Abstract
Background:
A small but growing body of evidence supports a relationship between neighborhood socioeconomic status (NSES) and cognitive decline. Additional work is needed to characterize this relationship controlling for risk factors such as cardiovascular, cerebrovascular, and genetic risk factors.
Methods:
Cognitive decline was assessed in association with NSES, and cardiovascular and cerebrovascular risk factors (heart disease, diabetes, hypertension, and stroke) in 8,198 individuals from the 1992–2010 waves of the Health and Retirement Study (HRS). Latent class trajectory analysis determined the number of cognitive trajectory classes that best fit the data, and a multinomial logistic regression model in the latent class framework assessed the risk for cognitive classes conferred by NSES index score and heart disease, diabetes, hypertension, and stroke across three trajectory classes of cognitive function. The analyses controlled for genetic risk for cognitive decline (including APOE genotype) and demographic variables, including education.
Results:
The HRS sample was 57.6% female and 85.5% White, with a mean age of 67.5(3.5) years at baseline. The three-quadratic-class model best fit the data, where higher classes represented better cognitive function. Those with better cognitive function were mainly younger white females. Those in the highest quartile of NSES had 57% higher odds of being in the high cognitive function class. Heart disease, diabetes, hypertension, and stroke each increased the odds having of lower cognitive function.
Conclusions:
In examining the relationship of cognitive status with various variables, neighborhood socioeconomic status, cardiovascular risk, and cerebrovascular risk persisted across the cognitive trajectory classes.
Keywords: Cognition, APOE, NSES, risk factors
Introduction
Cognitive impairment that does not meet the threshold for dementia affects 22% of American adults over age 70 (Plassman et al., 2008), and the prevalence of dementia in this group is nearly 14% (Plassman et al., 2007). Understanding factors that accelerate cognitive decline is critical in protecting the health of an aging population. Aging increases risk for heart disease, diabetes, hypertension, and stroke, which independently increase the likelihood of cognitive dysfunction (Haroon et al., 2015; Walker et al., 2017). Carriage of APOE ε4 and other relevant alleles, compounds the risk for cognitive decline in late life (Harold et al., 2009; Hayden et al., 2015).
Traditionally, research has targeted physiological contributors to cognitive decline, but recent work has examined associations between cognition and various measures of neighborhood physical environment and neighborhood socioeconomic status (SES) (Mungas et al., 2018; Rosso et al., 2016; Worn et al., 2017) To date a number of studies have reported that lower neighborhood SES is associated with lower baseline or cross-sectional cognitive status (Besser et al., 2017; Aneshensel et al., 2011; Sisco and Marsiske, 2012; Shih et al., 2011). But the findings on cognitive decline have been mixed. In a systematic review (Besser et al., 2017), among six longitudinal studies on U.S. samples (Boardman et al., 2012; Kovalchik et al., 2015; Martinez, 2007; Meyer et al., In press; Rej et al., 2015; Sheffield and Peek, 2009) that examined SES, only one study reported incident cognitive decline associated with greater neighborhood economic disadvantage (Sheffield and Peek, 2009).
The same systematic review (Besser et al., 2017) identified three longitudinal studies examining various physical aspects of neighborhoods and cognitive decline (Martinez, 2007; Clarke et al., 2015; Watts et al., 2015). Two of these studies (Clarke et al., 2015; Watts et al., 2015) reported that design attributes such as deterioration of public spaces and the lack of sidewalks was associated with greater cognitive decline. One study reported that the presence of a neighborhood community center and public transit stop was associated with slower cognitive decline (Clarke et al., 2015). The diversity of measures of SES factors and physical characteristics of neighborhoods in this systematic review limited the synthesis of the results, but the findings suggest that further investigation is needed to understand the influence of neighborhood characteristics on cognitive decline.
Given the inconsistent prior findings, we aimed to understand whether neighborhood SES and a range of genetic, health, and demographic factors differentially influenced subpopulations, defined by cognitive trajectory classes, within the larger heterogeneous population of individuals.
Our proposed model is an extension of a theoretical framework proposed by others (Wu et al., 2015). This framework has two components or levels that contribute, sequentially, to cognitive function trajectories in later life: a community level that is comprised of factors that are associated with the neighborhood where an individual resides and the individual level, which consists of lifestyle, health, and other characteristics of the individual person. For our model, the community level factors are the neighborhood socioeconomic status index (NSES) and geographic region. The individual level factors include genetics (APOE ε4 carrier status and a polygenic risk score of Alzheimer’s disease [AD] risk genes), cerebrovascular risk factors, vascular risk factors, education, and demographic characteristics. First, we hypothesized that there would be more than one subpopulation or trajectory class of cognitive change. Second, we hypothesized that NSES would be associated with cognitive function such that individuals living in less affluent neighborhoods would have a higher likelihood of belonging to lower cognitive trajectory classes than their wealthier counterparts. Third, we hypothesized that heart disease, diabetes, hypertension, and stroke would contribute to cognitive decline and that fewer years of education would be an important contributor to cognitive decline.
Method
Data source
Data for the current analyses was drawn from the 1992–2010 waves of the HRS, a longitudinal panel study surveying a nationally representative sample of approximately 26,000 Americans over the age of 50 (Juster and Suzman, 1995). Data were collected every two years. The protocol, methods, and consent procedures for the original study were approved by the University of Michigan Institutional Review Board (IRB); the present secondary data analysis was approved by the Wake Forest University School of Medicine and Duke University Medical Center IRBs. All participants provided informed consent prior to participation in the original study.
Participants
For the current study, data from 8,198 HRS participants who met the following criteria were included: (Plassman et al., 2008) aged 65 and older, (Plassman et al., 2007) provided genetic samples, and (Haroon et al., 2015) completed an abbreviated version of the modified Telephone Interview for Cognitive Status (HRS TICS (Ofstedal et al., 2005)) (described below). The analysis was limited to individuals aged 65 and older because the HRS TICS is not administered biannually until a participant reaches the age of 65. If an individual was initially evaluated before 1998 and prior to age 65, only data from visits occurring after age 65 were used.
Measures
Cognitive class (dependent variable)
Cognitive performance was measured using the modified Telephone Interview for Cognitive Status (TICS-m) (Brandt et al., 1988; Welsh et al., 1993), which was shortened for use with the HRS (HRS TICS). Prior versions of the TICS-m have been shown to discriminate well between cases of dementia and normal cognitive function (Plassman et al., 1994). The HRS TICS had a score range from 0 to 35, which included 20 points for immediate and delayed recall of a word list; 10 points for counting backwards, naming, and orientation; and 5 points for serial 7s. Higher scores represent better cognitive performance.
Cardiovascular and cerebrovascular disease
HRS collected participant data on cardiovascular and cerebrovascular history and risk from 1996 forward. History of cardiovascular risk factors (diabetes, hypertension); CVD (heart attack, angina, congestive heart failure, or other heart problems); and cerebrovascular disease (stroke) was assessed with the questions, “Has a doctor ever told you that you had [condition]?” and, for follow-up visits, “Since [date of previous assessment], has a doctor told you that you had [condition]?” All affirmative responses were coded as 1, with negative responses coded as 0. Participant reports of diabetes or hypertension were coded as 1 only if the participant also affirmed that medication was being taken for the condition. These items were time-varying, and all were coded positive from the first time they were endorsed and thereafter.
Neighborhood socioeconomic status (NSES)
We used a continuous (scaled 0 to 100) index comprised of data from six Census domains: percentage of adults aged 25 or older without a high school diploma; percentage of males unemployed; percentage of households with income below the poverty line; percentage of households receiving public assistance; percentage of female heads of household; and median household income (Escarce et al., 2011). Composite scores were z-transformed and divided into quartiles, higher scores indicated more affluent neighborhoods.
Demographic information
Demographic information, including age in years, sex, race (White, Other), and years of education was obtained during the baseline interview. We collapsed African Americans, American Indians, Asians, and people who did not identify with any of these groups into a single “other” category due to small numbers.
Genetic data
Saliva samples for DNA analysis were obtained in 2006 and 2008 from all HRS participants who consented to the procedure. Genetic analyses were completed in 2013 using Illumina Human Omni-2.5 Quad bead chips, and genetic data were added to the Database of Genotypes and Phenotypes (dbGaP). To ensure data would be comparable to those from other platforms, imputation was used to amplify available markers, and single nucleotide polymorphisms (SNPs) closest to the genes of interest were identified. Full details of these genotyping methods have been reported elsewhere (Saykin et al., 2010).
The following genes were selected based on empirical findings of Alzheimer’s disease (AD) risk (Harold et al., 2009; Bertram et al., 2007; Hollingworth et al., 2011; Lambert et al., 2009; Naj et al., 2011) to develop a genetic risk score: CLU, CR1, PICALM, MS4A6A/MS4A4E, CD33, CD2AP, ABCA7, CR1, and PICALM. APOE was examined separately to prevent its robust association with cognitive function from masking the signals of other genetic factors. A genetic risk score was derived using odds ratios (ORs) reported for AD risk from meta-analyses of previously published studies available on the AlzGene website (Bertram et al., 2007). A continuous risk score for each participant was calculated by ensuring all odds were reported in the same direction by inverting ORs as needed, then multiplying the OR for each risk gene by the number of risk alleles each participant possessed.
Geographic region
We controlled for geographic region as there is some evidence of regional variation in cognitive decline (Wadley et al., 2011). Geographic region of the United States (U.S.) in which participants lived was derived using U.S. Census Bureau definitions of divisions and regions. (Bureau, U. C., n.d.) Of the four regions (South, Northeast, Midwest, and West), the South region was treated as the reference group, as it contained the largest number of participants.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). To characterize the demographic and health risk factor variables, percentages (for categorical variables) or means and standard deviations (for continuous variables) were calculated and compared across latent trajectory classes using χ2 tests or generalized linear models, as appropriate.
One of the analytical tools to determine the existence of one or more latent (unobserved) trajectory classes is the group-based latent-class trajectory modeling (Jones et al., 2001). This pattern-centered approach was used to examine and understand the stability and change in the grouping of participants based on their cognitive trajectory. The approach identified different latent trajectory classes and simultaneously examined predictors of class membership within the same modeling framework. The number of trajectories were not fixed a priori but rather the method examined the number of trajectory classes, each with its own growth pattern over time. In this study, the classes of cognitive trajectories were estimated using a group-based trajectory model and PROC TRAJ in SAS. We used the Bayesian Information Criterion (BIC) to select the optimal number of trajectory classes; the number of classes was varied until the best-fitting model was obtained as indicated by having the lowest BIC. We determined a priori, that the smallest trajectory class should include >10% of the participants from the sample to provide a clinically representative pattern of change. Intercept-only, linear, and quadratic polynomial terms were modeled. Posterior probabilities of group membership from each individual and high probability of membership to a single group represented a good model fit.
We then examined the univariate association of trajectory classes and demographic, neighborhood socioeconomic, cerebrovascular, and cardiovascular variables using chi-square tests (for categorical variables) or ANOVA/Wilcoxon tests (for continuous variables). Finally, the predictors of cognitive trajectory classes, adjusted for all other variables in the model, were examined using multinomial logistic regression for time-invariant risk factors. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for membership in each cognitive trajectory class with the lowest class as reference, and for each of the predictors. In this latent modelling framework, time-varying and time-invariant predictors within each of the trajectory classes were modeled through uncensored ordinary least squares regression (Jones et al., 2001; Jones and Peskin, 2010; Nagin, 2005).
Results
Demographics and univariate models
The analytic sample consisted of 8,198 participants with a mean age of 67.5 years (standard deviation [SD]: 3.5 years) at baseline, were mostly female (57.6%), white (85.5%), and on average had 12.4 years of education. The largest proportion of participants came from the South (Table 1). On average, participants had 6.04 (SD: 2.4, range: 2–10) observations. The mean genetic risk score was 9.6 (SD: 2.0), with a range of 2.2 to 16.1.
Table 1.
Baseline characteristics of study sample by cognitive trajectory class membership (N = 8198).
| Cognitive class | |||||
|---|---|---|---|---|---|
|
| |||||
| 1=Low(N=1115) | 2=Medium(N=3453) | 3=High(N =3630) | Total (N=8198) | p value | |
| Age (in Years) | |||||
| Mean (SD) | 68.3 (4.4) | 67.8 (3.6) | 67.0 (2.8) | 67.5 (3.4) | <0.00011 |
| Median | 66.0 | 66.0 | 66.0 | 66.0 | |
| Q1, Q3 | 65.0, 71.0 | 65.0, 70.0 | 65.0, 68.0 | 65.0, 69.0 | |
| Sex [n (%)] | <0.00012 | ||||
| 1 = Male | 494 (44.3%) | 1644 (47.6%) | 1335 (36.8%) | 3473 (42.4%) | |
| 2 = Female | 621 (55.7%) | 1809 (52.4%) | 2295 (63.2%) | 4725 (57.6%) | |
| Race [n (%)] | <0.00012 | ||||
| Other | 453 (40.6%) | 526 (15.2%) | 211 (5.8%) | 1190 (14.5%) | |
| Whites | 662 (59.4%) | 2927 (84.8%) | 3419 (94.2%) | 7008 (85.5%) | |
| Region of United States [n (%)] | <0.00012 | ||||
| Midwest | 217 (19.5%) | 866 (25.1%) | 1031 (28.4%) | 2114 (25.8%) | |
| Northeast | 145 (13.0%) | 584 (16.9%) | 622 (17.1%) | 1351 (16.5%) | |
| South | 598 (53.6%) | 1377 (39.9%) | 1265 (34.8%) | 3240 (39.5%) | |
| West | 155 (13.9%) | 626 (18.1%) | 712 (19.6%) | 1493 (18.2%) | |
| Education (in Years) | <0.00011 | ||||
| Mean (SD) | 9.0 (3.8) | 12.0 (2.8) | 13.8 (2.3) | 12.4 (3.2) | |
| Median | 10.0 | 12.0 | 13.0 | 12.0 | |
| Q1, Q3 | 7.0, 12.0 | 11.0, 13.0 | 12.0, 16.0 | 12.0, 14.0 | |
| Neighborhood Socioeconomic Status | <0.00011 | ||||
| First quartile | 555 (48.8%) | 915 (26.5%) | 578 (15.9%) | 2048 (25.0%) | |
| Second quartile | 259 (23.2%) | 940 (27.2%) | 853 (23.5%) | 2052 (25.0%) | |
| Third quartile | 192 (17.2%) | 879 (25.5%) | 977 (26.9%) | 2248 (25.0%) | |
| Fourth quartile | 109 (9.8%) | 719 (20.8%) | 1222 (33.7%) | 2050 (25.0%) | |
| Genetic Risk Score | 0.00011 | ||||
| Mean (SD) | 9.8 (2.1) | 9.6 (2.0) | 9.5 (2.0) | 9.6 (2.0) | |
| Median | 9.3 | 9.3 | 9.2 | 9.3 | |
| Q1, Q3 | 8.1, 11.5 | 8.1, 11.4 | 8.1, 10.4 | 8.1, 11.4 | |
| APOE ɛ4 [n (%)] | <0.00012 | ||||
| No | 737 (66.1%) | 2477 (71.7%) | 2793 (76.9%) | 6007 (73.3%) | |
| Yes | 378 (33.9%) | 976 (28.3%) | 837 (23.1%) | 2191 (26.7%) | |
| Any heart condition [n (%)] | <0.00012 | ||||
| No | 808 (72.5%) | 2583 (74.8%) | 2892 (79.7%) | 6283 (76.6%) | |
| Yes | 307 (27.5%) | 870 (25.2%) | 738 (20.3%) | 1915 (23.4%) | |
| Diabetes [n (%)] | <0.00012 | ||||
| No | 832 (74.6%) | 2924 (84.7%) | 3313 (91.3%) | 7069 (86.2%) | |
| Yes | 283 (25.4%) | 529 (15.3%) | 317 (8.7%) | 1129 (13.8%) | |
| Hypertension [n (%)] | <0.00012 | ||||
| No | 473 (42.4%) | 1689 (48.9%) | 1987 (54.7%) | 4149 (50.6%) | |
| Yes | 642 (57.6%) | 1764 (51.1%) | 1643 (45.3%) | 4049 (49.4%) | |
| Stroke [n (%)] | <0.00011 | ||||
| No | 1020 (91.5%) | 3319 (96.1%) | 3570 (98.3%) | 7909 (96.5%) | |
| Yes | 95 (8.5%) | 134 (3.9%) | 60 (1.7%) | 289 (3.5%) | |
| HRS TICS Score (Baseline) | <0.00011 | ||||
| Mean (SD) | 16.4 (4.1) | 22.2 (3.0) | 26.6 (2.9) | 23.3 (4.6) | |
| Median | 16.0 | 22.0 | 26.0 | 24.0 | |
| Q1, Q3 | 14.0, 19.0 | 20.0, 24.0 | 25.0, 28.0 | 21.0, 26.0 | |
Kruskal Wallis.
Chi-Square.
Note. APOE ε4: Apolipoprotein E, allele ε4. HRS TICS: Health and Retirement Study version of the Telephone Interview for Cognitive Status. NSES: Neighborhood socioeconomic status. Q1: First quartile. Q3: Third quartile. SD: Standard deviation.
Trajectories of cognitive function
Using BIC criteria, the three-quadratic-class model provided the best fit (Figure 1). The three trajectory classes identified are displayed in Figure 2. These trajectory classes include low [14.0% (N = 1,115)], medium [42.1% (N = 3,453)], and high cognitive function [43.8% (N = 3,630)] (Figure 2). The overall mean HRS TICS score at baseline was 23.3 (SD: 4.6).
Figure 1.

Plot of number of classes vs BIC.
Figure 2.
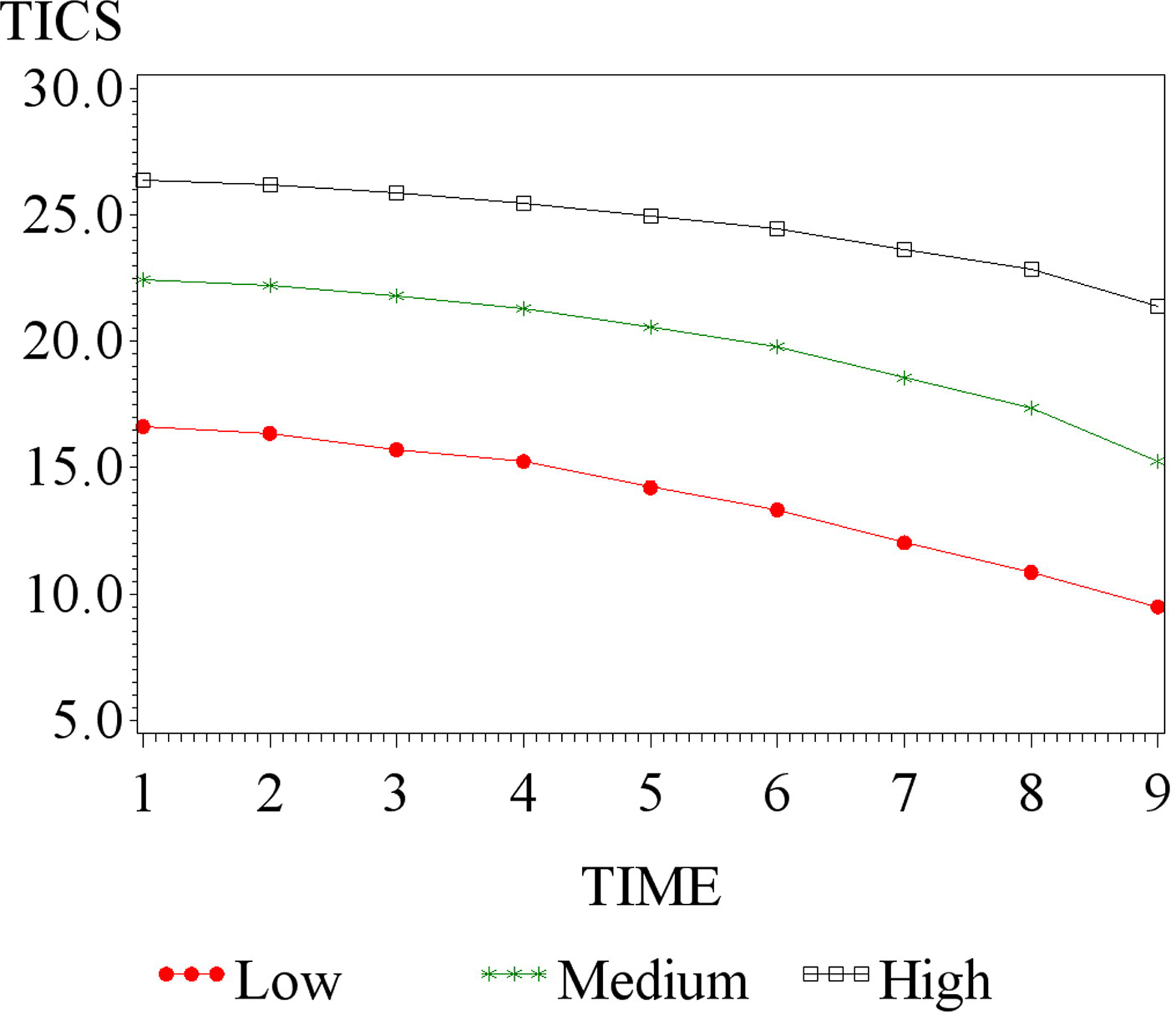
Trajectory classes of TICS.
The omnibus test of equality of intercepts, linear and quadratic terms across the classes reveal that there is significant difference in the intercept (p < .0001), linear (p < .008) and quadratic terms (p < .0001) between the classes as well as on their pairwise comparisons. The intercepts are significantly different from each other (p-values of the chi-square- test for various pairwise comparisons of intercepts are all p-value < .0001). The linear slope comparison of low vs medium (p-value = 0.011) and medium vs high (p-value = 0.008) are significant while low vs high (p = 0.418) is not significant. There is significant difference in the quadratic slopes between medium and high (p-value < .0001) while the quadratic slope comparison between low and medium groups (p-value = .080) and the low and high groups (p-value < .135) are not significant.
Characteristics by cognitive trajectory classes
Table 1 presents the descriptive characteristics of the sample according to cognitive trajectory class. On average, individuals in the higher cognitive trajectory classes were younger (p < 0.0001), more likely to be white (p < 0.0001), more likely to be female (p < 0.0001), and better educated (p < 0.0001) compared to those in the low cognitive trajectory class.
Mean NSES levels were positively associated with cognitive trajectory class, with the highest cognitive trajectory class having the likelihood of higher NSES. APOE ε4 and cardiovascular and cerebrovascular risk factors for cognitive decline [heart disease, diabetes, hypertension, and stroke] were less prevalent in the higher cognitive classes (all p-values <0.0001). There was a significant association between trajectory class and region of residence in the U.S., with the lowest cognitive trajectory class having more participants from the South.
Association of demographic, health, and genetic characteristics with cognitive trajectory class membership
Table 2 provides the results of multinomial logistic regression of the factors predicting membership in the three cognitive classes. Demographic factors, NSES quartile [reference: lowest], and genetic risk factors were treated as time-invariant. Cardiovascular and cerebrovascular risk factors (heart disease, diabetes, hypertension, and stroke) were time-varying. These variables were positive from the first endorsement forward.
Table 2.
Demographic, genetic, socioeconomic, regional, and time-varying predictors of cognitive trajectory class (n = 8,198).
| Variable | Cognitive Trajectory Class Odds Ratio (95% Confidence Limits) |
||
|---|---|---|---|
| Low | Medium | High | |
| Demographics | |||
| Age in years | 1.0 (REFERENCE) | 0.94 (0.91–0.96) | 0.83 (0.81–0.86) |
| Sex | 1.0 (REFERENCE) | 0.98 (0.81–1.18) | 2.42 (1.96–2.99) |
| Female [Reference: Male] | |||
| Race | 1.0 (REFERENCE) | 4.19 (3.35–5.25) | 18.50(13.63–25.13) |
| [White [Reference: Othera] | |||
| Education (Years) | 1.0 (REFERENCE) | 1.41 (1.36–1.46) | 2.09 (1.99–2.18) |
| Genetic Factors | |||
| APOE ɛ4 [reference: no APOE ɛ4] | 1.0 (REFERENCE) | 0.68 (0.56–0.83) | 0.40 (0.32–0.50) |
| Genetic Risk Score | 1.0 (REFERENCE) | 1.01 (0.96–1.06) | 0.98 (0.93–1.03) |
| Socioeconomic Factors | |||
| Neighborhood socioeconomic status (NSES) | |||
| Q2 vs Q1 | 1.0 (REFERENCE) | 1.07 (0.85–1.36) | 1.80 (0.89–1.55) |
| Q3 vs Q1 | 1.0 (REFERENCE) | 1.32 (0.99–1.73) | 1.45 (1.07–1.97) |
| Q4 vs Q1 | 1.0 (REFERENCE) | 1.06 (0.77–1.45) | 1.57 (1.12–2.19) |
| Regional Factors | |||
| Region of United States | |||
| West [Reference: South] | 1.0 (REFERENCE) | 1.21 (0.92–1.60) | 1.24 (0.93–1.68) |
| Mid-west [Reference: South] | 1.0 (REFERENCE) | 1.15 (0.90–1.46) | 1.51 (1.16–1.96) |
| North-East [Reference: South] | 1.0 (REFERENCE) | 1.39 (1.06–1.85) | 1.57 (1.16–2.14) |
| Time-Varying Covariates |
β (SD)
|
||
| Any Heart Condition | −0.28 (0.13)* | −0.28 (0.07)* | −0.29 (0.06)* |
| Diabetes | −0.64 (0.14)* | −0.67 (0.09)* | −0.48 (0.09)* |
| Hypertension | −0.76 (0.12)* | −0.47 (0.07)* | −0.09 (0.06) |
| Stroke | −1.75 (0.21)* | −1.45 (0.14)* | −1.01 (0.13)* |
Note. APOE ε4: Apolipoprotein E, allele ε4. b: Beta (regression coefficient). NSES: Neighborhood socioeconomic status. Q1: First quartile. Q2: Second quartile. Q3: Third quartile. Q4: Fourth quartile. SD: Standard deviation.
The “Other” category for race included African Americans, American Indians, Asians, and individuals who did not consider themselves to be part of any of these groups.
p < 0.05.
With every additional year of age, the odds of belonging to the medium [odds ratio (OR): 0.94 (95% confidence interval [CI]: 0.91, 0.96)] and high 0.83 (CI: 0.81, 0.86)] cognitive trajectory classes were lower. Females were 2.42 times more likely to belong to the high cognitive class [OR: 2.42 (1.96, 2.99)] than males. Whites had progressively higher odds of belonging to the medium [OR: 4.19 (3.35, 5.25)] or high 18.50 (13.63, 25.13)] cognitive trajectory classes, compared to non-white counterparts. For every additional year of education attained, odds of being in the medium cognitive trajectory class increased by 41% [OR: 1.41 (1.36, 1.46)], and odds of belonging to the high class more than doubled [OR: 2.09 (1.99, 2.18)]. Living in the South was associated with higher risk for being in the low cognitive trajectory class.
Though there was no significant association between the genetic risk score and cognitive trajectory class membership, the presence of an APOE ε4 allele reduced the likelihood of membership in the medium [OR: 0.68 (0.56, 0.83)] and high 0.40 (0.32, 0.50)] cognitive trajectory classes.
NSES and cognitive trajectory class
The odds that individuals belonging to the second, third, and fourth quartiles of NSES would be in the medium cognitive trajectory class were statistically no different than the odds of being in the low class. However, those with NSES in the third [OR: 1.45 (1.07, 1.97)] and fourth [OR: 1.57 (1.12, 2.19)] quartiles had significantly higher odds for being in the high cognitive trajectory class. Being in the second quartile for NSES did not significantly predict membership in the high cognitive trajectory class.
Cardiovascular and cerebrovascular risk (Time-varying predictors)
The cardiovascular and cerebrovascular risk factors were each associated with significantly lower cognitive function within each of the three classes of cognitive function, except for hypertension in the high trajectory class. Mean HRS TICS scores were 0.28 points ((SD): 0.13) lower for people who were in the low cognitive trajectory class and had CVD, as compared to those who did not have CVD. The mean HRS TICS score for individuals in the low cognitive trajectory class who had had a stroke was 1.75 (SD: 0.21) units lower than for those who had not had a stroke.
Interactions with NSES
The analyses tested for interactions between NSES and each of the cardiovascular (CVD, hypertension and diabetes) and cerebrovascular (stroke) risk factors and were found to be not significant. In addition, there was no interaction between NSES, and APOE ε4 carrier status and gender.
Discussion
Our goal was to examine the relationship between cognitive function and NSES, controlling for demographic, genetic, cardiovascular, and cerebrovascular risk factors. The finding that the relationship between NSES and cognitive trajectory class remained robust after controlling for demographic factors (including education) and genetic risk (including APOE-ε4 carrier status) is significant and contributes new knowledge to the body of empirical literature, as will be addressed below.
Role of neighborhood socioeconomic status
NSES did not predict class membership for the medium cognitive class, but the odds of being in the highest cognitive trajectory class were best for individuals’ in the highest quartile of NSES scores. These findings persisted above and beyond the effects of cardiovascular, cerebrovascular, genetic, and demographic risk factors.
This study contributes to a growing body of research about the importance of the association between neighborhood SES and cognitive outcomes. In an analysis of participants from the Cardiovascular Health Study, an association was found between baseline cognitive function, measured with the Modified Mini-Mental State Examination (3MS), and a similar multidimensional neighborhood SES index used in this study. The effect of NSES on cognition became non-significant in longitudinal analyses (Rosso et al., 2016).
In a study using a linear mixed effects approach to model baseline and longitudinal cognitive function, researchers found no effects of neighborhood affluence, measured as a multidimensional construct derived from four Census tract variables, on rate of cognitive decline, measured using the Spanish and English Neuropsychological Assessment Scales (SENAS). (Mungas et al., 2018) In contrast, the Hispanic Established Populations for Epidemiologic Studies of the Elderly used different measures of neighborhood SES and cognitive function, but still found that residents living in economically disadvantaged neighborhoods had faster rates of cognitive decline than those in more advantaged neighborhoods (Besser et al., 2017; Sheffield and Peek, 2009).
The importance of education
Education emerged as a strong predictor of cognitive trajectory class such that those with higher education performed better on the HRS TICS, on average. The estimated association between education and cognitive function—that is, the finding that, for every year of education attained, the likelihood that the individual will belong to the high cognitive trajectory class, was more than double the effect of NSES on cognitive function (57% increase in likelihood of belonging to the high cognitive class for having the most affluent quartile of NSES). Similar findings have been reported in the empirical literature; a recent meta-analysis found that having a low education level approximately doubled risk for dementia (Beydoun et al., 2014). Education may confer resilience against dementia among APOE ε4 carriers and may have a cumulative effect over the life course; in a study of 1,995 individuals without dementia, high lifetime educational enrichment delayed cognitive impairment by 8.7 years on average (Vemuri et al., 2014; McDermott et al., 2017).
Other studies have shown that, while NSES is associated with cognitive function at baseline, this relationship does not persist when individual SES variables, such as education, and other control variables are added to the model (Mungas et al., 2018; Worn et al., 2017). In contrast, we found an effect for NSES on cognitive status that persisted after controlling for education.
Role of cardiovascular and cerebrovascular factors
We examined whether increase in risk for membership in a lower cognitive trajectory class was associated with cardiovascular disease (CVD), its risk factors (diabetes and hypertension), and cerebrovascular disease (stroke) in a longitudinal, population-based cohort. In unadjusted models, heart disease, diabetes, hypertension, and stroke independently contributed to cognitive trajectory class membership. After including all other variables, participants with any of these factors were less likely to belong to the medium and high cognitive trajectory classes, compared to those without the risk factor(s). This observation is congruous with those from other studies demonstrating the relationship between cardiovascular risk factors, cardiovascular events, and cerebrovascular events and cognitive function (Haroon et al., 2015; Walker et al., 2017).
Trajectory class modelling
The trajectory analysis used in this study allowed for the detection of class membership effects where other approaches could not, but it is difficult to say whether the differences in the findings were due to differences in methodology or differences in the sample under study. Yet, the large size of the nationally representative, population-based cohort assessed in the present study should be generalizable.
Strengths and limitations
This study has several strengths. The HRS is a large nationally representative sample with up to 16 years of follow-up, spanning much of the crucial period of cognitive decline in later life. Latent class trajectory analysis allowed the identification of distinct subgroups based on trajectories rather than assuming one trajectory of cognitive decline with random variation around the mean over time. Using NSES and the time-varying health variables individually allowed us to estimate the association of each with the cognitive trajectory classes. The NSES measure used in this study was a continuous composite of six factors that span the social and economic breadth of the construct.
There are some design and methodological limitations to note. The HRS TICS is a global measure of cognitive function that is sensitive enough to detect change, but it is not equivalent to a comprehensive cognitive assessment. (de Jager et al., 2003) A design-related concern inherent to all NSES measures is the inability to distinguish definitively between causation, in which the neighborhood environment impacts people who move into specific areas, and selection, in which someone’s characteristics dictate where they can live, especially when NSES is low. (Mungas et al., 2018) This study did not statistically control for individual SES at baseline, which may be an important predictor of cognitive decline. (Mungas et al., 2018; Rosso et al., 2016) However, this model included education, an individual-level SES variable demonstrated to be more important in predicting cognitive decline than NSES. (Glymour and Manly, 2008) The index of NSES we used does not account for duration of residence in neighborhoods; thus, individuals transitioning between areas with different NSES scores may not fully capture the hazards or protective benefits of one or the other. However, recent work using a similar measure of NSES suggests NSES is likely to be fairly stable over time. (Miles et al., 2016) Finally, the study does not have measure of the physical aspects of neighborhoods, a factor that may also contribute to cognitive decline.
Conclusions and future directions
Medical (cardiovascular, cerebrovascular), socioeconomic/environmental (education, NSES), and genetic (APOE) factors contribute significantly to cognitive trajectory class membership. The finding that both individual and neighborhood-level SES contribute to cognitive risk emphasizes that cognition is not only a product of a behavior, trait, or medical condition, but also where an individual lives. Future studies should not only include new methodologies and more sophisticated measures of social environment, NSES or place of residence, but they should seek to identify the causal mechanisms behind the influence of these variables on longitudinal cognitive health.
Acknowledgements
We are very thankful to Bobby Jones (Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh PA) for assistance with ‘PROC TRAJ’ in SAS.
Funding
This work was funded by the National Institute on Aging (grant number R01 AG042633). The Health and Retirement Study is supported jointly by the National Institute on Aging (NIA U01AG009740) and the United States Social Security Administration. K.M.H. was also supported by funding from the NIA grant P30 AG049638.
Footnotes
Disclosure statement
M.K. was a statistical consultant for Scion NeuroStim, LLC. M.W.L. received consulting fees from Zinfandel Pharmaceutics and Cabernet Pharmaceutics.
References
- Aneshensel CS, Ko MJ, Chodosh J, & Wight RG (2011). The urban neighborhood and cognitive functioning in late middle age. Journal of Health and Social Behavior, 52(2), 163–179. doi: 10.1177/0022146510393974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, & Tanzi RE (2007). Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics, 39(1), 17–23. [DOI] [PubMed] [Google Scholar]
- Besser LM, McDonald NC, Song Y, Kukull WA, & Rodriguez DA (2017). Neighborhood environment and cognition in older adults: A systematic review. American Journal of Preventive Medicine, 53(2), 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, & Wang Y (2014). Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health, 14, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Barnes LL, Wilson RS, Evans DA, & Mendes de Leon CF (2012). Social disorder, APOE-Ε4 genotype, and change in cognitive function among older adults living in Chicago. Social Science & Medicine, 74(10), 1584–1590. doi: 10.1016/j.socscimed.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, & Folstein M (1988). The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 1, 111–117. [Google Scholar]
- Bureau UC (n.d.) Census Regions and Divisions of the United States In: US Department of Commerce Economics, and Statistics Administration, editor. [Google Scholar]
- Clarke PJ, Weuve J, Barnes L, Evans DA, & Mendes de Leon CF (2015). Cognitive decline and the neighborhood environment. Annals of Epidemiology, 25(11), 849–854. doi: 10.1016/j.annepidem.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager CA, Budge MM, & Clarke R (2003). Utility of TICS-M for the assessment of cognitive function in older adults. International Journal of Geriatric Psychiatry, 18(4), 318–324. [DOI] [PubMed] [Google Scholar]
- Escarce JJ, Lurie N, & Jewell A (2011). RAND Center for Population Health and Health Disparities (CPHHD) Data Core Series: Neighborhood Socioeconomic Status (SES) Index, 1990–2000 [United States]. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; Contract No.: ICPSR27865-v1
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18(3), 223–254. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, … Williams J (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature Genetics, 41(10), 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon NN, Austin PC, Shah BR, Wu J, Gill SS, & Booth GL (2015). Risk of dementia in seniors with newly diagnosed diabetes: a population-based study. Diabetes Care, 38(10), 1868–1875. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Lutz MW, Kuchibhatla M, Germain C, & Plassman L (2015). Effect of APOE and CD33 on Cognitive Decline. PLoS One, 10(6), e0130419. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, … Williams J (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nature Genetics, 43(5), 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, & Roeder K (2001). A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research, 29(3), 374–393. [Google Scholar]
- Jones CJ, & Peskin H (2010). Psychological health from the teens to the 80s: Multiple developmental trajectories. Journal of Adult Development, 17(1), 20. [Google Scholar]
- Juster FT, & Suzman R (1995). An overview of the Health and Retirement Study. Journal of Human Resources, 30, S7–S56. [Google Scholar]
- Kovalchik SA, Slaughter ME, Miles J, Friedman EM, & Shih RA (2015). Neighbourhood racial/ethnic composition and segregation and trajectories of cognitive decline among U.S. older adults. Journal of Epidemiology and Community Health, 69(10), 978–984. doi: 10.1136/jech-2015-205600. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, … Amouyel P (2009). Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature Genetics, 41(10), 1094–1099. [DOI] [PubMed] [Google Scholar]
- Martinez DM (2007). Racial disparities in cognitive performance over time among older adults: A multilevel analysis of neighborhood effects. Doctoral Dissertation Ann Arbor: University of California Los Angeles; 2007.
- McDermott KL, McFall GP, Andrews SJ, Anstey KJ, & Dixon RA (2017). Memory Resilience to Alzheimer’s genetic risk: Sex effects in predictor profiles. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 72(6), 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer OL, Sisco SM, & Harvey D (In press). Neighborhood Predictors of Cognitive Training Outcomes and Trajectories in ACTIVE. Res Aging. In press Online December 13, 2015. doi: 10.1177/0164027515618242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JN, Weden MM, Lavery D, Escarce JJ, Cagney KA, & Shih RA (2016). Constructing a Time-Invariant Measure of the Socio-economic Status of U.S. Census Tracts. Journal of Urban Health, 93(1), 213–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, King J, Hinton L, Farias S, & Reed B (2018). Neighborhood socioeconomic status and cognitive trajectories in a diverse longitudinal cohort. Clinical Gerontologist, 41, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D (2005). Group-based modeling of development Cambridge, MA: Harvard University Press; [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang L-S, Vardarajan BN, Buros J, … Schellenberg GD (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nature Genetics, 43(5), 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofstedal MB, Fisher GG, & Herzog AR (2005). Documentation of Cognitive Functioning Measures in the Health and Retirement Study – https://hrs.isr.umich.edu/publications/biblio/5620
- Plassman BL, Newman TT, Welsh KA, Helms M, & Breitner JCS (1994). Properties of the telephone interview for cognitive status: Application in epidemiological and longitudinal Studies. Neuropsychology, 7(3), 235–241. [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB (2008). Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine, 148(6), 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB (2007). Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology, 29(1–2), 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rej S, Begley A, Gildengers A, Dew MA, Reynolds CF, & Butters MA (2015). Psychosocial risk factors for cognitive decline in late-life depression: Findings from the MTLD-III Study. Canadian Geriatrics Journal : Cgj, 18(2), 43–50. doi: 10.5770/cgj.18.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Flatt JD, Carlson MC, Lovasi GS, Rosano C, Brown AF, … Gianaros PJ (2016). The urban neighborhood and cognitive functioning in late middle age. American Journal of Epidemiology, 183(12), 1088–1179. doi: 10.1177/0022146510393974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, … Weiner MW (2010). Alzheimer’s disease neuroimaging initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimers Dementia, 6(3), 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield KM, & Peek MK (2009). Neighborhood context and cognitive decline in older Mexican Americans: Results from the Hispanic established populations for epidemiologic studies of the elderly. American Journal of Epidemiology, 169(9), 1092–1101. doi: 10.1093/aje/kwp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih RA, Ghosh-Dastidar B, Margolis KL, Slaughter ME, Jewell A, Bird CE, … Espeland MA (2011). Neighborhood socioeconomic status and cognitive function in women. American Journal of Public Health, 101(9), 1721–1728. doi: 10.2105/AJPH.2011.300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisco SM, & Marsiske M (2012). Neighborhood influences on late life cognition in the ACTIVE Study. Journal of Aging Research, 2012, 1. doi: 10.1155/2012/435826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, … Jack CR, et al. (2014). Association of life-time intellectual enrichment with cognitive decline in the older population. JAMA Neurology, 71(8), 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley VG, Unverzagt FW, McGuire LC, Moy CS, Go R, Kissela B, … Howard G (2011). Incident cognitive impairment is elevated in the stroke belt: The REGARDS Study. Annals of Neurology, 70(2), 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Power MC, & Gottesman RF (2017). Defining the relationship between hypertension, cognitive decline, and dementia: A review. Current Hypertension Reports, 19(3), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A, Ferdous F, Moore KD, & Burns JM (2015). Neighborhood integration and connectivity predict cognitive performance and decline. Gerontology and Geriatric Medicine, 1, 1–9. doi: 10.1177/233372141559914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Breitner JCS, & Habib-Magruder KM (1993). Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychology, 62(2), 103–110. [Google Scholar]
- Worn J, Ellwardt L, Aartsen M, & Huisman M (2017). Cognitive functioning among Dutch older adults: Do neighborhood socioeconomic status and urbanity matter? Social Science & Medicine, 187, 29–38. [DOI] [PubMed] [Google Scholar]
- Wu YT, Prina AM, & Brayne C (2015). The association between community environment and cognitive function: a systematic review. Social Psychiatry and Psychiatric Epidemiology, 50(3), 351–362. doi: 10.1007/s00127-014-0945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


