Key Points
Question
What is the feasibility and clinical utility of creating simple and highly accurate prediction models for breast cancer–related lymphedema that incorporate known racial differences in disease incidence?
Findings
In this prognostic study including 1882 patients, preoperative and postoperative prediction models were developed that include basic, patient-facing data points with area under the receiver operating characteristic curve values of 0.78 and 0.86, respectively, and accuracy of 73.0% and 81.1% at optimal probability thresholds.
Meaning
In this study, the preoperative model accurately targeted high-risk patients for perioperative risk reduction measures, while the postoperative model allowed for screening patients without limb volume measurements and repeated clinic visits.
This prognostic study evaluates prediction models for breast cancer–related lymphedema (BCRL) that can be used to estimate preoperative or postoperative risk.
Abstract
Importance
Breast cancer–related lymphedema (BCRL) is a common complication of axillary lymph node dissection (ALND) but can also develop after sentinel lymph node biopsy (SLNB). Several models have been developed to predict the risk of disease development before and after surgery; however, these models have shortcomings that include the omission of race, inclusion of variables that are not readily available to patients, low sensitivity or specificity, and lack of risk assessment for patients treated with SLNB.
Objective
To create simple and accurate prediction models for BCRL that can be used to estimate preoperative or postoperative risk.
Design, Setting, and Participants
In this prognostic study, women with breast cancer who underwent ALND or SLNB from 1999 to 2020 at Memorial Sloan Kettering Cancer Center and the Mayo Clinic were included. Data were analyzed from September to December 2022.
Main Outcomes and Measures
Diagnosis of lymphedema based on measurements. Two predictive models were formulated via logistic regression: a preoperative model (model 1) and a postoperative model (model 2). Model 1 was externally validated using a cohort of 34 438 patients with an International Classification of Diseases diagnosis of breast cancer.
Results
Of 1882 included patients, all were female, and the mean (SD) age was 55.6 (12.2) years; 80 patients (4.3%) were Asian, 190 (10.1%) were Black, 1558 (82.8%) were White, and 54 (2.9%) were another race (including American Indian and Alaska Native, other race, patient refused to disclose, or unknown). A total of 218 patients (11.6%) were diagnosed with BCRL at a mean (SD) follow-up of 3.9 (1.8) years. The BCRL rate was significantly higher among Black women (42 of 190 [22.1%]) compared with all other races (Asian, 10 of 80 [12.5%]; White, 158 of 1558 [10.1%]; other race, 8 of 54 [14.8%]; P < .001). Model 1 included age, weight, height, race, ALND/SLNB status, any radiation therapy, and any chemotherapy. Model 2 included age, weight, race, ALND/SLNB status, any chemotherapy, and patient-reported arm swelling. Accuracy was 73.0% for model 1 (sensitivity, 76.6%; specificity, 72.5%; area under the receiver operating characteristic curve [AUC], 0.78; 95% CI, 0.75-0.81) at a cutoff of 0.18, and accuracy was 81.1% for model 2 (sensitivity, 78.0%; specificity, 81.5%; AUC, 0.86; 95% CI, 0.83-0.88) at a cutoff of 0.10. Both models demonstrated high AUCs on external (model 1: 0.75; 95% CI, 0.74-0.76) or internal (model 2: 0.82; 95% CI, 0.79-0.85) validation.
Conclusions and Relevance
In this study, preoperative and postoperative prediction models for BCRL were highly accurate and clinically relevant tools comprised of accessible inputs and underscored the effects of racial differences on BCRL risk. The preoperative model identified high-risk patients who require close monitoring or preventative measures. The postoperative model can be used for screening of high-risk patients, thus decreasing the need for frequent clinic visits and arm volume measurements.
Introduction
Breast cancer–related lymphedema (BCRL) is a common condition that lacks a widely used predictive framework to identify women who are at high risk. Female breast cancer is the most frequently diagnosed cancer worldwide, with 2.3 million new cases annually, including 300 000 in the US alone.1,2 Pooled estimates suggest that approximately 16.6% of women develop lymphedema following breast cancer treatment,3 with women of Black race disproportionately affected.4 Similar to hypertension, cardiovascular disease, and other diseases with substantial global burden and the potential to develop into chronic conditions,5,6 BCRL prediction is critical to identifying at-risk patients and instituting preventive strategies.
Several studies indicate that early diagnosis and management of BCRL can prevent the development of chronic lymphedema and its associated physical and economic costs.7,8 Close monitoring of high-risk women, use of compression garments in the immediate postoperative period, and prompt intervention with complex decongestive therapy have all been shown to slow the progression of early-stage disease.7,9,10,11 BCRL imposes an increasingly heavy toll on patients, health care professionals, and health care systems as severity of disease worsens. Women who reach later stages of disease are at higher risk of cellulitis, functional impairment, physical disfigurement, and lack of response to treatment.12,13 Accordingly, these women exhibit diminished health-related quality of life,14,15 which is felt most acutely among the Black population.16 Overall, women with late-stage BCRL consume more health care resources and represent a larger cost to the health care system17; the annual cost of managing patients with late-stage BCRL is nearly 5-fold the cost of patients with early-stage disease.18
Multiple predictive models for BCRL have appeared in the literature over the past 2 decades, although to our knowledge, none have gained widespread acceptance. Importantly, despite recent studies demonstrating the effects of racial disparities in lymphedema,4,19 no previously published model includes race as a predictive factor. Some models have favorable predictive ability, as measured by the area under the receiver operating characteristic curve (AUC), but include variables that are not readily available in a preoperative and/or postoperative setting; these variables include radiation field and fractionation type,20,21,22,23 number of cycles of chemotherapy in the ipsilateral arm,20 chemotherapeutic agent,23,24 proportion of lymph flow above the level of the axillary vein,24 mammographic breast density,25 and number of lymph nodes removed or positive nodes.21,22,23,25,26 Many models neglect or have a low AUC on external validation.7,20,21,25,26,27,28,29,30,31 An additional problem is the omission of sentinel lymph node biopsy (SLNB) from nearly all models, despite de-escalation of axillary surgery following the ACOSOG Z0011 and AMAROS trials,32,33 increasing rates of SLNB,34 and an approximate 5% to 6% rate of lymphedema after SLNB.3 Finally, some models define lymphedema based on patient-reported symptoms21,23; this may obscure BCRL diagnosis, since these symptoms may reflect nerve injury rather than lymphedema.
The purpose of this study was to leverage data from a multi-institutional experience with breast cancer and lymphedema to develop simple and highly accurate prediction models for BCRL, with particular attention to racial differences. We used clinically relevant, patient-facing variables to inform models with the preoperative objective of predicting risk, and the postoperative objective of screening patients without limb volume measurements and repeated clinic visits.
Methods
Study Design and Data Collection
This prospective cohort study was conducted at 2 institutions: the Memorial Sloan Kettering Cancer Center (MSKCC) and Mayo Clinic. The MSKCC cohort was composed of 2 previously published data sets of women with breast cancer who underwent SLNB, axillary lymph node dissection (ALND), or SLNB followed by ALND: 936 women from 1999 to 2003 (data set 1)35,36 and 268 women from 2016 to 2020 (data set 2).4 The Mayo Clinic cohort (data set 3) added 678 patients with breast cancer treated with ALND and/or SLNB from 2008 to 2021. The MSKCC and Mayo Clinic institutional review boards approved this study, and written informed consent was obtained from all patients. Data were analyzed from September to December 2022.
Data collected encompassed demographic and clinical information and patient-reported outcomes. Demographic variables included self-reported race, height, weight, and age; the latter 3 variables were recorded both at the time of surgery and at final follow-up. Clinical variables included type of breast surgery (lumpectomy or mastectomy), lymphadenectomy (SLNB or ALND), number of nodes removed, number of positive nodes, any radiation therapy, neoadjuvant chemotherapy, any chemotherapy, pathological tumor size, final T stage, and final N stage. Patients who underwent both SLNB and ALND were exclusively counted in the ALND group. Patients’ answers to the questions, “Do you have arm swelling now?” and “Have you had any infections in your arm?” at follow-up visits were also noted. Follow-up time in years was recorded. The primary outcome measure was development of lymphedema, defined as either a more than 2-cm difference in arm circumference 10 cm above or 5 cm below the olecranon (data set 1) or a relative volume change of 10% or greater based on perometer (data set 2) or circumferential arm measurements at 4-cm intervals along the length of the extremity (data set 3), all compared with the contralateral arm and adjusting for baseline measurements.
Statistical Analysis
Demographic and clinical characteristics were summarized descriptively using means and SDs for continuous variables and counts and percentages for categorical variables. Characteristics were compared between patients who developed lymphedema and those who did not using Wilcoxon rank sum tests for continuous variables, as Shapiro-Wilk tests indicated nonnormal distributions. Categorical data were compared using χ2tests or Fisher exact tests for variables with a single cell count fewer than 10. Univariable and multivariable logistic regression were used to model associations of demographic and clinical variables with the development of lymphedema. Receiver operating characteristic curves were plotted for combinations of clinically meaningful variables; the combination of preoperative variables that optimized the AUC was designated the preoperative model (model 1). The combination of variables in the postoperative setting that optimized the AUC was designated the postoperative model (model 2). Adjusted predicted margins at means and average marginal effects were calculated to estimate predicted probabilities for categorical and continuous variables, respectively.
For each model, goodness of fit was quantified via several measures in addition to AUC, including pseudo R2 (Tjur, McFadden, Cox-Snell, or Nagelkerke), Brier score, and Hosmer-Lemeshow test.37 The Youden index (J) was computed for each model,38 with 95% CIs generated by bootstrapping with 100 replications. A probability cutoff was selected based on consideration of the maximum J value in addition to the desired features of the model as a clinical test. Calibration plots modeled observed and predicted values for risk of lymphedema for each model.39 A sensitivity analysis was performed using Bayesian logistic regression given use of Bayes theorem for construction of alternative predictive models,7,37 where model coefficients and performance metrics were compared with those of the standard logistic model.
External (ie, independent) validation of model 1 was performed using a data set of all patients at MSKCC who underwent SLNB or ALND for breast cancer from 1995 to 2021 with or without an International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10 diagnosis of BCRL (postmastectomy lymphedema syndrome; ICD-9 code 457.0; ICD-10 code I97.2). This data set was constructed by retrospective query of electronic medical records using a modification of the methods reported by Basta et al.28 Internal validation of model 2 was achieved using a 10-fold cross validation with bootstrap-corrected 95% CIs.40 Analyses were conducted using Stata/SE version 17.0 (StataCorp) and Prism version 9.4.1 (GraphPad Software). Two-tailed P values less than .05 were considered statistically significant.
Results
Of 1882 included patients (1204 from MSKCC and 678 from Mayo Clinic), all were female, and the mean (SD) age was 55.6 (12.2) years; 80 patients (4.3%) were Asian, 190 (10.1%) were Black, 1558 (82.8%) were White, and 54 (2.9%) were another race (including American Indian and Alaska Native, other race, patient refused to disclose, or unknown). A total of 218 patients (11.6%) were diagnosed with BCRL at a mean (SD) follow-up of 3.9 (1.8) years (Table 1). A total of 819 patients (43.5%) underwent ALND (22.7% rate of BCRL [186 of 819]) and 1063 (56.5%) underwent SLNB (3.0% rate of BCRL [32 of 1063]). The lymphedema rate was 22.1% (42 of 190) for Black women compared with 12.5% (10 of 80) for Asian women, 10.1% (158 of 1558) for White women, and 14.8% (8 of 54) for women of another race (P < .001). Based on the multivariable model, the odds of developing lymphedema were 6.12-fold (95% CI, 3.45-10.85) as great for patients who underwent ALND compared with SLNB (P < .001) and 1.85-fold (95% CI, 1.20-2.86) greater for Black patients compared with White patients (P = .005) (eTable 1 in Supplement 1).
Table 1. Patient Demographic and Clinical Data for Study Cohort With Univariable Comparison by Lymphedema Diagnosis.
| Variable | No. (%) | Univariable P value | ||
|---|---|---|---|---|
| Overall (N = 1882) | No lymphedema (n = 1664) | Lymphedema (n = 218) | ||
| Age at surgery, mean (SD), y | 55.6 (12.2) | 55.7 (12.2) | 54.3 (12.1) | .08 |
| Height, mean (SD), cm | 162.7 (6.8) | 162.7 (6.8) | 162.6 (6.8) | .02 |
| Weight at surgery, mean (SD), kg | 71.0 (16.6) | 70.5 (16.4) | 75.0 (17.5) | <.001 |
| Body mass index at surgery, mean (SD)a | 26.8 (6.1) | 26.6 (6.0) | 28.4 (6.3) | <.001 |
| Weight change after surgery, mean (SD), kg | 1.1 (6.6) | 1.2 (6.4) | 1.0 (7.7) | .37 |
| Follow up, mean (SD), y | 3.9 (1.8) | 4.0 (1.7) | 3.8 (1.7) | .22 |
| Raceb | ||||
| Asian | 80 (4.3) | 70 (87.5) | 10 (12.5) | <.001 |
| Black | 190 (10.1) | 148 (77.9) | 42 (22.1) | |
| White | 1558 (82.8) | 1400 (89.9) | 158 (10.1) | |
| Other race | 54 (2.9) | 46 (85.2) | 8 (14.8) | |
| Breast surgery | ||||
| Mastectomy | 835 (55.6) | 706 (84.6) | 129 (15.5) | <.001 |
| BCT | 1047 (44.4) | 958 (91.5) | 89 (8.5) | |
| Lymphadenectomy | ||||
| ALND | 819 (43.5) | 633 (77.3) | 186 (22.7) | <.001 |
| SLNB | 1063 (56.5) | 1031 (97.0) | 32 (3.0) | |
| LNs removed, mean (SD) | 10.6 (10.0) | 9.5 (9.4) | 18.6 (10.2) | <.001 |
| Positive LNs, mean (SD) | 1.4 (3.8) | 1.2 (3.5) | 3.1 (5.0) | <.001 |
| Tumor size, mean (SD), cm | 1.8 (1.7) | 1.7 (1.6) | 2.4 (2.3) | <.001 |
| T stage | ||||
| T0 | 128 (6.8) | 122 (95.3) | 6 (4.7) | <.001 |
| T1 | 1105 (58.7) | 1012 (91.6) | 93 (8.4) | |
| T2 | 455 (24.2) | 378 (83.1) | 77 (28.4) | |
| T3 | 95 (5.1) | 68 (71.6) | 27 (28.4) | |
| T4 | 49 (2.6) | 42 (85.7) | 7 (14.3) | |
| NA | 50 (2.7) | 42 (84.0) | 8 (16.0) | |
| N stage | ||||
| N0 | 1130 (60.0) | 1079 (95.5) | 51 (4.5) | <.001 |
| N1 | 546 (29.0) | 436 (79.9) | 110 (20.2) | |
| N2 | 102 (5.4) | 74 (72.6) | 28 (27.5) | |
| N3 | 75 (4.0) | 52 (69.3) | 23 (30.7) | |
| NA | 29 (1.5) | 23 (79.3) | 6 (20.7) | |
| Any RT | 1412 (75.0) | 1232 (87.3) | 180 (12.8) | .006 |
| Any chemotherapy | 1226 (65.1) | 1034 (84.3) | 192 (15.7) | <.001 |
| Neoadjuvant chemotherapy | 391 (20.8) | 302 (77.2) | 89 (22.8) | <.001 |
| Current swelling | ||||
| Yes | 315 (16.7) | 168 (53.3) | 147 (46.7) | <.001 |
| No | 1468 (78.0) | 1403 (95.6) | 65 (4.4) | |
| Unknown | 99 (5.3) | 93 (93.9) | 6 (6.1) | |
| Previous infections | ||||
| Yes | 70 (3.7) | 53 (75.7) | 17 (24.3) | <.001 |
| No | 1704 (90.5) | 1508 (88.5) | 196 (11.5) | |
| Unknown | 108 (5.7) | 103 (95.4) | 5 (4.6) | |
Abbreviations: ALND, axillary lymph node dissection; BCT, breast-conserving therapy; LN, lymph node; NA, not available; RT, radiation therapy; SLNB, sentinel lymph node biopsy.
Calculated as weight in kilograms divided by height in meters squared.
Race data were self-reported. The other race category included American Indian and Alaska Native, other race, patient refused to disclose, or unknown.
Preoperative (model 1) and postoperative (model 2) logistic models are shown in Table 2 (eFigures 1 and 2 in Supplement 1). Receiver operating characteristic curves for both models are shown in Figure 1A. The AUC was 0.78 (95% CI, 0.75-0.81) for model 1 and 0.86 (95% CI, 0.83-0.88) for model 2. Figure 1B shows additional goodness of fit and pseudo R2 values. Adding the data set of origin as a variable in the models did not sufficiently alter the AUC to warrant inclusion.
Table 2. Preoperative and Postoperative Model Statistics.
| Variable | OR (95% CI) | P value | Measure, % |
|---|---|---|---|
| Preoperative model (model 1) | |||
| Baseline age (per 1-y increase) | 1.01 (1.00-1.02) | .13 | MEM, 0.1 |
| Baseline weight (per 1-kg increase) | 1.01 (1.00-1.02) | .009 | MEM, 0.1a |
| Height (per 1-cm increase) | 0.98 (0.96-1.00) | .10 | MEM, −0.1 |
| Raceb | |||
| Asian | 0.99 (0.48-2.06) | .99 | APM, 6.9a |
| Black | 1.53 (1.01-2.32) | .047 | APM, 10.2a |
| White | 1 [Reference] | NA | APM, 6.9a |
| Other race | 1.11 (0.49-2.51) | .80 | APM, 7.6a |
| Lymphadenectomy | |||
| SLNB | 1 [Reference] | NA | APM, 3.1a |
| ALND | 7.89 (5.14-12.10) | <.001 | APM, 20.0a |
| Any radiation therapy | |||
| No | 1 [Reference] | NA | APM, 6.5a |
| Yes | 1.17 (0.79-1.73) | .43 | APM, 7.5a |
| Any chemotherapy | |||
| No | 1 [Reference] | NA | APM, 5.3a |
| Yes | 1.66 (1.03-2.68) | .04 | APM, 8.5a |
| Postoperative model (model 2) | |||
| Current age (per 1-y increase) | 1.02 (1.01-1.04) | .002 | MEM, 0.1a |
| Current weight (per 1-kg increase) | 1.00 (0.99-1.01) | .50 | MEM, 0 |
| Raceb | |||
| Asian | 1.22 (0.54-2.72) | .63 | APM, 6.9a |
| Black | 1.26 (0.79-2.00) | .34 | APM, 7.1a |
| White | 1 [Reference] | NA | APM, 5.7a |
| Other race | 0.94 (0.39-2.29) | .89 | APM, 5.4a |
| Lymphadenectomy | |||
| SLNB | 1 [Reference] | NA | APM, 3.4a |
| ALND | 3.62 (2.25-5.83) | <.001 | APM, 11.4a |
| Any chemotherapy | |||
| No | 1 [Reference] | NA | APM, 4.2a |
| Yes | 1.68 (1.01-2.81) | .047 | APM, 6.9a |
| Any swelling | |||
| No | 1 [Reference] | NA | APM, 4.0a |
| Yes | 10.85 (7.48-15.74) | <.001 | APM, 31.0a |
| Unknown | 1.14 (0.47-2.76) | .77 | APM, 4.5a |
Abbreviations: ALND, axillary lymph node dissection; APM, adjusted predictive margin; MEM, marginal effect at the mean; SLNB, sentinel lymph node biopsy.
P < .05.
Race data were self-reported. The other race category included American Indian and Alaska Native, other race, patient refused to disclose, or unknown.
Figure 1. Receiver Operating Characteristic (ROC) Curves and Performance Metrics for the Preoperative (Model 1) and Postoperative (Model 2) Models.
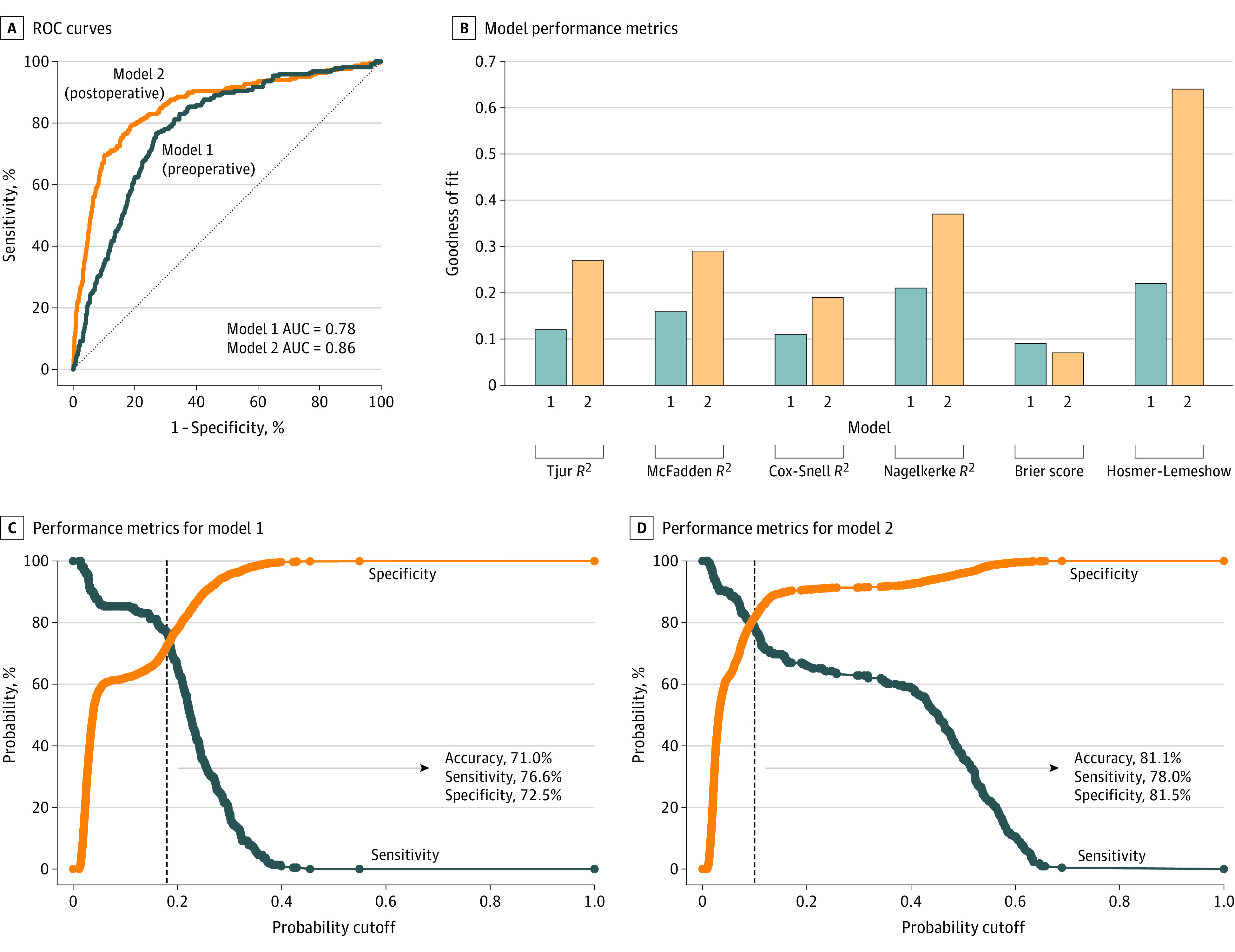
A, ROC curves for model 1 and model 2. B, Goodness of fit and pseudo R2 values for both models. R2 measures differ based on underlying method of calculation but are interpreted similarly; R2 represents the amount of variance explained by the model, where a higher R2 value indicates a stronger model. Brier score is the mean squared error, where a lower score indicates a better model. The Hosmer-Lemeshow test yields a P value representing whether predicted and observed values of a logistic regression are concordant; small values (typically less than 0.05, depending on the significance level) are unfavorable. C, Performance metrics as a function of probability cutoff for model 1. D, Performance metrics as a function of probability cutoff for model 2. Dashed black lines mark the optimal cutoff based on the Youden index (J; model 1, 0.18; model 2, 0.10). The Youden index is a method of assessing the performance of a diagnostic/prognostic test, where J = sensitivity + (specificity − 1). AUC indicates area under the ROC curve.
Sensitivity and specificity of each model as a function of probability cutoffs are illustrated in Figure 1C and D. The optimal cutoff was 0.18 (95% CI, 0.16-0.20; P < .001) for model 1 and 0.10 (95% CI, 0.07-0.13; P < .001) for model 2. At these cutoffs, the negative predictive value (NPV) was 96.0% and 95.6% for models 1 and 2, respectively. Bayesian model coefficients and performance metrics were consistent with those based on logistic regression (eTable 2 in Supplement 1). Figure 2A and B shows calibration plots of observed vs expected values for both models. Model nomograms are displayed in Figure 3.
Figure 2. Calibration and Validation Receiver Operating Characteristic (ROC) Plots.
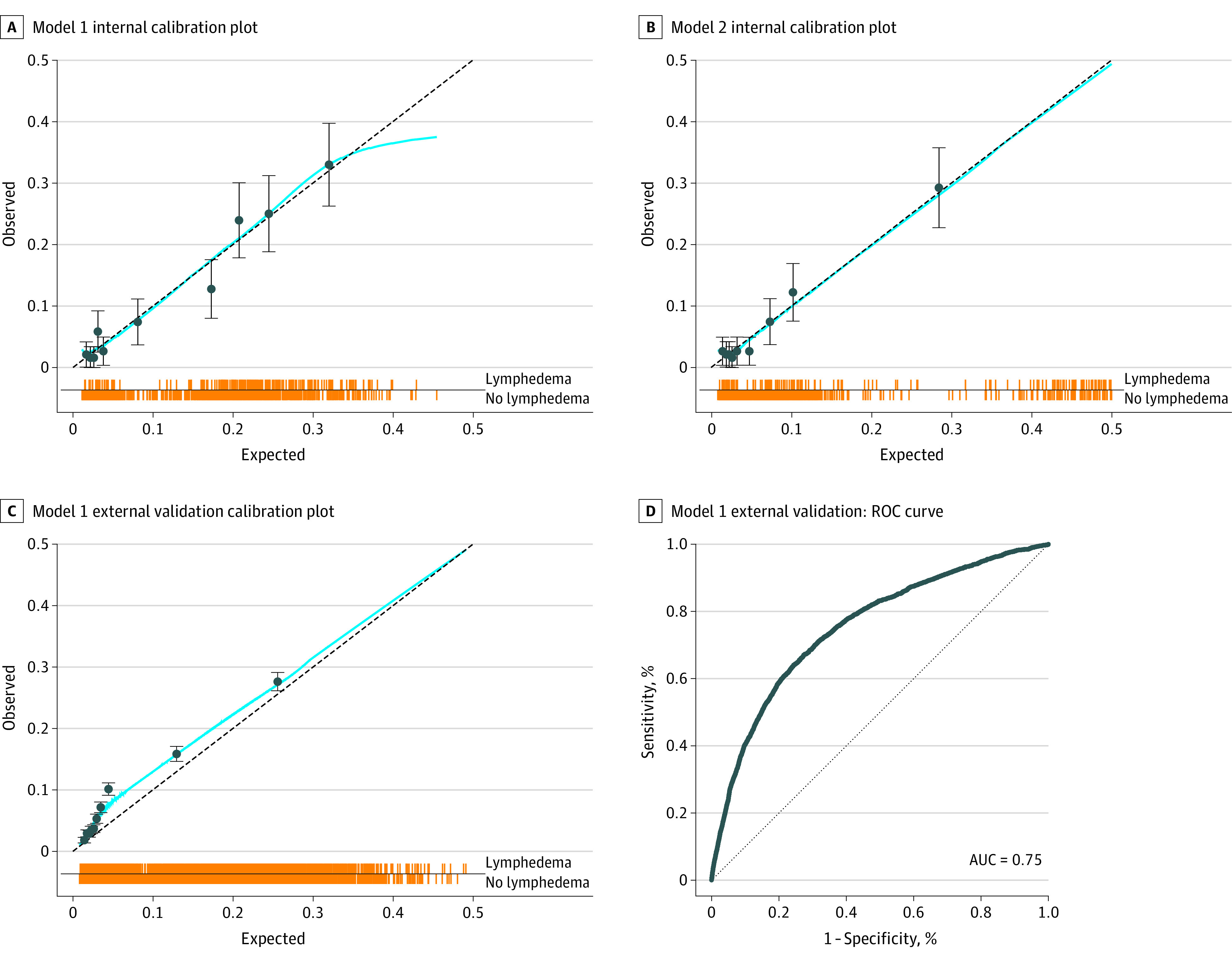
A, Calibration plot of observed vs expected values for model 1. B, Calibration plot for model 2. C, Calibration plot for model 1 external validation with the Memorial Sloan Kettering Cancer Center International Classification of Diseases data set. D, Receiver operating characteristic (ROC) curve for model 1 external validation with the Memorial Sloan Kettering Cancer Center International Classification of Diseases data set. Each line of the spike plots directly above the x-axis represents an individual data point. Data are subdivided into 10 groups based on deciles within each calibration plot. Error bars indicate 95% CIs. AUC indicates area under the ROC curve.
Figure 3. Nomograms for Preoperative and Postoperative Breast Cancer–Related Lymphedema Prediction Models.
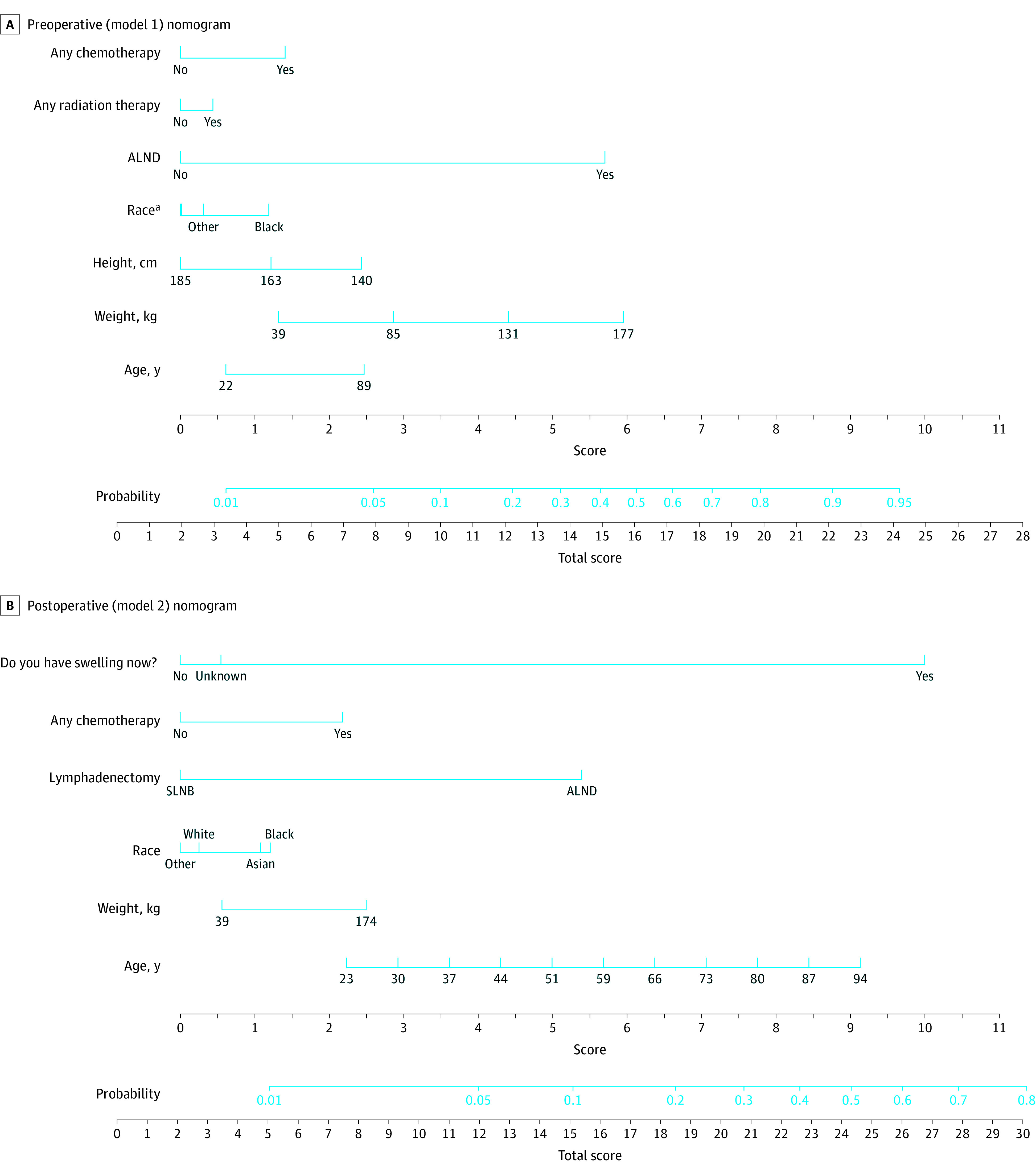
ALND indicates axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
aWhite race is counted as 0 points and Asian race as 0.3 points. The other race category includes American Indian and Alaska Native, other race, patient refused to disclose, or unknown.
The external validation cohort for model 1 consisted of 34 438 MSKCC patients who underwent breast cancer treatment including SLNB or ALND with a mean (SD) follow up of 6.2 (5.0) years. The BCRL rate was 8.1% (eTable 3 in Supplement 1). The calibration plot is shown Figure 2C. The AUC was 0.75 (95% CI, 0.74-0.76) (Figure 2D). Performance metrics for external validation of model 1 at a cutoff of 0.18 were 86.5% for accuracy, 38.7% for sensitivity, 90.7% for specificity, and 94.4% for NPV. Internal validation of model 2 yielded a cross-validated mean AUC of 0.82 (95% CI, 0.79-0.85) (eFigure 3 in Supplement 1).
Discussion
In this study, we developed and validated clinically relevant, patient-facing prediction models for BCRL that incorporate the racial differences of nearly 2000 patients. Both the preoperative and postoperative models demonstrated high discriminative ability and specificity, reflecting high model sensitivity and a high likelihood that patients with negative predictions did not develop lymphedema. The model 1 AUC of 0.78 (95% CI, 0.75-0.81; AUC of 0.75 [95% CI, 0.74-0.76] on external validation) and model 2 AUC of 0.86 (95% CI, 0.83-0.88) compare favorably with AUCs for prior models that range from 0.68 to 0.89 based on training or internal validation and 0.60 to 0.80 on external validation. For the preoperative model, we used 18% as a cutoff for lymphedema as a binary variable; patients who scored higher than 18% were predicted to have lymphedema and patients who scored lower than 18% were predicted to not have lymphedema. Using this cutoff, the model accuracy was 73.0% (86.5% on external validation). Similarly, the postoperative model identified patients who scored higher than a 10% risk as having lymphedema; the accuracy of the model at this cutoff was 81.1%. Patients who screen positive on either model should be targeted for perioperative risk reduction measures, while those that screen positive on the postoperative model can also be called in for further evaluation using arm measurements or bioimpedance.
At least 13 BCRL predictive models have been published (eTable 4 in Supplement 1),21,22,23,24,25,26,27,29,30,31,41,42,43,44,45 although all have at least 1 conceptual issue that inhibits functionality. In addition to inclusion of data points that are not readily available, omission of SLNB, and diagnoses based on patient-reported symptoms, some studies define lymphedema based on retrospective identification via ICD-9 or ICD-10 diagnosis codes28 or treatment associated with lymphedema,30 both of which introduce bias. Specifically, models based on ICD-defined cohorts of patients with lymphedema may include patients with mild or subclinical lymphedema; this may explain the relatively low sensitivity of our preoperative model (38.7%)—a model that was trained on a cohort with a more stringent definition of lymphedema—when validated with an ICD-based cohort.
Variables in our models have established associations with BCRL and are included within the models in a simplified and patient-friendly form. Age and high body mass index have consistently been associated with greater BCRL risk3,4,20,22,25,26,28,31,46,47,48; in our model, height and weight were included as independent inputs to avoid the need for body mass index calculation. Our model includes any (neoadjuvant or adjuvant) chemotherapy in addition to any radiation therapy, since both have been associated with BCRL risk in prospective cohort studies.3 Despite strong evidence that neoadjuvant therapy alone is associated with BCRL risk,4 this variable did not improve the accuracy of our model, potentially due to exclusion bias as neoadjuvant chemotherapy was rarely used during the study period of the 1999 to 2003 MSKCC cohort (data set 1). ALND was the strongest predictor in our preoperative model with a predicted probability of 20.0%, consistent with Level I evidence showing a nearly 4-fold greater risk of lymphedema following ALND vs SLNB.3 ALND was also strongly correlated with the number of lymph nodes removed, thus obviating the need for including this as a variable in our model. Lastly, self-reported arm swelling was the strongest predictor in our postoperative model, with a predicted probability of 31.0%, in line with prior evidence of an association between lymphedema and postoperative edema.20,29 Of note, not all patients who reported arm swelling had lymphedema, underscoring the importance of objective criteria for lymphedema diagnosis in place of patient-reported symptoms that may reflect perioperative nerve injury.
To our knowledge, race has not yet been included in any BCRL predictive model, despite emerging evidence that Black women have an elevated BCRL risk. Studies that base lymphedema diagnosis on patients’ self-reported arm swelling have found both insignificant associations49,50 and positive associations51,52 between Black race and lymphedema risk, although these findings are questionable given aforementioned reservations about subjective diagnoses. Using diagnosis codes to identify cases of lymphedema, Black et al19 demonstrated that Black patients have higher rates of ALND vs SLNB and higher rates of BCRL following ALND compared with White patients. Our group has similarly shown that Black race and Hispanic ethnicity are independent risk factors for BCRL on multivariable analysis.4 In this study, we found that the average Black woman treated for breast cancer had a 10.2% risk of lymphedema compared with a 6.9% risk for the average White woman based on our preoperative model, with a similarly significant increase in risk based on our postoperative model. The mechanism of increased risk among Black women remains unknown, with hypothesized relations to biological factors, such as dysregulated scarring and connective tissue overgrowth,53 and irregular inflammatory pathways.54 Differential BCRL incidence among racial groups may also be a downstream effect of disparities in health care quality and access.55 Additional work is needed to clarify the mechanism of increased BCRL risk among racial and ethnic minority groups.
Although AUC has been the most widely and often only used metric for prior BCRL predictive models, we report several additional relevant measures of discriminative performance.37 These measures include pseudo R2 values, which were relatively low for both models, suggesting that the models explain a minority of the variation in lymphedema development. These values, in addition to accuracy statistics that fall short of 100%, highlight the fact that the tradeoff for simplifying model inputs is omission of potential predictors of BCRL, such as genetic information, type of chemotherapy, field of radiation, and postoperative complications.22,23,24,42,56 Sensitivity and specificity were reported in a single prior study of BCRL predictive models29 yet are key features of clinical prediction models that are needed to calculate NPV. With all prediction models, performance is a balance between sensitivity and specificity that varies according to the chosen cutoff threshold.37 By selecting the maximum of the Youden index as the cutoff for each model, we chose to round our model predictions to jointly optimize sensitivity and specificity without substantially sacrificing accuracy. Based on this methodology, both models have high NPVs. Given that the purpose of these models is to direct high-risk patients to low-risk interventions, our model was designed to overidentify patients that may be at risk while also being confident that patients who screen negative are truly at low risk. In clinical practice, the cutoff of the models can be adjusted to achieve alternative performance metrics depending on the intended model use.
Predictive modeling is key to prioritizing patients with breast cancer for lymphedema intervention and achieving primary and secondary preventive goals. Preoperatively, identification of patients at high risk for lymphedema development can facilitate interventions on modifiable risk factors (eg, weight reduction measures for obesity)57 and selection of patients for immediate lymphatic reconstruction (ILR) using lymphovenous bypass and/or early postoperative complex decongestive therapy. While early evidence suggests that ILR may be a clinically effective and cost-effective preventive strategy,58,59,60 ILR is time intensive both in operative planning and utilization of medical resources. Prioritizing ILR for patients who are most likely to benefit based on our preoperative model would not only facilitate more efficient use of health care resources but would also increase the potential to see a positive effect from this intervention. A similar approach may be applied to preventive drug therapies. In terms of postoperative surveillance, standard surveillance measures, such as arm circumference measurements and bioimpedance spectroscopy, are similarly resource and time intensive.61 Use of our postoperative model can decrease the number of clinic visits that patients need to make for repeated arm measurements. Patients’ predicted risk of BCRL can be calculated based on a simple 1-question survey about arm swelling; those that screen above the desired risk threshold can be called in for additional evaluation.
Limitations
Limitations of this study include lack of data regarding time to lymphedema development, which prohibited time-to-event analysis. In addition, our preoperative model assumes that the need for ALND and for adjuvant chemotherapy and radiation therapy in patients not undergoing neoadjuvant therapies is known preoperatively, though this information is commonly unknown, particularly among women undergoing mastectomy.62,63 However, even in these circumstances, the preoperative model can be used for clinical decision-making by inputting the most likely data points or various combinations of data points based on possible scenarios. The definition of lymphedema in data set 1 (more than 2-cm difference above or below the olecranon) is not as sensitive as the 10% relative volume change definition used for data sets 2 and 3, and thus we may underestimate the incidence of lymphedema in that subset.64 We acknowledge that basing our data set for external validation of the preoperative model on ICD-9 and ICD-10 codes may introduce bias. In addition, we are unable to externally validate the postoperative model since we did not have access to an independent cohort with data on patient-reported arm swelling. Future work should externally validate our models in more diverse patient populations.
Conclusions
In conclusion, we have developed and validated prediction models for BCRL that are highly accurate, racially sensitive, and comprised of inputs that are easily accessible to patients and clinicians. The preoperative model can be used to target high-risk patients for perioperative risk reduction measures and early institution of postoperative preventive therapies. The postoperative model decreases the need for lymphedema surveillance with in-person measurements. Our understanding of lymphedema and its management has substantially improved over the past several years, outpacing our ability to efficiently connect patients with available interventions. By implementing these predictive models in a patient-facing format within clinical care, our goal is to direct patients to appropriate preoperative and postoperative interventions in a manner that balances precision and practicality.
eTable 1. Univariable and Multivariable Logistic Regression Modeling Lymphedema Development
eFigure 1. Margins Plots for Model 1 by Variable
eFigure 2. Margins Plots for Model 2 by Variable
eTable 2. Sensitivity Analysis: Bayesian Logistic Regression
eTable 3. Patient Demographic and Clinical Data for MSKCC ICD Validation Cohort
eFigure 3. Ten-Fold ROC Curves for Model 2 Internal Validation
eTable 4. Literature Review of Predictive Models for Breast Cancer-Related Lymphedema (BCRL)
Data Sharing Statement
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500-515. doi: 10.1016/S1470-2045(13)70076-7 [DOI] [PubMed] [Google Scholar]
- 4.Montagna G, Zhang J, Sevilimedu V, et al. Risk factors and racial and ethnic disparities in patients with breast cancer-related lymphedema. JAMA Oncol. 2022;8(8):1195-1200. doi: 10.1001/jamaoncol.2022.1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damen JAAG, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun D, Liu J, Xiao L, et al. Recent development of risk-prediction models for incident hypertension: an updated systematic review. PLoS One. 2017;12(10):e0187240. doi: 10.1371/journal.pone.0187240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12(4):289-294. doi: 10.1089/lrb.2014.0035 [DOI] [PubMed] [Google Scholar]
- 8.Ostby PL, Armer JM, Dale PS, Van Loo MJ, Wilbanks CL, Stewart BR. Surveillance recommendations in reducing risk of and optimally managing breast cancer-related lymphedema. J Pers Med. 2014;4(3):424-447. doi: 10.3390/jpm4030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112(12):2809-2819. doi: 10.1002/cncr.23494 [DOI] [PubMed] [Google Scholar]
- 10.Mondry TE, Riffenburgh RH, Johnstone PAS. Prospective trial of complete decongestive therapy for upper extremity lymphedema after breast cancer therapy. Cancer J. 2004;10(1):42-48. doi: 10.1097/00130404-200401000-00009 [DOI] [PubMed] [Google Scholar]
- 11.Smile TD, Tendulkar R, Schwarz G, et al. A review of treatment for breast cancer-related lymphedema: paradigms for clinical practice. Am J Clin Oncol. 2018;41(2):178-190. doi: 10.1097/COC.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 12.Schaverien MV, Dayan JH. Introduction. In: Schaverien MV, Dayan JH, eds. Multimodal Management of Upper and Lower Extremity Lymphedema. Springer International Publishing; 2022:1-6. doi: 10.1007/978-3-030-93039-4_1 [DOI] [Google Scholar]
- 13.Schaverien MV, Dayan JH. Key topic: patient selection and evidence-based algorithmic approach to surgical management of lymphedema. In: Schaverien MV, Dayan JH, eds. Multimodal Management of Upper and Lower Extremity Lymphedema. Springer International Publishing; 2022:47-52. doi: 10.1007/978-3-030-93039-4_7 [DOI] [Google Scholar]
- 14.Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. 2013;22(7):1466-1484. doi: 10.1002/pon.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pusic AL, Cemal Y, Albornoz C, et al. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv. 2013;7(1):83-92. doi: 10.1007/s11764-012-0247-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naughton MJ, Liu H, Seisler DK, et al. Health-related quality of life outcomes for the LEAP study-CALGB 70305 (Alliance): a lymphedema prevention intervention trial for newly diagnosed breast cancer patients. Cancer. 2021;127(2):300-309. doi: 10.1002/cncr.33184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basta MN, Fox JP, Kanchwala SK, et al. Complicated breast cancer-related lymphedema: evaluating health care resource utilization and associated costs of management. Am J Surg. 2016;211(1):133-141. doi: 10.1016/j.amjsurg.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 18.Stout NL, Pfalzer LA, Springer B, et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92(1):152-163. doi: 10.2522/ptj.20100167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black DM, Jiang J, Kuerer HM, Buchholz TA, Smith BD. Racial disparities in adoption of axillary sentinel lymph node biopsy and lymphedema risk in women with breast cancer. JAMA Surg. 2014;149(8):788-796. doi: 10.1001/jamasurg.2014.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevilacqua JLB, Kattan MW, Changhong Y, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012;19(8):2580-2589. doi: 10.1245/s10434-012-2290-x [DOI] [PubMed] [Google Scholar]
- 21.Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):498-503. doi: 10.1016/j.ijrobp.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 22.Gross JP, Whelan TJ, Parulekar WR, et al. Development and validation of a nomogram to predict lymphedema after axillary surgery and radiation therapy in women with breast cancer from the NCIC CTG MA.20 randomized trial. Int J Radiat Oncol Biol Phys. 2019;105(1):165-173. doi: 10.1016/j.ijrobp.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 23.Byun HK, Chang JS, Im SH, et al. Risk of lymphedema following contemporary treatment for breast cancer: an analysis of 7617 consecutive patients from a multidisciplinary perspective. Ann Surg. 2021;274(1):170-178. doi: 10.1097/SLA.0000000000003491 [DOI] [PubMed] [Google Scholar]
- 24.Yuan Q, Hou J, Zhou R, et al. Development and validation of an intraoperative nomogram to predict breast cancer-related lymphedema based on the arm lymphatics distribution. Ann Surg Oncol. 2021;28(12):7319-7328. doi: 10.1245/s10434-021-09982-0 [DOI] [PubMed] [Google Scholar]
- 25.Kwan JYY, Famiyeh P, Su J, et al. Development and validation of a risk model for breast cancer-related lymphedema. JAMA Netw Open. 2020;3(11):e2024373. doi: 10.1001/jamanetworkopen.2020.24373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Jaimez P, Armora Verdú M, Forero CG, et al. Breast cancer-related lymphoedema: risk factors and prediction model. J Adv Nurs. 2022;78(3):765-775. doi: 10.1111/jan.15005 [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Li HP, Liu AN, et al. A scoring system to predict arm lymphedema risk for individual Chinese breast cancer patients. Breast Care (Basel). 2016;11(1):52-56. doi: 10.1159/000443491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basta MN, Wu LC, Kanchwala SK, et al. Reliable prediction of postmastectomy lymphedema: the Risk Assessment Tool Evaluating Lymphedema. Am J Surg. 2017;213(6):1125-1133.e1. doi: 10.1016/j.amjsurg.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 29.Wei X, Lu Q, Jin S, et al. Developing and validating a prediction model for lymphedema detection in breast cancer survivors. Eur J Oncol Nurs. 2021;54:102023. doi: 10.1016/j.ejon.2021.102023 [DOI] [PubMed] [Google Scholar]
- 30.Konishi T, Tanabe M, Michihata N, et al. Risk factors for arm lymphedema following breast cancer surgery: a Japanese nationwide database study of 84,022 patients. Breast Cancer. 2023;30(1):36-45. doi: 10.1007/s12282-022-01395-5 [DOI] [PubMed] [Google Scholar]
- 31.Liu YF, Liu JE, Zhu Y, et al. Development and validation of a nomogram to predict the risk of breast cancer-related lymphedema among Chinese breast cancer survivors. Support Care Cancer. 2021;29(9):5435-5445. [DOI] [PubMed] [Google Scholar]
- 32.Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918-926. doi: 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303-1310. doi: 10.1016/S1470-2045(14)70460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rescigno J, Zampell JC, Axelrod D. Patterns of axillary surgical care for breast cancer in the era of sentinel lymph node biopsy. Ann Surg Oncol. 2009;16(3):687-696. doi: 10.1245/s10434-008-0195-5 [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213-5219. doi: 10.1200/JCO.2008.16.3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol. 2008;26(32):5220-5226. doi: 10.1200/JCO.2008.16.3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dankers FJ, Traverso A, Wee L, van Kuijk SM. Prediction modeling methodology. In: Kubben P, Dumontier M, Dekker A, eds. Fundamentals of Clinical Data Science. SpringerOpen; 2019:101-120. doi: 10.1007/978-3-319-99713-1_8 [DOI] [Google Scholar]
- 38.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. doi: [DOI] [PubMed] [Google Scholar]
- 39.Esnor J, Snell KI, Martin EC. PMCALPLOT: Stata module to produce calibration plot of prediction model performance. Accessed September 13, 2022. https://ideas.repec.org/c/boc/bocode/s458486.html
- 40.Luque-Fernandez M, Maringe C, Nelson P. CVAUROC: Stata module to compute cross-validated area under the curve for ROC analysis after predictive modelling for binary outcomes. Accessed September 17, 2022. https://ideas.repec.org/c/boc/bocode/s458324.html
- 41.Soran A, Wu WC, Dirican A, Johnson R, Andacoglu O, Wilson J. Estimating the probability of lymphedema after breast cancer surgery. Am J Clin Oncol. 2011;34(5):506-510. doi: 10.1097/COC.0b013e3181f47955 [DOI] [PubMed] [Google Scholar]
- 42.Li X, Huang H, Lin Q, et al. Validation of a breast cancer nomogram to predict lymphedema in a Chinese population. J Surg Res. 2017;210:132-138. doi: 10.1016/j.jss.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 43.Soran A, Menekse E, Girgis M, DeGore L, Johnson R. Breast cancer-related lymphedema after axillary lymph node dissection: does early postoperative prediction model work? Support Care Cancer. 2016;24(3):1413-1419. doi: 10.1007/s00520-015-2933-0 [DOI] [PubMed] [Google Scholar]
- 44.Byun HK, Kim JS, Chang JS, et al. Validation of a nomogram for predicting the risk of lymphedema following contemporary treatment for breast cancer: a large multi-institutional study (KROG 20-05). Breast Cancer Res Treat. 2022;192(3):553-561. doi: 10.1007/s10549-021-06507-x [DOI] [PubMed] [Google Scholar]
- 45.Lin Q, Yang T, Yongmei J, Die YM. Prediction models for breast cancer-related lymphedema: a systematic review and critical appraisal. Syst Rev. 2022;11(1):217. doi: 10.1186/s13643-022-02084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16(4):775-782. doi: 10.1158/1055-9965.EPI-06-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen TWF, Fan X, Sparapani R, Laud PW, Walker AP, Nattinger ABA. A contemporary, population-based study of lymphedema risk factors in older women with breast cancer. Ann Surg Oncol. 2009;16(4):979-988. doi: 10.1245/s10434-009-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basta MN, Fischer JP, Kanchwala SK, et al. A propensity-matched analysis of the influence of breast reconstruction on subsequent development of lymphedema. Plast Reconstr Surg. 2015;136(2):134e-143e. doi: 10.1097/PRS.0000000000001417 [DOI] [PubMed] [Google Scholar]
- 49.Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat. 2009;113(2):383-391. doi: 10.1007/s10549-008-9940-5 [DOI] [PubMed] [Google Scholar]
- 50.Togawa K, Ma H, Sullivan-Halley J, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. 2014;16(4):414. doi: 10.1186/s13058-014-0414-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwan ML, Yao S, Lee VS, et al. Race/ethnicity, genetic ancestry, and breast cancer-related lymphedema in the Pathways study. Breast Cancer Res Treat. 2016;159(1):119-129. doi: 10.1007/s10549-016-3913-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren Y, Kebede MA, Ogunleye AA, et al. Burden of lymphedema in long-term breast cancer survivors by race and age. Cancer. 2022;128(23):4119-4128. doi: 10.1002/cncr.34489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellwege JN, Torstenson ES, Russell SB, Edwards TL, Velez Edwards DR. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PLoS One. 2017;12(8):e0182791. doi: 10.1371/journal.pone.0182791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. J Eur Acad Dermatol Venereol. 2020;34(4):e161-e164. doi: 10.1111/jdv.16097 [DOI] [PubMed] [Google Scholar]
- 55.Schmitz KH, Neuhouser ML, Agurs-Collins T, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105(18):1344-1354. doi: 10.1093/jnci/djt223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visser J, van Geel M, Cornelissen AJM, van der Hulst RRWJ, Qiu SS. Breast cancer-related lymphedema and genetic predisposition: a systematic review of the literature. Lymphat Res Biol. 2019;17(3):288-293. doi: 10.1089/lrb.2017.0083 [DOI] [PubMed] [Google Scholar]
- 57.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer–related lymphedema. Cancer. 2007;110(8):1868-1874. doi: 10.1002/cncr.22994 [DOI] [PubMed] [Google Scholar]
- 58.Johnson AR, Fleishman A, Granoff MD, et al. Evaluating the impact of immediate lymphatic reconstruction for the surgical prevention of lymphedema. Plast Reconstr Surg. 2021;147(3):373e-381e. doi: 10.1097/PRS.0000000000007636 [DOI] [PubMed] [Google Scholar]
- 59.Johnson AR, Asban A, Granoff MD, et al. Is immediate lymphatic reconstruction cost-effective? Ann Surg. 2021;274(6):e581-e588. doi: 10.1097/SLA.0000000000003746 [DOI] [PubMed] [Google Scholar]
- 60.Granoff MD, Hamaguchi R, Singhal D. Step-by-step instruction: immediate lymphatic reconstruction for lymphedema risk reduction in breast cancer management. In: Schaverien MV, Dayan JH, eds. Multimodal Management of Upper and Lower Extremity Lymphedema. Springer International Publishing; 2022:169-173. doi: 10.1007/978-3-030-93039-4_23 [DOI] [Google Scholar]
- 61.Stout N, Armer J. Lymphedema prospective surveillance and risk reduction. In: Schaverien MV, Dayan JH, eds. Multimodal Management of Upper and Lower Extremity Lymphedema. Springer International Publishing; 2022:23-27. doi: 10.1007/978-3-030-93039-4_4 [DOI] [Google Scholar]
- 62.American Society of Breast Surgeons . Consensus statement on axillary management for patients with in-situ and invasive breast cancer: a concise overview. Accessed September 17, 2022. https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-the-Management-of-the-Axilla-Concise-Overview.pdf
- 63.Shah C, Al-Hilli Z, Vicini F. Advances in breast cancer radiotherapy: implications for current and future practice. JCO Oncol Pract. 2021;17(12):697-706. doi: 10.1200/OP.21.00635 [DOI] [PubMed] [Google Scholar]
- 64.Sun F, Hall A, Tighe MP, et al. Perometry versus simulated circumferential tape measurement for the detection of breast cancer-related lymphedema. Breast Cancer Res Treat. 2018;172(1):83-91. doi: 10.1007/s10549-018-4902-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Univariable and Multivariable Logistic Regression Modeling Lymphedema Development
eFigure 1. Margins Plots for Model 1 by Variable
eFigure 2. Margins Plots for Model 2 by Variable
eTable 2. Sensitivity Analysis: Bayesian Logistic Regression
eTable 3. Patient Demographic and Clinical Data for MSKCC ICD Validation Cohort
eFigure 3. Ten-Fold ROC Curves for Model 2 Internal Validation
eTable 4. Literature Review of Predictive Models for Breast Cancer-Related Lymphedema (BCRL)
Data Sharing Statement


