Abstract
Background:
Pulmonary embolism severity index and simplified pulmonary embolism severity index have been utilized in initial risk evaluation in patients with acute pulmonary embolism. However, these models do not include any imaging measure of right ventricle function. In this study, we proposed a novel index and aimed to evaluate the clinical impact.
Methods:
Our study population comprised retrospectively evaluated 502 patients with acute pulmonary embolism managed with different treatment modalities. Echocardiographic and computed tomographic pulmonary angiography evaluations were performed at admission to the emergency room within maximally 30 minutes. The formula of our index was as follows: (right ventricle diameter × systolic pulmonary arterial pressure-echo)/(right ventricle free-wall diameter × tricuspid annular plane systolic excursion).
Results:
This index value showed significant correlations to clinical and hemodynamic severity measures. Only pulmonary embolism severity index, but not our index value, independently predicted in-hospital mortality. However, an index value higher than 17.8 predicted the long-term mortality with a sensitivity of 70% and specificity of 40% (areas under the curve = 0.652, 95% CI, 0.557-0.747, P = .001). According to the adjusted variable plot, the risk of long-term mortality increased until an index level of 30 but remained unchanged thereafter. The cumulative hazard curve also showed a higher mortality with high-index value versus low-index value.
Conclusions:
Our index composed from measures of computed tomographic pulmonary angiography and transthoracic echocardiography may provide important insights regarding the adaptation status of right ventricle against pressure/wall stress in acute pulmonary embolism, and a higher value seems to be associated with severity of the clinical and hemodynamic status and long-term mortality but not with in-hospital mortality. However, the pulmonary embolism severity index remained as the only independent predictor for in-hospital mortality.
Keywords: Acute pulmonary embolism, computed tomography, pulmonary embolism severity index, risk prediction, right ventricle, transthoracic echocardiography
Highlights
Initial findings on computed tomographic pulmonary angiography and transthoracic echocardiography in the setting of acute pulmonary embolism reflect severity of the disease.
However, pulmonary embolism severity index and its simplified version scorings which are the most commonly utilized scoring systems for initial risk prediction in acute pulmonary embolism do not include any imaging parameter.
Our novel index derived from computed tomographic pulmonary angiography and transthoracic echocardiography or at the time of the diagnosis was described as: (right ventricle diameter × systolic pulmonary arterial pressure-echo) / (right ventricle free-wall diameter × tricuspid annular plane systolic excursion).
This index value showed significant correlations to clinical and hemodynamic severity measures.
Only pulmonary embolism severity index, but not our index value, independently predicted in-hospital mortality. However, an index value higher than 17.8 predicted the long-term mortality with a sensitivity of 70% and specificity of 40% (areas under the curve = 0.652, 95% CI; 0.557-0.747, P = .001).
Introduction
Acute pulmonary embolism (APE) has been reported to be the third most frequent cause of cardiovascular mortality in the developing countries and has a wide clinical and prognostic spectrum from subclinical low-risk APE to acute cardiogenic shock.1,2 Therefore, assessment of the severity of the disease and individualized prognosis are essential, immediately after diagnosis, for determining the risk-based optimal treatment modality. Pulmonary embolism severity index (PESI) and its simplified version sPESI have been the most widely used scoring tools in risk prediction.3,4 However, these scoring models do not include any imaging parameter reflecting the right ventricle (RV) dysfunction derived from transthoracic echocardiography (TTE) or computed tomographic pulmonary angiography (CTPA), which have been confirmed to predict 30-day adverse clinical outcome in patients with APE.5 In a large meta-analysis comprising 49 studies, an increased right ventricle to left ventricle diameter ratio (RV/LVr) derived from CTPA was found to be associated with a 2.5-fold (95% CI: 1.8-3.5) increase in the risk for all-cause mortality and a 5-fold (95% CI: 2.7-9.2) increase in the risk for APE-related mortality.6 Moreover, TTE provides many information about RV impairment and enables measuring pulmonary arterial (PA) pressure from tricuspid regurgitation.7-9 The end-diastolic RV/LVr more than 0.9 ratio as assessed by TTE is reported to be associated with poor prognosis, and systolic PA pressure estimated (sPAP) higher than 50 mm Hg at initial TTE predicts the progression to chronic thromboembolic pulmonary hypertension and increased mortality.8,9 Nevertheless, corresponding pooled negative and positive likelihood ratios were inadequate for risk prediction in these patients.10 Even in low-risk APE patients, the presence of RV dysfunction on TTE or CTPA is associated with short-term mortality.11 European Society of Cardiology/European Respiratory Society (ESC/ERS) 2014 and revised version in 2019 Pulmonary Embolism (PE) Guidelines have recommended an updated risk stratification regarding the patient’s initial hemodynamic status, PESI/sPESI score, RV dysfunction on TTE or CTPA, and blood troponin levels and classified APE patients into 4 risk groups as high risk (HR), intermediate to high risk (IHR), intermediate to low risk (ILR), and low risk (LR).12,13
Although the prognostic value of CTPA and TTE has been independently investigated in many studies before, there is no composite research using these 2 diagnostic tools together and evaluating their value for APE severity. Acute thrombotic obstruction in PA bed causes a sudden increase in pulmonary vascular resistance and PA pressure which results in RV strain and dilation with decreased RV free-wall systolic function. In this study, we proposed a novel composite risk stratification index composed of RV diameter, sPAP-echo, tricuspid annular plane systolic excursion (TAPSE), and RV free-wall diameter (RVFWD) as measures of RV pressure burden in this setting. This index was as follows: (RV diameter × sPAP-echo)/(RVFWD × TAPSE). While diameters of RV and RVFWD were acquired from the initial CTPA study, sPAP-echo and TAPSE were calculated from TTE examination during hospital admission. Moreover, we investigated the relationship between former risk stratification models and in-hospital and long-term mortality outcomes.
Methods
Study Population
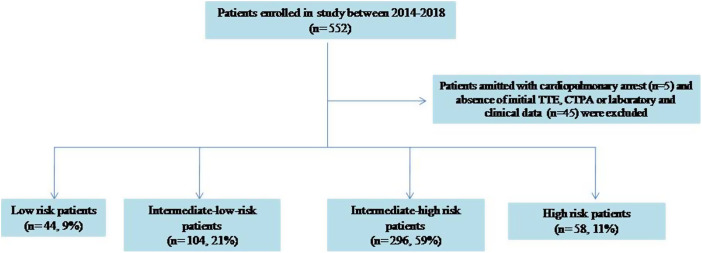
Retrospectively evaluated 502 patients (mean age 61.9 ± 16.9 years, and 58.2% female) with APE who were referred to our tertiary center and managed with different treatment modalities from 2014 to 2018 were enrolled in this study. The flowchart of the study was given in Supplementary Figure 1. The diagnosis of APE, definitions of the risk groups, and provoked or unprovoked APE were based on the criteria as recommended by the 2014 ESC/ERS PE Guidelines12 and was revised after the publication of 2019 ESC/ERS PE Guidelines.13 Initial physical examination and vital signs including blood pressure, heart rate, and pulse oximetric percentage saturation were noted for all patients during admission. The PESI, sPESI, and shock index were calculated in all patients at initial assessment of clinical and hemodynamic status. Echocardiographic and CTPA evaluations were performed at admission to the emergency room within maximally 30 minutes. Patients admitted to the emergency department because of the cardiopulmonary arrest before recording their initial data were excluded from the study.
Supplementary Figure 1.
Flowchart of the study.
Bedside Transthoracic Echocardiography
Transthoracic echocardiography (Philips HD11XE, SONOS 4500, Andover, Mass, USA) was performed according to American Society of Echocardiography and the European Association of Cardiovascular Imaging Guidelines,14 and measures consisting of TAPSE, tricuspid lateral annulus systolic tissue velocity (St), and sPAP-echo from tricuspid regurgitation were acquired by experienced cardiologists.
Computed Tomography Assessment
Using a 64-slice helical CT scanner (Aquilion 64™; Toshiba Medical Systems Corp., Tokyo, Japan) with angiographic contrast material (Omnipaque 350; GE Healthcare, Chicago, IL, USA) were evaluated before and after treatment. Qanadli score (QS) was used for evaluation of PA obstruction,15 and main, right and left PA diameters, RV and LV diameters, and RVFWD were measured from transverse plane 4-chamber view. Right ventricle free-wall diameter and RV transverse diameter were measured at diastole from the basis of RV just above the tricuspid valve. All CT measurements were performed by experienced radiologists.
Definition of Our Composite Index
The index is composed of RV diameter × sPAP-echo / RVFWD × TAPSE. The RV diameter and RVFWD were acquired from initial CTPA, while sPAP-echo and TAPSE were calculated from TTE examination at admission.
Invasive Assessment
Systolic, diastolic, and mean PA pressures were noted in patients who underwent catheter-directed treatment (CDT), including ultrasound-assisted thrombolysis (USAT) and rheolytic thrombectomy (RT).
Treatments
In accordance with currently available ESC/ERS PE Guidelines, all patients in HR and patients at IHR showing findings consistent with clinical and hemodynamic deterioration were treated using systemic thrombolysis (ST) or USAT with different dose and period of recombinant tissue-type plasminogen activator (t-PA). Patients with HR or IHR and having absolute or relative contraindications for t-PA or patients with inadequate response to USAT or ST were treated with RT. The t-PA dose and infusion duration were individually determined for each patient based on the patient’s clinical and hemodynamic status and risk of bleeding. The most widely used CDT modality was USAT with EKOS (EkoSonic® Endovascular System, EKOS Corporation; Bothell, WA, USA) device in our study group, otherwise RT was performed via Angiojet (AngioJet™ Peripheral Thrombectomy System-Boston Scientific, USA) system. The LR and ILR PE groups were mainly treated with anticoagulation alone by intravenous or low-molecular-weight heparin. Long-term follow-up was made in the outpatient clinic within a 3-month period. Follow-up mortality was confirmed from the National Health System registry.
Primary Endpoint
The study has 2 co-primary endpoints. The first one is the in-hospital mortality and the second is the long-term mortality.
Definition of Bleeding
Major bleeding was defined as overt bleeding associated with a fall in the hemoglobin level of at least 2 g/dL or with transfusion of 2 units of packed red blood cells or involvement of a critical site. Clinically overt bleeding not fulfilling the criteria of a major bleeding was classified as a minor bleeding complication.16
Statistical Analyses
The details of the statistical method used in our study are as follows: Whether the continuous variables were normally distributed or not was determined by the Kolmogorov–Smirnov test. Normally distributed continuous variables were expressed as mean ± SD, non-normally distributed continuous variables were expressed as median and interquartile range, and categorical variables were expressed as the number of patients and percentage. Student’s t-test was used for normally distributed continuous variables and Mann–Whitney U-test was used for non-normally distributed variables in comparisons of means between 2 groups. Chi-square or Fisher’s exact test was used for categorical variables. Power analysis was performed to determine the number of patients required for the study. Based on previous studies, the effective power for testing and evaluation was determined as 0.7. Type I error rate is accepted as 0.05 and type II error rate is accepted as 0.2, and it was planned to evaluate 552 patients with PE, assuming 20% data loss might occur. However, it was planned to perform a final analysis with 502 patients after exclusion due to inappropriate data image quality, missing or incomplete data, and retrospective nature of the study. A value of P < .05 was considered to indicate a statistically significant difference. The receiver operating characteristic (ROC) curves were drawn for in-hospital and long-term prognosis and mortality estimation of this proposed formula, and the areas under the curve (AUC) were calculated. Logistic regression analysis was performed for in-hospital events and bleeding, and COX proportional regression analysis was used to determine long-term mortality predictors. Correlation analysis was performed with Pearson and Spearman tests. Statistical Package for Social Sciences for Windows, Version 20.0 (SPSS Inc., Chicago, Ill, USA) and R (Vienna, Austria) programs were used for statistical analysis.
The informed consent form was obtained from each patient enrolled in the study, and the study protocol was approved by the Local Ethics Committee (number: 2018.6/16-132, date: 25/09/2018).
Results
Study group comprised 44 LR (9%), 104 ILR (21%), 296 IHR (59%) and 58 HR (11%) APE patients. Baseline demographic and clinical characteristics of patients were given in Table 1. Systolic blood pressure (mm Hg) (SBP) and pulse oximetry oxygen saturation at room air (%) of combined HR/IHR group were lower than ILR/LR group (115.5 ± 24.8 vs. 129 ± 21.8, P < .001 and 87.5 ± 7 vs. 91.3 ± 4.2 P < .001, respectively) whereas heart rate (bpm) of HR/IHR group was higher than ILR/LR group (108.9 ± 19.8 vs. 96.3 ± 18.4, P < .001). Comparisons of other baseline vital signs, risk stratification parameters, initial TTE, CTPA, and invasive hemodynamic features in terms of APE risk groups are given in Table 2. Beyond the increased RV/LVr as a component of risk definition, patients at HR/IHR compared with those at ILR/LR had significantly higher d-dimer and hs-troponin levels, QS, and sPAP, PA, and RV diameters, but significantly decreased RV free-wall thickness (Table 2).
Table 1.
Baseline Characteristics of Study Group
| Variable | Mean ± SD or Absolute Percentage (%) |
|---|---|
| Age (years) | 61.9 ± 16.9 |
| Female sex [n (%)] | 292 (58.2) |
| Syncope [n (%)] | 150 (29.8) |
| Previous VTE episode [n (%)] | 55 (10.9) |
| Hypertension [n (%)] | 216 (43.2) |
| Diabetes mellitus [n (%)] | 80 (16) |
| Coronary artery disease [n (%)] | 37 (7.5) |
| Atrial fibrillation [n (%)] | 30 (5.9) |
| Chronic lung disease [n (%)] | 55 (11) |
| Acute DVT [n (%)] | 282 (56) |
| Malignancy [n (%)] | 66 (13.1) |
| Postoperative status or permanent immobility [n (%)] | 189 (37.6) |
| Extremity fracture [n (%)] | 28 (5.6) |
| Previous stroke [n (%)] | 36 (7.2) |
| Oral contraceptive drug utilization [n (%)] | 21 (4.3) |
| History of traveling [n (%)] | 17 (3.3) |
| Known thrombophilia [n (%)] | 5 (1) |
| Provoked APE [n (%)] | 387 (72.1) |
| Unprovoked APE [n (%)] | 115 (22.9) |
| Symptom duration before referral to our center (days) | 5.0 (3-9) |
Continuous variables given as mean and SD or median and interquartile range, and categorical variables are given as number and percentage.
APE, acute pulmonary embolism; DVT, deep vein thrombosis; VTE, venous thromboembolism.
Table 2.
Comparison of Baseline Vital Signs, Risk Parameters, Initial TTE, CTPA, and Invasive Hemodynamic Measures in Terms of APE Risk Groups as HR/IHR Versus LR/ILR
| Variable | Overall Population | HR and IHR Groups | LR and ILR Groups | P |
|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | 119.5 (105-133) | 115.5 (100-131) | 129 (112.2-139.7) | <.001 |
| Diastolic blood pressure (mm Hg) | 72.2 (60-82) | 70.7 (60-80) | 75.8 (63.2-85) | .02 |
| Heart rate (bpm) | 105.2 (91-118) | 108.9 (96-120) | 96.3 (82-109.7) | <.001 |
| Oxygen saturation at room air (%) | 88.7 (85.7-93) | 87.5 (82-92) | 91.3 (88-95) | <.001 |
| Shock index (HR/SBP) | 0.94 (0.71-1.06) | 1.02 (0.75-1.13) | 0.77 (0.62-0.9) | <.001 |
| PESI score | 104.8 (78-127) | 113.1 (88.7-131.2) | 85 (60-108) | <.001 |
| PESI class | 3.2 (2-5) | 3.5 (3-5) | 2.5 (1-4) | <.001 |
| sPESI score | 1.4 (1-2) | 1.6 (1-2) | 0.9 (0-1) | <.001 |
| sPAP-echo (mm Hg) | 52.7 (40-60) | 56.2 (45-65) | 44.3 (35-50) | <.001 |
| TAPSE (mm) | 18.6 (15-22) | 17.6 (15-20) | 21.3 (19-24) | <.001 |
| St (cm/s) | 11.3 (9-13) | 10.7 (9-12) | 13.4 (10.4-15) | .17 |
| sPAP (catheter) (mm Hg) | NA | 56.2 ± 15 | NA | |
| dPAP (catheter) (mm Hg) | NA | 16.9 ± 6.7 | NA | |
| mPAP (catheter) (mm Hg) | NA | 30.9 ± 8.6 | NA | |
| RV/LV diameter ratio | 1.18 (1-1.3) | 1.25 (1.1-1.3) | 0.96 (0.85-1) | <.001 |
| RV diameter (mm) | 42.7 (38.6-46.8) | 44.4 (40.2-47.9) | 38 (34-42) | <.001 |
| RVFWD (mm) | 5.77 (4.4-6.6) | 5.47 (4.2-6.2) | 6.66 (5.3-7.5) | <.001 |
| RA/LA diameter ratio | 1.34 (1.13-1.49) | 1.39 (1.2-1.53) | 1.1 (0.97-1.22) | <.001 |
| Qanadli score | 21.8 (17-28) | 24.1 (20-29) | 14.3 (9-20) | <.001 |
| Main PA diameter (mm) | 30.1 (27.1-32.6) | 30.7 (27.9-33) | 28.1 (25-30.3) | <.001 |
| Main PA/aortic diameter ratio | 0.89 (0.79-0.98) | 0.9 (0.8-0.98) | 0.88 (0.76-0.97) | <.001 |
| d-Dimer | 10 (3.6-16) | 11.1 (4.2-18) | 7 (2.6-8.4) | <.001 |
| High-sensitivity troponin | 0.42 (0.04-0.4) | 0.52 (0.06-0.5) | 0.18 (0.009-0.1) | .02 |
Continuous variables given as median and interquartile range.
APE, acute pulmonary embolism; CTPA, computed tomographic pulmonary angiography; dPAP, diastolic pulmonary arterial pressure; HR, heart rate; IHR, intermediate to high risk; ILR, intermediate to low risk; LA, left atrium; LR, low risk; LV, left ventricle; mPAP, mean pulmonary arterial pressure; PA, pulmonary artery; PESI, pulmonary embolism severity index; RA, right atrium; RV, right ventricle; RVFWD, right ventricle free-wall diameter; SBP, systolic blood pressure; sPAP, systolic pulmonary arterial pressure; sPAP-echo, systolic pulmonary arterial pressure measured from echocardiography; sPESI, simplified PESI; St, systolic tissue velocity; TAPSE, tricuspid annular plane systolic excursion; TTE, transthoracic echocardiography.
The ST was documented to be utilized in 83 (20 of them were HR, 63 of them IHR) out of 502 patients with different doses and infusion duration. While 23 patients received 100 mg full dose t-PA, the mean t-PA administration dose was 58.7 ± 29.6 mg and infusion duration was 6.4 ± 4.9 hours in the remainder. In-hospital mortality, major, and minor bleeding rates in the ST group were 10 (12%), 9 (10.8%), and 4 (4.8%), respectively. The USAT was the most frequently preferred CDT modality and was noted in 195 patients [26 (13.3%) HR and 169 (86.7%) IHR], and it was used bilaterally and unilaterally in 161 (82.5%) and 22 (17.5%) of the cases, respectively. Mean 26 ± 6.9 mg dose of t-PA was given in mean 24.1 ± 7.2 hours infusion period. In-hospital mortality, major, and minor bleeding rates in the USAT group were 12 (6.1%), 13 (6.6%), and 23 (11.7%), respectively. In 36 (18.4%) out of 195 patients treated with USAT, neoadjuvant ST with mean t-PA dose of 48.2 ± 27.9 mg and 7.9 hours infusion duration was also utilized. Moreover, 3 patients were additionally treated with RT after USAT because of the inadequate response. The RT as other CDT modality was also performed in 40 patients with HR (2, 5%) or IHR (38, 95%). The bilateral and unilateral RT was performed in 52.5% and 47.5% of patients, and mean RT activation duration was 290.2 ± 85.7 seconds. Mean 15.7 ± 4.9 mg dose of adjuvant t-PA was given via system catheter in 11 (27.5%) cases during the RT procedure. In-hospital mortality, major, and minor bleeding rates in RT group were 4 (10%), 4 (10%), and 3 (7.5%), respectively. Contrast-induced nephropathy was observed in 3 (7.5%) patients treated with RT and all of them resolved without the need of hemodialysis. Other 187 patients were treated with anticoagulation alone with unfractionated heparin or low-molecular-weight heparin, and in-hospital mortality, major, and minor bleeding rates in this group were 11 (5.8%), 4 (2.1%), and 2 (1%), respectively. Pre- and post-treatment clinical, hemodynamic, TTE, and CTPA changes are summarized in Supplementary Table 1. Overall number of in-hospital and long-term mortality was 37 (7.3%) and 54 (10.7%), respectively. In-hospital and long-term mortality and major and minor bleeding rates among 4 different treatment arms are summarized in Supplementary Table 2.
Supplementary Table 1.
Pre and post-treatment clinical, hemodynamic, TTE and CTPA changes in four different treatment arms
| ST | USAT | RT | Anticoagulation alone | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Systolic BP (mm Hg) | 110±30.9 | 123.4±14.4 | 116.2±21.7 | 122.3±17 | 122.5±21.4 | 120.9±12.6 | 126.2±23.3 | 124±15.9 |
| Diastolic BP (mm Hg) | 64.5±23.5 | 71.5±12.8 | 73.5±13.3 | 72.6±9.3 | 70.3±16.6 | 72.6±14.2 | 75±19.6 | 75.3±11.4 |
| Heart rate (bpm) | 112.8±24.1 | 80.4±15.5 | 106.7±17.5 | 81.1±12.5 | 109.7±20.8 | 82±9.8 | 99.5±19.7 | 81.4±11.3 |
| Oxygene saturation (%) | 85.6±10.6 | 94±11.8 | 88.1±4.8 | 93.9±7.8 | 87.6±7.5 | 94.8±2.2 | 90.7±4.6 | 95.6±8.5 |
| sPAP – echo (mm Hg) | 54.8±13 | 35.6±13.1 | 55.7±12.5 | 35.9±9.4 | 59.4±14.4 | 40±16.4 | 47.1±14.4 | 35±14.9 |
| TAPSE (mm) | 17.4±4.4 | 23±3.6 | 17.9±3.9 | 22.5±3.9 | 17.6±3.4 | 22.1±2.9 | 20.3±4.9 | 22.7±5 |
| St (cm/sec) | 10.2±1.9 | 13.3±2.4 | 10.8±2.6 | 13.9±2.4 | 10.7±2.2 | 13.5±2 | 11.9±2.5 | 13.4±2.9 |
| RV/LV ratio | 1.26±0.19 | 0.94±0.13 | 1.21±0.19 | 0.92±0.11 | 1.31±0.21 | 0.96±0.17 | 1.07±0.21 | 0.93±0.13 |
| RA/LA ratio | 1.13±0.1 | 0.87±0.14 | 1.38±0.27 | 1.17±0.19 | 1.44±0.51 | 1.19±0.18 | 1.17±0.25 | 0.99±0.15 |
| RVFWD (mm) | 5.67±2.04 | 7.54±2 | 5.45±1.68 | 6.84±1.73 | 6.14±2.12 | 7.73±2 | 6.16±1.86 | 6.88±2 |
| Qanadli score | 23.3±7.7 | 7.8±5.4 | 24.9±6.2 | 9.9±5.6 | 23.6±6.3 | 12.2±7.2 | 16.1±7.5 | 8.6±7.1 |
| MPA diameter (mm) | 30.3±4.7 | 27.1±4.6 | 30.9±4.3 | 29.3±16.3 | 30.7±3.6 | 28.6±4.3 | 28.6±4.5 | 27.2±4 |
| RPA diameter (mm) | 22.8±3.8 | 20.7±4 | 25.3±19.6 | 21.7±3.4 | 24.2±3.1 | 22.8±3.6 | 22.6±3.2 | 21.7±3.2 |
| LPA diameter (mm) | 22.2±3.1 | 20.3±3.3 | 23.1±3 | 21.2±3.3 | 22.9±2.7 | 21.8±3.1 | 21.9±3.1 | 20.8±2.9 |
| sPAP-catheter (mm Hg) | NA | NA | 55.3±15.3 | 40.5±12 | 60.4±12.9 | 46.8±10.5 | NA | NA |
| dPAP-catheter (mm Hg) | NA | NA | 16.7±6.7 | 12.7±5.5 | 18±6.6 | 15.4±5.2 | NA | NA |
| mPAP-catheter (mm Hg) | NA | NA | 30.4±8.8 | 22.6±7.1 | 33.3±7.5 | 25.8±7.2 | NA | NA |
Continuous variables given as mean and standard deviation.
ST, systemic thrombolysis, USAT, ultrasound-assisted thrombolysis, RT, rheolytic thrombectomy, BP, blood pressure, sPAP-echo, systolic pulmonary arterial pressure measured from echocardiography, TAPSE, tricuspid annular plane systolic excursion, St, systolic tissue velocity, RV, right ventricle, LV, left ventricle, RA, right atrium, LA, left atrium, RVFWD, right ventricle free wall diameter, MPA, main pulmonary artery, RPA, right pulmonary artery, LPA, left pulmonary artery, sPAP, systolic pulmonary arterial pressure, dPAP, diastolic pulmonary arterial pressure, mPAP, mean pulmonary arterial pressure
Supplementary Table 2.
In-hospital and long-term mortality, major and minor bleeding rates in terms of four different treatment methods
| ST (n=83) | USAT (n=195) | RT (n=40) | Anticoagulation alone (n=187) | |
|---|---|---|---|---|
| Major bleeding | 9 (10.8%) | 13 (6.6%) | 4 (10%) | 4 (2.1%) |
| Minor bleeding | 4 (4.8%) | 23 (11.7%) | 3 (7.5%) | 2 (1%) |
| In-hospital mortality | 10 (12%) | 12 (6.1%) | 4 (10%) | 11 (5.8%) |
| Long-term mortality | 11 (13.2%) | 15 (7.6%) | 5 (12.5%) | 23 (12.2%) |
ST, systemic thrombolysis, USAT, ultrasound-assisted thrombolysis, RT, rheolytic thrombectomy
The index value was found to be significantly correlated with the clinical, hemodynamic, and CTPA measures of APE severity (Table 3). In univariable logistic regression analysis, the value of our composite index was not associated with increased in-hospital mortality [adjusted OR: 1.52 (0.71-3.24), P = .17] (Table 4). Although PESI, RV/LVr, and QS were significantly associated with in-hospital mortality in univariable analysis, only PESI remained as significant in multivariable analysis (adjusted OR: 5.08; 1.44-17.89, P < .001). However, the higher index value related to a higher long-term mortality both in univariable Cox regression (HR: 1.62; 1.07-2.46, P = .03) and multivariable Cox regression (HR: 1.53; 1.01-2.35, P = .04) analyses. The relation between all variables and long-term mortality is given in Table 5. The ROC curve analysis showed that 17.8 was the optimum value of the index in predicting the long-term mortality with a sensitivity of 70% and specificity of 40% (AUC = 0.652, 95% CI; 0.557-0.747, P = .001) (Figure 1). Adjusted variable plot demonstrated that the risk increased until an index level of 30, and after this threshold, there was no change in long-term mortality risk (Figure 2). The maximally selected rank statistics cut-points, which provide us, the classification of index into 2 groups for prediction mortality (Figure 3A). The cumulative hazard curve showed a higher mortality in high-index value group compared with low-index value group (log-rank test P-value .03) (Figure 3B).
Table 3.
Correlation Between Current Formula and Patient’s Baseline Characteristics
| Variable | r (Correlation Factor) | P |
|---|---|---|
| Age | 0.131 | .033 |
| Systolic BP | −0.396 | <.001 |
| Diastolic BP | −0.254 | <.001 |
| Heart rate | 0.380 | <.001 |
| Oxygen saturation | −0.408 | <.001 |
| PESI | 0.488 | <.001 |
| PESI class | 0.456 | <.001 |
| sPESI | 0.421 | <.001 |
| Shock index | 0.450 | <.001 |
| St | −0.456 | <.001 |
| Qanadli score | 0.602 | <.001 |
| RV/LV diameter ratio | 0.572 | <.001 |
| RA/LA diameter ratio | 0.321 | <.001 |
| Main PA diameter | 0.335 | <.001 |
| RVFWD | −0.574 | <.001 |
| d-Dimer | 0.243 | .001 |
| Troponin | 0.238 | <.001 |
BP, blood pressure; LA, left atrium; LV, left ventricle; PA, pulmonary artery; PESI, pulmonary embolism severity index; RA, right atrium; RV, right ventricle; RVFWD, right ventricle free-wall diameter; sPESI, simplified PESI; St, systolic tissue velocity.
Table 4.
Univariable and Multivariable Logistic Regression Analyses for In-Hospital Mortality
| Variable | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| PESI (from 78 to 127) | 6.27 (1.90-20.64) | <.001 | 5.08 (1.44-17.89) | <.001 |
| Admission RV/LV ratio (1.05-1.31) | 1.50 (1.03-2.21) | .03 | 0.80 (0.46-1.40) | .44 |
| Qanadli score (from 17 to 28) | 2.34 (1.29-4.24) | .004 | 1.84 (0.88-3.84) | .10 |
| Formula (from 14.4 to 36.6) | 1.47 (0.76-2.86) | .26 | 1.52 (0.71-3.24) | .17 |
| sPAP-echo (from 40 to 62) | 1.53 (0.85-2.75) | .15 | 1.50 (0.76-2.94) | .23 |
LV, left ventricle; OR, odds ratio; PESI, pulmonary embolism severity index; RV, right ventricle; sPAP-echo, systolic pulmonary arterial pressure measured from echocardiography.
Table 5.
Univariable and Multivariable Cox Proportional Regression Analyses for Long-term Mortality
| Univariable Cox | Multivariable Cox | |||
|---|---|---|---|---|
| Variable | Crude HR (95% CI) | P | Adjusted HR (95% CI) | P |
| PESI (from 78 to 127) | 3.73 (2.20-6.31) | <.001 | 3.58 (2.08-6.16) | <.001 |
| Admission RV/LV ratio (1.05 to 1.31) | 1.17 (0.92-1.47) | .18 | 0.90 (0.66-1.20) | .45 |
| Qanadli score (from 17 to 28) | 1.30 (0.93-1.82) | .11 | 1.08 (0.72-1.61) | .70 |
| Formula (from 14.4 to 36.6) | 1.62 (1.07-2.46) | .03 | 1.53 (1.01-2.35) | .04 |
| sPAP-echo (from 40 to 62) | 1.23 (0.87-1.76) | .23 | 1.09 (0.73-1.61) | .67 |
HR, hazard ratio; LV, left ventricle; PESI, pulmonary embolism severity index; RV, right ventricle; sPAP-echo, systolic pulmonary arterial pressure measured from echocardiography.
Figure 1.
ROC curve analysis of the formula for predicting long-term mortality.
Figure 2.
Adjusted variable plot analysis of the formula.
Figure 3.
The maximally selected rank statistics cut-points, which provide us the classification of the index into 2 groups for prediction mortality (A). Cumulative hazard curve showed a higher mortality in high-index value group compared with low-index value group (log-rank test P-value .03) (B).
Discussion
Our novel composite index which is acquired from initial TTE and CTPA parameters was significantly correlated with the severity measures of clinical and hemodynamic status and associated with long-term mortality but not with in-hospital mortality in patients with APE. Only PESI was found to be an independent predictor for in-hospital mortality, as expected. A value of our index higher than 17.8 predicted the long-term mortality in these patients with a sensitivity of 70% and specificity of 40%. The risk of long-term mortality increased until an index level of 30. Moreover, beyond the increased RV/LVr as a component of risk evaluations, HR/IHR compared with ILR/LR were associated with significantly higher QS, right atrial/left atrial ratio, sPAP, PA, and RV diameters, and d-dimer and hs-troponin levels but significantly decreased RV free-wall thickness.
Selecting the optimal treatment strategy between different options regarding patients’ clinical characteristics bleeding risk, and hemodynamic and imaging features has remained a pivotal issue in APE. Despite the presence of various risk prediction models and combination of some clinical and imaging parameters, the optimal model for predicting in-hospital and long-term outcomes with adequate sensitivity and specificity in patients with APE remains to be determined.17,18 Although CTPA is the gold standard diagnostic tool for APE, TTE is also a useful method for initial risk stratification and follow-up after selected treatments. In some previous meta-analyses, even in hemodynamically stable patients at the time of admission, the presence of RV dysfunction on TTE was found to be associated with an increased short-term mortality.10,19 In parallel, it was reported that both RV/LVr ≥1 and TAPSE <16 mm predicted poor prognosis.20 In our study, RV/LVr, QS, and main PA diameter were higher in IHR/HR group as compared to those in patients at ILR/LR. The most commonly utilized scoring system for predicting the mortality of APE patients is PESI and its simplified version sPESI. The 30-day mortality for patients with PESI class ≥3 and sPESI ≥1 versus PESI class I-II and sPESI class were 10.9% and 1%, respectively. The well-known main limitation of this validated system is being compounded with only patients’ initial clinical characteristics such as heart rate, blood pressure, oxygen saturation, respiratory rate, and consciousness status. However, it is known that TTE and CTPA features also widely influence the prognosis in APE.21 Moreover a low sPESI score solely may not always ensure a good prognosis for a patient with APE.22 A previous multi-center randomized clinical trial in which safety of early discharge was investigated, including 132 LR APE patients was prematurely terminated because of the high mortality.23 In a large retrospective analysis, thrombus volume as measured on CTPA was found to be strongly associated with Qanadli (r = 0.841, P < .001) and Mastora (r = 0.863, P < .001) scores and moderately associated with RV/LVr (r = 0.378, P < .001). Moreover, in multivariate analysis, RV/LVr >1.0 was found as an independent predictor for in-hospital mortality.24 A subgroup analysis of RIETE registry revealed the importance of early TTE examination, and right atrial dilatation (OR: 3.74, 95% CI: 2.10-6.66) and RV hypokinesia (OR: 3.11, 95% CI: 1.85-5.21) were reported to be associated with increased 30-day mortality.25 In addition to the absence of imaging features in PESI, syncope which reflects the severity of APE is not a part of this scoring system.26,27
Individualized risk stratification has also been pivotal in determining the evidence-based treatment modalities in patients with APE. In the setting of HR APE with hemodynamic instability, ST is recommended in order to achieve rapid PA reperfusion, unless any relative or absolute contraindication for t-PA.28,29 On the other hand, making a decision for patients with IR APE and HR APE with high bleeding risk is more confusing regarding many different clinical settings. Although in a large randomized PEITHO trial, ST showed no benefit for IR APE,30 a group of patients in IR PE especially concomitant clinical severity criteria and RV dysfunction may need further treatment beyond anticoagulation alone. Therefore, improved risk assessment strategies are required among the patients with IR PE for discriminating accurate candidates having benefit from ST or CDTs.31 Catheter-directed treatments are basically recommended for patients with HR and IHR APE.13,32 The most commonly utilized CDT modality both worldwide and in our current study is USAT via EKOS system, and its safety and efficacy issues have been documented in a randomized controlled trial, consecutive prospective single- or multi-center studies, and meta-analyses.33-38 Catheter-directed embolectomy such as Flowtriever®39 and AngioJet® RT system40 is also another favorable option for selected patients with IHR and HR PE with absolute contraindication for ST or surgical embolectomy and as an adjuvant therapy after failure of ST.
In our study, comparisons of the HR/IHR versus ILR/LR APE groups provided highly consistent data regarding the severity of the acute thrombo-obstructive burden, pressure mismatch, and strain over RV. Not only RV/LVr is already a component of the initial risk evaluation but also significantly higher QS, right atrial/left atrial ratio, sPAP, PA and RV diameters, and d-dimer and hs-troponin levels and a lower RV free-wall thickness were found to discriminate the patients at HR/IHR from those at ILR/LR status. Our novel index is composed of RV diameter and RVFWD acquired from initial CTPA and sPAP-echo and TAPSE calculated by TTE at initial evaluation. In our hypothesis inspired from the original Laplace formula, numerator (RV diameter × sPAP-echo) and denominator (RVFWD × TAPSE) were assumed to represent 2 sides of pressure/wall stress relationship, respectively. It has been considered that multiplying the RVFWD and TAPSE might provide additional information regarding the radial and longitudinal endurance of RV free-wall against acute-onset pressure overload. Recently, TAPSE/sPAP ratio (mm/mm Hg) was introduced into the diagnostic and prognostic evaluation algorithms in ESC/ERS 2022 Pulmonary Hypertension Guidelines.41 This index showed significant and clinically relevant correlations to the severity measures of clinical and hemodynamic status, and a higher value was found to be associated with long-term mortality but not with in-hospital mortality. Only PESI independently predicted in-hospital mortality. This discrepancy between in-hospital and long-term outcome predictions may be caused by a low number of early outcomes because CI is wide [adjusted OR: 1.52 (0.71-3.24, P = .17)]. According to the adjusted variable plot, the risk of long-term mortality increased until an index level of 30 but remained unchanged thereafter. The cumulative hazard curve showed a higher mortality with high-index value versus low-index value.
Study Limitations
Absence of the prospective follow-up should be considered as the first important limitation of this study. However, nearly complete follow-up in the registered APE patients may be considered as an important factor in a retrospective analysis. Despite some possible explanations, the aforementioned discrepancy regarding the in-hospital outcome prediction remains as another limitation of this study. The RV and RWFWD assessed by CTPA may not coincide with the phasic changes in RV volume and wall thickening. Simultaneous invasive and echocardiographic assessments might provide more robust data regarding the relation RV function against acute pressure strain in APE. Additionally, 70% of the patients were at HR or IHR status as a result of the referral pattern to our tertiary APE center, and this may lead to some difficulties in the comparison of 4 risk groups.
Conclusion
In addition to RV/LVr, the higher QS, sPAP, PA, and RV diameters and d-dimer and hs-troponin levels and a lower RV free-wall thickness seem to discriminate the HR/IHR versus ILR/LR status in patients with APE. Our index derived from initial TTE and CTPA assessments showed significant correlations to severity measures and was associated with long-term mortality but not with in-hospital mortality. An index value higher than 17.8 predicted long-term mortality, and this risk seemed to increase until an index level of 30. Only PESI remained as the independent predictor for in-hospital mortality. The issue of whether this index may provide additional benefit in the risk prediction of patients with APE needs to be determined with further studies.
Footnotes
Ethics Committee Approval: Informed consent form was obtained from each patient enrolled in the study for both medical and interventional treatments, and the study protocol was approved by Local Institutional Ethics Committee (number: 2018.6/16-132, date: 25/09/2018).
Informed Consent: Written informed consent was obtained from the patients/patient who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.H., C.K.; Design – A.K., C.K.; Supervision – İ.H.T., N.Ö.; Resources – S.T., A.T.; Materials – B.K., Ş.K.; Data Collection and/or Processing – A.T., S.T.D.; Analysis and/or Interpretation – A.K., İ.H.T., S.Ç.E.; Literature Search – A.H., Ö.Y.A.; Writing – A.H., C.K.; Critical Review – H.C.T.D., Z.B., C.D.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340 1347. ( 10.1161/CIRCRESAHA.115.306841) [DOI] [PubMed] [Google Scholar]
- 2. Minges KE, Bikdeli B, Wang Y, et al. National trends in pulmonary embolism hospitalization rates and outcomes for adults aged ≥65 Years in the United States (1999 to 2010). Am J Cardiol. 2015;116(9):1436 1442. ( 10.1016/j.amjcard.2015.07.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donzé J, Le Gal G, Fine MJ, et al. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008;100(5):943 948. ( 10.1160/th08-05-0285) [DOI] [PubMed] [Google Scholar]
- 4. Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383 1389. ( 10.1001/archinternmed.2010.199) [DOI] [PubMed] [Google Scholar]
- 5. Jia D, Zhou XM, Hou G. Estimation of right ventricular dysfunction by computed tomography pulmonary angiography: a valuable adjunct for evaluating the severity of acute pulmonary embolism. J Thromb Thrombolysis . 2017;43(2):271 278. ( 10.1007/s11239-016-1438-0) [DOI] [PubMed] [Google Scholar]
- 6. Meinel FG, Nance JW, Schoepf UJ, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med. 2015;128(7):747 59.e2. ( 10.1016/j.amjmed.2015.01.023) [DOI] [PubMed] [Google Scholar]
- 7. Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest. 2004;125(4):1539 1545. ( 10.1378/chest.125.4.1539) [DOI] [PubMed] [Google Scholar]
- 8. Ribeiro A, Lindmarker P, Johnsson H, Juhlin-Dannfelt A, Jorfeldt L. Pulmonary embolism: one-year follow-up with echocardiography Doppler and five-year survival analysis. Circulation. 1999;99(10):1325 1330. ( 10.1161/01.cir.99.10.1325) [DOI] [PubMed] [Google Scholar]
- 9. Frémont B, Pacouret G, Jacobi D, Puglisi R, Charbonnier B, de Labriolle A. Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: results from a monocenter registry of 1,416 patients. Chest. 2008;133(2):358 362. ( 10.1378/chest.07-1231) [DOI] [PubMed] [Google Scholar]
- 10. Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care. 2011;15(2):R103. ( 10.1186/cc10119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40(11):902 910. ( 10.1093/eurheartj/ehy873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033 3069. ( 10.1093/eurheartj/ehu283) [DOI] [PubMed] [Google Scholar]
- 13. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41(4):543 603. ( 10.1093/eurheartj/ehz405) [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor-Avi V, et. al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1 39.e14. ( 10.1016/j.echo.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 15. Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176(6):1415 1420. ( 10.2214/ajr.176.6.1761415) [DOI] [PubMed] [Google Scholar]
- 16. Schulman S. Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692 694. ( 10.1111/j.1538-7836.2005.01204.x) [DOI] [PubMed] [Google Scholar]
- 17. Konstantinides SV, Barco S, Lankeit M, Meyer G. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016;67(8):976 990. ( 10.1016/j.jacc.2015.11.061) [DOI] [PubMed] [Google Scholar]
- 18. Meyer G, Planquette B, Sanchez O. Pulmonary embolism: whom to discharge and whom to thrombolyze? J Thromb Haemost. 2015;13(suppl 1):S252 S258. ( 10.1111/jth.12944) [DOI] [PubMed] [Google Scholar]
- 19. Sanchez O, Trinquart L, Colombet I, et. al. Prognostic value of right ventricular dysfunction in patients with 3069ème ESC Guidelines Downloaded from by guest on August 1, 2015 haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569 1577. ( 10.1093/eurheartj/ehn208) [DOI] [PubMed] [Google Scholar]
- 20. Pruszczyk P, Goliszek S, Lichodziejewska B, et al. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc Imaging. 2014;7(6):553 560. ( 10.1016/j.jcmg.2013.11.004) [DOI] [PubMed] [Google Scholar]
- 21. Dentali F, Cei M, Mumoli N, Gianni M. How to predict short- and long-term mortality in patients with pulmonary embolism? Pol Arch Med Wewn. 2015;125(1-2):82 88. ( 10.20452/pamw.2652) [DOI] [PubMed] [Google Scholar]
- 22. Hellenkamp K, Kaeberich A, Schwung J, Konstantinides S, Lankeit M. Risk stratification of normotensive pulmonary embolism based on the sPESI – does it work for all patients? Int J Cardiol. 2015;197:162 163. ( 10.1016/j.ijcard.2015.06.065) [DOI] [PubMed] [Google Scholar]
- 23. Otero R, Uresandi F, Jiménez D, et al. Home treatment in pulmonary embolism. Thromb Res. 2010;126(1):e1 e5. ( 10.1016/j.thromres.2009.09.026) [DOI] [PubMed] [Google Scholar]
- 24. Furlan A, Aghayev A, Chang CCH, et. al. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265(1):283 293. ( 10.1148/radiol.12110802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bikdeli B, Lobo JL, Jiménez D, et al. Early use of echocardiography in patients with acute pulmonary embolism: findings from the RIETE registry. J Am Heart Assoc. 2018;7(17):e009042. ( 10.1161/JAHA.118.009042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Winter MA, van Bergen EDP, Welsing PMJ, Kraaijeveld AO, Kaasjager KHAH, Nijkeuter M. The prognostic value of syncope on mortality in patients with pulmonary embolism: A systematic review and meta-analysis. Ann Emerg Med. 2020;76(4):527 541. ( 10.1016/j.annemergmed.2020.03.026) [DOI] [PubMed] [Google Scholar]
- 27. Keskin B, Tokgöz HC, Akbal ÖY, et. Al. Clinical, imaging and hemodynamic correlates and prognostic impact of syncope in acute pulmonary embolism: A single-center study. Turk Gogus Kalp Damar Cerrahisi Derg. 2022;30(3):317 326. ( 10.5606/tgkdc.dergisi.2022.22798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H, Kasper W. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol. 1998;82(8):966 970. ( 10.1016/s0002-9149(98)00513-x) [DOI] [PubMed] [Google Scholar]
- 29. Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341(8844):507 511. ( 10.1016/0140-6736(93)90274-k) [DOI] [PubMed] [Google Scholar]
- 30. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402 1411. ( 10.1056/NEJMoa1302097) [DOI] [PubMed] [Google Scholar]
- 31. Barco S, Vicaut E, Klok FA, et al. Improved identification of thrombolysis candidates amongst intermediate-risk pulmonary embolism patients: implications for future trials. Eur Respir J. 2018;51(1):1701775. ( 10.1183/13993003.01775-2017) [DOI] [PubMed] [Google Scholar]
- 32. Rivera-Lebron B, McDaniel M, Ahrar K, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037. ( 10.1177/1076029619853037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479 486. ( 10.1161/CIRCULATIONAHA.113.005544) [DOI] [PubMed] [Google Scholar]
- 34. Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8(10):1382 1392. ( 10.1016/j.jcin.2015.04.020) [DOI] [PubMed] [Google Scholar]
- 35. Kaymaz C, Akbal OY, Tanboga IH, et al. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: A meta-analysis. Curr Vasc Pharmacol. 2018;16(2):179 189. ( 10.2174/1570161115666170404122535) [DOI] [PubMed] [Google Scholar]
- 36. Kaymaz C, Öztürk S, Akbal Ö, et al. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: results from a single-center cohort. Angiology. 2017;68(5):433 440. ( 10.1177/0003319716661446) [DOI] [PubMed] [Google Scholar]
- 37. Kaymaz C, Akbal OY, Hakgor A, et al. A five-year, single-centre experience on ultrasound-assisted, catheter-directed thrombolysis in patients with pulmonary embolism at high risk and intermediate to high risk. EuroIntervention. 2018;14(10):1136 1143. ( 10.4244/EIJ-D-18-00371) [DOI] [PubMed] [Google Scholar]
- 38. Kaymaz C, Akbal OY, Keskin B, et al. An eight-year, single-center experience on ultrasound assisted thrombo-lysis with moderate-dose, slow-infusion regimen in pulmonary embolism. Curr Vasc Pharmacol. 2022;20(4):370 378. ( 10.2174/1570161120666220428095705) [DOI] [PubMed] [Google Scholar]
- 39. Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. 2019;12(9):859 869. ( 10.1016/j.jcin.2018.12.022) [DOI] [PubMed] [Google Scholar]
- 40. Akbal ÖY, Keskin B, Tokgöz HC, et al. A seven-year single-center experience on AngioJet rheolytic thrombectomy in patients with pulmonary embolism at high risk and intermediate-high risk. Anatol J Cardiol. 2021;25(12):902 911. ( 10.5152/AnatolJCardiol.2021.28303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618 3731. ( 10.1093/eurheartj/ehac237) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a