Abstract
Background:
Dietary and lifestyle changes are the first line of therapy for non-alcoholic fatty liver disease (NAFLD), the most prevalent liver disease in the western world. Nutrition literacy is the ability to understand nutrition information and implement that knowledge. We aimed to compare indicators of nutrition literacy in subjects with and without NAFLD in a representative US cohort.
Methods:
In a cross-sectional study using data from the NHANES 2017-2018 cycle, we included 2,938 adult subjects with complete dietary and vibration-controlled transient elastography data and no alternative reason for hepatic steatosis. Nutrition literacy was assessed using questionnaires. Diet perception accuracy was assessed by comparing self-reported diet quality to objective diet quality scores - the Healthy Eating Index (HEI) and alternative Mediterranean diet score (aMED) - to assess real world application of nutrition knowledge.
Results:
Nutrition literacy was not different between subjects with or without NAFLD (p=0.17): over 90% of subjects reported using nutrition labels and most correctly identified the meaning of daily value. Subjects with NAFLD had a lower quality diet (HEI, p=0.018; aMED p=0.013) and rated their diet as poorer (p<0.001). On self-assessment, only 27.8% of subjects overestimated their diet quality while 37.5% consumed more calories than their self-assessed needs. Both accuracy measures were similar between subjects with NAFLD and those without (p=0.71 & 0.63, respectively). Subjects with NAFLD were more likely to report being advised to lose weight (42.1% vs. 16.5%, p <0.001) or to attempt losing weight (71.9% vs. 60.9%, p<0.001). Diet quality was not better in subjects with NAFLD who received dietary recommendations.
Conclusion:
Subjects with NAFLD have poor diet quality despite receiving medical recommendations to lose weight and having nutrition literacy and perception that are comparable to subjects without NAFLD. Educational approaches may not be sufficient to promote weight loss and improve diet quality in NAFLD.
Keywords: Non-alcoholic Fatty Liver Disease, Nutrition, National Health and Nutrition Examination Survey
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), the excess accumulation of fat in the liver, is associated with obesity and insulin resistance.1 NAFLD is estimated to have a global prevalence of about 25%, making it the most prevalent liver disease in the western world.2 There is currently no approved medication for the treatment of NAFLD. Instead, diet and lifestyle changes are the first line therapy recommended in guideline statements from every major global society.3 Adherence to the Mediterranean diet and diets limiting fats, carbohydrates and overall calorie consumption are common recommendations for the treatment of NAFLD.4-6
Nutrition literacy is the ability to understand nutrition information and implement that knowledge to reach dietary goals.7 Lower nutrition literacy has been linked to poorer diet quality in patients with nutrition-related chronic disease.8 Recent work has demonstrated that in a representative US cohort, NAFLD is associated with lower education and poorer diet quality.9 However, whether nutrition literacy affects diet quality and risk for NAFLD is unknown. No previous study has looked at NAFLD and nutrition literacy in both its components of functional knowledge and real-world application in a representative US cohort.
In this study we aimed to evaluate the nutrition literacy of subjects with NAFLD on multiple levels of literacy: the functional nutrition knowledge (tools), the desire to implement nutritional change (intention), and the ability to apply knowledge to real-world action (translation). Our secondary aim was to better understand how subjects with and without NAFLD understand and rate their own diet.
METHODS
Data sources and study population
Data for this study are from the publicly available National Health and Nutrition Examination Survey (NHANES) from the years 2017-2018.10 NHANES is a continuous program run by the National Center for Health Statistics (NCHS) that aims to capture health and nutrition data representative of all non-incarcerated Americans. The NHANES survey includes questionnaire data about health and lifestyle, physical examination, imaging studies and laboratory tests performed in Mobile Examination Centers (MEC). Ethical review of the survey protocol and data sharing was conducted and approved by the NCHS Research Ethics Review Board (continuation protocol #2011-17 and protocol #2018-01).
The 2017-2018 NHANES cohort was comprised of 9,254 subjects. Subjects younger than 20 years old and those without full interview information were excluded (n= 3,989). Subjects without valid FibroScan data were excluded (n = 755), as well as those with other possible causes of steatosis: excessive alcohol intake defined as over 20g/day for women and over 30g/day for men (n = 415), evidence of hepatitis C or B by serologic assay (n = 70), or more than 6 months of potentially steatogenic medications such as amiodarone, valproate, methotrexate, tamoxifen, and systemic corticosteroids (n = 45). Finally, subjects without both days of dietary recall completed (n = 787) or those who did not answer follow up nutritional surveys (n = 255) were excluded to create a study cohort of 2,938 subjects (Supplementary Figure 1).
The poverty income ratio in NHANES is calculated by dividing the reported family monthly income by the department of Health and Human Services poverty threshold.11 We divided the poverty income ratio into approximate terciles with a lower cutoff of 1.3, which is the eligibility threshold for supplemental nutritional assistance program (SNAP) by the US Federal government.12 The upper cutoff was set at 3.5 to form approximately equal-sized categories, as previously described.9
Definition of NAFLD and clinically significant fibrosis
Vibration-controlled transient elastography was performed using the FibroScan device with the Controlled Attenuation Parameter (CAP) module (Echosens) by trained operators with multiple quality checks in place, as detailed in the NHANES data files.10 We defined NAFLD as CAP ≥ 263 dB/m.13 Sensitivity analysis was performed using a higher cutoff of 285 dB/m to define NAFLD.14 Clinically significant fibrosis (CSF) was defined as transient elastography ≥ 8.6 kPa, corresponding to stage 2 or greater fibrosis grading on liver biopsy.9
Diet Quality Indices
Dietary intake was obtained by averaging two 24-hour dietary recalls, performed as part of the NHANES survey. The 24-hour dietary recalls were performed using the USDA Automated Multiple Pass Method (AMPM) instrument.15,16 The AMPM has been biologically validated by comparing the reported energy intake to the total energy expenditure using the doubly labeled water technique,16 and by correlating reported 24-hour sodium intake and simultaneously collected 24-hour urine sodium samples.17 The first day of dietary recall was performed in-person at a MEC and the second was performed 3-10 days later through a telephone interview. We did not exclude subjects with extreme low or high caloric intake.
The Healthy Eating Index (HEI) is a score that quantifies an individual’s diet quality according to the Dietary Guidelines for Americans produced and updated by the US Department of Health and Human Services (HHS) and US Department of Agriculture (USDA) and ranges from 0 (worst) to 100 (best). The latest iteration of the HEI (HEI-2015) is comprised of 13 categories. In 9 categories, points are awarded for consuming adequate amounts of healthier food groups, while in the other 4 categories, points are awarded for moderation of the consumption of less healthy components (Supplementary Table 1).18 HEI was calculated from both days of NHANES dietary recall using the SAS code provided by the National Cancer Institute (NCI).19
The alternative Mediterranean Diet Score (aMED), outlined by Fung et al20 as an adaptation of the original Mediterranean Diet adherence score by Trichpoulou et al,21 captures dietary patterns that adhere to the Mediterranean diet and are protective against chronic disease. aMed is graded on a scale from 0 (worst adherence to the Mediterranean diet) to 9 (best adherence). Points are awarded for exceeding the population median consumption of beneficial foods and falling beneath the median consumption of detrimental foods. The Food Patterns Equivalent Database (FPED) was used to categorize the individual foods recorded in the NHANES Dietary Recall Interview into simplified food categories to identify dietary patterns.22 The schema mapping the USDA’s FPED food categories to the 9 aMED categories and the median consumption for this study’s cohort is outlined in Supplementary Table 2.22
Questions used to assess participants’ nutrition literacy were derived from the Consumer Behavior Phone Follow-up Module, Diet Behavior and Nutrition and Medical Conditions questionnaires administered as part of NHANES.
Diet Perception Accuracy
To compare subjects’ perception of their diet quality with its objective quality as measured by HEI and aMED, we designed a Diet Perception Accuracy Rank (DPAR). Perception of diet quality was derived from the response to the question ‘how healthy is your diet?’ on the NHANES Diet Behavior and Nutrition (DBQ) questionnaire, scored using a five-point Likert scale ranging from ‘poor’ to ‘excellent’. Self-reported ratings were re-classified to three categories: low (including ‘poor’ and ‘fair’), mid (including ‘good’) and high (including ‘very good’ and ‘excellent’). Objective diet quality scores were divided into terciles: HEI (below 44.7, 44.7-57.1 and above 57.1) and aMED (0-3, 4-5, and 6-9). DPAR was constructed by contrasting HEI or aMED terciles with the self-reported quality category. Over estimators were those who considered their diet heathier than it was (self-reported diet quality > HEI or aMED). Under estimators were those whose diet was better than they thought (self-reported diet quality < HEI or aMED). Those whose self-reported diet quality tercile matched the objective diet quality tercile were labelled as accurate. For each subject an HEI-based score (HEI-DPAR) and aMED-based score (aMED-DPAR) were calculated.
To calculate the caloric accuracy rank (CAR), self-reported daily caloric needs were divided into three categories: less than 1500 calories, 1500-2500 calories and more than 2500 and compared to the actual caloric consumption, calculated as average of both days of dietary recall. This caloric range was chosen based on the Food and Drug Administration’s general guideline to consume 2,000 calories per day.23 Over consumers were those who ate more calories than their stated daily need. Under consumers were those who consumed fewer calories than their stated daily need. Subjects who consumed a quantity of calories that fell within their self-reported caloric need were labelled accurate.
Statistical Analysis
Analysis of the NHANES complex survey data was performed using the ‘survey’ package in R.24 The dietary two-day sample weight was used for our analysis as the second day of dietary recall represents the variable with the smallest number of respondents used in this study. Descriptive statistics of demographic information, baseline characteristics and survey responses are presented as mean and 95% confidence interval for continuous variables and percentage for categorical variables. Student’s t-test was performed for continuous variables. Kruskal-Wallis rank sum test was used for ordinal variables and chi-squared was performed on nominal variables.
RESULTS
Subject Characteristics & Demographics
Of the 2938 subjects in the study cohort, NAFLD was present in 1554 (52%), who were more likely to be Hispanic, older, male, have a higher BMI and hemoglobin A1c (Table 1). Subjects with NAFLD were less likely to have attended college (p = 0.034) but did not differ in poverty income ratio from those without NAFLD (Table 1). Reported daily caloric intake did not differ between groups (p=0.35, Table 1).
Table 1.
Baseline characteristics & demographics of the NAFLD cohort
| NAFLD N =1554 |
No NAFLD N = 1384 |
p value | |
|---|---|---|---|
| Age [Years] | 51.6 (50.3-53.0) | 44.8 (43.4-46.2) | <0.001 |
| Female Gender | 48.2% | 56.3% | 0.043 |
| BMI [kg/m2] | 33.2 (32.5-33.9) | 26.7 (26.1-27.4) | <0.001 |
| Race/ethnicity | |||
| Mexican American | 12.1% | 6.1% | 0.004 |
| Other Hispanic | 7.1% | 7.0% | |
| NH White | 61.3% | 63.5% | |
| NH Black | 9.4% | 13.9% | |
| NH Asian | 5.6% | 5.8% | |
| Other/Multiracial | 4.3% | 3.9% | |
| Poverty income ratio | 0.67 | ||
| Less than 1.3 | 20.2% | 18.4% | |
| 1.3-3.5 | 38.2% | 34.8% | |
| Over 3.5 | 41.6% | 46.7% | |
| Education level | 0.034 | ||
| Less than 9th grade | 3.7% | 2.4% | |
| 9-11th grade | 7.2% | 5.5% | |
| High school graduate/GED | 27.6% | 24.6% | |
| Some college or AA degree | 32.4% | 29.2% | |
| College graduate or above | 29.1% | 38.3% | |
| VCTE measurements | |||
| CAP [dB/m] | 316 (312-319) | 214 (211-217) | <0.001 |
| Liver stiffness [kPa] | 6.4 (5.9-7.0) | 4.9 (4.7-5.2) | <0.001 |
| Laboratory Values | |||
| AST [U/L] | 23 (22-24) | 21 (20-23) | 0.12 |
| ALT [U/L] | 26 (25-28) | 19 (18-21) | <0.001 |
| GGT [IU/L] | 33 (31-35) | 22 (21-24) | <0.001 |
| Platelets [1000 cells/uL] | 251 (243-258) | 244 (238-251) | 0.10 |
| Hemoglobin A1c [%] | 6.0 (5.9-6.1) | 5.4 (5.4-5.5) | <0.001 |
| Daily Caloric Intake [kcal] | 2054 (1984-2124) | 2002 (1924-2080) | 0.35 |
| HEI total score | 50.6 (48.8-52.4) | 53.3 (51.4-55.1) | 0.018 |
Data presented as mean (95% confidence intervals) unless otherwise stated
Subjects with NAFLD eat a lower quality diet
Diet quality, as measured by the HEI score, was lower in subjects with NAFLD compared to those without (50.6 [48.8-52.4] vs. 53.3 [51.4-55.1], p = 0.018, Table 1), as previously reported.9 Of the individual component scores, the scores for total vegetables (p = 0.018), greens and beans (p = 0.003) and the ratio of unsaturated to saturated fatty acids (p = 0.020) were all significantly lower in subjects with NAFLD (Supplementary Table 3). There were no significant differences between the NAFLD and no NAFLD groups in individual moderation component scores but subjects with NAFLD scored lower than those without NAFLD in both the total adequacy (p = 0.021) and total moderation scores (p = 0.024, Supplementary Table 3).
Subjects with NAFLD are as nutrition literate as those without NAFLD
More than half of subjects were able to correctly identify 1500-2500 calories per day as a reasonable amount for a person to consume and knowledge did not differ between subjects with or without NAFLD (p = 0.40, Table 2). Similarly, most subjects were able to correctly interpret the meaning of daily value on a nutrition label (p = 0.31, Table 2). Impressively, over 90% of all subjects, with and without NAFLD, reported using the nutrition label (p = 0.27, Supplementary Table 4). There was no difference in nutrition literacy between subjects with and without CSF (Supplementary Table 5).
Table 2:
Responses to nutrition literacy questions in subjects with and without NAFLD
| NAFLD | No NAFLD | P value | |
|---|---|---|---|
| Number of calories a person needs in a day | 0.38 | ||
| < or = 1500 | 36.0 % | 36.8% | |
| 1501-2500 (Correct) | 52.2% | 53.4% | |
| Over 2500 | 11.8% | 9.8% | |
| What does a 5% daily value of Vit A mean? | 0.17 | ||
| 5% of the calories in one serving come from Vit A | 25.6% | 21.0% | |
| One serving contains 5% Vit A by weight | 14.7% | 17.4% | |
| One serving is 5% of the Vit A for the day (Correct) | 59.7% | 61.6% | |
| What does ‘serving size’ mean to you? | |||
| The amount of this food that people should eat | 61.3% | 59.0% | 0.32 |
| The amount of this food that people usually eat | 16.9% | 16.7% | 0.93 |
| Something that makes it easier to compare foods | 27.9% | 35.5% | 0.01 |
Subjects with NAFLD slightly differed in their interpretation of a serving size on the nutrition label (p = 0.01, Table 2) and used the calorie information on food labels less frequently (p = 0.004, Supplementary Table 4). Nevertheless, most subjects with and without NAFLD were using the label calorie information.
We performed a secondary analysis to examine the impact of social determinants of health on nutrition literacy. Nutrition literacy was higher in subjects with higher education levels and higher income (Supplementary Tables 6-7) and differed by race and ethnicity (Supplementary Table 8).
Subjects with and without NAFLD have the same diet perception accuracy
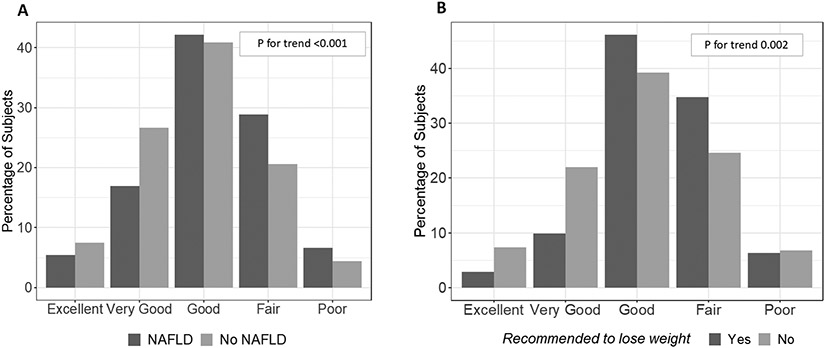
Subjects with NAFLD rated their diet as less healthy than those without NAFLD (Figure 1A, p<0.001). Subjects’ self-reported diet quality was compared to the objective diet quality as measured by HEI and caloric consumption. Overall, 27.8% of subjects overestimated their diet quality and 37.5% consumed more calories than their self-assessed needs. There was no difference between subjects with NAFLD and those without it in accuracy of perception of diet quality (p = 0.71) or caloric consumption (p = 0.63, Table 3). The accuracy of perception of diet quality or caloric consumption did not differ between NAFLD subjects with or without CSF (Supplementary Table 9).
Figure 1. Subjects with NAFLD are more likely to rate their diet as poorer quality.
(A) Histogram of the response to the question: “In general, how healthy is your overall diet?” by NAFLD status. (B) In subjects with NAFLD, histogram of the response to the question: “In general, how healthy is your overall diet?” by whether or not their doctor had told them to lose/reduce their weight. P-values for trend calculated using the Kruskal-Wallis test.
Table 3.
The Diet Perception Accuracy Score
| NAFLD | No NAFLD | P value | |
|---|---|---|---|
| Diet Accuracy Scores | |||
| HEI vs self-reported diet quality | 0.71 | ||
| Over estimate | 27.1% | 28.6% | |
| Accurate | 41.6% | 41.8% | |
| Under estimate | 31.3% | 29.7% | |
| Calories consumed vs self-reported caloric need | 0.63 | ||
| Over consuming | 39.7% | 35.1% | |
| Accurately consuming | 42.8% | 45.8% | |
| Under consuming | 17.4% | 19.1% |
The Mediterranean diet has benefits in NAFLD.25 We used the aMED score, which measures adherence to the Mediterranean diet, as an alternative measure of diet quality. Similar to HEI, subjects with NAFLD scored lower on the aMED diet quality score compared to those without NAFLD (p = 0.013, Supplementary Table 10). When self-reported diet quality was compared to the aMED score, there was no difference between NAFLD and non-NAFLD in the proportions of over estimators, accurate perceivers, and under estimators (p=0.29, Supplementary Table 10).
To determine if our results are affected by the CAP cutoff for defining NAFLD, we performed a sensitivity analysis using a higher cutoff of 285 dB/m and found similar results. There were 1134 subjects with CAP>=285 dB/m (39%) and 1804 without. NAFLD status of subjects did not result in significantly different DPAR scores, as measured using HEI (p = 0.77), aMED (p = 0.11), or caloric consumption (p = 0.37, Supplementary Table 11).
To account for BMI as a possible confounding variable, a sensitivity analysis was performed by comparing participants across BMI categories. There was no difference in diet perception between groups (Supplementary Table 12). Furthermore, subjects with NAFLD and obesity (BMI ≥ 30 kg/m2) did not differ from subjects with obesity without NAFLD in their accuracy of diet quality perception, although they showed a trend towards consuming more than their self-estimated caloric need (p=0.07, Supplementary Table 13). Similarly, in subjects without obesity, perception of diet quality and intake did not differ between those with NAFLD and those without.
Subjects with NAFLD report trying to make positive lifestyle changes
Weight loss is the therapeutic goal of many of the dietary and lifestyle changes recommended for NAFLD. Indeed, subjects with NAFLD were more than twice as likely to be told by a doctor to lose or control their weight or to reduce fat and calories in their diet (p <0.001 for both, Table 4). More than 2/3 of subjects with NAFLD were actively attempting to lose weight or to reduce fat or calories (Table 4).
Table 4.
Recommendations and attempts to control weigh and intake
| NAFLD | No NAFLD | P value | |
|---|---|---|---|
| Has a doctor told you to lose/control weight? (Yes) | 42.1% | 16.5% | <0.001 |
| Are you now controlling or losing weight? (Yes) | 71.9% | 60.9% | 0.001 |
| Has a doctor told you to reduce fat/calories? (Yes) | 44.1% | 19.7% | <0.001 |
| Are you now reducing fat/calories? (Yes) | 67.2% | 52.8% | <0.001 |
Subjects with NAFLD rate their diet quality as lower than those without NAFLD
Despite medical advice and their attempts, HEI diet quality scores, caloric intake and fat intake were not better in NAFLD subjects who were told to lose weight compared to those who were not told and were similarly not affected by a physician recommendation to decrease fat and calories (Supplementary Table 14). This is unlikely to reflect poor understanding, as subjects with NAFLD who were told to lose or control their weight did rate their diet as less healthy (p = 0.002, Fig 1B) compared to those who were not. Similarly, those who had been advised to reduce fat/calories in their diet were more also likely to rate their diet quality as poorer (p<0.001, data not shown).
DISCUSSION
In this study of a representative US cohort, we show that subjects with NAFLD have the same tools to make quality diet choices as those without NAFLD, are more likely to be attempting positive lifestyle change and are equally capable of translating these skills into their real-world food choices. Yet, subjects with NAFLD are eating lower quality diets. Importantly, subjects with NAFLD are as aware of their diet quality as those without, but even when dietary intervention is recommended, appear unable to translate this awareness to an effective change. Our findings suggests that, although diet and lifestyle changes are the mainstay of therapy for NAFLD, education and recommendations may not be sufficient in supporting people with NAFLD who are attempting to make a positive dietary change.
Every major practice guideline recommends providing people with NAFLD with dietary and lifestyle counseling, suggesting a consensus belief in the benefit of nutrition education and knowledge among patients with NAFLD.3 Indeed, nutrition literacy can be improved by counselling, as shown in a recent randomized controlled trial (RCT).26 However, data from interventional studies suggests that improving nutrition knowledge does not lead to NAFLD improvement. For example, in a 48-week RCT in patients with NAFLD, Promrat et al provided the control arm with nutrition education alone and these subjects did not lose weight or have a histological response.27 Similarly, Lee et al demonstrated that subjects in a control group who received written nutrition education did not achieve a significant change in ALT, CAP score or NAFLD fibrosis score after 48 weeks.28 The lack of success for education-alone approaches in the highly controlled and standardized environment of clinical trials predicts even lower success rates in real life scenarios. In this current epidemiological study we have demonstrated that subjects with NAFLD have similar nutrition literacy, in both functional knowledge and real world translation, to subjects without NAFLD. Taken together, the results from this current study and the recent literature suggest that nutrition literacy by itself may not be sufficient to achieve the dietary changes needed to improve NAFLD.
We found a discrepancy between nutrition knowledge and diet quality, even in subjects with NAFLD who are attempting to lose weight following medical advice. One possible explanation for this discrepancy is the behavioral component of food choice and diet behavior. Multiple studies have previously demonstrated that behavioral elements including stress,29 social norms,30,31 and lack of self-efficacy32 negatively affect food choice. Haigh et al identified stress, detrimental environmental factors and lack of convenience as barriers to adopting the Mediterranean diet in a cohort of NAFLD patients.33 There is a need for more research on the impacts of behavioral health and ability to make dietary changes among patients with NAFLD. However, our work and the context provided by the literature suggests emphasis should be put on understanding what affects dietary choice after nutrition knowledge is provided to patients with NAFLD.
Unequal access to healthy foods is another possible explanation for the discrepancy between nutrition knowledge and diet quality in subjects with NAFLD. Food access can be affected on multiple levels, from income to location. In low-income households, food-insecurity increased the risk of having NAFLD.34 Whether living in a “food desert” affects the risk of having NAFLD is unknown. Regardless, only about 11% of the United States was food insecure in 2018 and from 2010-2016 only 5.6% of the population lived in a limited-supermarket-access area. 35,36 While it is plausible there is a relationship between food access and higher rates of NAFLD, food access alone cannot fully account for the difference between knowledge and consumption shown in this study.
Multiple strengths of this study should be noted. First, to our knowledge this is the first report that examines nutrition literacy in a nationally representative cohort. While most nutritional studies are performed on a smaller scale, this study was able to capture information from a snapshot of the non-incarcerated United States population. Second, in this study NAFLD was identified using imaging data, rather than surrogates such as the Faty Liver Index (FLI), increasing its accuracy. Third, this is the first study to compare self-assessed and objective diet quality in the context of NAFLD.
There are also several limitations to this study. First, the use of 24-hour dietary recall questionnaires, albeit practical, is an imperfect measure of true dietary consumption.37 However, 24-hour recall was shown to be more accurate than other recall tools,38 the AMPM method has been well validated 16,17 and the use of two separate recalls likely improved accuracy. In addition, it has been suggested that underreporting of energy intake is more common in people with obesity,39 although though this has been brought into question.40 However, the perceived quality and quantity of dietary intake did not differ by obesity status (Supplementary Table 12), suggesting that, even if there is an obesity-driven bias in reported intake, it does not translate to bias in literacy or perception. Second, the Nutrition Literacy Assessment Instrument was recently described as a validated measure of nutrition literacy41 but this measure not available during the NHANES 2017-2018 cycle. We therefore had to assess literacy from existing questions. Third, the cross-sectional nature of the study does not allow us to assess dynamic changes (i.e. whether current nutrition literacy leads to future nutrition change), nor assess the actual efficacy of weight loss attempts. Finally, BMI is a potentially significant confounder in this study. Subjects with NAFLD are much more likely to have a higher BMI, and while NAFLD is not visible to the outside world, higher subject BMI might lead to stigmatization and increased lifestyle counselling that might be contributing to the differences observed between the NAFLD and non-NALFD groups.
In conclusion, we have summarized the nutrition literacy of subjects with NAFLD in a representative US cohort. We have found that subjects with and without NAFLD have similar levels of nutrition literacy, and report applying those skills in their everyday lives to a similar extent, however when asked about the quality of their diet those with NAFLD report they are eating lower quality diets. Overall, this suggests that while subjects with NAFLD have the knowledge to make nutritious choices, having this knowledge is not sufficient to achieve the lifestyle changes that are required for disease management, and that additional support beyond education is necessary.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Amber Courville for helpful advice.
Source of Funding:
This study was funded by the Intramural Research Program of NIDDK. AMC was supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the American Association for Dental Research and the Colgate-Palmolive Company.
Abbreviations:
- NAFLD
Non-alcoholic Fatty Liver Disease
- NHANES
National Health and Nutrition Examination Survey
- NCHS
National Center for Health Statistics
- MEC
Mobile Examination Center
- CAP
Controlled attenuation parameter
- CSF
Clinically significant fibrosis
- HEI
Healthy Eating Index
- HHS
Health and Human Services
- USDA
US Department of Agriculture
- aMED
alternative Mediterranean Diet Score
- FPED
Food Patterns Equivalent Database
- DPAR
Diet Perception Accuracy Rank
- RCT
Randomized Controlled Trial
Footnotes
Conflicts of Interest: The authors report no conflict of interest.
REFERENCES
- 1.Yki-Järvinen H Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. The Lancet Diabetes & Endocrinology. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4 [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. Mar 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 3.Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol. Aug 14 2018;24(30):3361–3373. doi: 10.3748/wjg.v24.i30.3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. Jun 2016;64(6):1388–402. doi: 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 6.Non-alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Eat Weight Disord. Jun 2022;27(5):1603–1619. doi: 10.1007/s40519-021-01287-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause C, Sommerhalder K, Beer-Borst S, Abel T. Just a subtle difference? Findings from a systematic review on definitions of nutrition literacy and food literacy. Health Promot Int. Jun 1 2018;33(3):378–389. doi: 10.1093/heapro/daw084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor MK, Sullivan DK, Ellerbeck EF, Gajewski BJ, Gibbs HD. Nutrition literacy predicts adherence to healthy/unhealthy diet patterns in adults with a nutrition-related chronic condition. Public Health Nutrition. 2019;22(12):2157–2169. doi: 10.1017/S1368980019001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilar-Gomez E, Nephew LD, Vuppalanchi R, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. Oct 20 2021;doi: 10.1002/hep.32207 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, Statistics. NCfH. Data from: National Health and Nutrition Examination Survey Data. 2017-2018. Hyattsville, MD. [Google Scholar]
- 11.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Income. US Department of Health and Human Services, Centers for Disease Control and Prevention. Accessed December 28, 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/P_INQ.htm [Google Scholar]
- 12.Food and Nutrition Service. Cost of Living Adujstement (COLA) Information. Accessed Nov 30, 2022, https://www.fns.usda.gov/snap/allotment/COLA
- 13.Chan W-K, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. Journal of Gastroenterology and Hepatology. 2014;29(7):1470–1476. doi: 10.1111/jgh.12557 [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clinical Gastroenterology and Hepatology. 2019/01/January/ 2019;17(1):156–163.e2. doi: 10.1016/j.cgh.2018.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raper N An overview of USDA's Dietary Intake Data System. Journal of food composition and analysis. 0000 2004;v. 17(no. 3-4):pp. 545–555-2004 v.17 no.3-4. [Google Scholar]
- 16.Moshfegh AJ. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. American journal of clinical nutrition. 0000 2008;v. 88(no. 2):pp. 324–332-2008 v.88 no.2. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes DG, Murayi T, Clemens JC, Baer DJ, Sebastian RS, Moshfegh AJ. The USDA Automated Multiple-Pass Method accurately assesses population sodium intakes. The American Journal of Clinical Nutrition. 2013;97(5):958–964. doi: 10.3945/ajcn.112.044982 [DOI] [PubMed] [Google Scholar]
- 18.Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. Sep 2018;118(9):1591–1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute. The Healthy Eating Index - Per Person Scoring Algorithm. https://epi.grants.cancer.gov/hei/sas-code.html
- 20.Fung TT, McCullough ML, Newby PK, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. Jul 2005;82(1):163–73. doi: 10.1093/ajcn.82.1.163 [DOI] [PubMed] [Google Scholar]
- 21.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean Diet and Survival in a Greek Population. New England Journal of Medicine. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- 22.USDA. Food Patterns Equivalent Database: Databases and Data Sets. USDA. Accessed May 2022, https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-databases/ [Google Scholar]
- 23.Food and Drug Administration. Calories on the New Nutrition Facts Label. Accessed December 6, 2022, https://www.fda.gov/food/new-nutrition-facts-label/how-understand-and-use-nutrition-facts-label
- 24.survey: analysis of complex survey samples. Version 4.0. Lumley, Thomas; 2020. [Google Scholar]
- 25.Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. May 21 2018;24(19):2083–2094. doi: 10.3748/wjg.v24.i19.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchello NJ, Daley CM, Sullivan DK, Nelson-Brantley HV, Hu J, Gibbs HD. Nutrition Literacy Tailored Interventions May Improve Diet Behaviors in Outpatient Nutrition Clinics. J Nutr Educ Behav. Dec 2021;53(12):1048–1054. doi: 10.1016/j.jneb.2021.07.013 [DOI] [PubMed] [Google Scholar]
- 27.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WM, Bae JH, Chang Y, et al. Effect of Nutrition Education in NAFLD Patients Undergoing Simultaneous Hyperlipidemia Pharmacotherapy: A Randomized Controlled Trial. Nutrients. Dec 13 2021;13(12)doi: 10.3390/nu13124453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zellner DA, Loaiza S, Gonzalez Z, et al. Food selection changes under stress. Physiol Behav. Apr 15 2006;87(4):789–93. doi: 10.1016/j.physbeh.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 30.Higgs S Social norms and their influence on eating behaviours. Appetite. Mar 2015;86:38–44. doi: 10.1016/j.appet.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 31.Robinson E, Blissett J, Higgs S. Social influences on eating: implications for nutritional interventions. Nutr Res Rev. Dec 2013;26(2):166–76. doi: 10.1017/s0954422413000127 [DOI] [PubMed] [Google Scholar]
- 32.Hardcastle SJ, Thøgersen-Ntoumani C, Chatzisarantis NL. Food Choice and Nutrition: A Social Psychological Perspective. Nutrients. Oct 2015;7(10):8712–5. doi: 10.3390/nu7105424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haigh L, Bremner S, Houghton D, et al. Barriers and Facilitators to Mediterranean Diet Adoption by Patients With Nonalcoholic Fatty Liver Disease in Northern Europe. Clinical Gastroenterology and Hepatology. 2019/06/January/ 2019;17(7):1364–1371.e3. doi: 10.1016/j.cgh.2018.10.044 [DOI] [PubMed] [Google Scholar]
- 34.Golovaty I, Tien PC, Price JC, Sheira L, Seligman H, Weiser SD. Food Insecurity May Be an Independent Risk Factor Associated with Nonalcoholic Fatty Liver Disease among Low-Income Adults in the United States. J Nutr. Jan 1 2020;150(1):91–98. doi: 10.1093/jn/nxz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpyn AE, Riser D, Tracy T, Wang R, Shen YE. The changing landscape of food deserts. UNSCN Nutr. Summer 2019;44:46–53. [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household Food Security in the United States in 2018. 2019. [Google Scholar]
- 37.Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. doi: 10.4178/epih/e2014009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burrows TL, Ho YY, Rollo ME, Collins CE. Validity of Dietary Assessment Methods When Compared to the Method of Doubly Labeled Water: A Systematic Review in Adults. Front Endocrinol (Lausanne). 2019;10:850. doi: 10.3389/fendo.2019.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wehling H, Lusher J. People with a body mass index ⩾30 under-report their dietary intake: A systematic review. J Health Psychol. Dec 2019;24(14):2042–2059. doi: 10.1177/1359105317714318 [DOI] [PubMed] [Google Scholar]
- 40.Waterworth SP, Kerr CJ, McManus CJ, Costello R, Sandercock GRH. Obese individuals do not underreport dietary intake to a greater extent than nonobese individuals when data are allometrically-scaled. American Journal of Human Biology. 2022;34(7):e23743. doi: 10.1002/ajhb.23743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbs HD, Ellerbeck EF, Gajewski B, Zhang C, Sullivan DK. The Nutrition Literacy Assessment Instrument is a Valid and Reliable Measure of Nutrition Literacy in Adults with Chronic Disease. J Nutr Educ Behav. Mar 2018;50(3):247–257.e1. doi: 10.1016/j.jneb.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.