Abstract
The effects of dietary supplementation of essential oil on growth performance, carcass yield, meat quality, serum antioxidant capacity, and intestinal tight junctions of broilers with or without Eimeria challenge were investigated. A total of 576 one-day-old male broilers were randomly separated into 8 treatments (6 replication floor-pens per treatment, 12 broilers per pen) in a 4 × 2 factorial design. The 4 diets consisted of 1) a corn and soybean meal basal diet, 2) an anticoccidial diet (60 g nicarbazin and 60 g narasin per ton of feed), 3) an oregano oil diet (500 ppm oregano oil), and 4) a clove oil diet (500 ppm clove oil). On d 10, half chicks were challenged with 1 × 104 sporulated oocysts of E. tenella, E. acervulina, and E. maxima per chick, whereas the others were inoculated with an equal amount of dilution (0.5 mL). The Eimeria challenge induced a higher fecal oocyst output on d 18, a lower duodenum Occludin expression level on d 28, a lower serum catalase level, and a higher cook loss and protein loss in thigh muscle on d 42. The anticoccidial diet lowered fecal Eimeria output and increased d 1 to 42 BW gain as compared to the control diet. The clove oil treatment enhanced duodenum ZO-1 expression level in nonchallenged birds, increased BW gain from d 1 to 14 and breast yield on d 42. The oregano oil treatment enhanced ZO-1 expression of challenged birds, reduced feed intake from 15 to 28 d, and helped broilers gain more tender meat. For those Eimeria-challenged broilers, both clove and oregano oil treatments recovered drip loss in breast muscle. Our results suggested that Eimeria challenge in broiler early age could interrupt later serum antioxidant capacity and damage meat quality. The dietary supplementation of clove or oregano essential oils could improve broiler growth performance and partially relieve the coccidial damage in gut integrity and meat quality.
Key words: broiler, essential oil, Eimeria, meat quality, tight junction
INTRODUCTION
Avian coccidiosis, a common parasitosis disease caused by Eimeria protozoa, results in a significant economic loss in the poultry industry globally, with an estimated amount of 12.96 billion dollars annually including the cost of prevention, clinical treatment, nutrition compensatory, and performance depression (Blake et al., 2020). The major coccidial control practices are vaccination (live oocyst) and feed additives (ionophores or chemicals), such as monensin, narasin, nicarbazin, and toltrazuril. Increasing drug resistance was reported in floor-raising systems in China (Lan et al., 2017; Zhang et al., 2022a), the United States (Bafundo et al., 2008), and Iran (Arabkhazaeli et al., 2013). Although shuttle (alteration of anticoccidials within a single flock) and rotation (alteration of anticoccidials between flocks) programs have been implemented to limit the resistance development (Peek and Landman 2011), other nutritional strategies to restore Eimeria anticoccidial sensitivity and maintain broiler performance gained more attention (Snyder et al., 2021).
Plant essential oils, with principal antioxidant activity (Wei and Shibamoto, 2010) and anticoccidial attribute (Remmal et al., 2011), were proposed to be considered as nondrug anticoccidial candidates (Sidiropoulou et al., 2020). Recently, diverse essential oils were reported to improve growth performance and gut health of Eimeria-challenged broilers in the absence of anticoccidial drugs (Sidiropoulou et al., 2020; Stefanello et al., 2020; Han et al., 2022; Zheng et al., 2023). In those studies, essential oils were applied as a complex formula such as the combination of oregano and garlic essential oils (Sidiropoulou et al., 2020), lauric acid monoglyceride and cinnamaldehyde (Zheng et al., 2023), or as adjunctive components with other feed additives such as organic acid (Stefanello et al., 2020), 8 plant extracts (Han et al., 2022 ). According to Rodriguez-Garcia et al. (2015), the antimicrobial efficacy of different essential oils can be ranked as follows: oregano = clove = cilantro = cinnamon > thyme > peppermint > rosemary > mustard > cilantro. Oregano oil, with bioactive compounds of carvacrol and thymol, exhibits a strong antioxidative ability due to its hydrogen donor merged with oxygen-free radicals (Kulisic et al., 2004). Dietary oregano oil and its bioactive components were reported to improve growth performance, serum antioxidant capacity, and meat quality in poultry and swine (Cheng et al., 2017; Zhang et al., 2021). Additionally, the oregano oil was also demonstrated to limit coccidial intracellular sporozoite infection through an in vitro platform (Sidiropoulou et al., 2020). Another potential anticoccidial alternative is clove oil which has a high antioxidant capacity due to the effective eugenol compound with a phenolic ring (Bezerra et al., 2017). The eugenol ingredient was reported to penetrate bacterial (Wang et al., 2018) and coccidial (Remmal et al., 2011) cell membranes, resulting in a leakage of intracellular components. However, limited research was conducted to evaluate the anticoccidial efficacy of pure essential oil in live broiler trials. In the current study, the inclusion of oregano oil or clove oil in broiler diets was used to catch up with the growth retarded by the Eimeria challenge and restore potential detrimental effects on meat quality and gut integrity.
MATERIALS AND METHODS
The experiment was conducted at a commercial research institute (Sichuan Tequ Agriculture and Animal Husbandry Technology Group Co., Ltd., Chengdu, China). All the handlings and procedures were approved by the Institutional Animal Care and Use Committee at Southwest Minzu University (2021NQNCZ15).
Treatments and Bird Management
A total of 576 one-day-old Arbor Acres male broilers were obtained from a commercial hatchery and received Newcastle and infectious bronchitis vaccines at hatch. Twelve birds were weighed and randomly assigned to 1 of 48 floor-pens (1.7 × 0.9 m). The 48 floor-pens were divided into 6 blocks according to their locations in the chick house (block served as replicate), and 8 treatments were randomly assigned into the 8 floor-pens in each block. Eight treatments were arranged in a 2 (Eimeria challenged vs. aliquot of dilution) × 4 (a corn and soybean-meal control diet, an anticoccidial diet (CON + 60 g of nicarbazin and 60 g of narasin per ton of feed, an oregano oil diet (a CON + 500 ppm oregano oil), and a clove oil diet (CON + 500 ppm clove oil) factorial setting. All diets were formulated to meet requirements recommended by National Research Council (1994) and Aviagen Company (Aviagen, 2022). Essential oils were prepared in a form of a powder with 5% effective ingredients and 95% natural feed grade inert carrier by the DDC company (DadHank Chengdu Biotech Corp., Chengdu, China). Effective ingredients were determined by the gas chromatography-mass spectrometry method. Feed ingredients and nutrient compositions of basal diets were presented in Table 1. On d 10, each challenged broiler chick was orally gavaged (to crop) with a 0.5 mL PBS of 104 sporulated oocysts of E. tenella, E. acervulina, and E. maxima (20 × commercial vaccine, Foshan Standard Biotech, Guangzhou, China), whereas unchallenged broilers received an aliquot of PBS dilution.
Table 1.
Composition and nutrient levels of the basal diet (air-dry basis, %).
| Items % | 0–14 d | 15–28 d | 29–42 d |
|---|---|---|---|
| Ingredients | |||
| Corn | 51.26 | 56.48 | 60.75 |
| Soybean meal | 41.34 | 36.58 | 30.45 |
| Soybean oil | 1.99 | 1.95 | 3.50 |
| Choline chloride | 0.19 | 0.21 | 0.22 |
| L-lysine sulfate | 0.11 | 0.13 | 0.47 |
| DL-methionine | 0.32 | 0.29 | 0.30 |
| L-threonine | 0.13 | 0.12 | 0.50 |
| Multivitamin1 | 0.40 | 0.40 | 0.40 |
| Premix2 | 0.20 | 0.20 | 0.20 |
| CaHPO4 | 2.43 | 2.16 | 1.83 |
| Sodium chloride | 0.33 | 0.33 | 0.33 |
| Limestone | 1.29 | 1.16 | 1.04 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated nutrients3 | |||
| ME kcal/kg | 2,977 | 3,032 | 3,153 |
| Crude protein | 23.09 | 20.92 | 19.24 |
| Ca | 0.94 | 0.84 | 0.73 |
| Available phosphorus | 0.61 | 0.57 | 0.51 |
| Na | 0.16 | 0.16 | 0.16 |
| Digestible lysine | 1.26 | 1.16 | 1.28 |
| Digestible methionine | 0.63 | 0.58 | 0.56 |
| Digestible threonine | 0.86 | 0.79 | 1.09 |
| Digestible TSAA4 | 0.94 | 0.87 | 0.82 |
Multivitamin provided the following ingredients per kilogram of diet: 19,000 IU of vitamin A, 4,000 IU of vitamin D3, 24 IU of vitamin E, 2.00 mg of vitamin K3, 2.6 mg of vitamin B1, 1.12 mg of vitamin B2, 5.6 mg of vitamin B6, and 0.02 mg of B12.
Premix provided the following trace elements per kilogram of diet: 110 mg of Mn, 100 mg of Zn, 80 mg of Fe, 10 mg of Cu, 0.6 mg of I, and 0.35 mg of Se.
Values were calculated from data provided by the Feed Database in China (2019).
TSAA: total sulfur-containing amino acid.
Boilers had ad libitum access to water and feed through 1 to 42 d. A commercial light program and temperature setting and used litter with top-dressed wood shavings were applied to simulate commercial broiler production (Arbor Acres, 2018). In detail, the house temperature was set at 32°C during 1 to 7 d, decreased by 3°C per wk, and kept at 25°C from d 21 to the end of the experiment. A light program of 23L:1D and 20L:4D was applied from d 1 to 7 and d 8 to 42, respectively. A round feeder and 3 nipple drinkers per floor-pen were provided.
Growth Performance and Carcass Yield
Feed intake and body weight (BW) were recorded by pen on d 1, 14, 28, and 42. Daily mortality was recorded. BW gain, feed intake, and the feed conversion ratio (FCR) were calculated from 1 to 14 d, 15 to 28 d, 29 to 42 d, and overall period of 1 to 42 d.
After 12 h of fasting, 2 broilers with median BW per pen were selected and processed for carcass yield on d 42. Birds were weighed individually, de-feathered, and eviscerated. Carcass yield (dressing percentage) was calculated by the carcass weight by live BW. Then, a pair of wings, drumsticks, thighs, and breast (pectoralis major) muscle without skin, and a pair of tender muscle (pectoralis minor) were weighed to determine each part yield (part weight/carcass weight in percentage).
Meat Quality
At harvest, breast and thigh muscles were collected for pH, color, quality, and nutrition evaluation. Each measurement was conducted with triplicates. The pH value at 45 min and 24 h postmortem was recorded using a pH meter (HI98163, Hanna Instruments, Woonsocket, RI). According to the Schanda (2007) method, the L*, a*, and b* values, representing lightness, redness, and yellowness, respectively, were measured by a colorimeter (CR-400, Konica Minolta Investment Co., Japan). A piece of breast, thigh, and tender muscle samples was weighed, hung in a sealed plastic bag at 4°C for 24 h, dried with absorbent paper, and then weighed for the terminal weight. Drip loss was calculated as the percentage of weight loss (initial weight minus terminal weight) to initial weight. Another muscle piece was weighed for the initial weight, sealed in a plastic bag, and cooked at 80°C until the central muscle reached at 75°C, dried, and then weighed for terminal weight. Cook loss was expressed the percentage of weight loss to initial weight (Huang et al., 2016). The nutrition compositions including moisture, protein, fat, and ash of breast and thigh muscles were measured according to AOAC standards (AOAC, 2019).
Fecal and Tissue Sampling
Fresh fecal samples from multiple birds in each pen were mixed homogenously and collected on d 10 (prechallenge) and d 18 (postchallenge). The oocysts count was determined using the method of Mengistu et al. (2021). Briefly, 2 g of homogenized samples were taken from each pen and diluted with 58 mL of saturated NaCl solution. After mixing thoroughly, oocyst counts were determined using the McMaster chamber at the 10 × objective magnification.
On d 18, 28, and 42, blood and intestine samples were taken from 2 birds with median BW from each pen via the brachial vein. After clotting at ambient temperature for 4 h, serum samples were collected after centrifuging at 1,900 × g for 15 min. Intestine samples were deep-frozen in liquid nitrogen and kept on dried ice. Samples were stored at −80°C for further tests.
Serum Antioxidative Ability
The activity of glutathione peroxidase, total superoxide dismutase, and catalase, and the content of malonaldehyde in serum were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Briefly, the serum glutathione peroxidase, total superoxide dismutase, catalase, and malonaldehyde levels were determined through the colorimetric method, hydroxylamine method, ammonium molybdate method, and thiobarbituric acid method, respectively.
Tight Junction Protein mRNA Levels
Expression levels of tight junctions of broilers at 28 d of age were determined. Total RNA was extracted from the mucosa tissues of duodenum, jejunum and ileum using RNAiso Plus kit (Takara Biotechnology, Dalian, China). The integrity of total RNA was confirmed by agarose gel electrophoresis. The purity of total RNA was measured by using Nanodrop 1000 (Thermo Fisher, Waltham, MA). Reverse transcription was performed utilizing a commercial kit (Transcript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR Kit, TransGen Biotech, Beijing, China). The reverse transcription reaction mixtures contained 1 µL of total RNA, 5 µL of commercial mix, and 12 µL of nuclease-free water in a final volume of 20 µL. The reverse transcription reactions were performed for 15 min at 37°C and inactivated by heating to 85°C for 5 s. The resulting complementary DNA was diluted to 1:1 with ddH2O. The RT-qPCR was performed on a CFX96 Real-Time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA). The 25 µL of PCR reaction mixture contained 12.5 µL of TB Green Premix Ex Taq II (Takara Biotechnology Co., Ltd., Dalian, China)., 2 µL of primers, 2 µL of cDNA, and 8.5 µL of ddH2O. Primer sequences were synthesized by Sangon Biotech (Shanghai, China) and listed in Table 2. The RT-PCR included an initial denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, 60°C for 30 s. The relative expression levels of target genes were normalized to that of reference gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and calculated using the 2−ΔΔCT method.
Table 2.
Primer sequences for the qPCR.
| Target gene1 | Primer sequence | Size (bp) | GenBank accession number |
|---|---|---|---|
| ZO-1 | Forward: 5′-GCCAACTGATGCTGAACCAA-3′ Reverse: 5′-GGGAGAGACAGGACAGGACT-3′ |
141 | XM_015278975 |
| Oocludin | Forward: 5′-GATGGACAGCATCAACGACC-3′ Reverse: 5′-CTTGCTTTGGTAGTCTGGGC-3′ |
142 | NM_205128.1 |
| Claudin-1 | Forward: 5′-CTGATTGCTTCCAACCAG-3′ Reverse: 5′-CAGGTCAAACAGAGGTACAAG-3′ |
140 | NM_001013611.2 |
| GAPDH | Forward: 5′-TGCTGCCCAGAACATCATCC-3′ Reverse: 5′-ACGGCAGGTCAGGTCAACAA-3′ |
142 | NM_204305.2 |
ZO-1: zona occludens-1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
A randomized complete block design was applied in the study. The prechallenge fecal oocyst count was analyzed using a 1-way ANOVA analysis. Growth performance, carcass yield, meat quality, serum antioxidants, postchallenge fecal oocyst counts, and the relative mRNA expression levels of tight junction protein were analyzed by a 2-way ANOVA analysis. The dietary essential oil supplementation, the Eimeria challenge, and their interaction were considered fixed factors and the block was considered a random factor. Data analysis was conducted by a mixed model in the MIX procedure of SAS (Version 9.4, SAS Institute Inc., Cary, NC; SAS Institute, 2016). Additionally, least square means were used to compare the means of treatments. A significance level was considered at P ≤ 0.05.
RESULTS
Oocyst Shedding
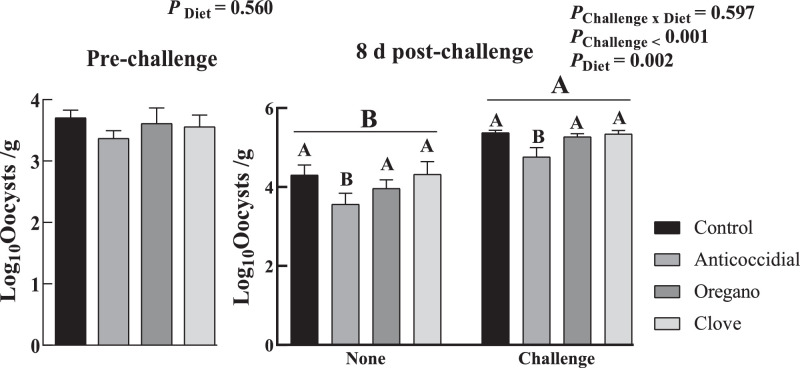
Before the Eimeria challenge, fecal oocyst count was not affected by dietary treatments (P = 0.560, Figure 1). At 7 d postchallenge, the oocyst number was higher in the challenged group compared with the nonchallenged group (P < 0.001). The anticoccidial diets reduced oocysts shedding as compared to other diets (P = 0.002).
Figure 1.
Effects of dietary essential oil supplementation and Eimeria challenge on fecal oocyst output. None: unchallenged broilers received aliquot of dilution at d 10. Challenge: challenged broilers received sporulated 104 oocysts of E. tenella, E. acervulina, and E. maxima at d 10. Control: a corn and soybean meal basal diet, anticoccidial: control diet + 60 g nicarbazin and 60 g narasin per ton of feed, oregano: control diet + 500 ppm oregano oil, and clove: control diet + 500 ppm clove oil. Fecal samples were taken prechallenge and 8 d postchallenge with 12 and 6 replicates per treatment, respectively. A, BCapitalized subscripts noted the major effects of Eimeria challenge and diet on fecal oocyst output.
Growth and Carcass Performances
The Eimeria challenge on d 10 tended to decrease feed intake (P = 0.066) and BW gain (P = 0.054) from 15 to 28 d (Table 3). The clove oil supplementation increased BW gain from 1 to 14 d (P = 0.030) and subsequently increased the overall BW gain from 1 to 42 d (P = 0.013) as compared to the control diet. The oregano oil supplementation decreased the feed intakes from 15 to 28 d (P = 0.004) and from 1 to 42 d (P = 0.034) and tended to decrease overall FCR (P = 0.060). During d 15 to 28, dietary essential oil supplementation and Eimeria challenge interactively affected the BW gain (Pinteraction < 0.001). For nonchallenged birds, the clove oil diet lowered their BW gain, and for challenged birds, the anticoccidial diet increased their BW gain.
Table 3.
Effects of dietary essential oil supplementation and Eimeria challenge on growth performance of broilers (n = 6).
| 1–14 d2 |
15–28 d |
29–42 d |
1–42 d |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet1 | Eimeria1 | Feed intake (g) | BW gain (g) | FCR | Feed intake (g) | BW gain (g) | FCR | Feed intake (g) | BW gain (g) | FCR | Feed intake (g) | BW gain (g) | FCR |
| Control | 322 | 270b | 1.18 | 999a | 528b | 1.91 | 1529b | 752 | 1.99 | 2613ab | 1391c | 1.78 | |
| Anticoccidial | 343 | 293ab | 1.16 | 1039a | 583a | 1.73 | 1671a | 806 | 2.04 | 2711a | 1566a | 1.69 | |
| Oregano | 324 | 275b | 1.17 | 888b | 506b | 1.76 | 1448b | 775 | 1.91 | 2463b | 1453bc | 1.70 | |
| Clove | 342 | 306a | 1.16 | 969a | 551ab | 1.84 | 1524b | 774 | 1.99 | 2610ab | 1508ab | 1.74 | |
| SEM | 7.9 | 8.8 | 0.015 | 27.3 | 16.6 | 0.049 | 47.9 | 28.3 | 0.053 | 56.4 | 34.9 | 0.024 | |
| No | 331 | 283 | 1.17 | 1000 | 558 | 1.80 | 1538 | 784 | 1.98 | 2597 | 1479 | 1.73 | |
| Yes | 335 | 290 | 1.16 | 948 | 525 | 1.82 | 1549 | 770 | 1.99 | 2602 | 1479 | 1.72 | |
| SEM | 5.6 | 6.2 | 0.010 | 19.3 | 11.7 | 0.034 | 33.9 | 20.0 | 0.037 | 39.8 | 24.8 | 0.017 | |
| Control | No | 313 | 258 | 1.19 | 1016 | 536c | 1.92 | 1515 | 775 | 1.97 | 2584 | 1423 | 1.78 |
| Anticoccidial | No | 343 | 282 | 1.18 | 1072 | 559bc | 1.80 | 1651 | 813 | 2.04 | 2673 | 1500 | 1.70 |
| Oregano | No | 327 | 271 | 1.18 | 878 | 504cd | 1.75 | 1397 | 763 | 1.89 | 2445 | 1432 | 1.71 |
| Clove | No | 342 | 319 | 1.15 | 1032 | 635a | 1.74 | 1587 | 784 | 2.02 | 2689 | 1563 | 1.72 |
| Control | Yes | 331 | 282 | 1.17 | 982 | 521cd | 1.91 | 1544 | 729 | 2.02 | 2642 | 1360 | 1.78 |
| Anticoccidial | Yes | 343 | 305 | 1.13 | 1006 | 606ab | 1.66 | 1691 | 801 | 2.04 | 2750 | 1632 | 1.69 |
| Oregano | Yes | 342 | 279 | 1.17 | 898 | 508cd | 1.77 | 1499 | 786 | 1.94 | 2481 | 1474 | 1.69 |
| Clove | Yes | 343 | 294 | 1.17 | 906 | 467d | 1.94 | 1463 | 764 | 1.95 | 2533 | 1452 | 1.75 |
| SEM | 11.2 | 12.4 | 0.021 | 38.6 | 23.48 | 0.069 | 67.8 | 39.9 | 0.075 | 79.6 | 49.3 | 0.034 | |
| P value | |||||||||||||
| Diet | 0.131 | 0.030 | 0.699 | 0.004 | 0.017 | 0.055 | 0.019 | 0.598 | 0.415 | 0.034 | 0.013 | 0.060 | |
| Eimeria | 0.646 | 0.409 | 0.315 | 0.066 | 0.054 | 0.706 | 0.810 | 0.626 | 0.894 | 0.946 | 0.998 | 0.847 | |
| Eimeria × Diet | 0.771 | 0.224 | 0.347 | 0.306 | <0.001 | 0.110 | 0.398 | 0.874 | 0.827 | 0.428 | 0.063 | 0.881 | |
No: unchallenged broilers received aliquot of dilution at d 10. Yes: challenged broilers received sporulated 104 oocysts of E. tenella, E. acervulina, and E. maxima at d 10. Control: a corn and soybean meal basal diet, anticoccidial: control diet+ 60 g nicarbazin and 60 g narasin per ton of feed, oregano: control diet + 500 ppm oregano oil, and clove: control diet + 500 ppm clove oil.
BW gain: body weight gain, and FCR: feed conversion ratio. Growth performance data were collected and calculated from 6 replication floor-pens of broilers.
Means in a column with different superscript letters are different at а = 0.05 level.
There was no interaction effect between essential oil supplementation or Eimeria challenge on carcass or part yields (all P > 0.050, Table 4). The challenged broilers exhibited a lower wing yield than the healthy broilers (P = 0.018). Adding oregano or clove oil in broiler diet improved the breast and tender yields (P = 0.028 and 0.020, respectively) as compared to the control diet.
Table 4.
Effects of dietary essential oil supplementation and Eimeria challenge on the carcass performance of broilers at 42 d of age (%, n = 6).
| Diet1 | Eimeria1 | Carcass/BW | Breast/carcass | Tender/carcass | Tight/carcass | Drumstick/carcass | Wing/carcass |
|---|---|---|---|---|---|---|---|
| Control | 68.31 | 23.00c | 4.83b | 13.95 | 14.56 | 11.11 | |
| Anticoccidial | 68.19 | 25.36a | 5.62a | 14.13 | 13.97 | 11.03 | |
| Oregano | 69.96 | 24.89ab | 5.23ab | 13.97 | 14.52 | 11.27 | |
| Clove | 68.30 | 23.65bc | 5.30a | 14.66 | 14.00 | 11.14 | |
| SEM | 0.935 | 0.594 | 0.164 | 0.295 | 0.228 | 0.215 | |
| No | 69.31 | 24.48 | 5.26 | 14.09 | 14.24 | 11.41a | |
| Yes | 68.07 | 23.98 | 5.23 | 14.26 | 14.28 | 10.87b | |
| SEM | 0.664 | 0.420 | 0.116 | 0.209 | 0.162 | 0.152 | |
| Control | No | 68.75 | 22.94 | 4.77 | 13.76 | 14.22 | 11.31 |
| Anticoccidial | No | 67.57 | 24.34 | 5.36 | 14.31 | 14.17 | 11.48 |
| Oregano | No | 71.47 | 25.93 | 5.34 | 14.33 | 14.45 | 11.54 |
| Clove | No | 69.45 | 24.70 | 5.59 | 13.97 | 14.13 | 11.29 |
| Control | Yes | 67.87 | 23.07 | 4.90 | 14.14 | 14.89 | 10.90 |
| Anticoccidial | Yes | 68.81 | 26.39 | 5.87 | 13.94 | 13.78 | 10.59 |
| Oregano | Yes | 68.46 | 23.85 | 5.13 | 13.61 | 14.59 | 10.99 |
| Clove | Yes | 67.15 | 22.60 | 5.02 | 15.36 | 13.87 | 10.99 |
| SEM | 1.319 | 0.839 | 0.232 | 0.418 | 0.322 | 0.303 | |
| P value | |||||||
| Diet | 0.524 | 0.028 | 0.020 | 0.319 | 0.135 | 0.894 | |
| Eimeria | 0.199 | 0.406 | 0.843 | 0.570 | 0.864 | 0.018 | |
| Eimeria × Diet | 0.388 | 0.052 | 0.146 | 0.082 | 0.380 | 0.785 | |
No: unchallenged broilers received aliquot of dilution at d 10. Yes: challenged broilers received sporulated 104 oocysts of E. tenella, E. acervulina, and E. maxima at d 10. Control: a corn and soybean meal basal diet, anticoccidial: control diet+ 60 g nicarbazin and 60 g narasin per ton of feed, oregano: control diet + 500 ppm oregano oil, and clove: control diet + 500 ppm clove oil.
Means in a column with different superscript letters are different at а = 0.05 level.
Meat Quality
The Eimeria challenge to broilers at their early age reduced their thigh muscle yellowness on d 42 (b*, P = 0.027, Table 5), lowered pH24h (P = 0.026), and increased cooking loss (P = 0.020). Furthermore, the Eimeria challenge increased thigh moisture content (P = 0.006) and reduced crude protein content (P = 0.012, Table 7). Feeding the anticoccidial additive, the oregano oil, or the clove oil reduced the drip loss of breast muscle in those challenged broilers, however, there were no dietary differences when broilers were unchallenged (Pinteraction = 0.001, Table 6). The clove oil supplementation increased the thigh redness (a* value) and decreased its cook loss (P = 0.040 and 0.022, Table 5). Feeding oregano oil to those unchallenged broilers increased their ash content in breast muscle as compared to that of the anticoccidial treatment (Pinteraction = 0.035, Table 7).
Table 5.
Effects of dietary essential oil supplementation and Eimeria challenge on broiler thigh quality at 42 d of age (n = 6).
| Diet1 | Eimeria1 | pH45min | pH24h | L* | a* | b* | Drip loss % | Cook loss % |
|---|---|---|---|---|---|---|---|---|
| Control | 6.36 | 6.11 | 55.53 | 5.80 | 5.84 | 1.49 | 32.31a | |
| Anticoccidial | 6.46 | 6.27 | 53.44 | 6.55 | 5.61 | 1.47 | 29.85ab | |
| Oregano | 6.36 | 6.22 | 55.18 | 4.78 | 4.15 | 1.57 | 31.01a | |
| Clove | 6.42 | 6.23 | 53.96 | 5.99 | 5.26 | 1.24 | 27.87b | |
| SEM | 0.064 | 0.054 | 0.786 | 0.605 | 0.512 | 0.152 | 0.994 | |
| No | 6.44 | 6.27a | 54.76 | 6.20 | 5.81a | 1.44 | 29.05b | |
| Yes | 6.36 | 6.15b | 54.29 | 5.35 | 4.63b | 1.44 | 31.47a | |
| SEM | 0.045 | 0.038 | 0.556 | 0.428 | 0.362 | 0.107 | 0.703 | |
| Control | No | 6.41 | 6.20 | 56.62 | 5.36bc | 5.60 | 1.48 | 31.91a |
| Anticoccidial | No | 6.48 | 6.29 | 53.69 | 6.36abc | 5.97 | 1.76 | 29.87a |
| Oregano | No | 6.32 | 6.23 | 55.85 | 5.18bc | 4.72 | 1.47 | 30.60a |
| Clove | No | 6.56 | 6.37 | 52.90 | 7.92a | 6.93 | 1.06 | 23.83b |
| Control | Yes | 6.31 | 6.03 | 54.45 | 6.25abc | 6.07 | 1.50 | 32.71a |
| Anticoccidial | Yes | 6.44 | 6.25 | 53.19 | 6.73abc | 5.25 | 1.17 | 29.83a |
| Oregano | Yes | 6.40 | 6.21 | 54.52 | 4.38bc | 3.59 | 1.68 | 31.43a |
| Clove | Yes | 6.28 | 6.09 | 55.01 | 4.05c | 3.59 | 1.42 | 31.90a |
| SEM | 0.090 | 0.076 | 1.111 | 0.856 | 0.724 | 0.214 | 1.406 | |
| P value | ||||||||
| Diet | 0.637 | 0.231 | 0.209 | 0.235 | 0.113 | 0.492 | 0.023 | |
| Eimeria | 0.186 | 0.026 | 0.550 | 0.168 | 0.027 | 0.989 | 0.020 | |
| Eimeria × Diet | 0.243 | 0.281 | 0.263 | 0.040 | 0.083 | 0.148 | 0.022 | |
No: unchallenged broilers received aliquot of dilution at d 10. Yes: challenged broilers received sporulated 104 oocysts of E. tenella, E. acervulina, and E. maxima at d 10. Control: a corn and soybean meal basal diet, anticoccidial: control diet + 60 g nicarbazin and 60 g narasin per ton of feed, oregano: control diet + 500 ppm oregano oil, and clove: control diet + 500 ppm clove oil.
Means in a column with different superscript letters are different at а = 0.05 level.
Table 7.
Effects of dietary essential oil supplementation and Eimeria challenge on nutrient compositions of broiler breast and thigh at 42 d of age (%, n = 6).
| Breast2 |
Thigh |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diet1 | Eimeria1 | Moisture | Protein | Fat | Ash | Moisture | Protein | Fat | Ash |
| Control | 75.19 | 22.19 | 0.79 | 1.27 | 77.13 | 19.17 | 2.28 | 1.23 | |
| Anticoccidial | 75.15 | 22.21 | 0.86 | 1.27 | 77.06 | 19.04 | 2.44 | 1.26 | |
| Oregano | 74.96 | 22.69 | 0.74 | 1.30 | 77.24 | 18.83 | 2.42 | 1.26 | |
| Clove | 74.78 | 22.85 | 0.81 | 1.31 | 76.79 | 19.44 | 2.22 | 1.27 | |
| SEM | 0.343 | 0.305 | 0.083 | 0.024 | 0.227 | 0.189 | 0.180 | 0.023 | |
| No | 75.11 | 22.44 | 0.85 | 1.29 | 76.72b | 19.37a | 2.44 | 1.27 | |
| Yes | 74.93 | 22.53 | 0.74 | 1.28 | 77.39a | 18.87b | 2.24 | 1.24 | |
| SEM | 0.243 | 0.216 | 0.059 | 0.017 | 0.160 | 0.134 | 0.127 | 0.016 | |
| Control | No | 75.26 | 22.07 | 0.87 | 1.28ab | 76.72 | 19.45 | 2.63 | 1.26 |
| Anticoccidial | No | 75.82 | 21.67 | 0.86 | 1.23b | 76.82 | 19.01 | 2.57 | 1.27 |
| Oregano | No | 74.65 | 23.09 | 0.74 | 1.36a | 76.94 | 19.43 | 2.09 | 1.29 |
| Clove | No | 74.69 | 22.96 | 0.95 | 1.30ab | 76.41 | 19.60 | 2.46 | 1.26 |
| Control | Yes | 75.13 | 22.32 | 0.70 | 1.26ab | 77.53 | 18.89 | 1.93 | 1.20 |
| Anticoccidial | Yes | 74.47 | 22.76 | 0.85 | 1.30ab | 77.31 | 19.07 | 2.32 | 1.26 |
| Oregano | Yes | 75.27 | 22.30 | 0.74 | 1.23b | 77.54 | 18.22 | 2.75 | 1.23 |
| Clove | Yes | 74.86 | 22.75 | 0.68 | 1.32ab | 77.17 | 19.28 | 1.98 | 1.29 |
| SEM | 0.485 | 0.432 | 0.117 | 0.034 | 0.321 | 0.267 | 0.254 | 0.032 | |
| P value | |||||||||
| Diet | 0.818 | 0.316 | 0.763 | 0.564 | 0.568 | 0.163 | 0.779 | 0.547 | |
| Eimeria | 0.616 | 0.773 | 0.188 | 0.556 | 0.006 | 0.012 | 0.289 | 0.247 | |
| Eimeria × Diet | 0.210 | 0.179 | 0.586 | 0.035 | 0.955 | 0.145 | 0.066 | 0.482 | |
No: unchallenged broilers received aliquot of dilution at d 10. Yes: challenged broilers received sporulated 104 oocysts of E. tenella, E. acervulina, and E. maxima at d 10. Control: a corn and soybean meal basal diet, anticoccidial: control diet + 60 g nicarbazin and 60 g narasin per ton of feed, oregano: control diet + 500 ppm oregano oil, and clove: control diet + 500 ppm clove oil.
Means in a column with different superscript letters are different at а = 0.05 level.
Table 6.
Effects of dietary essential oil supplementation and Eimeria challenge on broiler breast quality at 42 d of age (n = 6).
| Diet1 | Eimeria1 | pH45min | pH24h | L* | a* | b* | Drip loss % | Cook loss % |
|---|---|---|---|---|---|---|---|---|
| Control | 6.61 | 5.86 | 47.83 | 3.01 | 3.20 | 2.46a | 28.03 | |
| Anticoccidial | 6.66 | 5.97 | 47.60 | 2.62 | 3.11 | 1.74b | 27.09 | |
| Oregano | 6.60 | 5.89 | 47.16 | 1.99 | 2.72 | 1.51b | 29.07 | |
| Clove | 6.69 | 5.91 | 48.00 | 2.73 | 3.22 | 1.76b | 28.17 | |
| SEM | 0.065 | 0.041 | 0.652 | 0.335 | 0.349 | 0.162 | 0.727 | |
| No | 6.62 | 5.91 | 47.89 | 2.47 | 3.12 | 1.73 | 27.98 | |
| Yes | 6.66 | 5.91 | 47.40 | 2.71 | 3.00 | 2.01 | 28.19 | |
| SEM | 0.046 | 0.029 | 0.461 | 0.237 | 0.247 | 0.114 | 0.514 | |
| Control | No | 6.62 | 5.86 | 48.67 | 2.78 | 3.03 | 1.74b | 27.31 |
| Anticoccidial | No | 6.64 | 5.96 | 47.59 | 3.19 | 3.83 | 1.94b | 27.28 |
| Oregano | No | 6.59 | 5.83 | 47.17 | 1.65 | 2.44 | 1.68b | 29.39 |
| Clove | No | 6.65 | 5.99 | 48.15 | 2.26 | 3.20 | 1.58b | 27.95 |
| Control | Yes | 6.61 | 5.85 | 47.00 | 3.25 | 3.38 | 3.17a | 28.74 |
| Anticoccidial | Yes | 6.69 | 5.98 | 47.61 | 2.05 | 2.38 | 1.55b | 26.89 |
| Oregano | Yes | 6.61 | 5.96 | 47.16 | 2.32 | 3.00 | 1.35b | 28.75 |
| Clove | Yes | 6.73 | 5.83 | 47.86 | 3.21 | 3.24 | 1.95b | 28.40 |
| SEM | 0.092 | 0.058 | 0.921 | 0.473 | 0.494 | 0.229 | 1.027 | |
| P value | ||||||||
| Diet | 0.715 | 0.308 | 0.812 | 0.191 | 0.711 | 0.002 | 0.295 | |
| Eimeria | 0.593 | 0.915 | 0.459 | 0.486 | 0.719 | 0.102 | 0.777 | |
| Eimeria × Diet | 0.960 | 0.115 | 0.781 | 0.124 | 0.181 | 0.001 | 0.754 | |
No: unchallenged broilers received aliquot of dilution at d 10. Yes: challenged broilers received sporulated 104 oocysts of E. tenella, E. acervulina, and E. maxima at d 10. Control: a corn and soybean meal basal diet, anticoccidial: control diet + 60 g nicarbazin and 60 g narasin per ton of feed, oregano: control diet + 500 ppm oregano oil, and clove: control diet + 500 ppm clove oil.
Means in a column with different superscript letters are different at а = 0.05 level.
Antioxidative Ability
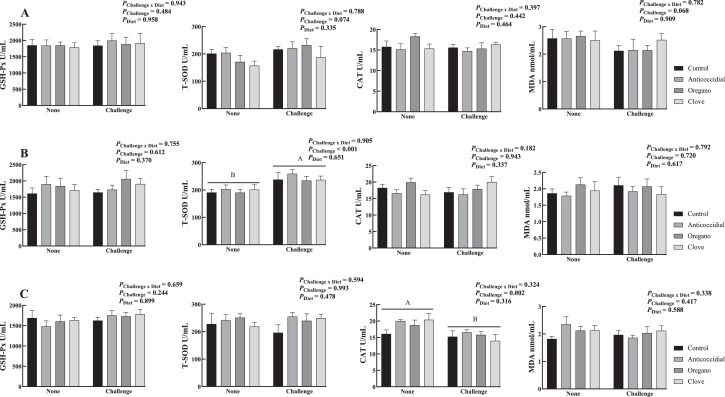
The Eimeria challenge increased serum total superoxide dismutase activity of broilers on d 28 (P < 0.001, Figure 2) and decreased their catalase activity on d 42 (P = 0.002). However, dietary treatments did not affect the serum antioxidant activity in the current study.
Figure 2.
Effects of dietary essential oil supplementation and Eimeria challenge on serum antioxidants of broilers at different age (A: 18 d, B: 28 d, C: 42 d, U/mL for all except for MDA, n = 6). None: unchallenged broilers received aliquot of dilution at d 10. Challenge: challenged broilers received sporulated 104 oocysts of E. tenella, E. acervulina, and E. maxima at d 10. Control: a corn and soybean meal basal diet, anticoccidial: control diet + 60 g nicarbazin and 60 g narasin per ton of feed, oregano: control diet + 500 ppm oregano oil, and clove: control diet + 500 ppm clove oil. Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malonaldehyde; T-SOD, total superoxide dismutase. A, BCapitalized subscripts noted the major effect of Eimeria challenge on fecal oocyst output.
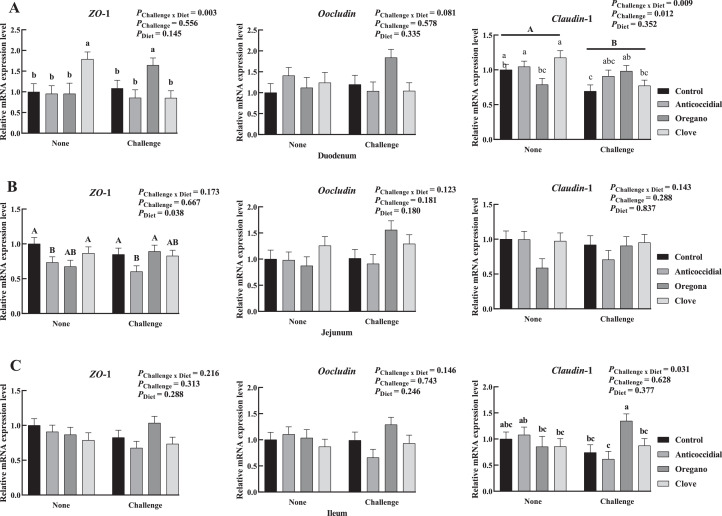
Tight Junctions
The relative mRNA expression levels of tight junction proteins including ZO-1, Oocludin, and Claudin-1 were presented in Figure 3. Our data indicated that the Eimeria challenge decreased the expression level of Claudin-1 in broiler duodenum (Figure 3A, P = 0.012). The anticoccidial diet reduced the relative mRNA expression level of jejuna ZO-1 in health broilers at 28 d of age (P = 0.038, Figure 3B). The Eimeria and diet treatment interactively affected the relative expression levels of duodenal ZO-1, Claudin-1, and ileal Claudin-1 (P = 0.003, 0.009, and 0.031, respectively). Briefly, the clove oil supplementation enhanced the expression level of duodenum ZO-1 of health broilers and the expression level of ileal Claudin-1 of challenged broilers. The oregano oil treatment enhanced the expression levels of duodenal ZO-1, Claudin-1, and ileal Claudin-1 of challenged broilers.
Figure 3.
Effects of Eimeria challenge and dietary essential oil supplementation on the relative mRNA expression levels of intestinal tight junction proteins of broiler at 28 d of age (A: duodenum, B: jejunum, C: ileum, and n = 12 birds). a–c, A, BMeans not sharing the same subscripts were different at α = 0.05 significant level. The a–c subscripts distinguished interactive differences and the A and B subscripts distinguished the main effect.
DISCUSSION
Eimeria spp. oocysts, utilized as a spray vaccine on day-hatch chicks, are widely distributed in poultry litter (Chapman et al., 2016). Although broilers in this study were reared on the used litter which contained oocysts to simulate the commercial condition, fecal oocyte counts of different dietary groups were statistically similar ahead of the extra challenge. A high dose of live Eimeria vaccine (20×) was given to half birds to exaggerate coccidial burden at 10 d of age. Then, a higher oocyst output, a depressed growth rate, and a lower carcass yield were observed in those extra-challenged broilers at 18 d of age. These results agreed with previous studies (Abdelhady et al., 2021; Nawarathne et al., 2022). Furthermore, the current study indicated that the coccidial challenge at an early age has a detrimental effect on meat quality at a later age (color, pH, protein content, cook loss). The thigh muscle of challenged broilers exhibited a lower pH and crude protein content and a higher cook loss. The low pH might cause protein denaturation and subsequently resulted in a poor water-holding capacity (Qaid et al., 2022). Mir et al. (2017) revealed that a low pH interfered with the space between myosin and actin filaments, which results in a low capacity to bind water. Qaid et al. (2022) also reported that the Eimeria challenge reduces the lightness (L*) of tender meat (pectoralis minor) in broilers. The poor meat quality was also reported in a previous Salmonella challenge study (Al-Owaimer et al., 2014), Clostridium perfringens studies (Hussein et al., 2020; Nicholds et al., 2021), and heat stress studies (Chen et al., 2022; Liao et al., 2022). Oxidative stress could impair meat quality by causing mitochondrial dysfunction (Chen et al., 2022), which has strong implications for the development of pale-soft-exudative meat (Liao et al., 2022). From the above research, factors in the broiler production system which contribute to birds’ oxidative stress and welfare, such as stress, disease, stock density, and fiber-enriched diet, would detrimentally impact meat quality (Marchewka et al., 2023).
The control of avian coccidiosis mainly rotates between vaccinations and anticoccidial drugs (Williams, 2002; Quiroz-Castaneda and Dantan-Gonzalez, 2015). However, coccidial vaccination caused an irreversible side effect on the growth of broilers in the early period, which might hinder intestine development and function (Chapman et al., 2005). The prolonged use of dietary anticoccidials resulted in the wide spread of drug-resistant Eimeria spp. (Xie et al., 2020; Zhang et al., 2022a). Therefore, essential oil and its bioactive components, mainly extracted from natural herbs, have gained more attention to defend against the negative effects caused by avian coccidiosis in recent studies (Betancourt et al., 2019; Sidiropoulou et al., 2020; Gordillo-Jaramillo et al., 2021; Youssefi et al., 2023).
Our data indicated that both oregano oil and clove oil improved broiler growth performance including weigh gain and feed conversion ability, which was consistent with previous studies (Nawarathne et al., 2022; Zaazaa et al., 2022; Youssefi et al., 2023). Although broilers are considered to have less sense of taste than other mammalian production animals due to the shortage of sweet sensing gene T1R2 (Shi and Zhang, 2006), essential oils could improve broiler nutrient utilization through other mechanisms. Bozkurt et al. (2016) reported that broilers fed an oregano oil diet had a higher activity of amylase and lipase in the intestine than those fed a basal diet. Additionally, supplementation of essential oils could modulate intestinal microbiota in poultry, inhibit the growth of specific bacterial pathogens, save immune costs, and drive nutrients and energy toward meat production (Seidavi et al., 2022). Furthermore, our results indicated that essential oil supplementation enhanced the tight junction protein of gut epithelial cells which may help birds maintain a functional nutrient digestion and absorption process under different stress or disease challenge.
Although dietary inclusion of essential oil did not improve the serum antioxidant capacity, they improved meat yield (breast) and quality (thigh). In theory, the phenol ring in essential oil could improve the antioxidant ability by degrading oxyradical in vitro (Bhalla et al., 2013). The improvement of antioxidant capacity was also reported in live broiler trials (Paraskeuas et al., 2017; Zhang et al., 2022b; Youssefi et al., 2023). Nevertheless, the inconsistent effects in serum oxidant capacity were also reported in other trials (Gordillo-Jaramillo et al., 2021; Choi et al., 2022). Serum biochemical indicators could be modified by multiple factors. Even though serum antioxidant capacity was not improved by dietary essential oils in the current studies, improvements in meat yield and quality were observed. The increased breast yields were also reported in cashew nutshell oil and castor oil supplementations (Torrent et al., 2019). Hong et al. (2012) found out broiler meat was tender and juicier when feeding diets supplemented with oregano or anise essential oils. Hernandez-Coronado et al. (2019) studied 2 Mexican oregano essential oils (Poliomintha longiflora Gray PLG and Lippia berlandieri Schauer) through drinking water application and found out both improved broiler meat quality including cook loss, redness, shear force, cohesiveness, and resilience. Lack of response on meat pH, color, cook loss, and drip loss were reported in fennel (Ghiasvand et al., 2021), a commercial mixture of thymol, eugenol, and piperine essential oil (Kim and Park, 2018), and peppermint and menthol supplementations (Abdel-Wareth et al., 2019). The inconsistent effects of essential oils on the meat quality of broiler chicken could be attributed to differences in effective ingredients, concentration, and combination of essential oils used, as well as the mode of administration and duration of treatment. Moreover, differences in the genetic background, feed type, and rearing conditions of broiler chickens could also influence the response to essential oils.
Tight junctions play a crucial role in maintaining the gut barrier by preventing the leakage of solutes and water between epithelial cells. Our data also indicated that the dietary inclusion of essential oil enhanced tight junction between enterocytes, especially in Eimeria-challenged broilers. Oregano oil supplementation upregulated the mRNA expression of intestinal barrier gene ZO-1 and Claudin-1 in the duodenum and Claudin-1 in the ileum of Eimeria-challenged broilers, which was consistent with the findings from Ruan et al. (2021). The upregulation of tight junction in the epithelial barrier was also reported in colorectal cancer mice model (Leong et al., 2022), female pigs (Hofmann et al., 2022), Escherichia coli-challenged mice (Zhao et al., 2022), and colitis mice (Raka et al., 2023). It is remarkable how essential oil interacts with the monolayer of enterocytes, probably due to their antioxidant capacity (Toschi et al., 2022). Intestinal oxidative stress plays a pivotal role in triggering intestine barrier breakdown (Wang et al., 2020). By neutralizing these free radicals caused by disease or stress, essential oils could maintain the function of tight junctions between enterocytes. Furthermore, transcriptome analysis and Western blot results indicated that a rose essential oil could ameliorate intestinal barrier dysfunction via a TLR4-NF-kB signaling pathway (Raka et al., 2023). Therefore, with benefits in preserving gut integrity and tight junctions, essential oils could be considered candidates to protect against inflammation and restore gut permeability damaged by Eimeria.
CONCLUSIONS
In summary, the Eimeria challenge in broilers at an early age could interrupt their antioxidant capacity and damage intestine integrity and meat quality at a later age. The dietary supplementation of clove or oregano essential oils could improve broilers performance and partially relieve the coccidial damage in gut integrity and meat quality. The findings of this study shed light on the dietary supplementation of essential oils as anticoccidial drug substitute to improve the performance and meat quality of broilers under Eimeria challenge.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (32102591), the Natural Science Foundation of Sichuan Province (2022NSFSC1745), and the Fundamental Research Funds for the Central Universities of Southwest Minzu University (2021PTJS26). Additionally, we appreciate the donation of essential oils from the DDC Company (DadHank Chengdu Biotech Corp., Chengdu, China).
DISCLOSURES
The authors declare that they have no conflicts of interest.
REFERENCES
- Abdelhady A.Y., El-Safty S.A., Hashim M., Ibrahim M.A., Mohammed F.F., Elbaz A.M., Abdel-Moneim A.E. Comparative evaluation of single or combined anticoccidials on performance, antioxidant status, immune response, and intestinal architecture of broiler chickens challenged with mixed Eimeria species. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Wareth A.A.A., Kehraus S., Sudekum K. Peppermint and its respective active component in diets of broiler chickens: growth performance, viability, economics, meat physicochemical properties, and carcass characteristics. Poult. Sci. 2019;98:3850–3859. doi: 10.3382/ps/pez099. [DOI] [PubMed] [Google Scholar]
- Al-Owaimer A.N., Suliman G.M., Alyemni A.H., Abudabos A.M. Effect of different probiotics on breast quality characteristics of broilers under Salmonella challenge. Ital. J. Anim. Sci. 2014;13:450–454. [Google Scholar]
- AOAC (Association of Official Analytical Chemists) 21st ed. Association of Official Analytical Chemists; Washington, DC: 2019. Official Methods of Analyses. [Google Scholar]
- Arabkhazaeli F., Modrisanei M., Nabian S., Mansoori B., Madani A. Evaluating the resistance of Eimeria spp. field isolates to anticoccidial drugs using three different indices. Iran. J. Parasitol. 2013;8:234–241. [PMC free article] [PubMed] [Google Scholar]
- Arbor Acres. 2018. Arbor Acres Broiler Management Handbook. Accessed Apr. 2023. http://eu.aviagen.com/assets/Tech_Center/AA_Broiler/AA-BroilerHandbook2018-EN.pdf.
- Aviagen. 2022. Arbor Acres Broiler Nutrition Specification. Accessed Apr. 2023. https://ap.aviagen.com/assets/Tech_Center/AA_Broiler/AA-BroilerNutritionSpecifications2022-EN.pdf.
- Bafundo K.W., Cervantes H.M., Mathis G.F. Sensitivity of Eimeria field isolates in the United States: responses of nicarbazin-containing anticoccidials. Poult. Sci. 2008;87:1760–1767. doi: 10.3382/ps.2008-00129. [DOI] [PubMed] [Google Scholar]
- Betancourt L., Hume M., Rodríguez F., Nisbet D., Sohail M.U., Afanador-Tellez G. Effects of Colombian oregano essential oil (Lippia origanoides Kunth) and Eimeria species on broiler production and cecal microbiota. Poult. Sci. 2019;98:4777–4786. doi: 10.3382/ps/pez193. [DOI] [PubMed] [Google Scholar]
- Bezerra D.P., Militao G.C.G., de Morais M.C., de Sousa D.P. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients. 2017;9:1367. doi: 10.3390/nu9121367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla Y., Gupta V.K., Jaitak V. Anticancer activity of essential oils: a review. J. Sci. Food Agric. 2013;93:3643–3653. doi: 10.1002/jsfa.6267. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Ayoade S., Gilbert W., Adebambo A.O., Jatau I.D., Raman M., Parker D., Rushton J., Tomley F.M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:115–129. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt M., Ege G., Aysul N., Akşit H., Tuzun A.E., Kucukyılmaz K., Borum A.E., Uygun M., Aksit D., Aypak S., Şimsek E., Seyrek K., Kocer B., Bintas E., Orojpour A. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 2016;95:1858–1868. doi: 10.3382/ps/pew077. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Hafeez M.A., Matsler P., Rathinam T., Raccoursier M. The epizootiology of Eimeria infections in commercial broiler chickens where anticoccidial drug programs were employed in six successive flocks to control coccidiosis. Poult. Sci. 2016;95:1774–1778. doi: 10.3382/ps/pew091. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Roberts B., Shirley M.W., Williams R.B. Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathol. 2005;34:279–290. doi: 10.1080/03079450500178378. [DOI] [PubMed] [Google Scholar]
- Chen Z., Xing T., Li J., Zhang L., Jiang Y., Gao F. Oxidative stress impairs the meat quality of broiler by damaging mitochondrial function, affecting calcium metabolism and leading to ferroptosis. Anim. Biosci. 2022;35:1616–1627. doi: 10.5713/ab.22.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Liu Z., Zhou Y., Wei H., Zhang X., Xia M., Deng Z., Zou Y., Jiang S., Peng J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017;33:103–109. doi: 10.1016/j.meatsci.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Choi J., Singh A.K., Chen X., Lv J., Kim W.K. Application of organic acids and essential oils as alternatives to antibiotic growth promoters in broiler chickens. Animals. 2022;12:2178–2187. doi: 10.3390/ani12172178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feed Database in China Tables of feed composition and nutritive values in China. 30th rev. ed. China Feed. 2019;22:111–116. [Google Scholar]
- Ghiasvand A.R., Khatibjoo A., Mohammadi Y., Gharaei M.Akbari, Shirzadi H. Effect of fennel essential oil on performance, serum biochemistry, immunity, ileum morphology and microbial population, and meat quality of broiler chickens fed corn or wheat-based diet. Br. Poult. Sci. 2021;62:562–572. doi: 10.1080/00071668.2021.1883551. [DOI] [PubMed] [Google Scholar]
- Gordillo-Jaramillo F.X., Kim D., Lee S.H., Kwon S., Jha R., Lee K. Role of oregano and citrus species-based essential oil preparation for the control of coccidiosis in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:47–55. doi: 10.1186/s40104-021-00569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Hu W., Chen T., Guo H., Zhu J., Chen F. Anticoccidial activity of natural plants extracts mixture against Eimeria tenella: an in vitro and in vivo study. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.1066543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Coronado A.C., Silva-Vazquez R., Rangel-Nava Z.E., Hernandez-Martinez C.A., Kawas-Garza J.R., Hume M.E., Mendez-Zamora G. Mexican oregano essential oils given in drinking water on performance, carcass traits, and meat quality of broilers. Poult. Sci. 2019;98:3050–3058. doi: 10.3382/ps/pez094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H.H., Heusler K., Roth K., Pröll-Cornelissen M.J., Große-Brinkhaus C., Schellander K., Neuhoff C. Oregano essential oil showed limited effects on pigs' carcass quality and haematology whereas a transcriptome analysis revealed significant modulations in the jejunum and the ileum. J. Anim. Physiol. Anim. Nutr. 2022;106:1017–1035. doi: 10.1111/jpn.13639. [DOI] [PubMed] [Google Scholar]
- Hong J., Steiner T., Aufy A., Lien T. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 2012;144:253–262. [Google Scholar]
- Huang Y., Yang J., Xiao F., Lloyd K., Lin X. Effects of supplemental chromium source and concentration on growth performance, carcass traits, and meat quality of broilers under heat stress conditions. Biol. Trace Elem. Res. 2016;170:216–223. doi: 10.1007/s12011-015-0443-z. [DOI] [PubMed] [Google Scholar]
- Hussein E.O.S., Ahmed S.H., Abudabos A.M., Suliman G.M., Abd El-Hack M.E., Swelum A.A., Alowaimer A.N. Ameliorative effects of antibiotic-, probiotic- and phytobiotic-supplemented diets on the performance, intestinal health, carcass traits, and meat quality of Clostridium perfringens-infected broilers. Animals. 2020;10:669. doi: 10.3390/ani10040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I.H., Park J.H. Effects of a protease and essential oils on growth performance, blood cell profiles, nutrient retention, ileal microbiota, excreta gas emission, and breast meat quality in broiler chicks. Poult. Sci. 2018;97:2854–2860. doi: 10.3382/ps/pey151. [DOI] [PubMed] [Google Scholar]
- Kulisic T., Radonic A., Katalinic V., Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. [Google Scholar]
- Lan L., Sun B., Zuo B., Chen X., Du A. Prevalence and drug resistance of avian Eimeria species in broiler chicken farms of Zhejiang province, China. Poult. Sci. 2017;96:2104–2109. doi: 10.3382/ps/pew499. [DOI] [PubMed] [Google Scholar]
- Leong W., Huang G., Liao W., Xia W., Li X., Su Z., Liu L., Wu Q., Wong V.K.W., Law B.Y.K., Xia C., Guo X., Khan I., Wendy Hsiao W.L. Traditional Patchouli essential oil modulates the host's immune responses and gut microbiota and exhibits potent anti-cancer effects in ApcMin/+ mice. Pharmacol. Res. 2022;176 doi: 10.1016/j.phrs.2022.106082. [DOI] [PubMed] [Google Scholar]
- Liao H., Zhang L., Li J., Xing T., Gao F. Acute stress deteriorates breast meat quality of Ross 308 broiler chickens by inducing redox imbalance and mitochondrial dysfunction. J. Anim. Sci. 2022;100:skac221. doi: 10.1093/jas/skac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchewka J., Sztandarski P., Solka M., Louton H., Rath K., Vogt L., Rauch E., Ruijter D., de Jong I.C., Horbanczuk J.O. Linking key husbandry factors to the intrinsic quality of broiler meat. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengistu B.M., Bitsue H.K., Huang K. The effects of selenium-enriched probiotics on growth performance, oocysts shedding, intestinal cecal lesion scores, antioxidant capacity, and mRNA gene expression in chickens infected with Eimeria tenella. Biol. Trace Elem. Res. 2021;199:278–291. doi: 10.1007/s12011-020-02118-7. [DOI] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nawarathne S.R., Kim D., Cho H., Hong J., Kim Y., Yu M., Yi Y., Lee H., Wan V., Ng N.K.J., Tan C.H., Heo J. Combinatorial effect of dietary oregano extracts and 3,4,5-trihydroxy benzoic acid on growth performance and elimination of coccidiosis in broiler chickens. J. Poult. Sci. 2022;59:233–246. doi: 10.2141/jpsa.0210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholds J.F., McQuain C., Hofacre C.L., Mathis G.F., Fuller A.L., Telg B.E., Montoya A.F., Williams S.M., Berghaus R.D., Jones M.K. The effect of different species of Eimeria with Clostridium perfringens on performance parameters and induction of clinical necrotic enteritis in broiler chickens. Avian Dis. 2021;65:132–137. doi: 10.1637/aviandiseases-D-20-00106. [DOI] [PubMed] [Google Scholar]
- Paraskeuas V., Fegeros K., Palamidi I., Hunger C., Mountzouris K.C. Growth performance, nutrient digestibility, antioxidant capacity, blood biochemical biomarkers and cytokines expression in broiler chickens fed different phytogenic levels. Anim. Nutr. 2017;3:114–120. doi: 10.1016/j.aninu.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Qaid M.M., Al-Mufarrej S.I., Azzam M.M., Al-Garadi M.A., Alqhtani A.H., Al-Abdullatif A.A., Hussein E.O., Suliman G.M. Dietary cinnamon bark affects growth performance, carcass characteristics, and breast meat quality in broiler infected with Eimeria tenella oocysts. Animals (Basal) 2022;12:160. doi: 10.3390/ani12020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Castaneda R.E., Dantan-Gonzalez E. Control of avian coccidiosis: future and present natural alternatives. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raka R.N., Xiao J., Wu H., Lv W., Ding Z., Cao Y., Li X., Sun J., Luan K. Pingyin rose essential oil restores intestinal barrier integrity in DSS-induced mice colitis model. Food Res. Int. 2023;164 doi: 10.1016/j.foodres.2022.112362. [DOI] [PubMed] [Google Scholar]
- Remmal A., Achahbar S., Bouddine L., Chami N., Chami F. In vitro destruction of Eimeria oocysts by essential oils. Vet. Parasitol. 2011;182:121–126. doi: 10.1016/j.vetpar.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia I., Silva-Espinoza B.A., Ortega-Ramirez L.A., Leyva J.M., Siddiqui M.W., Cruz-Valenzuela M.R., Gonzalez-Aguilar G.A., Ayala-Zavala J.F. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 2015;56:1717–1727. doi: 10.1080/10408398.2013.800832. [DOI] [PubMed] [Google Scholar]
- Ruan D., Fan Q., Fouad A.M., Sun Y., Huang S., Wu A., Lin C., Kuang Z., Zhang C., Jiang S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021;99:1–11. doi: 10.1093/jas/skab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute . SAS Institute. Inc.; Cary, NC: 2016. SAS Proprietary Software Release 9.4. [Google Scholar]
- Schanda J. John Wiley & Sons; Hoboken, NJ: 2007. Pages 467 in Colorimetry: Understanding the CIE System. [Google Scholar]
- Seidavi A., Tavakoli M., Asroosh F., Scanes C.G., Abd El-Hack M.E., Naiel M.A.E., Taha A.E., Aleya L., El-Tarabily K.A., Swelum A.A. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ. Sci. Pollut. Res. 2022;29:5006–5031. doi: 10.1007/s11356-021-17401-w. [DOI] [PubMed] [Google Scholar]
- Shi P., Zhang J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol. Biol. Evol. 2006;23:292–300. doi: 10.1093/molbev/msj028. [DOI] [PubMed] [Google Scholar]
- Sidiropoulou E., Skoufos I., Marugan-Hernandez V., Giannenas I., Bonos E., Aguiar-Martins K., Lazari D., Blake D.P., Tzora A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Vet. Sci. 2020;7:2020. doi: 10.3389/fvets.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R.P., Guerin M.T., Hargis B.M., Kruth P.S., Page G., Rejman E., Rotolo J.L., Sears W., Zeldenrust E.G., Whale J., Barta J.R. Restoration of anticoccidial sensitivity to a commercial broiler chicken facility in Canada. Poult. Sci. 2021;100:663–674. doi: 10.1016/j.psj.2020.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanello C., Rosa D.P., Dalmoro Y.K., Segatto A.L., Vievira M.S., Moraes M.L., Santin E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci. 2020;6:491. doi: 10.3389/fvets.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrent J., Arce-Menocal J., Lopez-Coello C., Avila-Gonzalez E. Effects of functional oils on performance and carcass characteristics of broilers under two different temperature environments. Poult. Sci. 2019;98:5855–5861. doi: 10.3382/ps/pez235. [DOI] [PubMed] [Google Scholar]
- Toschi A., Piva A., Grilli E. Phenol-rich botanicals modulate oxidative stress and epithelial integrity in intestinal epithelial cells. Animals (Basel) 2022;12:2188. doi: 10.3390/ani12172188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen Y., Zhang X., Lu Y., Chen H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: a review. J. Funct. Food. 2020;75 [Google Scholar]
- Wang Y.M., Kong L.C., Liu J., Ma H.X. Synergistic effect of eugenol with colistin against clinical isolated colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control. 2018;7:17. doi: 10.1186/s13756-018-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei A., Shibamoto T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010;58:7218–7225. doi: 10.1021/jf101077s. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 2002;31:317–353. doi: 10.1080/03079450220148988. [DOI] [PubMed] [Google Scholar]
- Xie Y., Huang B., Xu L., Zhao Q., Zhu S., Zhao H., Dong H., Han H. Comparative transcriptome analyses of drug-sensitive and drug-resistant strains of Eimeria tenella by RNA-sequencing. J. Eukaryot. Microbiol. 2020;67:406–416. doi: 10.1111/jeu.12790. [DOI] [PubMed] [Google Scholar]
- Youssefi M.R., Alipour R., Fakouri Z., Shahavi M.H., Nasrabadi N.T., Tabari M.A., Crescenzo G., Zizzadoro C., Centoducati G. Dietary supplementation with eugenol nanoemulsion alleviates the negative effects of experimental coccidiosis on broiler chicken's health and growth performance. Molecules. 2023;28:2200–2216. doi: 10.3390/molecules28052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaazaa A., Mudalal S., Alzuheir I., Samara M., Jalboush N., Fayyad A., Petracci M. The impact of thyme and oregano essential oils dietary supplementation on broiler health, growth performance, and prevalence of growth-related breast muscle abnormalities. Animals (Basal) 2022;12:3065–3075. doi: 10.3390/ani12213065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Li W., Chen L., Chen Z., Wang X., Xu Q., Zhang H., Chen H., Liu J. Oregano oil combined with Macleaya cordata oral solution improves the growth performance and immune response of broilers. Animals (Basal) 2022;12:2480–2491. doi: 10.3390/ani12182480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.Y., Peng Q.Y., Liu Y.R., Ma Q.G., Zhang J.Y., Guo Y.P., Xue Z., Zhao L.H. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang L., Si H., Liu X., Suo X., Hu D. Early transcriptional response to monensin in sensitive and resistant strains of Eimeria tenella. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.934153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhang Z., Nie D., Li Y. Protective effect of lemon essential oil and its major active component, D-limonene, on intestinal injury and inflammation of E. coli-challenged mice. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.843096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Xiao G., Yan X., Qiu T., Liu S., Ou J., Cen M., Gong L., Shi J., Zhang H. Complex of lauric acid monoglyceride and cinnamaldehyde ameliorated subclinicl necrotic enteritidis in yellow-feathered broilers by regulation gut morphology, barrier, inflammation and serum biochemistry. Animals (Basal) 2023;13:516. doi: 10.3390/ani13030516. [DOI] [PMC free article] [PubMed] [Google Scholar]