Abstract
Background and Aim:
Joint pain afflicts millions of adults worldwide. The effect of a bone morphogenetic protein complex on joint pain is assessed in this study.
Methods:
We compared the impact of a dietary supplement protein complex (Cyplexinol®) and placebo in 18 men and women (aged 43 ± 10 years) with self-reported joint pain. Subjects were randomly assigned to each condition, consumed twice daily for 14 days (900 mg/day). Subjects completed questionnaires (e.g., Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and subjective pain using a visual analog scale [VAS]) at the start and end of each treatment phase. Blood samples were analyzed for bone morphogenic protein (BMP), alkaline phosphatase, and cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-6, IL-10, IL-1β, and TGF-β). Blood was also collected on days 1 and 15 to determine the acute impact of treatment on these measures.
Results:
Pain and discomfort scores improved (P ≤ 0.05) for subjects following use of Cyplexinol® but not placebo. Improvements were noted for WOMAC pain (P = 0.05), stiffness (P = 0.039), and total pain (P = 0.026), as well as VAS pain (P = 0.015), recreational activity interference (P = 0.023), mood interference (P = 0.012), and total pain (P = 0.024). A trend was noted for WOMAC physical function (P = 0.052). An approximate 50% increase in BMP5 was noted following Cyplexinol® (P = 0.01), with a similar increase noted for placebo (P = 0.022). A near doubling in TGF-β (P = 0.001) was noted for Cyplexinol®. No other changes of significance were noted across time, nor were any differences noted in cytokines following acute intake of the conditions (P > 0.05).
Conclusions:
Cyplexinol® can alleviate joint pain in middle-aged men and women, while elevating BMP5 and TGF-β. Cyplexinol® does not influence cytokines, at least within a short 2-week supplementation period or within the 2-h post-ingestion period.
Relevance for Patients:
Individuals suffering with joint pain in the knee and/or hip may benefit from daily use of Cyplexinol®, as we observed decreased pain and stiffness following treatment.
Keywords: Bone morphogenic protein, Cyplexinol®, Cytokines, Joint pain
1. Introduction
Chronic inflammation routinely leads to joint pain, which is a common problem among men and women, with millions of adults suffering from chronic pain, including those with and without arthritis [1]. In fact, it is estimated that approximately 17.5% of American adults report joint pain not associated with arthritis [2]. With the high prevalence of joint pain among adults in the United States, it is not surprising that a variety of options are available for treatment.
Specific to this, dietary supplements are routinely used for joint pain reduction [3], with multiple mechanisms of action noted [4]. Those that are most popular and with evidence for effect include methylsulfonylmethane [5-7] and the combination or isolated use of glucosamine and chondroitin [8-10]. Methylsulfonylmethane inhibits nuclear factor kappa B resulting in down regulation of mRNA (interleukin [IL]-1, IL-6, and tumor necrosis factor [TNF]-α) as well as decreases in cytokine levels of IL-1 and TNFα. Glucosamine and chondroitin sulfate are thought to promote hyaluronic acid and proteoglycan synthesis important for joint health [9]. In addition, glucosamine degrades liposomal enzymes and chondroitin sulfate inhibits proteolytic enzyme and nitric oxide synthesis.
Cyplexinol® (a Bone Morphogenetic Protein [BMP] Complex) is another agent with potential effects. BMPs are growth factors that have been shown to activate mesenchymal stem cells to help the body regenerate osteoblasts and chondrocytes [11,12]. BMPs were initially identified in the 1970s as osteogenic factors that stimulate activation, proliferation, and differentiation of osteoprogenitor cells by binding BMP receptors and subsequent signaling through the SMAD pathway [13,14]. BMPs have also been shown to reduce inflammation and promote healthy inflammatory signaling in joints and other tissues.
Initial studies have been conducted to evaluate the safety and efficacy of Cyplexinol® as a dietary supplement for joint health [15-18]. In these studies, Cyplexinol® has been compared to a placebo alone or in combination with glucosamine/chondroitin or Boswellia serrata resin in randomized controlled trials lasting 4–12 weeks, with positive findings related to reductions in joint pain.
Anecdotal reports from additional users indicate that joint pain is relieved within as little as a few days of use of Cyplexinol®. However, very few laboratory data are available with regards to the alleviation of joint pain (or the measurement of BMPs), in particular following a short time-course of treatment. In addition, no acute studies have evaluated the impact of Cyplexinol® on cytokine concentrations during the acute post-ingestion period. Therefore, we determined the impact of Cyplexinol® on joint pain and associated measures over a period of 14 days, in comparison with a placebo, using a design with a 13-day wash-out period. In addition, we measured cytokine production, alkaline phosphatase, and BMPs that have previously been associated with inflammation and bone health [19,20]. We hypothesized that perceived pain and discomfort would be reduced when subjects used Cyplexinol®, with a possible alteration in BMPs and cytokines over the same time.
2. Materials and Methods
Eighteen subjects were recruited, provided informed consent, and completed the study. Subjects were between the ages of 30 and 60 years, non-tobacco users, with a BMI between 18 and 40 kg/m2, with self-reported joint pain at a minimum rating of 3/10 for at least the 30 days before study enrollment. Subjects did not have a rheumatic or osteoarthritic diagnosis and engaged in exercise at least 2 days/week for the past 6 months or longer. Therefore, they were considered to be recreationally active. Subjects were required to be non-users of anti-inflammatory medicines, pain medications, or dietary supplements (or willing to cease for 1-month before participation and throughout the study). Therefore, we are confident that other potential products did not interfere with the findings of the assigned condition. Women were not pregnant. Subjects were compensated $200 for their full participation. Subject descriptive characteristics are presented in Table 1. All procedures were approved by the University of Memphis Institutional Review Board for Human Subjects Research (protocol PRO-FY2021-6), and the study was registered through clinicaltrials.gov.
Table 1. Baseline subject characteristics (N = 18).
| Characteristics | Values (mean (SD) or n (%)) |
|---|---|
| Age (yrs) | 43.5 (10.3) |
| Gender, n (%) | |
| Male | 5 (27.8) |
| Female | 13 (72.2) |
| Height (cm) | 170.6 (9.7) |
| Weight (kg) | 86.4 (20.2) |
| BMI (kg/m2) | 29.6 (5.8) |
| Waist (cm) | 97.0 (18.3) |
| Hip (cm) | 112.5 (15.3) |
| Waist/Hip | 0.86 (0.08) |
| Systolic Blood Pressure (mm Hg) | 118.2 (10.5) |
| Diastolic Blood Pressure (mm Hg) | 75.3 (12.0) |
| Heart Rate (bpm) | 73.9 (9.1) |
| Exercise (min/week) | 291.9 (277.2) |
| Joint Health (WOMAC) | |
| WOMAC Pain | 7.1 (3.1) |
| WOMAC Stiffness | 3.3 (1.6) |
| WOMAC Physical Function | 21.1 (12.1) |
| WOMAC Total | 31.5 (15.5) |
| VAS Pain (100 mm scale) | |
| VAS Joint Pain | 65.3 (21.9) |
| VAS Work Interference | 45.0 (28.8) |
| VAS Recreational Activity Interference | 63.7 (22.0) |
| VAS Mood Interference | 53.0 (25.5) |
| VAS Total Average | 56.7 (20.7) |
Subjects were required to be non-users of anti-inflammatory medicines, pain medications, or dietary supplements that may have impacted the outcome measures
During the first laboratory visit, subjects completed the informed consent form, health history, medication and dietary supplement usage, physical activity, and joint pain questionnaires. The heart rate, blood pressure, height, weight, and waist and hip circumferences of each subject were measured. Female subjects were provided with a urine pregnancy test kit (AccuMed®, Houston, Texas, USA), escorted to a private restroom (within the lab), and asked to perform the test. The result was then confidentially confirmed by the investigators. Eligible subjects were scheduled for testing visits after all screening was completed.
Subjects reported to the laboratory approximately every 2 weeks for a total of four laboratory visits (2 weeks on, 2 weeks off [washout], and 2 weeks on). They were randomly assigned to either Cyplexinol® (ZyCal Bioceuticals Healthcare Co., Inc., Westborough, MA) or placebo (cellulose) for a period of 14 days. Cyplexinol® is an oral, demineralized bone matrix extract which contains bone morphogenetic proteins and growth factors, within a partially hydrolyzed collagen. The dosage delivered was 900 mg/day, taken in capsule form twice daily (450 mg in the morning and 450 mg in the afternoon/evening). All capsules were produced in accordance with Good Manufacturing Practices. Capsules were of similar appearance and provided to investigators in unidentified bottles labeled A or B for double-blinding.
2.1. Evaluation
On day one of each condition, subjects had their resting blood pressure and heart rate measured using an automated unit (OMRON HEM 907XL, OMRON Healthcare, Tokyo, Japan), following a 10-min seated rest period, with the average of duplicate measures recorded at each time. Subjects completed the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), as used previously [21-24], as well as a questionnaire developed to assess joint pain and how this interferes with their lifestyle. The latter questionnaire used a visual analog scale (VAS) that asked subjects to mark on a scale from 0 to 100 how much they disagreed (0) or agreed (100) with each of four statements: (1) In the past 2 weeks, I have experienced significant knee/hip joint pain. (2) In the past 2 weeks, my joint pain has interfered with my work. (3) In the past 2 weeks, my joint pain has interfered with my recreational activities. (4) In the past 2 weeks, my knee/hip joint pain has negatively impacted my mood/attitude. A blood sample was taken at baseline, following a 10-min rest period. Additional blood samples were taken at 1 and 2 h following condition ingestion (on both days 1 and 15). Details for blood analysis are provided below. On day 15 of each condition, subjects reported to lab again and completed the exact testing procedures as described above. A 13-day wash-out period separated each condition assignment, subjects then crossed into the other condition, and the procedures were repeated.
2.2. Blood collection and analysis
Single venipunctures were used to collect blood samples from subjects before ingestion of the assigned condition and at 1 h and 2 h following ingestion during testing visits. Approximately 10 mL samples of blood were collected in vacuum tubes (BD Vacutainer 366430, Franklin Lakes, NJ), processed in a refrigerated centrifuge, and stored in multiple aliquots at −80°C until analyzed for cytokines (TNF-α, IL-6, and IL-1β [Milliplex HCYTA-60K-03 Human Cyto/Chem/GF Panel A, Millipore Sigma, Burlington, MA], IL-10 [Human IL-10 High Sensitivity ELISA BMS215HS, Invitrogen, Vienna, Austria], TGF-β [custom Quantibody Human Cytokine Antibody Array; protocol number 092721 Cust-H7 SA72 Memphis; test procedure SOP-TF-QAH-001, SOP-TF-QAH-003 by Raybiotech, Peachtree Corners, GA], alkaline phosphatase [Alkaline Phosphatase Assay Kit, Colorimetric; ab83369, Abcam, Waltham, MA], and Bone Morphogenetic Protein [BMP2, BMP4, BMP5, BMP6, BMP7, and BMP9] through the Raybiotech custom array described above). Samples collected at 1 and 2 h following acute ingestion of the conditions were analyzed only for TNF-α, IL-6, IL-10, IL-1β, and alkaline phosphatase.
2.3. Physical activity and dietary intake
Subjects followed their usual activity patterns over the course of the study period but refrained from strenuous activity for the 48 h preceding each laboratory test day. Dietary intake was to remain similar over the entire study period. However, subjects consumed the same standard prepackaged meals during the day before each test day. These included three meal replacement drinks (Orgain, Irvine, CA), three food bars (e.g., Clif Builder, Emeryville, CA), 2 – 3 servings of fresh fruit, and two packages of mixed nuts (Emerald, S-L Snacks National; Charlotte, NC. Each subject received an allotment of these items, based on preference. They were then given the same items for each visit; following the same food plan for the days before all four laboratory test days. No other food should have been consumed during the day before each laboratory visit other than what was provided to subjects by the investigators. Subjects were allowed to consume as much water as they preferred during the days before each laboratory test day but should have consumed no other beverages. Subjects recorded all food and drink consumed during the 3 days before each test day. The diet records were analyzed for nutrient content using Food Processor Pro software (Esha Research, Salem, OR).
2.4. Statistical analysis
Descriptive statistics, including means, standard deviations, frequencies, and percentages were calculated for all screening variables. Next, two-way repeated measures ANOVAs were used to determine whether any changes occurred in the outcome variables for nutrition, WOMAC, VAS, and BMP as the result of the interaction between conditions (Cyplexinol® versus placebo) and time (day 1 compared to day 15). In the event of significant main effects or interactions, planned pairwise comparisons were made to identify differences among mean value time points. Separate pairwise comparisons were conducted using repeated measures t-tests to evaluate univariate differences from day 1 to day 15 for the outcome variables for Cyplexinol® condition and placebo condition, respectively. Three-way repeated measures ANOVAs were used to determine whether any changes occurred in the outcome variables for blood pressure, heart rate, alkaline phosphatase, and cytokines as the result of the interaction between conditions (Cyplexinol® versus placebo) and time (day 1 compared to day 15) and hour (0, 1, or 2). If interactions existed, follow-up analyses were conducted on simple main effects and comparisons. The significance level for all statistical tests was set at P < 0.05. Statistical analyses were completed using the SPSS software (Version 26.0, IBM, Inc. Chicago, IL). Data are presented as mean ± SD unless otherwise noted.
3. Results
Eighteen subjects completed both visits for each of the two test conditions, as seen in Figure 1. Of the 18 subjects, five were men and 13 were women. Two subjects withdrew from the research study and were replaced. No adverse events were noted, and subjects appeared to tolerate the treatment well. The screening data indicated that subjects experienced regular pain and discomfort in various joints, which interfered with their work, recreational activities, and mood (Table 1). With the exception of vitamin A (P = 0.019), no dietary variable was different between conditions or between days (day 1 vs. day 15) for any variable (P > 0.05). Dietary data are provided in Table 2. As expected, no significant effects were noted for heart rate, systolic blood pressure, or diastolic blood pressure (P > 0.05). These data are presented in Table 3.
Figure 1. CONSORT flow diagram.
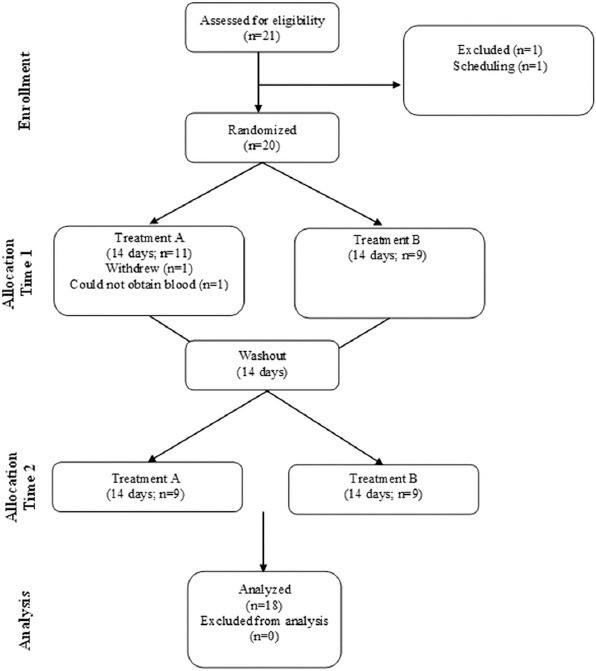
Table 2. Multivariate nutrition outcomes for Day 1 compared to Day 15 for Cyplexinol® versus placebo.
| Outcome measurement | Cyplexinol® (mean ± SD) | Placebo (mean ± SD) | P-value |
|---|---|---|---|
| Calories | 0.189 | ||
| Day 1 | 5575.88 ± 1807.96 | 5435.23 ± 1937.09 | |
| Day 15 | 5457.21 ± 1931.22 | 5854.41 ± 2004.71 | |
| Protein (g) | 0.260 | ||
| Day 1 | 297.04 ± 98.87 | 296.63 ± 74.95 | |
| Day 15 | 276.83 ± 89.48 | 303.59 ± 95.26 | |
| Carbohydrates (g) | 0.281 | ||
| Day 1 | 639.18 ± 283.57 | 585.28 ± 264.31 | |
| Day 15 | 618.83 ± 279.87 | 640.56 ± 300.74 | |
| Fat (g) | |||
| Day 1 | 220.59 ± 78.19 | 216.14 ± 100.08 | |
| Day 15 | 222.34 ± 99.62 | 234.67 ± 78.73 | 0.455 |
| Vitamin C (mg) | 0.741 | ||
| Day 1 | 466.90 ± 179.78 | 399.09 ± 152.49 | |
| Day 15 | 434.01 ± 197.55 | 380.83 ± 139.31 | |
| Vitamin A (RE) | 0.019* | ||
| Day 1 | 25299.72 ± 18070.33 | 14680.55 ± 5718.91 | |
| Day 15 | 19751.92 ± 12489.25 | 23067.14 ± 17563.48 | |
| Vitamin B6 (mg) | 0.146 | ||
| Day 1 | 5.44 ± 2.69 | 4.77 ± 1.86 | |
| Day 15 | 4.73 ± 2.33 | 5.21 ± 1.70 | |
| Vitamin B12 (mg) | 0.278 | ||
| Day 1 | 12.46 ± 8.77 | 11.10 ± 4.92 | |
| Day 15 | 10.98 ± 6.18 | 12.16 ± 7.66 | |
| Vitamin D (IU) | 0.391 | ||
| Day 1 | 514.39 ± 657.59 | 443.73 ± 251.41 | |
| Day 15 | 407.16 ± 377.11 | 448.64 ± 339.37 |
P < 0.05. Values are the total of the three days before each test day
Table 3. Descriptive statistics for blood pressure and heart rate before and after 14 days of treatment with Cyplexinol® and placebo (N=18).
| Condition/ Day/Time | Systolic blood pressure (mm Hg) | Diastolic blood pressure (mm Hg) | Heart rate (bpm) |
|---|---|---|---|
| Cyplexinol®, Day 1, Hour 0 | 114.83 (10.25) | 76.33 (9.44) | 69.17 (9.14) |
| Cyplexinol®, Day 1, Hour 1 | 114.89 (9.98) | 75.72 (10.14) | 66.67 (7.90) |
| Cyplexinol®, Day 1, Hour 2 | 118.50 (13.39) | 76.78 (10.43) | 64.00 (9.41) |
| Cyplexinol®, Day 15, Hour 0 | 116.11 (11.95) | 76.94 (10.75) | 69.28 (10.31) |
| Cyplexinol®, Day 15, Hour 1 | 113.17 (10.60) | 74.44 (11.36) | 65.67 (9.78) |
| Cyplexinol®, Day 15, Hour 2 | 115.06 (11.47) | 77.50 (10.19) | 66.06 (11.56) |
| Placebo, Day 1, Hour 0 | 115.44 (10.59) | 75.83 (9.30) | 70.67 (10.19) |
| Placebo, Day 1, Hour 1 | 113.94 (10.58) | 75.61 (10.74) | 64.11 (10.37) |
| Placebo, Day 1, Hour 2 | 116.50 (12.83) | 77.22 (10.66) | 65.72 (11.70) |
| Placebo, Day 15, Hour 0 | 116.22 (11.41) | 78.33 (8.67) | 69.61 (10.57) |
| Placebo, Day 15, Hour 1 | 116.72 (13.22) | 74.94 (10.16) | 64.11 (11.35) |
| Placebo, Day 15, Hour 2 | 118.94 (11.59) | 76.33 (10.49) | 64.17 (12.97) |
Values are mean ± (SD)
3.1. WOMAC
No condition or condition by time interaction effects were noted for any variable (p > 0.05) on the WOMAC. However, time effects were noted as follows: The main effect of time on WOMAC stiffness score was statistically significant (F (1, 16) = 5.30, P = 0.035). The Bonferroni-adjusted paired t-test revealed that pairwise differences on WOMAC stiffness score were statistically significant, with the WOMAC stiffness score being reduced by 0.676 between day 1 (M = 3.27) and day 15 (M = 2.59) (P = 0.035) for all participants. The main effect of time on WOMAC physical function score was statistically significant (F (1, 17) = 7.67, P = 0.013). The Bonferroni-adjusted paired t-test revealed that pairwise differences on WOMAC physical function score were statistically significant, with the WOMAC physical function score being reduced by 3.74 between day 1 (M = 20.81) and day 15 (M = 17.07) (P = 0.013) for all participants. The effect of time on the WOMAC total score was statistically significant (F (1, 16) = 10.90, p = 0.005). The Bonferroni-adjusted paired t-test revealed that pairwise differences on WOMAC total scores were statistically significant, with the WOMAC total score being reduced by 6.18 between day 1 (M = 30.65) and day 15 (M = 24.47) (P = 0.005).
In addition, multiple differences were noted between day 1 and day 15 when subjects were assigned to Cyplexinol®. Specifically, improvements were noted for pain (P = 0.05; 22% reduction; effect size = 0.498), stiffness (P = 0.039; 27% reduction; effect size = 0.546), and total (P = 0.026; 23% reduction; effect size = 0.595). A trend was noted for physical function (P = 0.052; 22% reduction; effect size = 0.493). No variable was improved in a statistically significant manner for placebo; however, a trend was noted for physical function (P = 0.074), with values improving approximately 16%. WOMAC data are presented in Table 4 (for Cyplexinol®) and Table 5 (for placebo).
Table 4. WOMAC and VAS outcomes for repeated measures t-test for Cyplexinol® (Day 1 vs. Day 15).
| Outcome measurement | Mean ± SD | P-value | Effect size |
|---|---|---|---|
| WOMAC pain | 0.050* | 0.498 | |
| Day 1 | 6.69 ± 3.80 | ||
| Day 15 | 5.19 ± 4.14 | ||
| WOMAC Stiffness | 0.039* | 0.546 | |
| Day 1 | 3.29 ± 1.76 | ||
| Day 15 | 2.41 ± 1.97 | ||
| WOMAC Physical Function | 0.052 | 0.493 | |
| Day 1 | 21.50 ± 14.11 | ||
| Day 15 | 17.31 ± 13.58 | ||
| WOMAC Total | 0.026* | 0.595 | |
| Day 1 | 31.59 ± 19.50 | ||
| Day 15 | 24.41 ± 19.28 | ||
| VAS1 Joint Pain | 0.015* | 0.637 | |
| Day 1 | 63.68 ± 15.44 | ||
| Day 15 | 48.36 ± 23.05 | ||
| VAS2 Work Interference | 0.413 | 0.198 | |
| Day 1 | 44.54 ± 27.39 | ||
| Day 15 | 39.10 ± 25.99 | ||
| VAS3 Recreational Activity Interference | 0.023* | 0.589 | |
| Day 1 | 62.89 ± 22.77 | ||
| Day 15 | 48.93 ± 24.96 | ||
| VAS4 Mood Interference | 0.012* | 0.667 | |
| Day 1 | 57.92 ± 18.69 | ||
| Day 15 | 40.85 ± 23.32 | ||
| VAS Total | 0.024* | 0.586 | |
| Day 1 | 229.02 ± 69.04 | ||
| Day 15 | 177.24 ± 91.86 |
P ≤ 0.05. WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index, VAS: Visual analog scale
Table 5. WOMAC and VAS outcomes for repeated measures t-test for placebo (Day 1 vs. Day 15).
| Outcome measurement | Mean ± SD | P-value | Effect size |
|---|---|---|---|
| WOMAC Pain | 0.636 | 0.114 | |
| Day 1 | 6.22 ± 3.75 | ||
| Day 15 | 5.94 ± 3.83 | ||
| WOMAC Stiffness | 0.562 | 0.139 | |
| Day 1 | 3.17 ± 1.72 | ||
| Day 15 | 2.89 ± 1.94 | ||
| WOMAC Physical Function | 0.074 | 0.449 | |
| Day 1 | 20.11 ± 13.00 | ||
| Day 15 | 16.83 ± 13.63 | ||
| WOMAC Total | 0.144 | 0.361 | |
| Day 1 | 29.50 ± 17.30 | ||
| Average Days 2-15 | 25.67 ± 18.95 | ||
| VAS1 Joint Pain | 0.252 | 0.409 | |
| Day 1 | 58.38 ± 28.11 | ||
| Day 15 | 52.54 ± 26.21 | ||
| VAS2 Work Interference | 0.456 | 0.280 | |
| Day 1 | 45.76 ± 29.62 | ||
| Day 15 | 40.83 ± 28.24 | ||
| VAS3 Recreational Activity Interference | 0.720 | 0.345 | |
| Day 1 | 56.76 ± 30.33 | ||
| Day 15 | 54.53 ± 31.78 | ||
| VAS4 Mood Interference | 0.793 | 0.063 | |
| Day 1 | 50.92 ± 30.13 | ||
| Day 15 | 49.60 ± 26.17 | ||
| VAS Total | 0.475 | 0.172 | |
| Day 1 | 211.82 ± 114.09 | ||
| Day 15 | 197.50 ± 104.20 |
*P ≤ 0.05. WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index, VAS: Visual analog scale
3.2. VAS
For the VAS variables (Joint Pain, Work Interference, Recreational Activity Interference, Mood Interference, and Total), no significant condition or condition by time interactions were noted (P > 0.05). However, time effects were noted as follows: The effect of time on the VAS joint pain score was statistically significant (F (1, 17) = 8.23, P = 0.011). The Bonferroni-adjusted paired t-test revealed that pairwise difference on VAS joint pain score was statistically significant, with the VAS joint pain score being reduced by 10.57 between day 1 (M = 61.03) and day 15 (M = 50.45) (P = 0.011). The effect of time on the VAS mood interference score was statistically significant (F (1, 17) = 7.42, P = 0.014). The Bonferroni-adjusted paired t-test revealed that pairwise difference on VAS Mood Interference score was statistically significant, with the VAS Mood Interference score being reduced by 9.19 between day 1 (M = 54.42) and day 15 (M = 45.22) (P = 0.014). The effect of time on the VAS total average score was statistically significant (F (1, 17) = 7.295, P = 0.015). The Bonferroni-adjusted paired t-test revealed that pairwise difference on VAS total average score was statistically significant, with the VAS total average score being reduced by 33.05 between day 1 (M = 220.42) and day 15 (M = 187.37) (P = 0.015).
In addition, multiple differences were noted between day 1 and day 15 when subjects were assigned to Cyplexinol®. Specifically, improvements were noted for pain (P = 0.015; 24% reduction; effect size = 0.637), recreational activity interference (P = 0.023; 22% reduction; effect size = 0.589), mood interference (P = 0.012; 29% reduction; effect size = 0.667), and total (P = 0.024; 23% reduction; effect size = 0.586). No variable significantly improved for placebo. The VAS data are presented in Table 4 (for Cyplexinol®) and Table 5 (for placebo).
3.3. Biochemical outcomes
Two subjects were removed from the BMP analysis due to unusually elevated values (e.g., BMP5 values were close to 280,000 pg/mL, while other subjects were less than 10,000 pg/mL). No condition or time effects were noted for BMPs (P > 0.05). An approximate 50% increase in BMP5 was noted following Cyplexinol® treatment (P = 0.01), while a similar increase was noted for placebo (P = 0.022). A trend for increase in BMP6 (15%; P = 0.079) was noted for Cyplexinol®. Data for BMPs are presented in Table 6 (for Cyplexinol®) and Table 7 (for placebo).
Table 6. BMP outcomes for repeated measures t-test for Cyplexinol® (Day 1 vs. Day 15).
| Outcome measurement | Mean ± SD | P-value | Effect size |
|---|---|---|---|
| BMP2 (pg/mL) | 0.215 | 0.203 | |
| Day 1 | 16.14 ± 13.15 | ||
| Day 15 | 18.31 ± 12.80 | ||
| BMP5 (pg/mL) | 0.010* | 0.654 | |
| Day 1 | 2861.37 ± 2665.21 | ||
| Day 15 | 4376.02 ± 2814.02 | ||
| BMP6 (pg/mL) | 0.079 | 0.372 | |
| Day 1 | 221.13 ± 148.22 | ||
| Day 15 | 255.71 ± 168.67 | ||
| BMP7 (pg/mL) | 0.148 | 0.271 | |
| Day 1 | 3515.08 ± 4083.14 | ||
| Day 15 | 4422.65 ± 4711.48 | ||
| BMP9 (pg/mL) | 0.112 | 0.317 | |
| Day 1 | 6.68 ± 6.81 | ||
| Day 15 | 5.05 ± 3.38 | ||
| TGFb1 (pg/mL) | 0.001* | 1.09 | |
| Day 1 | 7692.55 ± 6753.52 | ||
| Day 15 | 14675.23 ± 8055.54 |
P < 0.05
Table 7. BMP outcomes for repeated measures t-test for placebo (Day 1 vs. Day 15).
| Outcome measurement | Mean ± SD | P-value | Effect size |
|---|---|---|---|
| BMP2 (pg/mL) | 0.421 | 0.051 | |
| Day 1 | 21.04 ± 14.07 | ||
| Day 15 | 21.79 ± 20.89 | ||
| BMP5 (pg/mL) | 0.022* | 0.551 | |
| Day 1 | 3687.57 ± 3804.56 | ||
| Day 15 | 5854.46 ± 2779.40 | ||
| BMP6 (pg/mL) | 0.197 | 0.220 | |
| Day 1 | 246.95 ± 181.51 | ||
| Day 15 | 287.56 ± 240.37 | ||
| BMP7 (pg/mL) | 0.143 | 0.276 | |
| Day 1 | 5434.12 ± 6679.55 | ||
| Day 15 | 6524.68 ± 7745.08 | ||
| BMP9 (pg/mL) | 0.102 | 0.332 | |
| Day 1 | 7.87 ± 8.61 | ||
| Day 15 | 5.90 ± 4.90 | ||
| TGFb1 (pg/mL) | 0.162 | 0.255 | |
| Day 1 | 10678.28 ± 7182.89 | ||
| Day 15 | 12572.98 ± 8248.81 |
P < 0.05
With regard to TGF-β, a significant treatment by time interaction was noted (F (1, 15) = 5.695, P = 0.031). Therefore, simple main effects were run. For Cyplexinol®), there was a significant difference between day 1 (M = 7692.55, SE = 1688.38) and day 15 (M = 14675.23, SE = 2013.88) (P < 0.001). There was not a significant difference between day 1 and day 15 for the placebo (P = 0.162). Data are presented in Table 6 (for Cyplexinol®) and Table 7 (for placebo).
For the alkaline phosphatase and cytokines (Table 8), which were measured before and at 1 and 2 h post ingestion of Cyplexinol® and placebo on days 1 and 15, findings are as follows: For alkaline phosphatase, there was no statistically significant three-way interaction between condition, day, and hour (F (2, 28) = 0.554, P = 0.581). In addition, no statistically significant two-way interactions were found for condition by day (F (1, 14) = 0.278, P = 0.606); condition by hour (F (2, 28) = 2.643, P = 0.118); day by hour (F (2, 28) = 1.318, P = 0.280). Mauchly’s test of sphericity showed that the assumption was not met for condition by hour (P = 0.001) and day by hour (P = 0.035), so the Greenhouse–Geisser degrees of freedom correction were used when interpreting those two-way interactions.
Table 8. Descriptive statistics for alkaline phosphatase and cytokines.
| Condition/day/time | Alkaline phosphatase (μM) | TNF-α (pg/mL) | IL-10 (pg/mL) | IL-6 (pg/mL) | IL-1β (pg/mL) |
|---|---|---|---|---|---|
| Cyplexinol®, Day 1, Hour 0 | 393.20 (177.90) | 22.46 (10.65) | 1.17 (0.57) | 2.06 (0.83) | 4.24 (3.06) |
| Cyplexinol®, Day 1, Hour 1 | 384.80 (171.28) | 22.96 (13.02) | 1.22 (0.61) | 1.86 (0.77) | 4.30 (3.18) |
| Cyplexinol®, Day 1, Hour 2 | 388.53 (176.53) | 21.40 (10.76) | 0.97 (0.58) | 1.62 (0.69) | 4.01 (3.28) |
| Cyplexinol®, Day 15, Hour 0 | 422.47 (241.51) | 20.90 (10.80) | 1.17 (0.60) | 1.89 (0.50) | 3.97 (2.72) |
| Cyplexinol®, Day 15, Hour 1 | 407.93 (201.83) | 20.79 (9.62) | 1.68 (0.59) | 1.64 (0.60) | 4.07 (3.26) |
| Cyplexinol®, Day 15, Hour 2 | 398.53 (185.60) | 21.34 (10.66) | 1.10 (0.49) | 1.52 (0.60) | 4.51 (3.50) |
| Placebo, Day 1, Hour 0 | 383.40 (167.41) | 21.57 (9.77) | 1.24 (0.56) | 1.96 (0.74) | 4.54 (3.65) |
| Placebo, Day 1, Hour 1 | 382.00 (161.21) | 21.29 (9.97) | 1.08 (0.59) | 1.78 (0.58) | 4.73 (3.71) |
| Placebo, Day 1, Hour 2 | 391.67 (171.23) | 21.77 (9.52) | 1.16 (0.77) | 1.78 (0.74) | 4.82 (3.88) |
| Placebo, Day 15, Hour 0 | 388.20 (167.94) | 21.22 (10.33) | 1.42 (0.99) | 1.94 (0.83) | 4.22 (3.57) |
| Placebo, Day 15, Hour 1 | 384.27 (164.62) | 21.32 (9.56) | 1.16 (0.78) | 1.82 (0.69) | 4.20 (3.09) |
| Placebo, Day 15, Hour 2 | 396.13 (182.47) | 20.83 (8.24) | 1.20 (0.94) | 1.82 (0.89) | 4.34 (3.06) |
Values are mean ± (SD)
For TNF-α, there was no statistically significant three-way interaction between condition, day, and hour (F (2, 30) = 0.179, P = 0.837). In addition, no statistically significant two-way interactions were found for treatment by week (F (1, 15) = 0.726, P = 0.407); treatment by hour (F (2, 30) = 0.385, p = 0.684); and week by hour (F (2, 30) = 2.308, P = 0.244). Mauchly’s test of sphericity showed that the assumption was met for all two-way interactions, except week by hour. The Greenhouse–Geisser degrees of freedom correction were used to interpret results for that interaction.
For IL-10, there was no statistically significant three-way interaction found between condition, day, and hour (F (2, 30) = 0.924, P = 0.408). In addition, no statistically significant two-way interactions were found for treatment by week (F (1, 15) = 0.076, P = 0.786); treatment by hour (F (2, 30) = 2.154, P = 0.134); and week by hour (F (2, 30) = 0.233, P = 0.794). Mauchly’s test of sphericity showed that the assumption was met for all two-way interactions.
For IL-6, no statistically significant three-way interaction between condition, day, and hour was found (F (2, 30) = 0.179, P = 0.837). In addition, no statistically significant two-way interactions were found for treatment by week (F (1, 15) = 2.335, P = 0.147); treatment by hour (F (2, 30) = 2.180, P = 0.131); and week by hour (F (2, 30) = 0.213, P = 0.809). Mauchly’s test of sphericity showed that the assumption was met for all two-way interactions.
For IL-1β, no statistically significant three-way interaction between condition, day, and hour was found (F (2, 28) = 2.44, P = 0.105). In addition, no statistically significant two-way interactions were found for treatment by week (F (1, 14) = 0.927, P = 0.352); treatment by hour (F (2, 28) = 0.016, P = 0.985); and week by hour (F (2, 28) = 2.074, P = 0.145). Mauchly’s test of sphericity showed that the assumption was met for all two-way interactions.
4. Discussion
The present study determined the effect of the dietary supplement Cyplexinol® on the alleviation of joint pain in men and women. Results demonstrated a moderate to large effect for Cyplexinol® to reduce joint pain and stiffness, as well as to improve mood and allow for a reduction in recreational activity interference. An increase was observed in BMP5, but no additional biochemical measures were affected by treatment with Cyplexinol®. These findings are specific to a sample of middle-aged men and women who were supplemented with Cyplexinol® for a period of 14 days at a dosage of 900 mg/day. Additional longer-term studies are needed to more fully understand the benefit of supplemental Cyplexinol® to reduce joint pain and impact related biochemical measures.
Our findings for a reduction in joint pain agree with prior studies of Cyplexinol®. For example, in a non-controlled study, 44 subjects ages 55 or older with self-reported osteoarthritis in the hip, knee, or ankle received 150 mg of Cyplexinol® once daily for 4 weeks and reported decreased pain intensity (45%) and frequency (55%), as well as increased activity and strength [17]. Favorable effects of treatment were also noted in an open label study of 25 subjects diagnosed with moderate-to-severe osteoarthritis in the hip or knee and provided 150 mg of Cyplexinol® with 1500 mg of glucosamine hydrogen chloride and 1200 mg of chondroitin sulfate daily. Subjects reported reductions in pain and pain frequency over a 4 week period, with overall pain reduced by 54.7% and frequency of pain reduced 58.8% [15]. In a separate study with a subject population over the age of 55 with self-reported osteoarthritis, 34 subjects who ingested 135 mg of 2-Beta Coxatene (containing Cyplexinol® and Boswellia serrata resin) daily for 3 months reported improved WOMAC scores [18].
BMPs are members of the TGF-β superfamily that regulate articular cartilage through multiple mechanisms, including remodeling of the extracellular matrix, chondrocyte proliferation, hypertrophy-like differentiation, and countering inflammatory signals including IL-1 and IL-6 [25]. Osteoarthritis occurs when these processes are altered due to inflammation and aging. Increases in circulating IL-1, IL-6, and TNF-α are associated with osteoarthritis and rheumatoid arthritis [26-29]. Multiple BMPs are involved in signaling within cartilage and have been identified as potential therapeutic targets for cartilage regeneration following damage due to inflammation or injury, namely, BMP2/4/6/7/9 [25,30]. Mouse models have also previously found that BMP5 contributes to both osteoblast and chondrocyte signaling pathways [13].
The use of natural BMPs, recombinant BMPs, and viral vectors encoding for BMP genes have been studied in a variety of cells and osteochondral defect models [30]. A study using a rabbit articular cartilage defect model found that long-term treatments (8 weeks) with rhBMP2 in a heparin-conjugated fibrin carrier resulted in regeneration of hyaline-like cartilage [31]. Knee injections of BMP7 led to increased cartilage and IL-10 and decreases to IL-1β in rats with zymosan-induced arthritis [32] and delayed cartilage degeneration in rats undergoing strenuous running [33]. Similarly, experimental knee injuries in sheep treated with 340 μg of rhBMP7 protein in a collagen particle carrier immediately following or 3 weeks after injury were significantly improved over the controls [34]. In a separate study, full-thickness articular cartilage defects were treated with various conditions including rhBMP7, microfracture (a treatment that forms a fibrocartilage), and the combination of the above within in a collagen scaffold. It was noted that rhBMP7 resulted in high-quality repair while the combination led to higher quality and quantity of repair [35]. Porous tantalum with BMP7 resulted in a rabbit osteochondral defect model led to improved osteochondral regeneration compared to the porous tantalum alone [36]. Similarly, while not significant, a minipig osteochondral defect model found that the addition of rhBMP7 to collagen scaffolds inserted into the injury site led to improved cellular and extracellular matrix organization and mechanical properties [37]. Multiple BMPs have individually been shown to have the potential to regenerate cartilage in humans based on preliminary experiments in vitro, including BMP2, BMP4, BMP6, and rhBMP9 [30].
While animal and cell studies have demonstrated the potential of BMPs in the treatment of injuries to cartilage and joints, therapeutic use of BMPs in humans has focused mainly on bone. At least, two BMP treatments are on the market in the United States for clinical uses, including open long bone or non-union fractures and spinal fusions [38,39]. Recombinant human BMP7 either alone or in conjunction with bone grafts has been used successfully in the treatment of fractures non-unions for 20 years [40]. One clinical trial exploring BMP-7 injections at various dosages between 0 and 1.0 mg for the treatment of osteoarthritis with small cohorts led to no identified dose limiting toxicity but had otherwise modest results [41].
The summarized animal studies that employed BMPs to treat osteochondral defects and clinical applications of BMPs administer the BMP treatment directly to the injury site, unlike Cyplexinol®, which is ingested orally. The previous studies with Cyplexinol®, however, highlight it’s efficacy with repeated improvements to pain intensity and frequency when administered alone or in combination with other joint supplements [15-18]. The present study demonstrates the effectiveness of the Cyplexinol® treatment in a sample of middle-aged men and women, when used at a daily dosage of 900mg. Results are specific to the alleviation of joint pain and discomfort, with minimal changes noted in the measured biochemical variables. While prior studies have noted that arthritis and joint pain are multifactorial conditions involving various cells and cytokines [42], based on the present investigation – in particular the short-term nature of the intervention in our relatively small sample of men and women – it does not appear that Cyplexinol® is altering cytokines and must be providing pain reduction through another mechanism.
We note three main limitations of this study. First, the subject sample was relatively small and consisted primarily of overweight/obese men and women (with a much higher percentage of women as compared to men; Table 1), who may have presented with elevated inflammation/cytokine production and pain, as compared to those of normal weight. Further study will be needed to determine how those of normal weight respond to Cyplexinol® treatment, while making attempts to include similar numbers of men and women for comparison. Second, we included a relatively short timeframe of treatment, as subjects only used the Cyplexinol® for a period of 14 days before the post-treatment evaluation. While other longer-term trials of Cyplexinol® have been conducted, the dosage in those studies was far less than the 900 mg/day dose used in the present study. It is possible that the continued use of the higher dosage may provide more effective. For example, in a previous study where subjects received 150 mg Cyplexinol along with 1500 mg glucosamine sulfate and 1200 mg of chondroitin sulfate, the weekly average response continued to improve week over week [15]. It is unknown what impact Cyplexinol® at 900 mg/day would have on joint pain and physical function if used for several weeks or months. This is certainly an area of interest that deserves attention. Third, the subjects enrolled in this study were much younger (Mean: 43.5 years old) compared to previous studies with an average age in the 60s. Subjects in the present study also did not report having osteoarthritis, unlike previous Cyplexinol® studies, in which subjects had self-reported or diagnosed osteoarthritis. The inclusion of older adults, with and without osteoarthritis, may be considered in future, longer-term studies of Cyplexinol®. Finally and perhaps not considered a limitation, the present study did not make an effort to compare the Cyplexinol® to other agents known to alleviate joint pain, such as NSAIDs, opioids, serotonin-norepinephrine reuptake inhibitors, human serum albumin, interleukin-1 inhibitor, and others [43]. Hence, it is presently unknown how exactly Cyplexinol® compares to these agents – aside from contrasting results across different studies, in which case Cyplexinol® appears to perform quite well.
5. Conclusion
Cyplexinol® may provide relief to men and women suffering from chronic joint pain and may be considered as an alternative to over-the-counter and off-the-shelf products marketed as joint pain remedies. Future studies that include a larger sample size, those of normal body weight, and a longer course of treatment should be considered in an attempt to extend these initial findings.
Acknowledgments
None.
Funding
Funding for this work was provided in part by ZyCal Bioceuticals and the University of Memphis.
Conflicts of Interest
No author declares a specific conflict of interest related to this work. However, in the past three years, in addition to receiving research funding from ZyCal Bioceuticals, RJB has received research funding from the following companies: Liquid IV, Mannatech, Nuun & Company, CalerieHealth, DSE Healthcare, Zycal Bioceuticals, Yeahhh Baby, and Deerland Probiotics and Enzymes. He has served as a consultant to Mannatech, CalerieHealth, and BAT. JP and MS have received research funding from USANA Health Sciences. The sponsor had no role in the execution of the study or in the interpretation of the study data.
References
- [1].Barbour KE, Boring M, Helmick CG, Murphy LB, Qin J. Prevalence of severe joint pain among adults with doctor-diagnosed arthritis-United States, 2002-2014. MMWR Morb Mortal Wkly Rep. 2016;65:1052–6. doi: 10.15585/mmwr.mm6539a2. [DOI] [PubMed] [Google Scholar]
- [2].Clarke TC, Nahin RL, Barnes PM, Stussman BJ. Use of complementary health approaches for musculoskeletal pain disorders among adults:United States, 2012. Natl Health Stat Report. 2016;98:1–12. [PubMed] [Google Scholar]
- [3].Henrotin Y, Mobasheri A. Natural products for promoting joint health and managing osteoarthritis. Curr Rheumatol Rep. 2018;20:72. doi: 10.1007/s11926-018-0782-9. [DOI] [PubMed] [Google Scholar]
- [4].Colletti A, Cicero AF. Nutraceutical approach to chronic osteoarthritis:From molecular research to clinical evidence. Int J Mol Sci. 2021;22:12920. doi: 10.3390/ijms222312920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Butawan M, Benjamin RL, Bloomer RJ. Methylsulfonylmethane:Applications and safety of a novel dietary supplement. Nutrients. 2017;9:290. doi: 10.3390/nu9030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Debbi EM, Agar G, Fichman G, Ziv YB, Kardosh R, Halperin N, et al. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee:A randomized controlled study. BMC Complement Altern Med. 2011;11:50. doi: 10.1186/1472-6882-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim LS, Axelrod LJ, Howard P, Buratovich N, Waters RF. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee:A pilot clinical trial. Osteoarthritis Cartilage. 2006;14:286–94. doi: 10.1016/j.joca.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [8].Lubis AM, Siagian C, Wonggokusuma E, Marsetyo AF, Setyohadi B. Comparison of glucosamine-chondroitin sulfate with and without methylsulfonylmethane in grade I-II knee osteoarthritis:A double blind randomized controlled trial. Acta Med Indones. 2017;49:105–11. [PubMed] [Google Scholar]
- [9].Simental-Mendía M, Sánchez-García A, Vilchez-Cavazos F, Acosta-Olivo CA, Peña-Martínez VM, Simental-Mendía LE. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis:A systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol Int. 2018;38:1413–28. doi: 10.1007/s00296-018-4077-2. [DOI] [PubMed] [Google Scholar]
- [10].Zhu X, Sang L, Wu D, Rong J, Jiang L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis:A meta-analysis of randomized controlled trials. J Orthop Surg Res. 2018;13:170. doi: 10.1186/s13018-018-0871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6:32–52. doi: 10.4236/jbise.2013.68A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Urist MR, Lietze A, Mizutani H, Takagi K, Triffitt JT, Amstutz J, et al. A bovine low molecular weight bone morphogenetic protein (BMP) fraction. Clin Orthop Relat Res. 1982;162:219–32. [PubMed] [Google Scholar]
- [13].Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12:203–21. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- [14].Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50:1392–406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- [15].Garian R, Donar A, DeFabio D, Gahles N, Scaffidi J. An osteoinductive protein complex that stimulates regeneration of bone and cartilage for treatment of moderate to severe osteoarthritis. Integr Med. 2012;11:16–21. [Google Scholar]
- [16].Scaffidi JJ, Vieira KF. Cyplexinol:A natural BMP complex with osteoinductive and anti-inflammatory activity promotes De novo bone and joint tissue growth. J Stem Cell Res Ther. 2017;7:387. [Google Scholar]
- [17].Spinks K, Walker M, Scaffidi J. Clinical assessment of low-dose osteoinductive protein as a stand-alone regimen in self-reported osteoarthritis. Integr Med (Encinitas) 2015;14:23–32. [PMC free article] [PubMed] [Google Scholar]
- [18].Spinks K, Scaffidi JJ. In vivo osteoinduction:Evaluating 2-beta coxatene as an immunoinductive compound and novel ingredient for joint support. Integr Med (Encinitas) 2016;15:34–44. [PMC free article] [PubMed] [Google Scholar]
- [19].Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Visconti R, Iversen T, Cottrell J. Dysregulated osteoblast and osteoclast coupling in bone disease and failure. J Bone Res. 2019;7:201. [Google Scholar]
- [21].Basiri Z, Zeraati F, Esna-Ashari F, Mohammadi F, Razzaghi K, Araghchian M, et al. Topical effects of Artemisia absinthium ointment and liniment in comparison with piroxicam gel in patients with knee joint osteoarthritis:A randomized double-blind controlled trial. Iran J Med Sci. 2017;42:524–31. [PMC free article] [PubMed] [Google Scholar]
- [22].Cohen M, Wolfe R, Mai T, Lewis D. A randomized, double blind, placebo controlled trial of a topical cream containing glucosamine sulfate, chondroitin sulfate, and camphor for osteoarthritis of the knee. J Rheumatol. 2003;30:523–8. [PubMed] [Google Scholar]
- [23].Deal CL, Schnitzer TJ, Lipstein E, Seibold JR, Stevens RM, Levy MD, et al. Treatment of arthritis with topical capsaicin:A double-blind trial. Clin Ther. 1991;13:383–95. [PubMed] [Google Scholar]
- [24].Kosuwon W, Sirichatiwapee W, Wisanuyotin T, Jeeravipoolvarn P, Laupattarakasem W. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. J Med Assoc Thai. 2010;93:1188–95. [PubMed] [Google Scholar]
- [25].Thielen NG, van der Kraan PM, van Caam AP. TGFb/BMP signaling pathway in cartilage homeostasis. Cells. 2019;8:969. doi: 10.3390/cells8090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–46. [PubMed] [Google Scholar]
- [27].Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- [28].Nishimura R, Hata K, Takahata Y, Murakami T, Nakamura E, Ohkawa M, et al. Role of signal transduction pathways and transcription factors in cartilage and joint diseases. Int J Mol Sci. 2020;21:1340. doi: 10.3390/ijms21041340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–7. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- [30].Deng ZH, Li YS, Gao X, Lei GH, Huard J. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthritis Cartilage. 2018;26:1153–61. doi: 10.1016/j.joca.2018.03.007. [DOI] [PubMed] [Google Scholar]
- [31].Yang HS, La WG, Bhang SH, Kim HJ, Im GI, Lee H, et al. Hyaline cartilage regeneration by combined therapy of microfracture and long-term bone morphogenetic protein-2 delivery. Tissue Eng Part A. 2011;17:1809–18. doi: 10.1089/ten.TEA.2010.0540. [DOI] [PubMed] [Google Scholar]
- [32].Takahashi T, Muneta T, Tsuji K, Sekiya I. BMP-7 inhibits cartilage degeneration through suppression of inflammation in rat zymosan-induced arthritis. Cell Tissue Res. 2011;344:321–32. doi: 10.1007/s00441-011-1154-1. [DOI] [PubMed] [Google Scholar]
- [33].Sekiya I, Tang T, Hayashi M, Morito T, Ju YJ, Mochizuki T, et al. Periodic knee injections of BMP-7 delay cartilage degeneration induced by excessive running in rats. J Orthop Res. 2009;27:1088–92. doi: 10.1002/jor.20840. [DOI] [PubMed] [Google Scholar]
- [34].Hurtig M, Chubinskaya S, Dickey J, Rueger D. BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res. 2009;27:602–11. doi: 10.1002/jor.20787. [DOI] [PubMed] [Google Scholar]
- [35].Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006;14:1126–35. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [36].Wang Q, Zhang H, Gan H, Wang H, Li Q, Wang Z. Application of combined porous tantalum scaffolds loaded with bone morphogenetic protein 7 to repair of osteochondral defect in rabbits. Int Orthop. 2018;42:1437–48. doi: 10.1007/s00264-018-3800-7. [DOI] [PubMed] [Google Scholar]
- [37].Gavenis K, Heussen N, Hofman M, Andereya S, Schneider U, Schmidt-Rohlfing B. Cell-free repair of small cartilage defects in the Goettinger minipig:The effects of BMP-7 continuously released by poly(lactic-co-glycolid acid) microspheres. J Biomater Appl. 2014;28:1008–15. doi: 10.1177/0885328213491440. [DOI] [PubMed] [Google Scholar]
- [38].Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- [39].Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the united states using administrative data. Spine (Phila Pa 1976) 2010;35:1794–800. doi: 10.1097/BRS.0b013e3181ecf6e4. [DOI] [PubMed] [Google Scholar]
- [40].Giannoudis PV, Tzioupis C. Clinical applications of BMP-7:The UK perspective. Injury. 2005;36(suppl:S47):50. doi: 10.1016/j.injury.2005.07.035. [DOI] [PubMed] [Google Scholar]
- [41].Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2010;11:232. doi: 10.1186/1471-2474-11-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang W, Robertson WB, Zhao J, Chen W, Xu J. Emerging trend in the pharmacotherapy of osteoarthritis. Front Endocrinol (Lausanne) 2019;10:431. doi: 10.3389/fendo.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]


