Summary
Vessels that encapsulate tumour clusters (VETC) is a distinct histologic vascular pattern associated with a novel mechanism of metastasis. First described in human cancers in 2004, its prevalence and prognostic significance in hepatocellular carcinoma (HCC) has only been appreciated in the past decade with a rapidly increasing body of literature. A robust biomarker of aggressive disease, the VETC pattern is easy to recognise but relies on histologic examination of tumour tissue for its diagnosis. Radiological recognition of the VETC pattern is an area of active research and is becoming increasingly accurate. As a prognostic marker, VETC has consistently proven to be an independent predictor of disease recurrence and overall survival in patients with HCC undergoing resection and liver transplantation. It can also guide treatment by predicting response to other therapies such as transarterial chemoembolisation and sorafenib. Without prospective randomised-controlled trials or routine evaluation of VETC in clinical practice, there are currently no firm treatment recommendations for VETC-positive tumours, although some perspectives are provided in this review based on the latest knowledge of their pathogenesis – a complex interplay between tumour angiogenesis and the immune microenvironment. Nevertheless, VETC has great potential as a future biomarker that could take us one step closer to precision medicine for HCC.
Keywords: Vessels that encapsulate tumour clusters (VETC), angiogenesis, biomarker, metastasis, liver transplantation, epithelial mesenchymal transition (EMT)
Key points.
-
•
Vessels that encapsulate tumour clusters (VETC) is a novel and prevalent histologic pattern seen in HCC.
-
•
VETC-positive HCCs form under the influence of angiopoietin 2 and metastasise via an invasion-independent mechanism where tumour clusters directly embolise into the bloodstream.
-
•
Compared to VETC-negative HCCs, VETC-positive HCCs are consistently larger in size, higher in number and have higher levels of serum tumour markers such as α-fetoprotein.
-
•
VETC-positive HCCs exhibit distinctive features on contrast-enhanced ultrasound, CT, and MRI scans. With the help of radiomics and machine learning, VETC may be diagnosed non-invasively in the future.
-
•
VETC positivity has been repeatedly shown to be an independent negative prognostic factor in patients with HCC undergoing resection, liver transplantation and systemic therapy.
-
•
Knowing the VETC status of a patient’s tumour may have treatment implications in the future in terms of closer surveillance, the addition of (neo)adjuvant therapy, and/or the choice of systemic therapy. However, more research is needed.
Introduction
Hepatocellular carcinoma (HCC) is a major cause of clinical and economic burden globally owing to its high incidence and mortality rate. In 2020, liver cancer (85% HCC) was the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide.1 Despite advances in treatment across all disease stages over the past decade, its prognosis remains poor with 5-year relative survival rates of only 20%.2 For patients with HCC presenting with early-stage disease, curative therapies are associated with 5-year survival rates of ≥70%.3 However, their long-term benefit is limited by disease recurrence, which is either common in the case of resection and ablation (up to 80%) or incurable in the case of liver transplant(ation) (LT).4 At the other end of the disease spectrum, several combination systemic therapies have demonstrated superiority to sorafenib in recent phase III clinical trials of patients with unresectable HCC(5-8). Despite these promising results, an objective response is seen in a minority of patients (any response in 20-27% and complete response in 1-5%) and the search for predictive biomarkers in this cohort is ongoing.9 Therefore, robust prognostic biomarkers which can predict disease recurrence and response to systemic therapies would be highly valuable to guide patient selection for HCC treatment.
A focus of recent interest in the HCC literature has been a frequently observed vascular pattern known as vessels that encapsulate tumour clusters (VETC). The significance of this distinct histological finding is increasingly being recognised as reflected by an exponentially growing number of studies reporting on it (one study in 2015, 16 studies in 2022). Importantly, VETC has been shown to be an independent poor prognostic factor both in early-stage (patients undergoing resection and LT) and more advanced (patients receiving sorafenib) disease.10,11 In this review, we synthesise the published data regarding the epidemiology, pathogenesis, and prognostic value and clinical utility of VETC in patients with HCC and provide perspective on its potential as a future biomarker.
Angiogenesis in HCC
HCCs are typically hypervascular tumours. Like other solid tumours, HCC cannot grow beyond a few millimetres in size without angiogenesis.12 During the progression of a regenerative nodule to a dysplastic nodule to HCC, there is an increasing degree of arterial neovascularisation from recruitment of unpaired arteries driven by angiogenic factors such as vascular endothelial growth factor (VEGF).13 These tumour vessels are both functionally and structurally abnormal.
The clinical importance of angiogenesis in HCC is reflected by the use of treatments targeting tumour vasculature as one of the main strategies in intermediate- and advanced-stage HCC. These include transarterial chemoembolisation (TACE), small molecule kinase inhibitors (all of which inhibit key mediators of angiogenesis) and most recently, monoclonal antibodies directed against VEGF or its receptor.6,13,14 Additionally, markers of angiogenesis such as microvessel density (MVD), vasculogenic mimicry and VEGF expression have demonstrated prognostic value in some studies.[15], [16], [17] However, these markers have not been incorporated into routine clinical practice due to difficulty in obtaining measurements and mixed data regarding their prognostic value.
Nomenclature, definition and prevalence of VETC
Nomenclature
Although the term VETC was first used by Fang et al. in 2015(11), Sugino et al. first described “tumor nests surrounded by blood vessels” or “sinusoidal tumor angiogenesis” in humans as early as 2004(18). The authors reported an invasion-independent pathway of metastasis (i.e., without the need for epithelial-mesenchymal transition [EMT]) in HCC involving intravasation of tumour nests which travel to secondary sites as tumour emboli without penetration of the vascular wall (discussed in detail later). Although this phenomenon can be seen in at least ten common human cancers, its prevalence was highest among HCC, follicular thyroid carcinoma and renal cell carcinoma.18 In work arising from the same research group that coined the term VETC, but several years earlier, Ding et al. used the term “endothelium coated tumour clusters” to portray VETC and were the first to demonstrate its prognostic value in HCC after curative resection.19 Experimentally, VETC has also been observed in tumours arising from murine Hepa1-6 (but not H22) HCC cell lines, as well as human melanoma and murine mammary cell lines (as early as 1993).11,20,21 Since 2015, the nomenclature has been consistent in using the term VETC to describe this distinct vascular pattern.
Definition
The VETC pattern is a histological diagnosis and is relatively simple to stain for, identify and quantify on sections of HCC tissue. In most studies, the VETC pattern is defined as a continuous lining of sinusoid-like vessels that isolate and encapsulate individual tumour clusters, forming a cobweb-like pattern.11,22 This is in contrast to the classically described capillary vessels (Fig. 1A-D). Compositionally, VETC consists of endothelial cells (CD31 or CD34 positive), hepatic stellate cells (alpha-smooth muscle actin positive), and basement membrane,11 similar to that of capillary vessels. While it is possible to recognise the VETC pattern on routine H&E-stained sections,23 almost all studies highlight the endothelium with immunohistochemical staining using anti-CD34 antibody. VETC can also be easily distinguished using antibodies against CD31 (five studies) or CD105 (one study).19 The pattern is easy to recognise by pathologists with excellent interobserver agreement (kappa values 0.823-0.912) reported consistently across several studies both on H&E-stained and immunohistochemistry-stained sections.19,[23], [24], [25] In our experience, the addition of immunohistochemistry enables the VETC pattern to be screened for more rapidly at low magnification than on H&E sections.
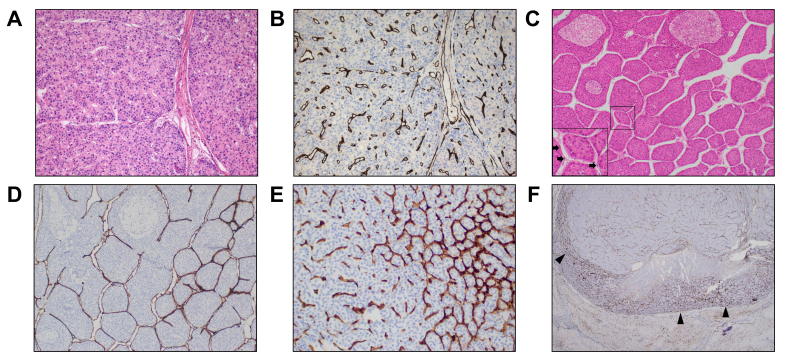
Fig. 1.
Different vascular patterns in HCC.
(A) H&E and (B) CD34 immunostaining of HCC tissue sections from explants of patients who have undergone liver transplantation showing a VETC-negative HCC with CD34 staining of capillaries only and no cluster formation; (C) Routine H&E section of a macrotrabecular massive, VETC-positive HCC with black arrows in the inset pointing to endothelial cells; (D) The same macrotrabecular massive, VETC-positive HCC after CD34 immunostaining highlighting a distinct, continuous CD34 endothelial lining around individual tumour clusters; (E) A mixed HCC with an area of VETC (right of image) transitioning to an adjacent area of HCC consisting of capillary staining (left of image); (F) An example of an HCC with marginal VETC pattern where the main locus of VETC is located at the tumour margins (black arrowheads). A-D: 100x magnification; E: 200x magnification; F: 20x magnification.
Although most studies report VETC as a dichotomous (positive or negative) variable, based on the presence of any (≥1%) VETC pattern seen within a tumour, several variations deserve mention. One study designated a third “intermediate” group of HCCs with features between VETC-positive and VETC-negative cases consisting of “gyrus-like vessels with sporadic distribution of VETC”(26). True to its name, this group had a prognosis between that of VETC-positive and VETC-negative tumours. As VETC is usually not homogeneous throughout an entire tumour (Fig. 1E), another study makes the distinction between cases where the VETC pattern is fully distributed across the HCC section, cases with a “mixed” vascular pattern and those without VETC seen in the HCC section, which also has prognostic significance.27 Finally, several studies adopted a value of ≥55% tumour area as the “optimal” cut-off to define VETC-positive vs. VETC-negative phenotypes to predict prognosis.24,26,[28], [29], [30], [31], [32] However, the original reference for this cut-off comes from a study which evaluated VETC using tissue microarrays24 which may not have the same applicability in the other studies which used whole sections. Indeed, the true prevalence and severity of VETC can only be quantified using resection or explant specimens and not tissue microarrays or liver biopsies (discussed later). For the remainder of this review, the term VETC-positive tumours will refer to HCCs containing any VETC structures within them while VETC-negative tumours refer to HCCs with a complete absence of VETC structures unless otherwise stated. However, this highlights the need for future studies to identify or validate an optimal cut-off to define VETC positivity on whole sections, tissue microarrays and liver biopsies.
Prevalence
The reported prevalence of VETC positivity in HCC tends to vary with the study population and differences in definitions. Indeed, there can be a two-to eight-fold difference in VETC prevalence depending on the cut-offs used to define it (based on percentage of tumour area exhibiting the VETC pattern).24 Among resection with curative-intent cohorts (using presence of any VETC as the cut-off), the reported prevalence of VETC-positive HCCs ranges from 14.2-56.3% (mostly 30-40%).11,19,24,25,27,29,[33], [34], [35], [36] In patients presenting with recurrent disease after their initial R0 (microscopically margin-negative) resection, the prevalence of VETC positivity was slightly higher: 55.5% for those with early recurrence undergoing repeat resection or radiofrequency ablation (RFA) and 48.9% in those with recurrence requiring sorafenib treatment.10,28 Two studies have reported on VETC in patients with unresectable HCC undergoing LT. Our group observed a VETC-positive prevalence of 76.5% on liver explants of patients undergoing deceased-donor LT (DDLT) for HCC (<4% outside Milan LT criteria at the time of listing).37 In contrast, a cohort study of living-donor LT (LDLT) recipients reported a VETC-positive HCC prevalence of 22%, even though the majority of patients had tumour burdens beyond any current transplant criteria (either DDLT or LDLT).38
Thus, the prevalence of VETC appears to broadly correlate with tumour stage and aggressiveness: 30-40% in patients receiving resection, 50-55% in patients with recurrent disease post-resection and up to 76% in patients with unresectable disease undergoing LT (Table 1). The notable exception is the aforementioned 22% prevalence seen in the Japanese cohort of LDLT recipients. A multinational cohort study of resection patients also observed a lower VETC prevalence among Japanese individuals when using the ≥55% of tumour area cut-off to define VETC positivity: 23.5% prevalence in Italians, 21.5% in Koreans and 8.7% in Japanese patients.24 Indeed, the reported VETC prevalence among studies of Japanese patients undergoing curative resection (using a cut-off of any VETC seen) is 8.7-23.8% which is lower than that seen in other (mostly Chinese) studies.23,24,26 Whether this observation is related to race or the aetiology of liver disease (predominately chronic hepatitis C infection in Japanese cohorts vs. chronic hepatitis B infection in Chinese cohorts) is currently unclear.
Table 1.
Clinical studies evaluating outcomes according to VETC pattern in patients with HCC.
| Study | Patient cohort (n) | Treatment setting | Prevalence (definition of VETC positivity) | Outcome measures | Prognostic value c.f. VETC-negative |
|---|---|---|---|---|---|
| Ding et al. 2011(19) | Chinese (239) 88% CHB |
Curative-intent resection | 45.2% (any VETC) | OS TTR |
Yes on MV analysis (HR 1.949) Yes on MV analysis (HR 2.085) |
| He et al. 2017(27) | Chinese (168) 89% CHB |
Surgical candidates without extrahepatic metastases | 26.2% (any VETC)∗
|
OS RFS |
Yes on MV analysis (HR 1.674) Yes on MV analysis (HR 1.625) |
| Fang et al. 2019(10) | Chinese (457) 92% CHB |
Recurrence and/or metastases after initial resection | 48.5% (any VETC)
|
OS PRS |
Yes on MV analysis (HR 1.495) Yes on MV analysis (HR 1.409) |
| Renne et al. 2020(24) | Italian (98) Korean (316) Japanese (127) Total (541) 52% CHB, 35% HCV |
Curative-intent resection | 39.0% (≥5% VETC)∗ 18.9% (≥55% VETC)
|
OS DFS Early recurrence (≤2 years) |
Yes on MV analysis (HR 2.26). Yes on MV analysis (HR 1.66) Yes on MV analysis (HR 1.52) |
| Kawasaki et al. 2021(38) | Japanese (150) 71% HCV |
Living-donor liver transplant | 22.0% (any VETC) | OS RFS |
Yes on UV analysis Yes on UV analysis Combination of VETC-positive and low CD3+ was independent predictor of OS on MV analysis (HR 2.760) |
| Lu et al. 2021(32) | Chinese (498) 87% CHB |
Curative-intent resection | 22.3% (≥55% VETC) | OS DFS |
Yes for VETC-positive/MVI-positive on MV analysis (HR 3.39 compared to VETC-negative/MVI-negative) Yes for VETC-positive/MVI-positive on MV analysis (HR 3.53 compared to VETC-negative/MVI-negative) |
| Chen et al. 2021(28) | Chinese (326) 87% CHB |
RFA or repeat resection for early-stage recurrence after initial curative-intent resection | 55.5% (any VETC) 36.5% (≥55% VETC) |
OS DFS |
Yes on MV analysis (HR 1.486) Yes on MV analysis (HR 1.454) |
| Akiba et al. 2021(23) | Japanese (985) Aetiology not stated |
Resection (unspecified) | 23.8% (≥5% VETC)
|
OS DFS |
Yes on UV analysis, not on MV analysis Yes on UV analysis, not on MV analysis |
| Lin et al. 2021(31) | Chinese (498) 87% CHB |
Curative-intent resection | 22.2% (≥55% VETC) | RFS | Yes on MV analysis (HR 1.853) |
| Ridder et al. 2022(48) | German (561) Alcohol 31%, HCV 20%, CHB 19% |
Resection (unspecified) | Not stated∗ | OS | Yes on MV analysis (HR 1.5) |
| Dennis et al. 2022(37) | Australian (158) 55% HCV |
Deceased-donor liver transplant | 76.5% (any VETC) | RFS TTR |
Yes for number of VETC-positive HCCs on explant on MV analysis (HR 1.267). Yes for number of VETC-positive HCCs on explant on MV analysis (HR 1.411). |
| Yu et al. 2022(25) | Chinese (182) 88% viral hepatitis |
Resection (unspecified) | 56.3% (any VETC) | Early recurrence (<2 years)-free survival PFS |
Yes on UV analysis. Yes on UV analysis |
| Huang et al. 2022(34) | Chinese (174) 51% HCV, 49% CHB |
Curative-intent resection | 30.5% (any VETC) | DFS | Yes on MV analysis (HR 2.066) |
| Feng et al. 2022(33) | Chinese (170) 83% CHB |
Resection or liver transplant (unspecified) | 30.6% (any VETC) | Early recurrence (<2 years)-free survival | Yes on MV analysis (HR 1.9) |
| Zhang et al. 2022(26) | Japanese66 44% HCV, 29% CHB |
Resection (unspecified) | 24.2% (≥50% VETC) | OS Metastasis RFS |
Yes on UV analysis Yes on UV analysis. No on UV analysis |
| Wang et al. 2022(36) | Chinese (262) 82% CHB |
Resection (unspecified) | 44.2% (any VETC) | OS TTR |
Yes on UV analysis Yes on UV analysis |
CHB, chronic hepatitis B; DFS, disease-free survival; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; K-M, Kaplan-Meier; MV, multivariable; MVI, microvascular invasion; OS, overall survival; PRS, post-recurrence survival; RFA, radiofrequency ablation; RFS, recurrence-free survival; TACE, transarterial chemoembolisation; TTR, time-to-recurrence; UV, univariable; VETC, vessels that encapsulate tumour clusters.
Studies using tissue microarrays.
Pathogenesis of VETC-positive HCCs
Formation of VETC
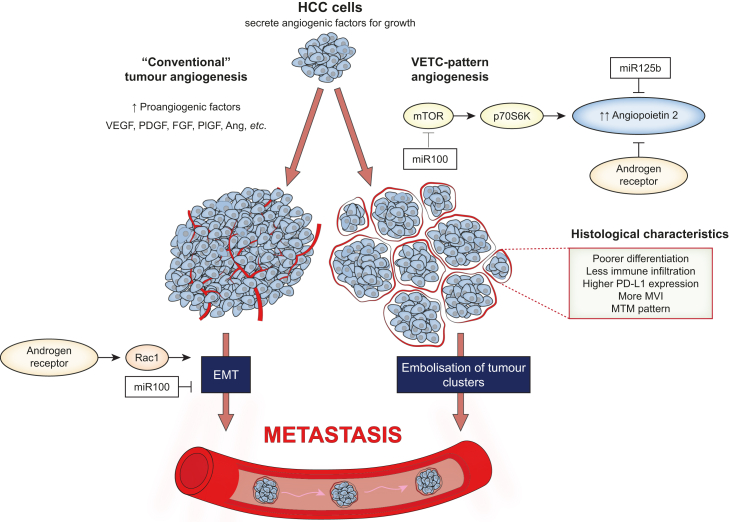
VETC formation is particularly dependent on angiopoietin 2 (Ang2), a growth factor belonging to the angiopoietin/Tie signalling pathway which controls the later events of angiogenesis such as vessel assembly, maturation and quiescence11 (Fig. 2). It is normally secreted by endothelial cells in non-cancer tissue but it is also secreted by cancer cells in HCC. Fang et al. demonstrated that VETC-positive HCCs had significantly higher Ang2 levels than VETC-negative HCCs in humans and knockdown of tumour-cell derived Ang2 expression disrupted VETC formation and reduced metastases (but not growth of the primary tumour) in mouse xenograft models.11 Subsequently, the association between VETC formation and high Ang2 levels has been confirmed by several other human studies.22,[38], [39], [40], [41]
Fig. 2.
Pathogenesis of VETC-positive HCCs.
VETC formation is particularly dependent on angiopoietin 2 which is influenced by miR-100, miR-125b, and the androgen receptor. Instead of the traditional EMT pathway for metastasis, VETC-positive HCCs can metastasise in an invasion-independent manner via embolisation of tumour clusters. Ang, angiopoietins; EMT, epithelial mesenchymal transition; FGF, fibroblast growth factor; HCC, hepatocellular carcinoma; mTOR, mammalian target of rapamycin; MVI, microvascular invasion; MTM, macrotrabecular massive; PDGF, platelet-derived growth factor; PD-L1, programmed death-ligand 1; PlGF, placental growth factor; VEGF, vascular endothelial growth factor; VETC, vessels that encapsulate tumour clusters.
Several factors have also been shown to influence VETC formation via its interaction with Ang2. Expression of microRNAs miR-125b and miR-100 were found to be inversely related to VETC formation in both human HCC specimens and mouse xenograft models.41 Further investigation revealed that miR-125b supressed Ang2 expression via direct binding while miR-100 achieved the same indirectly by inhibiting the mammalian target of rapamycin (mTOR) signalling pathway. Recently, the same research group went on to discover a relationship between androgen receptor expression and VETC formation.22 The androgen receptor was shown to decrease Ang2 transcription, VETC formation and invasion-independent metastases by directly binding to the Ang2 promoter. Interestingly, the authors revealed the androgen receptor to be a double-edged sword as it also promoted cellular migration and invasion-dependent metastases by upregulating Rac1 expression in VETC-negative HCCs.
Aside from Ang2, fibroblast growth factor (FGF) and its receptors (FGFR) 3 and 4 are also significantly elevated in VETC-positive HCC tissue.26,39 Other angiogenic factors such as angiopoietin 1, Tie-2, VEGF receptor (VEGFR) 2 and VEGF-A are present at similar levels in VETC-positive and VETC-negative tumours,11,26 although VEGF-A appears to be upregulated in the subset of VETC-positive tumours with a macrotrabecular massive (MTM) pattern on histology.39 C-X-C chemokine receptor type 4 expression on tumour endothelial cells has been demonstrated to promote vessel sprouting and also correlates with VETC formation.42 Although VETC prevalence correlates with the expression of angiogenic factors, not all HCCs with high angiogenic factors reliably form VETC patterns and those which fail to do so exhibit a T helper 1/cytotoxic T lymphocyte-related inflammatory infiltrate.39 Thus, the immune system may also play a role in VETC formation by inhibiting angiogenic signals.
Mechanism of metastasis
As mentioned above, metastasis of VETC-positive tumours can occur through the release of whole tumour clusters as endothelium-coated microemboli into the bloodstream.11 This is in contrast to the traditional method of metastasis through the process of EMT where a tumour cell loses its cell-cell adhesion, dissociates from the primary tumour, acquires the capacity to migrate and invade and gains entry into the bloodstream. Indeed, metastasis of VETC-positive HCCs has been shown to be independent of EMT and its indicators (loss of E-Cadherin, and increased Snail, Slug, and Twist).11,19,27 Of note, in tumours with both VETC-positive and VETC-negative components, the vast majority (>80%) of metastases are VETC-negative or “uncoated” microemboli.27 Since HCC metastases retain the VETC status of their primary tumour (discussed below), this suggests that metastases from mixed HCCs come from tumour cells outside of the VETC structures and it is likely that EMT is still the main pathway for metastasis. Interestingly, in a study of the histopathology of 130 extrahepatic metastases, the most common pathological pattern seen in lung metastases was VETC positivity, while most lymph node metastases had features of EMT(43).
Sugino et al. first noticed that metastases from VETC-positive primary tumours conserved their tissue structure (i.e., retained their VETC positivity).44 Interestingly, xenografts of human VETC-positive cells in nude mice retain their original VETC pattern, even as the human endothelial cells are replaced by mouse endothelial cells.11 It has been hypothesised that the VETC coating provides an “integrated ecosystem” that can protect or shield tumour cells from anoikis (apoptosis upon loss of attachment with the extracellular matrix), shearing forces in the bloodstream and immunological attack.44 Accordingly, it has been observed that VETC-positive metastases exhibited higher proliferation (as measured by Ki-67 expression) and lower apoptosis (as determined by TUNEL) compared to VETC-negative metastases.19 This protection of tumour cells by VETC may partly explain the poorer prognosis associated with it.
Immunosuppressive tumour microenvironment
There exists a reciprocal interaction between tumour angiogenesis and the anti-tumour response.13,39,45 Neovascularisation produces leaky vessels with abnormal flow which give rise to interstitial hypertension, oedema and tumour hypoxia. This hostile environment is associated with an impaired anti-tumour response characterised by reduced infiltration of cytotoxic CD8+ T cells, recruitment of immunosuppressive cells (regulatory T cells, myeloid-derived suppressor cells, M2-like macrophages) and upregulation of immune checkpoints.13
An immunosuppressive tumour microenvironment is also seen in VETC-positive tumours. A reduction in inflammatory (T cell) intratumoural infiltrate has been consistently described in VETC-positive tumours.24,[38], [39], [40],43 They also exhibit almost five-fold higher rates of programmed death-ligand 1 (PD-L1) positivity.35 Gene set-enrichment analysis of HCCs using RNA-seq data showed gene sets related to CD8+ T cell antigen receptor, B cell antigen receptor, interferon-γ and interferon-α pathways were significantly enriched in VETC-negative compared to VETC-positive tumours, suggesting a phenotype with lower immune activation in the latter.26 Using hierarchical cluster analysis of immunohistochemistry and gene expression readouts of both immune and angiogenesis variables, Kurebayashi et al. developed a novel immunovascular classification of HCC, stratifying cases into four distinct subtypes: immune-high/angiostatic, immune-mid/angio-mid, immune-low/angiogenic and immune-low/angio-low.39 Akin to the other results, the VETC pattern was almost entirely observed only in the immune-low subtypes (angiogenic > angio-low) whereas it was infrequently seen (<10% prevalence) in the immune-mid/angio-mid group and did not exist at all in the immune-high/angiostatic group. As the name suggests, the immune-low/angiogenic group was associated with lower intratumoural lymphocytic infiltration. In contrast, primary and metastatic HCCs high in EMT-related markers exhibit high immune infiltration.43
The mechanism behind the heightened immunosuppressive tumour microenvironment in VETC-positive tumours remains unresolved from these initial studies. Although tumour hypoxia may play a role, one study found no difference in hypoxia inducible factor 1-α (the main regulator of cellular response to hypoxia) between VETC-positive and VETC-negative HCCs.36 Conversely, increased expression of carbonic anhydrase IX (a hypoxia marker) has been observed in tumours with a sinusoid-like microvascular (i.e., VETC-positive) pattern compared to those with a capillary-like microvascular pattern.46 Clearly, more investigative work is required in this space.
Association with existing HCC subclasses
Several groups have examined where VETC-positive HCCs fit into existing HCC molecular classification systems. VETC-positive tumours are more often associated with CTNNB1 mutations and activation of the Wnt/β-catenin signalling pathway than with other subclasses, although only a minority (26-37%) of Wnt/β-catenin-positive tumours are VETC positive.24,26,39,40 Wnt/β-catenin-positive HCCs typically exhibit glutamine synthetase positivity, a “cold” immunosuppressive microenvironment, and high FGF2 expression all of which are characteristic of VETC-positive tumours.24,39,40 Correspondingly, VETC-positive tumours are also associated with Wnt/β-catenin subclasses in current molecular classification systems (i.e., Hoshida S1 and Boyault G5/6).24,39 Although Wnt/β-catenin-positive tumours are typically well-differentiated, VETC positivity appears to select out those with poorer differentiation and worse prognosis.40 That said, one group found no correlation between VETC, Wnt/β-catenin signalling and any of the Hoshida, Chiang and Fujita subtypes. However, this study was limited by the small numbers of patients with HCC (n = 66) for whom whole-genome sequencing and RNA-seq data were available for analysis.26 The same authors also reported a higher frequency of mutations in the RAS/phosphatidylinositol 3-kinase/mTOR pathway and fewer mutations in chromatin remodelling in VETC-positive tumours – findings which require further validation. Other molecular markers associated with VETC include transforming growth factor-activated kinase 147.
Clinicopathologic features of VETC-positive tumours
Clinical features
In both resection and LT cohorts, VETC positivity is associated with greater tumour burden. Compared to VETC-negative tumours, VETC-positive tumours are consistently larger in size (in particular, more tumours >5 cm) and appear in higher numbers (Table 2).10,19,27,30,34,37,38 Correspondingly, VETC-positive tumours are associated with a more advanced stage and significantly higher levels of serum tumour markers (α-fetoprotein [AFP], des-γ-carboxy prothrombin, protein induced by vitamin K absence II).11,19,24,30,40,48 Among LT recipients, those with VETC-positive tumours spent longer on the waitlist and were more likely to be outside standard transplant listing criteria based on explant histology.37 There seems to be no correlation between VETC status and aetiology of liver disease, cirrhosis status and degree of liver dysfunction. At least three studies have reported an association between elevated aspartate aminotransferase (AST) and VETC positivity.30,49,50
Table 2.
Clinicopathologic associations with VETC-positive HCCs.
| Category | Feature associated with VETC |
|---|---|
| Tumour burden | Tumour size >5 cm Higher tumour number/multiplicity More intrahepatic and lung metastases More advanced stage: BCLC, TNM, AJCC Outside of established LT listing criteria |
| Laboratory | AST >40 U/L Higher tumour markers in serum: AFP, DCP, PIVKA-II |
| Radiology | Increased tumour size Irregular rim-like enhancement Intratumoural necrosis Various MRI quantitative features not consistent across all studies |
| Histology | Poorly differentiated (Edmondson grade III-IV) Reduced inflammatory (T cell) infiltration PD-L1 positivity Increased microvascular density Microvascular invasion Macrotrabecular and MTM pattern |
AFP, α-fetoprotein; AJCC, American Joint Committee on Cancer; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; DCP, des-γ-carboxy prothrombin; HCC, hepatocellular carcinoma; MTM, macrotrabecular massive; PD-L1, programmed death-ligand 1; PIVKA-II, protein induced by vitamin K absence II; VETC, vessels that encapsulate tumour clusters.
In terms of clinical predictors of the VETC pattern, a large study of 385 patients with HCC (24% VETC-positive using a cut-off of ≥55% tumour area) aimed to determine independent predictors using routine peripheral blood and imaging results. These included: AFP level, tumour diameter, lymphocyte to monocyte ratio >7.75, neutrophil count >7x109/L, AST to alanine aminotransferase (ALT) ratio >0.86, and ALT to lymphocyte ratio index >21.7. Based on these variables, the authors went on to develop a nomogram which achieved acceptable areas under the receiver operating characteristic curve (AUROC) of 0.70-0.75 in training and validation cohorts.30 Another group confirmed both AFP and tumour diameter (but no other clinical parameters) were independent predictors of VETC in their cohort of 182 Chinese patients.25 Through the use of machine learning algorithms, these authors constructed clinical models using these two independent predictors and achieved AUROCs of 0.73-0.85 for VETC prediction. While these initial clinical models appear promising, none are currently sufficient for routine clinical practice.
Radiologic features
Radiological prediction of the VETC pattern is a flourishing area of active research with nine retrospective studies (seven using pre-operative MRI, one using pre-operative CT, and one using pre-operative contrast-enhanced ultrasound [CEUS]) of resection cohorts published in the last three years.25,29,33,46,[49], [50], [51], [52], [53] All MRI studies except one used the hepatobiliary-specific contrast agent gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA).
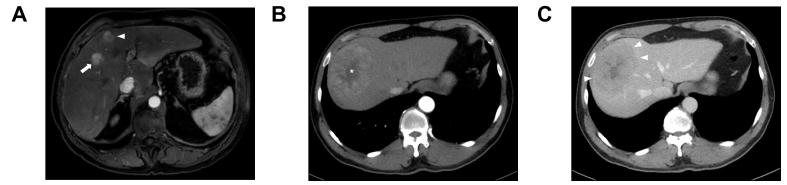
The earliest study investigated an atypical enhancement pattern known as “irregular rim-like enhancement” (IRE) seen on the arterial phase of an MRI scan46 (Fig. 3A). The authors demonstrated that 55% of tumours with sinusoid-like microvascular pattern (another name for the VETC pattern) had IRE on the arterial phase of an MRI scan compared to 0% with the traditional capillary-like microvascular pattern. IRE was also associated with other histologic features of VETC-positive tumours including poor differentiation, MTM pattern, and microvascular invasion. Prognostically, like VETC, patients with IRE HCCs had significantly worse recurrence-free survival after curative resection compared to those with non-IRE HCCs. On CEUS, VETC-positive HCCs exhibit a “crack and tendon-like” filling character and significantly longer filling times during the arterial phase compared to a “large scale and diffuse-like” filling character seen in VETC-negative tumours.53
Fig. 3.
Radiological features of VETC-positive HCCs.
(A) An axial arterial phase image of two HCCs on Gd-EOB-DTPA-enhanced MRI: one with peripheral irregular rim-like enhancement which has been associated with VETC positivity (white arrowhead) next to one with a more homogeneous enhancement associated with VETC negativity (white arrow). The patient subsequently received a liver transplant and explant pathology confirmed the respective VETC statuses associated with these two HCCs. (B) An axial arterial and (C) portal venous phase image of an 8 cm HCC on a contrast-enhanced CT scan with radiological features associated with the VETC pattern: size >5 cm, intratumoural necrosis (asterisk) and well-defined capsule (white arrowheads). The HCC was subsequently resected with histology demonstrating a VETC-positive HCC.
The largest radiology study of VETC to date (n = 320, 156 with any intratumoural VETC present), reported tumour size, heterogeneous enhancement with either septum-like or irregular ring-like structures and the presence of necrosis as independent MRI predictors of the VETC pattern on histology.49 A nomogram constructed based on these variables achieved a C-index of 0.86-0.87 and prognostic value in predicting the VETC pattern. Similarly, the only study to examine VETC using contrast-enhanced CT also demonstrated increased tumour size and presence of intratumoural necrosis to be independent predictors of VETC-positive tumours (Fig. 3B,C). The authors combined these into a predictive size-necrosis score with AUROCs of 0.69-0.72(33).
Several other promising VETC predictive models achieving AUROCs >0.80 using largely quantitative MRI features (i.e., not discernible by human eyes) have been created.25,29,50,51 Perhaps reflecting differences in patient demographics and/or MRI protocols between institutions, no quantitative MRI feature has been consistently reported to be associated with VETC. Two of these radiomic studies employed machine learning to help develop their models. The first study applied a three-dimensional convolutional neural network to Gd-EOB-DTPA-enhanced MRI images from a cohort of only 133 patients with HCC (44 VETC-positive) and their deep learning algorithm was able to predict VETC with an AUROC of 0.86(29). Another study extracted 1,316 quantitative features from intratumoural and peritumoral regions of HCCs on Gd-EOB-DTPA-enhanced MRI and used four different machine learning algorithms to generate impressive AUROCs (>0.90).25 In an interesting series of comparisons, the authors demonstrated that the model using peritumoral radiomics features (AUROC 0.97) outperformed the intratumoural model (AUROC 0.92) at diagnosing VETC, while combining the two provided no added predictive value. Additionally, all radiomics models (peritumoral, intratumoural, or both combined) outperformed the clinical machine learning model based on AFP and tumour diameter discussed in the section above (AUROCs 0.92-0.97 vs. 0.73). This highlights the likely key role for radiology in non-invasive VETC assessment in the future.
Histologic features
Aside from having less immune infiltration,24,[38], [39], [40] several other histologic features are associated with VETC. In keeping with their aggressiveness, VETC-positive tumours are more poorly differentiated (Edmondson grade III-IV) than VETC-negative tumours.19,24,27,38 Unsurprisingly, since VETC formation results from tumour angiogenesis and vascular remodelling, its presence also correlates with tumour MVD(18, 34). An intratumoural MVD cut-off of >40 vessels/mm2 could predict the VETC pattern with an AUROC of 0.69, sensitivity of 86.3% and specificity of 46.1%.34 In contrast, VETC does not appear to impact the vasculature of the surrounding non-tumour liver tissue. The significance of VETC was first recognised due to its association with increased rates of microvascular invasion, which can be seen in up to 80% of VETC-positive tumours.10,24,28,34 As discussed previously, these metastases retain their endothelial coating and represent tumour microemboli rather than direct vessel invasion.
Another common histologic association with the VETC pattern is the macrotrabecular pattern (trabeculae ≥6 cells thick in ≥20% of tumour area) and its more severe subtype the MTM pattern (macrotrabecular pattern in ≥50% of tumour area) (Fig. 1C,D).24,32,37,39 VETC-positive tumours and MTM tumours often overlap and share many markers of aggressiveness such that some studies have analysed them as one phenotype.39 However, unlike VETC, the independent prognostic value of MTM remains controversial.24,32,37 MTM tumours are also different at a molecular level, being significantly associated with TP53 mutations while VETC-positive tumours are associated with Wnt/β-catenin pathway activation.24
Prognostic value of VETC in HCC
Patients undergoing resection
Multiple studies have established VETC as a robust prognostic marker in patients undergoing hepatectomy independent of other clinical and pathologic variables (Table 1). Of note, the patients in these studies were retrospectively recruited from pre-existing resection cohorts. Although the majority (70-85%) had early-stage (Barcelona Clinic Liver Cancer [BCLC] stage 0 or A) disease, some included patients had intermediate- (BCLC stage B) or even advanced-stage (BCLC stage C) disease where surgical resection is not recommended according to current guidelines.54 Nonetheless, VETC was found to be a significant predictor of not only HCC recurrence, but also overall survival (OS), with reported hazard ratios (HRs) of between 1.5-2.5 for patients with VETC-positive vs. VETC-negative tumours.19,24,27,31,32,34 This prognostic value is also true of surrogate (radiological) markers of VETC as discussed above.25,29,33,46 There appears to be a dose-response relationship between the amount of VETC and poorer outcomes. When VETC was evaluated as a continuous variable in increments of 5% of tumour area on tissue microarrays instead of a categorical variable (yes vs. no), it was independently associated with both disease-free survival and OS (HR 1.02 per 5% area increase for both).24 A possible exception to the above are Japanese patients and/or patients with hepatitis C infection, in whom the prognostic value of VETC is uncertain. There are three Japanese cohorts (predominantly patients with hepatitis C) which did not demonstrate VETC to be an independent predictor of recurrence-free survival or OS on multivariable analyses.23,24,26 A Taiwanese cohort study showed that the presence or absence of the VETC pattern was associated with significantly different disease-free survival in patients with hepatitis B but not hepatitis C infections.34
VETC positivity portends a similar prognosis to having microvascular invasion and the combination of these two factors (diagnosed either histologically or radiologically) improves the predictive performance for HCC recurrence and OS compared to either factor alone.29,31,32,52 In a study of 498 patients with HCC who received curative resection, the presence of VETC, microvascular invasion, tumour number and maximum tumour size were found to be independent predictors of recurrence-free survival.31 A nomogram constructed based on these four variables (VMNS score) achieved time-dependent AUROCs of >0.71 in both internal and external validation cohorts across the first 5 years post-resection. Furthermore, the VMNS score outperformed seven current HCC prognostic scoring systems.
Other enhancements have been made to the way VETC patterns are classified to improve their prognostic accuracy. One group found prognostic significance in the localisation pattern of the VETC in the tumour section.40 Patients with a marginal VETC pattern (where VETC touches tumour margins and the main locus is at the margin, Fig. 1F) had worse disease-free survival compared to internal and combined patterns. The addition of MTM assessment to VETC (VETC/MTM-positive tumours) has also been shown to be an independent poor prognostic factor regardless of histological grade or subclass of HCC(39). In intrahepatic cholangiocarcinoma, combining a tumour’s VETC and PD-L1 status into a VETC/PD-L1 index led to better delineation of OS and time-to-recurrence rates.55 This index was an independent predictor of survival and recurrence on multivariable analysis. In HCC, PD-L1 expression in the tumour has been associated with poorer clinical outcomes and also significantly correlated with the VETC pattern.35 Therefore, a VETC/PD-L1 index should perform similarly well in HCC although this is yet to be tested.
Patients with unresectable disease undergoing LT
To date, there have been two studies evaluating the VETC pattern in LT recipients. A Japanese study examined 150 patients (33 VETC-positive) who underwent LDLT. The inclusion criteria for LT in this study was exceptionally liberal (absence of extrahepatic metastases and “major vascular invasion”). Correspondingly, the mean number of tumours in the VETC-positive group was 26.9 with a mean AFP level of 2,697 kIU/ml. Even the mean number of tumours in the VETC-negative group was 7.1. Nonetheless, the authors found that VETC positivity was associated with significantly worse OS and recurrence-free survival.38 Like others, VETC was shown to correlate inversely with intratumoural lymphocytic immune infiltrate and the combination of VETC (positive or negative) and CD3 (low or high) could further stratify patient prognosis post-LT compared to evaluating VETC alone. Our group studied 158 patients (121 VETC-positive) undergoing DDLT in Australia. We found that although VETC positivity alone was not associated with time-to-recurrence or recurrence-free survival, the total number of VETC-positive HCCs seen on the explant was an independent predictor of both on multivariable analysis (HR 1.3-1.4 per VETC-positive tumour present).37 This supports the notion of a dose-response relationship between the extent of VETC and tumour recurrence.
Patients with advanced-stage (recurrent) disease
There are no studies on VETC specifically in patients with advanced (BCLC stage C) HCC. Although some resection cohorts mentioned above included a small proportion of these patients,24 their outcomes were not reported separately. The closest study to examine this population followed a cohort of patients with HCC and “recurrent and/or metastatic disease” after their initial hepatectomy who received sorafenib and a well-matched control cohort who did not receive sorafenib.10 The assessment of VETC was based on histology from the initial hepatectomy and therefore the VETC status of the recurrent and/or metastatic disease was not known. Again, VETC proved to be an independent predictor of mortality after adjusting for BCLC stage, AFP, age and sorafenib treatment. Interestingly, sorafenib treatment was associated with significant improvements in OS in patients with VETC-positive but not VETC-negative HCCs. The greatest benefit was seen in those with more aggressive cancers (microvascular invasion, size >5 cm, AFP >400 kIU/ml, and BCLC stage B or C). Even though this suggests VETC might be a useful biomarker to select patients who may benefit from sorafenib (or similar multikinase inhibitors), care needs to be taken in interpreting these results. Since the evaluation of VETC is based on the original resection specimen in patients who presented with resectable HCC which subsequently recurred (and not on biopsies before sorafenib treatment), these results cannot be extrapolated to patients who present initially with advanced-stage disease. It is possible these recurrences are not VETC-positive, especially if the original tumour had mixed VETC-positive and VETC-negative components, as discussed previously. Furthermore, these retrospective associations require validation in a prospective randomised-controlled trial.
Treatment considerations for VETC-positive HCCs
All the research on VETC so far has been retrospective and focussed on its use as a prognostic marker. However, some therapeutic strategies can still be deduced for VETC-positive HCCs (Table 3).
Table 3.
Potential treatment considerations in patients with VETC-positive HCC for validation or exploration with future research.
| Stage of disease | VETC assessment | Treatment consideration for VETC-positive tumour |
|---|---|---|
| Early stage resectable for hepatectomy | Radiology from CT or MRI | Neoadjuvant therapy to treat micrometastases |
| Histology from resection specimen | More intense surveillance for recurrence post-resection Adjuvant therapy to reduce recurrence (e.g., TACE∗) More aggressive treatment of early recurrences (e.g., repeat resection >RFA∗) |
|
| Early stage unresectable for LT | Radiology from CT or MRI | Neoadjuvant therapy to treat micrometastases |
| Histology from liver explant | More intense surveillance for recurrence post-LT Introduction of mTOR inhibitor Adjuvant therapy to reduce recurrence |
|
| Intermediate stage | Radiology from CT or MRI Histology from liver biopsy# |
? Earlier transition to systemic therapy |
| Advanced stage | Radiology from CT or MRI Histology from liver biopsy# |
? Preference for multikinase inhibitors over immunotherapy |
Currently no data based on liver biopsy diagnosis of VETC, only resection specimens and tissue microarrays).
Already some evidence to support this approach but needs validation.
Multikinase inhibitors (sorafenib and lenvatinib)
As mentioned above, recurrences derived from originally resectable VETC-positive tumours demonstrated improved survival on sorafenib compared with control.10 The mechanism of benefit is unclear but it does not appear to result from activation of Raf/MEK/ERK and VEGF-A/VEGFR 2/ERK signalling or induction of autophagy.10 Lenvatinib is a multikinase inhibitor whose targets include VEGFR 1-3, and FGFR 1-4 among others.56 According to gene expression analyses of VETC-positive tumours, FGF2 and FGFR 3 and 4 (but not VEGF-A or VEGFR 1-3) are significantly elevated compared to VETC-negative tumours.26,40 This suggests that VETC-positive tumours might respond to lenvatinib treatment. Furthermore, a recent study of patients with renal cell carcinoma receiving multikinase inhibitors (sunitinib or pazopanib) also demonstrated much longer progression-free survival in those with VETC-positive tumours compared to VETC-negative tumours.57
Therapies targeting vasculature
VETC results from abnormal tumour angiogenesis. Therefore, it would seem logical to target the vasculature. Because VEGF-A and VEGFR expression are not significantly different based on VETC pattern,11,26 the effectiveness of anti-VEGF/VEGFR therapies such as bevacizumab or ramucirumab for VETC-positive HCCs is doubtful. Instead, the multikinase inhibitors mentioned above have anti-angiogenic actions (mediated by non-VEGF pathways e.g., inhibition of FGF/FGFR or platelet-derived growth factor receptor) which may partially explain their effectiveness in VETC-positive but not VETC-negative cancers.
Since VETC is dependent on Ang2 for its formation, Ang2 inhibitors may hold promise in treating VETC-positive HCCs. Ang2 inhibitors have already been tested either alone or in combination with cytotoxic chemotherapies, bevacizumab or immune checkpoint inhibitors in phase I clinical trials of advanced melanoma and solid malignancies.58,59 As these trials and recent positive phase III data in HCC suggest,[5], [6], [7], [8] it is likely that a combination approach is necessary. Although VETC-positive tumours have high levels of angiogenic factor expression, high levels of angiogenic factors on their own do not portend poorer prognosis.39 Thus, other oncological factors also play a role and merely inhibiting Ang2 alone is unlikely to improve outcomes. Since most tumours are not entirely VETC positive,27 treatments which inhibit Ang2 and the VETC component of HCC may paradoxically promote tumour progression and metastasis in the non-VETC component of the tumour via the traditional EMT pathway.22 Indeed, the EMT pathway has been shown to be the dominant mode of metastases in mixed tumours with both VETC-positive and VETC-negative components, suggesting traditional HCC treatments are still required in addition to anti-VETC agents.27 This may partially explain why mixed tumours have demonstrated worse prognosis compared to tumours which are wholly VETC positive.27
Immune checkpoint inhibitors
VETC-positive tumours are associated with CTNNB1 mutations and the Wnt/β-catenin signalling pathway activation.24,26,39,40,60 In HCC, so called “hot” tumours containing tumour-infiltrating lymphocytes are known to be more responsive to immunotherapies. Conversely, Wnt/CTNNB1 mutations characterise “cold” tumours from the immune-excluded class, which are typically resistant to immunotherapies.61 Indeed, VETC-positive tumours exhibit reduced immune infiltrate and low immune activation (i.e., immune-low/angiogenic subtype) which would suggest they may derive reduced benefit from immune checkpoint inhibitors.24,26,39 Of recent interest, the vast majority of patients with HCC included in VETC studies have viral hepatitis as their underlying liver disease which is associated with better response and improved outcomes when treated with checkpoint inhibitors compared to patients with non-viral aetiology.62 Therefore, the VETC pattern may be a useful biomarker to further stratify patients with HCC and viral hepatitis in terms of response to immunotherapies.
The immunosuppressive microenvironment of HCC can be manipulated via its aforementioned reciprocal relationship with tumour vasculature.45 Many groups have previously demonstrated that normalising (rather than starving) tumour vessels can increase intratumoural lymphocyte infiltration and enhance the therapeutic effects of immune checkpoint inhibitors.[63], [64], [65], [66] Thus, checkpoint inhibitors may still be beneficial for VETC-positive HCCs if they are given in combination with bevacizumab (which can normalise tumour vasculature initially before pruning it67) or another vascular normalising therapy.39 This again points to the need for combination therapies.
Treatment and prevention of recurrent disease
VETC status may offer some guidance on the prevention and treatment of HCC recurrence. Recently, a Chinese group investigated the effectiveness of prophylactic adjuvant TACE in the remnant liver within 2 months after curative-intent hepatectomy in a retrospective cohort study. The authors demonstrated significant benefits with adjuvant TACE in terms of time-to-recurrence and OS for patients with VETC-positive (but not VETC-negative) HCCs. Adjuvant TACE was an independent predictor of OS among the former. Although the role of TACE post-hepatectomy is controversial, with reported experiences largely limited to Asia,68 this study suggests a subgroup of patients may benefit. It also provides the basis for further evaluating VETC in those with intermediate-stage (BCLC stage B) HCC. In another retrospective study of 326 patients with early-stage (BCLC stage A) recurrence after hepatectomy with curative intent, the outcomes of patients who underwent repeat hepatic resection vs. RFA were compared according to their VETC status.28 The patients across the four arms were well-matched. Among patients with VETC-positive HCCs at their initial resection, repeat hepatic resection was associated with significantly better OS and disease-free survival compared to RFA. Although resection is known to be associated with lower recurrence compared to ablation overall,4 it is interesting that this difference was not seen in patients with VETC-negative HCCs at initial resection. Despite inherent limitations with selection bias in the two retrospective studies just discussed, they both support the notion that the VETC pattern may identify an aggressive subset of HCC such that adjuvant therapy (i.e., TACE) and more aggressive therapy (i.e., repeat resection over RFA) should be considered for the prevention and treatment of recurrences, respectively.
mTOR inhibitors have been recommended for LT recipients at high risk of HCC recurrence.69 This may be particularly beneficial in patients with VETC-positive tumours on explant since mTOR inhibition has been shown to disrupt the mTOR-p70S6K signalling pathway which downregulates Ang2 expression to inhibit VETC formation (or re-formation in this setting).
Finally, given the observed responses of VETC-positive tumours to multikinase inhibitors,10 they may be considered before and after curative resection or LT as (neo)adjuvant therapy to treat micrometastases and reduce recurrence. Although current literature does not support this approach,70 none of the existing studies assess or stratify patients based on their VETC status. At the very least, increased intensity of surveillance post-resection or LT needs to be considered. Ultimately, all of the above treatment possibilities require exploration and confirmation through prospective randomised-controlled trials.
Future directions
One of the main limitations of VETC assessment is its reliance on histology, particularly resection or explant specimens. Although there are typical imaging features and predictive nomograms (both clinical and radiologic) that can be applied to diagnose VETC non-invasively with reasonable accuracy,30,49,50 these are still not ready for routine clinical practice. Furthermore, Gb-EOB-DTPA MRI (on which almost all radiomic studies are based) is not universally accessible, especially in low- and middle-income countries where HCC is most prevalent.1 We look forward to seeing the potential benefits of artificial intelligence, which could be applied to improving the diagnostic accuracy of MRI assessments for VETC or perhaps to enabling the diagnosis to be made using more readily available investigations (e.g., a panel of blood biomarkers, CT scan or CEUS). Indeed, research groups have already started exploring this with promising results that require external validation.25,29
Although the VETC pattern has proven to be a robust prognostic marker in patients undergoing resection and LT, its utility in other stages of HCC remains unclear. Its ability to predict treatment response in patients undergoing adjuvant TACE and those receiving sorafenib for recurrence post-resection holds promise for patients with intermediate-stage HCC undergoing TACE and advanced-stage HCC undergoing systemic therapy, respectively.10,36 Therefore, future studies of VETC are needed in these settings. As most cases of HCC can be diagnosed radiologically without the need to obtain tissue, this would require biopsies of intermediate- and advanced-stage HCC to assess a patient’s VETC status until radiological diagnosis of VETC becomes sufficiently reliable (discussed above). Furthermore, the role of liver biopsy in evaluating VETC has not been established and remains a question for future research. Almost all prior studies diagnosed VETC by histological examination of whole sections of resection specimens. However, at least three studies have also reliably assessed VETC using 1-2 mm wide tissue microarrays.24,27,48 A reasonable correlation coefficient of 0.642 has been reported when comparing VETC quantification between surgical specimens and tissue microarrays, which suggests that liver biopsy may be a feasible (albeit less accurate) substitute for the diagnosis of VETC.24
To date, there have only been three HCC cohorts outside of Asia in which VETC was examined: an Italian resection cohort, a German resection cohort and an Australian LT cohort.24,37,48 As such, the vast majority of patients studied have been from China, Japan and Korea, and have viral HCC. Therefore, to improve the generalisability of the current VETC findings to other ethnicities and non-viral liver diseases, further studies also need to be conducted in these populations.
Ultimately, the goal is to be able to apply precision medicine to HCC treatment and VETC is a promising biomarker that can take us one step closer. The next phase of studies calls for a shift towards prospective trials investigating interventions specific for VETC-positive (and VETC-negative) HCCs, including some of those discussed in the section above. This will also require VETC (or its surrogate marker) to be included among the baseline variables documented in major HCC clinical trials of new and existing treatments.
The crosstalk between tumour vasculature and the immune microenvironment has already been discussed. Although there is some evidence of an immunosuppressive tumour microenvironment in VETC-positive HCCs, this is based on simple, low-dimensional assessments (i.e., lower numbers of T cell lymphocyte infiltration) which fails to capture the complexity of the immune landscape and its cellular interactions.24,35,[38], [39], [40] Therefore, more sophisticated and comprehensive techniques (e.g., imaging mass cytometry, spatial transcriptomics) are needed to adequately study the VETC-positive tumour microenvironment and enhance our understanding of tumour biology.71 The more recent studies are already moving toward this with the use of multiplex immunohistochemistry and hierarchical cluster analyses of both immune and angiogenic factors to help phenotype HCC into distinct subtypes.39
Finally, as the clinical value of VETC is realised in HCC, there is also growing interest in studying VETC across other cancers (e.g., renal cell carcinoma, follicular thyroid carcinoma, adrenal carcinoma, breast cancer and melanoma). Human studies have already demonstrated the prognostic value of VETC in intrahepatic cholangiocarcinoma and renal cell carcinoma, and further studies are planned for patients with adrenal carcinoma.55,57,60
Conclusion
VETC is a common histological vascular pattern found in HCC which can also be predicted radiologically. It is associated with an aggressive phenotype owing to its unique mechanism of metastasis and immunosuppressive tumour microenvironment. VETC holds robust prognostic value and can guide treatment decisions in patients undergoing resection, LT and potentially systemic therapy. On these bases, this novel biomarker demands the attention of the hepatology community. Further research opportunities exist, especially regarding its specific treatment and performance in certain populations.
Financial support
FM-W is supported by the International Society for the Advancement of Cytometry (ISAC) Marylou Ingram Scholars programme. Otherwise, the authors received no financial support to produce this manuscript.
Authors’ contributions
KL and GWM conceived the article. KL and CD drafted the initial manuscript. All authors critically revised the manuscript and approved the final version.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
Dr. Samer Ghattas helped select radiology images of VETC-positive HCCs.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://10.1016/j.jhepr.2023.100792.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Pinna A.D., Yang T., Mazzaferro V., De Carlis L., Zhou J., Roayaie S., et al. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann Surg. 2018;268:868–875. doi: 10.1097/SLA.0000000000002889. [DOI] [PubMed] [Google Scholar]
- 4.Vogel A., Meyer T., Sapisochin G., Salem R., Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Alfa G.K., Lau G., Kudo M., Chan S.L., Kelley R.K., Furuse J., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1:1–12. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 6.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 7.Ren Z., Xu J., Bai Y., Xu A., Cang S., Du C., et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 8.Qin S., Chan L.S., Gu S., Bai Y., Ren Z., Lin X., et al. LBA35 - Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. 2022;33:S808–S869. [Google Scholar]
- 9.Xu W., Liu K., Chen M., Sun J.Y., McCaughan G.W., Lu X.J., et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11 doi: 10.1177/1758835919862692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang J.H., Xu L., Shang L.R., Pan C.Z., Ding J., Tang Y.Q., et al. Vessels that encapsulate tumor clusters (VETC) pattern is a predictor of sorafenib benefit in patients with hepatocellular carcinoma. Hepatology. 2019;70:824–839. doi: 10.1002/hep.30366. [DOI] [PubMed] [Google Scholar]
- 11.Fang J.H., Zhou H.C., Zhang C., Shang L.R., Zhang L., Xu J., et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62:452–465. doi: 10.1002/hep.27760. [DOI] [PubMed] [Google Scholar]
- 12.Hlatky L., Hahnfeldt P., Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 13.Liu K., Zhang X., Xu W., Chen J., Yu J., Gamble J.R., et al. Targeting the vasculature in hepatocellular carcinoma treatment: starving versus normalizing blood supply. Clin Transl Gastroenterol. 2017;8:e98. doi: 10.1038/ctg.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu A.X., Kang Y.K., Yen C.J., Finn R.S., Galle P.R., Llovet J.M., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 15.Pang R., Poon R.T. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151–167. doi: 10.1016/j.canlet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Sun B., Zhang S., Zhang D., Du J., Guo H., Zhao X., et al. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16:693–698. [PubMed] [Google Scholar]
- 17.Poon R.T., Ng I.O., Lau C., Yu W., Yang Z., Fan S., et al. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775–1785. doi: 10.1200/JCO.2002.07.089. [DOI] [PubMed] [Google Scholar]
- 18.Sugino T., Yamaguchi T., Ogura G., Saito A., Hashimoto T., Hoshi N., et al. Morphological evidence for an invasion-independent metastasis pathway exists in multiple human cancers. BMC Med. 2004;2:9. doi: 10.1186/1741-7015-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding T., Xu J., Zhang Y., Guo R.P., Wu W.C., Zhang S.D., et al. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117:4878–4889. doi: 10.1002/cncr.26137. [DOI] [PubMed] [Google Scholar]
- 20.Sugino T., Kawaguchi T., Suzuki T. Sequential process of blood-borne lung metastases of spontaneous mammary carcinoma in C3H mice. Int J Cancer. 1993;55:141–147. doi: 10.1002/ijc.2910550125. [DOI] [PubMed] [Google Scholar]
- 21.Küsters B., Kats G., Roodink I., Verrijp K., Wesseling P., Ruiter D.J., et al. Micronodular transformation as a novel mechanism of VEGF-A-induced metastasis. Oncogene. 2007;26:5808–5815. doi: 10.1038/sj.onc.1210360. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H.C., Liu C.X., Pan W.D., Shang L.R., Zheng J.L., Huang B.Y., et al. Dual and opposing roles of the androgen receptor in VETC-dependent and invasion-dependent metastasis of hepatocellular carcinoma. J Hepatol. 2021;75:900–911. doi: 10.1016/j.jhep.2021.04.053. [DOI] [PubMed] [Google Scholar]
- 23.Akiba J., Nakayama M., Sadashima E., Kusano H., Kondo R., Mihara Y., et al. Prognostic impact of vessels encapsulating tumor clusters and macrotrabecular patterns in hepatocellular carcinoma. Pathol Res Pract. 2022;238:154084. doi: 10.1016/j.prp.2022.154084. [DOI] [PubMed] [Google Scholar]
- 24.Renne S.L., Woo H.Y., Allegra S., Rudini N., Yano H., Donadon M., et al. Vessels encapsulating tumor clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology. 2020;71:183–195. doi: 10.1002/hep.30814. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y., Fan Y., Wang X., Zhu M., Hu M., Shi C., et al. Gd-EOB-DTPA-enhanced MRI radiomics to predict vessels encapsulating tumor clusters (VETC) and patient prognosis in hepatocellular carcinoma. Eur Radiol. 2022;32:959–970. doi: 10.1007/s00330-021-08250-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P., Ono A., Fujii Y., Hayes C.N., Tamura Y., Miura R., et al. The presence of vessels encapsulating tumor clusters is associated with an immunosuppressive tumor microenvironment in hepatocellular carcinoma. Int J Cancer. 2022;151:2278–2290. doi: 10.1002/ijc.34247. [DOI] [PubMed] [Google Scholar]
- 27.He C., Zhou Z., Jiang H., Yin Z., Meng S., Zhang J., et al. Epithelial-mesenchymal transition is superior to vessels-encapsulate tumor cluster in promoting metastasis of hepatocellular carcinoma: a morphological evidence. J Cancer. 2017;8:39–47. doi: 10.7150/jca.16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z.Y., Guo Z.X., Lu L.H., Mei J., Lin W.P., Li S.H., et al. The predictive value of vessels encapsulating tumor clusters in treatment optimization for recurrent early-stage hepatocellular carcinoma. Cancer Med. 2021;10:5466–5474. doi: 10.1002/cam4.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu T., Zhao C., Zhang J., Duan K., Li M., Zhang T., et al. Application of a convolutional neural network for multitask learning to simultaneously predict microvascular invasion and vessels that encapsulate tumor clusters in hepatocellular carcinoma. Ann Surg Oncol. 2022;29:6774–6783. doi: 10.1245/s10434-022-12000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan R., Lin W., Zou J., Mei J., Wen Y., Lu L., et al. Development and validation of a novel nomogram for predicting vessels that encapsulate tumor cluster in hepatocellular carcinoma. Cancer Control. 2022;29:1–13. doi: 10.1177/10732748221102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin W.P., Xing K.L., Fu J.C., Ling Y.H., Li S.H., Yu W.S., et al. Development and validation of a model Including distinct vascular patterns to estimate survival in hepatocellular carcinoma. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L., Wei W., Huang C., Li S., Zhong C., Wang J., et al. A new horizon in risk stratification of hepatocellular carcinoma by integrating vessels that encapsulate tumor clusters and microvascular invasion. Hepatol Int. 2021;15:651–662. doi: 10.1007/s12072-021-10183-w. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z., Li H., Zhao H., Jiang Y., Liu Q., Chen Q., et al. Preoperative CT for characterization of aggressive macrotrabecular-massive subtype and vessels that encapsulate tumor clusters pattern in hepatocellular carcinoma. Radiology. 2021;300:219–229. doi: 10.1148/radiol.2021203614. [DOI] [PubMed] [Google Scholar]
- 34.Huang C.W., Lin S.E., Huang S.F., Yu M.C., Tang J.H., Tsai C.N., et al. The vessels that encapsulate tumor clusters (VETC) pattern is a poor prognosis factor in patients with hepatocellular carcinoma: an analysis of microvessel density. Cancers. 2022;14:5428. doi: 10.3390/cancers14215428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh S., Yoshizumi T., Yugawa K., Imai D., Yoshiya S., Takeishi K., et al. Impact of immune response on outcomes in hepatocellular carcinoma: association with vascular formation. Hepatology. 2020;72:1987–1999. doi: 10.1002/hep.31206. [DOI] [PubMed] [Google Scholar]
- 36.Wang J.H., Li X.S., Tang H.S., Fang R.Y., Song J.J., Feng Y.L., et al. Vessels that encapsulate tumor clusters (VETC) pattern predicts the efficacy of adjuvant TACE in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2022 doi: 10.1007/s00432-022-04323-4. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Dennis C., Prince D.S., Moayed-Alaei L., Remash D., Carr-Boyd E., Bowen D.G., et al. Association between vessels that encapsulate tumour clusters vascular pattern and hepatocellular carcinoma recurrence following liver transplantation. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.997093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki J., Toshima T., Yoshizumi T., Itoh S., Mano Y., Wang H., et al. Prognostic impact of vessels that encapsulate tumor cluster (VETC) in patients who underwent liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2021;28 doi: 10.1245/s10434-021-10209-5. [DOI] [PubMed] [Google Scholar]
- 39.Kurebayashi Y., Matsuda K., Ueno A., Tsujikawa H., Yamazaki K., Masugi Y., et al. Immunovascular classification of HCC reflects reciprocal interaction between immune and angiogenic tumor microenvironments. Hepatology. 2022;75:1139–1153. doi: 10.1002/hep.32201. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda K., Kurebayashi Y., Masugi Y., Yamazaki K., Ueno A., Tsujikawa H., et al. Immunovascular microenvironment in relation to prognostic heterogeneity of WNT/β-catenin-activated hepatocellular carcinoma. Hepatol Res. 2023;53:344–356. doi: 10.1111/hepr.13869. [DOI] [PubMed] [Google Scholar]
- 41.Zhou H.C., Fang J.H., Shang L.R., Zhang Z.J., Sang Y., Xu L., et al. MicroRNAs miR-125b and miR-100 suppress metastasis of hepatocellular carcinoma by disrupting the formation of vessels that encapsulate tumour clusters. J Pathol. 2016;240:450–460. doi: 10.1002/path.4804. [DOI] [PubMed] [Google Scholar]
- 42.Xu J., Liang J., Meng Y.M., Yan J., Yu X.J., Liu C.Q., et al. Vascular CXCR4 expression promotes vessel sprouting and sensitivity to sorafenib treatment in hepatocellular carcinoma. Clin Cancer Res. 2017;23:4482–4492. doi: 10.1158/1078-0432.CCR-16-2131. [DOI] [PubMed] [Google Scholar]
- 43.Woo H.Y., Rhee H., Yoo J.E., Kim S.H., Choi G.H., Kim D.Y., et al. Lung and lymph node metastases from hepatocellular carcinoma: comparison of pathological aspects. Liver Int. 2021;42:199–209. doi: 10.1111/liv.15051. [DOI] [PubMed] [Google Scholar]
- 44.Sugino T., Yamaguchi T., Hoshi N., Kusakabe T., Ogura G., Goodison S., et al. Sinusoidal tumor angiogenesis is a key component in hepatocellular carcinoma metastasis. Clin Exp Metastasis. 2008;25:835–841. doi: 10.1007/s10585-008-9199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santhakumar C., Gane E.J., Liu K., McCaughan G.W. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol Int. 2020;14:947–957. doi: 10.1007/s12072-020-10104-3. [DOI] [PubMed] [Google Scholar]
- 46.Rhee H., An C., Kim H.Y., Yoo J.E., Park Y.N., Kim M.J. Hepatocellular carcinoma with irregular rim-like arterial phase hyperenhancement: more aggressive pathologic features. Liver Cancer. 2019;8:24–40. doi: 10.1159/000488540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridder D.A., Urbansky L.L., Witzel H.R., Schindeldecker M., Weinmann A., Berndt K., et al. Transforming growth factor-β activated kinase 1 (Tak1) is activated in hepatocellular carcinoma, mediates tumor progression, and predicts unfavorable outcome. Cancers. 2022;14:430. doi: 10.3390/cancers14020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridder D.A., Weinmann A., Schindeldecker M., Urbansky L.L., Berndt K., Gerber T.S., et al. Comprehensive clinicopathologic study of alpha fetoprotein-expression in a large cohort of patients with hepatocellular carcinoma. Int J Cancer. 2022;150:1053–1066. doi: 10.1002/ijc.33898. [DOI] [PubMed] [Google Scholar]
- 49.Chen F.M., Du M., Qi X., Bian L., Wu D., Zhang S.L., et al. Nomogram estimating vessels encapsulating tumor clusters in hepatocellular arcinoma from peroperatie gadoxetate disodium-enhanced MRI. J Magn Reson Imaging. 2023;57:1893–1905. doi: 10.1002/jmri.28488. [DOI] [PubMed] [Google Scholar]
- 50.Fan Y., Yu Y., Hu M., Wang X., Du M., Guo L., et al. Imaging features based on Gd-EOB-DTPA-enhanced MRI for predicting vessels encapsulating tumor clusters (VETC) in patients with hepatocellular carcinoma. Br J Radiol. 2021;94 doi: 10.1259/bjr.20200950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y., Yu Y., Wang X., Hu M., Du M., Guo L., et al. Texture analysis based on Gd-EOB-DTPA-enhanced MRI for identifying vessels encapsulating tumor clusters (VETC)-positive hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:349–359. doi: 10.2147/JHC.S293755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J., Dong X., Wang G., Chen J., Zhang B., Pan W., et al. Preoperative MRI features for characterization of vessels encapsulating tumor clusters and microvascular invasion in hepatocellular carcinoma. Abdom Radiol. 2023;48:554–566. doi: 10.1007/s00261-022-03740-w. [DOI] [PubMed] [Google Scholar]
- 53.Lan C.Y., Ling B., Guo W.W., Yin W., Zhong X.G., Han Y.M., et al. The relationship between vimentin protein expression in endothelial cells and contrast-enhanced ultrasound chatacters in VETC (+) hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2018;40:105–109. doi: 10.3760/cma.j.issn.0253-3766.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado A., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao P., Ma L., Xue R., Wang H., Zhang S. Clinicopathological and prognostic implications of vessels encapsulate tumor clusters with PD-L1 in intrahepatic cholangiocarcinoma patients. Transl Cancer Res. 2020;9:3550–3563. doi: 10.21037/tcr.2020.04.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 57.Renne S.L., Valeri M., Perrino M., Di Tommaso L., Terracciano L., Colombo P., et al. Prognostic and predictive value of vessels encapsulating tumor clusters (VETC) in renal cell carcinoma (RCC) J Clin Oncol. 2021;39 [Google Scholar]
- 58.Hyman D.M., Rizvi R., Natale R., Armstrong D.K., Birrer M., Recht L., et al. Phase I study of MEDI3617, a selective angiopoietin-2 inhibitor alone and combined with carboplatin/paclitaxel, paclitaxel, or bevacizumab for advanced solid tumors. Clin Cancer Res. 2018;24:2749–2757. doi: 10.1158/1078-0432.CCR-17-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ott P.A., Nazzaro M., Pfaff K.L., Gjini E., Felt K.D., Wolff J.O., et al. Combining CTLA-4 and angiopoietin-2 blockade in patients with advanced melanoma: a phase I trial. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29 - Identifier NCT04666220, VETC, prognostic and predictive value in renal cell carcinoma and adrenal carcinoma; 2021 Feb 12 [cited 2023 Jan 23]; Available from: https://clinicaltrials.gov/ct2/show/NCT04666220.
- 61.Pinyol R., Sia D., Llovet J.M. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Res. 2019;25:2021–2023. doi: 10.1158/1078-0432.CCR-18-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfister D., Núñez N.G., Pinyol R., Govaere O., Pinter M., Szydlowska M., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian L., Goldstein A., Wang H., Lo H.C., Kim I.S., Welte T., et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shigeta K., Datta M., Hato T., Kitahara S., Chen I.X., Matsui A., et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71:1247–1261. doi: 10.1002/hep.30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu K., Zhao Y., Chen J., Boland J., Ting K.K., Gamble J.R., et al. Novel miRNA-based drug CD5-2 reduces liver tumour growth in diethylnitrosamine (DEN)-treated mice by normalising tumour vasculature and altering immune infiltrate. Gut. 2021;69:A2–A3. doi: 10.3389/fimmu.2023.1245708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y., Li J., Ting K.K., Chen J., Coleman P., Liu K., et al. The VE-Cadherin/β-catenin signalling axis regulates immune cell infiltration into tumours. Cancer Lett. 2021;496:1–15. doi: 10.1016/j.canlet.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 67.Jain R.K. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 68.Esagian S.M., Kakos C.D., Giorgakis E., Burdine L., Barreto J.C., Mavros M.N. Adjuvant transarterial chemoembolization following curative-intent hepatectomy versus hepatectomy alone for hepatocellular carcinoma: a systematic review and meta-analysis of randomized controlled trials. Cancers. 2021;13:2984. doi: 10.3390/cancers13122984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan P.S., Muthiah M.D., Koh T., Teoh Y.L., Chan A., Kow A., et al. Asian liver transplant network clinical guidelines on immunosuppression in liver transplantation. Transplantation. 2019;103:470–480. doi: 10.1097/TP.0000000000002532. [DOI] [PubMed] [Google Scholar]
- 70.Verna E.C., Patel Y.A., Aggarwal A., Desai A.P., Frenette C., Pillai A.A., et al. Liver transplantation for hepatocellular carcinoma: management after the transplant. Am J Transpl. 2020;20:333–347. doi: 10.1111/ajt.15697. [DOI] [PubMed] [Google Scholar]
- 71.Marsh-Wakefield F., Ferguson A.L., Liu K., Santhakumar C., McCaughan G., Palendira U. Approaches to spatially resolving the tumour immune microenvironment of hepatocellular carcinoma. Ther Adv Med Oncol. 2022;14:1–15. doi: 10.1177/17588359221113270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.