Abstract
Azetidines are of particular interest in medicinal chemistry for their favorable properties, including increased resistance to oxidative metabolism and lower lipophilicity. The recent development of [2+2] reactions has significantly expanded the limited repertoire of methods for azetidine synthesis, but access to more complex architectures still requires further development. Herein we report a visible-light enabled intramolecular [2+2] cycloaddition of unactivated alkenes that proved previously unreactive to access tricyclic azetidines with 3D complex structures and high levels of saturation.
Graphical Abstract

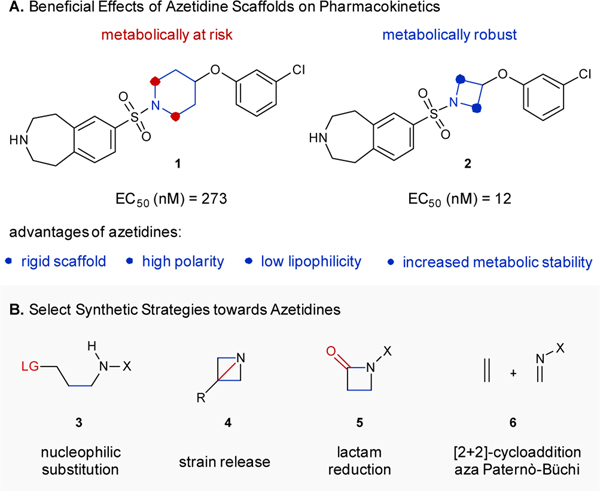
Incorporation of highly substituted, three-dimensional scaffolds into target structures is an essential strategy in medicinal chemistry to facilitate the development of future pharmaceuticals.1 Current discovery efforts are shifting towards the inclusion of more saturated, complex frameworks to increase the likelihood of identifying promising lead structures.1 Benefits from higher saturation and higher complexity range from increased solubility to greater specificity, and lower toxicity, which are desirable qualities for a drug.2 Azetidines are 4-membered nitrogen-containing heterocycles that recently garnered a lot of attention as medicinally desirable scaffolds in the quest for new lead compounds in drug design and development.3 Specifically, azetidines exhibit favorable pharmacokinetic properties and biological activities including decreased lipophilicity, high polarity, and rigidity, in addition to facilitating the rapid build-up of molecular complexity.1, 2, 4–9 Implementation of azetidines as alternatives for 5- and 6-membered nitrogen-containing heterocycles in current potential therapeutic candidates is known to improve their metabolic stability by enhancing their overall structural rigidity, resulting in enhanced resistance to oxidative metabolism.3–6 For example, 5-HT2C agonists, 1 and 2 identified by Pfizer, differ only in the ring size of the N-heterocycle, yet the azetidine results in a more potent compound with an EC50 of 12 nM compared to 273 nM (Fig. 1A).4
Figure 1.
A. Azetidines as important scaffolds for current drug design and development. B. Currently available synthetic strategies to access functionalized azetidines.
However, despite these desirable characteristics, the incorporation of azetidines in current pharmaceutical lead compounds remains limited. Importantly, less than 1% of all nitrogen-heterocycles included in current FDA-approved pharmaceuticals are functionalized azetidines.10 This divide is due to a dearth in efficient synthetic methods to access these desirable 4-membered ring nitrogen-containing heterocycles.11–15
Among the synthetic strategies most often applied are nucleophilic substitutions (3),13, 14 strain release of azabicyclobutanes (4),13–15 and the reduction of lactams (5, Fig. 1B).13, 14 However, all of these approaches require the synthesis of specific precursors and are prone to limitations in scope. Arguably, the most efficient and direct strategy towards functionalized azetidines relies on [2+2]-cycloaddition reactions between imines and alkenes, also referred to as aza Paternò-Büchi reactions (6).16
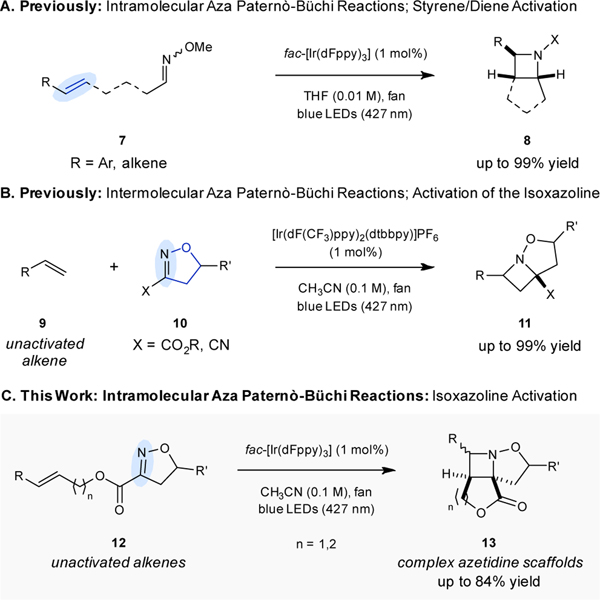
Additional efficient procedures for these aza Paternò-Büchi reactions have recently been developed, relying on visible light-mediated energy transfer (Fig. 2). These methods harness the triplet energy excited states of either alkenes or oximes, generated upon energy transfer from the triplet excited states of suitable photocatalysts.17–26 Following this approach, intramolecular aza Paternò-Büchi reactions proceed upon accessing the triplet excited states of activated alkenes (7) to undergo the desired [2+2]-cycloaddition with pendant oximes in up to 99% yield to form azetidines (8) (Fig. 2A).17An intermolecular variant of the aza Paternò-Büchi was subsequently developed that relies on unactivated alkenes 9 and the triplet excited states of cyclic oximes 10 to promote the formation of azetidines 11 in up to 99% yield (Fig. 2B).19
Figure 2.
A. Intramolecular methodology using excited alkenes. B. Intermolecular methodology using excited oximes and unactivated alkenes. C. Novel intramolecular methodology using excited oximes and unactivated alkenes.
Herein we expand the repertoire of efficient aza Paternò-Büchi reactions to report the development of an intramolecular transformation of unactivated alkenes by relying on the design principle of oxime sensitization to access complex azetidine scaffolds. Specifically, cyclic oximes react with the unactivated alkene moieties in 12 to result in the formation of tricyclic azetidines 13 to access substrates with opposite substitution patterns in comparison to the previous intermolecular approach. Notably, both metal-based and organic photocatalysts are shown to promote the desired transformation in yields up to 84% and 41% respectively. Additionally, N–O bond cleavage of the tricyclic azetidine initially formed upon [2+2]-cycloaddition is shown to proceed readily to result in the formation of the free azetidine products. To identify optimal conditions for intramolecular aza Paternò-Büchi reactions of unactivated alkenes, we evaluated a range of photocatalysts varying in triplet energies upon irradiation of oxime 14 with blue LED lights (Table 1). Specifically, conversion of oxime 14 with catalytic amounts of photocatalysts displaying a high triplet energy, around 60 kcal mol−1, including Ir(dF(Me)ppy)2(dtbbpy)] and Ir1·PF6 resulted in the formation of azetidine 15 in 73% and 78% yield, respectively (entries 1–2, Table 1). Under otherwise identical reaction conditions, conversion of oxime 14 with Ir2 gave rise to azetidine 15 with increased yields of 90% (entry 3, Table 1). Using a catalyst with a lower triplet energy of 58.6 kcal mol−1, fac-[Ir(Fppy)3, resulted in a decreased yield of 61% for 15 (entry 4, Table 1). Catalysts with triplet energies below 57 kcal mol−1 including [Ir(dFppy)2(dtbbpy)]PF6, fac-[Ir(ppy)3], and fac-[Ir(4’-CF3-ppy)3] resulted in diminished formation of azetidine 15 in yields of less than 29% (entries 5–6, Table 1). Organic photocatalysts were also considered under similar conditions, varying the triplet energy of the catalyst used. In particular, utilizing thioxanthone with a higher triplet energy than the optimal iridium catalyst, Ir2, no product was observed (entry 8, Table 1).
Table 1.
Evaluation of photocatalysts in intramolecular, visible light-mediated aza Paternò-Büchi reactions.

|
Conditions: 0.1 mmol 22 and photocatalyst are dissolved in acetonitrile (0.1 M) and the solution was sparged for 10 min. The sample was irradiated with blue LED lamps 16 hours. Percent yield was determined by NMR with mesitylene as internal standard.
Ran in toluene for increased solubility of some organic catalysts.
Using organic catalysts with lower triplet energies than thioxanthone, 2-MeOTx and 2CzPN, gave rise to azetidine 15 in 41% yield for both catalysts (entries 9–10, Table 1). However, conversion of oxime 14 relying on 4CzIPN exhibiting a triplet energy between 2-MeOTx and 2CzPN failed to provide the desired azetidine 15 (entry 12, Table 1). Suspecting that the solubility of the organic photocatalyst may be influencing the yield, the solvent was switched to toluene. Under these modified reaction conditions, thioxanthone produced trace amounts of azetidine 15 in 6% yield (entry 12, Table 1). 2-MeOTx did not perform as well in toluene as it did in acetonitrile, resulting in a decreased yield of 26%, while 2CzPN performed similarly, producing 15 in 38% yield (entries 13–14, Table 1). 4CzIPN in toluene produced an increased yield of 19% (entry 15, Table 1). The optimal organic catalyst conditions were identified as 2-MeOTx or 2CzPN in acetonitrile, which is the first instance of organic catalysts being used in a visible light-mediated [2+2] cycloaddition between alkenes and oximes to access azetidine products by exciting the oxime to its triplet state.
With an optimal photocatalyst identified, we subsequently focused on further improving the reaction conditions, with a particular emphasis on concentration (Table 2). Specifically, at 0.2 M a conversion of 98% was observed, however the desired product 15 was formed in diminished yields of only 76% (entry 16, Table 1). In comparison, decreasing the concentration to 0.1 M resulted in the formation of azetidine 15 with improved yields of 84% while complete consumption of oxime 14 was observed (entry 17, Table 1). Notably, decreasing the concentration further to 0.05 M or 0.01 M did not result in an improvement in yield (entries 18–19, Table 1). Without photocatalyst, no formation of azetidine 15 was observed when oxime 14 was irradiated with blue LED light (entry 20, Table 1).
Table 2.
Evaluation of the scope of intramolecular, visible light-mediated aza Paternò-Büchi reactions.

|
Conditions: 0.25 mmol isoxazoline and Ir2 (1 mol %) are dissolved in acetonitrile (0.1 M) and the solution is sparged for 10 min. The reaction is irradiated with blue LED lamps (427 nm) for 16–20 hours.
Reaction run at 0.01 M on 0.05 mmol scale, yield by HNMR.
Reaction was run on a 1 mmol scale using standard reaction conditions.
Utilizing the optimized conditions, the substrate scope for intra molecular aza Paternò-Büchi reactions of oximes incorporating unactivated alkenes was explored. The optimal reaction conditions identified proved to be compatible with a variety of alkene substitution patterns, chain lengths, and oxime backbone substituents, providing access to azetidines in up to 84% yield. The trisubstituted alkene substrate resulted in the formation of tricyclic azetidine 15 in 84% yield, while disubstituted alkenes (19, 20, and 23) produced yields up to 69% yield. The optimal reaction conditions were found to tolerate terminal alkenes, which was surprising as the postulated reaction mechanism for this intramolecular aza Paternò-Büchi reaction relied on the formation of a primary radical. Employing substrates incorporating terminal alkenes allows access to azetidines with methylene groups adjacent to the nitrogen, which were previously only accessible using ethylene gas following the former intermolecular reaction protocol. Terminal alkene 16 resulted in a yield of 27%, however this was improved to 80% by decreasing the concentration of the reaction. Substitution adjacent to the ester moiety resulted in a higher yield of 69% for the dimethyl substrate 17 although a decrease in yield to 9% was observed for azetidine 18, bearing a mono-methyl substituent. Conversely, the crotyl ester resulted in a 68% yield of azetidine 19 with a 1.3:1 mixture of diastereomers. Increasing the size of the substituent to a n-propyl group did not improve the diastereoselectivity, however did produce 20 in 61% yield. Importantly, azetidines incorporating 6-membered ring-lactones were similarly readily accessible, albeit in slightly diminished yields compared to their 5-membered ring analogs. The substrate utilizing a terminal alkene resulted in 21 being formed in 6% yield while the trisubstituted alkene resulted in 22 being formed in an increased 41% yield due to the increased substitution. By decreasing the reaction concentration for 21 and 22, the yields increased to 25% and 71%, respectively. An acrylate was also tolerated under these optimal reaction conditions, resulting in 27% yield of 23. Different substitution patterns on the oxime backbone were amenable, including the unsubstituted oxime 26 resulting in 80% yield, which is comparable to the 87% yield of 15 obtained from the corresponding dimethyl substituted oxime. Monosubstituted oximes were tolerated, however yields varied based on the substituent. Specifically, the n-butyl substituent azetidine 24 was produced in 72% yield, the highest yield for monosubstituted substrates while phenyl (27) and ethyl ether (28) resulted in 58% and 28% yields, respectively. Bulkier disubstituted oxime substituents, specifically diphenyl 25 and piperidine 29, resulted in 22% and 57% yield respectively. Dilution of the reaction for 25 to 0.01 M resulted in an improved yield of 61%. Diluting the reaction for select low yielding substrates resulted in the yields improving. Importantly, the increasing of the yield for terminal alkene 17 demonstrates that terminal alkenes can work just as well as trisubstituted alkenes under more dilute conditions.
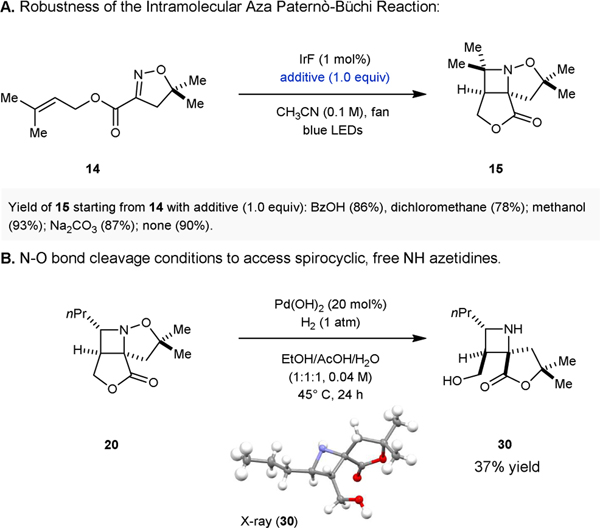
We subsequently evaluated the robustness27 of the intramolecular aza Paternò-Büchi reaction by subjecting oxime 14 to the optimized reaction conditions in the presence of one equivalent of various additives (Figure 3A). The addition of equimolar amounts of benzoic acid, chloroalkanes, methanol, and sodium carbonate resulted in azetidine 15 with less than 12% decrease in overall yield, suggesting a high level of functional group tolerance. Notably, the free azetidines are accessible upon N-O bond cleavage. Specifically, under hydrogenolysis relying on catalytic amounts of Pd(OH)2, spirocyclic azetidine 30 was obtained in 37% yield (Fig. 3B).
Figure 3:
A. Robustness evaluation. B. N–O bond cleavage conditions to access spirocyclic, free NH azetidines.
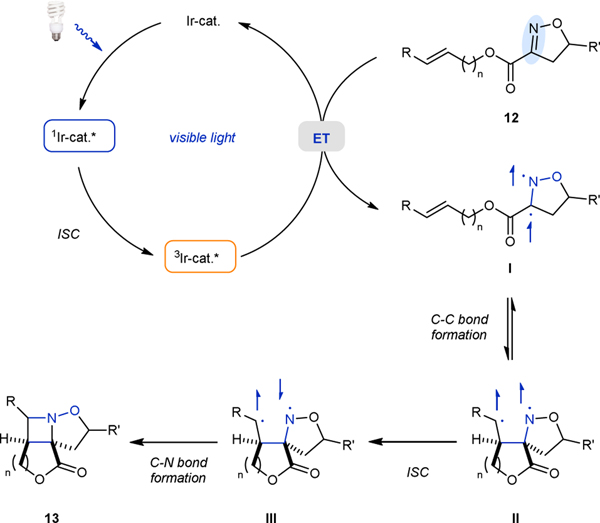
The previously proposed mechanism for the intermolecular aza Paternò-Büchi reaction utilizing unactivated alkenes and cyclic oximes is suspected to be operative for the intramolecular [2+2] reaction.19 The triplet-state photoexcited iridium catalyst sensitizes isooxazoline 12 through energy transfer to its triplet state I. The triplet-state isooxazoline I can then undergo C–C bond formation with the proximal alkene carbon to form 1,4-biradical II, which can then undergo intersystem crossing to intermediate III and form azetidine 13 through C–N bond formation.
To conclude, we have reported the development of an intramolecular visible light-enabled [2+2]-cycloaddition reaction between unactivated alkenes and cyclic oximes to access tricyclic azetidines in yields up to 84%. This transformation complements existing reports for aza Paternò-Büchi reactions to expand the repertoire of currently available efficient procedure for the formation of complex azetidine products. Furthermore, this transformation relies on an iridium photocatalyst but can similarly be promoted by an organic photocatalyst to achieve yields of the desired product of up to 41%, resulting in complex, highly saturated scaffolds. Notably, this method gives rise to a reverse substitution pattern in comparison to previous methods developed,17,19 allowing access to molecules that occupy distinct chemical space. We anticipate this new strategy to be used for the rapid build-up of molecular complexity when incorporating functionalized azetidines in current drug design and development.
Supplementary Material
Figure 4:
Mechanistic Hypothesis for the intramolecular aza Paternò-Büchi reaction.
ACKNOWLEDGMENT
We thank the Alfred P. Sloan Foundation, the David and Lucile Packard Foundation the Camille and Henry Dreyfus Foundation, and NIH (R01-GM141340) for funding. D.E.B. thanks Emily R. Wearing and Katie A. Rykaczewski for many helpful discussions.
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website and includes experimental procedures, characterization (1H-NMR, 13C-NMR, IR, and MS data), additional optimization and control experiments (pdf format).
The authors declare no competing financial interests.
REFERENCES
- (1).Lovering F; Bikker J; Humblet C, Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- (2).Lovering F, Escape from Flatland 2: complexity and promiscuity. Med. Chem. Commun 2013, 4, 515–519. 10.1039/C2MD20347B. [DOI] [Google Scholar]
- (3).Bauer MR; Di Fruscia P; Lucas SCC; Michaelides IN; Nelson JE; Storer RI; Whitehurst BC, Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem 2021, 12, 448–471. 10.1039/D0MD00370K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Fish PV; Brown AD; Evrard E; Roberts LR, 7-Sulfonamido-3-benzazepines as potent and selective 5-HT2C receptor agonists: hit-to-lead optimization. Bioorg. Med. Chem. Lett 2009, 19, 1871–5. 10.1061/j.bmcl.2009.02.071. [DOI] [PubMed] [Google Scholar]
- (5).St Jean DJ Jr.; Fotsch C, Mitigating heterocycle metabolism in drug discovery. J. Med. Chem 2012, 55, 6002–6020. 10.1021/jm300343m. [DOI] [PubMed] [Google Scholar]
- (6).Shu YZ; Johnson BM; Yang TJ, Role of biotransformation studies in minimizing metabolism-related liabilities in drug discovery. AAPS J. 2008, 10, 178–192. 10.1208/s12248-008-9016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lowe JT; Lee M. D. t.; Akella LB; Davoine E; Donckele EJ; Durak L; Duvall JR; Gerard B; Holson EB; Joliton A; Kesavan S; Lemercier BC; Liu H; Marie JC; Mulrooney CA; Muncipinto G; Welzel-O’Shea M; Panko LM; Rowley A; Suh BC; Thomas M; Wagner FF; Wei J; Foley MA; Marcaurelle LA, Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries. J. Org. Chem 2012, 77, 7187–7211. 10.1021/jo300974j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Brown A; Brown TB; Calabrese A; Ellis D; Puhalo N; Ralph M; Watson L, Triazole oxytocin antagonists: Identification of an aryloxyazetidine replacement for a biaryl substituent. Bioorg. Med. Chem. Lett 2010, 20, 516–520. 10.1016/bmcl.2009.11.097. [DOI] [PubMed] [Google Scholar]
- (9).Maetani M; Zoller J; Melillo B; Verho O; Kato N; Pu J; Comer E; Schreiber SL, Synthesis of a Bicyclic Azetidine with In Vivo Antimalarial Activity Enabled by Stereospecific, Directed C(sp(3))-H Arylation. J. Am. Chem. Soc 2017, 139, 11300–11306. 10.1021/jacs.7b06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Vitaku E; Smith DT; Njardarson JT, Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- (11).Cromwell NH; Phillips B, The Azetidines. Recent Synthetic Developments. Chem. Rev 1979, 79, 331–358. 10.1021/cr60320a003. [DOI] [Google Scholar]
- (12).Richardson AD; Becker MR; Schindler CS, Synthesis of azetidines by aza Paterno-Buchi reactions. Chem. Sci 2020, 11, 7553–7561. 10.1039/D0SC01017K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Brani A; Cicchi S; Cordero FM, Novel syntheses of azetidinesand azetidinones. Chem. Rev 2008, 108, 3988–4035. 10.1021/cr800325e. [DOI] [PubMed] [Google Scholar]
- (14).Antermite D; Degennaro L; Luisi R, Recent advances in the chemistry of metallated azetidines. Org. Biomol. Chem 2016, 15, 34–50. 10.1039/C6Ob01665K. [DOI] [PubMed] [Google Scholar]
- (15).Gianatassio R; Lopchuk JM; Wang J; Pan CM; Malins LR; Prieto L; Brandt TA; Collins MR; Gallego GM; Sach NW; Spangler JE; Zhu H; Zhu J; Baran PS, Organic chemistry. Strain-release amination. Science 2016, 351, 241–246. 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For an example of an aza Paternò-Büchi reaction using an enamide and an oxime under UV-light, see: Kumarasamy E; Kandappa SK; Raghunathan R; Jockusch S; Sivaguru J, Realizing an Aza Paterno-Buchi Reaction. Angew. Chem. Int. Ed. Engl 2017, 56, 7056–7061. 10.1002/anie.201702273 [DOI] [PubMed] [Google Scholar]
- (17).Becker MR; Richardson AD; Schindler CS, Functionalized azetidines via visible light-enabled aza Paterno-Buchi reactions. Nat. Commun 2019, 10, 5095. 10.1038/s41467-019-13072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhu M; Zhang X; Zheng C; You S-L, Visible-Light-Induced Dearomatization via [2+2] Cycloaddition or 1,5-Hydrogen Atom Transfer: Divergent Reaction Pathways of Transient Diradicals. ACS Catalysis 2020, 10, 12618–12626. 10.1021/acscatal.0c03808. [DOI] [Google Scholar]
- (19).Becker MR; Wearing ER; Schindler CS, Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions. Nat. Chem 2020, 12, 898–905. 10.1038/s41557-020-0541-1. [DOI] [PubMed] [Google Scholar]
- (20).Li X; Grosskopf J; Jandl C; Bach T, Enantioselective, Visible Light Mediated Aza Paterno-Buchi Reactions of Quinoxalinones. Angew. Chem. Int. Ed. Engl 2021, 60, 2684–2688. 10.1002/anie.202013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wearing ER; Blackmun DE; Schindler CS, 1- and 2-Azetines via Visible Light-Mediated [2+2]-Cycloadditions of Alkynes and Oximes. J. Am. Chem. Soc 2021, 143, 16235–16242. 10.1021/jacs.1c07523. [DOI] [PubMed] [Google Scholar]
- (22).Rykaczewski KA; Schindler CS Visible-Light-Enabled Paternò-Büchi Reaction via Triplet Energy Transfer for the Synthesis of Oxetanes. Org. Lett 2020, 22, 6516–6519. 10.1021/acsorglett.0c02316. [DOI] [PubMed] [Google Scholar]
- (23).D’Auria M, The Paterno-Buchi reaction - a comprehensive review. Photochem. Photobiol. Sci 2019, 18, 2297–2362. 10.1039/C9PP00148D. [DOI] [PubMed] [Google Scholar]
- (24).Kandappa SK; Valloli LK; Ahuja S; Parthiban J; Sivaguru J, Taming the excited state reactivity of imines - from non-radiative decay to aza Paterno-Buchi reaction. Chem. Soc. Rev 2021, 50, 1617–1641. 10.1039/d0cs00717j. [DOI] [PubMed] [Google Scholar]
- (25).Latrache M; Hoffmann N, Photochemical radical cyclization reactions with imines, hydrazones, oximes and related compounds. Chem. Soc. Rev 2021, 50, 7418–7435. 10.1039/d1cs00196e. [DOI] [PubMed] [Google Scholar]
- (26).Rykaczewski KA; Wearing ER; Blackmun DE; Schindler CS, Reactivity of oximes for diverse methodologies and synthetic applications. Nature Synthesis 2022, 1, 24–36. 10.1038/s44160-021-00007-y. [DOI] [Google Scholar]
- (27).Collins KD; Glorius F, A robustness screen for the rapid assessment of chemical reactions. Nat Chem 2013, 5, 597–601. 10.1038/nchem.1669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.