This cohort study investigates the association between pathologic response outcomes and the time interval between neoadjuvant therapy completion and total mesorectal excision in patients with rectal cancer.
Key Points
Question
Is the time interval between neoadjuvant therapy completion and total mesorectal excision associated with pathologic response outcomes in patients with rectal cancer?
Findings
In this cohort study of 1506 patients with rectal cancer treated with neoadjuvant therapy, different time intervals were not associated with variations in pathologic complete response rates. However, a significant risk reduction in bad response (tumor regression grade, 2-3) and systemic recurrence was observed at intervals longer than 12 weeks; additionally, mesorectal quality and perioperative events, such as conversion and minor postoperative complications, were worse in this group.
Meaning
Findings suggest that delaying surgery may improve tumor regression and decrease risk of distant metastasis but increase surgical complexity.
Abstract
Importance
The treatment for extraperitoneal locally advanced rectal cancer (LARC) is neoadjuvant therapy (NAT) followed by total mesorectal excision (TME). Robust evidence on the optimal time interval between NAT completion and surgery is lacking.
Objective
To assess the association of time interval between NAT completion and TME with short- and long-term outcomes. It was hypothesized that longer intervals increase the pathologic complete response (pCR) rate without increasing perioperative morbidity.
Design, Setting, and Participants
This cohort study included patients with LARC from 6 referral centers who completed NAT and underwent TME between January 2005 and December 2020. The cohort was divided into 3 groups depending on the time interval between NAT completion and surgery: short (≤8 weeks), intermediate (>8 and ≤12 weeks), and long (>12 weeks). The median follow-up duration was 33 months. Data analyses were conducted from May 1, 2021, to May 31, 2022. The inverse probability of treatment weighting method was used to homogenize the analysis groups.
Exposure
Long-course chemoradiotherapy or short-course radiotherapy with delayed surgery.
Main outcome and Measures
The primary outcome was pCR. Other histopathologic results, perioperative events, and survival outcomes constituted the secondary outcomes.
Results
Among the 1506 patients, 908 were male (60.3%), and the median (IQR) age was 68.8 (59.4-76.5) years. The short-, intermediate-, and long-interval groups included 511 patients (33.9%), 797 patients (52.9%), and 198 patients (13.1%), respectively. The overall pCR was 17.2% (259 of 1506 patients; 95% CI, 15.4%-19.2%). When compared with the intermediate-interval group, no association was observed between time intervals and pCR in short-interval (odds ratio [OR], 0.74; 95% CI, 0.55-1.01) and long-interval (OR, 1.07; 95% CI, 0.73-1.61) groups. The long-interval group was significantly associated with lower risk of bad response (tumor regression grade [TRG] 2-3; OR, 0.47; 95% CI, 0.24-0.91), systemic recurrence (hazard ratio, 0.59; 95% CI, 0.36-0.96), higher conversion risk (OR, 3.14; 95% CI, 1.62-6.07), minor postoperative complications (OR, 1.43; 95% CI, 1.04-1.97), and incomplete mesorectum (OR, 1.89; 95% CI, 1.02-3.50) when compared with the intermediate-interval group.
Conclusions and Relevance
Time intervals longer than 12 weeks were associated with improved TRG and systemic recurrence but may increase surgical complexity and minor morbidity.
Introduction
The criterion standard treatment for mid and distal locally advanced rectal cancer is total mesorectal excision (TME).1 Evidence supports the addition of neoadjuvant therapy (NAT) in selected cases, which include extramural venous invasion (EMVI), threatened circumferential resection margin (CRM), cancer stages cT3 to T4, or node-positive tumors. The main objective of NAT is to reduce tumor burden and facilitate downstaging, increasing the possibility of achieving higher rates of R0 (ie, surgical margin microscopically negative for residual tumor) resections.2,3 Patients with good response to NAT may benefit from sphincter-saving procedures or even an active surveillance strategy (watch and wait), thus avoiding surgical comorbidities.4,5,6,7
Pathologic complete response (pCR) is observed in 10% to 30% of rectal cancers after NAT8,9 and is an important predictive factor affecting long-term survival and recurrence.10 The appropriate interval between NAT completion and surgery, seeking the highest pCR rates, remains a matter of controversy. Traditionally, surgery is performed 6 to 8 weeks after NAT completion.11 However, the potential association between an extended interval and an increase in pCR rates has become a central issue.3,12 Conversely, some studies have shown that extended intervals may induce fibrosis around the mesorectal plane, leading to technical difficulties during surgery, more intraoperative and postoperative complications, and worse quality of resected specimens,13 although none of these hypotheses have been confirmed.
A watch-and-wait strategy for rectal cancer is a nonstandard approach.14 However, according to the International Watch & Wait Database, it is currently being practiced in more than 15 countries all over the world.15 The benefits of the nonoperative strategy outline the need for determining the adequate time interval between NAT completion and primary surgery. Additionally, assessment of the factors associated with tumor regression and the optimal evaluation of complete clinical response are of great importance.6 This national, multicenter, retrospective, cohort study at multiple Spanish regions aimed to define the optimal time interval between NAT completion and surgery that allows achieving the highest pCR rate. We hypothesized that longer intervals would increase the pCR rate without increasing perioperative morbidity.
Methods
Study Design and Participants
This study was conducted according to the ethical principles for medical research involving human participants stated in the Declaration of Helsinki and approved by the institutional review board of the Hospital Clinic of Barcelona. Written informed consent was obtained for all TME surgeries, including authorization for collection and analysis of patients’ clinical data. All participating centers were tertiary referral hospitals in different Spanish regions. Ethical approval was obtained from the local authorities, and a data-sharing agreement was signed before transferring data. This study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.16
All consecutive patients with biopsy-proven rectal adenocarcinoma who underwent neoadjuvant chemoradiotherapy or short-course radiotherapy and delayed TME between January 1, 2005, and December 31, 2020, were identified and reviewed. Clinical staging was performed using thoracoabdominal computed tomography to rule out metastatic disease, and endoscopic ultrasound (before 2007) or pelvic magnetic resonance imaging (MRI) was performed to assess the local stage. Exclusion criteria were age younger than 18 years, metastatic disease, tumors previously treated with local excision, patients undergoing short-course radiotherapy and immediate surgery (<2 weeks), patients who did not finish NAT for any reason, pelvic malignancy within the previous 5 years, and surgery performed in an emergency setting. Data were extracted through a medical record review, pseudo-anonymized, and entered into a standardized uniform database. Collected patient characteristics included sex, age, body mass index, American Society of Anesthesiologists classification, tumor characteristics at the time of diagnosis (location from anal verge and preoperative T and N stage), baseline carcinoembryonic antigen levels, NAT type, NAT completion date, radiologic reassessment after NAT (if the case), pathologic outcomes, and follow-up data. Data on patient race and ethnicity was not available, as this information is not usually gathered from participating centers in Spain.
Cancer Treatment Definitions
The indication and selection of NAT type were made in accordance with the local policy of the participating institutions and included the following: (1) long-course chemoradiation consisting of a total dosage of 45 Gy, at a daily dose of 1.8 Gy for a total of 5 weeks, with sensitizing continuous 5-fluorouracil infusion (225 mg/m2/day for 5 days per week) or capecitabine (825 mg/m2 twice daily for 5 days per week) administered throughout the radiation course; and (2) short-course radiotherapy, comprising 1-week radiotherapy administered at 25 Gy in 5 daily fractions with delayed surgery. Conversion was defined as an incision longer than or different from the planned incision, excluding the incision necessary for specimen extraction.
Groups According to Timing From the End of Neoadjuvant Therapy to Surgery
Three waiting interval groups were defined in the study protocol based on the time interval between the end of NAT and surgery: short interval (≤8 weeks), intermediate interval (>8 and ≤12 weeks), and long interval (>12 weeks). This division was made for several reasons: most available literature indicates that a waiting time of 8 weeks is necessary for a tumor to respond, 11 and in current clinical practice, a waiting period of 8 to 12 weeks is widely used. Nevertheless, there is increasing research on the benefits of waiting longer than 12 weeks.17
Outcomes
The primary outcome was the pCR, defined as the complete disappearance of the tumor in the resected specimen (ypT0N0). Other histopathologic features were assessed as secondary outcomes and include the following: tumor regression grade (TRG) assessed using the American Joint Committee on Cancer/College of American Pathologists TRG classification18 and subgrouped into good response (TRG 0-1) and bad response (TRG 2-3), mesorectum completeness, CRM (considered positive when the deepest part of the tumor was ≤1 mm of the resection margin or when a positive lymph node was ≤1 mm of the radical dissection plane) and distal resection margin (DRM) status (considered positive if tumor cells were present ≤1 mm from the lower border of the tumor to the cut edge of the specimen), perioperative events, and survival outcomes including locoregional recurrence (within the pelvis, including lymph nodes below the common iliac artery bifurcation), distant recurrence, and overall survival (OS).
Statistical Analysis
Categorical variables are expressed as absolute frequencies and percentages. Continuous variables are reported as medians with IQR (25th-75th percentile). To allow for an unbiased comparison, an inverse probability of treatment weighting (IPTW) method was used.19 Given that 3 interval groups were compared, a multinomial logistic regression model was applied to estimate the propensity score with its subsequent transformation to the IPTW, stabilized by the proportion of patients in each group. This model included available demographic and clinical preoperative variables including hospital, age, sex, smoking status, presence of previous malignancies, American Society of Anesthesiologists classification, body mass index, baseline carcinoembryonic level, distance of tumor from the anal verge, cT and cN stage, baseline radiologic CRM and EMVI, type of chemotherapy or radiotherapy, and comorbidities such as hypertension, diabetes, and chronic diseases. As the goal was to develop a balanced population independent of the outcome assessment, postoperative variables were not included. Following the guidelines,20 a well-balanced distribution of the covariates in the weighted sample was confirmed using standardized differences less than 0.10 and no inferential analysis was performed to compare waiting intervals. The intermediate interval was set as a reference category because it is the most commonly used in clinical practice. Prognostic estimation of response was performed by calculating the odds ratio (OR) and 95% CI for the outcome variables. For the primary outcome, the OR for obtaining a pCR was calculated. The association of waiting intervals with survival outcomes was estimated by calculating the hazard ratio (HR) and 95% CI using Cox proportional hazards models. Individual IPTW calculations were used as weights for all the described models. Concordance between restaging MRI-defined tumor response and histopathologic response was evaluated using the κ index. Statistical tests were 2-sided with a 5% type I error. All analyses were performed using SPSS, version 26 (IBM Corp).
Results
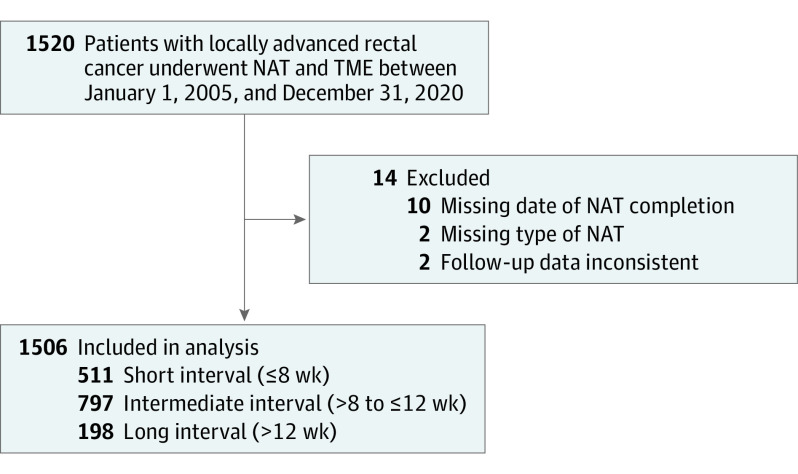
Between January 1, 2005, and December 31, 2020, 1520 patients with resectable nonmetastatic rectal adenocarcinoma underwent NAT and TME in 6 Spanish tertiary referral centers and met the inclusion criteria. Among these patients, 14 (0.9%) were excluded because of missing data on NAT and follow-up variables. Therefore, the final study population consisted of 1506 patients (median [IQR] age, 68.8 [59.4-76.5] years; 908 male [60.3%]; 598 female [39.7%]). The short-, intermediate-, and long-interval groups included 511 patients (33.9%), 797 patients (52.9%), and 198 patients (13.1%), respectively. The median (IQR) interval times were as follows: short, 7.1 (6.0-7.8) weeks, 9.4 (8.7-10.3) weeks, and 14.3 (12.8-17.4) weeks (Figure 1).
Figure 1. Flow Diagram of Study Population.
All patients underwent neoadjuvant therapy (NAT) and total mesorectal excision (TME).
Table 1 summarizes patient baseline characteristics, tumor features at the time of diagnosis, NAT type, and selected covariates of patients with a well-balanced distribution with standardized difference less than 0.10 in the pseudo-population after IPTW application. In the 3 interval groups, similar types of NAT were administered. Short-course radiotherapy was administered to 65 patients (12.7%) in the short-interval group, 98 patients (12.3%) in the intermediate-interval group, and 14 patients (7.1%) in the long-interval group. Long-course chemoradiotherapy was administered to 446 patients (87.3%) in the short-interval group, 699 patients (87.7%) in the intermediate-interval group, and 184 patients (92.9%) in the and long-interval group. In the entire cohort, only 351 patients (23.3%) were restaged using MRI after completing the NAT. The median (IQR) interval from NAT completion to restaging was 5 (4-7) weeks. A poor concordance was observed between MRI-defined tumor response and histopathologic response with a raw agreement of 34.5% and κ of 0.06 (eTable 1 in Supplement 1).
Table 1. Selected Covariates of Patients With Standardized Differences Before and After Inverse Probability of Treatment Weighting.
| Variable | Entire cohort | Short interval, ≤8 wk | Intermediate interval, >8 to ≤12 wk | Long interval, >12 wk | Standardized differencesa | |
|---|---|---|---|---|---|---|
| Before IPTW | After IPTW | |||||
| Center, No. (%) | ||||||
| Hospital Clínic de Barcelona | 271 (17.9) | 84 (16.4) | 133 (16.6) | 54 (27.2) | 0.8636 | 0.0680 |
| Hospital Clínico Universitario de Santiago | 538 (35.7) | 101 (19.7) | 404 (50.6) | 33 (16.6) | ||
| Hospital del Mar | 201 (13.3) | 73 (14.2) | 109 (13.6) | 19 (9.6) | ||
| Hospital Universitari Son Espases | 214 (14.2) | 122 (23.8) | 88 (11) | 4 (2) | ||
| Consorci Sanitari Integral–Hospital General de Hospitalet | 163 (10.8) | 120 (23.4) | 36 (4.5) | 7 (3.5) | ||
| Hospital Clínico Universitario Virgen de la Arrixaca | 119 (7.9) | 11 (2.1) | 27 (3.3) | 81 (40.9) | ||
| Age, median (IQR), y | 68.8 (59.4-76.5) | 68 (59-77) | 69 (60-77) | 66.6 (59-76) | 0.0145 | 0.0377 |
| Sex, No. (%) | ||||||
| Male | 908 (60.3) | 293 (57) | 492 (61.7) | 123 (62.1) | 0.0896 | 0.0261 |
| Female | 598 (39.7) | 218 (42.7) | 305 (38.3) | 75 (37.9) | ||
| BMI, No. (%)b | ||||||
| <20 | 56 (3.7) | 11 (2.2) | 34 (4.3) | 11 (5.6) | 0.1512 | 0.0228 |
| 20-24.9 | 383 (25.4) | 128 (25.1) | 196 (24.6) | 59 (29.8) | ||
| 25-30 | 615 (40.8) | 210 (41.1) | 329 (41.3) | 76 (38.4) | ||
| >30 | 284 (18.9) | 105 (20.6) | 136 (17.1) | 43 (21.7) | ||
| Missing | 168 (11.2) | 57 (11.2) | 102 (12.8) | 9 (4.6) | ||
| Smoker, No. (%) | ||||||
| No | 1245 (82.7) | 450 (88.1) | 660 (82.8) | 135 (68.2) | 0.1493 | 0.010 |
| Yes | 261 (17.3) | 61 (11.9) | 137 (17.2) | 63 (31.8) | ||
| Hypertension, No. (%) | ||||||
| No | 809 (53.7) | 274 (53.6) | 420 (52.7) | 115 (58.1) | 0.0185 | −0.001 |
| Yes | 697 (46.3) | 237 (46.4) | 377 (47.3) | 83 (41.9) | ||
| Diabetes, No. (%) | ||||||
| No | 1180 (78.4) | 406 (79.4) | 628 (78.8) | 146 (73.7) | 0.0162 | −0.017 |
| Yes | 326 (21.7) | 105 (20.6) | 169 (21.2) | 52 (26.3) | ||
| Chronic kidney disease, No. (%) | ||||||
| No | 1457 (96.7) | 494 (96.7) | 769 (96.5) | 194 (98) | 0.0162 | −0.017 |
| Yes | 49 (3.3) | 17 (3.3) | 28 (3.5) | 4 (2) | ||
| Chronic heart disease, No. (%) | ||||||
| No | 1279 (84.9) | 441 (86.3) | 678 (85.1) | 160 (80.8) | 0.0162 | −0.017 |
| Yes | 227 (15.1) | 70 (13.7) | 119 (14.9) | 38 (19.2) | ||
| Chronic obstructive pulmonary disease, No. (%) | ||||||
| No | 1365 (90.6) | 469 (91.8) | 716 (89.8) | 180 (90.9) | 0.0673 | 0.0246 |
| Yes | 141 (9.4) | 42 (8.2) | 81 (10.2) | 18 (9.1) | ||
| Chronic liver disease, No. (%) | ||||||
| No | 1474 (97.9) | 500 (97.9) | 782 (98.1) | 192 (97) | 0.0673 | 0.0246 |
| Yes | 32 (2.1) | 11 (2.1) | 15 (1.9) | 6 (3) | ||
| Vasculopathy, No. (%) | ||||||
| No | 1452 (96.4) | 493 (96.5) | 770 (96.6) | 189 (95.5) | −0.007 | 0.0178 |
| Yes | 54 (3.6) | 18 (3.5) | 27 (3.4) | 9 (4.5) | ||
| Previous malignancies, No. (%)c | ||||||
| No | 1429 (95) | 485 (95.1) | 766 (96.2) | 178 (89.9) | 0.0664 | 0.0653 |
| Hematologic | 7 (0.5) | 2 (0.4) | 4 (0.5) | 1 (0.5) | ||
| Colorectal | 5 (0.3) | 1 (0.2) | 1 (0.1) | 3 (1.5) | ||
| Otherd | 63 (4.2) | 22 (4.3) | 25 (3.1) | 16 (8) | ||
| ASA classification, No. (%) | ||||||
| I-II | 1029 (68.4) | 370 (72.7) | 524 (65.7) | 135 (68.2) | 0.1508 | −0.045 |
| III-IV | 475 (31.6) | 139 (27.3) | 273 (34.3) | 63 (31.8) | ||
| CEA, mean (SD), ng/mL | 8.99 (20.1) | 10.44 (27.8) | 8.24 (14.4) | 7.82 (11.4) | −0.10 | −0.083 |
| Height from AV, No. (%), cm | ||||||
| <5 | 480 (31.9) | 162 (31.7) | 244 (30.6) | 74 (37.4) | 0.0257 | 0.0368 |
| 5-10 | 774 (51.4) | 260 (50.9) | 415 (52.1) | 99 (50) | ||
| >10 | 252 (16.7) | 89 (17.4) | 138 (17.3) | 25 (12.6) | ||
| Clinical tumor (T) classification, No. (%) | ||||||
| cT1 | 9 (0.6) | 1 (0.2) | 4 (0.5) | 4 (2) | 0.1678 | 0.0797 |
| cT2 | 99 (6.7) | 26 (5.2) | 64 (8.2) | 9 (4.5) | ||
| cT3 | 1157 (76.5) | 404 (80.2) | 598 (74) | 155 (78.3) | ||
| cT4 | 241 (16.2) | 73 (14.5) | 138 (17.6) | 30 (15.2) | ||
| Clinical nodal (N) classification, No. (%) | ||||||
| cN0 | 372 (24.7) | 113 (22.1) | 184 (23.1) | 75 (37.9) | 0.0667 | 0.0438 |
| cN1 | 791 (52.5) | 272 (53.2) | 437 (54.8) | 82 (41.4) | ||
| cN2 | 312 (20.7) | 114 (22.3) | 162 (20.3) | 36 (18.2) | ||
| cNx | 31 (2.1) | 12 (2.4) | 14 (1.8) | 5 (2.5) | ||
| Baseline threatened CRM, No. (%) | ||||||
| No | 478 (31.7) | 189 (37) | 216 (27.1) | 73 (36.9) | 0.3834 | 0.0089 |
| Yes | 620 (41.2) | 223 (43.6) | 294 (36.9) | 103 (52) | ||
| Missing | 408 (27.1) | 99 (19.4) | 287 (36) | 22 (11.1) | ||
| Baseline EMVI, No. (%) | ||||||
| No | 860 (57.1) | 328 (64.2) | 396 (49.7) | 136 (68.7) | 0.4141 | 0.0192 |
| Yes | 257 (17.1) | 94 (18.4) | 120 (15.1) | 43 (21.7) | ||
| Missing | 389 (25.8) | 89 (17.4) | 281 (35.3) | 19 (9.6) | ||
| Clinical stage, No. (%) | ||||||
| I | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.0257 | 0.0368 |
| II | 398 (26.4) | 122 (23.9) | 197 (24.7) | 79 (39.9) | ||
| III | 1108 (73.6) | 389 (76.1) | 600 (75.3) | 119 (60.1) | ||
| Radiotherapy, No. (%) | ||||||
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | −0.004 | 0.0233 |
| Yes | 1506 (100) | 511 (100) | 797 (100) | 198 (100) | ||
| Chemotherapy, No. (%) | ||||||
| No | 177 (11.8) | 65 (12.7) | 98 (12.3) | 14 (7.1) | −0.004 | 0.0233 |
| Yes | 1329 (88.2) | 446 (87.3) | 699 (87.7) | 184 (92.9) | ||
| Type of chemotherapy, No. (%) | ||||||
| Capecitabine | 485 (36.1) | 151 (33.7) | 225 (31.6) | 109 (58.8) | −0.004 | 0.0233 |
| 5F | 844 (63.9) | 295 (66.3) | 474 (68.4) | 75 (41.2) | ||
| Type of radiotherapy, No. (%) | ||||||
| Short course | 177 (11.8) | 65 (12.7) | 98 (12.3) | 14 (7.1) | −0.012 | −0.035 |
| Long course | 1329 (88.2) | 446 (87.3) | 699 (87.7) | 184 (92.9) | ||
Abbreviations: ASA, American Society of Anesthesiologists; AV, anal verge; BMI, body mass index; CEA, carcinoembryonic antigen; CRM, circumferential resection margin; EMVI, extramural venous invasion; IPTW, inverse probability of treatment weighting.
SI conversion factor: To convert CEA to microgram per liter, multiply by 1.
Propensity score for IPTW calculation was performed by multinomial logistic regression model including all the variables shown in this table.
Calculated as weight in kilograms divided by height in meters squared.
Pelvic malignancy within the previous 5 years was an exclusion criterion.
Other malignancy includes skin cancer, breast cancer, and liver cancer.
pCR was obtained in 255 patients (17.2% [259 of 1506]; 95% CI, 15.4%-19.2%). When compared with the intermediate-interval group, no association between the time interval and pCR was demonstrated in the short-interval group (OR, 0.74; 95% CI, 0.55-1.01; P = .05) and long-interval group (OR, 1.07; 95% CI, 0.73-1.61; P = .70) (Table 2). However, a long interval was significantly associated with lower risk of bad response (TRG, 2-3; OR, 0.47; 95% CI, 0.24-0.91; P = .03) than the reference category. Regarding other histopathologic outcomes, the long-interval group was associated with a high risk of incomplete mesorectum (OR, 1.89; 95% CI, 1.02-3.50; P = .04) without increasing the risk of positive circumferential or distal resection margin (OR, 1.35; 95% CI, 0.74-2.45; P = .33 and OR, 0.56; 95% CI, 0.19-1.63; P = .29, respectively).
Table 2. Clinical, Pathologic, and Oncologic Outcomes Adjusted for Interval From End of Neoadjuvant Therapy to Surgery.
| Variable | >8 to ≤12 wk (Reference) | P value | |
|---|---|---|---|
| OR (95% CI) | HR (95% CI) | ||
| pCR | |||
| ≤8 wk | 0.74 (0.55-1.01) | NA | .05 |
| >12 wk | 1.07 (0.73-1.61) | .70 | |
| Bad response measured by histopathologic TRG (AJCC/CAP system)a | |||
| ≤8 wk | 1.27 (0.90-1.78) | NA | .17 |
| >12 wk | 0.47 (0.24-0.91) | .03 | |
| Incomplete mesorectal excision | |||
| ≤8 wk | 0.89 (0.51-1.53) | NA | .66 |
| >12 wk | 1.89 (1.02-3.50) | .04 | |
| Positive CRM | |||
| ≤8 wk | 1.22 (0.79-1.89) | NA | .37 |
| >12 wk | 1.35 (0.74-2.45) | .33 | |
| Positive DRM | |||
| ≤8 wk | 1.05 (0.59-1.89) | NA | .86 |
| >12 wk | 0.56 (0.19-1.63) | .29 | |
| Intraoperative complications | |||
| ≤8 wk | 0.76 (0.52-1.11) | NA | .15 |
| >12 wk | 1.35 (0.85-2.15) | .21 | |
| 30-d postoperative complications (Clavien-Dindo ≤II) | |||
| ≤8 wk | 1.00 (0.80-1.26) | NA | .97 |
| >12 wk | 1.43 (1.04-1.97) | .03 | |
| 30-d postoperative complications (Clavien-Dindo >II) | |||
| ≤8 wk | 0.94 (0.64-1.38) | NA | .75 |
| >12 wk | 1.25 (0.76-2.07) | .38 | |
| Conversion | |||
| ≤8 wk | 0.84 (0.42-1.70) | NA | .63 |
| >12 wk | 3.14 (1.62-6.07) | .001 | |
| Locoregional recurrence | |||
| ≤8 wk | NA | 1.33 (0.86-2.08) | .20 |
| >12 wk | 0.53 (0.21-1.33) | .18 | |
| Systemic recurrence | |||
| ≤8 wk | NA | 1.04 (0.79-1.34) | .79 |
| >12 wk | 0.59 (0.36-0.96) | .04 | |
| Overall survival | |||
| ≤8 wk | NA | 1.30 (0.99-1.70) | .06 |
| >12 wk | 1.38 (0.94-2.04) | .11 | |
Abbreviations: AJCC/CAP, American Joint Committee of Cancer and College of American Pathologists; CRM, circumferential resection margin; DRM, distal resection margin; HR, hazard ratio; NA, not applicable; OR, odds ratio; pCR, pathological complete response; TRG, tumor regression grade.
TRG stratified as good response (TRG 0-1) and bad response (TRG 2-3).
The time interval was not associated with intraoperative complications (short interval: OR, 0.76; 95% CI, 0.52-1.11; P = .15; long interval: OR, 1.35; 95% CI, 0.85-2.15; P = .21) or 30-day serious postoperative complications measured as Clavien-Dindo classification greater than II (short interval: OR, 0.94; 95% CI, 0.64-1.38; P = .75; long interval: OR, 1.25; 95% CI, 0.76-2.07; P = .38). Nonetheless, when compared with the reference category, the long-interval group was associated with a higher risk of minor postoperative complications (Clavien-Dindo ≤II: OR, 1.43; 95% CI, 1.04-1.97; P = .03), conversion to open surgery (OR, 3.14; 95% CI, 1.62-6.07; P = .001), and longer operative time (difference from <8 weeks: 15 minutes, 95% CI, 2.4-27.6; P = .001) (eTable 2 in Supplement 1).
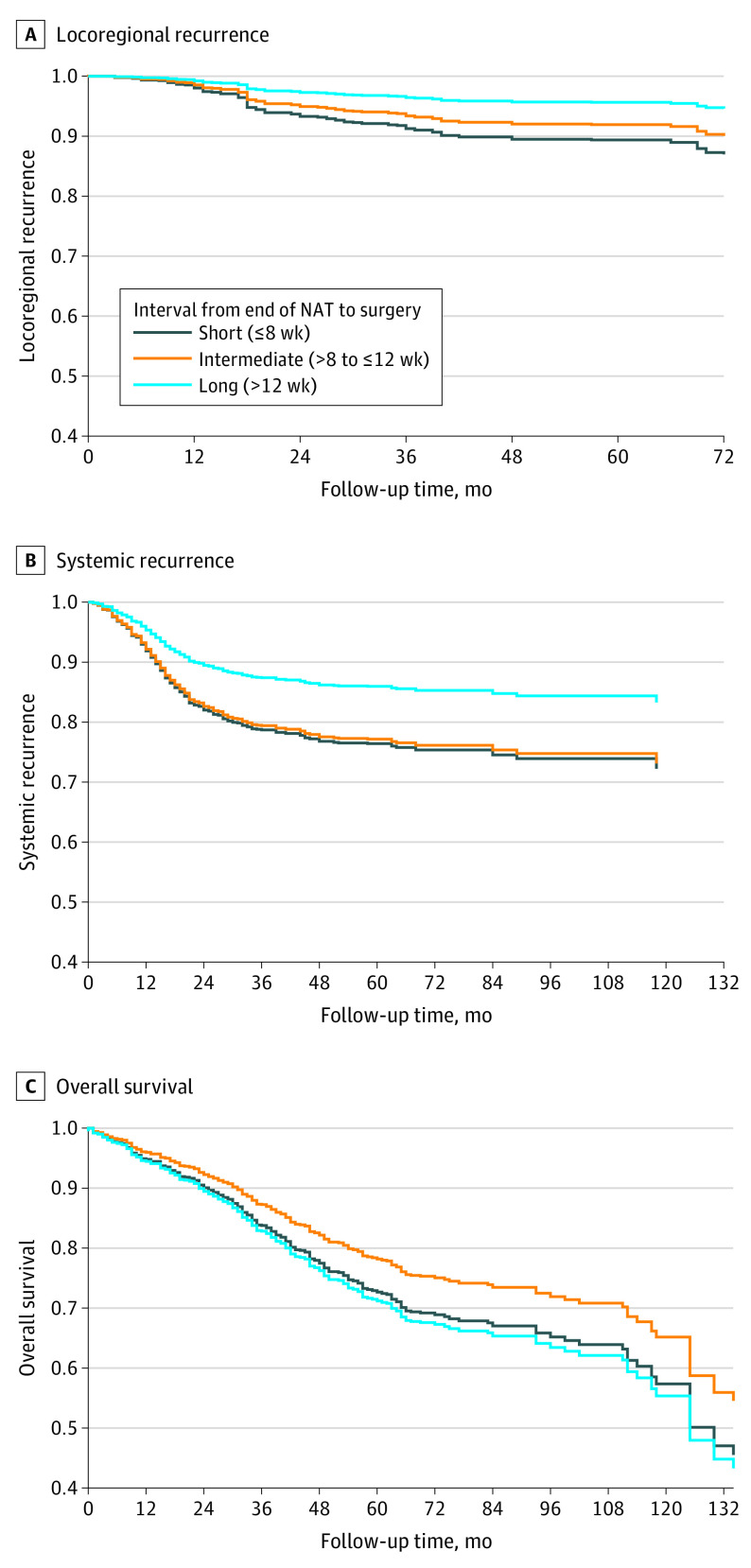
The median (IQR) follow-up period was 33 (15-58) months. No significant association between the time interval and locoregional recurrence was found (short interval: HR, 1.33; 95% CI, 0.83-2.08; P = .20; long interval: HR, 0.53; 95% CI, 0.21-1.33; P = .18) (Table 2). Remarkably, the long-interval group was associated with a lower risk of systemic recurrence than the reference category (HR, 0.59; 95% CI, 0.36-0.96; P = .04) (Figure 2). No association of the interval with OS was observed (short interval: HR, 1.30; 95% CI, 0.99-1.70; P = .06; long interval: HR, 1.38; 95% CI, 0.94-2.04; P = .11).
Figure 2. Estimated Survival/Recurrence Functions for Patients With Locally Advanced Rectal Cancer Treated With Neoadjuvant Therapy (NAT).
Locoregional recurrence (A), systemic recurrence (B), and overall survival (C) proportional Cox models in patients with locally advanced rectal cancer treated with NAT and total mesorectal excision.
Discussion
In this cohort study, the hypothesis of an increased pCR rate with a greater than 12-week delay in surgery was not met. Nevertheless, a trend toward a lower probability of obtaining a pCR in patients who waited less than 8 weeks should be noted, consistent with previously reported data.18,21 Notably, a long interval between NAT completion and TME was associated with a 53% risk reduction of bad response (histopathologic TRG) when compared with an intermediate interval. These findings follow the principle of radiotherapy, which involves a cellular lysis phenomenon within weeks after starting irradiation and once the DNA is damaged,22 thereby reinforcing the idea that tumor downstaging is a time-dependent process.21,22,23
Additionally, one of the main findings of the present study was a 41% risk reduction in systemic recurrence in the long-interval group compared with the intermediate group. This can be attributed to the fact that in patients with rectal cancer who were treated with NAT, the risk of distant metastases decreases progressively with tumor regression.9,24 Longer intervals are required to achieve not only tumor regression but also nodal downstaging,25 with the consequent minor risk of developing distant metastases.5 Indeed, in the German CAO/ARO/AIO-94 phase 3 randomized clinical trial,9 a significant association was found between TRG and systemic recurrence, with the patients having poor response to NAT being the ones with the highest rate of distant metastases. Several hypotheses on this matter have emerged, such as an aggressive tumor biology in poor responders, leading to an intrinsic resistance to NAT and high propensity to distant seeding.9 Our study results suggest that delaying surgery may reduce metastasis risk by favoring tumor regression sufficiently.
Nonetheless, the reported improvements in tumor regression and systemic recurrence in the long-interval group were unexpectedly not followed by improved OS. Although pCR by itself is associated with a better long-term outcome, and delaying surgery is one of the strategies that play a role in increasing pCR rates,10,26 no consistent data exist supporting improved survival as a consequence of an extended interval.2,3,27 In the Lyon trial,21 despite an improved pathologic response in the 6- to 8-week interval compared with the 2-week interval, no significant difference in OS was found in the 17-year follow-up.28 The results of the GRECCAR-6 trial13,29 showed that, although the pCR rates were similar between a 7- and 11-week waiting period, the interval had no impact on OS. This was reinforced in a pooled analysis, including more than 3000 patients from 7 randomized clinical trials, which showed that longer intervals may be associated with improved pathologic response but not improved survival.30 It is plausible that pCR is not the direct cause of good prognosis in rectal cancer as is largely claimed but, when achieved, is rather a good indicator. Ultimately, a favorable tumor biological profile is the key to a favorable prognosis, regardless of the interval time.
Additionally, tumor regression and waiting periods were not the only factors that appeared to be weakly correlated with treatment efficacy (OS). Petrelli et al31 analyzed 22 randomized clinical trials and concluded that other end points, such as disease-free survival, are also not surrogates of OS in rectal cancer studies. The poor correlation observed between surrogate end points and survival is a well-known issue in oncologic research, including the metastatic setting.32 This may explain our finding that improved systemic recurrence was not associated with better OS.
Another widely discussed topic is the association between the waiting interval, some clinicopathologic results, and early surgical outcomes. We found that a longer interval was associated with a higher risk of conversion to open surgery, minor postoperative complications, and longer operative time. The GRECCAR-6 trial delivered similar results for the 11-week interval, with mostly mild medical complications (44.5% vs 32%; P = .04) compared with the 7-week interval.13 Worse quality of mesorectal resection was found in the 11-week interval group13 (complete mesorectum 78.7% vs 90%; P = .01), similar to our data. All these indicators suggest higher surgical complexity due to radiation-induced pelvic fibrosis and/or mesorectal fragility. Interestingly, the worse mesorectal plane of resection found in the long-interval groups in both the GRECCAR-6 trial and our study did not result in a higher locoregional recurrence rate, with similar follow-up periods. As indicated by Quirke et al,33,34 the surgical plane is a strong prognostic factor for local relapse. However, in their study, only 42% of the patients had been treated with NAT, compared with all patients in both the GRECCAR-6 trial and our study. Further investigation of risk factors for local recurrence in patients treated with NAT and delayed surgery is warranted. Nevertheless, until future evidence suggests otherwise, mesorectal quality should continue to be considered of the greatest importance in all patients undergoing surgery for rectal cancer.
Lastly, assessment of tumor response is advisable, even if radical resection is the planned strategy. An adequate time frame for performing tumor restaging after NAT should be determined. Our results showed poor concordance between MRI findings and pathologic outcomes, probably because tumor restaging was not an extended practice among different centers throughout the years, and when performed, the timing was heterogeneous. Moreover, most patients were restaged with standard T2-weighted MRI, which has difficulties in distinguishing fibrosis from viable tumor.35 Despite this, different studies support the role of MRI as one of the best tools to assess tumor response, particularly when diffusion-weighted imaging techniques are implemented.36 Currently, in the new paradigm of rectal cancer treatment with organ-sparing strategies at the top, efforts should be directed to improve tumor response evaluation, taking special care of the appropriate time for restaging, and ultimately to describe noninvasive biomarkers that can improve diagnostic accuracy.
Strengths and Limitations
Our study has several strengths, including the large number of patients representative of routine clinical practice among Spanish tertiary referral hospitals. We used statistical techniques to avoid allocation bias and the influence of confounders in the database. The technique used is based on the IPTW calculation, which retains most participants for analysis, unlike matching techniques. Overall, these results are comparable with those obtained in a randomized clinical trial.37 However, despite our thorough corrections, the presence of hidden confounders, such as genetic alterations, cannot be excluded. The main limitation of this study was its retrospective nature, which could result in missing or inconsistent data, leading to potential bias. Additionally, due to the lack of consensus over time, the interval depended much more on teams’ preferences than on specific tumor characteristics. Therefore, the patients may have been assigned to a wide variety of intervals. This allocation bias increases with heterogeneity inherent to the different participating centers in the Spanish region. Another limitation is the relatively short follow-up period, which might not have captured the late recurrences.
Conclusions
In this cohort study, intervals greater than 12 weeks were associated with improved TRG and reduced risk of systemic recurrence. However, this did not translate into better OS. Furthermore, waiting for longer intervals may lead to more difficult surgical resection and higher minor morbidity.
eTable 1. Concordance Between MRI and Histopathological Outcomes
eTable 2. Operative Time
Data Sharing Statement
References
- 1.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457-460. doi: 10.1016/0140-6736(93)90207-W [DOI] [PubMed] [Google Scholar]
- 2.Rombouts AJM, Hugen N, Elferink MAG, Nagtegaal ID, de Wilt JHW. Treatment interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients: a population-based study. Ann Surg Oncol. 2016;23(11):3593-3601. doi: 10.1245/s10434-016-5294-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg. 2016;263(3):458-464. doi: 10.1097/SLA.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 4.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241(5):829-836. doi: 10.1097/01.sla.0000161980.46459.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23(34):8688-8696. doi: 10.1200/JCO.2005.02.1329 [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711-717. doi: 10.1097/01.sla.0000141194.27992.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hupkens BJP, Maas M, Martens MH, et al. Organ preservation in rectal cancer after chemoradiation: should we extend the observation period in patients with a clinical near-complete response? Ann Surg Oncol. 2018;25(1):197-203. doi: 10.1245/s10434-017-6213-8 [DOI] [PubMed] [Google Scholar]
- 8.Detering R, Borstlap WAA, Broeders L, et al. ; Dutch Snapshot Research Group . Cross-sectional study on MRI restaging after chemoradiotherapy and interval to surgery in rectal cancer: influence on short- and long-term outcomes. Ann Surg Oncol. 2019;26(2):437-448. doi: 10.1245/s10434-018-07097-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32(15):1554-1562. doi: 10.1200/JCO.2013.54.3769 [DOI] [PubMed] [Google Scholar]
- 10.Zorcolo L, Rosman AS, Restivo A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19(9):2822-2832. doi: 10.1245/s10434-011-2209-y [DOI] [PubMed] [Google Scholar]
- 11.Jeong DH, Lee HB, Hur H, Min BS, Baik SH, Kim NK. Optimal timing of surgery after neoadjuvant chemoradiation therapy in locally advanced rectal cancer. J Korean Surg Soc. 2013;84(6):338-345. doi: 10.4174/jkss.2013.84.6.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):582-589. doi: 10.1097/SLA.0b013e3181b91e63 [DOI] [PubMed] [Google Scholar]
- 13.Lefevre JH, Mineur L, Kotti S, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. 2016;34(31):3773-3780. doi: 10.1200/JCO.2016.67.6049 [DOI] [PubMed] [Google Scholar]
- 14.Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5(4):e185896. doi: 10.1001/jamaoncol.2018.5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Valk MJM, Hilling DE, Bastiaannet E, et al. ; IWWD Consortium . Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537-2545. doi: 10.1016/S0140-6736(18)31078-X [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 17.Terzi C, Bingul M, Arslan NC, et al. Randomized controlled trial of 8 weeks’ vs 12 weeks’ interval between neoadjuvant chemoradiotherapy and surgery for locally advanced rectal cancer. Colorectal Dis. 2020;22(3):279-288. doi: 10.1111/codi.14867 [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396. doi: 10.1200/JCO.1999.17.8.2396 [DOI] [PubMed] [Google Scholar]
- 22.Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol). 2013;25(10):578-585. doi: 10.1016/j.clon.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 23.Dhadda AS, Zaitoun AM, Bessell EM. Regression of rectal cancer with radiotherapy with or without concurrent capecitabine–optimising the timing of surgical resection. Clin Oncol (R Coll Radiol). 2009;21(1):23-31. doi: 10.1016/j.clon.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 24.Ryan ÉJ, O’Sullivan DP, Kelly ME, et al. Meta-analysis of the effect of extending the interval after long-course chemoradiotherapy before surgery in locally advanced rectal cancer. Br J Surg. 2019;106(10):1298-1310. doi: 10.1002/bjs.11220 [DOI] [PubMed] [Google Scholar]
- 25.Mihmanlı M, Kabul Gürbulak E, Akgün İE, et al. Delaying surgery after neoadjuvant chemoradiotherapy improves prognosis of rectal cancer. World J Gastrointest Oncol. 2016;8(9):695-706. doi: 10.4251/wjgo.v8.i9.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835-844. doi: 10.1016/S1470-2045(10)70172-8 [DOI] [PubMed] [Google Scholar]
- 27.Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56(7):921-930. doi: 10.1097/DCR.0b013e31828aedcb [DOI] [PubMed] [Google Scholar]
- 28.Cotte E, Passot G, Decullier E, et al. Pathologic response, when increased by longer interval, is a marker but not the cause of good prognosis in rectal cancer: 17-year Follow-up of the Lyon R90-01 randomized trial. Int J Radiat Oncol Biol Phys. 2016;94(3):544-553. doi: 10.1016/j.ijrobp.2015.10.061 [DOI] [PubMed] [Google Scholar]
- 29.Lefèvre JH, Mineur L, Cachanado M, et al. ; The French Research Group of Rectal Cancer Surgery (GRECCAR) . Does a longer waiting period after neoadjuvant radiochemotherapy improve the oncological prognosis of rectal cancer—3 years’ follow-up results of the GRECCAR-6 randomized multicenter trial. Ann Surg. 2019;270(5):747-754. doi: 10.1097/SLA.0000000000003530 [DOI] [PubMed] [Google Scholar]
- 30.Gambacorta MA, Masciocchi C, Chiloiro G, et al. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials. Radiother Oncol. 2021;154:154-160. doi: 10.1016/j.radonc.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 31.Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, Barni S. Pathologic complete response and disease-free survival are not surrogate end points for 5-year survival in rectal cancer: an analysis of 22 randomized trials. J Gastrointest Oncol. 2017;8(1):39-48. doi: 10.21037/jgo.2016.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389-1398. doi: 10.1001/jamainternmed.2015.2829 [DOI] [PubMed] [Google Scholar]
- 33.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection—histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2(8514):996-999. doi: 10.1016/S0140-6736(86)92612-7 [DOI] [PubMed] [Google Scholar]
- 34.Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH; Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group . Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20(7):1729-1734. doi: 10.1200/JCO.2002.07.010 [DOI] [PubMed] [Google Scholar]
- 35.Napoletano M, Mazzucca D, Prosperi E, et al. Locally advanced rectal cancer: qualitative and quantitative evaluation of diffusion-weighted magnetic resonance imaging in restaging after neoadjuvant chemoradiotherapy. Abdom Radiol (NY). 2019;44(11):3664-3673. doi: 10.1007/s00261-019-02012-4 [DOI] [PubMed] [Google Scholar]
- 36.Maas M, Lambregts DM, Nelemans PJ, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol. 2015;22(12):3873-3880. doi: 10.1245/s10434-015-4687-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres F, Ríos J, Saez-Peñataro J, Pontes C. Is propensity score analysis a valid surrogate of randomization for the avoidance of allocation bias? Semin Liver Dis. 2017;37(3):275-286. doi: 10.1055/s-0037-1606213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Concordance Between MRI and Histopathological Outcomes
eTable 2. Operative Time
Data Sharing Statement