 | 12 | |||
| With F. gonidiaformans | 33 | 9 | 2 | 1.2292 –3.2542 | 0.0053 |
| Without F. gonidiaformans | 11 | 17 | |||
| Coinfection with P. bivia and F. gonidiaformans | 27 | 7 | 9.5 | 1.4478–62.7231 | 0.019 |
| Without both bacteria | 1 | 11 | |||
Our results and previous studies provide strong evidence to propose P. bivia and F. gonidiaformans as relevant factors that increases susceptibility to anal epithelial lesions in a cohort of HIV-related MSM and TGW.
CD4+ T-cell counts and HIV viral load are two biomarkers routinely collected from HIV-positive patients to monitor infection and treatment response. We treated the CD4+ T-cell variable as categorical to define taxa associated with the CD4+ T-cell counts group (<500 cells/mm3/>500 cells/mm3). Eight species were identified, six of them increased in the lower CD4+ T-cell counts group (Peptostreptococcus anaerobius, Bacteroides fragilis, Campylobacter ureolyticus, Desulfovibrionaceae bacterium, and Prevotella disiens) and one (Anaerostipes hadrus) enriched in the higher CD4+ T-cell counts group (Supplementary Table 4). Figure 4a shows four relevant enriched species in the lower CD4+ T-cell count group. P. anaerobius has been shown to modulate the immune microenvironment by recruiting immune-suppressive cells that could directly suppress CD4+ and CD8+ T cell activities39. In addition, P. anaerobius and P. disiens were shown to recruit HIV-susceptible CD4+ T cells to the inner foreskin, and were associated with HIV acquisition36. C. ureolyticus has been identified as an emerging gastrointestinal pathogen causative of inflammation, gastroenteritis and diarrhea in patients with HIV infection40,41. B fragilis has been shown to exert potent immunomodulatory activity to protect against a lethal viral neuroinflammatory disease42. Our results define a distinctive group of anal bacteria that would have immunomodulatory effects in HIV-positive patients.
Fig. 4. Relevant bacterial species associated to CD4+ T-cell counts and Viral Load groups.
a Box plots showing the significant relationship of four anal bacterial species with CD4+ T-cell counts. The p-values displayed on the box plots were calculated using the Wilcoxon test. b Relative abundance of H. biformis and C. comes as the most relevant bacterial species associated with detectable VL in HIV-positive patients. The p-values displayed on the boxp lots were obtained from the multivariate analysis. c Venn diagram showing common species positively associated with detectable VL and ART untreated variable groups. det. = detectable, undet. = undetectable. To determine whether the observed intersection in the Venn diagram is statistically significant Fisher’s exact test was used.
For the VL group our analysis identified seven species positively associated with detectable HIV (Holdemanella biformis, Coprococcus comes, Ruthenibacterium lactatiformans, Desulfovibrionaceae bacterium, Bifidobacterium longum, Gemmiger formicilis and Roseburia faecis) and three negatively associated (Neisseria flavescens, Anaerostipes hadrus and Actinomyces turicensis) (Supplementary Table 4). C. comes (p = 0.004; Pr > 0.3) and H. biformis (p = 0.002; Pr > 0.3) were the most prevalent and positively associated with the anal samples (Fig. 4b), while N. flavescens (p < 0.003; Pr > 0.4) was the most prevalent and negatively associated with the oral samples (Supplementary Table 4).
C. comes and H. biformis were among the depleted bacteria in HIV-positive compared with HIV-negative (Fig. 2c), indicating that HIV-positive patients stratify into two groups regarding the enrichment of C. comes and H. biformis in detectable VL. To investigate whether this difference could be ascribed to the administration of ART treatment, we compared the abundance of bacteria in treated and untreated patients. By utilizing Venn diagrams, we found that the prevalence of C. comes, H. biformis, and three other species (B. longum, R. faecis, and R. lactatiformis) were consistently associated with both detectable viral load and ART-untreated patients (Fig. 4c). In this regard, it was reported that H. biformis is enriched in MSM where most individuals were not on treatment. In addition, H. biformis was enriched in untreated HIV-positive MSM or on a diversity of different ART drugs43. Similarly, C. comes was found to be enriched in a group of HIV-infected patients categorized as immunological ART non-responders compared to the group of patients immunologically responsive to treatment44. Together, our results and similar findings from other studies suggest that both H. biformis and C. comes could be relevant markers of immune response to ART treatment in HIV-infected patients.
Microbial pathways and functional genes
The relative abundance of pathways (PWYs) concerning the different groups was analyzed by MaAsLin2. We identified fifty-two pathways associated with oral and anal sites contributed by fifty-four species, of which twenty-six were prevalent in the oral site and twenty-eight in the anal site (Fig. 5a; Supplementary Table 6). The most overrepresented pathways (>50% species) were adenosine ribonucleotides de novo biosynthesis (PWY-7219), pyruvate fermentation to isobutanol (PWY-7111), 5-aminoimidazole ribonucleotide biosynthesis II (PWY-6122; PWY-6277), L.valinebiosynthesis (VALSYN.PWY) and guanosine ribonucleotides de novo biosynthesis (PWY-7221). The top five contributing species were H haemolyticus, H parainfluenzae, S mitis, S oralis, and N mucosa for the oral site, and F prausnitzii, P copri, P sp AM42 24, C mitsuokai, and F gonidiaformans for the anal site (Fig. 5a).
Fig. 5. Pathway profile in a multivariable association between phenotypes and microbial species.
a Heatmap representation of the fifty-two PWYs associated with oral and anal sites contributed by fifty-four species. b Scatter plot showing the relationship of the top significant differentially abundant PWYs associated with the different groups of variables, represented by their reference values (highlighted in bold): gender, TGW; age, und33yo; CSW, yes; HIV, positive; ART, yes; VL, detectable (det.); Lesions, yes; CD4+ T-cell counts (<500cells/mm3/>500cells/mm3). The full name of PWYs is available in Supplementary Table 6.
The multivariate analysis showed an enrichment of numerous pathways in TGW, CSW, younger subjects (under33yo), and subjects with lesions (Supplementary Table 7). In contrast, depletion of several distinctive pathways was observed for the groups on ART, HIV-positive and low CD4+ T-cell counts (Fig. 5b). In this sense, our results reveal two related but different settings: on one side the enrichment of several metabolic PWYs associated with younger subjects, TGW, Commercial Sex Workers, and subjects with precancerous anal lesions; on the other a predominant decrease in various pathways associated with people with HIV, detectable VL, ART treatment, and low CD4 cell counts, variables more related to the HIV condition (Fig. 5b). These pathways involved the biosynthesis of the basic amino acids arginine, histidine, and lysine and the hydrophobic one’s valine, methionine and isoleucine. We also found a depletion of oxidative and energetic processes such as pyruvate metabolism, glycolysis, and lipid biosynthesis (Supplementary Table 7). The few enriched pathways identified in HIV-infected cases were related to the ubiquinol biosynthesis (PWY-5855, PWY-5856, PWY-5857, PWY-6708); remarkably, they were found depleted in the group of detectable VL (Fig. 5b).
The taxa´s contribution to pathways differences were attributable to a few species associated with the defined conditions (Supplementary Table 7). These results would be consistent with the hypothesis that HIV-associated dysbiosis is independent of sexual practice or sexual preferences45,46.
Furthermore, to assess the potential functionality of oral and anal microbiota in a multivariable analysis based on bacterial genomes, microbial gene families obtained from MaAsLin2 (p < 0.05; Pr > 0.2; Supplementary Table 8) were annotated and analyzed using different databases and functional systems: UniProt, KEGGREST, KEGGMapper, MinPath. We were able to define gene signatures related to HIV infection, the presence of lesions and detectable viral load (Fig. 6).
Fig. 6. Differentially abundant bacterial genes between the groups of the variables: HIV, viral load and presence of lesions, and their associated KEGG pathways names.
a HIV-positive vs. HIV-negative differentially abundant genes. Red color indicates a positive association with the reference value (HIV-positive); green color indicates a negative association with the reference value. b VL detectable vs. VL undetectable; reference value = VL detectable. In the bottom, it is indicated which genes are also differentially abundant among ART treated vs. ART untreated. Orange color indicates a positive association with the reference value (ART untreated) Blue color indicates a negative association with the reference value. c Presence of lesions_yes vs presence of lesions_no. Red color indicates a positive association with the reference value (lesions_yes); green color indicates a negative association to the reference value. d Venn diagram showing common genes among signatures of genes.
HIV-positive was mainly enriched by genes related to RNA metabolism (pnp. DNAk, ENO, rpoB, rpoC), glycolysis/glyconeogenesis (ENO, PGK), amino acid metabolism (gatB, ilvD, PGK, pnp, PFAS, mnmA, etc.) and HIF1-signaling (ENO, PGK) (Fig. 6a). Of these genes, the so-called moonlighting genes (which have more than one biological function), DNAk and ENO, stand out. DnaK is a plasminogen-binding protein that upregulates APOB3G expression in CD4+ T cells and, more importantly, competes with HIV for binding to CCR5, and can block HIV binding47,48. ENO (enolase) is a prototypic moonlighting protein, in both prokaryotes and eukaryotes, with putative roles in a variety of human diseases49. Remarkably, human enolase ENO1 has recently been shown to suppress HIV-1 integration in viral target cells50. Although more studies are needed, bacterial DnaK and ENO could have some therapeutic potential in HIV-positive subjects.
Among HIV-positive with detectable VL, we identified a group of enriched genes (Tetx, nisG, VicR, hmo, phoB) associated with antimicrobial resistance and sensing of stressful conditions (Quorum sensing, two-component system) (Fig. 6b). Tetx monooxygenase showed the highest significant difference (p = 0.009). This enzyme catalyzes efficient degradation of a broad range of tetracycline analogs and conferred resistance to these antibiotics in vivo51. Recently, it was shown that the ART drug zidovudine decreases Tetx-mediated bacterial resistance52. Consistent with this, we found that Tetx along with genes enriched in the detectable VL group (VicR, hmo, phoB), were also enriched in the group of patients who did not receive treatment compared with the ART-treated group, and vice versa (Fig. 6b), suggesting these genes could be relevant markers of ART treatment response.
On the other hand, genes identified in the group of subjects with lesions were all enriched in this group (Fig. 6c). These genes are involved in several processes related to amino acids metabolism (glyA, leuD, glyQ, trpE, aroC, etc.) carbohydrates metabolism (ENO, por, PGK, PGAM) and nucleotide metabolism (purN, ushA, pyrG, etc.), among others (Fig. 6c). Finally, we found the ENO gene common to all three signatures reinforcing the idea of the potential relevancy of enolase as a gene marker to be investigated in the context of HIV and related diseases (Fig. 6d).
Discussion
Anal and oral Virome of HIV-infected patients and related factors
Virome of anal samples was mainly represented by species of Alphapapillomavirus, being Alphapapillomavirus 10 (HPV6, HPV11), 9 (HPV16), 3 (HPV72), and 7 (HPV18) among the most prevalent. It is established that HIV infection is associated with a greater HPV capacity to persist and increase the risk and progression of precancerous anal intraepithelial lesions. We found a significant enrichment of the oncogenic HPV16 in HIV-infected subjects with anal intraepithelial lesions, reinforcing the fact these patients are at an increased risk of anal cancer53. Furthermore, lesions also correlated with enrichment of HPV18, HPV6, and HPV72, independently of HIV condition. In addition, HPV18 and HPV72 were more prevalent in TGW compared with MSM. Overall, the results indicate that HPV16 would be the main driver in the progression of anal lesions in HIV-infected patients. TGW showed a higher susceptibility to HPV infection.
Human alphaherpesvirus 2 (HSV-2) was detected in anal samples associated with decreased CD4+ T-cell counts independently of HIV infection. It has been postulated that HSV-2 and HIV coinfection accelerate HIV disease, characterized by a persistent decline of CD4+ T cells24,25,54. Whether HSV-2 and HIV synergistically contribute to CD4+ T cell depletion, our results suggest that HSV-2 might be a marker of immunocompromised individuals in the context of HIV.
The oral virome of our cohort showed enrichment in bacteriophages, KSHV (HHV-8) and HHV-7, among others, being KSHV significantly predominant in HIV-positive and TGW independently of sexual behavior, compared with HIV-negative and MSM, respectively. Since KHSV is highly oncogenic in HIV-infected patients, the TGW of our cohort would be at higher risk of Kaposi sarcoma development. These results also suggest that non-sexual routes may explain the transmission of KSHV in the TGW cohort55.
HIV-associated microbiome and related factors
Previous studies have demonstrated that HIV infection and related factors exert profound changes in the gut microbiome. Most agree on a decrease in the richness and diversity indices with consequences in inflammatory processes and immune activation34,56–58. Consistent with those findings, our results showed lower indices in the anal samples of older subjects, HIV-positive patients, and the group of patients with lesions. Aging, HIV, and lesions are known to affect immunological status with an impact on the gut microbiome9,32,59,60. In particular, aging and HIV share features of intestinal damage and alterations in the communities of gut bacteria conducting to dysbiosis60. Less is known about diversity variations and the presence of anal SILs. However, several studies have suggested that microbiome variations contribute to cervical intraepithelial neoplasia (CIN) development9,61.
HIV infection significantly correlated with a decrease in species of Lachnospiraceae, Ruminococcaceae, Erysipelotrichaceae, Porphyromonadaceae or Rikenellaceae. In previous studies, taxa of these families have been found mostly depleted in HIV infection62–66.
Among the species found decreased, C. comes (Lachnospiraceae) and H. biformis (Erysipelotrichaceae) were associated with VL and ART treatment. It has been shown that C. comes has immunostimulatory properties in the acute phase response, which is the first immune response signal to HIV-1 infection with the increase of acute-phase reactants67,68. Additionally, C. comes was found to negatively correlate with the production of IL-22, a cytokine that downregulates CCR5 expression through induction of acute phase proteins68,69. These studies suggest C. comes plays a relevant role in the initial phase of HIV infection, modulating acute phase response with pro- or anti-inflammatory factors to manipulate the immune system.
Similarly, H. biformis was found to be enriched in untreated MSM patients and associated with an increased abundance of CCR5+CD4+ T cells, increasing the risk of HIV transmission32,43.
Our analysis identified that both C. comes and H. biformis were significantly enriched in untreated patients with detectable VL compared to individuals on ART and undetectable VL. Together, our results and those found in other studies indicate that C. comes and H. biformis could potentially accelerate HIV progression while the patient is not receiving treatment, placing them as potential markers of the immune response to ART treatment in HIV-infected patients.
While the microbiome has been well documented in the gut mucosa, less is known about the oral mucosa in the HIV context, particularly in TGW31,70. Another important finding that emerged from our analysis is the enrichment of bacterial species associated with periodontal pathological processes in the oral mucosa of HIV-positive. We identified common residents of the oral cavity such as S. salivarius, S. parasanguinis, and S. cristatus, as well as an enrichment of anaerobic bacteria such as A. odontolyticus, L. hofstadii, L. wadei, C. concisus, P. salivae, and V. atypica. Although many of these taxa have been previously characterized in the context of periodontal processes, little is known about their association with HIV. In this regard, compared with negative controls, S. anginosus and A. odontolyticus were found enriched in the gut of HIV-positive subjects on ART19. S. cristatus has been linked to the inhibition of HIV replication through the induction of APOBEC3 expression, while elevated levels of C. concisus were associated with detectable plasmatic VL in HIV-positive individuals71,72.
Interestingly, we also identified a signature of oral species associated with gender and/or sexual behavior independently of HIV condition. These species differed from those found associated with HIV infection, and were all enriched in TGW compared with MSM, and many were also prevalent in the group of CSW. Similarly, three species were found significantly enriched in the anal samples of both TGW and CSW groups. Importantly, many species enriched in TGW have been related to female genitalia and/or sexual transmission. G. asaccharolytica has been described as a vaginal bacteria associated with an increased risk of HIV infection in unprotected sex73. M. indolicus is a recently described species originally isolated from the female genital tract74. H. parainfluenzae has been postulated to be a sexually transmitted genitourinary pathogen among MSM75. G. vaginalis is mainly detected in women with bacterial vaginosis; conversely, it is uncommon in men or at sites other than the female genitalia; however, people with more sex partners and multiple sex partners are more at risk for getting infections of G. vaginalis76.
The fact that TGW of our cohort are most CSW led to the inescapable assumption that microbiome differences observed in this group are linked to sexual behavior. However, it is important to consider that TGW are often exposed to feminizing hormone therapy, which can impact microbiome composition77. In this regard, the higher abundance of bacteria usually found in the female genital tract, in the anal samples of TGW, compared with MSM, allows us to justify further studies to investigate this approach.
Furthermore, among the most enriched bacteria in the oral mucosa of TGW was F. nucleatum, a periodontal pathogen that can promote cancer by several mechanisms. It has been related to oral squamous cell carcinoma and colorectal cancer78. This finding, along with the higher prevalence of the oncogenic viruses KSHV and HPV-18 in TGW compared with MSM, suggest that this population is not only at increased risk of HIV infection but also of oncogenic infections.
Microbiome changes associated with HPV-related precancerous anal lesions
A recent study revealed that mucosal bacteria could predict the presence of anal lesions in HIV-infected MSM79. Here, we provided important evidence of the association of bacterial taxa at species level and the presence of anal lesions in both MSM and TGW subjects.
Two anal species were significantly enriched: P. bivia and F. gonidianformans. Remarkably, P. bivia has been related to high-risk HPV persistent infection in vaginal microbiome80. Moreover, in a later study P. bivia was found significantly higher in samples of pre-menopausal, non-pregnant women with CIN before and after excision treatment compared with control samples with normal cytology37. Furthermore, we estimated the RR of P. bivia and found that subjects with the infection had 2.5 times more likely to have anal intraepithelial lesions, compared with those who did not have detectable P. bivia. In addition, the RR increased to 9.5 in subjects with both P. bivia and F. gonidaformans, the latter related to colorectal malignancies. Therefore, we consider that P. bivia and F. gonidiaformans would be two relevant bacterial infections associated with the development of precancerous anal lesions.
In this study, we also defined a distinctive group of anal bacteria associated with low CD4+ T-cell counts (P. anaerobious, C. ureolyticus, P. dissiens and B. fragilis) that would have immunomodulatory effects in HIV-positive patients37,39–42.
Functional pathways and metabolic genes in HIV and related factors
We next analyzed the functional consequences of HIV infection and related factors. Pathways analysis revealed a significant decrease of several pathways in HIV-positive patients, mainly contributed by anal bacteria. These pathways involve, the biosynthesis of amino acids, pyruvate metabolism, glycolysis and lipid biosynthesis among others. It has been reported that an impaired metabolic capacity of the HIV gut microbiota to produce amino acids, which would be in line with our results81. Furthermore, the depletion of energetic bioprocesses would be related to the nutritional deficits observed in HIV disease82.
Interestingly, of the few enriched pathways in HIV-positive, the synthesis of ubiquinol (coenzyme Q7-10) stands out since these pathways were found to be depleted in the group of patients with detectable VL. Bacterial coenzyme Q contributes to the overall anti-oxidative stress system, antibiotics resistance, and modulation of bacterial virulence83. Diverse studies have suggested that HIV infection is characterized by oxidative stress contributing to several aspects of HIV disease pathogenesis, including viral replication, inflammatory response, loss of immune function, and chronic weight loss84. In addition, HIV may contribute to a decreased ability of the antioxidant system to control oxidative stress and increase HIV replication85. Thus, it is possible that the enrichment of bacteria that synthesize coenzyme Q benefits HIV patients, contributing to a relief of oxidative stress caused by the virus.
Pathways multivariable analysis revealed a significant enrichment of several metabolic processes in younger subjects, TGW, CSW, and subjects with precancerous anal lesions, all groups characterized by subjects with high frequency of sexual intercourse. While a predominant decrease of others bioprocess was associated with aging, HIV infection, detectable VL, ART treatment, and low CD4+ T-cell counts, variables more related to HIV pathogenesis. These results indicate the differential impact caused on the metabolic pathways of the microbiota by different but related factors. In addition, it would be in line with the fact that sexual practice has been revealed as a major source of microbiota variation, confounding prior interpretations of gut microbiota alterations among subjects with HIV45,46. Such studies have suggested that the differences between the gut microbiota of HIV-positive and negative subjects can be attributed in part to sexual preference, thus increasing the possibility that both scenarios, despite being closely related, contribute to a distinctive microbial microenvironment.
We finally described microbial gene richness associations with HIV infection, viral load, and the presence of lesions. For HIV-positive, we identified gene signatures related to oxidative and energy metabolism. Although some of these genes belong to bacterial proteins that are still poorly characterized, others deserve special attention since they have been previously associated with HIV as being involved in mechanisms of response to the infection (DNAk, ENO) or to the ART treatment (tetX)47–51 In addition, it is worth mentioning vicR and phoB, genes associated with response to nutritional stress, which were identified along with Tetx significantly more abundant in patients with detectable viral load. Together, these genes may constitute relevant markers of the presence of bacteria that respond to the stressful conditions generated by HIV disease.
Similarly, we defined a signature of genes enriched in the group of patients with lesions, many of them contributed by F. gonidiaformans and P. bivia, thus constituting potential prognostic biomarkers of developing anal intraepithelial lesions.
Overall, this study provides a comprehensive characterization of the oral and anal microbiome in a cohort of high-risk HIV-exposed MSM and TGW subjects from Argentina. Shotgun metagenomics sequencing allowed us to define viral and bacterial taxa associated with the subject´s gender, sex behavior, HIV infection, and related variables such as CD4+ T-cell counts, HIV viral load, and the development of precancerous anal lesions. Our results confirm the occurrence of oncogenic viromes in this high HIV-risk population. We also showed that TGW in our cohort may be at a greater risk of acquiring sexually transmitted infections explained in part by the sexual behavior of this group. However, we acknowledge that engagement in sex work and the influence of different sexual behaviors on the microbiome is complex, and care should be taken in interpreting the data. As we did not include a group of TGW who do not participate in sex work, we cannot generalize our findings to all TGW. We propose C. comes and H. biformis as potential markers of the immune response to ART treatment in HIV-infected patients. Moreover, we identified P. bivia and F. gonidiaformans as two relevant components that predispose to anal intraepithelial lesions. We showed that metabolic pathways are distinctively affected according to whether the predominant factors are associated with sexual behavior or HIV pathogenesis. Gene family analysis identified bacterial gene signatures associated with HIV status, viral load, ART, and the presence of lesions, which may have potential as prognostic and predictive biomarkers of HIV/AIDS-associated malignancies. Furthermore, our results confirm previous results from other studies and provide new insights that need to be explored in future studies.
Methods
Study participants
This study included an Argentinian cohort of HIV-positive and HIV-negative cases of TGW and MSM recruited at Fundación Huésped, Buenos Aires, Argentina. Participants were age > 18 at the beginning of the study (median: 33 years; range: 19–58 years).
Ethics approval and consent to participate
All participants included in this study signed informed consent before being involved in the project. The study was approved by the institutional review board (Comité de Bioética, Fundación Huésped).
Samples collection and DNA purification
A total of 130 samples (59 oral swabs and 71 anal swabs) were obtained from 47 MSM and 31 TGW.
Samples were collected in Qiagen specimen collection device (Qiagen, USA) by qualified staff at Fundación Huésped. DNA purification was performed with QIAMP DNA kits (Qiagen, USA). DNA integrity and concentration was evaluated and measured on Agarose (0.7%) gels electrophoresis stained with GelGreen® and Nanodrop spectrophotometer, respectively.
Data collection and variables
The study collected data from participants on clinical variables, sexual practices and consumption habits, which are displayed in Tables 1 and 2, respectively. Clinical data included gender, age, HIV status, current CD4+ T-cell counts, current viral load (VL), use of antiretroviral therapy (ART), and anal cytology results indicating the presence of epithelial lesions, specifically Atypical Squamous Cells of Undetermined Significance (ASCUS), Low-grade Squamous Intraepithelial Lesion (LSIL), and High-Grade Squamous Intraepithelial Lesion (HSIL). Sexual behavior variables comprised Age at First Sexual Intercourse (AFSI), Age of Anal Sex Initiation (AASI), Age of Oral Sex Initiation (AOFSI), Number of Sexual Partners in the Last Month (NSPLM), Number of Sexual Partners in Life (NSPL), Condom Use in Anal (CUAS) and Oral Sex (CUOS), and whether participants are or have been Commercial Sex Workers (CSW). It is important to note that none of the TGW included in the study have undergone gender-affirming surgery. Consumption habits included Alcohol, Tobacco, and intravenous or non-intravenous drug use. All metadata of samples and participants is available in Supplementary Table 1.
Shotgun metagenomics sequencing and data pre-processing
DNA samples were subject to cleanup and then processed for shotgun metagenomics sequencing using the PCR-free KAPA HyperPLUS (Roche, France) library preparation kit at the Onco-Genomics Shared Resource (OGSR, University of Miami, USA). We performed 100 nt paired-end sequencing using an Illumina NovaSeq 6000 System and obtained about 80 million reads per sample.
Raw data (510 GiB) was downloaded from Illumina BaseSpace to our dedicated server using BaseMount tools, and processed with the bioBakery toolkits86. Quality control on metagenomics sequencing data (Fastq files), were performed with KneadData tools, which separate the human (host) and the non-human (microbiome) reads for further QC-based filtering. The filtered reads from KneadData were then aligned and mapped to all reference microbiome genome sequences to determine the presence and abundance of different taxonomic groups among 130 DNA samples derived from oral and anal swabs. Host-removed metagenome sequences have been submitted to the SRA database with accession number PRJNA983219.
Taxonomic profiling, richness, and diversity
For profiling the composition of microbial communities at the species level, we run MetaPhlAn 3.0 using default parameters. MetaPhlAn relies on unique clade-specific marker genes identified from ~17,000 reference genomes (~13,500 bacterial and archaeal, ~3500 viral, and ~110 eukaryotic)86. This bioinformatics tool provides the relative abundances of each microbial clade with species-level resolution.
Species richness and diversity were calculated using R function estimate_richness from R package “phyloseq”. We considered the observed species and Chao1 indices for richness, and the Shannon and Simpson indices for diversity. Beta diversity was measured by Bray–Curtis, weighted UniFrac, and unweighted UniFrac. For Principal Coordinate Analysis, the Aitchison distance was used as the distance metric to analyze the compositional data87. To test whether the samples cluster beyond that expected by sampling variability we applied permutational multivariate analysis of variance (PERMANOVA). Differences in richness and diversity indices between the two groups were determined using the Wilcoxon rank sum test with a significance level of 0.05. For relative abundance analysis and visualization, we used R phyloseq and HMP packages87,88.
Differential abundance analysis
For determining the relative differential abundance and the multivariable association between subjects’ metadata and microbial features, we used the MaAsLin2 package from the bioBakery suite in R/Bioconductor89. We used default parameters for normalization (TSS method), transformation (Log), analysis method (LM), correction method (BH), and significance threshold (q-value < 0.25). Although association in MaAsLin 2.0 is considered significant at a q-value of below 0.25, a cut-off used in previous microbiome studies, for more stringency we selected the associated features (species, pathways, gene families) with a q-value < 0.15 (nominal p-value < 0.05)86,89–91. The minimum abundance for each feature was set to 0.001 (0.1%) while the minimum percent of samples for which a feature was detected (prevalence, Pr) at minimum abundance was used as follows: 0.05 (5%) for viruses, 0.1 (10%) for bacteria and pathways and 0.2 (20%) for gene families. To identify features that differ between sample categories, variables were defined and dichotomized as follows: site (oral/anal); gender (TGW/MSM); age (based on the median, under33yo/over33yo); CSW (yes/no); HIV status (positive/negative); current CD4+ T-cell counts (<500cel/mm3/>500cel/mm3); current VL (detectable: >50copies/ml, undetectable:<50copies/ml); for anal cytology, we grouped ASCUS (n = 3), LSILs (n = 40) and HSILs (n = 8) into the variable name presence of lesions (yes/no). For coefficients, referred to as the contrast between the category specified versus the reference category, we considered as reference category the first term or “numerator” of the dichotomous annotation89. Variables of consumption habits were included in the multivariate model to account for their potential impact on the microbiome as confounding factors. However, they were not the primary focus of this study and were only considered as covariates to adjust for additional factors.
Pathways and gene family analysis
Whole genome metagenomics pathway analysis was adopted in the HMP Unified Metabolic Analysis Network 3 (HUMAnN3) pipeline to assess the potential differences in metabolic pathways. HUMAnN3 identifies the species profile from metagenomics shotgun sequencing data, aligns reads to their pan-genomes, performs translated search on unclassified reads, and quantifies gene families and pathways86. By default, gene families are annotated using the comprehensive protein database UniRef9092 and metabolic pathways are annotated using MetaCyc database93. The UniRef90 gene family abundance from HUMAnN3 was then regrouped to Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology (KO)94. We used the KEGGREST package in R/Biconductor for KO identifiers95 and KEGG Mapper reconstruct tool for KEGG pathway maps and MinPath to biological pathway reconstruction/inference96,97.
Statistical analysis and data visualization
Heatmap representation of taxa abundances was done with the R package “phyloseq”. For the unsupervised ordination of samples, we applied the NMDS method and Bray distance in the plot_heatmap function. Heatmap visualization of differentially represented taxa and pathways was done with R/Bioconductor and the MultiExperiment Viewer software (MeV v4.9). For the different statistical comparisons outside of MaAsLin’s analysis, we used R/Bioconductor. In order to analyze continuous variables, we utilized either t tests or Wilcoxon tests as appropriate. For categorical data, we employed Chi-squared and Fisher tests.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors want to acknowledge the invaluable contributions from all study participants and from all the research team at Fundación Huésped, where participants were recruited. This work was supported by the NIH grant CA221208 (to E.A.M. and O.C.).
Author contributions
E.L.: conceptualization, investigation, methodology, formal analysis, writing—original draft, writing—review and editing. V.F.: resources; supervision and logistics of obtaining samples and clinical data of participants, manuscript review. M.E.S.: methodology, manuscript review. RC: methodology, manuscript review. J.N.: methodology, manuscript review. S.W.: methodology. O.C.: funding acquisition, manuscript review. O.S.: resources; logistics for obtaining samples, manuscript review. P.C.: funding acquisition, supervision, manuscript review. E.A.M.: funding acquisition, supervision, manuscript review M.C.A.: Conceived the study, funding acquisition, supervision, methodology, manuscript review, and editing. All authors contributed to the article and approved the submitted version. We want to express our heartfelt appreciation for the invaluable contributions of Dr. Enrique Mesri, the core and soul of the UM/SCCC—Argentina Consortium for research and training in Virally Induced AIDS Malignancies. Although he could not review the final version of this manuscript before submission, it is important to acknowledge his crucial role in reviewing all preliminary versions. Furthermore, Dr. Mesri’s expertise greatly aided in implementing methods, managing logistics, and allocating resources. We are sincerely grateful for his unwavering involvement and dedication to the study.
Data availability
Host-removed metagenomics sequences are available on the NCBI SRA database with the following link: https://www.ncbi.nlm.nih.gov/sra/PRJNA983219. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Code availability
All open-source codes used to analyze and visualize the data of this study are available in bioBakery and R/Bioconductor websites: GitHub - biobakery/biobakery: bioBakery tools for meta’omic profiling bioconductor.org/packages/release/bioc/vignettes/phyloseq/inst/doc/phyloseq-analysis.R; bioconductor.org/packages/release/bioc/vignettes/Maaslin2/inst/doc/maaslin2.R.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ezequiel Lacunza, Email: ez.lacunza@gmail.com.
Martín C. Abba, Email: mcabba@gmail.com
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-023-00413-4.
References
- 1.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogunrinola GA, Oyewale JO, Oshamika OO, Olasehinde GI. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020;12:8045646. doi: 10.1155/2020/8045646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewhirst FE, et al. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 5.Vujkovic-Cvijin I, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013;5:193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak P, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS. 2015;29:2409–2418. doi: 10.1097/QAD.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 7.Dillon SM, Frank DN, Wilson CC. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS. 2016;30:2737–2751. doi: 10.1097/QAD.0000000000001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curty G, et al. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci. Rep. 2017;7:17364. doi: 10.1038/s41598-017-17351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein C, et al. Relationship between the Cervical Microbiome, HIV Status, and Precancerous Lesions. mBio. 2019;10:e02785–18. doi: 10.1128/mBio.02785-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruffaz M, et al. Signatures of oral microbiome in HIV-infected individuals with oral Kaposi’s sarcoma and cell-associated KSHV DNA. PLoS Pathog. 2020;16:e1008114. doi: 10.1371/journal.ppat.1008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavallin LE, Goldschmidt-Clermont P, Mesri EA. Molecular and cellular mechanisms of KSHV oncogenesis of Kaposi’s sarcoma associated with HIV/AIDS. PLoS Pathog. 2014;10:e1004154. doi: 10.1371/journal.ppat.1004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabtree-Ramírez B, et al. The HIV epidemic in Latin America: a time to reflect on the history of success and the challenges ahead. J. Int. AIDS Soc. 2020;23:e25468. doi: 10.1002/jia2.25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabay P, et al. Lymphotropic viruses EBV, KSHV and HTLV in Latin America: epidemiology and associated malignancies. a literature-based study by the RIAL-CYTED. Cancers (Basel) 2020;12:2166. doi: 10.3390/cancers12082166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baral SD, et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect. Dis. 2013;13:214–222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 15.de Salud M., de la Nación P., 2020. Boletín sobre el VIH, sida e ITS en la Argentina. Año XXIII. 37. Diciembre 2020, Argentina.
- 16.Socías ME, et al. Factors associated with healthcare avoidance among transgender women in Argentina. Int J. Equity Health. 2014;13:81. doi: 10.1186/s12939-014-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radusky PD, et al. Examining factors associated with gender identity among individuals disengaged from HIV care in Argentina. Int J. Behav. Med. 2022;29:69–77. doi: 10.1007/s12529-021-09998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrera S, Martínez-Sanz J, Serrano-Villar S. HIV, cancer, and the microbiota: common pathways influencing different diseases. Front. Immunol. 2019;10:1466. doi: 10.3389/fimmu.2019.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai X, et al. Whole-genome metagenomic analysis of the gut microbiome in HIV-1-infected individuals on antiretroviral therapy. Front Microbiol. 2021;12:667718. doi: 10.3389/fmicb.2021.667718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birse KD, et al. The neovaginal microbiome of transgender women post-gender reassignment surgery. Microbiome. 2020;8:61. doi: 10.1186/s40168-020-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellsagué X, et al. Human papillomavirus detection in cervical neoplasia attributed to 12 high-risk human papillomavirus genotypes by region. Papillomavirus Res. 2016;2:61–69. doi: 10.1016/j.pvr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, He J. Distribution of high-risk HPV types among women in Sichuan province, China: a cross-sectional study. BMC Infect. Dis. 2019;19:390. doi: 10.1186/s12879-019-4038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clifford GM, et al. Determinants of high-grade anal intraepithelial lesions in HIV-positive MSM. AIDS. 2018;32:2363–2371. doi: 10.1097/QAD.0000000000001947. [DOI] [PubMed] [Google Scholar]
- 24.Looker KJ, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect. Dis. 2017;17:1303–1316. doi: 10.1016/S1473-3099(17)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan DH, et al. Herpes simplex virus type 2 coinfection does not accelerate CD4 count decline in untreated HIV infection. Clin. Infect. Dis. 2013;57(3):448–457. doi: 10.1093/cid/cit208. [DOI] [PubMed] [Google Scholar]
- 26.Garnett JA, et al. Structural insight into the role of Streptococcus parasanguinis Fap1 within oral biofilm formation. Biochem. Biophys. Res. Commun. 2012;417:421–426. doi: 10.1016/j.bbrc.2011.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langfeldt D, et al. Health- and disease-associated species clusters in complex natural biofilms determine the innate immune response in oral epithelial cells during biofilm maturation. FEMS Microbiol. Lett. 2014;360:137–143. doi: 10.1111/1574-6968.12596. [DOI] [PubMed] [Google Scholar]
- 28.Krzyściak W, et al. Effect of a Lactobacillus Salivarius probiotic on a double-species Streptococcus Mutans and Candida Albicans Caries biofilm. Nutrients. 2017;9:1242. doi: 10.3390/nu9111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eribe ERK, Olsen I. Leptotrichia species in human infections II. J. Oral. Microbiol. 2017;9:1368848. doi: 10.1080/20002297.2017.1368848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salliss ME, Maarsingh JD, Garza C, Łaniewski P, Herbst-Kralovetz MM. Veillonellaceae family members uniquely alter the cervical metabolic microenvironment in a human three-dimensional epithelial model. NPJ Biofilms Microbiomes. 2021;7:57. doi: 10.1038/s41522-021-00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coker MO, Cairo C, Garzino-Demo A. HIV-associated interactions between oral microbiota and mucosal immune cells: knowledge gaps and future directions. Front. Immunol. 2021;12:676669. doi: 10.3389/fimmu.2021.676669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman SL, et al. Can gut microbiota of men who have sex with men influence HIV transmission? Gut Microbes. 2020;11:610–619. doi: 10.1080/19490976.2019.1700756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillén Y, et al. Low nadir CD4+ T-cell counts predict gut dysbiosis in HIV-1 infection. Mucosal Immunol. 2019;12:232–246. doi: 10.1038/s41385-018-0083-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, et al. Signature changes in gut microbiome are associated with increased susceptibility to HIV-1 infection in MSM. Microbiome. 2021;9:237. doi: 10.1186/s40168-021-01168-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanavich C, et al. A pilot study of microbial signatures of liver disease in those with HIV mono-infection in Rio de Janeiro, Brazil. AIDS. 2022;36:49–58. doi: 10.1097/QAD.0000000000003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prodger JL, et al. Penile bacteria associated with HIV seroconversion, inflammation, and immune cells. JCI Insight. 2021;6:e147363. doi: 10.1172/jci.insight.147363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitra A, et al. The vaginal microbiota and innate immunity after local excisional treatment for cervical intraepithelial neoplasia. Genome Med. 2021;13:176. doi: 10.1186/s13073-021-00977-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussan H, Clinton SK, Roberts K, Bailey MT. Fusobacterium’s link to colorectal neoplasia sequenced: a systematic review and future insights. World J. Gastroenterol. 2017;23:8626–8650. doi: 10.3748/wjg.v23.i48.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long X, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019;4:2319–2323. doi: 10.1038/s41564-019-0541-3. [DOI] [PubMed] [Google Scholar]
- 40.Molina J, et al. Campylobacter infections in HIV-infected patients: clinical and bacteriological features. AIDS. 1995;9:881–885. doi: 10.1097/00002030-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 41.O’Donovan D, Corcoran GD, Lucey B, Sleator RD. Campylobacter ureolyticus: a portrait of the pathogen. Virulence. 2014;5:498–06. doi: 10.4161/viru.28776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramakrishna, et al. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019;10:2153. doi: 10.1038/s41467-019-09884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada E, et al. Intestinal microbial communities and Holdemanella isolated from HIV+/- men who have sex with men increase frequencies of lamina propria CCR5+ CD4+ T cells. Gut Microbes. 2021;13:1997292. doi: 10.1080/19490976.2021.1997292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu W, et al. Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front. Microbiol. 2018;9:1451. doi: 10.3389/fmicb.2018.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noguera-Julian M, et al. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine. 2016;5:135–146. doi: 10.1016/j.ebiom.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vujkovic-Cvijin I, et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat. Commun. 2020;11:2448. doi: 10.1038/s41467-020-16222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittall T, et al. Interaction between the CCR5 chemokine receptors and microbial HSP70. Eur. J. Immunol. 2006;36:2304–2314. doi: 10.1002/eji.200635953. [DOI] [PubMed] [Google Scholar]
- 48.Babaahmady K, Oehlmann W, Singh M, Lehner T. Inhibition of human immunodeficiency virus type 1 infection of human CD4+ T cells by microbial HSP70 and the peptide epitope 407-426. J. Virol. 2007;81:3354–3360. doi: 10.1128/JVI.02320-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson B, Martin A. Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011;79:3476–3491. doi: 10.1128/IAI.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kishimoto N, et al. Alpha-enolase in viral target cells suppresses the human immunodeficiency virus type 1 integration. Retrovirology. 2020;17:31. doi: 10.1186/s12977-020-00539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang W, et al. TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J. Biol. Chem. 2004;279:52346–52352. doi: 10.1074/jbc.M409573200. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, et al. Anti-HIV agent azidothymidine decreases Tet(X)-mediated bacterial resistance to tigecycline in Escherichia coli. Commun. Biol. 2020;3:162. doi: 10.1038/s42003-020-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2018;18:198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheth PM, et al. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J. Infect. Dis. 2008;197:1394–1401. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- 55.Minhas V, Wood C. Epidemiology and transmission of Kaposi’s sarcoma-associated herpesvirus. Viruses. 2014;6:4178–4194. doi: 10.3390/v6114178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mudd JC, Brenchley JM. Gut mucosal barrier dysfunction, microbial dysbiosis, and their role in HIV-1 disease progression. J. Infect. Dis. 2016;214:58–66. doi: 10.1093/infdis/jiw258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gootenberg DB, Paer JM, Luevano JM, Kwon DS. HIV-associated changes in the enteric microbial community: potential role in loss of homeostasis and development of systemic inflammation. Curr. Opin. Infect. Dis. 2017;30:31–43. doi: 10.1097/QCO.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villanueva-Millan MJ, Perez-Matute P, Recio-Fernandez E, Lezana Rosales JM, Oteo JA. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J. Int. AIDS Soc. 2017;20:1–13. doi: 10.7448/IAS.20.1.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin D, et al. Microbiome factors in HPV-driven carcinogenesis and cancers. PLoS Pathog. 2020;16:e1008524. doi: 10.1371/journal.ppat.1008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dillon SM, Wilson CC. What is the collective effect of aging and HIV on the gut microbiome? Curr. Opin. HIV AIDS. 2020;15:94–100. doi: 10.1097/COH.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitra A, et al. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4:58. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McHardy IH, et al. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1:26. doi: 10.1186/2049-2618-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lozupone CA, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14:329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mutlu EA, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dillon SM, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu G, et al. Anal microbiota profiles in HIV-positive and HIV-negative. MSM. 2014;28:753–760. doi: 10.1097/QAD.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 67.Kramer HB, et al. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 2010;6:e1000893. doi: 10.1371/journal.ppat.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schirmer M, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1125–1136.e8. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Missé D, et al. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J. Immunol. 2007;178:407–415. doi: 10.4049/jimmunol.178.1.407. [DOI] [PubMed] [Google Scholar]
- 70.Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL. The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol. 2000. 2021;87:107–131. doi: 10.1111/prd.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pereira VT, et al. The association between detectable plasmatic human immunodeficiency virus (HIV) viral load and different subgingival microorganisms in Brazilian adults with HIV: a multilevel analysis. J. Periodontol. 2014;85:697–05. doi: 10.1902/jop.2013.130273. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z, et al. Heat-stable molecule derived from Streptococcus cristatus induces APOBEC3 expression and inhibits HIV-1 replication. PLoS ONE. 2014;9:e106078. doi: 10.1371/journal.pone.0106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClelland RS, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect. Dis. 2018;18:554–564. doi: 10.1016/S1473-3099(18)30058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Austin MN, et al. Mageeibacillus indolicus gen. nov., sp. nov.: a novel bacterium isolated from the female genital tract. Anaerobe. 2015;32:37–42. doi: 10.1016/j.anaerobe.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu MS, Wu MY, Lin TH, Liao CH. Haemophilus parainfluenzae urethritis among homosexual men. J. Microbiol Immunol. Infect. 2015;48:450–452. doi: 10.1016/j.jmii.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 76.Plummer EL, et al. Gardnerella vaginalis clade distribution is associated with behavioral practices and nugent score in women who have sex with women. J. Infect. Dis. 2020;221:454–463. doi: 10.1093/infdis/jiz474. [DOI] [PubMed] [Google Scholar]
- 77.Krakowsky Y, et al. The effect of gender-affirming medical care on the vaginal and neovaginal microbiomes of transgender and gender-diverse people. Front. Cell Infect. Microbiol. 2022;11:769950. doi: 10.3389/fcimb.2021.769950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang S, et al. Fusobacterium nucleatum acts as a pro-carcinogenic bacterium in colorectal cancer: from association to causality. Front. Cell Dev. Biol. 2021;9:710165. doi: 10.3389/fcell.2021.710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ron R, et al. Exploiting the microbiota for the diagnosis of anal precancerous lesions in men who have sex with men. J. Infect. Dis. 2021;224:1247–1256. doi: 10.1093/infdis/jiab068. [DOI] [PubMed] [Google Scholar]
- 80.Chao X, et al. Research of the potential biomarkers in vaginal microbiome for persistent high-risk human papillomavirus infection. Ann. Transl. Med. 2020;8:100. doi: 10.21037/atm.2019.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vázquez-Castellanos JF, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015;8:760–772. doi: 10.1038/mi.2014.107. [DOI] [PubMed] [Google Scholar]
- 82.Serrano-Villar S, Moreno S, Ferrer M. The functional consequences of the microbiome in HIV: insights from metabolomic studies. Curr. Opin. HIV AIDS. 2018;13:88–94. doi: 10.1097/COH.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 83.Aussel L, et al. Biosynthesis and physiology of coenzyme Q in bacteria. Biochim. Biophys. Acta. 2014;1837:1004–1011. doi: 10.1016/j.bbabio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 84.Ivanov AV, et al. Oxidative stress during HIV Infection: mechanisms and consequences. Oxid. Med Cell Longev. 2016;2016:8910396. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin DH, et al. Relationship of oxidative stress with HIV disease progression in HIV/HCV co-infected and HIV mono-infected adults in Miami. Int. J. Biosci. Biochem. Bioinform. 2012;2:217–223. doi: 10.7763/ijbbb.2012.v2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beghini F, et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife. 2021;10:e65088. doi: 10.7554/eLife.65088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.La Rosa PS, et al. Hypothesis testing and power calculations for taxonomic-based human microbiome data. PLoS ONE. 2012;7:e52078. doi: 10.1371/journal.pone.0052078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mallick H, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021;17:e1009442. doi: 10.1371/journal.pcbi.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hall AB, et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017;9:103. doi: 10.1186/s13073-017-0490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swasrte JC, et al. Characteristics and dysbiosis of the gut microbiome in renal transplant recipients. J. Clin. Med. 2020;9:386. doi: 10.3390/jcm9020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzek BE, Wang Y, Huang H, McGarvey PB, Wu CH. UniProt Consortium. UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics. 2015;31:926–932. doi: 10.1093/bioinformatics/btu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caspi R, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016;44(D1):D471–D480. doi: 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tenenbaum D., Maintainer B. KEGGREST: Client-side REST access to the Kyoto Encyclopedia of Genes and Genomes (KEGG). R package version 1.40.0. Bioconductor—KEGGREST, 10.18129/B9.bioc.KEGGREST (2023).
- 96.Kanehisa M, Sato Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020;29:28–35. doi: 10.1002/pro.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu J, et al. Inside out: HIV, the gut microbiome, and the mucosal immune system. J. Immunol. 2017;198:605–614. doi: 10.4049/jimmunol.1601355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Host-removed metagenomics sequences are available on the NCBI SRA database with the following link: https://www.ncbi.nlm.nih.gov/sra/PRJNA983219. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
All open-source codes used to analyze and visualize the data of this study are available in bioBakery and R/Bioconductor websites: GitHub - biobakery/biobakery: bioBakery tools for meta’omic profiling bioconductor.org/packages/release/bioc/vignettes/phyloseq/inst/doc/phyloseq-analysis.R; bioconductor.org/packages/release/bioc/vignettes/Maaslin2/inst/doc/maaslin2.R.