Abstract
Introduction
The process of weaning patients off mechanical ventilation (MV) is difficult and complex. Critical care nurses must maintain continuity of care for patients undergoing MV weaning, assess patients’ overall condition, and meet patients’ needs.
Objectives
The study aimed to explore clinical factors of MV weaning success and 28-day survival among patients with acute respiratory distress syndrome secondary to COVID-19.
Methods
This prospective observational study was conducted on 90 newly admitted patients in the general intensive care unit Assiut Univeristy Main Hospital in Egypt from October 2021 to March 2022. The researchers applied a standard weaning protocol for all of the patients in this study and then assessed the outcome variables: success or failure of weaning trials from a mechanical ventilator, and 28-day survival.
Results
In total, 50 (55.6%) patients were successfully weaned from MV, and 45 (50%) patients survived at 28 days. In a multivariate regression analysis, dynamic compliance (OR, 1.115; 95% CI, 1.010–1.230, p = .031*), lymphocyte count (OR, 3.025; 95% CI, 1.322–6.923, p = .009*), urine output (OR, 1.002; 95% CI, 1.001–1.004, p = .002*), and alanine aminotransferase (ALT; OR, 0.993; 95% CI, 0.988–0.999, p = .017*) were significantly associated with weaning success. In addition, age (OR, 1.058; 95% CI, 1.015–1.102; p = .007*), lymphocyte count (OR, 3.304; 95% CI, 1.348–8.100; p = .009*), urine output (OR, 1.003; 95% CI, 1.001–1.004; p = .001*), and ALT (OR, 0.994; 95% CI, 0.989–0.99, p = .015*) were significantly associated with survival at 28 days.
Conclusion
Dynamic lung compliance, lymphocyte count, urine output, and ALT were found to be predictive parameters that may affect the success of weaning off MV. Additionally, it was found that age, lymphocyte count, urine output, and ALT are predictors of survival at 28 days. We recommend further studies with larger, more systematic samples and complete follow-up, focusing on pulmonary function and quality of life in postweaning patients with acute respiratory distress syndrome.
Keywords: mechanical ventilation, weaning failure, weaning success, outcome at 28 days, COVID-19, critical care nurses, ARDS
Introduction
Acute respiratory distress syndrome (ARDS) is marked by a severe inflammatory response and hypoxemia (Wawrzeniak et al., 2018). ARDS secondary to COVID-19 have similar pathophysiological, clinical features, and respiratory system responses to ARDS caused by other conditions. These patients need protective mechanical ventilation (MV) strategies that aim to reduce ventilator-induced lung injury (VILI) (Kondili et al., 2021). In the early days of MV, volume control is still the most common ventilation mode. Protective MV is intended to reduce stress, which is the primary cause of VILI when operating in controlled modes. In order to reduce the possibility of hyperoxia-induced consequences, the technique of permissive hypoxia had been suggested in patients with severe ARDS (Ferrando et al., 2020; Grasselli et al., 2021).
Review of Literature
The process of withdrawing MV for patients with ARDS is difficult and mortality is high, along with the potential of worsening lung injury (Kondili et al., 2021). Currently, there are few specific studies on the process of mechanical ventilator weaning in patients with ARDS (Chiumello et al., 2016; Wang et al., 2021). Weaning is described as a gradual reduction in ventilator support until the patient no longer requires ventilator assistance or until a further reduction is neither feasible nor realistic (European Federation of Critical Care Nursing Associations, 2012; Tingsvik et al., 2015). Weaning off MV accounts for approximately 40%–50% of total ventilation time. Weaning trials were standardized and documented prospectively. The weaning process for patients with ARDSs due to COVID-19 has multiple phases; the treatment of underlying causes, evaluation of the ability to wean, the application of a spontaneous breathing trial (SBT), and at the end extubation (Hur et al., 2020; Rouze et al., 2021). The first spontaneous breathing trial (SBT) occurs as soon as the criteria for weaning readiness are met, which are adequate oxygenation with positive end-expiratory pressure (PEEP) ≤ 8 cmH2O, hemodynamic stability without the need for vasopressors or inotropic agents, and normocapnia (PaCO2 ≤ 45.0 mmHg) on MV. At the time of the SBT, patients are not sedated (Attia et al., 2022; Beduneau et al., 2017; Blackwood et al., 2011; Shrestha et al., 2020). Patients with COVID-19 frequently experience challenging or drawn-out weaning. About half of these patients remained intubated for nearly 14 days. Elderly Patients and/or obese had an increased likelihood of having a difficult time weaning. The most frequent reason for weaning failure in COVID-19 is likely respiratory pump insufficiency, which is brought on by a decline in respiratory neuromuscular competence and/or an increase in respiratory muscle stress (Alqahtani et al., 2020; Bhatraju et al., 2020; Nseir et al., 2021).
Patients are classified at the end of the weaning process into two categories: patients with successful (weaning success) or unsuccessful weaning (weaning failure). The former is defined as spontaneous breathing for more than 7 days without concomitant clinical or laboratory signs of chronic ventilatory insufficiency (Lin et al., 2020). The latter is either death in the course of weaning or the transition to permanent noninvasive (by face mask) or invasive (by tracheostomy tube) home ventilation due to chronic ventilator insufficiency. Chronic ventilator insufficiency is defined as recurrent hypercapnia during the daily weaning trials, preventing the extension of spontaneous respiration, or hypercapnia occurring within 7 days after weaning completion, requiring resumption of MV (Ghiani et al., 2020).
Critical care nurses manage MV and weaning with a great deal of autonomy and responsibility (Rose et al., 2007). Nursing care includes ventilator care and weaning assessment, planning, implementation, evaluation, and documentation (Crocker & Scholes, 2009; Rose et al., 2011). According to the literature, nursing care allows for early prediction and detection of complications, which support accurate, efficient, and timely interventions to promote patients’ safety (Rocha et al., 2020). The nurse's role in weaning is to provide patient-centered care and support throughout the process. The nurse should assess the patient's condition, monitor how they place on the Glasgow Coma Scale and their vital signs, and look for signs of distress or discomfort. The nurse should also provide education to the patient and their family about the weaning process, including potential risks and benefits. Additionally, the nurse should collaborate with an interdisciplinary team to ensure that all aspects of care are coordinated and that any changes in the patient's condition are addressed promptly. Finally, the nurse should provide emotional support to both the patient and their family throughout this difficult process (Chawla et al., 2020; Lavelle & Dowling, 2018; Muzaffar et al., 2017).
In holistic critical care nursing, all aspects of the patient's condition and their effects on the MV weaning process are considered, along with the factors that contribute to successful weaning. Based on this approach, the physical and mental manifestations of ARDS are now treated holistically in medical practice (Marini & Gattinoni, 2020). However, only a few studies have analyzed the risk factors affecting weaning success and survival in ARDS COVID-19 patients. Therefore, the present study aimed to explore factors affecting mechanical ventilator weaning success and 28-day survival among patients with acute respiratory distress syndrome secondary to COVID-19.
Methods
Research Design, Setting, and Study Variables
A single-center retrospective design was used to investigate the COVID-19 patients who had severe ARDS and were admitted to the general ICU at Assiut Univeristy Main Hospital in Egypt between October 1, 2021, and March 31, 2022. The general ICU comprises 20 beds. Study variables included clinical characteristics, the Modified Sequential Organ Failure Assessment (SOFA) score, hemodynamic status, lung and ventilator settings, and laboratory findings at the time of hospitalization.
Research Questions
The endpoint was to answer the following questions at 28 days post-ICU admission:
What are the factors predicting successful weaning off the mechanical ventilator among patients with ARDS secondary to COVID-19?
What are the factors predicting 28-day survival among patients with ARDS secondary to COVID-19?
Patients
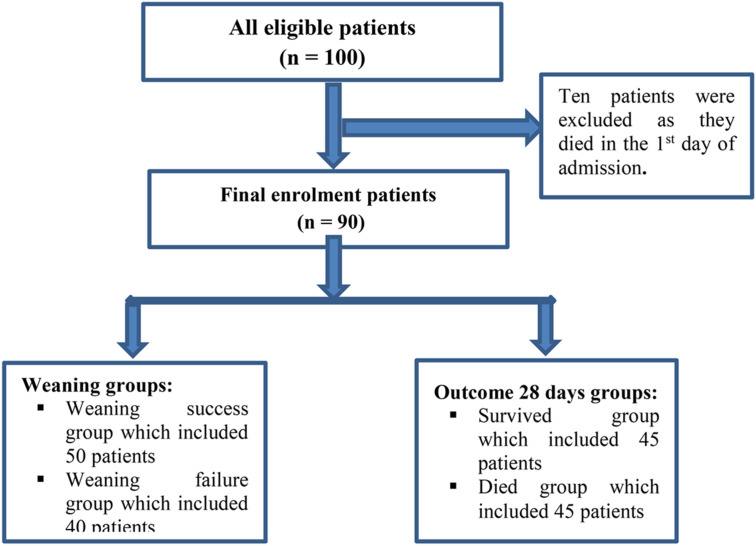
In total, 90 patients were included in the study. The inclusion criteria were age between 18 and 60 years; diagnosed COVID-19 with severe ARDS and required intubation and mechanical ventilation with standard criteria (PaO2/FiO2 < 200); and refractory hypoxemia and tachypnea (respiratory rate > 40 breaths/min) not relieved by a high-velocity nasal cannula or CPAP mask. Exclusion criteria were: pregnant women; patients who died within the first 24 h; and patients who had a negative COVID-19 RT-PCR (reverse transcription-polymerase chain reaction) result for two consecutive samples taken 48 h apart. According to the definitions provided by the WHO and the Egyptian Ministry of Health and Population (MOH), COVID-19 was identified and diagnosed using RT-PCR for viral RNA detection (Cobas 6800 System-Roche) (World Health Organization, 2020). The cases were divided into two groups based on their outcomes: successful weaning and failed weaning. At 28 days, the participants were divided into two groups: those who survived and those who were deceased.
Ethical Considerations
The study was carried out in accordance with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Research Committee of the Faculty of Nursing on July 27, 2021, Number 31-313. Permission was obtained from the hospital authorities after explaining the aim of the study and assuring the confidentiality and anonymity of the data.
Tools and Data Collection
The researchers analyzed the patients’ healthcare records consecutively from day 1 of admission to day 28 to collect data on the age, gender, comorbidities, body mass index, nursing notes, laboratory results, physicians’ notes, radiology reports, hemodynamic assessment, sedation protocol, medications, weaning success, ICU survival on day 28, antibiotics, anticoagulation, corticosteroids, and other ICU supportive care. The SOFA score was calculated on day 1 to assess organ dysfunction and the severity of illness. Oxygenation and ventilator parameters including the partial pressure of oxygen in arterial blood (PaO2), a fraction of inspired oxygen (FiO2), the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (P/F ratio), oxygen saturation (SaO2), alveolar–arterial O2 tension difference (PA_aDO2), partial pressure of carbon dioxide (PaCO2), peak airway pressure, plateau pressure, static compliance, dynamic compliance, required PEEP and airway resistance were used to assess the mechanics of the lungs. Intervention therapies such as circulatory/vasoactive support such as noradrenaline and sedation were assessed. Laboratory studies comprising the complete blood count, liver function test, renal function test, and coagulation studies were used to evaluate the body system's function and its effect on weaning and survival.
The weaning protocol was applied to all participant patients. First, the researchers assessed patient criteria for weaning, as follows: hemodynamic stability, ability to cough and effectively clear secretions, sufficient ventilation without the need for high levels of support, a strong enough respiratory effort to sustain breathing on their own, and successful spontaneous breathing trials. The criteria for the success or failure of a spontaneous breathing trial are summarized in Table 1.
Table 1.
Criteria of Success or Failure of Spontaneous Breathing Trial (SBT).
| Successful Spontaneous Breathing Trials | Failure of a Spontaneous Breathing Trial |
|---|---|
|
|
The weaning cycle was applied as follows:
- Spontaneous breathing mode (CPAP) on the lowest parameters:
- Respiratory rate < 30/min.
- Maximum inspiratory effort < −30 cmH2O
- Minute ventilation 6–12 L/min
- PEEP ≤ 5 cmH2O
- Rapid shallow breathing index (RSBI) (ratio of respiratory rate per minute to exhaled tidal volume in liters) < 105
- Pressure support needed for tidal volume ≥6 mL/breath < 8 cmH2O
- A spontaneous breathing trial (SBT) is conducted through one of the following methods:
- T-piece trial: Humidified oxygen connected directly to the endotracheal tube with a T-piece
- CPAP level equal to current PEEP level and pressure support kept at 5–8 cmH2O
- SBT conducted once daily for 60 min
A cuff leak test for extubation is conducted by deflating the cuff of the endotracheal tube, demonstrating air leakage around the cuff. If no leak is detected, then anti-edema measures are applied as follows: alpha-chymotrypsin, hydrocortisone injection, and nebulizing epinephrine (in a concentration of 1:10,000–100,000) until relief of obstructive edema of the trachea.
At the time of extubation, the patient is seated with head upright at an angle of 60°–80°.
Non-invasive NIV CPAP is considered in order to inhibit reintubation, especially for obese patients with a body mass index > 30.
Statistical Analysis
Data were computed and analyzed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). Categorical data were represented as numbers and percentages. The chi-squared test was applied to compare the two groups. Alternatively, the Monte Carlo correction test was applied when more than 20% of the cells had an expected count of less than five. Continuous data were tested for normality using the Shapiro–Wilk test. Quantitative data were expressed as the range (minimum and maximum), mean, standard deviation, and median. Student's t-test was used to compare two groups for normally distributed quantitative variables, and the Mann–Whitney test was used to compare two groups for non-normally distributed quantitative variables. Linear logistic regression (univariate and multivariate) was applied to detect the most affecting factor for successful weaning and 28-day survival. The significance of the obtained results was judged at the 5% level (Figure 1).
Figure 1.
Flowchart of patients’ enrolment.
Results
Sample Characteristics
Table 2 shows the characteristics of the study patients. The mean age was 63.07 ± 17.84 years, and more than half of the sample was female and/or overweight (55.6% and 55.3%, respectively). The average body surface area (BSA) was 2.0 ± 0.31, and 44.4% of the participants had hypertension. Of the total participants, 55.6% had been successfully weaned from the mechanical ventilator, and at 28 days, half of the patients had survived. To characterize the weaning success group, 60% were female, 58% were overweight, the mean BSA was 2.01 ± 0.30, about 46% of them had COPD or asthma, and the median SOFA score was 8. In general, there was homogeneity between the characteristics of the weaning success group and the weaning failure group, and no statistically significant differences were found among their characteristics. In terms of the outcome at 28 days, the survival group had a higher mean age (68.87 ± 14.95) and a higher percentage (51.1%) of COPD comorbidities than the deceased group, with a statistically significant difference. Moreover, the mean arterial blood pressure was 80.82 ± 14.14, with statistically significant differences between both (success and failure) weaning groups (p = .001) and between both (survival and death) 28-day outcome groups (p = .003).
Table 2.
Characteristics of the Studied Patients With ARDS Secondary to COVID-19.
| Characteristics | Total (n = 90) |
Weaning | p | Mortality Outcome at 28 Days | p | ||
|---|---|---|---|---|---|---|---|
| Weaning Success 50 (55.6%) |
Weaning Failure 40 (44.4%) |
Survival 45 (50%) |
Died 45 (50%) |
||||
| Age (years) | |||||||
| Mean ± SD | 63.07 ± 17.84 | 66.28 ± 16.88 | 59.05 ± 18.41 | .056 | 68.87 ± 14.95 | 57.27 ± 18.75 | .002* |
| Sex | |||||||
| Male | 40 (44.4%) | 20 (40.0%) | 20 (50.0%) | .343 | 18 (40.0%) | 22 (48.9%) | .396 |
| Female | 50 (55.6%) | 30 (60.0%) | 20 (50.0%) | 27 (60.0%) | 23 (51.1%) | ||
| BMI (kg/m2) | |||||||
| Mean ± SD | 35.26 ± 10.91 | 35.29 ± 9.28 | 35.22 ± 12.79 | .975 | 35.34 ± 9.59 | 35.18 ± 12.21 | .946 |
| ≤ 18.4 (underweight) | 1 (1.1%) | 1 (2.0%) | 0 (0%) |
MCp = 0.530 |
1 (2.2%) | 0 (0.0%) |
MCp = 0.883 |
| 18.5–24.9 (normal) | 18 (20.0%) | 8 (16.0%) | 10 (25.0%) | 8 (17.8%) | 10 (22.2%) | ||
| 25.0–39.9 (overweight) | 48 (53.3%) | 29 (58.0%) | 19 (47.5%) | 25 (55.6%) | 23 (51.1%) | ||
| ≥40 (obese) | 23 (25.6%) | 12 (24.0%) | 11 (27.5%) | 11 (24.4%) | 12 (26.7%) | ||
| Body surface area | |||||||
| Mean ± SD | 2.0 ± 0.31 | 2.01 ± 0.30 | 1.99 ± 0.33 | .703 | 2.01 ± 0.31 | 1.99 ± 0.32 | .737 |
| Co-morbidities | |||||||
| COPD | 36 (40.0%) | 23 (46.0%) | 13 (32.5%) | .194 | 23 (51.1%) | 13 (28.9%) | .031* |
| DM | 38 (42.2%) | 22 (44.0%) | 16 (40.0%) | .703 | 19 (42.2%) | 19 (42.2%) | 1.000 |
| HTN | 40 (44.4%) | 23 (46.0%) | 17 (42.5%) | .740 | 22 (48.9%) | 18 (40.0%) | .396 |
| Total SOFA score | |||||||
| Median (Min.–Max.) | 8 (4–18) | 8 (4–18) | 8.50 (4–18) | .378 | 8 (4–18) | 8 (4–18) | .833 |
| Temperature | |||||||
| Mean ± SD | 38.93 ± 1.08 | 38.87 ± 0.81 | 39.0 ± 1.34 | .579 | 38.80 ± 0.82 | 39.06 ± 1.28 | .254 |
| Respiratory rate | |||||||
| Mean ± SD | 37.22 ± 12.01 | 36.44 ± 11.70 | 38.20 ± 12.47 | .493 | 35.78 ± 11.02 | 38.67 ± 12.88 | .256 |
| Mean arterial blood pressure | |||||||
| Mean ± SD | 80.82 ± 14.14 | 85.12 ± 15.51 | 75.45 ± 10.04 | .001* | 85.20 ± 15.74 | 76.44 ± 10.83 | .003* |
Abbreviations: ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; HTN = hypertension; DM = diabetes mellitus; SOFA = sequential organ failure assessment; SD = standard deviation; χ2 = chi-square test; t: Student t-test.
U = Mann–Whitney test; MC: Monte Carlo; *Statistically significant at p ≤ .05.
Table 3 shows the results of oxygenation parameters among the studied patients. The median (minimum–maximum) PaO2 (arterial oxygen partial pressure) was 56 (32–92) mmHg. The mean FiO2 (fractional inspired oxygen) value was 0.83 ± 0.19, and the median P/F (PaO2/FiO2 ratio) value was 67 (37–135). The mean scores for SaO2 (arterial oxygen saturation) and ScvpO2 (central venous oxygen saturation) were 84.16 ± 8.70 and 64.81 ± 11.35, respectively. The median scores for PA_aDO2 (alveolar–arterial O2 tension difference) and PCO2 (carbon dioxide partial pressure) were 558.4 and 33.5 mmHg, respectively. The results showed no statistically significant differences regarding the oxygenation parameters between the weaning success group and the weaning failure group, or between the survival and death groups, except for PCO2 (p = .040*). Furthermore, 30% of the weaning success group versus 55% of the weaning failure group needed circulatory support (noradrenaline), with a statistically significant difference (p = .017). In addition, the mean urine output per day was 1197.8 ± 474.5 mL, with statistically significant differences between the two weaning groups (p = .001) and between the two mortality outcome groups (p = .001).
Table 3.
Comparison Between Weaning Groups, and Between the Mortality Outcome 28 Days Groups According to Oxygenation Parameters, Circulatory Support, and Urine Output.
| Parameters | Total (n = 90) |
Weaning | p | Mortality Outcome 28 days | p | ||
|---|---|---|---|---|---|---|---|
| Weaning Success 50 (55.6%) |
Weaning Failure 40 (44.4%) |
Survival 45 (50%) |
Died 45 (50%) |
||||
| PaO2 Median (Min.–Max.) |
56 (32–92) | 59 (32–88) | 54.5 (37–92) | .627 | 59 (36–88) | 55 (32–92) | .060 |
| FiO2 Mean ± SD | 0.83 ± 0.19 | 0.84 ± 0.19 | 0.82 ± 0.19 | .617 | 0.83 ± 0.20 | 0.83 ± 0.19 | .913 |
| P/F ratio Median (Min.–Max.) |
67 (37–135) | 67 (44–135) | 66.5 (37–130) | .410 | 67 (44–135) | 65 (37–130) | .208 |
| SaO2 Mean ± SD | 84.16 ± 8.70 | 84.88 ± 8.94 | 83.25 ± 8.41 | .380 | 85.24 ± 8.71 | 83.07 ± 8.64 | .237 |
| ScvpO2Mean ± SD | 64.81 ± 11.35 | 65.50 ± 10.46 | 63.95 ± 12.46 | .523 | 65.31 ± 10.63 | 64.31 ± 12.13 | .678 |
| PA_aDO2 Median (Min.–Max.) |
558.4 (229.8–647) |
575 (229.8–640) |
521.1 (272.8–647) |
.548 | 575 (229.8–640) |
556.7 (272.8–647) |
.289 |
| PCO2 Median (Min.–Max.) |
33.5 (17–117) | 44 (17–117) | 29 (19–97) | .059 | 52 (17–117) | 30 (19–97) | .040* |
| Lung compliance | |||||||
| Static (Mean ± SD) | 26.10 ± 6.64 | 26.38 ± 6.37 | 25.75 ± 7.03 | .657 | 26.36 ± 6.10 | 25.84 ± 7.20 | .717 |
| Dynamic (Mean ± SD) | 45.06 ± 7.06 | 46.54 ± 6.40 | 43.20 ± 7.47 | .025* | 46.64 ± 6.46 | 43.47 ± 7.34 | .032* |
| PEEP needed: Median (Min.–Max.) |
10 (0–12) | 10 (8–12) | 10 (0–12) | .261 | 10 (8–12) | 10 (0–12) | .240 |
| Raw (Mean ± SD) | 14.61 ± 5.36 | 14.62 ± 5.38 | 14.60 ± 5.39 | .986 | 14.87 ± 5.55 | 14.36 ± 5.21 | .653 |
| P. plat (Mean ± SD) | 20.02 ± 3.84 | 19.84 ± 3.51 | 20.25 ± 4.25 | .618 | 19.71 ± 3.57 | 20.33 ± 4.12 | .446 |
| P. peak (Mean ± SD) | 34.63 ± 4.71 | 34.46 ± 4.24 | 34.85 ± 5.29 | .699 | 34.58 ± 4.34 | 34.69 ± 5.11 | .912 |
| Circulatory support | |||||||
| Yes (noradrenaline) | 37 (41.1%) | 15 (30.0%) | 22 (55.0%) | .017* | 14 (31.1%) | 23 (51.1%) | .054 |
| Dose (mcg/kg) Median (Min.–Max.) |
0 (0–300) | 0 (0–300) | 50 (0–200) | .038* | 0 (0–300) | 50 (0–200) | .104 |
| Urine output | |||||||
| Mean ± SD | 1197.8 ± 474.5 | 1344 ± 453.6 | 1015 ± 440.0 | .001* | 1371.1 ± 435.2 | 1024.4 ± 452.4 | <.001* |
Abbreviations: PEEP = Positive End Expiratory Pressure; Raw = airway resistance; P. plat = plateau pressure; P peak = peak pressure.
PA_aDO2 = A-a Gradient (PA-aO2) (A-aDO2) Alveolar-arterial O2 Tension Difference.
PaO2/FiO2 ratio is the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen.
Table 4 shows that the median lymphocyte count was 1.40 for the survivors versus 0.90 for the deceased, with a statistically significant difference between them (p = .003*). The median international normalized ratio (INR) (minimum–maximum) was 1.12 (0.90–2.84), with statistically significant differences between both weaning groups and both 28-day outcome groups (p = .05). Regarding the liver function test, the median alanine aminotransferase (ALT) was 50 U/L and the mean albumin was 3.44 ± 0.77 g/dL, with statistically significant differences between both weaning groups and between both 28-day outcome groups (p = .05).
Table 4.
Comparison Between Weaning Groups, and Between the Mortality Outcome 28 Days Groups According to Laboratory Investigations.
| laboratory Investigations | Total (n = 90) |
Weaning | p | Mortality Outcome 28 days | p | ||
|---|---|---|---|---|---|---|---|
| Weaning Success (n = 50) |
Weaning Failure (n = 40) |
Survival (n = 45) |
Died (n = 45) |
||||
| Complete blood picture | |||||||
| Lymphocytic count: Median (Min.–Max.) |
1 (0.50–4.10) | 1.30 (0.60–4.10) | 0.95 (0.50–2.70) | .058 | 1.40 (0.60–4.10) | 0.90 (0.50 –2.70) | .003* |
| WBC: Median (Min.–Max.) | 7.30 (2.17–29.0) | 6.95 (2.17–27.0) | 7.45 (2.30–29.0) | .620 | 6.40 (2.17–27.0) | 7.50 (2.30 –29.0) | .958 |
| Coagulation studies | |||||||
| INR: Median (Min.–Max.) | 1.12 (0.90–2.84) | 1.21 (0.90–2.40) | 1.02 (0.94–2.84) | .006* | 1.20 (0.90–2.40) | 1.05 (0.94 –2.84) | .020* |
| PLT: Median (Min.–Max.) | 206 (46–473) | 216 (76–473) | 188 (46–435) | .111 | 214.0 (76–473) | 198 (46–435) | .292 |
| Renal function test | |||||||
| Creatinine Median (Min.–Max.) |
107 (40–498) | 110.5 (40.–498) | 107 (45–372) | .868 | 124 (40–498) | 106 (45–372) | .532 |
| Urea Median (Min.–Max.) | 24.1 (10–104) | 24.4 (10–57) | 23.2 (10–104) | .567 | 24.50 (10–57) | 23 (10–104) | .910 |
| Liver function test | |||||||
| ALT: Median (Min.–Max.) | 50 (11–1103) | 42.5 (11–498) | 68 (13–1103) | .014* | 43 (11–498) | 56 (13.– 1103) | .042* |
| AST: Median (Min.–Max.) | 66 (16–526) | 51 (16–337) | 78 (16–526) | .057 | 51 (16–337) | 78 (16–526) | .135 |
| Lactate Median (Min.–Max.) | 4.30 (1.70–15.0) | 4.30 (1.80–8.10) | 4.35 (1.70–15.0) | .482 | 4.30 (1.80–8.10) | 4.20 (1.70 –15.0) | .981 |
| Albumin: Mean ± SD | 3.44 ± 0.77 | 3.59 ± 0.70 | 3.25 ± 0.82 | .032* | 3.62 ± 0.69 | 3.26 ± 0.82 | .027* |
| BIL: Median (Min.–Max.) | 1 (0.60–3.30) | 1 (0.60–2.60) | 0.95 (0.60–3.30) | .365 | 1 (0.60–2.60) | 0.90 (0.60 –3.30) | .238 |
| IL6: Median (Min.–Max.) | 4.86 (0.54 −31.21) | 4.98 (0.54–15.08) | 4.72 (1.14 −31.21) | .868 | 4.73 (0.54 15.08) | 5.08 (1.14 31.21) | .591 |
Abbreviations: SD = standard deviation; χ2 = chi-square test; t = Student t-test; U = Mann Whitney test; p = p-value for comparing between weaned and not weaned.
*: Statistically significant at p ≤ .05.
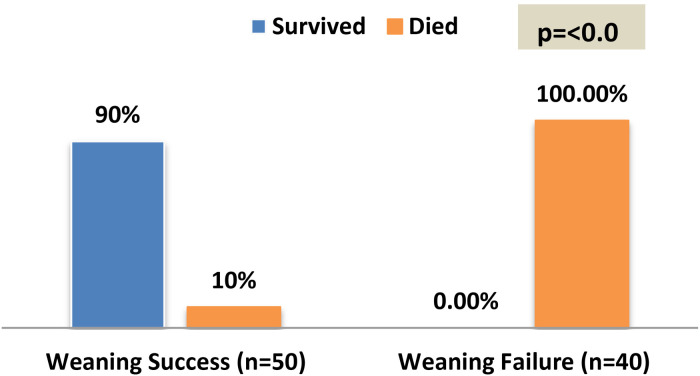
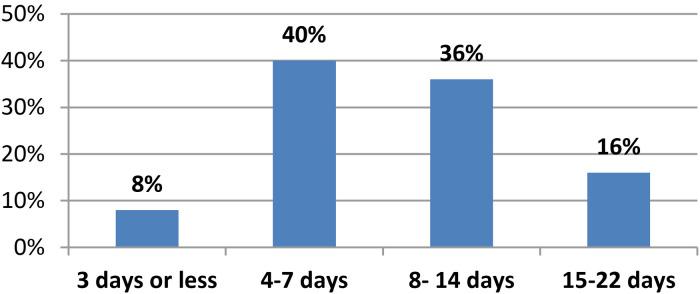
Figure 2 presents the outcome at 28 days among the participants. With a statistically significant difference of p = .001*, the majority of the weaning success group (90%) survived and the entire weaning failure group (100%) was deceased. Figure 3 clarifies that 40% of the weaning success patients stayed on MV for between 4 and 7 days, while 8% stayed on MV for 3 days or less.
Figure 2.
Outcome after 28 days among the weaning success and failure groups.
Figure 3.
Days on mechanical ventilation (MV) of weaning success patients n = 50.
To address the research question related to the factors predicting successful weaning off the mechanical ventilator among patients with ARDS secondary to COVID-19, the characteristics and clinical data were analyzed using univariate analysis. The significant independent variables were then analyzed using multivariate logistic regression to identify weaning success factors. Table 5 shows that the dynamic compliance (OR, 1.115; 95% CI, 1.010–1.230, p = .031*), lymphocyte count (OR, 3.025; 95% CI, 1.322–6.923, p = .009*), urine output (OR, 1.002; 95% CI, 1.001–1.004, p = .002*), and ALT (OR, 0.993; 95% CI, 0.988–0.999, p = .017*) were significantly associated with weaning success.
Table 5.
Univariate and Multivariate Logistic Regression Analysis for the Parameters Affecting Success Weaning Off Mechanical Ventilation.
| Independent Variables | Univariate | Multivariate a | ||
|---|---|---|---|---|
| p | OR (LL–UL 95% CI) | p | OR (LL–UL 95% CI) | |
| Dynamic compliance | .030* | 1.075 (1.007–1.146) | .031* | 1.115 (1.010–1.230) |
| Mean ABP | .003* | 1.065 (1.022–1.109) | .093 | 1.043 (0.993–1.096) |
| No needed noradrenalin | .018* | 2.852 (1.197–6.795) | .069 | 3.312 (0.912–12.034) |
| Lymphocytic count | .037* | 1.755 (1.034–2.979) | .009* | 3.025 (1.322–6.923) |
| Albumin | .036* | 1.850 (1.042–3.285) | .732 | 0.872 (0.397–1.914) |
| UOP | .002* | 1.002 (1.001–1.003) | .002* | 1.002 (1.001–1.004) |
| ALT | .021* | 0.994 (0.989–0.999) | .017* | 0.993 (0.988–0.999) |
| AST | .030* | 0.995 (0.990–0.999) | .221 | 1.005 (0.997–1.014) |
Abbreviations: AST = aspartate aminotransferase; ALT = alanine aminotransferase; UOP = urine output; OR = odd’s ratio; CI = confidence interval; LL = lower limit; UL = upper limit.
All variables with p < .05 were included in the multivariate.
* Statistically significant at p ≤ .05.
To address the research question related to the factors predicting 28-day survival among patients with ARDS secondary to COVID-19, univariate and multivariate Logistic regression analyses were performed. Table 6 shows that the age (OR, 1.058; 95% CI, 1.015–1.102, p = .007*), lymphocyte count (OR, 3.304; 95% CI, 1.348–8.100, p = .009*), urine output (OR, 1.003; 95% CI, 1.001–1.004, p = .001*), and ALT (OR, 0.994; 95% CI, 0.989–0.99, p = .015*) were significantly associated with the survival outcome at 28 days.
Table 6.
Univariate and Multivariate Logistic Regression Analysis for the Parameters Affecting Survival 28 Days.
| Univariate | Multivariate a | |||
|---|---|---|---|---|
| p | OR (LL–UL 95% CI) | p | OR (LL–UL 95% CI) | |
| Age (years) | .003* | 1.042 (1.014–1.071) | .007* | 1.058 (1.015–1.102) |
| Co-morbidities (COPD) | .033* | 2.573 (1.078–6.144) | .273 | 2.693 (0.457–15.861) |
| PCO2 | .035* | 1.022 (1.001–1.042) | .642 | 0.991 (0.954–1.030) |
| Dynamic compliance | .037* | 1.071 (1.004–1.142) | .175 | 1.069 (0.970–1.178) |
| Mean ABP | .006* | 1.054 (1.015–1.093) | .224 | 1.033 (0.980–1.089) |
| Lymphocytic count | .006* | 2.191 (1.256–3.823) | .009* | 3.304 (1.348–8.100) |
| Albumin | .030* | 1.881 (1.061–3.333) | .751 | 0.870 (0.369–2.053) |
| UOP | .001* | 1.002 (1.001–1.003) | .001* | 1.003 (1.001–1.004) |
| ALT | .038* | 0.995 (0.991–1.000) | .015* | 0.994 (0.989–0.999) |
Abbreviations: COPD = chronic obstructive pulmonary disease; ALT = alanine aminotransferase; ABP = ambulatory blood pressure; UOP = urine output; OR = odd's ratio; CI = confidence interval; LL = lower limit; UL = upper limit.
All variables with p < .05 were included in the multivariate.
*Statistically significant at p ≤ .05.
Discussion
Acute respiratory distress syndrome is a serious lung injury that develops in some COVID-19 patients and is a leading cause of morbidity and mortality in these patients (Swenson & Swenson, 2021; Tolossa et al., 2022). Weaning the patient off mechanical ventilation as soon as possible is one of the key objectives of ARDS treatment in order to enhance outcomes and lower the risk of complications. The pathophysiology of weaning failure is multifactorial (Kondili et al., 2021).
Since patients with COVID-19 present a number of difficulties and can develop ARDS, critical care nurses play a crucial role in the management of these patients. In order to reduce the potential complications, provide mechanical ventilator nursing care, and enhance the weaning process (Carter et al., 2020), our study aimed to identify the characteristics, respiratory and ventilator parameters, and laboratory status of 90 patients with ARDS secondary to COVID-19, then compare those between weaning success and failure groups and between survival and death groups, to determine the factors associated with weaning success and survival among ARDS COVID-19 patients.
Patients with COVID-19 frequently experience challenging or drawn-out weaning. In recent, United States and European cohorts (Hur et al., 2020, Rouze et al., 2021), roughly half of these patients remained intubated for nearly 14 days. Our findings showed that 55.6% of patients with ARDS COVID-19 were successfully weaned off MV, and the majority (90%) of them survived at 28 days. This is a relatively high success rate, which suggests that the weaning plan and protocols used were effective. However, while the weaning process was successful, the overall survival rate for this cohort of ARDS COVID-19 patients remained moderate. A retrospective study (Mughal et al., 2020) studied 30 patients with COVID-19 who were intubated with a mechanical ventilator and reported that 20% (n = 6) of them were successfully weaned from the ventilator.
We also observed in our study that the outcome at 28 days for all MV weaning failure patients was mortality, indicating a close relationship between failed weaning and mortality in patients with ARDS COVID-19. In our study, the overall mortality rate among those with ARDS caused by COVID-19 was 50%. It was potentially so high since invasive MV raises the danger of complications such as pneumonia, sepsis, and lung fibrosis, which can lower survival rates. This highlights the critical role of intensive care nurses in the ARDS COVID-19 weaning process. Nurses are responsible for monitoring a patient's vital signs and lung function, administering medications and treatments, and providing education and support to the patient and their family. They also collaborate with an interdisciplinary team, including physicians and respiratory therapists, to develop and implement a weaning plan. A large international epidemiological study, based on a convenience sample of 459 ICUs from 50 countries across five continents, reported that the mortality rate was 46.1% (95% CI, 41.9–50.4%) for those with severe ARDS (Bellani et al., 2016). In a prior study, the overall mortality rate for a prospective cohort of patients admitted to 361 ICUs was 30.7% (1590 patients), and the mortality rate for patients with ARDS who were on MV was higher, at 52.2% (120 patients) (Esteban et al., 2002). Additionally, a multicenter prospective trial discovered that patients who were successfully weaned from MV at 28 days had a lower death rate than those who were not (0.0% vs. 62.4%) (Gamberini et al., 2020).
In the current study, patients with COPD comorbidities had better survival chances than deceased patients. This can be attributed, as explained by two similar studies that investigated the relationship between COPD and mortality in ARDS COVID-19 patients. The first study found that patients with COPD had a lower mortality rate compared to those without COPD, despite having more severe symptoms and requiring more intensive care. The study suggested that the protective effect of COPD comorbidity on survival may be due to increased lung volume and reduced lung compliance, which can help prevent lung collapse and ventilator-induced lung injury. The second study also found that COPD comorbidity was not associated with increased mortality among ARDS COVID-19 patients. The study suggested that the use of corticosteroids and bronchodilators commonly used to treat COPD may have contributed to the lower mortality rate in the COPD group (Lacedonia et al., 2021; Russell et al., 2023). However, this contradicts a study published in 2020 that identified an association between COVID-19 infection and mortality rates in patients with COPD.
Critical care nurses play a key role in ensuring that patients transition safely from the acute stage of ventilation to weaning (Burns, 2005). According to a retrospective study published in 2021 under the title “The Risk Factors for Weaning Failure of Mechanically Ventilated Patients with COVID-19,” patients in the unsuccessful group had a worse outcome; their 28-day mortality rate was higher than that of the successful group (86.7% vs. 16.7%, p = .001), which is consistent with our findings (Zhao et al., 2021). Meanwhile, a retrospective study conducted in Saudi Arabia concluded that old age, active smoking, decreased SpO2/FiO2 ratio, pulmonary embolism, and increased lactate and d-dimers were predictors of 28-day mortality in critically ill COVID-19 patients (Alharthy et al., 2021).
In our study, the multivariate regression analysis revealed that dynamic lung compliance, lymphocyte count, urine output, and ALT are predictive parameters that may affect the success of weaning off mechanical ventilation. In addition, the regression study also revealed that age, lymphocyte count, urine output, and ALT are predictors of the survival outcome at 28 days.
Consistent with a prior study (Zhao et al., 2021), dynamic compliance was higher in successfully weaned patients than in unsuccessfully weaned patients. The lymphocyte count was another factor predicting weaning success and survival. A high lymphocyte count appeared to be a marker of a good prognosis in our patients. The possible explanation for this association is that a lower lymphocyte count may indicate a more severe and prolonged immune response to the underlying illness, such as COVID-19, which can lead to more severe lung injury, a higher risk of weaning failure, and a higher risk of death. In addition, the results indicated that a higher lymphocyte count is associated with higher odds of survival at 28 days after the onset of ARDS. Similarly, a previous study (Luo et al., 2018) discovered that the neutrophil/lymphocyte ratio may stand as a useful predictor of weaning failure. The liver enzyme alanine aminotransferase (ALT) can also be used to identify liver disease or injury. Our research showed that lower ALT is linked to better chances of successful MV weaning and survival at 28 days following the onset of ARDS. This is in agreement with a previous study (Senior, 2012). Finally, the regression analysis revealed a new factor affecting both weaning success and 28-day survival, the urine output. Patients with a higher urine output were more likely to be weaned off MV successfully, which indicates that higher urine output is associated with higher odds of survival at 28 days. Further research is needed to corroborate our findings.
Age, although not linked to weaning success in the current study, was associated with a chance of survival at 28 days, with an OR of 1.058 (95% CI, 1.015–1.102, p = .007*) for older age. According to this, elderly individuals had a higher chance of surviving for 28 days after the onset of ARDS. This is inconsistent with a previous study (Chiumello et al., 2022), which reported that elderly patients with COVID-19 who developed ARDS had a significantly higher mortality rate compared to those without COVID-19. Also, contrary to our findings (Yanez et al., 2020) found that the mortality rate of COVID-19 was more than 62 times higher among those aged 65 or older than among those aged 54 or younger. Moreover, recent research found that patients who were elderly (> 65 years) and/or obese (BM1 > 30) were at a greater risk of experiencing difficulty with weaning (Hur et al., 2020; Rouze et al., 2021). Overall, while the relationship between age and survival in COVID-19-associated ARDS is complex and multifactorial, it appears in the current study that older age may confer some protective benefits in terms of survival. However, further research is needed to fully understand this relationship.
In comparison, the results of our study showed that there were statistically significant differences in the median international normalized ratio (INR) between both weaning groups and between both 28-day outcome groups (p = .05), which suggests that there is a correlation between patients’ INR and their success at weaning as well as patients’ survival rate. INR is a measure of the blood's ability to clot; a higher INR value can indicate a greater risk of bleeding and can be an indicator of liver dysfunction, vitamin K deficiency, or other issues.
Increases in the level of plasma biomarker interleukin-6 (IL-6) were also linked to adverse ARDS outcomes in our study. This is in line with (Binnie et al., 2014; Ware et al., 2010), which noted that ARDS is characterized by an intense inflammatory response with the release of several inflammatory mediators during the course of this response, spanning the exudative, proliferative, and fibrotic phases of ARDS (Thompson et al., 2017). Yang et al. showed reduced levels of serum inflammatory cytokines, especially IL-6, with successful weaning in septic patients on ventilators (Yang et al., 2018).
It is important to note that the weaning process should be individualized for each patient and carried out gradually to minimize the risk of complications (Chatburn & Deem, 2007). Close monitoring of the patient's oxygenation, lung mechanics, and vital signs during the weaning process is crucial. Critical care nurses (CCNs) play a key role in helping to plan, initiate, assess, and establish a holistic weaning process. Additionally, nurses may also be responsible for monitoring a patient's response to weaning, adjusting the weaning plan as needed, and advocating for the patient's needs. Team collaboration among all healthcare practitioners is important, with CCNs playing a key role in prioritizing weaning and driving the process forward (Cederwall et al., 2014).
Strength and Limitation
The strength of the study is using multivariate statistical analyses to control for confounding variables and identify the unique contributions of different factors to MV weaning success and 28-day survival. However, the limitations of this study may have contributed to the inconsistent findings with previous studies. It can be due to the sample size being relatively small, and the participants were recruited from a single-center hospital setting, which may not be representative of the broader population. Furthermore, the retrospective design and the studied variables used in this study could account for some of the discrepancies in findings from previous studies. We recommend further studies with larger, more systematic samples and complete follow-up, which will better characterize the course of successful weaning, evaluate other factors related to successful weaning from MV, and validate the clinical impact of our findings. Future research is also suggested to focus on the pulmonary function and quality of life of postsurvival and postweaning patients with ARDS COVID-19.
Implication for Practice
The study emphasized the predictors that the healthcare team should be aware of when providing care for patients with ARDS secondary to COVID-19. Identifying the predictors of weaning success and survival, it could help assess the readiness for MV weaning, enhance weaning outcomes, and lower the rate of mortality.
Conclusion
The dynamic lung compliance, lymphocyte count, urine output, and ALT were found in this study's regression analysis to be predictive parameters that may affect the success of weaning off MV. The regression study also revealed that age, lymphocyte count, urine output, and ALT are predictors of the survival outcome at 28 days.
Acknowledgements
The authors acknowledge the Assiut University Main Hospital, for providing support in collecting data.
Footnotes
Ethical Considerations: The study was carried out in accordance with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Research Committee of the Faculty of Nursing on July 27, 2021, Number 31-313. Permission was obtained from the hospital authorities after explaining the aim of the study and assuring the confidentiality and anonymity of the data.
Data Availability Statement: Dr. Amal Isamel Abdelhafez can provide the data that support the study's results upon reasonable request at dramalcritical@aun.edu.eg.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Yousef Saad Aldabayan https://orcid.org/0000-0001-9498-946X
Amal Ismael Abdelhafez https://orcid.org/0000-0002-2416-2843
References
- Alharthy A., Aletreby W., Faqihi F., Balhamar A., Alaklobi F., Alanezi K., Jaganathan P., Tamim H., Alqahtani S. A., Karakitsos D., Memish Z. A. (2021). Clinical characteristics and predictors of 28-day mortality in 352 critically ill patients with COVID-19: A retrospective study. Journal of Epidemiology and Global Health, 11(1), 98–104. 10.2991/jegh.k.200928.001.Epub 2020 Oct 3. PMID: 33095982; PMCID: PMC7958266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqahtani J. S., Oyelade T., Aldhahir A. M., Alghamdi S. M., Almehmadi M., Alqahtani A. S., Quaderi S., Mandal S., Hurst J. R. (2020). Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS One, 15(5), e0233147. 10.1371/journal.pone.0233147.PMID: 32392262; PMCID: PMC7213702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia A., Abdullatif D., Desowky S. (2022). Factors affecting weaning of mechanically ventilated Patients. Egyptian Journal of Health Care, 13(2). 10.21608/ejhc.2022.228536 [DOI] [Google Scholar]
- Bellani G., Laffey J. G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D. F., Ranieri M., Rubenfeld G., Thompson B. T., Wrigge H., Slutsky A. S., Pesenti A.; LUNG SAFE Investigators; ESICM Trials Group. (2016). Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA, 315(8), 788–800. Erratum in: JAMA. 2016 Jul 19; 316(3):350. PMID: 26903337. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- Béduneau, G., Pham, T., Schortgen, F., Piquilloud, L., Zogheib, E., Jonas, M., Grelon, F., Runge, I., Nicolas Terzi, Grangé, S., Barberet, G., Guitard, P. G., Frat, J. P., Constan, A., Chretien, J. M., Mancebo, J., Mercat, A., Richard, J. M., Brochard, L., & WIND (Weaning according to a New Definition) Study Group and the REVA (Réseau Européen de Recherche en Ventilation Artificielle) Network ‡, (2017). Epidemiology of weaning outcome according to a new definition. The WIND study. American Journal of Respiratory and Critical Care Medicine, 195(6), 772–783. 10.1164/rccm.201607-1414OC [DOI] [PubMed] [Google Scholar]
- Bhatraju, P. K., Ghassemieh, B. J., Nichols, M., Kim, R., Jerome, K. R., Nalla, A. K., Greninger, A. L., Pipavath, S., Wurfel, M. M., Evans, L., Kritek, P. A., West, T. E., Luks, A., Gerbino, A., Dale, C. R., Goldman, J.D., O’Mahony, S., & Mikacenic, C. (2020). COVID-19 in critically ill patients in the Seattle region—case series. The New England Journal of Medicine, 382(21), 2012–2022. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnie A., Tsang J. L., dos Santos C. C. (2014). Biomarkers in acute respiratory distress syndrome. Current Opinion in Critical Care, 20(1), 47–55. 10.1097/MCC.0000000000000045 [DOI] [PubMed] [Google Scholar]
- Blackwood B., Alderdice F., Burns K. E. A., Cardwell C. R., Lavery G., O’Halloran P. (2011). Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis . BMJ , 342,c7237. 10.1136/bmj.c7237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S. M. (2005). Mechanical ventilation of patients with acute respiratory distress syndrome and patients requiring weaning: The evidence guiding practice. Critical Care Nurse, 25(4), 14–23. 10.4037/ccn2005.25.4.14 [DOI] [PubMed] [Google Scholar]
- Carter C., Osborn M., Agagah G., Aedy H., Notter J. (2020). COVID-19 disease: Invasive ventilation. Clinics in Integrated Care, 1, 100004. 10.1016/j.intcar.2020.100004 [DOI] [Google Scholar]
- Cederwall C. J., Plos K., Rose L., Dübeck A., Ringdal M. (2014). Critical care nurses management of prolonged weaning: An interview study. Nursing in Critical Care, 19(5), 236–242. 10.1111/nicc.12092 [DOI] [PubMed] [Google Scholar]
- Chatburn R. L., Deem S. (2007). Should weaning protocols be used with all patients who receive mechanical ventilation? Respiratory Care, 52(5), 609–621. 10.4187/respcare.02008 [DOI] [PubMed] [Google Scholar]
- Chawla R., Dixit S. B., Zirpe K. G., Chaudhry D., Khilnani G. C., Mehta Y., Khatib K.I, Jagiasi B.G., Chanchalani G., Mishra R., C., Samavedam S., Govil D., Gupta S., Prayag S., Ramasubban S., Dobariya J., Marwah V., Sehgal I., Jog S., A., Kulkarni A. P. (2020). ISCCM guidelines for the use of non-invasive ventilation in acute respiratory failure in adult ICUs. Indian Journal of Critical Care Medicine, 24(Suppl1), S61–S81. https://www.ijccm.org/doi/pdf/10.5005/jp-journals-10071-G23186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumello D., Coppola S., Froio S., Gotti M. (2016). What’s next after ARDS: Long-term outcomes. Respiratory Care, 61(5), 689–699. 10.4187/respcare.04632. [DOI] [PubMed] [Google Scholar]
- Chiumello D., Modafferi L., Fratti I. (2022). Risk factors and mortality in elderly ARDS COVID-19 compared to patients without COVID-19. Journal of Clinical Medicine, 11(17), 5180. https://doi.org/10.3390%2Fjcm11175180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker C., Scholes J. (2009). The importance of knowing the patient in weaning from mechanical ventilation. Nursing in Critical Care., 14(6), 289–296. 10.1111/j.1478-5153.2009.00355.x.[PubMed: 19840275] [DOI] [PubMed] [Google Scholar]
- Esteban A., Anzueto A., Frutos F., et al. (2002). Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA, 287(3), 345–355. 10.1001/jama.287.3.345 [DOI] [PubMed] [Google Scholar]
- European Federation of Critical Care Nursing Associations. (2012 [cited Feb 2]). Position statement on nurses’ role in weaning from ventilation 2012 Amsterdam: EfCCNa. Available from:http://www.efccna.org
- Ferrando, C., Suarez-Sipmann, F., Mellado-Artigas, R., Hernandez, M., Gea, A., Arruti, E., Aldecoa, C., Martinez-Palli, G., Martinez-Gonzalez, M. A., Slutsky, A. S., Villar, J., & COVID-19 Spanish ICU Network. (2020). Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ards. Intensive Care Medicine, 46(12), 2200–2211. 10.1007/s00134-020-06192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberini, L., Tonetti, T., Spadaro, S., Zani, G., Mazzoli, C. A., Capozzi, C., Giampalma, E., Reggiani, M. L. B., Bertellini, E., Castelli, A., Cavalli, I., Colombo, D., Crimaldi, F., Damiani, F., Fogagnolo, A., Fusari, M., Gamberini, E., Gordini, G., Laici, C., Lanza, M. C., … ICU-RER COVID-19 Collaboration. (2020). Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: Multicenter observational study in fifteen Italian ICUs. Journal Of Intensive Care, 8(1), 96. 10.1186/s40560-020-00499-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani A., Paderewska J., Sainis A., Crispin A., Walcher S., Neurohr C. (2020). Variables predicting weaning outcome in prolonged mechanically ventilated tracheotomized patients: A retrospective study. Journal of Intensive Care, 8(1), 1–10. 10.1186/s40560-020-00460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Cattaneo E., Florio G., Ippolito M., Zanella A., Cortegiani A., Huang J., Pesenti A., Einav S. (2021). Mechanical ventilation parameters in critically ill COVID-19 patients: A scoping review. Critical Care, 25, 115. 10.1186/s13054-021-03536-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur K., Price C. P. E., Gray E. L., Gulati R. K., Maksimoski M., Racette S. D., Schneider A. L., Khanwalkar A. R. (2020). Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngology–Head and Neck Surgery, 163(1), 170–178. 10.1177/0194599820936749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondili E., Makris D., Georgopoulos D., Rovina N., Kotanidou A., Koutsoukou A. (2021). COVID-19 ARDS: Points to be considered in mechanical ventilation and weaning. Journal of Personalized Medicine, 11(11), 1109. 10.3390/jpm11111109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacedonia D., Scioscia G., Santomasi C., Fuso P., Carpagnano G. E., Portacci A., Mastroianni F., Larizza G., Sabato E., Profilo E., Resta E., Foschino Barbaro M. P., Resta O., (2021). Impact of smoking, COPD and comorbidities on the mortality of COVID-19 patients. Scientific Reports, 11(1), 19251. 10.1038/s41598-021-98749-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle C., Dowling M. (2018). The factors which influence nurses when weaning patients from mechanical ventilation: Findings from a qualitative study. Intensive and Critical Care Nursing, 27(5), 244–252. 10.1016/j.iccn.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Lin S., Zhou D., Zhou F. (2020). Coronavirus disease 2019 (COVID-19): Cytokine storms, hyperinflammatory phenotypes, and acute respiratory distress syndrome. Genes & Diseases, 7(4), 520–527. 10.1016/j.gendis.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Zheng Y., Yang L., Liu S., Zhu J., Zhao N., Pang B., Cao Z., Ma Y. (2018). Neutrophil/lymphocyte ratio is helpful for predicting weaning failure: A prospective, observational cohort study. Journal of Thoracic Disease, 10(9), 5232–5245. PMID: 30416770; PMCID: PMC6196202.https://doi.org/10.21037%2Fjtd.2018.08.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J., Gattinoni L. (2020). Management of COVID-19 respiratory distress. JAMA, 323(22), 2329–2330. 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- Mughal M. S., Kaur I. P., Jaffery A. R., Dalmacion D. L., Wang C., Koyoda S., … Granet K. M. (2020). COVID-19 patients in a tertiary US hospital: Assessment of clinical course and predictors of the disease severity. Respiratory Medicine, 172, 106130. 10.1016/j.rmed.2020.106130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar S. N., Gurjar M., Baronia A. K., Azim A., Mishra P., Poddar B., Singh R. (2017). Predictors and pattern of weaning and long-term outcome of patients with prolonged mechanical ventilation at an acute intensive care unit in North India. Revista Brasileira De Terapia Intensiva, 29(1), 23–33. 10.5935/0103-507X.20170005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir, S., Martin-Loeches, I., Povoa, P., Metzelard, M., Du Cheyron, D., Lambiotte, F., Tamion, F., Labruyere, M., Makris, D., Boulle Geronimi, C., Pinetonde Chambrun, M., Nyunga, M., Pouly, O., Méègarbane, B., Saade, A., Gomà, G., Magira, E., Llitjos, J. F., Torres, A., Ioannidou, I. …, coVAPid study group. (2021). Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: A planned ancillary analysis of the covapid cohort. Critical Care, 25(1), 177. 10.1186/s13054-021-03588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M., Poeira A., Flamino R., Flamino R., Santos N. (2020). Nursing interventions in the extubation process: A scoping review. International Physical Medicine & Rehabilitation Journal, 5(6), 258–263. 10.15406/ipmrj.2020.05.00268 [DOI] [Google Scholar]
- Rose L., Blackwood B., Burns S. M., Frazier S. K., Egerod I. (2011). International perspectives on the influence of structure and process on weaning from mechanical ventilation. American Journal of Critical Care Nursing, 20(1), e10–e18. 10.4037/ajcc2011430. [DOI] [PubMed] [Google Scholar]
- Rose L, Nelson S., Johnston L., Presneill J. J. (2007). Decisions made by critical care nurses during mechanical ventilation and weaning in an Australian intensive care unit. American Journal of Critical Care, 16(5), 434–443. 10.4037/ajcc2007.16.5.434 [DOI] [PubMed] [Google Scholar]
- Rouzé, A., Martin-Loeches, I., Povoa, P., Makris, D., Artigas, A., Bouchereau, M., Lambiotte, F., Metzelard, M., Cuchet, P., Boulle Geronimi, C., Labruyere, M., Tamion, F., Nyunga, M., Luyt, C. E., Labreuche, J., Pouly, O., Bardin, J., Saade, A., Asfar, P., Baudel, J. L., … coVAPid study Group (2021). Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Medicine, 47(2), 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C. D., Lone N. I., Baillie J. K. (2023). Comorbidities, multimorbidity and COVID-19. Nature Medicine, 29(2), 334–343. 10.1038/s41591-022-02156-9 [DOI] [PubMed] [Google Scholar]
- Senior J. R. (2012). Alanine aminotransferase: A clinical and regulatory tool for detecting liver injury–past, present, and future. Clinical Pharmacology & Therapeutics, 92(3), 332–339. 10.1038/clpt.2012.50. [DOI] [PubMed] [Google Scholar]
- Shrestha G. S., Khanal S., Sharma S., Nepal G. (2020). COVID-19: Current understanding of pathophysiology. Journal of Nepal Health Research Council, 18(3), 351–359. 10.33314/jnhrc.v18i3.2459 [DOI] [PubMed] [Google Scholar]
- Swenson K. E., Swenson E. R. (2021). Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Critical Care Clinics, 37(4), 749–776. 10.1016/j.ccc.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. T., Chambers R. C., Liu K. D. (2017). Acute respiratory distress syndrome. New England Journal of Medicine, 377(6), 562–572. 10.1056/NEJMra1603418 [DOI] [PubMed] [Google Scholar]
- Tingsvik C., Johansson K., Martensson J. (2015). Weaning from mechanical ventilation: Factors that influence intensive care nurses’ decision making. Nursing in Critical Care, 20(1), 16–24. 10.1111/nicc.12116. [DOI] [PubMed] [Google Scholar]
- Tolossa, T., Merdassa Atomssa, E., Fetensa, G., Bayisa, L., Ayala, D., Turi, E., Wakuma, B., Mulisa, D., Seyoum, D., Getahun, A., Shibiru, T., Fekadu, G., Desalegn, M., & Bikila, H. (2022). Acute respiratory distress syndrome among patients with severe COVID-19 admitted to treatment center of Wollega University Referral Hospital, Western Ethiopia. PLoS ONE, 17(6), e0267835. 10.1371/journal.pone.0267835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang L., Xi X., Zhou J. X., & China Critical Care Sepsis Trial (CCCST) Workgroup. (2021). The association between etiologies and mortality in acute respiratory distress syndrome: A multicenter observational cohort study. Frontiers in Medicine, 8, 739596. 10.3389/fmed.2021.739596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware L. B., Koyama T., Billheimer D. D., Wu W., Bernard G. R., Thompson B. T., Brower R. G., Standiford T. J., Martin T. R., Matthay M. A. and NHLBI ARDS Clinical Trials Network (2010). Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest, 137(2), 288–296. 10.1378/chest.09-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzeniak I. C., Regina Rios Vieira S., Almeida Victorino J. (2018). Weaning from mechanical ventilation in ARDS: Aspects to think about for better understanding, evaluation, and management. Biomed Research International, 2018, 5423639. 10.1155/2018/5423639.PMID: 30402484; PMCID: PMC6198583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2020). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. Paediatrics and Family Medicine, 16(1), 9. https://www.who.int/europe/publications/i/item/WHO-2019-nCoV-clinical-2020-4 [Google Scholar]
- Yanez N. D., Weiss N. S., Romand J. A., et al. (2020). COVID-19 mortality risk for older men and women. BMC Public Health, 20(1), 1742. 10.1186/s12889-020-09826-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Hsiao J. L., Wu M. F., Lu M. H., Chang H. M., Ko W. S., Chiou Y. L. (2018). The declined levels of inflammatory cytokines related with weaning rate during period of septic patients using ventilators. The Clinical Respiratory Journal, 12(2), 772–778. 10.1111/crj.12593 [DOI] [PubMed] [Google Scholar]
- Zhao H., Su L., Ding X., Chen H., Zhang H., Wang J., Long Y., Zhou X., Zhang S. (2021). The risk factors for weaning failure of mechanically ventilated patients with COVID-19: A retrospective study in national medical team work. Frontiers in Medicine, 8(1), 678157. 10.3389/fmed.2021.678157 [DOI] [PMC free article] [PubMed] [Google Scholar]