Abstract
Objective.
ARIEL3 (NCT01968213) is a placebo-controlled randomized trial of the poly(ADP-ribose) polymerase inhibitor rucaparib as maintenance treatment in patients with recurrent high-grade ovarian carcinoma who responded to their latest line of platinum therapy. Rucaparib improved progression-free survival across all predefined subgroups. Here, we present an exploratory analysis of clinical and molecular characteristics associated with exceptional benefit from rucaparib.
Methods.
Patients were randomized 2:1 to receive rucaparib 600 mg twice daily or placebo. Molecular features (genomic alterations, BRCA1 promoter methylation) and baseline clinical characteristics were evaluated for association with exceptional benefit (progression-free survival ≥2 years) versus progression on first scan (short-term subgroup) and other efficacy outcomes.
Results.
Rucaparib treatment was significantly associated with exceptional benefit compared with placebo: 79/375 (21.1%) vs 4/189 (2.1%), respectively (p<0.0001). Exceptional benefit was more frequent among patients with favorable baseline clinical characteristics and with carcinomas harboring molecular evidence of homologous recombination deficiency (HRD). A comparison between patients who derived exceptional benefit from rucaparib and those in the short-term subgroup revealed both clinical markers (no measurable disease at baseline, complete response to latest platinum, longer penultimate platinum-free interval) and molecular markers (BRCA1, BRCA2, RAD51C, and RAD51D alterations and genome-wide loss of heterozygosity) significantly associated with exceptional benefit.
Conclusions.
Exceptional benefit in ARIEL3 was more common in, but not exclusive to, patients with favorable clinical characteristics or molecular features associated with HRD. Our results suggest that rucaparib can deliver exceptional benefit to a diverse set of patients with recurrent high-grade ovarian carcinoma.
Keywords: Ovarian carcinoma, Genomics, Rucaparib, Safety
1. Introduction
ARIEL3 (NCT01968213) is a double-blind, randomized, placebo-controlled study of the oral, small-molecule poly(ADP-ribose) polymerase (PARP) inhibitor rucaparib as maintenance treatment for recurrent high-grade ovarian carcinoma.1 In ARIEL3, rucaparib maintenance treatment improved progression-free survival across all predefined nested cohorts. The risk of disease progression or death in the overall intent-to-treat population was 0.36 (95% CI, 0.30–0.45; p<0.0001; median [95% CI] progression-free survival, 10.8 months [8.3–11.4] in the rucaparib group vs 5.4 months [5.3–5.5] in the placebo group).1 Outcomes, however, were not equivalent across all predefined molecular subgroups. Patients with BRCA1 or BRCA2 (BRCA)–mutant carcinoma derived the greatest benefit (HR, 0.23 [95% CI, 0.16–0.34]; p<0.0001; median progression-free survival, 16.6 months [13.4–22.9] in the rucaparib group vs 5.4 months [3.4–6.7] in the placebo group), followed by patients with a homologous-recombination-deficient carcinoma (HR, 0.32 [95% CI, 0.24–0.42], p<0.0001; median progression-free survival, 13.6 months [10.9–16.2] in the rucaparib group vs 5.4 months [5.1–5.6] in the placebo group), and those with BRCA–wild-type/low loss of heterozygosity (LOH) carcinomas (ie, without evidence of homologous recombination deficiency [HRD]; HR, 0.58 [95% CI 0.40–0.85], p=0.0049; median progression-free survival, 6.7 months [5.4–9.1] in the rucaparib group vs 5.4 months [5.3–7.4] in the placebo group).
Beyond characterizing median outcomes, analyses of patients who derive long-term benefit from rucaparib maintenance treatment may provide new insights that can help physicians in clinical decision making. While no established definition of exceptional benefit exists, survival duration that is 2 to 3 times the median has been used as a cutoff in prior studies.2, 3 Long-term benefit from maintenance treatment with the PARP inhibitor olaparib was previously investigated using such a cutoff (progression-free survival ≥2 years, twice the median),3 with complete response to most recent platinum-based chemotherapy emerging as the only significant clinical or molecular predictor of long-term benefit. BRCA mutations were common in patients who received long-term olaparib maintenance, but the frequency of BRCA mutations was not significantly different compared with those patients who received olaparib for <3 months.3 We previously showed that patients with recurrent high-grade ovarian carcinoma who achieved long-term responses (≥1 year) to rucaparib in the treatment setting were enriched for specific molecular characteristics, including the presence of reversion-resistant BRCA structural variants, high genome-wide LOH, and deleterious RAD51C and RAD51D alterations.4
Here, we present an exploratory analysis of the frequency of exceptional benefit (progression-free survival ≥2 years) in the overall ARIEL3 population as well as in patient subgroups defined by different clinical and molecular characteristics. We also explore the clinical and molecular characteristics associated with patients who derived exceptional benefit from rucaparib maintenance treatment as compared with those who progressed on or before their first scan (short-term subgroup) and all other patients.
2. Methods
2.1. Study design and population
The ARIEL3 study design and patient eligibility criteria have been described previously.1 Briefly, patients with recurrent, platinum-sensitive high-grade ovarian carcinoma who had responded to their last platinum-based regimen were randomized 2:1 to receive maintenance treatment with rucaparib 600 mg twice a day or placebo. The data cutoff date for efficacy and treatment-emergent adverse events was December 31, 2019. Patients were followed after treatment discontinuation for incidence of myelodysplastic syndrome or acute myeloid leukemia, adverse events of interest, and these data are reported as of December 19, 2020. The study was approved by national or local institutional review boards and performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Council for Harmonisation. Written informed consent was obtained from all patients, or the requirement for written informed consent was waived by the institutional review board.
2.2. Genomic characterization
Archival formalin-fixed paraffin-embedded neoplastic tissues, typically collected during debulking surgery prior to adjuvant chemotherapy treatment, were centrally analyzed to detect deleterious mutations in BRCA1, BRCA2, and other homologous-recombination-repair genes (ATM, ATR, ATRX, BARD1, BLM, BRIP1, CHEK1, CHEK2, FANCA, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, MRE11A, NBN, PALB2, RAD50, RAD51, RAD51B, RAD51C, RAD51D, RAD52, RAD54L, and RPA1), and to identify carcinomas with high genome-wide LOH (≥16%) using Foundation Medicine’s T5 NGS assay (Cambridge, MA, USA). Additional BRCA alterations were identified through local and central germline sequencing. Germline/somatic status for BRCA mutations was established through central germline sequencing using the BRCAnalysis CDx test (Myriad Genetics, Salt Lake City, UT, USA). The germline/somatic status of non-BRCA homologous-recombination-repair genes was determined by Color Genomics germline testing (Burlingame, CA, USA). Zygosity of non-BRCA homologous-recombination-repair genes was established computationally.5
Quantification of BRCA1 methylation levels in neoplastic tissues was performed by quantitative methylation-sensitive digital droplet polymerase chain reaction (Ambry Genetics, Aliso Viejo, CA, USA) and analyzed as previously described.6, 7 Samples were classified dichotomously as having “high” or “low” methylation levels based on a predefined cutoff of ≥70% for high methylation.
2.3. Analysis methods
Investigator-assessed progression-free survival, the primary endpoint of the ARIEL3 study, was defined as the time from randomization to investigator-assessed disease progression according to Response Evaluation Criteria in Solid Tumors v1.1 (RECIST) or death; patients without documented progression or death were censored as of their last tumor assessment.1 In this post hoc analysis, duration of investigator-assessed progression-free survival during ARIEL3 was used to define the outcome subgroups. Patients with progression-free survival ≥2 years (double the median in the intent-to-treat population [10.8 months1] rounded to the closest year) were classified as the exceptional benefit subgroup; patients with disease progression on, or before their first scan (≈12 weeks for most patients) were classified as the short-term subgroup; patients who did not fall in either of these categories were considered “all others.”
Univariate analysis of categorical variables was performed using Fisher’s exact test (for 2 categories) or chi-square test (for multiple categories); continuous data (age) were analyzed using the Mann-Whitney test. Median progression-free survival was determined using Kaplan-Maier survival analysis. No multiple hypothesis correction was performed; presented p values were not adjusted. All analyses were not prespecified and are exploratory in nature.
A stepwise multivariate logistics regression model was used to identify predictors of exceptional benefit by comparing the exceptional benefit patients versus everyone else (both the short-term and the all others subgroups) using the following baseline characteristics: age, body mass index, race (White vs other or missing), Eastern Cooperative Oncology Group performance status, type of ovarian cancer, number of prior chemotherapy regimens, number of prior platinum-based chemotherapy regimens, measurable disease at baseline, stratification variables of penultimate platinum-free interval and best response to last chemotherapy treatment, and molecular classifications based on HRD-based molecular status (BRCA mutant, BRCA wild-type/high LOH, BRCA wild-type/low LOH, BRCA wild-type/unknown LOH), mutations in the RAD51C or RAD51D genes, mutations in other homologous-recombination-repair genes, and archival methylation status in BRCA–wild-type patients (high methylation, low methylation, unmethylated, or not available).
3. Results
3.1. Frequency of exceptional benefit
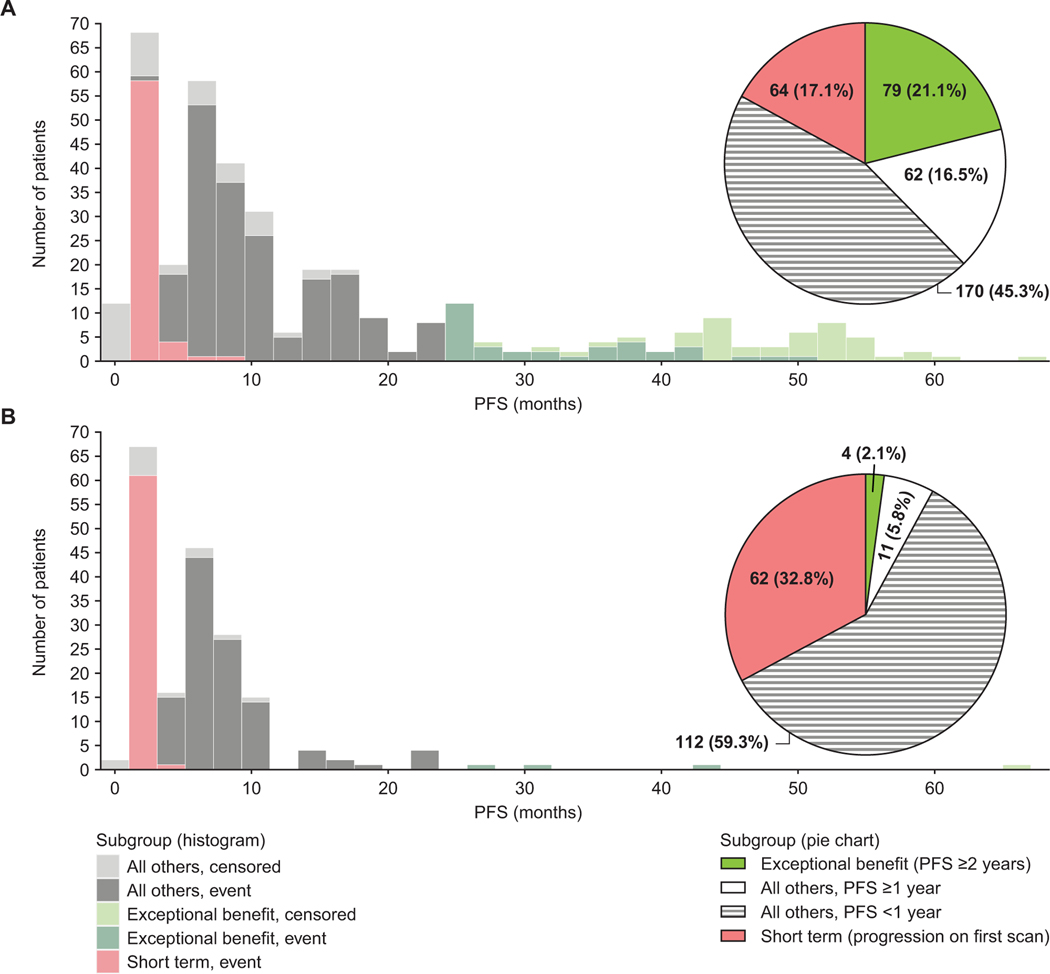
Overall, 564 patients were enrolled in ARIEL3, among whom 218 (38.7%) patients had BRCA-mutant carcinomas (143/375 [38.1%] in the rucaparib arm; 75/189 [39.7%] in the placebo arm) as identified by either central (tissue and germline) or local testing. As of the December 31, 2019, data cutoff date, with a median follow-up of 51.4 months, 33/375 (8.8%) and 1/189 (0.5%) patients were still receiving rucaparib or placebo, respectively. Within the rucaparib arm, 79/375 patients (21.1%) derived exceptional benefit (progression-free survival ≥2 years; Fig. 1A and 1C); 52/375 (13.9%) had progression-free survival ≥3 years, including 26/375 (6.9%) with progression-free survival ≥4 years. Placebo-arm patients were significantly less likely to achieve progression-free survival ≥2 years than those in the rucaparib arm (p<0.0001); only 4/189 patients (2.1%) showed exceptional benefit while 62/189 patients (32.8%) progressed at first scan (Fig. 1B and 1D). The median (range) progression-free survival was not reached among those in the rucaparib arm and was 37.1 months (27.4–66.0) among the four exceptional benefit patients in the placebo arm.
Fig. 1.
Distribution of PFS outcomes in ARIEL3 patients. (A) Frequencies of PFS outcomes in rucaparib-arm patients (pie chart) and distribution of PFS in the exceptional benefit, short-term, and all others subgroups in the rucaparib arm (histogram). (B) Frequencies of PFS outcomes in placebo-arm patients (pie chart) and distribution of PFS in the exceptional benefit, short-term, and all others subgroups in the placebo arm (histogram). Two patients who were included in the rucaparib short-term subgroup had a relapse on the first scan, but the gap in scan scheduling was longer than expected (at 6 months and 9 months after their first dose of rucaparib; protocol deviation). PFS, progression-free survival.
A majority (68/79 [86.1%]) of rucaparib-arm exceptional benefit patients achieved longer progression-free survival in ARIEL3 as compared with their penultimate platinum-free interval (Supplementary Fig. 1). The median (range) difference between progression-free survival in ARIEL3 and penultimate platinum-free interval was 21.3 months (−77.3 to 56.1), indicating that most exceptional benefit patients derived more durable benefit from rucaparib maintenance therapy after their most recent line of platinum-based treatment than from their penultimate treatment.
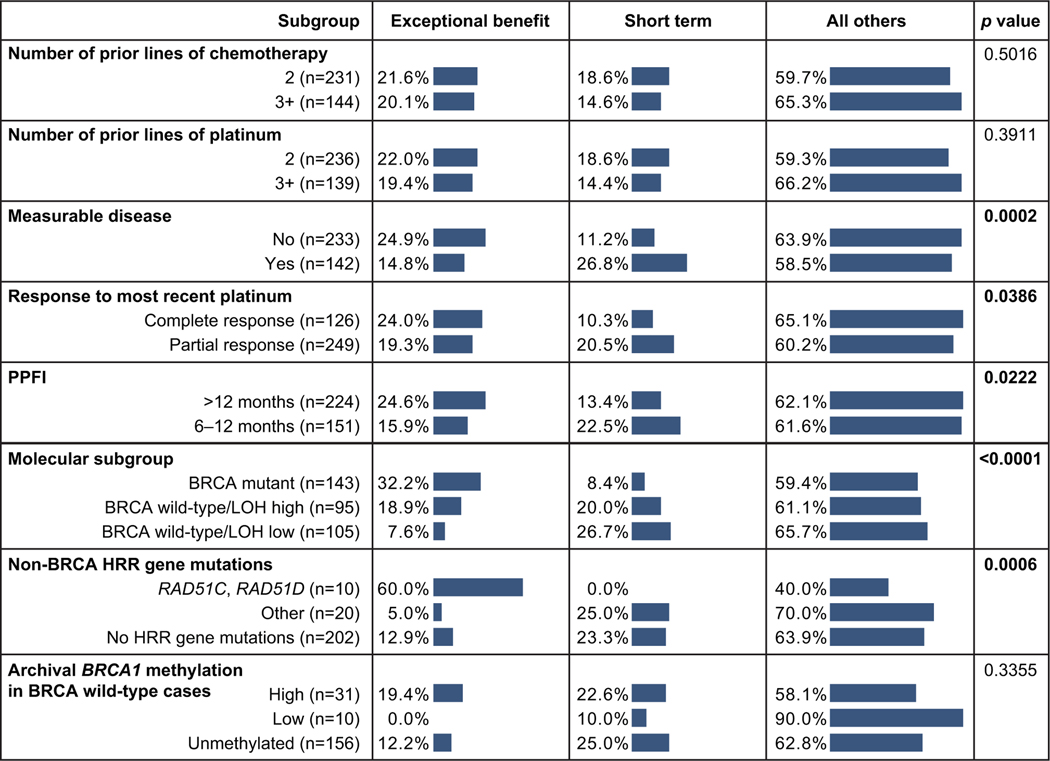
Exceptional benefit was significantly more common among patients with favorable clinical characteristics. Approximately 25% of patients with no measurable disease at baseline, complete response to most recent platinum, or penultimate platinum-free interval >12 months achieved exceptional benefit, while <15% of patients with these characteristics formed part of the short-term subgroup. In contrast, a smaller proportion of patients with less favorable clinical characteristics (measurable disease at baseline, partial response to most recent platinum, and penultimate platinum-free interval 6–12 months) derived exceptional benefit (Fig. 2). The number of prior lines of chemotherapy or platinum-based therapy was not differentially associated with exceptional benefit or progression at first scan. Similar trends were observed in the placebo arm (Supplementary Fig. 2).
Fig. 2.
Frequencies of outcomes in rucaparib-arm patients with different baseline clinical and molecular characteristics. p values based on chi-square tests; bold denotes significant results (p<0.05). BRCA, BRCA1 or BRCA2; ECOG PS, Eastern Cooperative Oncology Group performance status; HRR, homologous recombination repair; LOH, loss of heterozygosity; PPFI, penultimate platinum-free interval.
The molecular characteristics of the patient’s high-grade ovarian carcinoma also had a strong influence on whether they derived exceptional benefit from rucaparib maintenance. We observed a higher frequency of exceptional benefit among rucaparib-arm patients with homologous-recombination-deficient carcinomas; 32.2% of patients with high-grade ovarian carcinoma harboring a BRCA alteration experienced exceptional benefit (Fig. 2). Within the BRCA–wild-type population, exceptional benefit was more common among patients with high LOH carcinomas (18.9%) than among those with low LOH carcinomas (7.6%; Fig. 2). In ARIEL3, 2.3% of patients (13/564; 10 patients in the rucaparib arm and 3 patients in the placebo arm) had an alteration in RAD51C and RAD51D, known drivers of HRD; rucaparib-arm patients with a RAD51C or RAD51D alteration had very high frequency of exceptional benefit (6/10 [60.0%]), unlike patients harboring mutations in other homologous-recombination-repair genes (1/20 [5.0%]; Fig. 2). Archival BRCA1 promoter methylation status was not significantly associated with differential outcomes in ARIEL3. However, among patients with evidence of methylation, 19.4% of those with high archival methylation derived exceptional benefit from rucaparib; in contrast none of the patients with low archival methylation derived exceptional benefit (Fig. 2). None of the molecular characteristics summarized above were significantly associated with progression-free survival outcomes in the placebo arm (Supplementary Fig. 2).
3.2. Baseline clinical characteristics of exceptional benefit patients
To determine what clinical and molecular characteristics were significantly associated with exceptional benefit, we compared the exceptional benefit and the short-term subgroup patients within each treatment arm. In the rucaparib arm, those who experienced exceptional benefit were significantly more likely to have had more favorable clinical prognostic factors at baseline compared with those in the short-term subgroup, including no measurable disease at baseline (p<0.001), complete response to most recent platinum (p=0.018), and longer penultimate platinum-free interval (p=0.007; Table 1). Trends were similar in the placebo arm, although the small number of exceptional benefit patients precludes a meaningful analysis (Table 1).
Table 1.
Baseline characteristics in the exceptional benefit and short-term subgroups.
| Rucaparib arm | Placebo arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Exceptional benefit subgroup (n=79) | Short-term subgroup (n=64) | p value | Odds ratio (95% CI) | Exceptional benefit subgroup (n=4) | Short-term subgroup (n=62) | p value | Odds ratio (95% CI) |
| Age, median (range), y | 61 (42–79) | 61 (39–78) | 0.661 | — | 54 (48–62) | 62.5 (36–84) | 0.202 | — |
| ECOG PS, n (%) | 0.711 | >0.99 | ||||||
| 0 | 55 (69.6) | 47 (73.4) | 0.8 (0.4–1.8) | 3 (75.0) | 44 (71.0) | 1.2 (0.2–16.7) | ||
| 1 | 24 (30.4) | 17 (26.6) | 1.2 (0.6–2.5) | 1 (25.0) | 18 (29.0) | 0.8 (0.1–5.8) | ||
| Prior chemotherapy regimens, n (%) | 0.725 | >0.99 | ||||||
| 2 | 50 (63.3) | 43 (67.2) | 0.8 (0.4–1.7) | 3 (75.0) | 39 (62.9) | 1.8 (0.2–23.9) | ||
| ≥3 | 29 (36.7) | 21 (32.8) | 1.2 (0.6–2.4) | 1 (25.0) | 23 (37.1) | 0.6 (0.0–4.0) | ||
| Prior platinum regimens, n (%) | 0.725 | >0.99 | ||||||
| 2 | 52 (65.8) | 44 (68.8) | 0.9 (0.4–1.8) | 3 (75.0) | 40 (64.5) | 1.7 (0.2–22.3) | ||
| ≥3 | 27 (34.2) | 20 (31.3) | 1.1 (0.6–2.3) | 1 (25.0) | 22 (35.5) | 0.6 (0.0–4.3) | ||
| No measurable disease, n (%) | 58 (73.4) | 26 (40.6) | <0.001 | 4.0 (2.0–8.0) | 3 (75.0) | 33 (53.2) | 0.620 | 2.6 (0.4–35.3) |
| Complete response to latest platinum, n (%) | 31 (39.2) | 13 (20.3) | 0.018 | 2.5 (1.2–5.3) | 1 (25.0) | 11 (17.7) | 0.561 | 1.5 (0.1–11.2) |
| PPFI >12 mo, n (%) | 55 (69.6) | 30 (46.9) | 0.007 | 2.6 (1.3–5.2) | 4 (100) | 29 (46.8) | 0.114 | NA |
ECOG PS, Eastern Cooperative Oncology Group performance status; NA, not applicable; PPFI, penultimate platinum-free interval.
Bold denotes significant result (p<0.05). Statistical comparisons based on Fisher’s exact test for all cases except age, which was compared with the Mann-Whitney test.
3.3. HRD-based molecular characteristics associated with exceptional benefit
BRCA mutations were significantly enriched among rucaparib-arm patients who derived exceptional benefit compared with those in the short-term subgroup (p<0.001; Table 2, Fig. 3). Patients with BRCA mutations appeared to derive exceptional benefit from rucaparib regardless of which BRCA gene was mutated (BRCA1 vs BRCA2), mutation origin (germline vs somatic), or variant type (short variant vs rearrangement/loss; Supplementary Table 1). Similar trends were observed in the placebo-arm patients, but a low number of exceptional benefit cases hinders a meaningful statistical analysis (Supplementary Tables 2 and 3, Supplementary Fig. 3).
Table 2.
Genetic and epigenetic alterations in the rucaparib-arm exceptional benefit and short-term subgroups.
| Alteration | Exceptional benefit subgroup (n=79) | Short-term subgroup (n=64) | p value | Odds ratio (95% CI) |
|---|---|---|---|---|
| BRCA mutant | 46 (58.2) | 12 (18.8) | <0.001 | 6.0 (2.8–13.3) |
| BRCA wild-type + RAD51C/D mutation | 6 (7.6) | 0 | 0.033 | NA |
| BRCA wild-type + other HRR gene mutation | 1 (1.3) | 5 (7.8) | 0.090 | 0.2 (0.0–1.2) |
| BRCA wild-type + LOH high | 18 (22.8) | 19 (29.7) | 0.443 | 0.7 (0.3–1.5) |
| BRCA wild-type + LOH low | 8 (10.1) | 28 (43.8) | <0.001 | 0.14 (0.06–0.35) |
| BRCA wild-type + high BRCA1 methylation | 6/25 (24.0) | 7/47 (14.9) | 0.353 | 1.8 (0.5–6.0) |
BRCA, BRCA1 or BRCA2; HRR, homologous recombination repair; LOH, loss of heterozygosity; NA, not applicable.
Bold denotes significant result (p<0.05). Statistical comparisons based on Fisher’s exact test for all cases. Data are n (%) or n/N (%). Data for the placebo arm are available in Supplementary Table 1.
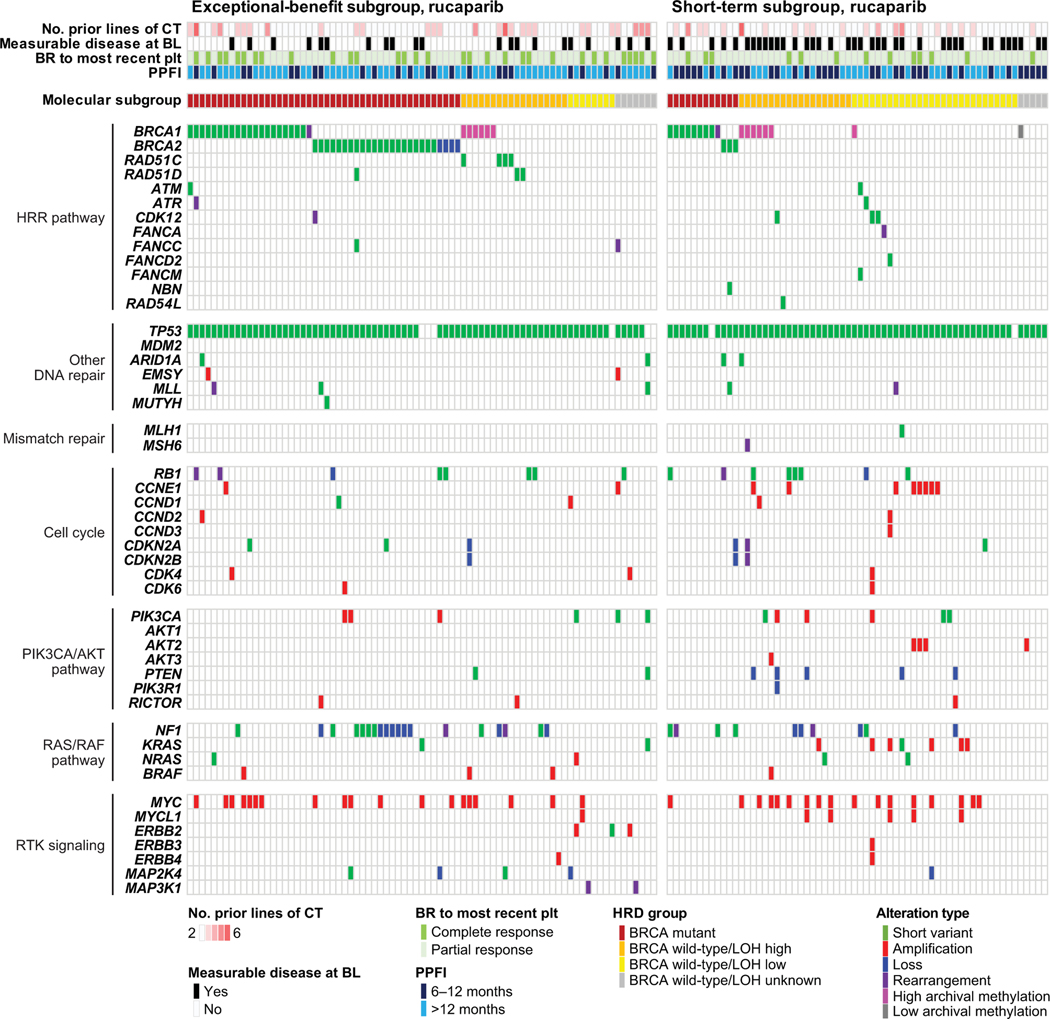
Fig. 3.
Genetic and epigenetic alterations in exceptional benefit (left) and short-term (right) subgroup patients in the rucaparib arm. BL, baseline; BR, best response; BRCA, BRCA1 or BRCA2; CT, chemotherapy; HRD, homologous recombination deficiency; HRR, homologous recombination repair; LOH, loss of heterozygosity; plt, platinum; PPFI, penultimate platinum-free interval.
Despite the strong association of BRCA mutations with positive outcomes, 33/79 (41.8%) of rucaparib-arm exceptional benefit patients had BRCA–wild-type carcinomas (Fig. 3, Table 2). Among those, RAD51C and RAD51D mutations were significantly associated with exceptional benefit (p=0.033). Germline and/or somatic mutations in these genes were present in 6/79 (7.6%) of exceptional benefit cases and completely absent from the short-term subgroup (Fig. 3, Table 2, Supplementary Table 4). Other non-BRCA homologous-recombination-repair genes were not significantly associated with exceptional benefit (Fig. 3, Table 2, Supplementary Table 4).
Genome-wide LOH was also significantly different between the exceptional benefit and short-term subgroups. Specifically, low LOH was more prevalent in the short-term subgroup, suggesting that patients harboring carcinomas without evidence of HRD are significantly less likely to derive durable benefit from rucaparib maintenance (p<0.001; Fig. 3, Table 2). Interestingly, however, a number of patients with BRCA–wild-type/low LOH carcinomas did derive exceptional benefit, although the mechanism of long-term sensitivity in this group was unclear. The frequency of high archival BRCA1 methylation (defined as ≥70% methylation) was similar among patients who derived exceptional benefit and those in the short-term subgroup (Fig. 3, Table 2).
A multivariate analysis comparing the exceptional benefit patients with all remaining patients enrolled in ARIEL3 identified both baseline clinical factors (treatment arm, penultimate platinum-free interval >12 months, no measurable disease at baseline) and molecular characteristics (eg, BRCA and RAD51C/D mutations) as significant independent predictors of exceptional benefit, confirming the findings from the univariate analyses described above across the entire ARIEL3 population (Supplementary Table 5, Supplementary Table 6).
3.4. Non-HRD alterations in exceptional benefit versus short-term subgroup patients
Beyond mutations in homologous-recombination-repair genes, rucaparib-arm patients in the exceptional benefit and short-term subgroups harbored alterations in other pathways commonly affected in high-grade ovarian carcinoma, including DNA-damage repair, cell cycle regulation, RAS/RAF signaling, and PIK3CA/PTEN signaling (Fig. 3). TP53 was the most frequently mutated gene in both subgroups, typical of high-grade ovarian carcinoma histology.8, 9 Among the few patients with TP53 wild-type who showed exceptional benefit while on rucaparib treatment, one harbored an activating KRAS mutation, suggesting a low-grade or mesonephric-like histology instead of high-grade ovarian carcinoma.7 Low-grade serous ovarian cancers are characterized by slower growth, which may account for the long progression-free survival experienced by this patient.7, 10 ARID1A mutations, which have been associated with preclinical PARP inhibitor sensitivity,11 were detected in two exceptional benefit cases, one of which had the co-occurring aforementioned KRAS mutation. RB1 deletions in the background of BRCA mutations have been associated with exceptional survival in high-grade ovarian carcinoma.12 Consistent with this observation, we identified a tumor in a patient with exceptional benefit having co-occurring BRCA2 mutation and RB1 loss. CCNE1 amplifications were significantly more common among rucaparib-arm patients in the short-term subgroup (p=0.043), which is consistent with reports linking this alteration with resistance to both platinum and PARP inhibitor treatment.13 In the placebo arm, patients in the exceptional benefit and short-term subgroups shared a similar array of nonhomologous-recombination-repair gene alterations as the rucaparib arm. For example, frequent CCNE1 amplifications were also observed in the short-term subgroup of the placebo arm (Supplementary Fig. 3).
3.5. Safety
Among rucaparib-arm patients, the incidence rates of the most common treatment-emergent adverse events were generally consistent between the exceptional benefit subgroup and the overall ARIEL3 patient population (Supplementary Tables 7 and 8).14 There was a higher incidence in certain safety parameters (grade ≥3 treatment-emergent adverse events, treatment interruption and/or dose reduction due to a treatment-emergent adverse event, and any-grade abdominal pain) in the exceptional benefit subgroup as compared with the overall population, which can be attributed to the length of time that patients remained on treatment (median treatment duration, 3.6 years). Most rucaparib-arm patients in the exceptional benefit subgroup (57/79 [72.2%]) had ≥1 dose reduction; 33/79 patients (41.8%) had ≥2 dose reductions; and median dose intensity was 0.83. As of December 19, 2020 (>6 years follow-up from first patient enrolled), 18 myelodysplastic syndrome/acute myeloid leukemia cases have been reported in the overall ARIEL3 patient population: 14 in the rucaparib arm (3.7%) and 4 in the placebo arm (2.1%; Supplementary Table 9). Of the cases in the rucaparib arm, 9 (11.4%) were reported among the 79 patients in the exceptional benefit subgroup (3 during treatment and 6 during long-term follow-up). No cases of myelodysplastic syndrome/acute myeloid leukemia were observed in the placebo-arm exceptional benefit subgroup (Supplementary Table 9).
4. Discussion
In ARIEL3, 21.1% of patients in the rucaparib arm derived exceptional benefit (progression-free survival ≥2 years) versus only 2.1% of those in the placebo arm. This 10-fold difference suggests that rucaparib maintenance treatment not only improves median progression-free survival for patients with recurrent high-grade ovarian carcinoma1 but leads to exceptional durable benefit for a large fraction of these patients.
The clinical characteristics associated with exceptional outcomes on rucaparib in the univariate analysis were all related to platinum sensitivity, including durable benefit from their penultimate platinum (subsequent platinum-free interval >12 months), no measurable disease at ARIEL3 baseline, and complete response to last platinum prior to initiating rucaparib. Platinum-based chemotherapies and PARP inhibitors both take advantage of HRD present in some high-grade ovarian carcinomas,15–17 and platinum sensitivity is a strong clinical correlate for rucaparib efficacy in the treatment setting.7 A complete response to last platinum did not emerge as a statistically significant variable in the multivariate analysis, likely due to its close relationship with the absence of measurable disease at baseline, which was a more powerful predictor for deriving exceptional benefit from maintenance with rucaparib than degree of response to platinum.
As expected, patients with BRCA-mutant high-grade ovarian carcinoma were most likely to derive exceptional benefit from rucaparib maintenance treatment. Both BRCA1 and BRCA2 mutations (germline or somatic) correlated with exceptional benefit. Although structural variant alterations (eg, deletions or rearrangements) in the BRCA genes were previously associated with more durable responses in the ARIEL2 treatment setting, which was likely due to their inability to revert to wild-type functionality,7 we detected no such link in ARIEL3. In contrast to the ARIEL2 population, cancers from ARIEL3 patients were less heavily pretreated and remained platinum sensitive; as a result, the lower likelihood of reversion mutations may explain the observed exceptional benefit across all classes of BRCA mutations in ARIEL3.
Despite being more common among BRCA-mutant cases, long-term benefit was not limited to this molecular subgroup, with approximately 40% of patients with exceptional benefit in the rucaparib arm having BRCA–wild-type carcinomas. Patients harboring RAD51C and RAD51D mutations had especially positive outcomes, with 60% of such patients deriving exceptional benefit with rucaparib. Alterations in RAD51C and RAD51D have been associated with improved responses to rucaparib in the treatment setting,7 and the detection of reversion mutations in these two genes has solidified their standing as drivers of HRD and synthetic lethality with PARP inhibitors.18 The number of patients with alterations in other homologous-recombination-repair genes was low, making it hard to conclude if additional homologous-recombination-repair genes may be associated with exceptional benefit from rucaparib maintenance. Notably, there were no cases with PALB2 mutations, a homologous recombination repair gene in which mutations have correlated with PARP inhibitor response in breast and pancreatic cancer.19, 20 Interestingly, of the 79 patients achieving exceptional benefit with rucaparib, 8 (10.1%) had carcinomas that were negative by HRD test (ie, were within the BRCA–wild-type/low LOH population), highlighting that some patients may benefit from maintenance with rucaparib even in the absence of a known PARP inhibitor-sensitizing genetic alteration and emphasizing the need for improved biomarkers of response.
Although high methylation of the BRCA1 promoter is a known driver of HRD,7 high archival BRCA1 methylation was not associated with increased likelihood of deriving exceptional benefit from rucaparib maintenance in ARIEL3. BRCA1 methylation is a reversible modification that can be lost during intermittent lines of platinum therapy as a resistance mechanism.7 Therefore, only methylation measured in biopsies obtained immediately prior to initiating rucaparib for measurable disease was predictive of rucaparib response.7 Pre-treatment biopsies were not collected as part of ARIEL3 and are usually difficult to obtain in the maintenance setting because treatment is initiated immediately after response to the most recent line of platinum, when many patients have no or minimal measurable residual disease. Archival methylation may prove to be an informative biomarker in the frontline setting, when only a single line of platinum treatment prior to initiating PARP inhibitor treatment likely lowers the chance for methylation loss as a resistance mechanism. Notably, none of the patients with low archival methylation experienced exceptional benefit, suggesting that incomplete BRCA1 promoter silencing is not a driver of HRD.
The incidence rates of treatment-emergent adverse events most frequently observed with rucaparib in exceptional benefit patients was generally consistent with that of the general ARIEL3 population. Therapy-related secondary myeloid neoplasms, including myelodysplastic syndrome and acute myeloid leukemia, have been observed after PARP inhibitor treatment.21 We identified 9 therapy-related secondary myeloid neoplasms cases among the exceptional benefit patients in the rucaparib arm of ARIEL3, 6 of which were identified during long-term follow-up after treatment discontinuation. While prior reports have suggested that longer duration of PARP inhibitor exposure may be associated with an increased risk of these neoplasms, the trend is confounded by the survival benefit of PARP inhibitor maintenance therapy21 and by prior and subsequent treatment. For example, ARIEL3 patients who developed therapy-related secondary myeloid neoplasms had longer overall exposure both to prior platinum therapies and to PARP inhibitor treatment compared with those who did not develop secondary myeloid neoplasms.22 Additionally, the presence of pre-existing TP53 clonal hematopoiesis mutations has been identified as a risk factor for the development of therapy-related secondary myeloid neoplasms in patients with high-grade ovarian carcinoma receiving rucaparib22; approximately 25% of exceptional benefit patients in ARIEL3 who developed therapy-related secondary myeloid neoplasms had such mutations prior to initiating maintenance treatment.22 Prospective trials investigating the interplay between platinum exposure, PARP inhibitor treatment duration and TP53 clonal hematopoiesis mutations are needed to parse out the contribution of each to the emergence of therapy-related secondary myeloid neoplasms. Clinicians and patients should consider the potential progression-free survival benefits and risks of rucaparib in the context of each patient’s disease status.
A strength of this study is that >60% of the enrolled patients had BRCA–wild-type high-grade ovarian carcinoma, which resulted in greater ability to evaluate additional molecular characteristics associated with exceptional benefit from rucaparib maintenance therapy, including the effects of RAD51C/D mutations and LOH status. These characteristics were not identified in prior studies of exceptional benefit from olaparib maintenance.3 Neither posttreatment tumor samples nor cell-free DNA were collected during ARIEL3. Only archival tissue was available, which was a limitation of our analysis that precluded identification of potential cross-resistance mechanisms, such as BRCA reversion mutations, that may explain why patients in the short-term subgroup had particularly poor outcomes.
These hypothesis-generating post hoc analyses provide additional insight into the relationship between platinum sensitivity, BRCA mutations, and HRD and the durability of response to PARP inhibitor maintenance therapy. Although these data are of interest clinically, prospectively designed studies would be needed to confirm the degree to which these characteristics confer enduring benefit in this setting and to determine which characteristics may be actionable. Further research for the development of tests to determine the methylation status of the BRCA1 and RAD51C promoter, eg, in minimally invasive plasma-derived cell-free DNA, could be useful given the difficulty in obtaining this type of information in the maintenance setting. In addition, evaluation of other types of biomarkers for HRD (eg, phenotypic or functional assays) may provide further insights into the tumor biology of exceptional benefit with PARP inhibitors.23
Rucaparib maintenance can deliver exceptional benefit to a diverse set of patients with high-grade ovarian carcinoma, especially to those with favorable clinical characteristics and those whose cancer shows evidence of HRD, including BRCA1, BRCA2, RAD51C, and RAD51D mutations.
Supplementary Material
Supplementary Fig. 1. Analysis of PFS-PPFI differences in exceptional benefit patients. (A) A schematic showing simplified typical patient clinical history in ARIEL3 and the events that define the PPFI and PFS lengths. (B) Histogram showing the distributions of PFS-PPFI differences in ARIEL3 exceptional benefit patients. PD, progressive disease; PFS, progression-free survival; PPFI, penultimate platinum-free interval.
Supplementary Fig. 2. Frequencies of outcomes in placebo-arm patients with different baseline clinical and molecular characteristics. p values based on chi-square tests; bold denotes significant results (p<0.05). BRCA, BRCA1 or BRCA2; HRR, homologous recombination repair; LOH, loss of heterozygosity; PPFI, penultimate platinum-free interval.
Supplementary Fig. 3. Genetic and epigenetic alterations in exceptional benefit (left) and short-term (right) subgroup patients in the placebo arm. BL, baseline; BR, best response; BRCA, BRCA1 or BRCA2; CT, chemotherapy; HRD, homologous recombination deficiency; HRR, homologous recombination repair; LOH, loss of heterozygosity; plt, platinum; PPFI, penultimate platinum-free interval.
Highlights:
Clinical/molecular characteristics associated with exceptional benefit from rucaparib maintenance in ARIEL3 were explored.
21% of patients in the rucaparib arm derived exceptional benefit (PFS ≥2 years) compared with only 2% in the placebo arm.
Clinical characteristics associated with exceptional outcomes on rucaparib were related to platinum sensitivity.
BRCA1, BRCA2, RAD51C, and RAD51D mutations were associated with exceptional benefit from rucaparib.
A diverse set of patients with high-grade ovarian carcinoma can derive exceptional benefit from rucaparib maintenance.
Acknowledgments
This study was funded by Clovis Oncology, Inc. Data from these analyses have been previously presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting (Kwan et al. J Clin Oncol. 2021;39[suppl 15]:abst 5537) and the 2021 European Society of Gynaecological Oncology (ESGO) Congress (Ledermann et al. Int J Gynecol Cancer. 2021;31[suppl 3]:abst 1). Dr. Aghajanian is supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. Dr. Swisher is supported in part by the NIH grant R01CA237600. Medical writing and editorial support funded by Clovis Oncology, Inc., were provided by Nathan Yardley and Stephen Bublitz of Ashfield MedComms, an Ashfield Health company.
Footnotes
Declaration of competing interest
DMO personal fees from consulting and/or advisory board participation from Clovis Oncology, AbbVie, Agenus, Ambry, Amgen, Arquer Diagnostics, AstraZeneca, Celsion Corp, Corcept Therapeutics, Eisai, Elevar, Genelux, Genentech/Roche, GOG Foundation, Immunogen, Inxmed, Iovance Biotherapeutics, Janssen/Johnson & Johnson, Merck, Mersana, Myriad Genetics, Novartis, Novocure, Regeneron, Roche Diagnostics MSA, Rubis, SeaGen, SDP Oncology (BBI), Takeda, and Tesaro/GlaxoSmithKline, Toray; research funding (all funding to institution) from Clovis Oncology, AbbVie, Agenus, Ajinomoto, Amgen, Array Biopharma, AstraZeneca, Bristol-Myers Squibb, Cerulean Pharma, Eisai, EMD Serono, Ergomed, Genentech/Roche, GenMab, GOG Foundation, Immunogen, INC Research, inVentiv Health Clinical, Iovance Biotherapeutics, Janssen/Johnson & Johnson, Ludwig Institute for Cancer Research, Merck, National Cancer Institute, New Mexico Cancer Care Alliance, Novocure, PRA International, Regeneron, SeaGen, Serono, Stemcentrx, Tesaro/GlaxoSmithKline, Tracon Pharmaceuticals, VentiRx, and Yale University; and has given a presentation on ovarian cancer at the National Comprehensive Cancer Network.
AMO reports grants to his institution from AstraZeneca; has served on steering committees for Clovis Oncology, AstraZeneca (uncompensated), and Tesaro (uncompensated); has served in an advisory role for AstraZeneca and GlaxoSmithKline (uncompensated); and has acted as a principal investigator on investigator-initiated trials with agents from Clovis Oncology, AstraZeneca, and GlaxoSmithKline.
DL reports institutional research funding from Clovis Oncology, AstraZeneca, Corcept Therapeutics, GlaxoSmithKline, Genmab, ImmunoGen, Merck Sharp & Dohme, Novartis, PharmaMar, Roche, and Seagen; consulting fees from Clovis Oncology, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and PharmaMar; payment or honoraria for lectures from Clovis Oncology, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, and Seagen; support for attending meetings and/or travel from AstraZeneca, PharmaMar, and Roche; participation on a data safety monitoring board or advisory board for Clovis Oncology, Agenus, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and Roche; and serves on the board of directors for GCIG and as chair of GCA for ENGOT.
CA has served on a steering committee for AbbVie and Genentech; has served as a principle investigator for studies by Clovis Oncology, AbbVie, AstraZeneca, and Genentech; served on advisory boards for AbbVie, AstraZeneca/Merck, Blueprint Medicine, Eisai/Merck, Mersana Therapeutics, Repare Therapeutics, and Roche/Genentech; and serves on the board of directors (unpaid) for GOG Foundation and NRG Oncology.
AO reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Clovis Oncology, AstraZeneca Farmaceutica Spain, AstraZeneca, Corcept Therapeutics, Deciphera Pharmaceutical, Eisai Europe Limited, EMD Serono, F. Hoffmann-La Roche, GlaxoSmithKline, Got It Consulting, Immunogen, KL Logistics, Medison Pharma, Merck Sharp & Dohme de España, Mersana Therapeutics, Novocure GmbH, PharmaMar, prIME Oncology, Roche Farma, Shattuck Labs, Sutro Biopharma, Tesaro Bio GmbH, Tesaro Bio Spain, and Tesaro; and support for attending meetings and/or travel from AstraZeneca, Roche, and PharmaMar.
AD has served in a consulting or advisory role for Precision Oncology Australia, Shire Pharmaceuticals, and Specialised Therapeutics Australia; and sits on the scientific advisory board for A2A Pharmaceuticals.
NC reports institutional research funding from Clovis Oncology; serving in a consulting or advisory role for Clovis Oncology, AstraZeneca, BIOCAD, Eisai, GlaxoSmithKline, Immunogen, Merck Sharp & Dohme, Mersana, Novartis, Nuvation Bio, Oncxerna, Pfizer, PharmaMar, Roche, and Tesaro; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Clovis Oncology, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, and Novartis; and support for attending meetings and/or travel from AstraZeneca.
JIW has received research support from AstraZeneca.
ARC reports consulting fees for advisory boards for AstraZeneca; payment or honoraria for speakers bureaus for Clovis Oncology and Merck Sharp & Dohme; support for attending meetings and/or travel from Tesaro; and institutional research funding from Clovis Oncology and AstraZeneca.
GS reports royalties or licenses from Merck Sharp & Dohme Italia; consulting fees from Tesaro Bio Italy and Johnson & Johnson; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Clovis Oncology Italy.
AL has served on advisory boards for Clovis Oncology, Ability Pharmaceuticals, AstraZeneca, BIOCAD, GamaMabs, Genmab/Seattle Genetics, GlaxoSmithKline, Gritstone, Merck Serono, Merck Sharp & Dohme, and Tesaro; steering committee for Merck Sharp & Dohme; reports institutional support for clinical trials or academic research from Clovis Oncology, Ability Pharmaceuticals, Agenus, AstraZeneca, Incyte, Inivata, Iovance, Merck Sharp & Dohme, Pfizer, Roche, Sanofi, and Tesaro; and reports boarding and travel expenses for congress activities from Clovis Oncology, AstraZeneca, and Roche.
RWH reports consulting fees from Clovis Oncology, AstraZeneca, and GlaxoSmithKline; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Clovis Oncology, AstraZeneca, and GlaxoSmithKline.
MAG has served on speakers’ bureaus for Clovis Oncology, AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, and PharmaMar; and reports support for attending meetings and/or travel from GlaxoSmithKline.
PCF reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck Sharp & Dohme; support for attending meetings and/or travel from Merck Sharp & Dohme and Pfizer.
JCG reports consulting fees from AstraZeneca, Bristol-Myers Squibb, Eisai, and Merck Sharp & Dohme; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Bristol-Myers Squibb, Ipsen, and Merck Sharp & Dohme Australia; support for attending meetings and/or travel from AstraZeneca; participation on a data safety monitoring board or advisory board for Bristol-Myers Squibb and Janssen; and stock or stock options for ICON Cancer Care, Australia.
EMS reports institutional research funding from Clovis Oncology.
LM, SG, KL, and TK are employees of Clovis Oncology and may own stock or have stock options in that company.
JAL has received lecture fees from Clovis Oncology, AstraZeneca, GlaxoSmithKline, and Pfizer; served on advisory boards for Clovis Oncology, Amgen, Artios Pharma, AstraZeneca, Bristol Myers Squibb, Eisai, Merck/Merck Sharp & Dohme, Nuvation, Pfizer, Regeneron, VBL Therapeutics, and Tesaro/GlaxoSmithKline; and received research grants from AstraZeneca and Merck/Merck Sharp & Dohme.
RLC reports grants or contracts from Clovis Oncology, AstraZeneca, Gateway Foundation, Genelux, Genmab, Janssen, Merck, and Roche/Genentech; consulting fees from Clovis Oncology, Agenus, Alkermes, AstraZeneca, Deciphera, Genelux, Genmab, GlaxoSmithKline, Immunogen, Janssen, OncoQuest, OncXerna, Onxeo, Regeneron, and Roche/Genentech.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949–61. doi: 10.1016/s0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saner FAM, Herschtal A, Nelson BH, et al. Going to extremes: determinants of extraordinary response and survival in patients with cancer. Nat Rev Cancer 2019;19:339–48. doi: 10.1038/s41568-019-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lheureux S, Lai Z, Dougherty BA, et al. Long-term responders on olaparib maintenance in high-grade serous ovarian cancer: clinical and molecular characterization. Clin Cancer Res 2017;23:4086–94. doi: 10.1158/1078-0432.CCR-16-2615. [DOI] [PubMed] [Google Scholar]
- 4.Swisher EM, Kristeleit RS, Oza AM, et al. Characterization of patients with long-term responses to rucaparib treatment in recurrent ovarian cancer. Gynecol Oncol 2021;163:490–7. doi: 10.1016/j.ygyno.2021.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Sun JX, He Y, Sanford E, et al. A computational approach to distinguish somatic vs. germline origin of genomic alterations from deep sequencing of cancer specimens without a matched normal. PLoS Comput Biol 2018;14:e1005965. doi: 10.1371/journal.pcbi.1005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondrashova O, Topp M, Nesic K, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun 2018;9:3970. doi: 10.1038/s41467-018-05564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swisher EM, Kwan TT, Oza AM, et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat Commun 2021;12:2487. doi: 10.1038/s41467-021-22582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Cancer Genome Atlas (TCGA) Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed AA, Etemadmoghadam D, Temple J, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaldawy A, Segev Y, Lavie O, et al. Low-grade serous ovarian cancer: a review. Gynecol Oncol 2016;143:433–8. doi: 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- 11.Shen J, Peng Y, Wei L, et al. ARID1A deficiency impairs the dna damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov 2015;5:752–67. doi: 10.1158/2159-8290.CD-14-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garsed DW, Alsop K, Fereday S, et al. Homologous recombination DNA Repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian cancer. Clin Cancer Res 2018;24:569–80. doi: 10.1158/1078-0432.CCR-17-1621. [DOI] [PubMed] [Google Scholar]
- 13.Gorski JW, Ueland FR, Kolesar JM. CCNE1 amplification as a predictive biomarker of chemotherapy resistance in epithelial ovarian cancer. Diagnostics (Basel) 2020;10. doi: 10.3390/diagnostics10050279. [DOI] [PMC free article] [PubMed]
- 14.Dean A, Oza AM, Lorusso D, et al. Timing of adverse events during maintenance treatment with rucaparib for recurrent ovarian cancer in the phase III ARIEL3 study. Ann Oncol 2020;31:abst 821P. doi: 10.1016/j.annonc.2020.08.960. [DOI] [Google Scholar]
- 15.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov 2015;5:1137–54. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dal Molin GZ, Omatsu K, Sood AK, Coleman RL. Rucaparib in ovarian cancer: an update on safety, efficacy and place in therapy. Ther Adv Med Oncol 2018;10:1758835918778483. doi: 10.1177/1758835918778483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 2014;20:764–75. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondrashova O, Nguyen M, Shield-Artin K, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov 2017;7:984–98. doi: 10.1158/2159-8290.cd-17-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiss KA, Mick R, O’Hara MH, et al. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol 2021;39:2497–505. doi: 10.1200/JCO.21.00003. [DOI] [PubMed] [Google Scholar]
- 20.Tung NM, Robson ME, Ventz S, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 2020;38:4274–82. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 21.Morice P-M, Leary A, Dolladille C, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol 2021;8:e122–e34. doi: 10.1016/S2352-3026(20)30360-4. [DOI] [PubMed] [Google Scholar]
- 22.Kwan TT, Oza AM, Tinker AV, et al. Preexisting TP53-variant clonal hematopoiesis and risk of secondary myeloid neoplasms in patients with high-grade ovarian cancer treated with rucaparib. JAMA Oncol 2021;7:1772–81. doi: 10.1001/jamaoncol.2021.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoppe MM, Sundar R, Tan DSP, Jeyasekharan AD. Biomarkers for Homologous recombination deficiency in cancer. J Natl Cancer Inst 2018;110:704–13. doi: 10.1093/jnci/djy085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Analysis of PFS-PPFI differences in exceptional benefit patients. (A) A schematic showing simplified typical patient clinical history in ARIEL3 and the events that define the PPFI and PFS lengths. (B) Histogram showing the distributions of PFS-PPFI differences in ARIEL3 exceptional benefit patients. PD, progressive disease; PFS, progression-free survival; PPFI, penultimate platinum-free interval.
Supplementary Fig. 2. Frequencies of outcomes in placebo-arm patients with different baseline clinical and molecular characteristics. p values based on chi-square tests; bold denotes significant results (p<0.05). BRCA, BRCA1 or BRCA2; HRR, homologous recombination repair; LOH, loss of heterozygosity; PPFI, penultimate platinum-free interval.
Supplementary Fig. 3. Genetic and epigenetic alterations in exceptional benefit (left) and short-term (right) subgroup patients in the placebo arm. BL, baseline; BR, best response; BRCA, BRCA1 or BRCA2; CT, chemotherapy; HRD, homologous recombination deficiency; HRR, homologous recombination repair; LOH, loss of heterozygosity; plt, platinum; PPFI, penultimate platinum-free interval.