Abstract
Background:
Sickle cell disease (SCD) is associated with poor neurocognitive outcomes due to biomedical and psychosocial factors. The aims of this study were to investigate associations between household and neighborhood socioeconomic status (SES) with cognitive and academic outcomes in SCD and to determine if these relationships were modified by sickle genotype, fetal hemoglobin, or age.
Procedure:
We prospectively recruited patients to complete a battery of neurocognitive and academic measures. Household SES was measured using the Barratt Simplified Measure of Social Status, a composite index of parent education and occupation. The Social Vulnerability Index was used to classify individuals based on social vulnerabilities at the neighborhood level.
Results:
Overall, 299 patients between the ages of 4–18 (Mean=11.4, Standard Deviation=4.3) years diagnosed with SCD (57% SS/SB0-thalassemia) completed testing. Stepwise multivariate models demonstrated that patients with low social vulnerability (i.e., high SES) at the neighborhood level displayed intelligence and math scores that were 4.70 and 7.64 points higher than those living in areas with moderate social vulnerability, respectively (p<0.05). Reading performance did not differ based on neighborhood SES; however, the effect of neighborhood SES was dependent on age, such that older participants living in neighborhoods with moderate or high levels of social vulnerability displayed poorer reading scores than those with low social vulnerability (p<0.05).
Conclusions:
This study identified patients with SCD at higher risk of poor academic performance based on SES. Interventions addressing academic difficulties should be offered to all children with SCD but should be emergently offered to this sub-population.
Keywords: sickle cell, anemia, neurocognitive, academic, socioeconomic status, social determinants
Introduction
Socioeconomic status (SES) is described as a measure of one’s combined economic and social status and is associated with health outcomes (1). Longitudinal studies in the general population demonstrate effects of SES on cognition as early as infancy, and these differences extend through adolescence and adulthood (2, 3). The effects of SES are documented on measures of cognitive performance and neuroimaging (4). Both household-level (e.g., familial educational attainment and occupation) and neighborhood-level measures of SES uniquely contribute to neurocognitive outcomes (5). Consistent with the general population, studies have documented the unique contribution of SES to neurocognition in patients with medical conditions who are at high risk for neurocognitive deficits. For example, in children born very preterm (<32 weeks’ gestation), SES has strong associations with cognitive outcomes, accounting for as much variance as a brain insult, such as severe intraventricular hemorrhage (6).
In the United States, racially and ethnically diverse populations experience disproportionate rates of social disparities, generational poverty, and systemic inequities (7). Specifically, Black/African Americans face several challenges including ongoing de facto segregation, education, crime, economic disadvantage, health issues, and discrimination, which place them at an increased risk for health issues (8). On a neighborhood level, Black/African-Americans living in underserved communities may have limited access to appropriate healthcare and possible underutilization of healthcare services (9). Structural inequities, such as neighborhood SES, may lead to negative perceptions and bias among healthcare providers, which may adversely impact treatment decisions and potentially limit a patient’s opportunities for health security and quality (9). For pediatric patients with sickle cell disease (SCD), an inherited hemoglobinopathy predominantly seen among individuals of African descent in the United States, the dual burden of living with a chronic disease and racial inequity increases this population’s vulnerability to social disparities (7). Environmentally, patients with SCD experience greater rates of poverty and fewer protective socioeconomic factors when compared with the Black/African-American population in the United States (10, 11). Patients from the most disadvantaged environments experience reduced health-related quality of life (12) and are at higher risk for in-hospital mortality (13).
SCD is associated with significant neurocognitive risk due to a combination of disease and environmental factors (7, 8). Neurological complications, including overt stroke, silent cerebral infarctions, and chronic insufficiencies in oxygen and/or glucose delivery to the brain contribute to the neurocognitive decline (14–16). There are several genotypes of SCD, with differing clinical presentations. Patients diagnosed with HbSS/HbSb0-thalassemia (also known as sickle cell anemia) are at higher risk for multiple complications (e.g., stroke, acute chest syndrome, pain episodes) compared to other genotypes (e.g., HbSC/HbSβ+-thalassemia). Disease status, along with neurocognitive functioning, in SCD typically worsens with age due to cumulative complications (17–19). To address disease complications, hydroxyurea therapy is considered standard of care for patients with HbSS/HbSβ0-thalassemia and is recommended on a case-by-case basis for other SCD genotypes (20). Hydroxyurea is a myelosuppressive agent that raises the level of fetal (HbF) and total hemoglobin (Hb). HbF protects the cell by inhibiting the polymerization of deoxy sickle hemoglobin. Hydroxyurea is known to ameliorate anemia and reduce the number of vaso occlusive events (21). Preliminary data suggests hydroxyurea treatment may provide neuroprotection and limit neurocognitive decline in patients with SCD (18).
Prior studies have demonstrated that SES is positively associated with cognitive and academic performance independent of disease complications in patients with SCD (22–25). Schatz and colleagues observed that the effect of SES on neurocognitive functioning depended on SCD severity (26). Children diagnosed with SCD who had mild to moderate degrees of anemia demonstrated a strong relationship between SES and neurocognition, but there was no association between SES and neurocognition among children with severe disease (26). Overall, studies have established that there is a relationship between measures of household SES (e.g., parent education, income, occupation) and neurocognitive outcomes in children and adolescents with SCD.
Additional research examining the relationship between SES and neurocognition in SCD is needed due to limitations of prior studies. Only a single study (26) with a small sample (N=36), examined modifiers of the relationship between SES and neurocognition to determine which patients are most impacted by socioeconomic disparities. There is minimal research examining how the relationship between household SES (i.e., parent education/occupation) and neurocognition extends into late adolescence or the unique contribution of neighborhood SES to the health outcomes in SCD. Lastly, the relative contribution of SES to neurocognitive outcomes compared to medical and treatment factors has yet to be thoroughly explored.
The primary objective of this study was to investigate associations between household and neighborhood SES with cognitive and academic (reading and math) outcomes in a large prospectively recruited sample of patients with SCD ranging from childhood to late adolescence. Given that SCD becomes more severe with aging, a secondary objective was to examine if the relationship between SES and neurocognitive/academic outcomes was moderated by disease genotype, HbF level, or age (i.e., if these factors would alter the effect of SES on neurocognition). Finally, we sought to measure the relative contribution of SES to neurocognitive/academic outcomes compared to medical and treatment factors. We hypothesized that both household and neighborhood SES would independently contribute to neurocognitive outcomes. We predicted that the effect of SES would not differ by disease genotype or HbF level. Rather, it was hypothesized that the association between age and neurocognitive/academic outcomes would differ by SES such that age would have a greater effect on patients with lower SES.
Method
The institutional review board (IRB) at St. Jude Children’s Research Hospital (Memphis, TN) approved the study. The legal guardian of each participant gave written informed consent and adolescents gave assent according to the requirements of the IRB.
Participants
Children and adolescents with SCD who participated in the Sickle Cell Clinical Research and Intervention Program (SCCRIP) study and received a routine neurocognitive assessment were eligible for this study. Briefly, SCCRIP is a longitudinal lifetime cohort study that collects retrospective and prospective data on clinical, neurocognitive, geographical, psychosocial and health outcomes of children, adolescents and adults with SCD (27). Neurocognitive assessments are performed approximately every four years between the ages of 4 and 18 years. These screening assessments are not clinical referrals, but systematic surveillance, as patients are not selected for disease severity, prior central nervous system findings, or existing cognitive concerns.
Medical and treatment variables
Medical and treatment variables were abstracted from the SCCRIP database. Participants with HbSS/HbSβ0-thalassemia received hydroxyurea according to established guidelines (28). For participants with HbSC/HbSβ+-thalassemia, initiation was guided by the frequency of acute disease complications (29). Lab values including HbF, Hb, and platelet count were collected on the day of neurocognitive testing or were the average value of measurements within three months prior to testing. Daytime Hb oxygen saturation was obtained on the day of the neurocognitive testing and >2 months from a blood transfusion. SCD genotype was split into 2 groups: HbSS/HbSβ0-thalassemia vs. HbSC/HbSβ+-thalassemia.
Socioeconomic Status
Household SES was measured using the Barratt Simplified Measure of Social Status (BSMSS)(30) based on Hollingshead’s Four Factor Index (31). Parents were asked to report their occupation, education level, and marital status as part of a clinical interview during the neurocognitive assessment. The BSMSS classification system codes occupations based on skill, power, and social position in society. Education was accounted for using level of school completed, with seventh grade and below receiving the lowest score and graduate degree or professional school beyond college receiving the highest score. A composite score is created by adding the scores for occupation and education for each parent. The average of the caregivers’ scores was used for households with multiple caregivers, whereas single-parent homes only included the individual parent’s scores. Total composite scores range from 8 (low) to 66 (high). The data used to calculate the BSMSS were collected at the same time as the most recent neurocognitive evaluation. The Social Vulnerability Index (SVI)(32, 33) was used to classify individuals based on social vulnerabilities at the neighborhood level. The SVI is comprised of 15 census variables collected by the U.S. Census Bureau. The 15 variables correspond to four themes: Socioeconomic Status, Household Composition and Disability, Minority Status and Language, and Housing and Transportation. Census tracts are ranked within each state to evaluate the relative vulnerability. A higher percentile score indicates higher social vulnerability, ranging from 0–100. SVI was categorized into three groups: low (0–33), moderate (33–66), and high (66–100). Geocodes to calculate the SVI are updated each year based on the patient’s reported address.
Neurocognitive Measures
Participants in SCCRIP completed a battery of neurocognitive tests. The administration of all measures was supervised by a licensed psychologist. The neurocognitive measures differed based on the patient’s age at the time of the assessment. In children older than 6 years of age, the Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II)(34) provided an estimated Full-Scale Intelligence Quotient (FSIQ; 4-subtest IQ). Academic achievement measures included Letter-Word Identification and Math Fluency from the Woodcock-Johnson Test of Achievement – Third Edition (35). Children younger than 6 years of age were administered the Wechsler Preschool and Primary Scale of Intelligence – Fourth Edition (36) as a measure of FSIQ (6-subtest IQ). Children younger than age 6 did not receive measures of academic achievement. All measures demonstrate appropriate reliability and validity and reference age-based normative samples.
Statistical Analyses
Participant demographics, clinical characteristics, and hydroxyurea treatment history were reported using means and standard deviations or frequencies and percentages. Differences between SCD genotype groups were compared using Chi-square or Fisher’s exact tests and two-sample t-tests or Wilcoxon rank sum tests. Normality of the data was checked using Shapiro-wilk test. Associations between demographic characteristics, clinical measures, and socioeconomic status with neurocognitive measures were found using simple linear regressions.
To address our primary objective, demographic and clinical characteristics with significant associations at p<0.10 or associated with intelligence or academic achievement in prior studies were used as covariates in multivariate analyses modeling the adjusted associations between neurocognitive measures and BSMSS or SVI. Interactions between SES measures with age, SCD genotype, and HbF were also included in multivariate analysis to examine potential modification of the relationships between SES and neurocognitive measures. The classical coefficient of determination (R2) was calculated to determine the contribution of household and neighborhood SES to cognitive and academic outcomes relative to other covariates for the fixed models. The correlation between SVI and BSMSS was assessed using Spearman correlation test. To investigate if the relationships between neurocognitive measures with household and neighborhood SES were independent of each other, an automated stepwise model with backward and forward variable selection based on Akaike information criterion (AIC) was used to incorporate patient demographics, SCD characteristics, BSMSS, SVI, and all SES interactions with age and genotype as possible model covariates. All covariates were tested for multicollinearity prior to entering the covariates in the multivariate model (a variance inflation factor <2).
False discovery rate (FDR) adjusted p-value (pFDR) or q-value was calculated to account for multiple comparisons. All p-values are two-sided and considered significant at pFDR <0.05 unless otherwise noted. Analyses were conducted in SAS 9.4, R version 3.6.3 (37), the MASS package (38), and the r2glmm package (39).
Results
Demographic and clinical characteristics
A total of 299 patients, ages 4–18, received neurocognitive testing at an average age of 11.42 (Standard Deviation [SD] = 4.25) years (Table 1). Consistent with standard of care, most patients (75%) diagnosed with HbSS/Hb Sβ0-thalassemia were treated with hydroxyurea, whereas only 18% of patients with HbSC/Hb Sβ+-thalassemia were taking hydroxyurea at the time of their cognitive evaluation. Household socioeconomic status based on the BSMSS was lower in those diagnosed with HbSS/HbSβ0-thalassemia compared to the group with HbSC/HbSβ+-thalassemia (pFDR=0.04). Based on the SVI, most patients lived in neighborhoods with high levels of social vulnerability (i.e., low socioeconomic status; Mean = 65.10, SD = 25.55). Neighborhood socioeconomic status did not differ based on sickle genotype (pFDR=0.23). Those that received neurocognitive testing were significantly older, more likely to have a mild SCD genotype (HbSC/SB+ thalassemia), and had lower social vulnerability compared to those without an assessment (Supplemental Table 1).
Table 1.
Demographic and clinical characteristics of patients by genotype
| Overall | HbSS/HbSβ0-thalassemia | HbSC/HbSβ+-thalassemia/Other | pFDRa | |
|---|---|---|---|---|
| N=299 | N=169 | N=130 | ||
|
| ||||
| Frequency (%) | Frequency (%) | Frequency (%) | ||
|
| ||||
| Sex |
0.17 |
|||
| Female | 147 (49%) | 76 (45%) | 71 (55%) | |
| Male | 152 (50%) | 93 (55%) | 59 (45%) | |
| Race |
0.23 |
|||
| African-American | 296 (99%) | 168 (99%) | 128 (98%) | |
| Other | 1 (0%) | 1 (1%) | 0 (0%) | |
| White | 2 (1%) | 0 (0%) | 2 (2%) | |
| Disease-modifying Therapy | ||||
| HU therapy alone | 148 (49%) | 125 (74%) | 23 (18%) | <.0001 |
| Chronic Transfusion alone | 3 (1%) | 3 (2%) | 0 (0%) | |
| Transfusions and HU | 2 (1%) | 2 (1%) | 0 (0%) | |
| None | 146 (49%) | 39 (23%) | 107 (82%) | |
| Current HU therapy | 150 (50%) | 127 (75%) | 23 (18%) | <.0001 |
| Age Started HU (years) | 6.27 (4.29) | 5.74 (3.81) | 9.26 (5.54) | 0.009 |
| Duration on HU (years) | 2.60 (3.52) | 4.22 (3.81) | 0.55 (1.53) | <.0001 |
| TCD* | 0.23 | |||
| Abnormal | 7 (4%) | 7 (4%) | 0 (0%) | |
| Conditional | 36 (22%) | 36 (23%) | 0 (0%) | |
| Normal | 124 (74%) | 113 (72%) | 11 (100%) | |
| SVI categories | ||||
| Low | 43 (14%) | 20 (12%) | 23 (18%) | |
| Moderate | 83 (28%) | 42 (25%) | 41 (32%) | 0.17 |
| High | 172 (58%) | 106 (63%) | 66 (51%) | |
| 504 Education Plan$ | ||||
| Yes | 102 (44%) | 59 (47%) | 43 (41%) | 0.53 |
| No | 114 (50%) | 61 (48%) | 53 (51%) | |
| Previously | 14 (6%) | 6 (5%) | 8 (8%) | |
| Individualized Education Plans& | ||||
| Yes | 39 (17%) | 26 (21 %) | 13 (13%) | 0.30 |
| No | 174 (78%) | 91 (74%) | 83 (82%) | |
| Previously | 11 (5%) | 6 (5%) | 5 (5%) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 11.42 (4.25) | 11.18 (4.20) | 11.73 (4.30) | 0.30 |
| Hemoglobin (g/dL) | 10.2 (1.7) | 9.2 (1.3) | 11.5 (1.2) | <.0001 |
| WBC (x109/L) | 8.7 (3.5) | 9.2 (3.5) | 7.9 (3.5) | 0.0004 |
| Fetal Hemoglobin (%) | 16.8 (10.0) | 18.9 (9.0) | 6.6 (7.9) | <.0001 |
| Platelet Count (x109/L) | 340 (169) | 392 (183) | 266 (112) | <.0001 |
| Oxygen Saturation (%) | 99 (1) | 99 (1) | 100 (1) | 0.10 |
| BSMSS | 30 (13) | 28 (12) | 32 (14) | 0.04 |
| SVI# | 65.10 (25.55) | 66.93 (24.98) | 62.73 (26.18) | 0.23 |
SD, standard deviation; HU, hydroxyurea; WBC, white blood cell count; TCD, transcranial doppler; BSMSS, Barratt Simplified Measure of Social Status; SVI, social vulnerability index; HbSS/HbSb0-thalassemia, sickle cell anemia; HbSC/Hb Sβ+-thalassemia, hemoglobinopathy. Values presented as mean (standard deviation) or frequency (group%) unless otherwise noted.
p-value adjusted for the false discovery rate (FDR). pFDR < 0.05 was in bold.
Missing data for 132 patients
Missing data for 69 patients
Missing data for 75 patients
Missing data for 1 patient
Univariate models
In univariate analyses (Table 2), the BSMSS was positively associated with measures of FSIQ, reading, and mathematics (pFDR<0.01). Consistently, greater SVI (continuous) was negatively associated with FSIQ, reading, and mathematics scores (pFDR≤0.01). Increased age was associated with poorer performance on measures of FSIQ, reading, and mathematics (pFDR<0.01). Higher levels of HbF were associated with improved mathematics performance (pFDR=0.01). A 1% increase in HbF was associated with an increase of 0.47 points in math performance (Standard Error = 0.15). Neurocognitive and academic scores did not differ by SCD genotype (all p≥0.1).
Table 2.
Univariate analyses of demographic, medical, and treatment factors with neurocognitive scores of overall cohort
| Overall |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Scale IQ (n=298) |
Letter-Word Identification (n=199) |
Math Fluency (n=194) |
|||||||||||||
| Est | Std B | SE | p | pFDR a | Est | Std B | SE | p | pFDR a | Est | Std B | SE | p | pFDR a | |
|
| |||||||||||||||
| Age | −0.65 | −0.21 | 0.17 | <.001 | <.001 | −1.98 | −0.44 | 0.29 | <.001 | <.001 | −1.75 | −0.38 | 0.31 | <.001 | <.001 |
| Sex | −0.25 | −0.01 | 1.49 | 0.87 | 0.87 | −0.87 | −0.03 | 2.23 | 0.70 | 0.99 | −2.32 | −0.07 | 2.30 | 0.31 | 0.45 |
| SCD genotype | 2.47 | 0.10 | 1.50 | 0.10 | 0.24 | 0.16 | 0.01 | 2.25 | 0.94 | 0.99 | −0.96 | −0.03 | 2.31 | 0.68 | 0.79 |
| Current HU therapy | 1.19 | 0.05 | 1.49 | 0.43 | 0.69 | 2.26 | 0.07 | 2.23 | 0.31 | 0.58 | 2.35 | 0.07 | 2.30 | 0.31 | 0.45 |
| Hemoglobin (g/dL) | 0.71 | 0.09 | 0.46 | 0.12 | 0.24 | 0.76 | 0.08 | 0.67 | 0.26 | 0.56 | 0.69 | 0.07 | 0.70 | 0.33 | 0.45 |
| WBC (x109/L) | −0.33 | −0.09 | 0.22 | 0.13 | 0.24 | 0.05 | 0.01 | 0.34 | 0.87 | 0.99 | −0.33 | −0.07 | 0.35 | 0.35 | 0.45 |
| Fetal Hemoglobin (%) | 0.07 | 0.05 | 0.10 | 0.51 | 0.74 | 0.18 | 0.12 | 0.14 | 0.20 | 0.51 | 0.47 | 0.30 | 0.15 | 0.002 | 0.01 |
| Platelet Count (x109/L) | 0.00 | 0.01 | 0.00 | 0.84 | 0.87 | −0.01 | −0.05 | 0.01 | 0.49 | 0.80 | −0.01 | −0.10 | 0.01 | 0.20 | 0.43 |
| Oxygen Saturation (%) | 0.27 | 0.02 | 0.70 | 0.71 | 0.83 | −0.01 | 0.00 | 0.99 | 0.99 | 0.99 | 0.32 | 0.02 | 1.06 | 0.76 | 0.79 |
| BSMSS | 0.35 | 0.34 | 0.05 | <.001 | <.001 | 0.38 | 0.31 | 0.08 | <.001 | <.001 | 0.31 | 0.25 | 0.09 | <.001 | 0.004 |
| SVI | −0.12 | −0.24 | 0.03 | <.001 | <.001 | −0.14 | −0.22 | 0.04 | 0.002 | 0.007 | −0.14 | −0.22 | 0.04 | 0.002 | 0.01 |
| Age at Initial HUb | −0.46 | −0.15 | 0.24 | 0.057 | 0.19 | −0.96 | −0.28 | 0.33 | 0.004 | 0.013 | −1.05 | −0.28 | 0.36 | 0.01 | 0.01 |
| Duration of HU therapy | −0.09 | −0.02 | 0.21 | 0.685 | 0.83 | −0.07 | −0.02 | 0.30 | 0.81 | 0.99 | −0.09 | −0.02 | 0.33 | 0.79 | 0.79 |
SE, standard error; SCD, sickle cell disease; HU, hydroxyurea; WBC, white blood cell count; BSMSS, Barratt Simplified Measure of Social Status; SVI, social vulnerability index; Std B, standardized regression coefficient. Reference categories were "Hb SC/SB+/Other" for SCD genotype, and "No" for current HU therapy. The Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) or Wechsler Preschool and Primary Scale of Intelligence, 4th Edition provided an estimated Full-Scale Intelligence Quotient. Children younger than age 6 completed the WPPSI-IV (6-subtest Full Scale IQ) and children older than age 6 completed the WASI-II (4-subtest IQ). Academic achievement was measured using Letter-Word Identification and Math Fluency from the Woodcock-Johnson Test of Achievement – Third Edition.
p-value adjusted for the false discovery rate (FDR). pFDR < 0.05 were considered significant and are in bold.
. this analysis was done only in HU treated patients.
Fixed multivariate models
We conducted separate multivariate models for the BSMSS and SVI controlling for hydroxyurea treatment, age, HbF and genotype (Table 3). The BSMSS was positively associated with performance on FSIQ, reading, and mathematics measures (p≤0.02). Greater SVI (continuous) was negatively associated with FSIQ and reading (p≤0.01). The effects of the continuous BSMSS and SVI on FSIQ, reading, and mathematics were not dependent on age, SCD genotype, or HbF (p>0.05).
Table 3.
Multivariate analysis of the effect of BSMSS and SVI on neurocognitive scores
| Overall |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Scale IQ (n=298) |
Letter-Word Identification (n=199) |
Math Fluency (n=194) |
||||||||||
| Effects | Est | Std B | SE | p | Est | Std B | SE | p | Est | Std B | SE | p |
| BSMSS a | 0.35 | 0.32 | 0.08 | <.001 | 0.33 | 0.28 | 0.10 | <.001 | 0.26 | 0.19 | 0.11 | 0.02 |
| BSMSS x SCD Genotype b | 0.17 | 0.18 | 0.19 | 0.39 | −0.01 | −0.01 | 0.22 | 0.98 | 0.33 | 0.29 | 0.25 | 0.19 |
| BSMSS x Age c | −0.01 | −0.10 | 0.02 | 0.67 | 0.01 | 0.12 | 0.03 | 0.72 | −0.03 | −0.37 | 0.03 | 0.28 |
| BSMSS x HbF d | 0.00 | 0.03 | 0.01 | 0.89 | 0.00 | −0.02 | 0.01 | 0.91 | −0.02 | −0.40 | 0.01 | 0.09 |
| SVI a | −0.10 | −0.19 | 0.04 | 0.01 | −0.15 | −0.24 | 0.05 | 0.004 | −0.10 | −0.15 | 0.06 | 0.08 |
| SVI x SCD Genotype b | −0.02 | −0.04 | 0.10 | 0.82 | 0.11 | 0.19 | 0.12 | 0.37 | 0.00 | 0.01 | 0.13 | 0.98 |
| SVI x Age c | 0.00 | 0.09 | 0.01 | 0.77 | −0.01 | −0.32 | 0.01 | 0.44 | 0.01 | 0.15 | 0.02 | 0.72 |
| SVI x HbF d | −0.01 | −0.40 | 0.00 | 0.09 | 0.00 | −0.23 | 0.00 | 0.41 | 0.00 | −0.24 | 0.01 | 0.41 |
SE, standard error; SES, socioeconomic status; SCD, sickle cell disease; BSMSS, Barratt Simplified Measure of Social Status; SVI, social vulnerability index; HbF, fetal hemoglobin; Std B, standardized regression coefficient. Reference categories were "Hb SC/SB+/Other" for SCD genotype and "No" for current HU use. Estimates are beta coefficients for SES parameter from the multivariate regression model. The Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) or Wechsler Preschool and Primary Scale of Intelligence, 4th Edition provided an estimated Full-Scale Intelligence Quotient. Children younger than age 6 completed the WPPSI-IV (6-subtest Full Scale IQ) and children older than age 6 completed the WASI-II (4-subtest IQ). Academic achievement was measured using Letter-Word Identification and Math Fluency from the Woodcock-Johnson Test of Achievement – Third Edition. p-value < 0.05 were considered significant and displayed in bold.
Model: NP ~ SES measure + current HU status + age at evaluation + genotype (for overall) + HbF, NP = neurocognitive performance
Model: NP ~ SES measure + current HU status + age at evaluation + genotype (for overall) + HbF + (SES x SCD genotype)
Model: NP ~ SES measure + current HU status + age at evaluation + genotype (for overall) + HbF + (SES x age)
Model: NP ~ SES measure + current HU status + age at evaluation + genotype (for overall) + HbF + (SES x HbF)
Table 4 displays the relative contribution (variance explained) of the BSMSS and SVI (examined separately) to FSIQ, reading, and mathematics scores compared to age, hydroxyurea treatment status, HbF, and SCD genotype. The BSMSS accounted for 8.05, 5.77, and 3.12 percent of the variance in FSIQ, reading, and mathematics scores, respectively. The SVI explained 0.81, 3.12, and 0.90 percent of the variance in FSIQ, reading, and math performance, respectively.
Table 4.
Contribution of BSMSS and SVI to neurocognitive performance relative to other factors of overall cohort
| Full Scale IQ | Letter-Word Identification | Math Fluency | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Socioeconomic Measure | R2all medical/treatment factors | R2Age | R2SES | R2all medical/treatment factors | R2Age | R2SES | R2all medical/treatment factors | R2Age | R2SES |
|
| |||||||||
| BSMSS | 2.40 | 3.26 | 8.05 | 1.46 | 23.02 | 5.77 | 5.50 | 18.77 | 3.12 |
| SVI | 0.81 | 3.12 | 0.90 | ||||||
BSMSS, Barratt Simplified Measure of Social Status; SVI, social vulnerability index; R2, coefficient of determination. All medical/treatment factors include current hydroxyurea use, fetal hemoglobin, and sickle cell disease genotype (for overall cohort). The Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) or Wechsler Preschool and Primary Scale of Intelligence, 4th Edition provided an estimated Full-Scale Intelligence Quotient. Children younger than age 6 completed the WPPSI-IV (6-subtest Full Scale IQ) and children older than age 6 completed the WASI-II (4-subtest IQ). Academic achievement was measured using Letter-Word Identification and Math Fluency from the Woodcock-Johnson Test of Achievement – Third Edition. R2 is reported as a percentage of variance from the model.
Models: NP = socioeconomic measure + age at evaluation + current hydroxyurea use + sickle cell disease genotype + fetal hemoglobin
Stepwise multivariate models
Stepwise multivariate models with SVI (categorical), BSMSS, and all potential covariates are displayed in Table 5. Household and neighborhood SES were moderately associated (ρ = −0.37). Higher FSIQ scores were positively associated with the BSMSS (p<0.001). Patients in the low SVI group displayed FSIQ scores that were 4.87 and 4.70 points higher than those in the high and moderate SVI groups, respectively (p<0.05), after controlling for current hydroxyurea treatment, BSMSS, SCD genotype, and age. Reading performance (Letter Word Identification) was positively associated with the BSMSS (p<0.001), but there were no overall group differences based on SVI status. The effect of SVI group status on reading scores was dependent on age, such that older participants from moderate and high SVI groups displayed poorer reading scores than those with low SVI (p<0.05; see Figure 1). Math scores were positively associated with BSMSS (p=0.05), and patients who lived in low SVI settings displayed math scores that were 7.64 points higher than those from a moderate SVI setting (p=0.03).
Table 5.
Multivariate analysis of the effect of BSMSS and SVI (categorical) on neurocognitive scores for overall cohort based on stepwise model selection
| Full Scale IQ (n=298) |
Letter-Word Identification (n=199) |
Math Fluency (n=194) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Estimate a | SE | p | Estimate b | SE | p | Estimate c | SE | p | |
|
| |||||||||
| Intercept | 92.25 | 3.76 | <.001 | 93.23 | 10.92 | <.001 | 106.60 | 6.22 | <.001 |
| Current HU use | 3.26 | 1.68 | 0.05 | ||||||
| BSMSS | 0.26 | 0.06 | <.001 | 0.26 | 0.08 | 0.001 | 0.18 | 0.09 | 0.05 |
| SCD genotype | −3.37 | 1.72 | 0.05 | 0.26 | 5.15 | 0.96 | |||
| Age at evaluation | −0.56 | 0.16 | 0.001 | 0.02 | 0.82 | 0.98 | −1.61 | 0.31 | <.001 |
| SVI- low vs. high | −4.87 | 2.17 | 0.03 | 20.40 | 11.35 | 0.07 | −5.11 | 3.21 | 0.11 |
| SVI- low vs. moderate | −4.70 | 2.28 | 0.04 | 10.97 | 12.42 | 0.38 | −7.64 | 3.55 | 0.03 |
| SCD genotype x SVI (low vs. high) | −3.04 | 5.72 | 0.60 | ||||||
| SCD genotype x SVI (low vs. moderate) | 6.02 | 6.40 | 0.35 | ||||||
| Age at evaluation x SVI (low vs. high) | −2.08 | 0.89 | 0.02 | ||||||
| Age at evaluation x SVI (low vs. moderate) | −2.03 | 0.99 | 0.04 | ||||||
SE, standard error; SCD, sickle cell disease; BSMSS, Barratt Simplified Measure of Social Status; SVI, social vulnerability index. Reference categories were "Hb SC/SB+/Other" for SCD genotype, "No" for current HU use, and “low” for categorized SVI. The Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) or Wechsler Preschool and Primary Scale of Intelligence, 4th Edition provided an estimated Full-Scale Intelligence Quotient. Children younger than age 6 completed the WPPSI-IV (6-subtest Full Scale IQ) and children older than age 6 completed the WASI-II (4-subtest IQ). Academic achievement was measured using Letter-Word Identification and Math Fluency from the Woodcock-Johnson Test of Achievement – Third Edition. p-value < 0.05 was considered significant and in bold. Social Vulnerability Index is categorized as low= 0 –33, moderate = 33–66, and high = 66 –100.
Model: Full Scale IQ ~ current HU status + genotype+ BSMSS + age at evaluation + SVI
Model: Letter-Word Identification~ BSMSS + age at evaluation + SVI + (Genotype x age at evaluation) + (SVI x age at evaluation)
Model: Math Fluency ~ BSMSS + age at evaluation + SVI
Figure 1. Interaction between age and social vulnerability on reading performance in patients with sickle cell disease.
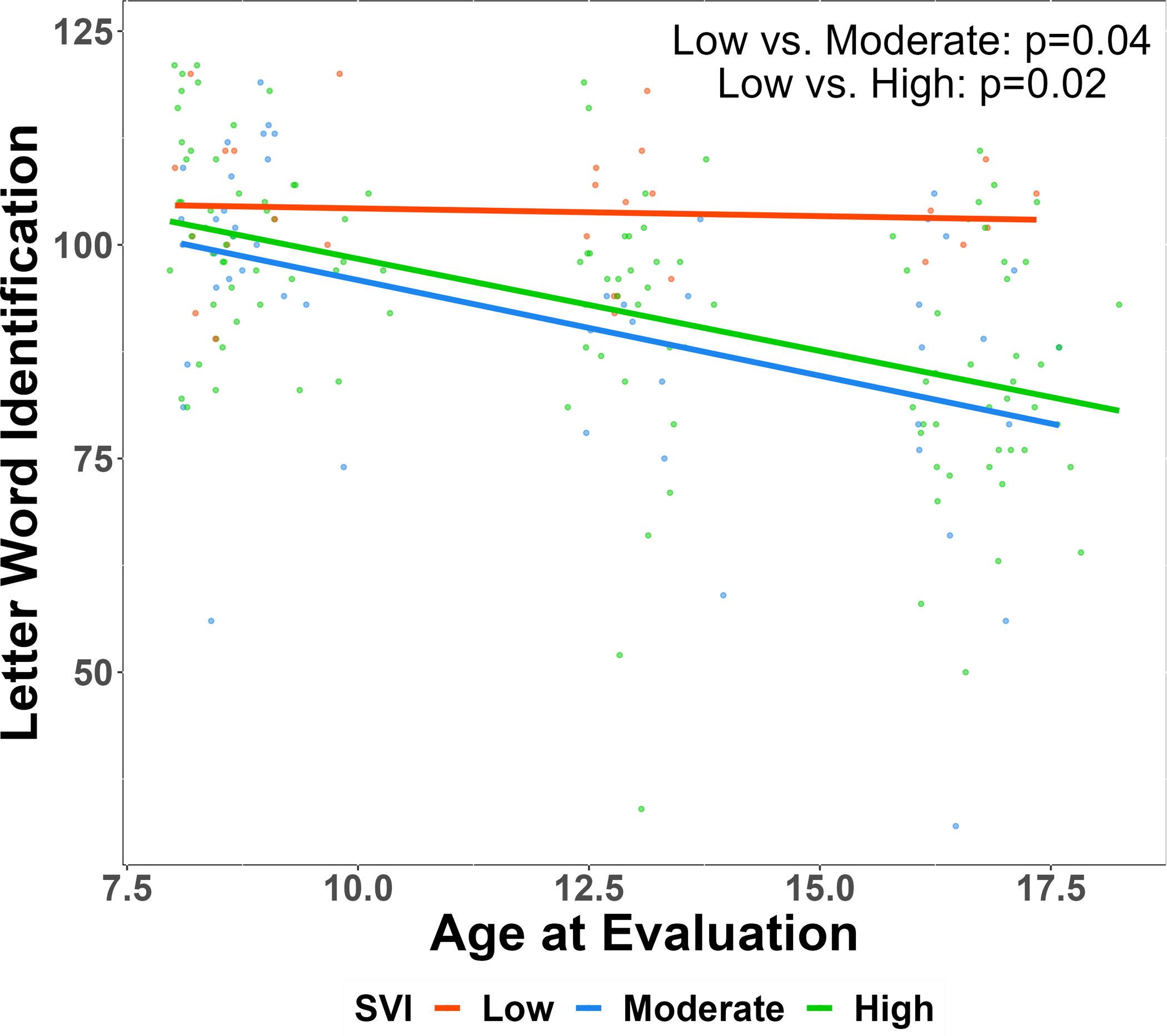
SVI, Social Vulnerability Index. Age displayed in years. HbSS/HbSb0-thalassemia, sickle cell anemia; HbSC/Hb Sβ+-thalassemia, hemoglobinopathy. Social Vulnerability Index categorized as low= 0–33, moderate = 33–66, high = 66–100. The Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II) provided an estimated Full-Scale Intelligence Quotient (4-subtest; Full Scale IQ). Academic achievement was measured using Letter-Word Identification and Math Fluency from the Woodcock-Johnson Test of Achievement – Third Edition. p-value was calculated using multivariate linear regression model with adjusting for BSMSS, SCD genotype, age at evaluation, categorical SVI, interactions between categorical SVI and age and between categorical SVI and SCD genotype.
Discussion
Patients with SCD experience greater rates of poverty and fewer protective SES factors when compared with the Black/African American population in the United States (10, 11). Patients from the most disadvantaged environments are at increased risk for poor health-related outcomes. As hypothesized, both household and neighborhood-level metrics of SES independently contributed to neurocognitive and academic performance in children and adolescents with SCD. Older age was associated with poorer performance across neurocognitive and academic domains. Consistent with our hypothesis, the negative association between age and reading performance was dependent on social vulnerability at the neighborhood level, such that older patients were at higher academic risk if living in neighborhoods with lower SES. Lastly, we confirmed our hypothesis that the contribution of household and neighborhood SES factors was greater than what is accounted for by medical and treatment factors (genotype, hydroxyurea treatment, and levels of HbF). These findings extend upon prior literature by demonstrating the unique contributions of neighborhood and household SES. Furthermore, we identified subgroups of SCD patients at greatest risk for neurocognitive/academic decline with age.
Measures of parental education, family income, and occupation status have consistently been documented as strong predictors of neurocognitive and academic performance in patients with SCD (22–25). We observed that neighborhood-level metrics of social vulnerability account for additional variance in neurocognitive and academic performance after accounting for parent education and occupation status as measured by the BSMSS. These findings are consistent with observations in the general population that neighborhood poverty levels are associated with cognitive performance even after accounting for family income (5). The SVI is comprised of 15 census variables measuring neighborhood-level poverty, transportation, housing, among other markers (32). Due to a lack of power, we were unable to examine the specific aspects of the SVI associated with neurocognitive and academic performance. It will be important to examine what aspects of neighborhood SES are contributing to neurocognitive and academic performance, which are not fully accounted for by household SES. Potential contributors include the quality of the school environment as well as healthcare and nutritional access.
Prior work by Schatz and colleagues observed that the effect of SES on overall cognition depended on SCD severity as measured by hematocrit lab values (i.e., degree of anemia) (26). In contrast to these findings, the effects of SES on neurocognitive and academic performance in our study were not modified by HbF level or sickle genotype. Low SES had a negative effect on SCD patients regardless of the sickle genotype or HbF level. Although we did not observe an interaction between HbF or genotype and neurocognitive performance, the negative effect of age on reading was dependent on SES. Patients living in areas with moderate or high SVI (low SES) demonstrated worse performance with older age whereas those patients living in low SVI environments displayed no age effects. This suggests that the slowed academic growth seen in patients diagnosed with SCD (18, 40) is limited to patients living in lower-resourced environments. These findings are consistent with the general population, where individuals from lower-resourced environments display slowed academic growth compared to peers from higher SES environments (41–43). Thus, resources should be allocated to prevent academic delays in young children diagnosed with SCD according to their degree of SES risk.
The influence of SES varied across neurocognitive and academic measures. After accounting for SCD genotype, age at evaluation, and current hydroxyurea treatment, 8.1% of the variance in FSIQ was accounted for by household SES as measured by the BSMSS, whereas the BSMSS only accounted for 5.8% and 3.1% of reading and math performance, respectively. Neighborhood level SES accounted for a smaller amount of variance in FSIQ and academic performance, ranging from 3.1% to 0.9%. The stronger influence of household SES is expected given the direct impact of the home environment on development. SCD genotype, HbF, and hydroxyurea treatment accounted for a relatively small amount of variance in neurocognitive and academic outcomes. There was a strong negative effect of age across measures, particularly measures of academic performance, accounting for 23.0% and 18.8% of the variance in reading and math outcomes, respectively. The negative effects of age on academic performance likely reflect a combination of worsening disease status but, according to our results, due to a strong effect of environmental factors (e.g., under-funded schools, lack of access to tutoring) hindering academic growth.
The study has several strengths including a large, representative, sample of patients that span school age to late adolescence. Information on medical, treatment, and demographic factors was collected allowing for analyses to isolate the influence of SES on intellectual function. Measurement of SES included both neighborhood and household level factors. The study included gold standard performance measures of neurocognitive and academic performance. Yet, several limitations exist. Cross-sectional analyses limited the conclusions that could be drawn from our data. The age effects and age by SES interactions may represent cohort effects rather than slowed development. Further, we were unable to assess how SES measured early in life affects the trajectory of neurocognitive and academic development as SES was measured at the time of the evaluation. The BSMSS composite score does not account for additional socioeconomic barriers that might impact single parents compared to homes with 2 parents. Future studies should account for the number of adults in the home as an additional sociodemographic factor. Only patients with a clinical indication received neuroimaging. Therefore, we could not control for the influence of brain insults, such as the presence of silent cerebral infarcts, that are known to influence neurocognitive performance (44–46).
To conclude, both household and neighborhood level metrics of SES contribute to the neurocognitive and academic difficulties observed in SCD. The influence of SES was independent of disease genotype, HbF, and disease treatment history. Performance across neurocognitive and academic measures worsened with increasing age; however, the negative effect of age on reading performance was limited to those with moderate to high levels of neighborhood social vulnerability. Intervening early in life to prevent academic delays in young children diagnosed with SCD is essential.
Supplementary Material
Acknowledgements
J.S.H. received funding from U01HL133996 during the conduct of this study. J.S.P was supported by K01HL125495 at the time of this project. This research was supported by the American Lebanese Syrian Associated Charities (ALSAC).
The authors would like to thank Jason Hodges, PhD, Pei-Lin Chen, MPH, Courtney Mays, Madelene Wilson, Tiana Thomas, Ruth Johnson, and Michelle Brignac, for support with data collection and regulatory matters.
Abbreviations Key
- AIC
Akaike information criterion
- BSMSS
Barratt Simplified Measure of Social Status
- FSIQ
Full Scale IQ
- Hb
Total Hemoglobin
- HbF
Fetal Hemoglobin
- IRB
Institutional Review Board
- NHLBI
National Heart, Lung, and Blood Institute
- pFDR
False Discovery Rate Adjusted p value
- PLT
Platelet Count
- R2
Coefficient of Determination
- SCCRIP
Sickle Cell Clinical Research Intervention Program
- SCD
Sickle Cell Disease
- SES
Socioeconomic Status
- SVI
Social Vulnerability Index
- WASI-II
Wechsler Abbreviated Scales of Intelligence, Second Edition
- WBC
White Blood Cell Count
- WPPSI-IV
Wechsler Preschool & Primary Scale of Intelligence, Fourth Edition
Footnotes
Conflicts of Interest
J.S.H. receives consultancy fees from Global Blood Therapeutics, CVS Health, Forma Therapeutics, and UpToDate. A.M.H. receives consultancy fees from Global Blood Therapeutics. There are no other conflicts of interest to report.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Baker EH. Socioeconomic status, definition. The Wiley Blackwell encyclopedia of health, illness, behavior, and society. 2014:2210–4. [Google Scholar]
- 2.Korous KM, Causadias JM, Bradley RH, Luthar SS, Levy R. A systematic overview of meta-analyses on socioeconomic status, cognitive ability, and achievement: The need to focus on specific pathways. Psychol Rep. 2022;125(1):55–97. [DOI] [PubMed] [Google Scholar]
- 3.Blums A, Belsky J, Grimm K, Chen Z. Building links between early socioeconomic status, cognitive ability, and math and science achievement. J Cogn Dev. 2017;18(1):16–40. [Google Scholar]
- 4.Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, et al. The Influence of Socioeconomic Status on Children’s Brain Structure. PLoS One. 2012;7(8):e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor RL, Cooper SR, Jackson JJ, Barch DM. Assessment of Neighborhood Poverty, Cognitive Function, and Prefrontal and Hippocampal Volumes in Children. JAMA Network Open. 2020;3(11):e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benavente-Fernández I, Synnes A, Grunau RE, Chau V, Ramraj C, Glass T, et al. Association of Socioeconomic Status and Brain Injury With Neurodevelopmental Outcomes of Very Preterm Children. JAMA Network Open. 2019;2(5):e192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Power-Hays A, Li S, Mensah A, Sobota A. Universal screening for social determinants of health in pediatric sickle cell disease: A quality-improvement initiative. Pediatr Blood Cancer. 2020;67(1):e28006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caucus CB, editor Ring the alarm: the crisis of Black youth suicide in America. A Report to Congress from The Congressional Black Caucus Emergency TaskForce on Black Youth Suicide and Mental Health; 2019. [Google Scholar]
- 9.Metzl JM, Hansen H. Structural competency: theorizing a new medical engagement with stigma and inequality. Social science & medicine. 2014;103:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farber MD, Koshy M, Kinney TR, Cooperative Study of Sickle Cell D. Cooperative study of sickle cell disease: demographic and socioeconomic characteristics of patients and families with sickle cell disease. J Chronic Dis. 1985;38(6):495–505. [DOI] [PubMed] [Google Scholar]
- 11.Khan H, Krull M, Hankins JS, Wang WC, Porter JS. Sickle cell disease and social determinants of health: A scoping review. Pediatric Blood & Cancer . 2023;70(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panepinto JA, Pajewski NM, Foerster LM, Sabnis S, Hoffmann RG. Impact of family income and sickle cell disease on the health-related quality of life of children. Qual Life Res. 2009;18(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aljuburi G, Laverty AA, Green SA, Phekoo KJ, Bell D, Majeed A. Socio-economic deprivation and risk of emergency readmission and inpatient mortality in people with sickle cell disease in England: observational study. Journal of Public Health. 2013;35(4):510–7. [DOI] [PubMed] [Google Scholar]
- 14.DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119(20):4587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prussien KV, Siciliano RE, Ciriegio AE, Anderson AS, Sathanayagam R, DeBaun MR, et al. Correlates of Cognitive Function in Sickle Cell Disease: A Meta-Analysis. J Pediatr Psychol. 2020;45(2):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatz J, McClellan CB. Sickle cell disease as a neurodevelopmental disorder. Mental retardation and developmental disabilities research reviews. 2006;12(3):200–7. [DOI] [PubMed] [Google Scholar]
- 17.Champlin G, Hwang SN, Heitzer A, Ding J, Jacola L, Estepp JH, et al. Progression of central nervous system disease from pediatric to young adulthood in sickle cell anemia. Exp Biol Med. 2021:153537022110357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heitzer AM, Longoria J, Okhomina V, Wang WC, Raches D, Potter B, et al. Hydroxyurea treatment and neurocognitive functioning in sickle cell disease from school age to young adulthood. Br J Haematol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561–73. [DOI] [PubMed] [Google Scholar]
- 20.McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Saf. 2015;14(11):1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B. Hydroxyurea in Sickle Cell Disease: Drug Review. Indian Journal of Hematology and Blood Transfusion. 2014;30(2):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarazi RA, Grant ML, Ely E, Barakat LP. Neuropsychological functioning in preschool-age children with sickle cell disease: the role of illness-related and psychosocial factors. Child Neuropsychol. 2007;13(2):155–72. [DOI] [PubMed] [Google Scholar]
- 23.Aygun B, Parker J, Freeman MB, Stephens AL, Smeltzer MP, Wu S, et al. Neurocognitive screening with the Brigance Preschool screen-II in 3-year-old children with sickle cell disease. Pediatr Blood Cancer. 2011;56(4):620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King AA, Rodeghier MJ, Panepinto JA, Strouse JJ, Casella JF, Quinn CT, et al. Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. Am J Hematol. 2014;89(10):E188–E92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, McKinstry RC, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014;89(2):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schatz J, Finke R, Roberts CW. Interactions of biomedical and environmental risk factors for cognitive development: a preliminary study of sickle cell disease. J Dev Behav Pediatr. 2004;25(5):303–10. [DOI] [PubMed] [Google Scholar]
- 27.Hankins JS, Estepp JH, Hodges JR, Villavicencio MA, Robison LL, Weiss MJ, et al. Sickle Cell Clinical Research and Intervention Program (SCCRIP): A lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer. 2018;65(9):e27228. [DOI] [PubMed] [Google Scholar]
- 28.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–48. [DOI] [PubMed] [Google Scholar]
- 29.Luchtman-Jones L, Pressel S, Hilliard L, Brown RC, Smith MG, Thompson AA, et al. Effects of hydroxyurea treatment for patients with hemoglobin SC disease. Am J Hematol. 2016;91(2):238–42. [DOI] [PubMed] [Google Scholar]
- 30.Barratt W The Barratt simplified measure of social status (BSMSS). Indiana State University. 2006. [Google Scholar]
- 31.Hollingshead AB. Four factor index of social status. 1975.
- 32.Cutter SL, Boruff BJ, Shirley WL. Social vulnerability to environmental hazards. Social science quarterly. 2003;84(2):242–61. [Google Scholar]
- 33.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention’s Social Vulnerability Index. J Environ Health. 2018;80(10):34. [PMC free article] [PubMed] [Google Scholar]
- 34.Wechsler D Wechsler Abbreviated Scale of Intelligence–Second Edition. San Antonio, TX: NCS Pearson. 2011. [Google Scholar]
- 35.Woodcock R, McGrew K, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 36.Wechsler D, Coalson D, Raiford S. Wechsler Preschool and Primary Scale of Intelligence - Fourth Edition (WPPSI-IV). San Antonio, TX: The Psychological Corporation; 2012. [Google Scholar]
- 37.R-Core-Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 38.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth ed. New York, NY: Springer; 2002. [Google Scholar]
- 39.Jaeger B _r2glmm: Computes R Squared for Mixed (Multilevel) Models. R package 0.1.2 ed2017. [Google Scholar]
- 40.Heitzer AM, Hamilton L, Stafford C, Gossett J, Ouellette L, Trpchevska A, et al. Academic Performance of Children With Sickle Cell Disease in the United States: A Meta-Analysis. Front Neurol. 2021;12:2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatrics. 2015;169(9):822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sirin SR. Socioeconomic status and academic achievement: A meta-analytic review of research. Review of educational research. 2005;75(3):417–53. [Google Scholar]
- 43.von Stumm S Socioeconomic status amplifies the achievement gap throughout compulsory education independent of intelligence. Intelligence. 2017;60:57–62. [Google Scholar]
- 44.Prussien KV, Jordan LC, DeBaun MR, Compas BE. Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: J Pediatr Psychol. 2019;44(8):948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schatz J, Brown R, Pascual J, Hsu L, DeBaun M. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56(8):1109–11. [DOI] [PubMed] [Google Scholar]
- 46.White DA, Moinuddin A, McKinstry RC, Noetzel M, Armstrong M, DeBaun M. Cognitive screening for silent cerebral infarction in children with sickle cell disease. J Pediatr Hematol Oncol. 2006;28(3):166–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


