Abstract
Currently, more than 170 modifications have been identified on RNA. Among these RNA modifications, various methylations account for two-thirds of total cases and exist on almost all RNAs. Roles of RNA modifications in cancer are garnering increasing interest. The research on m6A RNA methylation in cancer is in full swing at present. However, there are still many other popular RNA modifications involved in the regulation of gene expression post-transcriptionally besides m6A RNA methylation. In this review, we focus on several important RNA modifications including m1A, m5C, m7G, 2′-O-Me, Ψ and A-to-I editing in cancer, which will provide a new perspective on tumourigenesis by peeking into the complex regulatory network of epigenetic RNA modifications, transcript processing, and protein translation.
Subject terms: Cell biology, Cancer genetics
Background
Various types of RNA exist in the human transcriptome, including coding RNA (message RNA, mRNA) and non-coding RNA (including tRNA, rRNA, miRNA, lncRNA, circRNA, etc.). Each of these RNAs plays its own role to ensure cells function in an orderly manner [1–7]. There are a large number of chemical modifications on the bases and ribose of RNA that are involved in the post-transcriptional regulation of gene expression.
So far, more than 170 different types of modifications of RNA have been reported [8] and both mRNA and most non-coding RNAs can be modified [9, 10]. Similar to modifications on DNA and histone, RNA modifications can also be written, erased and recognised by corresponding enzymes (writer, eraser and reader). RNA modifications generally affect RNA splicing, stability, localisation, translation, and RNA–RNA/RNA–protein interactions, consequentially modulating life activities. Abnormalities in regulatory enzymes and changes in RNA modification patterns are closely associated with the occurrence and development of various diseases, including cancer [11, 12].
RNA modifications promote or inhibit tumourigenesis by regulating cell proliferation, differentiation, invasion, migration, stemness, metabolism, and drug resistance. Among these numerous RNA modifications, N6-methyladenosine (m6A), N1-methyladenosine (m1A), 5-methylcytidine (m5C), N7-methylguanosine (m7G), 2′-O-methylation (2′-O-Me), pseudouridine (Ψ), and adenosine-to-inosine (A-to-I) editing are notoriously linked to tumourigenesis. The functions, regulatory enzymes, detection methods of m6A modification and its role in tumours have been summarised in detail in previous reviews [13, 14]. Therefore, this review focuses on the molecular functions and regulatory enzymes of the other six RNA modifications (m1A, m5C, m7G, 2′-O-Me, Ψ and A-to-I) in RNAs (Table 1) and their roles in cancer.
Table 1.
Regulatory enzymes and molecular functions of RNA modifications.
| RNA modifications | RNA | Writer | Eraser | Reader | Function | References |
|---|---|---|---|---|---|---|
| m1A | mRNA | TRMT6/TRMT61A, TRMT10C, TRMT61B | ALKBH3 | YTHDF2, YTHDF3 | Promote/inhibit translation and degradation | [16–18, 97, 100] |
| tRNA | TRMT6/TRMT61A, TRMT10C/SDR5C1, TRMT61B | ALKBH1, ALKBH3, FTO | Stabilisation, promote translation initiation and elongation and inhibit tDRs production | [23, 24, 103, 105, 108] | ||
| rRNA | RRP8, TRMT61B | Promote ribosome assembly and translation | [28–30] | |||
| m5C | mRNA | DNMT2, NSUN2/4/6 | TET1 | YBX1, ALYREF, RAD52, LIN28B, FMRP | Nuclear export, stabilisation, promote/inhibit translation | [34, 36–41, 111, 118, 120] |
| tRNA | DNMT2, NSUN2/3/6 | TET2, ALKBH1 | Stabilisation, promote translation efficiency and fidelity | [103, 112, 116, 117, 119, 123] | ||
| rRNA | NSUN1/4/5 | YTHDF2 | Promote ribosome biogenesis, translation and pre-rRNA processing | [46–49] | ||
| m7G | mRNA | RNMT/RAM, METTL1/WDR4 | eIF4E family, Ago2 | Promote/inhibit translation and nuclear export | [53, 60, 131–133, 140, 143] | |
| tRNA | METTL1/WDR4 | stabilisation, promote translation | [135–139] | |||
| rRNA | WBSCR22/TRMT112 | ribosome biogenesis | [141] | |||
| Ψ | mRNA | RPUSD2/3/4, PUS1, PUS7, TRUB1/2, H/ACA snoRNPs | MetRS | Promote/inhibit translation, stabilisation, pre-mRNA processing | [166, 167, 169, 171, 180] | |
| tRNA | RPUSD4, PUS10, PUS7, PUS3, TRUB1/2 | MetRS | Stabilisation, promote/inhibit translation, tRFs generation | [168, 172, 174, 175, 180] | ||
| rRNA | RPUSD4, H/ACA snoRNPs | Promote translation fidelity and IRES-dependent translation | [174, 215, 238] | |||
| snRNA | H/ACA snoRNPs | Prp5 | Processing of pre-mRNA, RNA–RNA/protein interactions | [181] | ||
| 2′-O-Me | mRNA | CMTR1/2 | Processing of pre-mRNA, translation, stabilisation | [162, 163] | ||
| tRNA | hTrmt13, FTSJ1, TARBP1 | Affect tRNA stability, cellular translation, and inhibits tRFs production | [152–156] | |||
| rRNA | C/D-box snoRNPs, MRM1/2/3 | Promote IRES-dependent translation | [157, 213, 239] | |||
| A-to-I | mRNA | ADAR1, ADAR2 | Affect mRNA stability, nucleoplasmic localisation, circRNA formation, and amino acid changes | [76–87] | ||
| miRNA | ADAR1, ADAR2 | Affect miRNA biosynthesis and binding to targets | [88–95] |
Chemical structures and molecular functions of RNA modifications
RNA methylation
N1-methyladenosine (m1A)
N1-methyladenosine (m1A) modification adds a methyl group to the N1 position of adenosine, which blocks Watson–Crick base-pairing and introduces a positive charge on this position, affecting RNA secondary structure and RNA–RNA/RNA–protein interactions. In 1960s, the existence of m1A modification was first discovered in mammalian and plant RNA [15]. Initially, m1A was shown to be mainly present in tRNA and rRNA. With the progress of detection techniques, m1A in mRNA has been gradually confirmed [16]. m1A modification exists in the coding sequence (CDS), 5′UTR and 3′UTR of mRNA to regulate mRNA stability and translation [16–21]. m1A is reported on positions 9, 14, 22, 57 and 58 in tRNA [22] to influence tRNA self-assembly, stability, and regulate translation [23–27]. Similarly, m1A in rRNA is essential for ribosome assembly and regulates cellular translation [28–30].
5-methylcytidine (m5C)
5-methylcytidine (m5C) is a methylation modification on C5 of RNA cytosine ring [31]. This modification was reported as early as 1925, and subsequent studies proved that m5C indeed existed in animals and plants [32, 33]. m5C is enriched in 3′UTR, the GC-rich sequence, and around the start codon [34, 35] to modulate mRNA stability [36–38], nuclear export [34], translation [39], and DNA damage repair [40, 41]. m5C exists on positions 34, 38, 47–50 and 72 of tRNA [42] and mainly concentrated in the anticodon loop, between the variable loop and the TΨC loop, and near the amino acid accepting stem. m5C modification in tRNA helps to maintain tRNA stability [43], promote translation [44], and enhance translation accuracy [45]. In rRNA, m5C modification is associated with ribosome maturation [46–48] and translation efficiency [49].
N7-methylguanosine (m7G)
N7-methylguanosine (m7G) is the methylation of guanine N7 and adds a positive charge to the N atom, which affects the structure of RNA through electrostatic and spatial effects, but does not affect Watson–Crick base-pairing [50]. m7G is present in the 5′cap structure of mRNA and pre-tRNA [51, 52]. The m7G-cap is important for mRNA shearing and processing, nucleocytoplasmic transport, stability, and protein synthesis. m7G is also widely present in the interior of mRNA and tRNA [53–55] and on rRNA [56] and miRNA [57], affecting their processing and maturation [57], stability [58], nucleocytoplasmic transport [59], transcription extension and translation efficiency [53, 58, 60, 61].
2′-O-methylation (2′-O-Me)
2′-O-methylation (2′-O-Me) is adding a methyl group (-CH3) to the 2′-OH of RNA ribose. 2′-O-Me is not limited to bases and is collectively referred to Nm (Am, Um, Cm and Gm). The Nm sites are found in a variety of RNAs to affect RNA stability, flexibility, and RNA–protein interactions [62–66]. Nm is present in the mRNA cap structure and interior of mRNA to regulate mRNA stability, translation, and the interaction between mRNA and other RNAs [67–69]. A large number of sites on tRNA can be modified by 2′-O-Me, which is related to tRNA stability and translation efficiency. Among that Gm18, Cm32 and Nm34 are relatively conserved [70]. Similar to Ψ (see below), 2′-O-Me modification in rRNA are also mainly concentrated on important functional sites of the ribosome [71, 72], such as decoding centre (DC), intersubunit bridge and peptidyl transferase centre (PTC).
Other RNA modifications
Pseudouridine (Ψ)
With C3 and C6 as an axis, the pyrimidine ring of uridine (U) rotates 180° to form pseudouridine (Ψ). Compared with U, the base of Ψ is connected with ribose through C-C bond instead of N-C bond. Ψ has a more stable base arrangement than U and the N1 position can act as a proton donor to form additional hydrogen bonds, which helps improve RNA stability [73]. Ψ is commonly present in various RNAs [74], affecting RNA stability, RNA–RNA/RNA–protein interactions, tRNA fragments (tRFs) generation, splicing and translation.
Adenosine-to-inosine (A-to-I)
C6 hydrolytic deamination of adenosine (A) produces inosine (I), also known as hypoxanthine which is similar to guanosine (G) in structure. Inosine, with 2 fewer amino groups than guanosine, can be recognised as guanosine (G) and pairs with cytidine (C), leading to misreading of genetic information. Rate of A-to-I editing increases with intracellular acidification [75]. In cancer, A-to-I editing occurs frequently on mRNAs and miRNAs. A-to-I editing on mRNA modulates its stability, nucleocytoplasmic localisation, translation and circRNA formation [76–82]. In addition, A-to-I editing in the coding region of mRNA may lead to amino acid change, resulting in conformational change of protein, further affecting protein stability, protein-protein interaction, and subcellular localisation [76, 83–87]. Of note, A-to-I editing on miRNA affects its production and downstream targets [88–95].
In summary, RNA modifications regulate RNA splicing, stability, translation, nucleoplasmic localisation, base-pairing and RNA–RNA/RNA–protein interactions, further regulating life activities. The functional diversity of RNA modifications is closely related to localisation of modifications, types of RNA and reader proteins. For example, m1A and m7G occur on adenosine and guanosine, respectively, and although they both introduce positive charges, m1A blocks Watson–Crick base-pairing, whereas m7G does not affect Watson–Crick base-pairing, but rather affects the structure of the RNA through electrostatic interactions and spatial effects. 2′-O-Me adds methyl group to ribose and can be present on any base, increasing the hydrophobicity of RNAs, protecting them from nuclease attack as well as limiting the rotational freedom of 3′-phosphate. The pseudouridine (Ψ) and A-to-I editing introduce new bases. Compared to uridine (U), Ψ alters the chemical bond between bases and ribose and has a more stable base stacking arrangement. Compared to adenosine (A), inosine (I) pairs with cytidine (C), leading to the misreading of genetic information.
Regulatory enzymes for RNA modifications
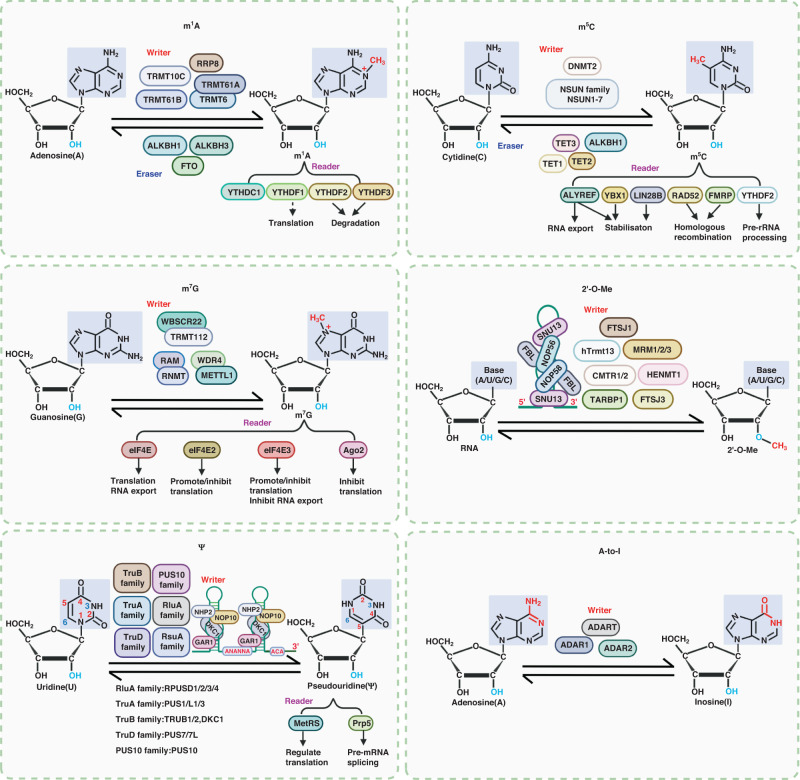
Most modifications are regulated by three types of enzymes, the “writer” which introduces the modification, the “eraser” for the removal of modification, and the “reader” for recognition of modification (Fig. 1). Balanced expression of these enzymes is necessary for the maintenance of normal cellular activities. Tumorigenesis and progression are usually due to dysregulation of these RNA-modifying enzymes, resulting in abnormal RNA modifications that further affect the expressions of oncogenes/tumour suppressor genes.
Fig. 1.
Chemical structures and regulatory enzymes for common RNA modifications
Regulatory enzymes for m1A
Till now, five writers (TRMT6, TRMT61A, TRMT61B, TRMT10C and RRP8), three erasers (ALKBH1, ALKBH3 and FTO), and four readers (YTHDF1, YTHDF2, YTHDF3 and YTHDC1) have been identified for m1A modification.
Writers
TRMT6, TRMT61A, TRMT61B and TRMT10C belong to the tRNA methyltransferase protein family. The TRMT6/TRMT61A complex was originally identified as a methyltransferase of cytoplasmic tRNA that recognises the motif GTTCRA and is responsible for m1A modification at position 58 in the TΨC loop of tRNA [96]. In this complex, TRMT61A has catalytic activity, while TRMT6 binds to RNA. Later, studies found the TRMT6/TRMT61A complex could also catalyse m1A modification on mRNA [97] and in the seed region of tRFs, disrupting the base-pairing of tRFs with their target genes [98]. TRMT61B resides in mitochondria. Initially, TRMT61B was shown to be responsible for m1A58 in mt-tRNA [99], and it was later found that TRMT61B was also able to catalyse m1A modification on mitochondrial mRNA [97] and 16 S rRNA [28]. TRMT10C is an m1A catalytic enzyme for mt-tRNA and mt-mRNA [100–102]. Specifically, TRMT10C interacts with SDR5C1 to catalyse m1A modification at position 9 of mt-tRNA [101, 102]. RRP8 (also known as nucleomethylin, NML), a nucleolar factor, mediates m1A modification on 28 S rRNA of mammals to contribute to the formation of 60 S ribosomal subunit [29, 30].
Erasers
AlkB family proteins ALKBH1, ALKBH3, and FTO act as erasers for m1A. ALKBH1 depletes m1A on tRNA to regulate protein translation [23, 103], affect mitochondrial unfolded protein response (UPRmt) [104], and enhance tRNA cleavage leading to decreased tRNA stability [105]. ALKBH3 is an m1A demethylase for mRNA [16, 17, 19, 20] and tRNA [24], regulating RNA stability and translation. ALKBH3 removes not only m1A on RNA, but also 1-meA on DNA to repair these abnormally methylated DNA/RNA [106, 107]. FTO has a wide range of substrates and can remove m6A and m6Am on mRNA and snRNA as well as m1A on tRNA [108]. As a demethylase for m1A, FTO displays strong substrate selectivity and can effectively remove m1A in loop structures (such as m1A58 in the TΨC loop of tRNA) rather than m1A in linear transcripts [108].
Readers
YTH domain family proteins recognise multiple methylation modifications. YTHDF1–3 and YTHDC1, key members of the YTH domain family, are identified as m1A readers [109]. YTHDF1 promotes translation of m1A-modified transcripts [109]. YTHDF2 and YTHDF3 recognise m1A on mRNA and reduce transcript stability [17, 18].
Regulatory enzymes for m5C
Writers for m5C include NSUN1–7, members of the NOL1/NOP2/SUN (NSUN) domains protein family and DNA methyltransferase (DNMT) homologue DNMT2. Erasers for m5C include the TET family (TET1–3) and ALKBH1, which oxidises m5C to 5-hydroxymethylcytidine (hm5C) or 5-formylcytidine (f5C). m5C in RNA is recognised by six readers, ALYREF, YBX1, LIN28B, YTHDF2, RAD52 and FMRP.
Writers
NSUN1–7 and DNMT2 utilise S-adenosyl-L-methionine (SAM) as methyl donor to catalyse m5C modification. Unlike other members of the family, DNMT2 does not methylate DNA, but mediates m5C modification in the anticodon loop of tRNA at position 38 [110] and on mRNA [40, 111]. NSUN1 (NOP2) and NSUN5 (Rcm1) catalyse m5C modification on rRNA to promote ribosome synthesis, processing, and global protein synthesis [46, 49]. NSUN2 (Trm4) mediates the methylation of various RNAs, including tRNA [112], mRNA [34, 36] and other ncRNAs [113–115] to regulate RNA maturation, stabilisation, nuclear export, translation, and affect mitochondrial function. NSUN3, a mitochondrial methylase, induces m5C modification at position 34 in the anticodon loop of mt-tRNA which is further oxidised into f5C34, promoting protein synthesis in mitochondria [116, 117]. NSUN4 mediates m5C modification on mt-rRNA and mRNA to enhance mitochondrial ribosome assembly and maturation, and protein translation [47, 118]. NSUN6 locates in the cytoplasm and catalyses cytoplasmic tRNA mC72 modification [119] as well as m5C modification in the 3′UTR of mRNA, implying higher translation efficiency [39, 120]. NSUN7 introduces m5C modification on enhancer RNA (eRNA), modulating cellular energy metabolism [121].
Erasers
Reported m5C erasers include TET1–3 and ALKBH1. TET1–3 belong to the Tet family, a group of DNA dioxygenases that oxidise 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC). Tet enzymes can oxidise m5C on RNA to hm5C [122]. For example, TET1 has been shown to remove m5C in DNA:RNA hybrids at DNA damage sites to promote homologous recombination (HR) [41]. TET2 oxidises m5C to hm5C in tRNA to promote cellular translation [123]. ALKBH1 is a member of the AlkB family, which functions on demethylation. ALKBH1 shows substrate diversity and can remove m1A, m6A, m5C, m3C, etc. [124]. As a dioxygenase, ALKBH1 oxidises m5C to hm5C or f5C on mt-RNA, which is involved in the regulation of mitochondrial translation and the activity of the respiratory chain [103].
Readers
Several proteins including ALYREF, YBX1, LIN28B, YTHDF2, RAD52 and FMRP can recognise m5C in RNA. Aly/REF export factor (ALYREF) is the first identified nuclear m5C reader, relying on its m5C recognition site K171 [34]. ALYREF recognises m5C in mRNA and viral RNA to promote mRNA export [60, 125] and improve mRNA stability [126]. Y-box binding protein 1(YBX1) relies on its cold-shock domain (CSD) to recognise m5C-modified RNAs in a sequence-dependent manner and enhance the mRNA stability by recruiting RNA stabilisers ELAVL1 or Pabpc1a [36, 37]. YBX1 can also directly improve mRNA stability by recognising m5C [127–129]. Lin-28 homologue B (LIN28B) has a conserved cold-shock domain (CSD) similar to YBX1, which recognises m5C in mRNA and improves the stability of mRNA [38]. YTHDF2 recognises m5C in RNA and participates in pre-rRNA processing and maturation [48]. DNA repair protein RAD52 and Fragile X mental retardation protein (FMRP) recognise m5C in mRNA at DNA damage sites to promote HR [40, 41].
Regulatory enzymes for m7G
The reported m7G writers include three protein complexes, RNMT/RAM, METTL1/WDR4 and WBSCR22/TRMT112. Notably, there is no report that m7G is a reversible modification. As for m7G readers, the eukaryotic translation initiation factor 4E (eIF4E) family proteins (eIF4E1/eIF4E, eIF4E2/4EHP and eIF4E3) and Ago2 are able to recognise m7G in the 5′UTR cap of mRNA.
Writers
m7G writers use S-adenosylmethionine (SAM) as methyl donor to add methyl group to N7 of guanine. ABD1 of Saccharomyces cerevisiae was the first identified m7G-cap writer [130]. Human RNMT, with the same catalytic subunit as ABD1, can form a complex with the cofactor RAM to catalyse methylation of the mRNA 5′cap structure to affect mRNA stability and translation [131–133]. There are specific writers to introduce m7G onto mRNAs and other RNAs. Trm8 and Trm82 are reported to be methyltransferases of tRNA m7G in yeast, and their homologues in humans are METTL1/WDR4 complexes [134]. METTL1/WDR4 mediate m7G modification in tRNA [135–139], mRNA [53, 140] and miRNA [57] to promote tRNA expression, mRNA translation efficiency, and miRNA processing. The Bud23–Trm112 complex in Saccharomyces cerevisiae, catalysing m7G at site 1575 of 18 S rRNA, is essential for 18 S rRNA processing and maturation [141]. The human homologue of Bud23 is WBSCR22 (MERM1), which mediates m7G modification at site 1639 in human 18 S rRNA [142].
Readers
The eIF4E family contains three members: eIF4E1 (eIF4E), eIF4E2 (4EHP) and eIF4E3, which bind to m7G in the 5′UTR cap structure of mRNA [143]. In general, eIF4E localised in the cytoplasm enhances translation, while eIF4E localised in the nucleus promotes nuclear export [144, 145]. 4EHP has been considered as a translation repressor [146]. However, under some specific environmental stimuli (such as hypoxia) or in combination with some cofactors (threonyl-tRNA synthetase (TRS), ariadne homologue 1 (ARIH1)), 4EHP can also activate translation [147–149]. Under normal physiological conditions, eIF4E3 competes with eIF4E1 for the same transcript and inhibits mRNA export and translation [150]. However, eIF4E3 can also regulate translation reprogramming during stress [151]. Ago2 contains a Cap-binding-like domain to compete with eIF4E for the m7G-cap and inhibits translation initiation [60].
Regulatory enzymes for 2′-O-Me
2′-O-Me writers include two types of enzymes: independent proteases and the C/D-box snoRNPs complex (SNORNP). The independent proteases in humans are CCDC76 (hTrmt13), FTSJ1, TARBP1, MRM1, MRM2 (FTSJ2), MRM3 (RNMTL1), FTSJ3, HENMT1 and CMTR1/2. But, no reader or eraser for 2′-O-Me has yet been reported.
Writers
The 2′-O-Me modification in tRNAs is mainly mediated by independent proteases, whereas 2′-O-Me of most rRNAs and snRNAs is mainly catalysed by C/D-box snoRNPs. C/D-box snoRNPs is a complex composed of small RNAs and proteins. Small RNAs have two highly conserved sequences: C-box (RUGAGA) and D-box (CUGA), which target proteins and modified RNAs, respectively [70]. Proteins include SNU13 (NHP2L1), NOP56/58, and fibrillin (FBL), among which FBL possesses catalytic activity [70].
hTrmt13 is a 2′-O-methyltransferase for position 4 of tRNA and may promote translation by inhibiting the production of tRFs [152]. FTSJ1 is the human homologue of S. cerevisiae Trm7 and is responsible for 2′-O-methylation at positions 32 and 34 on some tRNAs [153–155]. TARBP1, the human homologue of trmH (responsible for Gm18 in bacterial tRNA), introduces Gm18 in tRNA and is associated with immune stimulatory properties of tRNA [156]. MRM1, MRM2, and MRM3 are responsible for 2′-O-methylation of 16 S rRNA in mitochondria to maintain normal mitochondrial function and homeostasis [157]. FTSJ3 contains a RNA-methyl-transferase domain (FtsJ) in the N-terminal region [158], which introduces 2′-O-methylation and can be recruited to HIV-1 RNA by TRBP to introduce internal 2′-O-methylation on HIV-1 RNA to evade host immune surveillance [159]. HENMT1 catalyses 2′-O-methylation in miRNA and Piwi-interacting RNA (piRNA), which protects miRNA from exonuclease cleavage, enhances binding of miRNA to AGO2 [160], promotes piRNA stability, and maintains the length and quantity of piRNA [161]. Notably, there are also some Nm modifications that occur in the cap structure, catalysed by CMTR1 and CMTR2 [162, 163] to stabilise RNA and facilitate RNA processing and protein translation.
Regulatory enzymes for Ψ
Ψ modification is catalysed by two types of enzymes, independent proteases or a complex composed of snoRNA/scaRNA and proteins. Pseudouridine synthases include at least six families: TruA, TruB, TruD, RsuA, RluA and PUS10. The RsuA family has not been reported in eukaryotes [164]. There is no study indicating that Ψ is a reversible modification. Reported Ψ readers include the yeast methionine aminoacyl tRNAMet synthetase (MetRS) and the RNA ATPase Prp5 in yeast.
Writers
There are 12 independent pseudouridine synthases identified in humans: PUS1, PUSL1, PUS3, TRUB1, TRUB2, RPUSD1, RPUSD2, RPUSD3, RPUSD4, PUS7, PUS7L and PUS10 [165]. PUS1/L1/3 belong to the TruA family, TRUB1/2 to the TruB family, PUS7/7 L to the TruD family, and RPUSD1/2/3/4 to the RluA family. PUS1 is a pseudouridine synthase of mRNA that is associated with the processing and maturation of pre-mRNA [166, 167]. PUS3 catalyses pseudouridylation at positions 38 and 39 in the tRNA anticodon loop and is associated with intellectual disability in humans [168]. TruB1 catalyses Ψ modifications in tRNA and mRNA [169, 170]. TruB1 mediates nuclear tRNA Ψ55 modification, contributing to nuclear export of tRNA [170]. TruB2 mainly catalyses Ψ modification at position 55 of mt-tNRA and in mt-mRNA, which contributes to the assembly of mitochondrial ribosomes and protein synthesis [166, 170, 171]. PUS7 is a pseudouridine synthase for tRNA and mRNA that affects tRFs production, cellular translation, and pre-mRNA processing [167, 172, 173]. RPUSD2 catalyses Ψ modification in mRNA [166]. RPUSD3 has been reported as a pseudouridine synthase for mt-mRNA and is associated with mitochondrial protein synthesis [171]. RPUSD4 mediates pseudouridine in mt-tRNA, 16 S rRNA, and pre-mRNA [167, 171, 174] to facilitate pre-mRNA processing and maturation and maintain normal mitochondrial function. PUS10, localised in the cytoplasm, is responsible for pseudouridylation of tRNA at positions 54 and 55 to promote cell growth [175].
H/ACA snoRNPs are the complexes of box H/ACA snoRNA and four proteins, dyskerin (NAP57/DKC1), NOP10, NHP2 and GAR1 to target rRNA and snRNA for pseudouridylation. Only DKC1 is catalytically active and catalyses U-to-Ψ isomerization, and belongs to the TruB family. NOP10 and GAR1 enhance the catalytic activity of DKC1 [176]. In addition, GAR1 contributes to substrate recruitment and product release [177]. NHP2 and Box H/ACA help target RNAs to enter the catalytic centre of H/ACA RNPs [178]. scaRNA is small Cajal body-specific RNA, a specialised snoRNA in Cajal bodies (CBs), which can also guide Ψ modification in conjunction with the above four proteins [179].
Readers
MetRS can recognise Ψ in mRNA and tRNA to regulate the translation level in yeast cells [180]. The RNA ATPase Prp5 in yeast binds to Ψ42 and Ψ44 in U2 snRNA to facilitate splicing processing of pre-mRNA [181].
Regulatory enzymes for A-to-I editing
The known enzymes responsible for A-to-I editing are ADAR1 and ADAR2 of the ADAR family and ADAT family proteins [182]. To our knowledge, till now there is no specific enzyme that recognises this modification, and no report showing that A-to-I editing is reversible.
Writers
Adenosine deaminase includes ADAR family proteins and ADAT family proteins. The ADAR family contains three members: ADAR1, ADAR2 (ADARB1), and ADAR3 (ADARB2), which act on double-stranded RNA (dsRNA). These three proteins are highly conserved during evolution, and all contain a C-terminal catalytic deaminase domain and variable numbers of dsRNA binding domains (dsRBD) [183, 184]. However, only ADAR1 and ADAR2 exhibit catalytic activity and mediate A-to-I editing on dsRNAs. ADAR3 does not have deaminase activity and cannot catalyse A-to-I editing [185], and even competes with ADAR2 for target binding, inhibiting RNA editing [186]. ADAT family proteins, including ADAT1, ADAT2 and ADAT3, are deaminases that catalyse A-to-I editing in tRNA [187].
Roles of RNA modifications in cancer
RNA modifications directly promote/suppress the expression of oncogenes/tumour suppressor genes by affecting the transcription, splicing, stability, translation, nuclear export, and other processes of mRNA, and eventually affects the occurrence and development of cancer. In addition, modifications on non-coding RNAs can consequentially modulate the expression of oncogenes/tumour suppressor genes by affecting ncRNAs splicing, stability, translation, and interaction between RNA. For example, m1A, m5C, m7G, 2′-O-Me and Ψ modifications on tRNAs and rRNAs typically regulate the translation of downstream target genes, while m5C on lncRNAs, m7G, 2′-O-Me and A-to-I editing of miRNAs regulate the expression of target genes by affecting their own synthesis as well as stability or miRNA-mRNA interactions. Notably, A-to-I editing also affects tumourigenesis and cancer progression by directly altering amino acid sequence without affecting RNA expression. Of course, abnormalities in RNA modifications in cancer are closely associated with up- or downregulation of their regulatory enzymes.
m1A modification in cancer
m1A and its regulatory enzymes are abnormally expressed in cancer, and could be used as good diagnostic markers [188]. For instance, different m1A modification patterns are distinguished in ovarian, colon, and oral squamous cell carcinomas based on differences in expression of these regulatory enzymes [189–191]. Individual tumour m1A modification pattern predicts patient prognosis, cancer stage and grade, and has different immune infiltration characteristics, which will be used for individualised treatment in the future.
Specifically, m1A modification and its regulatory enzymes play important roles in tumour cell proliferation, apoptosis, invasion and migration, cancer stem cell (CSCs) self-renewal, and metabolism by regulating the stability or translation of oncogenes or tumour suppressor genes [20, 24, 25, 192–194] (Fig. 2a). For example, the TRMT6/TRMT61A complex enhances the translation of peroxisome proliferator-activated receptor (PPARδ) by catalysing m1A modification on tRNA and promoting cholesterol biosynthesis to initiate the Hedgehog signalling, resulting in self-renewal of liver CSCs and tumourigenesis of hepatocellular carcinoma [25]. ALKBH3, an eraser of m1A, raises the sensitivity of tRNA to ANG cleavage by removing m1A, thereby promoting the generation of tDRs to enhance ribosomal assembly and translation or interact with cytochrome c (Cytc) to inhibit apoptosis [24]. In ovarian and breast cancers, upregulation of ALKBH3 leads to m1A demethylation of cytokine CSF-1 mRNA to enhance its stability, and ultimately boosts tumour invasive capacity [20].
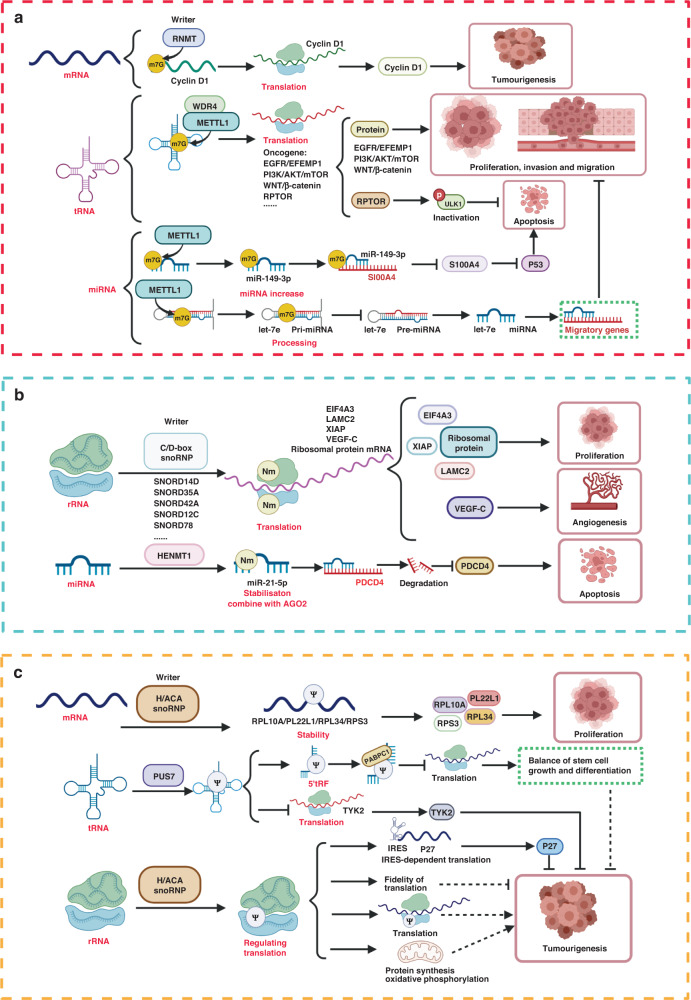
Fig. 2. Roles of m1A and m5C modifications in cancer.
a Role of m1A modifications in cancer. m1A on mRNA (e.g., CSF-1, COL1A1/2) affects tumour invasion and metastasis by regulating mRNA stability. m1A on tRNA affects tumourigenesis and apoptosis by promoting the translation of downstream target genes (such as PPARδ) and inhibiting the generation of tDRs. b Role of m5C modifications in cancer. m5C modifications on oncogenes (FTH1/FTL, PKM2, TEAD1, E2F5, YY1, RCC2, FOXC2, HDGF, KRT13, PIK3R1, PCYT1A and GRB2) enhance mRNA stability or translation, and then inhibit ferroptosis and promote tumour cell metabolic reprogramming, proliferation, invasion, and migration. The m5C modification of CDKN1C mRNA promotes CDKN1C degradation and induces cell proliferation. The m5C modifications of lncRNA (lncRNA H19 and lncRNA NKILA) enhance their stability, which further influences tumourigenesis and cancer progression. m5C on mRNA of DNA:RNA hybrids at DNA damage sites promotes DNA damage repair by recruiting DNA repair proteins and regulating the decomposition of R-loop.
m5C modification in cancer
RNA m5C modification is usually highly expressed in cancer tissues and circulating cancer cells [195, 196]. m5C affects tumour occurrence and development by regulating RNA function (Fig. 2b). m5C modification enhances oncogene mRNA stability and expression, promoting metabolic reprogramming, leading to proliferation, invasion, and migration of tumour cells [36, 38, 126, 128, 129, 197–200]. For example, NSUN2 catalyses m5C modification of HDGF [36] and KRT13 [197], which is recognised by YBX1 and stabilises HDGF and KRT13 to promote migration and invasion of bladder cancer cells and ovarian cancer cells, respectively. ALYREF is upregulated in bladder cancer and promotes glycolytic metabolism and cell proliferation by recognising m5C in 3′UTR of PKM2 and stabilising PKM2 [126]. NSUN5 promotes FTH1/FTL expression through mediating m5C modification in FTH1/FTL, resulting in decreased intracellular Fe2+ concentration and ultimately inhibiting cell ferroptosis [200]. Meanwhile, m5C also modifies the tumour suppressor genes to prompt their degradation, resulting in lower levels during tumourigenesis. For example, NSUN2 promotes gastric cancer cell proliferation by reducing CDKN1C (P57 Kip2) in an m5C-dependent manner [201].
In addition, m5C on mRNA of DNA:RNA hybrids at DNA damage sites promotes DNA damage repair by recruiting DNA repair proteins or regulating the decomposition of R-loop. TRDMT1 (DNMT2) induces the formation of m5C on mRNA at DNA damage sites, which is recognised by RAD52 or FMRP, and finally promotes HR repair. RAD52 recognises m5C and then recruits RAD51 to promote HR [40]. In the late stage of DNA repair, FMRP binds to m5C-modified mRNA and recruits TET1 to remove m5C, resulting in accelerated decomposition of R-loop (easy to break, causing genome instability), promoting the completion of DNA repair and maintaining genomic stability [41].
The tumour-promoting effect of m5C is not limited to mRNA, and many m5C-modified ncRNAs are also important for tumourigenesis [127, 202]. For example, NSUN2 enhances the stability of lncRNA NKILA in a YBX1-m5C-dependent manner. As a sponge for miR-582-3p, NKILA promotes the expression of YAP1 to promote the development of cholangiocarcinoma [127].
m7G modification in cancer
m7G modification and its regulatory enzymes affect the development of tumours by modulating cellular translation and miRNA biosynthesis (Fig. 3a). m7G-cap is associated with tumourigenesis. Cap methylation of some oncogene mRNAs boosts their nuclear export and translation [203–205]. For example, RNMT enhances the translation of cyclin D1 mRNA by promoting its cap methylation and ultimately promotes mammary epithelial cell transformation [204]. c-Myc activates the WNT/β-catenin signalling pathway by promoting cap methylation of transcripts of the WNT/β-catenin signalling pathway by RNMT [205].
Fig. 3. Role of m7G, 2′-O-Me, and Ψ modifications in cancer.
a Role of m7G modifications in cancer. mRNA (such as cyclin D1) cap methylation promotes mRNA translation and affects tumourigenesis. The increase of m7G modification on tRNA upregulates the translation of some oncogenes (e.g., EGFR/EFEMP1, RPTOR and relevant genes in PI3K/AKT/mTOR and WNT/β-catenin signalling pathways) to promote tumourigenesis, proliferation, invasion, and metastasis, and inhibit cell death. The m7G modification on miR-149-3p upregulates miRNA levels and inhibits the expression of oncogene S100A4. The m7G modification on let-7e pri-miRNA promotes its processing and further represses some genes associated with migration. b Role of 2′-O-Me modifications in cancer. Upregulation of C/D-box snoRNAs (e.g., SNORD14D, SNORD35A, SNORD42A, SNORD12C and SNORD78) in tumours enhances the translation of some oncogenes (e.g., VEGF, XIAP, EIF4A3 and LAMC2) by inducing 2′-O-Me modifications of rRNA. 2′-O-Me modification of miR-21-5p enhances miRNA stability and interaction with AGO2, which inhibits the expression of oncogene PDCD4 and ultimately promotes apoptosis. c Role of Ψ modifications in cancer. Ψ modifications on ribosomal protein mRNAs (e.g., RPL10A, RPL22L1, RPL34 and RPS3) enhance RNA stability, leading to increased ribosomal protein abundance, and promoting tumour cell proliferation. Ψ modifications on rRNA and tRNA affect tumourigenesis and cancer progression by regulating translation. The Ψ modifications on rRNA enhance IRES-dependent translation of the antioncogene p27 to suppress tumours, and also affects tumourigenesis by enhancing translation efficiency of ribosomes, improving translation fidelity, and enhancing mitochondrial function. Ψ modifications on tRNA affect tumourigenesis and cancer progression by promoting the production of 5′ tRFs or by inhibiting the translation of target genes (e.g., TYK2).
m7G modifications in mRNA interior and in some ncRNAs play an increasingly prominent role in cancer. m7G modification in tRNA upregulates translations of several oncogenes to promote tumourigenesis [135–139, 206, 207]. For example, METTL1 promotes the translation of EGFR/EFEMP1 by introducing m7G modification onto tRNA to promote proliferation, migration, and invasion in bladder cancer [139]. In esophageal squamous cell carcinoma (ESCC), METTL1/WDR4 prompts the expression of tRNA in an m7G-dependent manner, leading to abnormal activation of RPTOR, which further promotes the phosphorylation of ULK1 to inhibit autophagy [206]. m7G modifications in tumour suppressor miRNA upregulate the expression of miRNA by promoting the processing and maturation of pri-miRNA, resulting in decreased expression of downstream oncogenes [57, 208]. For example, METTL1 inhibits migration of A549 cells by inducing m7G in let-7e pri-miRNA to destroy the G-quadruplex structure and promote miRNA processing, which further inhibits several migration-related oncogenes [57].
2′-O-Me modification in cancer
2′-O-Me modification in rRNA and miRNA are closely linked to tumourigenesis (Fig. 3b). 2′-O-Me in rRNA is an important source of ribosomal heterogeneity in human cells, which is a stress response to external signals and dynamically regulates translation during tumourigenesis [209]. For example, upregulation of C/D-box snoRNA (SNORD14D, SNORD35A, and SNORD42A) enhances cellular translation by inducing 2′-O-Me on rRNA, which further promotes proliferation and self-renewal of leukaemia [210, 211]. Under hypoxic conditions, 2′-O-Me modified rRNA undergoes reprogramming and assembles into ribosomes that facilitates IRES recognition, leading to increased IRES-dependent translation of VEGF-C [212]. Many oncogenes such as VEGF and XIAP, have IRES sites that enhance translation in an IRES-dependent manner in the presence of increased 2′-O-Me on rRNA to promote tumourigenesis [212, 213]. The 2′-O-Me in rRNA is mainly introduced by box C/D snoRNP, and the assembly of box C/D snoRNP is a dynamic process which is regulated by a variety of factors. 2′-O-Me in miRNA helps to enhance its own stability which plays a role in cancer. For example, HENMT1 catalyses 2′-O-Me on miR-21-5p in lung cancer, which enhances miRNA stability through interacting with AGO2. Stabilised miR-21-5p inhibits the expression of programmed cell death protein 4 (PDCD4) and hinders apoptosis [160].
Ψ modification in cancer
Ψ modification affects tumourigenesis by regulating translation or RNA stability (Fig. 3c). Ψ modification in rRNA enhances the translation efficiency of ribosome to boost protein synthesis and ultimately promotes cell proliferation and invasion [214]. However, Ψ modification on rRNA can also prompt IRES-dependent translation of some antioncogenes (e.g., p27) and suppress tumourigenesis [215]. Ψ also helps to maintain the accuracy of translation. Abnormal Ψ modifications of certain parts of rRNA lead to mistranslation and tumourigenesis. In addition, Ψ modification in tRNA affects tumourigenesis by modulating cellular translation. For example, overexpression of PUS7 results in increased Ψ modification on tRNA to inhibit TYK2 translation and promote glioblastoma tumourigenesis [216]. Ψ8 in tRNA leads to the generation of 5′tRF (mTOG) and Ψ in mTOG inhibits translation initiation and maintains the balance of stem cell proliferation and differentiation [172]. Ψ modification in mRNA and tRNA also affects tumour progression by regulating self-stability. For example, Ψ-modified mRNAs of ribosomal proteins (RPL10A, RPL22L1, RPL34 and RPS3) are more stable, resulting in increased ribosomal protein abundance, which further promotes tumour cell proliferation [217].
Notably, RNA Ψ modification is critical for mitochondrial function. Ψ on mitochondrial 16 S rRNA and mRNA is essential for mitochondrial protein synthesis as well as oxidative phosphorylation reactions [171]. The enhanced mitochondrial function may help to meet the energy demands of rapid growth of tumour cells.
A-to-I editing in cancer
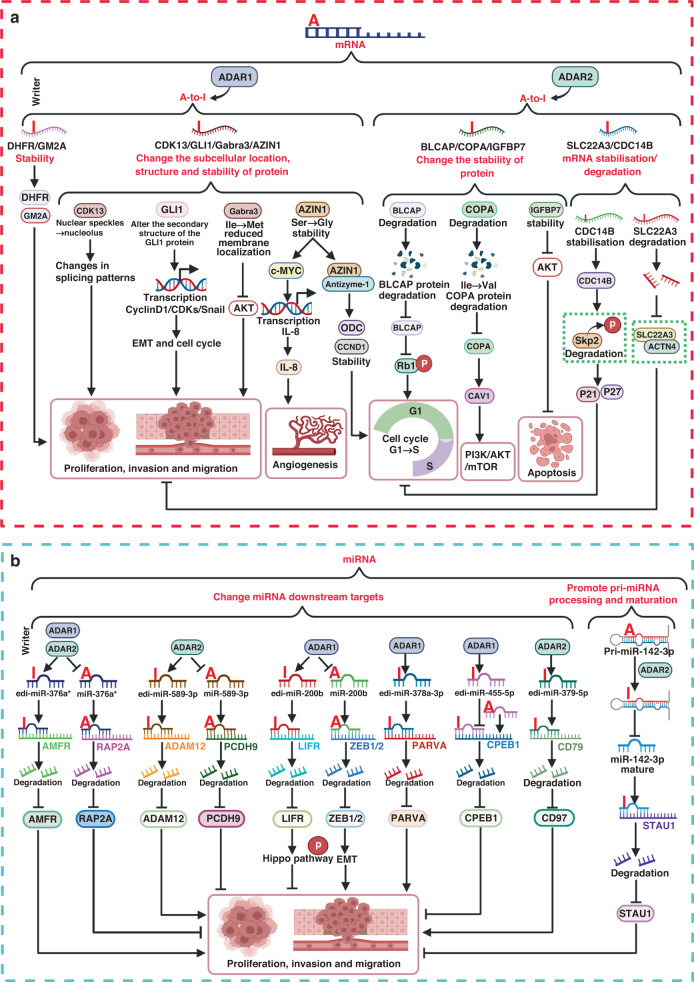
Compared with normal tissues, A-to-I editing in cancer is diverse, resulting in the heterogeneity of tumour proteomics [218]. Some specific editing sites are associated with cell viability and drug sensitivity [219]. Currently, the GPEdit website provides a global map of the genetics and pharmacogenomics of A-to-I editing in cancer, which helps A-to-I editing to be better utilised in cancer therapy [220]. In cancer, aberrant A-to-I editing mainly occurs on mRNA and miRNA (Fig. 4).
Fig. 4. Role of A-to-I editing in cancer.
a Role of A-to-I editing on mRNA in cancer. In mRNA, A-to-I editing regulates the expression of mRNA (such as GM2A, DHFR, SLC22A3, and CDC14B) to affect tumourigenesis. Meanwhile, A-to-I editing affects protein stability, binding to partners, and subcellular localisation through facilitating conformational changes in proteins such as CDK13, GLI1, Gabra3, AZIN1, COPA, BLCAP and IGFBP7 to regulate cell cycle, angiogenesis, proliferation, invasion, and migration. b A-to-I editing affects tumourigenesis and cancer progression by affecting pri-miRNA processing and maturation, and downstream targets of miRNA. For example, edited pri-miR-142-3p hinders its own processing and maturation, leading to upregulation of STAU1 and ultimately inhibiting cell migration and invasion. Unedited miR-376a* and miR-589-3p and edited miR-200b promote tumourigenesis, while edited miR-376a* and miR-589-3p and unedited miR-200b display tumour suppressor effects. Edited miR378a-3p and miR-379-5p suppressed the expression of oncogenes PAPVA and CD97, respectively. Edited miR-455-5p blocked the inhibitory effect of wild-type miR-455-5p on the tumour suppressor gene CPEB1.
A-to-I editing affects protein synthesis through regulating the stability of mRNA in cancer [79, 80, 82, 221]. For example, A-to-I editing of oncogenes GM2A and DHFR enhances their mRNA stability, leading to elevated protein synthesis and cell proliferation [79, 82]. Meanwhile, A-to-I editing also stabilises the expression of tumour suppressor genes and inhibit tumour progression under certain circumstances. For example, A-to-I edited CDC14B, with increased mRNA and protein expression, promotes the dephosphorylation and degradation of Skp2, leading to p27 and p21 accumulation, which inhibits the transition of cells from G1 phase to S phase and cell proliferation [221].
A-to-I editing may also cause amino acid change, leading to protein conformational change to regulate protein stability, partner binding, and subcellular localisation. The stability of the edited protein is altered, to affect tumourigenesis [85, 86, 222]. For example, in hepatocellular carcinoma, A-to-I edited COPA reduces CAV1 stability, resulting in declined PI3K/AKT/mTOR signalling pathway [85]. A-to-I editing alters the subcellular localisation of some edited proteins [83, 223]. For example, in breast cancer, edited Gabra3 reduces its membrane localisation and inhibits the activation of the AKT signalling pathway, which plays a tumour suppressor role [223]. In addition, A-to-I editing-induced changes in protein conformation may directly regulate expressions of downstream target genes. For example, A-to-I editing of transcriptional activator GLI1 leads to protein conformation change which activates the transcription of some target genes that positively regulate the cell cycle (e.g., Cyclin D1/CDKs) and the EMT pathway (e.g., Snail), resulting in proliferation and metastasis of pancreatic ductal adenocarcinoma [76].
Furthermore, A-to-I editing affects tumourigenesis and progression by influencing pri-miRNA processing and maturation, and the downstream targets of miRNAs. First, A-to-I editing in pri-miRNA hinders its processing and maturation [89, 224]. For example, A-to-I editing of let-7 pri-miRNA hinders its own processing and maturation, resulting in reduction of tumour suppressor let-7 miRNAs [89]. Second, A-to-I editing of miRNAs affects their recognition of downstream targets [91–94]. For example, wild-type miR-376a* plays a tumour-promoting role by targeting tumour suppressor gene RAP2A and promotes glioma cells migration and invasion, whereas A-to-I edited miR-376a* become a tumour suppressor to inhibit the expression of oncogene AMFR [94]. miR-200b plays a tumour suppressor role in a variety of tumours to inhibit the EMT progress by targeting ZEB1 and ZEB2, while the edited miR-200b inhibits the expression of metastasis suppressor LIFR [91, 92].
Conclusions and outlook
Hanahan and Weinberg characterised some common hallmarks of tumour cells that give them an advantage in growth [225–234]. In this review, we summarised six RNA modifications that regulate the proliferation, invasion, migration, metabolism, and apoptosis of tumour cells through affecting RNA processing, stability, nuclear export, and protein translation. In Table 2, we list the promoting or inhibitory effects of writers, readers, and erasers for RNA modification on these hallmarks.
Table 2.
Roles of RNA modification in human cancers.
| RNA modification | Cancer | Mechanism | References |
|---|---|---|---|
| m1A | Glioma | TRMT6/TRMT61A upregulation, promote proliferation, migration and invasion | [20, 25, 192, 240, 241] |
| Hepatocellular carcinoma | TRMT6/TRMT61A upregulation, drive self-renewal of liver CSCs and tumourigenesis | ||
| Ovarian and cervical cancer | TRMT10C upregulation, promote proliferation and migration | ||
| Breast and ovarian cancer | ALKBH3 upregulation, promote invasion | ||
| Hodgkin lymphoma | ALKBH3 downregulation, promote migration | ||
| m5C | Hepatocellular carcinoma | NSUN2 upregulation, promote proliferation, migration, invasion and angiogenesis/ALYREF upregulation, lymph node metastasis | [36, 38, 111, 126, 198, 199, 201, 202, 242] |
| Gastric carcinoma | NSUN2 upregulation, promote proliferation, migration and invasion | ||
| Oesophageal squamous cell carcinoma | NSUN2 upregulation, promote proliferation, migration, invasion and poor prognosis | ||
| Squamous cell carcinoma of the hypopharynx | NSUN2 upregulation, promote proliferation and invasion | ||
| Colorectal cancer | NSUN5 upregulation, promote proliferation | ||
| Pancreatic carcinoma | NSUN6 downregulation, inhibit migration | ||
| Bladder cancer | ALYREF upregulation, promote glycolysis and proliferation/YBX1 upregulation, promote proliferation and metastasis | ||
| m7G | Intrahepatic cholangiocarcinoma | METTL1/WDR4 upregulation, promote proliferation, migration, invasion, and inhibit apoptosis | [58, 136–139, 206, 208, 236, 237, 243–249] |
| Hepatocellular carcinoma | METTL1/WDR4 upregulation, promote proliferation, migration, invasion, and sorafenib resistance | ||
| Lung carcinoma | METTL1/WDR4 upregulation, promote proliferation, migration and invasion, inhibit autophagy | ||
| Oesophageal squamous cell carcinoma | METTL1/WDR4 upregulation, promote proliferation and inhibit apoptosis | ||
| Head and neck squamous cell carcinoma | METTL1/WDR4 upregulation, promote tumour growth and metastasis | ||
| Nasopharyngeal carcinoma | METTL1/WDR4 upregulation, promote tumour growth and metastasis | ||
| Bladder cancer | METTL1 upregulation, promote proliferation, migration and invasion | ||
| Glioma | WBSCR22 upregulation, promote proliferation, migration and invasion | ||
| Pancreatic carcinoma | WBSCR22 downregulation, inhibit proliferation, migration and invasion | ||
| Colon cancer | METTL1 downregulation, inhibit proliferation, migration, invasion and cisplatin resistance/WBSCR22 upregulation, oxaliplatin resistance and stemness maintenance | ||
| Ψ | Colorectal carcinoma | DKC1 upregulation, promote proliferation, migration, invasion and angiogenesis/PUS7 upregulation, promote proliferation, migration and invasion | [214, 216, 217, 250–255] |
| Lung adenocarcinoma | DKC1 upregulation, promote proliferation | ||
| Suprarenal epithelioma | DKC1 upregulation, promote proliferation, migration, invasion and angiogenesis | ||
| Neuroturbo chargeoma | DKC1 upregulation, promote proliferation | ||
| Glioblastoma | PUS7 upregulation, promote GSC growth and self-renewal | ||
| 2′-O-Me | Non-samll cell lung carcinoma | FTSJ1/FTSJ2 downregulation, inhibit proliferation, migration and promote cell apoptosis | [213, 256–259] |
| Breast cancer | FTSJ3 upregulation, promote proliferation | ||
| Colorectal cancer | NOP58 upregulation, promote proliferation and inhibit cell apoptosis | ||
| Prostatic carcinoma | FBL upregulation, promote proliferation | ||
| A-to-I | pancreatic ductal adenocarcinoma | ADAR1 upregulation, promote epithelial–mesenchymal transition and G2/M phase | [76, 79, 85, 92, 93, 106, 186, 222, 223, 260–263] |
| Melanoma | ADAR1 downregulation, inhibit tumour growth and metastasis | ||
| Triple-negative breast cancer | ADAR1 upregulation, promote proliferation and tumourigenesis | ||
| Breast cancer | ADAR1 downregulation, inhibit invasion and metastasis | ||
| Oral carcinoma | ADAR1 upregulation, promote proliferation, migration, invasion | ||
| Thyroid carcinoma | ADAR1 upregulation, promote proliferation, migration, invasion | ||
| Hepatic carcinoma | ADAR2 downregulation, inhibit proliferation | ||
| Oesophageal squamous cell carcinoma | ADAR2 downregulation, inhibit tumour growth and promote apoptosis | ||
| Glioma | ADAR3 downregulation, inhibit proliferation and angiogenesis | ||
| Glioblastoma | ADAR1 upregulation, promote proliferation and self-renewal/ ADAR2 downregulation, inhibit proliferation, migration, invasion/ADAR3 upregulation, promote migration and invasion |
Although RNA modification and its regulatory enzymes are abnormally expressed in a variety of cancers, most of the researches of these writers, erasers, and readers remains on tumour proliferation, invasion, and migration. Few studies investigate cancer immune escape, angiogenesis, and metabolism, which should be the focus of future work. In addition, the mechanism for up/downregulation of these regulatory enzymes remains elusive. It would be of great interest to know how hypoxia, virus infection, drug stimulation and other stress conditions [126, 200, 235] modulate the expression of these enzymes, which further affects gene expression through RNA modification, and ultimately impacts tumourigenesis. Elucidating the mechanism of up-/downregulation of these enzymes will help us to better prevent tumourigenesis. Furthermore, RNA modification is introduced by writer, removed by eraser, and recognised by reader to play a corresponding role. Writers for various RNA modifications have been extensively studied, but eraser and reader for some modifications have not been identified yet, which warrants further investigation.
Of note, due to the existence of tumour heterogeneity, it may not be accurate to designate a certain RNA modification or regulatory enzyme as a biomarker for a specific cancer. For example, m7G writer WBSCR22 promotes glioma growth whereas inhibits the proliferation of pancreatic carcinoma cells [236, 237]. In this direction, single-cell sequencing technology may aid in understanding the anomaly of RNA modifications and regulatory enzymes in different tumour subpopulations or tumour microenvironment immune cells for more accurate tumour diagnosis and treatment.
Studying the specific mechanism of RNA modification in cancers is only the first step. Importantly, it is promising to diagnose and treat cancers through exploring abnormal RNA modifications and its regulatory enzymes. For instance, thiram (TRMT6/TRMT61A complex inhibitor) [25] has been found effectively inhibits liver cancer growth. However, the research on small molecule drugs targeting RNA regulatory enzymes and modified RNA is still in its infancy. RNA modification targeting molecules in conjunction with other cancer therapies such as chemotherapy or immunotherapy may have prominent prospects for the treatment of cancers resistant to current therapeutics.
Acknowledgements
The figures are created with Biorender.com.
Author contributions
TQL, LLY, WYM, WP, HXC, OYJW, FCM, LZ, WFY, GC, ZM, LQJ and WH collected the relevant literature and drafted the manuscript. XB, JWH, LGY, ZZY and XW participated in the inception and revision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (U21A20382, U20A20367 and 82072374), the Overseas Expertise Introduction Project for Discipline Innovation (BP1221008), the Natural Science Foundation of Hunan Province (2021JJ30897).
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to publication.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang M, Mo Y, Ren D, Liu S, Zeng Z, Xiong W. Transfer RNA-derived small RNAs in tumor microenvironment. Mol Cancer. 2023;22:32. doi: 10.1186/s12943-023-01742-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Ge J, Wang Y, Xiong F, Guo J, Jiang X, et al. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat Commun. 2022;13:866. doi: 10.1038/s41467-022-28479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong F, Zhu K, Deng S, Huang H, Yang L, Gong Z, et al. AFAP1-AS1: a rising star among oncogenic long non-coding RNAs. Sci China Life Sci. 2021;64:1602–11. doi: 10.1007/s11427-020-1874-6. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Yan Q, Mo Y, Liu Y, Wang Y, Zhang S, et al. Splicing factor derived circular RNA circCAMSAP1 accelerates nasopharyngeal carcinoma tumorigenesis via a SERPINH1/c-Myc positive feedback loop. Mol Cancer. 2022;21:62. doi: 10.1186/s12943-022-01502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao M, Wang Y, Tan F, Liu L, Hou X, Fan C, et al. Circular RNA circCCNB1 inhibits the migration and invasion of nasopharyngeal carcinoma through binding and stabilizing TJP1 mRNA. Sci China Life Sci. 2022;65:2233–47. doi: 10.1007/s11427-021-2089-8. [DOI] [PubMed] [Google Scholar]
- 6.Ge J, Wang J, Xiong F, Jiang X, Zhu K, Wang Y, et al. Epstein-Barr virus-encoded circular RNA CircBART2.2 promotes immune escape of nasopharyngeal carcinoma by regulating PD-L1. Cancer Res. 2021;81:5074–88. doi: 10.1158/0008-5472.CAN-20-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mo Y, Wang Y, Wang Y, Deng X, Yan Q, Fan C, et al. Circular RNA circPVT1 promotes nasopharyngeal carcinoma metastasis via the β-TrCP/c-Myc/SRSF1 positive feedback loop. Mol Cancer. 2022;21:192. doi: 10.1186/s12943-022-01659-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccaletto P, Stefaniak F, Ray A, Cappannini A, Mukherjee S, Purta E, et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022;50:D231–d5. doi: 10.1093/nar/gkab1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ontiveros RJ, Stoute J, Liu KF. The chemical diversity of RNA modifications. Biochem J. 2019;476:1227–45. doi: 10.1042/BCJ20180445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, Song B, Tang Y, Wei Z, Xu Q, Su J, et al. RMDisease: a database of genetic variants that affect RNA modifications, with implications for epitranscriptome pathogenesis. Nucleic Acids Res. 2021;49:D1396–d1404. doi: 10.1093/nar/gkaa790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X, Li H, Liang J, Zhao Q, Xie Y, Ren J, et al. RMVar: an updated database of functional variants involved in RNA modifications. Nucleic Acids Res. 2021;49:D1405–d12. doi: 10.1093/nar/gkaa811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhi Y, Zhang S, Zi M, Wang Y, Liu Y, Zhang M, et al. Potential applications of N(6) -methyladenosine modification in the prognosis and treatment of cancers via modulating apoptosis, autophagy, and ferroptosis. Wiley Interdiscip Rev RNA. 2022;13:e1719. doi: 10.1002/wrna.1719. [DOI] [PubMed] [Google Scholar]
- 14.Tan F, Zhao M, Xiong F, Wang Y, Zhang S, Gong Z, et al. N6-methyladenosine-dependent signalling in cancer progression and insights into cancer therapies. J Exp Clin Cancer Res. 2021;40:146. doi: 10.1186/s13046-021-01952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn DB. The occurrence of 1-methyladenine in ribonucleic acid. Biochim Biophys Acta. 1961;46:198–200. doi: 10.1016/0006-3002(61)90668-0. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, et al. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol. 2016;12:311–6. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 17.Seo KW, Kleiner RE. YTHDF2 recognition of N(1)-methyladenosine (m(1)A)-modified RNA is associated with transcript destabilization. ACS Chem Biol. 2020;15:132–9. doi: 10.1021/acschembio.9b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Q, Gan H, Yang F, Yao Y, Hao F, Hong L, et al. Cytoplasmic m(1)A reader YTHDF3 inhibits trophoblast invasion by downregulation of m(1)A-methylated IGF1R. Cell Discov. 2020;6:12. doi: 10.1038/s41421-020-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuang W, Jin H, Yang F, Chen X, Liu J, Li T, et al. ALKBH3-dependent m(1)A demethylation of Aurora A mRNA inhibits ciliogenesis. Cell Discov. 2022;8:25. doi: 10.1038/s41421-022-00385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo HH, Chambers SK. Human ALKBH3-induced m(1)A demethylation increases the CSF-1 mRNA stability in breast and ovarian cancer cells. Biochim Biophys Acta Gene Regul Mech. 2019;1862:35–46. doi: 10.1016/j.bbagrm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–6. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong X, Li X, Yi C. N(1)-methyladenosine methylome in messenger RNA and non-coding RNA. Curr Opin Chem Biol. 2018;45:179–86. doi: 10.1016/j.cbpa.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Clark W, Luo G, Wang X, Fu Y, Wei J, et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:816–.e816. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Qi M, Shen B, Luo G, Wu Y, Li J, et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–45. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang J, Li X, Xiong X, Wang J, Zhou Z, et al. N(1)-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat Commun. 2021;12:6314. doi: 10.1038/s41467-021-26718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter U, Evans ME, Clark WC, Marttinen P, Shoubridge EA, Suomalainen A, et al. RNA modification landscape of the human mitochondrial tRNA(Lys) regulates protein synthesis. Nat Commun. 2018;9:3966. doi: 10.1038/s41467-018-06471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helm M, Giegé R, Florentz C. A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38:13338–46. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 28.Bar-Yaacov D, Frumkin I, Yashiro Y, Chujo T, Ishigami Y, Chemla Y, et al. Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biol. 2016;14:e1002557. doi: 10.1371/journal.pbio.1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma S, Hartmann JD, Watzinger P, Klepper A, Peifer C, Kötter P, et al. A single N(1)-methyladenosine on the large ribosomal subunit rRNA impacts locally its structure and the translation of key metabolic enzymes. Sci Rep. 2018;8:11904. doi: 10.1038/s41598-018-30383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waku T, Nakajima Y, Yokoyama W, Nomura N, Kako K, Kobayashi A, et al. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J Cell Sci. 2016;129:2382–93. doi: 10.1242/jcs.183723. [DOI] [PubMed] [Google Scholar]
- 31.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–33. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt GR. Occurrence of 5-methylcytosine in nucleic acids. Nature. 1950;166:237–8. doi: 10.1038/166237b0. [DOI] [PubMed] [Google Scholar]
- 33.Dubin DT, Taylor RH. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2:1653–68. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–25. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amort T, Rieder D, Wille A, Khokhlova-Cubberley D, Riml C, Trixl L, et al. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;18:1. doi: 10.1186/s13059-016-1139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan X, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. 2019;21:978–90. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Wang L, Han X, Yang WL, Zhang M, Ma HL, et al. RNA 5-methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol Cell. 2019;75:1188–.e1111. doi: 10.1016/j.molcel.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Su J, Wu G, Ye Y, Zhang J, Zeng L, Huang X, et al. NSUN2-mediated RNA 5-methylcytosine promotes esophageal squamous cell carcinoma progression via LIN28B-dependent GRB2 mRNA stabilization. Oncogene. 2021;40:5814–28. doi: 10.1038/s41388-021-01978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selmi T, Hussain S, Dietmann S, Heiß M, Borland K, Flad S, et al. Sequence- and structure-specific cytosine-5 mRNA methylation by NSUN6. Nucleic Acids Res. 2021;49:1006–22. doi: 10.1093/nar/gkaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Yang H, Zhu X, Yadav T, Ouyang J, Truesdell SS, et al. m(5)C modification of mRNA serves a DNA damage code to promote homologous recombination. Nat Commun. 2020;11:2834. doi: 10.1038/s41467-020-16722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Wang Y, Xiang Y, Yadav T, Ouyang J, Phoon L, et al. FMRP promotes transcription-coupled homologous recombination via facilitating TET1-mediated m5C RNA modification demethylation. Proc Natl Acad Sci USA. 2022;119:e2116251119. doi: 10.1073/pnas.2116251119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nombela P, Miguel-López B, Blanco S. The role of m(6)A, m(5)C and Ψ RNA modifications in cancer: Novel therapeutic opportunities. Mol Cancer. 2021;20:18. doi: 10.1186/s12943-020-01263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Li H, Long T, Dong H, Wang ED, Liu RJ. Archaeal NSUN6 catalyzes m5C72 modification on a wide-range of specific tRNAs. Nucleic Acids Res. 2019;47:2041–55. doi: 10.1093/nar/gky1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanmugam R, Fierer J, Kaiser S, Helm M, Jurkowski TP, Jeltsch A. Cytosine methylation of tRNA-Asp by DNMT2 has a role in translation of proteins containing poly-Asp sequences. Cell Discov. 2015;1:15010. doi: 10.1038/celldisc.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuorto F, Herbst F, Alerasool N, Bender S, Popp O, Federico G, et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. EMBO J. 2015;34:2350–62. doi: 10.15252/embj.201591382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S, Yang J, Watzinger P, Kötter P, Entian KD. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41:9062–76. doi: 10.1093/nar/gkt679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metodiev MD, Spåhr H, Loguercio Polosa P, Meharg C, Becker C, Altmueller J, et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10:e1004110. doi: 10.1371/journal.pgen.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai X, Gonzalez G, Li L, Li J, You C, Miao W, et al. YTHDF2 binds to 5-methylcytosine in RNA and modulates the maturation of ribosomal RNA. Anal Chem. 2020;92:1346–54. doi: 10.1021/acs.analchem.9b04505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heissenberger C, Liendl L, Nagelreiter F, Gonskikh Y, Yang G, Stelzer EM, et al. Loss of the ribosomal RNA methyltransferase NSUN5 impairs global protein synthesis and normal growth. Nucleic Acids Res. 2019;47:11807–25. doi: 10.1093/nar/gkz1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boulias K, Greer EL. Put the pedal to the METTL1: adding internal m(7)G increases mRNA translation efficiency and augments miRNA processing. Mol Cell. 2019;74:1105–7. doi: 10.1016/j.molcel.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Muthukrishnan S, Both GW, Furuichi Y, Shatkin AJ. 5′-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975;255:33–7. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- 52.Ohira T, Suzuki T. Precursors of tRNAs are stabilized by methylguanosine cap structures. Nat Chem Biol. 2016;12:648–55. doi: 10.1038/nchembio.2117. [DOI] [PubMed] [Google Scholar]
- 53.Zhang LS, Liu C, Ma H, Dai Q, Sun HL, Luo G, et al. Transcriptome-wide mapping of internal N(7)-Methylguanosine methylome in mammalian mRNA. Mol Cell. 2019;74:1304–.e1308. doi: 10.1016/j.molcel.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malbec L, Zhang T, Chen YS, Zhang Y, Sun BF, Shi BY, et al. Dynamic methylome of internal mRNA N(7)-methylguanosine and its regulatory role in translation. Cell Res. 2019;29:927–41. doi: 10.1038/s41422-019-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomikawa C. 7-Methylguanosine modifications in transfer RNA (tRNA) Int J Mol Sci. 2018;19:4080. doi: 10.3390/ijms19124080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu C, Yan Q, Weng C, Hou X, Mao H, Liu D, et al. Erroneous ribosomal RNAs promote the generation of antisense ribosomal siRNA. Proc Natl Acad Sci USA. 2018;115:10082–7. doi: 10.1073/pnas.1800974115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandolfini L, Barbieri I, Bannister AJ, Hendrick A, Andrews B, Webster N, et al. METTL1 promotes let-7 MicroRNA processing via m7G methylation. Mol Cell. 2019;74:1278–.e1279. doi: 10.1016/j.molcel.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai Z, Liu H, Liao J, Huang C, Ren X, Zhu W, et al. N(7)-methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Mol Cell. 2021;81:3339–.e3338. doi: 10.1016/j.molcel.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Volpon L, Culjkovic-Kraljacic B, Osborne MJ, Ramteke A, Sun Q, Niesman A, et al. Importin 8 mediates m7G cap-sensitive nuclear import of the eukaryotic translation initiation factor eIF4E. Proc Natl Acad Sci USA. 2016;113:5263–8. doi: 10.1073/pnas.1524291113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiriakidou M, Tan GS, Lamprinaki S, De Planell-Saguer M, Nelson PT, Mourelatos Z. An mRNA m7G cap binding-like motif within human Ago2 represses translation. Cell. 2007;129:1141–51. doi: 10.1016/j.cell.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Lin S, Liu Q, Lelyveld VS, Choe J, Szostak JW, Gregory RI. Mettl1/Wdr4-mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol Cell. 2018;71:244–.e245. doi: 10.1016/j.molcel.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adamiak DA, Milecki J, Popenda M, Adamiak RW, Dauter Z, Rypniewski WR. Crystal structure of 2′-O-Me(CGCGCG)2, an RNA duplex at 1.30 A resolution. Hydration pattern of 2′-O-methylated RNA. Nucleic Acids Res. 1997;25:4599–607. doi: 10.1093/nar/25.22.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lacoux C, Di Marino D, Boyl PP, Zalfa F, Yan B, Ciotti MT, et al. BC1-FMRP interaction is modulated by 2′-O-methylation: RNA-binding activity of the tudor domain and translational regulation at synapses. Nucleic Acids Res. 2012;40:4086–96. doi: 10.1093/nar/gkr1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbas YM, Laudenbach BT, Martínez-Montero S, Cencic R, Habjan M, Pichlmair A, et al. Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2′-O methylations. Proc Natl Acad Sci USA. 2017;114:E2106–e2115. doi: 10.1073/pnas.1612444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elliott BA, Ho HT, Ranganathan SV, Vangaveti S, Ilkayeva O, Abou Assi H, et al. Modification of messenger RNA by 2′-O-methylation regulates gene expression in vivo. Nat Commun. 2019;10:3401. doi: 10.1038/s41467-019-11375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurth HM, Mochizuki K. 2′-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. Rna. 2009;15:675–85. doi: 10.1261/rna.1455509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Picard-Jean F, Brand C, Tremblay-Létourneau M, Allaire A, Beaudoin MC, Boudreault S, et al. 2′-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO. PLoS ONE. 2018;13:e0193804. doi: 10.1371/journal.pone.0193804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai Q, Moshitch-Moshkovitz S, Han D, Kol N, Amariglio N, Rechavi G, et al. Nm-seq maps 2′-O-methylation sites in human mRNA with base precision. Nat Methods. 2017;14:695–8. doi: 10.1038/nmeth.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi J, Indrisiunaite G, DeMirci H, Ieong KW, Wang J, Petrov A, et al. 2′-O-methylation in mRNA disrupts tRNA decoding during translation elongation. Nat Struct Mol Biol. 2018;25:208–16. doi: 10.1038/s41594-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ayadi L, Galvanin A, Pichot F, Marchand V, Motorin Y. RNA ribose methylation (2′-O-methylation): occurrence, biosynthesis and biological functions. Biochim Biophys Acta Gene Regul Mech. 2019;1862:253–69. doi: 10.1016/j.bbagrm.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem Sci. 2002;27:344–51. doi: 10.1016/S0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- 72.Monaco PL, Marcel V, Diaz JJ, Catez F. 2′-O-methylation of ribosomal RNA: towards an epitranscriptomic control of translation? Biomolecules. 2018;8:106. doi: 10.3390/biom8040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newby MI, Greenbaum NL. Investigation of Overhauser effects between pseudouridine and water protons in RNA helices. Proc Natl Acad Sci USA. 2002;99:12697–702. doi: 10.1073/pnas.202477199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin TY, Mehta R, Glatt S. Pseudouridines in RNAs-switching atoms means shifting paradigms. FEBS Lett. 2021;595:2310–22. doi: 10.1002/1873-3468.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malik TN, Doherty EE, Gaded VM, Hill TM, Beal PA, Emeson RB. Regulation of RNA editing by intracellular acidification. Nucleic Acids Res. 2021;49:4020–36. doi: 10.1093/nar/gkab157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen P, Yang T, Chen Q, Yuan H, Wu P, Cai B, et al. CircNEIL3 regulatory loop promotes pancreatic ductal adenocarcinoma progression via miRNA sponging and A-to-I RNA-editing. Mol Cancer. 2021;20:51. doi: 10.1186/s12943-021-01333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L, Yang CS, Varelas X, Monti S. Altered RNA editing in 3′ UTR perturbs microRNA-mediated regulation of oncogenes and tumor-suppressors. Sci Rep. 2016;6:23226. doi: 10.1038/srep23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma CP, Liu H, Yi-Feng Chang I, Wang WC, Chen YT, Wu SM, et al. ADAR1 promotes robust hypoxia signaling via distinct regulation of multiple HIF-1α-inhibiting factors. EMBO Rep. 2019;20:e47107. doi: 10.15252/embr.201847107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang L, Hao Y, Shao C, Wu Q, Prager BC, Gimple RC, et al. ADAR1-mediated RNA editing links ganglioside catabolism to glioblastoma stem cell maintenance. J Clin Invest. 2022;132:e143397. doi: 10.1172/JCI143397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu L, Qin YR, Ming XY, Zuo XB, Diao YW, Zhang LY, et al. RNA editing of SLC22A3 drives early tumor invasion and metastasis in familial esophageal cancer. Proc Natl Acad Sci USA. 2017;114:E4631–e4640. doi: 10.1073/pnas.1703178114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brümmer A, Yang Y, Chan TW, Xiao X. Structure-mediated modulation of mRNA abundance by A-to-I editing. Nat Commun. 2017;8:1255. doi: 10.1038/s41467-017-01459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakano M, Fukami T, Gotoh S, Nakajima M. A-to-I RNA editing up-regulates human dihydrofolate reductase in breast cancer. J Biol Chem. 2017;292:4873–84. doi: 10.1074/jbc.M117.775684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramírez-Moya J, Miliotis C, Baker AR, Gregory RI, Slack FJ, Santisteban P. An ADAR1-dependent RNA editing event in the cyclin-dependent kinase CDK13 promotes thyroid cancer hallmarks. Mol Cancer. 2021;20:115. doi: 10.1186/s12943-021-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han F, Hu M, Zhang L, Fan X, Wang J, Lou Z, et al. A-to-I RNA editing of BLCAP promotes cell proliferation by losing the inhibitory of Rb1 in colorectal cancer. Exp Cell Res. 2022;417:113209. doi: 10.1016/j.yexcr.2022.113209. [DOI] [PubMed] [Google Scholar]
- 85.Song Y, An O, Ren X, Chan THM, Tay DJT, Tang SJ, et al. RNA editing mediates the functional switch of COPA in a novel mechanism of hepatocarcinogenesis. J Hepatol. 2021;74:135–47. doi: 10.1016/j.jhep.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 86.Chen L, Li Y, Lin CH, Chan TH, Chow RK, Song Y, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med. 2013;19:209–16. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han SW, Kim HP, Shin JY, Jeong EG, Lee WC, Kim KY, et al. RNA editing in RHOQ promotes invasion potential in colorectal cancer. J Exp Med. 2014;211:613–21. doi: 10.1084/jem.20132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shoshan E, Mobley AK, Braeuer RR, Kamiya T, Huang L, Vasquez ME, et al. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat Cell Biol. 2015;17:311–21. doi: 10.1038/ncb3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zipeto MA, Court AC, Sadarangani A, Delos Santos NP, Balaian L, Chun HJ, et al. ADAR1 activation drives leukemia stem cell self-renewal by impairing Let-7 biogenesis. Cell Stem Cell. 2016;19:177–91. doi: 10.1016/j.stem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, Shiekhattar R, et al. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat Struct Mol Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Xu X, Yu S, Jeong KJ, Zhou Z, Han L, et al. Systematic characterization of A-to-I RNA editing hotspots in microRNAs across human cancers. Genome Res. 2017;27:1112–25. doi: 10.1101/gr.219741.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramírez-Moya J, Baker AR, Slack FJ, Santisteban P. ADAR1-mediated RNA editing is a novel oncogenic process in thyroid cancer and regulates miR-200 activity. Oncogene. 2020;39:3738–53. doi: 10.1038/s41388-020-1248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cesarini V, Silvestris DA, Tassinari V, Tomaselli S, Alon S, Eisenberg E, et al. ADAR2/miR-589-3p axis controls glioblastoma cell migration/invasion. Nucleic Acids Res. 2018;46:2045–59. doi: 10.1093/nar/gkx1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choudhury Y, Tay FC, Lam DH, Sandanaraj E, Tang C, Ang BT, et al. Attenuated adenosine-to-inosine editing of microRNA-376a* promotes invasiveness of glioblastoma cells. J Clin Invest. 2012;122:4059–76. doi: 10.1172/JCI62925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paul D, Sinha AN, Ray A, Lal M, Nayak S, Sharma A, et al. A-to-I editing in human miRNAs is enriched in seed sequence, influenced by sequence contexts and significantly hypoedited in glioblastoma multiforme. Sci Rep. 2017;7:2466. doi: 10.1038/s41598-017-02397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozanick S, Krecic A, Andersland J, Anderson JT. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. Rna. 2005;11:1281–90. doi: 10.1261/rna.5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Xiong X, Zhang M, Wang K, Chen Y, Zhou J, et al. Base-resolution mapping reveals distinct m(1)A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. 2017;68:993–1005.e1009. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su Z, Monshaugen I, Wilson B, Wang F, Klungland A, Ougland R, et al. TRMT6/61A-dependent base methylation of tRNA-derived fragments regulates gene-silencing activity and the unfolded protein response in bladder cancer. Nat Commun. 2022;13:2165. doi: 10.1038/s41467-022-29790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chujo T, Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. Rna. 2012;18:2269–76. doi: 10.1261/rna.035600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–5. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 101.Vilardo E, Nachbagauer C, Buzet A, Taschner A, Holzmann J, Rossmanith W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase-extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40:11583–93. doi: 10.1093/nar/gks910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oerum S, Roovers M, Rambo RP, Kopec J, Bailey HJ, Fitzpatrick F, et al. Structural insight into the human mitochondrial tRNA purine N1-methyltransferase and ribonuclease P complexes. J Biol Chem. 2018;293:12862–76. doi: 10.1074/jbc.RA117.001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawarada L, Suzuki T, Ohira T, Hirata S, Miyauchi K, Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–15. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wagner A, Hofmeister O, Rolland SG, Maiser A, Aasumets K, Schmitt S, et al. Mitochondrial Alkbh1 localizes to mtRNA granules and its knockdown induces the mitochondrial UPR in humans and C. elegans. J Cell Sci. 2019;132:jcs223891. doi: 10.1242/jcs.223891. [DOI] [PubMed] [Google Scholar]
- 105.Rashad S, Han X, Sato K, Mishima E, Abe T, Tominaga T, et al. The stress specific impact of ALKBH1 on tRNA cleavage and tiRNA generation. RNA Biol. 2020;17:1092–103. doi: 10.1080/15476286.2020.1779492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aas PA, Otterlei M, Falnes PO, Vågbø CB, Skorpen F, Akbari M, et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421:859–63. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 107.Ougland R, Zhang CM, Liiv A, Johansen RF, Seeberg E, Hou YM, et al. AlkB restores the biological function of mRNA and tRNA inactivated by chemical methylation. Mol Cell. 2004;16:107–16. doi: 10.1016/j.molcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, et al. Differential m(6)A, m(6)A(m), and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell. 2018;71:973–.e975. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai X, Wang T, Gonzalez G, Wang Y. Identification of YTH domain-containing proteins as the readers for N1-methyladenosine in RNA. Anal Chem. 2018;90:6380–4. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–8. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 111.Xue S, Xu H, Sun Z, Shen H, Chen S, Ouyang J, et al. Depletion of TRDMT1 affects 5-methylcytosine modification of mRNA and inhibits HEK293 cell proliferation and migration. Biochem Biophys Res Commun. 2019;520:60–66. doi: 10.1016/j.bbrc.2019.09.098. [DOI] [PubMed] [Google Scholar]
- 112.Brzezicha B, Schmidt M, Makalowska I, Jarmolowski A, Pienkowska J, Szweykowska-Kulinska Z. Identification of human tRNA:m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA Leu (CAA) Nucleic Acids Res. 2006;34:6034–43. doi: 10.1093/nar/gkl765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sajini AA, Choudhury NR, Wagner RE, Bornelöv S, Selmi T, Spanos C, et al. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat Commun. 2019;10:2550. doi: 10.1038/s41467-019-10020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henry BA, Kanarek JP, Kotter A, Helm M, Lee N. 5-methylcytosine modification of an Epstein-Barr virus noncoding RNA decreases its stability. Rna. 2020;26:1038–48. doi: 10.1261/rna.075275.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yuan S, Tang H, Xing J, Fan X, Cai X, Li Q, et al. Methylation by NSun2 represses the levels and function of microRNA 125b. Mol Cell Biol. 2014;34:3630–41. doi: 10.1128/MCB.00243-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakano S, Suzuki T, Kawarada L, Iwata H, Asano K, Suzuki T. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNA(Met) Nat Chem Biol. 2016;12:546–51. doi: 10.1038/nchembio.2099. [DOI] [PubMed] [Google Scholar]
- 117.Van Haute L, Dietmann S, Kremer L, Hussain S, Pearce SF, Powell CA, et al. Deficient methylation and formylation of mt-tRNA(Met) wobble cytosine in a patient carrying mutations in NSUN3. Nat Commun. 2016;7:12039. doi: 10.1038/ncomms12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang L, Ren Z, Yan S, Zhao L, Liu J, Zhao L, et al. Nsun4 and Mettl3 mediated translational reprogramming of Sox9 promotes BMSC chondrogenic differentiation. Commun Biol. 2022;5:495. doi: 10.1038/s42003-022-03420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haag S, Warda AS, Kretschmer J, Günnigmann MA, Höbartner C, Bohnsack MT. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. Rna. 2015;21:1532–43. doi: 10.1261/rna.051524.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu J, Huang T, Zhang Y, Zhao T, Zhao X, Chen W, et al. Sequence- and structure-selective mRNA m(5)C methylation by NSUN6 in animals. Natl Sci Rev. 2021;8:nwaa273. doi: 10.1093/nsr/nwaa273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aguilo F, Li S, Balasubramaniyan N, Sancho A, Benko S, Zhang F, et al. Deposition of 5-methylcytosine on enhancer RNAs enables the coactivator function of PGC-1α. Cell Rep. 2016;14:479–92. doi: 10.1016/j.celrep.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huber SM, van Delft P, Mendil L, Bachman M, Smollett K, Werner F, et al. Formation and abundance of 5-hydroxymethylcytosine in RNA. Chembiochem. 2015;16:752–5. doi: 10.1002/cbic.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen H, Ontiveros RJ, Owens MC, Liu MY, Ghanty U, Kohli RM, et al. TET-mediated 5-methylcytosine oxidation in tRNA promotes translation. J Biol Chem. 2021;296:100087. doi: 10.1074/jbc.RA120.014226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, Wang C. Demethyltransferase AlkBH1 substrate diversity and relationship to human diseases. Mol Biol Rep. 2021;48:4747–56. doi: 10.1007/s11033-021-06421-x. [DOI] [PubMed] [Google Scholar]
- 125.Eckwahl M, Xu R, Michalkiewicz J, Zhang W, Patel P, Cai Z, et al. 5-Methylcytosine RNA modifications promote retrovirus replication in an ALYREF reader protein-dependent manner. J Virol. 2020;94:e00544–20. doi: 10.1128/JVI.00544-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang JZ, Zhu W, Han J, Yang X, Zhou R, Lu HC, et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 2021;41:560–75. doi: 10.1002/cac2.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zheng H, Zhu M, Li W, Zhou Z, Wan X. m(5) C and m(6) A modification of long noncoding NKILA accelerates cholangiocarcinoma progression via the miR-582-3p-YAP1 axis. Liver Int. 2022;42:1144–57. doi: 10.1111/liv.15240. [DOI] [PubMed] [Google Scholar]
- 128.Gao W, Chen L, Lin L, Yang M, Li T, Wei H, et al. SIAH1 reverses chemoresistance in epithelial ovarian cancer via ubiquitination of YBX-1. Oncogenesis. 2022;11:13. doi: 10.1038/s41389-022-00387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan J, Liu J, Huang Z, Huang W, Lv J. FOXC2-AS1 stabilizes FOXC2 mRNA via association with NSUN2 in gastric cancer cells. Hum Cell. 2021;34:1755–64. doi: 10.1007/s13577-021-00583-3. [DOI] [PubMed] [Google Scholar]
- 130.Mao X, Schwer B, Shuman S. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol Cell Biol. 1995;15:4167–74. doi: 10.1128/MCB.15.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trotman JB, Giltmier AJ, Mukherjee C, Schoenberg DR. RNA guanine-7 methyltransferase catalyzes the methylation of cytoplasmically recapped RNAs. Nucleic Acids Res. 2017;45:10726–39. doi: 10.1093/nar/gkx801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gonatopoulos-Pournatzis T, Dunn S, Bounds R, Cowling VH. RAM/Fam103a1 is required for mRNA cap methylation. Mol Cell. 2011;44:585–96. doi: 10.1016/j.molcel.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bueren-Calabuig JA, G Bage M, Cowling VH, Pisliakov AV. Mechanism of allosteric activation of human mRNA cap methyltransferase (RNMT) by RAM: insights from accelerated molecular dynamics simulations. Nucleic Acids Res. 2019;47:8675–92. doi: 10.1093/nar/gkz613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alexandrov A, Martzen MR, Phizicky EM. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. Rna. 2002;8:1253–66. doi: 10.1017/S1355838202024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma J, Han H, Huang Y, Yang C, Zheng S, Cai T, et al. METTL1/WDR4-mediated m(7)G tRNA modifications and m(7)G codon usage promote mRNA translation and lung cancer progression. Mol Ther. 2021;29:3422–35. doi: 10.1016/j.ymthe.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Z, Zhu W, Zhu S, Sun K, Liao J, Liu H, et al. METTL1 promotes hepatocarcinogenesis via m(7) G tRNA modification-dependent translation control. Clin Transl Med. 2021;11:e661. doi: 10.1002/ctm2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J, Li K, Chen J, Wang X, Ling R, Cheng M, et al. Aberrant translation regulated by METTL1/WDR4-mediated tRNA N7-methylguanosine modification drives head and neck squamous cell carcinoma progression. Cancer Commun. 2022;42:223–44. doi: 10.1002/cac2.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen B, Jiang W, Huang Y, Zhang J, Yu P, Wu L, et al. N(7)-methylguanosine tRNA modification promotes tumorigenesis and chemoresistance through WNT/β-catenin pathway in nasopharyngeal carcinoma. Oncogene. 2022;41:2239–53. doi: 10.1038/s41388-022-02250-9. [DOI] [PubMed] [Google Scholar]
- 139.Ying X, Liu B, Yuan Z, Huang Y, Chen C, Jiang X, et al. METTL1-m(7) G-EGFR/EFEMP1 axis promotes the bladder cancer development. Clin Transl Med. 2021;11:e675. doi: 10.1002/ctm2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao Y, Kong L, Pei Z, Li F, Li C, Sun X, et al. m7G methyltransferase METTL1 promotes post-ischemic angiogenesis via promoting VEGFA mRNA translation. Front Cell Dev Biol. 2021;9:642080. doi: 10.3389/fcell.2021.642080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Létoquart J, Huvelle E, Wacheul L, Bourgeois G, Zorbas C, Graille M, et al. Structural and functional studies of Bud23-Trm112 reveal 18S rRNA N7-G1575 methylation occurs on late 40S precursor ribosomes. Proc Natl Acad Sci USA. 2014;111:E5518–5526. doi: 10.1073/pnas.1413089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haag S, Kretschmer J, Bohnsack MT. WBSCR22/Merm1 is required for late nuclear pre-ribosomal RNA processing and mediates N7-methylation of G1639 in human 18S rRNA. Rna. 2015;21:180–7. doi: 10.1261/rna.047910.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joshi B, Lee K, Maeder DL, Jagus R. Phylogenetic analysis of eIF4E-family members. BMC Evolut Biol. 2005;5:48. doi: 10.1186/1471-2148-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carroll M, Borden KL. The oncogene eIF4E: using biochemical insights to target cancer. J Interferon Cytokine Res. 2013;33:227–38. doi: 10.1089/jir.2012.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Culjkovic B, Topisirovic I, Borden KL. Controlling gene expression through RNA regulons: the role of the eukaryotic translation initiation factor eIF4E. Cell Cycle. 2007;6:65–69. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X, Chapat C, Wang P, Choi JH, Li Q, Luo J, et al. microRNA-induced translational control of antiviral immunity by the cap-binding protein 4EHP. Mol Cell. 2021;81:1187–.e1185. doi: 10.1016/j.molcel.2021.01.030. [DOI] [PubMed] [Google Scholar]
- 147.Christie M, Igreja C. eIF4E-homologous protein (4EHP): a multifarious cap-binding protein. FEBS J. 2023;290;266–85. [DOI] [PubMed]
- 148.Jeong SJ, Park S, Nguyen LT, Hwang J, Lee EY, Giong HK, et al. A threonyl-tRNA synthetase-mediated translation initiation machinery. Nat Commun. 2019;10:1357. doi: 10.1038/s41467-019-09086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Stechow L, Typas D, Carreras Puigvert J, Oort L, Siddappa R, Pines A, et al. The E3 ubiquitin ligase ARIH1 protects against genotoxic stress by initiating a 4EHP-mediated mRNA translation arrest. Mol Cell Biol. 2015;35:1254–68. doi: 10.1128/MCB.01152-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Volpon L, Osborne MJ, Culjkovic-Kraljacic B, Borden KL. eIF4E3, a new actor in mRNA metabolism and tumor suppression. Cell Cycle. 2013;12:1159–60. doi: 10.4161/cc.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weiss B, Allen GE, Kloehn J, Abid K, Jaquier-Gubler P, Curran JA. eIF4E3 forms an active eIF4F complex during stresses (eIF4FS) targeting mTOR and re-programs the translatome. Nucleic Acids Res. 2021;49:5159–76. doi: 10.1093/nar/gkab267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li H, Dong H, Xu B, Xiong QP, Li CT, Yang WQ, et al. A dual role of human tRNA methyltransferase hTrmt13 in regulating translation and transcription. EMBOJ. 2022;41:e108544. doi: 10.15252/embj.2021108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guy MP, Phizicky EM. Conservation of an intricate circuit for crucial modifications of the tRNAPhe anticodon loop in eukaryotes. Rna. 2015;21:61–74. doi: 10.1261/rna.047639.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagayoshi Y, Chujo T, Hirata S, Nakatsuka H, Chen CW, Takakura M, et al. Loss of Ftsj1 perturbs codon-specific translation efficiency in the brain and is associated with X-linked intellectual disability. Sci Adv. 2021;7:eabf3072. doi: 10.1126/sciadv.abf3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li J, Wang YN, Xu BS, Liu YP, Zhou M, Long T, et al. Intellectual disability-associated gene ftsj1 is responsible for 2′-O-methylation of specific tRNAs. EMBO Rep. 2020;21:e50095. doi: 10.15252/embr.202050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Freund I, Buhl DK, Boutin S, Kotter A, Pichot F, Marchand V, et al. 2′-O-methylation within prokaryotic and eukaryotic tRNA inhibits innate immune activation by endosomal Toll-like receptors but does not affect recognition of whole organisms. Rna. 2019;25:869–80. doi: 10.1261/rna.070243.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee KW, Bogenhagen DF. Assignment of 2′-O-methyltransferases to modification sites on the mammalian mitochondrial large subunit 16 S ribosomal RNA (rRNA) J Biol Chem. 2014;289:24936–42. doi: 10.1074/jbc.C114.581868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simabuco FM, Morello LG, Aragão AZ, Paes Leme AF, Zanchin NI. Proteomic characterization of the human FTSJ3 preribosomal complexes. J Proteome Res. 2012;11:3112–26. doi: 10.1021/pr201106n. [DOI] [PubMed] [Google Scholar]
- 159.Ringeard M, Marchand V, Decroly E, Motorin Y, Bennasser Y. FTSJ3 is an RNA 2′-O-methyltransferase recruited by HIV to avoid innate immune sensing. Nature. 2019;565:500–4. doi: 10.1038/s41586-018-0841-4. [DOI] [PubMed] [Google Scholar]
- 160.Liang H, Jiao Z, Rong W, Qu S, Liao Z, Sun X, et al. 3′-Terminal 2′-O-methylation of lung cancer miR-21-5p enhances its stability and association with Argonaute 2. Nucleic Acids Res. 2020;48:7027–40. doi: 10.1093/nar/gkaa504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lim SL, Qu ZP, Kortschak RD, Lawrence DM, Geoghegan J, Hempfling AL, et al. HENMT1 and piRNA stability are required for adult male germ cell transposon repression and to define the spermatogenic program in the mouse. PLoS Genet. 2015;11:e1005620. doi: 10.1371/journal.pgen.1005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bélanger F, Stepinski J, Darzynkiewicz E, Pelletier J. Characterization of hMTr1, a human Cap1 2′-O-ribose methyltransferase. J Biol Chem. 2010;285:33037–44. doi: 10.1074/jbc.M110.155283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Werner M, Purta E, Kaminska KH, Cymerman IA, Campbell DA, Mittra B, et al. 2′-O-ribose methylation of cap2 in human: function and evolution in a horizontally mobile family. Nucleic Acids Res. 2011;39:4756–68. doi: 10.1093/nar/gkr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rintala-Dempsey AC, Kothe U. Eukaryotic stand-alone pseudouridine synthases—RNA modifying enzymes and emerging regulators of gene expression? RNA Biol. 2017;14:1185–96. doi: 10.1080/15476286.2016.1276150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Borchardt EK, Martinez NM, Gilbert WV. Regulation and function of RNA pseudouridylation in human cells. Annu Rev Genet. 2020;54:309–36. doi: 10.1146/annurev-genet-112618-043830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carlile TM, Martinez NM, Schaening C, Su A, Bell TA, Zinshteyn B, et al. mRNA structure determines modification by pseudouridine synthase 1. Nat Chem Biol. 2019;15:966–74. doi: 10.1038/s41589-019-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martinez NM, Su A, Burns MC, Nussbacher JK, Schaening C, Sathe S, et al. Pseudouridine synthases modify human pre-mRNA co-transcriptionally and affect pre-mRNA processing. Mol Cell. 2022;82:645–.e649. doi: 10.1016/j.molcel.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shaheen R, Han L, Faqeih E, Ewida N, Alobeid E, Phizicky EM, et al. A homozygous truncating mutation in PUS3 expands the role of tRNA modification in normal cognition. Hum Genet. 2016;135:707–13. doi: 10.1007/s00439-016-1665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Safra M, Nir R, Farouq D, Vainberg Slutskin I, Schwartz S. TRUB1 is the predominant pseudouridine synthase acting on mammalian mRNA via a predictable and conserved code. Genome Res. 2017;27:393–406. doi: 10.1101/gr.207613.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mukhopadhyay S, Deogharia M, Gupta R. Mammalian nuclear TRUB1, mitochondrial TRUB2, and cytoplasmic PUS10 produce conserved pseudouridine 55 in different sets of tRNA. Rna. 2021;27:66–79. doi: 10.1261/rna.076810.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Antonicka H, Choquet K, Lin ZY, Gingras AC, Kleinman CL, Shoubridge EA. A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep. 2017;18:28–38. doi: 10.15252/embr.201643391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guzzi N, Cieśla M, Ngoc PCT, Lang S, Arora S, Dimitriou M, et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell. 2018;173:1204–.e1226. doi: 10.1016/j.cell.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 173.Zhang DY, Ming GL, Song H. PUS7: a targetable epitranscriptomic regulator of glioblastoma growth. Trends Pharmacol Sci. 2021;42:976–8. doi: 10.1016/j.tips.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 174.Zaganelli S, Rebelo-Guiomar P, Maundrell K, Rozanska A, Pierredon S, Powell CA, et al. The Pseudouridine synthase RPUSD4 is an essential component of mitochondrial RNA granules. J Biol Chem. 2017;292:4519–32. doi: 10.1074/jbc.M116.771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Song J, Zhuang Y, Zhu C, Meng H, Lu B, Xie B, et al. Differential roles of human PUS10 in miRNA processing and tRNA pseudouridylation. Nat Chem Biol. 2020;16:160–9. doi: 10.1038/s41589-019-0420-5. [DOI] [PubMed] [Google Scholar]
- 176.Kamalampeta R, Kothe U. Archaeal proteins Nop10 and Gar1 increase the catalytic activity of Cbf5 in pseudouridylating tRNA. Sci Rep. 2012;2:663. doi: 10.1038/srep00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Duan J, Li L, Lu J, Wang W, Ye K. Structural mechanism of substrate RNA recruitment in H/ACA RNA-guided pseudouridine synthase. Mol Cell. 2009;34:427–39. doi: 10.1016/j.molcel.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 178.Caton EA, Kelly EK, Kamalampeta R, Kothe U. Efficient RNA pseudouridylation by eukaryotic H/ACA ribonucleoproteins requires high affinity binding and correct positioning of guide RNA. Nucleic Acids Res. 2018;46:905–16. doi: 10.1093/nar/gkx1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Levi O, Arava YS. Pseudouridine-mediated translation control of mRNA by methionine aminoacyl tRNA synthetase. Nucleic Acids Res. 2021;49:432–43. doi: 10.1093/nar/gkaa1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu G, Adachi H, Ge J, Stephenson D, Query CC, Yu YT. Pseudouridines in U2 snRNA stimulate the ATPase activity of Prp5 during spliceosome assembly. EMBOJ. 2016;35:654–67. doi: 10.15252/embj.201593113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schaub M, Keller W. RNA editing by adenosine deaminases generates RNA and protein diversity. Biochimie. 2002;84:791–803. doi: 10.1016/S0300-9084(02)01446-3. [DOI] [PubMed] [Google Scholar]
- 183.Eisenberg E, Levanon EY. A-to-I RNA editing—immune protector and transcriptome diversifier. Nat Rev Genet. 2018;19:473–90. doi: 10.1038/s41576-018-0006-1. [DOI] [PubMed] [Google Scholar]
- 184.Keegan LP, Leroy A, Sproul D, OConnell MA. Adenosine deaminases acting on RNA (ADARs): RNA-editing enzymes. Genome Biol. 2004;5:209. doi: 10.1186/gb-2004-5-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Melcher T, Maas S, Herb A, Sprengel R, Higuchi M, Seeburg PH. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J Biol Chem. 1996;271:31795–8. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- 186.Oakes E, Anderson A, Cohen-Gadol A, Hundley HA. Adenosine deaminase that acts on RNA 3 (ADAR3) binding to glutamate receptor subunit B pre-mRNA inhibits RNA editing in glioblastoma. J Biol Chem. 2017;292:4326–35. doi: 10.1074/jbc.M117.779868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Torres AG, Piñeyro D, Filonava L, Stracker TH, Batlle E, Ribas de Pouplana L. A-to-I editing on tRNAs: biochemical, biological and evolutionary implications. FEBS Lett. 2014;588:4279–86. doi: 10.1016/j.febslet.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 188.Zheng Q, Yu X, Zhang Q, He Y, Guo W. Genetic characteristics and prognostic implications of m1A regulators in pancreatic cancer. Biosci Rep. 2021;41:BSR20210337. doi: 10.1042/BSR20210337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gao Y, Wang H, Li H, Ye X, Xia Y, Yuan S, et al. Integrated analyses of m(1)A regulator-mediated modification patterns in tumor microenvironment-infiltrating immune cells in colon cancer. Oncoimmunology. 2021;10:1936758. doi: 10.1080/2162402X.2021.1936758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu J, Chen C, Wang Y, Qian C, Wei J, Xing Y, et al. Comprehensive of N1-methyladenosine modifications patterns and immunological characteristics in ovarian cancer. Front Immunol. 2021;12:746647. doi: 10.3389/fimmu.2021.746647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gao L, Chen R, Sugimoto M, Mizuta M, Kishimoto Y, Omori K. The impact of m1A methylation modification patterns on tumor immune microenvironment and prognosis in oral squamous cell carcinoma. Int J Mol Sci. 2021;22:19. doi: 10.3390/ijms221910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Esteve-Puig R, Climent F, Piñeyro D, Domingo-Domènech E, Davalos V, Encuentra M, et al. Epigenetic loss of m1A RNA demethylase ALKBH3 in Hodgkin lymphoma targets collagen, conferring poor clinical outcome. Blood. 2021;137:994–9. doi: 10.1182/blood.2020005823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao Y, Zhao Q, Kaboli PJ, Shen J, Li M, Wu X, et al. m1A regulated genes modulate PI3K/AKT/mTOR and ErbB pathways in gastrointestinal cancer. Transl Oncol. 2019;12:1323–33. doi: 10.1016/j.tranon.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shi L, Yang XM, Tang DD, Liu G, Yuan P, Yang Y, et al. Expression and significance of m1A transmethylase, hTrm6p/hTrm61p and its related gene hTrm6/hTrm61 in bladder urothelial carcinoma. Am J Cancer Res. 2015;5:2169–79. [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang Q, Zheng Q, Yu X, He Y, Guo W. Overview of distinct 5-methylcytosine profiles of messenger RNA in human hepatocellular carcinoma and paired adjacent non-tumor tissues. J Transl Med. 2020;18:245. doi: 10.1186/s12967-020-02417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Huang W, Qi CB, Lv SW, Xie M, Feng YQ, Huang WH, et al. Determination of DNA and RNA methylation in circulating tumor cells by mass spectrometry. Anal Chem. 2016;88:1378–84. doi: 10.1021/acs.analchem.5b03962. [DOI] [PubMed] [Google Scholar]
- 197.Wang L, Zhang J, Su Y, Maimaitiyiming Y, Yang S, Shen Z, et al. Distinct roles of m(5)C RNA methyltransferase NSUN2 in major gynecologic cancers. Front Oncol. 2022;12:786266. doi: 10.3389/fonc.2022.786266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen L, Ding J, Wang B, Chen X, Ying X, Yu Z, et al. RNA methyltransferase NSUN2 promotes hypopharyngeal squamous cell carcinoma proliferation and migration by enhancing TEAD1 expression in an m(5)C-dependent manner. Exp Cell Res. 2021;404:112664. doi: 10.1016/j.yexcr.2021.112664. [DOI] [PubMed] [Google Scholar]
- 199.Hu Y, Chen C, Tong X, Chen S, Hu X, Pan B, et al. NSUN2 modified by SUMO-2/3 promotes gastric cancer progression and regulates mRNA m5C methylation. Cell Death Dis. 2021;12:842. doi: 10.1038/s41419-021-04127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu J, Ren Z, Yang L, Zhu L, Li Y, Bie C, et al. The NSUN5-FTH1/FTL pathway mediates ferroptosis in bone marrow-derived mesenchymal stem cells. Cell Death Discov. 2022;8:99. doi: 10.1038/s41420-022-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mei L, Shen C, Miao R, Wang JZ, Cao MD, Zhang YS, et al. RNA methyltransferase NSUN2 promotes gastric cancer cell proliferation by repressing p57(Kip2) by an m(5)C-dependent manner. Cell Death Dis. 2020;11:270. doi: 10.1038/s41419-020-2487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sun Z, Xue S, Zhang M, Xu H, Hu X, Chen S, et al. Aberrant NSUN2-mediated m(5)C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene. 2020;39:6906–19. doi: 10.1038/s41388-020-01475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Piserà A, Campo A, Campo S. Structure and functions of the translation initiation factor eIF4E and its role in cancer development and treatment. J Genet Genomics = Yi Chuan Xue Bao. 2018;45:13–24. doi: 10.1016/j.jgg.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 204.Cowling VH. Enhanced mRNA cap methylation increases cyclin D1 expression and promotes cell transformation. Oncogene. 2010;29:930–6. doi: 10.1038/onc.2009.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Posternak V, Ung MH, Cheng C, Cole MD. MYC mediates mRNA cap methylation of canonical Wnt/β-catenin signaling transcripts by recruiting CDK7 and RNA methyltransferase. Mol Cancer Res. 2017;15:213–24. doi: 10.1158/1541-7786.MCR-16-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Han H, Yang C, Ma J, Zhang S, Zheng S, Ling R, et al. N(7)-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat Commun. 2022;13:1478. doi: 10.1038/s41467-022-29125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Orellana EA, Liu Q, Yankova E, Pirouz M, De Braekeleer E, Zhang W, et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol Cell. 2021;81:3323–.e3314. doi: 10.1016/j.molcel.2021.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu Y, Yang C, Zhao Y, Chi Q, Wang Z, Sun B. Overexpressed methyltransferase-like 1 (METTL1) increased chemosensitivity of colon cancer cells to cisplatin by regulating miR-149-3p/S100A4/p53 axis. Aging. 2019;11:12328–44. doi: 10.18632/aging.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jansson MD, Häfner SJ, Altinel K, Tehler D, Krogh N, Jakobsen E, et al. Regulation of translation by site-specific ribosomal RNA methylation. Nat Struct Mol Biol. 2021;28:889–99. doi: 10.1038/s41594-021-00669-4. [DOI] [PubMed] [Google Scholar]
- 210.Zhou F, Liu Y, Rohde C, Pauli C, Gerloff D, Köhn M, et al. AML1-ETO requires enhanced C/D box snoRNA/RNP formation to induce self-renewal and leukaemia. Nat Cell Biol. 2017;19:844–55. doi: 10.1038/ncb3563. [DOI] [PubMed] [Google Scholar]
- 211.Pauli C, Liu Y, Rohde C, Cui C, Fijalkowska D, Gerloff D, et al. Site-specific methylation of 18S ribosomal RNA by SNORD42A is required for acute myeloid leukemia cell proliferation. Blood. 2020;135:2059–70. doi: 10.1182/blood.2019004121. [DOI] [PubMed] [Google Scholar]
- 212.Metge BJ, Kammerud SC, Pruitt HC, Shevde LA, Samant RS. Hypoxia re-programs 2′-O-Me modifications on ribosomal RNA. iScience. 2021;24:102010. doi: 10.1016/j.isci.2020.102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yi Y, Li Y, Meng Q, Li Q, Li F, Lu B, et al. A PRC2-independent function for EZH2 in regulating rRNA 2′-O methylation and IRES-dependent translation. Nat Cell Biol. 2021;23:341–54. doi: 10.1038/s41556-021-00653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guerrieri AN, Zacchini F, Onofrillo C, Di Viggiano S, Penzo M, Ansuini A, et al. DKC1 overexpression induces a more aggressive cellular behavior and increases intrinsic ribosomal activity in immortalized mammary gland cells. Cancers. 2020;12:3512. doi: 10.3390/cancers12123512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bellodi C, Krasnykh O, Haynes N, Theodoropoulou M, Peng G, Montanaro L, et al. Loss of function of the tumor suppressor DKC1 perturbs p27 translation control and contributes to pituitary tumorigenesis. Cancer Res. 2010;70:6026–35. doi: 10.1158/0008-5472.CAN-09-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cui Q, Yin K, Zhang X, Ye P, Chen X, Chao J, et al. Targeting PUS7 suppresses tRNA pseudouridylation and glioblastoma tumorigenesis. Nat Cancer. 2021;2:932–49. doi: 10.1038/s43018-021-00238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kan G, Wang Z, Sheng C, Chen G, Yao C, Mao Y, et al. Dual inhibition of DKC1 and MEK1/2 synergistically restrains the growth of colorectal cancer cells. Adv Sci. 2021;8:2004344. doi: 10.1002/advs.202004344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Peng X, Xu X, Wang Y, Hawke DH, Yu S, Han L, et al. A-to-I RNA editing contributes to proteomic diversity in cancer. Cancer Cell. 2018;33:817–.e817. doi: 10.1016/j.ccell.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Han L, Diao L, Yu S, Xu X, Li J, Zhang R, et al. The genomic landscape and clinical relevance of A-to-I RNA editing in human cancers. Cancer Cell. 2015;28:515–28. doi: 10.1016/j.ccell.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ruan H, Li Q, Liu Y, Liu Y, Lussier C, Diao L, et al. GPEdit: the genetic and pharmacogenomic landscape of A-to-I RNA editing in cancers. Nucleic Acids Res. 2022;50:D1231–d1237. doi: 10.1093/nar/gkab810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Galeano F, Rossetti C, Tomaselli S, Cifaldi L, Lezzerini M, Pezzullo M, et al. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene. 2013;32:998–1009. doi: 10.1038/onc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen YB, Liao XY, Zhang JB, Wang F, Qin HD, Zhang L, et al. ADAR2 functions as a tumor suppressor via editing IGFBP7 in esophageal squamous cell carcinoma. Int J Oncol. 2017;50:622–30. doi: 10.3892/ijo.2016.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gumireddy K, Li A, Kossenkov AV, Sakurai M, Yan J, Li Y, et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun. 2016;7:10715. doi: 10.1038/ncomms10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Peng L, Zhang H, Su Y, Shen Q, Du C, Xie H, et al. Lipopolysaccharide enhances ADAR2 which drives Hirschsprung′s disease by impairing miR-142-3p biogenesis. J Cell Mol Med. 2018;22:4045–55. doi: 10.1111/jcmm.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 226.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 227.Jiang X, Deng X, Wang J, Mo Y, Shi L, Wei F, et al. BPIFB1 inhibits vasculogenic mimicry via downregulation of GLUT1-mediated H3K27 acetylation in nasopharyngeal carcinoma. Oncogene. 2022;41:233–45. doi: 10.1038/s41388-021-02079-8. [DOI] [PubMed] [Google Scholar]
- 228.Mo Y, Wang Y, Zhang S, Xiong F, Yan Q, Jiang X, et al. Circular RNA circRNF13 inhibits proliferation and metastasis of nasopharyngeal carcinoma via SUMO2. Mol Cancer. 2021;20:112. doi: 10.1186/s12943-021-01409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhong Y, Yang L, Xiong F, He Y, Tang Y, Shi L, et al. Long non-coding RNA AFAP1-AS1 accelerates lung cancer cells migration and invasion by interacting with SNIP1 to upregulate c-Myc. Signal Transduct Target Ther. 2021;6:240. doi: 10.1038/s41392-021-00562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fan C, Zhang S, Gong Z, Li X, Xiang B, Deng H, et al. Emerging role of metabolic reprogramming in tumor immune evasion and immunotherapy. Sci China Life Sci. 2021;64:534–47. doi: 10.1007/s11427-019-1735-4. [DOI] [PubMed] [Google Scholar]
- 231.Tang L, Xiong W, Zhang L, Wang D, Wang Y, Wu Y, et al. circSETD3 regulates MAPRE1 through miR-615-5p and miR-1538 sponges to promote migration and invasion in nasopharyngeal carcinoma. Oncogene. 2021;40:307–21. doi: 10.1038/s41388-020-01531-5. [DOI] [PubMed] [Google Scholar]
- 232.Fan C, Qu H, Xiong F, Tang Y, Tang T, Zhang L, et al. CircARHGAP12 promotes nasopharyngeal carcinoma migration and invasion via ezrin-mediated cytoskeletal remodeling. Cancer Lett. 2021;496:41–56. doi: 10.1016/j.canlet.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 233.Wang M, Dai M, Wang D, Tang T, Xiong F, Xiang B, et al. The long noncoding RNA AATBC promotes breast cancer migration and invasion by interacting with YBX1 and activating the YAP1/Hippo signaling pathway. Cancer Lett. 2021;512:60–72. doi: 10.1016/j.canlet.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 234.Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20:7. doi: 10.1186/s12943-020-01288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dai DL, Li X, Wang L, Xie C, Jin Y, Zeng MS, et al. Identification of an N6-methyladenosine-mediated positive feedback loop that promotes Epstein-Barr virus infection. J Biol Chem. 2021;296:100547. doi: 10.1016/j.jbc.2021.100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chi Y, Liang Z, Guo Y, Chen D, Lu L, Lin J, et al. WBSCR22 confers cell survival and predicts poor prognosis in glioma. Brain Res Bull. 2020;161:1–12. doi: 10.1016/j.brainresbull.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 237.Khan AA, Huang H, Zhao Y, Li H, Pan R, Wang S, et al. WBSCR22 and TRMT112 synergistically suppress cell proliferation, invasion and tumorigenesis in pancreatic cancer via transcriptional regulation of ISG15. Int J Oncol. 2022;60:24. doi: 10.3892/ijo.2022.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jack K, Bellodi C, Landry DM, Niederer RO, Meskauskas A, Musalgaonkar S, et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell. 2011;44:660–6. doi: 10.1016/j.molcel.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nachmani D, Bothmer AH, Grisendi S, Mele A, Bothmer D, Lee JD, et al. Germline NPM1 mutations lead to altered rRNA 2′-O-methylation and cause dyskeratosis congenita. Nat Genet. 2019;51:1518–29. doi: 10.1038/s41588-019-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang B, Niu L, Wang Z, Zhao Z. RNA m1A methyltransferase TRMT6 predicts poorer prognosis and promotes malignant behavior in glioma. Front Mol Biosci. 2021;8:692130. doi: 10.3389/fmolb.2021.692130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang Q, Zhang Q, Huang Y, Zhang J. m(1)A regulator TRMT10C predicts poorer survival and contributes to malignant behavior in gynecological cancers. DNA Cell Biol. 2020;39:1767–78. doi: 10.1089/dna.2020.5624. [DOI] [PubMed] [Google Scholar]
- 242.Jiang Z, Li S, Han MJ, Hu GM, Cheng P. High expression of NSUN5 promotes cell proliferation via cell cycle regulation in colorectal cancer. Am J Transl Res. 2020;12:3858–70. [PMC free article] [PubMed] [Google Scholar]
- 243.Liu Y, Zhang Y, Chi Q, Wang Z, Sun B. Methyltransferase-like 1 (METTL1) served as a tumor suppressor in colon cancer by activating 7-methyguanosine (m7G) regulated let-7e miRNA/HMGA2 axis. Life Sci. 2020;249:117480. doi: 10.1016/j.lfs.2020.117480. [DOI] [PubMed] [Google Scholar]
- 244.Tian QH, Zhang MF, Zeng JS, Luo RG, Wen Y, Chen J, et al. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J Mol Med. 2019;97:1535–45. doi: 10.1007/s00109-019-01830-9. [DOI] [PubMed] [Google Scholar]
- 245.Wang C, Wang W, Han X, Du L, Li A, Huang G. Methyltransferase-like 1 regulates lung adenocarcinoma A549 cell proliferation and autophagy via the AKT/mTORC1 signaling pathway. Oncol Lett. 2021;21:330. doi: 10.3892/ol.2021.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xia P, Zhang H, Xu K, Jiang X, Gao M, Wang G, et al. MYC-targeted WDR4 promotes proliferation, metastasis, and sorafenib resistance by inducing CCNB1 translation in hepatocellular carcinoma. Cell Death Dis. 2021;12:691. doi: 10.1038/s41419-021-03973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yan D, Tu L, Yuan H, Fang J, Cheng L, Zheng X, et al. WBSCR22 confers oxaliplatin resistance in human colorectal cancer. Sci Rep. 2017;7:15443. doi: 10.1038/s41598-017-15749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhao H, Su W, Sun Y, Wu Z. WBSCR22 competes with long non-coding RNA Linc00346 for miR-509-5p binding site to regulate cancer stem cell phenotypes of colorectal cancer. Biochem Genet. 2020;58:384–98. doi: 10.1007/s10528-020-09949-y. [DOI] [PubMed] [Google Scholar]
- 249.Zhao H, Su W, Kang Q, Xing Z, Lin X, Wu Z. Natural killer cells inhibit oxaliplatin-resistant colorectal cancer by repressing WBSCR22 via upregulating microRNA-146b-5p. Am J Cancer Res. 2018;8:824–34. [PMC free article] [PubMed] [Google Scholar]
- 250.Hou P, Shi P, Jiang T, Yin H, Chu S, Shi M, et al. DKC1 enhances angiogenesis by promoting HIF-1α transcription and facilitates metastasis in colorectal cancer. Br J Cancer. 2020;122:668–79. doi: 10.1038/s41416-019-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kan G, Wang Z, Sheng C, Yao C, Mao Y, Chen S. Inhibition of DKC1 induces telomere-related senescence and apoptosis in lung adenocarcinoma. J Transl Med. 2021;19:161. doi: 10.1186/s12967-021-02827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang M, Pan Y, Jiang R, Hou P, Shan H, Chen F, et al. DKC1 serves as a potential prognostic biomarker for human clear cell renal cell carcinoma and promotes its proliferation, migration and invasion via the NF‑κB pathway. Oncol Rep. 2018;40:968–78. doi: 10.3892/or.2018.6484. [DOI] [PubMed] [Google Scholar]
- 253.O’Brien R, Tran SL, Maritz MF, Liu B, Kong CF, Purgato S, et al. MYC-driven neuroblastomas are addicted to a telomerase-independent function of dyskerin. Cancer Res. 2016;76:3604–17. doi: 10.1158/0008-5472.CAN-15-0879. [DOI] [PubMed] [Google Scholar]
- 254.Song D, Guo M, Xu S, Song X, Bai B, Li Z, et al. HSP90-dependent PUS7 overexpression facilitates the metastasis of colorectal cancer cells by regulating LASP1 abundance. J Exp Clin Cancer Res: CR. 2021;40:170. doi: 10.1186/s13046-021-01951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Du J, Gong A, Zhao X, Wang G. Pseudouridylate synthase 7 promotes cell proliferation and invasion in colon cancer through activating PI3K/AKT/mTOR signaling pathway. Dig Dis Sci. 2022;67:1260–70. doi: 10.1007/s10620-021-06936-0. [DOI] [PubMed] [Google Scholar]
- 256.He Q, Yang L, Gao K, Ding P, Chen Q, Xiong J, et al. FTSJ1 regulates tRNA 2′-O-methyladenosine modification and suppresses the malignancy of NSCLC via inhibiting DRAM1 expression. Cell Death Dis. 2020;11:348. doi: 10.1038/s41419-020-2525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu B, Li J, Zheng M, Ge J, Li J, Yu P. MiR-542-3p exerts tumor suppressive functions in non-small cell lung cancer cells by upregulating FTSJ2. Life Sci. 2017;188:87–95. doi: 10.1016/j.lfs.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 258.Manning M, Jiang Y, Wang R, Liu L, Rode S, Bonahoom M, et al. Pan-cancer analysis of RNA methyltransferases identifies FTSJ3 as a potential regulator of breast cancer progression. RNA Biol. 2020;17:474–86. doi: 10.1080/15476286.2019.1708549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wu H, Qin W, Lu S, Wang X, Zhang J, Sun T, et al. Long noncoding RNA ZFAS1 promoting small nucleolar RNA-mediated 2′-O-methylation via NOP58 recruitment in colorectal cancer. Mol Cancer. 2020;19:95. doi: 10.1186/s12943-020-01201-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Velazquez-Torres G, Shoshan E, Ivan C, Huang L, Fuentes-Mattei E, Paret H, et al. A-to-I miR-378a-3p editing can prevent melanoma progression via regulation of PARVA expression. Nat Commun. 2018;9:461. doi: 10.1038/s41467-018-02851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kung CP, Cottrell KA, Ryu S, Bramel ER, Kladney RD, Bao EA, et al. Evaluating the therapeutic potential of ADAR1 inhibition for triple-negative breast cancer. Oncogene. 2021;40:189–202. doi: 10.1038/s41388-020-01515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu X, Fu Y, Huang J, Wu M, Zhang Z, Xu R, et al. ADAR1 promotes the epithelial-to-mesenchymal transition and stem-like cell phenotype of oral cancer by facilitating oncogenic microRNA maturation. J Exp Clin Cancer Res. 2019;38:315. doi: 10.1186/s13046-019-1300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang Y, Wang K, Zhao Z, Sun S, Zhang K, Huang R, et al. ADAR3 expression is an independent prognostic factor in lower-grade diffuse gliomas and positively correlated with the editing level of GRIA2(Q607R) Cancer Cell Int. 2018;18:196. doi: 10.1186/s12935-018-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.