Abstract
Objective
Alteplase is a recombinant tissue plasminogen activator used for thrombolytic treatment in several indications and is currently approved in Europe under the brand name Actilyse®. The current manufacturing process for alteplase was recently modified to meet increasing global demands. The aim of this randomized, open-label, adaptive two-stage design, two-way crossover study was to establish bioequivalence of alteplase derived from the two manufacturing processes (modified versus current).
Methods
The two alteplase formulations (modified and current, 0.2 mg/kg body weight) were compared in healthy male volunteers after intravenous infusion over a period of 30 min. The trial was put on hold after treatment of 12 subjects (Part A) and restarted as Part B (n = 18) with design adaptations, including a heparin bolus.
Results
Pharmacokinetic parameters of alteplase were determined from plasma concentration–time profiles. The pharmacokinetic parameters tested (AUC0–tz, Cmax, and AUC0–∞) for alteplase after single intravenous infusion demonstrated no differences between alteplase obtained from the modified and current processes. An analysis of variance (ANOVA) model was applied to test for bioequivalence. The geometric means ratio and the respective 92.83% confidence intervals (CIs) for all primary and secondary pharmacokinetic endpoints were well within the prespecified equivalence boundaries of 80–125%. The CIs also included unity, suggesting no statistically significant differences between the two treatments.
Conclusions
The results show that alteplase exposure was virtually identical for the formulations tested, and statistical evaluation demonstrated bioequivalence of the formulations. Both formulations of alteplase were well tolerated by the subjects at the single intravenous doses in the trial.
Trial Registration
Trial registration number: NCT04419493, 2019-004932-40 (EudraCT Number).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-023-01253-3.
Key Points
| This study reports results from a recently completed phase I clinical trial (NCT04419493), which investigated the pharmacokinetics of two different formulations of alteplase (tissue plasminogen activator) using an adaptive trial design. |
| The study found that the two formulations, derived from two different manufacturing processes, were bioequivalent in healthy male volunteers. |
Introduction
Alteplase, a tissue plasminogen activator (t-PA) produced by recombinant DNA technology, binds to fibrin clots, and activates plasminogen, leading to generation of plasmin and degradation of clots [1, 2]. Alteplase is the active pharmaceutical ingredient of medicinal products approved in Europe for more than 30 years under the brand names Actilyse® and Actilyse Cathflo®. Actilyse® is indicated for thrombolytic treatment of acute myocardial infarction, acute massive pulmonary embolism and acute ischemic stroke; Actilyse Cathflo® is indicated for thrombolytic treatment of occluded central venous access devices [3, 4]. The mode of action and pharmacokinetic (PK) profile of alteplase after intravenous (IV) administration are well documented [3, 5–7].
The current manufacturing process for alteplase was recently modified to support increasing global demand for Actilyse®. Analytical comparability between the products resulting from the two manufacturing processes was demonstrated for quality attributes potentially affecting safety, efficacy, and immunogenicity. However, minor differences in the glycosylation pattern of the alteplase protein, which have the potential to influence plasma clearance, were identified between the two processes. Animal studies have demonstrated bioequivalence between alteplase obtained from both modified and current processes (Boehringer Ingelheim, Data on File); however, no clinical data have yet been generated to confirm this.
This trial aimed to address residual uncertainty with respect to PK properties by establishing the bioequivalence of alteplase manufactured via the modified and current processes (hereafter referred to as “alteplase mp” and “alteplase cp”, respectively) following a 30-min IV administration of 0.2 mg/kg of body weight in healthy male subjects.
Methods
Trial Population
This trial was performed in healthy males, aged 18–45 years, with a body mass index of 18.5–29.9 kg/m2. All participants provided signed and dated written informed consent prior to admission to the study, in accordance with Good Clinical Practice (GCP) and local legislation.
Trial Design
This was a phase I, randomized, open-label, adaptive two-stage design, two-way crossover trial.
Part A
The two-part trial design was not prespecified in the trial protocol, but was introduced following unexpected findings in the first cohort of volunteers, as described below.
In the first part of the trial, alteplase mp and alteplase cp (0.2 mg/kg body weight, IV infusion over 30 min) were administered to 12 healthy subjects (Supplementary Fig. 1). However, due to potential signs of coagulation activation, the study was paused (for further details, see Online Resource 1, Supplementary Section S2). Upon evaluation of the PK data from this cohort, the mean concentration–time profiles showed large fluctuation in plasma concentration and high interindividual variability. This prevented comparison between the two active ingredients. Retrospectively, this phase of the study was termed Part A, and several modifications to the trial design were implemented before reinitiation of the study (Part B). These modifications included administration of the anticoagulant heparin prior to alteplase infusion, flushing of venous catheters with heparin saline solution after blood withdrawal (and discarding a small amount of blood prior to the next withdrawal), and measurement of a panel of pharmacodynamic parameters assessing fibrinolysis, coagulation, and platelet activation (see Online Resource 1, Supplementary Section S1 for details).
Due to the high PK variability from Part A, the data generated could not be used to evaluate bioequivalence. Therefore, only data from Part B are presented in this manuscript.
Please see Online Resource 1, Supplementary Section S2 for additional information on Part A.
Part B
Part B of the trial followed the same adaptive, two-stage design. Unfractionated heparin was administered as IV bolus (5000 IU) to all subjects 5 min prior to the start of each alteplase infusion. The test treatment (T), 0.2 mg alteplase mp/kg body weight (IV infusion over 30 min), was compared with the reference treatment (R): 0.2 mg alteplase cp/kg body weight (IV infusion over 30 min).
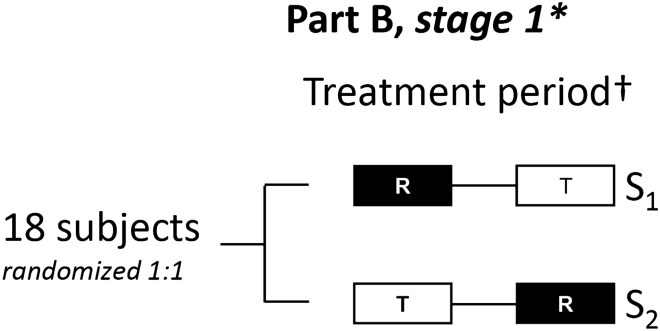
In stage 1, 18 subjects were randomly allocated in a 1:1 ratio to the two treatment sequences (T–R or R–T) with a washout period of at least 24 h between treatments (Fig. 1). In each of the two treatment periods, healthy volunteers received a single dose of medication.
Fig. 1.
Study design: Part B. Part B of the trial followed the same treatment periods and design as Part A (see Online Resource 1, Supplementary Fig. S1 for the Part A study design). *In case of proven bioequivalence at stage 1, the trial will be closed. Bioequivalence will be concluded when the 92.83% confidence intervals for the comparison of T versus R of all primary and secondary endpoints are within the prespecified boundaries of 80–125%. †The second treatment was administered 24 h after start of infusion of the first treatment. R reference treatment, S treatment sequence, T test treatment
For each subject, the schedule of trial participation included a screening examination (up to 21 days prior to first treatment), treatment period 1 (including follow-up; 1 day), treatment period 2 (including follow-up; 7 days), and a follow-up examination (up to 15 days). The expected total trial duration for a single subject was therefore 4–6 weeks (Fig. 1).
The study design was an adaptive, two-stage, sequential design scheme according to Lehmacher and Wassmer and Maurer et al. [8, 9]. A formal interim analysis to assess bioequivalence was prespecified to occur after stage 1 (Sect. 2.5). The alpha level (α1) for stage 1 was fixed to 0.03585 [resulting in a 92.83% confidence interval (CI) for stage 1]. The final analysis was to be performed according to the standard inverse normal approach [8, 9] with prespecified information fraction w = 0.8 n1*/n1, where n1 = 18, and n1* = the actual number of subjects contributing at least one evaluable PK parameter for at least one study period [8, 9]. A user-defined alpha spending function was to be used such that the full level of 0.05 was spent at the final analysis (after stage 2), depending on the value of w. It was expected that and therefore the planning values were w = 0.8 and α1 = α2 = 0.03585; these alpha values correspond to Pocock boundaries. Following the method by Maurer et al. [9], type 1 error control is guaranteed after stage 1 and stage 2. In the event that the 92.83% CIs for the comparison of T versus R for all primary and secondary endpoints were within the prespecified acceptance range of 80–125% after assessment of unblinded data from stage 1, the trial was to be closed and bioequivalence concluded. If bioequivalence was not observed for all endpoints, then a decision regarding continuation of the trial was to be made, as per Maurer et al. with the exception that no futility criterion with respect to the CI of the geometric means (gMean) ratio was implemented [9]. A sample size re-estimation procedure, as outlined in Maurer et al., would have been performed using observed values from unblinded data to calculate the number of subjects required for the second stage of the trial [9]. In doing so, a maximum overall sample size of 72 was specified.
There were limited data available to predict intraindividual variability of the primary endpoints. A crossover trial in rabbits (unpublished data) with alteplase mp and alteplase cp reported an intraindividual variability of approximately 15%; in the same study, a gMean ratio of ~ 100% was observed for maximum measured concentration of the analyte in plasma (Cmax) and of ~ 109% for area under the concentration–time curve of the analyte in plasma (AUC). On the basis of this animal study, it was assumed that the gMean ratios for these parameters in humans would range between 100% and 109%, respectively.
A weight of 0.8 for the inverse normal approach, a sample size n2 for stage 2 between 4 and 42, and usage of the observed geometric coefficient of variation (gCV) and gMean ratio were implemented. Using a sample size of n1 = 18 subjects for the first stage, the probability to conclude bioequivalence after the first stage was 79%, and the overall power to conclude bioequivalence was at least 95%, assuming an intraindividual gCV of 15% and a treatment difference of 9% (ratio scale). To investigate the sensitivity of the sample size, a range of gCVs around 15% and a range of gMean ratios around 109% were investigated. If, for example, the variability was 20% instead of 15%, the overall power was still ~ 89%.
Concomitant Therapy and Restrictions
In Part B, no concomitant therapy was allowed unless there were adverse events (AEs) requiring medical treatment. Intake of analgesics (e.g., ibuprofen and diclofenac) was forbidden from 1 week prior to the first administration of trial medication until discharge from the trial.
In addition to the investigational products (alteplase mp and alteplase cp), 0.2 mL (= 5000 IU) heparin saline solution was administered to each subject as a bolus injection, 5 min prior to the start of alteplase infusion.
Endpoints
The primary endpoints were AUC over the time interval from 0 until the last quantifiable data point (AUC0–tz) and the Cmax. The secondary endpoint was AUC over the time interval from 0 extrapolated to infinity.
The study endpoints were derived from measurement of plasma concentrations of the analyte, which were provided by a bioanalytical laboratory that was blinded to treatment allocation. When calculating PK parameters, endogenous levels of tissue-plasminogen activator were determined prior to administration in each treatment period and subtracted from the individual concentration measurements.
Safety and tolerability of alteplase mp and alteplase cp were assessed on the basis of AEs (including clinically relevant findings from physical examinations), safety laboratory tests, 12-lead electrocardiogram, and vital signs.
Statistical Plan
The Part B study had an adaptive, two-stage, sequential design, with a formal interim analysis to assess bioequivalence prespecified to occur after stage 1. The assessment of bioequivalence was based upon two-sided repeated CIs for the ratios of gMean (T versus R) for the primary and secondary endpoints using an acceptance range of 80–125%.
The statistical model used for the analysis of the primary and secondary endpoints for each stage of the trial was an analysis of variance (ANOVA) model on the logarithmic scale adjusted for the following fixed effects: sequence, subjects nested within sequences, period, and treatment. At stage 1, the point estimates for the ratio of gMeans (T versus R) including their (1–2α1) × 100% CI was calculated. If the study were to continue to stage 2, the repeated CI corresponding to the test decision was to be calculated.
Descriptive statistics were calculated for all endpoints. Safety analyses were descriptive and included all treated subjects.
Results
As PK data from Part A could not be used for bioequivalence testing, only data from Part B are presented in this manuscript. Please see Online Resource 1, Supplementary Section S2 for additional information on Part A.
Subject Disposition
Eighteen subjects were enrolled and treated in Part B of the trial. Seventeen subjects (94.4%) were treated with alteplase mp, and 18 (100.0%) were treated with alteplase cp. One subject reported a drug-related AE (hypotensive episode) after receiving alteplase mp leading to discontinuation from the trial. Another subject, receiving alteplase cp, was excluded from the final analysis because a paravenous infusion of study drug was suspected.
Subject demographics were generally similar in Parts A and B (Table 1).
Table 1.
Subject demographics in trial Part A and Part B
| Trial Part A | Trial Part B | |
|---|---|---|
| Number of subjects, N (%) | 12 (100.0) | 18 (100.0) |
| Sex, n (%) | ||
| Male | 12 (100.0) | 18 (100.0) |
| Race, n (%) | ||
| White | 12 (100.0) | 17 (94.4) |
| Black or African American | 0 | 1 (5.6) |
| Mean age, years (SD) | 33.3 (5.8) | 33.7 (6.0) |
| Mean BMI, kg/m2 (SD) | 25.88 (2.55) | 25.67 (2.66) |
| Smoking history, n (%) | ||
| Never smoked | 5 (41.7) | 10 (55.6) |
| Former smoker | 6 (50.0) | 4 (22.2) |
| Currently smokes | 1 (8.3) | 4 (22.2) |
BMI body mass index, SD standard deviation
Plasma Concentration–Time Profiles
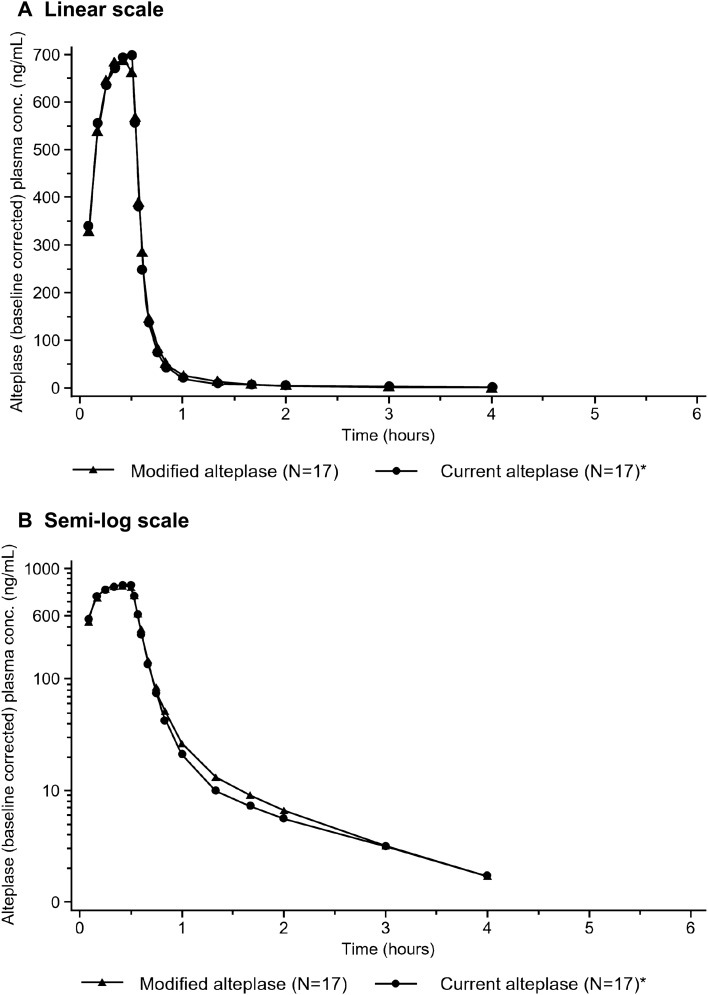
In Part B of the study, IV administration of 0.2 mg/kg of alteplase mp or alteplase cp resulted in increased plasma concentrations of alteplase, with peak concentrations observed around the end of infusion. When the infusion was stopped after 30 min, plasma concentrations declined rapidly and were below the lower limit of quantification in approximately one-third of the subjects at the 4-h timepoint, i.e., 3.5 h after the end of the infusion. A direct comparison of the gMean plasma concentration–time profiles of alteplase mp and alteplase cp is provided in Fig. 2. These profiles are virtually superimposable.
Fig. 2.
Comparison of gMean plasma concentration–time profiles of alteplase depicted on linear (A) and semi-log (B) scale. *N = 17 for subjects receiving alteplase cp as one subject was excluded from all calculations because a paravenous infusion of study drug was suspected. alteplase cp alteplase manufactured using current processes, conc concentration, gMean geometric mean
PK Endpoints
The adjusted gMean AUC0–tz (h⋅ng/mL) was 377 and 369 for alteplase mp and alteplase cp, respectively. The adjusted gMean Cmax was 781 and 738 ng/mL for alteplase mp and alteplase cp, respectively. Overall, the PK parameter values obtained for alteplase mp and alteplase cp were comparable.
Evaluation of Bioequivalence
An ANOVA model was applied to the data to test for bioequivalence, as prespecified. The model considered only Part B subjects with valid PK parameters in both treatment periods as required by a European Medicines Agency guideline on the investigation of bioequivalence from 2010 [10]. The gMean ratio and the 92.83% CIs for alteplase mp/alteplase cp are presented in Table 2.
Table 2.
Statistical analysis of primary and secondary PK endpoints in Part B
| Endpoint | Na | Comparison versus alteplase cp, solution | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted | Ratio (%) | gSE | 92.83% CI | p value | gCV (%) | ||||
| gMean | gSE | Lower | Upper | ||||||
| Primary endpoints | |||||||||
| AUC0–tz (h⋅ng/mL) | 10.8 | ||||||||
| Alteplase cp | 16 | 369.21 | 1.03 | ||||||
| Alteplase mp | 16 | 377.35 | 1.03 | 102.20 | 1.04 | 94.87 | 110.10 | < 0.0001 | |
| Cmax (ng/mL) | 9.3 | ||||||||
| Alteplase cp | 16 | 738.35 | 1.02 | ||||||
| Alteplase mp | 16 | 781.24 | 1.02 | 105.81 | 1.03 | 99.18 | 112.88 | < 0.0001 | |
| Secondary endpoint | |||||||||
| AUC0–∞ (h⋅ng/mL) | 10.8 | ||||||||
| Alteplase cp | 16 | 371.85 | 1.03 | ||||||
| Alteplase mp | 16 | 379.90 | 1.03 | 102.17 | 1.04 | 94.79 | 110.12 | < 0.0001 | |
| Further PK endpointsb | |||||||||
| t1/2 initial (h) | |||||||||
| Alteplase cp | 17 | 0.0694 | – | – | – | – | – | 12.4 | |
| Alteplase mp | 17 | 0.0730 | – | – | – | – | – | 14.9 | |
| t1/2 terminal (h) | |||||||||
| Alteplase cp | 17 | 0.963 | – | – | – | – | – | 36.1 | |
| Alteplase mp | 17 | 0.907 | – | – | – | – | – | 29.4 | |
| tmax (h) | |||||||||
| Alteplase cp | 17 | 0.410 | – | – | – | – | – | 22.2 | |
| Alteplase mp | 17 | 0.386 | – | – | – | – | – | 24.7 | |
alteplase cp alteplase manufactured using current processes, alteplase mp alteplase manufactured using modified processes, AUC0–∞ area under the concentration–time curve of the analyte in plasma over the time interval from 0 extrapolated to infinity, AUC0–tz area under the concentration–time curve of the analyte in plasma over the time interval from 0 up to the last quantifiable data point, Cmax maximum measured concentration of the analyte in plasma, gCV geometric coefficient of variation, gMean geometric mean, gSE geometric standard error, PK pharmacokinetic, t1/2 half-life of the analyte in plasma, tmax time from dosing to maximum measured concentration of the analyte in plasma
aAn ANOVA model was applied to the data to test for bioequivalence that only considered subjects with valid PK parameters in both treatment periods
bDescriptive statistics of noncompartmental PK parameters
The intrasubject variability was low, with gCV values of 10.8% and 9.3% for AUC0–tz and Cmax, respectively. The gMean ratio with respective 92.83% CI was 102.20% (94.87–110.10%) for AUC0–tz and 105.81% (99.18–112.88%) for Cmax. The CIs were well within the prespecified equivalence boundaries of 80–125% and included unity, suggesting no statistically significant differences between the two treatment arms, and thereby demonstrating bioequivalence between alteplase mp and alteplase cp.
A sensitivity analysis with a correction of the PK parameters for protein content was performed. The values obtained for alteplase mp and alteplase cp were comparable and demonstrated bioequivalence (Online Resource 1, Supplementary Table S1).
Adverse Events
Overall, 11 out of the 18 treated subjects (61.1%) were reported to have at least one treatment-emergent AE (TEAE) in any treatment period: 6/17 subjects (35.3%) receiving alteplase mp and 7/18 subjects (38.9%) receiving alteplase cp. The number of subjects with investigator-defined drug-related TEAEs in any treatment period was 7 (38.9%) in total: 5/17 subjects (29.4%) receiving alteplase mp and 3/18 (16.7%) receiving alteplase cp. The most frequent drug-related AE in Part B (including follow-up) was hematoma, reported by eight subjects (six during follow-up). There were no TEAEs of special interest, deaths, serious AEs or “other significant AEs” (according to ICH E3). An overview of subjects with TEAEs is given in Table 3.
Table 3.
Overall summary of subjects with TEAEs in Parts A and B
| Alteplase | Total | ||
|---|---|---|---|
| Alteplase cp (R) | Alteplase mp (T) | ||
| n (%) | n (%) | ||
| Part Aa | |||
| Number of subjects | 10 (100.0) | 11 (100.0) | 12 (100.0) |
| Subjects with any AE | 1 (10.0) | 4 (36.4) | 5 (41.7) |
| Subjects with severe AEs | 0 | 0 | 0 |
| Subjects with drug-related AEsb | 1 (10.0) | 2 (18.2) | 3 (25.0) |
| Part Ba | |||
| Number of subjects | 18 (100.0) | 17 (100.0) | 18 (100.0) |
| Subjects with any AE | 7 (38.9) | 6 (35.3) | 11 (61.1) |
| Subjects with severe AEs | 0 | 0 | 0 |
| Subjects with drug-related AEsb | 3 (16.7) | 5 (29.4) | 7 (38.9) |
AE adverse event, alteplase cp alteplase manufactured using current processes, alteplase mp alteplase manufactured using modified processes, ICH International Council for Harmonization, R reference treatment, T test treatment, TEAE treatment-emergent AE
aThere were no subjects with AEs leading to discontinuation of trial drug, AEs of special interest, serious AEs, or “other significant AEs” according to ICH E3 definition
bThe investigator assessed the possible causal relationship between the study medication and an AE
Discussion
This bioequivalence study was conducted to compare alteplase produced via a modified manufacturing process versus the current process (alteplase mp versus alteplase cp, 0.2 mg/kg body weight) in male healthy volunteers after IV infusion over a period of 30 min. Alteplase exposure was almost identical for the formulations tested, and statistical evaluation demonstrated bioequivalence of the formulations. Both formulations of alteplase were well tolerated by the subjects at the single IV dose.
Alteplase (rt-PA), a thrombolytic agent produced by recombinant DNA technology, is the active pharmaceutical ingredient of Actilyse and Actilyse Cathflo, which are used for thrombolytic treatment in several indications and occluded central venous access devices. Due to increasing global demand, the manufacturing process for alteplase has been modified to allow scale-up of production. To allow a post-approval change of the drug substance manufacturing process, this trial aimed to establish bioequivalence of alteplase produced by current and modified processes. The study showed that the two formulations of alteplase were bioequivalent, and both formulations were well tolerated at the dose administered.
In the first part of the study (retrospectively termed Part A), 12 healthy male subjects were dosed; however, it was put on hold as systemic effects of coagulation could not be excluded on the basis of clinical observations (e.g., occluded cannulas) and increased d-dimer values. Several modifications to the trial design were then implemented to allow re-initiation (Part B; please see Supplementary Section S1 for details). Part B of the trial followed the same treatment periods and design as Part A.
In Part B, 5000 IU heparin was given prior to alteplase infusion to mitigate the risk of potential thromboembolic complications, and peripheral venous catheters were flushed with heparin saline solution to reduce clotting within the cannulas. Adding heparin changed the character of drug-related AEs significantly. While occluded cannulas represented the only drug-related AE in Part A, bleeding (eight reported cases of hematoma) was the most frequent drug-related AE in Part B. A panel of pharmacodynamic parameters, including plasminogen and d-dimer, was measured to assess potential effects on the coagulation system. The coagulation parameters measured did not differ between the two formulations of alteplase when administered to the same subjects.
The adaptive trial design is an essential feature of this study, accounting for initial uncertainties with respect to variability and treatment difference, thereby allowing for an efficient study conduct which minimized the number of healthy subjects being exposed to the alteplase mp and alteplase cp formulations. As bioequivalence was proven in Part B stage 1, the trial was closed, and stage 2 was not required. This trial design achieved its study aims in a short period of time, and its methodology should be considered for future bioequivalence trials.
Conclusion
Alteplase exposure was almost identical for the two formulations of alteplase tested, and statistical evaluation demonstrated their bioequivalence. These results therefore address any residual uncertainty with respect to PK arising from the observed differences in the glycosylation pattern between the alteplase mp and alteplase cp formulations. Both formulations were well tolerated by subjects at the single IV dose.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Thomas Giessmann for his contributions to drafting the study protocol and report and reviewing this manuscript for scientific accuracy.
Declarations
Funding
The study was supported and funded by Boehringer Ingelheim. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. Medical writing support was provided by Shivani Singh of Meditech Media, a medical communications agency contracted and funded by Boehringer Ingelheim.
Conflict of interest
SG, BL, SC, MPN, CP, and MW are employees of Boehringer Ingelheim. JH is employed by Staburo, contracted by Boehringer Ingelheim.
Data availability statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on Vivli—Center for Global Clinical Research Data, and earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit Medical & Clinical Trials | Clinical Research | MyStudyWindow for further information.
Author contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). All authors approved the final version for submission. Study conceptualization and methodology: SG, CP, BL, and MW. Measurement and bioanalysis of pharmacokinetic concentration data: CP and SC. Calculation of pharmacokinetic parameters and data interpretation: SG. Bioanalysis of pharmacodynamic data: MW and MPN. Statistical analyses: JH and BL.
Consent for publication
Not applicable.
Consent to participate
All participants provided signed and dated written informed consent prior to admission to the study, in accordance with Good Clinical Practice (GCP) and local legislation.
Code availability
Not applicable.
Ethics approval
The trial was conducted and reported in accordance with the Declaration of Helsinki, the International Council for Harmonization Good Clinical Practice guidelines, and local regulations. Prior to the start of the trial, the subject information leaflet, the informed consent form, and other locally required documents were reviewed by the Independent Ethics Committee (IEC) of the participating center. The applicable IEC (Landesarztkammer Baden-Württemberg, Stuttgart, Germany) and medical regulatory authority (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM, Bonn, Germany) gave a favorable opinion and granted approval before the trial commenced at the investigational site.
References
- 1.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257(6):2912–2919. doi: 10.1016/S0021-9258(19)81051-7. [DOI] [PubMed] [Google Scholar]
- 2.Collen D. Molecular mechanism of action of newer thrombolytic agents. J Am Coll Cardiol. 1987;10(5 Suppl B):11B–B15. doi: 10.1016/S0735-1097(87)80422-9. [DOI] [PubMed] [Google Scholar]
- 3.Boehringer Ingelheim Limited. Actilyse® 10 mg powder and solvent for solution for injection and infusion summary of product characteristics. 2019 [cited 24 June 2022]. Available from: https://www.medicines.org.uk/emc/product/898/smpc.
- 4.Boehringer Ingelheim Limited. Actilyse Cathflo 2 mg powder for solution for injection and infusion. 2010 [cited 25 August 2022]. Available from: https://www.medicines.org.uk/emc/product/4617/smpc#gref.
- 5.Tanswell P, Seifried E, Stang E, Krause J. Pharmacokinetics and hepatic catabolism of tissue-type plasminogen activator. Arzneimittelforschung. 1991;41(12):1310–1319. [PubMed] [Google Scholar]
- 6.Tanswell P, Tebbe U, Neuhaus KL, Glasle-Schwarz L, Wojcik J, Seifried E. Pharmacokinetics and fibrin specificity of alteplase during accelerated infusions in acute myocardial infarction. J Am Coll Cardiol. 1992;19(5):1071–1075. doi: 10.1016/0735-1097(92)90297-Z. [DOI] [PubMed] [Google Scholar]
- 7.Tanswell P. Tissue-type plasminogen activator. In: Kung AHCBR, Larrick JW, editors. Therapeutic proteins: pharmacokinetics and pharmacodynamics. New York: W.H. Freeman & Co Ltd; 1992. pp. 255–281. [Google Scholar]
- 8.Lehmacher W, Wassmer G. Adaptive sample size calculations in group sequential trials. Biometrics. 1999;55(4):1286–1290. doi: 10.1111/j.0006-341X.1999.01286.x. [DOI] [PubMed] [Google Scholar]
- 9.Maurer W, Jones B, Chen Y. Controlling the type I error rate in two-stage sequential adaptive designs when testing for average bioequivalence. Stat Med. 2018;37(10):1587–1607. doi: 10.1002/sim.7614. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Committee for Human Medicinal Products (CHMP): guideline on the investigation of bioequivalence. 2010. 20 Jan 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on Vivli—Center for Global Clinical Research Data, and earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit Medical & Clinical Trials | Clinical Research | MyStudyWindow for further information.