Abstract
Background
Candida albicans causes high-mortality candidiasis. Antifungal drug resistance demands the development of virulence factor-targeting drugs, particularly antibiofilm. This study screened the effects of five invasive plants growing in Indonesia (Mimosa pudica, Lantana camara, Acacia mangium, Ageratina riparia, and Mikania micrantha) against C. albicans biofilms. Antifungal activity, antiphospholipase activity, biofilm morphology of C. albicans, and cytotoxic capacity were also evaluated.
Methods
Maceration was used to extract the plants, and the most active extract inhibiting the biofilms was fractionated using liquid–liquid fractionation. Antibiofilm activity was determined by a colorimetric assay, MTT. Antifungal activity was tested using the broth microdilution method. A phospholipase assay was performed using the egg-yolk agar method. Influence on the C. albicans morphology was assessed using scanning electron microscopy (SEM). The cytotoxic effect was carried out against Vero and HeLa cell lines.
Results
M. pudica extracts showed the most potent antifungal efficacy with minimum inhibitory concentration (MIC) of 15.62 µg/mL and 7.81 µg/mL for aerial parts and roots, respectively. At high concentrations (500 µg/mL and 250 µg/mL), ethanol extract of M. pudica aerial parts strongly inhibited the phospholipase activity. Ethyl-acetate fraction of M. pudica aerial parts demonstrated the most potent antibiofilm activity against 24 h old biofilm of C. albicans with an inhibitory concentration (53.89%) of 62.5 µg/mL showed no cytotoxicity in both Vero and HeLa cells. This fraction affected the morphology of C. albicans and contained promising compounds for inhibiting the 24 h old biofilm of C. albicans.
Conclusions
Invasive M. pudica plant inhibited the growth of planktonic C. albicans cells and its ethyl acetate fraction decreased the metabolic activity of C. albicans biofilms. This result demonstrates the potential of invasive M. pudica plant to reduce biofilm-associated candida infection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-023-04044-2.
Keywords: Invasive plants, Candida albicans, Antifungal, Antibiofilm, Mimosa pudica
Background
Candida albicans is the yeast that usually can be detected in healthy humans without causing health issues. However, when the immune system is compromised (e.g., long-term exposure to antibiotics, utilization of indwelling medical devices, and post-surgery), this yeast can penetrate the natural host barriers, invade the bloodstream, and intensely attack various organs, leading to invasive candidiasis (bloodstream infection/candidemia) and deep-seated infection with or without candidemia) that seriously threaten life [1, 2]. Indeed, the global burden of invasive candidiasis remains high, with candidemia-causing C. albicans being the most prevalent, either in the general population or in hospitals [3]. In Indonesia, approximately 7.7 million people have a serious fungal infection each year with the assumption of the candidemia incidence (the common invasive infection) was 10/100,000 [4]. In Europe, the number of candidemia between January 2000 and February 2019 was approximately 79 cases per day, with the fatal cases being around 29 patients on day thirty [5]. According to the Centers for Disease Control and Prevention’s (CDC) surveillance data, the mortality of candidemia in hospitals is around 25% [6].
The pathogenicity of C. albicans depends on some virulence factors such as biofilm formation, and secretion of extracellular enzymes [2]. Concerning biofilm formation, unicellular C. albicans cells must adhere to indwelling medical devices before infection, for then accumulate with other cells to form basal layers. Following initial adherence, most of the adherent yeast cells switch to the hyphal form, secrete extracellular polymeric substances, and get encapsulated in a layer of hydrogel, namely extracellular matrix, forming a physical barrier between the community and the extracellular environment. This process continues to thick and grows into a mature biofilm with a three-dimensional structure [7, 8]. Regarding phospholipase (one of the extracellular hydrolytic enzymes), it facilitates the adherence and invasion of C. albicans cells to the host epithelium by hydrolyzing phospholipids and peptide bonds, which play and regulate an essential role in multiple physiological processes on the human body such as immune system and stress tolerance [9–12].
The presence of virulence factors, especially biofilm formation, is associated with C. albicans resistant to the majority of antifungal drugs. Although biofilm resistance is multifactorial and mechanistically complex, the role of the extracellular matrix as a physical barrier may account for the high levels of resistance displayed by C. albicans biofilms [13, 14]. Al-Fattani and Douglas (2006) identified a correlation between matrix abundance and levels of fluconazole and amphotericin B resistance [15]. Moreover, the newest class of antifungal drugs, echinocandin, revealed the reduction susceptibility (resistance) against clinical and laboratory strains of Candida albicans [16, 17]. In the context of phospholipase enzyme, Ying and Chunyang, 2011 reported that there was a correlation between high phospholipase activity and resistance to antifungal drugs by increasing the expression of phospholipase B1 mRNA and protein [18]. Another study showed that some antifungal agents such as nystatin, fluconazole, and micafungin had a low reduction (approximately under 5%) of phospholipase activity [19].
Taken together, the high morbidity and mortality of invasive candidiasis and the great capability of C. albicans to resist antifungal agents demand the discovery of new drugs to protect humans against Candida infections, especially those associated with a biofilm. By incorporating traditional knowledge of plants as remedies into the drug discovery process, natural products can serve as a source of new drugs or active pharmaceutical ingredients. Indeed, the use of plants as medicine has a lengthy history, and remarkably, many drugs have already been derived from plants. However, global demand for medicinal plants has endangered native plants, contributing to biodiversity loss and depletion of natural resources critical to human health [20]. Moreover, the situation is worsened by the presence of invasive plants which entered and established in the new environment from outside of their natural habitat and caused environmental, economic, and/or human harm [21]. One of the most serious threats posed by invasive plants to the environment is the disruption of entire ecosystems. According to the United Nations (UN) Intergovernmental Platform for Biodiversity and Ecosystem Services (IPBES), the impacts of invasive plants are often severe for native species and especially for endemic species. Native species are estimated to have lost at least 20% of their original abundance, and even more in hotspots of endemic species [22]. Meanwhile, Indonesia, one of the world's richest nations in terms of biodiversity, with around 30,000 plant species and 9,600 medicinal plants [23], is known for the high rates of loss of diversity in the world that are caused by the introduction and spread of invasive plants in various Indonesian ecosystems [24]. Nevertheless, despite the negative effects caused by invasive plants, there are positive aspects, especially in the health sector. Numerous studies have documented the use of invasive plants in traditional medicine. For example, Mimosa pudica leaves (native of tropical America) are used to treat toothache and low libido in men, respectively, on Rodrigues Island of the Indian Ocean and Kurukshetra District, India [25, 26]. The leaves of Lantana camara (native to tropical America) have been reported to treat many diseases such as tuberculosis in South-Western Uganda, ulcers, swelling, and microbial infections in India [27–29]. Furthermore, Máximo et al., 2020 demonstrated the pharmaceutical potential of invasive plants that have produced compounds. They described the potential of invasive plants such as Carpobrotus edulis, Hakea salicifolia, Hakea sericea, Oxalis pes-caprae, Phytolacca americana, and Ageratina adenophora as sources of bioactive metabolites ranging from antioxidant, antimicrobial, and anticholinesterase to neuroprotective and antiproliferative [30]. Taking those matters into account, the authors put those main ideas of drug development into practice by utilizing five invasive plants growing in Indonesia and screening their antibiofilm activity against C. albicans. In addition, this study also screened for antifungal and antiphospholipase activity. Notably, these five plants are listed by the Ministry of Environment and Forestry as invasive plants which might become big threats to agriculture, forests, and other resources in Indonesia [31]. To the best of our knowledge, this study is the first to evaluate the efficacy of Indonesian invasive plants in inhibiting C. albicans virulence factors.
Materials and methods
Plant materials
M. pudica aerial parts (= M. pudica's structures above ground, including leaves, flowers, and stem), M. pudica roots, L. camara leaves, A. mangium leaves, A. riparia leaves, and M. micrantha leaves were used in this study and collected from the slopes of Merapi mountain, Indonesia (GPS positioning: between -7.5719346390002675, 110.43219680357387 and 7.5719346390002675, 110.43219680357387). The identification of plants was conducted by Dr. Djoko Santosa (Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Gadjah Mada) and by Anggityas Puspita Suci, S. Farm, Apt. (Merapi Farma Herbal) (identification number: 13.17.09). The voucher specimens of A. riparia, M. micrantha, A. mangium, M. pudica, and L. camara were 43AR-1, 43MM-2, 43AM-4, 43MP-5, and 43LC-6, respectively, and were deposited at the Department of Biology Pharmacy, Faculty of Pharmacy, Universitas Gadjah Mada.
Extraction of plant materials
The plants were shade-dried for seven days and powdered using a grinder. Afterward, the powder of each plant was processed for the preparation of ethanol (Merck, Darmstadt, Germany) or methanol extract (Merck, Darmstadt, Germany) by the maceration process as described previously with modification [32]. For this purpose, 100 g of plant powder were macerated in 500 mL of ethanol or methanol for three days with regular shaking. After filtration using the Buchner funnel, the residues were re-macerated using fresh solvents for three days. All filtrates obtained with the same solvent were pooled, filtered through filter papers (Whatman filter paper no. 1) in the Buchner funnel, and dried using a rotary evaporator (Heidolph, Schwabach, Germany). Samples were stored in a refrigerator at -20 °C until further experiments.
Liquid–liquid fractionation (LLF)
The extract with the highly active extract was subjected to liquid–liquid fractionation (LLF) according to the method as described previously with modifications [33]. For this purpose, the organic solvents (analytical grade, Merck, Darmstadt, Germany), in order of increasing polarity, were n-hexane, chloroform, ethyl-acetate, n-butanol, and double-distilled water (ddH20). Before partition, 5 g of extracts were solubilized in 10 mL of ethanol (Merck, Darmstadt, Germany) and 90 mL of ddH20. The solubilized extract was then partitioned with 100 mL of n-hexane, shaken, and then the n-hexane layer was separated. This process was carried out three times. Chloroform, ethyl-acetate, and n-butanol were processed following the same method. Each partition was performed three times, and the same eluents were pooled and dried using a rotary evaporator. Each obtained fraction was recorded as the total yield.
Phytochemical profiling
Liquid Chromatography-Mass Spectrometry (LC–MS)
The chemical composition of the best active extract was qualitatively screened and analyzed by Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC–MS-ESI–MS) using Acquity UPLC I-Class coupled with XEVO G2-XS QTOF (Waters, MA, USA) mass spectrometer. The column was ACQUITY UPLC® BEH C8, 1.7 µm, 2.1 × 100 mm. The mobile phase was composed of solvent A: water with 0.1% formic acid, and solvent B: acetonitrile containing 0.1% formic acid. The flow rate was set at 0.3 mL/min with a 1 μL injection volume. The parameters of MS/MS were optimized as follows: ionization type: ESI; Start Mass: 50.00 m/z; End Mass: 1200.00 m/z; Polarity: Positive. The screening process for constituents was performed with the UNIFI software, which contains a mass spectrum library of natural chemical constituents from the waters database [34].
High-Resolution Mass Spectrometer (HRMS)
Thermo ScientificTM DionexTM Ultimate 3000 RSLCnano UHPLC (ultra-high-performance liquid chromatography) and Q ExactiveTM High-Resolution Mass Spectrometer (ThermoFisher, MA, USA) were used to screen and discover non-targeted chemical compounds from the fraction showing the best antibiofilm activity. The mobile phase was composed of solvent A (water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The programming for the gradient mode was as follows: at t = 0–15 min, B 5%; at t = 16–20 min, B 90%; at t = 21–25 min, B 90%. The analytical column used was Phenyl-Hexyl 100 × 2.1 mm with a flow of 0.20 mL/min and an injection volume of 5 µl. MS1 was rendered at 70,000 FWHM, whereas MS2 was rendered at 17,500 FWHM. This experiment utilized Heated Electrospray Ionization (H-ESI) in both positive and negative modes. The spray voltage used was 3.8 kV. The flow rates for Sheath gas and Aux gas were 15 and 7, respectively. The capillary temperature was 250 °C. The mass range used was between 50 and 750 m/z. Thermo Scientific ™ Compound Discoverer Software was used for identifying the compounds.
Strains and growth conditions
C. albicans ATCC 10231 was used as a reference strain, and two isolates, CI-SPTM and CI-CVX which were recovered, respectively, from sputum isolated from pulmonary disease and from cervical swab specimens of vulvovaginal candidiasis patients at two different hospitals in Yogyakarta, Indonesia, were kindly provided by the Microbiology Department, Faculty of Medicine at Universitas Gadjah Mada, Indonesia. C. albicans were grown in Sabouraud dextrose agar (SDA) (Himedia, Maharashtra, India) at 37 °C for 24 h.
Antifungal susceptibility test
As per the M27-A3 protocol of the Clinical and Laboratory Standards Institute (CLSI), the broth microdilution method was used to evaluate the minimum inhibitory concentration (MIC) of the samples against C. albicans [35]. Each reference or clinical isolate of C. albicans was streaked on SDA plate. The plates were incubated for 24 h at 37 °C. Then several colonies from these cultures were picked up, and five colonies of around 1 mm diameter were suspended in sterile saline solution (0.85% NaCl) and adjusted to 0.5 McFarland standard (equivalent to 1–5 × 106 cells/mL) and then diluted at 1:50, followed by a 1:20 dilution in Roswell Park Memorial Institute medium (RPMI) (Himedia AT180, Maharashtra, India), which contained 0.165 M l−1 3-(N-morpholino)propanesulfonic acid (MOPS) buffer to obtain a suspension of approximately 1-5 × 103 cells/mL. The sample stocks (20 mg/mL) were prepared by weighing 20 mg of extract in a sterile Eppendorf tube and diluting it in 1 mL of 25% dimethyl sulfoxide (DMSO). The 100 µL of working yeast suspension was then added to wells of 96-well microtiter plates (Corning®, NY, USA) containing 100 µL of a serial two-fold dilution in RPMI 1640-MOPS medium of extracts. The final concentrations obtained ranged between 1000 and 3.9 µg / mL. Some wells were preserved for controls: non-treated yeasts (negative control), yeasts treated by fluconazole (positive control) (Sigma St. Louis, MO, USA), and yeasts treated by DMSO 2% (DMSO control). The test was run in triplicate and repeated at least twice. The determination of MIC was conducted according to the CLSI procedure: each well was assigned a numeric rating of 0 (visually clear), 1 (a rather foggy), 2 (significant decrease in visible growth), 3 (slight decrease in visible growth), or 4 (zero reduction in visible growth). Based on numerical scales, the lowest concentration that significantly inhibited visible growth was designated as the MIC50 (scale of 2) [35].
By subculturing one loopful (10 μL) of the solution from the wells without turbidity on SDA, the minimum fungicidal concentration (MFC) was ascertained. After an incubation period of 24 h, the minimal fungicidal concentration (MFC) was determined to be the lowest concentration that resulted in no growth or maximum three colonies growth (> 99.9%) on the subculture.
Antibiofilm assay
Colorimetric assays are tools that are reasonably simple to perform, very useful for determining yeast viability, and reveal a great association between cellular density and metabolic activity, allowing for semiquantitative evaluation of biofilm formation [36]. Therefore, to evaluate the metabolic activity of mature C. albicans biofilm, the colorimetric assay (MTT) was carried out according to the method of Prazynska and Gospodarek, 2014 with a few modifications [37]. For this purpose, yeast was first cultured on the SDA agar plate for 48 h. Thereafter, four loopfuls of this culture were transferred to 30 mL of Yeast Extract-Peptone-Dextrose (YPD) medium (Difco, Detroit, MI, USA) and cultured at 37 °C without shaking overnight. This culture was then centrifuged at 3000 g for 10 min, rinsed twice in 0.1 M phosphate-buffered saline (PBS, pH 7.2, GIBCO, New York, United States), standardized to 0.5OD600 (equivalent to 3 × 107 CFU/mL), and diluted to get a final concentration of 1 × 106 CFU/mL. The 100 µL of yeast suspension was transferred into a sterile, untreated 96-well polystyrene plate (Costar, Corning, USA), incubated for 24 h at 37 °C. Then, after 24 h of incubation, the non-adherent yeasts were removed by washing them twice with 0.2 mL sterile PBS. Two-fold serial dilutions of the extracts/fractions (between 1000 and 3.9 µg mL−1) prepared in YPD (Difco™ YPD Broth, USA) medium were added to each well-containing biofilm, and the microplates were incubated at 37 °C for 24 h. Further, the wells were washed twice with 0.2 mL PBS after 24 h incubation at 37 °C. Then, the wells were filled with 100 μL of MTT solution (5 mg/mL in PBS) and left at 37 °C for 90 min. Then, the solution was taken out of the incubation chamber, and the formed formazan was dissolved in 100 µL of isopropanol-HCl solution. Solubilized formazan color was measured using a microplate reader at a wavelength of 550 nm. The inhibition percentage for each concentration of the samples was calculated according to the following formula:
This study denoted a high or poor activity for above or under 50%, respectively. Inhibition percentages were calculated based on a minimum of two independent experiments with three replicates.
Qualitative analysis-scanning electron microscopy (SEM)
C. albicans ATCC 10231 biofilms (control and treated cells), as described previously in the antibiofilm assay section, were prepared on Thermanox™ polystyrene coverslips (Nunc™ Thermanox™). Briefly, after 24 h, the coverslips of control and treated biofilms were washed twice with PBS and fixed with glutaraldehyde and 0.1 N PBS for 1 h at room temperature. The coverslips were washed with PBS and dehydrated in ethanol solutions (50, 70, and 90% for 10 min). After that, coverslips were air-dried overnight in a desiccator before gold sputter coating. With a scanning electron microscope (JSM-6510LA, JEOL-USA), the morphology of C. albicans biofilms was examined. This procedure was modified from Pereira et al. 2016 [38].
Phospholipase assay
A phospholipase assay was performed using the egg-yolk agar method [39]. The egg-yolk agar medium contained 13 g of SDA, 11.7 g of NaCl, 0.11 g of CaCl2, and 8% of sterile egg-yolk emulsion (Merck, Darmstadt, Germany) in 184 mL of distilled water. After C. albicans ATCC 10231 was subcultured in SDA agar for 24 h, the cells (2 × 106) were cultured in YPD medium using the tested extract (treated cells) for 24 h at 37 °C. The cell control (not treated) was included in this assay. In a petri dish with a 90 mm diameter, a 5 μL suspension containing 106 yeast cells (treated and control cells) was plated on the surface of an egg-yolk medium and left to dry at room temperature. Afterward, the plates were incubated for seven days at 37 °C. When a precipitation zone (production of phospholipases) was visible around the C. albicans colony area, the phospholipase activity (Pz index) was established, and the formula employed to calculate the phospholipase production was: Pz = Diameter of the colony / (Diameter of the colony + precipitation zone).
The Pz values were categorized as follows: 1 (negative activity); 0.90–0.99 (very low activity); 0.80–0.89 (low activity); 0.70–0.79 (high activity); and ≤ 0.69 (very high activity).
All experiments were performed in duplicate, twice on different days.
Cytotoxicity assay
The fraction displaying the most potent antibiofilm activity was studied to evaluate its cytotoxicity via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay). For this purpose, the protocol of the cytotoxicity assay was modified from Nugroho et al., 2013 [40]. The Vero and HeLa cell lines were used in this assay to assess whether the fraction is more selective for antibiofilm activity or more toxic for both cells (normal and cancer cells). The Vero and HeLa cell lines were obtained from the Department of Parasitology, Faculty of Medicine, Universitas Gadjah Mada, Indonesia. The Vero and HeLa cells were grown in Dulbecco’s Modified Eagle’s Medium (Gibco, NY, USA) with 2 mM glutamine, containing 10% fetal bovine serum (FBS) (Gibco, NY, USA) and RPMI 1640 (Merck, Darmstadt, Germany) with 10% FBS (Gibco, NY, USA), respectively. Briefly, the cells were plated in 96-well microplate wells (2.0E + 04 cells per well) and incubated for 24 h (5% CO2; 37 °C). The two-fold serial dilutions of the studied fraction were added into each well (except for medium and control wells), with concentrations ranging between 500–7.8 µg/mL. After 24 h of incubation, the culture medium was removed, washed using PBS, and incubated (4 h; 37 °C; 5% CO2) with 20 µl MTT solution (5 mg/mL). Then, the MTT reaction was stopped by 10% Sodium Dodecyl Sulfate in 0.1 N HCL. Following overnight incubation in a dark environment with a plate covered with aluminum foil, the absorbance was measured using a microplate reader at a wavelength of 595 nm after 10 min of shaking. Three replicates were prepared for each of the three experimental sessions. The following formula calculated the cell viability:
Statistical analysis
All values were reported as the standard error of the mean (SEM) for all studies, which were conducted in a minimum of two independent experiments with three biological replicates. The Kruskal–Wallis and post hoc Dunn's multiple comparison tests were used to determine difference between mean of the control and treated samples (antibiofilm and antiphospholipase tests). The statistical analyses were performed using GraphPad Prism8 software (GraphPad Software, Inc., La Jolla, CA, USA), and a p-value ≤ 0.05 was considered statistically significant.
Results
The yields of plants extracts and fractions
The extraction yield reflects the efficiency of the solvent in extracting components from the original matter, a plant powder. Table 1 shows the methanol extract of A. mangium leaves with the highest yield at 18.35% and the ethanol extract of M. pudica roots with the lowest yield at 7.10%. Furthermore, the ethanol extract of M. pudica aerial parts was the most active extract against C. albicans biofilm. The ethanol extract (5 g) of M. pudica aerial parts was subjected to fractionation by a liquid–liquid partition. We obtained 4.40 g of n-hexane phase (88%), 0.16 g of chloroform phase (3.2%), 0.41 g of ethyl acetate phase (8.2%), 1.39 g of n-butanol phase (27.8%), and 1.26 g of ddH2O phase (25.2%). Among these fractions, the lowest yield was observed with the chloroform fraction, whereas the highest was associated with the n-hexane fraction.
Table 1.
Percentage yields of plant extracts
| Samples | Part of plants | Solvents | Powder weight (g) | Extracts weight (g) | Yields (%, w/w) |
|---|---|---|---|---|---|
| Ageratina riparia | Leaf | Ethanol | 100 | 10.02 | 10.02 |
| Mimosa pudica | Aerial parts | Ethanol | 100 | 7.36 | 7.36 |
| Mimosa pudica | Root | Ethanol | 100 | 7.10 | 7.10 |
| Lantana camara | Leaf | Methanol | 100 | 13.79 | 13.79 |
| Mikania micrantha | Leaf | Methanol | 100 | 15.85 | 15.85 |
| Acacia mangium | Leaf | Methanol | 100 | 18.35 | 18.35 |
Effects of studied invasive plant extracts against C. albicans planktonic cells
Table 2 shows the antifungal activity of the studied plant extracts against C. albicans ATCC 10231 and the clinical isolates (CI) SPTM and CVX. The ethanol extract of M. pudica roots displayed the highest activity against the C. albicans ATCC 10231 (MIC50 of 7.81 µg/mL) and clinical isolates (MIC50 of 15.62—31.25 µg/mL). The ethanol extract of M. pudica aerial parts and methanol extract of A. mangium leaves showed intermediate activity with MIC50 between 15.62 and 62.5 µg/mL. There was no activity of other extracts observed in the present study.
Table 2.
Susceptibility of C. albicans to the studied invasive plant extracts and to fluconazole (MIC and MFC in µg/mL)
| Extracts | Pathogens | |||||
|---|---|---|---|---|---|---|
| C. albicans ATCC 10231 | C. albicans CI-SPTM | C. albicans CI-CVX | ||||
| MIC50 | MFC | MIC50 | MFC | MIC50 | MFC | |
| A. mangium leaves | 62.5 | > 1000 | 31.25 | > 1000 | 125 | > 1000 |
| A. riparia leaves | > 1000 | NT | NA | NT | > 1000 | NT |
| M. pudica aerial parts | 15.62 | 250 | 62.5 | 250 | 62.5 | 1000 |
| M. pudica roots | 7.81 | 125 | 15.62 | 125 | 31.25 | 250 |
| L. camara leaves | > 1000 | NT | NA | NT | > 1000 | NT |
| M. micrantha leaves | > 1000 | NT | NA | NT | > 1000 | NT |
| Fluconazol | 0.78 | 100 | 1.56 | > 200 | 3.12 | > 200 |
| NA No Activity, NT Not Tested | ||||||
Effects of the studied invasive plant extracts against C. albicans biofilms
All the studied extracts were tested against 24 h old C. albicans biofilm. The ethanol extract of M. pudica aerial parts was the most active, with 51.11% inhibition at 125 μg mL−1 (p < 0.05) (Fig. 1). The inhibition of more than 50% was additionally demonstrated by the extract of L. camara leaves, however, this was only the case at the highest concentrations (≥ 250 µg/mL). The spectrum activity of a promising extract of M. pudica aerial parts was also evaluated against two clinical isolates, CI-SPTM and CI-CVX, and revealed inhibition activity of 53.83% at 125 μg mL−1 (p ≤ 0.05) and 50.81% at 250 μg mL−1, respectively.
Fig. 1.
Heat map of the inhibition percentages of the studied extracts against 24 h old biofilm of C. albicans ATCC 10231. Mp-A (M. pudica aerial parts); Mp-R (M. pudica roots); Lc-L (L. camara leaves); Am-L (A. mangium leaves); Mm-L (M. micrantha leaves); Ar-L (A. riparia leaves)
(see Additional file 1).
Effects of fractions of M. pudica aerial parts against 24 h old biofilm of C. albicans
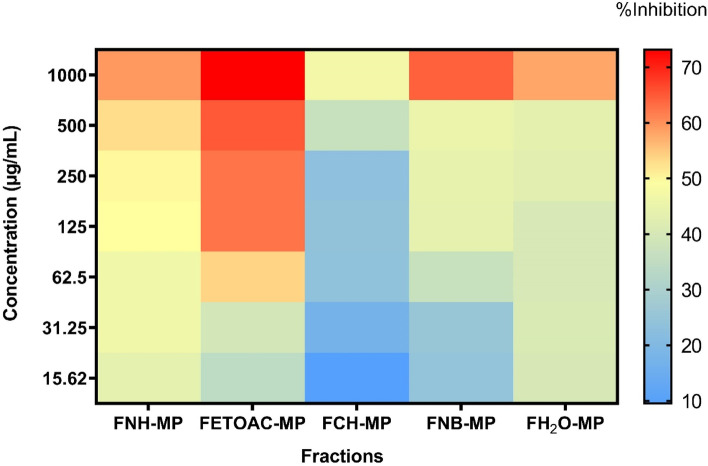
The five obtained fractions from the aerial parts of M. pudica were then evaluated against 24 h old biofilm of C. albicans ATCC 10231. The results showed that the ethyl acetate fraction of M. pudica had the highest activity and inhibited the preformed C. albicans biofilms as much as 53.89% at 62.5 μg/mL (p ≤ 0.05) (Fig. 2). At the highest concentration (1000 μg/mL), the ethyl acetate fraction inhibited biofilms by over 70%. The hexane fraction inhibited the biofilm cells at a higher concentration than ethyl acetate fraction, by 50.57% at 250 μg/mL. Whereas n-butanol and aqueous fractions showed inhibition of 64.33% and 57.94%, respectively, at the highest concentration (1000 μg/mL), and this activity decreased along with a decrease in the concentration of fractions.
Fig. 2.
Heat map of the inhibition percentage of all fractions of M. pudica aerial parts against 24 h old biofilm of C. albicans ATCC 10231. FNH-MP (n-hexane fraction of M. pudica); FETOAC-MP (ethyl acetate fraction of M. pudica); FCH-MP (chloroform fraction of M. pudica); FNB-MP (n-butanol fraction of M. pudica); FH2O-MP (ddH2O fraction of M. pudica)
The antibiofilm activity of ethyl acetate fraction of M. pudica aerial parts was also evaluated against mature biofilm of two clinical isolates: CI-SPTM and CI-CVX. The percentage inhibition on both clinical isolates was approximately 50% at different concentrations (125 vs 250 µg/mL), with CI-SPTM being the most susceptible isolate when treated with ethyl acetate fraction (Fig. 3). The antibiofilm activity is therefore retained in the clinical isolates, but for slightly higher concentrations than in the reference strain.
Fig. 3.
Metabolic activity (MTT assay) of ethyl acetate fraction of M. pudica aerial parts against 24 h old clinical isolates (CI-SPTM and C-CVX) biofilms. Asterisks denote statistically significant differences of treated biofilm versus non-treated (NT) biofilm. *p ≤ 0.05 was calculated by Kruskal–Wallis test, followed by Dunn’s multiple comparisons test
Alongside antibiofilm activity, we also evaluated the antifungal activity of ethyl acetate fraction of M. pudica aerial parts, and we found that the MIC50 and MFC were at 31.25 µg/mL and 250 µg/mL against C. albicans ATCC 10231, 62.5 µg/mL and 1000 µg/mL against both clinical isolates, respectively.
Phytochemical composition
Ethanol extract of M. pudica roots
Demonstrating the most potent antifungal activity, the ethanol extract of M. pudica roots was processed for identification and screening of bioactive compounds using LC–MS. Table 3 displays the list of the compounds from the ethanol extract of M. pudica roots that have been potentially identified, together with m/z, neutral mass, and retention time. The highest peak was detected at 4.61 retention time and corresponded to the alkaloid compound, 3α-(tigloyloxy) tropane (see Additional file 2). Then, the other ten peaks were chosen for the analysis. However, due to the limitation of the database library (UNIFI software) in the Advanced Characterization Laboratories Serpong, National Research and Innovation Institute (BRIN), some compounds could not be determined (denoted as Candidate mass).
Table 3.
Mass spectrometric analysis of ethanol extract of M. pudica root
| Component name | Observed m/z | Neutral mass (Da) | Retention time (min) |
|---|---|---|---|
| Pseudotropidine | 142.12 | 141.11 | 1.09 |
| Cyclo(Ala-Ala) | 143.08 | 142.07 | 1.12 |
| Epigallocatechin(4β,8)-gallocatechin | 611.14 | 610.13 | 1.25 |
| Candidate Mass C13H21NO3 | 240.16 | 239.15 | 4.03 |
| Meteloidine | 256.15 | 255.15 | 4.41 |
| Candidate Mass C18H27NO6 | 354.19 | 353.18 | 4.51 |
| 3α-(Tigloyloxy)tropane | 224.16 | 223.16 | 4.61 |
| Gallocatechin | 329.06 | 306.07 | 5.29 |
| 3,5,6-Trihydroxy-4',7-dimethoxyflavone | 331.08 | 330.07 | 5.57 |
| Candidate Mass C18H25NO4 | 320.19 | 319.18 | 6.26 |
| Candidate Mass C18H25NO5 | 336.18 | 335.17 | 6.33 |
Ethyl-acetate fraction of M. pudica aerial parts
The most potent antibiofilm activity of ethyl-acetate fraction of M. pudica aerial parts was analyzed for its chemical contents using HRMS. Twenty-three and fifty-three compounds were found using HRMS in the negative (Table 4) and positive modes, respectively (Table 5). Because catechin and adenine exhibited the highest peak areas in the negative and positive ionization modes, they were chosen as reference peak areas for calculating relative abundance percentages (RA) [41]. The following prominent compounds in the negative mode were quercetin-3β-D-glucoside (82.17%), (1ξ)-1,5-anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol (52.48%), luteolin (44.24%), and quercetin (24.27%). While in the positive mode were avicularin (47.45%), (1ξ)-1,5-anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol (46.24%), and kaempferol (44.50%).
Table 4.
Mass spectrometric analysis of ethyl-acetate fraction of M. pudica aerial parts using HRMS in negative mode
| No | Name | Formula | Calculated Molecular Weight (MW) | RT [min] | Peak Area (Max.) | % RA | mzCloud Best Match |
|---|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid | C16 H18 O9 | 354.095 | 4.284 | 10,131,770.68 | 1.65 | 99.5 |
| 2 | Catechin | C15 H14 O6 | 290.079 | 4.359 | 613,553,783.2* | 100.00 | 98 |
| 3 | (1ξ)-1,5-Anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol | C21 H20 O11 | 448.100 | 4.545 | 321,967,998.3 | 52.48 | 98 |
| 4 | (1S,3R,4R,5R)-1,3,4-trihydroxy-5-{[(2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoyl]oxy}cyclohexane-1-carboxylic acid | C17 H20 O9 | 368.110 | 5.22 | 8,509,566.354 | 1.39 | 97.6 |
| 5 | Quercetin-3β-D-glucoside | C21 H20 O12 | 464.095 | 5.373 | 504,131,364.8 | 82.17 | 98.4 |
| 6 | Rutin | C27 H30 O16 | 610.153 | 5.467 | 32,821,476.28 | 5.35 | 98.8 |
| 7 | Syringic acid | C9 H10 O5 | 198.052 | 5.673 | 28,560,131.71 | 4.65 | 84.3 |
| 8 | 4,5-Dicaffeoylquinic acid | C25 H24 O12 | 516.126 | 6.347 | 12,801,957.73 | 2.09 | 93.5 |
| 9 | Apigetrin | C21 H20 O10 | 432.105 | 6.439 | 17,355,630.28 | 2.83 | 85.4 |
| 10 | Genistein | C15 H10 O5 | 270.053 | 6.517 | 3,237,563.774 | 0.53 | 89 |
| 11 | 4-(3,4-dihydroxyphenyl)-7-hydroxy-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl]oxy}-2H-chromen-2-one | C21 H20 O11 | 448.100 | 6.571 | 9,208,855.303 | 1.50 | 98.4 |
| 12 | Juglalin | C20 H18 O10 | 418.090 | 6.589 | 27,689,699.26 | 4.51 | 93.1 |
| 13 | NP-015559 | C17 H14 O7 | 330.074 | 6.869 | 59,518,391.27 | 9.70 | 93.9 |
| 14 | 2,4,6-Trihydroxy-2-(4-hydroxybenzyl)-1-benzofuran-3(2H)-one | C15 H12 O6 | 288.063 | 6.971 | 7,881,929.5 | 1.28 | 92.4 |
| 15 | Luteolin | C15 H10 O6 | 286.047 | 7.355 | 271,424,429.2 | 44.24 | 92.4 |
| 16 | 3-tert-Butyladipic acid | C10 H18 O4 | 202.120 | 7.609 | 6,456,519.47 | 1.05 | 80.4 |
| 17 | Quercetin | C15 H10 O7 | 302.042 | 7.654 | 148,931,203.7 | 24.27 | 97.6 |
| 18 | Eriodictyol | C15 H12 O6 | 288.063 | 7.746 | 10,578,247.87 | 1.72 | 73.1 |
| 19 | 3-Methoxy-5,7,3',4'-tetrahydroxy-flavone | C16 H12 O7 | 316.058 | 8.023 | 47,968,532.18 | 7.82 | 99.1 |
| 20 | NP-019001 | C18 H12 O7 | 340.058 | 8.286 | 39,751,166.39 | 6.48 | 76.1 |
| 21 | Corchorifatty acid F | C18 H32 O5 | 328.225 | 8.355 | 80,920,096.63 | 13.19 | 97.1 |
| 22 | (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | C18 H34 O5 | 330.240 | 8.775 | 30,194,667.16 | 4.92 | 85.2 |
| 23 | 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) | C23 H32 O2 | 340.240 | 16.552 | 22,234,098.55 | 3.62 | 96.3 |
Relative percentage abundance (% RA) was measured by the ratio RA of the given peak area to RA of the *reference peak
Table 5.
Mass spectrometric analysis of the ethyl-acetate fraction of M. pudica aerial parts using high-resolution mass spectrometry in positive mode
| No | Name | Formula | Calc. MW | RT [min] | Area (Max.) | % RA | mzCloud Best Match |
|---|---|---|---|---|---|---|---|
| 1 | Choline | C5 H13 N O | 103.0999 | 0.963 | 38,986,744.01 | 5.70 | 95.7 |
| 2 | D-( +)-Proline | C5 H9 N O2 | 115.0634 | 1.016 | 19,894,456.59 | 2.91 | 92.2 |
| 3 | Adenine | C5 H5 N5 | 135.0543 | 1.028 | 684,067,887* | 100.00 | 99.4 |
| 4 | NP-019811 | C6 H7 N O2 | 125.0477 | 1.037 | 237,023,365 | 34.65 | 95.4 |
| 6 | Pyrrole-2-carboxylic acid | C5 H5 N O2 | 111.0322 | 1.047 | 54,258,960.28 | 7.93 | 95 |
| 7 | Tropine | C8 H15 N O | 141.1153 | 1.056 | 60,269,180.57 | 8.81 | 99.5 |
| 8 | 3-Hydroxypyridine | C5 H5 N O | 95.03736 | 1.064 | 178,293,700.5 | 26.06 | 100 |
| 9 | Pyridoxine | C8 H11 N O3 | 169.0737 | 1.112 | 48,875,824.48 | 7.14 | 99.4 |
| 10 | NP-000358 | C15 H14 O7 | 306.0737 | 1.139 | 24,522,474.57 | 3.58 | 99.5 |
| 11 | L-Isoleucine | C6 H13 N O2 | 131.0946 | 1.562 | 9,498,791.612 | 1.39 | 90.4 |
| 12 | Nicotinamide | C6 H6 N2 O | 122.048 | 1.616 | 23,108,176.84 | 3.38 | 91 |
| 13 | Nicotinic acid | C6 H5 N O2 | 123.032 | 1.618 | 32,845,375.37 | 4.80 | 95 |
| 14 | N, N-Dimethylaniline | C8 H11 N | 121.0892 | 3.791 | 31,350,071.07 | 4.58 | 86.7 |
| 15 | (1ξ)-1,5-Anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol | C21 H20 O11 | 448.0997 | 4.601 | 316,295,296.2 | 46.24 | 96.7 |
| 16 | 5-Methoxysalicylic acid | C8 H8 O4 | 168.0422 | 4.732 | 12,290,077.79 | 1.80 | 77.2 |
| 17 | Scopoletin | C10 H8 O4 | 192.0422 | 5.049 | 20,362,873.88 | 2.98 | 92.5 |
| 18 | Esculetin | C9 H6 O4 | 178.0265 | 5.105 | 14,215,495.18 | 2.08 | 89.9 |
| 19 | Cynaroside | C21 H20 O11 | 448.0997 | 5.288 | 122,295,357.9 | 17.88 | 99 |
| 20 | (1S)-1,5-Anhydro-2-O-(6-deoxy-α-L-mannopyranosyl)-1-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-6-yl]-D-glucitol | C27 H30 O14 | 578.1632 | 5.448 | 22,312,282.07 | 3.26 | 93.5 |
| 21 | NP-021018 | C12 H18 O4 | 226.1203 | 5.455 | 40,180,312.67 | 5.87 | 73.9 |
| 22 | Toliprolol | C13 H21 N O2 | 223.157 | 5.471 | 44,615,504.37 | 6.52 | 71.5 |
| 23 | Rutin | C27 H30 O16 | 610.1529 | 5.481 | 12,773,553.55 | 1.87 | 98.8 |
| 24 | Jasmonic acid | C12 H18 O3 | 210.1254 | 5.587 | 5,903,842.209 | 0.86 | 73.1 |
| 25 | 1,5-Anhydro-1-[5,7-dihydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]hexitol | C21 H20 O10 | 432.1053 | 5.614 | 170,435,176.3 | 24.91 | 72.9 |
| 26 | Quercetin-3β-D-glucoside | C21 H20 O12 | 464.095 | 5.737 | 163,476,631.8 | 23.90 | 99.6 |
| 27 | Quercetin | C15 H10 O7 | 302.0422 | 5.751 | 165,098,283.2 | 24.13 | 98.5 |
| 28 | NP-018720 | C27 H28 O16 | 608.1373 | 5.986 | 6,274,504.294 | 0.92 | 97 |
| 29 | Avicularin | C20 H18 O11 | 434.0843 | 6.131 | 324,561,610.4 | 47.45 | 99.7 |
| 30 | Aflatoxin G1 | C17 H12 O7 | 328.0578 | 6.187 | 200,501,817.2 | 29.31 | 86 |
| 31 | Diosmetin | C16 H12 O6 | 300.063 | 6.206 | 71,678,113.21 | 10.48 | 87 |
| 32 | 4-Coumaric acid | C9 H8 O3 | 164.0473 | 6.213 | 23,341,562.64 | 3.41 | 91.5 |
| 33 | NP-015559 | C17 H14 O7 | 330.0734 | 6.384 | 61,595,289.75 | 9.00 | 90 |
| 34 | Vitexin | C21 H20 O10 | 432.1053 | 6.443 | 36,425,664.32 | 5.32 | 76.5 |
| 35 | Galangin | C15 H10 O5 | 270.0525 | 6.531 | 9,846,563.576 | 1.44 | 98.7 |
| 36 | 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl β-L-xylofuranoside | C20 H18 O10 | 418.0895 | 6.602 | 28,743,146.4 | 4.20 | 94.5 |
| 37 | Isokaempferide | C16 H12 O6 | 300.063 | 6.784 | 4,774,062.327 | 0.70 | 87.4 |
| 38 | 5-O-Methylgenistein | C16 H12 O5 | 284.0682 | 6.838 | 11,697,238.21 | 1.71 | 98.7 |
| 39 | NP-003294 | C18 H16 O7 | 344.0893 | 6.859 | 6,715,576.313 | 0.98 | 83.1 |
| 40 | NP-000465 | C17 H14 O6 | 314.0787 | 6.941 | 30,654,410.59 | 4.48 | 90.7 |
| 41 | (-)-Caryophyllene oxide | C15 H24 O | 220.1826 | 7.153 | 25,344,135.34 | 3.70 | 83.2 |
| 42 | Chrysin | C15 H10 O4 | 254.0577 | 7.241 | 11,993,651.12 | 1.75 | 98.5 |
| 43 | Kaempferol | C15 H10 O6 | 286.0474 | 7.341 | 304,396,847.3 | 44.50 | 99.4 |
| 44 | NP-021018 | C12 H18 O4 | 226.1203 | 7.36 | 13,126,756.27 | 1.92 | 76.9 |
| 45 | Aflatoxin G2 | C17 H14 O7 | 330.0734 | 7.365 | 5,843,945.045 | 0.85 | 81.8 |
| 46 | N-(2,4-Dimethylphenyl) formamide | C9 H11 N O | 149.084 | 7.814 | 44,191,081.65 | 6.46 | 93.3 |
| 47 | 3-Methoxy-5,7,3',4'-tetrahydroxy-flavone | C16 H12 O7 | 316.0581 | 8.042 | 32,003,335.9 | 4.68 | 99 |
| 48 | 9S,13R-12-Oxophytodienoic acid | C18 H28 O3 | 292.2035 | 8.326 | 18,388,834.54 | 2.69 | 85.9 |
| 49 | Apigenin | C15 H10 O5 | 270.0525 | 8.45 | 53,092,646.63 | 7.76 | 99.8 |
| 50 | α-Pyrrolidinopropiophenone | C13 H17 N O | 203.1311 | 13.213 | 9,792,651.46 | 1.43 | 92.7 |
| 51 | Stearamide | C18 H37 N O | 283.2875 | 16.424 | 15,774,788.86 | 2.31 | 97.4 |
| 52 | Hexadecanamide | C16 H33 N O | 255.2561 | 14.821 | 8,241,911.601 | 1.20 | 86.5 |
| 53 | Oleamide | C18 H35 N O | 281.2717 | 15.321 | 8,537,558.88 | 1.25 | 96 |
Effects of the ethanol extract of M. pudica aerial parts against phospholipase activity
When C. albicans cells (control group) were cultured on the surface of egg-yolk emulsion agar (phospholipase induction), the average value of the phospholipases was 0.69 ± 0.013, demonstrating that the control group released many phospholipases. The mean extracellular phospholipases activity (Pz index) in the cells treated with the ethanol extracts of M. pudica aerial parts at 500 µg/mL and 250 µg/mL, were 0.94 ± 0.002 and 0.90 ± 0.012, respectively (Fig. 4). The extract decreased phospholipase activity significantly at these concentrations. At lower concentrations (125 µg/mL to 7.81 µg/mL), the reduction of phospholipase activity was statistically insignificant (p > 0.05).
Fig. 4.

The effect of ethanol extract of M. pudica aerial parts on the production of phospholipases secreted by C. albicans ATCC 10231. *p ≤ 0.05 and ns (not significant) were calculated by the Kruskal–Wallis test, followed by Dunn’s multiple comparison test
Scanning electron microscopy observations of the effects of the ethyl-acetate fraction of M. pudica aerial parts on C. albicans biofilm
This current work employed scanning electron microscopy (SEM) to investigate the effect of the ethyl acetate fraction of M. pudica aerial parts on the surface morphology of 24 h old C. albicans biofilm. The control cells (without fraction) showed a smooth, regular colony cell shape and a distinct bud morphology, as seen in Fig. 5 from A to C. In contrast to the untreated cells, those treated by the fraction showed an irregular cell shape, rough surface collapses, and disrupted hyphae (Fig. 5D–I).
Fig. 5.
Scanning electron microscopy of C. albicans ATCC 10231 24 h old biofilm with or without treatment with ethyl-acetate fractions of M. pudica aerial parts. The control group (A-B-C) was treated with 62.5 µg/mL (D-E–F) and 125 µg/mL (G-H-I). The arrows pointed to some surface morphological changes
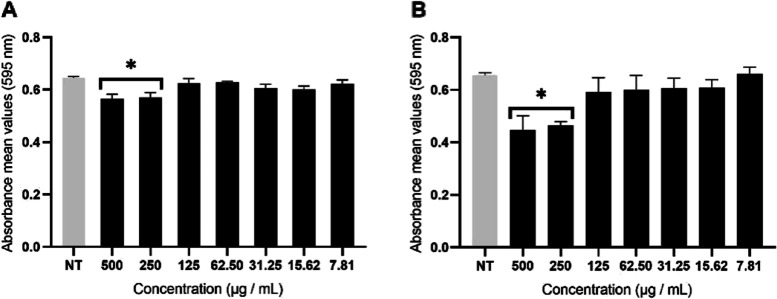
Cytotoxic activity of ethyl-acetate fraction of M. pudica aerial parts
It was evaluated that the ethyl acetate fraction of M. pudica aerial parts was not cytotoxic when tested at the concentration corresponding to its biofilm inhibitory concentration (Fig. 6). Result indicates that at the biofilm inhibitory concentration (50%), 96.77% of Vero cells and 89.91% of HeLa cells were viable. While at the greatest concentration, 500 μg/mL, the viability of Vero and HeLa cells was 61.86% and 85.66%, respectively.
Fig. 6.
Effect of ethyl acetate fraction of M. pudica aerial parts on the metabolic activity (MTT) of Vero (A) and HeLa (B) cells after 24 h treatment. Error bars indicate the standard error of the mean of three independent experiments performed in triplicate. *p < 0.05 calculated by Kruskal Wallis, followed by Dunn's multiple comparison test
Discussion
The ability of C. albicans to grow as a community of adherent cells encapsulated by extracellular matrix puts well-known antifungal drugs at risk of resistance, growing interest of drug discovery by utilizing plants that have existed for millions of years. Plants synthesize secondary metabolites or natural products that are generally divided into three classes, including phenolics, terpenoids, and alkaloids. Numerous studies employed natural products from native plants to combat biofim-induced fungal resistance [42–45]. However, exploring natural products from invasive plants as antibiofilm candidate drugs is still limited.
In this study, we used five invasive plants that were extracted by a maceration method using methanol or ethanol solvents. Furthermore, the most active extract was separated by the LLF method, and solvents were selected based on their degree of polarity. According to the results of the extraction method, methanol provided a greater yield contribution than ethanol. Even though we did not extract every studied plant with a variety of polar solvents, it is possible to hypothesize that increasing the polarity of the solvents might enhance the extraction yields. It means that in this study, methanol was more efficient at extracting phytochemicals of plants than ethanol. This is consistent with what was reported in previous research, which demonstrated that the yield of some plants, such as A. mangium leaf extract, M. pudica aerial parts, and Vernonia auriculifera Hiern leaves, was the greatest when it was extracted in a polar solvent [46–48]. However, the result of the fractionation yield of M. pudica in this study revealed that even though n-hexane is the lowest polarity solvent, it was associated with the highest yield. This finding indicates that most of the substances in M. pudica extract were non-polar substances. It needs to be emphasized, the differences in the extraction/fractionation yield are influenced by several factors, including not only by solvent polarity or typet, but also by extraction or fractionation method, the size of material, extraction time, and temperature [47, 49].
According to our results, the ethanol extracts of M. pudica (aerial parts and roots) exhibited great power of antifungal activity against C. albicans ATCC 10231 with MIC50 of 15.62 µg/mL and 7.81 µg/mL, respectively. Even though the MIC50 was 10–20 fold higher than that of fluconazole, both extracts exhibited comparable fungistatic rather than fungicidal properties to fluconazole. In previous studies, M. pudica extracts showed antifungal activity with various activity levels. It was reported that methanol extract from the leaves of invasive M. pudica growing in India showed an antifungal effect against C. albicans with MIC ranging between 0.394 and 0.398 mg/mL [50]. Two other studies evaluated the M. pudica antifungal activity by an agar disk diffusion method: the first one reported that ethanol extract of M. pudica leaves was effective against C. albicans at 30 mg/mL with a zone of inhibition of 17 mm [51] and the second study reported that M. pudica fractions and its diterpenoids, named 19-O-transferuloyl-labd-8(17)-en-15,19-diol and 19-O-[(E)-3’,4’-dimethoxy cinnamoyl]-labd-8(17)-en15,19-diol, inhibited C. albicans with an inhibition zone ranging from 9–15 mm [52]. Overall, the MICs of M. pudica extracts in our study against C. albicans were lower than those reported in the literature. It is noteworthy that it is difficult to compare our results with the reported literature because of the variations in the utilization of solvent, extraction/fractionation process, and antifungal method. In addition, it is conceivable that the chemical content of invasive M. pudica plants cultivated in Indonesia differs from those grown in other countries.
The antifungal activity of the root extract of M. pudica was mainly influenced by the bioactive compounds that either function independently, in synergy, or antagonistically with the other compounds. The LC–MS/MS analyses suggested that alkaloids are secondary plant metabolites that might be responsible for antifungal activity. However, we cannot disallow the possibility that existing flavonoids in the extract might also have this effect. To our best knowledge, no literature has yet reported these compounds present in the extract from the root of M. pudica. However, some compounds of tropane alkaloid were found in Datura stramonium, and particularly, 3α-tigloyloxytropane has been found in the variant D. Stramonium grown in Egypt [53]. But, no literature reported the antifungal activity of a 3α-tigloyloxytropane against C. albicans. The compounds of polyphenols (epigallocatechin(4β,8)-gallocatechin, and gallocatechin) and flavonoid (3,5,6-trihydroxy-4',7-dimethoxyflavone) which present in our study, might be contributing to the antifungal activity against C. albicans. The anti-Candida properties of polyphenol compounds have been reported in the literature: Evensen and Braun, 2009 demonstrated that phenolic compounds in green tea extracts reduced by 43% the growth of C. albicans when used at 5 mg/mL [54]. Other studies reported that proanthocyanidins (oligomeric flavonoids composed by derivatives of catechin and epicatechin and their gallic acid esters) polymer-rich fractions from the stem bark of Stryphnodendron adstringens revealed antifungal activity against C. albicans with MIC values of 15.6 μg/mL [55]. Finally, the mixture of epigallocatechin, gallocatechin, and epigallocatechin-(4β → 8)-gallocatechin in the subfractions from the stem bark of Stryphnodendron obovatum Benth showed antifungal activity against C. albicans and C. parapsilosis with MIC ranging from 31.5 µg/mL to 125 µg/mL [56].
The ethyl acetate fraction obtained from the aerial parts of M. pudica had good activity against planktonic cells of C. albicans ATCC 10231. The HR-MS analyses of this fraction revealed the presence of several kinds of compounds and, in particular, flavonoids such as avicularin, quercetin, luteolin, rutin, kaempferol, catechin, quercetin-3β-D-glucoside, and 1ξ-1,5-anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-8-yl]-D-galactitol. It is known that flavonoids have an intermediate polarity, making them extractable with ethyl-acetate [57]. They are also linked to multiple antifungal pathways, including disturbance of the plasma membrane, stimulation of mitochondrial malfunction, suppression of cell structural work, cell division, RNA and protein synthesis, and efflux mediated pumping systems [58, 59].
Several studies demonstrated the presence of flavonoids in invasive M. pudica plant: Sapkota et al. reported that an ethyl-acetate fraction of M. pudica growing in Nepal contained quercetin, catechin, and avicularin [60]; Yusof et al.determined orientin, kaempferol 7-rutinoside, and kaempferol 3-glucoside-7-rhamnoside in M. pudica aerial parts [61]; and Lobstein et al. isolated myricetin and two C-glycosylflavones, 4″-hydroxymaysin and cassiaoccidentalin B, from M. pudica aerial parts [62].
Literature reported that avicularin, kaempferol, luteolin, and quercetin inhibited the growth of planktonic Candida species [63–66]. Furthermore, it has been established that quercetin inhibited fatty acid synthase, an enzyme essential for endogenous fatty acid production in the fungal membrane, as part of its antifungal action [42]. In addition, quercetin induced apoptosis of C. albicans by increasing intracellular Mg2 + , mitochondrial Ca2 + , and mitochondrial dysfunction, which triggers the decline in mitochondrial redox levels and disruption in the mitochondrial antioxidant system [67].
Concerning the antibiofilm activity, the ethyl-acetate fraction of M. pudica aerial parts inhibited the metabolic activity of 24 h old biofilms of C. albicans. To the best of our knowledge, this is the first study describing the antibiofilm activity of M. pudica. The ability of M. pudica to inhibit biofilm formation was previously described only against a single-species bacterial biofilm, Streptococcus mutans biofilm [68]. Related to the effects of flavonoids against Candida biofilms, it has been reported that kaempferol inhibited C. albicans biofilm by reducing the hyphal formation and hydrophobicity of the fungal cell surface [43]. Another study showed that kaempferol and quercetin diminished the biomass of C. orthopsilosis and C. metapsilosis and the metabolic activity and biomass of developing biofilms of the C. parapsilosis complex [65]. Also, cathechin inhibited the biofilm formation of C. albicans involving proteasomal enzyme activity leading to metabolic instability and membrane cell disruption [54]. Concerning luteolin, this flavonoid doesn't seem like a good antibiofilm candidate, as a high concentration (625–5000 µg/mL) was required to prevent the formation of C. albicans biofilms [66]. Based on the literature, it was speculated that the activity of studied M. pudica against 24 h old C. albicans biofilm was due to the presence of flavonoids. However, the possible implications of other existing components in the present study are still required to determine the activity. For example, the presence of terpenes or terpenoids in our studied plant might play a role in the antibiofilm activity. Several studies have reported that terpenes showed antifungal [69] and antibiofilm activity [44, 70]. Indeed, work of Spengler et al., 2022 demonstrated the antibiofilm mechanism via efflux pump inhibitory on some bacteria [44].
Regarding the biofilm structure, SEM observations showed that the ethyl-acetate fraction of M. pudica aerial parts (at 62.5 and 125 µg/mL) influenced the surface morphology of C. albicans cells, and notably, no cytotoxic effect on the Hela and Vero cells evaluated at these concentrations. This finding demonstrated a qualitative correlation between the biofilm observed by microscopy and metabolic activity. After observing the effects of M. pudica on the growth of C. albicans cells in planktonic and biofilm modes, the possible action of the extract of M. pudica aerial parts in inhibiting the secretion of phospholipase enzyme was evaluated. The production of phospholipase enzyme is a fundamental event in the pathogenesis of C. albicans during the adhesion and invasion stages by damaging and penetrating host cell membranes, promoting blastospore hyphal development, etc.. [11, 71]. Our results showed that M. pudica aerial parts reduced phospholipase secretion, but it was only significant at high concentrations. To the best of our knowledge, this was the first study to report the effects of ethanol extract M. pudica on phospholipase enzyme.
Conclusions and future directions
The screening of the activity of five invasive plant extracts grown in Indonesia against C. albicans biofilms highlighted the interest in the ethyl acetate fraction of M. pudica aerial parts. To the best of our knowledge, this is the first study to investigate the effects of M. pudica on virulence factors, particularly against C. albicans biofilm. Even though ethanol extracts of M. pudica aerial parts and roots showed good antifungal activity, since the main objective of this work was to find antibiofilm compounds, we did not deeply investigate the antifungal potential of those extracts. However, this promising activity encourages further study, starting with testing it on other clinical strains and fungal species of Candida to clarify its spectrum of antifungal activity. Meanwhile, even though flavonoids in the ethyl acetate fraction of M. pudica aerial parts might exert antibiofilm activity, other components cannot be ignored. Thus, some steps can be taken, including 1} chemical characterization should be applied along with the evaluation of antibiofilm activity (bioassay-guided isolation). Therefore, the active pure compound can be determined. Another fast track to determining active pure compound is by 2} using bio-chemometric study, an interdisciplinary research field involving multivariate statistics, mathematical modeling, and computing, and is particularly applied to understanding chemical data, specifically in this term for the antibiofilm activity of bioactive compounds.
Supplementary Information
Acknowledgements
The authors would like to acknowledge the facilities, scientific and technical support of the Advanced Characterization Laboratories Serpong, National Research, and Innovation Institute through E- Layanan Sains, Badan Riset dan Inovasi Nasional and Laboratorium Penelitian dan Pengujian Terpadu (LPPT)-Universitas Gadjah Mada. The authors thank Rumbiwati from the Parasitology Department of Universitas Gadjah Mada for her assistance in performing cytotoxic assays.
Authors’ contribution
SD performed the experiments; SD, MM, TN conceived and designed the experiments; SD wrote the articles; MG, CI, MM, TN contributed materials tools; SD, MG, CI, MM, TN analyzed data. All authors have read and approved the final version of the manuscript.
Funding
The authors thank the Indonesia Endowment Fund for Education (LPDP, Lembaga Pengelola Dana Pendidikan) and the Final Project Recognition Grant Universitas Gadjah Mada Number 5075/UN1.PII/Dit-Lit/PT.01.01/2023 for financial support.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The study complies with relevant institutional, national, and international guidelines and legislation for plant ethics. Indeed, this study received approval from Medical and Health Research Ethics Commitee (MHREC)) of the Faculty of Medicine, Publich Health and Nursing, Universitas Gadjah Mada with number reference: KE/FK/0243/EC/2021.
Consent for application
Not applicable.
Competing interests
The authors have no competing financial interests or close personal ties that might influence the research described in this publication.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arshad H, Garcia S, Khaja M. Case report of invasive, disseminated candidiasis with peripheral nodular cavitary lesions in the lung. Respir Med Case Reports. 2017;20:34–37. doi: 10.1016/j.rmcr.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Prim. 2018;4(May):1–20. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 3.Tan BH, Chakrabarti A, Li RY, Patel AK, Watcharananan SP, Liu Z, et al. Incidence and species distribution of candidaemia in Asia: A laboratory-based surveillance study. Clin Microbiol Infect. 2015;21(10):946–953. doi: 10.1016/j.cmi.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Wahyuningsih R, Adawiyah R, Sjam R, Prihartono J, Ayu Tri Wulandari E, Rozaliyani A, et al. Serious fungal disease incidence and prevalence in Indonesia. Mycoses. 2021;64(10):1203–12. doi: 10.1111/myc.13304. [DOI] [PubMed] [Google Scholar]
- 5.Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJGT, Bohlius J, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25(10):1200–12. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Invasive Candidiasis Statistics. 2021 [cited 2022 Feb 17]. Available from: https://www.cdc.gov/fungal/diseases/candidiasis/invasive/statistics.html.
- 7.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce CG, Vila T, Romo JA, Montelongo-Jauregui D, Wall G, Ramasubramanian A, et al. The Candida albicans biofilm matrix: Composition, structure and function. J Fungi. 2017;3(1). [DOI] [PMC free article] [PubMed]
- 9.Hong Y, Zhao J, Guo L, Kim SC, Deng X, Wang G, et al. Plant phospholipases D and C and their diverse functions in stress responses. Prog Lipid Res. 2016;62:55–74. doi: 10.1016/j.plipres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Udayalaxmi J, Shenoy N. Comparison between biofilm production, phospholipase and haemolytic activity of different species of candida isolated from dental caries lesions in children. J Clin Diagnostic Res. 2016;10(4):DC21–3. doi: 10.7860/JCDR/2016/17019.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13(1):122–143. doi: 10.1128/CMR.13.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barman A, Gohain D, Bora U, Tamuli R. Phospholipases play multiple cellular roles including growth, stress tolerance, sexual development, and virulence in fungi. Microbiol Res. 2018;209(August 2017):55–69. doi: 10.1016/j.micres.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL, Nett JE, et al. Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci U S A. 2015;112(13):4092–4097. doi: 10.1073/pnas.1421437112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh R, Kumari A, Kaur K, Sethi P, Chakrabarti A. Relevance of antifungal penetration in biofilm-associated resistance of Candida albicans and non-albicans Candida species. J Med Microbiol. 2018;67(7):922–926. doi: 10.1099/jmm.0.000757. [DOI] [PubMed] [Google Scholar]
- 15.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J Med Microbiol. 2006;55(8):999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 16.Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother. 2006;50(6):2058–2063. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Kelly R, Kahn JN, Robles J, Hsu MJ, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49(8):3264–73. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying S, Chunyang L. Correlation between phospholipase of Candida albicans and resistance to fluconazole. Mycoses. 2012;55(1):50–55. doi: 10.1111/j.1439-0507.2011.02024.x. [DOI] [PubMed] [Google Scholar]
- 19.El-Houssaini HH, Elnabawy OM, Nasser HA, Elkhatib WF. Influence of subinhibitory antifungal concentrations on extracellular hydrolases and biofilm production by Candida albicans recovered from Egyptian patients. BMC Infect Dis. 2019;19(1):1–9. doi: 10.1186/s12879-019-3685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howes MJR, Quave CL, Collemare J, Tatsis EC, Twilley D, Lulekal E, et al. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet. 2020;2(5):463–481. doi: 10.1002/ppp3.10138. [DOI] [Google Scholar]
- 21.Lannone III B V, Bell EC, Carnevale S, Hill JE, Mcconnell J, Main M, et al. Standardized Invasive Species Terminology for Effective Outreach Education 1 Educating Stakeholders about Invasive Species : A Need for Standardized Terms Seven Terms to Use : A Standard Set of Terms to Communicate About Invasive Species across All. 2021;1–8.
- 22.IPBES 2019. IPBES (2019): Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Díaz S, Settele J, Brondízio ES, Ngo HT, Guèze M, Agard J, et al., editors. Bonn, Germany: IPBES secretariat; 2019. Available from: https://ipbes.net/global-assessment
- 23.Menteri Kesehatan RI 2007. Keputusan Menteri Kesehatan Republik Indonesia No : 381/MenKes/SK/III/2007. 2007.
- 24.KLHK. Strategi Nasional dan Arahan Rencana Aksi Pengelolaan Jenis Asing Invasif di Indonesia. 2015. 55 p.
- 25.Samoisy AK, Mahomoodally MF. Ethnopharmacological analysis of medicinal plants used against non-communicable diseases in Rodrigues Island. Indian Ocean J Ethnopharmacol. 2015;173:20–38. doi: 10.1016/j.jep.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad H, Sehgal S, Mishra A, Gupta R. Mimosa pudica L. (Laajvanti): An overview. Pharmacogn Rev. 2012;6(12):115–24. [DOI] [PMC free article] [PubMed]
- 27.Kirimuhuzya C, Waako P, Joloba M, Odyek O. The anti-mycobacterial activity of Lantana camara a plant traditionally used to treat symptoms of tuberculosis in South-western Uganda. Afr Health Sci. 2009;9(1):40–45. [PMC free article] [PubMed] [Google Scholar]
- 28.Deena MJ, Thoppil JE. Antimicrobial activity of the essential oil of Lantana camara. Fitoterapia. 2000;71(4):453–455. doi: 10.1016/S0367-326X(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 29.Verma RK, Verma SK. Phytochemical and termiticidal study of Lantana camara var. aculeata leaves. Fitoterapia. 2006;77(6):466–8. doi: 10.1016/j.fitote.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Máximo P, Ferreira LM, Branco PS, Lourenço A. Invasive plants: Turning enemies into value. Molecules. 2020;25(15). [DOI] [PMC free article] [PubMed]
- 31.Setyawati T, Narulita S, Bahri IP, Raharjo GT. A Guide Book to Invasive Plant Species in Indonesiae. 2015. 1–440 p.
- 32.Abubakar, Abdullahi R. Haque M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J Pharm Bioallied Sci. 2020;12(1):1–10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7398001/ [DOI] [PMC free article] [PubMed]
- 33.Ngouana V, Zeuko’O Menkem E, Youmbi DY, Yimgang LV, Toghueo RMK, Boyom FF. Serial Exhaustive Extraction Revealed Antimicrobial and Antioxidant Properties of Platycerium stemaria (Beauv) Desv. Biomed Res Int. 2021;2021. [DOI] [PMC free article] [PubMed]
- 34.Maryuni DR, Prameswari DA, Astari SD, Sari SP, Putri DN. Identification of active compounds in red onion (Allium ascalonicum) peel extract by LC-ESI-QTOF-MS/MS and determination of its antioxidant activity. J Teknol Has Pertan. 2022;15(1):20. doi: 10.20961/jthp.v15i1.55451. [DOI] [Google Scholar]
- 35.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard - third edition. Vol. 28, CLSI document M27-A3. 2008. 1–25 p.
- 36.Premamalini T, Anitha S, Mohanapriya K. Evaluation of 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyl tetrazolium bromide method for assessing biofilm formation in vitro by Trichosporon spp. J Lab Physicians. 2018;10:380–386. doi: 10.4103/JLP.JLP_37_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prażyńska M, Gospodarek E. In Vitro Effect of Amphotericin B on Candida albicans, Candida glabrata and Candida parapsilosis Biofilm Formation. Mycopathologia. 2014;177(1–2):19–27. doi: 10.1007/s11046-014-9727-7. [DOI] [PubMed] [Google Scholar]
- 38.Pereira JV, Freires IA, Castilho AR, da Cunha MG, Alves H da S, Rosalen PL. Antifungal potential of Sideroxylon obtusifolium and Syzygium cumini and their mode of action against Candida albicans. Pharm Biol. 2016;54(10):2312–9. [DOI] [PubMed]
- 39.Li X, Yu C, Huang X, Sun S. Synergistic effects and mechanisms of budesonide in combination with fluconazole against resistant candida albicans. PLoS ONE. 2016;11(12):1–20. doi: 10.1371/journal.pone.0168936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nugroho AE, Hermawan A, Putri DDP, Novika A, Meiyanto E. Combinational effects of hexane insoluble fraction of Ficus septica Burm. F. and doxorubicin chemotherapy on T47D breast cancer cells. Asian Pac J Trop Biomed. 2013;3(4):297–302. doi: 10.1016/S2221-1691(13)60066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karatt TK, Nalakath J, Perwad Z, Albert PH, Abdul Khader KK, Syed Ali Padusha M, et al. Mass spectrometric method for distinguishing isomers of dexamethasone via fragment mass ratio: An HRMS approach. J Mass Spectrom. 2018;53(11):1046–58. [DOI] [PubMed]
- 42.Bitencourt TA, Komoto TT, Massaroto BG, Miranda CES, Beleboni RO, Marins M, et al. Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Complement Altern Med. 2013;13. [DOI] [PMC free article] [PubMed]
- 43.Chen L, Shen J, Yan W, Fan L, Cao Y. Study on the antibiofilm activity of kaempferol in Candida albicans. J Pharm Pract. 2020;6:413–417. [Google Scholar]
- 44.Spengler G, Gajdács M, Donadu MG, Usai M, Marchetti M, Ferrari M, et al. Evaluation of the Antimicrobial and Antivirulent Potential of Essential Oils Isolated from Juniperus oxycedrus L. ssp. macrocarpa Aerial Parts. Microorganisms. 2022;10(4):1–19. [DOI] [PMC free article] [PubMed]
- 45.Li H, Kong Y, Hu W, Zhang S, Wang W, Yang M, et al. Litsea cubeba Essential Oil: Component Analysis, Anti-Candida albicans Activity and Mechanism Based on Molecular Docking. J Oleo Sci. 2022;71(8):1221–1228. doi: 10.5650/jos.ess22108. [DOI] [PubMed] [Google Scholar]
- 46.Shafiei SNS, Ahmad K, Fatin Mohd Ikhsan N, Izera Ismail S, Sijam K. Antibacterial Activity of Acacia spp. Leaves Extracts against Xanthomonas oryzae pv. oryzae and Screening for Active Phytochemical Contents. 2017;10(11):49–60.
- 47.Baharuddin NS, Roslan MAM, Bawzer MAM, Mohamad Azzeme A, Rahman ZA, Khayat ME, et al. Response surface optimization of extraction conditions and in vitro antioxidant and antidiabetic evaluation of an under-valued medicinal weed, Mimosa pudica. Plants. 2021;10(8). [DOI] [PMC free article] [PubMed]
- 48.Wado TE, Suleman S, Mohammed T. Antimicrobial Evaluation of Sequentially extracted Leaf of Vernonia auriculifera Hiern (Rejicho) BMC Complement Med Ther. 2022;22(1):1–10. doi: 10.1186/s12906-022-03690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunha IBS, Sawaya ACHF, Caetano FM, Shimizu MT, Marcucci MC, Drezza FT, et al. Factors that influence the yield and composition of Brazilian propolis extracts. J Braz Chem Soc. 2004;15(6):964–970. doi: 10.1590/S0103-50532004000600026. [DOI] [Google Scholar]
- 50.Mohan G, Doss A, Anand S. Efficacy of Aqueous and Methanol extracts of Caesalpinia sappan L. and Mimosa pudica L. for their potential Antimicrobial activity. South As J Biol Sci. 2011;1(2):48–57. [Google Scholar]
- 51.Kaur P, Kumar N, Shivananda TN, Kaur G. Phytochemical screening and antibacterial activity of Mimosa pudica L. and Mimosa invisa L. against selected microbes. J Med Plants Res. 2011;5(22):5356–9. [Google Scholar]
- 52.Shu W-J, Ho J-C. Two New Antimicrobial Diterpenoids from the Roots of Mimosa pudica. Chin Med J (Engl) 2013;24(2):223–229. [Google Scholar]
- 53.Berkov S, Zayed R, Doncheva T. Alkaloid patterns in some varieties of Datura stramonium. Fitoterapia. 2006;77(3):179–182. doi: 10.1016/j.fitote.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Evensen NA, Braun PC. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can J Microbiol. 2009;55(9):1033–1039. doi: 10.1139/W09-058. [DOI] [PubMed] [Google Scholar]
- 55.Luiz RLF, Vila TVM, de Mello JCP, Nakamura CV, Rozental S, Ishida K. Proanthocyanidins polymeric tannin from Stryphnodendron adstringens are active against Candida albicans biofilms. BMC Complement Altern Med. 2015;15(1):1–11. doi: 10.1186/s12906-015-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanches ACC, Lopes GC, Nakamura CV, Dias Filho BP, De Mello JCP. Antioxidant and antifungal activities of extracts and condensed tannins from Stryphnodendron obovatum Benth. Rev Bras Ciencias Farm J Pharm Sci. 2005;41(1):101–107. doi: 10.1590/S1516-93322005000100012. [DOI] [Google Scholar]
- 57.de Morais CB, Scopel M, Pedrazza GPR, da Silva FK, Dalla Lana DF, Tonello ML, et al. Anti-dermatophyte activity of Leguminosae plants from Southern Brazil with emphasis on Mimosa pigra (Leguminosae). J Mycol Med. 2017;27(4):530–8. Available from: http://dx.doi.org/10.1016/j.mycmed.2017.07.006 [DOI] [PubMed]
- 58.Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci. 2016;5. [DOI] [PMC free article] [PubMed]
- 59.Yun J, Lee H, Ko HJ, Woo ER, Lee DG. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim Biophys Acta - Biomemb. 2015;1848(2):695–701. doi: 10.1016/j.bbamem.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Sapkota BK, Khadayat K, Aryal B, Bashyal J, Jaisi S, Parajuli N. LC-HRMS-Based Profiling: Antibacterial and Lipase Inhibitory Activities of Some Medicinal Plants for the Remedy of Obesity. Sci Pharm. 2022;90(3):55. doi: 10.3390/scipharm90030055. [DOI] [Google Scholar]
- 61.Yusof UK, Abdullah F, Abdullah N, Itam K, Bakar B, Sukari M. Chemotaxonomic survey of Malaysian Mimosa species. Sains Malaysiana. 2003;32:121–129. [Google Scholar]
- 62.Lobstein A, Weniger B, Um BH, Steinmetz M, Declercq L, Anton R. 4″-Hydroxymaysin and cassiaoccidentalin B, two unusual C-glycosylflavones from Mimosa pudica (Mimosaceae) Biochem Syst Ecol. 2002;30(4):375–377. doi: 10.1016/S0305-1978(01)00086-2. [DOI] [Google Scholar]
- 63.Özçelik B, Kartal M, Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm Biol. 2011;49(4):396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 64.Da Silva Sa FA, De Paula JAM, Dos Santos PA, Oliveira LDAR, Oliveira GDAR, Liao LM, et al. Phytochemical analysis and antimicrobial activity of Myrcia tomentosa (Aubl.) DC. leaves. Molecules. 2017;22(7):1–10. [DOI] [PMC free article] [PubMed]
- 65.Rocha MFG, Sales JA, da Rocha MG, Galdino LM, de Aguiar L, Pereira-Neto WA, et al. Antifungal effects of the flavonoids kaempferol and quercetin: a possible alternative for the control of fungal biofilms. Biofouling. 2019;35(3):320–8. doi: 10.1080/08927014.2019.1604948. [DOI] [PubMed] [Google Scholar]
- 66.Ivanov M, Kannan A, Stojković DS, Glamočlija J, Calhelha RC, Ferreira ICFR, et al. Flavones, flavonols, and glycosylated derivatives—impact on candida albicans growth and virulence, expression of cdr1 and erg11, cytotoxicity. Pharmaceuticals. 2021;14(1):1–12. doi: 10.3390/ph14010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwun MS, Lee DG. Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol. 2020;124(2):83–90. doi: 10.1016/j.funbio.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Kavya B, Nagaraj NJ, Madhubala MM, Mahalaxmi S. Antibiofilm Efficacy of Mimosa pudica against Clinical Isolates of Streptococcus mutans as a Mouthrinse. World J Dent. 2022;13(6):582–586. doi: 10.5005/jp-journals-10015-2097. [DOI] [Google Scholar]
- 69.Cavaleiro C, Pinto E, Gonçalves MJ, Salgueiro L. Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J Appl Microbiol. 2006;100(6):1333–1338. doi: 10.1111/j.1365-2672.2006.02862.x. [DOI] [PubMed] [Google Scholar]
- 70.Lemos ASO, Florêncio JR, Pinto NCC, Campos LM, Silva TP, Grazul RM, et al. Antifungal Activity of the Natural Coumarin Scopoletin Against Planktonic Cells and Biofilms From a Multidrug-Resistant Candida tropicalis Strain. Front Microbiol. 2020;11(July):1–11. doi: 10.3389/fmicb.2020.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dalle F, Wächtler B, L’Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12(2):248–271. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.