Abstract
Background
Psoriasis is an immune‐mediated chronic inflammatory disease, and currently it is widely believed that the IL‐23/IL‐17 axis and Th17 cells play a critical and central role. However, increasing evidence suggests that neutrophils may interact with a variety of immune cells to play an indispensable role in psoriasis.
Materials and methods
We searched the recent literature on psoriasis and neutrophils through databases such as PubMed and CNKI, and summarized the findings to draw conclusions.
Results
Neutrophils can promote the development of psoriasis by secreting IL‐23, IL‐17, and cytokines with TH17 cell chemotaxis. Activated keratinocytes (KCs) can attract and activate neutrophils, induce the formation of neutrophil extracellular traps (NETs). KCs can also expose self‐antigens which lead to strong autoimmune reactions. The granule proteins secreted by activated neutrophils can activate IL‐36, which converts vulgaris psoriasis to generalized pustular psoriasis (GPP).
Conclusion
The function of neutrophils components and the interaction between neutrophils and immune cells play an essential role in the pathogenesis of psoriasis. The aim is to provide a theoretical basis for the exploration of targeted clinical treatments and fundamental research on the pathogenesis of psoriasis.
Keywords: granule proteins, neutrophil, neutrophil extracellular traps, psoriasis
1. INTRODUCTION
The incidence of psoriasis accounts for about 2% of the world's population, 1 , 2 affecting about 120 million people. 3 In China, the incidence is 0.47%, and it is estimated that there are about 6.5 million patients in China, with a trend of increase. 4 Psoriasis is an immune‐cell‐mediated chronic systemic inflammatory disease that mainly affects the skin. 1 Psoriasis can be divided into plaque‐type psoriasis, pustular psoriasis, erythrodermic psoriasis, arthritis psoriasis, and so on. 1 Psoriasis can be accompanied by various other diseases, such as atherosclerosis, chronic obstructive pulmonary disease, inflammatory bowel disease, type 2 diabetes, and kidney disease. 1 , 5 , 6 Previous studies have suggested that Th17 cells and the IL‐23/IL‐17 axis play a critical role in the pathogenesis of psoriasis. 7 Currently, biologic agents that antagonize TH17 cell‐related cytokines, such as Tumor necrosis factor (TNF) antagonists, 8 IL‐17 antagonists,IL‐23 receptor antagonists, IL‐23 antagonists, and IL‐36 antagonists, have been developed. 9 They can quickly clear skin lesions and bring a qualitative leap in the treatment of psoriasis, 10 but they can only achieve clinical remission. Some patients still experience relapse or worsening during or after treatment, which suggests that other factors may be involved in the mechanism of psoriasis development.
Neutrophils are the most abundant cells of innate immunity and the most prevalent white blood cells in the blood of healthy individuals. They play an indispensable role in anti‐infective and inflammatory processes through mechanisms such as antigen presentation, reactive oxygen species (ROS) generation, release of granular proteins, formation of neutrophil extracellular traps (NETs), and production and release of cytokines. 11 , 12 , 13 Changes in neutrophils within the skin lesions in patients with psoriasis suggest that neutrophils play an important role in the development of psoriasis. KCs, the skin's epithelial cells, release chemokines such as CXCL1, CXCL8, CXCL10, CCL2, CCL3, CCL5, and CCL20. Those chemokines attract neutrophils to psoriatic lesions. 14 Neutrophil‐keratinocyte interactions can result in the production of IL‐17, which initiates or exacerbates the skin lesions 15 (Figure 1). Recent studies have found that neutrophils are responsible for the most IL‐17 production in psoriatic lesions. 16 Suggesting that neutrophils play a key role in the development of psoriasis. The roles of IL‐17/IL‐23 axis have been widely reviewed otherwise for psoriasis, 7 we will focus on the new insights on the function of neutrophils and their potential mechanisms of action in interacting with other immune effectors during the pathogenesis of psoriasis in this review. We hope it will give a clue for exploring new treatment strategies for psoriasis in future.
FIGURE 1.
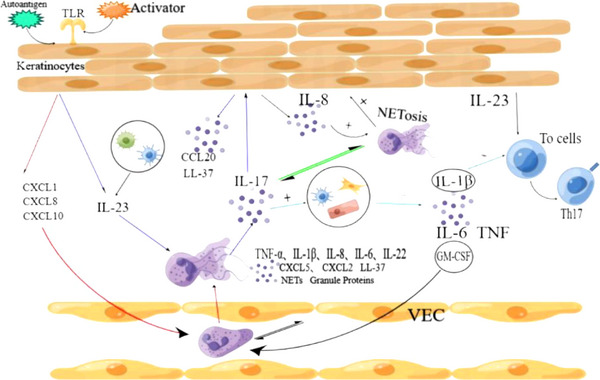
(1) Toll‐like receptor 3 (TLR3) on the membrane of keratinocytes (KCs) cells can be activated by activator (double‐stranded RNA), leading to the release of IL‐23. KCs can also be stimulated by other activators to release neutrophil‐derived chemokines, thereby attracting and activating neutrophils. (2) Activated neutrophils can produce TNF‐α, IL‐1β, IL‐8, IL‐6, IL‐22, CXCL5, CXCL2, LL‐37, NETs, granular protein, and so on. (3) There is a mutual activation between neutrophils and vascular endothelial cells (VECs). (4) IL‐17 can induce dendritic cells, epithelial cells, and fibroblasts to produce IL‐1β, IL‐6, TNF, and GM‐colony‐stimulating factor (CSF). (5) IL‐17 and IL‐8 promote The formation of extracellular traps by neutrophils (NETosis), and NETosis can release more IL‐17. (6) Products formed during neutrophil NETosis can also promote the proliferation and activation of KCs. (7) VECs and neutrophils have a mutual activation effect. (8) IL‐1β and IL‐23 can both induce the differentiation of initial naive T cell (T0 cell) into TH17 cells.
1.1. Neutrophil cytokines and psoriasis
Neutrophils, as a type of innate immune cell, are the first to reach the site of inflammation in the early stages of inflammation. Neutrophil lifespan is short, and apoptosis begins after entering the blood for 24 h, but during the period of psoriasis inflammation, the lifespan of neutrophils can be significantly extended. 13 For example, during inflammation, KCs can prolong neutrophil lifespan. 17 The chemokines released by skin KCs can attract neutrophils to migrate constantly to psoriasis lesions and prolong neutrophil lifespan during inflammation. 17 And then, neutrophils produce and secrete IL‐17 16 and other cytokines, which can participate in the occurrence and development of psoriasis.
1.1.1. IL‐23
IL‐23, composed of subunits p40 and p19. Macrophages, dendritic cells, and KCs in psoriatic skin tissue can secrete IL‐23 7 , 18 , 19 ; and neutrophils in the blood of healthy individuals also express IL‐23. 20 Membrane toll‐like receptor 8 (TLR8) on the surface of Neutrophil can be activated by single‐stranded RNA (ssRNA), resulting in promoting IL‐23 production by neutrophil 21 (Figure 2). This type of RNA may originate from viruses or NETs, indicating that viral infection may exacerbate psoriatic skin lesions.
FIGURE 2.
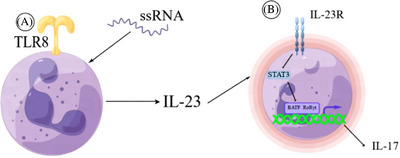
(A) Single‐stranded RNA (ssRNA) can activate the toll‐like receptor 8 (TLR8) receptor on the surface of neutrophils, promoting their production of IL‐23. (B) IL‐23 induces neutrophil production of IL‐17 through the STAT3‐dependent RORγt and BATF pathways. The ability of neutrophils to produce both IL‐23 and IL‐17 results in a pro‐inflammatory positive feedback loop.
After binding to IL‐23R, IL‐23 can exert various biological effects. IL‐23R is expressed on the surfaces of lymphocytes and myeloid cells (dendritic cell, macrophage, and monocyte). 22 The binding of IL‐23 to IL‐23R promotes immune cell activation, induces the formation of mast cell extracellular traps (MCETs), induces the production and release of IL‐17 and chemokines, and promotes the differentiation of naive T cell into Th17 and Th1 cells 16 , 23 (Figure 1). Recently, it was found that neutrophils also express IL‐23R, 24 and the release of IL‐23 by neutrophils can bind to its surface receptor IL‐23R, which lead to self‐activation of neutrophils and further exacerbate inflammation in psoriasis (Figure 2). Alternatively, IL‐23‐activated neutrophils may be capable of generating NETs, similar to mast cells (Figure 1). This remains to be further explored.
1.1.2. IL‐17 produced by neutrophils
It is currently believed that IL‐17 plays an important role in psoriasis, and IL‐17 antagonists such as secukinumab have been widely used in clinical practice. 25 The IL‐17 cytokine family consists of IL‐17A, IL‐17B, IL‐17C, IL‐17D, IL‐17E, and IL‐17F, among which IL‐17A and IL‐17F have the strongest pro‐inflammatory effects. 26 IL‐17A can be produced by T cells, innate lymphoid cells, mast cells, and neutrophils. In the pathogenesis of psoriasis, the Th17 cell is considered to be the main producer of IL‐17. 15 However, increasing evidence shows that neutrophils are the most abundant cell type producing IL‐17 in psoriatic lesions. 27 , 28 Studies have demonstrated that IL‐23 can specifically promote the transcription‐level production of IL‐17A, IL‐17F, and IL‐22 by neutrophils. IL‐23 induces to produce IL‐17 through the STAT3‐dependent RoRγt and BATF pathways by neutrophil. 24 (Figure 2). The IL‐17R, IL‐17 receptor, is expressed on monocytes, lymphocytes, fibroblasts, and KCs. 29 IL‐22 promotes the proliferation and hyperkeratination of KCs. 30 The function of IL‐17A in autoimmune disorders is to promote the release of cytokines, chemokines, and matrix metalloproteinases from tissue cells, 31 and to enhance the production of antimicrobial peptides and chemokines by KCs. 32 IL‐17A has been shown to drive the production of IL‐1β, IL‐6, TNF, and GM‐ colony‐stimulating factor (CSF) by fibroblasts, 33 epithelial cells, 34 and dendritic cells. 35 Among these factors, IL‐1β and IL‐17A cooperatively promote Th17 cell proliferation 36 ; IL‐17A can also induce the expression of chemokines CXCL2 and CCL20. 37 Although neutrophil recruitment is mainly influenced by the CXCL8 chemokine family, 38 IL‐17A can induce the release of granulocyte colony‐stimulating factor (G‐CSF) and GM‐CSF to promote the proliferation and survival of neutrophils, 39 which further recruit neutrophiles at the inflammatory site and resulting in a self‐amplifying effect (Figure 1).
Among other diseases comorbid with psoriasis, IL‐17‐producing neutrophils have also been observed. IL‐17+ neutrophils were found in atherosclerotic plaques. 40 These IL‐17+ neutrophils may be one of the mechanisms behind the comorbidity of psoriasis with other diseases.
1.2. The function of chemokines produced by neutrophils
Neutrophils can be recruited by various chemokines and migrate to the lesion site. KCs can produce neutrophil chemokines, but previous studies have shown that neutrophils themselves can also produce chemokines under the stimulation of inflammation, 47 resulting in a self‐amplifying effect. Moreover, this study showed that not only neutrophils, but also monocytes, macrophages, dendritic cells, NK cells, and T cell subsets can be recruited by neutrophil‐produced chemokines, including Th17 cells, closely related to psoriasis. This reflects the central role of neutrophils in immune inflammation.Various conditions can induce neutrophils to produce their own chemokines. Such as G‐CSF can induce neutrophils to produce CXCL5 48 and CXCL2 49 . Under the stimulation of microorganisms or their derivatives such as lipopolysaccharides, neutrophils can produce CCL20 and CCL19. 50 IL‐23 can promote the release of G‐CSF and indirectly promote neutrophil production by neutrophil. However, there is currently no evidence to confirm that IL‐23 can directly promote neutrophil production of chemokines. Further exploring whether IL‐23 can directly promote neutrophil to produce chemokines, which deserves a further study to clarify.
1.3. The role of neutrophil granule proteins in Psoriasis
The composition of neutrophil granule proteins is relatively complex and can be divided into three major categories, including primary azurophilic granules (myeloperoxidase (MPO) etc.), secondary specific granules, and gelatinase granules (matrix metallopeptidase 9 (MMP‐9) etc.). 51 Different particles have different functions, and those related to psoriasis include MPO, various neutrophil proteases, MMP‐9, etc. The granule proteins of these neutrophils promote the occurrence and development of psoriasis by activating cytokines released by other cells or damaging the VECs. Elastase, proteinase 3, and tissue proteinase G are all part of the neutrophil azurophilic granules. They not only activate the IL‐36 precursor into the more forms of IL‐36β and IL‐36γ, but also inactivate IL‐36Ra through their elastase, further enhancing inflammation. 52 In addition, it promotes the over‐proliferation of KCs in psoriatic lesions by activating the EGFR pathway, 11 as well as promoting the secretion of type I IFN by myeloid dendritic cells. 53 The proteinase 3 and tissue proteinase G can respectively activate neutrophils to produce chemokines CXCL‐8 and CXCL‐5. 54 This will cause self‐amplification of neutrophil chemotaxis. MMP‐9 belongs to the specific granules of neutrophils and is involved in cell apoptosis, innate immunity, and kidney development, and also has the function of limiting bacterial proliferation. 55 The further study demonstrates that MMP‐9 can disrupt the tight junctions and cytoskeletal integrity of endothelial cells, damage the vascular barrier function, and significantly increase vascular permeability, further enhancing inflammation. 56 Recently, it was found that neutrophils with MPO gene defects have decreased ability to produce NETs. 57 One possible mechanism is that the formation of NETs requires the participation of ROS. However, the same study also showed that the reduction of MPO leads to an increase in the activity of the serine protease (containing elastic enzyme, proteinase‐3, and cathepsin G) of neutrophils, which may result in the activation of more IL‐36. And the expression of CD47 on the cell membrane of MPO‐deficient neutrophils is increased, while CD47 can inhibit the phagocytosis of monocytes on neutrophils. 58 Therefore, MPO deficiency may be the cause of worsening IL‐36‐mediated GPP.
1.4. NETs
1.4.1. Generation and NETsis of NETs
NETs are fibrous networks released by neutrophils that contain various components such as neutrophil granule proteins, LL‐37, RNA, and cytokines. 59 , 60 Initially, NETs were believed to play a role in trapping and killing extracellular pathogens. 61 However, NETs are not only produced during states of infection but can also be produced in response to immune complexes and cytokines such as IL‐8 and IL‐23. 62 Indicating that NETs not only have anti‐infective effects but also play a role in autoimmunity. Eosinophils, mast cells, neutrophils, macrophages, and Th17 cells can all produce extracellular traps. 63 Compared to healthy controls, patients with psoriasis have higher levels of NETs in their skin lesions and peripheral blood. 63 Indicating that NETs play an important role in psoriasis.
The formation of extracellular traps by neutrophils is called NETosis. 64 There are three modes of NETosis, the first of which is suicidal. Neutrophils triggered by activators (such as carnitine, autoantibodies or uric acid crystals) 64 can induce ROS activation. Thus ROS activating peptidyl arginase deaminase 4, which leads to chromatin depolymerization. Neutrophil elastase and MPO are transferred to the nucleus to promote chromosome decondensation and nuclear membrane rupture. Ultimately, neutrophil death, and the release of NETs into the extracellular space. 64 The second mode, independent of ROS, is triggered by TLR and complement C3 receptors. It involves nuclear chromatin decondensation and nuclear membrane rupture, as well as the release of nuclear DNA. However, after this type of NETosis, neutrophils are still able to phagocytose pathogens, and neutrophil lifespan is not affected by DNA loss. 65 The third mode of NETosis also depends on ROS, but results in the release of mitochondrial DNA. 66
The uncontrolled release of NETs may contribute to various diseases such as psoriasis, systemic lupus erythematosus, 67 rheumatoid arthritis, 68 cardiovascular disease, inflammatory bowel disease, 69 etc. Therefore, NETs may not only be mechanisms of psoriasis but also mechanisms of comorbidities.
1.4.2. Functions of NETs
NETs contain MMP‐9, 11 , neutrophil elastase, MPO, and cathepsin G, 60 as well as proteases 3 and LL‐37. 70 In various autoimmune diseases, such as rheumatoid arthritis 71 and systemic lupus erythematosus, 72 NETs also contain self‐antigens, which can be recognized by immune cells to induce autoimmunity. 73 The antibacterial peptide (LL‐37) can also act as a self‐antigen, 65 and is found in many cells, especially in KCs and neutrophils, 74 LL‐37 can form complexes with nuclear DNA or RNA produced by NETs (DNA‐LL37 or RNA‐LL37). 74 DNA‐LL37 bind to TLR8 expressed on inactive neutrophils to produce further NETs and large amounts of cytokines, resulting in further inflammation. LL‐37 can also activate myeloid dendritic cells, Th17 cells, and KCs through TLRs to produce IFN‐β which ultimately cause further exacerbation of autoimmunity and induction of psoriasis. 75 Although the source of RNA required for formation of RNA‐LL37 has not been confirmed for neutrophils, NETs themselves can be produced through suicidal NETosis of neutrophils, and neutrophils contain large amounts of RNA. Perhaps the release of large amounts of RNA from lysed neutrophils binds to LL‐37 to form RNA‐LL37. In addition, certain RNAs do not require formation of RNA‐LL37 to promote psoriasis formation. For example, Hua et al. pointed out that lncRNA can promote psoriasis formation. 76
Both mast cells and neutrophils release IL‐17 during the formation of extracellular traps, but neutrophil NETs contain higher levels of IL‐17 than MCETs do. 16 This may be one reason why neutrophils rather than mast cells are dominant in psoriasis patients. NETs release IL‐17, which in turn promotes NETosis, forming a cytokine amplification loop. This indicates that NETs can promote the onset and maintenance of psoriasis through multiple pathways.
1.5. The interaction of neutrophils with other immune cells
In traumatized or microbially infected skin, mast cells recognize viruses, bacteria, and other pathogens through TLRs. 84 This may lead to mast cells releasing TNF‐a, IL‐17, and CXCL2 through degranulation or MCETs. 16 These mediators cause an increase in vascular permeability and the chemotaxis of neutrophils to the site of inflammation. IL‐23 produced by KCs 85 can rapidly induce mast cell degranulation and extracellular trap formation, promoting the release of IL‐17 by mast cells, etc. Mast cells, which are already resident in skin tissue, may reflect a synergistic interaction with neutrophils in early psoriatic skin. And may be one mechanism of the Koebner phenomenon in psoriasis patients. Interactions also exist between IL‐17‐producing T cells and neutrophils, as IL‐17‐producing T cells can produce cytokines that promote neutrophil development, recruitment, and survival. 13
KC in the skin has antigen‐presenting function. 86 Streptococcus is believed to be associated with psoriasis, 87 the antigen produced by Streptococcus can be presented by KC to T cells, thereby triggering psoriasis. Some studies have shown that KC's keratin 87 or carbohydrates 88 have common antigens with Streptococcus. After skin infection with Streptococcus, autoantibodies are formed. And due to genetic susceptibility, KCs are more likely to respond to damage, thus initiating psoriasis. In the initial stage of psoriasis, KCs also produce antimicrobial peptides (AMPs), such as β‐defensin 89 and LL‐37. 90 Among them, LL‐37 is recognized as the main autoantigen of psoriasis, 91 which ultimately induces Th17 cells to produce IL‐17 92 through a series of reactions. Increasing evidence shows that KCs can produce IL‐23, 19 , 28 , 93 triggering a cascade reaction (Figure 1). One of the pathways leading to the release of IL‐23 by KCs is the activation of TLR3, which can be dependent on double‐stranded RNA (dsRNA). 93 DsRNA can come from viruses or from the organism. 94 Perhaps dsRNA is a autoantigen that can induce psoriasis. Therefore, perhaps in the early stage, psoriasis is independent of Th17. As keratinocytes produce IL‐23, mast cells originally stationed in the skin tissue will release IL‐17 first. 16 Or in the early stages of inflammation, neutrophils arrive at the skin first, causing a series of reactions. This is because KCs simultaneously express and release both neutrophil chemokines and IL‐23, allowing neutrophils to be chemotactically activated. Studies have shown that co‐incubation of KCs and neutrophils for 4 hours can induce neutrophils to produce IL‐17 and IL‐22. 15 We can speculate that IL‐23 activates the IL‐23R on neutrophil membranes, causing neutrophils to release IL‐17. And IL‐17A is one of the most pro‐inflammatory cytokines in the IL‐17 family, which binds to the receptor IL‐17R on KCs, leading to an increase in the production of cytokines such as TNF‐α, IL‐1, IL‐8, and IL‐6 95 (Figures 1 and 2). Neutrophils can also induce KC proliferation and keratinization by producing IL‐22. 30 IL‐17 can also stimulate KCs to produce human β‐defensin 2 and other AMPs (including LL‐37). 30 Studies have shown that IL‐19 is downstream of the IL‐23/IL‐17 axis, and IL‐19 can be secreted by KCs. 42 This self‐secreted IL‐19 by KCs leads to keratin layer proliferation. If KCs can produce more IL‐36 when activated by IL‐17, 45 this may lead to a more severe type of psoriasis.
When activated by pathogenic factors in the internal and external environment, KCs release neutrophil‐derived chemokines, which results in the recruitment of neutrophils to the skin. When neutrophils arrive at the vessels in the skin, they interact with the VECs. The MMP‐9 produced by neutrophils destroys cell connections between VECs, causing vascular dilatation and increased permeability. Along with changes on vascular endothelial cells, neutrophils are activated, 96 and lifespan and migratory ability of neutrophils are increased. 17 Activated neutrophils form NETs, causing the release of additional inflammatory factors. On the other hand, neutrophils mainly adhere to atherosclerotic plaques through the formation of NETs. 97 The IL‐17 produced by activated neutrophils acts in synergy with TNF‐α to stimulate endothelial cells to express neutrophil‐derived chemokines (CXCL1, CXCL2, and CXCL5), 98 attracting more neutrophils to the affected area. This interaction between skin VECs and neutrophils further exacerbates the inflammation in psoriasis, and may explain why patients with psoriasis are prone to cardiovascular diseases. After migrating to the skin tissue, neutrophils promote the progression of psoriasis by producing IL‐23, IL‐17, granule proteins, NETs, and releasing other cytokines.
2. CONCLUSION AND PROSPECT
Neutrophils are innate immune cells that play a critical role in the early stages of psoriasis inflammation. They can interact with other immune cells such as KCs and mast cells through the production of IL‐17, formation of NETs, and release of granule proteins. Neutrophil lifespan is significantly extended, and KCs and TH17 cells can attract neutrophils, amplifying the inflammatory effect.
Previous studies have identified the role of NETs in psoriasis, atherosclerosis, uremia, inflammatory bowel disease, and chronic obstructive pulmonary disease. NETs also play an important role in the pathogenesis of comorbidities. Today several drugs that can inhibit NETs have been developed for possible blocking NETosis, including cannabidiol [99], 99 acting on the formation and the pro‐inflammatory components within NETs. NETosis inhibitor ascomycin, 100 and antagomiR‐155 that can inhibit PAD4 (an enzyme involved in NETosis). 101 We expected that their applications through combination with current biologicals or alone would provide more clinical solutions for psoriasis and comorbidities.The lifespan of activated neutrophil is then significantly extended with these effectors. KCs and TH17 cells can attract neutrophils, amplifying an overall inflammatory effect in psoriasis.
CONFLICT OF INTEREST STATEMENT
The authors have no relevant financial or nonfinancial interests to disclose.
ACKNOWLEDGMENTS
This work was supported by grants from the Traditional Chinese Medicine Science and Technology Program of Shandong Province (NM 2021M080) and Development Plan of Jining (NM 2021YXNS121).
Wang Z, Shi D. Research progress on the neutrophil components and their interactions with immune cells in the development of psoriasis. Skin Res Technol. 2023;29:e13404. 10.1111/srt.13404
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Griffiths C, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397(10281):1301‐1315. [DOI] [PubMed] [Google Scholar]
- 2. Barilo AA, Smirnova SV. The role of nutritional factors and food allergy in the development of psoriasis. Vopr Pitan. 2020;89(1):19‐27. [DOI] [PubMed] [Google Scholar]
- 3. Geller S, Xu H, Lebwohl M, et al. Malignancy risk and recurrence with psorTNFiasis and its treatments: a concise update. Am J Clin Dermatol. 2018;19(3):363‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li H, Hu L, Zheng Y, et al. Burden of Disease Study (GBD). Chin J Dermatovenereol. 2021;35(4):386‐392. [Google Scholar]
- 5. Li X, Kong L, Li F, et al. Association between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta‐analysis. PLoS One. 2015;10(12):e0145221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL‐17‐producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S, Li G, Li X, Wu F, Li L. Etanercept ameliorates psoriasis progression through regulating high mobility group box 1 pathway. Skin Res Technol. 2023. 29(4):e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weger W. Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. Br J Pharmacol. 2010;160(4):810‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(20):7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakabo S, Romo‐Tena J, Kaplan MJ. Neutrophils as drivers of immune dysregulation in autoimmune diseases with skin manifestations. J Invest Dermatol. 2022;142(3 Pt B):823‐833. [DOI] [PubMed] [Google Scholar]
- 12. Rosales C, Demaurex N, Lowell CA, et al. Neutrophils: their role in innate and adaptive immunity. J Immunol Res. 2016;2016:1469780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu XT, Shi ZR, Lu SY, et al. Enhanced migratory ability of neutrophils toward epidermis contributes to the development of psoriasis via crosstalk with keratinocytes by releasing IL‐17A. Front Immunol. 2022;13:817040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Towne JE, Garka KE, Renshaw BR, et al. Interleukin (IL)‐1F6, IL‐1F8, and IL‐1F9 signal through IL‐1Rrp2 and IL‐1RAcP to activate the pathway leading to NF‐kappaB and MAPKs. J Biol Chem. 2004;279(14):13677‐13688. [DOI] [PubMed] [Google Scholar]
- 15. Dyring‐Andersen B, Honoré TV, Madelung A, et al. Interleukin (IL)‐17A and IL‐22‐producing neutrophils in psoriatic skin. Br J Dermatol. 2017;177(6):e321‐e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL‐17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hind LE, Ingram PN, Beebe DJ, et al. Interaction with an endothelial lumen increases neutrophil lifetime and motility in response to P aeruginosa. Blood. 2018;132(17):1818‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199(1):125‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piskin G, Sylva‐Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL‐23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006. 176(3):1908‐1915. [DOI] [PubMed] [Google Scholar]
- 20. Kvedaraite E, Lourda M, Ideström M, et al. Tissue‐infiltrating neutrophils represent the main source of IL‐23 in the colon of patients with IBD. Gut. 2016;65(10):1632‐1641. [DOI] [PubMed] [Google Scholar]
- 21. Tamassia N, Arruda‐Silva F, Wright HL, et al. Human neutrophils activated via TLR8 promote Th17 polarization through IL‐23. J Leukoc Biol. 2019;105(6):1155‐1165. [DOI] [PubMed] [Google Scholar]
- 22. Awasthi A, Riol‐Blanco L, Jäger A, et al. Cutting edge: IL‐23 receptor gfp reporter mice reveal distinct populations of IL‐17‐producing cells. J Immunol. 2009;182(10):5904‐5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hile G, Kahlenberg JM, Gudjonsson JE. Recent genetic advances in innate immunity of psoriatic arthritis. Clin Immunol. 2020,214:108405. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Zhu L, Chu Z, et al. Characterization and biological significance of IL‐23‐induced neutrophil polarization. Cell Mol Immunol. 2018;15(5):518‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL‐23/IL‐17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cua DJ, Tato CM. Innate IL‐17‐producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479‐489. [DOI] [PubMed] [Google Scholar]
- 27. Katayama H. Development of psoriasis by continuous neutrophil infiltration into the epidermis. Exp Dermatol. 2018;27(10):1084‐1091. [DOI] [PubMed] [Google Scholar]
- 28. Tachibana K, Tang N, Urakami H, et al. Multifaceted analysis of IL‐23A‐ and/or EBI3‐including cytokines produced by psoriatic keratinocytes. Int J Mol Sci. 2021;22(23):12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaffen SL. Structure and signalling in the IL‐17 receptor family. Nat Rev Immunol. 2009;9(8):556‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aujla SJ, Chan YR, Zheng M, et al. IL‐22 mediates mucosal host defense against Gram‐negative bacterial pneumonia. Nat Med. 2008;14(3):275‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirata T, Osuga Y, Takamura M, et al. Recruitment of CCR6‐expressing Th17 cells by CCL 20 secreted from IL‐1 beta‐, TNF‐alpha‐, and IL‐17A‐stimulated endometriotic stromal cells. Endocrinology. 2010;151(11):5468‐5476. [DOI] [PubMed] [Google Scholar]
- 32. Cho KA, Suh JW, Lee KH, et al. IL‐17 and IL‐22 enhance skin inflammation by stimulating the secretion of IL‐1β by keratinocytes via the ROS‐NLRP3‐caspase‐1 pathway. Int Immunol. 2012;24(3):147‐158. [DOI] [PubMed] [Google Scholar]
- 33. Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin‐17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593‐2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin‐17A and ‐17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009,30(1):108‐119. [DOI] [PubMed] [Google Scholar]
- 35. Sutton CE, Lalor SJ, Sweeney CM, et al. Interleukin‐1 and IL‐23 induce innate IL‐17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331‐341. [DOI] [PubMed] [Google Scholar]
- 36. Martin BN, Wang C, Zhang CJ, et al. T cell‐intrinsic ASC critically promotes T(H)17‐mediated experimental autoimmune encephalomyelitis. Nat Immunol. 2016;17(5):583‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parsonage G, Filer A, Bik M, et al. Prolonged, granulocyte‐macrophage colony‐stimulating factor‐dependent, neutrophil survival following rheumatoid synovial fibroblast activation by IL‐17 and TNFalpha. Arthritis Res Ther. 2008;10(2):R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kullberg MC, Jankovic D, Feng CG, et al. IL‐23 plays a key role in Helicobacter hepaticus‐induced T cell‐dependent colitis. J Exp Med. 2006;203(11):2485‐2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwarzenberger P, Huang W, Ye P, et al. Requirement of endogenous stem cell factor and granulocyte‐colony‐stimulating factor for IL‐17‐mediated granulopoiesis. J Immunol. 2000;164(9):4783‐4789. [DOI] [PubMed] [Google Scholar]
- 40. de Boer OJ, van der Meer JJ, Teeling P, et al. Differential expression of interleukin‐17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol. 2010;220(4):499‐508. [DOI] [PubMed] [Google Scholar]
- 41. Adachi A, Honda T, Egawa G, et al. Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J Allergy Clin Immunol. 2022;150(4):909‐919.e8. [DOI] [PubMed] [Google Scholar]
- 42. Witte E, Kokolakis G, Witte K, et al. IL‐19 is a component of the pathogenetic IL‐23/IL‐17 cascade in psoriasis. J Invest Dermatol. 2014;134(11):2757‐2767. [DOI] [PubMed] [Google Scholar]
- 43. van Asseldonk EJ, Stienstra R, Koenen TB, et al. The effect of the interleukin‐1 cytokine family members IL‐1F6 and IL‐1F8 on adipocyte differentiation. Obesity (Silver Spring). 2010;18(11):2234‐2236. [DOI] [PubMed] [Google Scholar]
- 44. Farooq M, Nakai H, Fujimoto A, et al. Mutation analysis of the IL36RN gene in 14 Japanese patients with generalized pustular psoriasis. Hum Mutat. 2013;34(1):176‐183. [DOI] [PubMed] [Google Scholar]
- 45. Queen D, Ediriweera C, Liu L. Function and regulation of IL‐36 signaling in inflammatory diseases and cancer development. Front Cell Dev Biol. 2019;7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henry CM, Sullivan GP, Clancy DM, et al. Neutrophil‐derived proteases escalate inflammation through activation of IL‐36 family cytokines. Cell Rep. 2016;14(4):708‐722. [DOI] [PubMed] [Google Scholar]
- 47. Tecchio C, Cassatella MA. Neutrophil‐derived chemokines on the road to immunity. Semin Immunol. 2016;28(2):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suzuki S, Kobayashi M, Chiba K, et al. Autocrine production of epithelial cell‐derived neutrophil attractant‐78 induced by granulocyte colony‐stimulating factor in neutrophils. Blood. 2002,99(5):1863‐1865. [PubMed] [Google Scholar]
- 49. Nguyen‐Jackson HT, Li HS, Zhang H, et al. G‐CSF‐activated STAT3 enhances production of the chemokine MIP‐2 in bone marrow neutrophils. J Leukoc Biol. 2012;92(6):1215‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scapini P, Laudanna C, Pinardi C, et al. Neutrophils produce biologically active macrophage inflammatory protein‐3alpha (MIP‐3alpha)/CCL20 and MIP‐3beta/CCL19. Eur J Immunol. 2001;31(7):1981‐1988. [DOI] [PubMed] [Google Scholar]
- 51. Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503‐3521. [PubMed] [Google Scholar]
- 52. Macleod T, Doble R, McGonagle D, et al. Neutrophil elastase‐mediated proteolysis activates the anti‐inflammatory cytokine IL‐36 Receptor antagonist. Sci Rep. 2016;6:24880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skrzeczynska‐Moncznik J, Wlodarczyk A, Zabieglo K, et al. Secretory leukocyte proteinase inhibitor‐competent DNA deposits are potent stimulators of plasmacytoid dendritic cells: implication for psoriasis. J Immunol. 2012;189(4):1611‐1617. [DOI] [PubMed] [Google Scholar]
- 54. Van Spaendonk H, Ceuleers H, Witters L, et al. Regulation of intestinal permeability: the role of proteases. World J Gastroenterol. 2017;23(12):2106‐2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cassatella MA, Östberg NK, Tamassia N, et al. Biological roles of neutrophil‐derived granule proteins and cytokines. Trends Immunol. 2019;40(7):648‐664. [DOI] [PubMed] [Google Scholar]
- 56. Kvist‐Hansen A, Kaiser H, Wang X, et al. Neutrophil pathways of inflammation characterize the blood transcriptomic signature of patients with psoriasis and cardiovascular disease. Int J Mol Sci. 2021;22(19):10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haskamp S, Bruns H, Hahn M, et al. Myeloperoxidase modulates inflammation in generalized pustular psoriasis and additional rare pustular skin diseases. Am J Hum Genet. 2020;107(3):527‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schauer C, Janko C, Munoz LE, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20(5):511‐517. [DOI] [PubMed] [Google Scholar]
- 59. Lee KH, Kronbichler A, Park DD, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev. 2017;16(11):1160‐1173. [DOI] [PubMed] [Google Scholar]
- 60. Dos Santos Ramos A, Viana G, de Macedo Brigido M, et al. Neutrophil extracellular traps in inflammatory bowel diseases: implications in pathogenesis and therapeutic targets. Pharmacol Res. 2021;171:105779. [DOI] [PubMed] [Google Scholar]
- 61. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532‐1535. [DOI] [PubMed] [Google Scholar]
- 62. Vorobjeva NV, Pinegin BV. Neutrophil extracellular traps: mechanisms of formation and role in health and disease. Biochemistry (Mosc). 2014,79(12):1286‐1296. [DOI] [PubMed] [Google Scholar]
- 63. Ouyang K, Oparaugo N, Nelson AM, et al. T cell extracellular traps: tipping the balance between skin health and disease. Front Immunol. 2022;13:900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017,23(3):279‐287. [DOI] [PubMed] [Google Scholar]
- 65. Delgado‐Rizo V, Martínez‐Guzmán MA, Iñiguez‐Gutierrez L, et al. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol. 2017;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Czerwińska J, Owczarczyk‐Saczonek A. The role of the neutrophilic network in the pathogenesis of psoriasis. Int J Mol Sci. 2022,23(3):1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gestermann N, Di Domizio J, Lande R, et al. Netting neutrophils activate autoreactive B cells in lupus. J Immunol. 2018,200(10):3364‐3371. [DOI] [PubMed] [Google Scholar]
- 68. Goel RR, Kaplan MJ. Deadliest catch: neutrophil extracellular traps in autoimmunity. Curr Opin Rheumatol. 2020;32(1):64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bonaventura A, Vecchié A, Abbate A, et al. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5(8):577‐582. [DOI] [PubMed] [Google Scholar]
- 71. Sur Chowdhury C, Giaglis S, Walker UA, et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16(3):R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leffler J, Martin M, Gullstrand B, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188(7):3522‐3531. [DOI] [PubMed] [Google Scholar]
- 73. Garcia‐Romo GS, Caielli S, Vega B, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011,3(73):73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Herster F, Bittner Z, Archer NK, et al. Neutrophil extracellular trap‐associated RNA and LL37 enable self‐amplifying inflammation in psoriasis. Nat Commun. 2020;11(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ganguly D, Chamilos G, Lande R, et al. Self‐RNA‐antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206(9):1983‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hua X, Li J, Shang M, et al. Pathogenesis of psoriasis via miR‐149‐5p/AKT1axis by long noncoding RNA BLACAT1. Skin Res Technol. 2023;29(5):e13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu N, Qin H, Yu Y, et al. A distinct immature low‐density neutrophil population characterizes acute generalized pustular psoriasis. J Invest Dermatol. 2022;142(10):2831‐2835.e5. [DOI] [PubMed] [Google Scholar]
- 78. Cassatella MA, Scapini P. On the improper use of the term high‐density neutrophils. Trends Immunol. 2020;41(12):1059‐1061. [DOI] [PubMed] [Google Scholar]
- 79. Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284‐3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rodriguez‐Rosales YA, Langereis JD, Gorris M, et al. Immunomodulatory aged neutrophils are augmented in blood and skin of psoriasis patients. J Allergy Clin Immunol. 2021;148(4):1030‐1040. [DOI] [PubMed] [Google Scholar]
- 81. Tsuda Y, Takahashi H, Kobayashi M, et al. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin‐resistant Staphylococcus aureus. Immunity. 2004;21(2):215‐226. [DOI] [PubMed] [Google Scholar]
- 82. Albanesi C, Madonna S, Gisondi P, et al. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol. 2018;9:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Powner DJ, Pettitt TR, Anderson R, et al. Stable adhesion and migration of human neutrophils requires phospholipase D‐mediated activation of the integrin CD11b/CD18. Mol Immunol. 2007;44(12):3211‐3221. [DOI] [PubMed] [Google Scholar]
- 84. Yu Y, Blokhuis BR, Garssen J, et al. Non‐IgE mediated mast cell activation. Eur J Pharmacol. 2016;778:33‐43. [DOI] [PubMed] [Google Scholar]
- 85. Colonna M. Interleukin‐22‐producing natural killer cells and lymphoid tissue inducer‐like cells in mucosal immunity. Immunity. 2009;31(1):15‐23. [DOI] [PubMed] [Google Scholar]
- 86. Roopashree MR, Gondhalekar RV, Shashikanth MC, et al. Pathogenesis of oral lichen planus–a review. J Oral Pathol Med. 2010;39(10):729‐734. [DOI] [PubMed] [Google Scholar]
- 87. McFadden J, Valdimarsson H, Fry L. Cross‐reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125(5):443‐447. [DOI] [PubMed] [Google Scholar]
- 88. Shikhman AR, Cunningham MW. Immunological mimicry between N‐acetyl‐beta‐D‐glucosamine and cytokeratin peptides. Evidence for a microbially driven anti‐keratin antibody response. J Immunol. 1994;152(9):4375‐4387. [PubMed] [Google Scholar]
- 89. Hu SC, Yu HS, Yen FL, et al. Neutrophil extracellular trap formation is increased in psoriasis and induces human β‐defensin‐2 production in epidermal keratinocytes. Sci Rep. 2016;6:31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fuentes‐Duculan J, Bonifacio KM, Hawkes JE, et al. Autoantigens ADAMTSL5 and LL37 are significantly upregulated in active Psoriasis and localized with keratinocytes, dendritic cells and other leukocytes. Exp Dermatol. 2017;26(11):1075‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ni X, Lai Y. Keratinocyte: a trigger or an executor of psoriasis. J Leukoc Biol. 2020;108(2):485‐491. [DOI] [PubMed] [Google Scholar]
- 92. Sano S, Chan KS, Carbajal S, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 93. Ramnath D, Tunny K, Hohenhaus DM, et al. TLR3 drives IRF6‐dependent IL‐23p19 expression and p19/EBI3 heterodimer formation in keratinocytes. Immunol Cell Biol. 2015;93(9):771‐779. [DOI] [PubMed] [Google Scholar]
- 94. Chen YG, Hur S. Cellular origins of dsRNA, their recognition and consequences. Nat Rev Mol Cell Biol. 2022;23(4):286‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin‐17 and interferon‐gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111(4):645‐649. [DOI] [PubMed] [Google Scholar]
- 96. Chen J, Zhu Z, Li Q, et al. Neutrophils enhance cutaneous vascular dilation and permeability to aggravate psoriasis by releasing matrix metallopeptidase 9. J Invest Dermatol. 2021;141(4):787‐799. [DOI] [PubMed] [Google Scholar]
- 97. Acosta JB, del Barco DG, Vera DC, et al. The pro‐inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5(4):530‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Griffin GK, Newton G, Tarrio ML, et al. IL‐17 and TNF‐α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188(12):6287‐6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wójcik P, Garley M, Wroński A, et al. Cannabidiol modifies the formation of NETs in neutrophils of psoriatic patients. Int J Mol Sci. 2020,21(18): 6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gupta AK, Giaglis S, Hasler P, et al. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9(5):e97088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hawez A, Al‐Haidari A, Madhi R, et al. MiR‐155 regulates PAD4‐dependent formation of neutrophil extracellular traps. Front Immunol. 2019;10:2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


