Abstract
Either optic neuritis (neuropathy) or hypopituitarism has been known to occur separately after COVID-19 vaccination. In this report, we describe the rare combination of hypophysitis and optic neuritis which occurred after COVID-19 vaccination. A 74-year-old woman began to have thirst, polydipsia, and polyuria, and was diagnosed as central diabetes insipidus 1 month after the fourth COVID-19 mRNA vaccine. Head magnetic resonance imaging (MRI) disclosed the thickened pituitary stalk and enlarged pituitary gland with high contrast enhancement as well as the absence of high-intensity signals in the posterior pituitary lobe on the T1-weighted image, leading to the diagnosis of lymphocytic hypophysitis. She was well with desmopressin nasal spray until further 2 months later, when she developed bilateral optic neuritis, together with gait disturbance, intention tremor of the upper extremities, urinary retention, constipation, abnormal sensation in the distal part of the lower extremities, and moderate hemiplegia on the left side. Autoantibodies, including anti-aquaporin 4 (AQP4) and anti-myelin oligodendrocyte glycoprotein (MOG), were all negative. She showed multifocal spinal cord lesions on MRI and oligoclonal bands in the cerebrospinal fluid obtained by spinal tap, and so underwent steroid pulse therapy with methylprednisolone in the tentative diagnosis of multiple sclerosis, resulting in visual acuity recovery and alleviation of neurological symptoms. In the literature review, the combination of optic neuritis and hypophysitis, mostly with diabetes insipidus, was reported in 15 patients as case reports before the years of COVID-19 pandemic. The COVID-19 vaccination would trigger the onset of hypophysitis and optic neuritis in this patient.
Keywords: COVID-19 mRNA vaccine, lymphocytic hypophysitis, diabetes insipidus, optic neuritis, multiple sclerosis
Background
A great number of people worldwide have undergone vaccines for COVID-19, and the adverse events in the acute phase which occur in a week or so have been well reviewed. In contrast, the adverse events in the subacute phase which occur in a month or so are difficult to interpret whether they are related with the vaccination. Autoimmune diseases 1 and cardiovascular events 2 appear to be 2 major categories which occur in the subacute phase after the COVID-19 vaccination. In the field of ophthalmology, vaccine-associated uveitis has been known to occur after different types of vaccines for several diseases including COVID-19. 3 Unexpected retinal and vitreous hemorrhage are categorized as cardiovascular events in the subacute phase after the COVID-19 vaccination. 4
Optic neuritis or neuropathy is a clinical entity with immunological background which occurs in isolation or as a manifestation of multiple sclerosis or neuromyelitis optica spectrum disorder. In the acute phase within a week or in the subacute phase within a month or so after the COVID-19 vaccination, optic neuritis or neuropathy has been reported to occur as adverse events. 5 Hypopituitarism has been also noted after the infection of COVID-19 and vaccinations for COVID-19.6-9 In particular, hypophysitis in association with COVID-19 vaccination is presumed to have immunological background such as autoimmune reactions. 9 In this study, we reported a patient who developed bilateral optic neuritis and hypophysitis with central diabetes insipidus after COVID-19 mRNA vaccine inoculation. We also reviewed previous cases who showed the association of optic neuritis and central diabetes insipidus in the literature.
Case Report
A 74-year-old woman began to have thirst, polydipsia and polyuria, and was diagnosed as central diabetes insipidus in a local hospital. One month prior to the onset of symptoms, she had undergone the fourth inoculation of COVID-19 mRNA vaccine (elasomeran, Moderna) 8 months after the third vaccination. She had finished the first and second inoculations of COVID-19 mRNA vaccine (tozinameran, Pfizer-BioNTech) in the internal of 3 weeks and the third inoculation with the same vaccine in the interval of 8 months after the second vaccination. In the past history, she had been diagnosed tentatively as myelodysplastic syndrome 10 years previously and had been taking a stable course with observation.
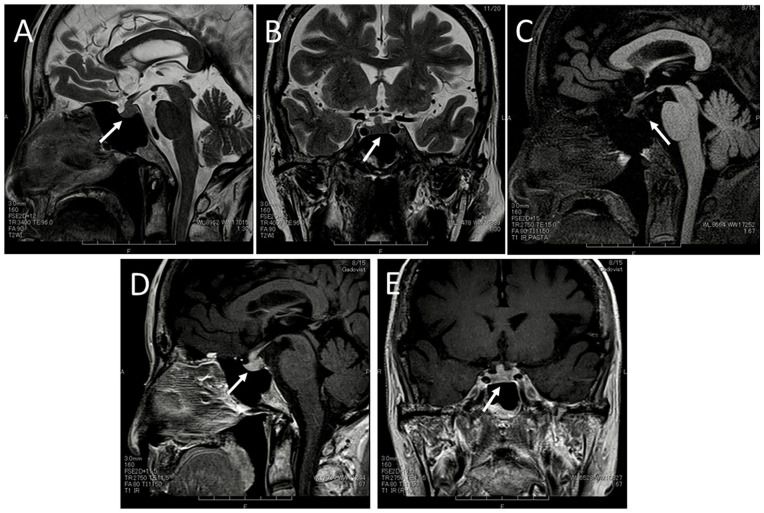
At the initial visit in the local hospital, she showed an elevated level of serum sodium ion at 147 mmol/L and a low level of antidiuretic hormone (arginine vasopressin, AVP) at 1.0 pg/mL. In hypertonic (5%) saline infusion test, 10 she showed the increase of plasma sodium ion from the basal level of 139 mmol/L up to 153 mmol/L, but no increase of antidiuretic hormone in the range of 0.6 to 0.9 pg/mL every 30 minutes in 120 minutes (Table 1). The urine osmolarity increased up to 383 mOsm/kg in 120 minutes from the baseline of 120 mOsm/kg by intravenous administration of desmopressin in vasopressin challenge test (Table 1). Her symptoms were relieved by the use of desmopressin nasal spray 5 µg twice daily. Anterior pituitary function tests with intravenous combined administration of 4 hypothalamic releasing hormones, thyrotropin-releasing hormone (TRH), growth hormone (GH)-releasing hormone (GHRH), corticotropin-releasing hormone (CRH), and luteinizing hormone (LH)-releasing hormone (LHRH), showed normal response curves from the normal baselines of TSH, prolactin, GH, adrenocorticotropic hormone (ACTH), cortisol, LH, and follicle-stimulating hormone (FSH) in 15, 30, 60, and 120 minutes (Table 1). Head magnetic resonance imaging demonstrated the thickening of the pituitary stalk and the relatively increased size of the pituitary gland (Figure 1A and B), together with the absence of high-intensity (bright) signals in the posterior pituitary lobe on the T1-weighted image (Figure 1C). Homogeneous high enhancement was noted with gadolinium DTPA contrast in the whole pituitary gland and stalk, and was extending continuously to the neighboring bilateral cavernous sinus (Figure 1D and E), leading to the diagnosis of lymphocytic hypophysitis.
Table 1.
Posterior and Anterior Pituitary Function Test at the Initial Visit in this Patient.
| Normal range (min-max) |
pretest | 15 minutes | 30 minutes | 60 minutes | 90 minutes | 120 minutes | |
|---|---|---|---|---|---|---|---|
| Hypertonic (5%) saline infusion test | |||||||
| Serum sodium (mmol/L) | 138-145 | 139 | Not tested | 145 | 147 | 149 | 153 |
| Plasma vasopressin (pg/mL) | 0.7 | Not tested | 0.8 | 0.8 | 0.9 | 0.6 | |
| Vasopressin challenge test | |||||||
| Urine osmolarity (mOsm/kg) | 50-1300 | 120 | Not tested | 244 | 310 | 360 | 383 |
| Anterior pituitary function test with intravenous combined administration of TRH, GHRH, CRH, and LHRH | |||||||
| Serum TSH (µIU/mL) | 0.465-4.68 | 0.956 | 4.369 | 4.818 | 3.921 | 2.940 | 2.122 |
| Serum prolactin (ng/mL) | 6.1-30.6 | 25.4 | 92.3 | 86.2 | 61.6 | 47.1 | 37.2 |
| Serum GH (ng/mL) | 0.93 | 19.20 | 16.50 | 10.10 | Not tested | 2.25 | |
| Plasma ACTH (pg/mL) | 7.2-63.3 | 6.4 | 63.6 | 67.8 | 44.3 | Not tested | 14.4 |
| Plasma cortisol (µg/dL) | 4.5-21.1 | 13.2 | 21.5 | 25.1 | 24.1 | Not tested | 18.5 |
| Serum LH (mIU/mL) | 13.9 | 30.7 | 36.4 | 41.2 | Not tested | 43.4 | |
| Serum FSH (mIU/mL) | 58.2 | 66.7 | 71.6 | 79.2 | Not tested | 80.7 | |
Abbreviations: TRH, thyrotropin-releasing hormone; GHRH, growth hormone (GH)-releasing hormone; CRH, corticotropin-releasing hormone; LHRH, luteinizing hormone–releasing hormone; TSH, thyroid-stimulating hormone; ACTH, adrenocorticotropic hormone; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
Figure 1.
Magnetic resonance imaging at the initial visit. The thickened pituitary stalk and the pituitary gland in relatively increased size (arrows) on T2-weighted sagittal (A) and coronal (B) images. Note the absence of high-intensity (bright) signals in the posterior pituitary lobe (arrow) on T1-weighted sagittal image with fat suppression (C) and marked homogeneous enhancement with gadolinium diethylenetriamine pentaacetic acid (DTPA) contrast in the whole pituitary gland and the stalk (arrows) on T1-weighted sagittal (D) and coronal (E) images.
White blood cell count was 2.7 × 103/µL with differentials of 68.8% neutrophils, 18.2% monocytes, and 13.0% lymphocytes, red blood cell count was 5.03 × 106/µL; and platelets count was 59 × 103/µL. Bone marrow biopsy confirmed the normal karyotype and her disease was designated as myelodysplastic syndrome at very low risk in IPSS-R (International Prognostic Scoring System–Revised). She showed normal liver and kidney function. Serum free T4 (thyroxine) was within the normal range at 1.08 ng/dL. Serum C-reactive protein (CRP) was negative, and rheumatoid factor was relatively elevated at 86.6 IU/mL while other autoantibodies such as anti-cyclic citrullinated peptide (CCP) antibody, antinuclear antibody, myeloperoxidase (MPO)-antineutrophil cytoplasmic antibody (ANCA), proteinase 3 (PR3)-ANCA, Sjogren syndrome (SS)-A, and SS-B were all negative. Serum IgG4 was low at 24 mg/dL, and plasma angiotensin-converting enzyme (ACE) activity was in the normal range at 12.2 U/L. Urinalysis detected no particular findings.
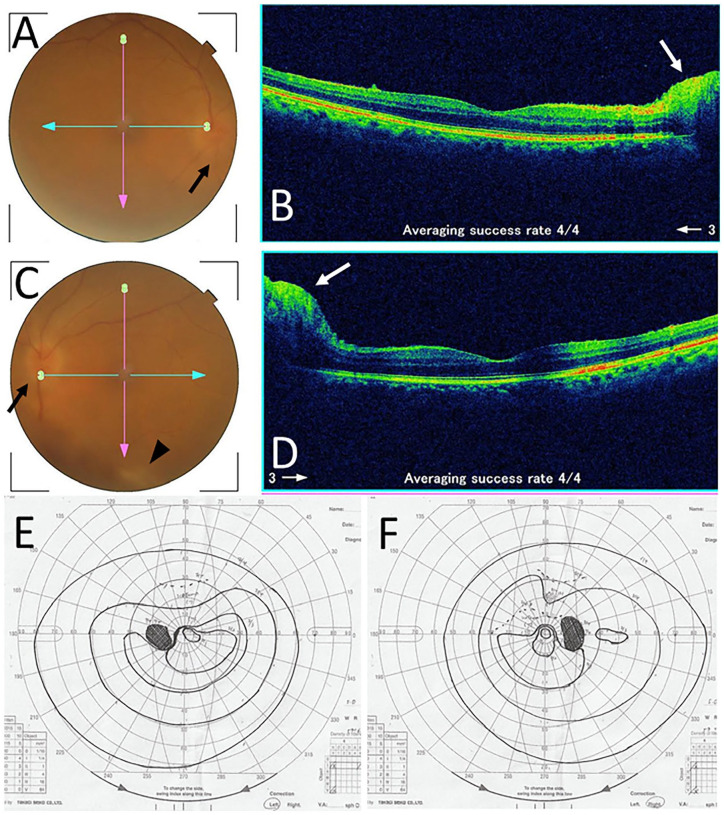
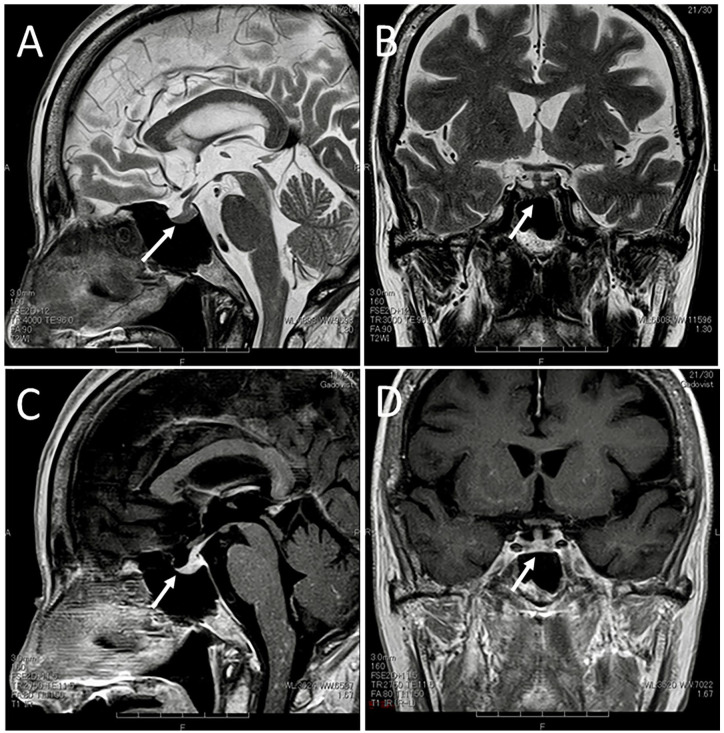
Two months after the initial visit, she experienced the deterioration of tremor of the hands at motion which had been stable for the previous 8 years and also had blurred vision. Four months after the initial visit, the best-corrected visual acuity in decimals was 0.8 in the right eye and 0.4 in the left eye. The intraocular pressure was 15 mm Hg in both eyes. The optic disks in both eyes were hyperemic, blurred, and swollen (Figure 2A-D), leading to the diagnosis of bilateral optic neuritis. The retinal manifestation was absent except for isolated retinal periphlebitis along the inferior vascular arcade in the posterior pole fundus of the left eye (Figure 2C). Goldmann periphery showed narrowing-down of the upper central visual field in both eyes (Figure 2E and F). Systemically, she had general fatigue, gait disturbance, and intentional tremor of the upper extremities. She experienced urinary retention, constipation, abnormal sensation in the distal part of the lower extremities, and moderate hemiplegia on the left side. Due to the difficulty in using desmopressin nasal spray, she began to have oral desmopressin 240 µg twice daily. At this time, serum free T4 was in the normal range at 1.39 ng/dL. Head magnetic resonance imaging showed that the pituitary gland and stalk decreased in size (Figure 3A and B), but showed persistent contrast enhancement (Figure 3C and D) as the same as the images 2 months previously (Figure 1D and F).
Figure 2.
Fundus photographs and optical coherence tomography in the right eye (A and B) and left eye (C and D) at the initial visit to an ophthalmologist. Note hyperemic and blurred optic disks (black arrows) in both eyes (A and C) and isolated retinal periphlebitis (arrowhead) in the left eye (C). Swollen optic disks (white arrows) in both eyes are evident in horizontal sections of optical coherence tomography (B and D). Goldmann perimetry in the right eye (F) and left eye (E), showing narrowing-down of the upper central visual field in both eyes, a week after the visit to an ophthalmologist.
Figure 3.
Magnetic resonance imaging 4 months after the initial visit. The pituitary stalk and the pituitary gland have decreased in size (arrows) on T2-weighted sagittal (A) and coronal (B) images. Note persistent enhancement with gadolinium diethylenetriamine pentaacetic acid (DTPA) contrast in the whole pituitary gland and the stalk (arrows) on T1-weighted sagittal (C) and coronal (D) images, as the same as the images 4 months previously (Figure 1D and E).
At referral to Department of Neurology at the University Hospital, she showed hyperesthesia in the distal part of the lower extremities, spastic hemiplegia on the left side, body ataxia, and positive Romberg sign of the inability to maintain the erect posture over 60 seconds with the eyes closed. The other physical examinations detected no particular findings. In additional blood tests, Serological tests for syphilis including rapid plasma reagin test and Treponema pallidum latex agglutination were negative, and interferon-γ-releasing assay with T-SPOT was negative. Soluble interleukin-2 receptor (sIL-2R) was elevated to 612 U/mL, vitamin B12 and folic acid were within the normal range, autoantibodies against aquaporin 4 (AQP4) and myelin oligodendrocyte glycoprotein (MOG) were negative. Magnetic resonance imaging disclosed several noncontinuous multifocal lesions with contrast enhancement, indicative of multiple demyelinating lesions, in the cervical and upper thoracic spinal cord, but showed no lesions in the brain. Spinal tap showed the increased number of cells at 36 cells/µL with 100% mononuclear cells, an elevated level of protein at 133 mg/dL, a relatively decreased level of glucose at 44 mg/dL, a higher level of sIL-2R at 325 U/mL, and an elevated level of IgG at 6.1 mg/dL with positive oligoclonal bands on electrophoresis.
With a tentative diagnosis of multiple sclerosis, she underwent 2 courses of steroid pulse therapy with methylprednisolone 1000 mg daily for 3 days. Neurological symptoms of the upper and lower extremities were alleviated while the hand tremor at rest on the left side remained unchanged. Magnetic resonance imaging also showed the subsidence of the lesions in the cervical and upper thoracic spinal cord. The optic disks in both eyes became normal and the best-corrected visual acuity was 0.9 in the right eye and 0.8 in the left eye. Her symptoms were stable with oral desmopressin 240 µg twice daily and weekly intramuscular injection of interferon-β-1a at 30 µg. In a month, she discontinued interferon-β-1a injection due to the ineffectiveness, and began to have oral prednisolone 20 mg daily after 1 dose of intravenous methylprednisolone 500 mg daily.
Discussion
To make the diagnosis of idiopathic lymphocytic hypophysitis, the other inflammatory, infectious, and neoplastic causes have to be excluded at first. Sarcoidosis and IgG4-related disease are listed as inflammatory diseases. Infectious diseases include syphilis and tuberculosis, and rarely aspergillosis. Lymphoma and meningioma are neoplastic diseases to be considered. In the present patient, blood examinations were done to exclude these infectious and inflammatory diseases. Magnetic resonance imaging is a key for diagnosing lymphocytic hypophysitis. The characteristic features are thickened pituitary stalk and enlarged pituitary gland in size as well as contrast enhancement of the stalk and gland. Central diabetes insipidus can be diagnosed by the loss of high-intensity signals in the posterior pituitary lobe, so called “posterior bright spot,” in the T1-weighted image.
The differential diagnoses in the present patient would include acute disseminated encephalomyelitis (ADEM) and multiple sclerosis. ADEM shows rapidly progressive demyelinating disorders in the brain and the spinal cord at the acute onset and occurs after viral or bacterial infection or vaccinations usually in children and young adults.11-13 COVID-19 vaccination is known as a precipitating factor for ADEM.12,13 Indeed, the present patient had a variety of neurological symptoms which might fit with the diagnosis of ADEM. One major point which is against the diagnosis of ADEM is the subacute to chronic onset of the neurological symptoms in this patient.
Multiple sclerosis is the ongoing diagnosis in this patient who showed spatial distribution of multifocal lesions in the spinal cord, in addition to bilateral optic neuritis. The patient, at first, showed hypophysitis, and then developed optic neuritis and other neurological symptoms caused by spinal cord lesions such as hyperesthesia and incomplete hemiplegia. Temporal distribution of multifocal lesions at the different timepoints in the disease course would remain somewhat questionable but would be aligned with the diagnosis of multiple sclerosis in this patient. The detection of oligoclonal bands in the cerebrospinal fluid in this patient would also support the diagnosis of multiple sclerosis. No autoantibodies were detected to support the other disease entities such as neuromyelitis optica spectrum disorder. COVID-19 vaccination has been recognized as a triggering factor for the onset or relapse of multiple sclerosis and other neurological disorders.14,15 Immunological aberrancy which would be probably induced by COVID-19 vaccination might result in demyelinating disorders in the brain and spinal cord. 16
Autoimmune diseases, including hypophysitis resulting in hypopituitarism, have been well recognized as immune-related adverse events which are sequel to immune checkpoint inhibitors for the treatment of cancers. 17 In the similar way, hypophysitis in the present patient would also have the background of immunological aberrancy which might be related with COVID-19 vaccination. 18 Hypophysitis is well known to occur as sequel to COVID-19 vaccination, 9 and optic neuritis has been also reported after COVID-19 vaccination. 5 Taken together, the sequence of events in the present patient who showed hypophysitis and then optic neuritis in the period of a few months after COVID-19 vaccination would be considered to be related with the vaccination. It should be noted that she had the different type of COVID-19 mRNA vaccine (elasomeran, Moderna) at the fourth inoculation, compared with the preceding 3-time inoculations (tozinameran, Pfizer-BioNTech). Indeed, the patient said she was relieved to hear that her symptoms would be related to the COVID-19 vaccination.
To analyze similar cases, PubMed and Google Scholar were searched for the key words: optic neuritis, hypophysitis, diabetes insipidus, COVID-19, and vaccine. The Japanese literature was searched for the same key words in the bibliographic database of medical literature in Japanese (Igaku Chuo Zasshi, Japana Centra Revuo Medicina, Ichushi-Web), published by the Japan Medical Abstracts Society (JAMAS, Tokyo, Japan). Old literatures were further collected from references cited in the articles which were identified during the literature search. There has been no report regarding the combination of optic neuritis and hypophysitis which would be related with COVID-19 or vaccination against COVID-19. Before the COVID-19 pandemic, the combination of optic neuritis and hypophysitis, especially with the diagnosis of diabetes insipidus, has been reported as case reports: 15 patients with sufficient description19-33 are summarized, together with the present case, in Table 2.
Table 2.
Review of 16 Patients With Idiopathic Hypophysitis and Optic Neuritis, Including the Present Patient.
| Case no./gender/age at onset | Main systemic diagnosis | Other systemic features | Pituitary biopsy | Pituitary biopsy pathology | Pituitary MRI findings | Other neurological and ocular features | Optic neuritis laterality | Systemic treatment and outcome | Authors |
|---|---|---|---|---|---|---|---|---|---|
| 1/Female/47 | Chronic thyroiditis with autoantibodies | Panhypopituitarism | No | Thick stalk and enlarged gland with contrast enhancement Bilateral cavernous sinus high signals |
Right abducens nerve palsy Bilateral incomplete oculomotor nerve palsy |
Bilateral | Prednisolone 80 mg Recovery |
Shimmyo and Izaki 19 | |
| 2/Female/43 | Diabetes insipidus | Panhypopituitarism | No | Thick stalk and enlarged gland with contrast enhancement Meningeal thickening |
Right abducens nerve palsy | Left | Prednisolone 60 mg | Hashimoto et al 20 | |
| 3/Female/53 | Secondary amenorrhea | Low serum levels of TSH, FSH, LH, prolactin, cortisol | Yes | Giant cell granulomatous hypophysitis | Thick stalk | Right abducens nerve palsy Right blepharoptosis |
Bilateral | Oral prednisolone Intravenous dexamethasone 16 mg Steroid pulse therapy, Recovery |
Arsava et al 21 |
| 4/Female/38 | Diabetes insipidus | High serum level of prolactin | No | Thick stalk Posterior lobe signal loss |
Right oculomotor and abducens nerve palsy | Bilateral | Intravenous prednisolone 240 mg Steroid pulse therapy Hormone replacement therapy |
Tamiya et al
22
Uemura et al 23 |
|
| 5/Female/23 | Secondary amenorrhea Diabetes insipidus |
Hypothyroidism | Yes | Lymphocytic hypophysitis | Mass-like enlarged hypothalamus and stalk | None | Bilateral | Oral dexamethasone Hormone replacement therapy |
Ouma and Farrell 24 |
| 6/Male/13 | Diabetes insipidus | Low serum levels of TSH, FT4, LH, FSH, testosterone | Yes | Lymphocytic hypophysitis | Mass-like enlarged gland Posterior lobe signal loss |
None | Bilateral | Intravenous and oral Prednisolone Azathioprine Hormone replacement therapy |
Al-Mujaini et al 25 |
| 7/Male/30 | Diabetes insipidus Hypogonadism | Low serum levels of TSH, ACTH, cortisol, LH, FSH High level of prolactin |
No | Thick stalk and enlarged gland with contrast enhancement Posterior lobe signal loss |
Oligoclonal bands in cerebrospinal fluid | Bilateral | Steroid pulse therapy | Saito et al 26 | |
| 8/Male/36 | Diabetes insipidus | Panhypopituitarism | No | Enlarged gland | Transient bilateral abducens nerve palsy | Left | Steroid pulse therapy | Ogasawara et al 27 | |
| 9/Female/51 | Diabetes insipidus | Panhypopituitarism | No | Posterior lobe signal loss Contrast enhancement of thick stalk and hypothalamus |
Bilateral lower extremities weakness Bilateral upper extremities numbness Left abducens nerve palsy |
Bilateral | Steroid pulse therapy | Ogasawara et al 27 | |
| 10/Male/41 | Hypogonadism | Low serum levels of TSH, LH, FSH, ACTH, cortisol | Yes | Lymphocytic hypophysitis | Mass-like enlarged gland with contrast enhancement | None | Bilateral | High-dose steroids Hormone replacement therapy |
Zoeller et al 28 |
| 11/Female/41 | None | None | Yes | Lymphocytic hypophysitis | Thick stalk and enlarged gland with contrast enhancement |
None | Bilateral | Oral corticosteroids Steroid pulse therapy Intravenous rituximab |
Schreckinger et al 29 |
| 12/Female/48 | Diabetes insipidus | Panhypopituitarism | Yes | Lymphocytic hypophysitis hypothalamitis |
Thick stalk and hypothalamic mass-like lesion with contrast enhancement Posterior lobe signal loss |
Bilateral uveitis | Bilateral | Oral prednisolone 25 mg Azathioprine High-dose intravenous dexamethasone |
Bianchi et al 30 |
| 13/Male/13 | Diabetes insipidus Multiple sclerosis |
Hypothyroidism Multifocal lesions in the brain on MRI |
No | Thick stalk and enlarged gland Posterior lobe signal loss | Left blepharoptosis Dysmetria and unstable gait Oligoclonal bands in cerebrospinal fluid |
Left | Steroid pulse therapy Intramuscular interferon β-1a Hormone replacement therapy |
Pena et al 31 | |
| 14/Female/52 | Diabetes insipidus | Panhypopituitarism | Yes | Lymphocytic hypophysitis | Thick stalk and enlarged gland Posterior lobe signal loss |
Bilateral scleritis and uveitis | Bilateral | Steroid pulse therapy Methotrexate Intravenous infliximab Intravenous rituximab Hormone replacement therapy |
Xu et al 32 |
| 15/Female/55 | Hypothyroidism | Low serum levels of TSH, FT4, LH, FSH, cortisol | No | Enlarged gland | None | Bilateral | Steroid pulse therapy Hormone replacement therapy |
Cozeto et al 33 | |
| 16/Female/74 | Diabetes insipidus | Oligoclonal bands in cerebrospinal fluid suggestive of multiple sclerosis | No | Thick stalk and enlarged gland with contrast enhancement Posterior lobe signal loss |
Left hemiplegia, Hand tremor Gait disturbance, Lower extremities abnormal sensation Retinal periphlebitis in left eye |
Bilateral | Steroid pulse therapy Oral prednisolone Hormone replacement therapy |
This case |
Steroid pulse therapy with methylprednisolone.
Abbreviations: MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; FT4, free thyroxine; ACTH, adrenocorticotropic hormone.
The 16 patients were 11 female and 5 male, with the age at the onset ranging from 13 to 74 years (median, 41 years). Pituitary biopsy or resection, suspicious of a tumor, was performed in 7 of the 16 patients, leading the pathological diagnosis of lymphocytic hypophysitis in 6 patients and giant cell granulomatous hypophysitis in one. The systemic presentation and diagnosis was diabetes insipidus in 11 patients. Panhypopituitarism at varying levels was concurrently diagnosed in 11 patients. Secondary amenorrhea was the main presentation in 2 women (Cases 3 and 5) and hypogonadism was in 2 men (Cases 7 and 10). Optic neuritis was bilateral in 13 patients while unilateral on the left side in 3. Two patients (Cases 13 and 16: this case) were diagnosed as multiple sclerosis based on multifocal brain or spinal cord lesions, together with the detection of oligoclonal bands in the cerebrospinal fluid. Another patient (Case 7) also showed oligoclonal bands in the cerebrospinal fluid but did not fulfill the diagnostic criteria of multiple sclerosis. No patients were reported to have autoantibodies to aquaporin 4, suggestive of the diagnosis of neuromyelitis optica spectrum disorder. Abducens nerve palsy which appeared to be transient was noted in 6 patients while uveitis, including retinal periphlebitis in the present patient (Case 16), was reported in 3 patients. Most patients, including the present patient, continued hormone replacement therapy in the course (Table 2), indicating that diabetes insipidus was irreversible.
In conclusion, the inoculation of COVID-19 mRNA vaccine in the present patient would serve as a precipitating factor to induce hypophysitis with diabetes insipidus in combination with bilateral optic neuritis. The other neurological symptoms such as muscle weakness, sensory abnormalities, and gait disturbance, as well as multifocal spinal cord lesions on magnetic resonance imaging and the detection of oligoclonal bands in cerebrospinal fluid, suggest the diagnosis of multiple sclerosis in this patient. The combination of optic neuritis and hypophysitis has been documented in the accumulation of case reports before the years of COVID-19 pandemic, and autoimmune mechanism was suggested to underlie the combination of manifestations which might be designated as a distinct clinical entity.34,35 To the best of our knowledge, the present patient appears to be the first case to show bilateral optic neuritis and hypophysitis which followed COVID-19 vaccination. The vaccination would be a triggering factor to induce immunological aberrancy and to develop the combination of manifestations.
Acknowledgments
None.
Footnotes
Authors’ Note: Data are available upon reasonable request to the corresponding author.
Author Contributions: T.M., as an ophthalmologist, diagnosed the patient and wrote the manuscript. K.O., as a neurologist, and T.I., as an internist, diagnosed, and treated the patient. H.M., as a radiologist, made the diagnosis on magnetic resonance imaging. All authors approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethics committee review was not applicable due to the case report design, based on the Ethical Guidelines for Medical and Health Research Involving Human Subjects, issued by the Government of Japan.
Informed Consent: Verbal informed consent was obtained from the patient for her anonymized information to be published in this article.
ORCID iD: Toshihiko Matsuo  https://orcid.org/0000-0001-6570-0030
https://orcid.org/0000-0001-6570-0030
References
- 1.Mahroum N, Lavine N, Ohayon A, et al. COVID-19 vaccination and the rate of immune and autoimmune adverse events following immunization: insights from a narrative literature review. Front Immunol. 2022;13:872683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu R, Pan J, Zhang C, Sun X.Cardiovascular complications of COVID-19 vaccines. Front Cardiovasc Med. 2022;9:840929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo T, Honda H, Tanaka T, Uraguchi K, Kawahara M, Hagiya H.COVID-19 mRNA vaccine-associated uveitis leading to diagnosis of sarcoidosis: case report and review of literature. J Investig Med High Impact Case Rep. 2022;10: 23247096221086450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuo T, Noda H.Temporal association of vitreous hemorrhage and hypertension after COVID-19 mRNA vaccines. Clin Case Rep. 2022;10(11):e6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elnahry AG, Al-Nawaflh MY, Eldin AAG, et al. COVID-19 vaccine-associated optic neuropathy: a systematic review of 45 patients. Vaccines (Basel). 2022;10:1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frara S, Loli P, Allora A, et al. COVID-19 and hypopituitarism. Rev Endocr Metab Disorders. 2022;23:215-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Racca G, D’Agnano S, Fasano N, Gianotti L.Pituitary apoplexy with transition to acute hypophysitis in a patient with Sars-CoV-2 pneumonia. JCEM Case Rep. 2023;1:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi M, Gunawardena S, Goenka A, Ey E, Kumar G.Post COVID-19 lymphocytic hypophysitis: a rare presentation. Child Neurol Open. 2022;9:2329048X221103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taieb A, Mounira EE.Pilot findings on SARS-CoV-2 vaccine-induced pituitary diseases: a mini review from diagnosis to pathophysiology. Vaccines (Basel). 2022;10:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takagi H, Hagiwara D, Handa T, et al. Diagnosis of central diabetes insipidus using a vasopressin radioimmunoassay during hypertonic saline infusion. Endocrine J. 2020;67:267-274. [DOI] [PubMed] [Google Scholar]
- 11.Tondo G, Virgilio E, Naldi A, Bianchi A, Comi C.Safety of COVID-19 vaccines: spotlight on neurological complications. Life (Basal). 2022;12:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabizadeh F, Noori M, Rahmani S, Hosseini H.Acute disseminated encephalomyelitis (ADEM) following COVID-19 vaccination: a systematic review. J Clin Neurosci. 2023;111:57-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad HR, Timmermans VM, Dakakni T.Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Am J Case Rep. 2022;23:e936574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabizadeh F, Ramezannezhad E, Kazemzadeh K, Khalili E, Ghaffary EM, Mirmosayyeb O.Multiple sclerosis relapse after COVID-19 vaccination: a case report-based systematic review. J Clin Neurosci. 2022;104:118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirmosayyeb O, Ghaffary EM, Vaheb S, Pourkazemi R, Shaygannejad V.Multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) following COVID-19 vaccines: a systematic review. Rev Neurol (Paris). 2023; 179(4):265-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo T, Takabatake R.Multiple sclerosis-like disease secondary to alpha interferon. Ocul Immunol Inflamm. 2002;10(4): 299-304. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36: 1714-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo T, Yamasaki O.Vogt-Koyanagi-Harada disease-like posterior uveitis in the course of nivolumab (anti-PD-1 antibody), interposed by vemurafenib (BRAF inhibitor), for metastatic cutaneous malignant melanoma. Clin Case Rep. 2017;5(5):694-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimmyo A, Izaki A.Lymphocytic adenohypophysitis with total ophthalmoplegia. Shinkei Ganka (Neuro-ophthalmol Jpn). 1997;14:38-43 [in Japanese]. [Google Scholar]
- 20.Hashimoto M, Ohtsuka K, Imaizumi H, et al. A case of presumed lymphocytic adenohypophysitis. Atarashii Ganka (J Eye). 1998;15:751-753 [in Japanese]. [Google Scholar]
- 21.Arsava EM, Uluç K, Kansu T, Dogulu CF, Soylemezoglu F, Selekler K.Granulomatous hypophysitis and bilateral optic neuropathy. J Neuroophthalmol. 2001;21(1):34-36. [DOI] [PubMed] [Google Scholar]
- 22.Tamiya A, Saeki N, Mizota A.Lymphocytic infundibulo-neurohypophysitis associated with recurrent optic neuritis. Br J Neurosurg. 2001;15(2):180-183. [DOI] [PubMed] [Google Scholar]
- 23.Uemura A, Mizota A, Usami-Adachi E, et al. Follow-up studies of optic neuritis with lymphocytic adenohypophysitis. Nippon Ganka Gakkai Zasshi (Jpn J Ophthalmol Soc). 2001;105:535-538 [in Japanese]. [PubMed] [Google Scholar]
- 24.Ouma JR, Farrell VJ.Lymphocytic infundibulo-neurohypophysitis with hypothalamic and optic pathway involvement: report of a case and review of the literature. Surg Neurol. 2002;57(1):49-53. [DOI] [PubMed] [Google Scholar]
- 25.Al-Mujaini A, Ganesh A, Al-Zuhaibi S, et al. Lymphocytic infundibulo-neurohypophysitis: an unusual cause of recurrent optic neuropathy in a child. J AAPOS. 2009;13(2):207-209. [DOI] [PubMed] [Google Scholar]
- 26.Saito S, Mori C, Toma K, et al. A case of optic neuritis associated with lymphocytic hypophysitis revealed by pattern-reversal VEP. Rinsho Shinkeigaku (Clin Neurol). 2011;51:27-31 [in Japanese]. [DOI] [PubMed] [Google Scholar]
- 27.Ogasawara M, Shikishima K, Sakai T, et al. Two cases of optic neuritis and hypophysitis. Shinkei Ganka (Neuro-ophthalmol Jpn). 2012;29:189-195 [in Japanese]. [Google Scholar]
- 28.Zoeller GK, Benveniste RJ, Farhadi FA, Bruce JH.Lymphocytic hypophysitis in a patient presenting with sequential episodes of optic neuritis. Pituitary. 2012;15(1):101-105. [DOI] [PubMed] [Google Scholar]
- 29.Schreckinger M, Francis T, Rajah G, et al. Novel strategy to treat a case of recurrent lymphocytic hypophysitis using rituximab. J Neurosurg. 2012;116:1318-1323. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi A, Mormando M, Doglietto F, et al. Hypothalamitis: a diagnostic and therapeutic challenge. Pituitary. 2014;17(3):197-202. [DOI] [PubMed] [Google Scholar]
- 31.Pena JA, Birchansky S, Lotze TE.Lymphocytic hypophysitis associated with pediatric multiple sclerosis. Pediatr Neurol. 2014;51(4):580-582. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, Ricciuti A, Caturegli P, Keene CD, Kargi AY.Autoimmune lymphocytic hypophysitis in association with autoimmune eye disease and sequential treatment with infliximab and rituximab. Pituitary. 2015;18(4):441-447. [DOI] [PubMed] [Google Scholar]
- 33.Cozeto IM, Zenerato LN, Maxta LA, et al. Optic neuritis and hypophysitis: partial remission after pulse therapy. Acta Sci Neurol. 2020;3:3-6. [Google Scholar]
- 34.Vernant JC, Cabre P, Smadja D, et al. Recurrent optic neuromyelitis with endocrinopathies: a new syndrome. Neurology. 1997;48:58-64. [DOI] [PubMed] [Google Scholar]
- 35.Petravic D, Habek M, Supe S.Recurrent optic neuromyelitis with endocrinopathies: a new syndrome or just a coincidence? Mult Scler. 2006;12:670-673. [DOI] [PubMed] [Google Scholar]