Abstract
Background
Striking geographic variations in prostate cancer incidence suggest an aetiological role for spatially-distributed factors. We assessed whether neighbourhood social deprivation, which can reflect limited social contacts, unfavourable lifestyle and environmental exposures, is associated with prostate cancer risk.
Methods
In 2005–2012, we recruited 1931 incident prostate cancer cases and 1994 controls in a case–control study in Montreal, Canada. Lifetime residential addresses were linked to an area-based social deprivation index around recruitment (2006) and about 10 years earlier (1996). Logistic regression estimated adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Men residing in areas characterised by greater social deprivation had elevated prostate cancer risks (ORs of 1.54 and 1.60 for recent and past exposures, respectively; highest vs lowest quintiles), independently from area- and individual-level confounders and screening patterns. The increase in risk with recent high social deprivation was particularly elevated for high-grade prostate cancer at diagnosis (OR 1.87, 95% CI 1.32–2.64). Associations were more pronounced for neighbourhoods with higher proportions of separated/divorced or widowed individuals in the past, and with higher percentages of residents living alone recently.
Conclusions
These novel findings, suggesting that neighbourhood-level social deprivation increases the risk of prostate cancer, point out to potential targeted public health interventions.
Subject terms: Risk factors, Social sciences
Introduction
Over 1.4 million men are diagnosed with prostate cancer annually [1]. It is the most common solid-tumour malignancy among men in Canada, representing about 10% of all male cancer deaths in this country [2]. Despite extensive research, elucidating the causes of prostate cancer has proven to be a major challenge. The only established risk factors, none of which are modifiable, are age, first-degree family history of this cancer and African ancestry, with genetic factors explaining over 30% of the familial risk [3, 4]. There is an urgent need to identify factors that can lead to public health interventions. Striking geographic variations in prostate cancer incidence, observed at international, national and local scales, which cannot be fully explained by detection patterns, suggest that spatially-distributed factors may play an aetiological role [5]. This is further supported by observations that men of Asian and Hispanic heritage who migrate to the United States tend to acquire the prostate cancer risks of their host countries [6, 7]. These unexplained spatial variations point out to a promising research avenue.
Townsend [8] distinguishes two forms of deprivation: material and social, which could be independently associated with prostate cancer. While the former refers to the concept of poverty and material resources, the latter refers to social disadvantage, including isolation, which could reflect restricted social networking, from the family to the community. There is mounting evidence that material deprivation, from childhood through adulthood, is associated with prostate cancer incidence and aggressiveness [9, 10]. In particular, it has been found that high educational attainment and high income were associated with an increased risk of this cancer [11–15]. At the neighbourhood level, material deprivation has also been recently linked to prostate cancer disparities, with men living in highly materially-deprived neighbourhoods being at lower risks of prostate cancer [15–18]. This was attributed, in part, to differential access to health care, including screening.
At the individual level, social isolation and limited social ties have been previously linked to greater prostate cancer incidence and/or poorer prognosis [19–21], as the presence of close ones can promote and facilitate medical follow-ups and a healthier lifestyle [22, 23]. To our knowledge, whether neighbourhood social deprivation relates to prostate cancer incidence has never been investigated.
We assessed here whether living in a socially-deprived environment, recently and in the prior decade, was associated with prostate cancer risk, independently from material deprivation. Findings could help clarify geographic variations in prostate cancer incidence and identify populations who could benefit from targeted community-based public health interventions.
Methods
Study design and population
We used data from the Prostate Cancer and Environment Study (PROtEuS), a population-based case-control study conducted in Montreal, Canada, in 2005-2012. Study details have been published elsewhere [24, 25]. Briefly, eligible subjects were males, aged less than 76 years at diagnosis or selection, Montreal residents, registered on Quebec’s electoral list and Canadian citizens. Cases were patients diagnosed with incident, histologically confirmed prostate cancer across French-language hospitals in Montreal between 2005 and 2009, covering 80% of all cases diagnosed in the Montreal area. Concurrently, controls were randomly selected from the electoral list of French-speaking men residing in an area of 39 electoral districts, corresponding to those of cases. These lists are continuously updated and are thought to include almost all Canadian citizens residing in Quebec. Controls were frequency-matched to cases by 5-year age groups, and those with a history of prostate cancer were excluded. Participation rates were 79% and 56% among cases and controls, respectively. Overall, 1931 cases and 1994 controls were included in the analyses. We had carried out sample size calculations a priori, before launching the study, under a range of hypothetical conditions. Fixing the alpha level at 5%, the statistical power to detect an odds ratio (OR) of 1.2 with our full study sample was expected to correspond to 80% for a prevalence of exposure of 20% (quintiles).
PROtEuS was approved by the ethics committees of all participating institutions: Institut national de la recherche scientifique, Centre de Recherche du Centre Hospitalier de l’Université de Montréal, Hôpital Jean-Talon, Hôpital Maisonneuve-Rosemont, Hôpital Charles-LeMoyne, and Hôpital Fleury. All subjects provided written informed consent.
Data collection
Face-to-face interviews were conducted between 2005 and 2012. For 3% of cases and 4% of controls, respondents were proxies, usually spouses. Information was collected on sociodemographic, lifestyle, environmental and medical factors. Lifetime residential addresses were elicited during in-person interviews or during a follow-up by telephone (73.9%). Addresses were geocoded using the ArcGIS 9.3 geographic information system (GIS) (ESRI, Redlands, CA) [26] and linked to geographical dissemination areas (DA) using a spatial join method. Prostate cancer aggressiveness, defined by the Gleason score (tumour grade), was extracted from diagnostic biopsy pathology reports. Information was elicited about prostate-specific antigen (PSA) and digital rectal examination (DRE) testing in the 5 years before diagnosis or interview (index date). We did not have information on the reason motivating these tests (routine screening, symptoms, confirmatory tests, etc.) and are referring to these here as screening tests, while some might in fact have been diagnostic tests.
Neighbourhood deprivation
To characterise neighbourhood-level deprivation, we used the Canadian Material and Social Deprivation Indices [27], shown to have good validity and reliability and to perform well in the identification of multiple health issues and social inequalities [28]. These indices are based on the smallest geographical unit at which census data are available (i.e., DA), corresponding to neighbouring blocks inhabited by approximately 400-700 individuals. Because the time elapsed between initiation and detection of prostate cancer can be as long as 10 years in men over 55 years old [29], we investigated the role of deprivation in 1996, which preceded recruitment date by about a decade. We also focused on the census year closest to diagnoses/interviews, i.e., 2006, to reflect exposure that could relate to tumour growth, and screening patterns, and thus prostate cancer diagnosis. This allowed us to consider changes in addresses and exposure over the ten-year period.
The two deprivation indices were constructed by the Institut national de la santé publique du Québec using principal component analysis. They incorporate six indicators extracted from the censuses of a corresponding year and standardised for the age and sex structure of the Canadian population. For the social deprivation index, these indicators are (1) the % of individuals aged ≥15 years living alone, (2) the % of individuals aged ≥15 years who are separated, divorced or widowed, and (3) the % of single-parent families. The material deprivation index was based on (1) the % of people aged ≥15 years without high school diploma, (2) the employment/population ratio of people aged ≥15 years, and (3) the average income of people aged ≥15 years.
For both types of deprivation (social and material), we classified census units into quintiles based on the distribution of deprivation scores among our controls’ population, ranging from the least deprived neighbourhood (quintile 1) to the most deprived one (quintile 5). The material and social components vary largely independently, as confirmed by the low correlation observed in our population (Pearson’s correlation coefficients were 0.12 at recruitment and 0.21 in 1996).
In addition, we examined patterns of change in neighbourhood social deprivation between 1996 and year of recruitment. For each of the two times points, a score below the median deprivation score among controls represented low deprivation, and high deprivation otherwise. Four patterns were investigated: subjects who lived in a neighbourhood with low deprivation at both time points were classified in the “low–low” category, those who changed from high to low deprivation or low to high deprivation were in the “high–low” or “low–high”, respectively, and those who stayed in a neighbourhood characterised by high deprivation were classified in the “high–high” category.
Statistical analysis
No sampling strategy across levels was performed for the PROtEuS study. As the number of individuals per geographic unit (median=1), and the intraclass correlation coefficients (<1%) were very low, we used unconditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between neighbourhood social deprivation and prostate cancer risk. We also applied generalised estimating equations models with exchangeable matrices, thereby accounting for participants clustering within geographic units, and results were virtually the same as those based on the classical logistic model (data not shown).
Polytomous models were applied to assess associations by cancer aggressiveness. A Gleason score <7 or 7 with a primary grade of 3 and a secondary grade of 4 (7 [3 + 4]) defined low-grade prostate cancer, whereas a score >7 or 7 [4 + 3] indicated high-grade cancer [30].
Exposure to neighbourhood social deprivation was assessed (1) in 1996, (2) around the time of recruitment (2006), and (3) by using social deprivation patterns between 1996 and recruitment. For the latter, subjects who resided in a neighbourhood characterised by low deprivation at both time points constituted the reference category. We also investigated whether some of the individual census-based components used to construct the social deprivation index had a stronger influence than others on findings by studying them separately (one model for each indicator).
For each exposure, three models were developed. Model 1 was age-adjusted only. For model 2, we identified potential confounders using a directed acyclic graph (DAG) (Supplementary Fig. 1). These included personal factors such as age (continuous), ancestry (European, African, Asian, Other), family income at index date (<$20,000, $20,000–29,999, $30,000–49,999, $50,000–79,999, ≥$80,000 Canadian dollars), educational level (Elementary, High School, College, University), to which we added the neighbourhood material deprivation index (quintiles) to single out the role of neighbourhood social deprivation. The DAG was constructed based on the current knowledge and expert hypotheses concerning the causal relationships of the various factors investigated.
To investigate neighbourhood-level deprivation independently from individual social isolation, model 3 added to the previous model the following personal covariates: marital status (Married or common law, Separated or Divorced, Single, Widower, Religious order), number of children, number of siblings, and number of persons living with the subject 2 years before the index date (continuous). Potential cross-level interactions were investigated between neighbourhood social deprivation and the aforementioned individual measures of social isolation.
Continuous covariates were included in the models as linear terms when linearity was confirmed and as polynomial functions if non-linearity was detected. Departure from linearity was investigated using likelihood ratio tests comparing linear models with second-order polynomial models.
Linear trend for neighbourhood social deprivation was tested by including the ordinal variable as continuous in the model.
The proportion of missing data for the covariates varied between 0.02% for the number of persons living with the subject two years before the index date to 8.8% for family income. Overall, 13.5% of subjects had at least one variable with missing data. Under the assumption that missing data were missing at random, we performed multiple imputations by the Markov chain Monte Carlo method [31] using 14 imputed data sets. The relationship between the presence of missing data in a variable and the observed data on other variables can be found in Supplementary Tables 1–5.
To provide insight into the potential mechanisms involved in the observed associations, we described personal lifestyle and medical characteristics of our study population according to the quintiles of neighbourhood social deprivation they belonged to around the period of recruitment. In addition, we described area-level characteristics of socially-deprived neighbourhood.
Finally, several sensitivity analyses were conducted: (1) restricting analyses to subjects who had been screened for prostate cancer within the 2 years preceding the date of diagnosis/interview, in order to reduce the likelihood of latent prostate cancers among controls and to assess the impact of screening history in our results; (2) using E-values, assessing how strongly an unmeasured confounder would have to be related to both exposures and outcome to fully explain the observed associations [32]; (3) using an alternative definition of aggressive cancers (Gleason scores ≥8) [33]; and (4) stratifying analyses on social deprivation patterns according to address changes between 1996 and recruitment, which can reflect a change in neighbourhood deprivation level and/or a lower (or greater) proximity to acquaintances.
Results
Study population and linkage to geographic areas
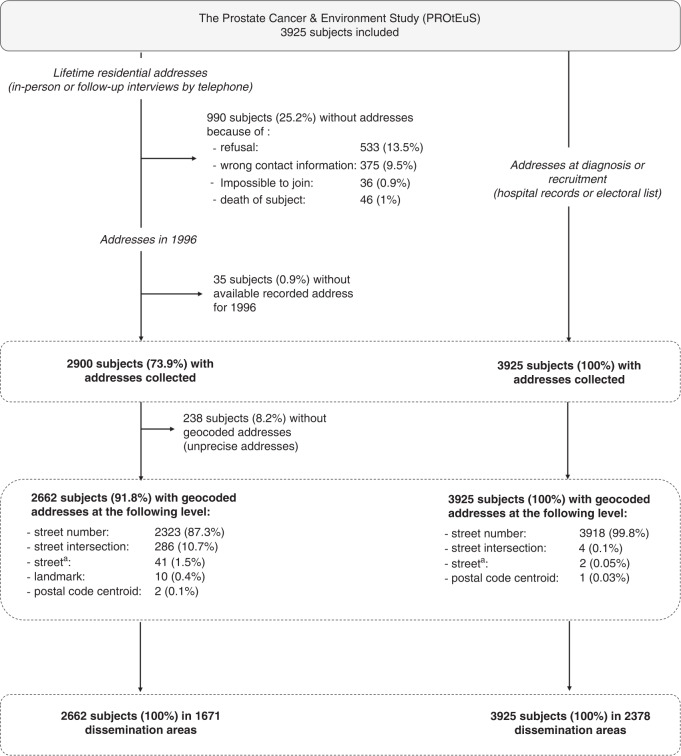
The PROtEuS study included 3925 subjects (Fig. 1). Addresses at diagnosis or recruitment were available for all and linked to 2378 DA. For 1996, 2662 addresses could be geocoded and linked to 1671 DA. Overall, 896 (33.7%) subjects had changed addresses between 1996 and recruitment.
Fig. 1. Collection and geocoding of residential addresses.
PROtEuS, Montreal, Canada, 2005–2012. aCentre of the street when <500 m long.
Characteristics of the study population
Selected characteristics of study participants at recruitment are displayed in Table 1. Controls were older, on average, than cases (64.8 vs 63.6 years), due to a longer recruitment time for controls. As expected, a greater proportion of cases than controls had a first-degree relative with history of prostate cancer. A higher proportion of cases than controls were of African ancestry, while a lower proportion were of Asian or other ancestry. Almost all cases (99%) and 76% of controls had been screened for prostate cancer in the 2-year period before the index date. Cases had a mean body mass index (BMI) of 26.8 kg/m2, vs 27.2 kg/m2 for controls. A higher proportion of controls lived with more than 3 people in the 2 years before the index date. Marginal differences between groups were observed in terms of education, family income, marital status, number of children and siblings, smoking and alcohol intake and daily frequency of fruits and vegetables consumption.
Table 1.
Individual-level characteristics of cases and controls at recruitment.
| Characteristics | Controls | Cases | ||
|---|---|---|---|---|
| n | % | n | % | |
| Ancestry | ||||
| European | 1686 | 84.6 | 1691 | 87.6 |
| African | 89 | 4.5 | 129 | 6.7 |
| Asian | 72 | 3.6 | 24 | 1.2 |
| Other | 133 | 6.7 | 75 | 3.9 |
| Missing | 14 | 0.7 | 12 | 0.6 |
| First degree relative with history of prostate cancer | ||||
| No | 1738 | 87.2 | 1411 | 73.1 |
| Yes | 196 | 9.8 | 449 | 23.3 |
| Missing | 60 | 3.0 | 71 | 3.7 |
| Timing of past prostate cancer screening (PSA and/or DRE) | ||||
| In the past 2 years | 1511 | 75.8 | 1911 | 99.0 |
| More than 2 years ago | 235 | 11.8 | 1 | 0.1 |
| Never screened | 191 | 9.6 | 3 | 0.2 |
| Do not know | 57 | 2.9 | 16 | 0.7 |
| Education | ||||
| Elementary | 431 | 21.6 | 451 | 23.4 |
| High School | 576 | 28.9 | 573 | 29.7 |
| College | 375 | 18.8 | 313 | 16.2 |
| University | 610 | 30.6 | 589 | 30.5 |
| Missing | 2 | 0.1 | 5 | 0.3 |
| Annual family income at index date (Canadian dollars) | ||||
| <20,000$ | 245 | 12.3 | 225 | 11.7 |
| 20,000–29,999$ | 252 | 12.6 | 264 | 13.7 |
| 30,000–49,999$ | 462 | 23.2 | 448 | 23.2 |
| 50,000–79,999$ | 410 | 20.6 | 423 | 21.9 |
| 80,000$ and more | 428 | 21.5 | 424 | 22.0 |
| Missing | 197 | 9.9 | 147 | 7.6 |
| Marital status | ||||
| Married or common law | 1503 | 75.4 | 1426 | 73.9 |
| Separated or divorced | 249 | 12.5 | 256 | 13.3 |
| Single | 151 | 7.6 | 164 | 8.5 |
| Widower | 83 | 4.2 | 73 | 3.8 |
| Religious order | 7 | 0.4 | 12 | 0.6 |
| Missing | 1 | 0.1 | 0 | 0.0 |
| Number of children | ||||
| 0 | 327 | 16.4 | 347 | 18.0 |
| 1 | 296 | 14.8 | 303 | 15.7 |
| 2 | 712 | 35.7 | 674 | 34.9 |
| ≥3 | 658 | 33.0 | 607 | 31.4 |
| Missing | 1 | 0.1 | 0 | 0.0 |
| Number of siblings | ||||
| 0 | 79 | 4.0 | 64 | 3.3 |
| 1-2 | 495 | 24.8 | 459 | 23.8 |
| 3-4 | 497 | 24.9 | 506 | 26.2 |
| ≥5 | 890 | 44.6 | 876 | 45.4 |
| Missing | 33 | 1.7 | 26 | 1.4 |
| Number of persons living with the subject 2 years before the index date | ||||
| 0 | 320 | 16.1 | 316 | 16.4 |
| 1 | 1108 | 55.6 | 1143 | 59.2 |
| 2 | 258 | 12.9 | 237 | 12.3 |
| ≥3 | 306 | 15.3 | 233 | 12.1 |
| Missing | 2 | 0.1 | 2 | 0.1 |
| Mean | SD | Mean | SD | |
|---|---|---|---|---|
| Age at index date (years) | 64.8 | 6.9 | 63.6 | 6.8 |
| Body mass index (kg/m2) | 27.2 | 4.4 | 26.8 | 4.0 |
| Smoking (pack-years) | 18.8 | 21.9 | 17.6 | 21.4 |
| Alcohol intake (drink-years) | 73.4 | 136.2 | 72.9 | 119.8 |
| Daily frequency of fruits and vegetables consumption | 4.5 | 2.1 | 4.4 | 2.1 |
PROtEuS, Montreal, Canada, 2005–2012.
Table 2 presents the personal characteristics of controls and cases based on interview information according to the quintiles of neighbourhood social deprivation. Overall patterns, based on a comparison of characteristics in the upper two deprivation quintiles (Q4/Q5), with those in the lowest quintile (Q1), are as follows. Compared to those living in a neighbourhood with the lowest social deprivation quintile, controls and cases who resided in the 4th or 5th deprivation quintiles tended to be heavier smokers and drinkers, to be more physically active, to report more often a history of depression treated with medication, to have had more often at least four physician visits five years earlier. In addition, controls living in areas characterised by greater levels of social deprivation tended to eat slightly fewer fruits or vegetables daily, were more likely to not have received a PSA test in the previous 5 years, to have a normal BMI, and they were less likely to be overweight/obese.
Table 2.
Selected personal characteristics of controls and cases according to the quintiles of neighbourhood social deprivation around the period of recruitment.
| Personal factors from interview | Controls | Cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Low deprivation) | 2 | 3 | 4 | 5 (High deprivation) | 1 (Low deprivation) | 2 | 3 | 4 | 5 (High deprivation) | |
| Lifetime number of drink-years (beer, spirits, wine), mean (SD) | 61.1 (103.6) | 62.4 (91.7) | 74.3 (163.4) | 92.3 (167.7) | 75.2 (134.6) | 49.1 (71.1) | 64.6 (102.3) | 70.7 (108.2) | 77.5 (127.3) | 91.4 (150.4) |
| Lifetime number of cigarette pack-years, mean (SD) | 15.4 (19.5) | 18.6 (20.5) | 19.8 (21.8) | 18.8 (23.5) | 21.1 (22.8) | 14.3 (18.8) | 15.0 (17.8) | 14.8 (18.7) | 19.8 (23.7) | 21.4 (23.5) |
| Number of fruits or vegetables/day 2 years earlier, mean (SD) | 4.7 (2.1) | 4.5 (2.1) | 4.5 (2.1) | 4.5 (2.1) | 4.2 (2.2) | 4.5 (2.8) | 4.6 (2.2) | 4.6 (2.0) | 4.3 (2.3) | 4.3 (2.8) |
| Overall physical activity level, n (%) | ||||||||||
| Not very active | 113 (29.3) | 101 (26.4) | 83 (21.6) | 79 (20.4) | 98 (25.7) | 82 (27.1) | 70 (19.9) | 80 (21.4) | 82 (22.7) | 104 (21.7) |
| Moderately active | 123 (31.9) | 106 (27.7) | 102 (26.6) | 103 (26.6) | 99 (25.9) | 88 (29.0) | 95 (27.1) | 107 (28.6) | 88 (24.3) | 133 (27.7) |
| Very active | 150 (38.9) | 176 (46.0) | 199 (51.8) | 205 (53.0) | 185 (48.4) | 133 (43.9) | 186 (53.0) | 187 (50.0) | 192 (53.0) | 243 (50.6) |
| Body mass index 2 years earlier, n (%) | ||||||||||
| Underweight (<18.5 kg/m2) | 1 (0.3) | 6 (1.6) | 4 (1.1) | 0 (0.0) | 5 (1.3) | 2 (0.7) | 1 (0.3) | 5 (1.3) | 2 (0.6) | 5 (1.1) |
| Normal (18.5–24.9 kg/m2) | 102 (26.4) | 105 (27.7) | 109 (28.6) | 122 (31.7) | 132 (34.7) | 102 (33.9) | 102 (29.2) | 127 (34.1) | 119 (33.2) | 157 (33.0) |
| Overweight (25.0–29.9 kg/m2) | 191 (49.5) | 177 (46.7) | 192 (50.4) | 175 (45.5) | 167 (44.0) | 151 (50.2) | 177 (50.7) | 177 (47.5) | 164 (45.7) | 223 (46.9) |
| Obesity (≥30 kg/m2) | 92 (23.8) | 91 (24.0) | 76 (20.0) | 88 (22.9) | 76 (20.0) | 46 (15.3) | 69 (19.8) | 64 (17.2) | 74 (20.6) | 91 (19.1) |
| Self-reported depression treated with medication, n (%) | ||||||||||
| No | 358 (92.8) | 352 (91.9) | 354 (92.2) | 349 (90.0) | 334 (87.4) | 273 (90.1) | 321 (91.5) | 333 (89.0) | 318 (87.9) | 410 (85.4) |
| Yes | 28 (7.3) | 31 (8.1) | 30 (7.8) | 39 (10.1) | 48 (12.6) | 29 (9.6) | 30 (8.6) | 39 (10.4) | 42 (11.6) | 67 (14.0) |
| Number of prostate specific antigen tests in the previous 5 years, n (%) | ||||||||||
| 0 | 37 (9.6) | 57 (14.9) | 65 (16.9) | 59 (15.2) | 67 (17.5) | 1 (0.3) | 1 (0.3) | 0 (0.0) | 1 (0.3) | 3 (0.6) |
| 1–4 | 120 (31.1) | 115 (30.0) | 127 (33.1) | 117 (30.2) | 119 (31.2) | 132 (43.6) | 130 (37.0) | 156 (41.7) | 142 (39.2) | 219 (45.6) |
| ≥5 | 201 (52.1) | 180 (47.0) | 153 (39.8) | 160 (41.2) | 160 (41.9) | 151 (49.8) | 195 (55.6) | 193 (51.6) | 191 (52.8) | 216 (45.0) |
| Don’t know | 28 (7.3) | 31 (8.1) | 39 (10.2) | 52 (13.4) | 36 (9.4) | 19 (6.3) | 25 (7.1) | 25 (6.7) | 28 (7.7) | 42 (8.8) |
| Number of physician visits, 5 years earlier, n (%) | ||||||||||
| Less than once a year | 65 (16.8) | 84 (21.9) | 85 (22.1) | 74 (19.1) | 75 (19.6) | 52 (17.2) | 66 (18.8) | 61 (16.3) | 70 (19.3) | 90 (18.8) |
| About 1–3 times a year | 289 (74.9) | 263 (68.7) | 256 (66.7) | 268 (69.1) | 259 (67.8) | 216 (71.3) | 253 (72.1) | 252 (67.4) | 254 (70.2) | 312 (65.0) |
| More than 3 times a year | 32 (8.3) | 36 (9.4) | 43 (11.2) | 46 (11.9) | 48 (12.6) | 34 (11.2) | 32 (9.1) | 60 (16.0) | 37 (10.2) | 76 (15.8) |
PROtEuS, Montreal, Canada, 2005–2012.
SD standard deviation.
Spatial distributions of the social deprivation index in Montreal were generally similar around the time of recruitment and in 1996 (Supplementary Figs. 2 and 3, respectively), with the most socially deprived areas located within the central area of the Island (city centre) and in the close periphery.
Table 3 presents the characteristics of the dissemination areas according to the quintiles of social deprivation at recruitment. Areas with high social deprivation tended to have higher population and dwellings density, a greater proportion of recent movers and a lower proportion of immigrants compared to low social deprivation areas. They were characterised by a higher proportion of multiple-unit buildings than houses and dwellings tended to be older. A similar proportion of persons aged ≥ 65 were found in the 5th quintile of deprivation compared to the 1st.
Table 3.
Selected characteristics of the dissemination areas according to the quintiles of neighbourhood social deprivation around the period of recruitment.
| Characteristics of the DA, median (IQR) | Social deprivation at recruitment | ||||
|---|---|---|---|---|---|
| 1 (Low deprivation) | 2 | 3 | 4 | 5 (High deprivation) | |
| Population density per square kilometre | 3547.2 (1776.3) | 3994.4 (4438.6) | 5865.6 (6963.5) | 8317.5 (7837.0) | 9924.5 (8610.2) |
| Density of dwellings per square kilometre | 665.8 (340.3) | 864.9 (950.8) | 1489.5 (1811.0) | 2227.4 (2264.8) | 2926.4 (3008.0) |
| % of immigrants | 23.6 (21.7) | 23.8 (26.2) | 24.0 (23.9) | 21.0 (20.9) | 19.2 (19.4) |
| % of movers in the previous 1 year | 5.6 (6.3) | 8.5 (7.7) | 11.3 (9.1) | 12.4 (10.4) | 17.2 (10.5) |
| % of persons aged ≥65 | 12.5 (9.5) | 14.4 (9.3) | 14.2 (8.1) | 14.3 (8.1) | 12.5 (8.9) |
| % of dwellings by period of construction | |||||
| Before 1970 | 34.5 (82.3) | 61.0 (71.3) | 66.0 (56.3) | 73.8 (34.8) | 72.0 (35.2) |
| Between 1970 and 1990 | 40.5 (68.9) | 24.4 (44.9) | 23.0 (39.8) | 17.6 (28.5) | 19.5 (29.4) |
| After 1990 | 0.0 (8.5) | 0.0 (11.8) | 2.7 (8.9) | 3.0 (7.1) | 3.6 (10.5) |
| % of dwellings by structural type | |||||
| House | 97.6 (11.5) | 65.6 (68.9) | 21.2 (51.9) | 8.5 (22.7) | 2.9 (11.3) |
| Apartment in building with <five storeys | 0.0 (10.3) | 29.9 (58.2) | 66.7 (61.2) | 86.7 (40.2) | 91.5 (25.8) |
| Apartment in building with ≥five storeys | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (10.2) |
| Movable dwelling | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
PROtEuS, Montreal, Canada, 2005–2012.
DA dissemination area, IQR interquartile range.
Associations between neighbourhood social deprivation and prostate cancer
Results for the association between neighbourhood social deprivation and prostate cancer risk in 1996 (approximately one decade prior to diagnosis) and around the time of recruitment are presented in Table 4. For 1996, based on the age-adjusted model, we observed that men residing in areas characterised by greater levels of social deprivation had higher odds of prostate cancer (p value for trend <0.0001). In model 2 (adjusted for potential confounders selected from the DAG describing our understanding of causal relationships, including neighbourhood material deprivation) and model 3 (further adjusted for individual indicators of social isolation), similar associations were found, albeit slightly attenuated. The OR for overall prostate cancer was 1.60 (95% CI 1.23–2.07) in model 3 for men living in areas in the upper quintile of social deprivation, compared to those in the lowest quintile. When investigating the three census-based indicators in model 3, we found positive associations for the proportion of population who were separated, divorced or widowed (OR 1.25, 95% CI 1.10–1.43), and for the proportion of population living alone (OR 1.14, 95% CI 1.05–1.25). Risk estimates were similar across models for overall and low-grade prostate cancers, and attenuated in models 2 and 3 for high-grade cancers. Nevertheless, for the latter, which included fewer cases, elevated risks persisted in the quintile characterised by greatest social deprivation.
Table 4.
Associations between neighbourhood social deprivation and prostate cancer risk in 1996 and around recruitment, overall and by cancer aggressiveness.
| 1996 | At recruitment (2006) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 Age only |
Model 2a Fully adjusted |
Model 3b Fully adjusted + personal factors of social isolation |
Model 1 Age only |
Model 2a Fully adjusted |
Model 3b Fully adjusted + personal factors of social isolation |
|||||||||||
| n Co | n Ca | OR | 95% CI | OR | 95% CI | OR | 95% CI | n Co | n Ca | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Overall | ||||||||||||||||
| Social deprivation index | ||||||||||||||||
| Q1 (low deprivation) | 256 | 216 | 1 | Reference | 1 | Reference | 1 | Reference | 386 | 303 | 1 | Reference | 1 | Reference | 1 | Reference |
| Q2 | 256 | 249 | 1.15 | 0.90–1.49 | 1.14 | 0.88–1.47 | 1.15 | 0.89–1.49 | 383 | 351 | 1.14 | 0.92–1.40 | 1.12 | 0.90–1.38 | 1.12 | 0.90–1.39 |
| Q3 | 255 | 277 | 1.30 | 1.01–1.67 | 1.25 | 0.97–1.61 | 1.23 | 0.95–1.59 | 384 | 374 | 1.25 | 1.02–1.54 | 1.21 | 0.98–1.50 | 1.18 | 0.96–1.46 |
| Q4 | 255 | 274 | 1.29 | 1.00–1.65 | 1.26 | 0.97–1.62 | 1.26 | 0.97–1.63 | 388 | 362 | 1.21 | 0.98–1.48 | 1.16 | 0.93–1.43 | 1.14 | 0.91–1.41 |
| Q5 (high deprivation) | 256 | 350 | 1.63 | 1.28–2.08 | 1.58 | 1.22–2.02 | 1.60 | 1.23–2.07 | 382 | 480 | 1.63 | 1.33–1.99 | 1.57 | 1.37–1.93 | 1.54 | 1.24–1.91 |
| p-trend | <0.0001 | 0.0004 | 0.0004 | <0.0001 | <0.0001 | 0.0003 | ||||||||||
| Census indicators | ||||||||||||||||
| % of the population aged 15 and over who are separated, divorced or widowed (per 10 % increase) | 1279 | 1370 | 1.28 | 1.13–1.45 | 1.26 | 1.10–1.43 | 1.25 | 1.10–1.43 | 1988 | 1925 | 1.14 | 1.04–1.25 | 1.11 | 1.01–1.22 | 1.09 | 0.99–1.20 |
| % of single-parent families (per 10 % increase) | 1277 | 1367 | 1.11 | 1.03–1.20 | 1.09 | 0.99–1.18 | 1.07 | 0.98–1.17 | 1982 | 1915 | 1.09 | 1.03–1.15 | 1.07 | 1.00–1.14 | 1.06 | 0.99–1.14 |
| % of the population aged 15 and over living alone (per 10 % increase) | 1278 | 1367 | 1.13 | 1.05–1.22 | 1.12 | 1.04–1.22 | 1.14 | 1.05–1.25 | 1982 | 1915 | 1.17 | 1.10–1.25 | 1.17 | 1.10–1.24 | 1.16 | 1.09–1.24 |
| Low grade | ||||||||||||||||
| Social deprivation index | ||||||||||||||||
| Q1 (low deprivation) | 256 | 167 | 1 | Reference | 1 | Reference | 1 | Reference | 386 | 247 | 1 | Reference | 1 | Reference | 1 | Reference |
| Q2 | 256 | 200 | 1.20 | 0.92–1.57 | 1.20 | 0.91–1.58 | 1.22 | 0.93–1.60 | 383 | 281 | 1.12 | 0.89–1.39 | 1.10 | 0.88–1.38 | 1.11 | 0.89–1.38 |
| Q3 | 259 | 219 | 1.31 | 1.00–1.72 | 1.28 | 0.97–1.68 | 1.27 | 0.97–1.66 | 384 | 281 | 1.16 | 0.93–1.45 | 1.14 | 0.91–1.43 | 1.12 | 0.90–1.40 |
| Q4 | 258 | 220 | 1.33 | 1.02–1.74 | 1.32 | 1.00–1.73 | 1.34 | 1.02–1.75 | 388 | 284 | 1.17 | 0.94–1.46 | 1.15 | 0.91–1.44 | 1.14 | 0.91–1.42 |
| Q5 (high deprivation) | 258 | 265 | 1.60 | 1.23–2.07 | 1.58 | 1.21–2.08 | 1.65 | 1.27–2.14 | 382 | 350 | 1.47 | 1.18–1.82 | 1.46 | 1.16–1.82 | 1.46 | 1.17–1.81 |
| p-trend | 0.0005 | 0.0011 | 0.0006 | 0.0008 | 0.0019 | 0.0015 | ||||||||||
| Census indicators | ||||||||||||||||
| % of the population aged 15 and over who are separated, divorced or widowed (per 10 % increase) | 1279 | 1066 | 1.28 | 1.12–1.46 | 1.27 | 1.11–1.46 | 1.28 | 1.11–1.47 | 1988 | 1487 | 1.14 | 1.03–1.26 | 1.12 | 1.01–1.24 | 1.10 | 0.99–1.22 |
| % of single-parent families (per 10 % increase) | 1277 | 1064 | 1.09 | 1.01–1.19 | 1.09 | 0.99–1.19 | 1.08 | 0.99–1.18 | 1982 | 1480 | 1.04 | 0.97–1.11 | 1.03 | 0.96–1.11 | 1.03 | 0.96–1.11 |
| % of the population aged 15 and over living alone (per 10 % increase) | 1278 | 1064 | 1.12 | 1.03–1.22 | 1.13 | 1.03–1.23 | 1.16 | 1.06–1.26 | 1982 | 1480 | 1.16 | 1.09–1.24 | 1.16 | 1.08–1.24 | 1.16 | 1.09–1.25 |
| High grade | ||||||||||||||||
| Social deprivation index | ||||||||||||||||
| Q1 (low deprivation) | 256 | 49 | 1 | Reference | 1 | Reference | 1 | Reference | 386 | 55 | 1 | Reference | 1 | Reference | 1 | Reference |
| Q2 | 256 | 49 | 1.00 | 0.65–1.54 | 0.94 | 0.61–1.46 | 0.92 | 0.60–1.42 | 383 | 70 | 1.26 | 0.86–1.85 | 1.19 | 0.81–1.75 | 1.18 | 0.81–1.73 |
| Q3 | 259 | 62 | 1.25 | 0.83–1.89 | 1.15 | 0.76–1.75 | 1.10 | 0.73–1.67 | 384 | 93 | 1.66 | 1.16–2.39 | 1.53 | 1.06–2.21 | 1.46 | 1.01–2.09 |
| Q4 | 258 | 55 | 1.11 | 0.73–1.70 | 1.03 | 0.66–1.57 | 0.96 | 0.63–1.47 | 388 | 77 | 1.37 | 0.94–1.98 | 1.21 | 0.83–1.78 | 1.16 | 0.80–1.69 |
| Q5 (high deprivation) | 258 | 85 | 1.73 | 1.17–2.56 | 1.49 | 1.00–2.24 | 1.40 | 0.95–2.07 | 382 | 129 | 2.32 | 1.64–3.28 | 2.01 | 1.40–2.87 | 1.87 | 1.32–2.64 |
| p-trend | 0.0033 | 0.0326 | 0.0837 | <0.0001 | 0.0003 | 0.0009 | ||||||||||
| Census indicators | ||||||||||||||||
| % of the population aged 15 and over who are separated, divorced or widowed (per 10 % increase) | 1279 | 301 | 1.28 | 1.04–1.56 | 1.18 | 0.95–1.46 | 1.15 | 0.93–1.44 | 1988 | 435 | 1.12 | 0.97–1.30 | 1.06 | 0.91–1.24 | 1.04 | 0.89–1.22 |
| % of single-parent families (per 10 % increase) | 1277 | 300 | 1.16 | 1.02–1.31 | 1.07 | 0.94–1.23 | 1.05 | 0.91–1.21 | 1982 | 432 | 1.26 | 1.15–1.38 | 1.17 | 1.05–1.30 | 1.16 | 0.99–1.29 |
| % of the population aged 15 and over living alone (per 10 % increase) | 1278 | 300 | 1.16 | 1.03–1.32 | 1.12 | 0.99–1.28 | 1.10 | 0.96–1.26 | 1982 | 432 | 1.22 | 1.10–1.34 | 1.20 | 1.08–1.32 | 1.16 | 1.04–1.29 |
PROtEuS, Montreal, Canada, 2005–2012.
aModel 2: adjusted for age, ancestry, family income at index date, highest level of education and neighbourhood material deprivation.
bModel 3: Model 2 + adjusted for marital status, number of children, number of siblings, number of persons living with the subject 2 years before the index date.
Around the time of recruitment, findings for overall prostate cancer were similar to those in 1996. For a 10% increase of the population living alone in the neighbourhood, the odds increased by 16% (95% CI 1.09–1.24), after adjustment for potential confounders and individual indicators of social isolation. Associations were elevated for high-grade cancers. Based on model 3, men living in neighbourhoods with the highest level of social deprivation had an 87% increase in odds of being diagnosed with an aggressive tumour, compared to those living in the lowest one (95% CI 1.32–2.64).
No cross-level interactions between neighbourhood social deprivation and individual measures of social isolation were found, neither for 1996 nor around the recruitment period (data not shown).
Table 5 presents associations for patterns of neighbourhood social deprivation between 1996 and recruitment. In total, 35% of subjects (N = 924) resided in a low-deprivation neighbourhood, and 39% (N = 1036) in a high-deprivation one in 1996 and at recruitment. Approximately 15% subjects (N = 388) moved from high- to low-deprivation neighbourhoods while 12% (N = 314) moved in the opposite direction.
Table 5.
Patterns of neighbourhood social deprivation between 1996 and recruitment and prostate cancer risk, overall and by cancer aggressiveness.
| Model 1 Age only |
Model 2a Fully adjusted |
Model 3b Fully adjusted + personal factors of social isolation |
||||||
|---|---|---|---|---|---|---|---|---|
| n Co | n Ca | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Overall | ||||||||
| Social deprivation index patterns | ||||||||
| Low–Low | 484 | 426 | 1 | Reference | 1 | Reference | 1 | Reference |
| High–Low | 161 | 206 | 1.44 | 1.13–1.83 | 1.42 | 1.11–1.81 | 1.41 | 1.10–1.80 |
| Low–High | 136 | 161 | 1.28 | 0.99–1.67 | 1.23 | 0.94–1.60 | 1.17 | 0.89–1.53 |
| High–High | 455 | 532 | 1.37 | 1.14–1.64 | 1.32 | 1.09–1.59 | 1.31 | 1.08–1.59 |
| Low grade | ||||||||
| Social deprivation index patterns | ||||||||
| Low–Low | 484 | 343 | 1 | Reference | Reference | 1 | Reference | |
| High–Low | 161 | 165 | 1.42 | 1.10–1.84 | 1.40 | 1.08–1.82 | 1.39 | 1.07–1.79 |
| Low–High | 136 | 122 | 1.21 | 0.92–1.60 | 1.17 | 0.88–1.55 | 1.11 | 0.84–1.47 |
| High–High | 455 | 398 | 1.28 | 1.06–1.55 | 1.26 | 1.03–1.54 | 1.27 | 1.05–1.54 |
| High grade | ||||||||
| Social deprivation index patterns | ||||||||
| Low–Low | 484 | 83 | 1 | Reference | 1 | Reference | 1 | Reference |
| High–Low | 161 | 40 | 1.46 | 0.97–2.20 | 1.44 | 0.95–2.18 | 1.44 | 0.96–2.18 |
| Low–High | 136 | 39 | 1.57 | 1.03–2.40 | 1.46 | 0.95–2.24 | 1.40 | 0.92–2.14 |
| High–High | 455 | 132 | 1.70 | 1.26–2.30 | 1.52 | 1.11–2.08 | 1.43 | 1.06–1.92 |
PROtEuS, Montreal, Canada, 2005–2012.
aModel 2: adjusted for age, ancestry, family income at index date, highest level of education and neighbourhood material deprivation.
bModel 3: Model 2 + adjusted for marital status, number of children, number of siblings, number of persons living with the subject 2 years before the index date.
Overall, no clear association with prostate cancer was found for the “low–high”, compared to the “low–low” social deprivation pattern. By contrast, men in the “high–low” and “high–high” deprivation patterns had a higher risk of prostate cancer, compared to those in the low deprivation category at both time points. For low-grade prostate cancer, a higher risk was found for the “high–low” pattern while for high-grade prostate cancer, similar increases in odds, by about 40%, were found for patterns involving high deprivation at one or both time points.
Sensitivity analyses
Restricting analyses to subjects who had been screened for prostate cancer within the two years preceding the diagnosis/interview resulted in stronger associations between social deprivation and prostate cancer than those observed in the main analyses (Supplementary Tables 6 and 7).
Through the E-value, we found that a factor would have to be associated with both the exposure and the outcome and yield an OR of 2.58 or more (lower limit 95% CI 1.74) to explain the OR of 1.60 (95% CI 1.22–2.07) for men in the upper quintiles of social deprivation in 1996. The corresponding E-value was 2.45 (lower limit 95% CI 1.79) for the risk estimate around the time of recruitment.
Complete-case analyses (data not shown) generated estimates analogous to those from our main analyses based on imputed data. Results were also similar when using a Gleason score of 8 or more to characterise aggressive cancers (Supplementary Table 8).
Supplementary Table 9 presents associations between levels of social deprivation over time and prostate cancer risk, separately for those who moved between 1996 and recruitment and those who did not. For those who had not moved, a higher risk of prostate cancer was found for all three patterns investigated compared to the “low–low” group. Associations for high-grade prostate cancer were particularly high for the “low–high” pattern. For those who moved, no clear associations emerged between the different patterns and prostate cancer risk, based on few subjects.
Discussion
We observed that men living in neighbourhoods characterised by high social deprivation had greater risks of prostate cancer, with evidence of dose–response patterns. Odds of the cancer were especially pronounced for individuals living in the top quintile of socially deprived areas. This held true for both exposure time points investigated. The area-based indicators most strongly related to cancer risk were the proportions of individuals who were separated, divorced or widowed in the neighbourhood (10 years earlier), and of individuals living alone (around recruitment). Men living in the most socially deprived neighbourhoods had a nearly twofold increase in odds of being diagnosed with aggressive tumours, compared to those living in the least socially deprived areas. Associations were independent from neighbourhood-level material deprivation and personal factors, including measures of social isolation. Findings persisted among subjects recently tested with PSA and/or DRE.
Literature
Previous studies addressing neighbourhood deprivation focused exclusively on socioeconomic circumstances, including area-based household income, education, housing, unemployment or occupation. According to these, men living in privileged socioeconomic areas had elevated prostate cancer risks [15–18], and cancers were more likely to be of low grade and stage at diagnosis [34, 35], possibly reflecting differences in cancer detection. This is in line with studies observing higher testing rates among men with higher socioeconomic status [18, 36]. Behaviours, such as eating habits, physical activity, or alcohol consumption, have also been invoked to partly explain differences in health risks across areas with varying socioeconomic deprivation levels [37–39], although this has yet to be explored with respect to prostate cancer specifically.
Material and social deprivation scores capture different concepts, as confirmed by the weak correlation observed here and previously [27, 40]. To the best of our knowledge, our study is the first to specifically investigate social deprivation of neighbourhoods in prostate cancer risk. Nevertheless, a recent study has assessed whether social capital, measured as the proportions of residents who voted in local government elections, is related to prostate cancer incidence [41]. The authors found that compared to men living in neighbourhoods with high linking social capital, those living in neighbourhoods with low and intermediate linking social capital presented lower odds of being diagnosed with aggressive tumours. Some aspects of social environment have also been investigated in relation with prostate cancer aggressiveness more specifically in a US study [42]. The authors identified two factors related to neighbourhood social deprivation that increased the risk of being diagnosed with high grade/stage prostate cancer (Surveillance, Epidemiology, and End Results (SEER) > 3 or Gleason >7) when compared to low grade/stage. These were the proportion of male householders living alone (OR 1.06, 95% CI 1.01–1.11) and the proportion of male householders over age 65 living alone (OR 1.07, 95% CI 1.02–1.13). We also found an excess risk of high-grade cancer for the proportion of residents living alone in the neighbourhood, in accordance with this prior research. Individual-level exposures were not measured in the US study, precluding the estimation of potential composition effects.
Mechanisms
Contextual social deprivation may play a role in prostate cancer risk, either directly or indirectly through other factors [43]. Associations with neighbourhood social deprivation differed to some extent between low- and high-grade cancers. The prostate cancer grade describes cell differentiation and does not necessarily reflect disease progression [44, 45]. Previous observations suggest that low- and high-grade prostate cancers can have different risk factor sets [46] and it may be that social deprivation would act through different mechanisms with respect to cancer grades.
The effect of social deprivation is likely to be the result of complex relationships between multi-level factors. One possible pathway could be through lifestyle factors such as alcohol consumption, or diet which are suspected causes of prostate cancer [3]. This is supported by a recent study of a large cohort of Canadian adults indicating that individuals who live in areas characterised by higher social deprivation have lower mean diet quality [47]. In addition, socially deprived areas may have greater availability of alcohol outlets. A study conducted in the province of Quebec found indeed that accessibility to alcohol outlets increases in high socially deprived neighbourhoods [48]. Neighbourhood social deprivation has also been associated with certain environmental exposures [49, 50] which have previously been linked to prostate cancer risk, such as air pollution [24].
In a recent Canadian study, Short et al. investigated neighbourhood material and social deprivation associations with health-related quality of life [51]. They found that individuals living in a highly socially deprived area were more likely to report difficulties in doing usual activities, taking care of themselves, and mobility, after adjustment for age, sex, and number of comorbidities. Of particular relevance here, they found that higher social deprivation, but not material deprivation, was associated with higher levels of anxiety and depression. Stress could lead to the chronic activation of neuroendocrine pathways [52], including the hypothalamic–pituitary–adrenal and sympathetic–adrenal–medullary axes, with consequences on cellular immune response, inflammatory processes, and thus on tumour pathogenesis [53]. In turn, stress can directly influence individual lifestyle behaviours, including alcohol consumption and diet [54, 55].
In the province of Quebec, where the present study was conducted, there is universal and free access to health care, including prostate cancer detection testing. Associations with social deprivation persisted in the subset of subjects recently tested with PSA and/or DRE suggesting that the deleterious effect of neighbourhood social deprivation might be independent from screening behaviour.
Strengths and limitations
Our study presents some limitations. First, census boundaries were used to represent neighbourhoods, and they may not accurately represent the boundaries of relevance here. We used the smallest geographic units for which census data were available (DA), which were found to be relatively homogeneous in terms of socioeconomic characteristics of residents [56]. Nevertheless, the use of the smallest and most homogeneous census area for a better approximation of neighbourhoods, when the information about the actual neighbourhood boundaries is unavailable, has been recommended elsewhere [57], and its utilisation is likely to lead to an underestimation of associations.
Recall errors in residential addresses in 1996 may have led to a misclassification of neighbourhood social deprivation, although most likely in a non-differential manner, thereby attenuating risk estimates. We did not consider places other than residential neighbourhoods, such as workplace neighbourhoods. However, Canadian adults living in urban areas similarly to ours reportedly spend over 65% of their time at home [58].
For analyses of social deprivation index in 1996 we excluded 1263 subjects having no addresses available for that year or having an address which could not be geocoded. In order to verify the presence of a potential selection bias, we evaluated whether the characteristics of this group differed from those with an available and geocodable address (Supplementary Table 10). Men with geocoded address tended to have a higher socioeconomic status and healthier lifestyles. These observations limit the generalisation of our findings for 1996. However, the comparability of the results obtained for both exposure time points investigated (1996 and 2006) reassures against the presence of a strong selection bias.
Finally, residual confounding by unmeasured factors related to neighbourhood social deprivation and prostate cancer risk, either at the contextual or individual level, remains possible, although few risk factors have been confirmed for this cancer. In order to investigate the robustness of our findings to residual confounding, E-values were calculated [32]. A single strong confounder or multiple unknown confounders would be necessary to explain our findings, which seems unlikely, although this cannot be completely excluded.
The main strengths of this study relate to its population-based design, large sample size and information on prostate cancer aggressiveness. Participation rates were comparable to studies of similar design, and comparisons of participants and non-participants yielded similar proportions in terms of contextual variables (education level, family income, proportion of recent immigrants, and proportion of unemployment), alleviating concerns about potential selection bias.
Lack of consideration of screening patterns can bias associations in studies of prostate cancer [59]. A notable strength of our work is that it was based on a largely screened population and information was available on prostate cancer screening practices. In the years that the study was conducted, screening for prostate cancer was commonly part of the men’s annual medical examinations, resulting in high screening rates; this allowed us to largely rule out the role of screening in the observed associations with neighbourhood social deprivation. We were also able to investigate exposures for neighbourhood deprivation 10 years before the recruitment. Past exposures are important to consider, especially for cancer with a long latency period, and to provide insights about possible long-term effects of neighbourhood deprivation.
Conclusion
Our study suggests that neighbourhood social deprivation is associated with an elevated risk of prostate cancer. Future studies should apply life course analyses to investigate potential cumulative effects of social deprivation and evaluate if there are critical periods, during childhood, young adulthood or late adulthood. Mediation analyses helping to better understand the underlying mechanisms involved are indicated. Our findings, largely novel, highlight the importance of considering neighbourhood characteristics for the implementation of public health policies and interventions.
Supplementary information
Acknowledgements
The authors would like to thank all members of the Epidemiology and Biostatistics Unit at INRS-Centre Armand-Frappier Santé Biotechnologie who were involved in the conduct of the study. In particular, we want to highlight the contribution of Hughes Richard in the preparation of the databases. We also thank the urologists from the participating hospitals for their collaboration in patients’ access.
Author contributions
CS conducted the analysis, interpreted the results and prepared the manuscript. M-EP supervised the work, designed and conducted the PROtEuS study and reviewed the manuscript. All authors participated in the interpretation of data and have read and approved the final version of the manuscript.
Funding
This work was supported financially by the Canadian Institutes of Health Research (grant CCP-155423) and the Canadian Cancer Society (grant 705562). CS held doctoral training awards from the Fonds de recherche du Québec – Santé.
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author, in keeping with ethics regulations. The data are not publicly available for confidentiality reasons.
Code availability
Code is available from the authors upon request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All individual studies were approved by local ethics committees and adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02299-7.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Société canadienne du cancer. Comité consultatif des statistiques canadiennes sur le cancer. Statistiques canadiennes sur le cancer 2021. Toronto (Ontario): Société canadienne du cancer; 2021.
- 3.Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D. Cancer epidemiology and prevention, fourth edition. Oxford: Oxford University Press; 2017.
- 4.Conti DV, Darst BF, Moss LC, Saunders EJ, Sheng X, Chou A, et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet. 2021;53:65–75. doi: 10.1038/s41588-020-00748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klassen AC, Platz EA. What can geography tell us about prostate cancer? Am J Prev Med. 2006;30:S7–15. doi: 10.1016/j.amepre.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend P. Deprivation. J Soc Policy. 1987;16:125–46. doi: 10.1017/S0047279400020341. [DOI] [Google Scholar]
- 9.Madathil S, Blaser C, Nicolau B, Richard H, Parent ME. Disadvantageous socioeconomic position at specific life periods may contribute to prostate cancer risk and aggressiveness. Front Oncol. 2018;8:515. doi: 10.3389/fonc.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolau B, Arekunnath Madathil S, Castonguay G, Rousseau MC, Parent ME, Siemiatycki J. Shared social mechanisms underlying the risk of nine cancers: a life course study. Int J Cancer. 2018;144:59–67. [DOI] [PubMed]
- 11.Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marså K, Johnsen NF, Bidstrup PE, Johannesen-Henry CT, Friis S. Social inequality and incidence of and survival from male genital cancer in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44:2018–29. doi: 10.1016/j.ejca.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Nilsen TIL, Johnsen R, Vatten LJ. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br J Cancer. 2000;82:1358–63. doi: 10.1054/bjoc.1999.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenland K, Rodriguez C, Mondul A, Calle EE, Thun M. Prostate cancer incidence and survival in relation to education (United States) Cancer Causes Control. 2004;15:939–45. doi: 10.1007/s10552-004-2231-5. [DOI] [PubMed] [Google Scholar]
- 15.Mihor A, Tomsic S, Zagar T, Lokar K, Zadnik V. Socioeconomic inequalities in cancer incidence in Europe: a comprehensive review of population-based epidemiological studies. Radio Oncol. 2020;54:1–13. doi: 10.2478/raon-2020-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeRouen MC, Schupp CW, Yang J, Koo J, Hertz A, Shariff-Marco S, et al. Impact of individual and neighborhood factors on socioeconomic disparities in localized and advanced prostate cancer risk. Cancer Causes Control. 2018;29:951–66. doi: 10.1007/s10552-018-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eylert MF, Bahl A, Hounsome L, Verne J, Jefferies ER, Persad RA. The impact of socio-economic deprivation on incidence, treatment and mortality from prostate cancer in England, 1990–2010. J Clin Urol. 2015;9:93–101. doi: 10.1177/2051415815594976. [DOI] [Google Scholar]
- 18.Rundle A, Neckerman KM, Sheehan D, Jankowski M, Kryvenko ON, Tang D, et al. A prospective study of socioeconomic status, prostate cancer screening and incidence among men at high risk for prostate cancer. Cancer Causes Control. 2013;24:297–303. doi: 10.1007/s10552-012-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmon C, Song L, Muir K, Pashayan N, Dunning AM, Batra J, et al. Marital status and prostate cancer incidence: a pooled analysis of 12 case-control studies from the PRACTICAL consortium. Eur J Epidemiol. 2021;36:913–25. doi: 10.1007/s10654-021-00781-1. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020;8:49–54. doi: 10.1016/j.prnil.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Nguyen NH, Wang D, Lynch BM, Hodge AM, Bassett JK, et al. Social connectedness and mortality after prostate cancer diagnosis: a prospective cohort study. Int J Cancer. 2019;147:766–76. [DOI] [PubMed]
- 22.Umberson D, Montez JK. Social relationships and health: a flashpoint for health policy. J Health Soc Behav. 2010;51:S54–66. doi: 10.1177/0022146510383501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tangen CM, Goodman PJ, Till C, Schenk JM, Lucia MS, Thompson IM., Jr Biases in recommendations for and acceptance of prostate biopsy significantly affect assessment of prostate cancer risk factors: results from two large randomized clinical trials. J Clin Oncol. 2016;34:4338–44. doi: 10.1200/JCO.2016.68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parent ME, Goldberg MS, Crouse DL, Ross NA, Chen H, Valois MF, et al. Traffic-related air pollution and prostate cancer risk: a case-control study in Montreal, Canada. Occup Environ Med. 2013;70:511–8. doi: 10.1136/oemed-2012-101211. [DOI] [PubMed] [Google Scholar]
- 25.Blanc-Lapierre A, Spence A, Karakiewicz PI, Aprikian A, Saad F, Parent M. Metabolic syndrome and prostate cancer risk in a population-based case-control study in Montreal, Canada. BMC public health. 2015;15:913. doi: 10.1186/s12889-015-2260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Environmental Systems Research Institute Inc. (ESRI). ArcGIS Desktop 9.3.1. Redlands: ESRI Inc.; 2009.
- 27.Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29:178–91. doi: 10.24095/hpcdp.29.4.05. [DOI] [PubMed] [Google Scholar]
- 28.Pampalon R, Hamel D, Gamache P, Simpson A, Philibert M. Validation of a deprivation index for public health: a complex exercise illustrated by the Quebec index. Chronic Dis Inj Can. 2014;34:12–22. doi: 10.24095/hpcdp.34.1.03. [DOI] [PubMed] [Google Scholar]
- 29.Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men: an important clinical entity. Nat Rev Urol. 2014;11:317–23. doi: 10.1038/nrurol.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright JL, Salinas CA, Lin DW, Kolb S, Koopmeiners J, Feng Z, et al. Prostate cancer specific mortality and Gleason 7 disease differences in prostate cancer outcomes between cases with Gleason 4 + 3 and Gleason 3 + 4 tumors in a population based cohort. J Urol. 2009;182:2702–7. doi: 10.1016/j.juro.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 33.Hurwitz LM, Agalliu I, Albanes D, Barry KH, Berndt SI, Cai Q, et al. Recommended definitions of aggressive prostate cancer for etiologic epidemiologic research. J Natl Cancer Inst. 2021;113:727–34. doi: 10.1093/jnci/djaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafique K, Oliphant R, Morrison DS. The impact of socio-economic circumstances on overall and grade-specific prostate cancer incidence: a population-based study. Br J Cancer. 2012;107:575–82. doi: 10.1038/bjc.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klassen AC, Curriero FC, Hong JH, Williams C, Kulldorff M, Meissner HI, et al. The role of area-level influences on prostate cancer grade and stage at diagnosis. Prev Med. 2004;39:441–8. doi: 10.1016/j.ypmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 36.Seo HS, Lee NK. Predictors of PSA screening among men over 40 years of age who had ever heard about PSA. Korean J Urol. 2010;51:391–7. doi: 10.4111/kju.2010.51.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fone DL, Farewell DM, White J, Lyons RA, Dunstan FD. Socioeconomic patterning of excess alcohol consumption and binge drinking: a cross-sectional study of multilevel associations with neighbourhood deprivation. BMJ Open. 2013;3:e002337. doi: 10.1136/bmjopen-2012-002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakshman R, McConville A, How S, Flowers J, Wareham N, Cosford P. Association between area-level socioeconomic deprivation and a cluster of behavioural risk factors: cross-sectional, population-based study. J Public Health. 2011;33:234–45. doi: 10.1093/pubmed/fdq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao Q, Keadle SK, Berrigan D, Matthews CE. A prospective investigation of neighborhood socioeconomic deprivation and physical activity and sedentary behavior in older adults. Prev Med. 2018;111:14–20. doi: 10.1016/j.ypmed.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curtis S, Setia MS, Quesnel-Vallee A. Socio-geographic mobility and health status: a longitudinal analysis using the National Population Health Survey of Canada. Soc Sci Med. 2009;69:1845–53. doi: 10.1016/j.socscimed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamano T, Li X, Sundquist J, Sundquist K. Neighborhood social capital and incidence and mortality of prostate cancer: a Swedish cohort study. Aging Clin Exp Res. 2021;33:3333–42. doi: 10.1007/s40520-021-01852-9. [DOI] [PubMed] [Google Scholar]
- 42.Lynch SM, Mitra N, Ross M, Newcomb C, Dailey K, Jackson T, et al. A Neighborhood-Wide Association Study (NWAS): example of prostate cancer aggressiveness. PLoS ONE. 2017;12:e0174548. doi: 10.1371/journal.pone.0174548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macintyre S, Ellaway A. Neighborhoods and health: an overview. Neighborhoods Health. 2003;20:42. [Google Scholar]
- 44.VanderWeele DJ, Brown CD, Taxy JB, Gillard M, Hatcher DM, Tom WR, et al. Low-grade prostate cancer diverges early from high grade and metastatic disease. Cancer Sci. 2014;105:1079–85. doi: 10.1111/cas.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penney KL, Stampfer MJ, Jahn JL, Sinnott JA, Flavin R, Rider JR, et al. Gleason grade progression is uncommon. Cancer Res. 2013;73:5163–8. doi: 10.1158/0008-5472.CAN-13-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Cancer Research Fund International, American Institute to Cancer Research. Diet, nutrition, physical activity and prostate cancer. 2014. https://dietandcancerreport.org.
- 47.Gilham K, Gu Q, Dummer TJB, Spinelli JJ, Murphy RA. Diet quality and neighborhood environment in the Atlantic Partnership for Tomorrow’s Health Project. Nutrients. 2020;12:3217. doi: 10.3390/nu12103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngamini Ngui A, Apparicio P, Philibert M, Fleury M-J. Neighborhood characteristics associated with the availability of alcohol outlets in quebec, Canada. J Addict. 2015;2015:876582-.. doi: 10.1155/2015/876582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crouse DL, Ross NA, Goldberg MS. Double burden of deprivation and high concentrations of ambient air pollution at the neighbourhood scale in Montreal, Canada. Soc Sci Med. 2009;69:971–81. doi: 10.1016/j.socscimed.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Pinault L, Crouse D, Jerrett M, Brauer M, Tjepkema M. Spatial associations between socioeconomic groups and NO2 air pollution exposure within three large Canadian cities. Environ Res. 2016;147:373–82. doi: 10.1016/j.envres.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 51.Short H, Al Sayah F, Ohinmaa A, Lahtinen M, Johnson JA. The relationship of neighbourhood-level material and social deprivation with health-related quality of life. Qual Life Res. 2018;27:3265–74.. doi: 10.1007/s11136-018-1962-9. [DOI] [PubMed] [Google Scholar]
- 52.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 53.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 54.Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS. Stress and alcohol: epidemiologic evidence. Alcohol Res Curr Rev. 2012;34:391–400. [PMC free article] [PubMed] [Google Scholar]
- 55.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, et al. Food selection changes under stress. Physiol Behav. 2006;87:789–93. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Gamache P, Hamel D, Blaser C. Material and social deprivation index: a summary. 2019. https://www.inspq.qc.ca/publications/2639.
- 57.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–78. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 58.Leech JA, Nelson WC, Burnett RT, Aaron S, Raizenne ME. It’s about time: a comparison of Canadian and American time–activity patterns. J Expo Sci Environ Epidemiol. 2002;12:427–32. doi: 10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Closas M, Berrington, de Gonzalez A. Invited commentary: Screening and the elusive etiology of prostate cancer. Am J Epidemiol. 2015;182:390–3. doi: 10.1093/aje/kwv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author, in keeping with ethics regulations. The data are not publicly available for confidentiality reasons.
Code is available from the authors upon request.