Highlights
-
•
Hydrodynamic cavitation (HC) is a highly effective, low-cost, and eco-friendly process.
-
•
The working principle of hydrodynamic cavitation is explained.
-
•
The recent applications of HC technique for food processing are discussed.
-
•
Application of HC for cell disruption and selective enzyme release has been discussed.
-
•
The HC processed foods showed improved physicochemical properties and bioactive stability.
-
•
The HC technology can be considered as a green technology for large-scale food processing applications.
Keywords: Hydrodynamic cavitation, Non-thermal food processing, Energy efficient, Cell disruption, Waste valorization
Abstract
Hydrodynamic cavitation (HC) is the process of bubbles formation, expansion, and violent collapse, which results in the generation of high pressures in the order of 100–5000 bar and temperatures in the range of 727–9727 °C for just a fraction of seconds. Increasing consumer demand for high-quality foods with higher nutritive values and fresh-like sensory attributes, food processors, scientists, and process engineers are pushed to develop innovative and effective non-thermal methods as an alternative to conventional heat treatments. Hydrodynamic cavitation can play a significant role in non-thermal food processing as it has the potential to destroy microbes and reduce enzyme activity while retaining essential nutritional and physicochemical properties. As hydrodynamic cavitation occurs in a flowing liquid, there is a decrease in local pressure followed by its recovery; hence it can be used for liquid foods. It can also be used to create stable emulsions and homogenize food constituents. Moreover, this technology can extract food constituents such as polyphenols, essential oils, pigments, etc., via biomass pretreatment, cell disruption for selective enzyme release, waste valorization, and beer brewing. Other applications related to food production include water treatment, biodiesel, and biogas production. The present review discusses the application of HC in the preservation, processing, and quality improvement of food and other related applications. The reviewed examples in this paper demonstrate the potential of hydrodynamic cavitation with further expansion toward the scaling up, which looks at commercialization as a driving force.
1. Introduction
Consumers are becoming more aware of the benefits of minimally processed, non-thermal foods that provide maximum nutrients while providing as few calories as possible. In fact, the consumption of polyphenol-rich foods, mainly fresh fruits and vegetables, has decreased due to increased demand for highly processed foods. Our diet should contain bioactive compounds such as polyphenols, flavonoids, alkaloids, tannins, essential oils, etc., because of their potent anti-inflammatory and strong antioxidant effects [1]. To meet this demand, food technologists must constantly look for new methods that produce desired results that are scalable and cost-effective. Consumer expectations must be addressed, but minimal processing comes with the risk to food safety, as taking care of all pathogens is crucial. With the evolution of emerging technologies, conventional technology can be replaced with encouraging results. These technologies include acoustic and hydrodynamic cavitation, irradiation, high-hydrostatic pressure, microwave, pulsed electric field, and ohmic heating [2]. The current barriers of high investment prices, insufficient control of process operation factors, and a lack of regulatory permission have been holding up wider industrial adoption of these technologies. In the United States, a survey of specialists in the field of food was conducted to understand the critical justifications for utilizing novel technologies, the barriers to their implementation, and the main forces driving innovation in non-thermal food processing methods [3]. Better nutritive and sensory qualities were cited as a primary reason for choosing non-thermal food processing technology by ˃70% of the consumers. The chief obstacle in adopting a non-thermal food process technology is the requirement of high investment (41%). Hydrodynamic cavitation technology is a unique, non-thermal, scalable, cost-effective method that can solve all the issues described.
Cavitation is a process in which bubbles or cavities emerge, expand, then implode in a short space of time, discharging a tremendous amount of energy in the process, with temperatures and pressures ranging from 727 to 9727 °C and 100–1000 bar, respectively [4]. These effects are observed simultaneously at multiple locations, resulting in higher mean energy pockets than traditional processes.
1.1. Types of cavitation generation
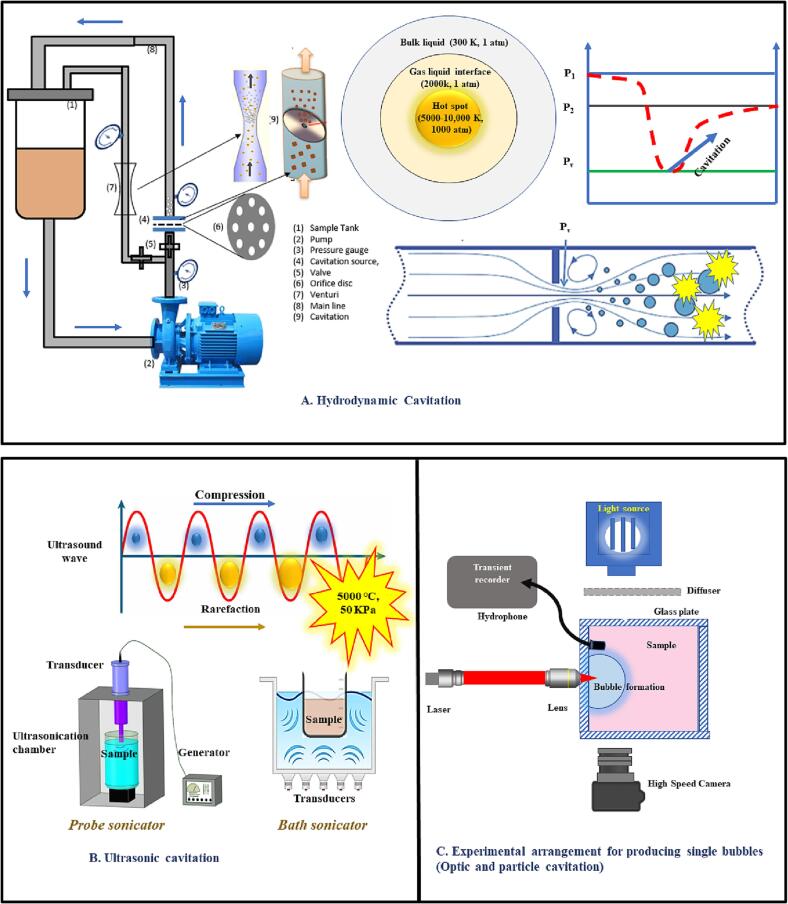
Tensions and local energy dissipation in a liquid are responsible for creating cavitation. The primary criterion for differentiating between different cavitation forms is the cavity creation technique. The four significant forms of cavitation displayed in Fig. 1 and their causes are summarized below (Fig. 1):
-
•
The pressure differential in a liquid flow induced by the system's flow velocity causes hydrodynamic cavitation.
-
•
Acoustic cavitation occurs when ultrasound waves cause a differential pressure in a liquid.
-
•
Optical cavitation happens when liquid bursts under the influence of laser or high-intensity light which is a consequence of the local deposition of energy.
-
•
Particle cavitation occurs when any subatomic particle, such as a proton, causes a liquid burst in a bubble trap to produce cavitation [5].
Fig. 1.
Types of cavitation A. hydrodynamic cavitation, B. acoustic cavitation, and C. optic and particle cavitation.
Optical and particle cavitation technology is used at the laboratory scale for fundamental cavitation studies such as bubble dynamics. Acoustic and hydrodynamic cavitation, on the other hand, was the first to be studied and widely applied in academia and industry, owing to ease of operation and the ability to generate required cavitation intensities. Hydrodynamic cavitation is particularly capable of extensive industrial applications compared to other cavitational techniques, such as optic, acoustic, and particle, as HC provides ease of scalability, cost-effectiveness, and potentially effective treatment.
1.1.1. Hydrodynamic cavitation
Science, engineering, and technology considerably use cavitation phenomena to accelerate chemical processes, microbial cell rupturing, and mass transfer [6]. When kinetic energy is converted to pressure energy using an aperture or a venturi, a fluid mechanics phenomenon known as hydrodynamic cavitation occurs; several other subtypes of hydrodynamic cavitation also exist, including traveling cavitation, fixed cavitation, vortex cavitation, and vibratory cavitation. Traveling cavitation occurs when individual transitory voids or bubbles arise in the liquid and move with it as they expand, contract, and eventually collapse. The scenario in which fluid flow disengages from the stiff border of a submerged body or a flow tube to produce a pocket or cavity connected to the boundary is referred to as “fixed cavitation” and occasionally arises after conception. The fixed or connected cavity is quasi-steadily stable. The cavities in vortex cavitation are discovered at centers of tornados which occur in high shear regions (Fig. 1A). The cavities might seem fixed or traveling cavities. When a liquid constituent is subjected to vibratory cavitation, the velocity is so low that there are numerous cavitation cycles rather than just one. When the liquid is through a cavitation reactor, it results in pressure and velocity fluctuations. A typical hydrodynamic cavitation can be achieved using high-pressure pumps, turbines, and propellers [5].
1.1.2. Acoustic cavitation
Sound waves, primarily ultrasound in the range of 16 KHz-1000 MHz, are employed to generate cavitation phenomenon. When sonic waves flow through a medium, cavitation bubbles fluctuate among compression and enlargement due to pressure differences in the medium, causing vapors to diffuse through and outside the bubble. This cavitation phenomenon is only seen in liquids and liquids containing solid components (Fig. 1B) [7]. Acoustic cavitation is characterized by two primary physical behaviors: diffusion and dispersion through the solid cell matrix and vibratory washing of cell compounds due to severe cellular membrane disruption [8]. The collapse and disintegration of cavities are responsible for cell wall disruption onto the matrix cells, subsequently generating microjets, which cause consequences such as surface peeling, erosion, and particle breakage [9].
Acoustic cavitation involves the use of two kinds of cavities: stable and transient. Stable cavities go through multiple sonic rounds and oscillate about a specific symmetric size; transient bubbles, on the other hand, can only go through just a few cycles and increase fast to roughly double their initial size before abruptly breaking apart. The cavities can expand by colliding with other bubbles with the diffusion of vapors in cavities throughout enlargement and partial expulsion through compression. The collapse of cavities into smaller bubbles whenever they reach a crucial limit size. Cavity collapse is a highly violent process that produces severe local circumstances. Pressures vary from 50 to 1000 bar, and temperatures can reach 5000 °C. Since the bubble collapse occurs relatively quickly, cooling occurs at a rate of 737 °C/sec, making it a quasi-adiabatic high-energy process [9]. Shear force and disintegration caused by cavitation explosion in the cellular structure promote the breakdown of matrix cells and the release of cell materials to the liquid as the surface area increases and particle size decreases [9]. However, several factors, such as sonication energy, frequency, power, and period of cavitation, can affect the efficacy and behavior of acoustic cavitation [10]. As a result, these factors must be controlled and optimized.
1.1.3. Optical and particle cavitation
When a large amount of energy is placed on a liquid, it causes optical and particle cavitation. Laser cavitation is caused by a shorter laser pulse directed in a cuvette containing a fluid with a weak absorption coefficient (typically water) (Fig. 1C). The initial energy in the pulse is received by laser-produced plasma, then transformed to light, heat, and mechanical energy, which are observed in the shock wave and bubble [11]. In this case, the light intensity at the point is so high that nonlinear absorption or snow slide ionization creates plasma, which can rapidly heat up owing to the laser, resulting in abrupt water vaporization and forming vapor bubbles [12]. The cavitation bubble expands to its maximum size before collapsing to restart the process. This bouncing motion is maintained in succeeding bubbles [13]. Optical cavitation may be created using both pulsed and continuous-wave lasers. However, it should be compressed into a highly absorbent liquid, frequently a mixture of water and some color [14]. The photonic flux is significantly absorbed by a small quantity of solution heated to the critical temperature of water (300 °C). At such temperatures, the restricted amount of superheated water is converted to vapor, forming rapidly growing bubbles termed as thermo-cavitation bubbles [14]. Optical fibers coated with metallic nanoparticles are commonly utilized to boost the optic cavitation effect [12].
In optic cavitation, photons are used to break the liquid. There is no reason why additional subatomic particles, such as protons and neutrons, might not dissolve in liquid. This type of cavitation is generally known as particle cavitation. In this type of cavitation, a charged particle is driven through a liquid, leaving an ionization path for a short time. A portion of the energy emitted by such ions is transformed into a few fast electrons, which can quickly release up to 1000 eV of energy, resulting in significant local heating. If the liquid is superheated because of expansion, boiling will occur, resulting in the development of a line of smaller bubbles. In another way, a threshold state is required for this sort of cavitation to occur. Bubble kinetics and the concurrent processes for a single cavity and its explosion, alongside the interactions amongst several cavities in a precise atmosphere, are primarily studied using optic and particle cavitation [5].
1.2. Principle of hydrodynamic cavitation (HC)
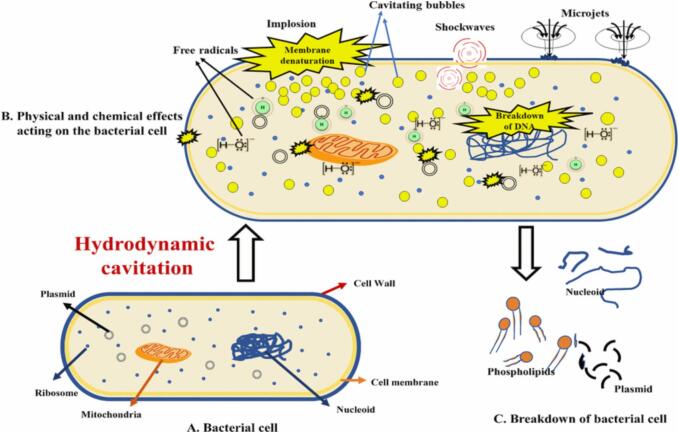
A simple method to generate cavities is passing the liquid through a flow constriction device such as an orifice or a venturi. As the liquid passes through these devices, velocity increases, which means the kinetic energy rises at the cost of pressure drop. When this pressure drop reaches below the liquid’s vapor pressure, it results in the formation of cavities. These cavities grow further at the expense of pressure recovery and start collapsing to generate colossal energy in the surrounding liquid. The cavitation pressure is usually the vapor pressure of the system. Hence, one can control the geometry and operating conditions of the cavitation reactor to obtain the desired cavitation effect. The HC is a fluid mechanics extravaganza generated by millions of small voids or microjets, which is analogous to acoustic cavitation. These spaces expand and burst in a liquid or liquid-matrix cell interface, resulting in mechanical properties such as frictional resistance and turbulent sound waves. This state creates significant pressures (500 MPa) and extreme heat (727 to 9727 °C), discharging a substantial amount of energy (about 1018 kW/m3) over a short time [6]. The concentration of hydraulic power and the turbulence's severe trembling causes cell enlargement or collapse. The intense inter-particle collision and turbulence in the cell-matrix initially allow for accelerated mass transfer rates, so after draining out and swiftly releasing the cell metabolites (plant bioactives, enzymes, proteins, etc.) in the liquid [15]. The mechanism of microbial cell destruction is represented in Fig. 2.
Fig. 2.
The mechanism of microbial cell destruction due to hydrodynamic cavitation and release of enzymes, cell organelles, and bioactives.
Theoretically, HC efficacy may be calculated using cavitation number (σ) [6], a derivative of Bernoulli's equation. It represents the pressure drop necessary to achieve vaporization. The specific kinetic energy at starting phase (σi) of cavitation is greater than that of the non-cavitating phase. The following equation (1) may be employed to compute the cavitation number.
| (1) |
where Pref (N/m2): Reference pressure (atmospheric pressure) before the cavitation device (venturi or orifice), Pv (N/m2): Liquid vapor pressure, σ (kg/m3): Liquid density, and V2 (m/s): starting flow rate of liquid at cavitation device, depends on Pref.
The cavitation number should be below 1 for better results but not too low to cause supercavitation, which could lead to the locking of vapors and no collapse of cavities. Cavitation originates from emerging cavitation numbers, above which the conditions are non-cavitating.
Hydrodynamic cavitation (HC) is achieved by passing a liquid through a small venturi or orifice device, which intensifies the kinetic energy. The cavitation effectiveness may be managed by adequately managing process factors like pressure and flow rate, as well as adjusting and employing modified orifice diameter or venturi size. Fig. 1A. depicts the hydrodynamic cavitation assembly and mechanism. The cavitation reactor comprises various components, including a (venturi or orifice), a sample storage tank, and a sample collecting outlet [16]. Tailored construction reduces surface damage, delays scaling caused by shock waves, and ensures correct and consistent thermal transfer through the equipment.
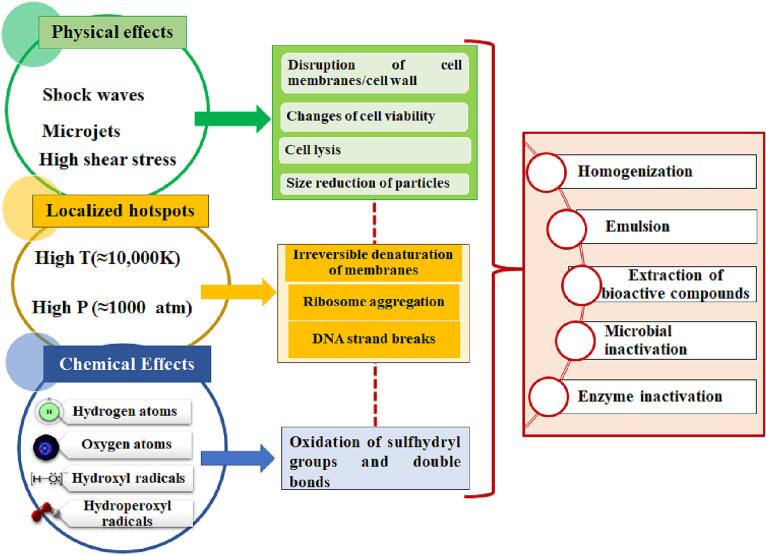
2. Physicochemical effects of cavitational collapse during hydrodynamic cavitation
To understand the physicochemical changes that occur during the cavitational collapse, the effect is studied in three parts, i.e., the interior region of cavity, the interfacial region between cavity and liquid, and the bulk liquid. The essential physicochemical effects of cavitational collapse during hydrodynamic cavitation are briefed in Fig. 3. A very high temperature and pressure exist in the interior region of cavity due to the inherent property to collapse adiabatically. Thus, the cavities could be considered high-temperature and pressure micro-reactors. Thus, free radicals are generated due to the breakage of constituents’ bonds inside the cavity. The cavitation bubble's radial motion concentrates energy and essentially generates the physical and chemical processes that cavitation causes. The system's pressure fluctuation profile is the primary source of the radial motion. In contrast to oscillatory behavior in non-turbulent situations, bubble behavior in turbulent conditions is transient and resembles the behavior of a cavity during acoustic cavitation [17]. Recovery pressure, the amount of permanent pressure head loss in the flow (which is manifested by the generation of turbulent pressure fluctuations in the flow), and cavitation number are the three main design variables that control the transient bubble dynamics in hydrodynamic cavitation that produces the sonochemical effect of generation of highly reactive radicals [17]. When the bubble transiently collapses, the trapped water molecules become exposed to the high temperatures and pressures that are generated. This causes thermal dissociation, which then produces radicals (such as hydrogen and hydroxyl radicals).
Fig. 3.
Physicochemical effects of cavitational collapse during hydrodynamic cavitation.
Water vapor movement in the bubble occurs in two stages: diffusion to the bubble wall and condensation at the bubble wall [18]. As bubble expands, at the bubble interface, liquid evaporates while vapor molecules diffuse inward towards the bubble's center. Vapor transport in the bubble is a diffusion-limited process, and the relative contribution of the diffusion mechanism to vapor entrapment is greater than the contribution of the condensation mechanism. At adiabatic collapse, the bubble's temperature and pressure reach their maximum levels, and the vapor and gas molecules inside go through thermal dissociation, creating a number of chemical species, some of which are radical species that cause the sonochemical effect [5].
Moreover, many physical effects are induced during the cavitational collapse, such as increasing the rate of heat and mass transfer, generation of shock waves, interfacial turbulence, and liquid microjet formation. The cavitational collapse impacts physicochemical changes depending on the systems in which it happens. In a homogeneous liquid and a heterogeneous system, respectively, symmetric collapse (chemical effect) and asymmetric collapse (physical effect) take place [19]. For a heterogeneous system, the collapse of a cavity will lead to forming micro-jets near the interface because of the asymmetric rush of liquid. These micro-jets are of high velocity, which causes disturbance in the boundary layer of the solid–liquid system and leads to the breakdown of the liquid film, which earlier led to the mass transfer resistance. Fragments of solid particles may be formed if the energy of the micro-jets is high enough. For a liquid–liquid system, liquid microjets break down the droplets [19].
3. Merits, demerits, and limitations of cavitation in food processing
Strangely, because of the difficulty in replacing thermal processes, other methods of food preparation have emerged, including microwave (MW), ultrasonic (US), hydrodynamic cavitation (HC), high-pressure processing (HPP), pulsed electric field (PEF), pulsed light (PL), and plasma therapy (PT). Each of the aforementioned technologies offers an alternative to traditional thermal processing and non-green processes, according to a comparative evaluation by Kamal et al. [20]. Because of its effective mixing capabilities operation, lower temperature application, simple operation (which eliminates several process stages), and selective extraction, HC is now one of the most researched sectors. The US, PEF, PL, HPP, and other technologies share some of the abovementioned benefits. However, because of the potential for large-scale execution in the future, HC continues to draw more researchers’ attention [20].
Compared to other technologies, HC has several benefits and process restrictions. For instance, HC may be used in a continuous cycle on a commercial basis and is simple to scale up [21]. Continuous processes immediately save costs by speeding up the process and using fewer solvents. However, HC reactors need regular and ongoing maintenance, which means hot spots and shock waves arise when cavitation bubbles rupture because they compress energetic liquid into minor areas [22]. In other terms, the equipment might deteriorate due to hot areas and ongoing shock waves. In addition, HC has a lower intensity of cavitation collapse than the US. Moreover, because of the excellent blending, there is equal cavitation throughout the reactor and, consequently, no direction sensitivity. Cavitation occurs in the bulk liquid's shear layer, making metal abrasion concerns less severe [4]. Another HC mode is negative pressure cavitation (NPC), which needs to apply negative pressure to the sample via a vacuum pump. NPC is mainly used to extract phenols, flavonoids, and bioactive compounds [23]. This surges intensive cavitation, turbulence, collisions, and mass transfer. In addition, the process is worked at ambient temperature and under the impact of a vacuum. Therefore, it can prevent the degradation of heat-sensitive compounds [23].
4. Applications of hydrodynamic cavitation (HC)
4.1. HC for microbial inactivation, cell disruption, selective release of enzyme, and detection
Microbial cell disintegration is identified as one of the fundamental requirements for microbial inactivation in food products. With HC, the cells experience physical stressors such as chock waves, bubble implosions, and microjets; that lead to a cellular breakdown (Fig. 2). This technique is used alone or in conjunction with other technological innovations for food preservation. Cell walls are most certainly the prime goal of high-pressure-driven spore destruction. High pressure will likely impact the outer spore membrane, although it is not required for spore germination. On the other hand, the inner spore membrane functions as the cell wall of the growing spore and guarantees spore core desiccation. As a result, a pressure-induced phase change or fluidity shift can modify the porousness characteristics of the inner cell wall, resulting in Ca2 + DPA (dipicolinic acid) release during the pressure treatment. As a result, following core hydration is accompanied by increased motion of core proteins and decreased temperature resistance of the spore [24]. According to this information, HC is involved with food decontamination at the appropriate moment. Aside from the detrimental impact on microbes under-regulated parameters, HC has several additional advantages, such as lower energy usage, freshness, and little processed food [4]. For instance, Milly et al. examined the use of HC to inactivate food spoilage microorganisms to pasteurize and sterilize liquid foods such as skimmed milk, apple, and tomato juices [25]. They found that cavitation generated in the food system effectively inactivated microorganisms, including yeast and lactic acid bacterium spores. In later years, Arya et al. researched the use of a thermal-assisted cavitation reactor to ensure the microbiological safety of foods (orange juice) containing high levels of acid (ascorbic acid) [26]. According to their testimony, lower temperatures and shorter HC treatment could result in a better deactivation of microorganisms with better retention of bioactives.
E. coli is a chief indicator of the safety of water and other food items. Staphylococcus aureus and Bacillus cereus are pathogenic organisms found in raw milk. Sun et al. found that the instantaneous temperature increase in the HC reactor and outlet temperature are essential factors in effectively inactivating pathogens [27]. The inactivation by continuous hydrodynamic cavitation is accomplished due to the mixture of mechanical, thermal, and chemical processes. During bubble collapse, the mechanical impact of shock waves with pressures as high as 6000 Giga Pa, micro-jets moving at 175 m/s, and shear stress as intense as 3.5 kPa can induce extensive membrane rupture, resulting in the loss of cytoplasmic and periplasmic components from the cell [27]. The continuous hydrodynamic cavitation treatment temperature (70 °C, 15–40 s) was lesser, and a shorter processing time (1–2 s) than the thermal treatment (71–74 °C, 15–40 s). Under ideal conditions, 5.9 for Escherichia coli (100%), 5.5 for Staphylococcus aureus (100%), and 3.0 for Bacillus cereus (99.9%) log reductions were attained with a processing rate of about 4.2 L/min [27]. Some macro (proteins and lipids), and micronutrients (vitamins and minerals), stay retained after the HC treatment (70 °C/2 s). It has also been discovered that HC processing security is equivalent to thermal processing for inactivation. However, the authors noted that while HC appears to be effective in keeping such characteristics in milk, more research into the process's influence on the shelf-life of the product in terms of lipid oxidation reactions is required [27]. The authors identified this study based on the detrimental impact of high pressure, shear forces, and temperature during bubble implosion on milk fat globules [28].
In reconstituted skim milk concentrate (SMC) (34–36 percent total solids), the microbial inactivation of the HC was tested [29]. Throughout the studies, the input pressure has been maintained constant (104000 Pa), whereas the outflow pressure increases as the pressure ratio grows. Within the experimental domain, the pressure ratio ranged from 1.21 to 0.491. After cavitation, the survival of B. Coagulans was not substantially different from the inoculation sample (4.4 and 4.8 log CFU/mL) for all treatments. After 48, 76, and 106 s, the final counts of the survivors after cavitation with HTST (high temperature short time) were 1.9, 1.3, and 1.1, respectively [29]. The cavitation-thermal treatment (75 °C) resulted in a 3.65 log reduction, whereas HTST alone resulted in a 3.06 log reduction [29].
Among the acoustic and hydrodynamic cavitation methods, venturi-based HC processing reduced total bacteria the most (approximately 1.2 log) at a pressure of 10 bar from a peanut milk sample [30]. The combined effect of HC and heat treatment on Bacillus coagulans reduction in SMC (skim milk concentrate) is expected to be synergetic at 75 °C. Though this explanation should be used with caution since the decrease of B. Coagulans is influenced by beginning counts of milk powder used to make the SMC, the resulting log reduction may not accurately reflect the method’s lethality. Cavitation followed by thermal treatment resulted in a 3.5 log reduction, whereas about 2.77 log reduction was attained when thermal treatment alone. Individual cavitation, on the other hand, did not result in any substantial reduction. This technique can be used in various processing conditions (50–300L/h, 600–3600RPM, 70–85 °C, and 10–110 s residence time). The microbiological burden of sugarcane juice was significantly reduced by orifice-based HC treatment, which is equivalent to other non-thermal high-pressure treatments. The designed system operates at a lower pressure than conventional higher-pressure systems. Although the color of HC-processed juice changes, this might not be satisfactory to specific customers [31].
Pathogen detection methods for food are frequently insufficient as it is difficult to entirely separate germs from food matrices. Nze et al. demonstrated a method for separating and detecting E. coli cells and C. parvum oocysts buried in crushed beef [32]. This was performed by enzymatic hydrolysis, HC to destroy microorganisms from the flesh, immunomagnetic isolation to clean meat, and passive electrochemical identification of specific pathogens. This method of pathogen isolation via HC is compared to an industry-standard separation approach. Hydrodynamic cavitation improves the detection abilities of a sensory system, which is similar or superior to standard sampling procedures. Due to the intricacy of a beef food matrix, this technology might be used to identify infections in other complex food matrices. An integrative and completely automized system for the identification and sensitive quantification of various pathogens in food and drinking water samples is being developed.
Considering all the aspects and applications discussed above, hydrodynamic cavitation is considered an effective and promising alternative technology for the inactivation of microbes during food processing. Compared to conventional thermal processes, the HC treatment has been efficient in destroying/inactivating microbes with the requirement of lesser treatment time and temperature conditions. Thus, HC can be utilized as an alternative to conventional thermal processes for the efficient degradation of microbes in food processing applications. A minimum log reduction of 3 has been observed and reported in most of the applications for microbial inactivation. Cavitation was found to be more effective in combination with thermal treatment and when operated in a continuous mode.
4.1.1. Hydrodynamic cavitation for the selective release of enzymes
The enzymes are considered one of the most important biocatalysts owing to their high catalytic activity, high specificity, reaction under milder environmental conditions, high product yield, and less contamination in the final product [33], [34]. Considering all these benefits, enzymes are used for numerous applications in various industries, such as food, biotechnology, pharmaceuticals, wastewater treatment, leather, and detergent [35], [36], [37], [38]. The enzymes are widely produced using fermentation processes. However, these biocatalysts are located at the intracellular locations of microbes such as membrane-bound, periplasm, or cytoplasm. The targeted recovery of enzymes is easier and more advantageous if located at the outer cell wall or periplasm location. This is due to the less contamination of unwanted materials, like other proteins, cell components, and nucleic acids, than in cytoplasmic locations. If the product location is in the cytoplasm, a complete cell disruption is required resulting in the release of other contaminating materials mentioned above along with cell debris [39], [40].
The presence of these unwanted materials adds an extra cost to the purification of the targeted enzymes. Therefore, tremendous efforts were made to genetically modify the microbes for the extracellular production of targeted products. But genetic manipulation is limited and cannot be done with every microbe, and hence some products are produced at the intracellular location only. Thus, the cell disruption process becomes unavoidable [41]. Hence an efficient large-scale disruption of microbial cells plays a vital role in the selective release of intracellular enzymes without hampering enzymatic activity. Over the last few decades, various methods have been used to develop efficient cell disruption technology, including cavitation technology.
The first application of cell rupture using hydrodynamic cavitation (HC) technology was reported by Harrison and Pandit in 1992. This was the first study on the successful application of HC for cell disruption. Followed by this, a considerable effort has been further made by both the authors in their respective research groups with more designs and improvements to enhance the effectiveness of cavitation reactors for intensified cell disruption operations. Different geometries of venturi and orifice plates were designed and used to generate cavitation of different intensities. An orifice plate gives far greater versatility than a venturi due to its geometrical design and operational requirements. Thus, a specific orifice plate can be selected based on the cavitational intensity and application required for the release of specific enzymes, operating conditions, and the geometry of the cavitation device.
The degree of enzyme release is determined by cavitation power, which Balasundaram and Pandit investigated using a term known as the location factor [41]. The location factor is the ratio of the targeted enzymes' release rate to the total protein release rate as a marker of cytoplasmic content. Many of these cell disruption processes follow first-order kinetics, and the release rate constant for specific enzyme release (kI) and total protein (kT) are as follows (equations (2), (3) [41].
| (2) |
where, RTot is the enzymes and total protein released in time ‘t’ second; Rmax is the maximum release of total proteins and enzymes. The values of release rate constants, kT or kI are obtained by plotting the values of ln D-1 against the time required for disruption of cells (t). Therefore, if the ratio of the targeted enzyme and total protein release rate constant is taken, it gives the location factor.
| (3) |
where, Ki = Release rate constant for enzyme; Kt = Release rate constant for total protein.
The release rate of enzymes located at the periplasmic location would be higher than the release rate of proteins. Thus, the ratio of enzyme to total protein release rate constant is equal to or greater than one. On the other hand, if an enzyme is present in the cytoplasm, the specific enzyme release rate could be less than the release rate of total proteins. This gives the release rate constant of enzymes to proteins less than or equal to one [42].
It has been reported that if targeted enzymes are located at the outer layer of the cell wall and periplasmic location, cell walls can be disrupted at much lower cavitational intensity, and enzymes can be recovered more quickly. On the other hand, if enzymes are located at the cytoplasmic location, a higher cavitational intensity is required for its release. Therefore, it is imperative to achieve cavitation intensity specifically for the release of targeted enzymes, and it can be achieved using a cavitation setup with an orifice plate. The orifice plates provide more significant benefits over a single-hole venturi. This is attributed to the fact that the multiple holes, and different holes diameter of the orifice, provides different flow area to the liquid [41]. This arrangement benefits generating different cavitational events and intensities. Therefore, a hydrodynamic cavitation reactor consisting of orifice plates provides greater flexibility in the operating and geometrical conditions.
The selective release of the invertase by the breakage of S. cerevisiae cells by hydrodynamic cavitation, HPH, and sonication were studied and compared by Balasundaram and Pandit [41]. The invertase enzyme is found to have tremendous applications in the food industry. This enzyme is located at the periplasmic location of S. cerevisiae cells. The cell disruption was carried out using an orifice plate device with 33 holes (1 mm diameter). Results indicated that the rate of invertase release was higher than that of total proteins, which is attributed to the periplasmic location of the invertase enzyme. Also, invertase can be selectively released at the beginning steps of cell disruption and before complete cell disruption, eventually releasing all proteins. The same study was conducted with acoustic cavitation, and the release rate of invertase was comparable with that of proteins. This is due to the generation of higher cavitational intensity by sonication compared to hydrodynamic cavitation. Therefore, the mild, intense cavities generated by hydrodynamic cavitation break only the cell wall and not the complete cells. It has been found that HC brings about 44 times higher release yield of enzyme than that of ultrasonication based on the total energy consumption of both systems.
The cavitation phenomenon is known to generate high pressure and temperature conditions and the formation of free radicals, the activity of invertase and glucosidase was unaltered, which was reported by Save et al. [43]. However, continued exposure to the severe conditions of cavitation has resulted in a decrease in enzyme activity. Therefore, it is essential to maintain the cavitational intensity by adjusting the system’s geometrical and operating parameters. Depending on the location of enzymes, cavitation devices can be used. For example, complete cell disruption leads to the release of intracellular enzymes using some cavitation devices in some cases, and it could be shear driven or with shock waves which can only break the cell wall, thus releasing the enzymes of the periplasmic location.
In the study investigated by Balasundaram and Harrison, the E. coli cells were partially disrupted for the selective release of specific proteins using hydrodynamic cavitation technology [44]. Different processing parameters, such as the specific growth rate of E. coli, number of passes, and cavitation number for the release of both cytoplasmic and periplasmic proteins, have been evaluated. It has been observed that 48% total soluble proteins, 67% β-galactosidase and 88% acid phosphatase release at the optimum cavitation number of 0.17. The higher release of acid phosphatase compared to total proteins confirmed its periplasmic location. This study concludes the lesser requirement of downstream operations to purify the enzymes due to their selective release using hydrodynamic cavitation.
Balasundaram and Harrison, in their subsequent work, studied the outcome of different processing factors like initial cell content, cavitation number, and the number of passes required for the selective release of invertase (cell wall bound), glucose-6-phosphate dehydrogenase (G6PDH; cytoplasmic), β-galactosidase and alcohol dehydrogenase (ADH; cytoplasmic) by disrupting yeast cells [44]. The results indicated a cavitation number of 0.13 for the optimum release of enzymes. Amongst all enzymes studied, the specific release rate of cell-bound enzyme, invertase, was found to be highest up to 86% compared to other enzymes at cytoplasmic and periplasmic locations.
4.1.2. Enzyme translocation using different pretreatment strategies
It has been studied that, before the process of cell disruption, the cell suspension can be pre-treated using various pre-treatments such as heating, changing the pH of suspension, solvent pre-treatment, reducing agents, hypoosmotic conditions, etc. These pre-treatment processes have shown a positive impact on the movement of enzymes from cytoplasmic to periplasmic location, and the concept is known as enzyme translocation. Thus, by translocating the enzymes, the process of cell disruption can be made much more energy efficient as the energy required to recover enzymes from the periplasmic location would be less than the energy required for complete cell disruption. Along with this, it is much easier to purify the enzymes further as there would be lesser contamination of other products that may be observed after complete cell disruption hence the requirement of fewer downstream processes hence higher purification. Some of the pre-treatment strategies followed by cavitation technology are discussed in the next section.
Balasundaram and Pandit have studied and compared the cell disruption processes using a sonicator, high-pressure homogenizer, and HC for the selective release of Penicillin acylase and alcohol dehydrogenase and invertase from yeasts from E. coli cells, respectively [45]. The quantification of enzyme release was calculated using the location factor concept. The location factor was found to be higher than 1 for the selective release of penicillin acylase and invertase using all processes. This confirms the location of these enzymes at the periplasmic location of E. coli and yeast, respectively. A higher location factor was achieved when cells were disrupted using hydrodynamic cavitation compared to the other two processes. In the case of ADH, the location factor was on the lower side (0.5), confirming ADH’s location at the cytoplasmic location [46]. As the pre-treatment strategy, the cell suspension was pre-treated by gentle heating. This pretreatment has resulted in the translocation of ADH enzyme. Also, from the fermentation time, it has been observed that longer fermentation time has resulted in the occurrence of penicillin acylase at the periplasmic location. On the other hand, penicillin acylase was found at the cytoplasmic location for the shorter fermentation time.
Recently, Mevada et al. have reported the selective release of enzymes using various pre-treatment strategies for yeast cell suspension before cell disruption [47]. The pre-treatment of cells using reducing agents and hypoosmotic acidic stress conditions has been studied. Results indicated that the maximum recovery of invertase enzyme was 55.78 U/mL with the selectivity of 495 U/mg at optimized pre-treatment conditions such as hypoosmotic acidic stress at pH 3.6 (0.05 M and 12 h) and 2 mM Dithiothreitol (2 h) as a reducing agent and cell disruption using hydrodynamic conditions. The pre-treatment of yeast cells before cell disruption has resulted in increased selectivity by 4.79 times with power efficacy of up to 63 times related to conventional cell disruption using high-pressure homogenization.
The same authors have carried out another study on the pre-treatment of yeast cells using hypoosmotic alkaline conditions for the selective, energy-saving, and scalable recovery of alcohol dehydrogenase (ADH) from the cytoplasmic location [48]. The yeast cells, when pre-treated at an alkaline condition at pH 8 for 6 h and cell disruption using hydrodynamic cavitation at 6 bar pressure for 5 passes, the selectivity of ADH (34.81 U/mg) increased up to 21 times as compared to cell disruption without pretreatment conditions. Also, the specific yield of ADH has increased by 58 times after pre-treatment and cell disruption using hydrodynamic cavitation compared with only hydrodynamic cavitation. This is attributed to the fact that alkaline pre-treatment leads to the translocation of ADH from the cytoplasmic location to periplasmic location. This has resulted in an increased selective release of enzymes.
In yet another study, Mevada et al. investigated a scalable method for the selective release of ADH from the cytoplasmic location of yeasts [49]. A pre-treatment approach in which changing the pH of cell suspension was used for the translocation of ADH from cytoplasmic position to periplasmic location. This pretreatment was followed by cell disruption using hydrodynamic cavitation using an orifice plate device (0.2 mm diameter). The pre-treatment of yeast cells at pH 8 (12 h) followed by disruption at the pressure of 6 bar with 5 times recirculation has resulted in the selective release of ADH 8.22 times and a specific yield of almost 12 times when compared to cell disruption with only cavitation, respectively.
Thus, hydrodynamic cavitation can be used as an alternative technology for the efficient disruption of microbial cells. The HC treatment is found to be highly energy efficient when compared with other cavitation processes, such as acoustic cavitation. The scale-up of HC reactors is quite easier as fluid dynamics at the downstream of cavitating devices are thoroughly studied and readily available. The only energy needed is to operate the pumps, which can be increased easily for large-scale applications. Compared to venturi, the orifice place cavitating devices are found to be more efficient as it provides greater flexibility in controlling the required cavitation intensity for specific applications. Hence, by controlling the cavitation intensity using an orifice device, the energy required for a specific application can be minimized. In general, hydrodynamic cavitation is a promising and well-established technology that can be used in laboratory and pilot-scale operations. With the help of a scale-up approach, this can be used for industrial-scale cell disruption operations.
4.2. HC for food enzymes inactivation
The physical characteristics of juices are influenced by enzymes like polyphenol oxidase (PPO), peroxidase (POD), and pectin methyl esterase (PME). Off-flavor, unwanted browning, and phase separation are prominent causes of quality deterioration in fruit juices caused by these enzymes, which substantially impact sensory quality. Hydrodynamic cavitation (HC) processing for fruit juices is a novel, emerging, and underexplored technique. Only a few reports are available describing the potential of HC in the inactivation of spoilage enzymes in foods which are summarized in Table 1.
Table 1.
Recent examples of application of hydrodynamic cavitation for different food processing operations.
| Food/matrix | Type of HC device and operation parameters | Major findings | References |
|---|---|---|---|
| 1. HC for microbial inactivation/sterilization/ cell disruption | |||
| Calcium-fortified apple juice, tomato juice, Skim milk |
Concentric cylinders with a 2.91 L volume annular space separating the stationary outer cylinder from the inner, rotating cylinder. Operating parameter: 3000 and 3600 rpm rotor speeds |
˃ 5 log (apple juice), 0.88 and 3.10 log (tomato juice),0.69 and 2.84 log (skim milk) reduction | [25] |
| Orange juice | HC system with cavitation device (orifice plate, 24 holes with 0.45 mm of diameter) Operating parameter: flow rate: 4 m3/h, fluid velocity through the orifice plate: 30 m/s and cavitation number: 0.191, pressure: 4 bar, temperature: 40–70 ℃ for 2–8 min. |
2.09 log reduction of TPC at 60 °C for 8 min and 3.92 log reduction at 70 °C for 6 min | [26] |
| Raw milk | 32 cone-cylinder-shaped cavitation generators fixed on surface of the rotor, front cover, and rear cover. Operating parameter: shock waves at pressure: 6000 GPa, micro-jets speed: 175 m/s, and shear stress: 3.5 kPa |
5.9 for E. coli (100%), 5.5 for S. aureus (100%), and 3.0 for B. cereus (99.9%) log reductions were attained at production rate of 4.2 L/min |
[27] |
| Reconstituted skim milk concentrate | Device consists of a stationary housing and an inner cylinder (rotor) with indentations drilled from the peripheral towards the center of the cylinder. The inner cylinder has 88 dents equidistantly located around the circumference, and they have cylindrical shaped dead-end bores. Operating parameter: flowrate: 50–300L/h, motor speed:600–3600RPM, at 70–85 °C, for 10–110 s time |
The cavitation-thermal treatment (75 °C) resulted in a 3.65 log reduction | [29] |
| Peanut milk | Venturi-based HC device with 5 mm throat diameter, 2 L holding tank, 0.5 Hp reciprocating pump, with a variable frequency up to 12 bar pressure and 600 LPH flow rate Operating parameter: 6, 8, and 10 bar for 15 min |
1.2 log reduction of total bacteria at 10 bar pressure | [30] |
| Sugarcane juice | Orifice-based HC device: The number of orifice plates (plate 1) 1 orifice × φ 2 mm, (plate 2) 9 orifices × φ 1 mm, (plate 3) 17 orifices × φ1 mm Operating parameter: inlet pressure: 2.5 to 3.5 bar, and treatment times 10 to 40 min. |
5.53 log10 CFU/mL to 3.3 log10 CFU/mL the highest pressure, processing time, and orifices. | [31] |
| Ground meat. | Orifice plate (diameter: 0.8 mm and 1.2 mm) connected to a peristaltic pump. Operating parameter: inlet pressure: 8, 14, and 20 PSI for 7.5 min. |
HC intensifies the detection capabilities of existing microbes sensing technique | [32] |
| 2. HC for enzyme inactivation | |||
| Orange juice | Single hole orifice plate (diameter: 2 mm) HC device connected to positive displacement peristaltic pump (1.1 kW) Operating parameter: inlet pressure: 3 to 15 bar for 5 to 25 min. |
21% and 13% reductions in PME and POD, respectively | [50] |
| Orange juice | HC system with cavitation device (orifice plate, 24 holes with 0.45 mm of diameter) Operating parameter: flow rate: 4 m3/h, fluid velocity through the orifice plate: 30 m/s and cavitation number: 0.191, pressure: 4 bar, temperature: 40–70 ℃ for 2–8 min. |
70% PME reduction at 70 °C for 8 min. | [26] |
| Aonla juice | Venturi-type cavitation device with holding tank with 2L capacity volume, reciprocating 0.5 HP pump with a variable frequency drive (max pressure: 5 psi) Operating parameter: inlet pressure 5 to 15 psi and time: 5 to 30 min |
Maximum of 13% PPO inactivation at 15 psi for 30 min | [52] |
| Sugarcane juice | Orifice plate (17 orifices × 1 mm) HC system (0.35 MPa) with a cooling arrangement. Operating parameter: temperatures: 4, 17, and 30 °C and time 10, 20, 30, and 40 min |
HC treatment reduced 84.64% PPO activity and 74.57% POD activity | [53] |
| Raw milk | Venturi-type cavitation device with 5 mm throat diameter and bypass line controlling the liquid flow Operating parameter: inlet pressure 4, 6, 8, and 10 psi, time: 5 to 15 min |
highest 21% alkaline phosphatase reduction was obtained at 10 psi pressure for 10 min | [54] |
| 3. HC for retention of nutrients | |||
| Tomato juice | HC system with cavitation device (orifice plate, 16 holes with 1 mm of diameter) Operating parameter: cavitation generated by pressure drop from 3 to 0.3 bar, temperature: 35–49 °C, 45–59 °C and 55–62 °C for 15 min. |
Phenolic compounds (40 to 44 mg/100 ml) and lycopene (38 to 42 mg/kg) retained. | [55] |
| Tomato juice | Venturi-type cavitation device with holding tank with 2L capacity volume, reciprocating 0.5 HP pump with a variable frequency drive (max pressure: 5 psi) Operating parameter: inlet pressure 5 to 15 psi and time: 5 to 30 min |
96.6% of total phenolic compounds and 93% of ascorbic acid retained.No change in soluble sugar content of juice. |
[56] |
| Cranberry puree | Pilot-scale HTD processor with venturi cavitator. Operating parameter: pressure 0.141 MPa and flow velocity: 0.45 m/s, reaching 7.5 m/s in the active area of cavitation, at 91 °C for 6 min |
Cranberry puree contained 26.8–86.8% more anthocyanins, 51.6–91.6% higher content 13 of PACs and 30.6–86.3% higher content of total phenolics | [57] |
| Aonla juice | Venturi-type cavitation device with holding tank with 2L capacity volume, reciprocating 0.5 HP pump with a variable frequency drive (max pressure: 5 psi) Operating parameter: inlet pressure 5 to 15 psi and time: 5 to 30 min |
88.01% of the vitamin C, and 96.80% of the total phenolic content were retained | [52] |
| Cow milk | Venturi-type cavitation device with 5 mm throat diameter and bypass line controlling the liquid flow Operating parameter: inlet pressure 4, 6, 8, and 10 psi, time: 5 to 15 min |
Increased protein availability from 0.72 to 3.8% and soluble sugars by 1% | [54] |
| Peanut milk | Venturi-based HC device with 5 mm throat diameter, 2 L holding tank, 0.5 Hp reciprocating pump, with a variable frequency up to 12 bar pressure and 600 LPH flow rate | Increased protein availability from 8.8 to 15.8% and soluble sugars from 9.1 to 14.2% | [30] |
| Raw milk | 32 cone-cylinder-shaped cavitation generators fixed on surface of the rotor, front cover, and rear cover. Operating parameter: shock waves at pressure: 6000 GPa, micro-jets speed: 175 m/s, and shear stress: 3.5 kPa |
Continuous HC effectively inhibit milk allergen production | [27] |
| 4. HC for physiochemical properties and quality improvement | |||
| Orange juice, tomato juice and aonla juice | HC system with cavitation device (orifice plate, 24 holes with 0.45 mm of diameter) and venturi-type cavitation device with holding tank with 2L capacity volume, reciprocating 0.5 HP pump with a variable frequency drive (max pressure: 5 psi) Operating parameter: pressure: 4 bar, temperature: 40–70 ℃ for 2–8 min and inlet pressure 5 to 15 psi and time: 5 to 30 min |
pH, acidity, TSS, and color of both the juices were stable. Sedimentation and viscosity were mildly affected in positive In orange juice reduced flow behavior index and cloudiness with improved flow consistency index. |
[26], [52], [56] |
| Sugarcane juice | Orifice-based HC device: The number of orifice plates (plate 1) 1 orifice × φ 2 mm, (plate 2) 9 orifices × φ 1 mm, (plate 3) 17 orifices × φ1 mm Operating parameter: inlet pressure: 2.5 to 3.5 bar, and treatment times 10 to 40 min. |
Increased acidity, while the pH, viscosity, TSS, and lightness and greenness value of sugarcane juice decreased | [31] |
| Peanut milk | Venturi-based HC device with 5 mm throat diameter, 2 L holding tank, 0.5 Hp reciprocating pump, with a variable frequency up to 12 bar pressure and 600 LPH flow rate | Particle size, fat globule size was significantly reduced. Raised consistency index while viscosity is decreased |
[30] |
| Tomato juice | HC system with cavitation device (orifice plate, 16 holes with 1 mm of diameter) Operating parameter: cavitation generated by pressure drop from 3 to 0.3 bar, temperature: 35–49 °C, 45–59 °C and 55–62 °C for 15 min. |
Reduced the particle size. Increased apparent viscosity Sedimentation affected in reduction |
[55] |
| Cow milk | Venturi-type cavitation device with 5 mm throat diameter and bypass line controlling the liquid flow Operating parameter: inlet pressure 4, 6, 8, and 10 psi, time: 5 to 15 min |
Number of fat globules increased with diameter Total particle size decreased No separation in the milk during storage |
[54] |
| Milk protein concentrate | Rotor cavitator. Operating parameter: flow rate of 100 L/h, and pressure of 2 bar. |
Viscosity decreased by 20% and 56%.Improved solubility (97.5%) Better tap and bulk densities |
[61] |
| Milk protein concentrate powder | Cavitator equipped with a proprietary dispersion head (300 mm diameter), consisting of 160 discrete fluid channels Operating parameter: flow rate: 850 L/h, at a motor frequency: 48.8 Hz, and system back-pressure: 2.4 bar |
Successful hydration of MPC powders Quick solubilization of MPC, decreased apparent viscosity, and reduced sedimentation |
[62] |
| SPI | Venturi tube cavitating device with 2L holding tank Operating parameter: pressure of 0.3 MPa for 5, 10, 20, and 30 min |
Enhanced surface hydrophobicity, solubility, and emulsifying abilities while diminishing the foam stability of SPI | [63] |
| Guar gum | Cavitating devices such as orifice plate, circular venturi and slit venturi and valve provided on the bypass line is used to control the liquid flow through the main line. Operating parameter: inlet pressure: 2–6 bar for 30 to 180 min |
Successful depolymerization of guar gum in aqueous solution.Reduced intrinsic viscosity (70%) |
[64] |
| 5. HC for extraction of bioactive compounds | |||
| Cocoa bean shell | A rotor/stator cavitational reactor of 25 L capacity Operating parameter: 3000 RPM rotor speed for 11 min |
Methylxanthines (theobromine: 160.2 mg/g and caffeine: 8.9 mg/g) and fatty acids (964 mg/g). | [68] |
| Orange peel | Venturi-shaped cavitation reactors, including a closed hydraulic loop (total volume capacity around 230 L) and a centrifugal pump Operating parameter: 2900 RPM rotor speed at 20 °C for 11 min |
Pectin (60%), Polyphenols and Terpenes (45%) | [69] |
| Silver Fir | Venturi-shaped cavitation reactors, including a closed hydraulic loop (total volume capacity around 230 L) and a centrifugal pump Operating parameter: 2900 RPM rotor speed at 67.5 °C for 60 min |
Flavanones and d-limonene (100%) | [70] |
| Equisetum palustre L. | Closed cylindrical vessel fitted with perforated plate at bottom. Operating parameter: 25/1: S/S ratio, at 60 °C and 0.7 bar pressure for 20 min |
Flavonoids (57 to 89%) | [98] |
| Pigeon pea leaves | Closed cylindrical vessel fitted with perforated plate at bottom. Operating parameter: 10/1: S/S ratio, N2 flowrate: 30 ml/min. at 3 cycles for 20 time of each cycle 60 °C. |
5 flavonoids (vitexin, isovitexin, luteolin, apigenin and isorhamnetin) were identified by LC–MS/MS | [99] |
| Pyrola | Closed cylindrical vessel fitted with perforated plate at bottom. Vessel connected to the homogenizer for pretreatment. Operating parameter: 22.74: S/S ratio, −0.5 bar pressure, at 2 min homogenization for 30 min |
Arbutin (2.718 mg/g), epicatechin (0.859 mg/g), hyperin (1.378 mg/g), 2′-O-galloylhyperin (5.132 mg/g) and chimaphilin (0.390 mg/g). | [23] |
| Pyrola | Closed cylindrical vessel fitted with perforated plate at bottom. NPHC device connected to microwave chamber. Operating parameter: 30/1 S/S ratio, −0.5 bar pressure, at 700 W microwave power, 50 °C for 12 min |
Hyperin, 2′-Ogalloylhyperin, (1.339 mg/g) and Chimaphilin (4.831 mg/g) | [100] |
| Lemon peel | Venturi-shaped cavitation reactors, including a closed hydraulic loop (total volume capacity around 230 L) and a centrifugal pump Operating parameter: 2900 RPM rotor speed at 20 °C for 11 min |
Pectin with high antioxidant activity and has no harmful effects on human epithelial cells even at concentration of 1.0 mg/mL | [72] |
| Sorghum flour and apple pomace | APV hydrodynamic cavitator with stator and rotor assembly. Rotor with surface indentations that influenced the flow trajectory inside the cavitator. Total of 44, 66 and 88 indentations placed equidistant from each other on the 200 mm diameter rotors with 2, 3 and 4 rows of holes, Operating parameter: 100 g/L and 87.5 g/L S/S ratio, 3 and 4 rows of rotor holes, 35 and 45 °C temperature |
Increased bound phenolics (39.5 and 42%) and antioxidant activity (38.6 and 97%) | [59] |
| 6. HC for beverages | |||
| Beer from pale malted barley with smaller fractions of Cara Pils and Cara Hell and hops, | Closed hydraulic loop with total volume capacity of 230 L, powered by a centrifugal pump with open impeller 0.174 m in diameter with rotation speed: 2900 rpm. Operating parameter: pressure: 4 atm and flow rate: 1,500 L/min |
Reduction of saccharification temperature. Acceleration in starch extraction efficiency, relevant energy saving, while retaining safety, reliability, scalability, virtually universal application to any brewing recipe |
[76], [79] |
| Gluten reduction in wort and finished beer from 100% barley malt Degradation of proline residues | |||
| Beer from pale malted barley and a mix of raw unmalted grains from typical old wheat | Wheat varieties could represent a viable support to the upscaling of brewing process | [77] | |
| Beer from pale malted barley with smaller fractions of Cara Pils and Cara Hell and hops, | Enhanced retention of the considered prenylflavonoids (xanthohumol, desmethylxanthohumol and 6-geranylnaringenin). Increased extraction from hops, reduced adsorption to insoluble malt proteins, and reduced isomerization |
[75] | |
| Coconut–palmyra wine |
Orifice-based HC device connected to tank of 10 L capacity Operating parameter: 3.45 bar pressure and 15–60 min of treatment (72 to 287 passes). |
Synergistic effect on phenols and Vit C and an antagonistic effect on color and antioxidants Functional compounds, physiochemical and sensory characteristics were stable with time during aging of sample wines |
[80] |
Note: abbreviations: HC: hydrodynamic cavitation, TPC: total plate count, PME: pectin methyl esterase, POD: peroxidase, PPO: polyphenol oxidase, PAC: proanthocyanin content, TSS: total soluble solids, MPC: milk protein concentrate, SPI: soy protein isolate, and S/S ratio: solid to solvent ratio.
Katariya et al. explored the influence of single hole orifice plate HC device (5 bar 15 min) on inhibiting the PME and POD enzymes in the orange juice [50]. They observed about 21% and 13% reductions in PME and POD, respectively, while maintaining natural bioactive compounds with excellent sensory properties due to the creation of cavities and shock waves. Later, Arya et al. demonstrated synergistic inhibition of PME in orange juice when HC was supplemented with moderate thermal treatment (40 to 70 °C) [26]. When HC processing was performed for 8 min at 70 °C, the highest PME decrease of 70% was obtained. In the case of orange fruit juices, around 35% of RA was evaluated, and Carbonell et al. determined that approximately 75% of inactivation owing to HPH was acceptable and sufficient to preserve cloudiness [51]. PME inactivation increased with increasing temperature and HC treatment time. Because PME is a heat stable enzyme, thermal assisted cavitation can effectively inactivate PME in orange juice. Unlike orange juice, at optimal 12.5 psi pressure for 10 min of processing, Annapoorna et al. discovered just 5.6% PPO inactivation, even though the authors observed a maximum of 13% PPO inactivation at 15 psi for 30 min [52]. PPO deactivation by HC might be attributed to structural changes in enzymes caused by cleavage of the protein backbone and unfolding processes caused by more significant shear stresses.
Furthermore, Bhukya et al. investigated the effect of ascorbic acid supplementation in fresh sugarcane juice during HC treatment [53]. The ascorbic acid supplementation to fresh juice reduced PPO by 6% and POD by 2.5%. This reduction may be due to the ascorbic acid, a powerful antioxidant, that might prevent polyphenols oxidation, therefore influencing enzymatic activity. Authors also observed considerably lower PPO and POD activities when the combination of temperature levels and HC treatment period was increased [22]. After a 40-minute HC treatment at 4 °C, about 85% and 75% of PPO and POD inactivation, respectively, in the ascorbic acid-enriched samples were dramatically decreased. The temperature of the precooled juice (30 °C) rapidly climbed throughout HC treatment, reaching up to 38 °C. A rise in juice temperature above the optimal range for enzyme activity might have inhibited PPO and POD activity. Similarly, increased enzyme inactivation at reduced treatment temperatures might be ascribed to enzyme loss at low temperatures. The HC processing time might be attributable to increased vibration over a prolonged treatment period. The HC-generated free radicals may oxidize the amino acids of several enzymes. As a result, enzyme inactivation due to HC could be caused by a range of biochemical and physical processes generated by cavitation, with free radicals created during bubble collapse [53].
In the instance of milk processing, Pegu et al. evaluated HC processing with a 21% greater cavitational yield than acoustic cavitation (AC) [54]. Alkaline phosphatase (ALP) activity initially surges about 1 to 2% in HC treatment at 4 psi (HC) and 200 W (AC), then declines when duration and intensity are increased. The sample treated with HC at 10 psi pressure for a duration of 10 min saw the maximum reduction (21%; 1.17 mol/mL), whereas AC treated (400 W, 8 min) sample had only a decrease of 14% (1.27 mol/mL). With more cycle passes available for the samples in the HC treatment due to the longer exposure time, more cavitation may have occurred with each cycle, deactivating the ALP enzyme, and reducing its activity.
Hence the HC technology can be exceptionally used for the inactivation of enzymes from fruit juices and milk. It was also reported that milder cavitation could slightly enhance the enzyme activity, and with a further increase in the cavitation intensity and treatment time, the enzyme activity reduces further. The orifice device has been shown to be more effective in enzyme deactivation. The HC technology can be combined with thermal treatment for the synergistic effect of both processes in enzyme deactivation.
4.3. HC for retention of nutrients
Retaining the natural nutritional content is essential when it comes to any food preservation technology (thermal, nonthermal, and chemical). Traditional thermal processing probably gives food safety from microbial and enzymatic spoilage, but it fails to retain the nutritional quality of foods. Recently, hydrodynamic cavitation has emerged as a technology that provides safety from food spoilage while improving food's nutritional and functional quality. Several researchers investigated the effect of HC on bioactive and nutrient retention in juices while deactivating the enzymes and microbes (Table 1). According to Helaris et al., the application of HC technology enhances the tomato juice's physical characteristics without changing the amount of overall phenolic compounds (40 to 44 mg/100 ml) and lycopene (38 to 42 mg/kg) [55]. Like these findings, a different study found that during HC processing, tomato juice retained 96.6% of total phenolic compounds and 93% of ascorbic acid [56]. The hydrodynamic cavitation technology was also used by Chen and Martynenko to prepare cranberry purée [57]. In that study, there was no discernible change between the pro-anthocyanidin content of samples that were left untreated (1.20 mg/g) and those that were treated for 5 min (1.16 mg/g) and 8 min (1.31 mg/g) at 70 °C. In addition, no appreciable anthocyanin content loss was seen when blueberries were treated with HC at temperatures below 70 °C. Additionally, Katariya et al. found that orange juice had increased cloud stability and palatability along with 94% and 91% of antioxidant capacity and ascorbic acid preservation under ideal processing conditions [50]. According to Annapoorna et al. optimum HC processing parameters for processing aonla juice at 10 psi for 15 min, 92.19% of the antioxidant activity, 88.01% of the vitamin C, and 96.80% of the total phenolic content were retained [52]. However, the treatment time and temperature invested in juices may impact their nutritional and bioactive composition.
Pegu et al. and Salve et al. compared the effect of HC and ultrasonication of protein and sugar bioavailability in cow milk and peanut milk [30], [54]. Both the reports suggested HC processing increased protein hydrolysis (0.72 to 3.8% in cow milk and 8.8 to 15.7% in peanut milk) and soluble sugars (10 to 11 °Brix in cow milk and 9.1 to 14.2 °Brix in peanut milk). However, unlike raw milk, HC has shown superior nutrient increment in peanut milk. Concerning the nutritional content of milk, continuous hydrodynamic cavitation treatment has a similar effect as HTST treatment. Heat treatment, on the other hand, can alter raw milk's allergy-protective qualities. When compared to HTST, CHC (continuous hydrodynamic cavitation) treatment with low temperature and short period might effectively inhibit milk allergens such as casein and whey protein by hydrolyzing them [58]. The results suggest that continuous hydrodynamic cavitation is a viable alternative or supplementary milk processing technique compared to typical continuous heating procedures. During this process, the nutritional value and flavor may be preserved [27]. Lohani et al. used HC to successfully increase the antioxidant activity of sorghum flour and apple pomace by releasing bound phenolics. The total phenolics and antioxidant activity of the sorghum flour were 39%, 39%, and for apple pomace, an increase of 42% and 97%, respectively [59]. This is good for reducing postprandial glucose in diabetic patients.
4.4. HC for improvement in physiochemical characteristics of foods
Several researchers are investigating hydrodynamic cavitation technology for its capacity to modification of physiochemical properties such as pH, acidity, total soluble solids (TSS), viscosity, cloudiness, color, etc., in liquid foods. These properties determine the shelf-life and palatability of food products which are summarized in Table 1. In this regard, Arya et al., Annapurna et al., and Vigneswaran et al. examined the effect of HC on these properties in different fruit juices [26], [52], [56]. The authors found that the pH, TSS, and titratable acidity remained unchanged throughout HC processing in orange, aonla, and tomato juices. Contrary to this, as the intake pressure, processing duration increased, and the number of orifices on the plates, the acidity and temperature of samples were increased, while the viscosity, pH, TSS, and color values (L, a, b) of sugarcane juice decreased [31]. In contrast, in a significant reduction in the sedimentation (70%) and viscosity (15%) in tomato juice, whereas there was a slight reduction in sedimentation (14%) and viscosity (11.33%) due to HC in aonla juice. A reduced flow behavior index and improved flow consistency index have been seen together with sedimentation, representing the ability of HC to alter the cloud stability of orange juice [26].
During the assessment of HC for the microbial reduction in peanut milk, Salve et al. discovered that HC significantly alters milk's microstructure by modifying the orientation of its constituent; in particular, the fat globule size was significantly reduced, suggesting the input of a well-homogenized product [30]. Similar results in the treatment of peach juice were revealed by other scientists [60]. They attributed the reduction in particle size to the high shear rates caused by cavitation, which to some degree, improved the gelling network and intermolecular interaction and forces. Additionally, the produced cavitation phenomena caused the consistency index to rise while the viscosity decreased [30]. In another study, Terán Hilares et al. found that the processing of tomato juice reduced the particle size [55]; however, in contrast to Salve's work, they also found that the apparent viscosity of the juice increased as a result of the release of cellular components as biopolymers (such as pectin and proteins), where it interacts with other constituents. This product's superior homogenization and viscosity rise resulted in a positive impact on sedimentation velocity. The HC-treated tomato juice stayed stable for 14 days without sedimentation, compared to a sedimentation index of 68% in control and pre-treatment samples. In addition, Pegu et al. reported that raw milk treated with HC had protein hydrolysis rise from 0.72 to 3.77. Additionally, as time and HC intensity expanded, the amount and size of fat globules enlarged, and total particle size decreased. No separation was seen in the HC-processed milk during storage, possibly related to the fat globules' smaller size (from 6.3 m to 2.2 m) [54]. Regarding the rotor speed used for spray drying, Li et al. showed that the HC processing on milk protein concentrate successfully lowered the viscosity up to 20 to 56% [61]. An average 97.5% solubility at 50 °C reconstitution temperature, greater tapped and bulk density and enhanced physicochemical attributes of SMP were also recorded, indicating that HC had no detrimental effects on powder solubility. The HC process, when compared to the widely utilized high-shear powder inductors/mixers procedure, Pathania et al. found that the HC was very successful in attaining total hydration of 20% w/w MPC powders at 50 °C [62]. Additionally, HC treatment resulted in enhanced and quick solubilization of milk protein concentrate powders, decreased apparent viscosity, and reduced sedimentation.
In another investigation, soy protein isolate (SPI) dispersions of 3% w/v were treated with hydrodynamic cavitation for 5, 10, 20, and 30 min with a venturi-type cavitation device with a processing volume of 2L considerably [63] in this investigation. After treatment with HC, SPI’s particle size and viscosity were reduced. HC treatments enhanced surface hydrophobicity, solubility, and emulsifying abilities while diminishing foam stability. The effect of HC on SPI was better with respect to acoustic cavitation under the same treatment time [63]. These findings show that using HC to enhance the functional characteristics of dietary proteins might be a viable option.
Guar gum has been utilized extensively in food, pharmaceutical, and other related sectors because of its chemical composition, biomedical applications, and lack of toxicity. Depolymerization is already done via physical, chemical, thermal, and enzymatic processes to provide changed viscosities. Prajapat and Gogate proposed the HC approach to speed up the depolymerization of guar gum in an aqueous solution since these procedures degrade the substance [64].
Depolymerization is aided by lower beginning concentrations, higher input pressures, the use of a split venturi as a cavitating device, and the inclusion of potassium persulfate. The use of hydrodynamic cavitation alone under optimal circumstances reduced intrinsic viscosity by about 70%, while with an increase of potassium persulfate loading, the viscosity decreased to 96 percent [64]. According to kinetic analysis, the kinetic rate constantly rose as the temperature and potassium persulfate concentration increased. Compared to ultrasound-based operation, HC was exposed to being more energy competent and suitable at an industry level [64].
4.5. HC for extraction of bioactive
Food industries are recognized to be the most significant global generators of waste and byproducts. Today, such leftovers are possible sources for getting molecules of interest for new applications and product development, according to prevailing “circular economy” and “zero waste” ideas [65]. In this regard, scientists are looking for new methods and ways to improve existing ones to acquire a higher yield when extracting molecules from such complex systems. Today, HC is envisioned as a green extraction technology option for this healing effort [66]. Compared to conventional solvent extraction techniques, hydrodynamic cavitation-assisted extraction (HCAE) offers a quick and efficient extraction process. Furthermore, this method is better suited for extracting temperature-sensitive bioactive due to its applicability at lower temperatures. One of the main advantages of the HCAE approach is its scalability at the industrial level, and it has used current advancements in cavitation processing [67]. The key benefits of the HCAE are removing unsafe solvents, shorter duration, and enhanced yield with maximum purity. It is an efficient technique for increasing plant tissue mixing or homogenization and hydrolysis, which improves the diffusion of intracellular chemicals into extraction solvent. The hydrodynamic cavitation-assisted extraction can be used in two different ways, including positively pressurized cavitation and negative pressure cavitation, as well as in combination with other extraction methods like HC in assistance with microwave extraction (HC + MAE), enzymatic-HC assisted extraction (EHCAE) [66].
To date, very few studies have been carried out which have demonstrated the viability of the HCAE system for the extraction of food bioactive compounds, either on its own or in combination with other extraction techniques. Recently, Grillo et al. has used a pilot-scale (25 L) HCAE reactor to extract different bioactives (flavanols, polyphenols, methylxanthines, cocoa butter, and fatty acids) from cocoa bean shells [68]. The research supported its effective extraction by demonstrating semi-continuous techniques with pilot-scale HCAE reactors. When compared to the entire weight of cocoa bean shell, which is made up of methylxanthines (theobromine: 160.2 mg/g of extract and caffeine: 8.9 mg/g of extract) and fatty acids (964 mg/g of extract), the overall yield by HC extraction was about 15.8% and 20.5% of lipophilic and hydrophilic bioactives respectively. Although there was only a minor amount of esterification in pectin extractions, flavanones and other derivatives of hydroxycinnamic acid and terpenes, particularly d-limonene, were highly successful and achieved significant yields in a short time [69]. According to the findings, roughly 60% of bioactive production was produced in 10 min, and, interestingly, over 53% of yield was reached in the first 2 min. The findings were encouraging, indicating that HCAE has a high potential. This was also demonstrated, with a good yield of flavonoids 0.14 mg GAE/mL and polyphenols 0.55 mg CE/mL extracted from Silver Fir (Abies alba Mill.) needles simply using water as the extraction solvent and a venturi device in the HC reactor [70].
Pectin is the most valuable natural hydrocolloid in the food business, where it is frequently utilized as a stabilizing addition and to improve the textural features of foods. Pectin generated via hydrodynamic cavitation in water and freeze-drying from discarded lemon peel has a high antibacterial effect against S. aureus, a Gram positive bacteria that often spoils food [71]. Lemon pectin, which was initially generated on a pre-industrial scale by hydrodynamic cavitation of discarded lemon peel in water, exhibits substantial antioxidant properties and has no harmful effects on humans, even at relatively large concentrations of 1.0 mg/mL [72]. These observations pave the way for the preparation of novel nutritional supplements and health-care applications based on a multi-functional copolymer imbued with new functions which can be acquired quickly from an ample by-product of the agri-food industry using a simple, cheap, robust, and effective HC extraction process applicable to waste lemon peel submerged in water while adhering to all-natural product green extraction principles [72].
The bound phenolics in sorghum flour and apple pomace were effectively liberated by natural fermentation followed by hydrodynamic cavitation, which resulted in increased total phenolic content (TPC) and antioxidant activity (AA) in both samples [59]. At 3490 rotor rpm, the cavitation numbers for sorghum flour and apple pomace were found to be 0.18 to 0.22 and 0.20 to 0.23, respectively [59]. TPC and antioxidants of sorghum flour and apple pomace were significantly affected by solid: liquid ratio, the number of rows of orifices in the rotor, and cavitation temperature. According to microstructural studies, HC induced increased cell breakdown, resulting in the release of polyphenolics from the complex structure [59].
Cravotto et al. uses the grape and olive production chains to demonstrate how cavitational reactors combine high-intensity ultrasound and rotating hydrodynamic units to generate important nutraceuticals, colorants, and food–beverage additives, which may help with food processing and waste recovery [73]. The waste orange peels, which are projected to weigh several million tons annually globally and burden the orange juice industry, are rich in bioactive compounds like polyphenols, terpenes, and pectin. Waste orange peel was treated in batches of several kilograms in a device with a venturi-shaped cavitation reactor in more than 100 L of water and no additional raw materials.
The sustainability of extraction technologies is crucial as far as health, environment, economy, and industrial scalability are concerned. Like any other HCAE should also demonstrate their maturity level via technological readiness levels (TRL) on a scale of 1 to 9 in order to support the development of sustainable technologies [74]. To date, the HCAE applications at the industrial scale for the extraction of bioactives are not reported and have only been studied at the pilot scale [66]. Although, the Italy-based ‘green technologies development platform’ are on the verge of developing cavitation reactors based on a flow mode extraction process (HCAE). This will ease the expansion of HCAE from pilot scale to industrial transformation with TRL of 6 to 7 [74].
4.6. HC for alcoholic beverages
Beer is among the ancient alcoholic beverages enjoyed in many nations. The primary materials used in beer production are water, malt, malt adjuncts, yeasts, and hops. Beer production includes mixing, grinding, extraction, heating, and boiling. Cooking enables starches to gelatinize, allowing malt enzymes to hydrolyze them into fermentable sugars. In addition to time, high temperatures and energy are required. Sterilization is essential to increase shelf life and eliminate microbiological contamination. Nevertheless, because of the high temperature, the bioactives and flavoring compounds derived from hops flowers generated during fermentation are lost. Albanese and his research group applied the HC approach more recently for brewing and alcoholic beverage manufacturing, which has been shown to have high efficiency for microbial disinfection and extraction operations [75], [76], [77], [78].
The authors indicated that malt's enzyme activity is intensified and accelerated by regulated HC. By doing this at a low temperature, more bioactives were retained, and flavour extraction didn't need boiling. The most notable advantages of the brewing process are a reduction in saccharification temperature of 35 °C, an increment and boost in peak starch extraction productivity of up to 30% with 12% less energy consumed, and a substantial decrease in processing times after conventional stages like dry milling and boiling are eliminated, saving up to 120 min for the boiling step alone [79]. Short cleaning intervals, an energy efficiency of 30% or more, and general simplicity of structural design and operational management of brewing processes are further noticeable benefits. Controlled HC, which was proven on a microbrewery scale, has the potency to have significant effects and spread quickly throughout the relevant large, expanding, and increasingly specialized industry.
While gluten levels in beers can be hazardous to celiac sufferers and the general population of gluten-intolerant persons, Albanese et al. demonstrated gluten elimination in wort and finished beer made from 100% barley malt, in accordance with appropriate cavitation regimens during both mashing and fermentation [76]. The gluten content in the beer is lower than the “gluten-free” (20 mg/L) or “extremely low gluten content” (100 mg/L) thresholds. This new gluten-free approach may be significant since it allows regular beer components and recipes to be used. Subsequently, Albanese et al. proved the application to raw unmalted grains, such as typical old wheat varieties, for brewing [77]. The observed findings are equivalent to those obtained using conventional approaches. According to preliminary results, HC-assisted brewing seems capable of maintaining or increasing quantities of several vital bioactive compounds of hope in the final beer, such as the prenylflavonoids xanthohumol, desmethylxanthohumol, and 6-geranylnaringenin [75]. The HC process was demonstrated to be a feasible method of producing healthier beers by using hops' distinctive natural compounds (α and β acids). It enables productive, more economical, secure, and reliable operations and significant gluten reduction without chemicals or sophisticated technological processes [75].
Recently, Kochadai et al. examined the outcome of HC on faster ageing of tender coconut-palmyra mixed wine at a pressure of 3.45 bar for a treatment time of 15–60 min [80]. HC processing exhibited a synergistic impact on phenols (3.1 mg GAE/ml) and vit C (61.1 mg/100 ml) and an antagonistic effect on color and antioxidants. Changes in functional components and physiochemical characteristics of HC-treated sample wines over time were equivalent to commercial wine. These studies highlight the use of HC in brewing and wine ageing as a rapid processing technique, which could be a game changer in the wine and beer industry.
4.7. HC for oil emulsification
Cavitation’s impact on food is among the efficient methods of intensifying chemical-technological, hydromechanical, and mass-exchange processes and the destruction of substances. The application of cavitation technologies in oil extraction and oil refining is evident in recent years. Power, inlet pressure in the HC system, and rotational speed in the high-speed homogenizer all enhance the size of the oil droplets in ultrasonic flow cells [81]. Ultrasonic baths use substantially less energy to produce stable emulsions than ultrasonic flow cells and HC with aperture circular and slit venturi [81]. The HC has been discovered to be a promising approach for manufacturing high-stability sub-100 nm oil in water emulsions with excellent stability [82]. The mean size of oil in water emulsion droplet size decreased as input pressure, cavitation number, and surfactant concentration increased. An emulsion with an average size of 27 nm was formed after six cavitation passes at a pressure of 120 psi [82]. The resulting oil in water emulsion exhibited outstanding physical stability for eight months [82]. The hydrodynamic cavitation emulsification method has much potential for emulsion creation since it uses less energy and is easy to scale up. In addition to emulsification, Stebeleva and Minakov summarized other applications of HC in oil processing, such as reduction of crude oil viscosity, demulsification of oil emulsion, treatment of oil sludge, oil desulfurization, etc. [83].
4.8. Other applications
4.8.1. HC for treating food sludge.
The waste sludge from the food processing sector is one of the environment-polluting factors. Traditionally such is treated chemically and enzymatically. In this regard, Şaǧban et al. mechanically disintegrated food sludge on a laboratory scale utilizing an orifice-based HC system. The effects of using NaOH, Ca(OH)2, and H2O2 in combination with HC treatment were also investigated. Disintegration degrees of 32 percent to 60 percent were reached after 150 min of cavitation [84]. The combination system of hydrogen peroxide addition with a dosage of 20 mg/L and HC produced the most significant results for solid disintegration and organic carbon release in terms of soluble COD [84]. According to the findings, HC treatment is a potential approach for the pretreatment of waste-activated food sludge and its demonstrated efficiency in wastewater treatment. Dindar et al. also reported that sludge disintegration could be aided by orifice-based hydrodynamic cavitation. The most effective sludge disintegration occurred when the cavitation number was 0.2 [85].
4.8.2. HC in biomass pretreatment
HC is a new and promising technology for enhancing many processes in moderate circumstances, such as low temperature and air pressure. Aside from low energy needs, increasing the material's porosity increases the carbohydrate's enzymatic digestibility yield [86]. Controlling the many factors that influence the HC process might result in a more effective pretreatment technique, making it more economically feasible in a lignocellulosic biorefinery. For the processing of lignocellulosic biomass, hydrodynamic cavitation was coupled with sodium percarbonate in this study. A venturi tube in a circular flow arrangement was used to achieve HC. A pretreatment system employing ultrasonication (US) and sodium percarbonate (SP) was compared to a combined system comprising hydrodynamic cavitation and sodium percarbonate. The removal of lignin to generate glucose and xylose was used to assess the success of each pretreatment method. Both systems removed the same amount of lignin, but the HC-SP system was more efficient in producing glucose and xylose than the US-SP system [87]. The HC-SP system's effectiveness was increased by increasing the venturi tube throat diameter. The HC-SP technology has promise for industrial lignocellulosic biomass pretreatment [87].
4.8.3. Hydrodynamic cavitation of water
For water treatment, various treatment approaches, including both physical and chemical treatments, are available. Because of their low cost and ease of use, sodium hypochlorite and UV disinfection are frequently used. Alternative treatments that do not involve addition of chemicals, such as electrolyzed or electrochemical water disinfection, may be intriguing. Because it is a mechanical treatment process, HC processing should be considered and researched further. This could also help reduce the disinfection-induced selection of resistant organisms that are frequently pathogenic, chiefly if it is proven to destroy resistance genes without causing them to a viable but non-culturable state.
The water generated by the fish processing industry has been contaminated by organic detritus. Biochemical oxygen demand (BOD) and chemical oxygen demand (COD) levels rise as the amount of organic matter in wastewater increases. To improve the efficiency of the HC technique, hydrogen peroxide (H2O2) and titanium dioxide (TiO2) were utilized. The biodegradability index and % BOD and COD degradation are used to assess the treatment of this effluent. At 8 bar inlet pressure, 30 °C temperature, and pH 4, 15 g/L of hydrogen peroxide resulted in a 69.50 percent reduction in COD in 120.0 min of treatment while simultaneously increasing the BI value to 0.93 [88]. COD reduction in the ultrasonic cavitation reactor is 68.70 % without TiO2 and 71.20% with TiO2. At the same operating conditions, this HC and UC reactor significantly lowered CFU count [88].
5. Techno-economic feasibility of hydrodynamic cavitation
For any technology to sustain in the market, cost plays a pivotal role. Numerous technologies or methods are available for food processing to carry out a variety of applications. For instance, for the extraction of bioactive, different techniques such as Soxhlet, ultrasonication, microwave, supercritical, hydrodynamic cavitation, etc., are available [8], [89]. The cell disruption for enzyme release can be carried out using high-pressure homogenization, ultrasonication, hydrodynamic cavitation, pulse field electricity, etc. [45]. The water treatment can be carried out using hydrodynamic cavitation, membrane separation, ultrasonication, etc. [90]. However, each of these techniques has its own benefits and limitations. Some of these techniques are highly efficient but energy intensive, while others are effective and energy efficient at the same time. As the energy required for a system is directly related to its cost, it is imperative to select a technology that can be effective and, at the same time, energy efficient. Regarding food processing and other applications discussed in this review, HC is well known for its high energy efficiency compared to other processes used for the same application.
The cavitation yield (CY) is the ratio of the amount of conversion (degradation/disruption /extraction) per unit of energy supplied to the system, and it is used to estimate the energy efficiency of a cavitation process [91]. To scale up the cavitation technology from lab to the industrial scale, several other parameters, such as energy consumption, treatment time, and cost, play a significant role. To operate the HC reactor, the only energy needed is to run the pump, and no additional energy is required to the system. In 1997, Save et al. studied and compared the energy efficiencies for yeast cell disruption using three techniques: ultrasonication, HC, and mixer blender (High pressure homogenization) [43]. The calculations were made based on the same amount of protein being released using the same level of cell concentration. For this, the actual power in terms of electricity has been considered and the efficiencies of the motor, pump, and ultrasound horn were neglected. Results showed HC set-up is simple to operate, cost-effective, and energy efficient; an order of magnitude compared to high pressure homogenizer and two orders of magnitude compared to sonication. Also, HC set-up can be operated continuously, making it easy to scale for handling large quantities of broth at the industrial level.
The energy efficiency for the cell disruption process using sonication, high pressure homogenization, and hydrodynamic cavitation were estimated and compared by Balasundaram and Pandit [45]. The energy calculation was calculated based on a specific yield of enzymes after cell disruption. The results indicated sonication (0.0019 mg/kJ) as the least energy efficient compared to the other two methods. For HC and high-pressure homogenization, the energy efficiencies were comparable; however, HC shows higher selectivity compared to high pressure homogenization. Lee and Han estimated the lipid yield and the specific energy input required for disrupting microalgae cells using HC, autoclave, and ultrasonication techniques [92]. The lipid yield obtained per specific energy consumption from hydrodynamic cavitation (25.9 to 99.0%) was higher than the autoclave (16.2–66.5%) and sonication (5.4–26.9%) methods, respectively. A similar study was carried out by Waghmare et al. in which the energy required for the disruption of microalgae cells has been estimated for lipids extraction and compared with that of ultrasound [93]. From the results, it has been estimated that HC (7.1 kWh/ kg) medicated cell disruption is almost 6.3 times more energy efficient than sonication (45 kWh/kg) for the disruption of algae cells. Besides, the HC technology can be easily scaled up at the industrial scale for cell disruption applications. The energy efficiency of HC for the pretreatment of sugarcane bagasse was calculated and compared with ultrasound assisted pretreatment by Hilares et al. Results showed higher energy efficiency of HC pretreatment over ultrasound-mediated pretreatment of the sugarcane bagasse [94].
Recently, Gholami et al. carried out a techno-economic analysis study for biodiesel production using hydrodynamic cavitation and traditional mechanical stirring [95]. From the results it was estimated that the overall cost of product capital investment for HC was around 10% and 65%, respectively. These values were less when compared to the mechanical stirring process respectively. The HC process also showed a positive net present value with an internal return rate of 25.61% and on the other side, mechanical stirring showed a negative net present value. Also, the HC process substantially reduces energy and materials consumption due to lower requirements of catalyst and alcohol and higher reactor yield. Lastly, the HC reactor costs only 18% compared to the reactor cost for mechanical stirring, which is attributed to the significant decrease in the residence time of the reaction. The HC technology is considered one of the promising technologies for wastewater treatment. It is evident that the HC process is one of the cost-effective technologies for wastewater treatment. The hydrodynamic cavitation-based techno-economic feasibility for the degradation of methylene blue has been estimated by Mukherjee et al. The study indicated that the energy consumption for methylene blue degradation using the combined effect of HC and hydrogen peroxide was only 1.24 kWh/m3 with an effective treatment cost of $0.12/m3 [96]. Other research groups reported similar energy and cost estimation trends for treating wastewater from different sources [90], [97].
Overall, from all comparisons and reported studies, hydrodynamic cavitation is found to be an efficient and cost-effective technology compared to other techniques to execute the above-mentioned applications. The requirements for HC-assisted applications are comparatively low. Thus, hydrodynamic cavitation could be recommended for many more possible food processing and other potential applications.
6. Industrial research gaps and future trends
HC is a novel technique for food processing, cell destruction and sterilization. It benefits from having no detrimental effects on the amount of bioactives, minerals, lipids, protein, and vitamins [6], [27], however, an additional investigation must be conducted to explore its possible changes in lipid oxidation processes and possible free radicals and other reactive species formed in cavitation. A particular consideration should be given to the dangers of nitro-derivative production and other by-products associated with the existence of usual anions in process streams. Furthermore, HC has enabled larger particle sizes in lipid globules and a rise in viscosity in dairy products [29]. Nevertheless, additional research into the impact of cavitated compositions on crystallization and structural formation is required. Cavitation devices (orifice plate, throttle valve, Venturi, stator-rotor) will be used to assess the quality along with the sensory properties of dairy products. The most crucial factor is consumer approval. While utilizing cavitation, post-processing nutrient inspection of food items is required to ensure the physicochemical qualities and retention of vital nutrients and bioactive [6]. The majority of studies fail this appraisal. Since water is the primary solvent in the food sector, the HC technique may be an option for water decontamination. As a result, it should lower operational and product costs, particularly in water-based food items such as alcoholic and non-alcoholic food products.
Furthermore, to recreate experimental results and compute necessary variables, investigators should provide an extensive explanation of the geometry of the cavitation devices. Furthermore, constant surveillance of the HC devices is required to avoid tube degradation and corrosion in valves and orifices. This is particularly crucial when the process is being scaled up to an industrial scale. The efficacy of cavitation systems in food processing is determined by a variety of parameters, including reactor geometry and design. It is critical to standardize the equipment and operational settings used in the investigations in order to achieve maximum efficacy. This will aid in understanding the true efficacy of the procedure and will allow for an accurate assessment of various studies. Although HC is still in its early stages in food processing, pressure variables, time intervals, sample size, and treatment time must be regulated and standardized for improved efficiency. Reactor optimization is critical for scaling up operations and calculating energy expenditure. Additional reactor building is also required.
7. Conclusion
The newest researchers exploring the application of hydrodynamic cavitation technology for processing of foods, food systems, and other relevant objects are discussed in this article. This process is energy efficient, easy to scale, and a non-thermal technology. The hydrodynamic cavitation has so far been shown to be most useful in modifying the physicochemical features of food systems and functional molecules, extracting biomolecules from complex systems, food sterilization, and helping typical food production procedures. They demonstrate the benefits of HC processing on minimally processed food in extremely short durations and concurrently with product sterilization. Moreover, HC devices have proved their persuasive capacity in cell disruption, not only for sterilization but also for releasing high-value-added metabolites stored inside the cell, such as lipids, enzymes, and other physiologically active chemicals. Another advantage of HC processing in typical food manufacturing processes is its flexibility to be combined with other unit activities. Because HC devices may be simply applied externally, this opens a new possibility for optimizing established manufacturing processes without altering the current plant structure and process design. Lastly, regardless of whether HC devices are used in food processing or adjusting the physicochemical parameters of food systems, researchers are strongly urged to publish a detailed description of the geometry of the cavitation device. This second component will let other researchers replicate experimental data, conduct a fair comparison of studies, and compute missing parameters. With lower energy inputs, lesser degradation, and improvement in nutritional and sensory qualities for liquid goods, HC stands out as an appealing option compared to the existing methods for food processing applications in food industries.
CRediT authorship contribution statement
Shalini S. Arya: Conceptualization, Writing – review & editing, Visualization, Supervision. Pavankumar R. More: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Mayur R. Ladole: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Kakoli Pegu: Investigation, Writing – original draft, Writing – review & editing. Aniruddha B. Pandit: Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Rubió L., Motilva M.J., Romero M.P. Recent advances in biologically active compounds in herbs and spices: a review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013;53:943–953. doi: 10.1080/10408398.2011.574802. [DOI] [PubMed] [Google Scholar]
- 2.Nonglait D.L., Chukkan S.M., Arya S.S., Bhat M.S., Waghmare R. Emerging non-thermal technologies for enhanced quality and safety of fruit juices. Int. J. Food Sci. Technol. 2022;57:6368–6377. doi: 10.1111/ijfs.16017. [DOI] [Google Scholar]
- 3.Khouryieh H.A. Novel and emerging technologies used by the U.S. food processing industry. Innov. Food Sci. Emerg. Technol. 2021;67 doi: 10.1016/j.ifset.2020.102559. [DOI] [Google Scholar]
- 4.Gogate P.R. Hydrodynamic cavitation for food and water processing. Food Bioprocess Technol. 2011;4:996–1011. doi: 10.1007/s11947-010-0418-1. [DOI] [Google Scholar]
- 5.Shah Y.T., Pandit A.B., Moholkar V.S. Sources and types of cavitation. 1999:1–14. doi: 10.1007/978-1-4615-4787-7_1. [DOI] [Google Scholar]
- 6.Arya S.S., Sawant O., Sonawane S.K., Show P.L., Waghamare A., Hilares R., Dos Santos J.C. Novel, Nonthermal, Energy efficient, industrially scalable hydrodynamic cavitation–Applications in food processing. Food Rev. Int. 2020;36:668–691. doi: 10.1080/87559129.2019.1669163. [DOI] [Google Scholar]
- 7.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 8.More P.R., Jambrak A.R., Arya S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022;128:296–315. doi: 10.1016/j.tifs.2022.08.016. [DOI] [Google Scholar]
- 9.Dash D.R., Pathak S.S., Pradhan R.C. Improvement in novel ultrasound-assisted extraction technology of high value-added components from fruit and vegetable peels. J. Food Process Eng. 2021;44:1–11. doi: 10.1111/jfpe.13658. [DOI] [Google Scholar]
- 10.Tiwari B.K. Ultrasound: a clean, green extraction technology. TrAC - Trends Anal. Chem. 2015;71:100–109. doi: 10.1016/j.trac.2015.04.013. [DOI] [Google Scholar]
- 11.Devia-Cruz L.F., Camacho-López S., Evans R., García-Casillas D., Stepanov S. Laser-induced cavitation phenomenon studied using three different optically-based approaches - an initial overview of results. Photonics Lasers Med. 2012;1:195–205. doi: 10.1515/plm-2012-0019. [DOI] [Google Scholar]
- 12.Guzmán-Barraza A., Ortega-Mendoza J.G., Zaca-Morán P., Toto-Arellano N.I., Toxqui-Quitl C., Padilla-Martinez J.P. Optical cavitation in non-absorbent solutions using a continuous-wave laser via optical fiber. Opt. Laser Technol. 2022;154:108330. [Google Scholar]
- 13.Petkovsek R., Gregorcic P., Mozina J. A beam-deflection probe as a method for optodynamic measurements of cavitation bubble oscillations. Meas. Sci. Technol. 2007;18:2972–2978. doi: 10.1088/0957-0233/18/9/030. [DOI] [Google Scholar]
- 14.Padilla-Martinez J.P., Berrospe-Rodriguez C., Aguilar G., Ramirez-San-Juan J.C., Ramos-Garcia R. Optic cavitation with CW lasers: A review. Phys. Fluids. 2014;26(12):122007. [Google Scholar]
- 15.Moholkar V.S., Senthil Kumar P., Pandit A.B. Hydrodynamic cavitation for sonochemical effects. Ultrason. Sonochem. 1999;6:53–65. doi: 10.1016/S1350-4177(98)00030-3. [DOI] [PubMed] [Google Scholar]
- 16.Tian H., Li W.Y., Xiao D., Li Z.M., Wang J.W. Negative-pressure cavitation extraction of secoisolariciresinol diglycoside from flaxseed cakes. Molecules. 2015;20:11076–11089. doi: 10.3390/molecules200611076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moholkar V.S., Pandit A.B. Bubble behavior in hydrodynamic cavitation: Effect of turbulence. AIChE J. 1997;43:1641–1648. doi: 10.1002/aic.690430628. [DOI] [Google Scholar]
- 18.Storey B.D., Szeri A.J. Water vapour, sonoluminescence and sonochemistry. Phys. Eng. Sci. 2000;456(1999):1685–1709. [Google Scholar]
- 19.Holkar C.R., Jadhav A.J., Pinjari D.V., Pandit A.B. Cavitationally driven transformations: a technique of process intensification. Ind. Eng. Chem. Res. 2019;58:5797–5819. doi: 10.1021/acs.iecr.8b04524. [DOI] [Google Scholar]
- 20.Kamal H., Ali A., Manickam S., Le C.F. Impact of cavitation on the structure and functional quality of extracted protein from food sources – an overview. Food Chem. 2023;407 doi: 10.1016/j.foodchem.2022.135071. [DOI] [PubMed] [Google Scholar]
- 21.Asaithambi N., Singha P., Dwivedi M., Singh S.K. Hydrodynamic cavitation and its application in food and beverage industry: A review. J. Food Process Eng. 2019;42:1–14. doi: 10.1111/jfpe.13144. [DOI] [Google Scholar]
- 22.Meletharayil G.H., Metzger L.E., Patel H.A. Influence of hydrodynamic cavitation on the rheological properties and microstructure of formulated Greek-style yogurts. J. Dairy Sci. 2016;99:8537–8548. doi: 10.3168/jds.2015-10774. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D.Y., Yao X.H., Duan M.H., Luo M., Zhao C.J., Zu Y.G., Fu Y.J. An effective homogenate-assisted negative pressure cavitation extraction for the determination of phenolic compounds in pyrola by LC-MS/MS and the evaluation of its antioxidant activity. Food Funct. 2015;6:3323–3333. doi: 10.1039/c5fo00727e. [DOI] [PubMed] [Google Scholar]
- 24.Reineke K., Mathys A. Endospore inactivation by emerging technologies: a review of target structures and inactivation mechanisms. Annu. Rev. Food Sci. Technol. 2020;11:255–274. doi: 10.1146/annurev-food-032519-051632. [DOI] [PubMed] [Google Scholar]
- 25.Milly P.J., Toledo R.T., Harrison M.A., Armstead D. Inactivation of food spoilage microorganisms by hydrodynamic cavitation to achieve pasteurization and sterilization of fluid foods. J. Food Sci. 2007;72:414–422. doi: 10.1111/j.1750-3841.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 26.Arya S.S., More P.R., Terán Hilares R., Pereira B., Arantes V., da Silva S.S., Santos J.C. Effect of thermally assisted hydrodynamic cavitation (HC) processing on physical, nutritional, microbial quality, and pectin methyl esterase (PME) inactivation kinetics in orange juice at different time and temperatures. J. Food Process. Preserv. 2021;45:1–18. doi: 10.1111/jfpp.15794. [DOI] [Google Scholar]
- 27.Sun X., Xuan X., Ji L.i., Chen S., Liu J., Zhao S., Park S., Yoon J.Y., Om A.S. A novel continuous hydrodynamic cavitation technology for the inactivation of pathogens in milk. Ultrason. Sonochem. 2021;71:105382. doi: 10.1016/j.ultsonch.2020.105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregersen S.B., Wiking L., Metto D.J., Bertelsen K., Pedersen B., Poulsen K.R., Andersen U., Hammershøj M. Hydrodynamic cavitation of raw milk: effects on microbial inactivation, physical and functional properties. Int. Dairy J. 2020;109 doi: 10.1016/j.idairyj.2020.104790. [DOI] [Google Scholar]
- 29.Sim J.Y., Beckman S.L., Anand S., Martínez-Monteagudo S.I. Hydrodynamic cavitation coupled with thermal treatment for reducing counts of B. coagulans in skim milk concentrate. J. Food Eng. 2021;293 doi: 10.1016/j.jfoodeng.2020.110382. [DOI] [Google Scholar]
- 30.Salve A.R., Pegu K., Arya S.S. Comparative assessment of high-intensity ultrasound and hydrodynamic cavitation processing on physico-chemical properties and microbial inactivation of peanut milk. Ultrason. Sonochem. 2019;59:104728. doi: 10.1016/j.ultsonch.2019.104728. [DOI] [PubMed] [Google Scholar]
- 31.Bhukya J., Naik R., Mohapatra D., Sinha L.K., Rao K.V.R. Orifice based hydrodynamic cavitation of sugarcane juice: changes in physico-chemical parameters and Microbiological load. LWT - Food Sci. Technol. 2021;150 doi: 10.1016/j.lwt.2021.111909. [DOI] [Google Scholar]
- 32.Nze U.C., Beeman M.G., Lambert C.J., Salih G., Gale B.K., Sant H.J. Hydrodynamic cavitation for the rapid separation and electrochemical detection of Cryptosporidium parvum and Escherichia coli O157:H7 in ground beef. Biosens. Bioelectron. 2019;135:137–144. doi: 10.1016/j.bios.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Sahu A., Badhe P.S., Adivarekar R., Ladole M.R., Pandit A.B. Synthesis of glycinamides using protease immobilized magnetic nanoparticles. Biotechnol. Reports. 2016;12:13–25. doi: 10.1016/j.btre.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talekar S., Joshi A., Kambale S., Jadhav S., Nadar S., Ladole M. A tri-enzyme magnetic nanobiocatalyst with one pot starch hydrolytic activity. Chem. Eng. J. 2017;325:80–90. doi: 10.1016/j.cej.2017.05.054. [DOI] [Google Scholar]
- 35.Ladole M.R., Muley A.B., Patil I.D., Talib M.I., Parate V.R. Immobilization of tropizyme-P on amino-functionalized magnetic nanoparticles for fruit juice clarification. J. Biochem. Technol. 2015;5:838–845. [Google Scholar]
- 36.Ladole M.R., Nair R.R., Bhutada Y.D., Amritkar V.D., Pandit A.B. Synergistic effect of ultrasonication and co-immobilized enzymes on tomato peels for lycopene extraction. Ultrason. Sonochem. 2018;48:453–462. doi: 10.1016/j.ultsonch.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Ladole M.R., Pokale P.B., Patil S.S., Belokar P.G., Pandit A.B. Laccase immobilized peroxidase mimicking magnetic metal organic frameworks for industrial dye degradation. Bioresour. Technol. 2020;317 doi: 10.1016/j.biortech.2020.124035. [DOI] [PubMed] [Google Scholar]
- 38.Ladole M.R., Pokale P.B., Varude V.R., Belokar P.G., Pandit A.B. One pot clarification and debittering of grapefruit juice using co-immobilized enzymes@chitosanMNPs. Int. J. Biol. Macromol. 2021;167:1297–1307. doi: 10.1016/j.ijbiomac.2020.11.084. [DOI] [PubMed] [Google Scholar]
- 39.Balasundaram B., Harrison S., Bracewell D.G. Advances in product release strategies and impact on bioprocess design. Trends Biotechnol. 2009;27:477–485. doi: 10.1016/j.tibtech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Liu D., Ding L., Sun J., Boussetta N., Vorobiev E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds — a review. Innov. Food Sci. Emerg. Technol. 2016;36:181–192. doi: 10.1016/j.ifset.2016.06.017. [DOI] [Google Scholar]
- 41.Balasundaram B., Pandit A.B. Selective release of invertase by hydrodynamic cavitation. Biochem. Eng. J. 2001;8:251–256. doi: 10.1016/S1369-703X(01)00114-0. [DOI] [Google Scholar]
- 42.Torner M.J., Asenjo J.A. Kinetics of enzyme release from breadmaking yeast cells in a bead mill. Biotechnol. Tech. 1991;5:101–106. doi: 10.1007/BF00159979. [DOI] [Google Scholar]
- 43.Save S.S., Pandit A.B., Joshi J.B. Use of hydrodynamic cavitation for large scale microbial cell disruption. Food Bioprod Process. Trans. Inst. Chem. Eng. Part C. 1997;75:41–49. doi: 10.1205/096030897531351. [DOI] [Google Scholar]
- 44.Balasundaram B., Harrison S.T.L. Study of physical and biological factors involved in the disruption of E. coli by hydrodynamic cavitation. Biotechnol. Prog. 2006;22:907–913. doi: 10.1021/bp0502173. [DOI] [PubMed] [Google Scholar]
- 45.Balasundaram B., Pandit A.B. Significance of location of enzymes on their release during microbial cell disruption. Biotechnol. Bioeng. 2001;75:607–614. doi: 10.1002/bit.10072. [DOI] [PubMed] [Google Scholar]
- 46.Keshavarz E., Bonnerjea J., Hoare M., Dunnill P. Disruption of a fungal organism, Rhizopus nigricans, in a high-pressure homogenizer. Enzyme Microb. Technol. 1990;12:494–498. doi: 10.1016/0141-0229(90)90064-W. [DOI] [Google Scholar]
- 47.Mevada J., Devi S., Pandit A. Large scale microbial cell disruption using hydrodynamic cavitation: energy saving options. Biochem. Eng. J. 2019;143:151–160. doi: 10.1016/j.bej.2018.12.010. [DOI] [Google Scholar]
- 48.Mevada J.S., Wanje S.G., Pandit A.B. Selective recovery of the intracellular enzyme using hydrodynamic cavitation: Scalable approach. Chem. Eng. Process. - Process Intensif. 2022;182 doi: 10.1016/j.cep.2022.109205. [DOI] [Google Scholar]
- 49.Mevada J., Shrung R., Khasgiwale V., Pandit A. Hybrid strategy for the selective recovery of the intracellular enzyme from the cytoplasmic location using hydrodynamic cavitation: Scalable approach. Chem. Eng. Process. - Process Intensif. 2022;181 doi: 10.1016/j.cep.2022.109152. [DOI] [Google Scholar]
- 50.Katariya P., Arya S.S., Pandit A.B. Novel, non-thermal hydrodynamic cavitation of orange juice: effects on physical properties and stability of bioactive compounds. Elsevier Ltd. 2020;62:102364. [Google Scholar]
- 51.Carbonell J.V., Navarro J.L., Izquierdo L., Sentandreu E. Influence of high pressure homogenization and pulp reduction on residual pectinmethylesterase activity, cloud stability and acceptability of Lane Late orange juice: a study to obtain high quality orange juice with extended shelf life. J. Food Eng. 2013;119:696–700. doi: 10.1016/j.jfoodeng.2013.06.041. [DOI] [Google Scholar]
- 52.Annapoorna R.P., More P.R., Arya S.S. Effect of pressure and time on bioactive content, PPO inactivation, physicochemical and sensory properties of aonla (Emblica officinalis) juice during hydrodynamic cavitation processing. Food Sci. Biotechnol. 2023;32(1):71–82. doi: 10.1007/s10068-022-01164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhukya J., Mohapatra D., Naik R. Hydrodynamic cavitation processing of ascorbic acid treated precooled sugarcane juice for physiochemical, bioactive, enzyme stability, and microbial safety. J. Food Process Eng. 2022:1–15. doi: 10.1111/jfpe.14209. [DOI] [Google Scholar]
- 54.Pegu K., Arya S.S. Comparative assessment of HTST, hydrodynamic cavitation and ultrasonication on physico-chemical properties, microstructure, microbial and enzyme inactivation of raw milk. Innov. Food Sci. Emerg. Technol. 2021;69:102640. [Google Scholar]
- 55.Terán Hilares R., dos Santos J.G., Shiguematsu N.B., Ahmed M.A., da Silva S.S., Santos J.C. Low-pressure homogenization of tomato juice using hydrodynamic cavitation technology: effects on physical properties and stability of bioactive compounds. Ultrason. Sonochem. 2019;54:192–197. doi: 10.1016/j.ultsonch.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 56.Vigneshwaran G., More P.R., Arya S.S. Non-thermal hydrodynamic cavitation processing of tomato juice for physicochemical, bioactive, and enzyme stability: effect of process conditions, kinetics, and shelf-life extension. Curr. Res. Food Sci. 2022;5:313–324. doi: 10.1016/j.crfs.2022.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y., Martynenko A. Effect of hydrothermodynamic (HTD) processing on physical and chemical qualities of American cranberry puree using response surface methodology (RSM) LWT - Food Sci. Technol. 2016;70:322–332. doi: 10.1016/j.lwt.2016.02.054. [DOI] [Google Scholar]
- 58.Sozańska B. Raw cow’s milk and its protective effect on allergies and asthma. Nutrients. 2019;11:469. doi: 10.3390/nu11020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lohani U.C., Muthukumarappan K., Meletharayil G.H. Application of hydrodynamic cavitation to improve antioxidant activity in sorghum flour and apple pomace. Food Bioprod. Process. 2016;100:335–343. doi: 10.1016/j.fbp.2016.08.005. [DOI] [Google Scholar]
- 60.Rojas M.L., Leite T.S., Cristianini M., Alvim I.D., Augusto P.E.D. Peach juice processed by the ultrasound technology: changes in its microstructure improve its physical properties and stability. Food Res. Int. 2016;82:22–33. [Google Scholar]
- 61.Li K., Woo M.W., Patel H., Metzger L., Selomulya C. Improvement of rheological and functional properties of milk protein concentrate by hydrodynamic cavitation. J. Food Eng. 2018;221:106–113. doi: 10.1016/j.jfoodeng.2017.10.005. [DOI] [Google Scholar]
- 62.Pathania S., Ho Q.T., Hogan S.A., McCarthy N., Tobin J.T. Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J. Food Eng. 2018;225:18–25. doi: 10.1016/j.jfoodeng.2018.01.005. [DOI] [Google Scholar]
- 63.Ren X., Li C., Yang F., Huang Y., Huang C., Zhang K., Yan L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2020;265 doi: 10.1016/j.jfoodeng.2019.109697. [DOI] [Google Scholar]
- 64.Prajapat A.L., Gogate P.R. Intensification of depolymerization of aqueous guar gum using hydrodynamic cavitation. Chem. Eng. Process. Process Intensif. 2015;93:1–9. doi: 10.1016/j.cep.2015.04.002. [DOI] [Google Scholar]
- 65.Castro-muñoz R., Boczkaj G., Jafari S.M. The role of hydrodynamic cavitation in tuning physicochemical properties of food items: a comprehensive review. Trends Food Sci. Technol. 2023;134:192–206. doi: 10.1016/j.tifs.2023.03.010. [DOI] [Google Scholar]
- 66.Panda D., Manickam S. Cavitation technology-the future of greener extraction method: a review on the extraction of natural products and process intensification mechanism and perspectives. Appl. Sci. 2019;9:766. doi: 10.3390/app9040766. [DOI] [Google Scholar]
- 67.Panda D., Saharan V.K., Manickam S. Controlled hydrodynamic cavitation: a review of recent advances and perspectives for greener processing. Processes. 2020;8:220. doi: 10.3390/pr8020220. [DOI] [Google Scholar]
- 68.Grillo G., Boffa L., Binello A., Mantegna S., Cravotto G., Chemat F., Dizhbite T., Lauberte L., Telysheva G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019;115:200–208. doi: 10.1016/j.foodres.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 69.Meneguzzo F., Brunetti C., Fidalgo A., Ciriminna R., Delisi R., Albanese L., Zabini F., Gori A., dos Santos Nascimento L., De Carlo A., Ferrini F., Ilharco L., Pagliaro M. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Processes. 2019;7(9):581. [Google Scholar]
- 70.Albanese L., Bonetti A., D’Acqui L.P., Meneguzzo F., Zabini F. Affordable production of antioxidant aqueous solutions by hydrodynamic cavitation processing of silver fir (Abies alba Mill.) needles. Foods. 2019;8:7–10. doi: 10.3390/foods8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Presentato A., Scurria A., Albanese L., Lino C., Sciortino M., Pagliaro M., Zabini F., Meneguzzo F., Alduina R., Nuzzo D., Ciriminna R. Superior antibacterial activity of integral lemon pectin extracted via hydrodynamic cavitation. ChemistryOpen. 2020;9:628–630. doi: 10.1002/open.202000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nuzzo D., Cristaldi L., Sciortino M., Albanese L., Scurria A., Zabini F., Lino C., Pagliaro M., Meneguzzo F., Di Carlo M., Ciriminna R. Exceptional Antioxidant, non-cytotoxic activity of integral lemon pectin from hydrodynamic cavitation. ChemistrySelect. 2020;5:5066–5071. doi: 10.1002/slct.202000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cravotto G., Mariatti F., Gunjevic V., Secondo M., Villa M., Parolin J., Cavaglià G. Pilot scale cavitational reactors and other enabling technologies to design the industrial recovery of polyphenols from agro-food by-products, a technical and economical overview. Foods. 2018;7:1–14. doi: 10.3390/foods7090130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belwal T., Chemat F., Venskutonis P.R., Cravotto G., Jaiswal D.K., Bhatt I.D., Devkota H.P., Luo Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC - Trends Anal. Chem. 2020;127:115895. [Google Scholar]
- 75.Ciriminna R., Albanese L., Di Stefano V., Delisi R., Avellone G., Meneguzzo F., Pagliaro M. Beer produced via hydrodynamic cavitation retains higher amounts of xanthohumol and other hops prenylflavonoids. Lwt. 2018;91:160–167. doi: 10.1016/j.lwt.2018.01.037. [DOI] [Google Scholar]
- 76.Albanese L., Ciriminna R., Meneguzzo F., Pagliaro M. Gluten reduction in beer by hydrodynamic cavitation assisted brewing of barley malts. LWT - Food Sci. Technol. 2017;82:342–353. doi: 10.1016/j.lwt.2017.04.060. [DOI] [Google Scholar]
- 77.Albanese L., Ciriminna R., Meneguzzo F., Pagliaro M. Innovative beer-brewing of typical, old and healthy wheat varieties to boost their spreading. J. Clean. Prod. 2018;171:297–311. doi: 10.1016/j.jclepro.2017.10.027. [DOI] [Google Scholar]
- 78.L. Albanese, F. Meneguzzo, Hydrodynamic cavitation-assisted processing of vegetable beverages: review and the case of beer-brewing, Elsevier Inc., 2019. doi:10.1016/b978-0-12-815260-7.00007-9.
- 79.Albanese L., Ciriminna R., Meneguzzo F., Pagliaro M. Beer-brewing powered by controlled hydrodynamic cavitation: theory and real-scale experiments. J. Clean. Prod. 2017;142:1457–1470. doi: 10.1016/j.jclepro.2016.11.162. [DOI] [Google Scholar]
- 80.Kochadai N., Hema V., S., Vadakkepulppara, Ramachandran, Nair Investigation of the effect of hydrodynamic cavitation treatment on the aging of tender coconut–palmyra wine. J. Food Process. Preserv. 2022;46:1–15. doi: 10.1111/jfpp.16788. [DOI] [Google Scholar]
- 81.Patil L., Gogate P.R. Ultrasound assisted synthesis of stable oil in milk emulsion: Study of operating parameters and scale-up aspects. Ultrason. Sonochem. 2018;40:135–146. doi: 10.1016/j.ultsonch.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z., Wang G., Nie Y., Ji J. Hydrodynamic cavitation as an efficient method for the formation of sub-100 nm O/W emulsions with high stability, Chinese. J Chem. Eng. 2016;24:1477–1480. doi: 10.1016/j.cjche.2016.04.011. [DOI] [Google Scholar]
- 83.Stebeleva O.P., Minakov A.V. Application of cavitation in oil processing: an overview of mechanisms and results of treatment. ACS Omega. 2021;6:31411–31420. doi: 10.1021/acsomega.1c05858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Şaǧban F.O.T., Dindar E., Cirakoglu C., Keskinler B. Hydrodynamic cavitation of waste-activated sludge. Environ. Eng. Sci. 2018;35:775–784. doi: 10.1089/ees.2016.0408. [DOI] [Google Scholar]
- 85.Dindar E., Topaç Şagban F.O., Etyam C. Variations of dissolved nitrogen and phosphorus levels in disintegration of active sludge using hydrodynamic cavitation. J. Fac. Eng. Archit. Gazi Univ. 2018;33:1145–1153. doi: 10.17341/gazimmfd.416416. [DOI] [Google Scholar]
- 86.Terán Hilares R., Ramos L., da Silva S.S., Dragone G., Mussatto S.I., dos Santos J.C. Hydrodynamic cavitation as a strategy to enhance the efficiency of lignocellulosic biomass pretreatment. Crit. Rev. Biotechnol. 2018;38:483–493. doi: 10.1080/07388551.2017.1369932. [DOI] [PubMed] [Google Scholar]
- 87.Nakashima K., Ebi Y., Shibasaki-Kitakawa N., Soyama H., Yonemoto T. Hydrodynamic Cavitation reactor for efficient pretreatment of lignocellulosic biomass. Ind. Eng. Chem. Res. 2016;55:1866–1871. doi: 10.1021/acs.iecr.5b04375. [DOI] [Google Scholar]
- 88.Patil A., Dhanke P., Kore V., Kanse N. Thumba methyl ester production using prepared novel TiO2 nano-catalyst in ultrasonic cavitation reactor. Mater. Today Proc. 2019;18:4322–4329. doi: 10.1016/j.matpr.2019.07.391. [DOI] [Google Scholar]
- 89.More P.R., Arya S.S. Intensification of bio-actives extraction from pomegranate peel using pulsed ultrasound: Effect of factors, correlation, optimization and antioxidant bioactivities. Ultrason. Sonochem. 2021;72 doi: 10.1016/j.ultsonch.2020.105423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patil P.B., Bhandari V.M., Ranade V.V. Wastewater treatment and process intensification for degradation of solvents using hydrodynamic cavitation. Chem. Eng. Process. - Process Intensif. 2021;166 doi: 10.1016/j.cep.2021.108485. [DOI] [Google Scholar]
- 91.Mukherjee A., Mullick A., Teja R., Vadthya P., Roy A., Moulik S. Performance and energetic analysis of hydrodynamic cavitation and potential integration with existing advanced oxidation processes: A case study for real life greywater treatment. Ultrason. Sonochem. 2020;66 doi: 10.1016/j.ultsonch.2020.105116. [DOI] [PubMed] [Google Scholar]
- 92.Lee I., Han J.I. Simultaneous treatment (cell disruption and lipid extraction) of wet microalgae using hydrodynamic cavitation for enhancing the lipid yield. Bioresour. Technol. 2015;186:246–251. doi: 10.1016/j.biortech.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 93.Waghmare A., Nagula K., Pandit A., Arya S. Hydrodynamic cavitation for energy efficient and scalable process of microalgae cell disruption. Algal Res. 2019;40 doi: 10.1016/j.algal.2019.101496. [DOI] [Google Scholar]
- 94.Terán Hilares R., dos Santos J.C., Ahmed M.A., Jeon S.H., da Silva S.S., Han J.I. Hydrodynamic cavitation-assisted alkaline pretreatment as a new approach for sugarcane bagasse biorefineries. Bioresour. Technol. 2016;214:609–614. doi: 10.1016/j.biortech.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Gholami A., Pourfayaz F., Saifoddin A. Techno-economic assessment and sensitivity analysis of biodiesel production intensified through hydrodynamic cavitation. Energy Sci. Eng. 2021;9:1997–2018. doi: 10.1002/ese3.941. [DOI] [Google Scholar]
- 96.Mukherjee A., Satish A., Mullick A., Rapolu J., Moulik S., Roy A., Ghosh A.K. Paradigm Shift toward developing a zero liquid discharge strategy for dye-contaminated water streams: a green and sustainable approach using hydrodynamic cavitation and vacuum membrane distillation. ACS Sustain. Chem. Eng. 2021;9:6707–6719. doi: 10.1021/acssuschemeng.1c00619. [DOI] [Google Scholar]
- 97.Das S., Bhat A.P., Gogate P.R. Degradation of dyes using hydrodynamic cavitation: Process overview and cost estimation. J. Water Process Eng. 2021;42 doi: 10.1016/j.jwpe.2021.102126. [DOI] [Google Scholar]
- 98.Qi X.L., Peng X., Huang Y.Y., Li L., Wei Z.F., Zu Y.G., Fu Y.J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crops Prod. 2015;70:142–148. doi: 10.1016/j.indcrop.2015.03.026. [DOI] [Google Scholar]
- 99.Liu W., Fu Y., Zu Y., Kong Y., Zhang L., Zu B., Efferth T. Negative-pressure cavitation extraction for the determination of flavonoids in pigeon pea leaves by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2009;1216:3841–3850. doi: 10.1016/j.chroma.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 100.Yao X.H., Zhang D.Y., Luo M., Jin S., Zu Y.G., Efferth T., Fu Y.J. Negative pressure cavitation-microwave assisted preparation of extract of Pyrola incarnata Fisch. rich in hyperin, 2′-O-galloylhyperin and chimaphilin and evaluation of its antioxidant activity. Food Chem. 2015;169:270–276. doi: 10.1016/j.foodchem.2014.07.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.