Abstract
Introduction:
Prior estimates of dementia prevalence in India were based on samples from selected communities, inadequately representing the national and state populations.
Methods:
From the Longitudinal Aging Study in India (LASI) we recruited a sample of adults ages 60+ and administered a rich battery of neuropsychological tests and an informant interview in 2018 through 2020. We obtained a clinical consensus rating of dementia status for a subsample (N = 2528), fitted a logistic model for dementia status on this subsample, and then imputed dementia status for all other LASI respondents aged 60+ (N = 28,949).
Results:
The estimated dementia prevalence for adults ages 60+ in India is 7.4%, with significant age and education gradients, sex and urban/rural differences, and cross-state variation.
Discussion:
An estimated 8.8 million Indians older than 60 years have dementia. The burden of dementia cases is unevenly distributed across states and subpopulations and may therefore require different levels of local planning and support.
Keywords: Alzheimer’s disease and related dementia, Clinical Dementia Rating, cognitive impairment, dementia prevalence, major neurocognitive disorder
1 ∣. INTRODUCTION
India is home to 1.37 billion people, comprising 18% of the total world population in 2019, and is set to surpass China as the world’s most populous country in 2023.1 Its population is also rapidly aging. The share of individuals aged 60 years or older is projected to increase to nearly 20% of the total Indian population by 2050 (319 million), accounting for 15.4% of individuals aged 60 and older worldwide.2 This demographic trend reflects rising longevity, as life expectancy in India has steadily increased from 42.9 years in 1960 to 70.4 years in 2020.3
Because age is the strongest and best-known risk factor for dementia,4 India faces an alarming potential increase in the number of people with dementia. An accurate national estimate of dementia prevalence is essential to understand the magnitude of the challenge the country is facing. In the absence of a nationally representative study in India, the Alzheimer’s and Related Disorders Society of India5 extrapolated dementia prevalence using estimates for South Asia reported by the 2015 World Alzheimer Report6 and six prior studies in Indian subregions conducted in 2010.7
Notably, all prior studies were based on samples from a few geographically confined communities, collectively covering only 6 out of 36 states and union territories of India, far short of representing the whole nation. Substantial heterogeneity in dementia prevalence rates across those six states has been also noted,5,7-8 but, because adopted dementia assessment and diagnostic criteria were heterogeneous, the extent of true differences in prevalence across states in India is unclear. The critical need for a nationwide study of dementia that captures the diversity of the country is widely recognized5,7-8 given significant regional variation in longevity and health. For example, life expectancy at birth ranges from 64.8 years in Bihar to 75.1 years in Kerala.2 Expected survival at age 60 is 17.4 years for men and 18.9 years for women9 (see Table S1 in supporting information for sex- and state-specific life expectancy). According to the India State-Level Disease Burden Initiative collaborators,10 the magnitudes of disease burden and risk factors vary significantly across the country, with the state of Kerala exhibiting better health indicators than the rest of India for the past several decades.
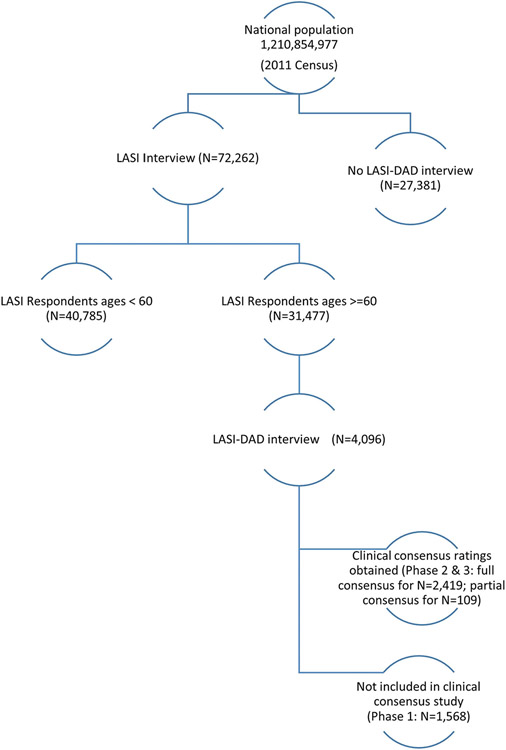
We developed the first national study of dementia in India, the Harmonized Diagnostic Assessment of Dementia for the Longitudinal Aging Study in India (LASI-DAD; N = 4096), with the aims of estimating dementia prevalence and incidence at national and state levels, investigating risk factors for dementia, and assessing the burden of dementia on families and society as a whole. LASI-DAD is an ancillary study of the LASI (N = 72,262), a multipurpose, longitudinal study on aging, representative of the country and of each state.2 In this paper, we report dementia prevalence rates at national and state levels, using the first wave of LASI and LASI-DAD. These estimates are crucial for national and state-specific health policy making in India.
2 ∣. METHODS
2.1 ∣. Research design
Our long-term goals are to establish a nationally representative, community-based cohort of individuals aged 60 and older in India to provide valid estimates of dementia prevalence and incidence in the country. With these goals in mind, we developed our sampling strategy and dementia assessment protocol.
2.1.1 ∣. Sampling strategy
We first drew a stratified, multi-stage, nationally and state-wise representative sample for the parent LASI study, using the 2011 Census. Specifically, we used the 2011 Census state-wise listing directory of districts, subdistricts (tehsils), which were the primary sampling units (PSUs), and villages/urban wards, which were the secondary sampling units (SSUs). As our field period was 2017 to 2019, the delimitation of PSUs and SSUs may have changed in the meanwhile and to capture such changes, we updated household sampling frame through a mapping and listing exercise in all selected SSUs. Through door-to-door household interviews, we identified all households with age-eligible (45+) individuals, and, from the resulting household list, LASI households were randomly selected. As the LASI was designed to provide reliable estimates of health, social and economic outcomes for the population age 45 and above in India and all of its 30 states and 6 union territories, a minimum sample size of 1000 age-eligible persons was considered appropriate for the smaller states/union territories with a population of less than 10 million people. For large states, a larger sample size proportionate to the population size of the state was allocated. In addition, we oversampled individuals aged 65 and older to achieve a better representation of this group in the sample. Within selected households, LASI enrolled all age-eligible individuals and their spouses regardless of age2 in 2017 through 2019 (N = 72,262).
Between 2018 and 2020, we recruited a subsample of age-eligible (60+) LASI respondents (N = 4096) for an in-depth dementia assessment (LASI-DAD11) which was conducted 6 to 7 months after the LASI interview. To maintain national representation and to ensure sufficient numbers of respondents with cognitive impairment, we used a two-stage stratified random sampling approach and oversampled respondents at a higher risk of cognitive impairment. Specifically, we first classified respondents into those at high and low risk of cognitive impairment based on LASI’s cognitive tests and on the proxy report for those who did not complete the cognitive tests. We then randomly drew the sample so that it would be equally split between individuals with high and low risk of cognitive impairment (this implies an oversample of individuals at high risk of cognitive impairment). We obtained consensus dementia ratings for 61.7% of the LASI-DAD sample.
Figure 1 illustrates this research design and sample sizes. To ensure representativeness of the sample within the practical limits of the fieldwork operation, stratified random sampling strategies were used for LASI. We constructed sampling weights that account for differential selection probabilities produced by the adopted sampling strategy and adjusted for differential nonresponse across demographic groups. The LASI-DAD sample was drawn from the LASI respondents ages 60 and older, also using a stratified random sampling strategy, from 18 states and union territories. The details of the sampling design and weight construction were reported previously2,11 (also summarized in S2 Methods in supporting information).
FIGURE 1.
Research design: Sampling frame and sample size for the Longitudinal Aging Study in India (LASI), the Harmonized Diagnostic Assessment of Dementia for LASI (LASI-DAD), and Clinical Consensus Study
2.1.2 ∣. Dementia assessment protocol
We purposefully developed a dementia assessment protocol for LASI and LASI-DAD with some overlapping tests to facilitate comparisons between LASI and LASI-DAD. Specifically, in both LASI and LASI-DAD, we administered commonly used cognitive screening tests, including orientation and object naming, which are part of the Hindi Mental State Examination,12 the Consortium to Establish a Registry for Alzheimer’s Disease word recall and retrieval fluency,13 and the Informant Questionnaire on Cognitive Decline in the Elderly.14 However, additional cognitive tests that have been validated in India15 were administered in LASI-DAD. LASI is a multipurpose survey. As such, even if cognition is an important focus of the study, the amount of interview time allocated to cognition assessment was limited. In contrast, the LASI-DAD protocol includes a richer battery of neuropsychological tests, a geriatric assessment, and an interview with an informant nominated by the respondent. It is important to note thatwe selected cognitive tests that are suitable for both literate and illiterate individuals, such as the Hindi Mental State Examination and retrieval fluency test,12,13 and we made modifications where necessary, for example, by administering the word recall test without presenting show cards. Detailed comparisons of the LASI and LASI-DAD protocols were reported previously16 (and summarized in TableS3 in supporting information). In addition, both LASI and LASI-DAD administered questions on difficulties with activities of daily living and instrumental activities of daily living and on depressive symptoms, health history (including stroke, depression, and psychiatric and neurological problems), and sensory impairments. See LASI Wave 1, 2017–2019, India Report2 and Lee et al.11 for details of the protocol details.
The instrument, including the cognitive tests, was translated into 12 local languages: Hindi, Kannada, Malayalam, Gujarati, Tamil, Punjabi, Urdu, Bengali, Assamese, Odia, Marathi, and Telugu. To minimize differences due to language, forward and backward translation was conducted, following the conceptual method.17 Specifically,the English version of the instrument was given to professional translators who translated it into another language. Each local team then examined the translated instrument to confirm proper translation, including whether intended concepts were captured accurately. This method was chosen over strict backward translation, as strict translation does not necessarily capture underlying concepts to be measured. Finally, comments from the local team were reviewed and a final version was agreed on for the different languages.
2.2 ∣. Clinical consensus rating of dementia and mild cognitive impairment
For clinical syndromes such as dementia, no single definitive diagnostic test exists. Many clinical researchers rely on a process of data review, adjudication, and consensus by a panel of expert clinicians.18 The panel meets in person to review detailed information on aspects of the clinical assessment of a given patient, discusses the findings, and renders a consensus diagnosis using standardized criteria, such as the Clinical Dementia Rating (CDR).19 This process allows the data of each study participant to be considered in detail, taking advantage of a wealth of collective clinical expertise and judgment. However, sending experienced specialists to visit participants’ homes throughout the study region is infeasible in large-scale, population-based studies in a country like India, where a significant shortage of clinical dementia experts20 coexists with a large linguistic diversity. To convene clinical specialists to diagnose dementia, LASI-DAD developed a cost-effective, web-based approach to facilitate the application of expert clinical judgment for a dementia rating. Under this approach, clinical researchers train non-clinician research interviewers to obtain key information from respondents and informants, using structured questions that are designed to address the key issues in the CDR, and then a group of clinical researchers reviews the standardized interview data collected by the trained interviewers. This approach resembles that used in prior epidemiological studies in which in-person clinical diagnosis is unavailable, such as the Cardiovascular Health Cognition Study,21 where a clinical researcher makes a clinical diagnosis based on standardized data collected by trained non-clinician interviewers, which is then discussed with other clinical researchers to reach a consensus diagnosis. A key feature of the consensus diagnosis process is that expert clinicians’ judgment is used to weigh variables that may have nonspecific contributions or may be part of complex interactions, contributing to the dementia syndrome. Our objective was to involve clinical experts in reviewing and rating standardized assessment data from the LASI-DAD interview and then to arrive at a consensus among the clinical experts for each participant. For the basis of clinical diagnosis, we used the CDR, which is comprised of six domains of (1) memory, (2) orientation, (3) judgment and problem solving, (4) community affairs, (5) home and hobbies, and (6) personal care. We designed the consensus portal to offer clinicians the relevant information to provide domain-specific ratings. We conducted a first feasibility study, inviting eight CDR-certified expert clinicians to the demonstration site that presented the information from the LASI-DAD interview of five recently diagnosed patients at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India. All clinicians agreed that the website provided sufficient information to develop a good understanding of the patient’s cognitive status and everyday functioning, but suggested including additional details about judgment and problem solving as well as self-reports of memory loss. Following this advice, we added additional questions assessing judgment and problem solving and eliciting self-reports of memory loss to the LASI-DAD instrument and implemented further refinements to the site, reflecting the expert clinicians’ suggestions.
We then conducted a validation study to examine the extent to which the online clinical consensus diagnosis, based on the interviews administered using the LASI-DAD protocol, yielded outcomes consistent with an in-person clinical consensus diagnosis, based on clinicians’ in-person assessment of patients. To do so, we recruited 60 patients from two hospitals in India, the All India Institutes of Medical Sciences (AIIMS), New Delhi and NIMHANS, where a number of CDR-certified clinicians were available for the gold standard, in-person clinical consensus diagnosis. Expert clinical teams of three to four CDR-certified clinicians at each institution conducted in-person assessments of patients and their informants, followed by a traditional in-person diagnostic consensus conference. The LASI-DAD interview team, consisting of trained non-clinician interviewers, conducted the LASI-DAD interview with the same patients and collected standardized data. We then invited the team of CDR-certified clinicians from AIIMS to rate patients from NIMHANS and vice versa, using the consensus website. A previously published paper discusses the validity and reliability of the web-based clinical consensus approach in great detail.22 Briefly, the online clinical consensus diagnosis based on the standardized LASI-DAD interview data collected by trained non-clinician interviewers exhibited a high consistency rate of 90.8% (z = 7.52, Prob > z = 0.00) with an in-person clinical consensus diagnosis after an in-person clinical assessment. To compare the in-person and online consensus diagnosis, we calculated inter-rater agreement measure. A kappa value of 0.75 is generally considered excellent, while values between 0.40 and 0.75 indicate fair to good agreement. The kappa statistic for our validation study was 0.76 with a standard error of 0.10, suggesting excellent agreement. For the cases with inconsistent in-person and online CDR, we further investigated domain-specific score differences and found that significant differences were most frequently observed in the social and community activities and home and hobbies domains. Hence, we further extracted relevant information from the core LASI survey data and uploaded them on the consensus website so that clinicians could more easily reach an agreement.22 We further evaluated the reliability of each clinician by comparing their individual ratings of the LASI-DAD interview data with the in-person clinical consensus diagnosis and found high reliability (kappa statistics ranging from 0.72 to 0.90). To reduce inter-rater differences in reliability, we first asked all interested clinicians to review 10 to 15 cases before inviting a clinician to participate in the online CDR. We compared each clinician’s rating on online platform to in-person clinical assessment and diagnosis and invited only clinicians with high reliability (kappa statistics higher than 0.8) to participate in the online clinical consensus procedure.
The structured questions were part of the LASI-DAD protocol and included the cognitive tests; informant reports of respondent’s cognitive status; demographics, such as age, sex, educational attainment, and occupation (see TableS4 in supporting information for detailed information); and health history, including medical comorbidity and sensory impairment. Clinicians evaluated whether cognitive state or other health conditions drove functional deficits based on all three sources of information: cognitive test performance, informant report of respondent’s cognitive state, and the presence or absence of medical morbidity and sensory impairment. When respondents had no known medical morbidity or sensory impairment, clinicians solely attributed functional deficits to cognitive state. When respondents had medical morbidity or sensory impairment, clinicians had to speculate whether cognitive state or another health condition drove functional deficits, and in such cases, clinicians weighed in informant reports. In particular, when sensory impairment influenced respondent’s cognitive test performance (in 19 cases in which respondents were unable to complete cognitive tests due to severe hearing loss), clinicians’ evaluation of cognitive status was solely based on informant reports.
Non-clinician interviewers were college graduates who majored in psychology, public health, or nursing; were fluent in English and one of 12 local languages; and competent in computer usage. They received 2 weeks of intensive training, and only those who met the clinical research team’s standards of competency for respondent and informant interview skills were certified and allowed to conduct the interviews. Non-clinician interviewers were asked to strictly follow the structured interview protocol and prohibited from asking additional questions for clarification in case of conflicting information, for example, when informant reports differed from respondent reports. While this strict protocol ensures the inter-interviewer reliability, it does not prevent ambiguity that could have been avoided if an expert clinician had performed the in-person clinical assessment.
The study’s expert clinicians undertook online CDR training and certification on the Washington University in St. Louis website (https://knightadrc.wustl.edu/overview-cdr-training-public-a-full/). Clinicians learned to score each of the six domains, and the global CDR score was obtained by applying the CDR scoring algorithm (https://knightadrc.wustl.edu/professionals-clinicians/cdr-dementia-staging-instrument/cdr-scoring-algorithm/).19 According to the CDR scoring rules, clinicians were trained to use all information available and make the best judgment.19 Particularly, each clinician was trained to make an educated rating rooted in their local cultural context. These clinicians, with expertise in geriatrics, neuropsychology, and psychiatry, were located at the AIIMS in New Delhi and NIMHANS in Bengaluru. Using an online platform, standardized data collected by trained interviewers were uploaded and presented to the expert clinicians to rate respondents on the CDR scale.19
Three to four CDR-certified clinicians independently rated each case on each CDR domain after reviewing the available information. After the initial assessment, cases in which the individual ratings differed across clinicians were discussed through a virtual consensus conference with the goal of achieving a consensus rating. If a consensus was not reached, a majority rating was recorded with a flag indicating the persistent differences in CDR. Out of the 2528 cases reviewed, 570 cases (22.6%) were discussed to resolve differences across individual CDR ratings. Out of the discussed cases, clinicians reached full consensus for 80.9% (461 cases) and partial consensus for 17.7% (101 cases). For eight cases (1.4% of 570 discussed cases or 0.3% of 2528 reviewed cases), no consensus was reached; all such cases involved a discrepancy between a CDR of 0 versus 0.5. On average, an individual rating took about 4 minutes. Resolving discrepant cases took about 6 minutes on a virtual consensus conference call, on average.
2.3 ∣. Imputation model
We fitted logistic regression models on the subset of LASI-DAD participants with a CDR (N = 2528) to probabilistically predict dementia status for all LASI respondents aged 60 and older without a clinical dementia classification (N = 28,949). We used the estimated models to impute dementia status for each participant without a CDR. Imputations have many desirable statistical properties.23,24 They do not ignore valuable information and provide consistent and efficient estimators. Moreover, we conducted multiple imputations (20 imputations per individual) to ensure that standard errors accurately reflect the uncertainty due to missingness.
The dependent variable in our models is a binary indicator of dementia, operationalized as a CDR total score of 1 or higher. The amount of information available varied across respondents, depending on whether the individual participated in LASI-DAD (where study participants underwent a more extensive cognitive assessment than those who only participated in the core LASI survey) and on whether the core interview was conducted with the individuals themselves (self-report and cognitive tests) or with an informant (proxy interview, without cognitive tests but with the Informant Questionnaire on Cognitive Decline in the Elderly). Specifically, out of 28,949 total imputed cases, 1568 individuals participated in the LASI-DAD interview; 26,883 individuals participated in the core LASI survey; and for 498 individuals, only the LASI core proxy interview was completed. Therefore, we used different model specifications to impute different subsamples. Depending on availability, the regressors in the models included cognition scores from the LASI-DAD and/or LASI core data; informant reports from the LASI-DAD and/or LASI core data; demographics (e.g., age, sex, education, etc.); and various physical health and disability measures, such as activities of daily living, and instrumental activities of daily living. See S5 Methods in supporting information for further details about the imputation procedure and for a robustness check that compares the results using the full, imputed sample to the results using only the subsample with observed dementia status based on the clinical consensus rating.
2.4 ∣. Prevalence estimation
The estimated prevalence rate (for the country, state, or subgroup) is the weighted mean of the (imputed) dementia status (for the relevant subsample; N = 31,477). Sampling weights (included in the data) ensure representativeness of the sample relative to the country and separately by state. Because dementia prevalence is strongly related to age, prevalence estimates for different populations are often difficult to compare if their age structure is different. Therefore, we also computed an age-standardized prevalence for India as a whole and by state, using the world standard population (i.e., World Health Organization reference age distribution for the period of 2000–2025).25
We assessed the population implications of our results by multiplying estimated prevalence rates by the number of adults aged 60 and older living in India and in each Indian state.26 We also estimated separate prevalence rates by age (60–64, 65–69, 70–74, 75–79, 80–84, and 85 years and older), sex (male vs. female), urbanicity (urban vs. rural), education (none, primary school or less, and middle school or more), and caste (scheduled caste, scheduled tribe, other backward group, and no or other caste). To investigate state-level patterns in prevalence we constructed heatmaps of India. To investigate multivariate relationships, we also estimated a logistic regression model for dementia status using the individual-level characteristics defined previously and state-level fixed effects as joint covariates. Analyses were performed in Stata versions 15.1 and 16.1. A P-value of 0.05 was considered statistically significant.
2.5 ∣. Ethics
We obtained ethics approval from the Indian Council of Medical Research and all collaborating institutions including the University of Southern California; the AIIMS, New Delhi; the International Institute of Population Sciences, Mumbai; the Harvard T.H. Chan School of Public Health; the University of Michigan; the AIIMS, Bhubaneshwar; Dr. Sampurnanand Medical College, Jodhpur; Government Medical College, Thiruvananthapuram; Grant Medical College and J.J. Hospital, Mumbai; Guwahati Medical College, Guwahati; the Institute of Medical Sciences, Banaras Hindu University, Varanasi; Madras Medical College, Chennai; Medical College, Kolkata; the NIMHANS, Bengaluru; Nizam’s Institute of Medical Sciences, Hyderabad; the Sher-e-Kashmir Institute of Medical Sciences, Srinagar; the Indira Gandhi Institute of Medical Sciences, Patna; the All India Institute of Medical Sciences, Rishikesh; and Government Medical College, Chandigarh. Written consents were obtained from each participating respondent and informant in the form of signature or thumb print.
3 ∣. RESULTS
Table 1 presents sample characteristics and dementia prevalence among individuals aged 60 and older. Our estimated dementia prevalence among individuals aged 60 and older in India is 7.4% (95% confidence interval [CI], 6.4 to 8.5), and age-standardized dementia prevalence is 8.0% (95% CI, 6.8 to 9.2). Columns 2 and 3 of Table 1 show the sample frequency (unweighted counts) and the weighted proportions of different demographic groups. Particularly noteworthy is the large fraction of individuals without any formal education (60%), which is a known correlate of dementia. Table 1 reveals large differences across population subgroups. As expected, a strong age gradient is evident, with prevalence sharply increasing with age. Prevalence among women is almost double that among men. It is also much higher in rural than in urban areas. Finally, dementia is considerably more prevalent among individuals with lower education.
TABLE 1.
Sample characteristics and dementia prevalence among adults 60 years and older in India
| LASI ages 60+ |
Dementia prevalencec |
|||||
|---|---|---|---|---|---|---|
| Na | %b | Population- representative |
95% CI | Age-standardized | 95% CI | |
| ALL | 31,477 | 100.0 | 7.43 | (6.35 to 8.51) | 8.01 | (6.83 to 9.18) |
| Age | ||||||
| 60–64 | 10,134 | 32.4 | 2.94 | (2.08 to 3.80) | n.a. | n.a. |
| 65–69 | 8845 | 29.4 | 4.01 | (2.93 to 5.09) | n.a. | n.a. |
| 70–74 | 5746 | 17.6 | 10.30 | (8.14 to 12.47) | n.a. | n.a. |
| 75–79 | 3362 | 10.3 | 13.34 | (10.93 to 15.75) | n.a. | n.a. |
| 80–84 | 1942 | 5.8 | 16.25 | (11.31 to 21.19) | n.a. | n.a. |
| 85+ | 1448 | 4.6 | 25.41 | (19.31 to 31.51) | n.a. | n.a. |
| Sex | ||||||
| Male | 15,106 | 49.2 | 5.77 | (4.40 to 7.14) | 6.30 | (4.81 to 7.79) |
| Female | 16,371 | 50.8 | 9.03 | (7.50 to 10.57) | 9.63 | (8.01 to 11.26) |
| Urbanicity | ||||||
| Urban | 10,756 | 30.7 | 5.34 | (4.32 to 6.37) | 5.98 | (4.80 to 7.16) |
| Rural | 20,721 | 69.4 | 8.35 | (7.08 to 9.61) | 8.91 | (7.58 to 10.24) |
| Education | ||||||
| None | 16,894 | 60.3 | 10.29 | (8.62 to 11.97) | 10.48 | (8.79 to 12.17) |
| Primary school or less (up to standard 7) | 7562 | 20.3 | 4.52 | (3.28 to 5.77) | 5.05 | (3.65 to 6.45) |
| Middle school or more (standard 8 and higher) | 7021 | 19.4 | 1.54 | (0.64 to 2.44) | 2.35 | (1.13 to 3.57) |
Abbreviations: CI, confidence interval; LASI, Longitudinal Aging Study in India; n.a., not applicable.
Refers to unweighted sample.
Values are weighted using survey weights to represent the population.
Survey weights are also applied to estimate the prevalence rate for the population.
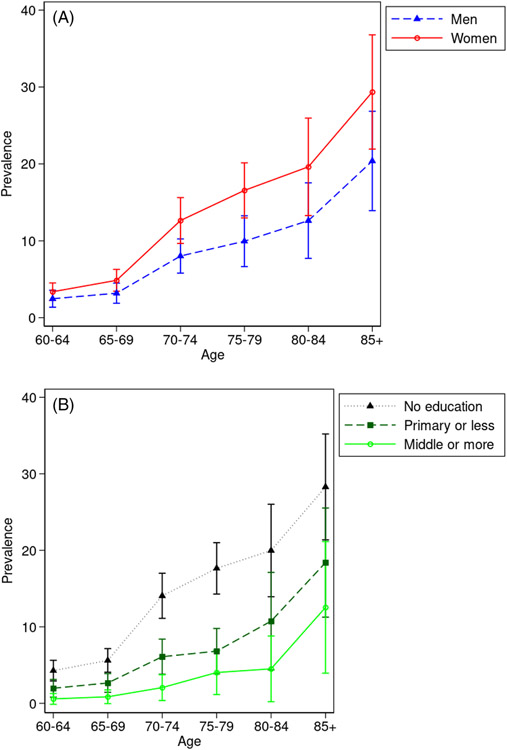
Figure 2 shows the age-specific prevalence among individuals with different demographic characteristics. Figure 2A shows that the sex gap in prevalence appears to increase with age, although the CIs are wide and therefore the differences, while meaningful in magnitude, are not statistically significant. The prevalence differences by education are large (Figure 2B). Individuals without formal education have much higher dementia prevalence relative to their more educated counterparts. This difference is especially apparent in the 70 to 84 age range and statistically significant within the 70 to 74 and 75 to 79 age groups. At older ages, differences remain large, but are not statistically significant due to larger standard errors.
FIGURE 2.
Age-specific dementia prevalence rates by sex, urbanity, and education. A, Mean age-specific dementia prevalence rate by sex (95% confidence interval [CI]). B, Mean age-specific dementia prevalence rate by education (95% CI)
Table 2 presents estimates of dementia prevalence by state or union territory. Cross-state variation in dementia prevalence is considerable, with lowest prevalence in Delhi at 4.5% (95% CI, 1.9 to 7.1) and highest in Jammu and Kashmir at 11.0% (95% CI, 7.3 to 14.8). The last two columns multiply these prevalence rates with population projections from the Indian government for 2016 (the year closest to the survey dates for which projections are available) and 2036.26 For India as a whole, this calculation suggests an increase from 8.8 million individuals aged 60 and older with dementia in 2016 to 16.9 million in 2036.
TABLE 2.
Estimated dementia prevalence and number of persons with dementia by selected states and union territories
| State/territory | Projected 60+ population in 2016 (×1000)a |
Estimated prevalence in 2017—2019c |
95% CI | Estimated/projected no. of persons with dementia (×1000) |
|
|---|---|---|---|---|---|
| 2016 | 2036 | ||||
| Jammu & Kashmir | 1047 | 11.04 | (7.27 to 14.82) | 116 | 253 |
| Himachal Pradesh | 829 | 8.43 | (4.71 to 12.15) | 70 | 129 |
| Punjab | 3335 | 5.19 | (3.25 to 7.12) | 173 | 310 |
| Uttarakhand | 1046 | 6.27 | (3.78 to 8.76) | 66 | 121 |
| Haryana | 2509 | 5.78 | (3.37 to 8.20) | 145 | 279 |
| Delhi | 1496 | 4.50 | (1.88 to 7.12) | 67 | 170 |
| Rajasthan | 5783 | 7.30 | (5.01 to 9.59) | 422 | 847 |
| Uttar Pradesh | 16,658 | 7.92 | (6.04 to 9.80) | 1319 | 2430 |
| Bihar | 8096 | 5.69 | (4.21 to 7.18) | 461 | 922 |
| Assam | 2367 | 8.47 | (5.76 to 11.18) | 201 | 456 |
| West Bengal | 9232 | 9.23 | (6.88 to 11.58) | 852 | 1734 |
| Jharkhand | 2704 | 7.17 | (4.60 to 9.75) | 194 | 398 |
| Odisha | 4522 | 9.87 | (7.23 to 12.51) | 446 | 824 |
| Chhattisgarh | 2210 | 6.96 | (4.49 to 9.44) | 154 | 317 |
| Madhya Pradesh | 6168 | 6.75 | (4.47 to 9.03) | 416 | 844 |
| Gujarat | 5836 | 6.47 | (4.10 to 8.83) | 377 | 812 |
| Maharashtra | 12,726 | 7.61 | (5.68 to 9.55) | 969 | 1780 |
| Andhra Pradesh | 5759 | 7.74 | (4.92 to 10.57) | 446 | 795 |
| Karnataka | 6640 | 7.61 | (4.38 to 10.83) | 505 | 941 |
| Kerala | 5011 | 8.27 | (5.66 to 10.88) | 414 | 696 |
| Tamil Nadu | 8923 | 6.13 | (4.03 to 8.23) | 547 | 996 |
| Telangana | 3675 | 8.27 | (6.15 to 10.40) | 304 | 558 |
| NE states excl. Assamb | 1124 | 7.35 | (5.29 to 9.41) | 83 | 199 |
| India | 118,185 | 7.43 | (6.35 to 8.51) | 8778 | 16,892 |
Source: Census of India 2011.26 Population projection information is not available from the Census for the following states and union territories: Chandigarh, Daman & Diu, Dadra & Nagar Haveli, Goa, Lakshadweep, Puducherry, Andaman & Nicobar.
Population size includes Sikkim, but the dementia prevalence rate estimate does not.
Author calculated based on LASI and LASI-DAD.
Abbreviations: LASI, Longitudinal Aging Study in India; LASI-DAD, Harmonized Diagnostic Assessment of Dementia for Longitudinal Aging Study in India.
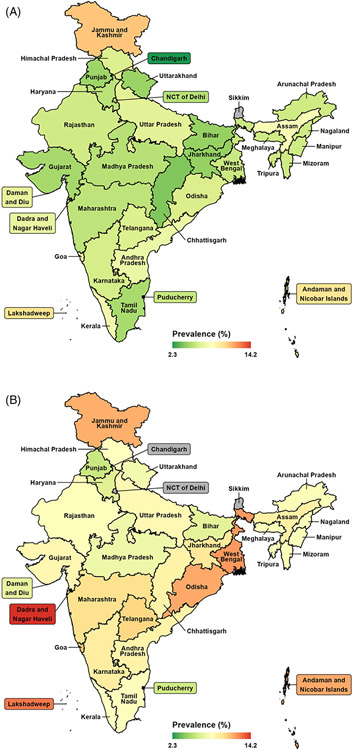
The estimated age-standardized dementia prevalence for individuals aged 60 and older in India is 8.0% (95% CI, 6.8 to 9.2), which differs only slightly from the non–age-standardized estimate (Table 1). The age-standardized prevalence is 6.3% (95% CI, 4.8 to 7.8) for men and 9.6% (95% CI, 8.0 to 11.3) for women. Figure 3 shows a map of India in which the colors represent the age-standardized prevalence rate per state or union territory by urbanicity. This map illustrates the substantial cross-state heterogeneity in prevalence rates in rural and urban areas. The estimates by state and the differences across states resemble those that were not age standardized (see TableS6 in the supporting information for age-standardized dementia prevalence by state). This suggests that the substantial cross-state heterogeneity in prevalence rates is not due to differences in age distributions.
FIGURE 3.
Age-standardized dementia prevalence among adults 60 years and older in the states of India, by (A) urban and (B) rural. Notes: No data for Sikkim; Delhi and Chandigarh are city-states that are completely urban. Age-standardized based on the World Health Organization reference age distribution for the period 2000–202525
We conducted a multivariable logistic regression analysis that confirmed the most salient patterns described in Table 1 (Table S7 in supporting information shows the results). We find that the risk of dementia appears to be strongly related to age and education. The odds ratios of other demographics tend to be statistically insignificant. Most strikingly, the odds ratio forfemale sex is fairly modest and statistically insignificant 1.2 (95% CI, 0.9 to 1.6), suggesting that the large sex difference in prevalence reported in Table 1 is likely attributable to females being older and less educated. Also, the coefficients of most state indicator variables are no longer statistically significant after adjustment for individual demographics. TableS8 in supporting information investigates whether sex and age interact and finds that all the interaction effects are small compared to the corresponding main age effects and that their odds ratios are not statistically significantly different from 1.
4 ∣. DISCUSSION
Using nationally representative data collected in India between 2017 and 2020, we found that an estimated 7.4% of people aged 60 years and older lived with dementia (8.8 million individuals). Dementia prevalence was higher among females than males (9.0% vs. 5.8%) and higher in rural than in urban areas (8.4% vs. 5.3%). We found substantial variation across states and territories, but cross-state differences in sociodemographic characteristics drive most of this variation. In a logistic regression model that controls for demographic characteristics, most odds ratios were not statistically different from 1, with age and education being the main exceptions. Different levels of educational attainment across states could contribute to cross-state differences in various dementia risk factors, such as under-nutrition, uncontrolled cardiovascular disease, and exposure to indoor air pollution. If prevalence stays the same, the number of people with dementia is projected to reach 16.9 million in 2036 due to the growth in the older Indian population. Considering the recent decline in dementia incidence in Europe and North America, this may be an overestimate,27 but the rapid rise in risk factors for cardiovascular disease in India28 may increase dementia risk.
Our state- and urbanicity-specific estimates are close to the estimate (within the 95% CI) from prior studies in urban Maharashtra29 and Kerala2 and in rural Tamil Nadu,30 but higher than other prior studies in urban Kerala31 and West Bengal32,33 and rural Haryana34 and Uttar Pradesh.35 Table 3 presents dementia prevalence estimates from prior studies, alongside information about where the study sample was drawn from, the criteria used for dementia diagnosis, and whether prior studies’ prevalence estimates are statistically different from our state- and urbanicity-specific estimates. The differences in prevalence estimates may stem from differences in the dementia ascertainment methods, the year of data collection, or the sampling frame. For example, for Tamil Nadu, Rodriguez et al.’s36 prevalence estimates based on 10/66 criteria are slightly higher than our estimates although they are within the 95% CI, but their estimates based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria43 are significantly lower than our estimates. Two large-scale urban studies, Vas et al.29 in Maharashtra and Banerjee et al.33 in West Bengal both used DSM-IV criteria, and Vas et al.’s estimates are within the 95% CI of our estimates, while Banerjee et al.’s estimates are significantly lower than ours. It is important to note that Banerjee et al. took a two-step approach. First, they screened individuals based on an informant’s report of memory decline and behavioral change in the past year, and then applied DSM-IV criteria to adults 50 years and older who were screened positive. In contrast, Vas et al.’s and our study took a one-step approach and conducted the same detailed assessment on all participants. Because informants tend to underreport memory decline and behavioral change,45 the two-step approach might have contributed to underestimate dementia prevalence. For urban Kerala, Shaji et al.’s31 2005 estimate for the sample from Emakulam is significantly lower than our estimate, but Mathuranath et al.’s37 2010 estimate for the sample from Trivandrum is within the 95% CI of our estimate. Both year of data collection and sample frame could have contributed to differences in prevalence estimates.
TABLE 3.
Comparison of state- and urbanicity-specific estimates of dementia prevalence to those of prior studies
| Authors | Year | Geographic location, state |
Age | Sample size |
Diagnostic criteria |
Prevalence | 95% CI | Diff (P < 0.05) |
|---|---|---|---|---|---|---|---|---|
| Urban | ||||||||
| Vas et al.29 | 2001 | Mumbai, Maharashtra | 65+ | 24,488 | DSM-IV | 2.3 | 1.8–2.8 | |
| Shaji et al.31 | 2005 | Ernakulam, Kerala | 65+ | 1934 | DSM-IV | 3.4 | 2.7–4.1 | * |
| Das et al.32 | 2006 | Kolkata, West Bengal | 60+ | 5430 | DSM-IV | 1.0 | 0.8–1.3 | * |
| Rodriguez et al.36 | 2008 | Chennai, Tamil Nadu | 65+ | 1005 | DSM-IV | 0.9 | 0.3–1.5 | * |
| 10/66 dementia | 7.5 | 5.8–9.1 | ||||||
| Mathuranath et al.37 | 2010 | Trivandrum, Kerala | 65+ | 1672 | DSM-IV | 4.9 | 3.8–5.9 | |
| Seby etal.38 | 2011 | Pune, Maharashtra | 65+ | 202 | ICD-10 | 14.9 | NR | |
| Banerjee et al.33 | 2017 | Kolkata, West Bengal | 50+ | 17,584 | DSM-IV | 1.3 | 1.1–1.6 | * |
| LASI | 2017–2020 | All India | 60+ | 10,756 | CDR>=1 | 5.3 | 4.3–6.4 | |
| Kerala | 60+ | 570 | CDR>=1 | 7.8 | 4.6–11.0 | |||
| Maharashtra | 60+ | 848 | CDR>=1 | 5.0 | 2.7–7.4 | |||
| Tamil Nadu | 60+ | 871 | CDR>=1 | 4.1 | 2.3–6.0 | |||
| West Bengal | 60+ | 776 | CDR>=1 | 5.6 | 3.6–7.7 | |||
| Rural | ||||||||
| Shaji et al.39 | 1996 | Ernakulam, Kerala | 60+ | 2067 | DSM-IIIR | 3.2 | Not Reported | |
| Rajkumar et al.30 | 1997 | Thiroporur, Tamil Nadu | 60+ | 750 | ICD-10 | 3.5 | 2.2–4.8 | |
| Chandra et al.34 | 1998 | Ballabgarh, Haryana | 65+ | 3869 | DSM-IV, CDR > = 0.5 | 1.4 | 1.0–1.9 | * |
| Rodriguez et al.36 | 2008 | Vellore, Tamil Nadu | 65+ | 999 | DSM-IV | 0.8 | 0.2–1.3 | * |
| 10/66 dementia | 10.6 | 8.6–12.6 | ||||||
| Tiwari et al.35 | 2013 | Lucknow, Uttar Pradesh | 60+ | 2146 | ICD-10 | 2.8 | 2.0–3.9 | * |
| Gurukartick et al.40 | 2016 | Villupuram district, Tamil Nadu | 65+ | 1300 | DSM-IV | 3.1 | NR | |
| LASI | 2017–2020 | All India | 60+ | 20,721 | CDR>=1 | 8.4 | 7.1–9.6 | |
| Haryana | 60+ | 600 | CDR>=1 | 6.2 | 3.5–8.8 | |||
| Kerala | 60+ | 640 | CDR>=1 | 8.7 | 5.0–12.4 | |||
| Tamil Nadu | 60+ | 663 | CDR>=1 | 7.8 | 4.6–11.0 | |||
| Uttar Pradesh | 60+ | 1740 | CDR>=1 | 8.4 | 6.2–10.7 |
Note:
Indicates prior study’s estimate is outside of the 95% CI of the state- and urbanicity-specific estimates from LASI.
Abbreviations: CDR, Clinical Dementia Rating;19 CI, confidence interval; DSM-IV, the Diagnostic and Statistical Manual of Mental Disorders, 4th edition;43 DSM-IIIR, Psychiatry Diagnostic & Statistical Manual of Mental Disorders, 3rd edition Revised;41 ICD-10, International Classification of Disease, the 10th revision;42 LASI, Longitudinal Aging Study in India; NR, not reported; 10/66 dementia, the 10/66 dementia algorithm.44
Also noteworthy is that our national-level dementia prevalence estimate, based on the entire LASI sample using imputations of dementia status for those for whom this was not observed, yielded the same point estimate of 7.4% as a machine learning study using only the LASI-DAD sample.46 Our age-standardized dementia prevalence (8.0% [95% CI, 6.8 to 9.2] for ages 60 years and older) also resembles dementia prevalence estimates in the United States (8.6% [95% CI, 8.1 to 9.3] for ages 65 years and older)47 and the UK (7.1% [95% CI, 6.3 to 8.1] for ages 65 and older).48
The first and main limitation of our study is that only 13% of the LASI sample aged 60 and older participated in the LASI-DAD study. The LASI-DAD sample provides national-level representation but does not provide state-level representation, and therefore our state-level prevalence estimates are based on the imputed probability estimates for the LASI sample. Although the cognitive test items, informant reports, and other covariates enabled us to impute the probability of dementia for all LASI respondents, this yields substantial standard errors.
Second, among the LASI-DAD respondents, modified CDR ratings were available for 61.7%; for the remaining one third of the sample, dementia status was also imputed, although considerably more information about individuals’ cognitive status was available compared to the LASI sample without the LASI-DAD interview. Not all LASI-DAD participants were assessed in the online consensus procedure because the original LASI-DAD cognitive test battery and informant report lacked enough information to adequately assess the judgment and problem-solving domains, with additional questions on those domains added in the later phases of the fieldwork.
Third, our dementia consensus rating was drawn from clinicians’ adjudication of the information collected from the LASI-DAD interview, without examining the respondents in person and without independently confirmed medical/health information. While the validity of our dementia consensus rating relative to an in-person dementia diagnosis was established,22 in some cases clinicians were unsure and could not reach conclusive decisions or consensus, especially when trying to distinguish between poor cognitive test performance due to lack of schooling and/or hearing impairment and mild cognitive impairment (CDR = 0 vs. 0.5). Such uncertainty likely led to potential overestimation of questionable dementia (CDR = −0.5), as the CDR guideline suggested clinicians to signify such potential, and therefore, interpreting CDR = 0.5 calls for caution. Expert clinicians can and often do disagree on this aspect of clinical rating with a given patient in a clinical setting as well, especially when trying to differentiate between normal and questionable dementia (CDR = 0 vs. 0.5).49 Even in the usual clinical setting, all an expert clinician can do is take the lack of schooling and hearing loss into account when interpreting a patient’s cognitive test performance. Hence, this is a more general issue that does not only pertain to the LASI-DAD study. But this kind of disagreement was uncommon for cases of dementia (CDR > = 1), providing a firmer ground for clinical assessment of dementia.
Our study illustrates the benefits and challenges of conducting quasi-clinical assessments of older adults in a nationwide representative sample as opposed to a community setting in a low- and middle-income country. The advantage of sampling from the population is that our study sample is not biased by selection factors that lead individuals to seek care in clinical settings, particularly for memory loss, which may be considered normative in older adults. Another benefit is that individuals are more likely to agree to participate if not required to travel, and more relaxed and comfortable in their own homes, reducing selection and performance-related biases. The challenge is that expert clinicians cannot be deployed in large numbers to travel to remote areas and perform assessments. This is particularly problematic in the context of India, where expert clinicians are also likely to face significant language barriers. Our approach represents a feasible and cost-effective way to assess individuals in a large-scale, representative study, via a predefined protocol strictly followed by trained interviewers, and the remote adjudication of individual cases by experts with full access to the collected data. While this classification will never be as accurate as a clinical, in-person diagnosis, it constitutes a significant step forward in obtaining a reliable assessment of cognitively impaired and dementia cases in a large, representative sample in a low- and middle-income country. It should also be noted that uncertain cases are likely to arise even in clinical settings, and are not necessarily a by-product of the approach we followed in this study. As in clinical settings, the validation of the research classification will come from follow-up re-assessment. Continuing to follow this cohort over time will allow us to validate our initial dementia ratings in prevalent cases, in addition to identifying newly developed incident cases. With technological advancement, telemedicine has emerged as an alternative to in-person assessment and presents a potential avenue for experts’ dementia ascertainment for future epidemiological studies of dementia. However, although virtual set-up can be arranged, linguistic diversity continues to be a significant barrier for a nationwide study in India.
In 2010, the Alzheimer’s and Related Disorders Society of India estimated that 3.7 million Indians had dementia and projected that this number would double by 2030.5 Our findings suggest this might have been an underestimate. The number doubled a decade earlier, reaching 8.8 million in 2019. Therefore, the need to scale up policies to prevent and manage dementia in India is urgent. We also found significant heterogeneity across states. This means that the burden of dementia cases is unevenly distributed across states and requires different levels of local planning and support.
As the current paper focuses on all-cause dementia for adults ages 60 and older, future research is needed to better assess early-onset dementia and subtypes of dementia. A top priority for future dementia research in India is a nationwide study of dementia incidence and its association with risk factors. Further, eliminating diagnostic uncertainty calls for future epidemiological study of dementia. In addition to bringing expert clinicians’ dementia adjudication through telemedicine as discussed earlier, algorithmic classification of dementia based on neuropsychological test performance and informant reports, which has been used in other epidemiological studies of dementia,50 is an alternative approach that can be adopted. Through follow-up repeated assessment of cognitive and functional status, the validity of alternative approaches to dementia ascertainment can be and needs to be evaluated.
The current recommendation for dementia prevention is based on evidence predominantly from high-income countries; however, the strength of association between risk factors and dementia may differ in low- and middle-income countries.51 A reliable assessment of cognitively impaired and dementia cases for India is a necessary condition to analyze these relationships in a large and rapidly aging low- and middle-income country. Our preliminary investigation based on cross-sectional data confirmed the existence of a strong association between education and dementia, consistent with prior literature.52 Lack of education is known to be significantly associated with several other risk factors, such as uncontrolled cardiovascular disease, so further investigation accounting for potential confounding factors is required to better understand the relationship between education and dementia.
Supplementary Material
Highlights.
The estimated dementia prevalence for adults ages 60+ in India is 7.4%.
About 8.8 million Indians older than 60 years live with dementia.
Dementia is more prevalent among females than males and in rural than urban areas.
Significant cross-state variation exists in dementia prevalence.
RESEARCH IN CONTEXT.
Systematic Review: We searched PubMed and the Alzheimer’s and Related Disorders Society of India website on August 17, 2020, and January 17, 2022, for any studies investigating prevalence of dementia in India using the search terms “dementia” and “India,” with no limits on language and date of publication.
Interpretation: We conducted a nationally representative study of late-life cognition and dementia in India and estimated 7.4% of people aged 60 years and older lived with dementia (8.8 million individuals). Dementia prevalence was higher among females than males and higher in rural than in urban areas. We found substantial variation across states, and cross-state differences in sociodemographic characteristics drive most of this variation.
Future Directions: Socioeconomic implications of dementia call for research attention. Given substantial heterogeneity in dementia prevalence across subpopulations, burden of dementia might be distributed unequally. Newly available epidemiological data offer opportunities to study risk factors for dementia.
ACKNOWLEDGMENTS
This work and LASI-DAD data collection were supported by the National Institute on Aging, National Institutes of Health (R01AG051125: J.L., E.M.C., M.A., D.E.B., K.M.L., A.B.D., M.V., U01AG065958: J.L., A.B.D.). LASI Data collection was supported by the National Institute on Aging, National Institutes of Health (R01AG042778: D.E.B., J.L., T.V.S.) and the Ministry of Health and Family Welfare, Government of India (T22011/02/2015-NCD: T.V.S., D.E.B., J.L.). LASI data processing of LASI was supported by the National Institute on Aging, National Institutes of Health (R01AG030153: J.L., E.M., M.A.). The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had final responsibility for the decision to submit for publication.
Footnotes
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest. Author disclosures are available in the supporting information.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
International Institute for Population Sciences, Harvard T.H. Chan School of Public Health, and the University of Southern California. Longitudinal Aging Study in India (LASI) Wave 1, 2017–2019, Gateway to Global Aging Data, 2020, https://g2aging.org. Jinkook Lee and A.B. Dey. Harmonized Diagnostic Assessment of Dementia for the Longitudinal Aging Study in India (LASI-DAD), Wave 1, 2018–2020, Gateway to Global Aging Data, 2020, https://g2aging.org.
REFERENCES
- 1.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results. UN DESA/POP/2022/TR/NO.3. New York: United Nations, New York; 2022. Accessed September 3, 2020. https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022 [Google Scholar]
- 2.International Institute for Population Sciences (IIPS), NPHCE, MoHFW, Harvard T.H. Chan School of Public Health (HSPH), and the University of Southern California (USC). Longitudinal Ageing Study in India (LASI) Wave 1, 2017-18, India Report. International Institute for Population Sciences; 2020. [Google Scholar]
- 3.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019 United Nations; 2020. Accessed September 3, 2020. https://population.un.org/wpp/DataQuery/ [Google Scholar]
- 4.Van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76(5):v2. doi: 10.1136/jnnp.2005.082867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar CTS, Shaji KS, Varghese M, Nair MKC, eds. Cochin, India: Dementia in India 2020. Alzheimer’s and Related Disorders Society of India (ARDSI), Cochin Chapter; 2019. [Google Scholar]
- 6.Prince M, Wimo A, Guerchet M, et al. World Alzheimer Report 2015, The Global Impact of Dementia. Alzheimer’s Disease International; 2015. [Google Scholar]
- 7.Shaji KS, Jotheeswaran AT, Girish N, et al. (eds.) The Dementia India Report: Prevalence, Impact, Costs and Services for Dementia. Alzheimer’s and Related Disorders Society of India; 2010. [Google Scholar]
- 8.Farina N, Ibnidris A, Alladi S, et al. A systematic review and meta-analysis of dementia prevalence in seven developing countries: a STRiDE project. Glob Public Health. 2020;15(12):1878–1893. doi: 10.1080/17441692.2020.1792527 [DOI] [PubMed] [Google Scholar]
- 9.Office of the Registrar General & Census Commissioner, India Ministry of Home Affairs Government of India SRS Based Abridged Life Tables 2014-18 New Delhi; 2020.
- 10.India State-Level Disease Burden Initiative Collaborators. Nations within a nation: variations in epidemiological transition across the states of India, 1990-2016 in the Global Burden of Disease Study. Lancet. 2017;390:2437–2460. doi: 10.1016/s0140-6736(17)32804-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Khobragade PY, Banerjee J, et al. Design and methodology of the Longitudinal Aging Study in India – Diagnostic Assessment of Dementia (LASI-DAD). J Am Geriatr Soc. 2020;68:S5–S10. doi: 10.1111/jgs.16737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganguli M, Ratcliff G, Chandra V, et al. A Hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatry. 1995;10(5):367–377. doi: 10.1002/gps.930100505 [DOI] [Google Scholar]
- 13.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 14.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x [DOI] [PubMed] [Google Scholar]
- 15.Tripathi R, Kumar JK, Bharath S, Marimuthu P, Varghese M. Clinical validity of NIMHANS neuropsychological battery for elderly: a preliminary report. Indian J Psychiatry. 2013;55(3):279–282. doi: 10.4103/0019-5545.117149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Banerjee J, Khobragade PY, et al. LASI-DAD study: a protocol for a prospective cohort study of late-life cognition and dementia in India. BMJ Open. 2019;9:e030300. doi: 10.1136/bmjopen-2019-030300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washington Group on Disability Statistics. Appendix 2: Translation Protocol in Protocols for Implementing Tests of the WG Short Set. Washington Group on Disability Statistics; 2006. Accessed September 3, 2020. https://www.cdc.gov/nchs/data/washington_group/meeting6/appendix2_translation.pdf [Google Scholar]
- 18.Weir DR, Wallace RB, Langa KM, et al. Reducing case ascertainment costs in US population studies of Alzheimer’s disease, dementia, and cognitive impairment-Part 1. Alzheimers Dement. 2011;7(1):94–109. doi: 10.1016/j.jalz.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 20.Dias A, Patel V. Closing the treatment gap for dementia in India. Indian J Psychiatry. 2009;51(l1):S93–S97. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3038542/ [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez OL, Kuller LH, Fitzpatrick A, et al. Evaluation of dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22(1):1–12. doi: 10.1159/000067110 [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Ganguli M, Weerman A, et al. Online clinical consensus diagnosis of dementia: development and validation. J AM Geriatr Soc. 2020;68:S54–S59. doi: 10.1111/jgs.16736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. Wiley; 2002. [Google Scholar]
- 24.Lee J, Meijer E, Phillips D. The Effect of Using Different Imputation Methods for Economic Variables in Aging Surveys. CESR–Schaeffer Working Paper Series, No. 2015-019. University of Southern California; 2015. [Google Scholar]
- 25.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age Standardization of Rates: A New WHO Standard. GPE Discussion Paper Series, No. 31. World Health Organization; 2001. Accessed September 5, 2020. http://www.who.int/healthinfo/paper31.pdf [Google Scholar]
- 26.Census of India, 2011. Population Projects for India and States 2011-2036: Report of the Technical Group on Population Projections; July, 2020. Accessed September 5, 2020. https://main.mohfw.gov.in/sites/default/files/Population%20Projection%20Report%202011-2036%20-%20upload_compressed_0.pdf [Google Scholar]
- 27.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.India State-Level Disease Burden Initiative CVD Collaborators. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health. 2018;6(12):e1339–e1351. doi: 10.1016/S2214-109X(18)30407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vas CJ, Pinto C, Panikker D, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13(4):439–450. doi: 10.1017/S1041610201007852 [DOI] [PubMed] [Google Scholar]
- 30.Rajkumar S, Kumar S, Thara R. Prevalence of dementia in a rural setting: a report from India. Int J Geriatr Psychiatry. 1997;12:702–707.doi: [DOI] [PubMed] [Google Scholar]
- 31.Shaji S, Bose S, Verghese A. Prevalence of dementia in an urban population in Kerala, India. Br J Psychiatry. 2005;186(2):136–140. doi: 10.1192/bjp.186.2.136 [DOI] [PubMed] [Google Scholar]
- 32.Das SK, Biswas A, Roy T, et al. A random sample survey for prevalence of major neurological disorders in Kolkata. Indian J Med Res. 2006;124(2):163–172. https://pubmed.ncbi.nlm.nih.gov/17015930/ [PubMed] [Google Scholar]
- 33.Banerjee TK, Dutta S, Das S, et al. Epidemiology of dementia and its burden in the city of Kolkata, India. Int J Geriatr Psychiatry. 2017;32(6):605–614. doi: 10.1002/gps.4499 [DOI] [PubMed] [Google Scholar]
- 34.Chandra V, Ganguli M, Pandav R, et al. Prevalence of Alzheimer’s disease and other dementias in rural India: the Indo-US study. Neurology. 1998;51(4):1000–1008.doi: 10.1212/WNL.51.4.1000 [DOI] [PubMed] [Google Scholar]
- 35.Tiwari SC, Srivastava G, Tripathi R, et al. Prevalence of psychiatric morbidity amongst the community dwelling rural older adults in northern India. Indian J Med Res. 2013;138(4):504–514. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3868063/ [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez LJ, Ferri C, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372(9637):464–474. doi: 10.1016/S0140-6736(08)61002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathuranath PS, Cherian PJ, Mathew R, et al. Dementia in Kerala, South India: prevalence and influence of age, education and gender. Int J Geriatr Psychiatry. 2010;25(3):290–297. doi: 10.1002/gps.2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seby K, Chaudhury S, Chakraborty R. Prevalence of psychiatric and physical morbidity in an urban geriatric population. Indian J Psychiatry. 2011;53(2):121. doi: 10.4103/0019-5545.82535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaji S, Promodu K, Abraham T, Roy KJ, Verghese A. An epidemiological study of dementia in a rural community in Kerala, India. Br J Psychiatry. 1996;168(6):745–749. doi: 10.1192/bjp.168.6.745 [DOI] [PubMed] [Google Scholar]
- 40.Gurukartick J, Dongre AR, Shah D. Social determinants of dementia and caregivers’ perspectives in the field practice villages of rural Health Training Centre, Thiruvennainallur. Indian Journal of Palliative Care. 2016;22(1):25–32.doi: 10.4103/0973-1075.173952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Revised. American Psychiatric Association; 1987. [Google Scholar]
- 42.World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems. 10th Revision. World Health Organization; 1992. [Google Scholar]
- 43.American Psychiatric Association. DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 44.Prince M, Acosta D, Chiu H, Scazufca M, Varghese M. 10/66 Dementia Research Group. Dementia diagnosis in developing countries: a cross-cultural validation study. Lance. 2003; 361(9361): 909–917. doi: 10.1016/S0140-6736(03)12772-9 [DOI] [PubMed] [Google Scholar]
- 45.Khobragade P, Nichols E, Meijer E, et al. Performance of the Informant Questionnaire on Cognitive Decline for the Elderly (IQCODE) in a nationally representative study in India: the LASI-DAD study. Int Psychogeriatr. 2022:1–11. doi: 10.1017/S1041610222000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin H, Chien S, Meijer E, Khobragade P, Lee J. Learning from clinical consensus diagnosis in India to facilitate automatic classification of dementia: machine learning study. JMIR Ment Health. 2021;8(5):e27113. doi: 10.2196/27113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prince M, Knapp M, Guerchet M, et al. Dementia UK: Update. 2nd ed. Alzheimer’s Society; 2014. [Google Scholar]
- 49.Tractenberg RE, Schafer K, Morris JC. Interobserver disagreements on clinical dementia rating assessment: interpretation and implications for training. Alzheimer Dis Assoc Disord. 2001;15(3):155–161. doi: 10.1097/00002093-200107000-00007 [DOI] [PubMed] [Google Scholar]
- 50.Manly JJ, Jones RN, Langa KM, Lindsay HR, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurology. 2022;79(12):1242–1249. doi: 10.1001/jamaneurol.2022.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019;7(5):e596–603. doi: 10.1016/S2214-109X(19)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
International Institute for Population Sciences, Harvard T.H. Chan School of Public Health, and the University of Southern California. Longitudinal Aging Study in India (LASI) Wave 1, 2017–2019, Gateway to Global Aging Data, 2020, https://g2aging.org. Jinkook Lee and A.B. Dey. Harmonized Diagnostic Assessment of Dementia for the Longitudinal Aging Study in India (LASI-DAD), Wave 1, 2018–2020, Gateway to Global Aging Data, 2020, https://g2aging.org.