Abstract
Background:
Adverse childhood experiences during key developmental periods have been shown to impact long-term health outcomes. Adverse childhood experiences may include psychological, physical, or sexual abuse; neglect; or socioeconomic factors. Adverse childhood experiences are linked with an increase in poor health behavior such as smoking and alcohol consumption, and may also influence epigenetic changes, inflammatory response, metabolic changes, and allostatic load.
Objective:
We sought to explore associations between adverse childhood experiences and allostatic load in adult female participants in the UK Biobank.
Design:
The UK Biobank is a multisite cohort study established to capture lifestyle, environment, exposure, health history, and genotype data on individuals in the United Kingdom.
Methods:
Adverse childhood experiences were assessed from the Childhood Trauma Screener, which measures abuse and neglect across five items. Biological measures at enrollment were used to construct allostatic load, including measures of metabolic, inflammatory, and cardiovascular function. Females with a cancer diagnosis prior to enrollment were removed as it may influence allostatic load. Poisson regression models were used to assess the association between adverse childhood experiences and allostatic load, accounting for a priori confounders.
Results:
A total of 33,466 females with complete data were analyzed, with a median age at enrollment of 54 (range = 40–70) years. Among the study sample, the mean allostatic load ranged from 1.85 in those who reported no adverse childhood experiences to 2.45 in those with all adverse childhood experiences reported. In multivariable analysis, there was a 4% increase in average allostatic load among females for every additional adverse childhood experience reported (incidence rate ratio = 1.04, 95% confidence interval = 1.03–1.05). Similar results were observed when assessing individual adverse childhood experience components.
Conclusion:
This analysis supports a growing body of evidence suggesting that increased exposure to early life abuse or neglect is associated with increased allostatic load in females.
Keywords: adverse childhood experiences, allostatic load, biological embedding, early life, life course
Introduction
Adverse childhood experiences (ACEs) during key developmental periods impact long-term health outcomes through various mechanisms. 1 ACEs may include psychological, physical, or sexual abuse; neglect; instability; and early life socioeconomic factors. ACEs are linked to behavioral changes1–5 as well as changes in epigenetic profiles, inflammatory response, metabolic changes, and increased allostatic load (AL).6–10
There are two proposed mechanisms by which ACEs may impact long-term health: behavior and biology. First, increased exposure to early stress has been demonstrated to influence poor health behaviors such as smoking, alcohol intake, and poor diet,11,12 and reduced odds of compliance with important interventions such as breast cancer screening recommendations in adulthood. 13 The second proposed mechanism is via biological embedding, the process by which chronic exposure to stress can lead to dysregulation of key physiological processes which manage stress hormones. 14 While behavioral changes may be due to coping mechanisms or learned behavior, it has been increasingly suggested that physiological changes in the brain may play a role. Although some stress during childhood is necessary for normal emotional and cognitive growth, chronic exposure to stress can disrupt healthy brain development, impacting pleasure and reward feedback, executive functioning, and response to fear. 15 Chronic exposure to stress in childhood is also believed to result in increased inflammation over the life course.12,16 Collectively, ACEs may lead to an increased risk of mental illness, chronic inflammation, and predisposition to chronic health issues. 17
The AL framework was proposed to describe the mechanisms by which chronic stress may impact physiological dysregulation. Allostasis is the process by which our bodies modulate the internal environment to accommodate exogenous stressors.10,18 Physiologically, three main mediators lead to dysregulation of internal systems, resulting in abnormal ranges in clinical biomarkers. 19 The first mediator is the neuroendocrine system. Perceived threats initiate the sympathetic–adrenal–medullary (SAM) axis release of catecholamines such as epinephrine and norepinephrine, and the hypothalamic–pituitary–adrenal (HPA) axis secretion of glucocorticoids such as cortisol. 20 The second mediator is neurophysiological, wherein chronic stress exposure can alter physiological function of the sympathetic and parasympathetic nervous systems, resulting in cardiovascular changes such as increased heart rate variability. 21 The third mediator is the inflammatory system, where chronic stress can lead to an increase in proinflammatory cytokines such as tumor necrosis factor α (TNFα). 22 These systems work to mobilize energy for fight-or-flight responses; however, in response to chronic stress, these mechanisms can be dysregulated, resulting in a shift in homeostasis toward abnormal ranges. 23 AL is a composite measure of biomarkers associated with the neuroendocrine, neurophysiological, and inflammatory systems that is intended to quantify damages to the body during repeated allostatic response to stress. 23
The mechanisms by which early life stress may be internalized have been posited to differ by sex. Animal studies suggest that the effect of chronic stress on neural pathways varies according to sex, which is further impacted by hormonal differences, 24 specifically the presence or absence of ovarian hormones estrogen and progesterone was shown to modify stress response in female rats. 25 Assessments of childhood trauma reporting show important differences between males and females, including the type and frequency of trauma reported, and their associations with long-term health.26,27 While several studies have identified associations between childhood adversity and AL in adulthood, 28 few studies have focused on female participants only. In studies assessing females in the United States, mixed results were observed.29,30 Among female veterans, AL was found to not differ between participants by reported sexual abuse, 29 whereas a study of African-American females found a history of ACEs was associated with increased AL after controlling for potential mediators. 30 With a focus on women’s health, this analysis is aimed at understanding the association between early life trauma as reported in the Childhood Trauma Screener (CTS)31,32 and AL in adulthood among female participants of the UK Biobank, with the hypothesis that an increased exposure to ACEs will be associated with a higher AL in adulthood.
Materials and methods
Population
The UK Biobank is a multisite cohort study established to collect lifestyle, environment, exposure, health history, and genotype data on individuals in the United Kingdom. During 2006–2010, the UK Biobank conducted its recruitment phase in which 502,419 participants aged 40 to 69 gave their written consent and completed their baseline assessment visit, including touch-screen questionnaires assessing lifetime exposure history, in-person interviews for physical measures, and blood, urine, and saliva sample collection. Detailed information on the study design, data capture, and variable definitions can be found on the UK Biobank website (https://www.ukbiobank.ac.uk/).
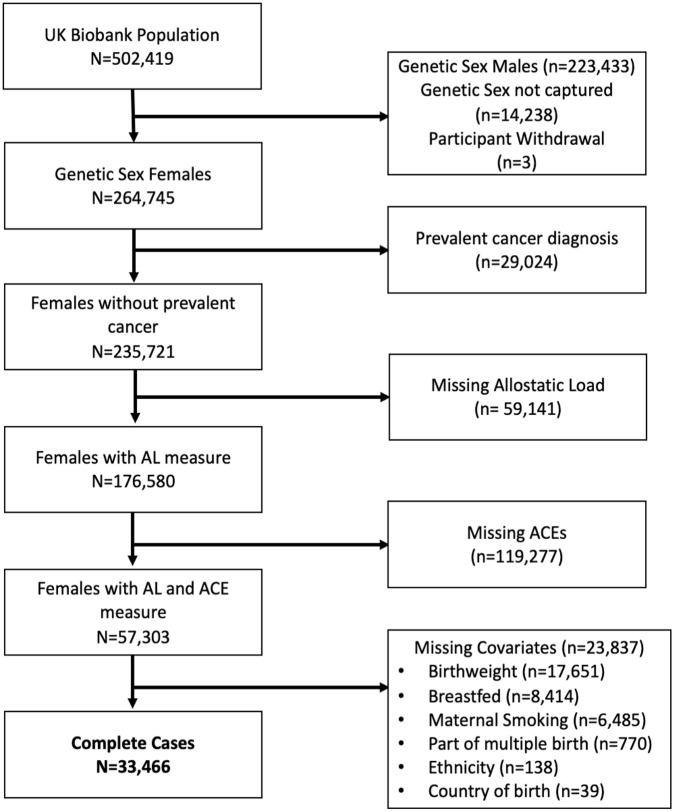
Our study sample was restricted to female participants identified by chromosomal sex, resulting in 264,748 females based on chromosomal status. Participants with a cancer diagnosis prior to enrollment were excluded from analysis since the stress of this prior diagnosis and subsequent therapies may influence AL. 33 Data on cancer diagnoses are provided to the UK Biobank by the Medical Research Information Service and the Information Services Division of NHS Scotland. The UK Biobank is provided with all cancer diagnoses preceding the initiation of the study dating back to 1970 and following enrollment. The latest cancer registry record linked to the UK Biobank data was on 16 November 2021. 34 The final analytic cohort consisted of complete cases with respect to AL, ACEs, and covariates identified via our prespecified directed acyclic graph (Figure 1). From eligible female participants, 59,141 were missing at least one measure needed for the AL score. In addition, 119,277 participants were missing or did not respond to ACE questions. Of females with both AL and ACE measures fully captured, 23,867 were missing at least one covariate. The final analysis set consisted of 33,466 complete cases (Figure 2).
Figure 1.
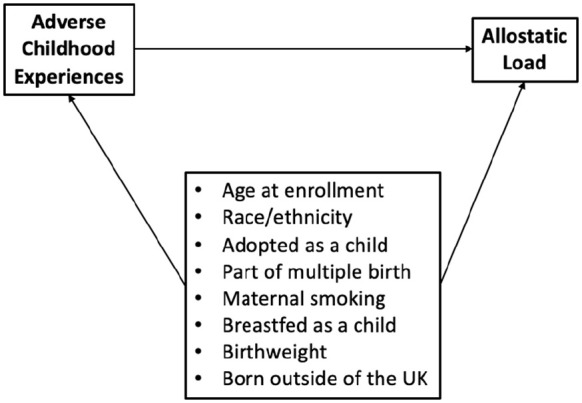
Directed acyclic graph.
Figure 2.
Participant flow chart.
AL: allostatic load; ACE: adverse childhood experience.
Exposure assessment
ACEs were measured from the CTS, 31 which measures exposure to abuse (physical, psychological, and sexual) and neglect (physical and emotional). These five items are measured on a Likert-type scale as “never,” “rarely,” “sometimes,” “often,” and “very often.” The presence of ACEs was assessed by the validated instrument, such that physical and psychological abuse were considered present if the respondent answered “sometimes,” “often,” or “very often”; sexual abuse was considered present if the respondent answered “rarely,” “sometimes,” “often,” or “very often”; and factors associated with neglect were considered present if the respondent answered “never” or “rarely” (Table 1). 35 Additional information on the CTS measure is available in Supplementary Table S1.
Table 1.
Adverse childhood experiences.
| Domain | Item | “When I was growing up. . .” |
|---|---|---|
| Abuse | Physical | People in my family hit me so hard that it left me with bruises or marks |
| Psychological | I felt that someone in my family hated me | |
| Sexual | Someone molested me (sexually) | |
| Neglect | Physical | There was someone to take me to the doctor if I needed it |
| Emotional | I felt loved |
The ACE score was calculated by summing the presence of factors associated with abuse and neglect. A score of 0 represented no reported ACEs, and a score of 5 represented a report of all ACE items.
Outcome definitions
AL was determined based on 10 available biomarkers assessed at the baseline visit associated with dysregulation of metabolic, cardiovascular, and inflammatory systems.19,36 Unfasted blood serum biomarkers included serum glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol, HbA1c, insulin-like growth factor 1 (IGF-1), and C-reactive protein. Trained staff collected physical measures, including waist and hip circumference, weight, height, and blood pressure. Methods for assays and physical measures are outlined in Table 2.
Table 2.
Biomarkers and clinical thresholds for the construct of allostatic load.
| Domain | Biomarker | Analysis methodology | Clinical threshold | % dysregulated |
|---|---|---|---|---|
| Metabolic | Glucose | Enzymatic | ⩾ 7.0 mmol/L (32) | 2% |
| Total cholesterol | Enzymatic | >6.2 mmol/L (33) | 35% | |
| HDL | Enzyme immuno-inhibition | <1.3 mmol/L (33) | 19% | |
| HbA1c | High-performance liquid chromatography | ⩾ 48 mmol/mol (34) | 11% | |
| IGF-1 | Chemiluminescent Immunoassay—one-step sandwich | Age-specific (35–37) | 19% | |
| Age 36–40 | >30.3 nmol/L | |||
| Age 41–45 | >29.1 nmol/L | |||
| Age 46–50 | >28.6 nmol/L | |||
| Age 51–55 | >27.5 nmol/L | |||
| Age 56–60 | >25.4 nmol/L | |||
| Age 61–65 | >23.1 nmol/L | |||
| Age 66–70 | >21.5 nmol/L | |||
| Age 71+ | >21.0 nmol/L | |||
| Waist-to-hip ratio | Wessex non-stretchable sprung tape measure, manual entry | >0.85 (38) | 24% | |
| BMI | Tanita BC-418 MA body composition analyzer, Seca 202 height measure, manual entry | >30.0 kg/m2 (39) | 18% | |
| Inflammatory | C-reactive protein | Immuno-turbidimetric | ⩾ 3 mg/L (40) | 19% |
| Cardiovascular | SBP | Omron HEM-7015IT digital blood pressure monitor, two readings, automatic entry | >140/90 (41) | 38% |
| DBP |
HDL: high-density lipoprotein; IGF: insulin-like growth factor; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Metabolic dysregulation was measured by serum glucose (mmol/L), total cholesterol (mmol/L), HDL cholesterol (mmol/L), HbA1c (mmol/mol), waist-to-hip ratio, and body mass index (BMI). Inflammatory dysregulation was assessed by C-reactive protein (mg/L), and cardiovascular dysregulation was assessed by systolic blood pressure (SBP; mmHg) and diastolic blood pressure (DBP; mmHg). Blood pressure dysregulation was estimated based on criteria for hypertension, requiring either SBP or DBP being beyond their thresholds.
A derived indicator for each biomarker was determined by clinical cutoff points identified in literature or clinical guidelines. An assigned value of 1 indicated the measure was beyond the clinically normal range (Table 2). In addition, self-reported use of medication for cholesterol, blood pressure, or diabetes (glucose) was imputed as HDL cholesterol, SBP/DBP, or glucose being beyond the clinical threshold, respectively. AL was estimated as a summation of these multi-system indicators. Because SBP and DBP were combined into a single blood pressure measurement, the possible range of scores is from 0 (no dysregulation) to 9 (all markers dysregulated).
AL was modeled as a continuous count measure, representing the number of dysregulated biomarkers. For descriptive purposes, a prespecified categorical variable was constructed with low AL representing approximately the bottom 80th percentile and high AL representing approximately the top 20th percentile. All measures were taken at or shortly following enrollment into the UK Biobank study, and for participants with more than one valid measure of any biomarker, the instance with the closest proximity to enrollment was used.
Covariates
Potential confounding factors between early life trauma and AL in adulthood were specified a priori based on plausible effects and availability in the UK Biobank database (Figure 1). As date of birth and date of enrollment are considered personal information and therefore not shared under General Data Protection Regulation (GDPR), participant age was estimated as year of enrollment – year of birth and is therefore susceptible to rounding error. Age was scaled per 10 years for modeling purposes. Race was self-reported and considered across four categories: Caucasian, Black, Asian, and Other. Early life factors as self-reported through questionnaires included the participant’s place of birth (United Kingdom or outside of the United Kingdom), whether the participant’s mother smoked around the time of their birth, whether the participant was breastfed as a baby, whether the participant was part of multiple births, and the participant’s weight at birth (kg). Adoption at birth was included as a prespecified covariate; however, the rate of reporting this factor was rare and not carried through into modeling. Responses of “don’t know” and “prefer to not respond” were treated as missing.
Statistical analysis
The final analytic data set included complete cases, and a STROBE diagram was constructed to display participant disposition. Population characteristics were described overall as well as those with low versus high AL. Continuous measures were displayed as mean (standard deviation (SD)) as well as median (minimum to maximum). Categorical variables were displayed as n (%). Comparison of population characteristics of complete cases and the full eligible population was explored to assess potential selection bias in the analytic population.
Because AL is a count of dysregulated systems, univariable and multivariable Poisson regression was used to assess the association between number of reported ACEs and AL. The association between individual components of AL and ACE measures was also explored. Multivariable models included a priori–defined confounding factors (Figure 1). Potential heterogeneity of the association between ACE and AL was assessed through inclusion of age, race, and early life factors as multiplicative terms with ACE count, with a 1% level of significance indicating heterogeneity. For all models, robust standard errors were estimated using the R package sandwich, 37 and exponentiated confidence intervals were derived using the delta method via the R package msm. 38 Model assumptions were visually assessed as any small divergence from the assumptions would come out significant due to the large sample size. With over 30,000 complete cases, this analysis is powered to detect at least a 5% increase in incidence rate ratio (IRR) at over 90% power.
Data management was conducted using SAS software, Version 9.4 (SAS Institute Inc, Cary, NC, USA). Analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria). STROBE Guidelines were used when preparing this article.
Results
Sample characteristics
The analysis sample had an average age of 54 years and was 97.8% White, and 93.6% were born in the United Kingdom. Maternal smoking at birth was reported by 26.8% of participants, and 29.0% reported not being breastfed. Median birth weight was reported at 3.29 kg. Comparison of the complete cases against the overall eligible population indicates those without missing data tended to be younger (median age 54 vs 57 years), a higher proportion were White (97.8% vs 94.4%), have higher level of education (46.2% college educated vs 29.4%), and lower index of multiple deprivation (median 11.0 vs 12.9; Supplementary Table S2).
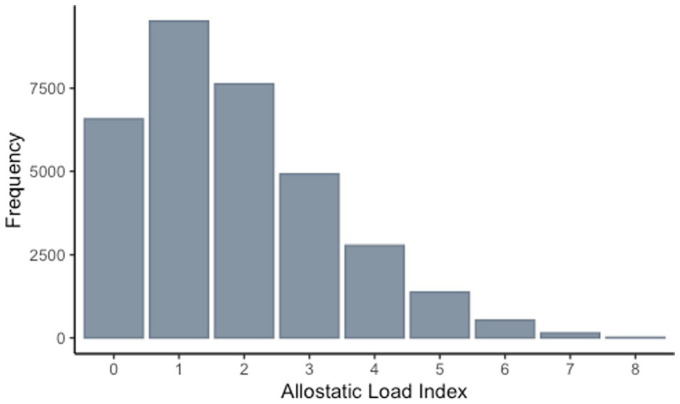
Across the nine components of AL, the percent of participants who were classified as dysregulated ranged from 2% in glucose to 51% in BP (Table 2). AL score ranged from 0 to 8 in the analysis sample, with a mean AL score of 1.85 (SD = 1.5). The distribution of AL scores is skewed right and approximately follows a Poisson distribution (λ≅2) (Figure 3). With 85% of individuals having an AL score of 0–3, the dichotomous AL measure was defined as low (AL ⩽ 3) and high (AL > 3). Table 3 describes participant characteristics by low versus high AL as well as overall. In bivariate analyses, age, race, birth origin, and maternal smoking were associated with a significant increase in average AL, while increase in reported birth weight was associated with reduced average AL (Supplementary Table S1).
Figure 3.
Distribution of allostatic load.
Table 3.
Patient characteristics by allostatic load.
| Allostatic load 0–3 (n = 28,626) |
Allostatic load 4–8 (n = 4840) |
Overall (N = 33,466) |
|
|---|---|---|---|
| Age at enrollment a | |||
| Mean (SD) | 53.5 (7.54) | 56.6 (7.37) | 54.0 (7.59) |
| Median (min, max) | 53.0 (40.0, 70.0) | 58.0 (40.0, 70.0) | 54.0 (40.0, 70.0) |
| Race | |||
| White | 28,019 (97.9%) | 4703 (97.2%) | 32,722 (97.8%) |
| Black | 113 (0.4%) | 39 (0.8%) | 152 (0.5%) |
| Asian | 196 (0.7%) | 36 (0.7%) | 232 (0.7%) |
| Other | 298 (1.0%) | 62 (1.3%) | 360 (1.1%) |
| Born in the United Kingdom | |||
| No | 1922 (6.7%) | 241 (5.0%) | 2163 (6.5%) |
| Yes | 26,704 (93.3%) | 4599 (95.0%) | 31,303 (93.5%) |
| Maternal smoking at birth | |||
| No | 21,090 (73.7%) | 3407 (70.4%) | 24497 (73.2%) |
| Yes | 7536 (26.3%) | 1433 (29.6%) | 8969 (26.8%) |
| Breastfed as baby | |||
| No | 8297 (29.0%) | 1401 (28.9%) | 9698 (29.0%) |
| Yes | 20,329 (71.0%) | 3439 (71.1%) | 23,768 (71.0%) |
| Part of multiple births | |||
| No | 27,993 (97.8%) | 4745 (98.0%) | 32,738 (97.8%) |
| Yes | 633 (2.2%) | 95 (2.0%) | 728 (2.2%) |
| Birth weight (kg) | |||
| Mean (SD) | 3.29 (0.572) | 3.23 (0.637) | 3.28 (0.582) |
| Median (min, max) | 3.29 (0.910, 10.0) | 3.23 (0.620, 6.35) | 3.29 (0.620, 10.0) |
SD: standard deviation.
Age is estimated as [year of enrollment – year of birth] and is, therefore, susceptible to rounding error.
The distribution of AL by reported ACEs are presented in Table 4. About 75.3% of the participants in the analytic sample had no reported ACEs, and 2.9% had three or more ACEs. An increased number of reported ACEs is associated with an increased average AL, with mean (SD) AL of 1.85 (1.49) in individuals with no ACEs versus 2.45 (1.71) in those with five ACEs. This trend is consistent across ACE categories, with higher average AL observed in respondents reporting the ACE domain as being present.
Table 4.
Allostatic load by reported adverse childhood experiences.
| n (%) | Mean AL (SD) | |
|---|---|---|
| Number of ACEs | ||
| 0 | 27,585 (75.3%) | 1.85 (1.49) |
| 1 | 6043 (16.5%) | 1.87 (1.54) |
| 2 | 1928 (5.3%) | 1.91 (1.56) |
| 3 | 750 (2.0%) | 1.99 (1.62) |
| 4 | 252 (0.7%) | 2.17 (1.63) |
| 5 | 74 (0.2%) | 2.45 (1.71) |
| Abuse—Physical | ||
| Never true | 30,250 (82.6%) | 1.85 (1.49) |
| Rarely true | 3559 (9.7%) | 1.90 (1.57) |
| Sometimes true | 2320 (6.3%) | 1.93 (1.55) |
| Often true | 314 (0.9%) | 2.10 (1.73) |
| Very often true | 189 (0.5%) | 2.19 (1.76) |
| Abuse—Psychological | ||
| Never true | 30,381 (82.9%) | 1.87 (1.50) |
| Rarely true | 2316 (6.3%) | 1.81 (1.50) |
| Sometimes true | 2821 (7.7%) | 1.84 (1.53) |
| Often true | 598 (1.6%) | 1.86 (1.59) |
| Very often true | 516 (1.4%) | 2.07 (1.72) |
| Abuse—Sexual | ||
| Never true | 32,706 (89.3%) | 1.86 (1.50) |
| Rarely true | 1994 (5.4%) | 1.84 (1.51) |
| Sometimes true | 1470 (4.0%) | 2.00 (1.61) |
| Often true | 257 (0.7%) | 1.85 (1.56) |
| Very often true | 205 (0.6%) | 2.32 (1.77) |
| Support—Emotional | ||
| Never true | 423 (1.2%) | 2.09 (1.63) |
| Rarely true | 1494 (4.1%) | 1.92 (1.58) |
| Sometimes true | 5640 (15.4%) | 1.88 (1.53) |
| Often true | 8540 (23.3%) | 1.90 (1.51) |
| Very often true | 20,535 (56.1%) | 1.84 (1.49) |
| Support—Physical | ||
| Never true | 662 (1.8%) | 2.11 (1.58) |
| Rarely true | 264 (0.7%) | 2.06 (1.47) |
| Sometimes true | 921 (2.5%) | 2.02 (1.59) |
| Often true | 3735 (10.2%) | 2.03 (1.55) |
| Very often true | 31,050 (84.8%) | 1.83 (1.50) |
AL: allostatic load; ACE: adverse childhood experience; SD: standard deviation.
Categories in italics define those used to indicate the presence of abuse or neglect. Row percentages are presented.
Association between ACEs and AL
Univariable and multivariable Poisson regression models assessing the association between ACEs are reported in Table 5. Assessment of interactions showed no evidence of heterogeneity of effect between ACE and AL (data not shown), and as such models report overall association between ACEs and AL. In univariable models, average AL is expected to increase by 3% per one ACE reported (IRR = 1.03, 95% confidence interval (CI) = 1.02–1.04). Multivariable models adjusting for age, race, and early life factors result in similar associations (IRR = 1.04, 95% CI = 1.03–1.05).
Table 5.
Poisson regression models of allostatic load.
| Covariate | Incidence rate ratio | 95% CI |
|---|---|---|
| ACE burden | ||
| Univariable model | 1.03 | 1.02–1.04 |
| Multivariable model a | 1.04 | 1.03–1.05 |
CI: confidence interval; ACE: adverse childhood experience.
Adjusted for age, race, birth origin, maternal smoking, history of being breastfed, part of multiple birth, and weight at birth.
Exploratory analyses of ACE and AL components
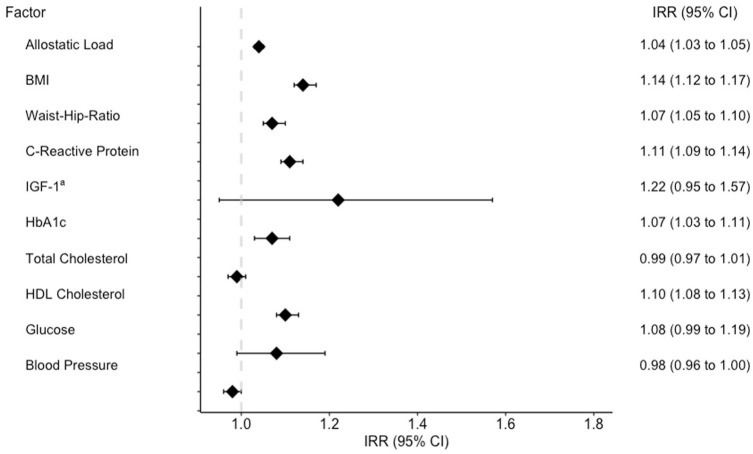
Individual components of AL were assessed for their association with ACEs. After adjustment for sex, race, and early life factors, individuals reporting a higher number of ACEs were found to have increased dysregulation in BMI (IRR = 1.14, 95% CI = 1.12–1.17), waist-to-hip ratio (IRR = 1.07, 95% CI = 1.07–1.10), C-reactive protein (IRR = 1.11, 95% CI = 1.09–1.14), HbA1c (IRR = 1.07, 95% CI = 1.03–1.11), and HDL Cholesterol (IRR = 1.10, 95% CI = 1.08–1.13) (Figure 4).
Figure 4.
Multivariable Poisson regression of AL (continuous) and individual AL components (yes vs no) by ACE burden. Models adjusted for age, race, birth origin, maternal smoking, history of being breastfed, part of multiple birth, and weight at birth.
AL: allostatic load; ACE: adverse childhood experience; BMI: body mass index; IGF-1: insulin-like growth factor 1; HDL: high-density lipoprotein; IRR: incidence rate ratio; CI: confidence interval.
aDue to age-specific patterns of dysregulation, IGF-1 model includes interaction with mean-centered age.
In multivariable models, the presence of individual components of the ACE score was found to be associated with increased AL. Across all domains, the reporting of that ACE was associated with an increased average AL with the highest magnitude of association in those with physical abuse (IRR = 1.10, 95% CI = 1.07–1.14), sexual abuse (IRR = 1.07, 95% CI = 1.04–1.10), and physical neglect (IRR = 1.08, 95% CI = 1.03–1.13). Physical abuse showed a dose-response trend, with increasing frequency of exposure leading to an increase in average AL ranging from an expected 9% and 10% increase in average AL in those reporting rarely and sometimes vs never, respectively, to a 18% and 23% increase in average AL in those reporting often and very often vs never, respectively. The dose-response trend was less evident in other ACE domains; however, across all ACE domains the reporting of any abuse or neglect with frequency of often or very often had the highest magnitude of association (Supplementary Figure S1).
Discussion
In this study of female participants in the UK Biobank, a significant association between reported ACEs and AL in adulthood is observed. Associations persisted after controlling for a priori confounding factors as defined by our directed acyclic graph. These results are in alignment with a growing body of evidence identifying an association between early life stress and physiological dysregulation in adulthood.
Several studies have explored the association between reported ACEs and AL. In a systematic review of articles assessing the association between ACEs and AL, 14 of 19 studies found a history of ACEs was associated with elevated AL in adulthood. 28 Two of the identified studies focused specifically on female populations. Among female veterans, Beckie et al. 29 reported that women with a history of sexual assault during childhood as well as civilian and military life presented with increased AL, but not those reporting either individually. An analysis by Berge et al. 39 found that among Black women, a reported history of childhood adversity was significantly associated with increased AL, with an estimated 11% increase in AL count per 1 standard deviation increase in early life adversity. Berge et al. 39 used a five-item questionnaire focusing on parental involvement, divorce, parental death, household violence, and marital conflict, which varies substantially from the ACE domains included in this current analysis.
Additional studies explored interactions with or stratification by sex, with mixed results with respect to sex-specific patterns. In an analysis of individuals living with HIV, Wallace et al. 40 identified a significant increase in mean AL with an increasing number of ACEs reported, though a non-significant interaction with sex. One study reported a significant association between stressful events and circumstances in childhood and AL in males but not females; however, three separate studies reported significant associations between childhood adversity and AL in females but not males.41,42 Importantly, each of these studies had varying definitions of childhood adversity and constructs of AL, which may contribute to inconsistent findings.
Several studies were identified which captured similar domains of ACEs and their association AL components or related clinical outcomes in adult women.1,42–45 In a German population with ACEs reported via the CTS, female participants with a higher CTS score were found to be at increased risk of obesity. 43 While the association was attenuated after controlling for socioeconomic factors in adulthood, it should be noted that these factors may be on the causal pathway or act as independent risk factors, rather than true confounders. Two separate analyses from the Nurses’ Health Study demonstrated a dose-response relationship between early life physical and sexual abuse with hypertension 44 and type II diabetes 45 in women, results that persisted after adjusting for the potential mediation of BMI. Each of these clinical outcomes is related to biomarkers included in our current study, with alignment in our finding of significant associations between BMI, waist-to-hip ratio, and HbA1c which serve as clinical markers for obesity and diabetes.
While the mechanism by which ACEs may lead to increased dysregulation of biomarkers is not well understood, research focused on physical and sexual abuse in women demonstrate an increased risk of mental illness and depressive symptoms,46–48 which may impact health behaviors and physical well-being in adulthood, thereby mediating the observed associations. For example, Barboza Solís et al. 1 reported a significant association between childhood adversity and AL in adult women, but after controlling for health behaviors at the age of 23 the association disappeared, indicating strong mediation by health behavior.
This analysis used the CTS to estimate exposure to ACEs. Approximately 75% of the analytic sample reported no ACEs, which may be high. For instance, only 34% of the sample reported no ACEs in the landmark CDC-Kaiser ACE Study compared with 75% in this analysis. 49 However, among the questions that closely overlap between the original ACE study and CTS, prevalence of exposure to physical and psychological abuse was similar (physical abuse: 10.8% vs 7.7%; psychological abuse: 11.1% vs 10.8%) but not sexual abuse (22.0% vs 7.7%). The CTS is an abbreviated version of the 28-item Childhood Trauma Questionnaire (CTQ) which is a commonly used tool in assessing childhood trauma. 32 In validation of the tool, CTS showed high correlation to CTQ, 31 suggesting that this abbreviated survey may capture similar information as the more extensive ACE tools, albeit across fewer domains.
Constructs of AL are inconsistent across studies, with varying biomarkers included and estimation methods observed. Review of studies constructing a measure of AL identified inconsistency of biomarkers included in the construct of AL. 50 In addition, estimation of AL varied with respect to what constituted high measures for each component. In some studies, the cutoff point for high biomarker measures was established via clinical criteria, while others used sample-specific quartiles as cutoffs. Across all studies, AL comprised the sum of all biomarkers falling above the specified threshold. 33 However, in systematic reviews of associations between constructs of AL and health outcomes, consistent results demonstrating higher AL is related to increased risk of poor health outcomes suggests that AL may be robust against these variations. 51
Many of the reviewed studies included adjustment for covariates that represent post-exposure factors such as educational attainment, occupation, or health behaviors like smoking and alcohol use. Through careful construction of directed acyclic graph, we considered these factors to be potential mediators of the association between ACEs and AL rather than confounders. This analysis instead adjusted for early life factors which may influence both the likelihood of childhood trauma and adult health, and found no impact on the associations between ACEs and AL.
This study had strengths in the design and analysis compared with other assessments of ACEs on adult health. We focused our analysis on individuals without a diagnosis of cancer prior to the assessment of AL as the internalized stress of such a diagnosis along with subsequent therapies may artificially influence physiological measurements. In addition, the UK Biobank provides consistent measures of biomarkers, with all measurements adhering to the same protocol. This reduces noise that may be introduced in a population-based analysis where measures may be collected in varying manners. Our prespecification of a directed acyclic graph reduced the introduction of potential mediating factors which would alter the interpretability of our models, and instead focus on early life factors which may act as confounders between ACEs and AL in adulthood. Finally, the assessment of exposure was captured via a validated questionnaire on ACEs, which has been demonstrated to efficiently capture information on early life adversity.
This study also has several limitations. The CTS is an abbreviated questionnaire which may be missing critical aspects of early life adversity such as socioeconomic status (SES) and household dysfunction, both of which have been established as having association with AL in adulthood.28,49,52 Research has found early life socioeconomic status to be an important explanatory factor in a child’s physiological response to stress, 53 as well as to AL later in life.28,54,55 Furthermore, a general recollection of a “difficult” childhood was found to be strongly associated with AL in adulthood. 56 In addition, respondents may suffer from recall bias when responding to these questions. However, in an assessment of the validity of retrospective measures of ACEs it was concluded that false negatives are more likely than false positives. 57 Nevertheless, results from this analysis may be impacted by such bias. The construct of AL in this analysis was limited to biomarkers available through the UK Biobank study. Importantly, biomarkers from the neuroendocrine system were not incorporated, which represents a primary mediator for biological systems. Finally, both the UK Biobank population and complete case sample may suffer from selection bias, resulting in a lack of diversity and reducing generalizability to a broader population. In addition, only 14% of the original sample of eligible genetic females without a prevalent cancer were included in the analyses, due to missing data on the exposure, outcome, and covariates. The large amount of missing data leave open the distinct possibility for selection bias. If individuals with more ACEs were more likely to refuse to answer these questions due to their sensitive nature, and, if individuals with high AL were more likely to miss study visits for health reasons, then we would expect this to attenuate our association between ACEs and AL. In assessment of differences between the eligible population and complete cases, minor differences were seen in distributions of race and some early life factors. Assessment of socioeconomic factors, including Index of Multiple Deprivation and Education, suggests that females belonging to higher socioeconomic status were more likely to have complete data, including the reporting of ACE questions. As such, the possibility of selection and reporting bias cannot be ruled out. In addition, analysis of the UK Biobank against other UK population data suggests that the study population differs in critical ways compared to the sampling frame, including belonging to higher socioeconomic groups, lower reporting of poor health behaviors such as smoking and alcohol use, lower rate of self-reported disease, and lower rate of all-cause mortality, 58 which may have influenced the observed distribution of AL in our sample.
Conclusion
These findings support growing literature demonstrating long-term health effects of ACEs, with particular emphasis on AL in adult females. With increasing evidence of associations between childhood trauma and AL, there is a demonstrated need for translational efforts to inform clinical interventions and monitoring in both pediatric and adult settings. 59 The ability to identify individuals with prior exposure to ACEs who may be at increased risk of physiological dysregulation may allow for improved screening programs for downstream chronic disease, or interventional efforts to improve health behaviors. In addition, research is needed to understand potential interventions in the pediatric setting. Given the high prevalence of ACEs in the general population, investing in community-based intervention or safety networks may lead to improved long-term health outcomes. Initial research has demonstrated the value of early interventions in reducing exposure to and/or the impact of childhood trauma.60–62
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057231184325 for Association between adverse childhood experiences and later-life allostatic load in UK Biobank female participants by Debbie Jakubowski, Caryn E Peterson, Jiehuan Sun, Kent Hoskins, Garth H Rauscher and Maria Argos in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057231184325 for Association between adverse childhood experiences and later-life allostatic load in UK Biobank female participants by Debbie Jakubowski, Caryn E Peterson, Jiehuan Sun, Kent Hoskins, Garth H Rauscher and Maria Argos in Women’s Health
Acknowledgments
The authors wish to acknowledge the UK Biobank study participants, without whom this research would not be possible.
Footnotes
ORCID iD: Debbie Jakubowski  https://orcid.org/0000-0002-3524-3761
https://orcid.org/0000-0002-3524-3761
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethics approval and consent to participate: Exemption from ethics approval was granted by the University of Illinois at Chicago Office for the Protection of Research Subjects under criteria as defined by the US Department of Health and Human Services Regulations for the Protection of Human Subjects (45 CFR 46.104(d)).
Consent for publication: Not applicable.
Author contribution(s): Debbie Jakubowski: Conceptualization; Data curation; Formal analysis; Methodology; Visualization; Writing—original draft; Writing—review & editing.
Caryn E Peterson: Conceptualization; Methodology; Writing—review & editing.
Jiehuan Sun: Conceptualization; Methodology; Writing—review & editing.
Kent Hoskins: Conceptualization; Methodology; Writing—review & editing.
Garth H Rauscher: Conceptualization; Methodology; Writing —review & editing.
Maria Argos: Conceptualization; Methodology; Writing—review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: UK Biobank data are available for access via application at https://www.ukbiobank.ac.uk/
References
- 1.Barboza Solís C, Kelly-Irving M, Fantin R, et al. Adverse childhood experiences and physiological wear-and-tear in midlife: findings from the 1958 British birth cohort. Proc Natl Acad Sci 2015; 112: E738–E746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lupien SJ, McEwen BS, Gunnar MR, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009; 10(6): 434–445. [DOI] [PubMed] [Google Scholar]
- 3.Bellis MA, Hughes K, Leckenby N, et al. Adverse childhood experiences and associations with health-harming behaviours in young adults: surveys in eight eastern European countries. Bull World Health Organ 2014; 92: 641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiehn J, Hornberg C, Fischer F.How adverse childhood experiences relate to single and multiple health risk behaviours in German public university students: a cross-sectional analysis. BMC Public Health 2018; 18: 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ports KA, Holman DM, Guinn AS, et al. Adverse childhood experiences and the presence of cancer risk factors in adulthood: a scoping review of the literature from 2005 to 2015. J Pediatr Nurs 2019; 44: 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aristizabal MJ, Anreiter I, Halldorsdottir T, et al. Biological embedding of experience: a primer on epigenetics. Proc Natl Acad Sci 2019; 117(38): 23261–23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly-Irving M, Mabile L, Grosclaude P, et al. The embodiment of adverse childhood experiences and cancer development: potential biological mechanisms and pathways across the life course. Int J Public Health 2013; 58(1): 3–11. [DOI] [PubMed] [Google Scholar]
- 8.Holman DM, Ports KA, Buchanan ND, et al. The association between adverse childhood experiences and risk of cancer in adulthood: a systematic review of the literature. Pediatrics 2016; 138(suppl 1): S81–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warner ET, Hargreaves MK, Mouton CP, et al. Abstract C66: adverse childhood experiences and breast cancer risk factors in black and white women. Cancer Epidem Biomar 2016;25: C66. [Google Scholar]
- 10.Kelly-Irving M.Allostatic load: how stress in childhood affects life-course health outcomes, 2019, https://www.health.org.uk/sites/default/files/upload/publications/2019/Allostatic%20load%20how%20stress%20in%20childhood%20affects%20life%20course%20health%20outcomes.pdf
- 11.Windle M, Haardörfer R, Getachew B, et al. A multivariate analysis of adverse childhood experiences and health behaviors and outcomes among college students. J Am Coll Health 2018; 66(4): 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E, Parker KJ.Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving towards a model of behavioral and biological mechanisms. Psychol Bull 2011; 137: 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcalá HE, Mitchell EM, Keim-Malpass J.Heterogeneous impacts: adverse childhood experiences and cancer screening. Cancer Causes Control 2018; 29(3): 343–351. [DOI] [PubMed] [Google Scholar]
- 14.Kalmakis KA, Meyer JS, Chiodo L, et al. Adverse childhood experiences and chronic hypothalamic–pituitary–adrenal activity. Stress 2015; 18(4): 446–450. [DOI] [PubMed] [Google Scholar]
- 15.Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of Child and Family Health, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012; 129: e232–e246. [DOI] [PubMed] [Google Scholar]
- 16.Miller GE, Chen E.Harsh family climate in early life presages the emergence of pro-inflammatory phenotype in adolescence. Psychol Sci 2010; 21: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danese A, Moffitt TE, Harrington H, et al. Adverse childhood experiences and adult risk factors for age-related disease. Arch Pediatr Adolesc Med 2009; 163(12): 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ScienceDirect. Allostasis—an overview, https://www.sciencedirect.com/topics/immunology-and-microbiology/allostasis (accessed 18 March 2020).
- 19.Goldman N, Turra CM, Glei DA, et al. Predicting mortality from clinical and nonclinical biomarkers. J Gerontol A Biol Sci Med Sci 2006; 61(10): 1070–1074. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS.Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism 2003; 52: 10–16. [DOI] [PubMed] [Google Scholar]
- 21.Kim HG, Cheon EJ, Bai DS, et al. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig 2018; 15(3): 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BS.Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci 2006; 8: 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juster RP, McEwen BS, Lupien SJ.Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010; 35(1): 2–16. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS.Neurobiological and systemic effects of chronic stress. Chronic Stress 2017; 1: 2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood GE, Shors TJ.Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A 1998; 95: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAnee G, Shevlin M, Murphy J, et al. Where are all the males? Gender-specific typologies of childhood adversity based on a large community sample. Child Abuse Negl 2019; 90: 149–159. [DOI] [PubMed] [Google Scholar]
- 27.Haahr-Pedersen I, Perera C, Hyland P, et al. Females have more complex patterns of childhood adversity: implications for mental, social, and emotional outcomes in adulthood. Eur J Psychotraumatol 2020; 11(1): 1708618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misiak B, Stańczykiewicz B, Pawlak A, et al. Adverse childhood experiences and low socioeconomic status with respect to allostatic load in adulthood: a systematic review. Psychoneuroendocrinology 2022; 136: 105602. [DOI] [PubMed] [Google Scholar]
- 29.Beckie TM, Duffy A, Groer MW.The relationship between allostatic load and psychosocial characteristics among women veterans. Womens Health Issues 2016; 26(5): 555–563. [DOI] [PubMed] [Google Scholar]
- 30.Berg CJ, Haardörfer R, McBride CM, et al. Resilience and biomarkers of health risk in Black smokers and nonsmokers. Health Psychol 2017; 36(11): 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grabe HJ, Schulz A, Schmidt CO, et al. [A brief instrument for the assessment of childhood abuse and neglect: the Childhood Trauma Screener (CTS)]. Psychiatr Prax 2012; 39: 109–115. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994; 151(8): 1132–1136. [DOI] [PubMed] [Google Scholar]
- 33.Mathew A, Doorenbos AZ, Li H, et al. Allostatic load in cancer: a systematic review and mini meta-analysis. Biol Res Nurs 2021; 23(3): 341–361, http://journals.sagepub.com/doi/10.1177/1099800420969898?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed (accessed 2 April 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UK Biobank cancer numbers summary report, https://biobank.ndph.ox.ac.uk/~bbdatan/CancerNumbersReport.html (2021, accessed 11 May 2021).
- 35.Glaesmer H, Schulz A, Häuser W, et al. [The childhood trauma screener (CTS)—development and validation of cut-off-scores for classificatory diagnostics]. Psychiatr Prax 2013; 40(4): 220–226. [DOI] [PubMed] [Google Scholar]
- 36.Szanton SL, Gill JM, Allen JK.Allostatic load: a mechanism of socioeconomic health disparities? Biol Res Nurs 2005; 7(1): 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeileis A.Object-oriented computation of sandwich estimators. J Stat Softw 2006; 16: 1–16. [Google Scholar]
- 38.Jackson C.Multi-state models for panel data: the msm package for R. J Stat Softw 2011; 38: 1–28. [Google Scholar]
- 39.Berg MT, Simons RL, Barr A, et al. Childhood/Adolescent stressors and allostatic load in adulthood: support for a calibration model. Soc Sci Med 2017; 193: 130–139. [DOI] [PubMed] [Google Scholar]
- 40.Wallace M, Felker-Kantor E, Madkour A, et al. Adverse childhood experiences, smoking and alcohol use, and allostatic load among people living with HIV. AIDS Behav 2020; 24(6): 1653–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horan JM, Widom CS.From childhood maltreatment to allostatic load in adulthood: the role of social support. Child Maltreat 2015; 20(4): 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Shields J, Gibbs JJ.Depressive symptoms, childhood maltreatment, and allostatic load: the importance of sex differences. Psychoneuroendocrinology 2021; 126: 105130. [DOI] [PubMed] [Google Scholar]
- 43.Fleischer T, Ulke C, Beutel M, et al. The relation between childhood adversity and adult obesity in a population-based study in women and men. Sci Rep 2021; 11: 14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riley EH, Wright RJ, Jun HJ, et al. Hypertension in adult survivors of child abuse: observations from the nurses’ health study II. J Epidemiol Commun Health 2010; 64(5): 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med 2010; 39(6): 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindert J, von Ehrenstein OS, Grashow R, et al. Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: systematic review and meta-analysis. Int J Public Health 2014; 59(2): 359–372. [DOI] [PubMed] [Google Scholar]
- 47.Chaplin AB, Jones PB, Khandaker GM.Sexual and physical abuse and depressive symptoms in the UK Biobank. BMC Psychiatry 2021; 21: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yapp E, Booth T, Davis K, et al. Sex differences in experiences of multiple traumas and mental health problems in the UK Biobank cohort. Soc Psychiatry Psychiatr Epidemiol. Epub ahead of print 10 May 2021. DOI: 10.1007/s00127-021-02092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am J Prev Med 1998; 14: 245–258. [DOI] [PubMed] [Google Scholar]
- 50.Johnson SC, Cavallaro FL, Leon DA.A systematic review of allostatic load in relation to socioeconomic position: poor fidelity and major inconsistencies in biomarkers employed. Soc Sci Med 2017; 192: 66–73. [DOI] [PubMed] [Google Scholar]
- 51.Guidi J, Lucente M, Sonino N, et al. Allostatic load and its impact on health: a systematic review. Psychother Psychosom 2021; 90(1): 11–27. [DOI] [PubMed] [Google Scholar]
- 52.Finlay S, Roth C, Zimsen T, et al. Adverse childhood experiences and allostatic load: a systematic review. Neurosci Biobehav Rev 2022; 136: 104605. [DOI] [PubMed] [Google Scholar]
- 53.Blair C, Raver CC, Granger D, et al. Allostasis and allostatic load in the context of poverty in early childhood. Dev Psychopathol 2011; 23(3): 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gruenewald TL, Karlamangla AS, Hu P, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med 2012; 74(1): 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lunyera J, Stanifer JW, Davenport CA, et al. Life course socioeconomic status, allostatic load, and kidney health in Black Americans. Clin J Am Soc Nephrol 2020; 15: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomasdottir MO, Sigurdsson JA, Petursson H, et al. Self reported childhood difficulties, adult multimorbidity and allostatic load. A cross-sectional analysis of the Norwegian HUNT study. PLoS ONE 2015; 10(6): e0130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hardt J, Rutter M.Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry 2004; 45(2): 260–273. [DOI] [PubMed] [Google Scholar]
- 58.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017; 186: 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shonkoff JP, Bales SN.Science does not speak for itself: translating child development research for the public and its policymakers. Child Dev 2011; 82(1): 17–32. [DOI] [PubMed] [Google Scholar]
- 60.Bethell CD, Newacheck P, Hawes E, et al. Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Aff 2014; 33(12): 2106–2115. [DOI] [PubMed] [Google Scholar]
- 61.Garner AS, Shonkoff JP, Committee on Psychosocial Aspects of Child and Family Health, et al. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics 2012; 129(1): e224–e231. [DOI] [PubMed] [Google Scholar]
- 62.Gilgoff R, Singh L, Koita K, et al. Adverse childhood experiences, outcomes, and interventions. Pediatr Clin North Am 2020; 67: 259–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057231184325 for Association between adverse childhood experiences and later-life allostatic load in UK Biobank female participants by Debbie Jakubowski, Caryn E Peterson, Jiehuan Sun, Kent Hoskins, Garth H Rauscher and Maria Argos in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057231184325 for Association between adverse childhood experiences and later-life allostatic load in UK Biobank female participants by Debbie Jakubowski, Caryn E Peterson, Jiehuan Sun, Kent Hoskins, Garth H Rauscher and Maria Argos in Women’s Health