Abstract
Purpose
Acute syphilitic posterior placoid chorioretinitis (ASPPC) is a rare form of ocular syphilis. However, its pathophysiology is not fully understood. Laser speckle flowgraphy (LSFG) can facilitate the non-invasive evaluation of blood flow and allow investigations into the effects of treatments in various ocular diseases. We report a case of ASPPC that presented with symptoms only in the right eye but showed bilateral disease in LSFG.
Observations
A 54-year-old man presented with decreased vision and visual field defects in the right eye 2 days prior to the initial visit. Fundoscopy images showed a typical yellowish placoid lesion in the macular area, and optical coherence tomography showed disruption of the outer retinal layers and nodular appearance of the retinal pigment epithelium. Fluorescence angiography showed excessive leakage of the placoid lesion characterized by hypofluorescent dots in the inner area (“leopard spotting”). The patient was diagnosed with unilateral ASPPC based on multiple imaging and serological tests. Penicillin was administered for 2 weeks, and the patient showed improvement in symptoms and restoration of the retinal structure. The mean blur rate of the right/left eye was 2.1/5.9 arbitrary units (AU) before treatment and increased to 4.5/9.3 AU 6 months after treatment.
Conclusions and importance
Despite the absence of typical imaging signs and symptoms in the left eye, both eyes may have been affected with different degrees of severity. Thus, LSFG may facilitate the evaluation of treatment effects and the prediction of ocular inflammatory diseases in the early stages.
Keywords: Syphilis, Acute syphilitic posterior placoid retinitis, Laser speckle flowgraphy, Syphilitic chorioretinitis, Choroidal blood flow
1. Introduction
Ocular syphilis is caused by the spirochete Treponema pallidum and can affect a wide range of ocular structures, leading to various symptoms that are also observed in other ocular diseases.1 The number of patients with syphilis, a sexually transmitted disease, has been increasing recently. Acute syphilitic posterior placoid retinitis (ASPPC) is a rare, specific type of syphilitic chorioretinitis presenting with characteristic ocular findings on multiple imaging tests. However, the detailed pathogenesis of this disease remains unclear. Cases of syphilitic uveitis, including ASPPC, are likely to not show significant clinical findings, especially in the early stages, making their diagnosis difficult unless the disease is suspected. For this reason, clinicians should be aware of the possibility of ASPPC when evaluating imaging tests, and conduct angiography examinations if needed.
Laser speckle flowgraphy (LSFG) is a technique involving non-invasive quantification of the relative blood flow changes in tissues and is used to investigate the changes in ocular blood flow in various ocular conditions.2,3 In LSFG, the mean blur rate (MBR) is calculated from the moving erythrocytes illuminated by an 830-nm wavelength diode laser beam. MBR is expressed in arbitrary units (AU) and is considered an indicator of the relative erythrocyte velocity.4 We report a case of ASPPC that was presumably affected bilaterally according to the LSFG findings, although the right eye was mainly affected.
2. Case report
A 54-year-old man presented with decreased vision and visual field defects in the right eye. The symptoms had appeared 2 days before the first visit to our hospital and had gradually worsened. Two months before presenting to our hospital, the patient had experienced an episode of skin rash on the anterior chest, abdomen, and both arms and thighs that had resolved spontaneously. The patient also had a medical history of hospitalization for the treatment of syphilis at 20 years of age with no relevant social history, including homosexuality.
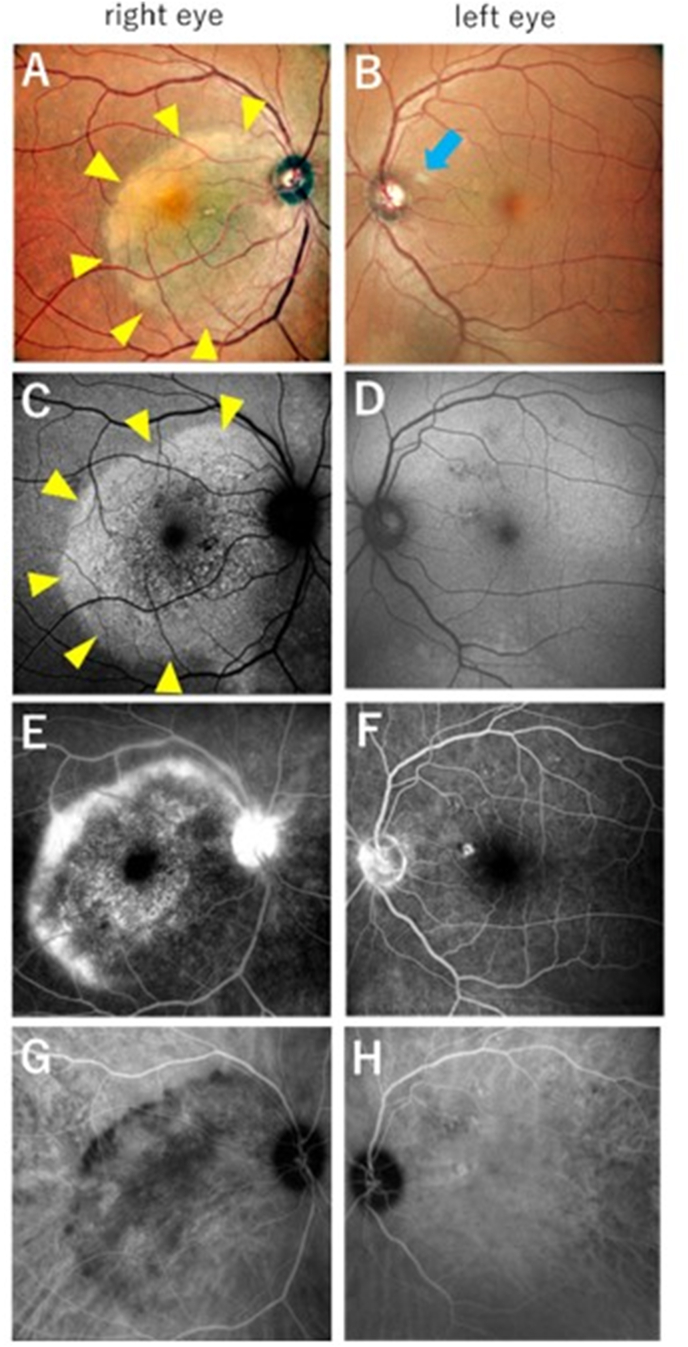
At the initial examination, the best-corrected visual acuity (BCVA) was 20/63 in the right eye and 20/15 in the left eye. The intraocular pressure (IOP) of the right/left eye was 12/13 mmHg. Inflammation in the anterior chamber and anterior vitreous was not apparent, and mild cataracts were observed in both eyes. Color fundoscopy showed a yellow placoid lesion in the macular area of the right eye, but not in the left eye (Fig. 1A and B). Swelling or redness of the optic nerve was not observed. Autofluorescence (AF) imaging showed enlargement of the hyperautofluorescent area corresponding to the macular placoid lesion (Fig. 1C). Optical coherence tomography (OCT) revealed nodular thickening of the retinal pigment epithelium (RPE), disruption of the ellipsoid zone (EZ), and a small amount of subretinal fluid in the right eye (Fig. 3A). These findings were not observed in the left eye (Fig. 3B). On fluorescein angiography (FA), the area of the placoid lesion was hyperfluorescent and showed progressive leakage from the late phase, especially at the edge of the lesion and the optic disc in the right eye (Fig. 1E).
Fig. 1.
Color fundoscopic images (right eye: A, left eye: B), autofluorescence (AF) images (right eye: C, left eye: D), and angiographic images (right eye: E, G; left eye: F, H) at the initial visit.
A) Yellowish placoids within a size of approximately 5 optic disc diameters are present in the macular area. The papilledema was unremarkable. B) Soft exudate was observed (blue arrow), presumably caused by diabetes. There were no placoid lesions or typical signs of acute syphilitic posterior placoid retinitis (ASPPC). C) AF imaging shows a hyperautofluorescent area corresponding to the placoid lesion. D, F, H) Small microvascular aneurysms are presumed to be the result of diabetes, and a hyperfluorescent area is observed in the upper nasal area of the fovea, but no typical signs of ASPPC are seen in the left eye. E) Fluorescein angiography (FA) shows excessive leakage in the placoid lesion and optic disc in the late phase before treatment, especially at the edge of the lesion. Hypofluorescent spots were observed in the inner area of placoid lesions. G) Indocyanine green angiography (ICGA). The hypofluorescent area corresponds to the hyperfluorescent area on FA, suggesting a blood flow deficit in the choroid. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
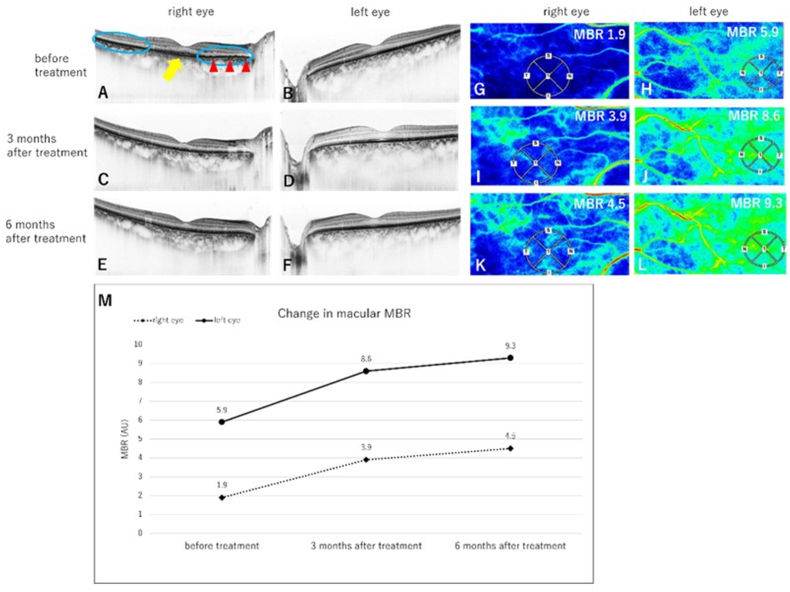
Fig. 3.
Optical coherence tomography (OCT) and LSFG images of the right eye (A, C, E, G, I, K) and left eye (B, D, F, H, J, L) at the initial visit (A, B, G, H), 3 months after treatment (C, D, I, J), and 6 months after treatment (E, F, K, L).
A) A nodular appearance of the retinal pigment epithelium (RPE) was seen, especially in the nasal region (red arrowhead), while disruption of the ellipsoid zone (EZ) is seen on both sides of the macula (blue circle), and a small amount of subretinal fluid (SRF) is apparent (yellow arrow). C) The EZ is recognizable, the nodular thickening of the RPE has improved, and the SRF had subsided. E) Reconstruction of the retina is persistent, with no recurrence of SRF or nodular lesions in the RPE. B, D, F) No abnormality is observed in the left eye during the follow-up period., M) A line chart showing the change in macular mean blur rate throughout the follow-up period. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The placoid lesion showed hypofluorescence on indocyanine green angiography (ICGA) (Fig. 1G), while AF and angiographic images of the left eye showed no significant findings (Fig. 1D, F, 1H). The patient showed no signs of optic neuritis or any other intracranial lesions on magnetic resonance imaging. He showed positive results in the rapid plasma reagin (RPR) test, with a titer of 1:64, and negative results for the HIV antibody. A cerebrospinal fluid examination performed by a neurologist revealed elevated cerebrospinal fluid cell counts and positive results for treponema pallidum latex-agglutination (TPLA) and fluorescent treponemal antibody absorption (FTA-ABS).
Based on these findings, the patient was diagnosed with unilateral ASPPC in the right eye. The cerebrospinal fluid test results indicated the possibility of active neurosyphilis. The patient was hospitalized and administered intravenous penicillin (24 million units per day) for 2 weeks.
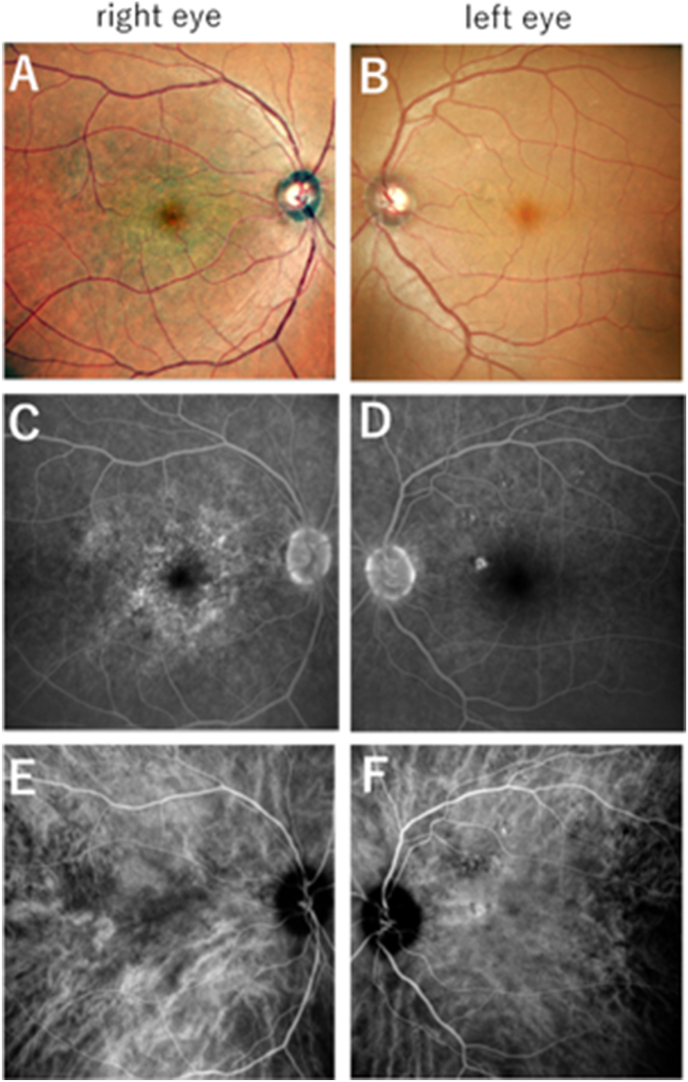
After 2 weeks of treatment, the BCVA improved to 20/20 and the placoid lesion regressed in the right eye (Fig. 2A). IOP of the right/left eye was 13/14 mmHg at 3 months and 13/12 mmHg at 6 months after treatment. During treatment, there were no significant changes in IOP. Disruption of the outer retinal layers was resolved, and OCT showed recovery of the retinal structure. Nodular thickening of the RPE was no longer visible at 3 months after treatment (Fig. 3C) and was not present even at 6 months after treatment (Fig. 3E). The left eye showed no abnormal structural changes on OCT throughout the study period (Fig. 3D and F). The distinctive findings seen on angiography regressed after treatment (Fig. 2C and E). The soft exudate disappeared in the left eye (Fig. 2B), and no significant changes in angiography were observed after treatment (Fig. 2D and F). The mean blur rate (MBR) in the macular area, which is an index of choroidal blood flow,5 was measured using the LSFG-NAVI system (Software Co., Ltd., Fukutsu, Japan) at the initial visit and 3 months and 6 months after treatment. The LSFG assessments indicated that the MBRs of the right and left eyes were, respectively, 2.1 AU (Figs. 3G) and 5.9 AU (Fig. 3H), and they respectively increased to 3.9 AU (Figs. 3I) and 8.6 AU (Fig. 3J) at 3 months after treatment and to 4.5 AU (Figs. 3K) and 9.3 AU (Fig. 3L) at 6 months after treatment (Fig. 3M). Recurrence was not observed during the follow-up period.
Fig. 2.
Color fundoscopy and angiographic images after treatment (right eye:A,C, and E, left eye: B,D, and F).
A) The yellowish placoid lesion had dissolved. B) The soft exudate next to the optic disc had disappeared. C) FA shows leakage of the outer side of the lesion is unnoticeable, although the hyperfluorescent area associated with hypofluorescent spots is partially persistent. E) ICGA shows that the hypofluorescent area is unremarkable. D, F) No significant differences in angiography test results are observed in the left eye before and after treatment. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
According to a study by Gass et al. ASPPC is considered to be inflammation of the photoreceptors-RPE-choriocapillaris complex. This condition is characterized by disruption of the outer retinal layers and nodular appearance of the RPE on optical coherence tomography (OCT), as well as the appearance of a hyperfluorescent area associated with hypofluorescent dots known as “leopard spotting”, on FA.6 The hyperautofluorescent areas in AF imaging are thought to indicate an accumulation of lipofuscin in the RPE or incomplete phagocytosis of the outer segments.7,8 ASPPC shows a good response to treatment with penicillin,9 as in this case, but the clinical course varies greatly among patients, indicating that the inflammation may be the result of an immune response.10,11
Previous clinical studies have reported changes in choroidal blood flow in ocular inflammatory diseases. Takemoto et al. reported an increase in the MBR in the macular area in Vogt-Koyanagi-Harada disease within one month of corticosteroid treatment.2 To our knowledge, this is the first report to evaluate the changes in choroidal blood flow in ASPPC with unilateral symptoms using LSFG. In our case, MBR increased gradually not only in the right eye, which showed typical characteristic findings for ASPPC, but also in the left eye during the 6 months of follow-up. Based on these findings, we estimated that the blood flow in the left eye was also impaired, although typical imaging signs of ASPPC were not detected. FA before treatment shows ONH hyperfluorescence in the left eye, which may imply some subclinical involvement. Moreover, the fact that the MBR increased in the left eye despite the absence of placoid lesions and abnormal OCT findings suggests that the signs of decreased blood flow could not be attributed to the blockage of the lesion. Changes in IOP are one factor that may affect MBR in LSFG; however, IOP was stable in this case, and no significant changes were seen throughout the follow-up period.12 Another factor that might affect MBR is blood pressure and ocular perfusion pressure; however, those were 127/89 mmHg and 55 mmHg before treatment and 128/85 mmHg and 57 mmHg at 1 month after treatment, respectively. Based on these pressures, we determined that the increase in MBR was due to the improvement of ASPPC in response to treatment, and other factors did not affect MBR. It is also reported that there are diurnal variations in retinal blood flow13; therefore, we conducted the measurement at the same time of the day. The choroidal thickness decreased in both eyes after treatment, which may suggest that choroidal circulation was impaired by inflammation caused by the spirochetes and consequently responded to antibiotics. This may be similar to the mechanism of acute posterior multifocal placoid pigment epitheliopathy (APMPPE) reported by Hirooka et al. which showed increased choroidal blood flow in LSFG and decreased choroidal thickness on OCT after regression of the disease, despite differences in the origin of inflammation.11 Some studies have hypothesized inflammatory involvement in the outer retina and inner choroid in ASPPC based on swept-source OCT angiography (SS-OCTA) findings showing choriocapillaris perfusion abnormalities.14 The degree of improvement in deficits may depend on the severity of the effects on choroidal perfusion.15 Villaret et al. reported that RPE may be primarily involved in ASPPC, according to the dynamics of ICG, leading to secondary damage to the photoreceptors.16 Although the pathophysiology of ASPPC remains under discussion, the findings in our case showed an improvement in the choroidal blood flow deficit in both eyes over the disease course and suggested that subclinical blood flow reduction occurs before the appearance of the placoid lesion and disorders of the outer retina and RPE. LSFG may be a more sensitive indicator of subclinical involvement, but there is a necessity for further verification in a larger series of patients.
4. Conclusion
In conclusion, the possibility of bilateral disease should be considered in patients showing unilateral symptoms or imaging findings, and LSFG may play an important role in detecting subclinical inflammation non-invasively.
Patient consent
Informed consent to publish this case was obtained from the patient.
Funding
This study received no funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the patient for his collaboration.
References
- 1.Margo C.E., Hamed L.M. Ocular syphilis. Surv Ophthalmol. 1992;37(3):203–220. doi: 10.1016/0039-6257(92)90138-j. [DOI] [PubMed] [Google Scholar]
- 2.Takemoto Y., Namba K., Mizuuchi K., et al. Choroidal circulation impairment during the anterior recurrence of Vogt-Koyanagi-Harada disease confirmed with indocyanine green angiography and laser speckle flowgraphy. Acta Ophthalmol. 2016;94(7):e629–e636. doi: 10.1111/aos.13024. [DOI] [PubMed] [Google Scholar]
- 3.Hanazaki H., Yokota H., Aso H., Yamagami S., Nagaoka T. Evaluation of ocular blood flow over time in a treated retinal arterial macroaneurysm using laser speckle flowgraphy. Am J Ophthalmol Case Rep. 2021;21 doi: 10.1016/j.ajoc.2021.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebuchi Y., Nagaoka T., Fukamachi D., et al. Comprehensive assessment of systemic arteriosclerosis in relation to the ocular resistive index in acute coronary syndrome patients. Sci Rep. 2022;12(1):2321. doi: 10.1038/s41598-021-04196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isono H., Kishi S., Kimura Y., Hagiwara N., Konishi N., Fujii H. Observation of choroidal circulation using index of erythrocytic velocity. Arch Ophthalmol. 2003;121(2):225–231. doi: 10.1001/archopht.121.2.225. [DOI] [PubMed] [Google Scholar]
- 6.Gass J.D., Braunstein R.A., Chenoweth R.G. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288–1297. doi: 10.1016/s0161-6420(90)32418-1. [DOI] [PubMed] [Google Scholar]
- 7.Eandi C.M., Neri P., Adelman R.A., et al. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina. 2012;32(9):1915–1941. doi: 10.1097/IAE.0b013e31825f3851. [DOI] [PubMed] [Google Scholar]
- 8.Pichi F., Ciardella A.P., Cunningham E.T., et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. 2014;34(2):373–384. doi: 10.1097/IAE.0b013e3182993f11. [DOI] [PubMed] [Google Scholar]
- 9.Neri P., Pichi F. Acute syphilitic posterior placoid chorioretinitis: when the great mimicker cannot pretend any more; new insight of an old acquaintance. J Ophthalmic Inflamm Infect. 2022;12(1):9. doi: 10.1186/s12348-022-00286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco M., Nogueira V. Severe acute syphilitic posterior placoid chorioretinitis with complete spontaneous resolution: the natural course. GMS Ophthalmol Cases. 2016;6:Doc02. doi: 10.3205/oc000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirooka K., Saito W., Saito M., et al. Increased choroidal blood flow velocity with regression of acute posterior multifocal placoid pigment epitheliopathy. Jpn J Ophthalmol. 2016;60(3):172–178. doi: 10.1007/s10384-016-0440-6. [DOI] [PubMed] [Google Scholar]
- 12.Iwase T., Akahori T., Yamamoto K., et al. Evaluation of optic nerve head blood flow in response to increase of intraocular pressure. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-35683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukami M., Iwase T., Yamamoto K., et al. Diurnal variation of pulse waveform parameters determined by laser speckle flowgraphy on the optic nerve head in healthy subjects. Medicine (Baltim) 2017;96(44) doi: 10.1097/MD.0000000000008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barikian A., Davis J., Gregori G., Rosenfeld P. Wide field swept source OCT angiography in acute syphilitic placoid chorioretinitis. Am J Ophthalmol Case Rep. 2020;18 doi: 10.1016/j.ajoc.2020.100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferro Desideri L., Rosa R., Musetti D., et al. The multifaceted presentation of syphilitic chorioretinitis examined by multimodal imaging: a case series. Am J Ophthalmol Case Rep. 2022;26 doi: 10.1016/j.ajoc.2022.101434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villaret J., Errera M.H., Sahel J.A., et al. Indocyanine green angiography features in acute syphilitic posterior placoid chorioretinitis. Am J Ophthalmol. 2022;241:40–46. doi: 10.1016/j.ajo.2022.02.008. [DOI] [PubMed] [Google Scholar]