Abstract
Background & Aims
Non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) affect 17–46% of Western countries, making coexistence with other liver diseases inevitable. We investigated the prevalence and clinical significance of NAFLD/NASH or the components of metabolic syndrome (MetS) in a large multicentric cohort of patients with autoimmune hepatitis (AIH).
Methods
Data from six academic centres (Greece, Canada, Japan, Germany, The Netherlands, and Spain) were evaluated. The presence of NAFLD/NASH in liver biopsy, MetS components, and clinical and laboratory parameters were recorded.
Results
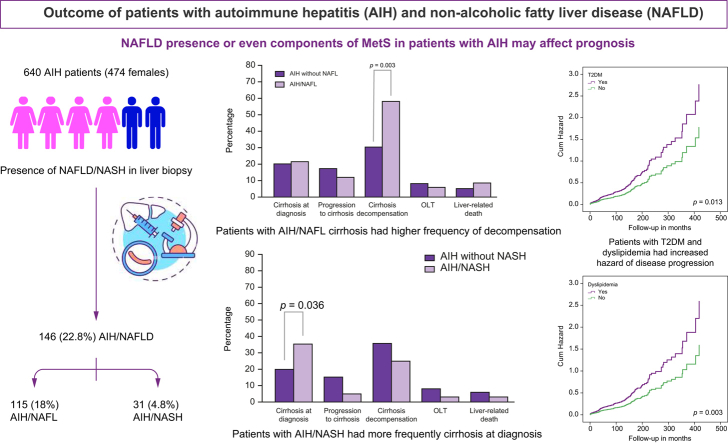
A total of 640 patients (474 females, age 49 [4–87] years; follow-up 78 [1–521] months) were included. NAFLD was present in 146 (22.8%) patients (AIH/non-alcoholic fatty liver [NAFL] 115 [18%], AIH/NASH 31 [4.8%]). AIH/NAFL patients were older (p = 0.017), more frequently overweight or obese (p = 0.002), had hypertension (p = 0.001), and had diabetes (p = 0.016), whereas they less frequently had acute presentation (p = 0.002) and soluble liver antigen/liver pancreas positivity (p <0.05), lower transaminases (p <0.001), ALP (p = 0.028) and IgG (p = 0.004) and higher albumin (p <0.001) than patients with AIH only. Patients with AIH/NASH more frequently had cirrhosis at diagnosis (p = 0.036) and higher IgG (p = 0.009). Response to treatment did not differ between groups. Patients with cirrhosis with AIH/NAFL had higher frequency of decompensation compared with patients with AIH only (p <0.05). Patients with type 2 diabetes mellitus and dyslipidaemia had increased hazard of disease progression (p <0.05 for each).
Conclusions
The prevalence of NAFLD in AIH is similar to the general population. Concurrence of NASH in patients with AIH signifies a more severe disease, whereas that of NAFL may indicate a worse prognosis in patients with cirrhosis. T2DM and dyslipidaemia in AIH patients are associated with dismal parameters of outcome. Our findings suggest that NAFLD presence or even components of MetS in patients with AIH may affect prognosis, so closer follow-up of such patients is warranted.
Impact and implications
Non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) affect many people, making coexistence with other liver diseases inevitable. We investigated the prevalence and clinical significance of NAFLD/NASH or the components of metabolic syndrome (MetS) in patients with autoimmune hepatitis (AIH). NAFLD and NASH presence in patients with AIH is as frequent as in the general population. The concurrence of NASH in patients with AIH seems to signify a more severe disease, whereas that of non-alcoholic fatty liver may indicate a worse prognosis in a specific subgroup of patients who already have cirrhosis at diagnosis. Diabetes or dyslipidaemia in patients with AIH were associated with worse prognosis. Therefore, it seems that closer follow-up of patients with concurrent AIH and NAFLD or AIH and components of MetS is needed.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Metabolic dysfunction-associated fatty liver disease, Autoimmune hepatitis
Graphical abstract
Highlights
-
•
NAFLD and NASH prevalence in patients with AIH is similar to that of the general population.
-
•
The presence of concomitant AIH and diabetes or dyslipidaemia may affect prognosis.
-
•
Patients with AIH/NASH more frequently have cirrhosis at diagnosis.
-
•
The presence of NAFLD does not affect response to treatment of patients with AIH.
-
•
The presence of overweight or obesity, or diabetes at diagnosis independently predicts AIH/NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a modern-day ‘pandemic’ that affects a quarter of the population worldwide with prevalence ranging according to geography from 13.5% in Africa, to 20–30% in Europe, and possibly over 30% in the Middle East and certain populations in Central and South America.[1], [2], [3], [4] There is a strong bidirectional association between NAFLD and components of metabolic syndrome (MetS), so NAFLD prevalence increases as high as 75% in individuals with MetS, especially in those with type 2 diabetes mellitus (T2DM) and overweight or obesity.5,6
NAFLD is defined by the accumulation of fat in >5% of hepatocytes, in association with insulin resistance.[2], [3], [4] Exclusion of secondary causes of hepatic steatosis and increased alcohol consumption (>20–30 g/day for men and >10–20 g/day for women) is necessary.2,4 Although most patients have non-alcoholic fatty liver (NAFL) defined by simple steatosis on liver histology, about 5–10% of the general population will develop non-alcoholic steatohepatitis (NASH), characterised by the presence of steatosis combined with lobular inflammation and hepatocyte ballooning.7,8 About one-third of patients with NASH will develop significant and/or severe fibrosis with one-third of them progressing to cirrhosis.[1], [2], [3], [4] The latter group bears an additional risk of decompensation (about 5–10% after 10 years from the establishment of cirrhosis) and development of hepatocellular carcinoma (HCC).
The high prevalence of NAFLD in the general population makes the coincidence with other chronic liver diseases almost inevitable.9,10 The co-existence of two chronic liver diseases might lead to a more severe liver disease phenotype and a worse prognosis. Under this context, the prevalence of NAFLD and NASH in patients with autoimmune hepatitis (AIH) is poorly defined.[10], [11], [12] AIH is a relatively rare, non-resolving chronic liver disease of unknown aetiology with an estimated prevalence of 20–25 cases per 100,000 in Europe.[13], [14], [15], [16] It affects all ages, both sexes, all ethnic groups and is characterised by a favourable response to immunosuppressive treatment.[13], [14], [15], [16], [17], [18], [19] Serological hallmarks include polyclonal hypergammaglobulinaemia and circulating non-organ specific autoantibodies, whereas its diagnosis is based on the combination of these serological findings, absence of viral hepatitis and presence of characteristic histological lesions.[13], [14], [15], [16],20 The diagnosis of AIH in patients with concurrent NAFLD is challenging because non-organ specific autoantibodies have been reported with increased prevalence among NAFLD patients (range: 12–48%).[21], [22], [23] Moreover, the significance of this concurrence remains largely unknown, as, up to the present, it has been assessed by only two retrospective clinical studies. The first, from the United States, included 73 patients with AIH and found that the prevalence of concomitant AIH/NAFLD and AIH/NASH was 14% and 16%, respectively, whereas it was shown that patients with concomitant AIH/NASH had more severe liver disease.24 A significant limitation of that study was the small number of participants. The second study from Japan included 1151 patients with AIH 17% of whom had NAFLD.25 However, this study did not assess the outcome of patients and there was no data regarding the individual impact of NAFL and NASH on response to treatment and the characteristics of patients.
In this context, the International Autoimmune Hepatitis Group (IAIHG) designed a large retrospective study to assess the prevalence and the potential impact of NAFL and NASH in the clinical course, response to treatment, and clinical outcomes of patients with AIH.
Patients and methods
Patients
Patients with well-established AIH according to the simplified criteria of the IAIHG20 between January 1, 2017 and December 31, 2019 were eligible for inclusion in the study. In addition, patients should have: (a) available liver biopsy with a detailed description of possible histological lesions of NAFLD, and (b) available clinical and follow-up data including response to treatment and clinical outcomes. Exclusion criteria involved: (a) alcohol consumption >20 g ethanol/day for men and >10 g/day for women, (b) coexistence of other liver diseases such as viral hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, haemochromatosis, etc.
Patients with AIH were divided into three groups: patients with AIH without any evidence of NAFLD on liver biopsy (AIH-only), patients with steatosis but without evidence of NASH (AIH/NAFL), and patients with NASH (AIH/NASH). MetS was defined according to the criteria proposed by the Adult Treatment Panel III.26 However, as waist circumference measurements were not available, MetS was defined by the presence of any three of the following: (a) serum triglycerides >150 mg/dl or specific drug treatment, (b) HDL <40 mg/dl for males and <50 mg/dl for females or specific drug treatment, (c) hypertension or specific drug treatment, (d) fasting glucose ≥100 mg/dl or drug treatment for elevated blood glucose. Finally, overweight or obesity was defined as BMI >25 kg/m2 for Caucasians and >23 kg/m2 for Japanese patients.
Data collection
The medical records of patients were reviewed retrospectively and the clinical, biochemical, serological, and histological characteristics at the time of diagnosis (before treatment initiation) and during follow-up were collected.
The study was conducted in accordance with the protocol and principles of the 1975 Declaration of Helsinki. The protocol was approved by the Institutional Research Board of each participating centre, in accordance with local regulations. At the time of initial evaluation, all patients agreed to the use of their data by anonymous analysis.
Clinical data
Clinical data concerning parameters associated with the MetS, such as weight, height, and BMI, history of hypertension, T2DM, dyslipidaemia (defined as the presence of either hypercholesterolaemia or hypertriglyceridaemia), and cardiovascular disease were recorded.
In addition, the presence of concomitant autoimmune diseases was recorded. The diagnosis of cirrhosis in patients who did not have a second liver biopsy was based on ultrasonography (coarse echo pattern of the liver parenchyma and irregular liver margins, splenomegaly, portal vein >16 mm), endoscopic evidence of portal hypertension (varices, portal gastropathy), and/or clinical findings of decompensation (ascites, variceal bleeding, hepatic encephalopathy) as we described previously.18,27 Furthermore, the outcome (orthotopic liver transplantation [OLT], liver-related death, progression of fibrosis as attested by follow-up liver biopsy or non-invasive tests [e.g. transient elastography], development of cirrhosis during follow-up, cirrhosis decompensation and overall disease progression defined as either liver-related death or OLT or progression of fibrosis, development of cirrhosis, decompensation of cirrhosis) was also included in the analyses.
Biochemical and serological data
Conventional laboratory tests were recorded from medical records, at the time of diagnosis and last follow-up visit. The laboratory data included platelets (PLT), prothrombin time, aspartate aminotransferase (AST, IU/L), alanine aminotransferase (ALT, IU/L), gamma-glutamyl transpeptidase (γ-GT, IU/L), alkaline phosphatase (ALP, IU/L), bilirubin (mg/dl), albumin (g/dl), immunoglobulin class G (IgG, mg/dl), cholesterol (Chol, mg/dl), high density cholesterol (HDL, mg/dl), triglycerides (TRG, mg/dl), fasting glucose levels (Glu, mg/dl), and glycosylated haemoglobulin (HbA1c %).
Autoantibodies testing
Smooth muscle antibodies (SMA) and anti-liver/kidney microsome antibodies (anti-LKM) were determined according to practice guidelines by standard methods such as indirect immunofluorescence (IIF) on rodent liver–kidney–stomach substrate and/or ELISAs or Western immunoblotting.13,14,16 Antinuclear antibodies (ANA) were detected by IIF on HEp2 cells or rodent liver–kidney–stomach substrate. Antibodies against soluble liver antigen/liver pancreas (anti-SLA/LP) were detected by ELISAs or Western immunoblotting.28,29
Histological evaluation
Biopsies were not evaluated centrally but only locally at each expertise centre by experienced liver pathologists. Data on the presence of NAFL and/or NASH features (amount and location of steatosis, presence/absence of Malory’s hyaline, hepatocyte ballooning, lobular inflammation, zone 3 fibrosis) at baseline and follow-up liver biopsy close to the last follow-up visit were recorded. Follow-up liver biopsies were performed to assess histological remission. NAFL and NASH were defined according to the current criteria.[2], [3], [4] The difficulty of assessing lobular inflammation as a component of the diagnosis of NASH in the context of AIH was addressed by the combined presence of steatosis or hepatocyte ballooning using the NASH Clinical Research Network Histologic Scoring System.3 Fibrosis was assessed according to the Metavir staging system.30
Treatment for AIH and response to treatment
Patients were treated according to current guidelines with corticosteroids alone or in combination with azathioprine or mycophenolate mofetil.[13], [14], [15],31,32 According to the study protocol and to achieve homogeneity between centres, complete biochemical response (CBR, normalisation of AST, ALT, and IgG levels) was assessed only at the last follow-up. Insufficient response was defined as a lack of CBR and no response (NR) was defined according to our previous publications and EASL guidelines.13,31,33 In addition, relapses during treatment after CBR achievement and withdrawal rates of corticosteroids were recorded. Treatment was withdrawn after at least 3 years of treatment, while on CBR for at least 2 years.[13], [14], [15]
Study endpoints
The study endpoints were: (1) the prevalence of biopsy-proven NAFL and NASH in AIH patients; (2) the prevalence of parameters associated with MetS in AIH patients; and (3) the potential impact of NAFL, NASH, or parameters of the MetS on response to treatment, withdrawal rates of corticosteroids, and outcome of patients with AIH.
Statistical analysis
Data were analysed using the SPSS version 24 package (IBM Corp., Armonk, NY, USA). Results were expressed as median (range) and mean ± standard deviation where appropriate. Data were analysed using the t test, Mann–Whitney U test, Χ2 test (two-by-two with Yates’ correction), Pearson’s Χ2 test, where applicable, and binary logistic regression analysis to examine multivariable interactions. For the comparison between two paired samples, the McNemar test and the paired sample T test were used. In addition, Cox regression analysis for outcome parameters was used. Two-sided p values <0.05 were considered statistically significant; 95% CIs were calculated by the Wilson procedure with a correction for continuity.
Results
Six hundred and forty patients (474 females, median age at diagnosis 49 [4–87] years) with AIH from six centres of the IAIHG, fulfilling the inclusion and exclusion criteria, were included in the analysis (179 from Greece, 309 from Canada, 56 from the Netherlands, 49 from Japan, 32 from Germany and 15 from Spain).
The baseline and at last follow-up clinical and laboratory characteristics of the patients are shown in Table S1. One hundred and ninety-eight patients (31%; 95% CI: 27–35%) had concurrent autoimmune diseases with Hashimoto thyroiditis being the most frequent (48/198; 24.2%). At last follow-up, significantly more patients had cirrhosis, dyslipidaemia, and T2DM compared with baseline (p <0.001 for each comparison; Table S1).
A second liver biopsy was performed in 192 (30%) patients at 60 (12–420) months from baseline biopsy. In these patients, there was a significant progression of fibrosis stage (F3–F4 at diagnosis 68/192 [35.4%] vs. 84/192 at last follow-up [43.8%]; p = 0.044) and steatosis overtime (steatosis at diagnosis 45/192 [23.4%] vs. 60/192 at last follow-up [31.2%]; p = 0.028). The other histological features of NAFL did not change (Table 1).
Table 1.
Histological characteristics of patients with autoimmune hepatitis.
| Liver biopsy at diagnosis n = 640 |
Last follow-up liver biopsy n = 192 |
p value | |
|---|---|---|---|
| Fibrosis | 0.009 | ||
| F0–F1 | 222 (35%) | 71 (37%) | |
| F2 | 147 (23%) | 37 (19.3%) | |
| F3 | 143 (22%) | 35 (18.2%) | |
| F4 | 128 (20%) | 49 (25.5%) | |
| Steatosis | 146/640 (21%) | 60/192 (31%) | 0.061 |
| Mild | 52 (31%) | 23 (38%) | |
| Moderate | 82 (60%) | 25 (42%) | |
| Severe | 12 (9%) | 12 (20%) | |
| Presence of Malory’s hyaline | 25/640 (4%) | 5/192 (2.6%) | NS |
| Hepatocyte ballooning | 52/640 (8%) | 12/192 (6.3%) | NS |
| Lobular inflammation | 147/640 (23%) | 52/192 (27%) | NS |
| Zone 3 fibrosis | 47/640 (7.4%) | 22/192 (11.5%) | NS |
All comparisons were performed by using the Χ2 test (two-by-two with Yates’ correction). NS, not statistically significant.
Six hundred and twenty-five patients received treatment. The remaining 15 patients who did not receive treatment had either burn-out cirrhosis or mild disease. The responses to treatment and outcome parameters in these 625 patients are shown in Table S2.
At diagnosis, NAFLD was present in 146 (22.8%; 95% CI: 19.6–26%) patients (Table 1 and Fig. 1) with AIH/NAFL in 115 (18%; 95% CI: 15–21%), and AIH/NASH in 31 patients (4.8%; 95% CI: 3–7%) (Fig. S1).
Fig. 1.
Frequency (%) of autoimmune hepatitis (AIH) only and AIH with non-alcoholic fatty liver disease (AIH/NAFLD) according to nationality.
On the second liver biopsy, the presence of NAFL and NASH was found in 59/192 (30.7%; 95% CI: 24–38%) and 6/192 (3.1%; 95% CI: 1.3–7) patients, respectively. Of note, by using the paired test the proportion of NAFLD within patients with AIH (n = 192) increased significantly at the end of follow-up compared with that at diagnosis (60/192, 31.2% vs. 45/192, 23.4%, respectively; p = 0.028), whereas the proportion of patients with NASH did not change.
Differences between patients with AIH with and without NAFL
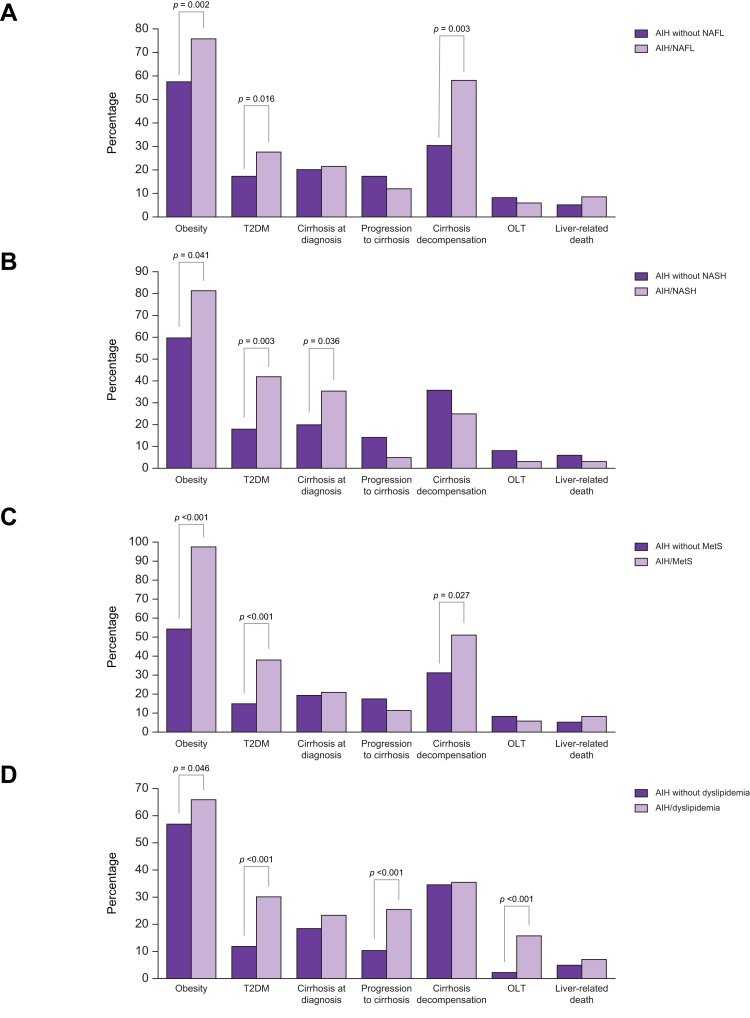
The baseline clinical, laboratory, and treatment outcome characteristics of the AIH-only and AIH/NAFL groups are shown in Table 2 and Table S2. Patients with AIH/NAFL were older (p = 0.017) and as expected, more frequently had overweight or obesity (p = 0.002, Fig. 2A), hypertension (p = 0.001), T2DM (p = 0.016, Fig. 2A), higher levels of triglycerides (p = 0.022), whereas they less frequently had acute presentation (p = 0.002) and SLA/LP positivity (p <0.05) compared with the AIH-only group (Table 2). In addition, they had lower AST (p <0.001), ALT (p <0.001), ALP (p = 0.028), and IgG (p = 0.004) levels and higher albumin (p <0.001) (Table 2).
Table 2.
Clinical and laboratory characteristics of patients with autoimmune hepatitis according to the presence or absence of non-alcoholic fatty liver at diagnosis.
| AIH patients without NAFL (n = 525) | AIH/NAFL (n = 115) | p value | |
|---|---|---|---|
| Sex (female/male) | 392/133 | 82/33 | NS |
| Age at diagnosis (years) | 48 (4–87) | 52 (6–76) | 0.017 |
| BMI (kg/m2) | n = 440 | n = 92 | |
| 26.5 ± 8 | 28.7 ± 8 | 0.021 | |
| Overweight or obesity | 254 (57.7%) | 70 (76%) | 0.002 |
| Disease duration (months) | 98.7 ± 83 | 120.4 ± 94 | 0.014 |
| Follow-up (months) | 74 (1–521) | 92.7 (1–418) | 0.069 |
| AIH simplified diagnostic score | 6.97 ± 1.2 | 6.72 ± 1.2 | 0.054 |
| Acute presentation | 237 (45.1%) | 33 (28.7%) | 0.002 |
| Concurrent autoimmune diseases | 159 (30.3%) | 40 (34.8%) | NS |
| Cirrhosis at diagnosis | 107 (20.4%) | 25 (21.7%) | NS |
| Hypertension | 129 (24.6%) | 46 (40%) | 0.001 |
| T2DM | 92 (17.5%) | 32 (27.8%) | 0.016 |
| Dyslipidaemia | 209 (39.8%) | 55 (47.8%) | NS |
| Cardiovascular disease | 15 (2.9%) | 6 (5.3%) | NS |
| Chronic kidney disease | 35 (6.7%) | 10 (8.7%) | NS |
| Platelets (×109/L) | 199 ± 88 | 208 ± 82 | NS |
| ALT (IU/L, ULN: 40) | 368 (10–4,070) | 160 (18–3,500) | <0.001 |
| AST (IU/L, ULN: 40) | 294 (9–5,490) | 112 (20–4,150) | <0.001 |
| γ-GT (IU/L, ULN: 40) | 200 ± 224 | 210 ± 281 | NS |
| ALP (IU/L, ULN: 120) | 186 ± 153 | 149 ± 117 | 0.028 |
| Albumin (g/dl) | 3.57 ± 0.7 | 3.9 ± 0.68 | <0.001 |
| Bilirubin (mg/dl, ULN: 1.1) | 5 ± 6.9 | 3.6 ± 6.5 | 0.066 |
| IgG (mg/dl, ULN: 1,500) | 2,463 ± 1130 | 2,119 ± 1048 | 0.004 |
| Cholesterol (mg/dl) | N = 322 185 ± 59 |
N = 74 184 ± 67 |
NS |
| HDL (mg/dl) | N = 328 52 ± 23 |
N = 74 50 ± 24 |
NS |
| Triglycerides (mg/dl) | N = 322 124 ± 69 |
N = 72 131 ± 65 |
0.022 |
| Glucose (mg/dl) | N = 356 104 ± 43 |
N = 76 106 ± 40 |
NS |
| HbA1c (%) | N = 204 5.7 ± 1.3 |
N = 51 5.8 ± 1.3 |
NS |
| ANA-positive | 345 (65.7%) | 66 (57.4%) | NS |
| SMA-positive | 389 (74%) | 81 (70.4%) | NS |
| Anti-SLA/LP-positive | N = 241 32 (13.3%) |
N = 101 6 (5.9%) |
0.049 |
| Anti-LKM-positive | N = 415 14 (3.4%) |
N = 103 7 (6.8%) |
NS |
Data were analysed using a t test, Mann–Whitney U test, Χ2 test (two-by-two with Yates’ correction) where applicable. γ-GT, gamma-glutamyl transpeptidase; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibodies; anti-LKM, liver-kidney microsomal antibodies; anti-SLA/LP, soluble liver antigen/liver pancreas antibodies; AST, aspartate aminotransferase; HbA1c, glycosylated haemoglobulin; NAFL, non-alcoholic fatty liver; SMA, smooth muscle antibodies; T2DM, type 2 diabetes mellitus.
Fig. 2.
Differences between AIH patients according to the NAFL, NASH or Mets status. (A) Patients with AIH/NAFL more frequently were overweight or obese and had T2DM compared with patients with AIH but without NAFL (p = 0.002 and p = 0.016, respectively; Χ2 test with Yates’ correction). In addition, patients with AIH/NAFL with cirrhosis at baseline decompensated more frequently compared with patients with AIH and cirrhosis but without NAFL (p = 0.003; Χ2 test with Yates’ correction). (B) Patients with AIH/NASH more frequently were overweight or obese, and had T2DM and cirrhosis at diagnosis compared with AIH patients without NASH (p = 0.041, p = 0.003, and p = 0.036, respectively; Χ2 test with Yates’ correction). (C) Patients with AIH/MetS more frequently were overweight or obese and had T2DM compared with patients with AIH but without MetS (p <0.001 for each; Χ2 test with Yates’ correction). In addition, patients with AIH/MetS with cirrhosis at baseline decompensated more frequently compared with patients with AIH and cirrhosis but without MetS (p = 0.027; Χ2 test with Yates’ correction). (D) Patients with AIH with dyslipidaemia more frequently were overweight or obese, had T2DM, developed cirrhosis more frequently during follow-up, and underwent OLT more frequently compared with patients with AIH without dyslipidaemia (p = 0.046, p <0.001, p <0.001 and p <0.001, respectively; Χ2 test with Yates’ correction). AIH, autoimmune hepatitis; MetS, metabolic syndrome; NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis; OLT, orthotopic liver transplantation; T2DM: type 2 diabetes mellitus.
Response to treatment and frequency of corticosteroid withdrawal did not differ between the two groups (Table S2). Although outcome parameters such as OLT, liver-related death, HCC development, fibrosis progression, development of cirrhosis and overall disease progression did not differ between groups, patients with established cirrhosis at baseline with AIH/NAFL decompensated more frequently compared with the patients with AIH only with cirrhosis at baseline (OR: 1.9; 95% CI: 1.3–2.7; p = 0.003, Table S2 and Fig. 2A). The same was true when the second liver biopsy was considered (cirrhosis decompensation: AIH/NAFL 13/22, 59% vs. AIH-only 2/39, 5.1%; OR: 11.5; 95% CI: 2.8–46.4; p <0.001). However, when Cox proportional hazard analysis was performed there was no difference in hazard ratios regarding the development of decompensation between groups.
Differences between patients with AIH with and without NASH
The baseline clinical, laboratory, and treatment outcome characteristics of the AIH patients with and without NASH are shown in Table 3 and Table S2. Patients with AIH/NASH were older (p = 0.003) and as expected, more frequently had overweight or obesity (p = 0.041, Fig. 2B), T2DM (p = 0.003, Fig. 2B) and higher triglyceride levels (p = 0.007). Of note, they more frequently had cirrhosis at diagnosis (p = 0.036, Fig. 2B) and higher IgG levels (p = 0.009) compared with the AIH-only patient group.
Table 3.
Clinical and laboratory characteristics of patients with autoimmune hepatitis according to the presence or absence of non-alcoholic steatohepatitis at diagnosis.
| AIH patients without NASH (n = 609) | AIH/NASH (n = 31) | p value | |
|---|---|---|---|
| Sex (female/male) | 454/155 | 20/11 | NS |
| Age at diagnosis (years) | 45.7 ± 18 | 55.8 ± 17.4 | 0.003 |
| BMI (kg/m2) | n = 505 | n = 27 | |
| 26.7 ± 8 | 28.7 ± 9 | NC | |
| Overweight or obesity | 302 (59.8%) | 22 (81.5%) | 0.041 |
| Disease duration (months) | 104 ± 86 | 76 ± 60 | NS |
| Follow-up (months) | 96 ± 83 | 71 ± 66 | NS |
| AIH simplified diagnostic score | 6.9 ± 1.22 | 6.7 ± 1.08 | NS |
| Acute presentation | 255 (41.9%) | 15 (48.4%) | NS |
| Concurrent autoimmune diseases | 191 (31.4%) | 8 (25.8%) | NS |
| Cirrhosis at diagnosis | 21 (20%) | 11 (35.5%) | 0.036 |
| Hypertension | 163 (26.8%) | 12 (38.7%) | NS |
| Diabetes mellitus | 111 (18%) | 13 (42%) | 0.003 |
| Dyslipidaemia | 250 (41%) | 14 (45%) | NS |
| Cardiovascular disease | 21 (3.5%) | 0 | NS |
| Chronic kidney disease | 42 (6.9%) | 3 (9.7%) | NS |
| Platelets ( × 109/L) | 201 ± 87 | 198 ± 89 | NS |
| ALT (IU/L, ULN: 40) | 556 ± 619 | 511 ± 548 | NS |
| AST (IU/L, ULN: 40) | 499 ± 607 | 571 ± 644 | NS |
| γ-GT (IU/L, ULN: 40) | 202 ± 238 | 188 ± 174 | NS |
| ALP (IU/L, ULN: 120) | 181 ± 151 | 158 ± 89 | NS |
| Albumin (g/dl) | 3.6 ± 0.7 | 3.5 ± 0.8 | NS |
| Bilirubin (mg/dl, ULN: 1.1) | 4.6 ± 6.8 | 5.9 ± 7.6 | NS |
| IgG (mg/dl, ULN: 1,500) | 2,369 ± 1,109 | 2921 ± 1244 | 0.009 |
| Cholesterol (mg/dl) | n = 370 183 ± 61 |
n = 26 200 ± 60 |
NS |
| HDL (mg/dl) | n = 375 52 ± 24 |
n = 27 49 ± 19 |
NS |
| Triglycerides (mg/dl) | n = 365 123 ± 67 |
n = 29 158 ± 73 |
0.007 |
| Glucose (mg/dl) (n = 432) | n = 402 105 ± 43 |
n = 30 110 ± 38 |
NS |
| HbA1c (%) (n = 255) | n = 240 5.7 ± 1.3 |
n = 15 6.02 ± 1 |
NS |
| ANA-positive | 390 (64%) | 21 (67.7%) | NS |
| SMA-positive | 450 (74%) | 20 (64.5%) | NS |
| Anti-SLA/LP-positive | n = 317 37 (11.7%) |
n = 25 1 (4%) |
NS |
| Anti-LKM-positive | n = 487 18 (3.7%) |
n = 31 3 (9.7%) |
NS |
Data were analysed using a t test, Mann–Whitney U test, Χ2 test (two-by-two with Yates’ correction) where applicable. γ-GT, gamma-glutamyl transpeptidase; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibodies; anti-LKM, liver-kidney microsomal antibodies; anti-SLA/LP, soluble liver antigen/liver pancreas antibodies; AST, aspartate aminotransferase; HbA1c, glycosylated haemoglobulin; NASH, non-alcoholic steatohepatitis; SMA, smooth muscle antibodies; T2DM, type 2 diabetes mellitus.
Regarding outcome, none of the outcome parameters differed significantly between the two groups (Table S2 and Fig. 2B) and this was also true when the second liver biopsy was considered (data not shown). However, interestingly, the presence of NASH on the second liver biopsy was associated with the presence of insufficient response/NR compared with the AIH-only group (5/6, 83.3% vs. 36/184, 19.6%, respectively; OR: 4.25; 95% CI: 2.7–6.7; p = 0.002).
Predictors of NAFLD
Binary logistic regression analysis showed that overweight or obesity at diagnosis (p = 0.026), presence of T2DM at diagnosis (p = 0.024), and lower AST and ALT (p = 0.009 and p = 0.008, respectively) independently predicted the presence of AIH/NAFLD (Table 4).
Table 4.
Binary logistic regression analysis on parameters which could predict the presence of autoimmune hepatitis/non-alcoholic fatty liver disease.
| Variables in the equation |
||||||
|---|---|---|---|---|---|---|
| B | SE | Wald | d.f. | Sig. | Exp(B) | |
| Age at diagnosis | -0.012 | 0.012 | 1,092 | 1 | 0.296 | 0.988 |
| BMI at diagnosis | -0.951 | 0.427 | 4,955 | 1 | 0.026 | 0.386 |
| T2DM | 0.615 | 0.938 | 0.430 | 1 | 0.512 | 1,849 |
| T2DM at diagnosis | -2,279 | 1,010 | 5,091 | 1 | 0.024 | 0.102 |
| Insidious presentation | -0.342 | 0.595 | 0.330 | 1 | 0.566 | 0.710 |
| AST at diagnosis | -0.002 | 0.001 | 6,753 | 1 | 0.009 | 0.998 |
| ALT at diagnosis | 0.003 | 0.001 | 6,952 | 1 | 0.008 | 10.003 |
| ALP at diagnosis | -0.002 | 0.002 | 1,295 | 1 | 0.255 | 0.998 |
| Albumin at diagnosis | -0.376 | 0.299 | 1,578 | 1 | 0.209 | 0.687 |
| Triglycerides at diagnosis | -0.003 | 0.003 | 1,664 | 1 | 0.197 | 0.997 |
| Anti-SLA/LP | -0.185 | 0.787 | 0.055 | 1 | 0.814 | 0.831 |
| Anti-LKM | -1,905 | 1,080 | 3,111 | 1 | 0.078 | 0.149 |
| Constant | 4,282 | 1,637 | 6,842 | 1 | 0.009 | 72,368 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; anti-LKM, liver-kidney microsomal antibodies; anti-SLA/LP, soluble liver antigen/liver pancreas antibodies; AST, aspartate aminotransferase; B, binary; d.f., degrees of freedom; Sig., significance; T2DM, type 2 diabetes mellitus.
Differences between patients with AIH with and without MetS components
The characteristics of patients with AIH/MetS are shown in Fig. 2C. When each component of the MetS (presence or absence of overweight or obesity, hypertension, T2DM, dyslipidaemia) was considered, the diagnosis of T2DM or dyslipidaemia at any time from diagnosis of AIH to the last follow-up, but not overweight or obesity or hypertension, was associated with more severe disease and worse outcome. In more detail, patients with AIH with T2DM more frequently had cirrhosis at diagnosis (37/124, 29.8% vs. 95/516 18.4%; OR: 1.6; 95% CI: 1.1–2.2; p = 0.007), developed cirrhosis more frequently during follow-up (24/87, 27.6% vs. without 60/421, 14.3%; OR: 1.9; 95% CI: 1.3–2.9; p = 0.004) (HR: 1.517; 95% CI: 1.127–2.043; p = 0.006), had higher frequency of liver-related death (13/124, 10.5% vs. without 25/516, 4.8%; OR: 2.16; 95% CI: 1.14–4; p = 0.03) (HR: 1.96; 95% CI: 1.001–3.836; p = 0.05), or OLT (18/124, 14.5% vs. without 33/516, 6.4%; OR: 2.27; 95% CI: 1.3–3.8; p = 0.005) (HR: 1.958; 95% CI: 1.089–3.521; p = 0.025) and had more frequently overall disease progression (46/124, 37.1% vs. without 111/516, 21.5%; OR: 1.7; 95% CI: 1.3–2.3; p <0.001) (HR: 1.552; 95% CI: 1.097–2.197; p = 0.013, Fig. 3).
Fig. 3.
Cox regression analysis regarding overall autoimmune hepatitis progression. Patients with type 2 diabetes mellitus (T2DM) had an increased hazard ratio of disease progression compared with patients without T2DM (p = 0.013).
In addition, patients with AIH with dyslipidaemia more frequently developed cirrhosis during follow-up (52/202, 25.7% vs. 32/306, 10.5%; OR: 2.46; 95% CI: 1.6–3.7; p <0.001), more frequently underwent OLT (42/264, 15.9% vs. 9/376, 2.4%; OR: 6.6; 95% CI: 3.3–13.4; p <0.001) (HR: 5.863; 95% CI: 2.847–12.073; p <0.001) (Fig. 2D) and had higher frequency of overall disease progression (88/264, 33.3% vs. 69/376, 18.4%; OR: 1.8; 95% CI: 1.4–2.4; p <0.001) (HR: 1.627; 95% CI: 1.184–2.237; p = 0.003, Fig. 4). Furthermore, patients with dyslipidaemia more frequently resulted in insufficient response/NR than patients without (73/260, 28.1% vs. 69/365 18.7%; OR: 1.5; 95% CI: 1.12–2; p = 0.008).
Fig. 4.
Cox regression analysis regarding overall autoimmune hepatitis progression. Patients with dyslipidaemia had an increased hazard ratio of disease progression compared with patients without dyslipidaemia (p = 0.003).
Apart from T2DM and dyslipidaemia, overall disease progression (compared with no progression) was associated with insufficient response/NR to treatment (67/149 [45%] vs. 75/476 [15.8%]; p <0.001), absence of acute hepatitis at diagnosis (105/157 [67%] vs. 265/483 [55%]; p = 0.011), decreased PLTs (147 ± 79 × 109/L vs. 217 ± 82 × 109/L, p <0.001) and albumin (3.2 ± 0.75 g/dl vs. 3.7 ± 0.67 g/dl, p <0.001) levels, increased ALT (453 ± 563 U/L vs. 580 ± 626 U/L, p = 0.049) and IgG (2,649 ± 1,118 mg/dl vs. 2320 ± 1113 mg/dl, p = 0.003) levels at baseline. When these variables along with age entered the logistic regression analysis, dyslipidaemia tended to independently predict disease progression (p = 0.063), whereas significant independent predictors were PLTs, albumin, and response to treatment (p <0.001, for each).
Discussion
This is the largest retrospective study to date, which assessed the prevalence and clinical significance, in terms of response to treatment and liver-related outcomes, of NAFL and NASH in patients with well-established AIH. Actually, this is a unique cohort gathered from six academic centres including Caucasian as well as Asian populations. The main findings of our study were: (a) the prevalence of NAFLD and NASH in AIH patients was similar to that reported in the general population; patients with AIH/NAFLD were older and more frequently overweight or obese and diabetic compared with the patients with AIH-only; (b) the presence of T2DM and dyslipidaemia were associated with disease progression and worse prognosis among patients with AIH; patients with AIH/NASH more frequently had cirrhosis at diagnosis, and those with established cirrhosis at diagnosis and AIH/NAFL decompensated more frequently than patients with AIH-only with cirrhosis; and (c) the presence of overweight or obesity and T2DM at diagnosis as well as lower AST and ALT levels, independently predicted the presence of AIH/NAFLD.
The prevalence of NAFLD in patients with AIH (23%) was within the expected rate reported in the general population.[1], [2], [3], [4] NASH was found in one-fifth of the NAFLD population. Of note, the Japanese patients with AIH had a higher prevalence of NASH (20%) than that reported in the general population,[34], [35], [36] even though it was the same with a previous study.24 In addition, contrary to the Japanese study on AIH/NAFLD,25 we found no female predominance in patients with AIH/NAFL or AIH/NASH. In parallel with this study, however, our patients with either AIH/NAFL or AIH/NASH were older at diagnosis than patients with AIH only, which could imply a delay in diagnosis of AIH in these patient groups. Indeed, this potential delay in diagnosis seems rational, as a high degree of suspicion is needed to diagnose AIH in patients with MetS and NAFLD. Furthermore, the presence of non-organ specific antibodies, even though in low titres, is not uncommon in NAFLD patients, making the situation even more complicated.21,22 Regarding the biochemical profile, patients with AIH/NAFL had lower transaminases and IgG levels indicating that the acute form of the disease was less frequent in these patients. This more insidious presentation of AIH/NAFLD may further explain the possible delay in the diagnosis of AIH in this specific group of patients. However, interestingly, patients with AIH/NASH had higher IgG levels at diagnosis than those in patients with AIH only. This finding could be explained by the increased prevalence of cirrhosis at diagnosis in patients with AIH/NASH or by an unspecific IgG production triggered by steatohepatitis but not by simple steatosis.
Patients with AIH with T2DM and dyslipidaemia, as components of the MetS, had more severe disease and worse prognosis than patients without. This is in line with previous studies in other autoimmune diseases showing that MetS was associated with increased disease activity and severity in patients with rheumatoid arthritis,37 increased inflammatory markers in those with systemic lupus erythematosus,38 and worse prognosis in patients with Sjögren.39
A previous study of De Luca-Johnson et al.24 showed that patients with AIH/NASH had an increased risk for liver-related mortality and liver-related adverse outcomes compared with the AIH-only cohort. However, this study included only 73 patients of whom 22 had AIH/NAFLD (10 patients AIH/NAFL and 12 AIH/NASH). Although we could not confirm the findings of De Luca-Johnson et al., the presence of NASH was associated with more severe disease, as patients with AIH/NASH more frequently had cirrhosis at diagnosis. The reason for this discrepancy could be that the results of the previous study have been based on only 22 patients with AIH/NAFLD making general conclusions very difficult and questionable. However, a finding that has been demonstrated quite safely by our study is that patients with AIH/NAFL who had cirrhosis at baseline decompensated during follow-up more frequently than those with cirrhosis from the AIH-only group, indicating worse disease prognosis in this specific subgroup of patients with cirrhosis. Moreover, a novel finding was that patients who developed NASH in the second liver biopsy were those with a worse response to treatment. However, as the response to treatment did not differ between patients with AIH/NASH (according to the first liver biopsy) and patients with AIH only, no safe conclusion on the impact of NASH presence on treatment response can be drawn.
In our study, contrary to the study of De Luca-Johnson et al.24 who could not perform multivariate analysis because of the small sample size, we could proceed to multivariate analysis to determine the independent factors which could predict the presence of AIH/NAFLD. Accordingly, we found that the presence of overweight or obesity, T2DM, and lower transaminases levels could predict the presence of concurrent AIH/NAFLD.
A major limitation of our study is its retrospective nature and that liver biopsies were not evaluated centrally for a second histopathological review, even though when biopsies are centrally reviewed by experts the variability of findings is also large. Second, CBR was assessed according to the study protocol only at the end of follow-up as gathering of the data was done before the publication of the new response criteria and endpoints by the IAIHG.40 Third, the number of patients with NASH is relatively small and half of them were Japanese. Fourth, a second liver biopsy was performed per protocol in only one third of the patients because this was done to assess the histological remission of the disease. Nevertheless, to date, this is the largest multicentre effort to address a clinically relevant issue including patients with AIH with and without NAFLD from different referral centres worldwide with expertise in liver pathology.
In conclusion, the prevalence of NAFL and NASH in patients with AIH is similar to the general population. The concurrence of NASH in patients with AIH seems to signify a more severe disease and probably decreased likelihood of response to treatment, whereas that of NAFLD may indicate a worse prognosis in patients with cirrhosis at diagnosis. The presence of T2DM and dyslipidaemia in patients with AIH is associated with dismal outcome parameters. Our findings suggest that concurrent AIH and NAFLD or components of MetS in AIH patients indicates increased surveillance and closer follow-up is warranted.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Study concept and design: KZ, GND. Acquisition of research data: KA, EL, RJALMS, ATak, MR, RJA, CS, AWL, ATan, JPHD, AJM-L. Analysis and interpretation of data: KZ, GND, KA, NKG. Drafting of the manuscript: KZ, GND. Critical revision and editing of the manuscript: all authors.
Data availability statement
All data used to support the findings of this study are included within the article and supplementary files.
Conflicts of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://10.1016/j.jhepr.2023.100778.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Younossi Z., Tacke F., Arrese M., Chander Sharma B., Mostafa I., et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 2.European association for the study of the liver (EASL), European association for the study of diabetes (EASD), and European association for the study of obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Brunt E.M., Kleiner D.E., Carpenter D.H., Rinella M., Harrison S.A., Loomba R., et al. NAFLD: reporting histologic findings in clinical practice. Hepatology. 2021;73:2028–2038. doi: 10.1002/hep.31599. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 5.Hallsworth K., Thoma C., Moore S., Ploetz T., Anstee Q.M., Taylor R., et al. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44–51. doi: 10.1136/flgastro-2014-100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N., et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Yeh M.M., Brunt E.M. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Giashuddin S., Alawad M. Histopathological diagnosis of nonalcoholic steatohepatitis (NASH) Methods Mol Biol. 2022;2455:1–18. doi: 10.1007/978-1-0716-2128-8_1. [DOI] [PubMed] [Google Scholar]
- 9.Cotter T.G., Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158:1851–1864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Dalekos G.N., Gatselis N.K., Zachou K., Koukoulis G.K. NAFLD and autoimmune hepatitis: do not judge a book by its cover. Eur J Intern Med. 2020;75:1–9. doi: 10.1016/j.ejim.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Dyson J.K., De Martin E., Dalekos G.N., Drenth J.P.H., Herkel J., Hubscher S.G., et al. Review article: unanswered clinical and research questions in autoimmune hepatitis-conclusions of the International Autoimmune Hepatitis Group Research Workshop. Aliment Pharmacol Ther. 2019;49:528–536. doi: 10.1111/apt.15111. [DOI] [PubMed] [Google Scholar]
- 12.Dalekos G.N., Gatselis N.K., Koukoulis G.K. Non-alcoholic steatohepatitis or autoimmune hepatitis? Sometimes a closer look under the surface is needed. BMJ Case Rep. 2020;21(13) doi: 10.1136/bcr-2020-238400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Dalekos G.N., Koskinas J., Papatheodoridis G.V. Hellenic association for the study of the liver clinical practice guidelines: autoimmune hepatitis. Ann Gastroenterol. 2019;32:1–23. doi: 10.20524/aog.2018.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack C.L., Adams D., Assis D.N., Kerkar N., Manns M.P., Mayo M.J., et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 16.Dalekos G.N., Gatselis N.K. Autoimmune serology testing in clinical practice: an updated roadmap for the diagnosis of autoimmune hepatitis. Eur J Intern Med. 2023;108:9–17. doi: 10.1016/j.ejim.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Grønbæk L., Vilstrup H., Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612–617. doi: 10.1016/j.jhep.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Dalekos G.N., Azariadis K., Lygoura V., Arvaniti P., Gampeta S., Gatselis N.K. Autoimmune hepatitis in patients aged 70 years or older: disease characteristics, treatment response and outcome. Liver Int. 2021;41:1592–1599. doi: 10.1111/liv.14900. [DOI] [PubMed] [Google Scholar]
- 19.Mieli-Vergani G., Vergani D. Autoimmune hepatitis in childhood. Clin Liver Dis. 2014;10(3):6–8. doi: 10.1002/cld.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennes E.M., Zeniya M., Czaja A.J., Parés A., Dalekos G.N., Krawitt E., et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 21.Tsuneyama K., Baba H., Kikuchi K., Nishida T., Nomoto K., Hayashi S., et al. Autoimmune features in metabolic liver disease: a single-center experience and review of the literature. Clin Rev Allergy Immunol. 2013;45:143–148. doi: 10.1007/s12016-013-8383-x. [DOI] [PubMed] [Google Scholar]
- 22.Vuppalanchi R., Gould R.J., Wilson L.A., Unalp-Arida A., Cummings O.W., Chalasani N., et al. Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatol Int. 2012;6:379–385. doi: 10.1007/s12072-011-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y.J., Zheng K.I., Ma H.L., Li G., Pan X.Y., Zhu P.W., et al. Association between positivity of serum autoantibodies and liver disease severity in patients with biopsy-proven NAFLD. Nutr Metab Cardiovasc Dis. 2021;31:552–560. doi: 10.1016/j.numecd.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 24.De Luca-Johnson J., Wangensteen K.J., Hanson J., Krawitt E., Wilcox R. Natural history of patients presenting with autoimmune hepatitis and coincident nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61:2710–2720. doi: 10.1007/s10620-016-4213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi A., Arinaga-Hino T., Ohira H., Abe K., Torimura T., Zeniya M., et al. Non-alcoholic fatty liver disease in patients with autoimmune hepatitis. JGH Open. 2018;2:54–58. doi: 10.1002/jgh3.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 27.Gatselis N.K., Zachou K., Giannoulis G., Gabeta S., Norman G.L., Dalekos G.N. Serum cartilage oligomeric matrix protein and Golgi protein-73: new diagnostic and predictive tools for liver fibrosis and hepatocellular cancer? Cancers (Basel) 2021;13:3510. doi: 10.3390/cancers13143510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zachou K., Gampeta S., Gatselis N.K., Oikonomou K., Goulis J., Manoussakis M.N., et al. Anti-SLA/LP alone or in combination with anti-Ro52 and fine specificity of anti-Ro52 antibodies in patients with autoimmune hepatitis. Liver Int. 2015;35:660–672. doi: 10.1111/liv.12658. [DOI] [PubMed] [Google Scholar]
- 29.Zachou K., Weiler-Normann C., Muratori L., Muratori P., Lohse A.W., Dalekos G.N. Permanent immunosuppression in SLA/LP-positive autoimmune hepatitis is required although overall response and survival are similar. Liver Int. 2020;40:368–376. doi: 10.1111/liv.14280. [DOI] [PubMed] [Google Scholar]
- 30.Bedossa P., Poynard T., The French METAVIR Cooperative Study Group An algorithm for grading activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 31.Zachou K., Gatselis N.K., Arvaniti P., Gabeta S., Rigopoulou E.I., Koukoulis G.K., et al. A real-world study focused on the long-term efficacy of mycophenolate mofetil as first-line treatment of autoimmune hepatitis. Aliment Pharmacol Ther. 2016;43:1035–1047. doi: 10.1111/apt.13584. [DOI] [PubMed] [Google Scholar]
- 32.Dalekos G.N., Arvaniti P., Gatselis N.K., Samakidou A., Gabeta S., Rigopoulou E., et al. First results from a propensity matching trial of mycophenolate mofetil vs. azathioprine in treatment-naïve AIH patients. Front Immunol. 2022;12 doi: 10.3389/fimmu.2021.798602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalekos G.N., Arvaniti P., Gatselis N.K., Gabeta S., Samakidou A., Giannoulis G., et al. Long-term results of mycophenolate mofetil vs. azathioprine use in individuals with autoimmune hepatitis. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto E., Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46:63–69. doi: 10.1007/s00535-010-0311-8. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Zou B., Yeo Y.H., Feng Y., Xie X., Lee D.H., et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 36.Chan J.C., Malik V., Jia W., Kadowaki T., Yajnik C.S., Yoon K.H., et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 37.Karvounaris S.A., Sidiropoulos P.I., Papadakis J.A., Spanakis E.K., Bertsias G.K., Kritikos H.D., et al. Metabolic syndrome is common among middle-to-older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross-sectional, controlled, study. Ann Rheum Dis. 2007;66:28–33. doi: 10.1136/ard.2006.053488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabio J., Zamora-Pasadas M., Jiménez-Jáimez J., Albadalejo F., Vargas-Hitos J., Rodríguez del Aguila M.D., et al. Metabolic syndrome in patients with systemic lupus erythematosus from Southern Spain. Lupus. 2008;17:849–859. doi: 10.1177/0961203308093554. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Casals M., Brito-Zerón P., Sisó A., Vargas A., Ros E., Bove A., et al. High prevalence of serum metabolic alterations in primary Sjögren's syndrome: influence on clinical and immunological expression. J Rheumatol. 2007;34:754–761. [PubMed] [Google Scholar]
- 40.Pape S., Snijders R., Gevers T., Chazouilleres O., Dalekos G.N., Hirschfield G., et al. Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group. J Hepatol. 2022;76:841–849. doi: 10.1016/j.jhep.2021.12.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used to support the findings of this study are included within the article and supplementary files.