Highlights
-
•
Stroke is the major cause of long-term disability; current therapies lack efficacy.
-
•
γ-aminobutyric acid (GABA) appears to play an important role in stroke recovery.
-
•
MRS, TMS & TMS-EEG have complementary translatability to measure GABA mechanisms.
-
•
Understanding GABA post-stroke roles could contribute “pro-plastic” interventions.
-
•
Determining how GABA is implicated in stroke recovery will impact patients’ care.
Keywords: GABA, Stroke recovery, TMS, MRS, EEG
Abstract
Stroke is a major cause of death and chronic neurological disability. Despite the improvements in stroke care, the number of patients affected by stroke keeps increasing and many stroke survivors are left permanently disabled. Current therapies are limited in efficacy. Understanding the neurobiological mechanisms underlying post-stroke recovery is therefore crucial to find new therapeutic options to address this medical burden. Long-lasting and widespread alterations of γ-aminobutyric acid (GABA) neurotransmission seem to play a key role in stroke recovery. In this review we first discuss a possible model of GABAergic modulation of post-stroke plasticity. We then overview the techniques currently available to non-invasively assess GABA in patients and the conclusions drawn from this limited body of work. Finally, we address the remaining open questions to clarify GABAergic changes underlying post-stroke recovery, we briefly review possible ways to modulate GABA post stroke and propose a novel approach to thoroughly quantify GABA in stroke patients, by integrating its concentration, the activity of its receptors and its link with microstructural changes.
1. Introduction
With more than 13 million cases worldwide annually, stroke is a major cause of death and chronic neurological disability (Feigin et al., 2021). Ischemic stroke accounts for about 85% of cases (Sedova et al., 2021). Despite improvements in prevention, control of risk factors and acute stroke care, the number of strokes almost doubled in the past years (Feigin et al., 2014), with an exponential increase in stroke incidence among young adults (Lindsay et al., 2019).
Stroke often disrupts motor systems, leaving half of stroke survivors permanently disabled and a third of them dependent on others for normal daily activities (Lawrence et al., 2001). To date, physical therapy remains the gold standard intervention to minimize functional disability (Hubbard et al., 2009). Several approaches have been shown to reduce patients’ deficits: robotic and mirror therapy ((Mehrholz et al.,) Thieme et al., 2012), constraint-induced movement therapy (Smania et al., 2012), electro-mechanical gait training (Mehrholz et al., 2020), treadmill training (Mehrholz et al., 2017), and brain stimulation (eg., Transcranial Magnetic Stimulation -TMS-, Grefkes & Fink, 2020; transcranial Direct Current Stimulation -tDCS-,Hordacre et al., 2021). Unfortunately, access to rehabilitation centers and stroke expert therapists is limited and expensive (Teasell et al., 2020). Physical protocols typically require extensive repetitions of motor exercises, which can be unengaging and tiring, jeopardizing compliance (Ward et al., 2019). Additionally, it is still debated whether these protocols improve motor recovery above what is expected due to spontaneous biological recovery mechanisms alone (Stinear et al., 2020). Therefore, there is a pressing need to develop adjunct therapies to promote brain plasticity and enhance the efficacy of physical therapy. To do so, the neurobiological mechanisms underlying post-stroke recovery must be thoroughly understood.
Ischemic stroke results from a reduction of blood supply, which initiates a cascade of metabolic failure, excitotoxicity and neuroinflammation, and eventually causes irreversible tissue damages (Doyle et al., 2008). While neurons die within minutes from lack of oxygen and nutrients in the ischemic region, connected regions can undergo various degrees of axonal degeneration, synapse loss and neuronal dysfunction (Baron et al., 2014). Once the immediate damage has been done, this acute phase is followed by a window of heightened neuroplasticity: the subacute recovery phase, which is typically said to last about 3 months (Bernhardt et al., 2017). During this period, endogenous processes including axonal sprouting and spine morphogenesis mediate the structural and functional changes underlying sensory and motor remapping (Nishibe et al., 2015). The plastic processes of the subacute phase progressively decline, leaving the recovery rate of the chronic phase about 10% of what is observed during the subacute phase (Murphy and Corbett, 2009, Wolf et al., 2010). In addition, there are critical differences between the nature of functional improvements in the sub-acute and chronic phases: true “recovery”, as opposed to adaption and functional improvements, can only be said to occur in the subacute phase (Krakauer & Carmichael, 2022). Interventions aimed at widening and lengthening the spontaneous plasticity marking the subacute phase are therefore foreseen to optimize post-stroke recovery.
A better understanding of the mechanisms underlying the spontaneous plasticity observed in the subacute phase is a prerequisite to tailor restorative therapies to the individual patient. Long-lasting and widespread alterations of inhibitory neurotransmission after stroke seems to be one crucial potential mechanism (Redecker et al., 2002). Indeed, a decrease in γ-aminobutyric acid (GABA) promotes neuroplasticity both in humans (Ziemann et al., 2001) and animals (Hess et al., 1996), and stimulates long-term potentiation, a cellular process of plasticity and learning (Hagemann et al., 1998).
In this review we first discuss a possible model of GABAergic modulation of post-stroke plasticity in the subacute phase. We then overview the techniques currently available to assess non-invasively GABA in patients and briefly review the conclusions drawn from this limited body of work as well as possible ways to modulate GABA post stroke. Finally, we address the remaining open questions to clarify GABAergic changes underlying post-stroke recovery and propose a novel approach to thoroughly quantify GABA in stroke patients, by integrating its sheer amount, the activity of its receptors and its link with microstructural changes.
2. Neurobiological basis of GABAergic modulation in post-stroke neuroplasticity
The brain has a remarkable capacity to reorganize its circuits following an intrinsic or extrinsic perturbation (Cramer et al., 2011). This neuroplasticity is expressed at various levels (e.g., cell, brain networks, behavior) throughout the lifespan (Murphy & Corbett, 2009). It is maximal during development (Hensch, 2005) but is also enhanced during the subacute phase of stroke. This post-stroke sensitive period (Dromerick et al., 2021) is associated with the expression of genes and proteins implicated in neuronal growth, synaptogenesis, and proliferation of dendritic spines (Hattiangady et al., 2005, Cramer and Chopp, 2000). Stroke animal models have suggested that the presence of critical periods and training-dependent effects after stroke might indicate that synapse-based learning rules, involved both in wiring and refining brain connections, are implicated in stroke recovery (Song et al., 2000). These learning rules are mainly mediated by Hebbian (Hebb, 1949b) and homeostatic (Turrigiano & Nelson, 2004) plastic mechanisms. In the next two sections we discuss the evidence from animal studies for these two forms of plasticity, highlighting the role played by GABA in neuroplastic phenomena.
2.1. Hebbian plasticity
Classical Hebbian plasticity arises from repeated coordinated firing of two connected neurons and results in the strengthening (long-term potentiation) or weakening (long-term depression) of the neurotransmission (Hebb, 1949a). Spike timing across synapses also conveys critical information as the order of coincident spikes can either strengthen or weaken a given synapse (a phenomenon referred to as spike timing dependent plasticity, Caporale & Dan, 2008). Both forms of synaptic plasticity result in long-lasting functional and structural changes which mediates task-relevant shaping of neuronal population activity (for more detail see previous reviews, e.g., Magee & Grienberger, 2020).
GABAergic modulation appears to be important in regulating synaptic plasticity and has thus been hypothesized to have a role in regulating many stages of memory formation and consolidation. GABAergic interneurons seem to set the temporal coincidence requirement for plasticity and integrate spatial context switching principal cells between plastic and non-plastic mode of operation (for a complete review on the role of inhibitory circuits in memory see Topolnik & Tamboli, 2022). Fast spiking parvalbumin + GABAergic interneurons are involved in certain forms of learning (Hu et al., 2014): suppression of these interneurons is necessary for visual plasticity (Kuhlman et al., 2013), associative fear learning (Wolff et al., 2014) and reward seeking behavior (Sparta et al., 2014). Moreover, GABAergic circuits have also been shown to shape spike-timing-dependent plasticity and operate as Hebbian/anti-Hebbian switch (Paille et al., 2013). GABA has therefore been proposed as a third factor acting on the classical two-factor Hebbian learning equation, where the two factors index the presynaptic and postsynaptic activities. This third factor may gate Hebbian plasticity by providing a global information on how well the whole network is performing (Kuśmierz et al., 2017).
Animal studies suggest that stroke rehabilitation relies on Hebbian plasticity (Plautz et al., 2003). Following stroke, surviving circuits with sufficient drive to produce coherent pre- and postsynaptic paired action potentials, and thus presumed behaviorally relevant, are strengthened (via Long Term Potentiation [LTP]). There is also some evidence that asynchronously active synapses are weakened (via Long Term Depression [LTD], Murphy & Corbett, 2009). However, complex systems based only on Hebbian mechanisms are intrinsically unstable. Indeed, Hebbian learning exhibits a positive feedback instability that could cause pathological runaway dynamics of neural activity. First, continuous synaptic potentiation would cause synaptic saturation, loss of selectivity and excess of excitability (Rochester et al., 1956). Second, spurious correlation between pre and postsynaptic firing would cause maladaptive Hebbian plasticity (Sejnowski, 1977). Therefore, Hebbian plasticity likely requires stabilization by additional compensatory processes. A diversity of homeostatic plasticity phenomena found in neural circuits could play this role.
2.2. Homeostatic plasticity
Homeostatic plasticity serves as a stabilizing mechanism that prevents neural circuits from becoming hyper- or hypoactive. It can broadly be defined as neuronal changes that optimize neuronal activity to sense and adapt to a changing environment: while neurons forced to rest become hyperexcitable, those potentiated by intense synaptic activity have to reduce their excitability (Nelson & Turrigiano, 2008). These adjustments are achieved by a combination of synaptic and intrinsic homeostatic phenomena that act on the balance between excitatory and inhibitory inputs (Turrigiano et al., 1998) or between inward/outward voltage-dependent current (Turrigiano et al., 1994), respectively.
Synaptic scaling is a global, cell-dependent process favoring synaptic homeostasis (Turrigiano, 2008) that allows neurons to detect changes in their own firing/depolarization rate and adjust it to a target value (Gainey et al., 2009). The same paradigm that scales up excitatory postsynaptic currents, scales down inhibitory ones via a combination of various changes in the accumulation of postsynaptic GABAA receptors, synapse number and GABA packaging and release (Hartman et al., 2006, Kilman et al., 2002). However, focusing on synaptic homeostasis only gives us a limited picture of how neural circuits evolve over time. To maintain an appropriate electrical activity, neurons need to alter their intrinsic electrical proprieties on top of regulating the strengths of their synaptic connections (Desai, 2003). Blocking GABAA receptors has been shown to dramatically impact intrinsic plasticity in cerebellar granule cells: firing frequencies increase and spike threshold decreases, indicating that GABA-mediated tonic inhibition may control the cell's excitability (Brickley et al., 2001).
Synaptic homeostasis adjusts the gain of the input thus modifying the excitation/inhibition balance. By contrast, intrinsic homeostasis modifies the contribution of a neuron to circuit function by changing the inward/outward current densities, enhancing firing, and promoting structural changes (e.g., in the axon initial segment). The interplay between synaptic and intrinsic plasticity is still debated although recent computational models have shown the efficacy of echo state networks with a synergistic plasticity learning rule, which simultaneously consider the regulation of synaptic weights and the adjustment of neuronal intrinsic excitability (Wang et al., 2021). Following network disruption, synaptic mechanisms might come first into play with intrinsic processes triggered in a second step (Turrigiano, 2011) to maintain synaptic strength in a functional range.
Normal pathways of synaptic activity are altered or interrupted after stroke both in the peri-infarct area (Carmichael et al., 2004) and in distant functionally related structures (Bütefisch et al., 2003). Homeostatic plasticity might promote a series of changes to restore synaptic activity and keep the network activity at a desired stable set point, by modulating synaptic homeostasis. Such changes could be suggested by the hyperexcitability observed throughout the subacute phase (Winship & Murphy, 2008), which results from a decrease in inhibition and altered intrinsic electrophysiological properties of the neurons (Schiene et al., 1996). More than just regulating synaptic activity, homeostatic mechanisms could also favor structural changes by increasing dendritic spine production, axonal sprouting, and the unmasking of horizontal connections in the peri-infarct area, which would compensate for lost circuits and help to reinstate the post-stroke synaptic activity to a certain set point (Stroemer et al., 1995). The interplay between Hebbian and homeostatic synaptic plasticity after stroke, however, is still under debate.
2.3. A possible model of stroke recovery and the role of GABA
Whether Hebbian and homeostatic mechanisms cooperate or compete in normal brain plasticity (Galanis & Vlachos, 2020), let alone in pathological brain conditions such as stroke, remains unsettled.
During the subacute phase of stroke, homeostatic mechanisms might initially lead to a transient loss of inhibition and the proliferation of new connections (Winship & Murphy, 2008) in such a way that the activity of surviving neurons is reset to the pre-stroke input (Murphy & Corbett, 2009). Cortical hyperexcitability, resulted by a relatively reduced inhibition and an increase in glutamate transmission, might facilitate the transmission of subthreshold inputs after stroke (Schiene et al., 1996), in particular in the peri-infarct cortex (Michalettos & Ruscher, 2022). Simultaneously, growth promoting genes might be upregulated (Carmichael, 2006), and create a favorable environment for axonal sprouting (Stroemer et al., 1995), synaptogenesis (Brown et al., 2007), and the unmasking of latent horizontal connections (Carmichael et al., 2001). This initial proliferation of new connections might restore neuronal circuitry and mediate sensory-motor remapping (Winship & Murphy, 2009). The increase in the excitation/inhibition ratio characterizing the subacute phase of stroke might be reminiscent of the environment observed during neurodevelopment when plasticity is maximal. The resulting novel connections might be secondarily refined by Hebbian-like mechanisms which strengthens and retains properly wired connections (behaviorally relevant ones) (Fig. 1). Later on, in the chronic phase, inhibition drive might thrive again as the potential for spontaneous recovery declines (Grigoras & Stagg, 2021) (for a complete review on the possible mechanisms favoring post-stroke plasticity see Murphy & Corbett, 2009).
Fig. 1.
Possible model of stroke recovery. Before the stroke, the human cortex has a normal network structure. Neurons fire in response to inputs and are interconnected. Connected neurons are here represented with the same color. During the first week after stroke, in the acute phase, neurons in the core stroke areas die, while the surviving neurons in the peri-lesional area undergo dendritic spines loss and connections disruption. This phase is characterized by oxidative stress, neuroinflammation and a glutamate-mediated excitotoxicity. An increase in inhibition might be necessary to quickly reverse this excitotoxicity and avoid irrevocable damages. Once the neurons survive the acute phase, homeostatic mechanisms might lead to a shift in the excitation/inhibition balance (reduced inhibition and increased excitation) to support neural repair and neuroplasticity. The promoted circuit excitability and the upregulation of growth promoting genes might favor axonal sprouting, synaptogenesis, and the unmasking of latent horizontal connections (early phase). This hyperexcitability and continuous response to external inputs might lead to coincidental presynaptic and postsynaptic activity. The resulting novel connections might be secondarily pruned by Hebbian-like mechanisms which optimize the restored neural circuits in response to relearning skills (late phase). The figure was created with BioRender.com.BioRender.com
Overall, animal evidence suggests a critical role of GABA in regulating the post-stroke plasticity. Nevertheless, the extent to which this hypothesis applies to humans remains undetermined.
3. Quantifying GABA neurotransmission in the human brain
Assessing how GABA affects neural repair and recovery during the subacute phase of stroke in human needs complementary non-invasive techniques: molecular imaging and clinical neurophysiology (Fig. 2).
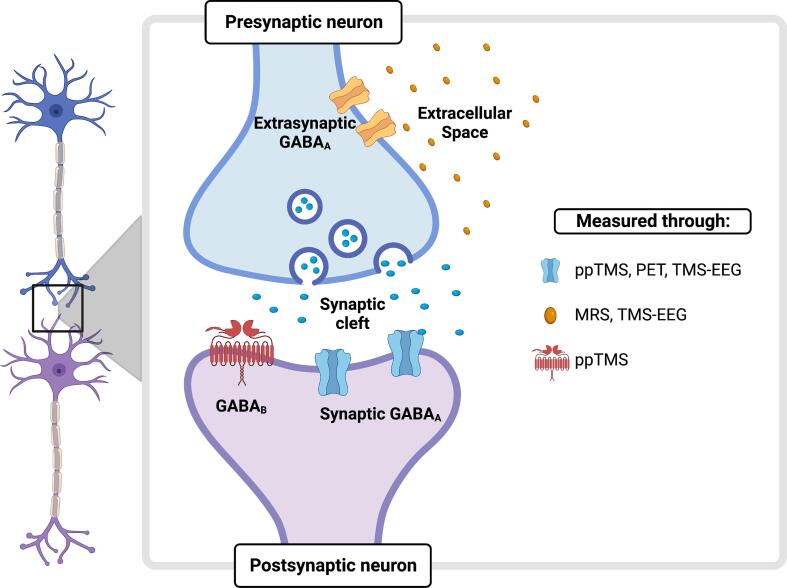
Fig. 2.
Integrated assessment of GABA in the human brain. A synaptic connection is zoomed in with the presynaptic and the postsynaptic terminal represented in light blue and violet, respectively. GABA is synthesized transported, stored, and released in the synaptic cleft. GABA inhibitory effects result from an interaction of a fast (phasic) and a slow (tonic) inhibition. Synaptically released GABA mediates the phasic effect through binding to two main classes of receptors: ionotropic fast-acting ligand-gated chloride GABAA receptors, or metabotropic GABAB receptors. For the purpose of this review, here we focus on GABAA receptors only. GABA spillover activates instead extrasynaptic GABAA receptors, which sense the GABA level in the extracellular space and modulate tonic inhibition. Magnetic Resonance Spectroscopy (MRS) measures non-invasively free extracellular GABA. However, by using MRS alone, one would picture just a part of the complex GABA dynamics. Other electrophysiological techniques, able to probe synaptic GABA activity, would therefore be ideal to complement the MRS measure. Two techniques might assess non-invasively in humans the activity of synaptic GABAA receptors: Positron emission tomography (PET) and Transcranial Magnetic Stimulation (TMS) both when coupled with electromyography (in paired-pulse, ppTMS, protocols) and with electroencephalography (TMS-EEG). PET can provide a direct quantification of GABAA activity by measuring the emissions from radioactive tracers specifically targeting GABAA receptors. ppTMS, instead, detects synaptic GABAA activity by measuring the reduction of a motor evoked potential after activating cortical interneurons. TMS-EEG, instead, provides a measure of synaptic GABAA activity by either measuring the late peaks of the TMS-evoked potential (TEP), known to be related to GABA function, or by modeling the TEP with a Neural Mass Model (NMM) which provides a mesoscopic description of GABAA receptor density (see Fig. 3 for a graphical representation of the NMM). MEG and fMRI are not represented in the figure as they provide indirect measures of GABA. The figure was created with BioRender.com.BioRender.com
3.1. Magnetic Resonance spectroscopy (MRS)
Proton MRS (H-MRS) provides a reliable quantification of neurochemicals, including GABA and the main excitatory neurotransmitter glutamate, usually in a discrete region of the brain, e.g., the primary motor cortex (M1) (Stagg & Rothman, 2013). MRS is one of the most widely used methods to study neuroplastic GABAergic changes in humans. GABA concentration has been shown to decrease in situations thought to elicit LTP-like plasticity, such as learning (Floyer-Lea et al., 2006) and tDCS (Stagg et al., 2009), and to closely correlate with the individual learning potential (Sumner et al., 2010, Stagg et al., 2011a). The few studies using MRS to investigate GABA alterations post-stroke revealed a decreased GABA concentration in chronic patients undergoing constraint-induced motor therapy (Blicher et al., 2015, Głodzik-Sobańska et al., 2004), and a significant lower GABA concentration in the contralateral primary motor cortex compared to healthy age-matched controls (Cirillo et al., 2020). In keeping with animal studies, these results might provide a twofold description of GABAergic role in plasticity: a low initial GABA concentration may indicate a high potential for cortical plasticity, while a further decrease in GABA may result from subsequent learning. Longitudinal studies are very difficult, but quantifying GABA throughout the acute, subacute, and chronic phases would be compelling to characterize GABA dynamics after stroke and determine their potential to predict individual recovery.
In spite of being a promising technique, one limitation of MRS is its current inability to distinguish between the various functional GABAergic pools (cytoplasmic, vesicular, free extracellular), which are believed to play different role in metabolism, synaptic neurotransmission (Martin & Rimvall, 1993), and cortical inhibition (Belelli et al., 2009). However, neural models recently provided a mechanistic account of the MRS-derived metrics: MRS seems to detect GABAergic tonic and neuromodulatory role in cortical inhibition by measuring free extracellular GABA acting on extrasynaptic GABAA receptors (Lea-carnall et al., 2021). Clearly, complementary electrophysiological techniques that probe specific aspects of GABAergic signaling (with a particular focus on GABAA activity), are needed.
Other MRI-based methods, particularly chemical exchange saturation transfer (CEST), can map endogenous molecules important for brain metabolism and function. Relative to MRS, CEST has the advantage of collecting data at the whole-brain level both faster and with higher spatial resolution (Wu et al., 2016). However, MRS can quantify multiple compounds simultaneously, while CEST can typically only acquire one or two compounds at a time, with higher specificity and spectral resolution (Shaffer Jr et al., 2020). Moreover, the gains in signal-to-noise-ratio and measurement precision at ultra-high field (7 T) MRI (Pradhan et al., 2015) and the recent advances in the field of magnetic resonance spectroscopy imaging (MRSI) might allow the high-resolution 3-D imaging of metabolic profiles over large regions of the brain (Lemke et al., 2015), which confer an enhanced clinical translatability to MRS.
3.2. Transcranial Magnetic stimulation (TMS)
TMS can probe the activity of different neurochemical systems if administered in paired-pulse (ppTMS) protocols. In ppTMS, two pulses are applied over the same scalp location, the first below the intensity needed to elicit a motor evoked potential (MEP) and the second above threshold. The first pulse stimulates the cortical interneurons, thus decreasing the amplitude of the MEP elicited by the second pulse (Stagg et al., 2011b). The activity within different neurotransmitter systems can be assessed by varying the inter-stimulus interval (ISI) between the two paired pulses. Intervals of 1 to 5 ms cause short intracortical inhibition (SICI), which is thought to be a putative measure of GABAA receptors activity (Stagg et al., 2011b), reflecting either synaptic (2–5 ms ISI) or extrasynaptic inhibition (1 ms ISI) (Stagg et al., 2011c). Inhibition can also be modulated by GABAB receptors through an enhancement of extrasynaptic GABAA receptors (Connelly et al., 2013). Postsynaptic GABAB receptor activity can be assessed by long intracortical inhibition (LICI), obtained by increasing the interval between the two TMS pulses of about 100–200 ms (McDonnell et al., 2006). Late cortical disinhibition (LCD), which is observed after LICI, is instead thought to be mediated by presynaptic GABAB receptor activity (Cash et al., 2010).
Several studies have used ppTMS to investigate GABAergic changes after stroke, mainly in the chronic phase. Broadly, they have revealed that SICI acquired at rest is reduced in the ipsilesional motor cortex (Swayne et al., 2008a) and, importantly, is inversely correlated with motor recovery (Talelli et al., 2006). However, during movement preparation, there is a blunting of the normal release of GABA as stroke survivors go to move (Hummel et al., 2009). Taken together this suggests that there is less resting inhibition in the cortex after stroke, but that it does not decrease to allow movements to occur. Likewise, in the subacute phase, GABAA activity (indexed by SICI) has been shown to be significantly reduced causing an overall higher excitation-inhibition ratio in the affected hemisphere (McDonnell & Stinear, 2017). Less conclusive is the interpretation of GABAB changes after stroke. A recent study reported a bi-hemispheric increase in GABAB activity (indexed by LICI) in the subacute phase of stroke (Cirillo et al., 2020) in contradiction to previous studies (Schambra et al., 2015, Swayne et al., 2008).
TMS is dependent on a measurable motor output (such as the MEP), making the use of TMS in stroke patients somewhat limited, as lesions of the corticospinal tract after stroke can impair MEPs. An alternative approach to provide a validated mesoscopic description of inhibitory activity (as indexed by GABAA receptor activity) in stroke patients without relying on a motor output is to concurrently register the brain response to the TMS pulse.
3.3. TMS coupled with electroencephalography (EEG)
A TMS pulse produces a time-locked depolarization of underlying neurons and trans-synaptic activation of local and distal cortical networks. This activity can be recorded by scalp electroencephalograpy (EEG) as deflections in the EEG signal, known as TMS-evoked potential (TEP) (Tremblay et al., 2019). A TEP can be recorded from few milliseconds after the stimulation and lasts around 200 to 300 ms spreading to the homologous contralateral areas around 20 ms later (Ilmoniemi et al., 1997). Pharmacological studies using TMS-EEG have suggested that TEP’s early peaks (such as the positive peak at 30 ms, P30), are likely to reflect excitatory activity, while later peaks (N45, N100), reflect cortical inhibition (Darmani & Ziemann, 2019). Pharmaco-TMS-EEG interventions, despite their recent development, have already provided important insights into the activity of GABAergic receptors. The N45 component of the TEP, for instance, is thought to be mediated by GABAA receptor activity (Darmani et al., 2016, Premoli et al., 2014) as its amplitude is increased by GABAA-mediated drugs (alprazolam, zolpidem and diazepam) only. Moreover, separate inhibitory mechanisms seem to differently affect the production and mediation of oscillatory activity in the human brain, such that early alpha-band synchronization seems to be increased by GABAA-mediated activity and decreased by GABAB-mediated one, whereas both GABAA and GABAB-mediated activity increased late beta-band desynchronization (Premoli et al., 2017).
TMS-EEG allows the study of cortical inhibition/excitation mechanisms also by applying an in-silico Neural Mass Model (NMM) (Friston et al., 2003) to the registered TEP. NMM is part of the Dynamic Causal Modeling (DCM) framework, which has mainly been used to investigate macroscopically the influence of one brain region over another in the context of multi-region network responses. However, recently, advanced versions of these models were applied to single region to describe molecular factors underlying local brain activity (Moran et al., 2011a). NMM models event-related potentials as the response of a network to exogenous inputs, by inferring that neuronal states within a given cortical area comprise up to 4 subpopulations of neurons, namely deep and superficial pyramidal cells, excitatory stellate cells, and inhibitory interneurons, each in a particular cortical layer. These subpopulations exhibit self-inhibition controlling neuronal gain and communicate through excitatory and inhibitory connections: inhibitory neurons, placed in the supragranular layer, receive inputs from excitatory pyramidal cells, which in turn are driven by excitatory spiny cells (For a more detailed explanation please refer to Moran et al., 2011a, (Moran et al., 2013)). The connectivity of each neural subpopulation is summarized in Fig. 3. More recent versions of the NMM include also active currents that describe ligand-gated excitatory and inhibitory ion flow, mediated through fast glutamatergic (α -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, AMPA, and N-methyl-D-aspartate, NMDA) and GABAergic (GABAA) receptors (Moran et al., 2011b). NMM can be applied to model the initial part of the TMS-evoked EEG response (e.g., the first 50 ms) and isolate simultaneously excitation and inhibition drives from different neuronal subpopulation in vivo and non-invasively by using forward models for evoked EEG responses and then fitting these models using a Bayesian inversion scheme (i.e., EEG source reconstruction) (Friston et al., 2003). A mesoscopic description of GABAergic activity can then be provided by either extracting a model-based metric of GABAA receptor function (based on its time constants) or a cell-to-cell connectivity parameter (obtained by calculating the inhibitory connections from inhibitory interneurons to stellate and superficial pyramidal cells) (Chellappa et al., 2016). Interestingly, NMM allows the distinction between inhibitory self-connections and inhibitory projections from interneurons to pyramidal cells, which are thought to mediate tonic background inhibition and phasic inhibition respectively (Adams et al., 2021). Support to the validity of NMM was provided by human studies using pharmacological intervention, sometimes compared to equivalent invasive interventions in animals (Moran et al., 2011, Muthukumaraswamy et al., 2013, Muthukumaraswamy et al., 2015).
Fig. 3.
Graphical representation of the Neural Mass Model (NMM). NMM in Dynamic Causal Modeling decomposes a cortical area into 4 neuronal subpopulations: superficial and deep pyramidal cells (represented in yellow), spiny stellate cells (represented in green) and inhibitory interneurons (represented in light grey). Each subpopulation is assigned to a particular cortical layer and projects to the other subpopulations via excitatory (red) and inhibitory (blue lines) connections and have inhibitory feedback-loops controlling neuronal gain. Furthermore, NMM also provides information on the density and the activity of three mains receptors: AMPA and NMDA, mediating excitatory connections and GABAA mediating inhibitory ones (Moran et al., 2013), therefore represented in red and blue respectively. GABAA, AMPA and NMDA measures are the rate constant of the receptors lumped over the entire circuit. A mesoscopic description of GABAergic activity can then be provided by 1) GABAA receptor density; 2) cell-to-cell connectivity parameter (connections from inhibitory interneurons to stellate and superficial pyramidal cells); 3) quantification of tonic (inhibitory self-connections) and phasic (inhibitory projections to pyramidal cells). The figure was created with BioRender.com.BioRender.com
TMS-EEG is a complex technique whose quality depends on the experimenter’s proficiency and that requires many measurements. Besides stimulating the cortex, TMS also stimulates peripheral somatosensory neurons and produces a loud click that, if not properly masked, elicits auditory evoked responses (Conde et al., 2019). This opened a debate focused on the importance of disentangling genuine cortical responses from concomitant sensory activations. This debate shed light on the challenges of TMS-EEG, whose resolution is an ongoing active research effort (see for instance Russo et al., 2022). When applied in standard procedures and in effective experimental designs (Belardinelli et al., 2019), TMS-EEG could provide a reproducible way to non-invasively investigate inhibitory activity while bypassing spinal-cord excitability and eventual lesion, which are known to influence MEP measurements.
3.4. Other non-invasive techniques to probe GABAergic activity: Positron Emission Tomography (PET), Magnetoencephalography (MEG) and functional Magnetic Resonance Imaging (fMRI)
PET allows the quantification of postsynaptic GABAergic receptors (for a complete review see (Andersson et al., 2019). GABAA receptors are widely distributed in the brain and can be targeted by various PET tracers such as [11C]flumazenil, [18F]flumazenil and [11C]Ro15-4513 (Kassenbrock et al., 2016). GABAA activity was found to be decreased 3 months post-stroke, and such decrease was significantly correlated with recovery (Kim et al., 2014). PET currently plays an important role as diagnostic tool but the potential hazard of ionizing radiations exposure has become an increasingly issue (Brix et al., 2009). PET creates a modest radiation-induced mortality risk especially when performed repeatedly (Nievelstein et al., 2012), which would be needed if one wants to assess GABAergic variations throughout the 3 months of the stroke subacute phase. Moreover, despite its ability of detecting GABAergic modulations several months after stroke, detecting GABAA receptor changes in density with PET short after stroke is much more complex and leads to contradictive results (Richter et al., 2020).
MEG can also probe some aspects of the GABAergic system. GABA-enhancing agents showed localized increases in occipital and frontal gamma, sensorimotor beta and gamma and occipitotemporal alpha rhythms (Jensen et al., 2005). In a single case study of a patient with a pathological increase in beta rhythm following unilateral temporal lobe lesion, a sub-sedative dose of zolpidem (a GABAA positive modulator) reduced the pathological rhythm thus improving cognitive performance (Hall et al., 2010). Driving gamma frequency oscillations using transcranial Alternate Current Stimulation (tACS) significantly decreases local resting GABAA inhibition, which is closely related with performance in a motor learning task (Nowak et al., 2017). Interestingly, the peak frequency of slow gamma sub-band (∼30-60 Hz) seems to be positively related with endogenous GABA signaling during movement preparation whereases the power of the mid gamma sub-band (60–90 Hz) predicted performance in a subsequent motor task (Zich et al., 2021). Some MRS- and PET-MEG studies showed a positive correlation between gamma oscillation frequency and both resting GABA concentration and GABAA receptor density in the visual cortex. However, these results are not consistently replicated (Cousijn et al., 2014, Gawne et al., 2020), which suggests that cortical gamma oscillations do not have a consistent relationship to excitatory/inhibitory network such that more research is needed to further clarify their role.
The significant expansion of multimodal MRS-fMRI studies has enabled a more detailed examination of the interaction between neurochemistry and neurophysiology in the human brain. GABA concentration was shown to be negatively correlated with regional neural activation within the occipital (Bednařík et al., 2015) and the temporal cortex (Jung et al., 2017) and the anterior cingulate cortex/medial prefrontal cortex (Chen et al., 2019). These findings could be supported by previous animal studies showing that pharmacological increases in GABA produced weaker neural activation or stronger deactivation (Chen et al., 2005). However, such clear relationship is not found anywhere in the brain. Indeed, the sensorimotor cortex, which would be one of the main regions of interest to investigate for motor recovery after stroke, exhibits no association between GABA level and neural activity (Bhattacharyya et al., 2017). More MRS-fMRI studies are needed to overcome the lack of substantial conclusions on the relationship between metabolites and neural activity and to increase the effect size, which, to date, is moderate at best only for glutamate (for a complete review on the topic see (Kiemes et al., 2021).
4. Future directions and conclusions
Given the increasing number of stroke survivors, post-stroke long-term disability represents an urgent clinical burden to address by developing new interventions aimed at augmenting and lengthening the plasticity naturally occurring during the subacute phase of stroke. Understanding the mechanisms underlying post-stroke plasticity is, therefore, essential. In this review, we provided convergent evidence that GABAergic activity is crucial for plasticity. However, its implication in stroke recovery, although promising, remains to be established. The pattern of inhibitory changes after stroke are complex and GABA could have a twofold role post-stroke: increased inhibition might be protective during the acute phase of stroke and decreased inhibition might support plasticity afterwards.
The acute phase of stroke (1–7 days post-stroke) is characterized by oxidative stress, neuroinflammation and a glutamate-mediated excitotoxicity, which needs to be quickly reversed to avoid irrevocable damages (Doyle et al., 2008). At this stage, countering excitotoxicity with an increase in inhibition, might be necessary to protect neurons from injury and death. Once neurons survive the acute phase, the excitotoxicity has subsided and the environment becomes predominantly inhibitory, a shift in the excitation/inhibition balance (reduced inhibition and increased excitation) might be needed to support neural repair and neuroplasticity (Kumar & Kitago, 2019). This hypothesis needs to be tested in stroke patients by measuring GABA at different stroke stages (especially acute and subacute). However, MRS, TMS and TMS-EEG, the main techniques with a clear clinical translatability to non-invasively measure GABA in stroke patients, can detect or probe just specific aspects of GABA dynamics. Therefore, integrative approaches are crucial to obtain a comprehensive description of GABA: its sheer concentration, its signaling and the activity of its receptors.
A first attempt of integration was proposed by linking the concentration of GABA in M1, as measured with MRS, with the activity of its receptors, as measured by ppTMS (Stagg et al., 2011c), even if this result has not been consistently replicated both in healthy participants (Mooney et al., 2017) and chronic stroke patients (Mooney et al., 2019). One reason for this lack of replication could be that MRS commonly quantifies GABA concentration across a relatively large region of tissue centered on the anatomical hand knob in M1, whereas ppTMS targets the functional hotspot, defined as the stimulation site providing the largest MEPs, which is much smaller (in the order of a few mm3) and is also sometimes outside M1, most frequently anteriorly over the precentral or middle frontal gyrus (Ahdab et al., 2016). Given that we do not know currently how consistent GABAergic function is between brain regions, the fact that the anatomical and the functional hotspot do not always overlap could account for the inconsistent link between MRS and ppTMS-based GABA measures. If true, this issue could be easily overcome by using MEP-free techniques where the measurements could be restricted over the same region targeted with MRS. Another possible reason could be that ppTMS and MRS quantify distinct GABAergic mechanisms and their relationship is complex, especially in the context of dynamic changes. In particular, MRS seems to depict tonic inhibition, which originates from extracellular GABA acting on extrasynaptic GABAA receptors, other than to vesicular GABA acting on synaptic GABAA (as mostly assessed by ppTMS) (Stagg et al., 2011c, Cuypers et al., 2021). In this sense, NMM applied on TMS-EEG data would be useful as it could allow the differentiation between tonic and phasic inhibition. If the different GABA metrics converge, they could offer a complete characterization of GABA. If they do not, they could still provide complementary information to comprehensively assess GABAergic activity. Moreover, the role of GABA in plasticity could be further developed by linking GABA with some microstructural signatures of plasticity. Advances in MRI now allows the quantification of microstructural parameters, such as myelin, iron and water content which bear unprecedented insight into the mechanisms grounding brain plasticity. Indeed, learning potentially increases the rate of remyelination resulting in an augmentation of intracortical myelin content (Matuszewski et al., 2021), which might be related to the decrease observed in GABA concentration and activity.
A comprehensive assessment of GABAergic changes after stroke will extend our current understanding of the pathophysiology of post-stroke plasticity. Determining if GABA is implicated in stroke recovery would impact patients’ care. For instance, the routinely administration of baclofen (GABAB agonist) to treat patients’ spasticity could be cautiously reconsidered, as it has been shown that a single dose of baclofen impairs visuomotor learning in healthy adults (Johnstone et al., 2021), though this relationship was not straightforwardly recapitulated in stroke survivors (Johnstone et al., 2022). Understanding the role of GABA post-stroke would also inform novel interventions aimed at modulating GABA to create a “pro-plastic” neurochemical milieu during the subacute phase of stroke, and hence improve functional recovery.
There are potentially several ways to modulating GABA in humans. Non-invasive brain stimulation (NIBS) has extensively been used to either increase or decrease cortical excitability. We have already discussed the potential of TMS as a tool to quantify GABAergic activity. However, when applied as a train of pulses at a set frequency, repetitive-TMS (rTMS) it can decrease GABA levels and improve learning (e.g., Chen et al., 2021), as have transcranial direct current stimulation (tDCS) (e.g, Antonenko et al., 2017) and transcranial alternate current stimulation (tACS). There is already a cumulative evidence of a beneficial effect of NIBS (especially of rTMS, Fisicaro et al., 2019 and tDCS, Kang et al., 2016) in stroke patients. However, many of the effect sizes of NIBS in clinical trials are moderate at best and are likely to be even smaller if one considers the high inter-subject variability of the effects. Further research is therefore needed to define stimulation protocols where the start, duration and frequency are tailored to the individual patients, thus maximizing the potential therapeutic effect of NIBS.
Some drugs can also modulate GABA and act on performance/learning, though drugs that decrease GABAergic signaling have high-risk of inducing seizures and therefore have to be cautiously considered. Benzodiazepine, for instance, can increase GABAergic signaling through direct interactions with the GABA receptor complex, and can have region-specific effects in vivo (Sigel, 2002). Gaboxadol is a selective extrasynaptic receptor agonist that acts on a δ-containing GABAA receptor subtype found exclusively outside of the synapse (Wafford & Ebert, 2006). Finally, the Z-drugs (e.g., zolpidem, zaleplon, and eszopiclone) have modulatory proprieties on GABAA receptors. Notably, zolpidem has shown to enhance motor recovery when given 1–3 days post-stroke in mice (Hiu et al., 2016) and rats (Oh et al., 2018), but not when given after 2 weeks (Richter et al., 2020). Pharmacological interventions of this type are rather scarce in humans given the non-specific, brain-wide effect of these drugs which have potential proconvulsive and psychomimetic properties. More specific effects may be achieved by administering compounds that target limited subsets of GABAA subunits.
An innovative and promising intervention could be focused ultrasound (FUS), which offers the unique ability to non-invasively and precisely intervene in key damaged circuits (Meng et al., 2021). The blood brain barrier (BBB) can be temporarily and reversibly disrupted through the intravascular injection of microbubbles coupled with low-intensity ultrasound (Hynynen et al., 2005). Such BBB permeabilization lasts a few hours and holds promise for targeted uncaging of nanodroplets for drug delivery (Marty et al., 2012). FUS has been used to open the BBB in either the visual (Constans et al., 2020) or the somatosensory cortex (Todd et al., 2019) of non-human primates and rats, respectively, followed by an intra-venous injection of GABA leading to a GABA-dose-dependent decrease in activity. Importantly, the neuromodulation induced by FUS has been shown to be spatially and temporally limited and to drive secondary changes in brain regions that are functionally connected to the sonicated area (Wang et al., 2018). Despite being relatively new FUS has the potential for a non– invasive, controllable, repeatable, and reversible neuromodulation of targeted brain areas.
To conclude, tailored and optimized interventions in stroke patient will not be possible without a detailed understanding of the cellular and network mechanisms underlying the plasticity of the subacute phase. Among these processes, changes in GABAergic neurotransmission seems to play a particularly important role in the functional recovery and deserve a special attention.
Funding sources
IP is supported by the FNRS and the GIGA Doctoral School for Health Sciences from the University of Liège. GV is supported by the FRNS, CJS holds a Senior Research Fellowship, funded by the Wellcome Trust (224430/Z/21/Z). IP, GV and PM have ongoing research projects related to the topic discussed in the manuscript. These research projects are funded by the FNRS, ULiège, Fondation Léon Frédéricq.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Prof. Vincent Seutin for his inputs on the different types of brain plasticity, and Paolo Cardone and Lindsay Vogel for valuable discussions on the topic.
Data availability
No data was used for the research described in the article.
References
- Adams N.E., Hughes L.E., Rouse M.A., Phillips H.N., Shaw A.D., Murley A.G., Cope T.E., Bevan-Jones W.R., Passamonti L., Street D., Holland N., Nesbitt D., Friston K., Rowe J.B. GABAergic cortical network physiology in frontotemporal lobar degeneration. Brain. 2021;144(7):2135–2145. doi: 10.1093/brain/awab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahdab R., Ayache S.S., Brugières P., Farhat W.H., Lefaucheur J.P. The Hand Motor Hotspot is not Always Located in the Hand Knob: A Neuronavigated Transcranial Magnetic Stimulation Study. Brain Topogr. 2016;29(4):590–597. doi: 10.1007/s10548-016-0486-2. [DOI] [PubMed] [Google Scholar]
- Andersson J.D., Matuskey D., Finnema S.J. Positron emission tomography imaging of the γ-aminobutyric acid system. Neuroscience Letters. 2019;691:35–43. doi: 10.1016/j.neulet.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Antonenko D., Schubert F., Bohm F., Ittermann B., Aydin S., Hayek D., Grittner U., Flöel A. tDCS-induced modulation of GABA levels and resting-state functional connectivity in older adults. J. Neurosci. 2017;37(15):4065–4073. doi: 10.1523/JNEUROSCI.0079-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J.-C., Yamauchi H., Fujioka M., Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J. Cereb. Blood Flow Metab. 2014;34(1):2–18. doi: 10.1038/jcbfm.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednařík P., Tkáč I., Giove F., DiNuzzo M., Deelchand D.K., Emir U.E., Eberly L.E., Mangia S. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J. Cereb. Blood Flow Metab. 2015;35(4):601–610. doi: 10.1038/jcbfm.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli P., Biabani M., Blumberger D.M., Bortoletto M., Casarotto S., David O., Desideri D., Etkin A., Ferrarelli F., Fitzgerald P.B., Fornito A., Gordon P.C., Gosseries O., Harquel S., Julkunen P., Keller C.J., Kimiskidis V.K., Lioumis P., Miniussi C., Rosanova M., Rossi S., Sarasso S., Wu W., Zrenner C., Daskalakis Z.J., Rogasch N.C., Massimini M., Ziemann U., Ilmoniemi R.J. Reproducibility in TMS–EEG studies: A call for data sharing, standard procedures and effective experimental control. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2019;12(3):787–790. doi: 10.1016/j.brs.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Belelli D., Harrison N.L., Maguire J., Macdonald R.L., Walker M.C., Cope D.W. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 2009;29(41):12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt J., Hayward K.S., Kwakkel G., Ward N.S., Wolf S.L., Borschmann K., Krakauer J.W., Boyd L.A., Carmichael S.T., Corbett D., Cramer S.C. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int. J. Stroke. 2017;12(5):444–450. doi: 10.1177/1747493017711816. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya P.K., Phillips M.D., Stone L.A., Lowe M.J. Activation volume vs BOLD signal change as measures of fMRI activation–Its impact on GABA–fMRI activation correlation. Magn. Reson. Imaging. 2017;42:123–129. doi: 10.1016/j.mri.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blicher J.U., Near J., Næss-Schmidt E., Stagg C.J., Johansen-Berg H., Nielsen J.F., Østergaard L., Ho Y.C.L. GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil. Neural Repair. 2015;29(3):278–286. doi: 10.1177/1545968314543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley S.G., Revilla V., Cull-Candy S.G., Wisden W., Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409(6816):88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brix G., Nekolla E.A., Nosske D., Griebel J. Risks and safety aspects related to PET/MR examinations. Eur. J. Nucl. Med. Mol. Imaging. 2009;36(S1):131–138. doi: 10.1007/s00259-008-0937-4. [DOI] [PubMed] [Google Scholar]
- Brown C.E., Li P., Boyd J.D., Delaney K.R., Murphy T.H. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci. 2007;27(15):4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch C.M., Netz J., Wessling M., Seitz R.J., Hömberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126(2):470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Caporale N., Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 2008;31(1):25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T. Cellular and molecular mechanisms of neural repair after stroke: making waves. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 2006;59(5):735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Wei L., Rovainen C.M., Woolsey T.A. New patterns of intracortical projections after focal cortical stroke. Neurobiol. Dis. 2001;8(5):910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- Carmichael S.T., Tatsukawa K., Katsman D., Tsuyuguchi N., Kornblum H.I. Evolution of diaschisis in a focal stroke model. Stroke. 2004;35(3):758–763. doi: 10.1161/01.STR.0000117235.11156.55. [DOI] [PubMed] [Google Scholar]
- Cash R.F.H., Ziemann U., Murray K., Thickbroom G.W. Late cortical disinhibition in human motor cortex: a triple-pulse transcranial magnetic stimulation study. J. Neurophysiol. 2010;103(1):511–518. doi: 10.1152/jn.00782.2009. [DOI] [PubMed] [Google Scholar]
- Chellappa S.L., Gaggioni G., Ly J.Q.M., Papachilleos S., Borsu C., Brzozowski A., Rosanova M., Sarasso S., Luxen A., Middleton B., Archer S.N., Dijk D.J., Massimini M., Maquet P., Phillips C., Moran R.J., Vandewalle G. Circadian dynamics in measures of cortical excitation and inhibition balance. Sci. Rep. 2016;6(May):1–13. doi: 10.1038/srep33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.i., Fan X., Hu Y., Zuo C., Whitfield-Gabrieli S., Holt D., Gong Q., Yang Y., Pizzagalli D.A., Du F., Ongur D. Regional GABA concentrations modulate inter-network resting-state functional connectivity. Cereb. Cortex. 2019;29(4):1607–1618. doi: 10.1093/cercor/bhy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Silva A.C., Yang J., Shen J. Elevated endogenous GABA level correlates with decreased fMRI signals in the rat brain during acute inhibition of GABA transaminase. J. Neurosci. Res. 2005;79(3):383–391. doi: 10.1002/jnr.20364. [DOI] [PubMed] [Google Scholar]
- Chen Q.M., Yao F.R., Sun H.W., Chen Z.G., Ke J., Liao J., Cai X.Y., Yu L.Q., Wu Z.Y., Wang Z., Pan X., Liu H.Y., Li L., Zhang Q.Q., Ling W.H., Fang Q. Combining inhibitory and facilitatory repetitive transcranial magnetic stimulation (rTMS) treatment improves motor function by modulating GABA in acute ischemic stroke patients. Restor. Neurol. Neurosci. 2021;39(6):419–434. doi: 10.3233/RNN-211195. [DOI] [PubMed] [Google Scholar]
- Cirillo J., Mooney R.A., Ackerley S.J., Alan Barber P., Borges V.M., Clarkson A.N., Mangold C., Ren A., Smith M.C., Stinear C.M., Byblow W.D. Neurochemical balance and inhibition at the subacute stage after stroke. J. Neurophysiol. 2020;123(5):1775–1790. doi: 10.1152/jn.00561.2019. [DOI] [PubMed] [Google Scholar]
- Conde V., Tomasevic L., Akopian I., Stanek K., Saturnino G.B., Thielscher A., Bergmann T.O., Siebner H.R. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage. 2019;185:300–312. doi: 10.1016/j.neuroimage.2018.10.052. [DOI] [PubMed] [Google Scholar]
- Connelly W.M., Fyson S.J., Errington A.C., McCafferty C.P., Cope D.W., Di Giovanni G., Crunelli V. GABAB receptors regulate extrasynaptic GABAA receptors. J. Neurosci. 2013;33(9):3780–3785. doi: 10.1523/JNEUROSCI.4989-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans C., Ahnine H., Santin M., Lehericy S., Tanter M., Pouget P., Aubry J.F. Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier. J. Control. Release. 2020;318(May 2019):223–231. doi: 10.1016/j.jconrel.2019.12.006. [DOI] [PubMed] [Google Scholar]
- Cousijn H., Haegens S., Wallis G., Near J., Stokes M.G., Harrison P.J., Nobre A.C. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. 2014;111(25):9301–9306. doi: 10.1073/pnas.1321072111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S.C., Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23(6):265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- Cramer S.C., Sur M., Dobkin B.H., O'Brien C., Sanger T.D., Trojanowski J.Q., Rumsey J.M., Hicks R., Cameron J., Chen D., Chen W.G., Cohen L.G., deCharms C., Duffy C.J., Eden G.F., Fetz E.E., Filart R., Freund M., Grant S.J., Haber S., Kalivas P.W., Kolb B., Kramer A.F., Lynch M., Mayberg H.S., McQuillen P.S., Nitkin R., Pascual-Leone A., Reuter-Lorenz P., Schiff N., Sharma A., Shekim L., Stryker M., Sullivan E.V., Vinogradov S. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(6):1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K., Hehl M., van Aalst J., Chalavi S., Mikkelsen M., Van Laere K., Dupont P., Mantini D., Swinnen S.P. Age-related GABAergic differences in the primary sensorimotor cortex: A multimodal approach combining PET, MRS and TMS. Neuroimage. 2021;226(October 2020) doi: 10.1016/j.neuroimage.2020.117536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani G., Ziemann U. Pharmacophysiology of TMS-evoked EEG potentials: A mini-review. Brain Stimul. 2019;12(3):829–831. doi: 10.1016/j.brs.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Darmani G., Zipser C.M., Böhmer G.M., Deschet K., Müller-Dahlhaus F., Belardinelli P., Schwab M., Ziemann U. Effects of the selective α5-GABAAR antagonist S44819 on excitability in the human brain: a TMS–EMG and TMS–EEG phase I study. J. Neurosci. 2016;36(49):12312–12320. doi: 10.1523/JNEUROSCI.1689-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N.S. Homeostatic plasticity in the CNS: synaptic and intrinsic forms. Journal of Physiology-Paris. 2003;97(4–6):391–402. doi: 10.1016/j.jphysparis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Doyle K.P., Simon R.P., Stenzel-Poore M.P. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55(3):310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromerick A.W., Geed S., Barth J., Brady K., Giannetti M.L., Mitchell A., Edwardson M.A., Tan M.T., Zhou Y., Newport E.L., Edwards D.F. Critical Period After Stroke Study (CPASS): A phase II clinical trial testing an optimal time for motor recovery after stroke in humans. PNAS. 2021;118(39) doi: 10.1073/pnas.2026676118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L., Truelsen T. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–255. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin V.L., Vos T., Alahdab F., Amit A.M.L., Bärnighausen T.W., Beghi E., Beheshti M., Chavan P.P., Criqui M.H., Desai R., Dhamminda Dharmaratne S., Dorsey E.R., Wilder Eagan A., Elgendy I.Y., Filip I., Giampaoli S., Giussani G., Hafezi-Nejad N., Hole M.K., Ikeda T., Owens Johnson C., Kalani R., Khatab K., Khubchandani J., Kim D., Koroshetz W.J., Krishnamoorthy V., Krishnamurthi R.V., Liu X., Lo W.D., Logroscino G., Mensah G.A., Miller T.R., Mohammed S., Mokdad A.H., Moradi-Lakeh M., Morrison S.D., Shivamurthy V.K.N., Naghavi M., Nichols E., Norrving B.o., Odell C.M., Pupillo E., Radfar A., Roth G.A., Shafieesabet A., Sheikh A., Sheikhbahaei S., Shin J.I., Singh J.A., Steiner T.J., Stovner L.J., Wallin M.T., Weiss J., Wu C., Zunt J.R., Adelson J.D., Murray C.J.L. Burden of neurological disorders across the US from 1990–2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165. doi: 10.1001/jamaneurol.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisicaro F., Lanza G., Grasso A.A., Pennisi G., Bella R., Paulus W., Pennisi M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther. Adv. Neurol. Disord. 2019;12 doi: 10.1177/1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A., Wylezinska M., Kincses T., Matthews P.M. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 2006;95(3):1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gainey M.A., Hurvitz-Wolff J.R., Lambo M.E., Turrigiano G.G. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J. Neurosci. 2009;29(20):6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis C., Vlachos A. Hebbian and Homeostatic Synaptic Plasticity—Do Alterations of One Reflect Enhancement of the Other? Front. Cell. Neurosci. 2020;14(March):1–8. doi: 10.3389/fncel.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne T.J., Overbeek G.J., Killen J.F., Reid M.A., Ellis C.A., Lahti A.C., Kraguljac N.V., Denney T.S. OPEN A multimodal magnetoencephalography 7 T fMRI and 7 T proton MR spectroscopy study in fi rst episode psychosis. NPJ Schizophr. 2020;6(1), 23:1–9. doi: 10.1038/s41537-020-00113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głodzik-Sobańska L., Słowik A., Kozub J., Sobiecka B., Urbanik A., Szczudlik A. GABA in ischemic stroke: proton magnetic resonance study. Med. Sci. Monit. 2004;10(Supplement 3) [PubMed] [Google Scholar]
- Grefkes C., Fink G.R. Recovery from stroke: current concepts and future perspectives. Neurological Research and Practice. 2020;2(1) doi: 10.1186/s42466-020-00060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoras I.-F., Stagg C.J. Recent advances in the role of excitation–inhibition balance in motor recovery post-stroke. Faculty Reviews. 2021;10(58) doi: 10.12703/r/10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann G., Redecker C., Neumann-Haefelin T., Freund H., Witte O.W. Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1998;44(2):255–258. doi: 10.1002/ana.410440217. [DOI] [PubMed] [Google Scholar]
- Hall S.D., Yamawaki N., Fisher A.E., Clauss R.P., Woodhall G.L., Stanford I.M. GABA (A) alpha-1 subunit mediated desynchronization of elevated low frequency oscillations alleviates specific dysfunction in stroke–a case report. Clin. Neurophysiol. 2010;121(4):549–555. doi: 10.1016/j.clinph.2009.11.084. [DOI] [PubMed] [Google Scholar]
- Hartman K.N., Pal S.K., Burrone J., Murthy V.N. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat. Neurosci. 2006;9(5):642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Hattiangady B., Rao M.S., Shetty G.A., Shetty A.K. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hebb D.O. Organization of behavior. new york: Wiley. J. Clin. Psychol. 1949;6(3):307–335. [Google Scholar]
- Hebb D.O. The first stage of perception: growth of the assembly. The Organization of Behavior. 1949;4:60–78. [Google Scholar]
- Hensch T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hess G., Aizenman C.D., Donoghue J.P. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J. Neurophysiol. 1996;75(5):1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hiu T., Farzampour Z., Paz J.T., Wang E.H.J., Badgely C., Olson A., Micheva K.D., Wang G., Lemmens R., Tran K.V., Nishiyama Y., Liang X., Hamilton S.A., O’Rourke N., Smith S.J., Huguenard J.R., Bliss T.M., Steinberg G.K. Enhanced phasic GABA inhibition during the repair phase of stroke: a novel therapeutic target. Brain. 2016;139(2):468–480. doi: 10.1093/brain/awv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordacre B., McCambridge A.B., Ridding M.C., Bradnam L.V. Can Transcranial Direct Current Stimulation Enhance Poststroke Motor Recovery? Neurology. 2021;97(4):170–180. doi: 10.1212/wnl.0000000000012187. [DOI] [PubMed] [Google Scholar]
- Hu H., Gan J., Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science. 2014;345(6196) doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- Hubbard I.J., Parsons M.W., Neilson C., Carey L.M. Task-specific training: evidence for and translation to clinical practice. Occup. Ther. Int. 2009;16(3–4):175–189. doi: 10.1002/oti.275. [DOI] [PubMed] [Google Scholar]
- Hummel F.C., Steven B., Hoppe J., Heise K., Thomalla G., Cohen L.G., Gerloff C. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009;72(20):1766–1772. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K., McDannold N., Sheikov N.A., Jolesz F.A., Vykhodtseva N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi R.J., Virtanen J., Ruohonen J., Karhu J., Aronen H.J., Näätänen R., Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8(16):3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Jensen O., Goel P., Kopell N., Pohja M., Hari R., Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26(2):347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Johnstone A., Grigoras I., Petitet P., Capitão L.P., Stagg C.J. A single, clinically relevant dose of the GABA B agonist baclofen impairs visuomotor learning. J. Physiol. 2021;599(1):307–322. doi: 10.1113/JP280378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone A., Brander F., Kelly K., Bestmann S., Ward N. Differences in outcomes following an intensive upper-limb rehabilitation program for patients with common central nervous system-acting drug prescriptions. Int. J. Stroke. 2022;17(3):269–281. doi: 10.1177/17474930211006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Williams S.R., Sanaei Nezhad F., Lambon Ralph M.A. GABA concentrations in the anterior temporal lobe predict human semantic processing. Sci. Rep. 2017;7(1):15748. doi: 10.1038/s41598-017-15981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N., Summers J.J., Cauraugh J.H. Transcranial direct current stimulation facilitates motor learning post-stroke: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2016;87(4):345–355. doi: 10.1136/jnnp-2015-311242. [DOI] [PubMed] [Google Scholar]
- Kassenbrock A., Vasdev N., Liang H., S. Selected PET radioligands for ion channel linked neuroreceptor imaging: focus on GABA, NMDA and nACh receptors. Curr. Top. Med. Chem. 2016;16(16):1830–1842. doi: 10.2174/1568026616666160315142457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiemes, A., Davies, C., Kempton, M. J., Lukow, P. B., Bennallick, C., Stone, J. M., & Modinos, G. (2021). GABA, Glutamate and Neural Activity: A Systematic Review With Meta-Analysis of Multimodal 1H-MRS-fMRI Studies. In Frontiers in Psychiatry (Vol. 12, Issue March). https://doi.org/10.3389/fpsyt.2021.644315. [DOI] [PMC free article] [PubMed]
- Kilman V., Van Rossum M.C.W., Turrigiano G.G. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABAA receptors clustered at neocortical synapses. J. Neurosci. 2002;22(4):1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.K., Yang E.J., Cho K., Lim J.Y., Paik N.-J. Functional recovery after ischemic stroke is associated with reduced GABAergic inhibition in the cerebral cortex: a GABA PET study. Neurorehabil. Neural Repair. 2014;28(6):576–583. doi: 10.1177/1545968313520411. [DOI] [PubMed] [Google Scholar]
- Krakauer J.W., Carmichael S.T. MIT Press; 2022. Broken movement: the neurobiology of motor recovery after stroke. [Google Scholar]
- Kuhlman S.J., Olivas N.D., Tring E., Ikrar T., Xu X., Trachtenberg J.T. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501(7468):543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kitago T. Pharmacological Enhancement of Stroke Recovery. Curr. Neurol. Neurosci. Rep. 2019;19(7) doi: 10.1007/s11910-019-0959-2. [DOI] [PubMed] [Google Scholar]
- Kuśmierz Ł., Isomura T., Toyoizumi T. Learning with three factors: modulating Hebbian plasticity with errors. Curr. Opin. Neurobiol. 2017;46:170–177. doi: 10.1016/j.conb.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Lawrence E.S., Coshall C., Dundas R., Stewart J., Rudd A.G., Howard R., Wolfe C.D.A. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32(6):1279–1284. doi: 10.1161/01.str.32.6.1279. [DOI] [PubMed] [Google Scholar]
- Lea-carnall C.A., El-deredy W., Williams S.R., Stagg C.J., Trujillo-barreto N.J. From synaptic activity to human in vivo quantification of neurotransmitter dynamics : a neural modelling approach. BioRxiv. 2021:1–33. [Google Scholar]
- Lemke C., Hess A., Clare S., Bachtiar V., Stagg C., Jezzard P., Emir U. Two-voxel spectroscopy with dynamic B0 shimming and flip angle adjustment at 7 T in the human motor cortex. NMR Biomed. 2015;28(7):852–860. doi: 10.1002/nbm.3328. [DOI] [PubMed] [Google Scholar]
- Lindsay M.P., Norrving B., Sacco R.L., Brainin M., Hacke W., Martins S., Pandian J., Feigin V. World Stroke Organization (WSO): Global Stroke Fact Sheet 2019. Int. J. Stroke. 2019;14(8):806–817. doi: 10.1177/1747493019881353. [DOI] [PubMed] [Google Scholar]
- Magee J.C., Grienberger C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020;43:95–117. doi: 10.1146/annurev-neuro-090919-022842. [DOI] [PubMed] [Google Scholar]
- Martin D.L., Rimvall K. Regulation of γ-aminobutyric acid synthesis in the brain. J. Neurochem. 1993;60(2):395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Marty B., Larrat B., Van Landeghem M., Robic C., Robert P., Port M., Le Bihan D., Pernot M., Tanter M., Lethimonnier F., Mériaux S. Dynamic study of blood–brain barrier closure after its disruption using ultrasound: a quantitative analysis. J. Cereb. Blood Flow Metab. 2012;32(10):1948–1958. doi: 10.1038/jcbfm.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewski J., Kossowski B., Bola Ł., Banaszkiewicz A., Paplińska M., Gyger L., Kherif F., Szwed M., Frackowiak R.S., Jednoróg K., Draganski B., Marchewka A. Brain plasticity dynamics during tactile Braille learning in sighted subjects: Multi-contrast MRI approach. Neuroimage. 2021;227:117613. doi: 10.1016/j.neuroimage.2020.117613. [DOI] [PubMed] [Google Scholar]
- McDonnell M.N., Orekhov Y., Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006;173(1):86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McDonnell M.N., Stinear C.M. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimul. 2017;10(4):721–734. doi: 10.1016/j.brs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Mehrholz, J., Hädrich, A., Platz, T., Kugler, J., & Pohl, M. (2012). Electromechanical and robot‐assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database of Systematic Reviews, 6. [DOI] [PubMed]
- Mehrholz J., Thomas S., Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017;8 doi: 10.1002/14651858.CD002840.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrholz J., Thomas S., Kugler J., Pohl M., Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020;10 doi: 10.1002/14651858.CD006185.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Hynynen K., Lipsman N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat. Rev. Neurol. 2021;17(1):7–22. doi: 10.1038/s41582-020-00418-z. [DOI] [PubMed] [Google Scholar]
- Michalettos G., Ruscher K. Crosstalk Between GABAergic Neurotransmission and Inflammatory Cascades in the Post-ischemic Brain: Relevance for Stroke Recovery. Front. Cell. Neurosci. 2022;16(March):1–16. doi: 10.3389/fncel.2022.807911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R.A., Cirillo J., Byblow W.D. GABA and primary motor cortex inhibition in young and older adults: a multimodal reliability study. J. Neurophysiol. 2017;118(1):425–433. doi: 10.1152/jn.00199.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R.A., Ackerley S.J., Rajeswaran D.K., Cirillo J., Barber P.A., Stinear C.M., Byblow W.D. The influence of primary motor cortex inhibition on upper limb impairment and function in chronic stroke: a multimodal study. Neurorehabil. Neural Repair. 2019;33(2):130–140. doi: 10.1177/1545968319826052. [DOI] [PubMed] [Google Scholar]
- Moran R.J., Symmonds M., Stephan K.E., Friston K.J., Dolan R.J. An in vivo assay of synaptic function mediating human cognition. Curr. Biol. 2011;21(15):1320–1325. doi: 10.1016/j.cub.2011.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R.J., Pinotsis D.A., Friston K.J. Neural masses and fields in dynamic causal modeling. Front. Comput. Neurosci. 2013;7:57. doi: 10.3389/fncom.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Carhart-Harris R.L., Moran R.J., Brookes M.J., Williams T.M., Errtizoe D., Sessa B., Papadopoulos A., Bolstridge M., Singh K.D., Feilding A., Friston K.J., Nutt D.J. Broadband cortical desynchronization underlies the human psychedelic state. J. Neurosci. 2013;33(38):15171–15183. doi: 10.1523/JNEUROSCI.2063-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Shaw A.D., Jackson L.E., Hall J., Moran R., Saxena N. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J. Neurosci. 2015;35(33):11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S.B., Turrigiano G.G. Strength through diversity. Neuron. 2008;60(3):477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievelstein R.A.J., Quarles van Ufford H.M.E., Kwee T.C., Bierings M.B., Ludwig I., Beek F.J.A., de Klerk J.M.H., Mali W.P.T.M., de Bruin P.W., Geleijns J. Radiation exposure and mortality risk from CT and PET imaging of patients with malignant lymphoma. Eur. Radiol. 2012;22(9):1946–1954. doi: 10.1007/s00330-012-2447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe M., Urban E.T.R., III, Barbay S., Nudo R.J. Rehabilitative training promotes rapid motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model. Neurorehabil. Neural Repair. 2015;29(5):472–482. doi: 10.1177/1545968314543499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M., Hinson E., Van Ede F., Pogosyan A., Guerra A., Quinn A., Brown P., Stagg C.J. Driving human motor cortical oscillations leads to behaviorally relevant changes in local GABAA inhibition: a tACS-TMS study. J. Neurosci. 2017;37(17):4481–4492. doi: 10.1523/JNEUROSCI.0098-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.-K., Yoon K.J., Lee Y.-T., Chae S.W., Choi H.Y., Shin H.S., Park Y.H., Chun S.-W., Park Y.S. Effect of zolpidem on functional recovery in a rat model of ischemic stroke. J. Int. Med. Res. 2018;46(1):249–257. doi: 10.1177/0300060517723799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paille V., Fino E., Du K., Morera-Herreras T., Perez S., Kotaleski J.H., Venance L. GABAergic circuits control spike-timing-dependent plasticity. J. Neurosci. 2013;33(22):9353–9363. doi: 10.1523/JNEUROSCI.5796-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz E.J., Barbay S., Frost S.B., Friel K.M., Dancause N., Zoubina E.V., Stowe A.M., Quaney B.M., Nudo R.J. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol. Res. 2003;25(8):801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- Pradhan S., Bonekamp S., Gillen J.S., Rowland L.M., Wijtenburg S.A., Edden R.A.E., Barker P.B. Comparison of single voxel brain MRS AT 3 T and 7 T using 32-channel head coils. Magn. Reson. Imaging. 2015;33(8):1013–1018. doi: 10.1016/j.mri.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli I., Castellanos N., Rivolta D., Belardinelli P., Bajo R., Zipser C., Espenhahn S., Heidegger T., Müller-Dahlhaus F., Ziemann U. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J. Neurosci. 2014;34(16):5603–5612. doi: 10.1523/JNEUROSCI.5089-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli I., Bergmann T.O., Fecchio M., Rosanova M., Biondi A., Belardinelli P., Ziemann U. The impact of GABAergic drugs on TMS-induced brain oscillations in human motor cortex. Neuroimage. 2017;163:1–12. doi: 10.1016/j.neuroimage.2017.09.023. [DOI] [PubMed] [Google Scholar]
- Redecker C., Wang W., Fritschy J.M., Witte O.W. Widespread and long-lasting alterations in GABAA-receptor subtypes after focal cortical infarcts in rats: Mediation by NMDA-dependent processes. J. Cereb. Blood Flow Metab. 2002;22(12):1463–1475. doi: 10.1097/01.WCB.0000034149.72481.BD. [DOI] [PubMed] [Google Scholar]
- Richter G., Liao V.W.Y., Ahring P.K., Chebib M. The Z-Drugs Zolpidem, Zaleplon, and Eszopiclone Have Varying Actions on Human GABAA Receptors Containing γ1, γ2, and γ3 Subunits. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.599812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester N., Holland J., Haibt L., Duda W. Tests on a cell assembly theory of the action of the brain, using a large digital computer. IRE Trans. Inf. Theory. 1956;2(3):80–93. [Google Scholar]
- Russo S., Sarasso S., Puglisi G.E., Dal Palù D., Pigorini A., Casarotto S., D’Ambrosio S., Astolfi A., Massimini M., Rosanova M., Fecchio M. TAAC-TMS Adaptable Auditory Control: A universal tool to mask TMS clicks. J. Neurosci. Methods. 2022;370 doi: 10.1016/j.jneumeth.2022.109491. [DOI] [PubMed] [Google Scholar]
- Schambra H.M., Ogden R.T., Martínez-Hernández I.E., Lin X., Chang Y.B., Rahman A., Edwards D.J., Krakauer J.W. The reliability of repeated TMS measures in older adults and in patients with subacute and chronic stroke. Front. Cell. Neurosci. 2015;9:335. doi: 10.3389/fncel.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiene K., Bruehl C., Zilles K., Qu M., Hagemann G., Kraemer M., Witte O.W. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J. Cereb. Blood Flow Metab. 1996;16(5):906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- Sedova P., Brown R.D., Zvolsky M., Belaskova S., Volna M., Baluchova J., Bednarik J., Mikulik R. Incidence of stroke and ischemic stroke subtypes: a community-based study in Brno. Czech Republic. Cerebrovascular Diseases. 2021;50(1):54–61. doi: 10.1159/000512180. [DOI] [PubMed] [Google Scholar]
- Sejnowski T.J. Statistical constraints on synaptic plasticity. J. Theor. Biol. 1977;69(2):385–389. doi: 10.1016/0022-5193(77)90146-1. [DOI] [PubMed] [Google Scholar]
- Sigel E. Mapping of the benzodiazepine recognition site on GABA-A receptors. Curr. Top. Med. Chem. 2002;2(8):833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- Smania N., Gandolfi M., Paolucci S., Iosa M., Ianes P., Recchia S., Giovanzana C., Molteni F., Avesani R., Di Paolo P., Zaccala M., Agostini M., Tassorelli C., Fiaschi A., Primon D., Ceravolo M.G., Farina S. Reduced-intensity modified constraint-induced movement therapy versus conventional therapy for upper extremity rehabilitation after stroke: a multicenter trial. Neurorehabil. Neural Repair. 2012;26(9):1035–1045. doi: 10.1177/1545968312446003. [DOI] [PubMed] [Google Scholar]
- Song S., Miller K.D., Abbott L.F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 2000;3(9):919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- Sparta D.R., Hovelsø N., Mason A.O., Kantak P.A., Ung R.L., Decot H.K., Stuber G.D. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J. Neurosci. 2014;34(10):3699–3705. doi: 10.1523/JNEUROSCI.0235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Wylezinska M., Matthews P.M., Johansen-Berg H., Jezzard P., Rothwell J.C., Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J. Neurophysiol. 2009;101(6):2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., Johansen-Berg H. The role of GABA in human motor learning. Curr. Biol. 2011;21(6):480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., Johansen-Berg H. What are we measuring with GABA Magnetic Resonance Spectroscopy? Commun. Integr. Biol. 2011;4(5):573–575. doi: 10.4161/cib.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Bestmann S., Constantinescu A.O., Moreno Moreno L., Allman C., Mekle R., Woolrich M., Near J., Johansen-Berg H., Rothwell J.C. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 2011;589(23):5845–5855. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C., Rothman D. Academic Press; 2013. Magnetic resonance spectroscopy: tools for neuroscience research and emerging clinical applications. [Google Scholar]
- Stinear C.M., Lang C.E., Zeiler S., Byblow W.D. Advances and challenges in stroke rehabilitation. The Lancet Neurology. 2020;19(4):348–360. doi: 10.1016/S1474-4422(19)30415-6. [DOI] [PubMed] [Google Scholar]
- Stroemer R.P., Kent T.A., Hulsebosch C.E. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26(11):2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Sumner P., Edden R.A.E., Bompas A., Evans C.J., Singh K.D. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat. Neurosci. 2010;13(7):825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- Swayne O.B.C., Rothwell J.C., Ward N.S., Greenwood R.J. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb. Cortex. 2008;18(8):1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P., Greenwood R.J., Rothwell J.C. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin. Neurophysiol. 2006;117(8):1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Teasell R., Salbach N.M., Foley N., Mountain A., Cameron J.I., Jong A.d., Acerra N.E., Bastasi D., Carter S.L., Fung J., Halabi M.-L., Iruthayarajah J., Harris J., Kim E., Noland A., Pooyania S., Rochette A., Stack B.D., Symcox E., Timpson D., Varghese S., Verrilli S., Gubitz G., Casaubon L.K., Dowlatshahi D., Lindsay M.P. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int. J. Stroke. 2020;15(7):763–788. doi: 10.1177/1747493019897843. [DOI] [PubMed] [Google Scholar]
- Thieme H., Mehrholz J., Pohl M., Behrens J., Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2012;3 doi: 10.1002/14651858.CD008449.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd N., Zhang Y., Power C., Becerra L., Borsook D., Livingstone M., McDannold N. Modulation of brain function by targeted delivery of GABA through the disrupted blood-brain barrier. Neuroimage. 2019;189(September 2018):267–275. doi: 10.1016/j.neuroimage.2019.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L., Tamboli S. The role of inhibitory circuits in hippocampal memory processing. Nat. Rev. Neurosci. 2022;23(8):476–492. doi: 10.1038/s41583-022-00599-0. [DOI] [PubMed] [Google Scholar]
- Tremblay S., Rogasch N.C., Premoli I., Blumberger D.M., Casarotto S., Chen R., Di Lazzaro V., Farzan F., Ferrarelli F., Fitzgerald P.B., Hui J., Ilmoniemi R.J., Kimiskidis V.K., Kugiumtzis D., Lioumis P., Pascual-Leone A., Pellicciari M.C., Rajji T., Thut G., Daskalakis Z.J. Clinical utility and prospective of TMS–EEG. Clin. Neurophysiol. 2019;130(5):802–844. doi: 10.1016/j.clinph.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Turrigiano G.G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135(3):422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano G., Abbott L.F., Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264(5161):974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Turrigiano G.G., Leslie K.R., Desai N.S., Rutherford L.C., Nelson S.B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391(6670):892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano G.G., Nelson S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wafford K.A., Ebert B. Gaboxadol - A new awakening in sleep. Curr. Opin. Pharmacol. 2006;6(1 SPEC. ISS.), 30–36 doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wang J.B., Aryal M., Zhong Q., Vyas D.B., Airan R.D. Noninvasive Ultrasonic Drug Uncaging Maps Whole-Brain Functional Networks. Neuron. 2018;100(3):728–738.e7. doi: 10.1016/j.neuron.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Jin Y., Hao K. Synergies between synaptic and intrinsic plasticity in echo state networks. Neurocomputing. 2021;432:32–43. [Google Scholar]
- Ward N.S., Brander F., Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J. Neurol. Neurosurg. Psychiatry. 2019;90(5):498–506. doi: 10.1136/jnnp-2018-319954. [DOI] [PubMed] [Google Scholar]
- Winship I.R., Murphy T.H. In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. J. Neurosci. 2008;28(26):6592–6606. doi: 10.1523/JNEUROSCI.0622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship I.R., Murphy T.H. Remapping the somatosensory cortex after stroke: insight from imaging the synapse to network. Neuroscientist. 2009;15(5):507–524. doi: 10.1177/1073858409333076. [DOI] [PubMed] [Google Scholar]
- Wolf S.L., Thompson P.A., Winstein C.J., Miller J.P., Blanton S.R., Nichols-Larsen D.S., Morris D.M., Uswatte G., Taub E., Light K.E. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41(10):2309–2315. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S.B.E., Gründemann J., Tovote P., Krabbe S., Jacobson G.A., Müller C., Herry C., Ehrlich I., Friedrich R.W., Letzkus J.J. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509(7501):453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Wu B., Warnock G., Zaiss M., Lin C., Chen M., Zhou Z., Mu L., Nanz D., Tuura R., Delso G. An overview of CEST MRI for non-MR physicists. EJNMMI Physics. 2016;3(1):1–21. doi: 10.1186/s40658-016-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zich C., Nowak M., Hinson E.L., Quinn A.J., Woolrich M.W., Stagg C.J. Human motor cortical gamma activity relates to GABAergic signalling and to behaviour. BioRxiv. 2021:2006–2021. [Google Scholar]
- Ziemann U., Muellbacher W., Hallett M., Cohen L.G. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124(6):1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.