Abstract
Background
With the increasing availability of chest computed tomography (CT), the detection of small pulmonary nodules has become more common, facilitating the development of lung segmental resection. However, anatomical variations of the bronchi are common, particularly in the right upper lobe of the lung.
Case presentation
We report a case of thoracoscopic resection of the posterior segment of the right upper lobe of the lung. Preoperatively, the nodule was believed to be located in the superior segment of the right lower lobe. However, intraoperative exploration revealed that the nodule was located in the posterior segment of the right upper lobe, further showing that the bronchi of the posterior segment of the right lung opened into the bronchus intermedius. The procedure was completed uneventfully. Postoperative retrospective three-dimensional (3D) reconstruction of the lung CT images confirmed that the bronchi of the posterior segment of the right upper lobe originated from the bronchus intermedius.
Conclusions
This rare case highlights the importance of 3D reconstruction to guide accurate segmentectomy in patients with anatomic variations.
Keywords: Three-dimensional reconstruction, Segmentectomy, Variations, Right upper lobe bronchus, Lung cancer
Background
The increasing application of video-assisted thoracoscopic surgery for lung surgery requires accurately determining lung anatomy to prevent severe complications. Postoperative lung function is better preserved during anatomic segmentectomy than lobectomy, with the former garnering great interest [1]. However, because of the anatomical complexity of the lung, segmentectomy is more technically difficult than standard lobectomy; therefore, proficient knowledge of anatomical variations becomes increasingly important for the general thoracic surgeon. Here, we report the case of a patient with a bronchial variation in the posterior segment (S2) of the right upper lobe who underwent thoracoscopic segmentectomy and lymph node sampling for lung cancer and a literature review.
Case presentation
A 74-year-old female patient presented with an abnormal shadow on chest computed tomography (CT) at a medical checkup and subsequently visited our hospital. Chest CT showed a 17 mm × 8 mm ground-glass opacity with approximately 30% solid component in the right superior segment (S6) (Fig. 1A-C). The patient had undergone radical treatment of right breast cancer staged at pT2N0M0 IIA, followed by four cycles of postoperative adjuvant chemotherapy 11 years prior. All other medical history was unremarkable.
Fig. 1.
A + B + C, Preoperative lung CT; a mixed ground-glass nodule (arrow) measuring approximately 17 mm × 8 mm with a CT value of -700 HU was observed in the posterior segment of the right upper lobe. D, Postoperative lung CT. CT, computed tomography
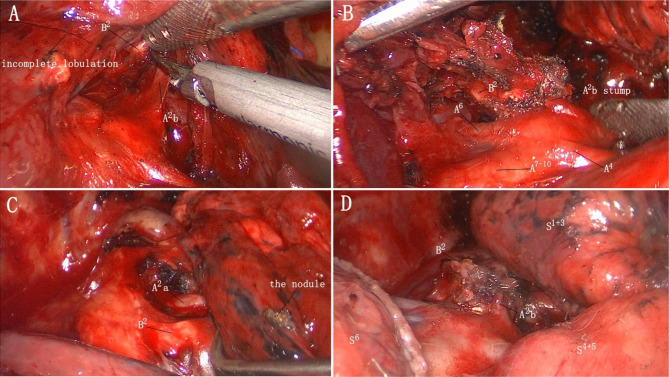
Relevant examinations were performed after admission, and no obvious contraindications to surgery were observed. Because it was difficult to palpable the lesion and the location of lesion was closed to the bronchus, a thoracoscopic right S6 segmentectomy was planned. We performed thoracoscopic surgery using two ports. We made a 2-cm incision in the 7th intercostal space of the right midaxillary line and a 4-cm incision in the 4th intercostal space of the right anterior axillary line as the observation and operating holes, respectively. Pleural adhesions were observed throughout the thoracic cavity perioperatively. The right lung was found to be divided into three lobes after being released. The horizontal and posterior oblique fissures were poorly developed, and the intersegmental plane could not be distinguished. Digital palpation indicated that the nodule was located in a high position, and lifting the right upper lobe revealed the suspected location of the nodule in the upper lobe. The interlobar fissures were separated, and the posterior segmental and superior segmental arteries were located following the pulmonary trunk arteries (Fig. 2A,B). After labelling the nodule on the pleural surface, multiple comparisons were performed, and the nodule was eventually found in the S2 of the right upper lobe (Fig. 2C). So the recurrent and ascending arteries were dissociated and resected. Then, the posterior segmental bronchus (B2) was exposed and transected. Finally, the right lung was reventilated with pure oxygen and the intersegmental plane was clear after 20 min. The intersegmental plane was divided along the inflation-deflation line using the endostaplers; thus, resection of the S2 of the right upper lobe was completed (Fig. 2D). The tumor was 3 cm away from the incisal margin. As intraoperative pathology analysis revealed minimally invasive adenocarcinoma (MIA), hilar lymph node sampling was performed. On postoperative day 2, the right lung was completely redilated on CT (Fig. 1D), and postoperative pathological examination revealed MIA with negative surrounding lymph nodes.
Fig. 2.
Patient anatomy during surgery
We reviewed lung CT images and performed three-dimensional (3D) reconstructions using Mimics Medical 21.0 software postoperatively. It revealed that the B2 originated from the bronchus intermedius, the posterior segmental artery (A2) of the right upper lung lobe bifurcated into the A2a and A2b branching from the recurrent and ascending arteries, respectively, and the right superior pulmonary vein had no central vein but a posterior intrasegmental vein ( V2t ) that travelled below the S2 (Fig. 3).
Fig. 3.
Three-dimensional reconstruction of the patient. The apical segmental bronchus (B1) and anterior segmental bronchus (B3) originate from the right upper lobe bronchus, while the posterior segmental bronchus (B2) originates from the bronchus intermedius. A2a originates from the posterior recurrent branching artery, A2b originates from the posterior ascending branching artery, and there is no central vein in the right upper pulmonary vein and only a V2t, which enters the left atrium from the posterior aspect of the right lower lobe bronchus. A, dorsal view; B, ventral view; C + D: lateral view
Discussion and conclusions
Pulmonary segmentectomy is one of the most discussed topics in thoracic surgery since it requires an accurate understanding of the targeted lung segment to be successful [2]. However, lung segments frequently exhibit anatomical variations, particularly in the bronchi of the right upper lobe [3, 4]. Here, we reported a case of bronchial variation in the right upper lobe and reviewed the literature on this variation to provide clinical guidance for pulmonary segmentectomy.
Trifurcated and bifurcated bronchi are common anatomical variations in the right upper lobe. The bifurcated type can be classified into the simple (B1 + 2, B3; B1 + 3, B2; and B2 + 3, B1) or intersecting subtypes (B1a + 2, B1b + 3 and B1 + 2a, B2b + 3) whereas the quadrifurcated type is open in the right upper lobe and relatively rare. Contrary to the numerous variations of lobar or segmental bronchial subdivisions, abnormal bronchi originating from the trachea or main bronchi are rarer. For example, the tracheal bronchus, which is a distal or proximal displacement of the bronchus in the right upper lobe, is a variant of proximal bronchial displacement that arises directly from the trachea or primary bronchi, proceeding towards the right upper lobe. Conversely, distal displacement of the bronchus in the right upper lobe has been reported [5]. In very rare cases, a segmental bronchus of the lung may be ectopic to the distal or proximal bronchi [6, 7]. Yaginuma previously reported 15 cases of apical segmental bronchus (B1), which is ectopic to the lateral wall of the trachea or the right main bronchus (Right B1 Type), and 7 and 11 cases of B2 and anterior segmental bronchus (B3), respectively, originating directly posterior to the bronchus intermedius (Right B2 Type and Right B3 Type) [7]. In the Right B2 and Right B3 Types, abnormal lung lobulations and top pulmonary veins were frequently noted. For the Right B2 Type, all patients had an incomplete lobulation between the upper and lower lobes; however, no abnormality of the pulmonary artery was observed. We reviewed these anatomical types of bronchi in the right upper lobe (Table 1) and described a Right B2 Type that was misdiagnosed.
Table 1.
Previously reported anatomical variations of the bronchi in the right upper lobe
| Right upper lobe | Inspection method | Author, Year | |
|---|---|---|---|
| Normal findings and most common bronchial variations | Trifurcation B1, B2, B3 |
3DCT; Bronchography |
Nagashima et al. (2015) [3]; Gonlugur et al. (2005) [8] |
| Bifurcation B1 + 2, B3 | 3DCT; Bronchography | Nagashima et al. (2015) [3]; Gonlugur et al. (2005) [8] | |
| Bifurcation B1 + 3, B2 | 3DCT; Bronchography | Nagashima et al. (2015) [3]; Gonlugur et al. (2005) [8] | |
| Bifurcation B2 + 3, B1 | 3DCT; Bronchography | Nagashima et al. (2015) [3]; Gonlugur et al. (2005) [8] | |
| Bifurcation B1a + 2, B1b + 3 | 3DCT; Bronchography | Nagashima et al. (2015) [3]; Zhang et al. (2021) [9] | |
| Bifurcation B1 + 2a, B2b + 3 | 3DCT; Bronchography | Nagashima et al. (2015) [3]; Zhang et al. (2022) [10] | |
| Quadrifurcation | 3DCT; Bronchography | Nagashima et al. (2015) [3]; Gonlugur et al. (2005) [8] | |
| Rare variations | Tracheal bronchus |
Thoracoscopy; Bronchography |
Yurugi et al. (2012) [11]; Martín-Ruiz et al. (2021) [4] |
| Right upper lobe bronchus ectopic to the intermediate bronchus | 3DCT | Huang et al. (2020) [5] | |
|
B1 ectopic to the lateral wall of the trachea or the right main bronchus (Right B1 Type)) |
CT | Yaginuma (2020) [7] | |
|
B2 ectopic to the intermediate bronchus (Right B2 Type) |
CT | Yaginuma (2020) [7] | |
|
B3 ectopic to the intermediate bronchus (Right B3 Type) |
3DCT |
Ghaye et al. (2001) [6]; Yaginuma (2020) [7] |
B1 = apical segmental bronchus; B2 = posterior segmental bronchus; B3 = anterior segmental bronchus; 3DCT = three-dimensional computed tomography; CT = computed tomography
The patient we reported also had an incomplete lobulation between the right upper and lower lobe, and no abnormality of the pulmonary artery was found. The right superior pulmonary vein had no central vein but a V2t that travelled below the S2. In this case, the patient had a nodule located in the S2 rather than the S6. The B2 originated from the bronchus intermedius, and no significant abnormalities were found in the apical and anterior segmental bronchi, whereas the bronchus, which was originally believed to be a subsuperior segment (S*), was the superior segmental bronchus (B6). Therefore, a right posterior segmentectomy was performed.
Although the overall surgical procedure was uneventful, it was time-consuming and difficult. However, if this anatomical variation had been better understood before surgery, the surgical approach selection and the intersegmental plane identification would have been more streamlined, and the duration of surgery could have been shortened.
Despite the rarity of bronchial displacement incidence, it may present the risks of bronchovascular injury and difficulties in different lung ventilation processes. Therefore, identifying the displaced bronchi and associated pulmonary lobulations and vascular course abnormalities is important before anatomic segmentectomy. Furthermore, 3D imaging technology can help reveal anatomical variations promptly, particularly the rare types of bronchial branching, and is effective at improving the accuracy and safety of pulmonary segmentectomy.
Abbreviations
- A2
posterior segmental artery
- A4
lateral segmental artery
- A6
superior segmental artery
- A7–10
basal segmental artery
- V2t
posterior intrasegmental vein
- B1
apical segmental bronchus
- B2
posterior segmental bronchus
- B3
anterior segmental bronchus
- B*
subsuperior bronchus
- B6
superior segmental bronchus
- S2
posterior segment
- S6
superior segment
- MIA
minimally invasive adenocarcinoma
- 3D
three-dimensional
- CT
computed tomography
Authors’ contributions
Tao Xu wrote the main manuscript text and Hui Ye prepared Figs. 1, 2 and 3. All authors reviewed the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tane S, Nishio W, Nishioka Y, Tanaka H, Ogawa H, Kitamura Y, et al. Evaluation of the residual lung function after thoracoscopic segmentectomy compared with lobectomy. Ann Thorac Surg. 2019;108:1543–50. doi: 10.1016/j.athoracsur.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 2.Bedetti B, Bertolaccini L, Rocco R, Schmidt J, Solli P, Scarci M. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2017;9:1615–23. doi: 10.21037/jtd.2017.05.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagashima T, Shimizu K, Ohtaki Y, Obayashi K, Kakegawa S, Nakazawa S, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg. 2015;63:354–60. doi: 10.1007/s11748-015-0531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martín-Ruiz S, Gutiérrez-Collar C, Forcén V, De Vera E, Bernabé-Barrios MJ, de Blas CS, Konschake M, et al. The bronchial segmentation and its anatomical variations. A clinical-anatomic and bronchoscopy study. Ann Anat. 2021;235:151677. doi: 10.1016/j.aanat.2021.151677. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Wu P, Li W, Chai Y. Combined ectopic variation of the right upper pulmonary vein and bronchus. Ann Thorac Surg. 2020;109:e353–5. doi: 10.1016/j.athoracsur.2019.07.081. [DOI] [PubMed] [Google Scholar]
- 6.Ghaye B, Szapiro D, Fanchamps JM, Dondelinger RF. Congenital bronchial abnormalities revisited. Radiographics. 2001;21:105–19. doi: 10.1148/radiographics.21.1.g01ja06105. [DOI] [PubMed] [Google Scholar]
- 7.Yaginuma H. Investigation of displaced bronchi using multidetector computed tomography: associated abnormalities of lung lobulations, pulmonary arteries and veins. Gen Thorac Cardiovasc Surg. 2019;68:342–9. doi: 10.1007/s11748-019-01223-2. [DOI] [PubMed] [Google Scholar]
- 8.Gonlugur U, Efeoglu T, Kaptanoglu M, Akkurt I. Major anatomical variations of the tracheobronchial tree: bronchoscopic observation. Anat Sci Int. 2005;80:111–5. doi: 10.1111/j.1447-073x.2005.00104.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Zhu Y, Li H, Yu C, Min W. VATS right posterior segmentectomy with anomalous bronchi and pulmonary vessels: a case report and literature review. J Cardiothorac Surg. 2021;16:60. doi: 10.1186/s13019-021-01420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Li H, Yu C, Zhu Y. Thoracoscopic segmentectomy for right upper lobe with unique anatomic variation. Ann Thorac Surg. 2022;114:e201–3. doi: 10.1016/j.athoracsur.2021.11.052. [DOI] [PubMed] [Google Scholar]
- 11.Yurugi Y, Nakamura H, Taniguchi Y, Miwa K, Fujioka S, Haruki T, et al. Case of thoracoscopic right upper lobectomy for lung cancer with tracheal bronchus and a pulmonary vein variation. Asian J Endosc Surg. 2012;5:93–5. doi: 10.1111/j.1758-5910.2011.00115.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.