Abstract
Spermidine have been reported a role in antioxidative, antiaging, and antiinflammatory. Oxidative stress causes granulosa cell (GC) apoptosis, follicular atresia, and impairs poultry reproductive functions. Studies have found that autophagy is the protective mechanism against antioxidant stress and apoptosis in cells. However, the relationship between spermidine-induced autophagy, oxidative stress, and apoptosis in goose GCs remains unclear. In this study, we investigated the autophagy mechanism to mediate spermidine effects on the alleviation of oxidative stress and apoptosis in goose GCs. Follicular GCs were treated with spermidine combination with 3-Nitropropanoic acid (3-NPA), rapamycin (RAPA), and chloroquine (CQ) or with hydrogen peroxide, RAPA, and CQ. Spermidine upregulated the ratio of LC3-II/I, inhibited the accumulation of p62 protein, and induced autophagy. 3-NPA treatment significantly increased ROS production, MDA content, SOD activity, cleaved CASPASE-3 protein expression, and decreased BCL-2 protein expression in follicular GCs. Spermidine inhibited oxidative stress and apoptosis induced by 3-NPA. In addition, hydrogen peroxide-induced oxidative stress was inhibited by spermidine. However, the inhibitory effect of spermidine was eliminated under chloroquine. Our results demonstrated that spermidine relieved oxidative stress and apoptosis of GCs by inducing autophagy, indicating that spermidine has a great potential to maintain proteostasis and sustain granulosa cell viability in geese.
Key words: apoptosis, autophagy, oxidative stress, granulosa cell, spermidine
INTRODUCTION
Spermidine is a natural autophagy inducer and antioxidant stress and has good ameliorating effects on neurological, cardiovascular and cancer-related diseases (Madeo et al., 2018; Omar et al., 2021) Recent studies have implicated spermidine in antioxidative stress, antiapoptosis, and induced autophagy (Eisenberg et al., 2009; Yousefi-Manesh et al., 2023). Therefore, the role of spermidine in inducing autophagy and antioxidative stress has been a hot topic in recent years.
Oxidative stress has adverse effects on animal reproductive function. It is also one of the major reasons of follicular atresia and decreased egg production in poultry (Devine et al., 2012; Wang et al., 2019). Autophagy can remove oxidized proteins, DNA and lipids in cells and alleviate the damage caused by oxidative stress damage (Filomeni et al., 2015; Gao, 2019). Through the p62-related pathway, the molecular chaperone-mediated related pathway and the mitotic phagocytosis pathway, autophagy can regulate reactive oxygen species (ROS) (Li et al., 2015; Ornatowski et al., 2020), but the exact mechanism remains unclear. Therefore, the mechanism by which autophagy regulates oxidative stress needs further study.
Spermidine can directly or indirectly upregulate the mRNA levels of NRF2, HO-1, NQO1, and other proteins through the NRF2-ARE signaling pathway(Das and Misra, 2004, Yang et al., 2013 ) and reduce the ROS and malondialdehyde (MDA) levels in cells and tissues to play an antioxidant role in the zebrafish body (Jeong et al., 2018), liver (Liu et al., 2019; Adhikari et al., 2021), brain (Yadav et al., 2018), myocardial tissue (Chai et al., 2019), cartilage tissue (Che et al., 2022), and renal tubular cells (Kim, 2017) or reduce oxidative stress damage to cells by improving polyamine metabolism and maintaining intracellular homeostasis (Kim et al., 2021). Exogenous spermidine supplementation can increase the expression of the ATG3, LC3B, and ULK1 genes in the brain tissue of aging rats, increase the activities of CAT and SOD, reduce the levels of lipid peroxide, protein carbonyl, nitric oxide, and ROS, and enhanced the activity of the electron transfer chain complex in the brain mitochondria of aging rats (Singh et al., 2021). Similarly, spermidine and rapamycin can increase SOD activity in aging model mice, reduce MDA levels in the brain tissue of aging model mice, and increase AMPK phosphorylation levels and LC3, Beclin-1, and p62 expression (Xu et al., 2020), suggesting that spermidine can improve aging-induced oxidative stress by regulating autophagy and antioxidant levels.
In a study by Zheng et al. (2018), spermidine induced autophagy in rat nucleus pulposus cells by promoting LC3-II, ATG7, and Beclin-1 protein expression and p62 protein degradation, which was inhibited by oxidative stress-induced apoptosis. Shortly afterwards, Yuan et al. (2021) found that spermidine could induce autophagy by upregulating LC3-II and p62 protein expression in female reproductive stem cells, increase autophagy flux and ameliorate oxidative stress-induced ageing. Moreover, after chloroquine (CQ) treatment blocked autophagy, spermidine could not exert the above protective effects. Similarly, H2O2 can induce oxidative stress in chondrocytes. Spermidine can increase the expression of the Beclin1 protein in chondrocytes, upregulate the expression of the LC3-II and p62 genes, induce mitochondrial autophagy, increase autophagy flux, and prevent chondrocyte death and DNA oxidative damage caused by oxidative stress (D'Adamo et al., 2020). This suggests that spermidine induces autophagy to exert an antiapoptotic function. However, in recent years there has been considerable evidence that autophagy can induce apoptosis and enhance cytotoxicity (He et al., 2021). These results show that autophagy has a dual role in inhibiting and promoting apoptosis.
Autophagy plays a crucial regulatory role in follicular development in geese (Yu et al., 2016a,b). Previous reports confirmed that H2O2 regulated autophagy by decreasing mTOR and increasing p53, while enhanced autophagy reduced ROS-induced damage. Both ROS and autophagy played important roles in regulating follicular development to control broodiness in geese (Lou et al., 2017). However, whether spermidine- induced autophagy plays an antioxidative stress and antiapoptotic role remains to be elucidated. Therefore, 3-NPA and hydrogen peroxide were used to establish an oxidative stress model, and spermidine, rapamycin and chloroquine were used to treat GCs to study and reveal the mechanism by which spermidine induces autophagy and the antioxidative stress and antiapoptotic functions of spermidine in GCs. Our data provide a reference for studying and clarifying the mechanism of spermidine-induced autophagy against oxidative stress and apoptosis.
MATERIAL AND METHODS
Culture and Treatment of Primary Follicular GCs
The Sichuan white goose experimental protocols were approved by the Animal Ethics Committee of the College of Animal Science and Technology at Sichuan Agricultural University (permit number: DKY-B2019202018). Sichuan white geese reared in the same environment and at the peak of egg production were selected. F2∼F4 hierarchical follicles were collected quickly after sacrifice and follicular GCs were isolated and cultured. Briefly, the layer of follicular granulosa cells was carefully removed from the follicular wall in a sterile manner. After 3 washes in PBS and dispersed with 0.1% type Ⅱ collagenase (Sigma-Aldrich, MI) for 5 min at 37°C, the cell were sieved with 200 mesh filter into new centrifuge tube and were centrifuged at 1,000 × g for 10 min. Follicular GCs were cultured in DMEM/F12 (HyClone, Shanghai, China) with fetal bovine serum (Gibco, South America) at a concentration of 10% in a 37%, 5% CO2 incubator after adjusting the cell density to 1 × 106. When the cell density per well reached more than 80%, the wells were pretreated with 3-NPA or hydrogen peroxide and incubated with spermidine (rapamycin or chloroquine).
Cell Viability Analysis
Cell viability was determined by Cell Counting (CCK-8) Kit-8 assay. Follicular GCs were seeded and treated in 96-well plates. After 24 h, 10 μL of CCK-8 solution (Beyotime, Shanghai, China) was added to each well, shaken and incubated for 2 h at 37°C. Finally, the well OD was measured at 450 nm using enzyme calibration (Molecular Devices, San Jose, CA).
Measurement of ROS Production
Cells were pretreated with 3-NPA and incubated with spermidine (rapamycin or chloroquine). The DCFH-DA reaction mixture was prepared according to the instructions (Beyotime), incubated with DMEM containing cells for 20 min at 37°C, and mixed upside down every couple of minutes. Next, we observed the cells by fluorescence microscopy and measured their fluorescence.
Detection of MDA Levels
Cells were pretreated with 3-NPA or hydrogen peroxide and incubated with spermidine (rapamycin or chloroquine). TBA storage solution and MDA working solution were combined, and each well was added according to the MDA instructions. The samples were treated according to the MDA instructions, and the MDA content in the tissues was measured and calculated.
Detection of SOD Activity
Cells were pretreated with 3-NPA or hydrogen peroxide and incubated with spermidine (rapamycin or chloroquine). The protein concentration was detected by BCA kit. The samples were prepared according to the instructions of the SOD kit, and the total SOD activity was detected and calculated.
Electromicrospy
The Follicular GCs were collected in a centrifuge tube by trypsinization and centrifugation at 1,000 × g for 5 min, and prefixed with 2.5% glutaraldehyde (12 h, 4°C). Each sample was postfixed with 1% osmium tetroxide, dehydrated sequentially with ethanol and acetone, and embedded in EP812. Sections were generated on an ultratome. After staining with uranyl acetate and alkaline lead citrate for 2 min, the sections were observed by TEM (JEM-1400FLASH, Tokyo, Japan).
Measurement of Cell Apoptosis
Cells were pretreated with 3-NPA or hydrogen peroxide and incubated with spermidine (rapamycin or chloroquine). Apoptosis was detected by Annexin V-FITC/PI (Beyotime). Each tube of the sample was added to 2.5 μL of PE and Annexin fuel, whisked and mixed, and incubated at room temperature for 20 min (dark). The apoptotic rate was calculated with a FACSCalibur flow cytometer (BD Biosciences, NJ).
Analysis of Autophagy Protein Expression
Cells were pretreated with 3-NPA or hydrogen peroxide and incubated with spermidine (rapamycin or chloroquine). Cells were lysed with RIPA buffer (including 0.1% phenylmethylsulfonyl fluoride), and the supernatant was collected after centrifugation. The protein concentration was measured by BCA Protein Assay Kit (Beyotime). Protein samples (30 μg) were separated on 12% SDS‒PAGE gels, transferred to PVDF membranes, and then incubated with primary antibody at 4°C overnight after blocking with 5% skim milk. Antibody dilution ratio: Anti-LC3 and auto-p62 (Proteintech, Wuhan, China) were diluted at 1:1,000, and anti-β-actin was diluted at 1:2,000 (Transgenic Biotech, Beijing, China). The cells were washed using TBST (Beyotime) and then incubated with goat anti-rabbit immunoglobulin (IgG) 1:1,000 (Beyotime), and the membranes were washed again. Finally, the target band was visualized by ECL (Beyotime) using a gel imaging system. The relative protein content was calculated after analyzing the optical density value using Image Lab (Bio-Rad, Shanghai, China).
Analysis of mRNA Expression
Cells were pretreated with 3-NPA or hydrogen peroxide and incubated with spermidine (rapamycin or chloroquine). The expression of related genes was quantified using RT‒qPCR. RNA was isolated using a total RNA (1 μg) extraction kit (Toyobo, Saka, Japan). According to the reverse transcription and SYBR Green q-PCR kit (Takara, Dalian, China), the mRNA levels of autophagy and oxidative stress-related genes were detected. The primer sets used are described in Table 1.
Table 1.
Primer sequences related to oxidative stress and autophagy.
| Primers | Sequences (5′-3′) | Tm (°C) | Size | |
|---|---|---|---|---|
| Beclin1 | F: CGCTGTGCCAGATGTGGAAGG R: CAGAAGGAATACTGCGAGTTCAAGAGG |
63.3°C | 151 bp | XM_048051865.1 |
| ULK1 | F:GCAAAGGCATCATCCATCGG R:GCAGGTATCGGGCAAATCCA |
59.0°C | 126 bp | XM_048063805.1 |
| AMPK | F:TTGAATGCACCGAAGAGGAA R:CAGGATGAGGGCGAGACAGA |
59.0°C | 186 bp | XM_048048443.1 |
| mTOR | F:TCATTTGTTACTACCTCCCA R:TCTAGAGCAGCTTTGCGAGCCAC |
61.4°C | 78 bp | XM_048067427.1 |
| EP300 | F:TTCACAACCTCAAACCACGAT R:TTCGTCCTGTTTTCTTTAGGC |
59.0°C | 188 bp | XM_048069898.1 |
| p62 | F:GCTAAATCCGTTTGAGTGGC R:GAGGCGGGCTGTTAGTAGAA |
59.0°C | 194 bp | XM_048057318.1 |
| NRF2 | F:CGCCTTGAAGCTCATCTCAC R:TTCTTGCCTCTCCTGCGTAT |
59.0°C | 176 bp | XM_013171578.2 |
| HO-1 | F:TGCCTACACTCGCTATCTGG R:AGGTCCATCTCAAGGGCATT |
59.0°C | 183 bp | XM_013181078.2 |
| GPX | F:AACCAATTCGGGCACCAG R:CCGTTCACCTCGCACTTCTC |
59.0°C | 149 bp | XM_035337923.1 |
| CAT | F:ATACAGTTCGTGACCCTCG R:CCAGAAGTCCCATACCAT |
55.8°C | 188 bp | XM_048058873.1 |
| GAPDH | F:GTGGTGCAAGAGGCATTGCTGAC R:GCTGATGCTCCCATGTTCGTGAT |
65.0°C | 86 bp | XM_013199522.2 |
Note: Beclin1: myosin-like; BCL-2 interacting protein; ULK1: Unc-51-like kinase 1; AMPK: AMP-activated protein kinase; mTOR: mammalian target of rapamycin; EP300: E1A-binding protein p300; p62: Sequestosome-1; NRF2: NFE2-related factor 2; HO-1: heme oxygenase-1; GPX: glutathione peroxidase; CAT: catalase enzymes. GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
The data were analyzed by SAS 9.0 and plotted using GraphPad Prism 9. The experimental data are represented as the mean ± SEM. ANOVA followed by Tukey's test was utilized for comparisons among groups. P < 0.05 was used to define statistical significance.
RESULTS
Spermidine Induced Autophagy in Follicular GCs
After follicular GCs were treated with spermidine (0, 20, 40, 80, and 160 μmol/L) for 24 h under the protection of 0.6 mmol/L AG, GC viability was not significantly changed (Figure 1A). Treatment with 80 and 160 μmol/L spermidine for 24 h significantly increased the LC3-II/I ratio and reduced p62 protein expression in GCs (P < 0.05; Figure 1B). Furthermore, an increased LC3-II/I ratio and reduced p62 were observed in follicular GCs treated with 80 μmol/L spermidine for 16 h and 24 h or 160 μmol/L spermidine for 8 h and 16 h (P < 0.05; Figures 1C and 1D). These results suggested that autophagy in GCs was induced by 80 μmol/L spermidine treatment for 16 h and 24 h and 160 μmol/L spermidine treatment for 8 h, 16 h, and 24 h.
Figure 1.
Effect of spermidine on the cell activity and autophagy of follicular GCs. GCs were treated with different concentrations of spermidine with 0, 20, 40, 80, and 160 μmol/L for 24 h. (A) Cell viability of follicular GCs. (B) The protein expression levels of LC3-II, LC3-I, and p62 in GCs. The protein expression levels of LC3-II, LC3-I, and p62 in GCs treated with 80 μmol/L (C) and 160 μmol/L (D) spermidine for different times. There was a significant difference in the representation of different letters (P < 0.05), but there was no significant difference in the representation of the same letter (P > 0.05).
Effect of Spermidine and on Autophagy in GCs
Spermidine and RAPA (autophagy inducer) reversed 3-NPA-induced p62 upregulation and ULK1 gene expression downregulation (P < 0.05). Although 3-NPA did not affect the mRNA expression of mTOR, EP300, or Beclin-1 (P > 0.05), 3-NPA combined with spermidine or RAPA significantly downregulated mTOR expression and upregulated Beclin-1 expression (P < 0.05; Figure 3A). Meanwhile, spermidine and RAPA reversed 3-NPA-induced p62 accumulation (P < 0.05) and did not affect the LC3-II/I ratio. However, the addition of chloroquine significantly increased the LC3-II/I ratio (P < 0.05; Figure 2B). In addition, autophagosomes were observed by transmission electron microscopy. 3-NPA combined with spermidine, RAPA, and chloroquine increased the number of lysosomes and autolysosomes compared with the 3-NPA group (Figure 2C).
Figure 3.
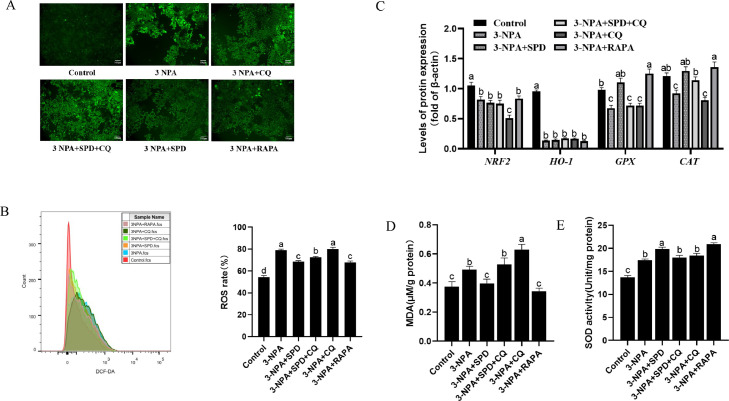
Effect of combined treatment with spermidine and 3-NPA on the oxidative stress of follicular GCs. GCs were treated with 80 μmol/L spermidine in combination with 7 μmol/L 3-NPA, 100 μmol/L CQ, 15 nmol/L RAPA for 24 h. (A) Immunofluorescence intensity of GCs. (B) Intracellular ROS levels in GCs. (C) The mRNA levels of NRF2, Ho-1, GPX, and CAT. The MDA levels (D) and SOD activity (E) in GCs. There was a significant difference in the representation of different letters (P < 0.05), but there was no significant difference in the representation of the same letter (P > 0.05). Abbreviations: CQ, chloroquine; RAPA, rapamycin; SPD, spermidine.
Figure 2.
Effect of combined treatment with spermidine and 3-NPA on autophagy in follicular GCs. GCs were treated with 80 μmol/L spermidine in combination with 7 μmol/L 3-NPA, 100 μmol/L CQ, 15 nmol/L RAPA for 24 h. (A) The mRNA levels of AMPK, mTOR, EP300, ULK1, Beclin-1, and p62. (B) The protein expression levels of LC3-I, LC3-II, and p62. (C) Changes in autolysosomes and autophagosomes (the red arrow represents autolysosomes, and the blue arrow represents autophagosomes). There was a significant difference in the representation of different letters (P < 0.05), but there was no significant difference in the representation of the same letter (P > 0.05). Abbreviations: CQ, chloroquine; RAPA, rapamycin; SPD, spermidine.
Spermidine Improved Oxidative Stress in GCs
We further investigated the effects of spermidine on 3-NPA-induced oxidative stress in follicular GCs. The results showed that 3-NPA combined with spermidine or RAPA significantly decreased ROS production compared with the 3-NPA group, but ROS significantly increased after the addition of chloroquine (P < 0.05; Figures 3A and 3B). The GPX and CAT mRNA levels in follicular GCs treated with 3-NPA in combination with spermidine or rapamycin were significantly higher than those in follicular GCs treated with 3-NPA alone (P < 0.05). Spermidine and rapamycin did not alleviate the down-regulation of NRF2 and HO-1 expression caused by 3-NPA (P > 0.05; Figure 3C). In addition, 3-NPA combined with spermidine or RAPA significantly decreased MDA levels and increased SOD viability compared with 3-NPA alone (P < 0.05). However, the effect of spermidine on improving oxidative stress induced by 3-NPA was inhibited by chloroquine (Figures 3D and 3E). These results indicated that spermidine-induced autophagy improved 3-NPA-induced oxidative stress.
Spermidine Improved Apoptosis in GCs
The effect of spermidine on 3-NPA-induced apoptosis in follicular GCs was determined. The results showed that 3-NPA combined with spermidine treatment significantly increased GC activity compared with that in the control group (P < 0.05; Figure 4A). Spermidine inhibited the increase in the apoptosis and necrosis, and the expression of cleaved CASPASE-3 induced by 3-NPA (P < 0.05). In addition, spermidine promoted BCL-2 protein expression (P < 0.05). However, the protective effect of spermidine was inhibited after the addition of chloroquine (P < 0.05; Figures 4B–4E). These results indicated that spermidine-induced autophagy to alleviate 3-NPA-induced apoptosis.
Figure 4.
Effect of combined treatment with spermidine and 3-NPA on the apoptosis of follicular GCs. GCs were treated with 80 μmol/L spermidine in combination with 7 μmol/L 3-NPA, 100 μmol/L CQ, 15 nmol/L RAPA for 24 h. (A) Cell viability of follicles. (B, C) Apoptosis rate. Apoptosis was defined as both annexin V(+)/PI(+), and annexin V(+)/PI(−). Necrosis was defined as both PI(+)/annexin V(+) and PI(+)/annexin V(−). (D–F) The expression of Cleaved CASPASE-3, BCL-2, BAX, and BCL-2/BAX in GCs was detected by Western blotting. There was a significant difference in the representation of different letters (P < 0.05), but there was no significant difference in the representation of the same letter (P > 0.05). Abbreviations: CQ, chloroquine; RAPA, rapamycin; SPD, spermidine.
Spermidine Improved Oxidative Stress in GCs
We further investigated the effect of spermidine on hydrogen peroxide-induced oxidative stress. Cell viability, MDA levels, SOD activity, and autophagy protein expression were detected. The results showed that spermidine reversed the decrease in cell viability, increased in MDA levels and accumulation of p62 protein induced by hydrogen peroxide and further increased SOD activity (P < 0.05). However, the effect of spermidine was inhibited after chloroquine was added (P < 0.05; Figure 5). This result showed that spermidine-induced autophagy could inhibit hydrogen peroxide-induced oxidative stress.
Figure 5.
Effect of combined treatment of spermidine and H2O2 on apoptosis of goose follicular GCs. GCs were pretreated with 1250 μmol/L hydrogen peroxide for 6 h and incubated with 160 μmol/L spermidine (or 100 μmol/L chloroquine) for 8 h. (A) The viability of GCs. (B, C) The intracellular MDA levels and SOD activity. (D, E) The protein expression levels of LC3-II, LC3-I and p62 in GCs. There was a significant difference in the representation of different letters (P < 0.05), but there was no significant difference in the representation of the same letter (P > 0.05). Abbreviations: CQ, chloroquine; RAPA, rapamycin; SPD, spermidine.
DISCUSSION
Spermidine is ubiquitous in organisms and has physiological functions such as stabilizing nucleic acids, regulating proteins, and promoting cell proliferation. Spermidine is also a natural autophagy inducer (Alavi et al., 2021). Exogenous spermidine supplementation can extend the life of mice, Drosophila, and nematodes and improve aging-related diseases (Fîlfan et al., 2017 ; Ni and Liu, 2021). Yuan et al. (2021) found that 100 μmol/L spermidine for 24 h significantly increased the number, activity and proliferation rate of human female reproductive stem cells. However, higher doses of spermidine are decomposed to produce toxic substances during cell culture, which can also induce cell damaging autophagy and cause cell death. Aminoguanidine (AG) can inhibit the action of amine oxidase in serum (Wang et al., 2018), and our previous study found that 0.6 mmol/L AG can prevent spermidine decomposition and lessen damage to cells. Therefore, in this experiment, 0.6 mmol/L of AG was added. After follicular GCs were treated with spermidine (0, 20, 40, 80, and 160 μmol/L) for 24 h, the GC activity was not significantly changed. Therefore, we further explored the relationship between spermidine and granulosa cell autophagy. The results showed that 80 μmol/L spermidine treatment for 16 h or 24 h or 160 μmol/L spermidine treatment for 8 h, 16 h, or 24 h induced autophagy in GCs.
Chloroquine interferes with autophagy initiation and autophagy formation (Nguyen Hoang et al., 2022 ). If autophagy is inhibited, LC3-II protein expression will not change significantly after adding chloroquine. In this experiment, we added 100 μmol/L chloroquine and 15 nmol/L rapamycin. We found that compared with the 3-NPA group, the LC3-II and p62 proteins did not change significantly after adding chloroquine, indicating that 3-NPA treatment may inhibit autophagy flow. In contrast, the expression of the ULK1 gene in the 3-NPA group significantly decreased, and the expression of the p62 gene significantly increased. We observed a small number of autolysosomes in the 3-NPA group through transmission electron microscopy, suggesting that autophagy occurred in cells in 3-NPA, but autophagy initiation may be blocked to some extent, and autophagy flow may be inhibited. In the spermidine combined with 3-NPA group, LC3-II protein expression increased in GCs and promoted the degradation of the p62 protein, indicating that spermidine promoted cell autophagy, and LC3-II protein expression further increased after the addition of chloroquine. The expression of the mTOR, p62, ULK1, and Beclin-1 genes in GCs treated with rapamycin was also consistent with that of spermidine, suggesting that spermidine plays a similar role as an autophagy inducer in GCs.
3-NPA can induce mitochondria to produce excessive ROS, causing oxidative stress, and can lead to a decline in the vitality of poultry follicular GCs and an increase in ROS levels (Silva-Palacios et al., 2017), suggesting that oxidative stress in GCs is induced by 3-NPA. In this experiment, granular cells were treated with 3-NPA for 24 h, GC activity was reduced, and ROS levels were increased, consistent with the above findings and the oxidative stress model established in the early laboratory. In addition, when oxidative stress causes cell lipid peroxidation and ROS accumulation, SOD decomposes ROS into hydrogen peroxide and O2− and then further decomposes hydrogen peroxide by antioxidant enzymes such as GPX and CAT. MDA, a derivative of lipid peroxidation produced by oxidative stress, can also damage cellular proteins and DNA ( Blokhina et al., 2003). In this experiment, 3-NPA treatment significantly increased MDA and ROS levels in GCs, while spermidine significantly reduced the accumulation of ROS and MDA caused by 3-NPA, increased the mRNA levels of GPX and CAT, and further increased SOD activity, indicating that spermidine could increase the activity of the SOD enzyme and the expression of the GPX and CAT genes to improve the antioxidant capacity of cells and eliminate ROS to play an antioxidant stress role. Similarly, spermidine decreased MDA accumulation in GCs induced by hydrogen peroxide treatment and further increased SOD activity.
As an inducer of cellular oxidative stress, 3-NPA may have some specificity, and the mechanism of 3-NPA induced oxidative stress in cells remains unclear. Therefore, hydrogen peroxide was used as a model drug to induce oxidative stress in cells in this experiment, which provides further evidence for the mechanism of action of spermidine against oxidative stress in GCs through the induction of autophagy. In this study, LC3-II and p62 protein expression in the hydrogen peroxide-treated group was significantly higher than that in the control group. There was no significant change in LC3-II protein expression in GCs in the hydrogen peroxide combined with spermidine group compared to the chloroquine group, while p62 protein expression was significantly higher in the hydrogen peroxide combined with spermidine group than in the hydrogen peroxide combined with chloroquine group. Suggesting that spermidine may increase autophagy synthesis, the effect of spermidine on decreasing the accumulation of p62 disappeared after the addition of chloroquine, indicating that spermidine may activate autophagic flow by enhancing autophagic synthesis. In summary, spermidine changes the autophagic levels of GCs under oxidative stress.
3-NPA can increased mitochondrial ROS in the skeletal muscle of male rats, decrease the GSH/GSSG ratio, reduced the activity of electron transfer chain enzymes, p53 and BCL-2 protein expression, and cause mitochondrial-dependent apoptosis. However, oxidative stress and mitochondrial damage can be improved through the antioxidant fullerene C60 (Gonchar et al., 2021). Similarly, Abdelfattah et al. (2020) found that intraperitoneal injection of 3-NPA can induce a reduction in SOD and CAT in the striatum and induce an apoptosis-promoting cascade reaction. Cleaved CASPASE-3 and BAX expression was upregulated, and BCL-2 gene expression was downregulated. However, combined administration of selenium and rutin can improve the antioxidant levels, downregulated cleaved CASPASE-3 expression, upregulated antiapoptosis gene expression and improve the damage caused by 3-NPA. An intraperitoneal injection of 3-NPA in mice caused ovarian oxidative stress, down-regulation of BCL-2 protein expression and up-regulation of CASPASE-3 and BAX protein expression (Yang et al., 2022), suggesting that 3-NPA causes oxidative stress and induces apoptosis at the same time, and some antioxidants could improve the oxidative stress and apoptosis caused by 3-NPA. This experiment found that 3-NPA caused apoptosis of GCs, significantly increased the apoptosis rate, Cleaved CASPASE-3 and BAX protein expression in GCs, and decreased the BCL-2/BAX ratio of GCs. These results are the same as those of previous studies. Studies have shown that spermidine has a similar antioxidant effect (Adhikari et al., 2021). Compared with the antioxidant effect of spermidine in this experiment, spermidine plays a similar antioxidant role in GCs, improving the oxidative stress and apoptosis of GCs caused by 3-NPA.
Autophagy inhibitors can enhance the apoptosis and ROS levels of osteoblasts injured by hydrogen peroxide (Yang et al., 2014), while rapamycin treatment can reduce the accumulation of ROS (Tai et al., 2017), and autophagy blockade can aggravate oxidative damage. This experiment showed that the MDA levels of GCs in the 3-NPA combined with chloroquine group was higher than that in the 3-NPA group, and the effect of spermidine on 3-NPA-induced oxidative stress in GCs disappeared after the addition of chloroquine. Similarly, after the addition of the autophagy inhibitor chloroquine, spermidine could not alleviate the increase in MDA levels induced by hydrogen peroxide, indicating that inhibition of autophagy affects the antioxidant effect of spermidine and that spermidine could improve the oxidative stress of GCs through autophagy.
Zhu et al. (2021) reported zearalenone treatment up-regulated BAX and downregulated BCL-2 expression, promoted cytochrome c release into the cytosol, and triggered mitochondria-mediated apoptosis, Pretreatment with chloroquine and rapamycin increased and decreased the rate of apoptosis, respectively, in contrast with other ZEA-treated groups. Therefore, autophagy may prevent apoptosis and promotes the survival of GCs. Similarly, autophagy inhibition by chloroquine enhances the apoptosis of neuronal cells induced by nonylphenol, while autophagy induced by rapamycin significantly inhibits apoptosis (Li et al., 2019), suggesting that autophagy can improve cell apoptosis to a certain extent. Our previous in vivo experiments have confirmed that spermidine can enhance the antioxidant level of goose ovary and alleviate 3-NPA-induced oxidative damage. This study found that the apoptosis rate of 3-NPA combined with chloroquine was increased compared with 3-NPA, and the GC apoptosis rate was reduced in 3-NPA combined with rapamycin compared to 3-NPA. Moreover, combined treatment with 3-NPA, spermidine, and chloroquine showed that the antiapoptotic effect of spermidine disappeared after inhibition of the granulosa cell autophagy pathway, suggesting that autophagy can alleviate granulosa cell apoptosis. In addition, rapamycin can reduce ROS levels induced by Pseudomonas aeruginosa through autophagy, reduced Cleaved-CASPASE-9, BAX, and CYTC protein expression in mouse macrophages, and increase BCL-2 protein expression (Han et al., 2020 ), suggesting that autophagy can improve oxidative stress and cell apoptosis. This experiment showed that CASPASE-3 and cleaved CASPASE-3 protein expression in the 3-NPA combined with rapamycin group was significantly lower than that in the 3-NPA group, and the BCL-2 protein expression was significantly increased, which was consistent with the effect of spermidine, suggesting that spermidine may have similar effects to rapamycin, inducing autophagy, playing an antioxidant role, and improving cell apoptosis.
CONCLUSIONS
Our results demonstrate that spermidine induces autophagy by upregulating the LC3-II/I ratio and downregulating the expression of p62 protein, further increasing SOD activity, reducing ROS production and MDA levels, inhibiting cleaved CASPASE-3 protein expression and promoting BCL-2 protein expression. In conclusion, spermidine alleviated oxidative stress and apoptosis by inducing autophagy in follicular GCs in Sichuan white geese. This study provides an entry point for improving oxidative stress and apoptosis in follicles.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (32172727, 31872358, and 31702116).
DISCLOSURES
All contributing authors declare no conflicts of interest.
REFERENCES
- Abdelfattah M.S., Badr S.E.A., Lotfy S.A., Attia G.H., Aref A.M., Abdel Moneim A.E., Kassab R.B. Rutin and selenium co-administration reverse 3-nitropropionic acid-induced neurochemical and molecular impairments in a mouse model of Huntington's disease. Neurotox. Res. 2020;37:77–92. doi: 10.1007/s12640-019-00086-y. [DOI] [PubMed] [Google Scholar]
- Adhikari R., Shah R., Reyes-Gordillo K., Arellanes-Robledo J., Cheng Y., Ibrahim J., Tuma P.L. Spermidine prevents ethanol and lipopolysaccharide-induced hepatic injury in mice. Molecules. 2021;26 doi: 10.3390/molecules26061786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi J., Miller D.M., Bagga I., Schempf A.M., Hsu Y.M., Woods B.D., Brown Mayfield S.M., Mitchell A.N., Tannady G., Talbot A.R., Dueck A.M., Barrera Ovando R., Parker H.D., Wang J., Schoeneweis J.K., Kennedy B.K. The autophagy inducer spermidine protects against metabolic dysfunction during overnutrition. Gerontol. A Biol. Sci. Med. Sci. 2021;76:1714–1725. doi: 10.1093/gerona/glab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O., Virolainen E., Fagerstedt K.V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 2003;2:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai N., Zhang H., Li L., Yu X., Liu Y., Lin Y., Wang L., Yan J., Nikolaevna S.E., Zhao Y. Spermidine prevents heart injury in neonatal rats exposed to intrauterine hypoxia by inhibiting oxidative stress and mitochondrial fragmentation. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/5406468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che H., Ma C., Li H., Yu F., Wei Y., Chen H., Wu J., Ren Y. Rebalance of the polyamine metabolism suppresses oxidative stress and delays senescence in nucleus pulposus cells. Oxid. Med. Cell Longev. 2022;2022 doi: 10.1155/2022/8033353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K.C., Misra H.P. Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol. Cell Biochem. 2004;262:127–133. doi: 10.1023/b:mcbi.0000038227.91813.79. [DOI] [PubMed] [Google Scholar]
- Devine P.J., Perreault S.D., Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012;86:27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamo S., Cetrullo S., Guidotti S., Silvestri Y., Minguzzi M., Santi S., Cattini L., Filardo G., Flamigni F., Borzì R.M. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free Radic. Biol. Med. 2020;153:159–172. doi: 10.1016/j.freeradbiomed.2020.03.029. [DOI] [PubMed] [Google Scholar]
- Eisenberg T., Knauer H., Schauer A., Büttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., Fussi H., Deszcz L., Hartl R., Schraml E., Criollo A., Megalou E., Weiskopf D., Laun P., Heeren G., Breitenbach M., Grubeck-Loebenstein B., Herker E., Fahrenkrog B., Fröhlich K.U., Sinner F., Tavernarakis N., Minois N., Kroemer G., Madeo F. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Fîlfan M., Sandu R.E., Zăvăleanu A.D., GreşiŢă A., Glăvan D.G., Olaru D.G., Popa-Wagner A. Autophagy in aging and disease. Rom. J. Morphol. Embryol. 2017;58:27–31. [PubMed] [Google Scholar]
- Filomeni G., De Zio D., Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q. Oxidative stress and autophagy. Adv. Exp. Med. Biol. 2019;1206:179–198. doi: 10.1007/978-981-15-0602-4_9. [DOI] [PubMed] [Google Scholar]
- Gonchar O.O., Maznychenko A.V., Klyuchko O.M., et al. C60 fullerene reduces 3-nitropropionic acid-induced oxidative stress disorders and mitochondrial dysfunction in rats by modulation of p53, Bcl-2 and Nrf2 targeted proteins. Int. J. Mol. Sci. 2021;22:5444. doi: 10.3390/ijms22115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Ma Q., Yu J., Gong Z., Ma C., Xu Y., Deng G., Wu X. Autophagy plays a protective role during Pseudomonas aeruginosa-induced apoptosis via ROS-MAPK pathway. Innate Immun. 2020;26:580–591. doi: 10.1177/1753425920952156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R., Wang Z., Cui M., Liu S., Wu W., Chen M., Wu Y., Qu Y., Lin H., Chen S., Wang B., Shao Z. HIF1A Alleviates compression-induced apoptosis of nucleus pulposus derived stem cells via upregulating autophagy. Autophagy. 2021;17:3338–3360. doi: 10.1080/15548627.2021.1872227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.W., Cha H.J., Han M.H., Hwang S.J., Lee D.S., Yoo J.S., Choi I.W., Kim S., Kim H.S., Kim G.Y., Hong S.H., Park C., Lee H.J., Choi Y.H. Spermidine protects against oxidative stress in inflammation models using macrophages and zebrafish. Biomol. Ther. (Seoul) 2018;26:146–156. doi: 10.4062/biomolther.2016.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Kim J.H., Hwangbo H., Kim S.Y., Ji S.Y., Kim M.Y., Cha H.J., Park C., Hong S.H., Kim G.Y., Park S.K., Jeong J.W., Kim M.Y., Choi Y.H., Lee H. Spermidine attenuates oxidative stress-induced apoptosis via blocking Ca2+ overload in retinal pigment epithelial cells independently of ROS. Int. J. Mol. Sci. 2021;22:1361. doi: 10.3390/ijms22031361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch. Pharm. Res. 2017;40:1197–1208. doi: 10.1007/s12272-017-0957-3. [DOI] [PubMed] [Google Scholar]
- Li L., Tan J., Miao Y., Lei P., Zhang Q. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol. Neurobiol. 2015;35:615–621. doi: 10.1007/s10571-015-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Jiang Z., Chai W., Xu Y., Wang Y. Autophagy activation alleviates nonylphenol-induced apoptosis in cultured cortical neurons. Neurochem. Int. 2019;122:73–84. doi: 10.1016/j.neuint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Liu P., de la Vega M.R., Dodson M., Yue F., Shi B., Fang D., Chapman E., Liu L., Zhang D.D. Spermidine confers liver protection by enhancing NRF2 signaling through a MAP1S-mediated noncanonical mechanism. Hepatology. 2019;70:372–388. doi: 10.1002/hep.30616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y., Yu W., Han L., Yang S., Wang Y., Ren T., Yu J., Zhao A. ROS activates autophagy in follicular granulosa cells via mTOR pathway to regulate broodiness in goose. Anim. Reprod. Sci. 2017;185:97–103. doi: 10.1016/j.anireprosci.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Madeo F., Eisenberg T., Pietrocola F., Kroemer G. Spermidine in health and disease. Science. 2018;359:6374. doi: 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- Nguyen Hoang A.T., Lee H., Lee S.J. Casein kinase I inhibitor D4476 influences autophagy and apoptosis in chloroquine-induced adult retinal pigment epithelial-19 cells. Exp. Eye Res. 2022;218 doi: 10.1016/j.exer.2022.109004. [DOI] [PubMed] [Google Scholar]
- Ni Y.Q., Liu Y.S. New insights into the roles and mechanisms of spermidine in aging and age-related diseases. Aging Dis. 2021;12:1948–1963. doi: 10.14336/AD.2021.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar E.M., Omar R.S., Shoela M.S., El Sayed N.S. A study of the cardioprotective effect of spermidine: a novel inducer of autophagy. Chin. J. Physiol. 2021;64:281–288. doi: 10.4103/cjp.cjp_76_21. [DOI] [PubMed] [Google Scholar]
- Ornatowski W., Lu Q., Yegambaram M., Garcia A.E., Zemskov E.A., Maltepe E., Fineman J.R., Wang T., Black S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Palacios A., Colín-González A.L., López-Cervantes S.P., Zazueta C., Luna-López A., Santamaría A., Königsberg M. Tert-buthylhydroquinone pre-conditioning exerts dual effects in old female rats exposed to 3-nitropropionic acid. Redox Biol. 2017;12:610–624. doi: 10.1016/j.redox.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Kumar R., Garg G., Singh A.K., Verma A.K., Bissoyi A., Rizvi S.I. Spermidine, a caloric restriction mimetic, provides neuroprotection against normal and D-galactose-induced oxidative stress and apoptosis through activation of autophagy in male rats during aging. Biogerontology. 2021;22:35–47. doi: 10.1007/s10522-020-09900-z. [DOI] [PubMed] [Google Scholar]
- Tai H., Wang Z., Gong H., Han X., Zhou J., Wang X., Wei X., Ding Y., Huang N., Qin J., Zhang J., Wang S., Gao F., Chrzanowska-Lightowlers Z.M., Xiang R., Xiao H. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 2017;13:99–113. doi: 10.1080/15548627.2016.1247143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Liu Y., Qi C., Shen L., Wang J., Liu X., Zhang N., Bing T., Shangguan D. Oxidative degradation of polyamines by serum supplement causes cytotoxicity on cultured cells. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-28648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang Z., Celi P., Yan L., Ding X., Bai S., Zeng Q., Mao X., Feng B., Xu S., Zhang K. Alteration of the antioxidant capacity and gut microbiota under high levels of molybdenum and green tea polyphenols in laying hens. Antioxidants (Basel) 2019;8:503. doi: 10.3390/antiox8100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T.T., Li H., Dai Z., Lau G.K., Li B.Y., Zhu W.L., Liu X.Q., Liu H.F., Cai W.W., Huang S.Q., Wang Q., Zhang S.J. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging (Albany NY) 2020;12:6401–6414. doi: 10.18632/aging.103035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M., Parle M., Jindal D.K., Sharma N. Potential effect of spermidine on GABA, dopamine, acetylcholinesterase, oxidative stress and proinflammatory cytokines to diminish ketamine-induced psychotic symptoms in rats. Biomed. Pharmacother. 2018;98:207–213. doi: 10.1016/j.biopha.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Yang H., Lee S.E., Kim G.D., Park H.R., Park Y.S. Hemeoxygenase-1 mediates an adaptive response to spermidine-induced cell death in human endothelial cells. Oxid. Med. Cell Longev. 2013;2013 doi: 10.1155/2013/238734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.H., Li B., Zheng X.F., Chen J.W., Chen K., Jiang S.D., Jiang L.S. Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway–implications for the treatment of osteoporosis. Free Radic. Biol. Med. 2014;77:10–20. doi: 10.1016/j.freeradbiomed.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Yang Y.H., Li B., Zheng X.F., Chen J.W., Chen K., Jiang S.D., Jiang L.S. Decrease in ovarian reserve through the inhibition of SIRT1-mediated oxidative phosphorylation. Aging (Albany NY) 2022;14:2335–2347. doi: 10.18632/aging.203942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi-Manesh H., Shirooie S., Noori T., Sheibani M., Tavangar S.M., Hemmati S., Sadeghi M.A., Akbarniakhaky H., Mohammadi Z., Foroutani L., Dehpour A.R. Spermidine reduced neuropathic pain in chronic constriction injury-induced peripheral neuropathy in rats [e-pub ahead of print] Fundam Clin. Pharmacol. 2023 doi: 10.1111/fcp.12880. [DOI] [PubMed] [Google Scholar]
- Yu J., Lou Y., He K., Yang S., Yu W., Han L., Zhao A. Goose broodiness is involved in granulosa cell autophagy and homeostatic imbalance of follicular hormones. Poult. Sci. 2016;95:1156–1164. doi: 10.3382/ps/pew006. [DOI] [PubMed] [Google Scholar]
- Yu J., Lou Y., Zhao A. Transcriptome analysis of follicles reveals the importance of autophagy and hormones in regulating broodiness of Zhedong white goose. Sci. Rep. 2016;6:36877. doi: 10.1038/srep36877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Tian G.G., Pei X., Hu X., Wu J. Spermidine induces cytoprotective autophagy of female germline stem cells in vitro and ameliorates aging caused by oxidative stress through upregulated sequestosome-1/p62 expression. Cell Biosci. 2021;11:107. doi: 10.1186/s13578-021-00614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Wang Z.G., Chen Y., Chen J., Khor S., Li J., He Z., Wang Q., Zhang H., Xu K., Fanghua G., Xiao J., Wang X. Spermidine promotes nucleus pulposus autophagy as a protective mechanism against apoptosis and ameliorates disc degeneration. J. Cell Mol. Med. 2018;22:3086–3096. doi: 10.1111/jcmm.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Wang H., Wang J., Han S., Zhang Y., Ma M., Zhu Q., Zhang K., Yin H. Zearalenone induces apoptosis and cytoprotective autophagy in chicken granulosa cells by PI3K-AKT-mTOR and MAPK signaling pathways. Toxins (Basel) 2021;13:199. doi: 10.3390/toxins13030199. [DOI] [PMC free article] [PubMed] [Google Scholar]