Abstract
Background & Aims
Numerous studies have evaluated the role of human albumin (HA) in managing various liver cirrhosis-related complications. However, their conclusions remain partially controversial, probably because HA was evaluated in different settings, including indications, patient characteristics, and dosage and duration of therapy.
Methods
Thirty-three investigators from 19 countries with expertise in the management of liver cirrhosis-related complications were invited to organise an International Special Interest Group. A three-round Delphi consensus process was conducted to complete the international position statement on the use of HA for treatment of liver cirrhosis-related complications.
Results
Twelve clinically significant position statements were proposed. Short-term infusion of HA should be recommended for the management of hepatorenal syndrome, large volume paracentesis, and spontaneous bacterial peritonitis in liver cirrhosis. Its effects on the prevention or treatment of other liver cirrhosis-related complications should be further elucidated. Long-term HA administration can be considered in specific settings. Pulmonary oedema should be closely monitored as a potential adverse effect in cirrhotic patients receiving HA infusion.
Conclusions
Based on the currently available evidence, the international position statement suggests the potential benefits of HA for the management of multiple liver cirrhosis-related complications and summarises its safety profile. However, its optimal timing and infusion strategy remain to be further elucidated.
Impact and implications
Thirty-three investigators from 19 countries proposed 12 position statements on the use of human albumin (HA) infusion in liver cirrhosis-related complications. Based on current evidence, short-term HA infusion should be recommended for the management of HRS, LVP, and SBP; whereas, long-term HA administration can be considered in the setting where budget and logistical issues can be resolved. However, pulmonary oedema should be closely monitored in cirrhotic patients who receive HA infusion.
Keywords: Decompensated, Human albumin, Kidney injury, Liver failure, Management, Portal hypertension, Sepsis
Graphical abstract
Highlights
-
•
Thirty-three investigators from 19 countries contributed to the position statement.
-
•
Twelve position statements were proposed on the use of human albumin (HA) in liver cirrhosis.
-
•
Short-term HA infusion should be recommended for managing hepatorenal syndrome, large volume paracentesis, and spontaneous bacterial peritonitis.
-
•
Long-term HA infusion can be considered for managing ascites in specific settings.
-
•
Pulmonary oedema should be closely monitored in patients receiving HA infusion.
Introduction
Liver cirrhosis is prevalent worldwide1 and imposes a substantial economic burden on many countries.2,3 Core approaches for reversing the course and progression of cirrhosis are treatment of its underlying aetiology and liver transplantation.1,4 Human albumin (HA) is one of the most commonly used treatment options in patients with decompensated cirrhosis.5 HA treatment of selected complications of cirrhosis, including hepatorenal syndrome (HRS), spontaneous bacterial peritonitis (SBP), and large volume paracentesis (LVP) (>5 L), has been well established.6 HA has been recently proposed as a disease-modifying agent in decompensated cirrhosis.7,8 Several recent large-scale randomised controlled trials (RCTs), including the ANSWER,9 MACHT,10 and ATTIRE11 studies, which aimed at expanding the therapeutic use of HA, have been published with conflicting conclusions. In particular, the effects of HA may depend on the time of infusion, indications, infusion strategy, baseline serum albumin level, and severity of cirrhosis.[9], [10], [11], [12], [13]
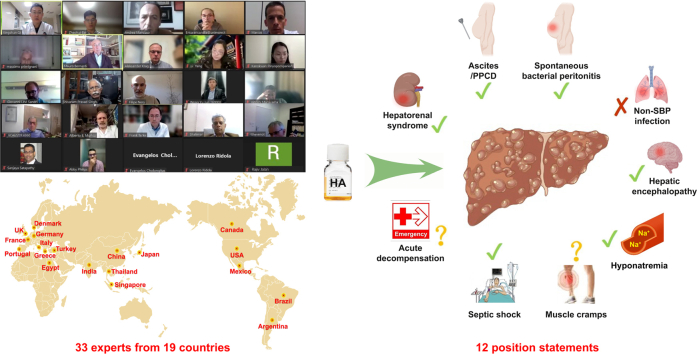
Recent data from clinical trials suggest that HA can decrease mortality in patients with cirrhosis.14 However, its survival benefit has not been sufficiently recognised by any of the current practise guidelines.6,15 Nonetheless, HA is widely prescribed in real-world clinical practice,[16], [17], [18], [19] and often beyond the approved indication of patients with cirrhosis.20 Two previous surveys conducted by the American Association for the Study of Liver Diseases (AASLD) and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF) showed that HA prescriptions in clinical practise concerned the treatment of HRS, SBP, ascites, LVP, non-SBP infections, hyponatremia, hepatic encephalopathy (HE), muscle cramps, hypoalbuminemia, peripheral oedema, and gastrointestinal bleeding.21,22 However, current guidelines6,15,[23], [24], [25] only recommend the use of HA to treat HRS, preventing acute kidney injury (AKI) induced by SBP, and preventing post-paracentesis circulatory dysfunction (PPCD) after LVP. Some national societies have identified additional indications for the use of HA. A joint position document by the Italian Association for the Study of the Liver (AISF) and the Italian Association of Transfusion Medicine and Immunohematology (SIMTI) focused on the use of HA in patients with cirrhosis was published in 201626 and updated in 2020,27 also recommended infusion of HA for paracentesis of <5 L, long-term treatment of patients with ascites, severe hyponatremia, and septic shock, but not HE or non-SBP infections. More recently, a position statement by the Mexican Association of Hepatology in 2022 on the use of HA in cirrhosis28 recommended HA for the treatment of LVP, HRS, SBP, hyponatremia, and muscle cramps, but not long-term treatment of ascites, non-SBP infections, or prevention of AKI. In summary, current recommendations regarding the use of HA in patients with cirrhosis remain inconsistent with those of previous guidelines, consensus statements from academic societies, or associations from various countries or regions. This work organised a panel of leading expert investigators in the treatment of liver cirrhosis-related complications and formulated an international position statement on the use of HA infusion in such conditions by comprehensively reviewing all available evidence.
Methods
A panel of investigators, skilled in managing liver cirrhosis-related complications, was invited to constitute an International Special Interest Group to draft and discuss the current position statement. Notably, the investigators were not selected by any specific pharmaceutical company, but through an international academic initiative. Due to the global scope of this position statement and the worldwide use of HA, investigators were selected to ensure representation from across the world. Consequently, the composition of this group was not restricted to only one or two scientific associations or societies. Additionally, senior authors of high-quality clinical studies on the use of HA in liver cirrhosis were given precedence to be invited. A total of 84 investigators were invited by the last author of the position statement via electronic emails. Finally, 31 investigators from 19 countries in five continents (i.e. North America, Europe, Asia, South America, and Africa) responded and accepted the invitation. All conflicts of interest related to this position statement were requested and disclosed.
Considering that the Delphi method can condense the knowledge and experience of experts to establish a broad scientific basis for a consensus on topics or procedures,29 a three-round Delphi consensus process was performed for the international position statement.30
First Delphi round (qualitative inventory of issues)
From May 8 to August 22, 2022, two members (ZB and XQ) of this group, who are skilled in literature search as well as systematic reviews and meta-analyses, were responsible for searching the literature in the PubMed database using the search items 'liver cirrhosis' AND 'albumin'. They systematically reviewed relevant evidence and any published guidelines/consensus statements, defined the main topics related to the position statement document, and elaborated the provisional statements. Subsequently, between August 23 and October 20, 2022, e-mails were sent to 13 additional members from multiple countries or regions to discuss the significance of these topics and to enquire about the revisions to the original document in terms of position statements, background, context, evidence, and references, as well as structure and writing.31
Second Delphi round (online consensus Delphi survey)
From October 21 to November 19, 2022, a link to an online questionnaire (www.wjx.cn/vm/P0xQCEr.aspx) was sent to 33 members of this group via an e-mail to assess the level of agreement with the position statements. The questionnaire consisted of 5-point Likert scales with an additional ‘do not know’ response option.29,32 In addition to the predefined response options, they could provide free-text comments to explain their responses or provide alternative suggestions. Point-to-point responses to each investigator’s comments were provided, as well as the corresponding revisions, if necessary. A summary of key results for the final discussion was reported at the position statement advisory meeting.
Quantitative analysis of the investigators’ ratings on the 5-point Likert scales was undertaken using IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA). The outcome measures were percentages and median values as indicators of agreement on the 5-point scales, as well as variances and interquartile ranges (IQRs) as indicators of consensus. Thresholds and consensus definitions were in accordance with previous studies.29 The level of agreement with an item was conceived as a composite measure and was rated either as ‘very high’ (median of 5, ≥80% agreement, IQR = 0), ‘high’ (median of 4 or 5, ≥80% agreement, IQR = 1), ‘moderate’ (median ≤4, 60–79% agreement, IQR ≥1), or ‘low’ (median <4, <60% agreement, IQR ≥2).
Third Delphi round (final advisory meeting)
From November 20 to November 25, 2022, a pre-voting link to an online questionnaire (https://www.wjx.cn/vm/hk2j2AS.aspx) was sent to 33 members in this group via an e-mail to reassess the level of agreement with the position statements.
On December 1, 2022, all members were invited to participate in an online meeting to make final corrections and comments on the position statement. All relevant comments and revisions were recorded to improve the quality of the position statements.
From December 2 to December 4 2022, a link to an online questionnaire (https://www.wjx.cn/vm/rZytuCB.aspx) was sent to the 33 members in this group via an e-mail to assess the level of agreement with the final position statements. Subsequently, the updated version was sent to all members for final approval of the submission.
The quality (level) of the evidence and the strength of each recommendation using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system were not rated in the current position statement.33 This was because a position statement is different from a clinical practice guideline. A practice guideline is developed by a multidisciplinary panel of experts who often rate the quality (level) of the evidence and the strength of each recommendation using the GRADE system. By comparison, a position statement is developed by a panel of experts in the topic, and position statements, but not recommendations, are put forward to help clinicians understand and implement the most recent evidence. Additionally, it should be acknowledged that these position statements may be updated, if more high-quality evidence is provided and accumulated.
Physical and chemical properties of HA
HA is a 66.5 kDa negatively charged protein with high solubility and stability.34 It is composed of 585 amino acids, of which a minority are tryptophan or methionine residues, and the majority are lysine and aspartic acids.35 HA has three homologous domains (I–III), each containing two subdomains (A and B), which help binding of substances.35 Some sites are responsible for the binding of pharmaceutical substances.36 Furthermore, HA contains 35 cysteine residues, of which 34 form disulphide bridges that contribute to the overall tertiary structure of the molecule, and one is a free cysteine (Cys-34), which is reactive and capable of thiolation and nitrosylation, contributing to antioxidation reactions.35,37
Physiology of HA and its potential effects on liver cirrhosis
In a healthy body, serum albumin levels range from 35 to 50 g/L. HA is synthesised by hepatocytes and is excreted into the systemic circulation at a rate of about 10–15 g daily.38 Its half-life in blood is about three weeks.39,40 Most albumin molecules are excreted through the kidneys, and minor amounts are excreted through the gut.41,42 In patients with cirrhosis, serum albumin synthesis is abnormal due to liver cell damage,34 thereby compromising its quantity and quality.[43], [44], [45] There are some changes in the quality of serum albumin, including an increase in the percentage of oxidised albumin, a functional alteration of the N-terminal portion of the serum albumin molecule, and serum albumin dimerisation.44 Protein-loss enteropathy can further decrease serum albumin levels in liver cirrhosis.46,47 Therefore, the concept of 'effective albumin concentration' has been proposed.43,48
Serum albumin is responsible for ∼75% of plasma colloid oncotic pressure due to its high concentration and net negative charge.35 Furthermore, HA exerts many functions, such as antioxidation, inhibition of systemic inflammation, immunomodulation, and endothelial stabilisation.35,43,49 Considering the importance of systemic inflammation, oxidative stress, circulatory dysfunction, and immune dysfunction in the development of complications and worse outcomes in cirrhosis,50 HA should theoretically be effective for the treatment of complications related to liver cirrhosis.
Use of HA in complications related to liver cirrhosis
Results of the Delphi consensus process
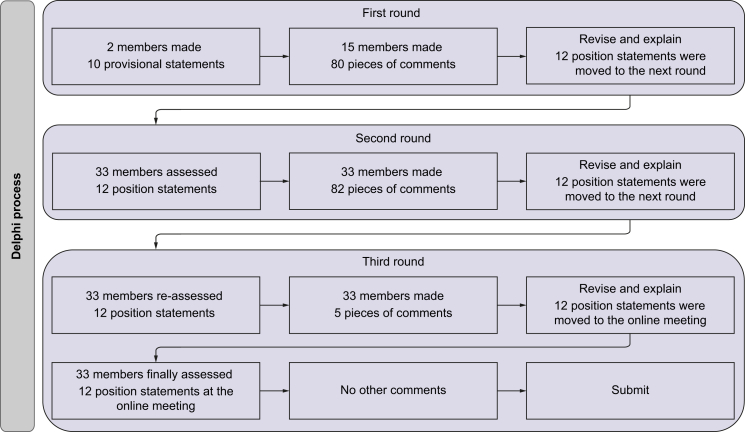
In the first round, 15 members from 10 countries provided 80 individual comments. After explanation, discussion, and revision, 12 provisional statements were approved. In the second round, 33 members from 19 countries made a further 82 individual comments. All these members responded to the online questionnaire and reached a consensus (levels of 'very high' or ‘high’) on eight position statements, but not for four others. After explanation, discussion, and revision, all the members approved to reassess the international position statement. In the third round, they provided another five comments. After explanation, discussion, and revision, the international position statement was approved. After the final advisory meeting, all the members responded to the online questionnaire, and reached a consensus on the 12 position statements without other comments (Fig. 1). Accordingly, three Delphi rounds are considered sufficient to achieve data saturation, and a consensus has been achieved for the international position statement (Table 1).
Fig. 1.
The flowchart of the Delphi process used in this study.
Table 1.
Degree of consensus for position statements during Delphi process.
| Position statements | Second round (33/33) |
Third round (33/33) |
Degree of consensus |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-voting |
Final voting |
|||||||||
| Median | Interquartile range | Agreement (%) | Median | Interquartile range | Agreement (%) | Median | Interquartile range | Agreement (%) | ||
| Position statement 1. HA infusion should be used for the differential diagnosis of HRS. | 5 | 0 | 97.0 | 5 | 1 | 97.0 | 5 | 0 | 97.0 | Very high |
| Position statement 2. HA infusion should be used for the management of HRS. | 5 | 0 | 100.0 | 5 | 0 | 100.0 | 5 | 0 | 100.0 | Very high |
| Position statement 3. HA infusion should be used for the prevention of PPCD in patients with liver cirrhosis and ascites undergoing LVP (>5 L). HA infusion could be considered in cirrhotic patients with ACLF/AKI undergoing paracentesis of <5 L. | 5 | 1 | 100.0 | 5 | 0 | 97.0 | 5 | 1 | 100.0 | High |
| Position statement 4. In the setting where budget and logistical issues can be resolved, long-term regular HA infusion can be used to improve ascites, prevent other complications of liver cirrhosis, and prolong survival in patients with uncomplicated ascites requiring diuretics. | 4 | 1.5 | 75.8 | 4 | 1 | 84.9 | 4 | 1 | 87.9 | High |
| Position statement 5. HA infusion should be used to prevent AKI and improve survival in cirrhotic patients with SBP, particularly those with a baseline serum bilirubin ≥4 mg/dl or a serum creatinine ≥1 mg/dl. | 5 | 1 | 100.0 | 5 | 0.5 | 100.0 | 5 | 0 | 100.0 | Very high |
| Position statement 6. HA infusion does not prevent renal impairment or improve survival in patients with cirrhosis affected by non-SBP infections other than septic shock. | 4 | 1 | 81.8 | 5 | 1 | 87.8 | 5 | 1 | 91.0 | High |
| Position statement 7. HA infusion may be considered for the management of overt HE, especially in patients with liver cirrhosis and hypoalbuminemia. | 4 | 2 | 66.7 | 4 | 1 | 81.8 | 4 | 1 | 87.9 | High |
| Position statement 8. HA infusion may be considered to improve and prevent hyponatremia in cirrhotic patients, but its effects needs to be further explored. | 4 | 1 | 78.8 | 5 | 1 | 100.0 | 4 | 1 | 100.0 | High |
| Position statement 9. More data are warranted to evaluate the use of HA to attenuate muscle cramps in patients with liver cirrhosis. | 5 | 1 | 87.9 | 5 | 1 | 97.0 | 5 | 1 | 97.0 | High |
| Position statement 10. HA may be considered for the management of cirrhotic patients with septic shock. | 4 | 2 | 69.7 | 4 | 1 | 93.9 | 5 | 1 | 91.0 | High |
| Position statement 11. Short-term HA infusion in general patients with acute decompensation of cirrhosis may not be beneficial. Further validation should be considered. | 4 | 1 | 91.9 | 5 | 1 | 84.8 | 5 | 1 | 100.0 | High |
| Position statement 12. HA infusion is generally safe but may induce the development of pulmonary oedema and severe allergic reactions. | 5 | 1 | 100.0 | 5 | 1 | 100.0 | 5 | 0 | 100.0 | Very high |
HA, human albumin; HE, hepatic encephalopathy; AKI, acute kidney injury; LVP, large volume paracentesis; SBP, spontaneous bacterial peritonitis; HRS, hepatorenal syndrome; ACLF, acute on chronic liver failure; PPCD, post-paracentesis circulatory dysfunction.
Hepatorenal syndrome
Position statement 1. HA infusion should be used for the differential diagnosis of HRS (agreement score: 5; degree of consensus: very high)
HRS, a form of AKI (HRS-AKI) that occurs in cirrhosis, requires differentiation from prerenal AKI.25 Prerenal AKI follows a decrease in blood pressure related to blood or fluid loss and consequent renal hypoperfusion.51 Therefore, it can be relieved by an adequate expansion of plasma volume. Instead, HRS-AKI, a functional renal failure secondary to cardiocirculatory dysfunction in advanced cirrhosis, does not improve after plasma volume expansion.52 Therefore, when AKI develops in cirrhosis, plasma volume expansion is recommended to differentiate HRS-AKI from prerenal AKI.25,53 As HA has a stronger effect and a longer duration than isotonic saline in expanding plasma volume,52 the current recommendation of the International Club of Ascites (ICA) is HA infusion at a dose of 1 g/kg up to a maximum of 100 g daily for at least 2 days with diuretic withdrawal.
Position statement 2. HA infusion should be used for the management of HRS (agreement score: 5; degree of consensus: very high)
Classically, a severe reduction in effective volume secondary to cardiovascular dysfunction in advanced cirrhosis is considered the most relevant pathophysiological mechanism of HRS. As a result, the consequent renal hypoperfusion and intrarenal vasoconstriction would lead to renal failure.54 This explains why the current well-established treatment of HRS includes a vasoconstrictor to counteract splanchnic arterial vasodilation and HA infusion as a plasma expander. In recent years, the role of persistent systemic inflammation and oxidative stress has gained relevance, so the treatment of HRS may also address different goals in the near future.55 Furthermore, it should be noted that nearly all studies on HRS treatment preceded the publication of the revised diagnostic criteria for ICA-AKI in cirrhosis. Many of these studies enrolled patients with HRS-1 and HRS-2 in accordance with the previous definitions. Therefore, they may not completely reflect the current definition of HRS-AKI.
HA is the first-choice plasma expander for the treatment of HRS.[56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66] There is evidence that HA could improve the beneficial effects of vasoactive drugs. In a study that included 21 patients with HRS (HRS-1:16, HRS-2:5), 13 received HA plus terlipressin and eight terlipressin alone.67 The dose of HA was 1 g/kg on day 1, then changed to 20-40 g daily. HA added to terlipressin significantly increased the complete response rate compared to terlipressin alone (77% vs. 25%, p = 0.03), and was the only predictor of complete response. Furthermore, the combination of HA and terlipressin was associated with a marked decrease in serum creatinine level, an increase in arterial pressure, and suppression of the renin-angiotensin-aldosterone system (RAAS). By contrast, these parameters did not significantly change with terlipressin alone. More importantly, HA was associated with a higher 90-day survival rate (p <0.03).
The recommended dosage of HA in patients with cirrhosis and HRS is heterogeneous in the current guidelines and consensus statements. According to the ICA recommendation, it should be 1 g/kg on day 1 with a maximum dosage of 100 g, followed by 20–40 g daily. HA infusion can be discontinued if the serum albumin level is >45 g/L, and should be withdrawn in the case of pulmonary oedema.52 The European Association for the Study of the Liver (EASL) recommends that after 2 days of administration of HA (1 g/kg) for differential diagnosis, a dose of 20–40 g daily should be maintained until a complete response (i.e. a serum creatinine <1.5 mg/dl) or 14 days.6 AASLD recommends 1 g/kg on day 1, followed by 40–50 g on all subsequent day.15 A previous meta-analysis showed a dose-response relationship between HA and survival in patients with HRS-1. The expected 90-day survival rate was 24.8%, 33.1%, and 41.6% in patients who received HA at a cumulative dosage of 200 g, 400 g, and 600 g, respectively. An increment of 100 g in the cumulative dosage of HA was associated with a 1.15-fold increase in survival rate.68 However, this meta-analysis did not clarify the type or dosage of vasoactive drugs combined with HA, nor did it consider the potential risks associated with the excessive daily dose of HA.
As far as the safety of HA is concerned, the current position statement recommends that the dose of HA for HRS treatment should be 20–40 g daily and the infusion of HA should be maintained until a complete response (i.e. a serum creatinine level <1.5 mg/dl) is achieved or for a maximum duration of 14 days. Central venous pressure monitoring can help prevent volume overload and pulmonary oedema when possible.
Ascites
Position statement 3. HA infusion should be used for the prevention of PPCD in patients with liver cirrhosis and ascites undergoing LVP (>5 L). HA infusion could be considered in cirrhotic patients with acute-on-chronic liver failure (ACLF)/AKI undergoing paracentesis of <5 L (agreement score: 5; degree of consensus: high)
Paracentesis is the first-line treatment option for severe/refractory ascites,6,15,23 and can quickly and effectively relieve symptoms.69,70 PPCD, a common complication of LVP, is secondary to an acute reduction in the effective volume due to enhanced arterial vasodilation.71 PPCD can accelerate the re-accumulation of ascites and induce complications, such as hyponatremia, renal dysfunction, and HE, thus increasing the risk of death.[72], [73], [74] In 2012, a meta-analysis, including 17 RCTs with 1,225 patients, explored the effects of HA administration after LVP.75 Among these included RCTs, the dose of HA ranged from 5 to 8 g/L of removed ascites, and HA significantly reduced the incidence of PPCD (OR = 0.39, 95% CI = 0.27–0.50). The prevention of PPCD ensured by HA is superior to that of plasma expanders (i.e. hypertonic saline, dextran, gelatin, and hydroxyethyl starch) and vasoconstrictors (i.e. norepinephrine, terlipressin, and midodrine).75 Additionally, HA could significantly decrease the incidence of hyponatremia (OR = 0.58, 95% CI = 0.39–0.87) and mortality (OR = 0.64, 95% CI = 0.41–0.98) after LVP.75 In 2019, another meta-analysis, which included 27 studies with 1,592 patients, explored the efficacy of plasma expanders in patients with cirrhosis and large ascites treated with abdominal paracentesis.76 Among them, 21 studies compared other plasma expanders versus HA. Other plasma expanders had a significantly higher incidence of PPCD than HA infusion (RR = 1.98, 95% CI = 1.31-2.99), but all-cause mortality was similar between the two groups (RR = 1.03, 95% CI = 0.82–1.30).76 In 2021, an updated meta-analysis, which included 21 RCTs with 1,584 patients receiving HA at a dosage of 5–8 g/L ascites removed, further confirmed the benefits of HA for the prevention of PPCD (OR = 0.40, 95% CI = 0.27–0.58) and hyponatremia after paracentesis (OR = 0.59, 95% CI = 0.39–0.88) in patients with cirrhosis and ascites undergoing paracentesis.77 In particular, the HA group had significantly lower mortality than other volume expanders (OR = 0.57, 95% CI = 0.36–0.89).77 The effects of HA on PPCD were also better than those of hypertonic saline, dextran, gelatin, and hydroxyethyl starch, but were similar to those of vasoactive drugs (OR = 0.93, 95% CI = 0.35–2.45).77 A recent meta-analysis also showed that HA infusion could not significantly decrease mortality in cirrhotic patients undergoing LVP (OR = 0.81, 95% CI = 0.59–1.10).14 It should be noted that a volume of 5 L ascites removed as a cut-off value for defining LVP and initiating HA infusion after LVP is in accordance with the design of the currently published studies, but is a bit arbitrary and does not consider the patient’s body weight. Furthermore, it is uncommon to remove 5 L of ascites at once during a single paracentesis in the real-world clinical practice. Therefore, in the future, an optimal threshold should be identified.
According to the EASL practice guideline, patients with ascites undergoing paracentesis of <5 L should still be treated with HA rather than with an alternative plasma expander,6 although they often have a low risk of PPCD. In contrast, according to the British Society of Gastroenterology (BSG) and British Association for the Study of the Liver (BASL) practice guidelines, plasma expansion is not necessary in patients with ascites undergoing paracentesis of <5 L, unless ACLF is evident.78 In 1997, a study evaluated haemodynamic and neurohumoral responses in 12 patients after paracentesis,79 and showed that a single paracentesis of 5 L without HA infusion is a safe and satisfactory option for short-term management of cirrhosis with tense ascites resistant to diuretics. In 2019, an RCT including 80 ACLF patients found that HA infusion significantly reduced the incidence of renal impairment, hyponatremia, and mortality after paracentesis of <5 L.80
The current position statement recommends the use of HA to prevent PPCD after LVP at a dose of 8 g/L of removed ascites. HA infusion at the same dose can be considered in cirrhotic patients with ACLF/AKI and ascites undergoing paracentesis of <5 L; however, more evidence is needed.
Position statement 4. In a setting where budget and logistical issues can be resolved, long-term regular HA infusion can be used to improve ascites, prevent other complications of liver cirrhosis, and prolong survival in patients with uncomplicated ascites requiring diuretics (agreement score: 4; degree of consensus: high)
Approximately 23% of patients with cirrhosis present with ascites at their first diagnosis.81 Ascites is the most frequent decompensating event and carries a poor prognosis, as approximately 20% of patients die within 1 year.81 It is primarily classified as uncomplicated and refractory ascites.53,78 Uncomplicated ascites refers to ascites that is neither infected nor associated with the development of HRS, and refractory ascites is defined as ascites that cannot be mobilised or is an early recurrence of ascites (i.e. after therapeutic paracentesis) which cannot be satisfactorily prevented by medical treatment.53,78 Diagnostic paracentesis is recommended in all patients with new-onset ascites, and can be used to exclude other aetiologies of ascites based on a serum-ascites albumin gradient.23 Portal hypertension is the predominant pathophysiological mechanism of ascites formation in cirrhosis. A hepatic venous pressure gradient of at least 10 mmHg defines clinically significant portal hypertension, which leads to portosystemic collaterals formation and peripheral arterial vasodilation at the compensated stage of cirrhosis.50 Several mechanisms contribute to arterial vasodilation. The most relevant include enhanced nitric oxide synthesis induced by vascular endothelial growth factor and inflammation promoted by abnormal gut translocation of bacteria and bacterial products.50,82 The impairment in effective volume secondary to arterial vasodilation evokes the compensatory activation of vasoconstrictor and sodium/water-retaining systems (i.e. RAAS, sympathetic nervous system, and arginine-vasopressin) so that the blood volume expands.83 Increased sinusoidal hydrostatic pressure secondary to portal hypertension compartmentalises hypervolemia by enhancing hepatic lymph formation, ultimately leading to the appearance of ascites.83 HA can alleviate circulatory dysfunction by improving effective volume, modulating systemic inflammation, and opposing hyperoxidation, so it may be effective in managing ascites.
The role of long-term HA infusion in patients with cirrhosis and ascites has been explored in several RCTs9,10,[84], [85], [86] and two meta-analyses.14,87 The latter study reported that long-term HA infusion could decrease the recurrence of ascites, but could not significantly decrease the mortality in patients with cirrhosis and ascites.14,87 However, the heterogeneity was significant among these included RCTs and the main characteristics of the patients should be further specified. Studies exploring the effect of HA in patients with cirrhosis and ascites date back to 1962. The first RCT enrolled 16 patients, who were randomly assigned to receive diuretics alone (n = 9) and diuretics plus HA (n = 7).84 The dosage of HA varied between 25 and 100 g weekly, and the target serum-colloid osmotic pressure was maintained between 35 and 40 cm of water. HA infusion did not reduce the requirement for diuretics nor mortality.84 In 1999, a second RCT enrolled 126 patients, who were randomly assigned to receive diuretics alone (n = 63) and diuretics plus HA (n = 63) at a daily dose of 12.5 g.85 The diuretics plus HA group had a significantly higher cumulative rate of response to diuretics treatment (90.5% vs. 74.7%, p <0.05) and lower rates of recurrence of ascites (69% vs. 82%, p <0.02) and re-admissions (69% vs. 79%, p <0.02) than the diuretics alone group. However, the 3-year mortality was similar between the two groups (82.5% vs. 85.7%).85 In 2006, a third RCT evaluated the effects of long-term HA infusion on the survival of 100 patients with cirrhosis and ascites.86 Patients were randomly assigned to receive diuretics plus HA (n = 54) and diuretics alone (n = 46). The HA dosage was 25 g weekly in the first year and 25 g every 2 weeks thereafter. HA significantly prolonged the median survival time (108 vs. 36 months p = 0.0079) and decreased the rate of ascites recurrence (38.88% vs. 84.78%, p <0.001).86 In 2018, the ANSWER study, a multicentre randomised open-label trial, explored the effects of long-term HA infusion in 431 cirrhotic patients with uncomplicated ascites requiring diuretic administration.9 Patients were randomly assigned to standard medical treatment (SMT) (n = 213) or SMT plus HA (n = 218). The HA dose was 40 g twice weekly for the first 2 weeks, followed by 40 g weekly. The SMT plus HA group had a significantly lower need for LVP (HR = 0.48, 62% vs. 34%, p <0.0001) and a lower incidence of complications related to cirrhosis, such as refractory ascites, bacterial infections, episodes of renal dysfunction, and severe HE (grades III and IV), HRS-AKI, hyponatremia, and hyperkalemia. Finally, patients who received HA had a significantly higher survival rate at 18 months (77% vs. 66%, p = 0.028).9 A few months later, the MACHT study, a randomised placebo-controlled trial that explored the effects of long-term HA infusion in 196 patients with cirrhosis and ascites and planned liver transplantation, was published.10 Patients were randomly assigned to SMT plus midodrine (15–30 mg daily according to mean arterial pressure) and HA (n = 99) or SMT plus placebo (n = 97). The HA dose was 40 g every 15 days. The incidence of cirrhosis-related complications (37% vs. 43%, p = 0.402) and 1-year mortality (7% vs. 5%, p = 0.527) were not significantly different between the HA and SMT groups.10
An important explanation for this controversy in these findings is that the median duration of HA treatment was longer than 1 year in the ANSWER study, but 63 days in the MACHT study due to a high proportion of patients who underwent liver transplantation, whereas the dosage of HA was double in the ANSWER study. Interestingly, a prospective non-randomised study that enrolled patients with cirrhosis and refractory ascites and used the same HA dosage of the ANSWER study (i.e. 20 g twice weekly) also obtained favourable results in terms of incidence of cirrhosis-related complications and mortality.88
The most recent EASL clinical practice guideline6 relative to the management of decompensated cirrhosis was published prior to the ANSWER study.9 A recent AASLD practice guideline did not recommend long-term HA treatment in cirrhotic patients with ascites.15 By contrast, a position paper from AISF and SIMTI strongly recommended that long-term HA treatment should be included among the medical treatment options for cirrhotic patients with ascites.27 Reasons for concern and debate are related to the availability of HA and its cost. Although the availability of HA may be challenging in some contexts, long-term HA infusion is considered cost-effective and even cheaper than SMT, as the ANSWER study showed that a lower incidence of complications in patients receiving HA infusion reduced the need for and the duration of hospitalisations. Certainly, an economic analysis of long-term HA infusion, including its indirect costs, would be worthwhile.
Based on available literature, the current position statement indicates long-term HA infusion as a treatment option for patients with cirrhosis and uncomplicated ascites requiring diuretic therapy. Specifically, a 40 g weekly dose of HA might be preferred,9 although the optimal dosage should be further explored. Furthermore, the target serum albumin level after 1 month of HA treatment might be reached ≥40 g/L.89
Bacterial infections
Position statement 5. HA infusion should be used to prevent AKI and improve survival in cirrhotic patients with SBP, particularly those with a baseline serum bilirubin ≥4 mg/dl or a serum creatinine ≥1 mg/dl (agreement score: 5; degree of consensus: very high)
Cirrhotic patients have a higher risk of developing bacterial infections than the general population,[90], [91], [92] which can significantly increase the risk of death.93 SBP, the most common type of bacterial infection in cirrhosis,94 is defined as infection of the ascitic fluid without any surgically treatable intra-abdominal source of infection.6 The 90-day mortality after SBP is approximately 20%.95 Based on available evidence, antibiotics plus HA have become the first-line treatment for SBP.96 In the context of SBP, the benefits of HA are attributable to its ability to inhibit inflammation and oxidative stress and improve haemodynamic status.5 In an early RCT, a total of 126 SBP patients were randomised to receive cefotaxime alone (n = 63) or in combination with HA (n = 63).97 The dose of HA was 1.5 g/kg at diagnosis and 1 g/kg on day 3. The rate in improvement of SBP was not significantly different between the two groups (98% vs. 94%, p = 0.36), but the cefotaxime plus HA group had a significantly lower incidence of AKI (10% vs. 33%, p = 0.002), mortality at 1 month (10% vs. 29%, p = 0.01), and mortality at 3 months (22% vs. 41%, p = 0.03) than the cefotaxime alone group. Furthermore, this RCT showed that HA infusion was particularly effective in patients with a baseline serum bilirubin level ≥4 mg/dl or a serum creatinine level ≥1 mg/dl. Another study explored the effects of HA on inflammatory mediators in 30 cirrhotic patients with SBP.98 Patients were randomly assigned to antibiotics alone (n = 15) and combined with HA groups (n = 15). HA was infused 3 days after the diagnosis of SBP. Antibiotics plus HA significantly decreased tumour necrosis factor (TNF)-α and interleukin (IL)-6 levels in blood and ascites.98 A randomised pilot study that enrolled 20 patients with cirrhosis and SBP assessed the impact of HA on systemic haemodynamics.99 Patients were randomly selected to receive HA (n = 10) or hydroxyethyl starch (n = 10). The dose of HA was 1.5 g/kg at diagnosis and 1 g/kg on day 3. In the HA group, none developed circulatory dysfunction or renal failure. Instead, three patients developed circulatory dysfunction and one renal failure in the hydroxyethyl starch group. In the HA group, but not in the hydroxyethyl starch group, systemic and pulmonary pressures increased significantly, systemic vascular resistance decreased, and the cardiac work index improved. These findings suggest that the effects of HA were not exclusively due to plasma volume expansion, but also mediated by non-oncotic properties.
The current position statement recommends that HA infusion be administered to prevent AKI and reduce mortality in cirrhotic patients with SBP, particularly those with a baseline serum bilirubin level ≥4 mg/dl or a serum creatinine level ≥1 mg/dl. Specifically, HA infusion is considered at a dose of 1.5 g/kg at diagnosis and 1 g/kg on day 3, but the optimal dosage of HA infusion in patients with SBP should be further explored.
Position statement 6. HA infusion does not prevent renal impairment or improve survival in patients with cirrhosis affected by non-SBP infections other than septic shock (agreement score: 5; degree of consensus: high)
Non-SBP infections, including urinary tract infections, pneumonia, skin infections, bacteraemia, and septic shock, account for 75% of all bacterial infections in cirrhosis.92,94 The complications of these infections can induce AKI, circulatory failure, and even multiple organ failure, significantly influencing the outcomes of patients with cirrhosis.100 Until now, the effects of HA on patients with cirrhosis and non-SBP infections remain controversial.[101], [102], [103] The INFECIR-2 study assessed the effect of 1-week HA (1.5 g/kg on day 1 and 1 g/kg on day 3) in patients with decompensated cirrhosis and bacterial infections unrelated to SBP.104 Seventy-eight patients were randomly assigned to HA plus antibiotics (n = 38) or antibiotics alone (n = 40). HA plus antibiotics significantly decreased the levels of TNF-α (p = 0.01), granulocyte colony-stimulating factor (p = 0.01), IL-6 (p = 0.003), and IL-10 (p = 0.03), but antibiotics alone did not. However, survival was not different between patients who received and did not receive HA. This unexpected phenomenon may be attributed to the fact that patients who received HA had a higher prevalence of ACLF/AKI at admission than those who did not. In 2020, a meta-analysis of three RCTs with 406 patients, including 202 in the HA group and 204 in the control group, explored the efficacy and safety of HA in cirrhotic patients with non-SBP bacterial infections.105 The dose of HA was 1.5 g/kg on day 1 and 1 g/kg on day 3. HA infusion could not decrease the risk of renal impairment (OR = 0.58, 95% CI = 0.28-1.23, p = 0.16), 30-day mortality (OR = 1.61, 95% CI = 0.87–3.00, p = 0.13), or 90-day mortality (OR = 1.30, 95% CI = 0.81–2.07, p = 0.28). It is worth noting that HA group had a significantly higher incidence of pulmonary oedema (OR = 4.38, 95% CI = 1.30–14.79, p = 0.02). A recent meta-analysis also showed that HA infusion could not significantly decrease mortality in patients with cirrhosis and non-SBP infections (OR = 1.01, 95% CI = 0.73–1.40).14
The current position statement does not recommend the use of HA for the treatment of non-SBP infections without septic shock in patients with cirrhosis.
Hepatic encephalopathy
Position statement 7. HA infusion may be considered for the management of overt HE, especially in patients with liver cirrhosis and hypoalbuminemia (agreement score: 4; degree of consensus: high)
HE, a severe complication of cirrhosis, significantly worsens the quality of life of patients and their outcomes.106,107 The incidence of covert and overt HE is 20–80% and 30–40%, respectively.108 Hyperammonaemia is considered the main pathogenesis of HE.109,110 Consequently, the main objectives of HE treatment are to reduce ammonia absorption and increase ammonia excretion, and the most common drugs include lactulose,111 rifaximin,112 and L-ornithine-L-aspartate.113 There is also evidence that systemic inflammation and oxidative stress are associated with the development of HE.5,82,114,115 Additionally, a significantly higher incidence of overt HE is observed in cirrhotic patients with hypoalbuminemia (i.e. a serum albumin level ≤31.6 g/L), and patients with overt HE and severe hypoalbuminemia (i.e. a serum albumin level ≤22.8 g/L) have a significantly higher mortality.12
In 2013, an RCT explored the effect of HA on 56 patients with cirrhosis and overt HE.116 Patients were randomised to receive HA (n = 26) or saline (n = 30). The mean serum albumin level at baseline was 29 g/L and 30 g/L in the HA and saline groups, respectively. The dose of HA was 1.5 g/kg on day 1 and 1 g/kg on day 3. The percentage of patients without HE on day 4 was not significantly different between the HA and saline groups (57.7% vs 53.3%; p = 0.7), but the HA group had a significantly higher 90-day survival rate than the saline group (69.2% vs 40.0%, p = 0.02). In 2017, another RCT further evaluated the addition of HA in 120 patients with cirrhosis and overt HE receiving lactulose.117 The mean serum albumin level at baseline was 23 g/L and 24 g/L in the HA and saline groups, respectively. The dose of HA was 1.5 g/kg daily. The HA plus lactulose group had a significantly higher rate of complete reversal of overt HE (75% vs. 53.3%, p = 0.03) and lower 10-day mortality (18.3% vs. 31.6%, p = 0.04) than the lactulose alone group. A retrospective cohort study, which included 708 cirrhotic patients, showed that HA significantly decreases the incidence of overt HE (4.20% vs. 12.70%, p <0.001), improves overt HE (84.60% vs. 68.10%, p = 0.009), and reduces in-hospital mortality (7.70% vs. 19.80%, p = 0.018).118 The mean serum albumin level at baseline was 26.55 g/L in the HA group and 27.03 g/L in the control group. More recently, the HEAL study has explored the role of HA infusion in cirrhotic patients with prior HE who had already used standard of care for the treatment of HE.119 Forty-eight patients were randomly assigned to the HA (n = 24) and saline (n = 24) groups. The dose of HA was 1.5 g/kg weekly over 5 weeks. The HA group had significantly higher rates of reversal and improvement of minimal HE, which was defined by the psychometric hepatic encephalopathy score, Stroop test, or critical clicker frequency, than the saline group. Additionally, there was a significant reduction of IL-1β and endothelial dysfunction markers after treatment in the HA group, but not in the saline group.119 To date, there have been at least three published meta-analyses regarding HA administration for the treatment of HE.14,120,121 Generally, they supported the benefits of HA in decreasing the incidence of HE, improving the severity of HE, and reducing the mortality of HE patients. Unfortunately, the quality of available evidence regarding HA infusion for the management of HE could have been influenced by the heterogeneity in the study design and subjectivity on the outcome assessment.122
The current position statement indicates that HA at a dose of 20–40 g daily can be considered to treat overt HE, especially in cirrhotic patients with hypoalbuminemia. A longer duration of HA infusion may be associated with a higher probability of improved HE based on the current evidence.
Hyponatremia
Position statement 8. HA infusion may be considered to improve and prevent hyponatremia in cirrhotic patients, but its effects need to be further explored (agreement score: 4; degree of consensus: high)
Hyponatremia, defined as a serum sodium level <135 mmol/L,6,23 is observed in 49% of patients with cirrhosis and ascites.123 The most frequent type of hyponatremia is hypervolemic hyponatremia in patients with cirrhosis, which accounts for 90% of cases.124 Hyponatremia is significantly associated with worse outcomes in patients with cirrhosis.125,126 However, effective treatment options for hyponatremia are limited.127 Hyperdynamic circulation, splanchnic vasodilation, and systemic inflammation concur in the development of hyponatremia in cirrhosis.127 Therefore, HA may be effective in correcting this abnormality. A cohort study included 1,126 patients with cirrhosis and hyponatremia, of whom 777 received HA infusion at a median cumulative dose of 225 g.128 HA infusion improved the resolution of hyponatremia (69% vs. 61%, p = 0.008). A post hoc analysis of the data from the ATTIRE trial11 showed that HA infusion increased serum sodium levels in hyponatraemic patients hospitalised with an acute decompensation of cirrhosis, but did not improve their survival.129 Among patients with a serum sodium level <130 mmol/L, the cumulative dose of HA was 239.4 g in the HA group and 123.3 g in the control group. HA significantly increased serum sodium levels on day 5 after treatment (p <0.0001). A post hoc analysis based on the ANSWER9 study database evaluated 149 patients with hyponatremia, of whom 75 had been assigned to SMT plus HA and 74 to SMT alone.130 The HA group had significantly higher rates of resolution of hyponatremia at 1 (45% vs. 28%, p = 0.042) and 3 months (61% vs. 38%, p = 0.006). Furthermore, HA-treated patients had a lower incidence of at least moderate hyponatremia than those who received SMT alone (incidence rate ratio = 0.245, 95% CI = 0.167–0.359, p <0.001). A meta-analysis explored the role of HA in the prevention and treatment of hyponatremia in patients with cirrhosis.131 Thirty studies, including 25 RCTs and five cohort studies, were included. Among cirrhotic patients without hyponatremia, the HA group had a significantly lower incidence of hyponatremia (OR = 0.55, 95% CI = 0.38–0.80, p = 0.001) and a higher serum sodium level (MD = 0.95, 95% CI = 0.47–1.43, p = 0.0001) than the control group. Among cirrhotic patients with hyponatremia, the HA group had a significantly higher rate of resolution of hyponatremia (OR = 1.50, 95% CI = 1.17–1.92, p = 0.001) than the control group. However, the quality of the available evidence is low.
In light of the current evidence, HA can improve hyponatremia in patients with cirrhosis. However, prospective randomised trials specifically evaluating this topic are warranted to validate the role of HA in the treatment of hyponatremia in cirrhosis. Additionally, the optimal dosage and administration schedule of HA in this context and its withdrawal rules must still need to be defined. The impact of hyponatremia correction on the prognosis of cirrhosis should also be clarified.
The current position statement recommends that HA may be considered for the management of hyponatremia in selected patients with cirrhosis.
Muscle cramps
Position statement 9. More data are warranted to evaluate the use of HA to attenuate muscle cramps in patients with liver cirrhosis (agreement score: 5; degree of consensus: high)
Muscle cramps, a complication of cirrhosis likely due to a diuretics-induced reduction in effective volume and disturbances in homeostasis, negatively influence quality of life.132,133 Current treatments for muscle cramps include baclofen134 and quinidine.135 In one study, 12 cirrhotic patients who developed muscle cramps more than three times weekly were observed.133 HA infusion was associated with a decrease in the frequency of muscle cramps (p <0.01). However, until more robust evidence is available, HA cannot be recommended for the treatment of muscle cramps in cirrhosis.
Septic shock
Position statement 10. HA may be considered for the management of cirrhotic patients with septic shock (agreement score: 5; degree of consensus: high)
Cirrhotic patients are at increased risk for the development of sepsis, sepsis-induced organ failure, and sepsis-related death.136 Sepsis is a complex pathophysiological state characterised by the release of many pro-inflammatory and anti-inflammatory and procoagulant and anticoagulant substances in response to pathogens.137 The severity of sepsis is often divided into sepsis, severe sepsis in which acute organ failure attributed to sepsis develops, and septic shock in which refractory hypotension requires vasopressor agents.138 The in-hospital mortality in patients with cirrhosis and septic shock can exceed 80%.139 However, the optimal management of patients with cirrhosis and sepsis/septic shock has not been well established. Current management mainly includes fluid resuscitation, vasopressors, steroids, antibiotics, and liver transplantation.140 A retrospective cohort study showed a close association of HA infusion with improved survival in cirrhotic patients with septic shock.141 Two RCTs also explored the role of HA infusion in patients with cirrhosis and sepsis-induced hypotension.142,143 In the FRISC study,142 a total of 308 cirrhotic patients with sepsis-induced hypotension were randomly assigned to the HA (n = 154) and saline (n = 154) groups. The dose of HA was 5% HA intravenous bolus, 250 ml over 15–30 min, followed by a maintenance infusion of 50 ml/h for a total of 3 h (20 g in total). The reversal of hypotension was higher in patients receiving HA than those receiving saline (11.7% and 3.2%, p = 0.008). There was a significantly higher proportion of patients who survived 1 week in the HA group than in the saline group (43.5% vs. 38.3%, p = 0.03). In the ALPS trial,143 a total of 100 cirrhotic patients with sepsis-induced hypotension were randomly assigned to the HA (n = 50) and plasmalyte (n = 50) groups. The dose of HA was 0.5–1.0 g/kg over 3 h. The proportion of patients with mean arterial pressure above 65 mmHg at 3 h was significantly higher in the HA group than in the plasmalyte group (62% vs. 22%, p <0.001). However, the 28-day mortality was not significantly different between the two groups (58% vs. 62%, p = 0.57), and HA caused more pulmonary complications than plasmalyte.
The current position statement recommends that HA infusion may be considered to treat septic shock. Considering the heterogeneity in safety profile among various HA infusion strategies, it is preferred that 5% HA solution is intravenously infused at a dosage of 250 ml over 15–30 min, followed by a maintenance infusion of 50 ml/h until haemodynamically stable.
Acute decompensation of cirrhosis
Position statement 11. Short-term HA infusion in general patients with acute decompensation of cirrhosis may not be beneficial. Further validation should be considered (agreement score: 5; degree of consensus: high)
The ATTIRE trial explored the target HA administration, where the serum albumin level should reach at least 35 g/L, in 777 cirrhotic patients with hypoalbuminemia (<30 g/L) who were hospitalised for acute decompensation.11 Patients were randomly assigned to the HA (n = 380) and SMT (n = 397) groups. Infusion of 20% HA was administered at a rate of 100 ml/h from day 1 of recruitment, with the aim of maintaining a serum albumin level of 35 g/L or more. The mean serum albumin level was 30 g/L or more after treatment in the HA group, but did not reach 35 g/L as planned. HA did not decrease the probability of developing a composite primary endpoint event (29.7% vs. 30.2%; OR = 0.98; 95% CI = 0.70–1.33, p = 0.87), which included any infection, kidney dysfunction, or death during hospitalisations. Additionally, the 28-day (14.0% vs. 15.6%), 3-month (24.2% vs. 23.2%), and 6-month (34.7% vs. 30.0%) mortality were similar between the two groups. Based on current evidence, populations and an optimal infusion strategy to guide HA infusion in cirrhotic patients with unspecified types of acute decompensated events should be further explored.
HA-related serious adverse events
Position statement 12. HA infusion is generally safe, but may induce the development of pulmonary oedema and severe allergic reactions (agreement score: 5; degree of consensus: very high)
Pulmonary oedema
HA plays an important role in maintaining plasma colloid osmotic pressure. However, infusion of high doses of HA in a short time can cause an excessive increase in circulating blood volume and increase the risk of pulmonary oedema. This risk is amplified by concomitant cardiomyopathy.144,145 In the ATTIRE trial, the median dose of 20% HA solution was 200 g during a median hospitalisation stay of 8 days in the HA group, but only 20 g during a median hospitalisation stay of 9 days in the control group. The HA group had a higher incidence of pulmonary oedema than the control group (6% vs. 2%).11 In the ALPS trial, the dose of 20% HA solution was 0.5–1.0 g/kg over 3 h. Six (12%) patients developed pulmonary oedema in the HA group, but none in the plasmalyte group.143 In a meta-analysis dealing with HA use in patients with non-SBP infections at a dose of 1.5 g/kg on day 1 and 1 g/kg on day 3, the HA group had a higher incidence of pulmonary oedema (OR = 4.38, 95% CI = 1.30–14.79).105 By comparison, in the ANSWER and MACHT studies, where the 20% HA solution was 40 g weekly and 40 g every 15 days, respectively, none of the patients developed pulmonary oedema. Additionally, in the FRISC study, where the dosage of 5% HA solution was 250 ml over 15–30 min, followed by a maintenance infusion of 50 ml/h for a total of 3 h, none of the patients developed pulmonary oedema.142 Collectively, high-dose HA infusion in a short time should be performed with caution, except in SBP patients,97 and a 'restrictive' HA infusion strategy may be considered to reduce the risk of pulmonary oedema,146 especially when a combination of HA with terlipressin has been administered.147
Anaphylactic shock
Anaphylactic shock is a rare adverse reaction to HA.[148], [149], [150], [151] It may be caused by impurities that are associated with HA production, storage, and transportation, such as albumin aggregates generated during the production phase.152,153 If an anaphylactic shock develops, immediate withdrawal from HA infusion and symptomatic treatment are necessary.
Portal hypertension-associated bleeding
Several anecdotal series have suggested that HA infusion could increase the risk of portal hypertension-related bleeding,84,154,155 where the dose of HA used was relatively high (100 g/day). However, this opinion is somewhat outdated. The ANSWER study,9 where HA infusion was employed at a dosage of 40 g/weekly, showed that long-term HA infusion did not increase the risk of bleeding from gastro-oesophageal varices, but caused a marginally higher incidence of other portal-hypertensive bleeding in the SMT plus HA group. Recently, a retrospective study included cirrhotic patients with gastrointestinal bleeding and explored the impact of HA infusion on rebleeding and death during hospitalisations.156 It showed that HA infusion was associated with a lower risk of rebleeding regardless of HA dosage (<40 g or >40 g), and an increased dosage of HA could reduce the risk of rebleeding (≤40 g vs. 0 g: OR = 0.500, 95% CI = 0.312–0.800, p = 0.004; >40 g vs. 0 g: OR = 0.279, 95% CI = 0.134–0.580, p <0.001). To date, there is no clear evidence supporting the association of HA infusion with an increased risk of portal hypertension-related bleeding.
Unresolved issues
HA can be considered a therapeutic option, to manage several complications of cirrhosis, because it improves circulatory dysfunction and may counteract systemic inflammation and oxidative stress. However, its indications and optimal infusion strategy remain controversial. Several topics warrant further investigation.
-
(1)
The concept of 'effective albumin concentration' has been proposed to distinguish the optimal time to initiate and stop HA infusion to manage decompensated cirrhosis. However, its clinical relevance in guiding HA infusion needs further validation. Furthermore, more convenient assays for determining 'effective albumin concentration' should be developed.
-
(2)
At present, the dosage of HA is often based on the agreement of expert opinions and clinical experience. The optimal dosage and duration of HA infusion for various complications of cirrhosis to maximise its efficacy and minimise the risk of adverse events should be clarified.
-
(3)
The development and aggravation of HE and hyponatremia negatively influence the quality of life and outcomes of patients with cirrhosis. Pilot studies have demonstrated the potential benefits of HA for managing the two complications. However, large-scale RCTs are warranted to confirm these findings.
-
(4)
Hepatic oedema other than ascites, such as hydrothorax and leg oedema, can also develop in cirrhosis. The role of HA infusion in such patients warrants further investigation in the future.
-
(5)
The potential benefits of HA in ACLF patients and its use in plasmapheresis need to be further explored.
-
(6)
The prevention of septic shock in cirrhotic patients with infections by infusion of HA needs further evaluation in multicentre collaboration.
-
(7)
The underlying aetiology of cirrhosis is associated with patient outcomes, and the aetiology of cirrhosis is also heterogeneous across regions. Notably, NAFLD will be the most prevalent cause of liver disease in Western countries. Therefore, it should be clarified whether the benefits of HA infusion differ across patients with various aetiologies of cirrhosis.
-
(8)
A threshold of HA dosage should be identified to predict the risk of pulmonary oedema in patients with cirrhosis.
Financial support
This work is partially funded by the Young and Middle-aged Scientific and Technological Innovation Talents Support Plan Project of Shenyang (RC210011) and the Outstanding Youth Foundation of Liaoning Province (2022-YQ-07).pt
Authors’ contributions
Conceptualisation: XQ, MB. Data Curation: ZB, NM-S, FGR, AM, CAP, FT, MB, MP, MI, YJW, FGN, RT, CNF, MB, XQ. Formal Analysis: ZB, XQ. Investigation and Discussion: ZB, NM-S, FGR, AM, CAP, FT, MB, MP, MI, YJW, FGN, RT, CNF, AEM, KP, TT, SPS, AM, SKS, LR, HM, EC, GBLS, LY, S, YY, EV, AK, FW, RJ, AOB, MB, XQ. Methodology: ZB, XQ. Writing-original draft: ZB, XQ. Writing-review & editing: ZB, NM-S, FGR, AM, CAP, FT, MB, MP, MI, YJW, FGN, RT, CNF, AEM, KP, TT, SPS, AM, SKS, LR, HM, EC,GBLS, LY, S, YY, EV, AK, FW, RJ, AOB, MB, XQ. Supervision: XQ, MB.
Data availability statement
Data sharing is not applicable for this study as no new data were created in this study.
Conflicts of interest
ZB, NM-S, FGR, AM, and CAP declare no potential conflicts of interest that pertain to this work. FT’s laboratory received research grants from Gilead, Allergan, Bristol-Myers Squibb and Inventiva. MB, MP, MI, YJW, FGN, RT, CNF, AEM, KP, TT, and SPS declared no potential conflicts of interest concerning the publication of this article. AM received grant funding from Gilead (clinical trials), Inventiva, Intercept, and Novo-Nordisk; and was a member of the advisory board (Gilead). SKS received research funding from Novartis, Durect, Fibronostics, and Gilead. LR, HM, EC, GBLS, LY, S, YY, EV, and AK declared no potential conflicts of interest concerning the publication of this article. FW received grant support from Grifols. RJ is the inventor of OPA, which has been patented by UCL and licensed to Mallinckrodt Pharma. He is also the founder of Yaqrit Discovery, a spin-off company from University College London, Hepyx Limited, and Cyberliver. He has research collaborations with Yaqrit Discovery, has received speaker fees from Grifols, and is a consultant for Ambys, Takeda, and Bioconvergent Health. AOB declared no potential conflicts of interest concerning the publication of this article. MB declared the following potential conflicts of interest with respect to the publication of this article: personal fees from CLS Behring GmbH (consultant, speaker), Grifols SA (consultant, speaker), Takeda (consultant, speaker), PPTA (speaker), and Octapharma (speaker). XQ declared no potential conflicts of interest concerning the publication of this article. He received a governmental grant from the Young and Middle-aged Scientific and Technological Innovation Talents Support Plan Project of Shenyang (RC210011), which does not influence the publication of this article, for holding the final advisory meeting of the position statement.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100785.
Contributor Information
Mauro Bernardi, Email: mauro.bernardi@unibo.it.
Xingshun Qi, Email: xingshunqi@126.com.
Supplementary data
The following are the supplementary data to this article.
References
- 1.Ginès P., Krag A., Abraldes J.G., Solà E., Fabrellas N., Kamath P.S. Liver cirrhosis. Lancet. 2021;398:1359–1376. doi: 10.1016/S0140-6736(21)01374-X. [DOI] [PubMed] [Google Scholar]
- 2.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Cirrhosis Collaborators The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge PS, Runyon BA. Treatment of patients with cirrhosis. N Engl J Med. 2016;375:767–777. doi: 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Caraceni P, Abraldes JG, Ginès P, Newsome PN, Sarin SK. The search for disease-modifying agents in decompensated cirrhosis: from drug repurposing to drug discovery. J Hepatol. 2021;75:S118–S134. doi: 10.1016/j.jhep.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2018;15:753–764. doi: 10.1038/s41575-018-0045-2. [DOI] [PubMed] [Google Scholar]
- 9.Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417–2429. doi: 10.1016/S0140-6736(18)30840-7. [DOI] [PubMed] [Google Scholar]
- 10.Solà E, Solé C., Simón-Talero M., Martín-Llahí M., Castellote J., Garcia-Martínez R., et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250–1259. doi: 10.1016/j.jhep.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 11.China L., Freemantle N., Forrest E., Kallis Y., Ryder SD, Wright G., et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384:808–817. doi: 10.1056/NEJMoa2022166. [DOI] [PubMed] [Google Scholar]
- 12.Bai Z., Guo X., Tacke F., Li Y., Li H., Qi X. Association of serum albumin level with incidence and mortality of overt hepatic encephalopathy in cirrhosis during hospitalization. Therap Adv Gastroenterol. 2019;12 doi: 10.1177/1756284819881302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CH, Lin CY, Sheen IS, Chen WT, Lin TN, Ho YP, et al. Recurrence of spontaneous bacterial peritonitis in cirrhotic patients non-prophylactically treated with norfloxacin: serum albumin as an easy but reliable predictive factor. Liver Int. 2011;31:184–191. doi: 10.1111/j.1478-3231.2010.02377.x. [DOI] [PubMed] [Google Scholar]
- 14.Bai Z., Wang L., Wang R., Zou M., Méndez-Sánchez N., Romeiro F.G., et al. Use of human albumin infusion in cirrhotic patients: a systematic review and meta-analysis of randomized controlled trials. Hepatol Int. 2022;16:1468–1483. doi: 10.1007/s12072-022-10374-z. [DOI] [PubMed] [Google Scholar]
- 15.Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74:1014–1048. doi: 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- 16.Tarín Remohí MJ, Sánchez Arcos A, Santos Ramos B, Bautista Paloma J, Guerrero Aznar MD. Costs related to inappropriate use of albumin in Spain. Ann Pharmacother. 2000;34:1198–1205. doi: 10.1345/aph.19385. [DOI] [PubMed] [Google Scholar]
- 17.Tanzi M, Gardner M, Megellas M, Lucio S, Restino M. Evaluation of the appropriate use of albumin in adult and pediatric patients. Am J Health Syst Pharm. 2003;60:1330–1335. doi: 10.1093/ajhp/60.13.1330. [DOI] [PubMed] [Google Scholar]
- 18.Yazdani MS, Retter A, Maggs T, Li P, Robson MG, Reid C, et al. Where does the Albumin go? Human Albumin Solution usage following the implementation of a demand management programme. Transfus Med. 2017;27:192–199. doi: 10.1111/tme.12406. [DOI] [PubMed] [Google Scholar]
- 19.Garioud A, Cadranel JF, Pauwels A, Nousbaum JB, Thévenot T, Dao T, et al. Albumin use in patients with cirrhosis in France: results of the “ALBU-LIVE” survey: a case for better EASL guidelines diffusion and/or revision. J Clin Gastroenterol. 2017;51:831–838. doi: 10.1097/MCG.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues DM, Djerboua M, Flemming JA. Intravenous albumin in patients with cirrhosis: evaluation of practice patterns and secular trends of usage in ontario 2000 to 2017. J Can Assoc Gastroenterol. 2021;4:179–185. doi: 10.1093/jcag/gwaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajaj JS, O’Leary JG, Wong F, Kamath PS. Variations in albumin use in patients with cirrhosis: an AASLD members survey. Hepatology. 2015;62:1923–1924. doi: 10.1002/hep.27789. [DOI] [PubMed] [Google Scholar]
- 22.Caraceni P, Pavesi M, Baldassarre M, Bernardi M, Arroyo V. The use of human albumin in patients with cirrhosis: a European survey. Expert Rev Gastroenterol Hepatol. 2018;12:625–632. doi: 10.1080/17474124.2018.1460203. [DOI] [PubMed] [Google Scholar]
- 23.Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9–29. doi: 10.1136/gutjnl-2020-321790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Runyon BA, AASLD Practice Guidelines Committee Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 25.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 26.Italian Association for the Study of the Liver. Italian Society of Transfusion. Medicine Immunohaematology AISF-SIMTI Position Paper: the appropriate use of albumin in patients with liver cirrhosis. Dig Liver Dis. 2016;48:4–15. doi: 10.1016/j.dld.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Caraceni P, Angeli P, Prati D, Bernardi M, Berti P, Bennardello F, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19:9–13. doi: 10.2450/2020.0414-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro-Narro G, Moctezuma-Velázquez C, Male-Velázquez R, Trejo-Estrada R, Bosques FJ, Moreno-Alcántar R, et al. Position statement on the use of albumin in liver cirrhosis. Ann Hepatol. 2022:100708. doi: 10.1016/j.aohep.2022.100708. [DOI] [PubMed] [Google Scholar]
- 29.Jünger S, Brearley S, Payne S, Mantel-Teeuwisse AK, Lynch T, Scholten W, et al. Consensus building on access to controlled medicines: a four-stage Delphi consensus procedure. J Pain Symptom Manage. 2013;46:897–910. doi: 10.1016/j.jpainsymman.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Helmer DO. An experimental application of the DELPHI method to the use of experts. Manag Sci. 1963;9:458–467. [Google Scholar]
- 31.Brouwers MC, Kerkvliet K, Spithoff K. Agree Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152. doi: 10.1136/bmj.i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esteban JPG, Rein L, Szabo A, Saeian K, Rhodes M, Marks S. Attitudes of liver and palliative care clinicians toward specialist palliative care consultation for patients with end-stage liver disease. J Palliat Med. 2019;22:804–813. doi: 10.1089/jpm.2018.0553. [DOI] [PubMed] [Google Scholar]
- 33.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–255. doi: 10.2147/IJGM.S102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 36.Kragh-Hansen U, Chuang VT, Otagiri M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull. 2002;25:695–704. doi: 10.1248/bpb.25.695. [DOI] [PubMed] [Google Scholar]
- 37.Dröge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp Gerontol. 2002;37:1333–1345. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 38.Moman RN, Gupta N, Varacallo M. StatPearls. Treasure Island (FL); 2021. Physiology, albumin. [PubMed] [Google Scholar]
- 39.Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta. 2013;1830:5526–5534. doi: 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Nilsen J, Trabjerg E, Grevys A, Azevedo C, Brennan SO, Stensland M, et al. An intact C-terminal end of albumin is required for its long half-life in humans. Commun Biol. 2020;3:181. doi: 10.1038/s42003-020-0903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr RM, Du Bois JJ, Holt PR. Use of 125-I- and 51-Cr-labeled albumin for the measurement of gastrointestinal and total albumin catabolism. J Clin Invest. 1967;46:2064–2082. doi: 10.1172/JCI105694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho JR, Verdelho Machado M. New insights about albumin and liver disease. Ann Hepatol. 2018;17:547–560. doi: 10.5604/01.3001.0012.0916. [DOI] [PubMed] [Google Scholar]
- 45.Baldassarre M, Naldi M, Zaccherini G, Bartoletti M, Antognoli A, Laggetta M, et al. Determination of effective albumin in patients with decompensated cirrhosis: clinical and prognostic implications. Hepatology. 2021;74:2058–2073. doi: 10.1002/hep.31798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahlqvist GE, Jamar F, Zech F, Geubel AP. In-111 transferrin scintigraphy in cirrhosis with hypoalbuminemia: evidence for protein-losing enteropathy in a small group of selected cases. Scand J Gastroenterol. 2012;47:1247–1252. doi: 10.3109/00365521.2012.696682. [DOI] [PubMed] [Google Scholar]
- 47.Davcev P, Vanovski B, Sestakov D, Tadzer I. Protein-losing enteropathy in patients with liver cirrhosis. Digestion. 1969;2:17–22. doi: 10.1159/000196916. [DOI] [PubMed] [Google Scholar]
- 48.Jalan R, Bernardi M. Effective albumin concentration and cirrhosis mortality: from concept to reality. J Hepatol. 2013;59:918–920. doi: 10.1016/j.jhep.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 50.Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75(Suppl 1):S49–S66. doi: 10.1016/j.jhep.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manzoor H, Bhatt H. StatPearls. Treasure Island (FL); 2022. Prerenal kidney failure. [PubMed] [Google Scholar]
- 52.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 54.Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020;370:m2687. doi: 10.1136/bmj.m2687. [DOI] [PubMed] [Google Scholar]
- 55.Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–822. doi: 10.1016/j.jhep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 57.Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O’Leary JG, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150:1579–1589. doi: 10.1053/j.gastro.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 58.Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818–828. doi: 10.1056/NEJMoa2008290. [DOI] [PubMed] [Google Scholar]
- 59.Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62:567–574. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 60.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63:983–992. doi: 10.1002/hep.28396. [DOI] [PubMed] [Google Scholar]
- 62.Saif RU, Dar HA, Sofi SM, Andrabi MS, Javid G, Zargar SA. Noradrenaline versus terlipressin in the management of type 1 hepatorenal syndrome: a randomized controlled study. Indian J Gastroenterol. 2018;37:424–429. doi: 10.1007/s12664-018-0876-3. [DOI] [PubMed] [Google Scholar]
- 63.Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689–1697. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 64.Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology. 2003;38:238–243. doi: 10.1053/jhep.2003.50276. [DOI] [PubMed] [Google Scholar]
- 65.Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh S, Choudhary NS, Sharma AK, Singh B, Kumar P, Agarwal R, et al. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver Int. 2013;33:1187–1193. doi: 10.1111/liv.12179. [DOI] [PubMed] [Google Scholar]
- 67.Ortega R, Ginès P, Uriz J, Cárdenas A, Calahorra B, De Las Heras D, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–948. doi: 10.1053/jhep.2002.35819. [DOI] [PubMed] [Google Scholar]
- 68.Salerno F, Navickis RJ, Wilkes MM. Albumin treatment regimen for type 1 hepatorenal syndrome: a dose-response meta-analysis. BMC Gastroenterol. 2015;15:167. doi: 10.1186/s12876-015-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quintero E, Ginés P, Arroyo V, Rimola A, Bory F, Planas R, et al. Paracentesis versus diuretics in the treatment of cirrhotics with tense ascites. Lancet. 1985;1:611–612. doi: 10.1016/s0140-6736(85)92147-6. [DOI] [PubMed] [Google Scholar]
- 70.Ginés P, Arroyo V, Quintero E, Planas R, Bory F, Cabrera J, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234–241. doi: 10.1016/0016-5085(87)91007-9. [DOI] [PubMed] [Google Scholar]
- 71.Ruiz-del-Arbol L, Monescillo A, Jimenéz W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–586. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 72.Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 73.Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 74.Solà R, Vila MC, Andreu M, Oliver MI, Coll S, Gana J, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282–288. doi: 10.1016/s0168-8278(05)80070-4. [DOI] [PubMed] [Google Scholar]
- 75.Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172–1181. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 76.Simonetti RG, Perricone G, Nikolova D, Bjelakovic G, Gluud C. Plasma expanders for people with cirrhosis and large ascites treated with abdominal paracentesis. Cochrane Database Syst Rev. 2019;6:CD004039. doi: 10.1002/14651858.CD004039.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shrestha DB, Budhathoki P, Sedhai YR, Baniya R, Awal S, Yadav J, et al. Safety and efficacy of human serum albumin treatment in patients with cirrhotic ascites undergoing paracentesis: a systematic review and meta-analysis. Ann Hepatol. 2021;26:100547. doi: 10.1016/j.aohep.2021.100547. [DOI] [PubMed] [Google Scholar]
- 78.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(Suppl 6):vi1–12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peltekian KM, Wong F, Liu PP, Logan AG, Sherman M, Blendis LM. Cardiovascular, renal, and neurohumoral responses to single large-volume paracentesis in patients with cirrhosis and diuretic-resistant ascites. Am J Gastroenterol. 1997;92:394–399. [PubMed] [Google Scholar]
- 80.Arora V, Vijayaraghavan R, Maiwall R, Sahney A, Thomas SS, Ali R, et al. Paracentesis-induced circulatory dysfunction with modest-volume paracentesis is partly ameliorated by albumin infusion in acute-on-chronic liver failure. Hepatology. 2020;72:1043–1055. doi: 10.1002/hep.31071. [DOI] [PubMed] [Google Scholar]
- 81.Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343–1350. doi: 10.1111/j.1365-2036.2010.04473.x. [DOI] [PubMed] [Google Scholar]
- 82.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 84.Wilkinson P, Sherlock S. The effect of repeated albumin infusions in patients with cirrhosis. Lancet. 1962;2:1125–1129. doi: 10.1016/s0140-6736(62)90895-4. [DOI] [PubMed] [Google Scholar]
- 85.Gentilini P, Casini-Raggi V, Di Fiore G, Romanelli RG, Buzzelli G, Pinzani M, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol. 1999;30:639–645. doi: 10.1016/s0168-8278(99)80194-9. [DOI] [PubMed] [Google Scholar]
- 86.Romanelli RG, La Villa G, Barletta G, Vizzutti F, Lanini F, Arena U, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12:1403–1407. doi: 10.3748/wjg.v12.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sandi BB, Leão GS, de Mattos AA, de Mattos  Z. Long-term albumin administration in patients with cirrhosis and ascites: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2021;36:609–617. doi: 10.1111/jgh.15253. [DOI] [PubMed] [Google Scholar]
- 88.Di Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019;39:98–105. doi: 10.1111/liv.13968. [DOI] [PubMed] [Google Scholar]
- 89.Caraceni P, Tufoni M, Zaccherini G, Riggio O, Angeli P, Alessandria C, et al. On-treatment serum albumin level can guide long-term treatment in patients with cirrhosis and uncomplicated ascites. J Hepatol. 2021;74:340–349. doi: 10.1016/j.jhep.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 90.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56:S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 91.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 92.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 93.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 94.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 95.Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology. 2016;63:1299–1309. doi: 10.1002/hep.27941. [DOI] [PubMed] [Google Scholar]
- 96.Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis--bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116–1131. doi: 10.1111/apt.13172. [DOI] [PubMed] [Google Scholar]
- 97.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 98.Chen TA, Tsao YC, Chen A, Lo GH, Lin CK, Yu HC, et al. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol. 2009;44:619–625. doi: 10.1080/00365520902719273. [DOI] [PubMed] [Google Scholar]
- 99.Fernández J, Monteagudo J, Bargallo X, Jiménez W, Bosch J, Arroyo V, et al. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627–634. doi: 10.1002/hep.20829. [DOI] [PubMed] [Google Scholar]
- 100.Terra C, Guevara M, Torre A, Gilabert R, Fernández J, Martín-Llahí M, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944–1953. doi: 10.1053/j.gastro.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 101.Guevara M, Terra C, Nazar A, Solà E, Fernández J, Pavesi M, et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012;57:759–765. doi: 10.1016/j.jhep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 102.Thévenot T, Bureau C, Oberti F, Anty R, Louvet A, Plessier A, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015;62:822–830. doi: 10.1016/j.jhep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 103.Fernández J, Angeli P, Trebicka J, Merli M, Gustot T, Alessandria C, et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2020;18:963–973. doi: 10.1016/j.cgh.2019.07.055. [DOI] [PubMed] [Google Scholar]
- 104.Fernández J, Clària J, Amorós A, Aguilar F, Castro M, Casulleras M, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157:149–162. doi: 10.1053/j.gastro.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 105.Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and Safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52:1137–1142. doi: 10.1016/j.dld.2020.05.047. [DOI] [PubMed] [Google Scholar]
- 106.Wijdicks EF. Hepatic encephalopathy. N Engl J Med. 2016;375:1660–1670. doi: 10.1056/NEJMra1600561. [DOI] [PubMed] [Google Scholar]
- 107.European Association for the Study of the Liver EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77:807–824. doi: 10.1016/j.jhep.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 108.American Association for the Study of Liver Diseases European association for the study of the liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European association for the study of the liver and the American association for the study of liver diseases. J Hepatol. 2014;61:642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 109.Brusilow SW, Koehler RC, Traystman RJ, Cooper AJ. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7:452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.González-Usano A, Cauli O, Agustí A, Felipo V. Hyperammonemia alters the modulation by different neurosteroids of the glutamate-nitric oxide-cyclic GMP pathway through NMDA- GABAA - or sigma receptors in cerebellum in vivo. J Neurochem. 2013;125:133–143. doi: 10.1111/jnc.12119. [DOI] [PubMed] [Google Scholar]
- 111.Gluud LL, Vilstrup H, Morgan MY. Nonabsorbable disaccharides for hepatic encephalopathy: a systematic review and meta-analysis. Hepatology. 2016;64:908–922. doi: 10.1002/hep.28598. [DOI] [PubMed] [Google Scholar]
- 112.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 113.Kircheis G, Nilius R, Held C, Berndt H, Buchner M, Görtelmeyer R, et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25:1351–1360. doi: 10.1002/hep.510250609. [DOI] [PubMed] [Google Scholar]
- 114.Bosoi CR, Rose CF. Oxidative stress: a systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2013;28:175–178. doi: 10.1007/s11011-012-9351-5. [DOI] [PubMed] [Google Scholar]
- 115.Jain L, Sharma BC, Sharma P, Srivastava S, Agrawal A, Sarin SK. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Dig Liver Dis. 2012;44:1027–1031. doi: 10.1016/j.dld.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Simón-Talero M, García-Martínez R, Torrens M, Augustin S, Gómez S, Pereira G, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: a randomized double-blind study. J Hepatol. 2013;59:1184–1192. doi: 10.1016/j.jhep.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 117.Sharma BC, Singh J, Srivastava S, Sangam A, Mantri AK, Trehanpati N, et al. Randomized controlled trial comparing lactulose plus albumin versus lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32:1234–1239. doi: 10.1111/jgh.13666. [DOI] [PubMed] [Google Scholar]
- 118.Bai Z, Bernardi M, Yoshida EM, Li H, Guo X, Méndez-Sánchez N, et al. Albumin infusion may decrease the incidence and severity of overt hepatic encephalopathy in liver cirrhosis. Aging (Albany NY) 2019;11:8502–8525. doi: 10.18632/aging.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fagan A, Gavis EA, Gallagher ML, Mousel T, Davis B, Puri P, et al. A double-blind randomized placebo-controlled trial of albumin in patients with hepatic encephalopathy: HEAL study. J Hepatol. 2023;78:312–321. doi: 10.1016/j.jhep.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Teh KB, Loo JH, Tam YC, Wong YJ. Efficacy and safety of albumin infusion for overt hepatic encephalopathy: a systematic review and meta-analysis. Dig Liver Dis. 2021;53:817–823. doi: 10.1016/j.dld.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 121.Is B, Bombassaro IZ, Tovo CV, de Mattos ÂZ, Ahlert M, Chiesa T, et al. Albumin in the management of hepatic encephalopathy: a systematic review and meta-analysis. Ann Hepatol. 2021;26 doi: 10.1016/j.aohep.2021.100541. [DOI] [PubMed] [Google Scholar]
- 122.Wong YJ, Loo JH. Albumin therapy for hepatic encephalopathy: current evidence and controversies. Metab Brain Dis. 2023;38:1759–1763. doi: 10.1007/s11011-022-01002-8. [DOI] [PubMed] [Google Scholar]
- 123.Angeli P, Wong F, Watson H, Ginès P, Investigators C. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 124.Attar B. Approach to hyponatremia in cirrhosis. Clin Liver Dis (Hoboken) 2019;13:98–101. doi: 10.1002/cld.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis. 2000;32:605–610. doi: 10.1016/s1590-8658(00)80844-0. [DOI] [PubMed] [Google Scholar]
- 127.Alukal JJ, John S, Thuluvath PJ. Hyponatremia in cirrhosis: an update. Am J Gastroenterol. 2020;115:1775–1785. doi: 10.14309/ajg.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 128.Bajaj JS, Tandon P, OʼLeary JG, Biggins SW, Wong F, Kamath PS, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113:1339. doi: 10.1038/s41395-018-0119-3. [DOI] [PubMed] [Google Scholar]
- 129.China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, et al. Targeted albumin therapy does not improve short-term outcome in hyponatremic patients hospitalized with complications of cirrhosis: data from the ATTIRE trial. Am J Gastroenterol. 2021;116:2292–2295. doi: 10.14309/ajg.0000000000001488. [DOI] [PubMed] [Google Scholar]
- 130.Zaccherini G, Baldassarre M, Tufoni M, Nardelli S, Piano S, Alessandria C, et al. Correction and prevention of hyponatremia in patients with cirrhosis and ascites - post hoc analysis of the ANSWER study database. Am J Gastroenterol. 2023;118:168–173. doi: 10.14309/ajg.0000000000001995. [DOI] [PubMed] [Google Scholar]
- 131.Bai Z, Wang L, Lin H, Tacke F, Cheng G, Qi X. Use of human albumin administration for the prevention and treatment of hyponatremia in patients with liver cirrhosis: a systematic review and meta-analysis. J Clin Med. 2022;11:5928. doi: 10.3390/jcm11195928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rogal SS, Hansen L, Patel A, Ufere NN, Verma M, Woodrell CD, et al. AASLD Practice Guidance: palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022 Sep;76:819–853. doi: 10.1002/hep.32378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Angeli P, Albino G, Carraro P, Dalla Pria M, Merkel C, Caregaro L, et al. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology. 1996;23:264–273. doi: 10.1002/hep.510230211. [DOI] [PubMed] [Google Scholar]
- 134.Elfert AA, Abo Ali L, Soliman S, Zakaria S, Shehab El-Din I, Elkhalawany W, et al. Randomized placebo-controlled study of baclofen in the treatment of muscle cramps in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:1280–1284. doi: 10.1097/MEG.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 135.Lee FY, Lee SD, Tsai YT, Lai KH, Chao Y, Lin HC, et al. A randomized controlled trial of quinidine in the treatment of cirrhotic patients with muscle cramps. J Hepatol. 1991;12:236–240. doi: 10.1016/0168-8278(91)90944-7. [DOI] [PubMed] [Google Scholar]
- 136.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124:1016–1020. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- 137.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 138.Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022–2033. doi: 10.1002/hep.23264. [DOI] [PubMed] [Google Scholar]
- 139.Plessier A, Denninger MH, Consigny Y, Pessione F, Francoz C, Durand F, et al. Coagulation disorders in patients with cirrhosis and severe sepsis. Liver Int. 2003;23:440–448. doi: 10.1111/j.1478-3231.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 140.Simonetto DA, Piccolo Serafim L, Gallo de Moraes A, Gajic O, Kamath PS. Management of sepsis in patients with cirrhosis: current evidence and practical approach. Hepatology. 2019;70:418–428. doi: 10.1002/hep.30412. [DOI] [PubMed] [Google Scholar]
- 141.Sauneuf B, Champigneulle B, Soummer A, Mongardon N, Charpentier J, Cariou A, et al. Increased survival of cirrhotic patients with septic shock. Crit Care. 2013;17:R78. doi: 10.1186/cc12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Philips CA, Maiwall R, Sharma MK, Jindal A, Choudhury AK, Kumar G, et al. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): a randomized controlled trial. Hepatol Int. 2021;15:983–994. doi: 10.1007/s12072-021-10164-z. [DOI] [PubMed] [Google Scholar]
- 143.Maiwall R, Kumar A, Pasupuleti SSR, Hidam AK, Tevethia H, Kumar G, et al. A randomized-controlled trial comparing 20% albumin to plasmalyte in patients with cirrhosis and sepsis-induced hypotension [ALPS trial] J Hepatol. 2022;77:670–682. doi: 10.1016/j.jhep.2022.03.043. [DOI] [PubMed] [Google Scholar]
- 144.Shasthry SM, Kumar M, Khumuckham JS, Sarin SK. Changes in cardiac output and incidence of volume overload in cirrhotics receiving 20% albumin infusion. Liver Int. 2017;37:1167–1176. doi: 10.1111/liv.13375. [DOI] [PubMed] [Google Scholar]
- 145.Umgelter A, Wagner K, Reindl W, Nurtsch N, Huber W, Schmid RM. Haemodynamic effects of plasma-expansion with hyperoncotic albumin in cirrhotic patients with renal failure: a prospective interventional study. BMC Gastroenterol. 2008;8:39. doi: 10.1186/1471-230X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bai Z, Cheng G, Méndez-Sánchez N, Qi X. Human albumin infusion strategy in liver cirrhosis: liberal or restrictive? Ann Transl Med. 2021;9:1114. doi: 10.21037/atm-21-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Allegretti AS, Subramanian RM, Francoz C, Olson JC, Cárdenas A. Respiratory events with terlipressin and albumin in hepatorenal syndrome: a review and clinical guidance. Liver Int. 2022;42:2124–2130. doi: 10.1111/liv.15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujita A, Kitayama M, Hirota K. Anaphylactoid shock in a patient following 5% human serum albumin infusion during off-pump coronary artery bypass grafting. J Anesth. 2007;21:396–398. doi: 10.1007/s00540-007-0512-3. [DOI] [PubMed] [Google Scholar]
- 149.Moreno Lozano L, Galindo Bonilla P, Borja Segade J, Extremera Ortega A, Gómez Torrijos E, García Rodríguez R. Human serum albumin induced anaphylaxis in a patient with good tolerance to human plasma. J Investig Allergol Clin Immunol. 2019;29:51–53. doi: 10.18176/jiaci.0325. [DOI] [PubMed] [Google Scholar]
- 150.Ring J, Stephan W, Brendel W. Anaphylactoid reactions to infusions of plasma protein and human serum albumin. Role of aggregated proteins and of stabilizers added during production. Clin Allergy. 1979;9:89–97. doi: 10.1111/j.1365-2222.1979.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 151.Stafford CT, Lobel SA, Fruge BC, Moffitt JE, Hoff RG, Fadel HE. Anaphylaxis to human serum albumin. Ann Allergy. 1988;61:85–88. [PubMed] [Google Scholar]
- 152.Dengler T, Stöcker U, Kellner S, Fürst G. Chemical and immunochemical characterization of polymers of aggregates in preparations of human serum albumin. Infusionstherapie. 1989;16:160–164. doi: 10.1159/000222371. [DOI] [PubMed] [Google Scholar]
- 153.Jensen LB, Dam J, Teisner B. Identification and removal of polymer- and aggregate-forming proteins in human plasma albumin preparations. Vox Sang. 1994;67:125–131. doi: 10.1111/j.1423-0410.1994.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 154.Faloon WW, Eckhardt RD, Murphy TL, Cooper AM, Davidson CS. An evaluation of human serum albumin in the treatment of cirrhosis of the liver. J Clin Invest. 1949;28:583–594. doi: 10.1172/JCI102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kunkel HG, Labby DH, Ahrens EH, Shank RE, Hoagland CL. The use of concentrated human serum albumin in the treatment of cirrhosis of the liver. J Clin Invest. 1948;27:305–319. doi: 10.1172/JCI101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Z, Xie Y, Lu Q, Yan H, Liu X, Long Y, et al. The impact of albumin infusion on the risk of rebleeding and in-hospital mortality in cirrhotic patients admitted for acute gastrointestinal bleeding: a retrospective study of a single institute. BMC Gastroenterol. 2020;20:198. doi: 10.1186/s12876-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable for this study as no new data were created in this study.