Summary
Background
The oncological safety of minimally invasive surgery has been questioned for several abdominal cancers. Concerns also exist regarding the use of minimally invasive distal pancreatectomy (MIDP) in patients with resectable pancreatic cancer as randomised trials are lacking.
Methods
In this international randomised non-inferiority trial, we recruited adults with resectable pancreatic cancer from 35 centres in 12 countries. Patients were randomly assigned to either MIDP (laparoscopic or robotic) or open distal pancreatectomy (ODP). Both patients and pathologists were blinded to the assigned approach. Primary endpoint was radical resection (R0, ≥1 mm free margin) in patients who had ultimately undergone resection. Analyses for the primary endpoint were by modified intention-to-treat, excluding patients with missing data on primary endpoint. The pre-defined non-inferiority margin of −7% was compared with the lower limit of the two-sided 90% confidence interval (CI) of absolute difference in the primary endpoint. This trial is registered with the ISRCTN registry (ISRCTN44897265).
Findings
Between May 8, 2018 and May 7, 2021, 258 patients were randomly assigned to MIDP (131 patients) or ODP (127 patients). Modified intention-to-treat analysis included 114 patients in the MIDP group and 110 patients in the ODP group. An R0 resection occurred in 83 (73%) patients in the MIDP group and in 76 (69%) patients in the ODP group (difference 3.7%, 90% CI −6.2 to 13.6%; pnon-inferiority = 0.039). Median lymph node yield was comparable (22.0 [16.0–30.0] vs 23.0 [14.0–32.0] nodes, p = 0.86), as was the rate of intraperitoneal recurrence (41% vs 38%, p = 0.45). Median follow-up was 23.5 (interquartile range 17.0–30.0) months. Other postoperative outcomes were comparable, including median time to functional recovery (5 [95% CI 4.5–5.5] vs 5 [95% CI 4.7–5.3] days; p = 0.22) and overall survival (HR 0.99, 95% CI 0.67–1.46, p = 0.94). Serious adverse events were reported in 23 (18%) of 131 patients in the MIDP group vs 28 (22%) of 127 patients in the ODP group.
Interpretation
This trial provides evidence on the non-inferiority of MIDP compared to ODP regarding radical resection rates in patients with resectable pancreatic cancer. The present findings support the applicability of minimally invasive surgery in patients with resectable left-sided pancreatic cancer.
Funding
Medtronic Covidien AG, Johnson & Johnson Medical Limited, Dutch Gastroenterology Society.
Keywords: Distal pancreatectomy, Pancreatic ductal adenocarcinoma, Minimally invasive surgery
Research in context.
Evidence before this study
Prior to the start of our trial, we performed an extensive systematic literature review using PubMed, Embase, and the Cochrane library database on studies published before March 9, 2018. We used search terms including “distal pancreatectomy”, “pancreatic adenocarcinoma”, and “minimally invasive surgery”. We only included comparative studies on minimally invasive vs open distal pancreatectomy for pancreatic cancer, published in English. We identified 21 retrospective cohort studies with a total of 11,246 patients. No randomised controlled trials were identified. Each study had a moderate to high risk of bias and several limitations (including selection bias, small number of patients, retrospective study design). Radical resection rate (odds ratio 1.24) and overall survival rate (hazard ratio 0.86) were comparable between groups, whereas tumor size (weighed mean difference −0.46 cm) and lymph node yield (weighed mean difference −1.3 lymph nodes) were lower in minimally invasive distal pancreatectomy.
Added value of this study
Our study is the first randomised trial to compare minimally invasive distal pancreatectomy with the current standard approach of open distal pancreatectomy in patients with resectable pancreatic cancer. Hence, our study is the first to provide level 1 evidence on the oncological safety and feasibility of minimally invasive distal pancreatectomy in patients with pancreatic cancer.
Implications of all the available evidence
Based on the results of this study, we expect that minimally invasive distal pancreatectomy will be incorporated in guidelines as a standard, valid approach in patients with resectable pancreatic cancer. Oncological outcome is non-inferior to the open approach without any evidence for impaired safety.
Introduction
Pancreatic cancer (i.e., pancreatic ductal adenocarcinoma) is currently the third most lethal cancer worldwide with a five-year survival rate of 5–10%.1 Mortality rates of pancreatic cancer have increased from 8.6/100.000 persons in 2000 to 9.5/100.000 in 2019.2 Patients with left-sided pancreatic cancer represent less than 10% of all patients with resectable pancreatic cancer.3 Distal pancreatectomy (formally ‘left radical pancreatectomy’) with splenectomy followed by adjuvant chemotherapy is the preferred treatment in these patients, with a five-year survival rate of approximately 20%.1,4
Traditionally, distal pancreatectomy was performed using an open approach. Since its first introduction in 1994,5 minimally invasive distal pancreatectomy (MIDP) has been gaining popularity. Several retrospective studies and two randomised trials demonstrated short-term benefits in terms of functional recovery and hospital stay after MIDP over ODP for all indications.6, 7, 8 However, concerns remain regarding the oncological safety of MIDP in patients with resectable pancreatic cancer. Those concerns were highlighted by an international survey study wherein one-third of pancreatic surgeons expected inferior oncological outcomes after MIDP for pancreatic cancer.9 A systematic review and meta-analysis including over 11.000 patients reported comparable radical resection rates and survival after MIDP and ODP, but a decreased lymph node yield with MIDP.10 Recently, these concerns regarding the safety of minimally invasive surgery for other abdominal cancers were highlighted by a randomised trial that reported worse survival outcomes after minimally invasive hysterectomy in patients with cervical cancer.1,11 Results of this randomised trial led to a decreased use of minimally invasive hysterectomy.12 Additionally, two randomised trials were not able to confirm the non-inferiority of minimally invasive surgery on pathological outcomes in patients with colorectal cancer.13,14
To address these concerns, a pragmatic international randomised trial was designed to investigate the oncological safety of MIDP as compared to ODP in patients with resectable pancreatic cancer in a non-inferior setup.
Methods
Study design and participants
The investigator-initiated international multicentre non-inferiority randomised DIPLOMA trial included patients aged 18 years and older with potentially resectable pancreatic cancer (i.e., ductal adenocarcinoma) of the body and tail. Procedures were performed in 35 high-volume hospitals across 12 countries collaborating in the European Consortium on Minimally Invasive Pancreatic Surgery (www.e-mips.com). Initially, 38 centres were planned to participate, however, three centres were unable to start patient inclusion due to delay in legal approval. To ascertain sufficient surgical experience and quality, each participating surgical team had to perform at least 15 distal pancreatectomies (any diagnosis) annually and should have performed at least 50 MIDPs prior to start of trial enrolment. Similar to current clinical practice, preoperative pathological proof of the diagnosis of pancreatic cancer was not mandatory, except for patients receiving neoadjuvant therapy. Pancreatic cancer was defined according to the World Health Organisation definition, including adenosquamous carcinoma, colloid carcinoma (mucinous non-cystic carcinoma), hepatoid carcinoma, medullary carcinoma, signet ring cell carcinoma, undifferentiated carcinoma and undifferentiated carcinoma with osteoclast-like giant cells.15 Patients who received neoadjuvant chemotherapy were considered eligible only if the tumor was upfront resectable. Initially, patients receiving neoadjuvant chemotherapy were excluded, but this was amended in the protocol after the enrolment of the first 11 patients. Patients with multivisceral involvement (i.e., beyond the pancreas and spleen) were considered eligible. Exclusion criteria were American Society of Anaesthesiology physical status >3, distant metastases, and tumor involvement or abutment of major vessels (excluding the splenic vessels),16 see Appendix (p 2).
All patients provided written informed consent before randomisation. The study protocol was initially approved by the Amsterdam UMC and Southampton University Hospital ethics committees and thereafter by all individual ethics committees of the participating centres. The study protocol was previously published.17
This study was performed and reported according to the CONSORT guidelines for randomised controlled trials (Appendix pp 26–27).
Randomisation and blinding
Patients were randomly assigned to the minimally invasive approach and open approach in a 1:1 ratio. Permuted-block randomisation with varying block sizes (4-6-8) and stratification for hospital volume (≤20, 21–40, >40 distal pancreatectomies annually) and multivisceral involvement was performed by the study coordinators (MK, LJ, FV, SL, AE) centrally in Amsterdam and Southampton initially, and since October 2019 in Amsterdam and Brescia using a web-based randomisation module (Castor EDC, CIWIT B.V., Amsterdam, the Netherlands).
Pathologists and patients were blinded for the assigned treatment group to minimize bias in the assessment of the primary endpoint and functional recovery. For pathologists, the allocated treatment was not disclosed. After skin closure, patients were blinded using a large abdominal dressing (appropriate for the size of the ventral abdomen) which was securely taped to obstruct the view of the abdomen. This dressing was removed on postoperative day 5 or earlier when all five criteria of the functional recovery checklist were met, or for clinical reasons (Appendix p 3). The efficacy of blinding was assessed by asking the pathologist which surgical approach they believed the patient had received and by asking the patient, prior to removal of the abdominal dressing, which approach they had received.
Procedures
Patients were screened for eligibility in each individual participating centre during a multidisciplinary tumor board meeting. At the time of distal pancreatectomy, an abdominal CT-scan of maximum 4 weeks old was required to minimize the risk of progression to vascular contact or metastases during the waiting period. After randomisation, the trial coordinator contacted the surgical team prior to surgery with information including quality of life questionnaires, functional recovery checklists, and documents containing surgery and histopathology webinars with standardised procedures.
Surgical procedures were performed according to the left radical pancreatectomy principles, rather than the distal pancreatectomy principles.18,19 Left radical pancreatectomy would be the preferred terminology considering the oncological magnitude and currently ongoing change in terminology. To obtain homogeneity in terminology with respect to the published trial protocol, distal pancreatectomy is used in this manuscript.
Standardised surgical and histopathological procedures had been agreed on during several physical central meetings with participating surgeons and pathologists prior to study initiation. Each centre was trained in the protocol during a physical initiation meeting with the trial coordinators prior to the first inclusion and thereafter through online webinars. Previously published surgical standards for oncological resection during distal pancreatectomy were followed: radical antegrade modular pancreatosplenectomy20 for open procedures and radical ‘no-touch’ left pancreatosplenectomy for minimally invasive procedures.18 Both surgical procedures included standardised pancreatic transection, standardised lymph node dissection, routine splenectomy with resection of Gerota's (i.e., perirenal) fascia with or without left adrenal gland based on the location of the tumour. Pancreatic transection was performed at the pancreatic neck and preferably performed using stapler devices, but other techniques were allowed as long as these were used in the same manner during ODP and MIDP per centre. The height of the staplers was left to the surgeon's discretion depending on intraoperative assessment of the pancreas. Surgical sutures were placed after specimen extraction at the transection and posterior margin to facilitate pathologist orientation during histopathology assessment. Histopathological procedures were previously published.21 This included standardised orientation, inking, fixation, block taking and macroscopic and microscopic assessment. Each pathologist was trained in this standardised pathology protocol prior to the first specimen assessment.
Postoperative care was according to an enhanced recovery protocol.22 Patients received quality of life questionnaires at 2 weeks and 1-, 3-, and 6-months postoperatively. Patients also received a questionnaire for the scar satisfaction score and pain at 12 months postoperatively. These scores ranged from 1 (completely unsatisfied) to 10 (completely satisfied) for scar scores and from 0 (no pain) to 10 (severe pain) for pain scores.
Data collection was performed by local physicians using web-based data collection software (Castor EDC, CIWIT B.V., Amsterdam, the Netherlands).
Outcomes
The primary outcome was radical resection (R0), defined as the minimum margin of ≥1 mm between tumor and surgical margin, measured at the transection and posterior margins in patients who underwent a resection (regardless of the ultimate histopathological diagnosis).19 Predefined secondary outcomes included time to functional recovery, length of hospital stay, overall and pancreas-specific complications, postoperative transfusions, surgical site infections, readmissions, mortality, histopathology outcomes (e.g., lymph node yield, tumor differentiation, and perineural and lymphovascular invasion), overall and disease-free survival, time and site of disease recurrence or progression, and quality of life up to 6 months postoperatively (i.e., generic and disease-specific health status by EQ-5D-5L and QLQ-C30 respectively, and scar satisfaction scores). Morbidity and mortality outcomes were assessed up to 90 days postoperatively. The definitions of outcomes in this trial are reported in the Appendix (p 3).
A blinded adjudication committee crosschecked the primary and secondary endpoints with the respective definitions. Disagreements were resolved during a plenary consensus meeting. After enrolment of 50, 100, and 258 patients, an independent data monitoring committee assessed patient accrual and safety endpoints including adverse events. Coordinating centres (University Hospital Southampton and Amsterdam UMC) had regular monitoring visits. Monitoring of each participating centre was according to local regulatory protocols.
Statistical analysis
The sample size calculation was based on the results of a previously published observational study of >1300 patients.23 Including results of this observational study, assuming a non-inferiority margin of −7% for radical resection rates (58% in the ODP group and 67% in the MIDP group), a power of 80% (1-β) and significance level of 5% (α), the minimum number of patients required was 226. The 7% ‘minimally clinically important difference’ margin was based on discussion within the larger study group, in the absence of a consensus or data in the literature. Considering an expected dropout rate of 2.5% and 10% rate of patients with occult metastatic disease detected at surgical exploration, we calculated a sample size of 258 patients.
Analyses were by intention-to-treat, unless stated otherwise. Patients who were randomised to MIDP but were converted to ODP were analysed in the MIDP group. Patients who received another procedure than distal pancreatectomy were also analysed in the assigned group. Analyses for the primary endpoint and for the secondary endpoints of histopathology outcomes, postoperative pancreas-specific complications, and functional recovery were by modified intention-to-treat. This included only patients who had ultimately undergone a resection of their tumour, as these data were not available for patients who did not. Besides, patients who did not undergo a resection were not blinded for allocation for ethical reasons. Patients with other diagnoses than pancreatic cancer, but in whom radicality of resection could be reliably assessed (e.g., neuroendocrine tumours, metastases of different primary tumor), were included in the modified intention-to-treat population. A best- and worst-case analysis was performed for the primary endpoint to correct for missing data; in best-case analysis, those with missing data in the MIDP group were imputed as radical resection (R0) and those in the ODP group as non-radical (R1) resection. The reverse was performed in the worst-case analysis.
The primary endpoint is presented as the difference between the two groups with corresponding two-sided 90% confidence interval (CI) based on Wilson's score method. The lower limit of this 90% CI was compared with the predefined −7% non-inferiority margin to confirm the non-inferiority of MIDP, with the corresponding pnon-inferiority following Dunnett and Gent.24 We compared dichotomous data with Chi-square or Fisher's exact test (as appropriate), and present these as proportions and percentages. We compared continuous data with Mann–Whitney U test or independent samples t-test as appropriate, and present these as median with interquartile range (IQR) or mean with standard deviation (SD). Time-to-event outcomes (i.e., length of hospital stay, time to functional recovery, disease-free and overall survival, and time until start of chemotherapy) were analysed using Kaplan–Meier estimates and log-rank tests. To facilitate the comparison with previous trials, time to functional recovery and hospital stay were also analysed using conventional Mann–Whitney U tests. We also performed exploratory per-protocol analysis, which included only patients who received the allocated treatment for pancreatic cancer. We also performed sensitivity analysis (excluding neoadjuvant treatment) and post-hoc subgroup analysis (laparoscopic vs robotic procedures). Moreover, we performed a sensitivity analysis for functional recovery by excluding centres from the United States of America as these patients did not receive blinding, and by excluding patients with a complicated postoperative course (i.e., POPF, DGE, PPH, Clavien-Dindo grade ≥3). We performed a Cox proportional hazard analysis to identify predictors for overall survival. All covariates with a p-value <0.1 in the univariable models were included in one multivariable model. Hazard ratios for overall survival and disease-free survival of MIDP compared to ODP were calculated using univariable cox regression analysis. Quality of life outcomes were compared using a linear mixed model (see Appendix p 4). Health resource costs were calculated in Euros (€), compared using nonparametric bootstrapping (5000 samples), and presented as mean differences with corresponding two-sided 95% bias corrected and accelerated confidence intervals (BCaCIs). See Appendix (pp 4–5) for a detailed description of quality of life and costs. All analyses were performed in SPSS for Macintosh, version 24.0 (IBM Corp., Orchard Road Armonk, New York, US), except for the time-to-event outcomes which were analysed using R (cran.r-project.org). A two-tailed p-value <0.05 was considered statistically significant. This trial is registered with the ISRCTN registry, number ISRCTN44897265.
Role of the funding source
Medtronic Covidien AG (Rheinfall, Switzerland) and Johnson & Johnson Medical Limited (Livingston, United Kingdom) funded the trial (investigator-initiated grant) to cover salary costs of the trial coordinators. The funders had no role in the design or conduct of the trial, the interpretation of the results, and the decision to publish. The first authors wrote the first draft of the manuscript; the senior authors revised and supervised this. All co-authors participated in study design, interpretation of data, and manuscript preparation.
Results
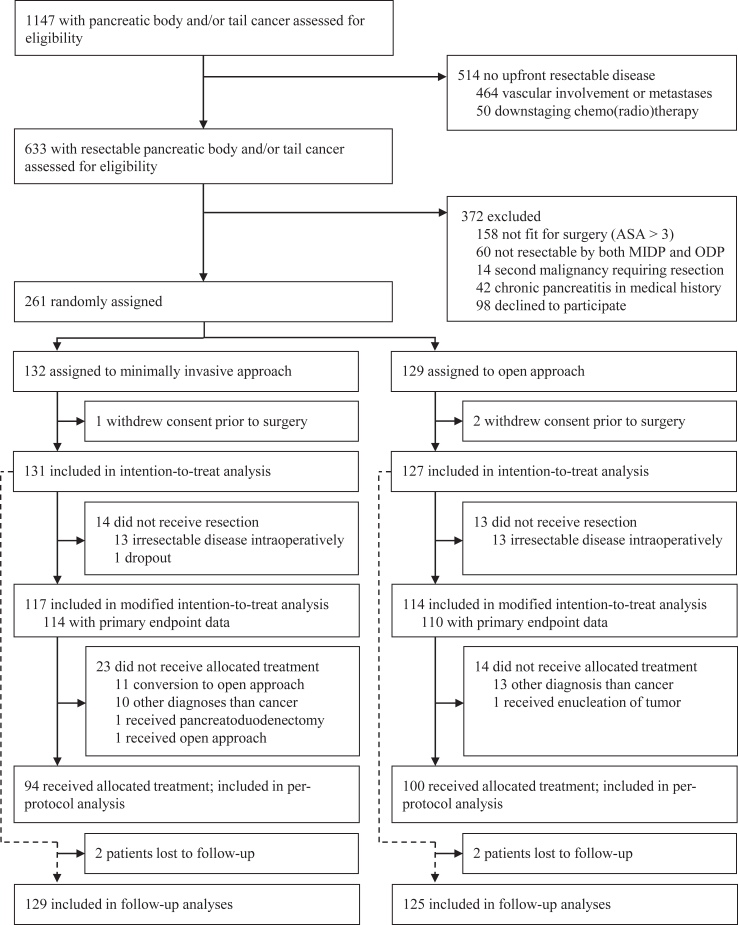
Between May 8, 2018 and May 7, 2021, 261 patients with resectable pancreatic cancer were randomly assigned to MIDP (132 patients) and ODP (129 patients) (Fig. 1). One patient in the MIDP group and two patients in the ODP group withdrew their consent for further study participation before surgery and were replaced according to protocol. Eventually, 131 patients in the MIDP group and 127 patients in the ODP group were included in the intention-to-treat population. The modified intention-to-treat population included 114 patients in the MIDP group and 110 patients in the ODP group after exclusion of 13 patients with unresectable disease at surgical exploration in each group, and one patient in the MIDP group who became inoperable before surgery due to impaired cardiac and pulmonary function, and 7 patients with missing data on primary endpoint (3 MIDP, 4 ODP). The per-protocol analyses included 94 patients in the MIDP group and 100 patients in the ODP group after the further exclusion of 37 patients: 20 patients because of non-PDAC diagnoses (8 in the MIDP group and 12 in the ODP group); 14 patients in the MIDP group because of conversion; one patient in the ODP group who received pancreatoduodenectomy because of tumor location; one patient in the ODP group who received enucleation because of intraoperative confirmation of a neuroendocrine tumor; and one patient in the MIDP group who was inoperable at time of surgery due to cardiac arrest and received ODP three months after initial surgery because of tumor progression. Baseline characteristics were well balanced for both the intention-to-treat, modified intention-to-treat, and per-protocol populations (Table 1 and Appendix p 8, respectively).
Fig. 1.
Trialprofile.
Table 1.
Baseline characteristics of 258 patients undergoing distal pancreatectomy in the intention-to-treat population.
| MIDP group (n = 131) | ODP group (n = 127) | |
|---|---|---|
| Age—yr. | 69.2 (±8.4) | 68.5 (±9.0) |
| Sex | ||
| Male | 63 (48.1) | 68 (53.5) |
| Female | 68 (51.9) | 59 (46.5) |
| BMI—kg/m2 | 25.2 (22.6–27.9) | 25.8 (23.0–28.7) |
| ASA classification | ||
| I | 13 (9.9) | 6 (4.7) |
| II | 74 (56.5) | 63 (49.6) |
| III | 44 (33.6) | 58 (45.7) |
| Prior abdominal surgery | 89 (67.9) | 77 (60.6) |
| Tumor location | ||
| Pancreatic body | 56 (42.7) | 54 (42.5) |
| Pancreatic tail | 56 (42.7) | 49 (38.6) |
| Body–tail junction | 19 (14.6) | 24 (18.9) |
| Tumor size—mm | 27.0 (20.0–36.0) | 26.5 (20.0–40.0) |
| Splenic vessel involvement on imaging | ||
| Vein involvement | 24 (18.3) | 20 (15.7) |
| Artery involvement | 12 (9.2) | 6 (4.7) |
| Both involved | 36 (27.5) | 38 (30.0) |
| Multivisceral involvement on imaging | 9 (6.9) | 12 (9.4) |
| Neoadjuvant treatment | 23 (17.6) | 28 (22.0) |
| Chemotherapy | 19 (14.5) | 22 (17.3) |
| Radiotherapy | 0 (0.0) | 1 (0.8) |
| Chemoradiation | 4 (3.1) | 5 (3.9) |
| Tumor markers at baseline | ||
| CEA—ng/mL | 2.8 (2.0–6.7) | 2.9 (1.9–6.5) |
| CA 19–9—u/mL | 50.0 (14.0–170.0) | 47.3 (10.8–287.8) |
| Resection performed | 117 (89.3) | 114 (89.8) |
| Received distal pancreatectomy | 116 (88.5) | 112 (88.2) |
| Received pancreatoduodenectomy | 1 (0.8) | 1 (0.8) |
| Received enucleation | 0 (0.0) | 1 (0.8) |
| Received assigned procedure for pancreatic cancer | 94 (71.8) | 100 (78.7) |
Categorical data are reported in numbers and frequencies.
Normally distributed continuous data are reported in mean (SD).
Non-normally distributed continuous data are reported in median (IQR).
MIDP, Minimally invasive distal pancreatectomy; ODP, Open distal pancreatectomy; SD, Standard deviation; IQR, Interquartile range; BMI Body mass index; ASA, American Society of Anesthesiologists; CEA, Carcinoembryonic antigen; CA, Carbohydrate antigen; mL, Millilitre.
The primary endpoint of R0 resection was achieved in 83 (73%) of 114 patients who underwent MIDP and in 76 (69%) of 110 patients who underwent ODP (Table 2) when analysed by modified intention-to-treat. The absolute difference in primary endpoint was 3.7% (90% CI −6.2% to 13.6%), thus confirming the non-inferiority of MIDP compared to ODP (pnon-inferiority = 0.039). Median lymph node yield was comparable between the two groups (22.0 [16.0–30.0] vs 23.0 [14.0–32.0], p = 0.86). The other histopathological outcomes also did not differ between the groups (Table 2).
Table 2.
Histopathology outcomes in the (modified) intention-to-treat population.
| MIDP group (n = 114)a | ODP group (n = 110)a | Risk difference (%) (90% CI) | p-value | |
|---|---|---|---|---|
| Radicality of resectionb | ||||
| Radical, R0 resection—≥1 mm | 83 (72.8) | 76 (69.1) | 3.7 (−6.2 to 13.6) | 0.039 (Pnon-inferiority) |
| Non-radical resection—<1 mm | 31 (27.2) | 34 (30.9) | ||
| Lymph node yield | 22.0 (16.0–30.0) | 23.0 (14.0–32.0) | 0.86 | |
| Positive lymph nodes | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.82 | |
| Histopathological tumor size—mm | 30.0 (23.0–42.0) | 29.0 (20.0–40.0) | 0.56 | |
| Poor tumor differentiation | 32 (31.7) | 38 (40.9) | 0.18 | |
| Perineural invasion | 90 (78.9) | 83 (75.5) | 0.53 | |
| Lymphovascular invasion | 77 (67.5) | 72 (65.5) | 0.74 | |
| T-stadium | 0.89 | |||
| T1 | 22 (19.3) | 26 (23.6) | ||
| T2 | 64 (56.1) | 59 (53.7) | ||
| T3 | 28 (24.6) | 25 (22.7) | ||
| T4 | 0 (0.0) | 0 (0.0) | ||
| N-stadium | 0.08 | |||
| N0 | 43 (37.7) | 47 (42.7) | ||
| N1 | 53 (46.5) | 36 (32.7) | ||
| N2 | 18 (15.8) | 27 (24.5) | ||
| M-stadium | NA | |||
| M0 | 114 (100.0) | 110 (100.0) | ||
| M1 | 0 (0.0) | 0 (0.0) | ||
| Histopathological diagnosis | 0.98 | |||
| Pancreatic cancer | 107 (93.9) | 101 (91.8) | ||
| NET | 3 (2.6) | 3 (2.7) | ||
| Other | 4 (3.5) | 6 (5.5) |
Categorical data are reported in numbers and frequencies.
Continuous data are reported in median (IQR).
MIDP, Minimally invasive distal pancreatectomy; ODP, Open distal pancreatectomy; IQR, Interquartile range; NET, Neuroendocrine tumor.
Analyses were performed according to modified intention-to-treat since histopathology data were not available for patients with unresectable disease (n = 27) and for 7 patients with missing data on primary endpoint (3 MIDP, 4 ODP) because of different diagnosis.
As measured at the transection and posterior margins.
Conversion was performed in 14 (12%) patients in the MIDP group. Most of them (n = 10) were non-urgent conversions because of unexpected tumor involvement of vascular structures (n = 7), adhesions (n = 2), or intraoperative conversion to pancreatoduodenectomy (n = 1). Urgent conversions occurred in four patients, which were all due to intraoperative bleeding. Robotic procedures were performed in 31 (27%) of 117 MIDP procedures. The median operative time was 31 min longer in the MIDP group (240 [IQR 175–309] vs 209 [IQR 158–257] minutes, p = 0.01), whereas blood loss was less (although not statistically significant) after MIDP (200 [IQR 100–300] vs 200 [IQR 100–400], p = 0.06, Table 3).
Table 3.
Intraoperative outcomes and postoperative outcomes up to 90 days in the intention-to-treat population.
| MIDP group (n = 131) | ODP group (n = 127) | p-value | |
|---|---|---|---|
| Operative time—min | 240.0 (175.3–308.8) | 209.0 (158.0–257.0) | 0.01 |
| Blood loss—mL | 200.0 (100.0–300.0) | 200.0 (100.0–400.0) | 0.06 |
| Multivisceral resection | 17 (14.5) | 21 (18.4) | 0.43 |
| Vascular resection | 6 (5.1) | 3 (2.6) | 0.33 |
| Conversion to ODP | 14 (12.0) | – | – |
| Type of MIDP procedurea | |||
| Laparoscopic | 86 (73.5) | – | – |
| Robotic | 31 (26.5) | – | – |
| Complications Clavien-Dindo grade ≥ III | 25 (19.1) | 26 (20.5) | 0.78 |
| Postoperative pancreatic fistulaa | 0.41 | ||
| Grade B | 25 (21.4) | 19 (16.7) | |
| Grade C | 0 (0.0) | 1 (0.9) | |
| Delayed gastric emptyinga | 0.83 | ||
| Grade B | 1 (0.9) | 1 (0.9) | |
| Grade C | 1 (0.9) | 2 (1.8) | |
| Postoperative pancreatic hemorrhagea | 0.43 | ||
| Grade B | 2 (1.7) | 4 (3.5) | |
| Grade C | 1 (0.9) | 0 (0.0) | |
| Reinterventions | 22 (16.8) | 21 (16.5) | 0.96 |
| Surgical reintervention | 4 (3.1) | 4 (3.1) | 0.94 |
| Radiological reintervention | 16 (12.2) | 12 (9.4) | 0.21 |
| Endoscopic reintervention | 7 (5.3) | 8 (6.3) | 0.67 |
| Postoperative transfusions | 9 (6.9) | 13 (10.2) | 0.33 |
| Surgical site infection | 7 (5.3) | 6 (4.7) | 0.82 |
| Unplanned ICU admission | 6 (4.6) | 13 (10.2) | 0.08 |
| Length of ICU admission—daysb | 1.0 (0.4–1.6) | 4.0 (2.6–5.4) | 0.02 |
| Total length of stay—daysb | 7.0 (6.4–7.6) | 7.0 (6.3–7.7) | 0.96 |
| Time to functional recovery—daysb | 5.0 (4.5–5.5) | 5.0 (4.7–5.3) | 0.22 |
| Readmission | 29 (22.1) | 27 (21.3) | 0.86 |
| 90-day mortality | 2 (1.5) | 3 (2.4) | 0.63 |
| Complication related | 0 (0) | 1 (0.8) | |
| Oncology related | 2 (1.5) | 2 (1.6) | |
| Mean total inpatient hospital costsc, euros, € | 18,067 (15,910–20,524) | 21,192 (15,804–30,633) | 0.44 |
Categorical data are reported in numbers and frequencies.
Continuous data are reported in median (IQR).
MIDP, Minimally invasive distal pancreatectomy; ODP, Open distal pancreatectomy; IQR, Interquartile range; ICU, Intensive care unit; US, United States.
Data on surgical parameters and pancreatic surgery specific complications were available for resected patients only (117 MIDP, 114 ODP).
Length of hospital stay and time to functional recovery are reported as medians with corresponding 95% confidence intervals, as calculated by Kaplan-Meier estimates and log-rank tests. For reference, when tested by Mann–Whitney U analyses, hospital stay was comparable (p = 0.43) and time to functional recovery was significantly shorter after MIDP (p = 0.03).
Costs are expressed for the year 2022, with corresponding 95% bias corrected and accelerated confidence intervals (BCaCIs).
Median time to functional recovery (5.0 [95% CI 4.5–5.5] days after MIDP vs 5.0 [95% CI 4.7–5.3] days after ODP, p = 0.22 when analysed by Kaplan–Meier estimates and log-rank test [for reference: p = 0.03 with Mann–Whitney U]) and total length of hospital stay (7.0 [95% CI 6.4–7.6] days after MIDP vs 7.0 [95% CI 6.3–7.7] days after ODP, p = 0.96 when analysed by Kaplan–Meier estimates and log-rank test [for reference p = 0.43 with Mann–Whitney U]) were comparable. Other postoperative outcomes also did not differ between the two groups (Table 4). The rate of unplanned intensive care admission was less (although not statistically significant) after MIDP (5% vs 10%, p = 0.08), but the length of unplanned ICU stay was shorter in the MIDP group (median 1.0 [95% CI 0.4–1.6] day vs 4.0 [95% CI 2.6–5.4] days, p = 0.02). The rate of readmission was comparable between groups (22.1% vs 21.3%, p = 0.86; see Appendix p 24–25). Serious adverse events were reported in 23 (18%) of 131 patients in the MIDP group vs 28 (22%) of 127 patients in the ODP group. Protocol deviations are summarised in the Appendix (p 23).
Table 4.
Oncological outcomes in the intention-to-treat population.
| MIDP group (n = 131)a | ODP group (n = 127)a | p-value | |
|---|---|---|---|
| Lost to follow-up | 2 (1.5) | 2 (1.6) | 0.96 |
| Survival ratesb | |||
| One-year survival | 96 (76.8) | 88 (72.1) | 0.40 |
| Two-year survival | 41 (45.6) | 42 (48.3) | 0.72 |
| Deceased during follow-up | 51 (39.5) | 49 (39.2) | 0.96 |
| Death cause during follow-up | 0.12 | ||
| Due to tumor-related causes | 37 (72.5) | 43 (87.8) | |
| Other causes | 9 (17.6) | 5 (10.2) | |
| Unknown cause | 5 (9.8) | 1 (2.0) | |
| Recurrence or progressionc | 58 (44.3) | 55 (43.3) | 0.98 |
| Local | 7 (12.1) | 7 (12.7) | |
| Distant | 43 (74.1) | 41 (74.5) | |
| Local and distant | 8 (13.8) | 7 (12.7) | |
| Intraperitoneal recurrence | 53 (40.5) | 48 (37.8) | 0.45 |
| Recurrence free survival—months | 9.0 (7.6–10.4) | 8.0 (5.5–10.5) | 0.10 |
| Adjuvant therapy | 0.85 | ||
| Adjuvant chemotherapy | 78 (59.5) | 73 (57.5) | |
| Adjuvant radiotherapy | 1 (0.8) | 0 (0.0) | |
| Adjuvant chemoradiotherapy | 7 (5.3) | 9 (7.1) | |
| Time to adjuvant therapy—days | 57 (51.5–62.5) | 57 (50.6–63.4) | 0.37 |
| Tumor markers at one-year follow-up | |||
| CEA—ng/mL | 3.0 (2.0–5.3) | 2.7 (1.5–5.4) | 0.46 |
| CA 19–9—u/mL | 26.5 (9.0–84.0) | 20.0 (5.3–137.0) | 0.44 |
Categorical data are reported in numbers and frequencies.
Normally distributed continuous data are reported in mean (SD).
Non-normally distributed continuous data are reported in median (IQR). Time-to-event data are reported in median and 95% confidence interval.
MIDP, Minimally invasive distal pancreatectomy; ODP, Open distal pancreatectomy; CEA, Carcinoembryonic antigen; CA, Carbohydrate antigen; mL, Millilitre.
Data were available for 254 patients (129 MIDP and 125 ODP), four patients were lost to follow-up.
In the intention-to-treat population, one-year survival was available for 247 patients (125 MIDP, 122 ODP), two-year survival was available in 177 patients (90 MIDP, 87 ODP).
Intraperitoneal progression or recurrence occurred in 33.6% in the MIDP group and in 33.1% in the ODP group (p = 0.93).
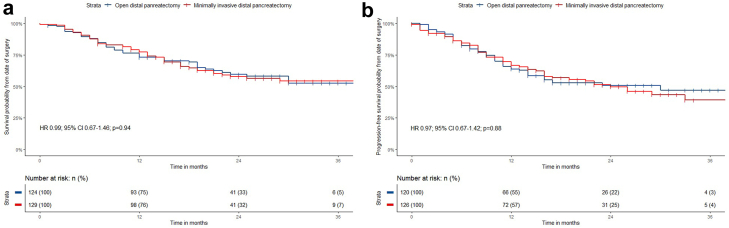
During a median follow-up time of 23.5 (IQR 17.0–30.0) months, 100 (39%) of 258 patients died: 51 (40%) of 131 patients in the MIDP group and 49 (39%) of 127 patients in the ODP group (Table 4). Four patients were lost-to-follow-up (two in each group). In evaluable patients, one-year (77% vs 72%) and two-year survival rates (46% vs 48%) were comparable. The hazard ratio for overall survival was 0.99 (95% CI 0.67–1.46, p = 0.94; Fig. 2a). Recurrence or disease progression occurred in 58 (44%) of 131 patients in the MIDP group and in 55 (43%) of 127 patients in the ODP group after a median follow-up of 9 months in both groups. The hazard ratio for disease-free survival was 0.97 (95% CI 0.67–1.42, p = 0.88; Fig. 2b). In patients with recurrence or disease progression, the rate of local recurrence or disease progression was 26% after MIDP and 25% after ODP. Adjuvant treatment was administered in 86 (66%) of 131 patients following MIDP and in 82 (65%) of 127 patients following ODP. Median time until start of adjuvant treatment was 57 (95% CI 51.5–62.5) days after MIDP and 57 (95% CI 50.6–63.4) days after ODP (p = 0.37). Age, radical resection, perineural invasion, and adjuvant chemotherapy were independent predictors for overall survival (Appendix p 7).
Fig. 2.
Overall and disease-free survival after MIDP and ODP in patients with resectable pancreatic cancer according to intention-to-treat. Survival analyses were performed with Kaplan–Meier estimated and log-rank tests. a) Kaplan–Meier curves for overall survival, hazard ratio was 0.99 (95% CI 0.67–1.46, p = 0.94). b) Kaplan–Meier curves for disease-free survival, hazard ratio was 0.97 (95% CI 0.67–1.42, p = 0.88).
The per-protocol analysis failed to confirm non-inferiority (pnon-inferiority = 0.06; Appendix p 9), as did the worst-case scenario (pnon-inferiority = 0.098; Appendix p 22). The best-case scenario confirmed the non-inferiority (pnon-inferiority = 0.011; Appendix p 22). Sensitivity and subgroup analyses did not affect results (Appendix pp 12–19). Functional recovery was shorter after MIDP compared to ODP when excluding patients with Clavien-Dindo grade ≥3 complications (5.0 [95% CI 4.6–5.4] days after MIDP vs 5.0 [95% CI 4.7–5.3] days after ODP, p = 0.04) and grade B/C POPF (4.0 [95% CI 3.5–4.5] days after MIDP vs 5.0 [95% CI 4.7–5.3] days after ODP, p = 0.045), see Appendix p 20. There were no significant differences in the global health status up to 6 months postoperatively (Appendix p 6). The median scar satisfaction score was improved in the MIDP group as compared to the ODP group (9.0 vs 8.0, p = 0.01, Appendix p 21). The mean costs of distal pancreatectomy and healthcare resources used up to 90 days postoperatively were comparable between the two groups (€18067 vs €21192; mean difference, €−3124; 95% BCaCI, −11056 to 4806).
Regarding the quality of patient blinding, 45 (38%) of 117 patients in the MIDP group and 67 (58%) of 114 patients in the ODP group reported to have undergone (i.e., guessed to have undergone) the opposite procedure. Patients who reported the other approach did not have a different time to functional recovery compared to patients who reported the correct approach. Pathologists reported the opposite procedure in 20 (17%) of 117 patients in the MIDP group and in 23 (22%) of 114 patients in the ODP group (Appendix p 21).
Discussion
This international multicentre randomised trial provides some evidence of the non-inferiority of MIDP (formally ‘left radical pancreatectomy’) for radical resection rate as compared to ODP in patients with resectable pancreatic cancer. Lymph node yield was comparable in both groups. MIDP was associated with longer operative time compared to ODP but better scar satisfaction scores at one year postoperatively. Other postoperative outcomes did not differ between groups.
Previous retrospective studies raised concerns regarding the oncological safety of MIDP compared to ODP, but were subject to well-known flaws of retrospective studies, such as treatment allocation bias, the presence of learning curves for MIDP, and differences in definitions and protocols between studies and specimen handling between MIDP and ODP.10 The present study demonstrated some evidence of non-inferior oncological outcomes in terms of radical resection rate after MIDP compared to ODP according to modified intention-to-treat and sensitivity analysis. The result of the per-protocol analysis (pnon-inferiority = 0.06) suggested to suspend judgment, but was hampered by a lack of power, because the point estimate of radicality of resection was in favour of MIDP over ODP. Although the worst-case scenario could not confirm non-inferiority and the best-case scenario could, these situations are considered not very probable. Hence, this study supports the use of MIDP as a safe and feasible approach in this patient category. As this study was not designed to confirm superiority of the minimally invasive approach, it remains at the surgeon's discretion whether MIDP or ODP is best suitable for an individual patient. This should consider the surgeon's surgical experience and training as MIDP for pancreatic cancer is a more challenging procedure than MIDP for other indications. This is highlighted by the relatively high 12% conversion rate in this study (compared to the 3–8% conversion rate in two previous randomised trials with only 18–21% of pancreatic cancer). This increase was mainly related to progression to vascular involvement prior to MIDP, as this was the case in 50% of conversions. However, general outcomes of MIDP were good with low rates of post-pancreatectomy fistula, haemorrhage and delayed gastric emptying.
In a recent randomised trial in patients with cervical cancer, demonstrating worse survival rates after minimally invasive hysterectomy compared to open hysterectomy, the authors questioned whether the insufflation gas (CO2) or different tumor manipulation in minimally invasive procedures might facilitate tumor spillage into the peritoneal cavity.11 The similar rate of intraperitoneal recurrences found after MIDP and ODP in the present study (41% vs 38%, p = 0.45) seems to refute this theory for pancreatic cancer. Accordingly, reports of randomised trials on minimally invasive esophagectomy for oesophageal cancer and minimally invasive resection for colon cancer showed comparable oncological outcomes to open surgical resection.25,26 Tumor biology rather than surgical approach is probably the main factor driving dissemination of cancer and prognosis.
The technical feasibility of MIDP for pancreatic cancer was also questioned in previous studies.10,27 Main concerns were raised since the reported lymph node yield of MIDP was lower compared to ODP in several studies,10 which were fuelled by a recent study that reported a minimum requirement of 19–20 nodes for adequate postoperative staging.28,29 The present study showed comparable lymph node yield in both groups, with a higher yield (22–23 nodes in both MIDP and ODP) compared to previous studies. The use of standardised surgery and histopathological protocols might have contributed to this finding. Moreover, the technical applicability of MIDP for radical procedures (e.g., radical antegrade modular pancreatosplenectomy) was questioned and other procedures were proposed in previous studies.18,27 In the present study, procedures in the MIDP group were performed using the standardised ‘no-touch’ left radical pancreatosplenectomy,18 which follows the same oncological principles as the radical antegrade modular pancreatosplenectomy procedure,20 permitting a local radicality and reducing the risk of intra-abdominal seeding. The outcomes of this study show that surgeons are able to obtain the minimum oncological criteria required during distal pancreatectomy for pancreatic cancer through MIDP.19
In this study, no benefit for time to functional recovery, estimated blood loss, and length of hospital stay were seen in the MIDP group as has been reported in two previous randomised trials for MIDP vs ODP, when performed for all indications.7,8 The authors feel that this, among others, could be related to the large groups of 35 participating centres from 12 countries with multiple surgeons, which could have diluted treatment effects. Furthermore, distal pancreatectomy for cancer is known to be more challenging than distal pancreatectomy for benign or premalignant disease. The two previous randomised trials7,8 only included a minority (45%) of patients with pancreatic cancer. Therefore, it could be that the advantages of minimally invasive surgery are less prominent in this subgroup of patients. Analyses for blood loss did show a trend towards less blood loss in MIDP (200 [IQR 100–300] vs 200 [IQR 100–400]), but this did not reach statistical significance, possibly due to type II error. When excluding patients with complicated postoperative course (i.e., Clavien-Dindo ≥3 and POPF), time to functional recovery was significantly shorter after MIDP (p = 0.04). Hence, in patients with an uncomplicated postoperative course, MIDP might provide benefits in terms of shorter time to functional recovery. Possible other benefits in the present study were shorter unplanned ICU admission, and improved scar satisfaction scores compared to ODP at one year postoperatively (also previously reported in the long-term report of the LEOPARD trial30). Furthermore, when analysing time to functional recovery after MIDP and ODP with a Mann–Whitney U test (as done previously in the LEOPARD and LAPOP trials7,8), rather than with a ‘time to event approach’ as done in the present study, we found a significant shorter time to functional recovery after MIDP compared to ODP (p = 0.03).
This study has several limitations that should be considered. First, the ideal primary endpoint for this trial would have been survival, but this would require >1000 patients to be enrolled and was therefore considered not feasible for this indication. Radicality of resection reflects oncological safety of surgical procedures and was associated with survival in several studies,31 which was therefore chosen as primary endpoint of this study. Second, primary endpoint assessment did not include the anterior surface, which has demonstrated prognostic significance in a recent study.32 However, the aim of the present study was to evaluate the feasibility of MIDP at ‘surgical margins’, which only included the posterior and transection margins. Third, the preoperative imaging interval of maximum 4 weeks was exceeded in 40 (16%) of 258 patients. This was, however, considered of small impact as four (10%) of these patients had unresectable disease (i.e., occult metastatic disease) at surgical exploration, whereas 23 (11%) of 218 patients with an interval <4 weeks had unresectable disease (i.e., occult metastatic disease) at surgical exploration. Fourth, 9% of patients had other diagnoses than pancreatic cancer in which primary outcome assessment of radical resection was not possible. Although undesirable, this reflects the diagnostic difficulties during the work-up of patients affected by suspected resectable pancreatic cancer. Moreover, this rate is comparable to previously reported in studies.33,34 Central pathology review was not performed but would not have changed this finding as preoperative pathological confirmation was not mandatory given the pragmatic design of the present study. Fifth, data on functional recovery were not available for 27 patients since these data were not collected in patients who did not undergo a resection. Although this somewhat limited the statistical power, it was considered of low impact. Sixth, neoadjuvant treatment was initially set as exclusion criteria for this study because of surgical difficulties and lack of consensus on resection margin reporting among different pathologists.35 Randomised evidence is lacking on the benefit of neoadjuvant treatment in patients with left-sided primary resectable pancreatic cancer. Several randomised trials are either recently completed (NCT02919787) or are currently underway on neoadjuvant therapy in resectable pancreatic cancer (e.g., NCT04340141 and NCT04927780). However, given the growing experience in minimally invasive surgery after neoadjuvant treatment, it was agreed that these patients could be included based on the surgeon's discretion as long as these patients had upfront resectable disease. Patients with neoadjuvant treatment were well balanced between the two groups (18% vs 22%) and sensitivity analyses showed no impact of the inclusion of neoadjuvant treatment on outcomes. Seventh, median survival was not reached. However, no significant differences in postoperative survival are expected considering the overlapping survival curves in this study. Eighth, due to the Covid pandemic operating theatre availability was reduced over the majority of the inclusion period. Nevertheless, on average 6 patients were included per centre per year out of on average 11 patients with upfront resectable pancreatic cancer per centre per year. Ninth, although the rates of grade B/C POPF did not differ between groups, differences between pancreatic transection methods in centres could have influenced outcomes. The study protocol included standardised transection at the pancreatic neck with a preference for stapling of the pancreas. Furthermore, two previous randomised trials did not find differences in rates of POPF between stapler transection, handsewn closure, and ultrasonic dissector.36,37 Stratification per centre could have minimised the impact of differences between centres, but was not performed as the resulting high number of strata would have hampered the randomisation process. The strengths of this trial lay in the well-standardised surgical and pathological procedures with blinded endpoint assessment (i.e., blinding of patients and pathologists) to minimize the impact of these confounding factors. This was considered successful, as 45% of patients and 77% of pathologist were not able to correctly guess the type of surgical approach (Appendix p 21). Moreover, outcomes in this study were comparable among participating centres, allowing the results to be universal and reproducible.
In conclusion, this randomised controlled trial provides some evidence of non-inferior radical resection rates after MIDP as compared to ODP in patients with resectable pancreatic body and tail cancer. This supports the use of minimally invasive surgery in this patient category. It remains at the surgeons’ discretion to decide whether a minimally invasive or open approach is suitable for an individual patient.
Contributors
Maarten Korrel.
Development and design of methodology including protocol writing, application of statistical techniques to analyse study data, conducting the research process, research management/administration (initiation of participating centres, data collection, data cleaning, annual reports to DMC and ethical committees), preparation, creation, and writing of the original draft and financial acquisition.
Leia Jones.
Application of statistical techniques to analyse study data, conducting the research process, research management/administration (data collection, data cleaning), preparation, creation, publications, and writing of the original draft.
Jony van Hilst.
Development and design of methodology including protocol writing, supervision of preparation and writing of the original draft, and financial acquisition.
Gianpaolo Balzano.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Bergthor Björnsson.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Ugo Boggi.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Svein Olav Bratlie.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Olivier Busch.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Giovanni Butturini.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Giovanni Capretti.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Riccardo Casadei.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Bjorn Edwin.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Anouk Emmen.
Conducting the research process, research management/administration (data collection, data cleaning), review and editing of original draft including approval of final version.
Alessandro Esposito.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Massimo Falconi.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Bas Groot Koerkamp.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Tobias Keck.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Ruben de Kleine.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Dyre Kleive.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Arto Kokkola.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Daan Lips.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Sanne Lof.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Misha Luyer.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Alberto Manzoni.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Ravi Marudanayagam.
Performing surgical procedures in the trial, ata collection from participating centre, review and editing of original draft including approval of final version.
Matteo de Pastena.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Nicolò Pecorelli.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
John Primrose.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Claudio Ricci.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Roberto Salvia.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Per Sandström.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Frederique Vissers.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Ulrich Wellner.
Performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Alessandro Zerbi.
Development and design of methodology, performing surgical procedures in the trial, data collection from participating centre, review and editing of original draft including approval of final version.
Marcel Dijkgraaf.
Development and design of methodology, development of statistical analysis plan, supervision of final statistical analysis and approval of final manuscript.
Marc Besselink.
Development and design of methodology including protocol writing, performing surgical procedures in the trial, supervision of research management/administration, supervision of preparation and writing the original draft, final judgement to submit manuscript and financial acquisition.
Mohammad Abu Hilal.
Development and design of methodology including protocol writing, performing surgical procedures in the trial, supervision of research management/administration, supervision of preparation and writing the original draft, final judgement to submit manuscript and financial acquisition.
Data sharing statement
Deidentified individual participant data collected in the DIPLOMA trial can be made available upon request. Please contact the principal investigators (MAH and MGB) who will review all requests. The DIPLOMA investigators will be allowed to approve all research performed with the shared data.
Declaration of interests
Tobias Keck is a member of the advisory board for Olympus, Medtronic, and Dexter. Daan Lips received a proctoring grant by Intuitive Surgical. Marc Besselink and Mohammad Abu Hilal received Investigator Initiated Research grants by Medtronic (DIPLOMA trial), Ethicon (DIPLOMA trial and E-MIPS registry), and Intuitive Surgical (E-MIPS registry) and proctoring grants for Dutch and European training programs in robotic pancreatoduodenectomy by Intuitive Surgical. The other authors have no conflicts of interest.
Acknowledgements
We thank Kevin Conlon, Antonio Benedetti, Patrick Maisonneuve, Tim Underwood, and Ed Juszczak for their participation in the Data Monitoring Committee of the DIPLOMA trial.
Collaborators of the European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS)
The E-MIPS consortium collaborators are:
Adnan Alseidi (Virginia Mason Medical Center, Seattle, United States of America), Constanza Aquilano (Niguarda Ca’Granda Hospital, Milan, Italy), Johanna Arola (Helsinki University Hospital, Helsinki, Finland), Denise Bianchi (Instituto Ospedaliero Fondazione Poliambulanza, Brescia, Italy), Rachel Brown (University Hospital Birmingham, Birmingham, United Kingdom), Daniela Campani (Universitá di Pisa, Pisa, Italy), Joanne ChinAleong (Royal London Hospital, London, United Kingdom), Jerome Cros (Beaujon Hospital, Clichy, France), Lyubomira Dimitrova (Sahlgrenska University Hospital, Gothenburg, Sweden), Claudio Doglioni (San Raffaele Hospital IRCCS, Università Vita-Salute, Milan, Italy), Safi Dokmak (Beaujon Hospital, Clichy, France), Russell Dorer (Virginia Mason Medical Center, Seattle, United States of America), Michael Doukas (Erasmus MC Cancer Institute, Rotterdam, the Netherlands), Jean Michel Fabre (Hopital Saint Eloi, Montpellier, France), Giovanni Ferrari (Niguarda Ca’Granda Hospital, Milan, Italy), Viacheslay Grinevich (Moscow Clinical Scientific Center, Moscow, Russian Federation), Stefano Gobbo (Pederzoli Hospital, Peschiera, Italy), Thilo Hackert (Heidelberg University Hospital, Heidelberg, Germany), Marius van den Heuvel (University Medical Centre Groningen, Groningen, the Netherlands), Clement Huijsentruijt (Catharina Ziekenhuis, Eindhoven, the Netherlands), Mar Iglesias (Hospital del Mar, Barcelona, Spain), Casper Jansen (Medisch Spectrum Twente, Enschede, the Netherlands), Igor Khatkov (Moscow Clinical Scientific Center, Moscow, Russian Federation), David Kooby (Emory University Hospital, Atlanta, United States of America), Marco Lena (San Raffaele Hospital IRCCS, Università Vita-Salute, Milan, Italy), Claudio Luchini (Pancreas Institute, University Hospital of Verona, Verona, Italy), Krishna Menon (King's College Hospital, London, United Kingdom), Patrick Michenet (Centre Hospitalier Regional Universitaire Orleans, Orleans, France), Quintus Molenaar (University Medical Center Utrecht, Utrecht, the Netherlands), Anna Nedkova (Linköping University, Linköping, Sweden), Andrea Pietrabissa (IRCCS Policlinico San Matteo Pavia, Pavia, Italy), Mihaela Raicu (University Medical Center Utrecht, Utrecht, the Netherlands), Rushda Rajak (University Hospital Southampton, Southampton, United Kingdom), Branislava Rankovic (University Medical Center Ljubljana, Ljubljana, Slovenia), Aniko Rendek (Oxford University Hospital, Oxford, United Kingdom), Benjamin Riviere (Hopital Saint Eloi, Montpellier, France), Antonio Sa Cunha (Paul-Brousse Hospital, Villejuif, France), Olivier Saint Marc (Centre Hospitalier Regional Universitaire Orleans, Orleans, France), Patricia Sanchez Velazquez (Hospital del Mar, Barcelona, Spain), Donatella Santini (S. Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy), Aldo Scarpa (Pancreas Institute, University Hospital of Verona, Verona, Italy), Mylene Sebagh (Paul-Brousse Hospital, Villejuif, France), Donald Sears (Emory University Hospital, Atlanta, United States of America), Mihir Shah (Emory University Hospital, Atlanta, United Sates of America), Zahir Soonawalla (Oxford University Hospital, Oxford, United Kingdom), Paola Spaggiari (IRCCS Humanitas Research Hospital, Rozzano, Italy), Lars Tharun (UKSH campus Lübeck, Lübeck, Germany), Tore Tholfsen (Oslo University Hospital, Oslo, Norway), Ales Tomazic (University Medical Center Ljubljana, Ljubljana, Slovenia), Alessandro Vanoli (IRCCS Policlinico San Matteo Pavia, Pavia, Italy), Caroline Verbeke (Oslo University Hospital, University of Oslo, Oslo, Norway), Joanne Verheij (Amsterdam UMC, University of Amsterdam, the Netherlands), Roeland de Wilde (Erasmus MC Cancer Institute, Rotterdam, the Netherlands), Moritz Von Winterfeld (Heidelberg University Hospital, Heidelberg, Germany), Vincent Yip (Royal London Hospital, London, United Kingdom), Yoh Zen (King's College Hospital, London, United Kingdom).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100673.
Contributor Information
Marc G. Besselink, Email: m.g.besselink@amsterdamUMC.nl.
Mohammad Abu Hilal, Email: abuhilal9@gmail.com.
European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS):
Adnan Alseidi, Constanza Aquilano, Johanna Arola, Denise Bianchi, Rachel Brown, Daniela Campani, Joanne ChinAleong, Jerome Cros, Lyubomira Dimitrova, Claudio Doglioni, Safi Dokmak, Russell Dorer, Michael Doukas, Jean Michel Fabre, Giovanni Ferrari, Viacheslay Grinevich, Stefano Gobbo, Thilo Hackert, Marius van den Heuvel, Clement Huijsentruijt, Mar Iglesias, Casper Jansen, Igor Khatkov, David Kooby, Marco Lena, Claudio Luchini, Krishna Menon, Patrick Michenet, Quintus Molenaar, Anna Nedkova, Andrea Pietrabissa, Mihaela Raicu, Rushda Rajak, Branislava Rankovic, Aniko Rendek, Benjamin Riviere, Antonio Sa Cunha, Olivier Saint Marc, Patricia Sanchez Velazquez, Donatella Santini, Aldo Scarpa, Mylene Sebagh, Donald Sears, Mihir Shah, Zahir Soonawalla, Paola Spaggiari, Lars Tharun, Tore Tholfsen, Ales Tomazic, Alessandro Vanoli, Caroline Verbeke, Joanne Verheij, Moritz Von Winterfeld, Roeland de Wilde, Vincent Yip, and Yoh Zen
Appendix A. Supplementary data
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Rose T.C., Pennington A., Kypridemos C., et al. Analysis of the burden and economic impact of digestive diseases and investigation of research gaps and priorities in the field of digestive health in the European Region-White Book 2: executive summary. United European Gastroenterol J. 2022;10(7):657–662. doi: 10.1002/ueg2.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neoptolemos J.P., Palmer D.H., Ghaneh P., et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 4.van Erning F.N., Mackay T.M., van der Geest L.G.M., et al. Association of the location of pancreatic ductal adenocarcinoma (head, body, tail) with tumor stage, treatment, and survival: a population-based analysis. Acta Oncol. 2018;57(12):1655–1662. doi: 10.1080/0284186X.2018.1518593. [DOI] [PubMed] [Google Scholar]
- 5.Cuschieri A. Laparoscopic surgery of the pancreas. J R Coll Surg Edinb. 1994;39(3):178–184. [PubMed] [Google Scholar]
- 6.Adams A.M., Russell D.M., Carpenter E.L., et al. Minimally invasive versus open distal pancreatectomy: a matched analysis using ACS-NSQIP. Surg Endosc. 2022;37:617. doi: 10.1007/s00464-022-09363-y. [DOI] [PubMed] [Google Scholar]
- 7.Bjornsson B., Larsson A.L., Hjalmarsson C., et al. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg. 2020;107:1281. doi: 10.1002/bjs.11554. [DOI] [PubMed] [Google Scholar]
- 8.de Rooij T., van Hilst J., van Santvoort H., et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269(1):2–9. doi: 10.1097/SLA.0000000000002979. [DOI] [PubMed] [Google Scholar]
- 9.de Rooij T., Besselink M.G., Shamali A., et al. Pan-European survey on the implementation of minimally invasive pancreatic surgery with emphasis on cancer. HPB (Oxford) 2016;18(2):170–176. doi: 10.1016/j.hpb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hilst J., Korrel M., de Rooij T., et al. Oncologic outcomes of minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2019;45(5):719–727. doi: 10.1016/j.ejso.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez P.T., Frumovitz M., Pareja R., et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 12.Lewicki P.J., Basourakos S.P., Qiu Y., et al. Effect of a randomized, controlled trial on surgery for cervical cancer. N Engl J Med. 2021;384(17):1669–1671. doi: 10.1056/NEJMc2035819. [DOI] [PubMed] [Google Scholar]
- 13.Fleshman J., Branda M., Sargent D.J., et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson A.R., Solomon M.J., Lumley J.W., et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 15.Cancer TiAfRo . 4th ed. 2010. WHO classification of tumours of the digestive system. [Google Scholar]
- 16.Lee S.H., Kang C.M., Hwang H.K., et al. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc. 2014;28(10):2848–2855. doi: 10.1007/s00464-014-3537-3. [DOI] [PubMed] [Google Scholar]
- 17.van Hilst J., Korrel M., Lof S., et al. Minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma (DIPLOMA): study protocol for a randomized controlled trial. Trials. 2021;22(1):608. doi: 10.1186/s13063-021-05506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu Hilal M., Richardson J.R., de Rooij T., et al. Laparoscopic radical 'no-touch' left pancreatosplenectomy for pancreatic ductal adenocarcinoma: technique and results. Surg Endosc. 2016;30(9):3830–3838. doi: 10.1007/s00464-015-4685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartwig W., Vollmer C.M., Fingerhut A., et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS) Surgery. 2014;156(1):1–14. doi: 10.1016/j.surg.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Strasberg S.M., Linehan D.C., Hawkins W.G. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg. 2007;204(2):244–249. doi: 10.1016/j.jamcollsurg.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Lof S., Rajak R., Vissers F., et al. DIPLOMA approach for standardized pathology assessment of distal pancreatectomy specimens. J Vis Exp. 2020;(156):e60343. doi: 10.3791/60343. [DOI] [PubMed] [Google Scholar]
- 22.Wilmore D.W., Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322(7284):473–476. doi: 10.1136/bmj.322.7284.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hilst J., de Rooij T., Klompmaker S., et al. Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a pan-European propensity score matched study. Ann Surg. 2017;269:10. doi: 10.1097/SLA.0000000000002561. [DOI] [PubMed] [Google Scholar]
- 24.Dunnett C.W., Gent M. Significance testing to establish equivalence between treatments, with special reference to data in the form of 2X2 tables. Biometrics. 1977;33(4):593–602. [PubMed] [Google Scholar]
- 25.Straatman J., van der Wielen N., Cuesta M.A., et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266(2):232–236. doi: 10.1097/SLA.0000000000002171. [DOI] [PubMed] [Google Scholar]
- 26.Colon Cancer Laparoscopic or Open Resection Study G. Buunen M., Veldkamp R., et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10(1):44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 27.Takagi K., Umeda Y., Yoshida R., et al. A systematic review of minimally invasive versus open radical antegrade modular pancreatosplenectomy for pancreatic cancer. Anticancer Res. 2022;42(2):653–660. doi: 10.21873/anticanres.15523. [DOI] [PubMed] [Google Scholar]
- 28.Wang W., Shen Z., Zhang J., et al. A novel criterion for lymph nodes dissection in distal pancreatectomy for ductal adenocarcinoma: a population study of the US SEER database. Ann Surg Oncol. 2022;29(3):1533–1539. doi: 10.1245/s10434-021-10797-2. [DOI] [PubMed] [Google Scholar]
- 29.Malleo G., Maggino L., Ferrone C.R., et al. Number of examined lymph nodes and nodal status assessment in distal pancreatectomy for body/tail ductal adenocarcinoma. Ann Surg. 2019;270(6):1138–1146. doi: 10.1097/SLA.0000000000002781. [DOI] [PubMed] [Google Scholar]
- 30.Korrel M., Roelofs A., van Hilst J., et al. Long-term quality of life after minimally invasive vs open distal pancreatectomy in the LEOPARD randomized trial. J Am Coll Surg. 2021;233(6):730–739.e9. doi: 10.1016/j.jamcollsurg.2021.08.687. [DOI] [PubMed] [Google Scholar]
- 31.Holm M.B., Verbeke C.S. Prognostic impact of resection margin status on distal pancreatectomy for ductal adenocarcinoma. Curr Oncol. 2022;29(9):6551–6563. doi: 10.3390/curroncol29090515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahakyan M.A., Verbeke C.S., Tholfsen T., et al. Prognostic impact of resection margin status in distal pancreatectomy for ductal adenocarcinoma. Ann Surg Oncol. 2022;29(1):366–375. doi: 10.1245/s10434-021-10464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Versteijne E., van Dam J.L., Suker M., et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022;40(11):1220–1230. doi: 10.1200/JCO.21.02233. [DOI] [PubMed] [Google Scholar]
- 34.Gerritsen A., Molenaar I.Q., Bollen T.L., et al. Preoperative characteristics of patients with presumed pancreatic cancer but ultimately benign disease: a multicenter series of 344 pancreatoduodenectomies. Ann Surg Oncol. 2014;21(12):3999–4006. doi: 10.1245/s10434-014-3810-7. [DOI] [PubMed] [Google Scholar]
- 35.Verbeke C., Lohr M., Karlsson J.S., et al. Pathology reporting of pancreatic cancer following neoadjuvant therapy: challenges and uncertainties. Cancer Treat Rev. 2015;41(1):17–26. doi: 10.1016/j.ctrv.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Diener M.K., Seiler C.M., Rossion I., et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet. 2011;377(9776):1514–1522. doi: 10.1016/S0140-6736(11)60237-7. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y., Fujino Y., Tanioka Y., et al. Randomized clinical trial of ultrasonic dissector or conventional division in distal pancreatectomy for non-fibrotic pancreas. Br J Surg. 1999;86(5):608–611. doi: 10.1046/j.1365-2168.1999.01120.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.