Abstract
Background
After neoadjuvant therapy, most of the lymph nodes (LNs) will shrink and disappear in patients with rectal cancer. However, LNs that are still detectable on MRI carry a risk of metastasis. This study aimed to evaluate the performance of the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) criterion (short-axis diameter ≥ 5 mm) in diagnosing malignant LNs in patients with rectal cancer after neoadjuvant therapy, and whether nodal morphological characteristics (including shape, border, signal homogeneity, and enhancement homogeneity) could improve the diagnostic efficiency for LNs ≥ 5 mm.
Methods
This retrospective study included 90 patients with locally advanced rectal cancer who underwent surgery after neoadjuvant therapy and performed preoperative MRI. Two radiologists independently measured the short-axis diameter of LNs and evaluated the morphological characteristics of LNs ≥ 5 mm in consensus. With a per node comparison with histopathology as the reference standard, a ROC curve was performed to evaluate the diagnostic performance of the size criterion. For categorical variables, either a χ2 test or Fisher’s exact test was used.
Results
A total of 298 LNs were evaluated. The AUC for nodal size in determining nodal status was 0.81. With a size cutoff value of 5 mm, the sensitivity, specificity, positive predictive value, negative predictive value and accuracy were 65.9%, 87.0%, 46.8%, 93.6% and 83.9%, respectively. No significant differences were observed in any of the morphological characteristics between benign and malignant LNs ≥ 5 mm (all P > 0.05).
Conclusions
The ESGAR criterion demonstrated moderate diagnostic performance in identifying malignant LNs in patients with rectal cancer after neoadjuvant therapy. It was effective in determining the status of LNs < 5 mm but not for LNs ≥ 5 mm, and the diagnostic efficiency could not be improved by considering nodal morphological characteristics.
Keywords: Rectal cancer, Neoadjuvant therapy, Lymph node, Magnetic resonance imaging, Histopathology
Background
Patients with locally advanced rectal cancer (LARC) and those who are unable to undergo radical resection are recommend to receive neoadjuvant therapy, which aims to increase the possibility of complete resection and reduce the risk of local recurrence by downstaging and downsizing [1]. After neoadjuvant therapy, most of the lymph nodes (LNs) shrink and disappear, but the LNs that can still be detected on MRI carry a risk of metastasis, with LNs larger than 5 mm having a 38.6% possibility of being pathologically metastatic [2]. Even in patients with a pathological complete response, 3.2-14% of visible LNs are malignant [3, 4]. Malignant LNs can lead to poor prognosis, including local recurrence and reduced long-term survival rate [1, 5]. Therefore, for patients after neoadjuvant therapy, particularly whose who are nearly clinical complete responders, MRI restaging of LNs is valuable in evaluating the feasibility of conservative treatment, such as a local excision or a watch-and-wait strategy.
Restaging of benign and malignant LNs is more challenging than primary staging in rectal cancer. Most studies recognize that MRI criteria for LNs involvement in primary staging include size, shape, border, signal homogeneity, and enhancement homogeneity [6–8]. However, these criteria are not entirely suitable for nodal restating after neoadjuvant therapy [9, 10]. Most research has primarily relied on the size criterion and has not considered whether morphological characteristics (including shape, border, signal homogeneity and enhancement homogeneity) can effectively distinguish benign and malignant LNs [4, 9, 11]. Only a few studies have shown that nodal morphological characteristics contribute to nodal restaging [12–14]. However, evaluating the details of morphological characteristics in small LNs (short-axis diameter < 5 mm) have been considered challenging [10].
In 2016, a new criterion was added to European Society of Gastrointestinal and Abdominal Radiology (ESGAR), considering LNs with a short-axis diameter < 5 mm as benign after neoadjuvant therapy [6]. To the best of our knowledge, no study has validated this criterion for a node-by-node evaluation. Thus, this study aimed to validate the ESGAR criterion and explore the diagnostic efficiency of nodal morphological characteristics for LNs ≥ 5 mm.
Methods
Patients
This retrospective study was approved by our Institutional Review Board, and written informed consent was obtained from all patients. A total of 262 consecutive patients with LARC treated with neoadjuvant therapy between March 2015 and June 2021 were considered for inclusion. Among these patients, 172 patients were excluded for the following reasons (a) no total mesorectal excision 6–8 weeks after neoadjuvant therapy (n = 108); (b) no post-treatment MRI within 2 weeks before surgery (n = 30); (c) poor image quality for evaluation (n = 12); (d) no LNs found on MRI (n = 20); and (e) the location of the LNs on MRI did not match the histopathologic results (n = 2). Finally, 90 patients (68 men, 22 women; median age: 55 years, range: 20–90 years) were included in this study (Fig. 1).
Fig. 1.
Flow diagram of patients included
Neoadjuvant therapy
Neoadjuvant therapy included preoperative chemoradiotherapy and preoperative chemotherapy. Preoperative chemoradiotherapy regimen involved long-term radiotherapy using volumetric modulated arc therapy with a total dose of 50–60 Gy for primary and nodal gross tumor volumes administered with conventional segmentation, along with concurrent chemotherapy. Chemotherapy regimens included the mFOLFOX regimen (leucovorin + fluorouracil + oxaliplatin), CapeOX regimen (capecitabine + oxaliplatin), or capecitabine monotherapy.
MRI examination
Patients, except those with lower and large rectal tumors, were infused with an appropriate amount (20–80 mL) of ultrasonic gel into the rectum. To reduce bowel motility, 20 mg of raceanisodamine hydrochloride was injected intramuscularly 10 min before MRI examination unless contraindicated.
MRI was performed using a 3.0-T scanner (Magnetom Verio, Siemens Healthcare) with a 6-channel phased-array body coil. Patients were positioned supine with feet first. The rectal MRI protocols included (a) sagittal, coronal and oblique axial (orthogonal to tumor base) non-fat-suppressed high-spatial-resolution T2- weighted imaging (HR-T2WI) using a turbo spin-echo sequence; and (b) coronal fat-suppressed isotropic contrast-enhanced three-dimensional high-spatial-resolution T1-weighted imaging (CE-3D-HR-T1WI) using a gradient-echo sequence. Axial, sagittal and coronal multiplanar reconstructions were performed on CE-3D-HR-T1WI with a slice thickness of 3 mm. An intravenous bolus of 0.2 mL/kg gadopentetate dimeglumine was injected at a rate of 3.0 mL/s, followed by a 25-mL saline flush at the same rate. Detailed protocols are listed in Table 1.
Table 1.
High-spatial-resolution MRI protocols for rectal cancer
| Sequences | TR/TE (ms) | Slice thickness/Gap (mm) | Slices | Base resolution | Phase resolution (%) | FOV (mm) | Voxel size (mm3) | Acquisition time |
|---|---|---|---|---|---|---|---|---|
| High-spatial-resolution T2-weighted imaging | ||||||||
| Sagittal | 3000/87 | 3/0 | 19 | 320 | 80 | 180 | 0.7 × 0.6 × 3.0 | 2 min 30 s |
| Coronal | 4000/77 | 3/0 | 25 | 384 | 80 | 220 | 0.7 × 0.6 × 3.0 | 2 min 52 s |
| Oblique axial | 3000/84 | 3/0 | 24 | 320 | 100 | 180 | 0.6 × 0.6 × 3.0 | 3 min 18 s |
| Contrast-enhanced three-dimensional high-spatial-resolution T1-weighted imaging | ||||||||
| Coronal | 10/4.9 | 1/0.2 | 144 | 384 | 100 | 380 | 1.0 × 1.0 × 1.0 | 3 min 10 s |
TR, repetition time; TE, echo time; FOV, field of view
Image interpretation
Two radiologists with 2 and 7 years of experience in rectal MRI, reviewed all MR images blinded to histopathologic findings. Firstly, all visible LNs on HR-T2WI were determined by the two radiologists in consensus. Secondly, the short-axis diameters of LNs were measured independently, and the average values were calculated for subsequent analyses. Thirdly, the two radiologists assessed the shape (oval/round), border (smooth/irregular), and signal homogeneity (homogeneous/heterogeneous) of LNs ≥ 5 mm on HR-T2WI and enhancement homogeneity (homogeneous/ heterogeneous) on CE-3D-HR-T1WI in consensus.
Radiologic–histopathologic comparison
All visible regional LNs on HR-T2WI were divided into three groups, including mesorectal, superior rectal and inferior mesenteric LNs. Based on the agreed-upon nodal position by the radiologist and surgeon, an experienced surgeon specializing in colorectal cancer successively localized and removed the regional LNs in different groups during surgery. In order to provide an accurate node-by-node comparison between MR images and histopathological findings, special attention was paid to nodal size and morphology, as well as nodal relative position to the tumor, rectal wall, mesorectal fascia, vessels and adjacent LNs [15]. The excised LNs were then sent to the pathology department and quickly placed in individual trays. All LNs were fixed in formalin and stained with hematoxylin and eosin. Thereafter, an experienced gastrointestinal pathologist analyzed and classified each LN as benign or malignant under light microscope. The LNs reported by histopathology were rematched with HR-T2WI in the corresponding groups and were excluded if they could not be matched.
Statistical analysis
Statistical analyses were performed using SPSS software (version 25.0, IBM). Figures were generated using GraphPad Prism (version 9.0, GraphPad Software) and MedCalc statistical software (version 15.8, MedCalc Software bvba, Ostend, Belgium). The normality of quantitative data was test by using Kolmogorov-Smirnov test. Normally distributed data were compared with the independent samples t-test, while nonnormally distributed data were presented as medians with ranges and compared with the Mann-Whitney U test. Categorical data were expressed as numbers with percentages and compared with the χ2 test or Fisher’s exact test. ROC curve was constructed, and the AUC with a 95% confidence interval was calculated to evaluate the diagnostic efficacy of nodal size. With a short-axis diameter cutoff value of 5 mm, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated. The morphological characteristics were compared for LNs ≥ 5 mm using the χ2 test or Fisher’s exact test. A two-tailed P value < 0.05 indicated a statistically significant difference.
Results
Clinicopathologic findings
A total of 90 patients were included, of which 27 (30.0%, 27/90) were confirmed to have malignant LNs. Malignant LNs were more likely occur in patients with pT3-4 tumors (P < 0.05) (Table 2). Histopathology of 1049 LNs harvested from the rectal specimens in 90 patients (median:11, range: 0–31) revealed that 72 (6.9%, 72/1049) were malignant. A total of 308 LNs were visualized on MRI in the 90 patients (median:3, range:1–8). For a node-by-node evaluation, 298 LNs were successfully matched between MRI and histopathology, of which 44 were malignant.
Table 2.
Relationship between clinicopathologic features and lymph nodes metastases in 90 patients
| Parameters | Total (n = 90) |
ypN0 (n = 63) |
ypN+ (n = 27) |
P value |
|---|---|---|---|---|
| Age (years) | 55 (24–82)* | 56(27–82) * | 55(24–75) * | 0.926 a |
| Sex | 0.830 b | |||
| Man | 68 (75.6%) | 48(76.2%) | 20(74.1%) | |
| Woman | 22 (24.4%) | 15(23.8%) | 7(25.9%) | |
| Tumor location# | 0.777 c | |||
| Upper | 11(12.2%) | 7(11.1%) | 4(14.8%) | |
| Middle | 51(56.7%) | 35(55.6%) | 16(59.3%) | |
| Lower | 28(31.1%) | 21(33.3%) | 7(25.9%) | |
| Histological subtype | 0.234 c | |||
| Nonmucinous adenocarcinoma | 82(91.1%) | 59(93.7%) | 23(85.2%) | |
| Mucinous adenocarcinoma | 8(8.9%) | 4(6.3%) | 4(14.8.%) | |
| Differentiation | 0.808 c | |||
| Well | 1(1.1%) | 1(1.6%) | 0(0.0%) | |
| Moderate | 78(86.7%) | 55(87.3%) | 23(85.2%) | |
| Poor | 11(12.2%) | 7(11.1%) | 4(14.8%) | |
| ypT stage | 0.004 b | |||
| ypT0-2 | 44(48.9%) | 37(58.7%) | 7(25.9%) | |
| ypT3-4 | 46(51.1%) | 26(41.3%) | 20(74.1%) |
* Date are medians and ranges in parentheses
# According to the distance from the most caudal border of the rectal tumor to the anal verge on MRI: upper, > 10 cm; middle, 5–10 cm; lower, < 5 cm
a Mann-Whitney U test, bχ2 test, c Fisher’s exact test
yp, post-neoadjuvant treatment pathological feature
Diagnostic value of nodal size
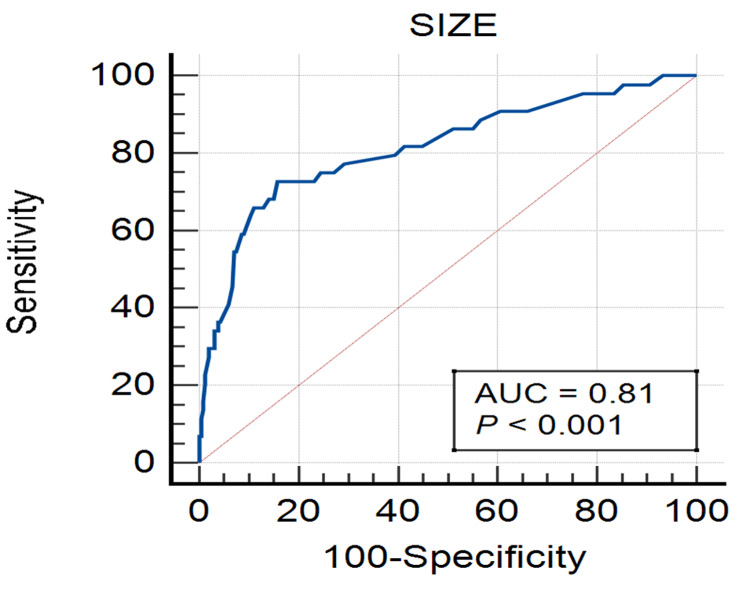
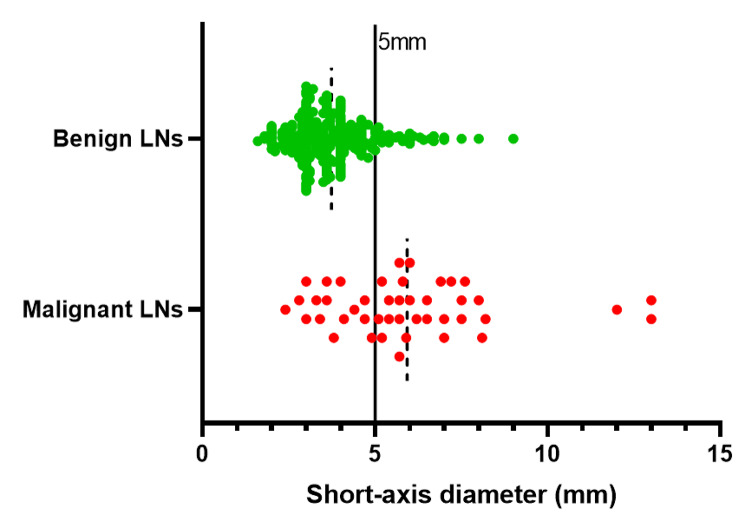
The median short-axis diameter was 3.7 mm (range: 1.6-9 mm) for the 254 benign LNs and 5.9 mm (range:2.4–13 mm) for the 44 malignant LNs. The AUC was 0.81 (95% confidence interval: 0.74–0.89), indicating that nodal size had moderate diagnostic performance in distinguishing malignant from benign LNs (Fig. 2). When LNs ≥ 5 mm were considered malignant, sensitivity, specificity, PPV, NPV and accuracy for determining malignant LNs were 65.9% (29/44), 87.0% (221/254), 46.8% (29/62), 93.6% (221/236) and 83.9% (250/298), respectively (Table 3). Among LNs < 5 mm, almost all (93.6%, 221/236) were benign, while less than half (46.8%, 29/62) LNs ≥ 5 mm were malignant (Fig. 3).
Fig. 2.
ROC curve showing the diagnostic performance of nodal size for malignant lymph nodes
Table 3.
Comparison of nodal size and histopathologic findings in 298 lymph nodes
| Histopathologic findings | |||
|---|---|---|---|
| Nodal size | ypLN- | ypLN+ | Total |
| LN- (< 5 mm) | 221 | 15 | 236 |
| LN+ (≥ 5 mm) | 33 | 29 | 62 |
| Total | 254 | 44 | 298 |
LNs, lymph nodes; yp, post-neoadjuvant treatment pathological feature
Fig. 3.
Short-axis diameter distribution of benign and malignant LNs. Dotted line: mean, solid line: cutoff value. 93.6% of the LNs < 5 mm were benign (green spots), and only 46.8% of the LNs ≥ 5 mm were malignant (red spots). LNs, lymph nodes
Nodal status compared with its morphological characteristics
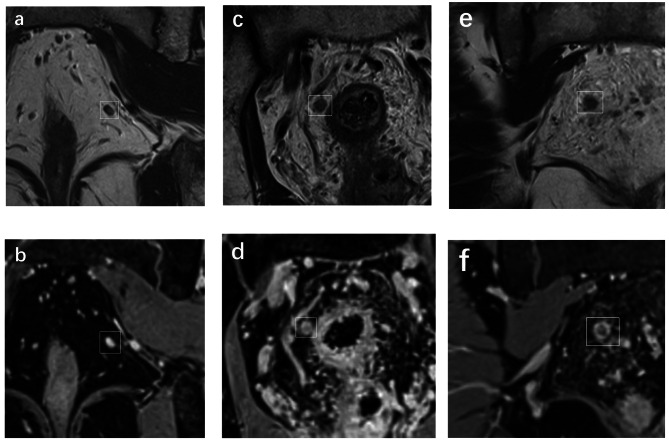
Among the 62 LNs ≥ 5 mm, 21.2% (7/33) of benign LNs showed an oval shape, while 86.2% (25/29) of malignant LNs were round. Smooth borders were observed in 69.7% (23/33) of benign LNs, compared to only 17.2% (5/29) of malignant LNs with irregular borders. Homogeneous signal intensity was present in 66.7% (22/33) of benign LNs, while 41.4% (12/29) of malignant LNs showed heterogeneous signal intensity. Furthermore, 21.2% (7/33) of benign LNs exhibited homogeneous enhancement, compared to 86.2% (25/29) of malignant LNs with heterogeneous enhancement (Fig. 4). However, none of these morphological characteristics were found to be statistically significant in differentiating benign and malignant LNs (P > 0.05) (Table 4).
Fig. 4.
Coronal non-fat-suppressed high-spatial-resolution T2-weighted imaging (a, c and e); coronal fat-suppressed isotropic contrast-enhanced three-dimensional high-spatial-resolution T1-weighted imaging (b, d and f). The lymph nodes were signed by white boxes. a-b, Benign node with 3.7 mm in short-axis diameter, showed oval, smooth border, homogeneous signal and homogeneous enhancement. c-d, Benign node with 5.6 mm in short-axis diameter, showed round, irregular border, heterogeneous signal and heterogeneous enhancement. e-f, Malignant node with 5.8 mm in short-axis diameter, showed round, irregular border, heterogeneous signal and heterogeneous enhancement
Table 4.
Comparison of nodal morphological characteristics and histopathologic findings in 62 lymph nodes ≥ 5 mm
| Histopathologic findings | |||
|---|---|---|---|
| Morphological characteristics | ypLN- (n = 33) |
ypLN+ (n = 29) |
P value |
| Shape | 0.445 a | ||
| Oval | 7 (21.2%) | 4 (13.8%) | |
| Round | 26 (78.8%) | 25 (86.2%) | |
| Border | 0.231 a | ||
| Smooth | 23 (69.7%) | 24 (82.8%) | |
| Irregular | 10 (30.3%) | 5 (17.2%) | |
| Signal homogeneity | 0.513 a | ||
| Homogeneous | 22 (66.7%) | 17 (58.6%) | |
| Heterogeneous | 11 (33.3%) | 12 (41.4%) | |
| Enhancement homogeneity | 0.445 a | ||
| Homogeneous | 7 (21.2%) | 4 (13.8%) | |
| Heterogeneous | 26 (78.8%) | 25 (86.2%) | |
LNs, lymph nodes; yp, post-neoadjuvant treatment pathological feature
aχ2 test
Discussion
For the evaluation of LNs in rectal cancer, many studies have focused on assessing the agreement between MRI and histopathology for N-staging [16, 17]. Although this method is easy to implement, it ignores the characteristics of each individual node. In our study, we conducted a node-by-node analysis, matching and verifying each LN found on MRI with the pathological results. This approach has been proven to be more specific and valuable in establishing diagnostic criteria for LNs [7, 18].
Our study validated that the ESGAR criterion had a moderate diagnostic performance (AUC = 0.81) for nodal restaging in rectal cancer after neoadjuvant therapy. With a short-axis diameter cutoff value of 5 mm, the sensitivity, specificity and accuracy were 65.9%, 87.0% and 83.9%, respectively. The size criterion is the most widely used indicator for nodal staging and restaging in current studies on rectal cancer LNs [9, 19]. According to the ESGAR consensus meeting in 2012, the size criterion after neoadjuvant therapy is more reliable than the baseline MRI assessment for diagnosing malignant LNs [19]. Many studies have shown that the number of LNs usually decreases, and the short-axis diameter becomes smaller or even disappears after neoadjuvant therapy. Downsized LNs have a very low chance of metastasis [2]. However, there is still no consensus on the optimal cutoff value. In the 2016 ESGAR, a new item was added for MRI nodal restaging: all LNs < 5 mm should be considered benign, but there are no reliable criteria for LNs ≥ 5 mm. Our study also showed that short-axis diameter cutoff value of 5 mm had a high NPV, as almost all (93.6%, 221/236) LNs < 5 mm were benign. Thus, we can confidently conclude that there are no malignant LNs if all LNs on MRI after neoadjuvant therapy are < 5 mm. However, among the 62 LNs ≥ 5 mm, malignant LNs accounted for only 46.8% (29/62), resulting a low PPV. Therefore, it is impossible to effectively distinguish benign and malignant LNs ≥ 5 mm.
Although we used HR-T2WI and CE-3D-HR-T1WI with thin thickness in our study, confidently observing the morphological features of small LNs remains challenging [9, 10]. Consequently, we only evaluated the morphological features of LNs ≥ 5 mm. Although morphological characteristics were effective in predicting the status of LNs before treatment [18], we found no significant differences in shape, border, signal homogeneity, and enhancement homogeneity between benign and malignant LNs after neoadjuvant therapy. Nodal shape is likely influenced by the scanning planes. Even benign LNs showed irregular border and heterogeneous signal intensity. This could be attributed to fibrous thickening of the capsule and fibrotic or mucinous changes after treatment, which make elevation more complex [20]. Regarding enhancement homogeneity evaluation, we applied fat-suppressed isotropic CE-3D-HR-T1WI, which has demonstrated good performance in nodal staging [7]. Compared with HR-T2WI, this sequence offers advantages such as higher spatial resolution, improved signal-to-noise ratio, and multiplanar reconstruction, providing better-detailed of LNs. However, heterogenous enhancement could still be observed in benign LNs ≥ 5 mm, likely due to nodal fibrosis or the presence of acellular mucin lakes caused by neoadjuvant therapy [20, 21]. Malignant LNs with smooth border, homogeneous signal intensity, and enhancement may contain micrometastases.
There were some limitations in our study. Firstly, we only evaluated the morphological features of LNs ≥ 5 mm because accurately assessing the morphological features of small LNs is challenging. Secondly, the number of metastatic LNs was lower than that of benign because patients with obvious LN metastasis usually do not undergo surgery. Finally, we did not evaluate iliac LNs as extended pelvic lymphadenectomy is not usually performed in total mesorectal excision.
Conclusions
In summary, our study demonstrates that the ESGAR has moderate diagnostic performance for nodal restaging in rectal cancer after neoadjuvant therapy. LNs < 5 mm can be effectively identified as benign using the size criterion alone, in line with the ESGAR recommendation. However, morphological features do not aid in the diagnosis of LNs ≥ 5 mm on MRI restaging. Future research should focus on refining the criteria for distinguishing benign and malignant LNs ≥ 5 mm and exploring alternative imaging techniques to improve the accuracy of nodal restaging in rectal cancer after neoadjuvant therapy.
Acknowledgements
None.
List of abbreviations
- LARC
locally advanced rectal cancer
- LNs
lymph nodes
- ESGAR
European Society of Gastrointestinal and Abdominal Radiology
- HR-T2WI
high-spatial-resolution T2-weighted imaging
- CE-3D-HR-T1WI
contrast-enhanced three-dimensional high-spatial-resolution T1-weighted imaging
- PPV
positive predictive value
- NPV
negative predictive value
Authors’ contributions
ZZ and YC contributed equally to this work. SY put forward the study concepts and ZZ designed the study. Data acquisition and interpretation were done by all authors. ZZ and YC wrote and edited the manuscript. All authors read and approved the final manuscript.
Funding
Support by Guangdong Basic and Applied Basic Research Foundation (2020A1515010796).
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by our institutional review board. Written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhiwen Zhang and Yan Chen contributed equally to this work.
Contributor Information
Zhiwen Zhang, Email: zhangzhw55@163.com.
Yan Chen, Email: cheny866@mail.sysu.edu.cn.
Ziqiang Wen, Email: wenzq6@mail.sysu.edu.cn.
Xuehan Wu, Email: wuxh78@mail2.sysu.edu.cn.
Yutao Que, Email: queyt@mail2.sysu.edu.cn.
Yuru Ma, Email: mayr8@mail2.sysu.edu.cn.
Yunzhu Wu, Email: yunzhu.wu@siemens-healthineers.com.
Quanmeng Liu, Email: liuqm28@mail2.sysu.edu.cn.
Wenjie Fan, Email: fanwj26@mail2.sysu.edu.cn.
Shenping Yu, Email: yushp@mail.sysu.edu.cn.
References
- 1.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl4):iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 2.Heijnen LA, Maas M, Beets-Tan RG, Berkhof M, Lambregts DM, Nelemans PJ, et al. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis. 2016;31(6):1157–62. doi: 10.1007/s00384-016-2576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan JE, Warrier SK, Lynch AC, Heriot AG. Assessing pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis. 2015;17(10):849–61. doi: 10.1111/codi.13081. [DOI] [PubMed] [Google Scholar]
- 4.Lahaye MJ, Beets GL, Engelen SM, Kessels AG, de Bruine AP, Kwee HW, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology. 2009;252(1):81–91. doi: 10.1148/radiol.2521081364. [DOI] [PubMed] [Google Scholar]
- 5.Dinaux AM, Leijssen L, Bordeianou LG, Kunitake H, Amri R, Berger DL. Outcomes of persistent lymph node involvement after neoadjuvant therapy for stage III rectal cancer. Surgery. 2018;163(4):784–8. doi: 10.1016/j.surg.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 european Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–75. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Yang X, Wen Z, Lu B, Xiao X, Shen B, et al. Fat-suppressed gadolinium-enhanced isotropic high-resolution 3D-GRE-T1WI for predicting small node metastases in patients with rectal cancer. Cancer Imaging. 2018;18(1):21. doi: 10.1186/s40644-018-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jao SY, Yang BY, Weng HH, Yeh CH, Lee LW. Evaluation of gadolinium-enhanced T1-weighted magnetic resonance imaging in the preoperative assessment of local staging in rectal cancer. Colorectal Dis. 2010;12(11):1139–48. doi: 10.1111/j.1463-1318.2009.01959.x. [DOI] [PubMed] [Google Scholar]
- 9.Perez RO, Pereira DD, Proscurshim I, Gama-Rodrigues J, Rawet V, Sao Juliao GP, et al. Lymph node size in rectal cancer following neoadjuvant chemoradiation–can we rely on radiologic nodal staging after chemoradiation? Dis Colon Rectum. 2009;52(7):1278–84. doi: 10.1007/DCR.0b013e3181a0af4b. [DOI] [PubMed] [Google Scholar]
- 10.Seo N, Kim H, Cho MS, Lim JS. Response Assessment with MRI after Chemoradiotherapy in rectal Cancer: current evidences. Korean J Radiol. 2019;20(7):1003–18. doi: 10.3348/kjr.2018.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DJ, Kim JH, Lim JS, Yu JS, Chung JJ, Kim MJ, et al. Restaging of rectal Cancer with MR Imaging after Concurrent Chemotherapy and Radiation Therapy. Radiographics. 2010;30(2):503–16. doi: 10.1148/rg.302095046. [DOI] [PubMed] [Google Scholar]
- 12.Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250(3):730–9. doi: 10.1148/radiol.2503080310. [DOI] [PubMed] [Google Scholar]
- 13.Nahas SC, Nahas CSR, Cama GM, de Azambuja RL, Horvat N, Marques CFS, et al. Diagnostic performance of magnetic resonance to assess treatment response after neoadjuvant therapy in patients with locally advanced rectal cancer. Abdom Radiol (NY) 2019;44(11):3632–40. doi: 10.1007/s00261-019-01894-8. [DOI] [PubMed] [Google Scholar]
- 14.Almlov K, Woisetschlager M, Loftas P, Hallbook O, Elander NO, Sandstrom P. MRI lymph node evaluation for prediction of metastases in rectal Cancer. Anticancer Res. 2020;40(5):2757–63. doi: 10.21873/anticanres.14247. [DOI] [PubMed] [Google Scholar]
- 15.Cho EY, Kim SH, Yoon JH, Lee Y, Lim YJ, Kim SJ, et al. Apparent diffusion coefficient for discriminating metastatic from non-metastatic lymph nodes in primary rectal cancer. Eur J Radiol. 2013;82(11):E662–E8. doi: 10.1016/j.ejrad.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Grone J, Loch FN, Taupitz M, Schmidt C, Kreis ME. Accuracy of various Lymph Node staging criteria in rectal Cancer with magnetic resonance imaging. J Gastrointest Surg. 2018;22(1):146–53. doi: 10.1007/s11605-017-3568-x. [DOI] [PubMed] [Google Scholar]
- 17.Koh DM, Chau I, Tait D, Wotherspoon A, Cunningham D, Brown G. Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2008;71(2):456–61. doi: 10.1016/j.ijrobp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227(2):371–7. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 19.Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 european Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23(9):2522–31. doi: 10.1007/s00330-013-2864-4. [DOI] [PubMed] [Google Scholar]
- 20.Prall F, Wohlke M, Klautke G, Schiffmann L, Fietkau R, Barten M. Tumour regression and mesorectal lymph node changes after intensified neoadjuvant chemoradiation for carcinoma of the rectum. APMIS. 2006;114(3):201–10. doi: 10.1111/j.1600-0463.2006.apm_304.x. [DOI] [PubMed] [Google Scholar]
- 21.Chetty R, McCarthy AJ. Neoadjuvant chemoradiation and rectal cancer. J Clin Pathol. 2019;72(2):97–101. doi: 10.1136/jclinpath-2018-205592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.