Abstract
目的
探讨乙酰紫堇灵(Ace)对大鼠脊髓损伤(SCI)后功能恢复的作用和机制。
方法
使用脊髓撞击仪制备SD大鼠中度挫伤性SCI模型(SCI组),给予不同浓度Ace(10、20、40 mg/kg)腹腔注射干预,以假手术大鼠(Sham组)为对照。通过BBB评分评估SCI大鼠运动功能恢复情况;利用HE染色观察脊髓组织损伤面积变化;采用PCR、ELISA与免疫荧光染色评估脊髓损伤后体内炎症水平(TNF-α、IL-6、IL-1β)变化和小胶质细胞的活化情况(CD11b/CD68)。使用脂多糖(LPS组,100 ng/mL)诱导小胶质细胞BV2活化,分别给予不同浓度Ace处理(Ace组,1、2、4 μmol/L),Control组给予等体积DMSO处理。免疫荧光和PCR验证Ace对BV2活化及炎症因子分泌的作用;利用网络药理学预测Ace抑制小胶质细胞活化的靶点蛋白和信号机制,采用AutoDock软件对Ace与预测的靶点蛋白进行分子对接。使用信号通路阻断剂(Osimertinib),在体干预SCI大鼠模型,体外干预LPS诱导的BV2细胞模型,以验证信号机制。
结果
大鼠体内实验显示,与SCI组相比,Ace组大鼠BBB评分增高、脊髓损伤面积减小、小胶质细胞活化数量和促炎因子水平减少(P < 0.05);体外LPS刺激BV2细胞的研究结果显示,Ace组BV2活化及炎症因子水平显著低于对照组(P < 0.05)。网络药理学预测EGFR是Ace的主要作用靶点,分子对接技术显示EGFR与Ace的结合能为-8.9 kJ/mol,二者之间存在连接氢键;免疫印迹证实Ace可抑制脊髓组织及BV2细胞中EGFR/MAPK信号通路活化,加入EGFR抑制剂Osimertinib干预后,其抑制效能与Ace相当。
结论
Ace可能通过调控EGFR/MAPK通路抑制小胶质细胞介导的炎症反应,从而促进脊髓损伤后组织修复和运动功能恢复,为SCI治疗药物选择提供新的方向。
Keywords: 脊髓损伤, 乙酰紫堇灵, 小胶质细胞, 炎症, 网络药理学
Abstract
Objective
To investigate the effect of acetylcorynoline (Ace) for promoting functional recovery of injured spinal cord in rats and explore the underlying mechanism.
Methods
Rat models of spinal cord injury (SCI) were treated with intraperitoneal injection of different concentrations of Ace, with the sham-operated rats as the control group. After the treatment, the changes in motor function of the rats and the area of spinal cord injury were assessed with BBB score and HE staining, and the changes in pro-inflammatory cytokine levels and microglial activation were determined using PCR, ELISA and immunofluorescence staining. In a lipopolysaccharide (LPS)-treated BV2 cell model, the effects of different concentrations of Ace or DMSO on microglial activation and inflammatory cytokine production were observed. Network pharmacology analysis was performed to predict the target protein and signaling mechanism that mediated the inhibitory effect of Ace on microglia activation, and AutoDock software was used for molecular docking between Ace and the target protein. A signaling pathway blocker (Osimertinib) was used to verify the signaling mechanism in rat models of SCI and LPS-treated BV2 cell model.
Results
In rat models of SCI, Ace treatment significantly increased the BBB score, reduced the area of spinal cord injury, and lowered the number of activated microglia cells and the levels of pro-inflammatory cytokines (P < 0.05). The cell experiments showed that Ace treatment significantly lower the level of cell activation and the production of inflammatory cytokines in LPS-treated BV2 cells (P < 0.05). Network pharmacology analysis suggested that EGFR was the main target of Ace, and they bound to each other via hydrogen bonds as shown by molecular docking. Western blotting confirmed that Ace inhibited the activation of the EGFR/MAPK signaling pathway in injured mouse spinal cord tissue and in LPS-treated BV2 cells, and its inhibitory effect was comparable to that of Osimertinib.
Conclusion
In rat models of SCI, treatment with Ace can inhibit microglia-mediated inflammatory response by regulating the EGFR/MAPK pathway, thus promoting tissue repair and motor function recovery.
Keywords: spinal cord injury, acetylcorynoline, microglia, inflammation, network pharmacology
脊髓损伤(SCI)是一种严重危害生命的神经系统疾病,中国SCI年患病率为37人次/100万,其高致残率大大降低患者的生活质量[1]。通过药物干预减轻损伤脊髓局部炎症等继发性病理损伤是当前的主要治疗手段,然而遗憾的是目前仅有甲基强的松龙被FDA批准用于SCI的治疗,且仅限于损伤后8 h内,同时还存在诸多副作用[2, 3],因此寻找新型治疗药物成为当下研究热点。脊髓组织损伤后局部炎症反应是机体固有的防御性反应[4]。SCI后小胶质细胞过度活化并释放大量促炎介质参与了损伤后的继发性病理过程,因而抑制小胶质细胞活化是减轻SCI损伤的关键[5-7]。目前,天然植物活性成分具有抑制巨噬细胞/小胶质细胞活化的作用,在SCI等多种炎症损伤性疾病中显示出抗炎和疾病治疗作用[8, 9]。紫堇提取物-乙酰紫堇灵(Ace)作为异喹啉生物碱,被报道具有抗炎活性,可抑制脂多糖(LPS)诱导的肝细胞炎性损伤[10]。但Ace对小胶质细胞介导的炎症反应及对SCI的作用均未见报道。本研究基于网络药理学和分子对接技术分析Ace治疗SCI的作用和可能的分子机制,观察Ace对SCI后小胶质细胞活化的影响,以期为临床药物治疗提供参考。
1. 材料和方法
1.1. 材料
1.1.1. 实验动物
本研究选取雌性SD大鼠(济南朋悦实验动物公司),6~8周龄,体质量200~220 g。饲养于无特定病原菌环境。所有实验经蚌埠医学院动物研究伦理委员会批准(伦理号:伦动科批字[2020]第044号)。
1.1.2. 主要试剂
Ace(源叶生物,上海),MEM培养基(索莱宝,北京),LPS(Sigma),苏木素染色液、伊红染色液(珠海贝索,武汉)、CD11b(Servicebio,武汉),反转录试剂盒、PCR试剂盒(Takara),CD68、β-actin、EGFR、pEGFR、p38、p-p38(Abcam),Osimertinib(陶术,上海),TNF-α试剂盒、IL-6试剂盒、IL-1β试剂盒(博士德,武汉)。
1.2. 方法
1.2.1. 动物模型和取材方法
将SD大鼠随机分为假手术组(Sham组)、模型组(SCI组)和Ace干预组(Ace组)。模型建立方法如下:大鼠经腹腔注射戊巴比妥钠(50 mg/kg)麻醉后,掀除T9节段椎板暴露脊髓,利用脊髓撞击仪(型号:IH-0400)制备出中度挫伤性损伤的模型,大鼠出现双下肢抽搐、尾巴摇摆则为造模成功[11]。假手术组仅掀除椎板不撞击脊髓。造模后,Ace干预组每日给予不同浓度的Ace(10、20、40 mg/kg[12])腹腔注射,其余两组给予等量的生理盐水。Ace采用DMSO溶解,生理盐水稀释到对应浓度。抑制剂组(Os组)给予0.4 mg/mL浓度的Osimertinib灌胃处理。
运动功能评估与组织学检测选取干预6周的大鼠,免疫学检测选取干预1周的大鼠,此时间点为脊髓损伤后的炎症高峰期[8]。不同取材处理方式简述如下:(1)大鼠经麻醉后采用PBS和4%PFA进行灌注,取出脊髓以损伤点为中心截取1 cm,放入4%PFA中4 ℃过夜,再用20%、30%蔗糖梯度脱水后,进行OCT包埋切片用于组织学检测;(2)大鼠经麻醉后直接取材,取出脊髓以损伤点为中心截取1 cm,冻于-80 ℃,用于后续的PCR、ELISA和Western blot检测。
1.2.2. 细胞培养及处理
将BV2细胞(2×105)接种于含有爬片的六孔板中待细胞生长至对数期时,更换新鲜培养基,分别加入1、2、4 μmol/L[13]的Ace预孵育1 h,而后加入LPS(100 ng/mL)诱导6 h,收集爬片加入4%细胞固定液后进行免疫荧光检测。正常培养的细胞加入等量的DMSO设为Control组,LPS诱导的细胞设为LPS组,Ace干预处理设为Ace组,抑制剂组(8 μmol/L)设为Os组。
1.2.3. 运动功能评估
运动功能按照巴索-贝蒂-布雷斯纳汉评分法进行(BBB):以术前BBB评分为基点,评估术后1、3、7、14、21、28、35、42 d运动功能恢复情况,包括关节与躯干运动、肢体协调等方面,21分为正常,0分为完全瘫痪[14];网格行走实验:将大鼠置于边长0.5~2.5 cm的金属网格上,待大鼠站稳后让其独立爬行4 min,其中需有2 min的连续行走过程,记录大鼠后肢行走总步数和从网格掉落的次数,计算错步率[15]。
1.2.4. 组织学评估
将脊髓组织制作成冰冻切片(8 μm),各组选择相同截点切片进行常规H & E染色,步骤如下:用双蒸水洗去OCT,苏木精染色6 min,10%醋酸乙醇分色3 s后,利用自来水返蓝10 min,85%乙醇浸泡3 min,伊红染色30 s。最后用乙醇依次梯度脱水后,二甲苯透明后封片。于40倍镜下观察脊髓组织病变面积,相对损伤面积(%)=(空洞面积/脊髓面积)×100%。
1.2.5. 实时荧光定量PCR
将脊髓放置冰上进行匀浆处理,利用Trizol提取总RNA,按照逆转录试剂盒说明书将RNA反转录为cDNA,并作为模板建立反应体系进行荧光定量PCR检测。以β-actin为内参,采用2-△△CT法计算各基因的相对表达量。细胞利用Trizol提取总RNA,与上述实验方法相同。引物序列详见(表 1)。
表 1.
qRT-PCR对差异表达mRNA的引物序列
Primer sequence for qRT-PCR of differentially expressed mRNAs
| Gene name | Forward primer (5'-3') | Reverse primer (5'-3') |
| Rat | ||
| β-actin | CTGGCTCCTAGCACCATGAAG | GAGCCACCAATCCACACAGA |
| TNF-α | CCCAGAAAAGCAAGCAACCA | CCTCGGGCCAGTGTATGAGA |
| IL-6 | TCCTACCCCAACTTCCAATGCTC | TTGGATGGTCTTGGTCCTTAGCC |
| IL-1β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC |
| Mouse | ||
| β-actin | GTGACGTTGACATCCGTAAAGA | GCCGGACTCATCGTACTCC |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | TCTATACCACTTCACAAGTCGGA | GAATTGCCATTGCACAACTCTTT |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
1.2.6. ELISA
脊髓组织裂解离心后(12 000 r/min,10 min)提取上清,细胞直接收集上清,根据ELISA试剂盒说明书进行操作,检测TNF-α、IL-6、IL-1β含量,通过酶标仪检测各组光密度值(A450 nm)。
1.2.7. 免疫荧光染色
将脊髓组织制作冰冻切片,PBS洗去包埋剂后采用山羊血清封闭2 h,加入CD11b (1∶500)和CD68(1∶200)4 ℃孵育过夜,再加二抗室温孵育30 min,最后滴加DAPI复染核后封片。每张切片随机挑选5个视野对双阳性细胞进行计数。细胞爬片经PBS洗去培养基后采用相同的免疫荧光染色方法。
1.2.8. 免疫印迹法
脊髓组织利用RIPA裂解液匀浆后提取总蛋白,进行BCA法蛋白定量,采用SDS-PAGE电泳并转膜至PVDF膜上,5%脱脂奶粉进行封闭,4 ℃过夜孵育一抗(β-actin、EGFR、p-EGFR、p38、p-p38),经洗涤后,孵育辣根过氧化物酶(HRP)偶联的二抗,滴加ECL超敏发光液,经凝胶成像系统成像系统曝光采集图片,最后经ImageJ软件分析目的条带的灰度值,并计算蛋白相对表达量。细胞每孔加入100 μL RIPA放至4 ℃裂解30 min后收集细胞,采取相同实验方法。
1.2.9. 网络药理学
从Pubchem数据库(https://pubchem.ncbi.nlm.nih.gov/)获得Ace的结构式,利用Swiss Target Prediction数据库(http://www.swisstargetprediction.ch/)预测Ace潜在作用靶点,并与SCI相关靶点(https://www.genecards.org/)取交集。将交集靶点导入String数据库(https://cn.string-db.org/)构建蛋白-蛋白互作网络图(PPI),后导入Cytoscapev3.7.2软件(https://cytoscape.org/)分析各个靶点的度中心性(DC)[16]。采用David数据库(https://david.ncifcrf.gov/) 对交集靶点基因进行基因本体论(GO)和京都基因和基因组数据库(KEGG)通路富集分析,以此来预测Ace的生物过程、分子功能、细胞成分以及相关信号通路。
1.2.10. 分子对接
从PDB数据库(http://www.rcsb.org/pdb)获取目标靶点的蛋白结构,采用AutoDock 4.2.6软件对Ace与目标蛋白进行分子对接,若结合能小于0 kJ/mol,提示Ace能较好地与该蛋白进行自发结合。最后利用Pymol软件对结果进行可视化分析。
1.2.11. 统计学
采用SPSS 26.0软件分析数据。计量资料用均数±标准差表示;两组间差异采用t检验。P < 0.05认为差异具有统计学意义。所有实验都是独立重复3次。
2. 结果
2.1. Ace改善脊髓损伤后运动功能的恢复
SCI组与Ace组BBB评分显著低于Sham组,4周内呈上升趋势。在造模第4周后,Ace(20和40 mg/kg)干预组大鼠BBB评分显著高于SCI组(P < 0.05,图 1A)。网格步行评分显示(图 1B),Ace(20和40 mg/kg)干预组的错步率明显低于SCI组(P < 0.05)。结果证明Ace对大鼠无毒副作用且20 mg/kg为合适剂量,后续实验采用该剂量进行干预。
图 1.

Ace干预促进SCI大鼠运动功能恢复
Ace treatment promotes motor function recovery in SCI rats (Mean±SD, n=6). A: BBB scale. B: Grid walking experiment. *P < 0.05 vs SCI.
2.2. Ace对脊髓组织病变面积的影响
H & E染色结果显示(图 2),SCI组存在大量炎性细胞浸润,脊髓空洞面积([39.20±10.65)%]显著高于Ace干预组([21.92±5.32)%,P < 0.05]。
图 2.

Ace干预减少SCI后脊髓空洞面积
Ace treatment reduces the area of spinal cavity after SCI (HE staining, scale bar: 200 μm).
2.3. Ace抑制脊髓组织中小胶质细胞活化和炎性因子表达
与SCI组相比,ELISA和qRT- PCR结果显示(图 3A、B):Ace组脊髓组织中TNF-α、IL-6、IL-1β水平显著低于SCI组(P < 0.05)。免疫荧光检测结果显示,Ace干预后小胶质细胞活化数量显著低于SCI组(P < 0.05,图 3C、D)。
图 3.

Ace干预可抑制脊髓组织中小胶质细胞活化和炎性因子表达
Ace intervention inhibits the activation of microglia and the expression of inflammatory factors in the spinal cord tissue. A, B: qRT-PCR and ELISA for detecting the expression levels of TNF-α, IL-6 and IL-1β mRNA and protein levels. C: Immunofluorescence staining results in each group (scale bar: 20 μm). D: Quantitative analysis of CD11b+CD68+ cells. Data are presented as Mean±SD (n=6). *P < 0.05 vs SCI.
2.4. Ace体外抑制BV2活化和炎症因子分泌
PCR(图 4A)与ELISA(图 4B)检测结果显示,Ace可显著降低LPS诱导的BV2炎症因子分泌(P < 0.05),且具有浓度依赖性,最终选择2 μmol/L进行后续实验。免疫荧光结果显示,Ace干预后BV2活化(CD11b+ CD68+)数量显著低于LPS诱导组(P < 0.05,图 4C、D)。
图 4.

Ace干预可抑制BV2活化和炎症因子分泌
Ace intervention inhibits activation and inflammatory factor secretion in LPS-treated BV2 cells. A, B: qRT-PCR and ELISA for detecting the expression levels of TNF-α, IL-6 and IL-1β mRNA and proteins in each group, C: Immunofluorescence staining results in each group. D: Quantitative analysis of CD11b+CD68+ cells. Data are presented as Mean±SD (n=3). #P < 0.05 vs LPS.
2.5. 网络药理学预测Ace可靶向EGFR
网络药理学分析发现Ace潜在靶点与SCI的相关作用靶点存在53个交集靶点(图 5A)。可视化分析筛选出DC值排名第1的靶蛋白为EGFR(图 5B、表 2)。分子对接结果显示(图 5C),EGFR与Ace的结合能为-8.9 kJ/mol,二者之间存在连接氢键,提示EGFR可能是Ace治疗SCI的关键靶蛋白。
图 5.

网络药理学预测结果
Prediction of the target protein of Ace using network pharmacology analysis. A: Venn diagram of the intersection genes of Ace and SCI. B: Core PPI network diagram. C: Diagram of molecular docking betweenAce and EGFR.
表 2.
Ace治疗SCI关键靶点信息表
Key targets ofAce in the treatment of SCI
| Key targets | Protein description | Degree | Energy (kcal/mol) |
| EGFR | Epidermal growth factor receptor | 34 | -8.9 |
| SRC | Tyrosine-protein kinase src | 31 | -7.1 |
| MTOR | Mechanistic target of rapamycin | 28 | -9 |
| PIK3CA | Phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha | 27 | -8.9 |
| MAPK1 | Mitogen-activated protein kinase 1 | 24 | -8.7 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | 23 | -10.2 |
2.6. GO功能富集与KEGG通路富集预测Ace作用的信号通路
对53个交集靶点进行GO(图 6A~C)和KEGG(图 6D)富集预测分析显示,EGFR tyrosine kinase inhibitor resistance可能是Ace调控小胶质细胞活化的关键信号通路。
图 6.
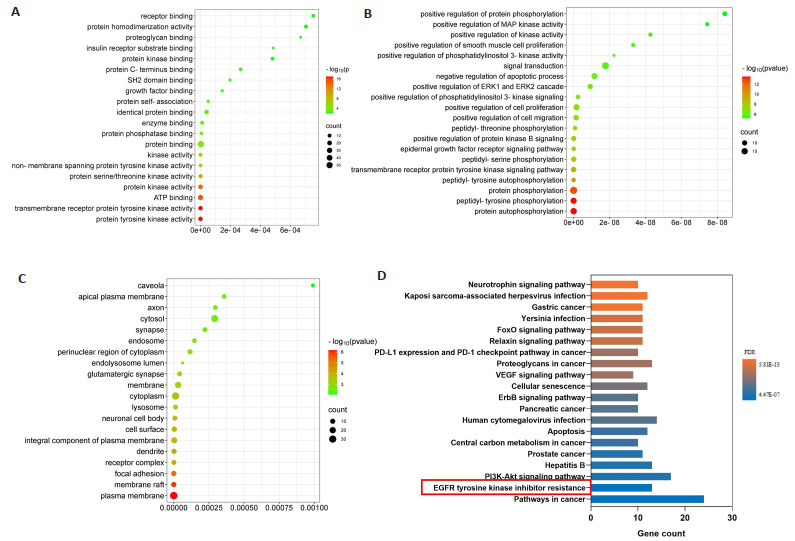
交集靶点GO与KEGG富集分析结果
GO and KEGG enrichment analysis of the intersection targets. A: GO-MF; B: GO-BP; C: GO-CC; D: KEGG pathway.
2.7. Ace调控损伤脊髓中EGFR/MAPK信号通路
与SCI组相比,Ace组和EGFR抑制剂Os组的脊髓组织中p-EGFR、p-p38表达均显著降低(P < 0.05,图 7A、C)。同样在体外细胞实验中也得到了相似的结果(P < 0.05,图 7B、D)。此外,免疫荧光结果显示(图 7E、F),Ace组与Os组均可抑制LPS诱导的小胶质细胞(BV2) 活化,但是二者间无统计学意义(P > 0.05)。
图 7.

Ace可调控EGFR/MAPK信号通路
Ace regulates EGFR/MAPK signaling pathway. A, B: Expressions of EGFR, p-EGFR, p38, and p-p38 in the spinal cord tissue in each group detected by Western blotting (*P < 0.05 vs SCI). C: CD11b+CD68+ cells detected by immunofluorescence assay. D: Quantitative analysis of the number of CD11b+CD68+ cells (Mean±SD, n=3; P > 0.05 vs Ace).
3. 讨论
本研究结果发现Ace可抑制SD大鼠SCI后脊髓组织中小胶质细胞介导的炎症反应,进而促进脊髓损伤后组织修复和运动功能恢复,并通过调控EGFR/MAPK信号通路发挥作用。
脊髓损伤病理过程复杂,大体可分为机械暴力直接作用下的原发性创伤和随后由炎症等病理反应导致的继发性损伤[17]。原发性创伤造成的脊髓神经功能障碍不可逆转,减轻脊髓继发性炎性损伤是研究重点[18]。随着中医药领域研究的发展,已有大量中药活性单体被证明具有潜在的SCI治疗作用[8, 11]。Ace是从紫堇中提取的生物碱,属于一种中药来源制剂,既往报道其具有抗炎和免疫调节的生物学活性[10, 19]。Ace被证明可以抑制LPS诱导的乳腺组织和人脐静脉内皮细胞炎症反应[13, 20]。Ace的抗炎作用提示其在SCI中可能具有缓解脊髓损伤后炎症反应的功能。基于此我们采用SCI大鼠模型,分析Ace能否减轻脊髓损伤后的炎症反应及功能障碍。研究结果显示,Ace干预组的BBB评分显著高于SCI组,且网格行走实验的错步率显著降低,BBB量表包括了SCI后大鼠后肢恢复过程中的所有行为变化,是评判运动功能恢复的有效指标[14],本研究结果证实Ace可改善SCI后的运动功能障碍。H & E染色结果显示,Ace干预组炎性细胞浸润程度显著低于SCI组,且脊髓空洞面积显著减小。同时,损伤脊髓组织中的促炎介质(TNF-α、IL-6、IL-1β)显著降低。我们的研究结果证实Ace具有拮抗SCI后脊髓组织炎症损伤的作用,并可以促进组织修复和运动功能恢复。
小胶质细胞作为神经系统中的常驻免疫细胞,在脊髓损伤后被活化并浸润到损伤部位,从而促进神经元的凋亡,参与SCI后继发性组织损伤[21-24]。随着目前对小胶质细胞功能及其参与脊髓损伤的发病机制的深入认识,调控小胶质细胞已被用于指导脊髓损伤治疗的临床前研究[25]。我们的结果证实,Ace可以抑制脊髓损伤后小胶质细胞活化,同时体外可以抑制LPS诱导小胶质细胞BV2活化,并降低小胶质细胞分泌的促炎介质(TNF- α、IL-6、IL-1β)水平。以上结果提示,Ace对脊髓局部免疫微环境具有调控作用。我们的结果证明了Ace除抑制肝细胞等实质细胞炎症反应外[26],也可以抑制免疫细胞介导的炎症反应,然而Ace抑制小胶质细胞活化的通路需进一步研究。
我们利用网络药理学和分子对接技术尝试分析Ace治疗脊髓损伤的可能分子机制。通过数据库筛选出Ace与SCI的交集靶点共53个,依据DC值,发现EGFR是最相关的靶点蛋白。将Ace与EGFR进行分子对接,结果提示二者具有较高的结合活性,由此我们推测EGFR为Ace在脊髓损伤中发挥保护效应的关键蛋白。EGFR为表皮生长因子受体,近年来因其可调节细胞活化的作用而受到广泛关注[27-29]。EGFR信号活化被证明参与激活小胶质细胞,增加SCI后的炎症级联反应,抑制EGFR信号通路可减少小胶质细胞炎症反应和相关继发性损伤,寻找以EGFR为靶点的信号抑制剂在脊髓损伤治疗中具有重要作用[30, 31]。另外,文献报道EGFR主要通过促进其下游MAPK通路p38的磷酸化调控炎症反应[32]。因此我们推测Ace可能通过调控EGFR/MAPK信号通路抑制小胶质细胞活化而发挥其免疫调节作用。我们的研究结果显示Ace可抑制脊髓组织和BV2中EGFR和p38的磷酸化水平,且该抑制效果与经典的EGFR抑制剂(奥希替尼)作用相当。提示Ace可作为EGFR的潜在抑制药物。本研究表明Ace通过靶向EGFR/MAPK信号通路调控脊髓局部免疫微环境,阐明了Ace抑制小胶质细胞活化的分子机制。
综上所述,本研究证明Ace可促进脊髓损伤后组织修复和运动功能恢复,可能通过调控EGFR/MAPK通路抑制小胶质细胞介导的炎症反应,为SCI治疗药物选择提供新的方向。
Biography
孙洋,在读硕士研究生,E-mail: sunyangSYANG@163.com
Funding Statement
国家自然科学基金(82071360);研究生科研创新计划项目(Byycx22031,Byycx22010)
Supported by National Natural Science Foundation of China (82071360)
Contributor Information
孙 洋 (Yang SUN), Email: sunyangSYANG@163.com.
胡 建国 (Jianguo HU), Email: jghu9200@bbmc.edu.cn.
References
- 1.陈 星月, 陈 栋, 陈 春慧, et al. 中国创伤性脊髓损伤流行病学和疾病经济负担的系统评价. 中国循证医学杂志. 2018;18(2):143–50. [Google Scholar]
- 2.Stokes S, Drozda M, Lee C. The past, present, and future of traumatic spinal cord injury therapies: a review. Bone Jt Open. 2022;3(5):348–58. doi: 10.1302/2633-1462.35.BJO-2021-0177.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karsy M, Hawryluk G. Modern medical management of spinal cord injury. Curr Neurol Neurosci Rep. 2019;19(9):65. doi: 10.1007/s11910-019-0984-1. [DOI] [PubMed] [Google Scholar]
- 4.Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15(3):541–53. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–68. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53(1):14–8. doi: 10.1038/sc.2014.184. [DOI] [PubMed] [Google Scholar]
- 7.Lukacova N, Kisucka A, Kiss Bimbova K, et al. Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int J Mol Sci. 2021;22(24):13577. doi: 10.3390/ijms222413577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li FZ, Song X, Xu JX, et al. Morroniside protects OLN-93 cells against H2O2-induced injury through the PI3K/Akt pathwaymediated antioxidative stress and antiapoptotic activities. Cell Cycle. 2021;20(7):661–75. doi: 10.1080/15384101.2021.1889186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue MT, Sheng WJ, Song X, et al. Atractylenolide Ⅲ ameliorates spinal cord injury in rats by modulating microglial/macrophage polarization. CNS Neurosci Ther. 2022;28(7):1059–71. doi: 10.1111/cns.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L iu, Y an. Corynoline attenuates LPS-induced acute lung injury in mice by activating Nrf2. Int Immunopharmacol. 2017;48:96–101. doi: 10.1016/j.intimp.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Duan FX, Shi YJ, Chen J, et al. Neuroprotective effects of P7C3 against spinal cord injury in rats. Exp Biol Med (Maywood) 2019;244(18):1680–7. doi: 10.1177/1535370219888620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng WF, Wang FY, He XJ, et al. Protective effect of Corynoline on the CFA induced Rheumatoid arthritis via attenuation of oxidative and inflammatory mediators. Mol Cell Biochem. 2021;476(2):831–9. doi: 10.1007/s11010-020-03948-8. [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Su K, Wang JX, et al. Corynoline exhibits anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cells through activating Nrf2. Inflammation. 2018;41(5):1640–7. doi: 10.1007/s10753-018-0807-6. [DOI] [PubMed] [Google Scholar]
- 14.Bhimani AD, Kheirkhah P, Arnone GD, et al. Functional gait analysis in a spinal contusion rat model. Neurosci Biobehav Rev. 2017;83:540–6. doi: 10.1016/j.neubiorev.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Zhang L, Ndong JC, et al. Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J Neuroinflammation. 2019;16(1):238. doi: 10.1186/s12974-019-1630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.石 娅, 刘 文, 刘 兴德, et al. 基于PI3K/Akt信号通路探究当归补血汤干预大鼠实验性脑缺血再灌注损伤的作用机制. 中草药. 2022;53(16):5052–65. [Google Scholar]
- 17.Li XY, Li M, Tian LG, et al. Reactive astrogliosis: implications in spinal cord injury progression and therapy. Oxid Med Cell Longev. 2020;2020:9494352. doi: 10.1155/2020/9494352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cofano F, Boido M, Monticelli M, et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. doi: 10.3390/ijms20112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plazas E, Muñoz DR, et al. Natural isoquinoline alkaloids: Pharmacological features and multi-target potential for complex diseases. Pharmacol Res. 2022;177:106126. doi: 10.1016/j.phrs.2022.106126. [DOI] [PubMed] [Google Scholar]
- 20.Wu YH, He T, Fu YH, et al. Corynoline protects lipopolysaccharideinduced mastitis through regulating AKT/GSK3β/Nrf2 signaling pathway. Environ Toxicol. 2021;36(12):2493–9. doi: 10.1002/tox.23362. [DOI] [PubMed] [Google Scholar]
- 21.Fan BY, Wei ZJ, Yao X, et al. Microenvironment imbalance of spinal cord injury. Cell Transplant. 2018;27(6):853–66. doi: 10.1177/0963689718755778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ya ng, L uo. M1 macrophages impair tight junctions between endothelial cells after spinal cord injury. Brain Res Bull. 2022;180:59–72. doi: 10.1016/j.brainresbull.2021.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Guo XY, Duan FX, Chen J, et al. Subcutaneous administration of PDGF-AA improves the functional recovery after spinal cord injury. Front Neurosci. 2019;13:6. doi: 10.3389/fnins.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 25.Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159(3):595–602. doi: 10.1097/j.pain.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 26.Liu RJ, Zhou F, He H, et al. Metabolism and bioactivation of corynoline with characterization of the glutathione/cysteine conjugate and evaluation of its hepatotoxicity in mice. Front Pharmacol. 2018;9:1264. doi: 10.3389/fphar.2018.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li ZW, Li JJ, Wang L, et al. Epidermal growth factor receptor inhibitor ameliorates excessive astrogliosis and improves the regeneration microenvironment and functional recovery in adult rats following spinal cord injury. J Neuroinflammation. 2014;11:71. doi: 10.1186/1742-2094-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Kim SJ, Jeong HR, et al. Inhibiting EGFR/HER-2 ameliorates neuroinflammatory responses and the early stage of tau pathology through DYRK1A. Front Immunol. 2022;13:903309. doi: 10.3389/fimmu.2022.903309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jian CD, Wei L, Mo RK, et al. Microglia mediate the occurrence and development of alzheimer's disease through ligand-receptor axis communication. FrontAging Neurosci. 2021;13:731180. doi: 10.3389/fnagi.2021.731180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan PH, Wu XJ, Liu XK, et al. A causal relationship in spinal cord injury rat model between microglia activation and EGFR/MAPK detected by overexpression of microRNA-325-3p. J Mol Neurosci. 2019;68(2):181–90. doi: 10.1007/s12031-019-01297-w. [DOI] [PubMed] [Google Scholar]
- 31.Qu WS, Tian DS, Guo ZB, et al. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J Neuroinflammation. 2012;9:178. doi: 10.1186/1742-2094-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li ZW, Zhao JJ, Li SY, et al. Blocking the EGFR/p38/NF-κB signaling pathway alleviates disruption of BSCB and subsequent inflammation after spinal cord injury. Neurochem Int. 2021;150:105190. doi: 10.1016/j.neuint.2021.105190. [DOI] [PubMed] [Google Scholar]


