Abstract
Statins are commonly prescribed medications widely investigated for their potential actions on the brain and mental health. Pre-clinical and clinical evidence suggests that statins may play a role in the treatment of depressive disorders, but only the latter has been systematically assessed. Thus, the physiopathological mechanisms underlying statins’ putative antidepressant or depressogenic effects have not been established. This review aims to gather available evidence from mechanistic studies to strengthen the pharmacological basis for repurposing statins in depression. We used a broad, well-validated search strategy over three major databases (Pubmed/MEDLINE, Embase, PsychINFO) to retrieve any mechanistic study investigating statins’ effects on depression. The systematic search yielded 8068 records, which were narrowed down to 77 relevant papers. The selected studies (some dealing with more than one bodily system) described several neuropsychopharmacological (44 studies), endocrine-metabolic (17 studies), cardiovascular (6 studies) and immunological (15 studies) mechanisms potentially contributing to the effects of statins on mood. Numerous articles highlighted the beneficial effect of statins on depression, particularly through positive actions on serotonergic neurotransmission, neurogenesis and neuroplasticity, hypothalamic-pituitary axis regulation and modulation of inflammation. The role of other mechanisms, especially the association between statins, lipid metabolism and worsening of depressive symptoms, appears more controversial. Overall, most mechanistic evidence supports an antidepressant activity for statins, likely mediated by a variety of intertwined processes involving several bodily systems. Further research in this area can benefit from measuring relevant biomarkers to inform the selection of patients most likely to respond to statins’ antidepressant effects while also improving our understanding of the physiopathological basis of depression.
Subject terms: Depression, Biomarkers, Pathogenesis, Clinical pharmacology, Molecular neuroscience
Introduction
Statins are among the most prescribed medications worldwide [1, 2]. Thanks to their established safety [3], statins are considered prototype candidates for ’drug repurposing’—an approach to find new therapeutic uses for well-known molecules; this approach can be useful in areas at high risk of failure in drug discovery such as psychiatry [4]. One strategy behind drug repurposing in psychiatry is based on the advances in our understanding of biological determinants of mental illness, which can then be targeted with molecules known to express the relevant pharmacological activity [4]. A classic example involves the repositioning of anti-inflammatory medications for the treatment of depression [5], which was initially promoted by the observation that depressive symptoms seen in chronic inflammatory disorders seem to respond to immune-active drugs regardless of concomitant physical health improvement [6]. Following this, a ’depressive-inflammatory’ subtype of depression has been increasingly established, and the same occurred for a variety of treatments aiming to benefit this subgroup of patients [7]. Among these, statins have been extensively investigated because of their recognised anti-inflammatory activity [8]. However, these medications also have several other molecular targets—primarily the reduction of cholesterol, that could argue against their use in depression: for example, previous data suggesting that low cholesterol, suicidality and low mood can be associated [9].
Overall, while statins’ general pharmacological actions are well-established, their broader effects—especially neuropsychopharmacological ones, are less clear and increasingly explored. The general pharmacology and neuropsychopharmacology of statins are now briefly summarised.
General pharmacology of statins
Statins’ primary mechanism of action involves the competitive, reversible antagonism of liver 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis [10]. By inhibiting HMG-CoA reductase, statins thwart the physiological production of cholesterol with a subsequent decline of its blood levels [11]. The ensuing reduction in cholesterol concentration within hepatocytes triggers the upregulation of low-density lipoprotein (LDL)-receptor via sterol regulatory element binding proteins [12], leading to increased uptake of LDL cholesterol from systemic circulation [13]. In other words, statins’ cholesterol-lowering properties depend not only on the reduction of cholesterol biosynthesis from the liver, but also on the consequent substantial upsurge in LDL clearance from plasma. Ancillary mechanisms of cholesterol reduction comprise inhibition of hepatic synthesis of apolipoprotein B100 [14] and decreased production and secretion of triglyceride-rich lipoproteins [15]. Overall, the effects on lipid profile include substantial contractions in total cholesterol, LDL, and triglycerides, as well as an accrue in high-density lipoproteins (HDLs) [16]. Additionally, statins differ from other lipid-lowering agents because their upstream inhibition of the mevalonate pathway affects several end-products other than cholesterol, which are responsible for numerous homoeostatic processes, including Coenzyme Q (mitochondrial respiratory chain), farnesyl- and geranyl-geranyl pyrophosphate moieties (protein post-translational modifications), isopentenyl tRNAs (RNA transcription), and dolichol (protein N-glycosylation) [17]. On this basis, statins are described as possessing ’pleiotropic effects’ [18].
Neuropsychopharmacology of statins
There is increasing—though not definitive evidence that all statins, regardless of their lipophilicity, can reach the central nervous system (CNS) [19–22]. These molecules are detected in rodent brains after a few hours following a single dose administration [19]. Both lipophilic and hydrophilic statins can be found in the neuroparenchyma of animals [20] and humans [21], with functional magnetic resonance imaging (fMRI) studies displaying their effect on neural activity [22]. Consistent data indicate that statins can affect brain function both directly and indirectly [23, 24].
The local CNS effects of statins involve brain lipids, neurotransmission, neurogenesis and neuroprotection from trauma, inflammation, and oxidative stress [24]. Firstly, it should be noted that cholesterol and other end-products of the mevalonate pathway are especially abundant in the CNS, where they serve many essential physiological functions [25]. These molecules are rather metabolically inert in the adult brain: their half-life spans from months to years [26], and only some 0.02% undergo daily turnover through de novo synthesis mainly by astrocytes [27], meaning that there is no need to rely on uptake from systemic circulation [28]. Nevertheless, even short-term statins administration seems to cause acute disruption in the homoeostasis of these metabolites in the CNS [20], whereas chronic statin use determines further reductions in brain cholesterol [29] and other mevalonate end-products [30], either directly through HMG-CoA reductase inhibition, or secondarily via a ’sink effect’. Modulation of these lipids within the CNS leads to changes in brain function and behaviour, and is therefore associated with neuropsychological diseases [25] and their treatment [31].
Statins have widespread effects on neurotransmission, involving the monoaminergic, cholinergic and glutamatergic systems that have been implicated in a variety of neuropsychiatric disorders: both cholesterol-dependent and unrelated (e.g. anti-inflammatory and antioxidant) mechanisms can explain such alterations in neurotransmitters levels [32]. Statins are also ligands of peroxisome proliferator-activated receptor (PPAR)α, which drives the expression of neurotrophins such as brain-derived neurotrophic factor (BDNF) [33]. Furthermore, statin-dependent inhibition of the mevalonate pathway stimulates hippocampal neurogenesis via Wnt signalling [34] and promotes neurite outgrowth [35], though also appearing to inhibit synaptic spurring [36].
Finally, statins can be neuroprotective against a variety of stressors. Following traumatic injury, statin use is associated with reduced neuronal loss [37] and increased tissue recovery via vascular endothelial growth factor (VEGF) and activation of the PI3K/Akt-BDNF pathway [38]. Likewise, the suppression of certain mevalonate metabolites mediated by statins dampens the production of pro-inflammatory cytokines [39] and free radicals [40] such as reactive oxygen species (ROS) and nitric oxide (NO), thus protecting neurons from leaky blood-brain barrier (BBB) [41] and overly activated microglia [42] (i.e. neuroinflammation), as well as oxidative stress [43].
The peripheral effects of statins involve a wealth of systems, part of their dubbed ’pleiotropy’ [23]. In addition to, and independently from their established activity on the metabolism of bodily lipids [44, 45], statins can regulate critical functions of endocrine (e.g. cortisol [46, 47] and insulin secretion [48, 49]), cardiovascular (e.g. endothelial function, platelet activation and atherogenesis [50]), and immune (e.g. regulation of innate immunity via pro- and anti-inflammatory cytokines [51, 52] and of adaptive immunity via inhibition of antigen-1 leucocytes (LFA-1) [53], T-cell activation [54] and regulatory T-cells induction [55]) systems. All these processes share profound interactions with each other [56–59], not to mention their substantial crosstalk with the neurobiological mechanisms described above [23, 24].
Aim of the review
Despite considerable research probing statins in a variety of neuropsychiatric disorders, and the growing amount of literature available on this topic, the effects of statins in neuropsychiatric disorders remain controversial [60]. Clinical studies show that statins are promising candidate molecules to repurpose in depression [61], but while evidence from trials and observational studies has been extensively summarised, both descriptively [62, 63] and quantitatively [64–72], the same cannot be said for mechanistic studies. A prior paper had described the neurobiological underpinnings potentially targeted by statins in mood disorders [73], but evidence had not been systematically drawn from studies that directly assessed statins’ use in depression—or models thereof.
The large amount of original research investigating the use of statins in depression, and the several articles attempting to summarise such evidence over the last few years, highlight that this is a topic of ongoing debate within the scientific community [62]. In this context, the design of further clinical research may benefit from a comprehensive overview of relevant translational findings.
Evidence from in vitro, animal, and human translational research is usually gathered and presented by means of narrative reviews. Because these studies are abundant yet less methodically organised on search engines and databases than their clinical counterparts, systematically searching for relevant mechanistic evidence can be daunting, though profitable [74]— and machine learning approaches have been developed to support the task [75]. In this paper, we, therefore, provide an overview of the mechanistic evidence that defines the pharmacological bases for repurposing statins in depression.
Materials and methods
In this review, we used a broad search strategy conducted on three major databases (i.e. Pubmed/MEDLINE, Embase, PsychINFO) via OvidSP on 8 April 2022, updated then on 22 April 2023 following peer-review. The search algorithms combined index terms and free-text words for statins, depression or depressive symptoms, and depression-like models used in animals (Supplementary Material, S1). As advised for reviews of mechanistic studies, a web-based software (i.e. Rayyan) [76] for semi-automated text mining, and extensive forward/backward searching were employed to support de-duplicating and screening records. Two researchers (RDG, NRP) independently screened titles and abstracts for relevance, assessed the full texts for eligibility, and extracted relevant data. Disagreements were discussed with the other authors and resolved by consensus. Eventually, we only included mechanistic studies that reported original data on the pharmacological effects of statins in depression, with no restriction to their design and language.
Results
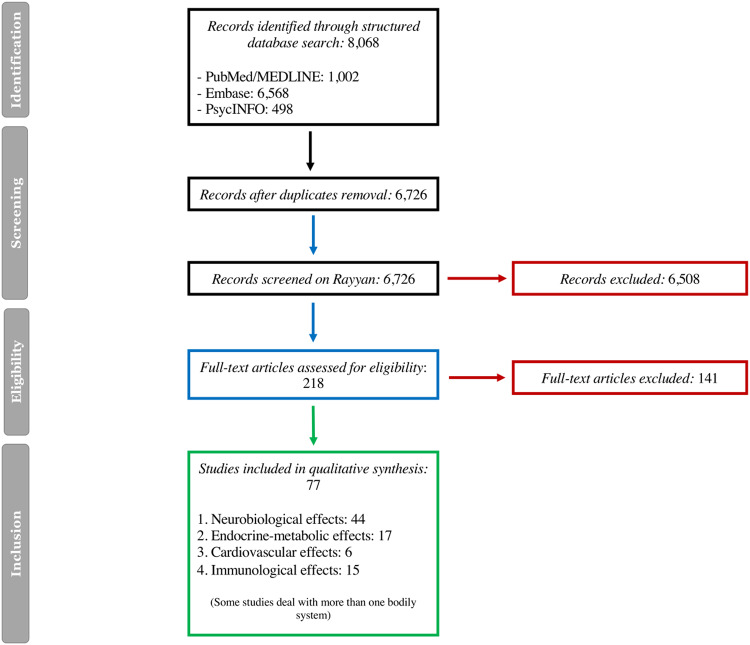
The search flow chart is in Fig. 1. The initial search yielded 6806 records, of which 2080 were duplicates. Screening of titles and abstracts led to the removal of 4548 non-relevant studies. Further 107 articles were excluded from the eligibility assessment of their full texts. Eventually, 77 studies were included in the review. Of these, the majority included animal models of depression (50 studies), six involved in vitro investigations and 21 were translational studies in human participants.
Fig. 1.
Flow chart of the search for mechanistic studies.
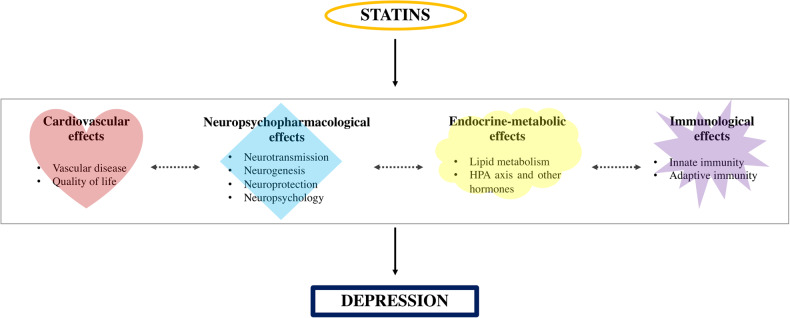
Overall, mechanistic evidence showed that several intertwined neuropsychopharmacological (44 studies), endocrine-metabolic (17 studies), cardiovascular (6 studies) and immunological (15 studies) processes may contribute to the effects of statins in depression (Fig. 2).
Fig. 2.
Current mechanisms explaining the effects of statins in depression.
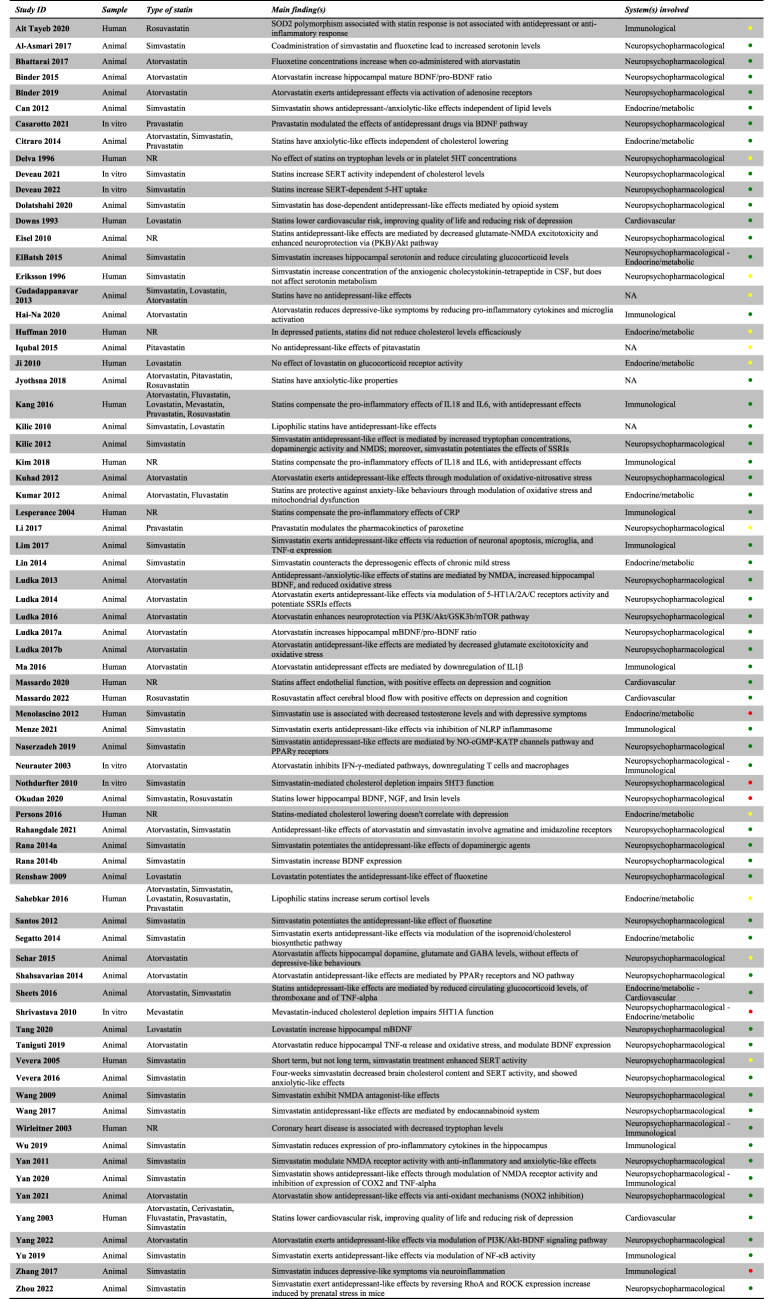
Each included study was described in its relevant section(s) and summarised in Table 1 (see also Supplementary Material, S2).
Table 1.
Summary of included studies.
Green: positive effect; yellow: no effect; red: negative effect.
Further four studies assessed the putative antidepressant activity of statins in animal models of depression without further investigating their underlying mechanism of action and are here briefly reported. These showed that the highly-lipophilic simvastatin and lovastatin have antidepressant-like effects in rats or mice [77], while the less lipophilic atorvastatin [78] and pitavastatin [79] fail to do so. However, atorvastatin, pitavastatin and hydrophilic rosuvastatin display antianxiety properties [80].
Neuropsychopharmacological effects of statins in depression
These include effects on neurotransmission, neurogenesis, neuroprotection and neuropsychology.
Neurotransmission
The pathophysiology of depression is classically associated with anomalies in monoaminergic (i.e. serotonin or 5-hydroxytryptamine, 5HT; noradrenaline, NA; dopamine, DA) neurotransmission [81], though more recently glutamatergic, γ-aminobutyric acid (GABA)ergic, and cholinergic receptors have been implicated [82]. Numerous studies indicate that statins can alter synaptic transmission by modulating the function of several of these neurotransmitter receptors and their ligands [32].
Serotonin
In vitro, statin-induced cholesterol depletion impairs 5HT1A [83] and 5HT3 [84] receptor function. Simvastatin also increases serotonin reuptake by augmenting serotonin transporter (SERT) activity via both cholesterol-mediated [85] and independent [86] pathways. These effects would apparently decrease serotonin activity. Nevertheless, animal models have shown an antidepressant-like effect of simvastatin which may be linked to an increase in the availability of tryptophan, the serotonin precursor, through the inhibition of indoleamine 2,3-dioxygenase (IDO) [87], and increases in hippocampal serotonin [88], as well as reduced SERT activity [89]. Conversely, serotonin depletion or 5HT1A and 5HT2A/C receptor antagonism abolish the antidepressant effect of atorvastatin [90]. These findings have not been replicated in human studies assessing 5HT neuroendocrine function and plasma tryptophan in hypercholesterolaemic patients receiving statins [91]. Furthermore, simvastatin appeared to increase SERT function in the short-, but not long-term in humans [92].
Statins can also modulate the serotonergic effects of some antidepressants in vitro, via the tyrosine kinase receptor 2 (TRKB) domain of BDNF receptor [93]. In animals, the antidepressant effect of selective serotonin reuptake inhibitors (SSRIs) seems potentiated by several statins [87, 90, 94, 95], possibly involving pharmacokinetics interactions [96–98], but the same does not apply to tricyclic antidepressants (TCAs) [87].
Dopamine
Because dopamine neural circuitry, difficult-to-treat depressive symptoms (especially anhedonia), and inflammation appear reliably related [99, 100], statins might be ideally placed to modulate these mechanisms at the same time. Indeed, the dopaminergic system appears affected by statins administration, but while certain studies demonstrate the occurrence of concomitant dopaminergic and antidepressant- or anxiolytic-like effects [87], perhaps mediated by interaction with BDNF function [101, 102] or via potentiation of dopaminergic mechanisms [103] for simvastatin, others fail to show any concurrent changes in animal depressive or anxiety behaviour for atorvastatin [104].
Glutamate and GABA
The most recent and successful developments in depression therapeutics have not been confined to monoaminergic systems but have focussed instead on molecules capable of targeting the glutamatergic and GABAergic pathways [105](e.g., ketamine and esketamine for depression and suicidality [106], brexanolone for post-partum depression [107]). The antidepressant and anxiolytic properties of simvastatin [87, 108–111] and atorvastatin [112] in rats seem linked to glutamate N-methyl-D-aspartate (NMDA) receptor expression and blockade, especially in the hippocampus and amygdala. However, another study showed that while atorvastatin seems to affect hippocampal glutamate and GABA, no concurrent effect on depression or anxiety can be observed in mice [104].
Other neurotransmitters
Less conventional pathways have also been explored in animal models of depression, showing that simvastatin may elicit antidepressant-like action via opioid- [113] and endocannabinoid-mediated [114] neurotransmission, while atorvastatin does so via adenosine-dependant pathways [115]. Simvastatin might also increase the concentration of the anxiogenic cholecystokinin-tetrapeptide in the cerebrospinal fluid (CSF) of healthy human subjects [116] while no effect on CSF serotonin or its metabolite 5-HIAA was found.
Neurogenesis
Processes of hippocampal neurogenesis and neuroplasticity, largely controlled by neurotrophins such as BDNF [117], are considered today a hallmark of depressive disorder and antidepressant action [118].
Emerging evidence from animal studies suggests that lovastatin and atorvastatin may enhance the proteolytic cleavage of pro-BDNF [119–121], BDNF hippocampal concentrations [112, 122] and α7nAChR-mediated activation of the PI3K/Akt-BDNF pathway [123], with a consequent positive influence on depressive-like behaviour. Agmatine and imidazoline receptors, whose function broadly relates to BDNF neurogenesis, NMDA neuroprotection and monoamine regulation, have also been involved in the antidepressant-like effect of simvastatin and atorvastatin [124]. On the other hand, simvastatin or rosuvastatin administration seem associated with lower hippocampal BDNF and anxiogenic response in rats [125].
Neuroprotection
Excitotoxicity [126] and oxidative stress due to reactive oxygen and nitrogen species in the brain [127] are strictly related, to highly depressogenic triggers.
Numerous animal studies show that the antidepressant effect of statins may occur because of decreasing glutamate-NMDA excitotoxicity [128], PPARγ-mediated [129, 130] or inflammation-related [131, 132] nitrosative and oxidative stress, or all the above [112, 133], while also inducing neuroprotective pathways such as protein kinase B (PKB)/Akt [128], PI3K/Akt-GSK3b/mTOR [134] and RhoA/ROCK [135] signalling.
Neuropsychology
The cognitive neuropsychological model of depression uses changes in emotional processing as a biomarker for depressive disorders and the assessment of antidepressant or depressogenic responses [136]. Negative bias in emotional processing has long been recognised as a core feature of depression, leading to a vicious circle of negative feelings, thoughts and behaviour which triggers and maintains depressive symptoms [137]. These emotional biases can occur across several cognitive domains, including perception, attention and memory [138]: for example, people with depression are more likely to perceive and categorise facial expressions as negative or to attend and recall negative information in emotional word-based tasks [139].
The cognitive effects of statins have been investigated for several years [140], but only a few, recent studies have done so in humans in the context of depression. Firstly, an observational study shows a favourable association between statins use and lower recognition of negative faces, with increased misclassification of these expressions as positive, predicting increased depression and anxiety symptoms at later assessments [141]. Conversely, two experimental medicine trials respectively find that atorvastatin [142] and simvastatin [143] have negative or no effects on emotional processing.
Endocrine-metabolic effects of statins in depression
These include effects on lipid metabolism and on the hypothalamic-pituitary-adrenal (HPA) axis and other hormones.
Lipid metabolism
Lipids in the CNS and peripheral circulation interact with biological pathways implicated in depression [144] and antidepressant action [145]. Intriguingly, lipid homoeostasis is critical to several interconnected mechanisms involved in mood regulation, anxiety and suicidal behaviour, including serotonin neurotransmission [146–148], neurogenesis [149], neuroprotection from excitotoxicity [109] and systemic inflammation [148]. From a clinical standpoint, dyslipidaemia and depression, its severity and prospective course appear associated [150], while SSRI-induced increase in cholesterol has been argued to be protective against depression [151]. Correlations between depressive symptomatology and both raised [152, 153] and diminished [154–157] concentrations of circulating lipids, including total cholesterol, LDL, HDL, triglycerides, ω-3 polyunsaturated fatty acids (PUFA), can be observed. These associations may differ between men and women [158]. Some studies highlight a link between cholesterol and anxiety, rather than an effect on mood [159]. Nevertheless, changes in lipid metabolism have been proposed as potential biomarkers for depressive disorders [160].
In keeping with the variable findings above, the effects of statins on lipid metabolism and thus depression seem conflicting. An in vitro study shows that statin-mediated cholesterol depletion inhibits 5HT1A receptor dynamics [83]. High-fat diet induces depressive and anxiety behaviours in rats, but these effects are counteracted by simvastatin [161]. Also, simvastatin administration affects mevalonate metabolites within the hippocampus and prefrontal cortex of rodents, with consequent modulation of emotional cognition [17], though another study highlights a detrimental association between cholesterol-lowering and altered behaviour, weight loss, and circadian disruption [162]. On the other hand, the antidepressant- and anxiolytic-like effects of several statins are observed in rats in the absence of concurrent changes in plasma cholesterol [163]. An intriguing study in humans reports that, despite low LDL cholesterol levels correlating with depression, as also described above, such association is not observed when cholesterol-lowering is achieved via statins [164]. Furthermore, failure to improve the lipid profile, following statin therapy, in patients who suffered a myocardial infarction, seems associated with a higher incidence of depression at 6 months [165].
Hypothalamic-pituitary-adrenal axis and other hormones
Disturbances of glucocorticoids and the HPA or ’stress’ axis, closely related to abnormal inflammatory response, are known to play a major role in the pathophysiology of depression [166], with elevated plasmatic cortisol potentially predicting the development of depressive disorders [167].
Some reviews have hypothesised that statins may mediate the relationship between lipid metabolism, stress, inflammation, and depression in animals [168] and humans [169, 170]. Simvastatin [88] and atorvastatin [171] reduce glucocorticoid levels while expressing antidepressant-like effects in rats. Equally, the depressive- [172] and anxiety-like [173] behaviours caused by chronic mild stress are neutralised with statins use. In humans however statins have been observed to have no effect on glucocorticoid receptors [174] or to even increase serum cortisol [46], although neither study specifically addresses whether these events eventually lead to the development of depression. A case report instead describes the onset of depressive symptomatology in a male whose simvastatin initiation was associated with a reduction of testosterone levels [175].
Cardiovascular effects of statins in depression
These include effects on vascular disease and overall quality of life.
Vascular disease
Atherosclerosis and endothelial dysfunction, which statins lessen via both cholesterol-mediated and other mevalonate-dependant pathways, appear involved in depression [176, 177], especially in late-life according to the ’vascular depression hypothesis’ [178]. A recent meta-analysis has indeed identified a pattern of increased hyperintensity burden on magnetic resonance imaging (MRI) in people whose depression has a late onset [179]. Furthermore, there is a clear bidirectional association between depression and cardiovascular morbidity and mortality [180, 181], therefore interventions that are capable of targeting both mechanisms could yield particular benefit.
Statins are considered excellent candidates for reducing vascular dysfunction of the small white matter vessels in the neuroparenchyma, with consequent positive effects on depression [182]. In obese rats, atorvastatin administration reduces thromboxane and improves vascular reactivity while decreasing depressive-like behaviour [171]. One recent human study shows that low doses of statins in depressed participants determine blood flow changes in key brain areas of mood and cognitive control as well as an improvement in depressive symptoms and markers of endothelial function [183, 184].
Quality of life
On the back of strong bidirectional links between depression, cardiovascular disorders and quality of life [185], some authors argue the ability of statins to prevent cardiovascular and cerebrovascular accidents can lead to improved quality of life and thus lower onset of depressive disorders [186, 187]. However, no studies that explicitly investigate this issue in humans could be retrieved.
Immunological effects of statins in depression
These include effects on innate immunity or inflammation and adaptive immunity.
Innate immunity (inflammation)
Extensive evidence suggests that immune processes, especially inflammatory ones, are prominent in depression pathophysiology [7]. Both peripheral and CNS inflammation appear causally involved [188].
Simvastatin [189–193] and atorvastatin [194] reduce depressive-like symptoms in animals by decreasing neuroinflammation thanks to the suppression of pro-inflammatory cytokines, P2X7-inflammasome complex, and microglia activation. In addition, the reduction of circulating tumour necrosis factor (TNF)α by simvastatin [171] and atorvastatin [195] is likewise associated with improved depressive-like behaviour. Some translational human studies indicate that statins might positively affect mood by offsetting the peripheral pro-inflammatory effects of interleukin (IL)1β [196], IL6 and IL18 [197, 198] and C-reactive protein (CRP) [199]. Nonetheless, a study on a functional genetic polymorphism of superoxide dismutase (SOD)2, an enzyme responsible for the anti-inflammatory activity of rosuvastatin, could not observe any association with an antidepressant response or CRP [200].
Adaptive immunity
Though with less consistency, disruptions in adaptive immunity (i.e. acquired humoral and cell-mediated immune system) have been implicated in depression [201].
No studies could be identified that directly assessed the effect of statins on these mechanisms. One study shows that atorvastatin can inhibit interferon (IFN)γ-dependant cellular immunity, which is related to increased tryptophan availability [202]. Since tryptophan is the precursor of serotonin, it is suggested that statins might reduce the risk of depression by decreasing immune-mediated tryptophan degradation [203, 204].
Discussion
In this article, after recapitulating the general pharmacological and neuropsychopharmacological activities of statins, we reviewed the mechanistic evidence for the effects of these drugs in depression. While a few studies only assessed the behavioural consequences of statins administration in animal models of depression, the great majority (67 studies) of the investigations were mechanistic in nature, thus providing valuable insights on the interactions between statin use, depressive and anxiety symptoms, and numerous biological and psychological mechanisms.
Overall, most studies pointed toward an antidepressant and anxiolytic effect of statins by means of neurobiological, endocrine-metabolic, cardiovascular, and immunological mechanisms largely communicating with each other. A minority of investigations reported no effect, or even depressogenic and anxiogenic ones. Among the few in vitro studies, most identified a modulatory role of statins on serotoninergic pathways, possibly supporting some clinical evidence that statins’ effects in depression might be related to their ability to augment traditional antidepressants [64]. Evidence from the numerous studies in animal models of depression appears particularly suggestive of statins’ benefit: 32/36 studies showed a positive effect by influencing neurotransmitters turnover, neuroreceptors function, and neuroplasticity (two studies showed no effect [98, 104] and one a negative effect [125]), 7/7 studies via lipid metabolism and HPA axis regulation, and 7/8 studies via modulation of circulating molecules involved in immunological and cardiovascular function (one study showed, however, an increase in neuroinflammatory markers [190]). Findings from human translational studies were instead mixed: 10/201studies identified a potentially beneficial effect mainly mediated by anti-inflammatory and cardioprotective mechanisms, while the remaining showed either no effect or indeed a negative one on neurobiological, neuropsychological, and endocrine-metabolic processes – the latter perhaps in keeping with well-documented literature about the associations between low levels of cholesterol and some depressive symptoms [205]. Nevertheless, negative pre-clinical findings are less frequently published [206], therefore the dearth of the latter associations might not reflect a lack of harmful effects for statins. It is also important to notice that several other bodily systems probably affected by statins administration, such as the gut-brain axis [207], have not been assessed in the context of depression yet, and warrant further investigation.
Meanwhile, a few new clinical studies have recently been completed [208] or are ongoing (NCT04301271, NCT04685642), which may provide important insights not only on establishing the clinical efficacy of statins in depression, but also on mechanistic aspects of such effects (or lack thereof). Specifically, the last published clinical trial [208] has investigated the putative antidepressant effect of adjunctive (i.e., in addition to standard care) simvastatin in a large sample of 150 adults with treatment-resistant depression followed up for 12 weeks. This study design includes several features (e.g., use of the most lipophilic simvastatin, focus on a subgroup of patients with treatment-resistant depression, measurement of baseline lipid and inflammatory markers) that both pre-clinical and clinical evidence would support [61]—which is why the lack of any beneficial effect of statin compared to placebo, regardless of the mediating effect of lipid and inflammatory markers, appears disappointing [208] in contrast with earlier promising, yet smaller trials [64, 68]. Nevertheless, a large amount of clinical evidence (extensively reviewed elsewhere [63], see also Supplementary Material, S3 for an up-to-date list of studies) continues supporting the value of identifying subgroups of patients whose specific depression phenotype (as based on neuropsychopharmacological, endocrine-metabolic, cardiovascular, immunological or other markers) may be more responsive to, or preventable with, targeted statin treatment [208].
Limitations
This review has several limitations. Although we used a broad and systematised approach to literature searching, it is possible that some records may have been missed, especially from grey literature, because pre-clinical studies are generally much more numerous and less methodically organised in databases than their clinical counterparts [209]. Overall, our work remains a narrative overview of mechanistic evidence, which includes a variety of heterogeneous studies including in vitro, animal, and human (both in clinically depressed and healthy populations) investigations. As such, the review was not been pre-registered, there was no attempt at pooling results to produce new evidence, and we did not systematically assess for sources of bias in the studies included—though we followed available advice on narrative reviews reporting [210] (Supplementary Material, S4). In this context, it should be noted that the internal validity of many pre-clinical experiments is sometimes poor, while publication bias is common [74]—meaning that caution is required when drawing any conclusion from the evidence reported.
Conclusion
The translation of findings from in vitro, animal, and indeed human studies to medical practice remains a particular challenge for mental illnesses [211]. Consequently, the repurposing of medications based on the targeting of molecular pathways shown to be associated with the course of psychiatric diseases [4], such as mood disorders, has thus far produced modest results [212]. Mechanistic reasoning or “pathophysiologic rationale”—as compared to evidence produced via clinical trials, has often led to unjustified interpretations, to the extent that most evidence-based medicine proponents are legitimately sceptical about using such reasoning as evidence for efficacy or harm [213]. Nevertheless, the design of further pre-clinical and clinical studies investigating the effects of statins—or of any molecule targeting the physiopathological pathways examined above, as well as measurement of related biomarkers for depression and antidepressant response, may be informed by the evidence presented in this review.
Supplementary information
Acknowledgements
This project was funded by the Wellcome Trust, award: 102176/Z/13/Z, grant: 216452/Z/19/Z, title: ’The effects of anti-inflammatory drugs on emotional and reward processing’ to Dr Riccardo De Giorgi and supported by the NIHR Oxford Health Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the Wellcome Trust, the NIHR, the NHS or the Department of Health.
Author contributions
RDG conceived the study and acquired funding for it. RDG and NRP designed the study methodology, organised the database, performed the literature search and extracted the data. GR, GM, PJC and CJH validated the data, supported the interpretation of the findings and supervised the overall project. RDG wrote the first draft of the paper and NRP devised the figures and tables. All authors critically revised the manuscript and approved the final version. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Conflict of interest
CJH has received consultancy fees from P1vital, Lundbeck, Servier, UCB, Zogenix, J&J and Syndesi outside of the current work. GR has been a speaker and/or consultant from Angelini, Janssen, Lundbeck and Otsuka outside of the current work. GM has been a consultant and/or speaker and/or has received research grants from Angelini, Boehringer Ingelheim, FB-Health, Janssen, Lundbeck, Otsuka and Innova Pharma, outside of the current work. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/9/2023
In the original version of this article, the given and family names of Nicola Rizzo Pesci were incorrectly structured. Given name is Nicola and family name is Rizzo Pesci.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-023-02533-z.
References
- 1.Urquhart, L. Top drugs and companies by sales in 2018. Nat Rev Drug Discov. 2019. 10.1038/d41573-019-00049-0. [DOI] [PubMed]
- 2.Simons J. The $10 billion pill. Fortune. 2003;147:58–62. [PubMed] [Google Scholar]
- 3.Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 4.Fava M. The promise and challenges of drug repurposing in psychiatry. World Psychiatry. 2018;17:28–29. doi: 10.1002/wps.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbott R, Whear R, Nikolaou V, Bethel A, Coon JT, Stein K, et al. Tumour necrosis factor-α inhibitor therapy in chronic physical illness: a systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res. 2015;79:175–84. doi: 10.1016/j.jpsychores.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Miller AH, Haroon E, Felger JC. Therapeutic implications of brain-immune interactions: treatment in translation. Neuropsychopharmacology. 2017;42:334–59. doi: 10.1038/npp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SW, Kang HJ, Jhon M, Kim JW, Lee JY, Walker AJ, et al. Statins and inflammation: new therapeutic opportunities in psychiatry. Front Psychiatry. 2019;10:103. doi: 10.3389/fpsyt.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law M. Having too much evidence (depression, suicide, and low serum cholesterol) BMJ. 1996;313:651–2. doi: 10.1136/bmj.313.7058.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;72:323–6. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- 11.Trapani L, Segatto M, Pallottini V. Regulation and deregulation of cholesterol homeostasis: the liver as a metabolic "power station". World J Hepatol. 2012;4:184–90. doi: 10.4254/wjh.v4.i6.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet. 2007;41:401–27. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- 13.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg HN, Le NA, Short MP, Ramakrishnan R, Desnick RJ. Suppression of apolipoprotein B production during treatment of cholesteryl ester storage disease with lovastatin. Implications for regulation of apolipoprotein B synthesis. J Clin Invest. 1987;80:1692–7. doi: 10.1172/JCI113259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM. Consensus statement: role of therapy with "statins" in patients with hypertriglyceridemia. Am J Cardiol. 1998;81:1b–6b. doi: 10.1016/s0002-9149(98)00030-7. [DOI] [PubMed] [Google Scholar]
- 16.McFarland AJ, Anoopkumar-Dukie S, Arora DS, Grant GD, McDermott CM, Perkins AV, et al. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. 2014;15:20607–37. doi: 10.3390/ijms151120607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segatto M, Manduca A, Lecis C, Rosso P, Jozwiak A, Swiezewska E, et al. Simvastatin treatment highlights a new role for the isoprenoid/cholesterol biosynthetic pathway in the modulation of emotional reactivity and cognitive performance in rats. Neuropsychopharmacology. 2014;39:841–54. doi: 10.1038/npp.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu D, Liao JK. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc Res. 2022;118:413–23. doi: 10.1093/cvr/cvab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson-Anuna LN, Eckert GP, Keller JH, Igbavboa U, Franke C, Fechner T, et al. Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharm Exp Ther. 2005;312:786–93. doi: 10.1124/jpet.104.075028. [DOI] [PubMed] [Google Scholar]
- 20.Lütjohann D, Stroick M, Bertsch T, Kühl S, Lindenthal B, Thelen K, et al. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 2004;69:431–8. doi: 10.1016/j.steroids.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Botti RE, Triscari J, Pan HY, Zayat J. Concentrations of pravastatin and lovastatin in cerebrospinal fluid in healthy subjects. Clin Neuropharmacol. 1991;14:256–61. doi: 10.1097/00002826-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Parker BA, Polk DM, Rabdiya V, Meda SA, Anderson K, Hawkins KA, et al. Changes in memory function and neuronal activation associated with atorvastatin therapy. Pharmacotherapy. 2010;30:625. [Google Scholar]
- 23.Farooqui AA, Ong WY, Horrocks LA, Chen P, Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev. 2007;56:443–71. doi: 10.1016/j.brainresrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Fracassi A, Marangoni M, Rosso P, Pallottini V, Fioramonti M, Siteni S, et al. Statins and the brain: more than lipid lowering agents. Curr Neuropharmacol. 2019;17:59–83. doi: 10.2174/1570159X15666170703101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartocci V, Servadio M, Trezza V, Pallottini V. Can cholesterol metabolism modulation affect brain function and behavior? J Cell Physiol. 2017;232:281–6. doi: 10.1002/jcp.25488. [DOI] [PubMed] [Google Scholar]
- 26.Andersson M, Elmberger PG, Edlund C, Kristensson K, Dallner G. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 1990;269:15–18. doi: 10.1016/0014-5793(90)81107-y. [DOI] [PubMed] [Google Scholar]
- 27.Nieweg K, Schaller H, Pfrieger FW. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem. 2009;109:125–34. doi: 10.1111/j.1471-4159.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- 28.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipido. 2001;12:105–12. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Locatelli S, Lütjohann D, Schmidt HH, Otto C, Beisiegel U, von Bergmann K. Reduction of plasma 24S-hydroxycholesterol (cerebrosterol) levels using high-dosage simvastatin in patients with hypercholesterolemia: evidence that simvastatin affects cholesterol metabolism in the human brain. Arch Neurol. 2002;59:213–6. doi: 10.1001/archneur.59.2.213. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowski SM, Johnson K, Siefert M, Shank S, Sironi L, Wolozin B, et al. Simvastatin inhibits protein isoprenylation in the brain. Neuroscience. 2016;329:264–74. doi: 10.1016/j.neuroscience.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider M, Levant B, Reichel M, Gulbins E, Kornhuber J, Müller CP. Lipids in psychiatric disorders and preventive medicine. Neurosci Biobehav Rev. 2017;76:336–62. doi: 10.1016/j.neubiorev.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Kosowski M, Smolarczyk-Kosowska J, Hachula M, Maligłówka M, Basiak M, Machnik G, et al. The effects of statins on neurotransmission and their neuroprotective role in neurological and psychiatric disorders. Molecules. 2021;26:2838.. doi: 10.3390/molecules26102838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, et al. HMG-CoA reductase inhibitors bind to PPARα to upregulate neurotrophin expression in the brain and improve memory in mice. Cell Metab. 2015;22:253–65. doi: 10.1016/j.cmet.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robin NC, Agoston Z, Biechele TL, James RG, Berndt JD, Moon RT. Simvastatin promotes adult hippocampal neurogenesis by enhancing Wnt/β-catenin signaling. Stem Cell Rep. 2014;2:9–17. doi: 10.1016/j.stemcr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pooler AM, Xi SC, Wurtman RJ. The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor pravastatin enhances neurite outgrowth in hippocampal neurons. J Neurochem. 2006;97:716–23. doi: 10.1111/j.1471-4159.2006.03763.x. [DOI] [PubMed] [Google Scholar]
- 36.Mailman T, Hariharan M, Karten B. Inhibition of neuronal cholesterol biosynthesis with lovastatin leads to impaired synaptic vesicle release even in the presence of lipoproteins or geranylgeraniol. J Neurochem. 2011;119:1002–15. doi: 10.1111/j.1471-4159.2011.07474.x. [DOI] [PubMed] [Google Scholar]
- 37.Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–46. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25:130–9. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 39.McFarland AJ, Davey AK, Anoopkumar-Dukie S. Statins reduce lipopolysaccharide-induced cytokine and inflammatory mediator release in an in vitro model of microglial-like cells. Mediators Inflamm. 2017;2017:2582745. doi: 10.1155/2017/2582745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mancini G, Martins WC, de Oliveira J, de Bem AF, Tasca CI. Atorvastatin improves mitochondrial function and prevents oxidative stress in hippocampus following amyloid-β(1-40) intracerebroventricular administration in mice. Mol Neurobiol. 2020;57:4187–201. doi: 10.1007/s12035-020-02026-w. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto T, Morofuji Y, Kovac A, Erickson MA, Deli MA, Niwa M, et al. Pitavastatin ameliorates lipopolysaccharide-induced blood-brain barrier dysfunction. Biomedicines. 2021;9:837.. doi: 10.3390/biomedicines9070837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng X, Liao Y, Wang J, Hu S, Rudramurthy GR, Swamy MK, et al. The antineuroinflammatory effect of simvastatin on lipopolysaccharide activated microglial cells. Evid Based Complement Altern Med. 2018;2018:9691085.. doi: 10.1155/2018/9691085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadollah-Damavandi S, Sharifi ZN, Arani HZ, Jangholi E, Karimi A, Parsa Y, et al. Atorvastatin prevents the neuron loss in the hippocampal dentate gyrus region through its anti-oxidant and anti-apoptotic activities. CNS Neurol Disord Drug Targets. 2021;20:76–86. doi: 10.2174/1871527319666200922160627. [DOI] [PubMed] [Google Scholar]
- 44.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharm. 2005;19:117–25. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 45.Tibrewala A, Jivan A, Oetgen WJ, Stone NJ. A comparative analysis of current lipid treatment guidelines: nothing stands still. J Am Coll Cardiol. 2018;71:794–9. doi: 10.1016/j.jacc.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Sahebkar A, Rathouska J, Simental-Mendía LE, Nachtigal P. Statin therapy and plasma cortisol concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016;103:17–25. doi: 10.1016/j.phrs.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Lecanu L, Han Z, Yao Z, Greeson J, Papadopoulos V. Inhibition of adrenal cortical steroid formation by procaine is mediated by reduction of the cAMP-induced 3-hydroxy-3-methylglutaryl-coenzyme A reductase messenger ribonucleic acid levels. J Pharmacol Exp Ther. 2003;307:1148–57. doi: 10.1124/jpet.103.055178. [DOI] [PubMed] [Google Scholar]
- 48.Abbasi F, Lamendola C, Harris CS, Harris V, Tsai MS, Tripathi P, et al. Statins are associated with increased insulin resistance and secretion. Arterioscler Thromb Vasc Biol. 2021;41:2786–97. doi: 10.1161/ATVBAHA.121.316159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grunwald SA, Haafke S, Grieben U, Kassner U, Steinhagen-Thiessen E, Spuler S. Statins aggravate the risk of insulin resistance in human muscle. Int J Mol Sci. 2022;23:2398.. doi: 10.3390/ijms23042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milajerdi A, Larijani B, Esmaillzadeh A. Statins influence biomarkers of low grade inflammation in apparently healthy people or patients with chronic diseases: a systematic review and meta-analysis of randomized clinical trials. Cytokine. 2019;123:154752. doi: 10.1016/j.cyto.2019.154752. [DOI] [PubMed] [Google Scholar]
- 52.Ferro D, Parrotto S, Basili S, Alessandri C, Violi F. Simvastatin inhibits the monocyte expression of proinflammatory cytokines in patients with hypercholesterolemia. J Am Coll Cardiol. 2000;36:427–31. doi: 10.1016/s0735-1097(00)00771-3. [DOI] [PubMed] [Google Scholar]
- 53.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 54.Bu DX, Tarrio M, Grabie N, Zhang Y, Yamazaki H, Stavrakis G, et al. Statin-induced Krüppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J Clin Invest. 2010;120:1961–70. doi: 10.1172/JCI41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YC, Kim KK, Shevach EM. Simvastatin induces Foxp3+ T regulatory cells by modulation of transforming growth factor-beta signal transduction. Immunology. 2010;130:484–93. doi: 10.1111/j.1365-2567.2010.03269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morris G, Puri BK, Olive L, Carvalho A, Berk M, Walder K, et al. Endothelial dysfunction in neuroprogressive disorders-causes and suggested treatments. BMC Med. 2020;18:305.. doi: 10.1186/s12916-020-01749-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris G, Berk M, Walder K, O’Neil A, Maes M, Puri BK. The lipid paradox in neuroprogressive disorders: causes and consequences. Neurosci Biobehav Rev. 2021;128:35–57. doi: 10.1016/j.neubiorev.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 58.van Diepen JA, Berbée JF, Havekes LM, Rensen PC. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. 2013;228:306–15. doi: 10.1016/j.atherosclerosis.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 59.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 60.Avan R, Sahebnasagh A, Hashemi J, Monajati M, Faramarzi F, Henney NC, et al. Update on statin treatment in patients with neuropsychiatric disorders. Life. 2021;11:1365.. doi: 10.3390/life11121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Giorgi R, Cowen PJ, Harmer CJ. Statins in depression: a repurposed medical treatment can provide novel insights in mental health. Int Rev Pÿsychiatry. 2022;34:699–714. doi: 10.1080/09540261.2022.2113369. [DOI] [PubMed] [Google Scholar]
- 62.Köhler-Forsberg O, Otte C, Gold SM, Østergaard SD. Statins in the treatment of depression: hype or hope. Pharm Ther. 2020;215:107625.. doi: 10.1016/j.pharmthera.2020.107625. [DOI] [PubMed] [Google Scholar]
- 63.De Giorgi R, Rizzo Pesci N, Quinton A, De Crescenzo F, Cowen PJ, Harmer CJ. Statins in depression: an evidence-based overview of mechanisms and clinical studies. Front Psychiatry. 2021;12:702617.. doi: 10.3389/fpsyt.2021.702617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Giorgi R, De Crescenzo F, Rizzo Pesci N, Martens M, Howard W, Cowen PJ, et al. Statins for major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2021;16:e0249409.. doi: 10.1371/journal.pone.0249409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Giorgi R, De Crescenzo F, Cowen PJ. Effects of various statins on depressive symptoms: is there enough evidence. J Affect Disord. 2021;295:1093–4. doi: 10.1016/j.jad.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 66.De Giorgi R, Waters S, Pesci NR, Rosso G, Cowen PJ, Harmer CJ. The effects of statin monotherapy on depressive symptoms: A systematic review and meta-analysis. J Affect Disord. 2022;311:336–43. doi: 10.1016/j.jad.2022.05.113. [DOI] [PubMed] [Google Scholar]
- 67.Köhler-Forsberg O, C NL, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404–19. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 68.Yatham MS, Yatham KS, Ravindran AV, Sullivan F. Do statins have an effect on depressive symptoms? A systematic review and meta-analysis. J Affect Disord. 2019;257:55–63. doi: 10.1016/j.jad.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Lee MC, Peng TR, Chen BL, Lee CH, Wang JY, Lai CP, et al. Effects of various statins on depressive symptoms: a network meta-analysis. J Affect Disord. 2021;293:205–13. doi: 10.1016/j.jad.2021.06.034. [DOI] [PubMed] [Google Scholar]
- 70.Hang X, Li J, Li Z, Zhang Y, Ye X, Tang Q, et al. Comparative efficacy and acceptability of anti-inflammatory agents on major depressive disorder: a network meta-analysis. Front Pharmacol. 2021;12:691200. doi: 10.3389/fphar.2021.691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee MC, Peng TR, Lee CH, Wang JY, Lee JA, Chen SM, et al. Statin use and depression risk: a systematic review and meta-analysis. J Affect Disord. 2021;282:308–15. doi: 10.1016/j.jad.2020.12.164. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L, Bao Y, Tao S, Zhao Y, Liu M. The association between cardiovascular drugs and depression/anxiety in patients with cardiovascular disease: a meta-analysis. Pharm Res. 2022;175:106024.. doi: 10.1016/j.phrs.2021.106024. [DOI] [PubMed] [Google Scholar]
- 73.Walker AJ, Kim Y, Borissiouk I, Rehder R, Dodd S, Morris G, et al. Statins: neurobiological underpinnings and mechanisms in mood disorders. Neurosci Biobehav Rev. 2021;128:693–708. doi: 10.1016/j.neubiorev.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 74.Russell AAM, Sutherland BA, Landowski LM, Macleod M, Howells DW. What has preclinical systematic review ever done for us? BMJ Open Sci. 2022;6:e100219.. doi: 10.1136/bmjos-2021-100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bannach-Brown A, Przybyła P, Thomas J, Rice ASC, Ananiadou S, Liao J, et al. Machine learning algorithms for systematic review: reducing workload in a preclinical review of animal studies and reducing human screening error. Syst Rev. 2019;8:23.. doi: 10.1186/s13643-019-0942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kilic FS, Ozatyk Y, Kaygisiz B, Baydemyr C, Erol K. Focused Conference Group: P17 - Newapproaches and targets in psychiatry investigation of antidepressant and anxiolytic effects of simvastatine in rats. Basic Clin Pharmacol Toxicol. 2010;107:374.. [Google Scholar]
- 78.Gudadappanavar AM, Hiremath SV, Shashikant T. Evaluation of antidepressant activity of simvastatin, lovastatin and atorvastatin in male swiss mice - An experimental study. Int J Drug Dev Res. 2013;5:102–8. [Google Scholar]
- 79.Iqubal A, Iqubal MK, Kumar R, Ahmad S. A step closer to lookup alternate or adjuvant therapy for epilepsy: intranasal delivery of solid-lipid nanoparticles of pitavastatin to appraise antiepileptic properties in mice. Int Res J Pharm. 2015;6:820–4. [Google Scholar]
- 80.Jyothsna VC, Balaji O, Chogtu B. Effect of different statins in animal model of anxiety in wistar rats. Asian J Pharm Clin Res. 2018;11:369–71. [Google Scholar]
- 81.Pytka K, Podkowa K, Rapacz A, Podkowa A, Żmudzka E, Olczyk A, et al. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharm Rep. 2016;68:263–74. doi: 10.1016/j.pharep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Pytka K, Dziubina A, Młyniec K, Dziedziczak A, Żmudzka E, Furgała A, et al. The role of glutamatergic, GABA-ergic, and cholinergic receptors in depression and antidepressant-like effect. Pharm Rep. 2016;68:443–50. doi: 10.1016/j.pharep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin 1A receptors. Biochemistry. 2010;49:5426–35. doi: 10.1021/bi100276b. [DOI] [PubMed] [Google Scholar]
- 84.Nothdurfter C, Tanasic S, Di Benedetto B, Rammes G, Wagner EM, Kirmeier T, et al. Impact of lipid raft integrity on 5-HT3 receptor function and its modulation by antidepressants. Neuropsychopharmacology. 2010;35:1510–9. doi: 10.1038/npp.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deveau CM. A new mechanism of serotonin transporter regulation by simvastatin and the isoprenylation pathway [dissertation]. Indiana University; 2022.
- 86.Deveau CM, Rodriguez E, Schroering A, Yamamoto BK. Serotonin transporter regulation by cholesterol-independent lipid signaling. Biochem Pharm. 2021;183:114349.. doi: 10.1016/j.bcp.2020.114349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kilic FS, Ozatik Y, Kaygisiz B, Baydemir C, Erol K. Acute antidepressant and anxiolytic effects of simvastatin and its mechanisms in rats. Neurosci. 2012;17:39–43. [PubMed] [Google Scholar]
- 88.ElBatsh MM. Antidepressant-like effect of simvastatin in diabetic rats. Can J Physiol Pharm. 2015;93:649–56. doi: 10.1139/cjpp-2014-0560. [DOI] [PubMed] [Google Scholar]
- 89.Vevera J, Vales K, Fisar Z, Hroudova J, Singh N, Stuchlik A, et al. The effect of prolonged simvastatin application on serotonin uptake, membrane microviscosity and behavioral changes in the animal model. Physiol Behav. 2016;158:112–20. doi: 10.1016/j.physbeh.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 90.Ludka FK, Constantino LC, Kuminek G, Binder LB, Zomkowski ADE, Cunha MP, et al. Atorvastatin evokes a serotonergic system-dependent antidepressant-like effect in mice. Pharmacol Biochem Behav. 2014;122:253–60. doi: 10.1016/j.pbb.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 91.Delva NJ, Matthews DR, Cowen PJ. Brain serotonin (5-HT) neuroendocrine function in patients taking cholesterol-lowering drugs. Biol Psychiatry. 1996;39:100–6. doi: 10.1016/0006-3223(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 92.Vevera J, Fisar Z, Kvasnicka T, Zdenek H, Starkova L, Ceska R, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133:197–203. doi: 10.1016/j.psychres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299. doi: 10.1016/j.cell.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Renshaw PF, Parsegian A, Yang CK, Novero A, Yoon SJ, Lyoo IK, et al. Lovastatin potentiates the antidepressant efficacy of fluoxetine in rats. Pharmacol Biochem Behav. 2009;92:88–92. doi: 10.1016/j.pbb.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos T, Baungratz MM, Haskel SP, de Lima DD, da Cruz JN, Dal Magro DD, et al. Behavioral interactions of simvastatin and fluoxetine in tests of anxiety and depression. 2012;8:413–22. [DOI] [PMC free article] [PubMed]
- 96.Al-Asmari AK, Ullah Z, Al Masoudi AS, Ahmad I. Simultaneous administration of fluoxetine and simvastatin ameliorates lipid profile, improves brain level of neurotransmitters, and increases bioavailability of simvastatin. J Exp Pharm. 2017;9:47–57. doi: 10.2147/JEP.S128696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhattarai B, Swamy S, Baral SR, Acharya CP. A study on the effect of atorvastatin on the pharmacokinetic and antidepressant activity of fluoxetine. Natl J Physiol Pharm Pharmacol. 2017;7:1224–9. [Google Scholar]
- 98.Li F, Ling ZL, Wang ZJ, Zhong ZY, Shu N, Zhang M, et al. Differential effects of pravastatin on the pharmacokinetics of paroxetine in normal and diabetic rats. Xenobiotica. 2017;47:20–30. doi: 10.3109/00498254.2016.1154999. [DOI] [PubMed] [Google Scholar]
- 99.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–65. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bekhbat M, Li Z, Mehta ND, Treadway MT, Lucido MJ, Woolwine BJ, et al. Functional connectivity in reward circuitry and symptoms of anhedonia as therapeutic targets in depression with high inflammation: evidence from a dopamine challenge study. Mol Psychiatry. 2022;27:4113–21. doi: 10.1038/s41380-022-01715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rana DG, Patel AK, Joshi CG, Jhala MK, Goyal RK. Alteration in the expression of exon IIC transcripts of brain-derived neurotrophic factor gene by simvastatin [correction of simvastain] in chronic mild stress in mice: a possible link with dopaminergic pathway. Can J Physiol Pharmacol. 2014;92:985–92. doi: 10.1139/cjpp-2014-0125. [DOI] [PubMed] [Google Scholar]
- 102.Rana DG, Patel A, Joshi CG, Goyal RK. Possible involvement of altered expression of BDNF exon II gene and specific dopamine receptors in simvastatin induced beneficial effects in depression. Mol Cytogenet. 2014;7:P67. [Google Scholar]
- 103.Rana DG, Upadhyay UM, Goyal RK. The preferential effect of simvastatin on dopaminergic agents induced behavioral effect in experimental models of depression. Int J Pharm Res. 2014;6:55–60. [Google Scholar]
- 104.Sehar N, Agarwal NB, Vohora D, Raisuddin S. Atorvastatin prevents development of kindling by modulating hippocampal levels of dopamine, glutamate, and GABA in mice. Epilepsy Behav. 2015;42:48–53. doi: 10.1016/j.yebeh.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Fasipe OJ, Agede OA, Enikuomehin AC. Announcing the novel class of GABA-A receptor selective positive allosteric modulator antidepressants. Future Sci OA. 2020;7:Fso654.. doi: 10.2144/fsoa-2020-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pochwat B. Ketamine - a long way from anesthetic to a prototype antidepressant: review of potential mechanisms of action. Psychiatr Pol. 2022;56:1017–32. doi: 10.12740/PP/OnlineFirst/134330. [DOI] [PubMed] [Google Scholar]
- 107.Edinoff AN, Odisho AS, Lewis K, Kaskas A, Hunt G, Cornett EM, et al. Brexanolone, a GABA(A) modulator, in the treatment of postpartum depression in adults: a comprehensive review. Front Psychiatry. 2021;12:699740. doi: 10.3389/fpsyt.2021.699740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan J, Xu Y, Zhu C, Zhang L, Wu A, Yang Y, et al. Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PLoS ONE. 2011;6:e20945. doi: 10.1371/journal.pone.0020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.da Cruz JN, Dal Magro DD, de Lima DD, da Cruz JGP. Simvastatin treatment reduces the cholesterol content of membrane/lipid rafts, implicating the N-methyl-D-aspartate receptor in anxiety: a literature review. Braz J Pharm. Sci. 2017;53.
- 110.Wang Q, Zengin A, Deng C, Li Y, Newell KA, Yang GY, et al. High dose of simvastatin induces hyperlocomotive and anxiolytic-like activities: The association with the up-regulation of NMDA receptor binding in the rat brain. Exp Neurol. 2009;216:132–8. doi: 10.1016/j.expneurol.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 111.Yan J, Liu A, Fan H, Qiao L, Wu J, Shen M, et al. Simvastatin improves behavioral disorders and hippocampal inflammatory reaction by NMDA-mediated anti-inflammatory function in MPTP-treated mice. Cell Mol Neurobiol. 2020;40:1155–64. doi: 10.1007/s10571-020-00804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ludka FK, Zomkowski AD, Cunha MP, Dal-Cim T, Zeni AL, Rodrigues AL, et al. Acute atorvastatin treatment exerts antidepressant-like effect in mice via the L-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur Neuropsychopharmacol. 2013;23:400–12. doi: 10.1016/j.euroneuro.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 113.Dolatshahi M, Davoudi S, Paridar Y, Naserzadeh R, Ghorbanzadeh B. Pharmacological evidence for the involvement of the opioid system in the antidepressant-like effect of simvastatin in mice: Without tolerance and withdrawal syndrome. Neurosci Lett. 2020;714:134578.. doi: 10.1016/j.neulet.2019.134578. [DOI] [PubMed] [Google Scholar]
- 114.Wang H, Zhou J, Liu QZ, Wang LL, Shang J. Simvastatin and Bezafibrate ameliorate Emotional disorder Induced by High fat diet in C57BL/6 mice. Sci Rep. 2017;7:2335.. doi: 10.1038/s41598-017-02576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Binder LB, Ludka FK, Marques NF, Constantino LC, Tasca CI. Adenosine A1 and A2A receptors modulation by atorvastatin: Neuroprotective and antidepressant-like effects. J Neurochemistry. 2019;150:125.. [Google Scholar]
- 116.Eriksson M, Eklundh T, Sjoberg S, Angelin B, Nordin C. Cholesterol lowering and cerebrospinal fluid neurotransmitters: increased levels of the anxiogenic cholecystokinin-tetrapeptide during simvastatin administration to healthy male volunteers. Biol Psychiatry. 1996;40:302–4. doi: 10.1016/0006-3223(96)00008-X. [DOI] [PubMed] [Google Scholar]
- 117.Yang T, Nie Z, Shu H, Kuang Y, Chen X, Cheng J, et al. The role of BDNF on neural plasticity in depression. Front Cell Neurosci. 2020;14:82.. doi: 10.3389/fncel.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tartt AN, Mariani MB, Hen R, Mann JJ. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27:2689–99. doi: 10.1038/s41380-022-01520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsai SJ. Statins may enhance the proteolytic cleavage of proBDNF: implications for the treatment of depression. Med Hypotheses. 2007;68:1296–9. doi: 10.1016/j.mehy.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 120.Ludka FK, Cunha MP, Dal-Cim T, Binder LB, Constantino LC, Massari CM, et al. Atorvastatin Protects from Aβ1-40-Induced Cell Damage and Depressive-Like Behavior via ProBDNF Cleavage. Mol Neurobiol. 2017;54:6163–73. doi: 10.1007/s12035-016-0134-6. [DOI] [PubMed] [Google Scholar]
- 121.Binder LB, Ludka FK, Cunha MP, Dal-Cim T, Constantino LC, Massari C, et al. Atorvastatin prevents cell death and depressive-like behaviour induced by Abeta1-40 peptide via BDNF cleavage. J Neurochemistry. 2015;134:332–3. [Google Scholar]
- 122.Tang C-R, Yu X-B, Zhang H-N, Cao Y-C, Yang F, Xu L-M, et al. Lovastatin Prevents Depressive Behaviors and Increased Hippocampal Neurogenesis in Streptozotocin-Induced Diabetic Mice. Pharmacology. 2020;105:339–48. doi: 10.1159/000503865. [DOI] [PubMed] [Google Scholar]
- 123.Yang C, Ni HY, Yin JJ, Zhou T, Gu QX, Chen TT, et al. Atorvastatin ameliorates depressive behaviors via regulation of alpha7nAChR expression by PI3K/Akt-BDNF pathway in mice. Biochem Biophys Res Commun. 2022;593:57–64. doi: 10.1016/j.bbrc.2022.01.034. [DOI] [PubMed] [Google Scholar]
- 124.Rahangdale S, Fating R, Gajbhiye M, Kapse M, Inamdar N, Kotagale N, et al. Involvement of agmatine in antidepressant-like effect of HMG-CoA reductase inhibitors in mice. Eur J Pharmacol. 2021;892:173739.. doi: 10.1016/j.ejphar.2020.173739. [DOI] [PubMed] [Google Scholar]
- 125.Okudan N, Belviranli M. High dose simvastatin and rosuvastatin impair cognitive abilities of healthy rats via decreasing hippocampal neurotrophins and irisin. Brain Res Bull. 2020;165:81–89. doi: 10.1016/j.brainresbull.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 126.Olloquequi J, Cornejo-Córdova E, Verdaguer E, Soriano FX, Binvignat O, Auladell C, et al. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: therapeutic implications. J Psychopharmacol. 2018;32:265–75. doi: 10.1177/0269881118754680. [DOI] [PubMed] [Google Scholar]
- 127.Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270–6. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 128.Eisel ULM, Dolga A, Granic I, Dobos N, Naude P, Nyakas C, et al. Inflammation as a target in neurodegenerative disease and related depression. Acta Physiol Hung. 2010;97:437–8. [Google Scholar]
- 129.Naserzadeh R, Abad N, Ghorbanzadeh B, Dolatshahi M, Mansouri MT. Simvastatin exerts antidepressant-like activity in mouse forced swimming test: Role of NO-cGMP-KATP channels pathway and PPAR-gamma receptors. Pharmacol Biochem Behav. 2019;180:92–100. doi: 10.1016/j.pbb.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 130.Shahsavarian A, Javadi S, Jahanabadi S, Khoshnoodi M, Shamsaee J, Shafaroodi H, et al. Antidepressant-like effect of atorvastatin in the forced swimming test in mice: the role of PPAR-gamma receptor and nitric oxide pathway. Eur J Pharmacol. 2014;745:52–8. doi: 10.1016/j.ejphar.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Kuhad A, Kishor S, Chadha R. Psychopharmacological studies on atrovastatin and its solvatomorphs in experimental paradigm of depression. Int J Neuropsychopharmacol. 2012;15:188. [Google Scholar]
- 132.Taniguti EH, Ferreira YS, Stupp IJV, Fraga-Junior EB, Doneda DL, Lopes L, et al. Atorvastatin prevents lipopolysaccharide-induced depressive-like behaviour in mice. Brain Res Bull. 2019;146:279–86. doi: 10.1016/j.brainresbull.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 133.Ludka FK, Dal-Cim T, Binder LB, Constantino LC, Massari C, Tasca CI. Atorvastatin and Fluoxetine Prevent Oxidative Stress and Mitochondrial Dysfunction Evoked by Glutamate Toxicity in Hippocampal Slices. Mol Neurobiol. 2017;54:3149–61. doi: 10.1007/s12035-016-9882-6. [DOI] [PubMed] [Google Scholar]
- 134.Ludka FK, Constantino LC, Dal-Cim T, Binder LB, Zomkowski A, Rodrigues ALS, et al. Involvement of PI3K/Akt/GSK-3β and mTOR in the antidepressant-like effect of atorvastatin in mice. J Psychiatr Res. 2016;82:50–7. doi: 10.1016/j.jpsychires.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 135.Zhou J, Ma Y, Chen J, Yao D, Feng C, Dong Y, et al. Effects of RhoA on depression-like behavior in prenatally stressed offspring rats. Behav Brain Res. 2022;432:113973.. doi: 10.1016/j.bbr.2022.113973. [DOI] [PubMed] [Google Scholar]
- 136.Godlewska BR, Harmer CJ. Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment. Psychopharmacology (Berl) 2021;238:1265–78. doi: 10.1007/s00213-019-05448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Elliott R, Zahn R, Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36:153–82. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roiser JP, Sahakian BJ. Hot and cold cognition in depression. CNS Spectr. 2013;18:139–49. doi: 10.1017/S1092852913000072. [DOI] [PubMed] [Google Scholar]
- 139.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–36. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mospan CM. Are statins protective or harmful to cognitive function? JAAPA. 2016;29:11–12. doi: 10.1097/01.JAA.0000475471.02134.37. [DOI] [PubMed] [Google Scholar]
- 141.Gillespie AL, Wigg C, van Assche I, Murphy SE, Harmer, CJ. Associations between statin use and negative affective bias during COVID-19: an observational, longitudinal UK study investigating depression vulnerability. Biol Psychiatry.2022;92:543–51. [DOI] [PMC free article] [PubMed]
- 142.De Giorgi R, Martens M, Rizzo Pesci N, Cowen PJ, Harmer CJ. The effects of atorvastatin on emotional processing, reward learning, verbal memory and inflammation in healthy volunteers: an experimental medicine study. J Psychopharmacol. 2021;35:1479–87. doi: 10.1177/02698811211060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Giorgi R, Quinton AGM, Waters S, Cowen PJ, Harmer CJ. An experimental medicine study of the effects of simvastatin on emotional processing, reward learning, verbal memory, and inflammation in healthy volunteers. Psychopharmacology. 2022;239:2635–45. doi: 10.1007/s00213-022-06156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walther A, Cannistraci CV, Simons K, Dur n C, Gerl MJ, Wehrli S, et al. Lipidomics in Major Depressive Disorder. Front Psychiatry. 2018;9:459.. doi: 10.3389/fpsyt.2018.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, et al. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med. 2013;19:934–8. doi: 10.1038/nm.3214. [DOI] [PubMed] [Google Scholar]
- 146.Park YM, Lee BH, Lee SH. The association between serum lipid levels, suicide ideation, and central serotonergic activity in patients with major depressive disorder. J Affect Disord. 2014;159:62–65. doi: 10.1016/j.jad.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 147.Sun S, Yang S, Mao Y, Jia X, Zhang Z. Reduced cholesterol is associated with the depressive-like behavior in rats through modulation of the brain 5-HT1A receptor. Lipids Health Dis. 2015;14:22.. doi: 10.1186/s12944-015-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patel JS, Polanka BM, Stewart JC. Association of blood lipids fractions with depressive symptom clusters and individual symptoms in men and women in the United States: National health and nutrition examination survey (NHANES) 2005-2012. Psychosom Med. 2016;78:A25. [Google Scholar]
- 149.Lütjohann D. Brain cholesterol and suicidal behaviour. Int J Neuropsychopharmacol. 2007;10:153–7. doi: 10.1017/S1461145706007048. [DOI] [PubMed] [Google Scholar]
- 150.Wagner CJ, Musenbichler C, Böhm L, Färber K, Fischer AI, von Nippold F, et al. LDL cholesterol relates to depression, its severity, and the prospective course. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:405–11. doi: 10.1016/j.pnpbp.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 151.Goldstein MR, Mascitelli L, Pezzetta F. Is the increase in LDL cholesterol induced by selective serotonin reuptake inhibitor therapy a blessing in disguise? Med Hypotheses. 2010;74:955–6. doi: 10.1016/j.mehy.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 152.Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of hyperlipidemia in patients with major depressive disorder: a population-based study. J Psychosom Res. 2013;75:270–4. doi: 10.1016/j.jpsychores.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 153.Fabre LF, Smith LC. Total cholesterol levels in subjects with major depression. J Clin Lipidol. 2012;6:267–8. [Google Scholar]
- 154.Persons JE, Fiedorowicz JG. Depression and serum low-density lipoprotein: a systematic review and meta-analysis. J Affect Disord. 2016;206:55–67. doi: 10.1016/j.jad.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shin JY, Suls J, Martin R. Are cholesterol and depression inversely related? A meta-analysis of the association between two cardiac risk factors. Ann Behav Med. 2008;36:33–43. doi: 10.1007/s12160-008-9045-8. [DOI] [PubMed] [Google Scholar]
- 156.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–7. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 157.Flicker L, Almeida O, Yeap B, Hankey G, Golledge J. HDL cholesterol and the risk of depression over 5 years among older men. Int Psychogeriatr. 2013;25:S70.. doi: 10.1038/mp.2013.113. [DOI] [PubMed] [Google Scholar]
- 158.Ancelin ML, Carrière I, Boulenger JP, Malafosse A, Stewart R, Cristol JP, et al. Gender and genotype modulation of the association between lipid levels and depressive symptomatology in community-dwelling elderly (the ESPRIT study). Biol Psychiatry. 2010;68:125–32. [DOI] [PubMed]
- 159.Coutu MF, Dupuis G, D’Antono B. The impact of cholesterol lowering on patients’ mood. J Behav Med. 2001;24:517–36. doi: 10.1023/a:1012980909550. [DOI] [PubMed] [Google Scholar]
- 160.Parekh A, Smeeth D, Milner Y, Thure S. The role of lipid biomarkers in major depression. Healthcare. 2017;5:5. [DOI] [PMC free article] [PubMed]
- 161.Can ÖD, Ulupinar E, Özkay ÜD, Yegin B, Öztür Y. The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav Pharmacol. 2012;23:582–92. doi: 10.1097/FBP.0b013e328356c3f2. [DOI] [PubMed] [Google Scholar]
- 162.Ferris HA, Davis F, Kahn CR. Insulin action at astrocytes is important for regulation of circadian rhythm and metabolism. Diabetes. 2016;65:A13.. [Google Scholar]
- 163.Citraro R, Chimirri S, Aiello R, Gallelli L, Trimboli F, Britti D, et al. Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia. 2014;55:1284–91. doi: 10.1111/epi.12686. [DOI] [PubMed] [Google Scholar]
- 164.Persons JE, Robinson JG, Coryell WH, Payne ME, Fiedorowicz JG. Longitudinal study of low serum LDL cholesterol and depressive symptom onset in postmenopause. J Clin Psychiatry. 2016;77:212–20. doi: 10.4088/JCP.14m09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huffman JC, SmithFA, Fricchione GL, Januzzi JL, Nadelman S, Pirl WF. Depression and failure of cholesterol lowering after acute myocardial infarction. Prim Care Companion J Clin Psychiatry. 2010;12:PCC.08m00766. [DOI] [PMC free article] [PubMed]
- 166.Horowitz MA, Zunszain PA, Anacker C, Musaelyan K, Pariante CM. Glucocorticoids and inflammation: a double-headed sword in depression? How do neuroendocrine and inflammatory pathways interact during stress to contribute to the pathogenesis of depression? Mod Trends Pharmacopsychiatry. 2013;28:127–43. doi: 10.1159/000343980. [DOI] [PubMed] [Google Scholar]
- 167.Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:321–38. doi: 10.1038/s41380-019-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pellosmaa H, Dougall AL. A systematic review of the role statins and mood on plasma lipids in animal models. Psychosom Med. 2018;80:A17. [Google Scholar]
- 169.Pellosmaa H, Orsak G, Tebbe D, Jenney C, Das S, Thompson J, et al. Managing high cholesterol: Relationships among stress, depression, adherence and inflammatory markers. Psychosom Med. 2015;77:A136. [Google Scholar]
- 170.Pellosmaa H, Dougall AL. Examining the role of statins and mood on plasma lipids and health outcomes: a systematic review. Psychosom Med. 2016;78:A94.. [Google Scholar]
- 171.Sheets WJ, Brooks SD, Chantler P, Frisbee J. Interventions blunting systemic inflammation and oxidant stress improve depressive symptoms and vascular function in a model of metabolic syndrome. FASEB J. 2016;30:A16.. [Google Scholar]
- 172.Lin PY, Chang AY, Lin TK. Simvastatin treatment exerts antidepressant-like effect in rats exposed to chronic mild stress. Pharmacol Biochem Behav. 2014;124:174–9. doi: 10.1016/j.pbb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 173.Kumar A, Vashist A, Kumar P, Kalonia H, Mishra J. Protective effect of HMG CoA reductase inhibitors against running wheel activity induced fatigue, anxiety like behavior, oxidative stress and mitochondrial dysfunction in mice. Pharm Rep. 2012;64:1326–36. doi: 10.1016/s1734-1140(12)70930-1. [DOI] [PubMed] [Google Scholar]
- 174.Ji H, Guo WZ, Yan ZH, Li D, Lu CL. Influence of drug treatment on glucocorticoid receptor levels in patients with coronary heart disease. Chin Med J. 2010;123:1685–9. [PubMed] [Google Scholar]
- 175.Menolascino M, Nelson-Hawks J. Potential mood effect of statin medications. J Altern Complement Med. 2012;18:635–6. [Google Scholar]
- 176.Pereira J, Massardo T, Saez C, Olivares N, Valenzuela J, Risco L, et al. Endothelial dysfunction, oxidative stress and activation of Rho A/Rho kinase pathway in major depressive disorder. Res Pract Thromb Haemost. 2019;3:488.. [Google Scholar]
- 177.Chrysohoou C, Kollia N, Tousoulis D. The link between depression and atherosclerosis through the pathways of inflammation and endothelium dysfunction. Maturitas. 2018;109:1–5. doi: 10.1016/j.maturitas.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 178.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salo KI, Scharfen J, Wilden ID, Schubotz RI, Holling H. Confining the concept of vascular depression to late-onset depression: a meta-analysis of MRI-defined hyperintensity burden in major depressive disorder and bipolar disorder. Front Psychol. 2019;10:1241.. doi: 10.3389/fpsyg.2019.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rajan S, McKee M, Rangarajan S, Bangdiwala S, Rosengren A, Gupta R, et al. Association of symptoms of depression with cardiovascular disease and mortality in low-, middle-, and high-income countries. JAMA Psychiatry. 2020;77:1052–63. doi: 10.1001/jamapsychiatry.2020.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meng R, Yu C, Liu N, He M, Lv J, Guo Y, et al. Association of depression with all-cause and cardiovascular disease mortality among adults in China. JAMA Netw Open. 2020;3:e1921043. doi: 10.1001/jamanetworkopen.2019.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van Agtmaal MJM, Houben A, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:729–39. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Massardo T, Quintana J, Pereira J, Saez C, Risco L, Corral S, et al. Effect of statins on brain perfusion in major depression as an addition to standard therapy. SPM analysis of inflammation, platelet activation, endothelial dysfunction and neurocognitive parameters. Eur J Nucl Med Mol Imaging. 2020;47:S446–7. [Google Scholar]
- 184.Massardo T, Quintana JC, Risco L, Corral S, Spuler J, Vicentini D, et al. Effect of low-dose statins in addition to standard therapy on brain perfusion and neurocognitive performance in patients with major depressive disorder. Neuropsychobiology. 2022;81:271–85. [DOI] [PubMed]
- 185.Ishak WW, Edwards G, Herrera N, Lin T, Hren K, Peterson M, et al. Depression in heart failure: a systematic review. Innov Clin Neurosci. 2020;17:27–38. [PMC free article] [PubMed] [Google Scholar]
- 186.Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163:1926–32. doi: 10.1001/archinte.163.16.1926. [DOI] [PubMed] [Google Scholar]
- 187.Downs JR, Oster G, Santanello NC. HMG CoA reductase inhibitors and quality of life. JAMA. 1993;269:3107–8. doi: 10.1001/jama.269.24.3107. [DOI] [PubMed] [Google Scholar]
- 188.Lamers F, Milaneschi Y, Vinkers CH, Schoevers RA, Giltay EJ, Penninx BWJH. Depression profilers and immuno-metabolic dysregulation: longitudinal results from the NESDA study. Brain Behav Immun. 2020;88:174–83. doi: 10.1016/j.bbi.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 189.Wu H, Lv W, Pan Q, Kalavagunta PK, Liu Q, Qin G, et al. Simvastatin therapy in adolescent mice attenuates HFD-induced depression-like behavior by reducing hippocampal neuroinflammation. J Affect Disord. 2019;243:83–95. doi: 10.1016/j.jad.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 190.Zhang HN, Xu H, Yu XB, Wang CZ, Xu QQ, Dai GX, et al. Effect of simvastatin on lipopolysaccharides induced depressive behaviors and neuroinflammation in mice. Chin Pharmacol Bull. 2017;33:373–8. [Google Scholar]
- 191.Lim S-W, Shiue Y-L, Liao J-C, Wee H-Y, Wang C-C, Chio C-C, et al. Simvastatin therapy in the acute stage of traumatic brain injury attenuates brain trauma-induced depression-like behavior in rats by reducing neuroinflammation in the hippocampus. Neurocrit Care. 2017;26:122–32. doi: 10.1007/s12028-016-0290-6. [DOI] [PubMed] [Google Scholar]
- 192.Yu X-B, Zhang H-N, Dai Y, Zhou Z-Y, Xu R-A, Hu L-F, et al. Simvastatin prevents and ameliorates depressive behaviors via neuroinflammatory regulation in mice. J Affect Disord. 2019;245:939–49. doi: 10.1016/j.jad.2018.11.086. [DOI] [PubMed] [Google Scholar]
- 193.Menze ET, Ezzat H, Shawky S, Sami M, Selim EH, Ahmed S, et al. Simvastatin mitigates depressive-like behavior in ovariectomized rats: Possible role of NLRP3 inflammasome and estrogen receptors’ modulation. Int Immunopharmacol. 2021;95:107582.. doi: 10.1016/j.intimp.2021.107582. [DOI] [PubMed] [Google Scholar]
- 194.Hai-Na Z, Xu-Ben Y, Cong-Rong T, Yan-Cheng C, Fan Y, Lei-Mei X, et al. Atorvastatin ameliorates depressive behaviors and neuroinflammatory in streptozotocin-induced diabetic mice. Psychopharmacology (Berl) 2020;237:695–705. doi: 10.1007/s00213-019-05406-w. [DOI] [PubMed] [Google Scholar]
- 195.Yan J, Huang J, Liu A, Wu J, Fan H, Shen M, et al. Atorvastatin improves motor function, anxiety and depression by NOX2-mediated autophagy and oxidative stress in MPTP-lesioned mice. Aging. 2020;13:831–45. doi: 10.18632/aging.202189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ma W, Shen D, Pan J, Yu L, Shi W, Deng L, et al. Statin function as an anti-inflammation therapy for depression in patients with coronary artery disease by downregulating interleukin-1beta. J Cardiovasc Pharmacol. 2016;67:129–35. doi: 10.1097/FJC.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 197.Kang HJ, Bae KY, Kim SW, Kim JT, Park MS, Cho KH, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. 2016;72:156–60. doi: 10.1016/j.psyneuen.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 198.Kim S-W, Kang H-J, Bae K-Y, Shin I-S, Hong YJ, Ahn Y-K, et al. Interactions between pro-inflammatory cytokines and statins on depression in patients with acute coronary syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:250–4. doi: 10.1016/j.pnpbp.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 199.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–7. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 200.Ait Tayeb AEK, Becquemont L, El-Asmar K, Mahmoudi K, Colle R, Trabado S, et al. SOD2 genetic polymorphism (rs4880) has no impact on 6-month response to antidepressant treatment and inflammatory biomarkers in depressed patients. Basic Clin Pharmacol Toxicol. 2020;126:289–95. doi: 10.1111/bcpt.13385. [DOI] [PubMed] [Google Scholar]
- 201.Haapakoski R, Ebmeier KP, Alenius H, Kivimäki M. Innate and adaptive immunity in the development of depression: an update on current knowledge and technological advances. Prog Neuropsychopharmacol Biol Psychiatry. 2016;66:63–72. doi: 10.1016/j.pnpbp.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Neurauter G, Wirleitner B, Laich A, Schennach H, Weiss G, Fuchs D. Atorvastatin suppresses interferon-gamma -induced neopterin formation and tryptophan degradation in human peripheral blood mononuclear cells and in monocytic cell lines. Clin Exp Immunol. 2003;131:264–7. doi: 10.1046/j.1365-2249.2003.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wirleitner B, Rudzite V, Neurauter G, Murr C, Kalnins U, Erglis A, et al. Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest. 2003;33:550–4. doi: 10.1046/j.1365-2362.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 204.Wirleitner B, Sperner-Unterweger B, Fuchs D. Statins to reduce risk of depression. J Am Coll Cardiol. 2004;43:1132–3. doi: 10.1016/j.jacc.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 205.Kim EJ, Hong J, Hwang JW. The association between depressive mood and cholesterol levels in Korean adolescents. Psychiatry Investig. 2019;16:737–44. doi: 10.30773/pi.2019.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.van der Naald M, Wenker S, Doevendans PA, Wever KE, Chamuleau SAJ. Publication rate in preclinical research: a plea for preregistration. BMJ Open Sci. 2020;4:e100051.. doi: 10.1136/bmjos-2019-100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–5. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- 208.Husain MI, Chaudhry IB, Khoso AB, Kiran T, Khan N, Ahmad F, et al. Effect of adjunctive simvastatin on depressive symptoms among adults with treatment-resistant depression: a randomized clinical trial. JAMA Netw Open. 2023;6:e230147. doi: 10.1001/jamanetworkopen.2023.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Soliman N, Rice ASC, Vollert J. A practical guide to preclinical systematic review and meta-analysis. Pain. 2020;161:1949–54. doi: 10.1097/j.pain.0000000000001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5.. doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bartoli F, Cavaleri D, Bachi B, Moretti F, Riboldi I, Crocamo C, et al. Repurposed drugs as adjunctive treatments for mania and bipolar depression: A meta-review and critical appraisal of meta-analyses of randomized placebo-controlled trials. J Psychiatr Res. 2021;143:230–8. doi: 10.1016/j.jpsychires.2021.09.018. [DOI] [PubMed] [Google Scholar]
- 213.Howick J, Glasziou P, Aronson JK. Evidence-based mechanistic reasoning. J R Soc Med. 2010;103:433–41. doi: 10.1258/jrsm.2010.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.