Graphical abstract
Keywords: Areca nut seed polyphenol, Ultrasonic-assisted extraction, Response surface methodology, Osteoblast, Proliferation, Differentiation
Highlights
-
•
An improved method to extract polyphenol from areca nut (Areca catechu L.) seeds.
-
•
Areca nut seed polyphenol promoted the proliferation of osteoblasts.
-
•
Areca nut seed polyphenol enhanced the differentiation of osteoblasts.
-
•
Areca nut seed polyphenol increased the mineralization of osteoblasts.
Abstract
Areca nut (Areca catechu L.) seeds are rich in polyphenols, while few studies focused on it. This study was designed to obtain the maximum extraction yield of areca nut seed polyphenol (ACP). An ultrasonic-assisted extraction method optimized by response surface methodology (RSM) was established to extract ACP. Under the optimal conditions (ultrasonic power of 87 W, ethanol concentration of 65%, extraction temperature of 62℃, and extraction time of 153 min), the actual extraction yield of ACP was 139.62 mg/g. Then we investigated the effects of ACP on the proliferation, differentiation and mineralization of MC3T3-E1 pre-osteoblasts. Results suggested that ACP notably promoted the proliferation of MC3T3-E1 cells without cytotoxicity, and the contents of collagen type Ⅰ (COL-Ⅰ) and osteocalcin (OCN) were rising. Meanwhile, the alkaline phosphatase (ALP) activity and mineralized nodules were enhanced. These findings demonstrated that ACP could induce the proliferation, differentiation and mineralization of osteoblasts in vitro. This work provided a certain experimental basis for the developing and utilization of polyphenols from Areca nut seeds.
1. Introduction
Areca nut (Areca catechu L.) is a crucial cash crop in the tropics. In recent years, the Areca nut industry has developed rapidly and has become an important pillar of economic development in Hainan Island [1]. The prime active components in Areca nut are alkaloids and phenolic compounds. However, studies have fully proved that alkaloids are the main cause of Areca nut addiction and carcinogenesis [2]. As another functional component of Areca nut, phenolic compounds are rarely studied and reported at present. The phenolic compounds have the highest content in Areca nut seeds, accounting for about 30% [3]. They have antibacterial, antioxidant, hypoglycemic, antithrombotic and other biological activities [4]. Our previous study reported that the polyphenol extracted from Areca nut seeds could alter the gut microbiome and immune system to improve osteoporosis in osteoporosis rats induced by estrogen deficiency [5]. Lee et al. [6] found that the phenolic compounds in Areca nut can significantly inhibit skin aging and inflammation. Wetwitayaklung et al. [7] revealed that the phenolic compounds in Areca nut seeds had the strongest free radical scavenging ability, which had great development potential in the food or medical field. Hence, it is vital to establish an effective, safe, and environmentally friendly extraction technology in the development of polyphenols from Areca nut.
Ultrasound is an emerging green, safe and low-cost technology that is widely used in the food industry, such as modification, extraction, degassing, and emulsification [8], [9]. Ultrasonic-assisted extraction can destroy the tissue or cells by the “cavitation effect”, enhancing the mass transfer rate and facilitating the release of polyphenol [10], [11]. Nowadays, the ultrasonic wave has been extensively adapted to assist the extraction of active substances from plant materials. Compared with traditional methods like Soxhlet extraction, hot reflux extraction and immersion extraction, ultrasonic-assisted extraction has attracted research interest recently because of its characteristics of higher yield, shorter processing time and less solvent [12], [13]. Several physical-assisted extraction polyphenols methods, such as microwave-assisted extraction, high-pressure-assisted extraction, and supercritical carbon dioxide extraction, have the disadvantages of longer extraction time, lower extraction efficiency, higher power consumption, and more organic solvent pollution than ultrasonic-assisted extraction [14]. Thus, these methods limit the extraction of polyphenols from hard seeds. The benefits of ultrasonic-assisted extraction include promoting the hydration of dried seeds, destroying the cell walls to increase the mass transfer and causing no changes in the structure and function of polyphenols, which significantly improves the efficiency of extraction [15]. Belwal et al. [16] found that under the optimal ultrasonic-assisted extraction conditions, significantly higher polyphenolic contents and antioxidant activity were recorded than conventional extraction. Serna et al. [17] reported that the extraction yield was as high as 85%, at a markedly lower energy requirement using ultrasound-assisted extraction instead of conventional solid–liquid extraction. The antioxidant activity of polyphenol from citrus peels using ultrasound-assisted extraction was higher by about 28% than that when using microwave-assisted extraction [18].
Postmenopausal osteoporosis is a common chronic disease with a severe physical and financial impact on patients. At present, the clinical treatment mainly uses metabolic drugs that inhibit bone resorption and promote bone formation to treat osteoporosis. However, long-term clinical treatment has brought serious side effects, such as hypercalcemia, breast cancer, uterine bleeding and other adverse reactions [19]. Numerous reports have proved that functional components originating from organisms can be safe and reliable routes for disease treatment and prevention.
To our knowledge, ultrasonic-assisted extraction using ethanol to extraction ACP has not been reported before, and there is no report about ACP on bone metabolism in vitro. Herein, ultrasonic-assisted extraction of ACP was established to develop and optimize via response surface methodology, then the effects of ACP on pre-osteoblast proliferation, differentiation, and mineralization were examined using mouse pre-osteoblasts in vitro.
2. Materials and methods
2.1. Materials and reagents
The Areca nut seeds were acquired from Hainan Huachuang Areca catechu Research Institute (Hainan, China). The gallic acid standard was acquired from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). MC3T3-E1 cell line, purchased from American Type Culture Collection (Rockville, USA). Alpha-minimum essential medium (ɑ-MEM), phosphate-buffered saline (PBS), Fetal bovine serum (FBS), trypsin and Penicillin-Streptomycin Nystatin Solution were purchased from Shanghai Lifei Biotechnology Co., Ltd. (Shanghai, China). Alizarin Red S solutions, cetylpyridinium chloride, β-sodium glycerophosphate, and L-Ascorbic acid were obtained from Sigma-Aldrich Co. (Shanghai, China). Alkaline Phosphatase Assay Kit and BCA Protein Assay Kit were obtained from Shanghai Biyuntian Biotechnology Co., Ltd. (Shanghai, China). Collagen Type Ⅰ (COL-Ⅰ) enzyme-linked immunosorbent assay (ELISA) Assay kit and Osteocalcin ELISA Assay Kit were obtained from Nanjing Jiancheng Biological Engineering Research Institute (Nanjing, China). Other chemicals were analytical grade.
2.2. Extraction of ACP
The Areca nut seeds were ground and sieved with a 100-mesh sieve. The powder (0.2 g) was mixed with ethanol aqueous solution (50 mL) in a 50 mL centrifuge tube. The suspension was extracted by a 40 kHz ultrasonic cleaner (XO-5200DTD, Nanjing Xianou Instruments Manufacture Co., Ltd., Jiangsu, China). Then the crude extract was centrifuged at 3500 rpm for 10 min (Hunan Xiang Yi Laboratory Instrument Development Co., Ltd., Hunan, China). After that, the supernatant was filtered through a 0.45 μm filter membrane, the filtrate was collected for further study. The extraction yield (mg/g) of ACP was figured out by equation (1):
| (1) |
where, Y is the extraction yield (mg/g) of ACP; C is the concentration (mol/L) of ACP; d is the dilution factor; V is the volume (L) of the sample, m is the mass (g) of the sample.
2.3. Single-factor experiment
The single-factor extraction experiment was performed under the following conditions: the ultrasonic power of 40–180 W, ethanol concentration of 50–90%, extraction temperature of 40–80℃, and extraction time of 0.5–3 h. The experiment design is shown in Table 1.
Table 1.
Single-factor experimental design.
| Single factor | Power (W) | Concentration (%) | Temperature (℃) | Time (h) |
|---|---|---|---|---|
| Power | 40, 80, 120, 180 | 120 | 120 | 120 |
| Concentration | 70 | 50, 60, 70, 80, 90 | 70 | 70 |
| Temperature | 70 | 70 | 40, 50, 60, 70, 80 | 70 |
| Time | 1 | 1 | 1 | 0.5, 1, 1.5, 2, 3 |
2.4. Response surface design
The four-factor and three-level experimental designs were determined on the basis of the single-factor experimental design. Ultrasonic power (A), ethanol concentration (B), extraction temperature (C), and extraction time (D) were the independent variables, the extraction yield of ACP was the dependent variable. The experiment was optimized by Box-Bohnken (B-B) design. Table 2 exhibited the factors and coding levels.
Table 2.
Factors and their coding levels in experimental design for RSM.
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: Ultrasonic power (W) | 40 | 80 | 120 |
| B: Ethanol concentration (%) | 60 | 70 | 80 |
| C: Extraction temperature (℃) | 60 | 70 | 80 |
| D: Extraction time (h) | 1.5 | 2.0 | 3.0 |
2.5. Measurement of ACP content
The ACP content was measured by Folin-Ciocalteu (FC) reagent method selecting gallic acid as a standard, following the second method of GB/T 8313–2018 with slight modifications. Using the gallic acid standard curve [A = 0.005C + 0.0861, r2 = 0.9968] to calculate the ACP content. The outcome was presented in mg of gallic acid equivalent per g of Areca nut seed powder.
2.6. Culture of pre-osteoblast MC3T3-E1
Removed the old medium from the culture flask, added PBS to clean cell fragments and metabolites, then removed PBS and added 2 mL trypsin containing 0.25% EDTA and digested 2 min. After removing the trypsin, 2 mL of the complete medium (α-MEM including 10% fetal bovine serum and 100 U/mL streptomycin-penicillin) was added to the culture flask to pipette the cells until they are completely suspended. Finally, 1 mL of the medium including cells was absorbed and shifted to a T25 culture flask containing 7 mL of complete medium, and cultured in a 37℃, 95% humidity, 5% carbon dioxide incubator.
2.7. Proliferation assay of pre-osteoblast MC3T3-E1
Cells at a density of 2 × 104 cells/mL were added into a 96-well plate at 100 μL per well and incubated for 1 day. Then the medium containing ACP (0, 25, 50, 100, 150 μg/mL) was added and cultured for 2 days. The proliferation of cells was measured by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) method. Added 20 µL, 5 mg/mL MTT to each well and cultured for 4 h in an incubator. After that, removed the supernatant and added 150 µL of DMSO to each well, then shaken for 10 min. The absorbance (A490 nm) was detected on a microplate reader.
2.8. Determination of alkaline phosphatase (ALP) activity
MC3T3-E1 cells were laid in 24-well plates at a density of 2 × 104 cells/mL, with 1 mL in each well. After incubated in the incubator for 1 day, the medium was substituted with α-MEM differentiation medium containing 10 mM β-sodium glycerophosphate, 50 μg/mL ascorbic acid, 10% fetal bovine serum, 100 U/mL streptomycin-penicillin, and ACP (0, 25, 50, 100, 150 μg/mL). The medium was replaced every 2 days. The cells were gathered on the 7th day, the medium was discarded, and washed twice with precooled PBS. Then added 300 μL 1% TritonX-100 lysate to each well, and the cells were lysed at 4℃ for 20 min. After that, centrifuged at 12,000 r/min 4℃ and collected the supernatant. The relative enzyme activity was detected referring to the directions of the alkaline phosphatase assay kit. The total cell protein was detected by the BCA protein assay kit.
2.9. Determination of COL-Ⅰ and osteocalcin (OCN) content
The cell culture was the same as 2.8, and the supernatant was gathered on the 7th day and 14th day, respectively. The content of COL-Ⅰ was measured referring to the indications of the COL-Ⅰ assay kit. And the content of OCN was determined by referring to the indications of the OCN assay kit.
2.10. Determination of mineralized nodules
The medium of cell culture and differentiation medium was the same as 2.8. Changed the medium every 2 days and detected on the 17th day. Then removed the supernatant, and washed twice using PBS, then the cells were fixed with 300 µL 4% neutral formaldehyde for 15 min. After that, washed twice with precooled PBS, adding 300 μL 1% alizarin red S solution to stain for 30 min at 37℃ in the darkness. Finally, washed the cells with running water 3 times and observed.
After recording, added 300 µL of 100 mM cetylpyridinium chloride solution to each well, and reacted at 37℃ for 15 min in the darkness for semi-quantitative detection. Absorbed 100 µL of the supernatant from each well and added it to the 96-well plate. The absorbance was tested at 570 nm.
2.11. Statistical analysis
All the experiments were repeated for 3 times, the data were shown in mean ± standard deviations (SD). The data were analyzed by ANOVA followed by Duncan’s post hoc testing SPSS 25.0. The significance level was designated as p < 0.05. The outcomes derived from the RSM design were computed through Design-Expert 13.0 software.
3. Results
3.1. Single-factor experiment
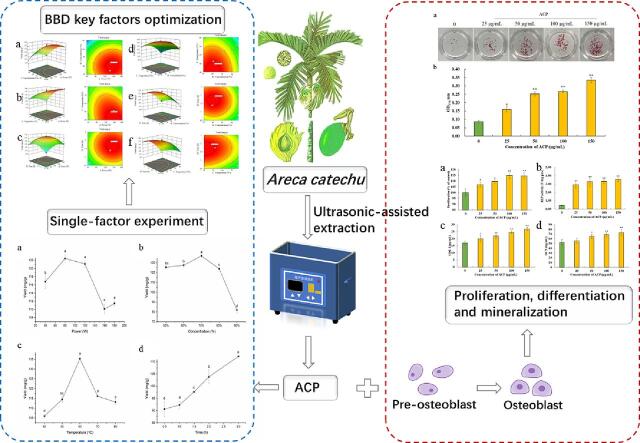
3.1.1. Effect of ultrasound power on the extraction yield of ACP
As shown in Fig. 1a, ultrasonic power played a significant role in enhancing the extraction yield of ACP. With the elevation of ultrasonic power, the yield initially increased and then decreased at higher power (120 W). The extraction yield reached the peak (122.45 ± 0.87 mg/g) at 80 W. Since the cavitation effect generates shear force, mechanically breaks the cell walls and conduces to the migration of substances, the yield was increased after ultrasonic treatment [20]. As the ultrasonic power kept increasing, the extraction yield reduced. A high power could generate multiple bubbles, which hindered the transmission of ultrasonic waves [21]. In addition, it could also be explained by the damage of the polyphenol structure, the dissolution of impurities and the reduction of active components caused by the significant high power [22], [23]. Taken together, 80 W was considered to be the optimal ultrasonic power.
Fig. 1.
Effect of ultrasonic power (a), ethanol concentration (b), temperature (c), and time (d) on the extraction yield of ACP. Values are expressed as means ± SD (n = 3). Different lowercase letters (a, b, c, and d) in the same figure indicate a statistically significant difference (p < 0.05).
3.1.2. Effect of ethanol concentration on the extraction yield of ACP
As exhibited in Fig. 1b, with the rise of ethanol concentration between 50% and 70%, the yield was rising, while the yield declined as the concentration exceeded 70%. The extraction yield reached the highest value (136.95 ± 1.32 mg/g) at 70%. The trend was corresponding to the report of Wang et al. [24]. The hydroxyl groups of phenolic compounds could interact with the hydroxyl, amidogen and carboxyl groups to form stable compounds in proteins and polysaccharides [25], [26]. When the concentration of ethanol was increased, non-covalent interactions like hydrogen bonds and hydrophobic interactions were destroyed [27], contributing to the dissolution of ACP. Afterward, the concentration had an adverse influence on the yield of ACP. This result may be ascribed to the transformation of solvent polarity as the concentration was continuously increased [28]. Moreover, this behavior resulted from the competitive interactions between ethanol, water, and polyphenol molecules [29]. As a result, the optimum ethanol concentration is 70%.
3.1.3. Effect of extraction temperature on the extraction yield of ACP
Within 40–80℃, the extraction yield increased substantially and then decreased significantly when the temperature was over 60℃ (Fig. 1c). The yield reached a maximum (135.28 ± 0.29 mg/g) at 60℃. It indicated that elevated temperature exerted an adverse effect on the extraction of ACP. The hydroxyl groups of phenols are prone to oxidation and condensation reactions, which was key responsible for the extraction yield of ACP [30]. Furthermore, when the temperature exceeded the boiling point of ethanol, the evaporation of ethanol was accelerated. Consequently, 60℃ was chosen to be the best extraction temperature.
3.1.4. Effect of extraction time on the extraction yield of ACP
According to Fig. 1d, the yield showed an increasing time-dependent effect. Though the high yield of ACP was relevant to the extension of extraction time, immoderate extraction time should be averted because of the thermal degradation of ACP [31]. For instance, while the tea polyphenols were extracted with ethanol over 60 min, the total content was reduced [32]. Additionally, long-time extraction would increase the cost. Therefore, 3 h was selected to be the optimal extraction time.
3.2. Optimization of ACP extraction process
3.2.1. RSM model fitting
According to the analysis of the single-factor experiment, the four-factor and three-level test was proceeded by RSM. A total of 29 experimental combinations were presented in Table 3. The results were fitted via multiple regression using Design-Expert, and the relationship between the extraction yield of ACP and variables was expressed by the following Equation (2):
| (2) |
where Y is the predicted value of the extraction yield of ACP; A, B, C, and D are coding values of the independent variables as described above.
Table 3.
Box-Behnken design and resultant responses.
| Run | A: Power (W) | B: Concentration (%) | C: Temperature (℃) | D: Time (h) | Y: Yield(mg/g) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 128.95 |
| 2 | 1 | −1 | 0 | 0 | 131.12 |
| 3 | −1 | 1 | 0 | 0 | 114.12 |
| 4 | 1 | 1 | 0 | 0 | 117.95 |
| 5 | 0 | 0 | −1 | −1 | 128.62 |
| 6 | 0 | 0 | 1 | −1 | 116.78 |
| 7 | 0 | 0 | −1 | 1 | 133.78 |
| 8 | 0 | 0 | 1 | 1 | 123.62 |
| 9 | −1 | 0 | 0 | −1 | 120.12 |
| 10 | 1 | 0 | 0 | −1 | 120.62 |
| 11 | −1 | 0 | 0 | 1 | 125.62 |
| 12 | 1 | 0 | 0 | 1 | 130.45 |
| 13 | 0 | −1 | −1 | 0 | 138.12 |
| 14 | 0 | 1 | −1 | 0 | 128.95 |
| 15 | 0 | −1 | 1 | 0 | 129.12 |
| 16 | 0 | 1 | 1 | 0 | 118.45 |
| 17 | −1 | 0 | −1 | 0 | 125.12 |
| 18 | 1 | 0 | −1 | 0 | 139.78 |
| 19 | −1 | 0 | 1 | 0 | 124.45 |
| 20 | 1 | 0 | 1 | 0 | 127.12 |
| 21 | 0 | −1 | 0 | −1 | 129.12 |
| 22 | 0 | 1 | 0 | −1 | 115.95 |
| 23 | 0 | −1 | 0 | 1 | 134.62 |
| 24 | 0 | 1 | 0 | 1 | 120.28 |
| 25 | 0 | 0 | 0 | 0 | 135.28 |
| 26 | 0 | 0 | 0 | 0 | 133.95 |
| 27 | 0 | 0 | 0 | 0 | 135.95 |
| 28 | 0 | 0 | 0 | 0 | 137.45 |
| 29 | 0 | 0 | 0 | 0 | 137.95 |
3.2.2. RSM analysis of the extraction yield of ACP
The data were analyzed by ANOVA to assess the significance of these factors and their interactions in this model. In Table 4, the model was remarkably significant (p < 0.0001). The credible predictive value (R2 = 0.9412) showed a high correlation between the predicted value and the experimental value. The adjusted coefficient of determination was R2Adj = 0.8623. The difference between R2 (0.9412) and R2Adj (0.8623) was<0.2, suggesting reasonable agreement between them. The absence of fit term (p = 0.1543 > 0.05) was not significant and the coefficient of variation (CV = 1.97%) was<5%, indicating the data had fine fit and reproducibility. Moreover, A, B, C, D, AC, A2, B2, and D2 had a remarkable effect (p < 0.0001) on the yield of ACP, showing the rationality of the single-factor experiment.
Table 4.
Analysis of variance of RSM of extraction yield of ACP.
| Source | Sum of Squares | df | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|
| Model | 1416.12 | 14 | 101.15 | 16.01 | < 0.0001*** |
| A-Power (W) | 100.11 | 1 | 100.11 | 15.84 | 0.0014** |
| B-Concentration (%) | 473.14 | 1 | 473.14 | 74.87 | < 0.0001*** |
| C-Temperature (℃) | 250.53 | 1 | 250.53 | 39.64 | < 0.0001*** |
| D-Time (h) | 80.91 | 1 | 80.91 | 12.80 | 0.0030** |
| AB | 0.6889 | 1 | 0.6889 | 0.1090 | 0.7462 |
| AC | 35.94 | 1 | 35.94 | 5.69 | 0.0318* |
| AD | 0.6972 | 1 | 0.6972 | 0.1103 | 0.7447 |
| BC | 0.5625 | 1 | 0.5625 | 0.0890 | 0.7698 |
| BD | 0.3422 | 1 | 0.3422 | 0.0542 | 0.8194 |
| CD | 0.7056 | 1 | 0.7056 | 0.1116 | 0.7432 |
| A2 | 184.39 | 1 | 184.39 | 29.18 | < 0.0001*** |
| B2 | 226.98 | 1 | 226.98 | 35.92 | < 0.0001*** |
| C2 | 41.21 | 1 | 41.21 | 6.52 | 0.0230* |
| D2 | 238.34 | 1 | 238.34 | 37.71 | < 0.0001*** |
| Residual | 88.48 | 14 | 6.32 | ||
| Lack of Fit | 77.92 | 10 | 7.79 | 2.95 | 0.1543 |
| Pure Error | 10.56 | 4 | 2.64 | ||
| Cor Total | 1504.60 | 28 |
*, the difference is significant at 0.05 level (p < 0.05); * *, the difference is significant at 0.01 level (p < 0.01); ***, the difference is significant at 0.001 level (p < 0.001)
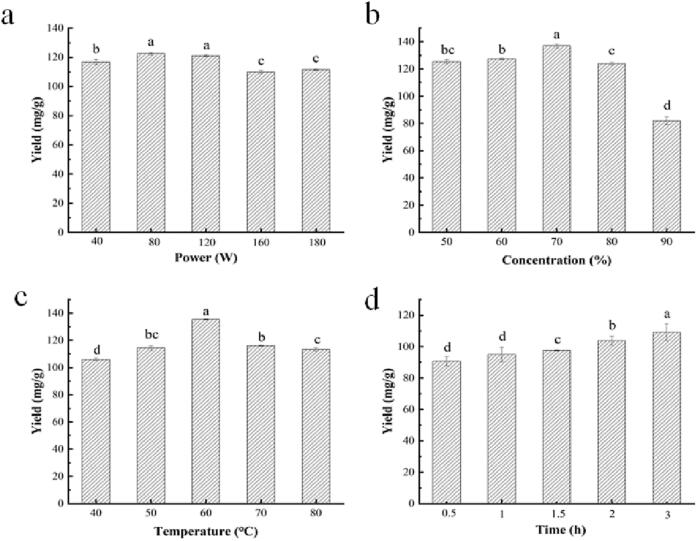
Fig. 2 depicted three-dimensional (3D) surface plots and contour plots those reflected the influence of each factor and the interaction between any two factors on the yield of ACP. In the 3D surface plot, the steeper of the surface plot, the more obvious the interaction between the variables. The contour plot is the bottom projection of the response surface and represents the interaction between two factors. If the contour plot tends to be elliptical, the interaction between the two factors is significant [33], [34]. In contrast, if it tends to circles, the interaction is not significant. With the increase of four factors, the yield of ACP tended to increase, but when the four factors exceeded certain values, their influence on the yield of ACP showed a downward tendency.
Fig. 2.
Response surface plots and contour plots for interaction between various factors on the yield of ACP, ultrasonic power and ethanol concentration (a), ultrasonic power and extraction temperature (b), ultrasonic power and extraction time (c), ethanol concentration and extraction temperature (d), ethanol concentration and extraction time (e), and extraction temperature and extraction time (f).
Ultrasonic power and extraction temperature exhibited notable interaction (p < 0.05) on the yield of ACP (Fig. 2b). While the temperature was lower than 70℃, the yield was rising markedly with the rise of ultrasonic power. The powerful cavitation effect could account for this result [1]. However, the yield tended to decrease with the elevation of power as the extraction temperature was over 75℃. Excessive power led to the degradation of phenolic compounds [3], thereby reducing the yield of ACP. Except for the above two factors, the interaction between the other two factors is not remarkable (p > 0.05). Comparing the F-values of varied factors and the slopes of the 3D response surface plots, we inferred the most important factor affecting the yield was ethanol concentration, followed by extraction temperature, ultrasonic power, and extraction time.
3.2.3. Verification of optimally predicted extraction yield of ACP
Based on the statistical analysis with Design-Expert 13.0, the optimal extraction conditions of ACP were obtained: ultrasonic power 87.12 W, ethanol concentration 64.58%, extraction temperature 61.96℃, extraction time 152.82 min, and the yield of ACP 139.878 mg/g. For the feasibility of the experiment, the actual conditions were: ultrasonic power of 87 W, ethanol concentration of 65%, extraction temperature of 62℃, and extraction time of 153 min. The verification assay was carried out based on the above conditions, representing the yield of ACP was 139.62 mg/g with a small deviation (RSD = 0.18%) compared with the model prediction. Consequently, the model had good veracity and reproducibility.
3.3. Effects of ACP on MC3T3-E1 cells proliferation
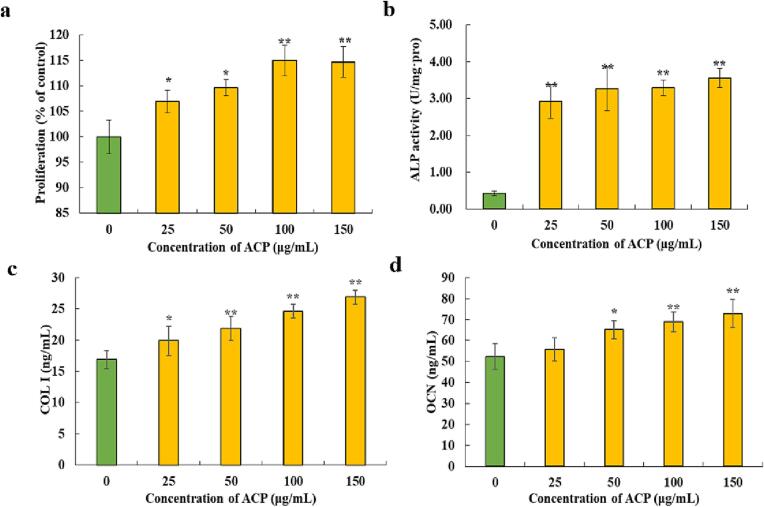
To verify the influence of ACP on pre-osteoblast proliferation, the MC3T3-E1 cells were treated with different concentrations of ACP (25–150 μg/mL). From Fig. 3a, the proliferation rate of MC3T3-E1 cells rose with the increase of the concentration of ACP (0, 25, 50, 100, 150 μg/mL), and the proliferation rate of the 100 µg/mL and 150 µg/mL groups were the highest, with no remarkable difference (p > 0.05). These consequences manifested ACP can significantly induce the proliferation of MC3T3-E1 pre-osteoblast cells without cytotoxicity. Similarly, Patel et al. [35] reported that the primary calvarial osteoblasts had a significant increase in proliferation after the polyphenol extracted from S.cordifolia was treated. And low concentration quercetin could significantly increase (143.14%) the number of osteoblasts compared with the untreated group [36].
Fig. 3.
Effect of ACP on proliferation (a), ALP activity (b), COL-Ⅰ content (c), and OCN content (d) of pre-osteoblast MC3T3-E1. Values are expressed as means ± standard deviations (n = 3). *, the difference is significant at 0.05 level (p < 0.05); **, the difference is significant at 0.01 level (p < 0.01).
3.4. Effects of ACP on ALP activity
When induced by β-glycerophosphate and L-ascorbic acid, the MC3T3-E1 cells, as the precursor of osteoblasts, can differentiate into osteoblasts [37]. To evaluate the effect of ACP on cell differentiation, we measured the activity of ALP. ALP is a significant marker of the early differentiation of osteoblasts, and its activity reflects the degree of osteoblast differentiation [38]. Compared with the control group, the expression of ALP in the ACP groups was notably rising (p < 0.01), with no notable difference in the four ACP concentrations of 25 µg/mL, 50 µg/mL, 100 µg/mL, and 150 µg/mL (p > 0.05). (Fig. 3b). As the ACP concentration was 50 μg/mL, the ALP activity was 8.26 times that of the control group. Therefore, ACP could stimulate osteoblasts to grow and differentiate.
3.5. The effect of ACP on the COL-Ⅰ and OCN content
COL-Ⅰ is recognized as a representative marker protein in the middle osteoblast differentiation, which is in charge of the formation of extracellular matrix and involved in osteogenesis [39]. As seen in Fig. 3c, the ACP-treated groups showed higher COL-Ⅰ than in the control group (p < 0.01). The COL-Ⅰ level in the experimental groups showed an increasing dose-dependent effect, and no obvious difference was noted in ACP concentration between 50 μg/mL and 150 μg/mL. The result revealed that ACP could induce MC3T3-E1 cells to secret COL-Ⅰ.
OCN is a specific symbol of late osteoblast differentiation [40]. It participates to regulate the deposition of bone matrix, the calcification of bone tissue and the content of bone calcium, which is the basis of mineralization [41]. As shown in Fig. 3d, ACP had a notable dose-dependent effect on OCN content. It could notably stimulate the OCN content when compared with the control group (p < 0.01). As a result, ACP could markedly stimulate the late differentiation and mineralization of osteoblasts.
3.6. The effect of ACP on the formation of osteoblast mineralized nodules
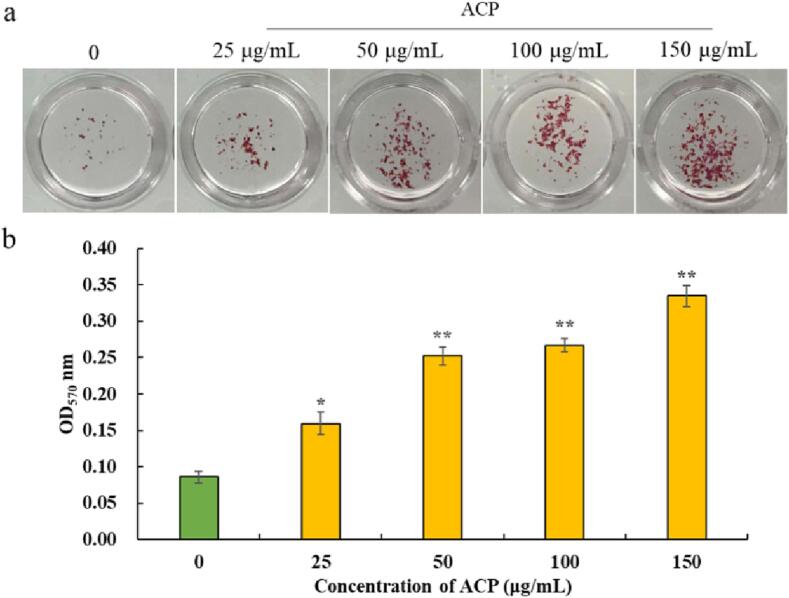
Mineralized bone nodules are the most intuitive manifestation of osteogenesis. Ca2+ could specifically combine with alizarin red S to turn red, and its absorbance is positively correlated with calcium deposition. These results could suggest the extent of mineralization of osteoblasts directly [42]. The number of calcium nodules in ACP groups increased markedly when comparing with the control group (p < 0.01), and it also had a notable dose-dependent effect (Fig. 4a).
Fig. 4.
Effect of ACP on mineralized nodules of pre-osteoblast MC3T3-E1. Values are shown by means ± SD (n = 3). *, the difference is significant at 0.05 level (p < 0.05); **, the difference is significant at 0.01 level (p < 0.01).
The calcium nodules were dissolved in cetylpyridinium chloride solution for semi-quantitative analysis, and the results were shown in Fig. 4b. ACP could significantly induce the mineralization of MC3T3-E1 cells. Compared with the control group, the calcium nodule increased the most (289.53%) when the dose of ACP was 150 μg/mL.
4. Discussion
As one of the “Three trees” in Hainan Province, Areca nut is one of the main sources of income for>2 million farmers. However, there are few studies on Areca nut polyphenols at present. This work optimized the ultrasound-assisted extraction conditions for maximizing the yield of Areca nut seed polyphenol. Under the optimal conditions (ultrasonic power of 87 W, ethanol concentration of 65%, extraction temperature of 62℃, and extraction time of 153 min), the actual extraction yield of polyphenols was 139.62 mg/g with a small deviation (RSD = 0.18%) compared with the model prediction. The statistical analysis of high coefficients of correlation proved the effectiveness and reproducibility of the proposed model.
In vivo, osteoblasts participate in proliferation and differentiation in response to various factors. During differentiation, pre-osteoblasts express extracellular matrix proteins, such as COL-Ⅰ, ALP, and OCN, then osteoblasts are mineralized [43], [44]. MC3T3-EI cells are murine calvarial pre-osteoblast cell lines, which could differentiate into osteoblasts and deposit minerals in vitro [45]. It is a common cell model to investigate the mechanism of osteogenesis in the world. In the previous research, we set up an ovariectomized model of osteoporosis rats to explore the mechanism of action of ACP on osteoporosis by regulating gut microbiota [5]. Our main finding revealed that the ACP treatment contributed to the bone formation via modulating gut microbiota relevant to maintained Paneth cells and lysozyme. In this research, we measured the ALP activity, the extracellular matrix calcium nodules, the content of COL-I and OCN. The experimental results revealed that ACP could promote the proliferation, differentiation and mineralization of MC3T3-E1 pre-osteoblasts.
ALP is a homologous dimer glycoprotein, whose main function is to promote osteoblast maturation and mineralization. It is an early symbol of osteoblast differentiation and extracellular matrix maturation [46]. We found that ACP remarkably enhanced the content of ALP in osteoblasts, which promoted the differentiation of osteoblasts. COL-Ⅰ is one of the symbols of the early differentiation of osteoblasts, accounting for 90% of the protein secreted by osteoblasts. And OCN is a marker of late differentiation of osteoblasts. It is the staple extracellular matrix non-collagen protein specifically composited by osteoblasts, which has a significant effect in maintaining the stability of mineralization of bone tissue in the late differentiation [47]. The results manifested that ACP could notably promote the protein content of COL-Ⅰ and OCN in osteoblasts, and further motivated the differentiation and mineralization of osteoblasts. The formation of mineralized nodules is a sign of osteogenic differentiation and maturity and a morphological manifestation of osteogenesis. In this study, used alizarin red staining to detect the bone nodules, and the results further confirmed that ACP had the effect of promoting the mineralization of osteoblasts. Taken together, ACP could contribute to the differentiation of MC3T3-E1 cells into mature osteoblasts and mineralization. The previous study reported that the polyphenol extracted from Hibiscus syriacus L. had effects on osteogenic and anti-osteoporotic by enhancing calcification and ALP activity in MC3T3-E1 and MG-63 cells [48]. Patel et al. [35] revealed that the polyphenol in the roots and leaves of Sida cordifolia significantly increased the osteoblast cell proliferation, differentiation, mineralization, and mRNA expression of osteogenic genes. Moreover, our previous study found that ACP could downregulate 5-HT and increase bone mass by inhibiting bone resorption and inducing bone formation [49]. Therefore, the effect of ACP on bone formation in this paper is consistent with the above results. The results of this work could offer ideas for improving the added value of Areca nut polyphenol.
5. Conclusions
In this work, RSM was successfully used for optimizing the extraction process of ACP. Four-factor and three-level design were adopted, then the optimum parameters were obtained by RSM optimization (ultrasonic power 87 W, ethanol concentration 65%, extraction temperature 62℃, extraction time 153 min). On the optimum conditions, the actual yield of ACP was up to 139.62 mg/g which was in fine conformity with the predicted values of the model, and the error was small (RSD = 0.18%). The findings showed that the model fitted well and the predicted ACP yield data had good accuracy. In addition, results from our work indicated that ACP could induce the proliferation, differentiation and mineralization of MC3T3-E1 pre-osteoblasts. This work could provide basic data for optimizing the extraction process of ACP. And ACP has the potential to be an effective ingredient in the treatment of osteoporosis.
CRediT authorship contribution statement
Ying Sun: Project administration, Methodology, Software, Writing – original draft. Jinfeng Lu: Project administration, Visualization, Writing – review & editing. Jiaqi Li: Writing – review & editing. Peng Li: Formal analysis, Investigation. Meihui Zhao: Supervision, Writing – review & editing. Guanghua Xia: Conceptualization, Investigation, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by Primary Research & Development Plan of Hainan Province (no: ZDYF2020224).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2023.106511.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Li J., Jia X., Liu L., Cao X., Xiong Y., Yang Y., Zhou H., Yi M., Li M. Comparative biochemical and transcriptome analysis provides insights into the regulatory mechanism of striped leaf albinism in arecanut (Areca catechu L.) Indust. Crop. Prod. 2020;154:112734. [Google Scholar]
- 2.Volgin A.D., Bashirzade A., Amstislavskaya T.G., Yakovlev O.A., Demin K.A., Ho Y.-J., Wang D., Shevyrin V.A., Yan D., Tang Z., Wang J., Wang M., Alpyshov E.T., Serikuly N., Wappler-Guzzetta E.A., Lakstygal A.M., Kalueff A.V. DARK Classics in Chemical Neuroscience: Arecoline. ACS Chem. Nerosci. 2019;10:2176–2185. doi: 10.1021/acschemneuro.8b00711. [DOI] [PubMed] [Google Scholar]
- 3.Fan X., Jiang C., Dai W., Jing H., Du X., Peng M., Zhang Y., Mo L., Wang L., Chen X., Lou Z., Wang H. Effects of different extraction on the antibacterial and antioxidant activities of phenolic compounds of areca nut (husks and seeds) J. Food Meas. Charact. 2022;16:1502–1515. doi: 10.1007/s11694-021-01244-7. [DOI] [Google Scholar]
- 4.Wang C.K., Lee W.H. Separation, Characteristics, and Biological Activities of Phenolics in Areca Fruit. S. Afr. J. Educ. 2009;29:2014–2019. doi: 10.1021/jf950611o. [DOI] [Google Scholar]
- 5.Mei F., Meng K., Gu Z., Yun Y., Zhang W., Zhang C., Zhong Q., Pan F., Shen X., Xia G., Chen H. Arecanut (Areca catechu L.) Seed Polyphenol-Ameliorated Osteoporosis by Altering Gut Microbiome via LYZ and the Immune System in Estrogen-Deficient Rats. J. Agric. Food Chem. 2021;69:246–258. doi: 10.1021/acs.jafc.0c06671. [DOI] [PubMed] [Google Scholar]
- 6.Lee K.-K., Cho J.-J., Park E.-J., Choi J.-D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001;23(6):341–346. doi: 10.1046/j.0412-5463.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 7.Wetwitayaklung P., Phaechamud T., Limmatvapirat C., Keokitichai S. The study of antioxidant capacity in various parts of Areca catechu L. Naresun Univers. J. 2006;14:1–14. [Google Scholar]
- 8.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan S., Li C., Zhang Y., Yu H., Xie Y., Guo Y., Yao W. Ultrasound as an emerging technology for the elimination of chemical contaminants in food: A review. Trends Food Sci. Technol. 2021;109:374–385. doi: 10.1016/j.tifs.2021.01.048. [DOI] [Google Scholar]
- 10.Caldas T.W., Mazza K.E.L., Teles A.S.C., Mattos G.N., Brígida A.I.S., Conte-Junior C.A., Borguini R.G., Godoy R.L.O., Cabral L.M.C., Tonon R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crop. Prod. 2018;111:86–91. doi: 10.1016/j.indcrop.2017.10.012. [DOI] [Google Scholar]
- 11.Roselló-Soto E., Galanakis C.M., Brnčić M., Orlien V., Trujillo F.J., Mawson R., Knoerzer K., Tiwari B.K., Barba F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015;42:134–149. doi: 10.1016/j.tifs.2015.01.002. [DOI] [Google Scholar]
- 12.Heng M.Y., Tan S.N., Yong J.W.H., Ong E.S. Emerging green technologies for the chemical standardization of botanicals and herbal preparations. TrAC Trends Anal. Chem. 2013;50:1–10. doi: 10.1016/j.trac.2013.03.012. [DOI] [Google Scholar]
- 13.Vilkhu K., Mawson R., Simons L., Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry — A review. Innov. Food Sci. Emerg. Technol. 2008;9:161–169. doi: 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]
- 14.Qin L., Yu J., Zhu J., Kong B., Chen Q. Ultrasonic-assisted extraction of polyphenol from the seeds of Allium senescens L. and its antioxidative role in Harbin dry sausage. Meat Sci. 2021;172:108351. doi: 10.1016/j.meatsci.2020.108351. [DOI] [PubMed] [Google Scholar]
- 15.Zhong X., Zhang S., Wang H., Yang J., Li L., Zhu J., Liu Y. Ultrasound-alkaline combined extraction improves the release of bound polyphenols from pitahaya (Hylocereus undatus 'Foo-Lon') peel: Composition, antioxidant activities and enzyme inhibitory activity. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belwal T., Bhatt I.D., Cravotto G. Effect of ultrasound on extraction and stability of polyphenols from Berberis jaeschkeana C.K. Schneid fruits: A comparative study. Sustain. Chem. Pharm. 2022;27:100649. [Google Scholar]
- 17.Serna-Jiménez J.A., Torres-Valenzuela L.S., Sanín Villarreal A., Roldan C., Martín M.A., Siles J.A., Chica A.F. Advanced extraction of caffeine and polyphenols from coffee pulp: Comparison of conventional and ultrasound-assisted methods. LWT. 2023;177:114571. [Google Scholar]
- 18.Nayak B., Dahmoune F., Moussi K., Remini H., Dairi S., Aoun O., Khodir M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015;187:507–516. doi: 10.1016/j.foodchem.2015.04.081. [DOI] [PubMed] [Google Scholar]
- 19.Wang C., Meng M.X., Tang X.L., Chen K.M., Zhang L., Liu W.N., Zhao Y.Y. The proliferation, differentiation, and mineralization effects of puerarin on osteoblasts in vitro. Chin. J. Nat. Med. 2014;12:436–442. doi: 10.1016/S1875-5364(14)60068-6. [DOI] [PubMed] [Google Scholar]
- 20.Fang X., Wang J., Wang Y., Li X., Zhou H., Zhu L. Optimization of ultrasonic-assisted extraction of wedelolactone and antioxidant polyphenols from Eclipta prostrate L using response surface methodology. Sep. Purif. Technol. 2014;138:55–64. doi: 10.1016/j.seppur.2014.1010.1007. [DOI] [Google Scholar]
- 21.Contamine R.F., Wilhelm A.M., Berlan J., Delmas H. Power measurement in sonochemistry. Ultrason. Sonochem. 1995;2:S43–S47. doi: 10.1016/1350-4177(1094)00010-P. [DOI] [Google Scholar]
- 22.Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021;70 doi: 10.101016/j.ultsonch.102020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.W. Wu, S. Jiang, M. Liu, S. Tian, Simultaneous process optimization of ultrasound-assisted extraction of polyphenols and ellagic acid from pomegranate (Punica granatum L.) flowers and its biological activities. Ultrason. Sonochem. 80 (2021) 105833, Doi: 105810.101016/j.ultsonch.102021.105833. [DOI] [PMC free article] [PubMed]
- 24.Wang X., Liu X., Shi N., Zhang Z., Chen Y., Yan M., Li Y. Response surface methodology optimization and HPLC-ESI-QTOF-MS/MS analysis on ultrasonic-assisted extraction of phenolic compounds from okra (Abelmoschus esculentus) and their antioxidant activity. Food Chem. 2023;405 doi: 10.1016/j.foodchem.2022.134966. [DOI] [PubMed] [Google Scholar]
- 25.Liu F., Ma C., Gao Y., McClements D.J. Food-Grade Covalent Complexes and Their Application as Nutraceutical Delivery Systems: A Review. Compr. Rev. Food Sci. Food Saf. 2017;16:76–95. doi: 10.1111/1541-4337.12229. [DOI] [PubMed] [Google Scholar]
- 26.Al-Shabib N.A., Khan J.M., Malik A., Alsenaidy M.A., Rehman M.T., AlAjmi M.F., Alsenaidy A.M., Husain F.M., Khan R.H. Molecular insight into binding behavior of polyphenol (rutin) with beta lactoglobulin: Spectroscopic, molecular docking and MD simulation studies. J. Mol. Liq. 2018;269:511–520. doi: 10.1016/j.molliq.2018.1007.1122. [DOI] [Google Scholar]
- 27.Rohn S. Possibilities and limitations in the analysis of covalent interactions between phenolic compounds and proteins. Food Res. Int. 2014;65:13–19. doi: 10.1016/j.foodres.2014.05.042. [DOI] [Google Scholar]
- 28.Karacabey E., Mazza G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 2010;119:343–348. doi: 10.1016/j.foodchem.2009.1006.1029. [DOI] [Google Scholar]
- 29.Huaman-Castilla N.L., Martinez-Cifuentes M., Camilo C., Pedreschi F., Mariotti-Celis M., Perez-Correa J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carmenere Pomace Extracts. Molecules. 2019;24:3145. doi: 10.3390/molecules24173145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezk B.M., Haenen G.R.M.M., van der Vijgh W.J.F., Bast A. The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 2002;295:9–13. doi: 10.1016/S0006-1291X(1002)00618-00616. [DOI] [PubMed] [Google Scholar]
- 31.Hang L., Huan G., Qiong L., DingTao W., Liang Z., Yi L., HuaBin L., RenYou G. Current extraction, purification, and identification techniques of tea polyphenols: An updated review. Crit. Rev. Food Sci. Nutr. 2021:11–19. doi: 10.1080/10408398.10402021.11995843. [DOI] [PubMed] [Google Scholar]
- 32.Imran A., Arshad M.U., Arshad M.S., Imran M., Saeed F., Sohaib M. Lipid peroxidation diminishing perspective of isolated theaflavins and thearubigins from black tea in arginine induced renal malfunctional rats. Lipids Health Dis. 2018;17:157. doi: 10.1186/s12944-018-0808-3. 110.1186/s12944-12018-10808-12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Song H., Dan J., Li J., Du J., Xiao J., Xu J. Experimental study on the cutting force during laser-assisted machining of fused silica based on the Taguchi method and response surface methodology. J. Manuf. Process. 2019;38:9–20. doi: 10.1016/j.jmapro.2018.1012.1038. [DOI] [Google Scholar]
- 35.Patel K., Mangu S.R., Sukhdeo S.V., Sharan K. Ethanolic extract from the root and leaf of Sida cordifolia promotes osteoblast activity and prevents ovariectomy-induced bone loss in mice. Phytomedicine. 2022;99 doi: 10.1016/j.phymed.2022.154024. [DOI] [PubMed] [Google Scholar]
- 36.Lezcano V., Morelli S., Gonzalez-Pardo V. Molecular and cellular outcomes of quercetin actions on healthy and tumor osteoblasts. Biochimie. 2022;199:46–59. doi: 10.1016/j.biochi.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Alcantara E.H., Shin M.-Y., Sohn H.-Y., Park Y.-M., Kim T., Lim J.-H., Jeong H.-J., Kwon S.-T., Kwun I.-S. Diosgenin stimulates osteogenic activity by increasing bone matrix protein synthesis and bone-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells. J. Nutr. Biochem. 2011;22:1055–1063. doi: 10.1016/j.jnutbio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Orimo H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon Med. Sch. 2010;77:4–12. doi: 10.1272/jnms.77.4. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Luo S., Zhang D., Qu X., Tan Y. Sika pilose antler type I collagen promotes BMSC differentiation via the ERK1/2 and p38-MAPK signal pathways. Pharm. Biol. 2017;55:2196–2204. doi: 10.1080/13880209.2017.1397177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J.-L., Kang S.-W., Kang M.-K., Gong J.-H., Lee E.-S., Han S.J., Kang Y.-H. Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts. J. Cell. Biochem. 2012;113(1):247–259. doi: 10.1002/jcb.23351. [DOI] [PubMed] [Google Scholar]
- 41.A. Patti, L. Gennari, D. Merlotti, F. Dotta, R. Nuti, Endocrine Actions of Osteocalcin. International Journal of Endocrinology. 2013 (2013) 846480, Doi: 10.1155/2013/846480. [DOI] [PMC free article] [PubMed]
- 42.Sardar A., Ansari A., Koneni S., Trivedi R. Identification of a new class of BMP-2 targeted bone anabolic agent. Endocr. Rev. 2020;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [Google Scholar]
- 43.Canalis E., Economides A.N., Gazzerro E. Bone Morphogenetic Proteins, Their Antagonists, and the Skeleton. Endocr. Rev. 2003;24:218–235. doi: 10.1021/jp076582p. [DOI] [PubMed] [Google Scholar]
- 44.Stein G.S., Lian J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993;14:424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 45.Sudo H., Kodama H.A., Amagai Y., Yamamoto S., Kasai S. In vitro differentiation and calcification in a newclonal osteogenic cell line derived from new born mouse calvaria. J. Cell Biol. 1983;96(1):191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song L., Zhao J., Zhang X., Li H., Zhou Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013;714:15–22. doi: 10.1016/j.ejphar.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 47.Park K., Ju W.C., Yeo J.H., Kim J.Y., Seo H.S., Uchida Y., Cho Y. Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation. Int. J. Mol. Med. 2014;33:178–184. doi: 10.3892/ijmm.2013.1557. [DOI] [PubMed] [Google Scholar]
- 48.W. Karunarathne, I.M.N. Molagoda, K.T. Lee, Y.H. Choi, C.Y. Jin, G.Y. Kim, Anthocyanin-enriched polyphenols from Hibiscus syriacus L. (Malvaceae) exert anti-osteoporosis effects by inhibiting GSK-3beta and subsequently activating beta-catenin. Phytomedicine. 91 (2021) 153721, http://doi.org/10.1016/j.phymed.2021.153721. [DOI] [PubMed]
- 49.Meng K., Mei F., Zhu L., Xiang Q., Quan Z., Pan F., Xia G., Shen X., Yun Y., Zhang C., Zhong Q., Chen H. Arecanut (Areca catechu L.) seed polyphenol improves osteoporosis via gut-serotonin mediated Wnt/β-catenin pathway in ovariectomized rats. J. Funct. Foods. 2021;84:104598. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.