Abstract
Coffee ground waste from the coffee beverage preparation is mainly discarded and consequently ends up in landfill, which cause the contamination of caffeine in various environmental compartments. This study focuses on the upcycling of coffee-ground waste to carbon quantum dots (CQDs) for use as a modifying material to improve the visible light activity of titanium dioxide (TiO2). The CQD solution was synthesized by hydrothermal method, which has an average size of 2.80 ± 0.63 nm. The CQDs/TiO2 photocatalysts were prepared by combining CQD solutions at various amounts with sol-gel TiO2 and then coated on the fiberglass cloths (FGCs). The photocatalytic application mainly focuses on the removal of caffeine from the water. The photocatalytic experiment was preliminary run in a simple batch reactor under visible light. The 5CQDs/TiO2 coated FGC (5 mL of CQD solution/g of Ti-based on sol-gel) showed the best performance, and it was selected for the removal of caffeine and other pharmaceuticals (i.e., carbamazepine and ibuprofen) in the recirculating reactor. The removals of caffeine, carbamazepine, and ibuprofen after irradiation for 9 h were 82%, 88%, and 84%, respectively. The residual concentrations were significantly lower than the reported toxicity levels based on specific species. The changes in total organic carbon were observed, indicating the mineralization of pharmaceuticals in water. The 5CQDs/TiO2 coated FGC showed good flexible performance. No obvious loss of activity was observed for five runs. The actual wastewater from the coffee pot cleaning process was also tested. The removal was 80% for caffeine and 86% for color in the unit of the American Dye Manufacturers Institute (ADMI).
Keywords: Coffee ground waste, Carbon quantum dots, Caffeine, Titanium dioxide, Fiberglass cloth, Visible-light photocatalysis
Graphical abstract
Highlights
-
•
The coffee ground waste from coffee brewing was upcycled to CQD solutions.
-
•
A novel CQDs/TiO2-coated FGC was developed as a photocatalyst for water treatment.
-
•
The CQD solution content of 5 mL/g of Ti showed the highest caffeine removal efficiency (72.66%).
-
•
The photocatalyst could be easily regenerated by exposure to sunlight.
-
•
The photocatalyst efficiently removed caffeine and color from actual wastewater.
1. Introduction
Coffee is one of the most popular beverages, with a worldwide consumption rate of 2.25 billion cups per day [1]. Coffee beverage preparation, especially coffee brewing at home, in cafeterias, and in manufacturing industries, generates much coffee ground waste. The worldwide production of coffee ground waste is approximately 6–8 million tons annually [2], mainly in landfill sites [3].
CAF is a dominant constituent in coffee ground waste, which is hazardous to the environment [4]. Disposing of coffee ground waste in a landfill may cause CAF-containing leachate to flow through the soil and groundwater [5]. The recycling of coffee ground waste is a practical approach to reducing the environmental impact and also saving disposal costs. The current strategies for recycling coffee ground waste mainly involve upgrading to adsorbent and biofuel because it contains high organic content [3].
CAF, CBZ, and IBP are frequently detected in the same water bodies because of their enormous consumption. Besides coffee, there are many caffeinated food, beverages, and medicines (e.g., cold medicines, analgesics, and weight-loss drugs) worldwide [6]. CBZ and IBP have been listed in the Model List of Essential Medicines 2019 by World Health Organization (WHO) as anticonvulsant and non-steroidal anti-inflammatory drugs (NSAIDs), respectively [7]. The observed concentrations of pharmaceuticals in their manufacturing wastewater were 0.022–3.594 mg/L for CAF [8], 0.077–0.575 mg/L for CBZ [9], and 0.703–1.673 mg/L for IBP [10]. These concentrations significantly exceed the lowest observed effect concentration (LOEC), which has been reported in chronic toxicity studies for specific organisms such as fish (0.91 mg/L CAF) [6], water fleas (0.1 mg/L CBZ) [11], and diatoms (1 mg/L IBP) [12].
A large portion of pharmaceutical wastewater originates from the manufacturing process in the pharmaceutical industry [13]. The pharmaceutical industry in many areas still discharges untreated effluents into the sewers [9]. Most pharmaceuticals are resistant to sewage treatment plants because of their low biodegradability and high solubility in water [14], so they could eventually contaminate the aquatic environment. Consequently, it is reasonable to apply advanced technology for the treatment of pharmaceuticals in water to minimize toxicological risk to the ecosystem.
Semiconductor photocatalysis is one of the promising options for the advanced treatment of refractory organic compounds such as pharmaceuticals in water [15]. This technology is a cost-effective approach because it can harvest solar energy, does not require external oxidants (e.g., ozone, hydrogen peroxide), does not require waste disposal (e.g., sludge disposal), and allows high reusability of the photocatalyst film without complex processes [16,17]. Titanium dioxide (TiO2) has been reported as an efficient photocatalyst for both fundamental research and practical applications. However, there are some limitations of TiO2, including (i) the intrinsic wide band gap of TiO2 (3.2 eV for anatase), which limits its application under visible light, and (ii) the rapid recombination of photo-excited electrons and holes, which lead to the decrease of its photocatalytic activity [18].
CQDs are a new class of fluorescent carbon nanomaterials with a size below 10 nm [19,20]. CQDs have received much attention because of their attractive properties, such as good water solubility, large specific surface area, low toxicity, and excellent photoluminescence behavior [19]. CQDs can be applied in many fields, such as bio-imaging, sensing, drug delivery, and photocatalysis [21]. CQDs have been used as modifying materials to improve visible light harvesting of TiO2 photocatalysts. In the previous studies on CQDs/TiO2, the CQDs were usually synthesized from chemical precursors such as citric acid [20,22], sodium citrate [23], and L-ascorbic acid [24], and in most studies, the CQDs/TiO2 powder was dispersed in wastewater as a photocatalyst.
Food waste is a cost-effective raw material for the synthesis of CQDs. In recent years, CQDs have been successfully synthesized using different kinds of food waste, such as coffee ground waste [[25], [26], [27]], onion peel [28], crab shells [29], and tea waste [30]. Some researchers have already used coffee ground waste as a starting material for synthesizing CQDs during 2016–2022. The obtained CQDs were efficiently used for bio-imaging [25], detection of heavy metals including Fe3+ and Cu2+ [25,26], and detection of noxious nitroanilines [27]. However, these previous studies did not investigate the potentiality of coffee ground waste-derived CQDs for other applications such as photocatalysis.
This study presents an alternative way to take advantage of the coffee ground waste-derived CQDs. The CQDs were used to modify the visible light activity of TiO2. The objectives of this study are to upcycle the coffee ground waste as CQDs and develop a visible light-active photocatalyst using coffee ground waste-derived CQDs composite with TiO2 for the removal of pharmaceuticals from water. The CQDs were combined with sol-gel TiO2 and coated on the FGC to overcome the difficulty in catalyst separation and recovery at the end of the photocatalytic process. CAF was the primary target pollutant to investigate the visible-light activity of FGC coated with CQDs/TiO2 in a simple batch reactor and recirculating reactor. The optimum photocatalyst was also applied to remove other pharmaceuticals, including CBZ and IBP, and to remove CAF from the actual wastewater generated from the coffee-pot cleaning process. The reusable and flexible photocatalysts which are beneficial for practical applications were investigated and reported.
2. Materials and methods
2.1. Materials and chemicals
Ultrapure water from the water purification system (Millipore, resistivity of 18.2 mΩ cm) was used in all preparations and experiments. The coffee ground waste (Arabica, average moisture content of 36.8 ± 3.2%) was dried at 105 °C for 24 h before use for the synthesis of CQD solution. FGC (C-glass type, HT800) was supplied by Zaftec company, Thailand. Commercial TiO2 powder (pure anatase, 98.5%), acetylacetone (ACAC, ≥99.5%), hydrogen peroxide solution (H2O2, 30% w/v), dipotassium phosphate (K2HPO4, ≥98%) and potassium dihydrogen phosphate (KH2PO4, 99%) were purchased from Carlo Erba Reagents. Tetrabutyl titanate (TBOT, 97%), CAF (99%), CBZ (98%), and IBP (98%) were purchased from Sigma-Aldrich. Sodium hydroxide pellets (NaOH, 99%) were purchased from QRëC. Methanol (HPLC grade), water (HPLC grade), acetonitrile (HPLC grade), and dichloromethane (99.8%) were purchased from RCI Labscan.
2.2. Synthesis and characterizations of CQD solutions
CQDs were synthesized by the one-pot hydrothermal method. NaOH (3.2 g) was stirred with ultrapure water (400 mL) for 10 min. The dried coffee ground waste (15 g) was dispersed in NaOH solution under constant stirring for 1 h H2O2 (100 mL) was slowly added to the mixture and manually stirred using a glass rod. The obtained mixture was heated at 300 °C for 4 h in a 500-mL hydrothermal reactor equipped with 4848 reactor controllers (Parr instrument company, USA). After cooling to room temperature, the solid part was separated using 6 μm and 0.2 μm filter papers, while the liquid part was then purified by dichloromethane three times. The purified CQD solutions were characterized using a transmission electron microscope (TEM, JEOL model JEM 2100) and a photoluminescence spectrometer (HORIBA Scientific, model: FLUOROMAX-4). The CQDs concentration in the solution was measured as total carbon in triplicate using a total organic carbon (TOC) analyzer (Analytik Jena, Multi N/C 3100). The average total carbon in CQD solution was 5.72 mg/mL.
2.3. Preparation of CQDs/TiO2 powders and CQDs/TiO2 coated FGC
CQDs/TiO2 was prepared by ultrasonic-assisted sol-gel method. Firstly, a mixture of TBOT (8 g), ACAC (2.35 g), and methanol (40 mL) was magnetically stirred for 30 min. After that, a certain volume of CQD solutions (1, 2, 3, 4, 5, 6, 9, or 12 mL/g of Ti) was dropped into the mixture under stirring for another 30 min. The obtained mixture was sonicated in an ultrasonic bath (Crest, 690HTAE) for 30 min and aged for 24 h. The reference TiO2 (without CQDs) was also prepared by this procedure without the addition of any CQD solution. For the preparation of CQDs/TiO2 powder, sol-gel CQDs/TiO2 was dried at 105 °C for 24 h, milled and sieved with mesh (No. 200), and finally calcined at 500 °C for 1 h under N2 gas.
For the preparation of CQDs/TiO2 coated FGC, the FGC pieces were heated at 500 °C for 1 h (heating rate of 10 °C/min) to eliminate the organic compounds on their surface. There are two coating steps as follows: (i) the FGC pieces were firstly coated by a mixture of commercial anatase TiO2 powder and ultrapure water (2 g/L) using a paintbrush, followed by drying for 1 h (105 °C) and calcined in the air for 2 h (200 °C, heating rate of 2 °C/min), and (ii) the sol-gel-derived CQDs/TiO2 was gently dropped over the prior layer followed by drying at room temperature for 24 h, dried in the oven for 1 h (105 °C), and finally calcined under 1 mL/min nitrogen gas for 1 h (500 °C, heating rate of 10 °C/min). In each step, the TiO2 mixture was coated on FGC with a fixed ratio of 0.06 mL/cm2. The obtained photocatalysts were labeled as FGC coated with xCQDs-TiO2 (x = mL of CQD solution/g of Ti-based on sol-gel).
2.4. Characterizations of photocatalysts
The as-synthesized CQDs/TiO2 powders without coating on FGC were characterized by X-ray diffractometer (XRD, BRUKER AXS, D8DISCOVER), Fourier transform infrared spectroscopy (FTIR, Thermo, Nicolet 6700), and photoluminescence spectrometer (HORIBA Scientific, model: FLUOROMAX-4) to avoid the noise of FGC as recommended in previous studies [31,32]. The surface morphology of the FGCs coated with CQDs/TiO2 was investigated using a field emission scanning electron microscope (FESEM, model: NOVA NANOSEM 450). The absorption and reflection spectra were measured using a UV-VIS-NIR spectrophotometer (Shimadzu SolidSpec-3700) to determine the band gap energy using the Kubelka-Munk equation and the Tauc plot method [33].
2.5. Photocatalytic experiments
For all experiments, the reactions were initially carried out for 3 h in the dark to ensure the adsorption/desorption equilibrium. Then the visible light was irradiated by a double-ended halogen lamp (Philips, Plusline, R7s, 300W). The distance of the light source from the surface of FGC was fixed at 25 cm. CAF was the main target pollutant for the photocatalytic experiment. Fundamentally, the wastewater temperature directly affects the degradation rate of organic pollutants in water and solvent evaporation. Therefore, it was controlled at around 28.5 ± 1.5 °C using a water-cooling system throughout the experiments.
Firstly, the optimization of CQDs content in sol-gel CQDs/TiO2 was studied in a simple batch reactor through photocatalytic degradation of CAF. A piece of photocatalyst-coated FGC (10 cm × 10 cm) was soaked in pharmaceutical solution (60 mL) inside a glass container (10 cm × 10 cm × 6 cm) under horizontal shaking (60 rpm).
The optimal photocatalyst-coated FGC was obtained and further used in the recirculating reactor (Fig. 1) to remove CAF and other pharmaceuticals (i.e., CBZ and IBP) at the concentrations found in their production effluents [[8], [9], [10]]. The photocatalysis experiments were performed by stretching a piece of FGC coated with optimum photocatalyst (12 cm × 28 cm) through the surface of a glass box. The wastewater was pumped into a glass spray bar and subsequently ran down to the water reservoir by gravity. The solution was recirculated at a 450 mL/min flow rate using a diaphragm pump. Magnetic stirring was performed throughout the experiments to ensure that the solution was completely mixed. At desired reaction intervals, 1 mL of samples were taken and filtrated with a 0.22 μm nylon syringe filter. The influences of irradiation time and initial concentration of pharmaceuticals on the removal of CAF, CBZ, and IBP were investigated. The reuse and regeneration of the photocatalyst-coated FGC were also investigated for five cycles through the photocatalytic degradation of CAF. The removal of CAF from actual wastewater was investigated using wastewater collected from the coffee-pot cleaning process.
Fig. 1.
A recirculating reactor with an inset showing the direction of visible light irradiation.
2.6. Analytical procedures
The quantification of pharmaceuticals in water was performed by high-performance liquid chromatography (HPLC) using the Alliance-e2695 Separations Module. A stationary phase was a C18-column (Vertisep™, 4.6 mm × 150 mm, particle size of 5 μm) with a column temperature of 30 °C. The mobile phases consisted of methanol and ultrapure water (50:50 v/v, flow rate = 1 mL/min) for CAF, acetonitrile, and ultrapure water (60:40 v/v, flow rate = 1 mL/min), and 20 mM phosphate buffer (pH = 7.4) and acetonitrile (70:30 v/v, flow rate = 1.2 mL/min). The injection volume of each sample was set at 100 μL. CAF and CBZ were detected by a photodiode array detector (Waters 2998) at 280 nm for CAF and 285 nm for CBZ. IBP was detected by a fluorescent detector (FP-2020, JASCO) at the excitation and emission wavelengths of 263 nm and 288 nm, respectively. Besides HPLC measurements, the mineralization of pharmaceuticals in water was also estimated using a total organic carbon (TOC) analyzer (Analytik Jena, Multi N/C 3100). NPOC method was used for TOC measurement with the sample volume of 500 mL and the furnace temperature of 800 °C. The color of wastewater was measured in the unit of the American Dye Manufacturers Institute (ADMI) using a UV-VIS spectrophotometer (Hach, DR6000).
3. Results and discussion
3.1. Properties of CQD solutions
The morphology of coffee ground waste-derived CQDs was characterized by TEM. The representative TEM image is shown in Fig. 2a. CQDs are spherical and mono-dispersed in the as-prepared solution. The inset of Fig. 2a shows the crystal plane with a lattice spacing of 0.217 nm, which corresponds to graphitic carbon [34]. The size distribution histogram (Fig. 2b) showed that the particle size of CQDs ranged from 1.6 to 4.4 nm. The average size is 2.80 ± 0.63 nm (obtained from the statistical analysis of 70 particles in the TEM image). These results confirmed the formation of CQDs.
Fig. 2.
(a) TEM image of the coffee ground waste-derived CQDs (inset is a zoomed-in image of individual CQD) and (b) particle size distribution of CQDs.
The optical properties of the as-prepared CQD solution were studied through UV–visible absorption and photoluminescence (PL) emission. Fig. 3a shows the adsorption spectrum of the CQD solution in the range of 300–700 nm. The presence of broad adsorption in the visible region represented the photocatalytic activity of CQDs under visible light [24]. The as-prepared CQD solution was light brown under natural daylight and exhibited strong blue-green PL under UV irradiation at 365 nm (Fig. 3b).
Fig. 3.
Optical properties of the coffee ground waste-derived CQD solutions: (a) UV–visible absorption spectrum, (b) photographs of the CQD solution, (c) PL emission spectra, and (d) up-conversion PL emission spectra.
From Fig. 3c–d, the PL emission spectra of the as-prepared CQD solution were dependent on excitation wavelengths. This is attributed to their small particle sizes, the electrostatic repulsions between the particles, and the abundance of a hydroxyl group on the surface [35,36]. With the increase of excitation wavelength from 320 to 650 nm (Fig, 3c), the emission peak shifted to the longer wavelengths. The maximum emission peak was observed at 537 nm under the excitation wavelength of 470 nm. This result indicated the down-conversion PL behavior of the CQD solution. The CQD solution also showed the up-conversion PL behavior under the excitation wavelength of 700–900 nm (Fig. 3d). The up-converted emission peak covered the visible light range of 420–700 nm. This result demonstrated that CQDs could expose to near-infrared light and then emit light with shorter wavelengths. The multi-photon absorption process can explain the up-conversion PL behavior, which leads to anti-stokes emission [37].
3.2. Properties of TiO2 photocatalysts
XRD was used to analyze the crystalline structure and phase of the TiO2 photocatalysts. XRD patterns of the sol-gel-derived TiO2 powder are presented in Fig. 4. All samples showed the diffraction peak of anatase. No characteristic peak of CQDs was observed in the diffraction patterns, which is consistent with the previous studies [23,38,39]. This is due to the relatively low diffraction intensity of the CQDs as compared to TiO2. The crystallite sizes of the TiO2 powder obtained by sol-gel were calculated by the Scherrer equation [40] using the characteristic peak of anatase TiO2 (2θ = 25.2°). They are 10.71, 9.75, 8.94 and 8.57 nm for reference TiO2, 3CQDs/TiO2, 5CQDs/TiO2 and 9CQDs/TiO2, respectively. This is implied that the crystallite size of the sol-gel-derived TiO2 powder is decreased with increasing CQD solution content. In addition, the XRD pattern of the commercial TiO2 powder (titania source for coating on FGC in the first step) is also shown in Fig. S1. The samples before and after calcined (500 °C for 1 h) contain pure anatase phase with crystallite sizes of 27.18, and 27.88 nm, respectively. The crystalline phase is still anatase and the crystallite size is not much change because anatase transforms to rutile at the calcination temperature of ∼600 °C. Rutile phase has a large crystallite size and high electron-hole recombination rate as compared to anatase [41].
Fig. 4.
XRD patterns of the sol-gel derived TiO2 powder.
The morphologies of the reference TiO2 powder (Fig. 5a) and the 5CQDs/TiO2 powder (Fig. 5b) were characterized by TEM. The lattice spacing of 0.349–0.352 nm corresponded to the (101) crystal plane of anatase TiO2. The lattice spacing of 0.212–0.218 nm corresponded to the (002) crystal plane of graphitic carbon, indicating the presence of CQDs [36]. This result confirms the heterostructure of 5CQD/TiO2, which involves the presence of CQDs on the surface of TiO2.
Fig. 5.
TEM images of the sol-gel derived TiO2 powder: (a) reference TiO2 powder and (b) 5CQDs/TiO2 powder.
The surface functional groups of sol-gel-derived TiO2 powder were analyzed by FTIR spectroscopy. From Fig. 6a, the absorption band at 3450 and 1632 cm−1 indicated the presence of hydroxyl group (-OH) on the surface of sol-gel-derived TiO2, which helps to promote the photocatalytic activity by increasing the electron transportability [42,43]. For the broad absorption bands in the 400-900 cm−1 range, the band is attributed to Ti–O–Ti in the case of the reference TiO2 (without CQDs) [44]. For the CQDs/TiO2 samples, the bands show a slight blue shift in wavenumber compared to that of the reference TiO2. This is attributed to the presence of Ti–O–C in combination with Ti–O–Ti vibrations [[45], [46], [47]].
Fig. 6.
FTIR spectra of the sol-gel derived TiO2 powder in the wavenumber range of (a) 4000-480 cm−1 and (b) 2200-1200 cm−1.
From Fig. 6b, the peaks of the C–H (1407 cm−1) and COO− (1387 cm−1) appeared in all samples [34,48], which related to the residue of TiO2 precursor (titanium butoxide and acetylacetone) from the sol-gel process [49]. Compared to the reference TiO2, the CQDs/TiO2 samples showed some new functional groups, including C=O of carbonyl groups (1760 cm−1) and C=C (1562, 1532 and 1448 cm−1) [42,48,50,51]. This observation is due to the surface functional groups of CQDs that can interact with TiO2. The blue shift of the broad absorption bands in the range of 400–900 cm−1 indicates the interaction between TiO2 and CQDs with the formation of Ti–O–C [[45], [46], [47]].
Photoluminescence measurement is well known technique to investigate the behavior of the photo-excited electrons and holes. The previous works well reported that the PL spectra are caused by the electron-hole recombination process [38,52]. PL spectra of the reference TiO2 powder and the CQDs/TiO2 powder at the excitation wavelength of 300 nm are presented in Fig. 7. A lower PL intensity indicates a hindrance to the recombination of the photo-excited electrons and holes in the photocatalyst, which is beneficial to the photocatalytic activity. All samples exhibited similar shapes with different intensities. A broad PL emission in each sample was observed at around 390–470 nm. The PL intensity of all CQDs/TiO2 samples was lower than that of the reference TiO2 powder. For CQDs/TiO2 samples, the PL intensity was on the order of 3CQDs/TiO2 > 9CQDs/TiO2 > 5CQDs/TiO2. This result indicates that the composite of TiO2 with appropriate amount of CQDs helps to suppress the recombination of the photo-excited electrons and holes in the photocatalyst. However, further increasing the amount of CQDs increase the electron-hole recombination because the excessive CQDs loading reduced the surface active sites of TiO2 thus increase the probability of collision between the photo-excited electrons and holes [45].
Fig. 7.
PL emission spectra of the sol-gel derived TiO2 powder.
3.3. Properties of TiO2-coated FGCs
FESEM analysis was used to study the morphologies of TiO2-coated FGCs. Fig. 8a–d shows the FESEM images of reference TiO2-coated FGC and CQDs/TiO2-coated FGC. The FESEM images show that TiO2 can be coated on flexible FGC using two-step coating procedures without an additional binding agent, which is consistent with the previous report [53]. Fig. 8e shows the schematic illustration of TiO2-coated FGC. The sol-gel TiO2 helps to attach the commercial TiO2 powder to the FGC surface. Meanwhile, the commercial TiO2 powder provides the surface roughness of TiO2 film, which helps to enhance the photocatalytic activity [54].
Fig. 8.
FESEM images of the FGC coated with (a) reference TiO2, (b) 3CQDS/TiO2, (c) 5CQDS/TiO2, and (d) 9CQDS/TiO2, and (e) schematic illustration of the TiO2-coated FGC.
UV–Visible absorption spectra of the FGC coated with reference TiO2 and CQDs/TiO2 are presented in Fig. 9. The FGC coated with reference TiO2 showed strong absorption in the UV region, and an absorption edge was observed at ∼410 nm. The previous studies regarding anatase TiO2-coated FGC also found an absorption edge in the range of 410–420 nm [55,56]. In the visible region, the absorbances of all FGCs coated with CQDs/TiO2 were slightly higher than the FGC coated with reference TiO2. For the bandgap of the samples, only crystalline anatase was identified by XRD in the previous section. Anatase TiO2 has only an indirect bandgap, which can be estimated by the Kubelka-Munk equation coupled with the Tauc plot methodology (inset of Fig. 9) [33]. The indirect bandgaps were estimated to be 3.08, 3.04, 2.96, and 2.99 eV for the FGC coated with reference TiO2, 3CQDs/TiO2, 5CQDs/TiO2, and 9CQDs/TiO2, respectively. As compared to the FGC coated with reference TiO2, the FGCs coated with CQDs/TiO2 showed a small bandgap narrowing. In addition, the indirect bandgap of reference TiO2 power was also estimated to be 3.2 eV (Fig. S2), which is slightly higher than that of the reference TiO2-coated FGC. The value of 3.2 eV is consistent with the theoretical bandgap of anatase TiO2 [57]. These results demonstrate that the visible light absorption of TiO2-coated FGC could improve due to the interaction of both FGC and CQDs with TiO2, which is expected to enhance the photocatalytic activity under visible light.
Fig. 9.
UV–visible absorption spectra of the TiO2-coated FGCs with an inset showing the Tauc plots for indirect bandgaps.
3.4. Photocatalytic removal of pharmaceuticals from water
In the experiments, the initial concentrations of CAF, CBZ, and IBP were individually set based on real values found in their manufacturing wastewater, as reported in the literature [[8], [9], [10]]. Each experiment was conducted in duplicate. The solution evaporation during the experiment was negligible (≤1.2%) because the wastewater temperature was controlled at around 28.5 ± 1.5 °C using a water-cooling system throughout the experiments.
3.4.1. Optimization of CQD contents for CQDs/TiO2-coated FGC
To optimize CQD content in the CQDs/TiO2-coated FGC, the photocatalytic experiments were conducted in a simple batch reactor using CAF (3.6 mg/L) as a target pollutant. Before irradiation, the photocatalyst-coated FGC (10 cm × 10 cm) was soaked in CAF solution (60 mL) under horizontal shaking for 3 h to provide an adsorption-desorption desorption equilibrium. Fig. 10a presents the removal of CAF through adsorption in the dark for 3 h and photocatalytic degradation under visible light irradiation for 6 h. The adsorption of CAF on the TiO2-coated FGCs exhibited efficiencies of 1–3%. Photocatalytic degradation of CAF under visible light irradiation showed that all CQDs/TiO2-coated FGCs exhibited much higher removal efficiency compared with the TiO2-coated reference FGCs (without CQDs). Fig. S3 shows that the removal of CAF from water in the presence of a pure CQD solution did not exhibit photocatalytic activity, indicating that the CQD solution cannot act individually as a photocatalyst under visible light irradiation. The results suggest that the interaction between TiO2 and CQDs significantly enhances the photocatalytic activity, which is consistent with previous reports [19,24].
Fig. 10.
Visible light-driven photocatalytic degradation of CAF in the simple batch reactor for 6 h using CQDs/TiO2-coated FGC with different CQD contents: (a) degradation ratio respecting the time and (b) removal efficiency respecting the ratio of mL CQD solution to g Ti.
From Fig. 10b, the most efficient photocatalyst was 5CQDs/TiO2-coated FGC, with an overall removal efficiency of 72.66%. Therefore, the 5CQDs/TiO2-coated FGC were selected for further studies in the recirculating reactor. However, the removal efficiency cannot be directly compared to previous reports because of the different preparation methods for photocatalysts and the different operational conditions for the photocatalytic processes.
According to the mechanism reported in the literature, the visible-light-driven photocatalytic activity of the CQDs/TiO2 composite is enhanced due to the up-conversion PL of CQDs and the efficient electron-hole pair separation [37,[58], [59], [60]]. From the up-conversion PL spectra of the CQD solution (Fig. 3d), the emission wavelengths covered 420–700 nm, which cannot provide enough energy to excite the TiO2 without CQDs. From Fig. 9, the FGCs coated with CQDs/TiO2 have better light absorption and lower bandgaps than the FGCs coated with reference TiO2. Therefore, the lower PL intensity of the PL spectra of CQDs/TiO2 (Fig. 7) is due to an obstruction of the recombination of the photo-excited electrons and holes in the photocatalyst [38,61]. The electron-hole recombination rate was in the order of reference TiO2 > 3CQDs/TiO2 > 9CQDs/TiO2 > 5CQDs/TiO2.
For photocatalytic degradation of CAF using the CQDs/TiO2-coated FGCs under visible light irradiation, a trend of removal efficiency (Fig. 10b) is consistent with the electron-hole recombination result. The photocatalyst with lower electron-hole recombination can provide higher photocatalytic activity. From Fig. 10a and b, 5CQDs/TiO2-coated FGC showed the highest removal efficiency (72.66%) because the electron-hole recombination in 5CQDs/TiO2 was lower than other photocatalysts. Therefore, a plausible mechanism is presented in Fig. 11. CQDs play the role of photosensitizers, which can inject the excited electron into TiO2. When the CQDs/TiO2 composite is irradiated by visible light, the ground state electrons of CQDs can be excited to their LUMO level and leave the holes at the ground state. The excited electrons can transfer to the conduction band (CB) of TiO2 and then react with the adsorbed oxygen molecules (O2) to produce the superoxide radicals (•O2−). Meanwhile, the holes can react with the adsorbed water molecules (H2O) or the hydroxide ions (OH−) to produce the hydroxyl radicals (•OH). The superoxide radicals (•O2−) and hydroxyl radicals (•OH) can eventually degrade the organic pollutants (such as pharmaceutical pollutants) [37,[58], [59], [60],62].
Fig. 11.
Proposed photocatalytic mechanism of CQDs/TiO2 composite.
3.4.2. Photocatalytic degradation of pharmaceuticals in the recirculating reactor
3.4.2.1. Influence of irradiation time
The effect of irradiation time on the photocatalytic degradation of CAF, CBZ, and IBP from water was studied individually using the initial concentrations of each pharmaceutical as found in their production effluents (CAF = 3.6 mg/L, CBZ = 0.6 mg/L, and IBP = 1.7 mg/L). The experiments were conducted at the inherent solution pH of 7.2 ± 0.1 for CAF, 7.3 ± 0.1 for CBZ, and 6.1 ± 0.1 for CBZ. It is beneficial to reduce operating costs if the wastewater can be treated without any pH adjustment, especially in large-scale applications.
From Fig. 12a, the experiments include adsorption in the dark for 3 h followed by photocatalysis under visible light irradiation for 9 h. The overall removal efficiencies of CAF, CBZ, and IBP were 82%, 88%, and 84%, corresponding to the residual concentrations of 0.63 mg/L, 0.08 mg/L, and 0.27 mg/L, respectively. In comparison with the toxicity values reported in the literature, these residual concentrations are significantly below their lowest observed effect concentrations (LOEC) for fish (0.91 mg/L CAF) [6], water flea (0.1 mg/L CBZ) [11], and diatoms (1 mg/L IBP) [12].
Fig. 12.
Photocatalytic degradation of pharmaceuticals in the recirculating reactor: (a) degradation ratio respecting the time and (b) pharmaceutical removal efficiencies and TOC removal efficiencies after 9 h of visible light irradiation.
From Fig. 12b, the mineralizations of CAF, CBZ, and IBP were studied through the measurement of total organic carbon (TOC), which are 59%, 43%, and 48%, respectively. The results demonstrated that the 5CQDs/TiO2-coated FGC has the potential for degradation and mineralization of pharmaceutical pollutants from water. The mineralization rates were found to be slower than the pharmaceutical degradation rates, agreeing with the previous reports [63,64]. This result indicates the presence of some intermediate species. However, the toxicity of the intermediates from the photocatalytic degradation of CBZ and IBP was lower than that of the parent compounds. Based on fish, daphnia, and green algae, the intermediates from the photocatalytic degradation of CAF showed the same toxicity as CAF [65].
3.4.2.2. Influence of initial pharmaceutical concentration
Kinetic studies on the photocatalytic degradation of pharmaceutical pollutants are essential to understand the condition for the future design of the large-scale application. The influences of initial concentration of CAF (3–5 mg/L), CBZ (0.6–1.2 mg/L), and IBP (1.4–2.4 mg/L) were investigated for kinetic studies using the method of initial rates (Equations (1), (2))) coupled with Langmuir-Hinshelwood model (Equations (3), (4))).
| (1) |
| ln (C/Ce,0) = -kappt | (2) |
| (3) |
| (4) |
where re,0 is the initial photocatalytic reaction rate (mg/L·h), kapp is the apparent rate constant (h−1), C is the initial concentration of pharmaceutical (mg/L), Ce,0 is the equilibrium concentration after adsorption in the dark (mg/L), t is irradiation time (h), k is the reaction rate constant (mg/L·h), and K is the dynamic Langmuir adsorption constant (L/mg) [66,67].
All photocatalytic experiments were run for 6 h under visible light irradiation, as shown in Figs. S4a–S4c. The order of reaction was determined by the method of initial rates using the experimental data during the first 2 h under visible light irradiation. Fig. 13a–c shows the kinetic fitting results for four different initial concentrations of each pharmaceutical. The photocatalytic reaction was well-described by the pseudo-first-order kinetic model in accordance with those reported in previous studies [66,68,69]. The slope of the line represents the apparent rate constants (kapp). The initial reaction rates (re,0) were calculated from Equation (1) and sequentially fitted by the linearity equation of the Langmuir-Hinshelwood model (Equation (4)). The values of k and K were determined from the plots of 1/re0 against 1/Ce,0 (Insets of Fig. 13a–c). The obtained kinetic parameters are summarized in Table 1.
Fig. 13.
Pseudo-first-order kinetic plots for the photocatalytic degradation of (a) CAF, (b) CBZ, and (c) IBP using 5CQDs/TiO2-coated FGC under visible light irradiation. The insets show the linearization of the Langmuir-Hinshelwood model.
Table 1.
Kinetic parameters for the photocatalytic degradation of CAF, CBZ, and IBP.
| Pollutant | C0 (mg/L) | kapp(h−1) | re,0(mg/L·h) | k (mg/L·h) | K (L/mg) |
|---|---|---|---|---|---|
| CAF | 3 | 0.1865 | 0.5614 | 0.7452 | 0.9791 |
| 3.6 | 0.1640 | 0.5691 | |||
| 4 | 0.1475 | 0.5915 | |||
| 5 | 0.1246 | 0.6230 | |||
| CBZ | 0.6 | 0.1413 | 0.0876 | 0.1809 | 1.5413 |
| 0.8 | 0.1255 | 0.1029 | |||
| 1 | 0.1126 | 0.1092 | |||
| 1.2 | 0.0978 | 0.1144 | |||
| IBP | 1.4 | 0.1795 | 0.2567 | 0.4573 | 0.8866 |
| 1.7 | 0.1643 | 0.2727 | |||
| 2 | 0.1433 | 0.2866 | |||
| 2.4 | 0.1327 | 0.3145 |
In the studied concentration ranges, the values of kapp decreased with increasing the initial concentrations of CAF, CBZ, and IBP. This could be explained that the increase in pharmaceutical concentration leads to (i) low photon penetration into the solutions and (ii) a large number of pharmaceutical molecules adsorbed on the photocatalyst surface, reducing the surface-adsorbed hydroxide ions and oxygen molecules, resulting in the decrease of free radical formation [70]. The photocatalytic degradation of CAF, CBZ, and IBP could be described by substituting the values of k and K in the Langmuir-Hinshelwood model (Equation (3)). The relationship between the equilibrium concentration of pharmaceuticals and the initial photocatalytic reaction rate could be written as Equations (5), (6), (7)).
| (5) |
| (6) |
| (7) |
3.4.2.3. Reusable and flexible performance of FGC coated with CQDs/TiO2
In order to investigate the reusable and flexible performance of 5CQDs/TiO2-coated FGC, the photocatalytic degradation of CAF (3.6 mg/L) was repeated for five cycles under visible light irradiation. After each cycle, the 5CQDs/TiO2-coated FGC were thoroughly washed with ultrapure water, exposed to the sun for one day, and sequentially rolled and kept in a cylindrical container for 12 h before reuse. The photocatalytic reaction was run for 7 h in each cycle to achieve the lowest observed effect concentration (LOEC) for fish (0.91 mg/L) [6]. From Fig. 14, the removal efficiencies were relatively stable (77–79%) after five runs. The result demonstrates a good reusable and flexible performance of 5CQDs/TiO2-coated FGC. Therefore, it can be folded into many shapes, which provides the opportunity to use in various types of photocatalytic reactors in practical applications.
Fig. 14.
Photocatalytic degradations of CAF under visible light irradiation for five cycles.
3.4.2.4. CAF removal in actual wastewater
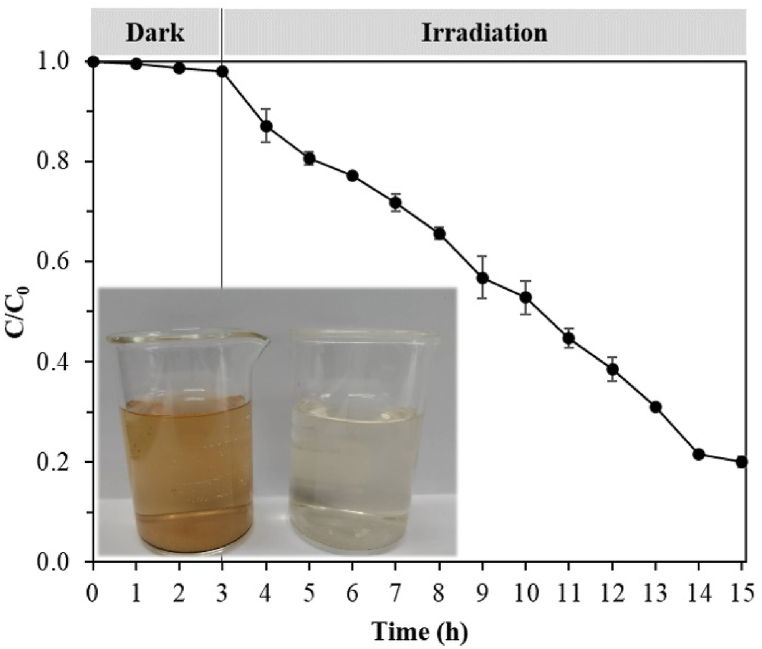
The actual wastewater was collected from the coffee pot cleaning process. The photocatalytic degradation of CAF in actual wastewater was conducted to evaluate the applicability of 5CQDs/TiO2-coated FGC in the recirculating reactor (Fig. 15). The initial concentration of CAF in actual wastewater was 3.92 mg/L, and the initial color was 388 ADMI. The experiments were run through adsorption in the dark for 3 h followed by visible light irradiation for 12 h. The treated water showed a remaining CAF concentration of 0.79 mg/L (removal efficiency ∼80%), which is below the LOEC for fish (0.91 mg/L) [6]. The remaining color was 54 ADMI, which is significantly lower than the industrial effluent standard (300 ADMI) [71].
Fig. 15.
Removal of CAF from actual wastewater. Inset is a photograph of actual wastewater before (left) and after treatment (right) (adsorption for 3 h followed by visible light irradiation for 12 h).
4. Conclusion
CQD solutions have been successfully prepared by hydrothermal method using the coffee ground waste as a raw waste material. The visible light-sensitive photocatalysts were synthesized by composite TiO2 with the coffee ground-derived CQDs, and then coated on FGCs. The main results can be concluded as follows:
-
(1)
The optimum photocatalyst was 5CQDs/TiO2-coated FGC (5 mL of CQD solution/g of Ti), which efficiently removed CAF, CBZ, and IBP from water in the recirculating reactor. The residual concentrations of CAF, CBZ, and IBP achieved their reported toxicity levels based on fish, water fleas, and diatoms.
-
(2)
The 5CQDs/TiO2-coated FGC showed good reusability and flexibility in use, and it could be easily regenerated by exposure to natural sunlight.
-
(3)
The treatment of coffee-pot cleaning wastewater (actual wastewater) showed high removal efficiencies of 80% for CAF and 86% for ADMI color, which was not harmful to the environment.
Author contribution statement
Conceived and designed the experiments; Patiya Kemacheevakul, Surawut Chuangchote
Performed the experiments; Rattana Muangmora
Analyzed and interpreted the data; Rattana Muangmora, Patiya Kemacheevakul, Surawut Chuangchote
Contributed reagents, materials, analysis tools; Surawut Chuangchote, Patiya Kemacheevakul
Wrote the paper; Rattana Muangmora, Patiya Kemacheevakul, Surawut Chuangchote.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The author (R. Muangmora) would like to acknowledge the Petchra Pra Jom Klao Ph.D. Research Scholarship from King Mongkut’s University of Technology Thonburi. This research project was supported by Thailand Science Research and Innovation. Basic Research Fund: The fiscal year 2022 under project number FRB650048/0164. Technical support from the Department of Environmental Engineering, Department of Tool and Materials Engineering, and Department of Food Engineering, King Mongkut’s University of Technology Thonburi, is also gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17693.
Abbreviation
- CAF
Caffeine
- CBZ
Carbamazepine
- IBP
Ibuprofen
- CQDs
Carbon quantum dots
- FGC
Fiberglass Cloth
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mayson S., Williams I. Applying a circular economy approach to valorize spent coffee grounds. Resour. Conserv. Recycl. 2021;172:105659–105671. [Google Scholar]
- 2.Bekirogullari M. Hydrogen production from sodium borohydride by ZnCl2 treated defatted spent coffee ground catalyst. Int. J. Hydrogen Energy. 2020;45:9733–9743. [Google Scholar]
- 3.Kim M.J., Choi S.W., Kim H., Mun S., Lee K.B. Simple synthesis of spent coffee ground-based microporous carbons using K2CO3 as an activation agent and their application to CO2 capture. Chem. Eng. J. 2020;397:125404–125414. [Google Scholar]
- 4.Fernandes A.S., Mello F.V.C., Thode Filho S., Carpes R.M., Honório J.G., Marques M.R.C., Felzenszwalb I., Ferraz E.R.A. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017;141:30–36. doi: 10.1016/j.ecoenv.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Sui Q., Cao X., Lu S., Zhao W., Qiu Z., Yu G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: a review. Emerg. Contam. 2015;1:14–24. [Google Scholar]
- 6.Li M., Mei Q., Han D., Wei B., An Z., Cao H., Xie J., He M. The roles of HO•, ClO• and BrO• radicals in caffeine degradation: a theoretical study. Sci. Total Environ. 2021;768:144733–144742. doi: 10.1016/j.scitotenv.2020.144733. [DOI] [PubMed] [Google Scholar]
- 7.Sim W.J., Lee J.W., Lee E.S., Shin S.K., Hwang S.R., Oh J.E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere. 2011;82:179–186. doi: 10.1016/j.chemosphere.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Tran N.H., Li J., Hu J., Ong S.L. Occurrence and suitability of pharmaceuticals and personal care products as molecular markers for raw wastewater contamination in surface water and groundwater. Environ. Sci. Pollut. Res. 2014;21:4727–4740. doi: 10.1007/s11356-013-2428-9. [DOI] [PubMed] [Google Scholar]
- 9.Kleywegt S., Payne M., Ng F., Fletcher T. Environmental loadings of active pharmaceutical ingredients from manufacturing facilities in Canada. Sci. Total Environ. 2019;646:257–264. doi: 10.1016/j.scitotenv.2018.07.240. [DOI] [PubMed] [Google Scholar]
- 10.Ashfaq M., Khan K.N., Rehman M.S.U., Mustafa G., Nazar M.F., Sun Q., Iqbal J., Mulla S.I., Yu C.P. Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan, Ecotoxicol. Environ. Saf. 2017;136:31–39. doi: 10.1016/j.ecoenv.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Hai F.I., Yang S., Asif M.B., Sencadas V., Shawkat S., Sanderson-Smith M., Gorman J., Xu Z.Q., Yamamoto K. Carbamazepine as a possible anthropogenic marker in water: occurrences, toxicological effects, regulations and removal by wastewater treatment technologies. Water. 2018;10:107–138. [Google Scholar]
- 12.Ding T., Yang M., Zhang J., Yang B., Lin K., Li J., Gan J. Toxicity, degradation, and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard Mater. 2017;330:127–134. doi: 10.1016/j.jhazmat.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Patel M., Kumar R., Kishor K., Mlsna T., Pittman J.C.U., Mohan D. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019;119:3510–3673. doi: 10.1021/acs.chemrev.8b00299. [DOI] [PubMed] [Google Scholar]
- 14.Olasupo A., Suah F.B.M. Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: a case of polymer inclusion membranes. J. Hazard Mater. 2021;406 doi: 10.1016/j.jhazmat.2020.124317. [DOI] [PubMed] [Google Scholar]
- 15.Fattahi A., Arlos M.J., Bragg L.M., Kowalczyk S., Liang R., Schneider O.M., Zhou N., Servos M.R. Photodecomposition of pharmaceuticals and personal care products using P25 modified with Ag nanoparticles in the presence of natural organic matter. Sci. Total Environ. 2021;752:142000–142010. doi: 10.1016/j.scitotenv.2020.142000. [DOI] [PubMed] [Google Scholar]
- 16.Isari A.A., Mehregan M., Mehregan S., Hayati F., Kalantary R.R., Kakavandi B. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations: a novel hybrid system. J. Hazard Mater. 2020;390:122050–122063. doi: 10.1016/j.jhazmat.2020.122050. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q., Du R., Tan C., Chen P., Yu G., Deng S. Efficient degradation of typical pharmaceuticals in water using a novel TiO2/ONLH nano-photocatalyst under natural sunlight. J. Hazard Mater. 2021;403:123582–123591. doi: 10.1016/j.jhazmat.2020.123582. [DOI] [PubMed] [Google Scholar]
- 18.Shayegan Z., Lee C.S., Haghighat F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase–A review. Chem. Eng. J. 2018;334:2408–2439. [Google Scholar]
- 19.Xu N., Huang H., Ouyang H., Wang H. Preparation of the heterojunction catalyst N-doping carbon quantum dots/P25 and its visible light photocatalytic activity. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-46277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva V., Invêncio I., Silva C.P., Otero M., Lima D.L. Photodegradation of oxolinic acid in aquaculture effluents under solar irradiation: is it possible to enhance efficiency by the use of TiO2/carbon quantum dots composites? Chemosphere. 2022;308:136522–136532. doi: 10.1016/j.chemosphere.2022.136522. [DOI] [PubMed] [Google Scholar]
- 21.Kang C., Huang Y., Yang H., Yan X.F., Chen Z.P. A review of carbon dots produced from biomass wastes. Nanomaterials. 2020;10:2316–2339. doi: 10.3390/nano10112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P., Wang F., Chen Z.F., Zhang Q., Su Y., Shen L., Yao K., Liu Y., Cai Z., Lv W. Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: the significant roles of reactive oxygen species. Appl. Catal. B Environ. 2017;204:250–259. [Google Scholar]
- 23.Wang W., Ni Y., Xu Z. One-step uniformly hybrid carbon quantum dots with high-reactive TiO2 for photocatalytic application. J. Alloys Compd. 2015;622:303–308. [Google Scholar]
- 24.Miao R., Luo Z., Zhong W., Chen S.Y., Jiang T., Dutta B., Nasr Y., Zhang Y., Suib S.L. Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst. Appl. Catal. B Environ. 2016;189:26–38. [Google Scholar]
- 25.Wang L., Li W., Wu B., Li Z., Wang S., Liu Y., Pan D., Wu M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016;300:75–82. [Google Scholar]
- 26.Crista D.M., El Mragui A., Algarra M., Esteves da Silva J.C., Luque R., Pinto da Silva L. Turning spent coffee grounds into sustainable precursors for the fabrication of carbon dots. Nanomaterials. 2020;10:1209–1225. doi: 10.3390/nano10061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa A.I., Barata P.D., Moraes B., Prata J.V. Carbon dots from coffee grounds: synthesis, characterization, and detection of noxious nitroanilines. Chemosensors. 2022;10:113–129. [Google Scholar]
- 28.Bankoti K., Rameshbabu A.P., Datta S., Das B., Mitra A., Dhara S. Onion derived carbon nanodots for live cell imaging and accelerated skin wound healing. J. Mater. Chem. B. 2017;5:6579–6592. doi: 10.1039/c7tb00869d. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y.Y., Gedda G., Girma W.M., Yen C.L., Ling Y.C., Chang J.Y. Magnetofluorescent carbon dots derived from crab shell for targeted dual-modality bioimaging and drug delivery. ACS Appl. Mater. Interfaces. 2017;9:13887–13899. doi: 10.1021/acsami.7b01599. [DOI] [PubMed] [Google Scholar]
- 30.Gunjal D.B., Naik V.M., Waghmare R.D., Patil C.S., Shejwal R.V., Gore A.H., Kolekar G.B. Sustainable carbon nanodots synthesised from kitchen derived waste tea residue for highly selective fluorimetric recognition of free chlorine in acidic water: a waste utilization approach. J. Taiwan Inst. Chem. Eng. 2019;95:147–154. [Google Scholar]
- 31.Liu Z., Fang P., Wang S., Gao Y., Chen F., Zheng F., Liu Y., Dai Y. Photocatalytic degradation of gaseous benzene with CdS-sensitized TiO2 film coated on fiberglass cloth. J. Mol. Catal. Chem. 2012;363:159–165. [Google Scholar]
- 32.Pham T.D., Lee B.K. Feasibility of silver doped TiO2/glass fiber photocatalyst under visible irradiation as an indoor air germicide. Int. J. Environ. Res. Publ. Health. 2014;11:3271–3288. doi: 10.3390/ijerph110303271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roongraung K., Chuangchote S., Laosiripojana N. Enhancement of photocatalytic oxidation of glucose to value-added chemicals on TiO2 photocatalysts by a zeolite (Type y) support and metal loading. Catalysts. 2020;10:423–438. [Google Scholar]
- 34.He M., Zhang J., Wang H., Kong Y., Xiao Y., Xu W. Material and optical properties of fluorescent carbon quantum dots fabricated from lemon juice via hydrothermal reaction. Nanoscale Res. Lett. 2018;13:1–7. doi: 10.1186/s11671-018-2581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam A.M., Park B.Y., Ghouri Z.K., Park M., Kim H.Y. Synthesis of carbon quantum dots from cabbage with down-and up-conversion photoluminescence properties: excellent imaging agent for biomedical applications. Green Chem. 2015;17:3791–3797. [Google Scholar]
- 36.Deng Y., Chen M., Chen G., Zou W., Zhao Y., Zhang H., Zhao Q. Visible-ultraviolet upconversion carbon quantum dots for enhancement of the photocatalytic activity of titanium dioxide. ACS Omega. 2021;6:4247–4254. doi: 10.1021/acsomega.0c05182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ke J., Li X., Zhao Q., Liu B., Liu S., Wang S. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017;496:425–433. doi: 10.1016/j.jcis.2017.01.121. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S., Umar A., Mehta S.K., Ibhadon A.O., Kansal S.K. Solar light driven photocatalytic degradation of levofloxacin using TiO2/carbon-dot nanocomposites. New J. Chem. 2018;42:7445–7456. [Google Scholar]
- 39.Choi D., Ham S., Jang D.J. Visible-light photocatalytic reduction of Cr (VI) via carbon quantum dots-decorated TiO2 nanocomposites. J. Environ. Chem. Eng. 2018;6:1–8. [Google Scholar]
- 40.Sirirerkratana K., Kemacheevakul P., Chuangchote S. Color removal from wastewater by photocatalytic process using titanium dioxide-coated glass, ceramic tile, and stainless-steel sheets. J. Clean. Prod. 2019;215:123–130. [Google Scholar]
- 41.Yuangpho N., Le S.T.T., Treerujiraphapong T., Khanitchaidecha W., Nakaruk A. Enhanced photocatalytic performance of TiO2 particles via effect of anatase-rutile ratio. Physica E Low Dimens. Syst. Nanostruct. 2015;67:18–22. [Google Scholar]
- 42.Fu Y., Gao G., Zhi J. Electrochemical synthesis of multicolor fluorescent N-doped graphene quantum dots as a ferric ion sensor and their application in bioimaging. J. Mater. Chem. B. 2019;7:1494–1502. doi: 10.1039/c8tb03103g. [DOI] [PubMed] [Google Scholar]
- 43.John A.K., Palaty S., Sharma S.S. Greener approach towards the synthesis of titanium dioxide nanostructures with exposed {001} facets for enhanced visible light photodegradation of organic pollutants. J. Mater. Sci. Mater. Electron. 2020;31:20868–20882. [Google Scholar]
- 44.Gohari G., Mohammadi A., Akbari A., Panahirad S., Dadpour M.R., Fotopoulos V., Kimura S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-57794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H., Wang X., Li N., Xia J., Meng Q., Ding J., Lu J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018;8:34241–34251. doi: 10.1039/c8ra06681g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kocijan M., Ćurković L., Ljubas D., Mužina K., Bačić I., Radošević T., Podlogar M., Bdikin I., Otero-Irurueta G., Hortigüela M.J. Graphene-based TiO2 nanocomposite for photocatalytic degradation of dyes in aqueous solution under solar-like radiation. Appl. Sci. 2021;11:3966–3980. [Google Scholar]
- 47.Nasir A., Khalid S., Yasin T., Mazare A. A Review on the progress and future of TiO2/graphene photocatalysts. Energies. 2022;15:6248–6281. [Google Scholar]
- 48.Thangaraj B., Chuangchote S., Wongyao N., Solomon P.R., Roongraung K., Chaiworn W., Surareungchai W. Flexible sodium-ion batteries using electrodes from Samanea saman tree leaf-derived carbon quantum dots decorated with SnO2 and NaVO3. Clean Energy. 2021;5:354–374. [Google Scholar]
- 49.Khodaparast P., Ounaies Z. Influence of dispersion states on the performance of polymer-based nanocomposites. Smart Mater. Struct. 2014;23:104004–104018. [Google Scholar]
- 50.Lauria A., Lizundia E. Luminescent carbon dots obtained from polymeric waste. J. Clean. Prod. 2020;262:121288–121296. [Google Scholar]
- 51.Yan X., Rahman S., Rostami M., Tabasi Z.A., Khan F., Alodhayb A., Zhang Y. Carbon quantum dot-incorporated chitosan hydrogel for selective sensing of Hg2+ ions: synthesis, characterization, and density functional theory calculation. ACS Omega. 2021;6:23504–23514. doi: 10.1021/acsomega.1c03557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sui Y., Wu L., Zhong S., Liu Q. Carbon quantum dots/TiO2 nanosheets with dominant (001) facets for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019;480:810–816. [Google Scholar]
- 53.Horikoshi S., Watanabe N., Onishi H., Hidaka H., Serpone N. Photodecomposition of a nonylphenol polyethoxylate surfactant in a cylindrical photoreactor with TiO2 immobilized fiberglass cloth. Appl. Catal. B Environ. 2002;37:117–129. [Google Scholar]
- 54.Kuo C.C., Chen Y.R. A new method to characterizing surface roughness of TiO2 thin films. Opt Laser. Eng. 2011;49:410–414. [Google Scholar]
- 55.Lin S., Zhang X., Sun Q., Zhou T., Lu J. Fabrication of solar light induced Fe-TiO2 immobilized on glass-fiber and application for phenol photocatalytic degradation. Mater. Res. Bull. 2013;48:4570–4575. [Google Scholar]
- 56.Yang S.B., Chun H.H., Tayade R.J., Jo W.K. Iron-functionalized titanium dioxide on flexible glass fibers for photocatalysis of benzene, toluene, ethylbenzene, and o-xylene (BTEX) under visible-or ultraviolet-light irradiation. J. Air Waste Manag. Assoc. 2015;65:365–373. doi: 10.1080/10962247.2014.995838. [DOI] [PubMed] [Google Scholar]
- 57.Zhou T., Chen S., Li L., Wang J., Zhang Y., Li J., Bai J., Xia L., Xu Q., Rahim M. Carbon quantum dots modified anatase/rutile TiO2 photoanode with dramatically enhanced photoelectrochemical performance. Appl. Catal. B Environ. 2020;269:118776–118785. [Google Scholar]
- 58.Zhang Y.Q., Ma D.K., Zhang Y.G., Chen W., Huang S.M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy. 2013;2:545–552. [Google Scholar]
- 59.Markad G.B., Kapoor S., Haram S.K., Thakur P. Metal free, carbon-TiO2 based composites for the visible light photocatalysis. Sol. Energy. 2017;144:127–133. [Google Scholar]
- 60.Xie C., Fan T., Wang A., Chen S.L. Enhanced visible-light photocatalytic activity of a TiO2 membrane-assisted with N-doped carbon quantum dots and SiO2 opal photonic crystal. Ind. Eng. Chem. Res. 2018;58:120–127. [Google Scholar]
- 61.Jin X., Che R., Yang J., Liu Y., Chen X., Jiang Y., Liang J., Chen S., Su H. Activated carbon and carbon quantum dots/titanium dioxide composite based on waste rice noodles: simultaneous synthesis and application in water pollution control. Nanomaterials. 2022;12:472–492. doi: 10.3390/nano12030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Awfa D., Ateia M., Fujii M., Johnson M.S., Yoshimura C. Photo-degradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: a critical review of recent literature. Water Res. 2018;142:26–45. doi: 10.1016/j.watres.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 63.Carabin A., Drogui P., Robert D. Photocatalytic oxidation of carbamazepine: application of an experimental design methodology. Water Air Soil Pollut. 2016;227:1–16. [Google Scholar]
- 64.Vaiano V., Jaramillo-Paez C.A., Matarangolo M., Navío J.A., Hidalgo M.D.C. UV and visible-light driven photocatalytic removal of caffeine using ZnO modified with different noble metals (Pt, Ag and Au) Mater. Res. Bull. 2019;112:251–260. [Google Scholar]
- 65.Muangmora R., Roongraung K., Kemacheevakul P., Chuangchote S. Photocatalytic degradation of pharmaceuticals from water using nitrogen-doped titanium dioxide coated on fiberglass cloth. J. Clean. Prod. 2023;397:136487–136497. [Google Scholar]
- 66.Chong M.N., Jin B. Photocatalytic treatment of high concentration carbamazepine in synthetic hospital wastewater. J. Hazard Mater. 2012;199:135–142. doi: 10.1016/j.jhazmat.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 67.Atitar M.F., Bouziani A., Dillert R., El Azzouzi M., Bahnemann D.W. Photocatalytic degradation of the herbicide imazapyr: do the initial degradation rates correlate with the adsorption kinetics and isotherms? Catal. Sci. Technol. 2018;8:985–995. [Google Scholar]
- 68.Castañeda C., Martínez J.J., Santos L., Rojas H., Osman S.M., Gómez R., Luque R. Caffeine photocatalytic degradation using composites of NiO/TiO2–F and CuO/TiO2–F under UV irradiation. Chemosphere. 2022;288:132506–132514. doi: 10.1016/j.chemosphere.2021.132506. [DOI] [PubMed] [Google Scholar]
- 69.Muangmora R., Kemacheevakul P., Punyapalakul P., Chuangchote S. Enhanced photocatalytic degradation of caffeine using titanium dioxide photocatalyst immobilized on circular glass sheets under ultraviolet C irradiation. Catalysts. 2020;10:964–978. [Google Scholar]
- 70.Raha S., Ahmaruzzaman M. Novel magnetically retrievable In2O3/MoS2/Fe3O4 nanocomposite materials for enhanced photocatalytic performance. Sci. Rep. 2021;11:1–23. doi: 10.1038/s41598-021-85532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suwanboriboon J., Meesiri W., Wongkokua W. An application of spectrophotometer for ADMI color measurement. J. Phys. Conf. Ser. 2018;1144 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.