Abstract
Kidney disease is a leading cause of morbidity and mortality across the globe. Current interventions for kidney disease include dialysis and renal transplantation, which have limited efficacy or availability and are often associated with complications such as cardiovascular disease and immunosuppression. There is therefore a pressing need for novel therapies for kidney disease. Notably, as many as 30% of kidney disease cases are caused by monogenic disease and are thus potentially amenable to genetic medicine, such as cell and gene therapy. Systemic disease that affects the kidney, such as diabetes and hypertension, might also be targetable by cell and gene therapy. However, although there are now several approved gene and cell therapies for inherited diseases that affect other organs, none targets the kidney. Promising recent advances in cell and gene therapy have been made, including in the kidney research field, suggesting that this form of therapy might represent a potential solution for kidney disease in the future. In this Review, we describe the potential for cell and gene therapy in treating kidney disease, focusing on recent genetic studies, key advances and emerging technologies, and we describe several crucial considerations for renal genetic and cell therapies.
Introduction
Chronic kidney disease (CKD) is estimated to affect approximately 13% of the global population and is associated with high levels of morbidity and mortality1-3. For instance, among patients with end-stage kidney disease caused by diabetes, the 5-year mortality rate for a patient is almost as high as that for lung cancer and worse than that for colon cancer4. Nonetheless, cancer receives a huge investment from federal funding agencies and biotechnology and pharmaceutical companies in the cell and gene therapy fields, whereas kidney-targeted cell and gene therapy clinical trials are almost nonexistent5. Despite its severity and a continuously evolving understanding of kidney disease pathophysiology, however, end-stage treatments are limited. Dialysis and renal transplantation are required for patients with end-stage kidney disease, although these interventions involve risk of major complications such as cardiovascular disease and immunosuppression6. In addition, dialysis provides only 5–10% of normal kidney function and necessitates considerable lifestyle changes involving three times-per-week or daily, multiple-hour appointments for dialysis treatment. Moreover, the number of patients with end-stage kidney disease far outstrips the supply of organs available for transplant, meaning that many patients can wait years for an organ and die while waiting. There is therefore an urgent need for new and innovative therapies for CKD, with the aim to lessen the burden on patients and the wider health-care ecosystem.
Recent advances in cell and gene therapy have translated into successful therapies for a range of clinical diseases, but none that targets the kidney. Cell and gene therapies aim to prevent, treat or even cure diseases by introducing cells or genetic material into a patient, and they often require an understanding of the underlying disease pathophysiology. Notably, many of the genetic causes of kidney disease are known7, with up to 30%of CKD attributed to inherited monogenic disorders8,9, including polycystic kidney disease (PKD), Alport syndrome, cystinosis, Fabry disease, tuberous sclerosis, Gitelman syndrome and cystinuria10,11 (Table 1). Clinicians can frequently diagnose these monogenic renal diseases in childhood before the onset of CKD, sometimes allowing for intervention before kidney damage becomes irreparable12. Genetic testing can also identify affected patients before onset of symptoms or irreversible kidney damage13,14. Unfortunately, the few effective treatments for monogenic diseases affecting the kidney, such as enzyme replacement therapy for Fabry disease, are often expensive, have short-term effects and are inaccessible to a large number of patients15-17. The clear treatment gap for patients with monogenic renal disorders therefore needs to be addressed with innovative strategies, such as cell and gene therapy. Of note, recent successes in the cell and gene therapy field have occurred in cells and organs more easily targeted and less complex than the kidney; nonetheless, as technologies advance with improved vector design and gene delivery, hope for cell and gene therapy for kidney disease seems just on the horizon.
Table 1 ∣.
Prevalence and pathogenesis for genetic kidney diseases
| Disease group | Disease | Gene | cDNA size (bp) | Prevalence |
|---|---|---|---|---|
| Glomerular diseases | Alport syndrome | COL4A3 | 8,097 | 1 in 5,000–10,000 in the USA |
| COL4A4 | 9,895 | |||
| COL4A5 | 6,483 | |||
| Fabry disease | GLA | 1,290 | 1 in 15,000 worldwide | |
| Familial amyloidosis | FGA | 2,110 | 50,000 patients worldwide | |
| APOA1 | 1,009 | |||
| LYZ | 447 | |||
| B2M | 360 | |||
| Congenital nephrotic syndrome | NPHS1 | 3,762 | 1 in 100,000 worldwide | |
| NPHS2 | 1,152 | |||
| Proximal tubule diseases | Autosomal dominant PKD | PKD1 | 14,138 | 1 in 1,000 worldwide |
| PKD2 | 5,056 | |||
| GANAB | 3,906 | |||
| Autosomal recessive PKD | PKHD1 | 16,282 | 1 in 20,000 worldwide | |
| Tubulointerstitial kidney disease | MUC1 | Variable | 500 families in the USA; 2–5% of CKD due to monogenic disorders |
|
| UMOD | 2,477 | |||
| REN | 1,462 | |||
| HNF1B | 2,790 | |||
| SEC61A1 | 1,871 | |||
| Cystinuria | SLC3A1 | 1,737 | 1 in 7,000 in the USA | |
| SLC7A9 | 1,752 | |||
| Proximal renal tubular acidosis | SLC4A4 | 3,108 | <1 in 1,000,000 worldwide | |
| Cystinosis | CTNS | 2,866 | 1 in 100,000–200,000 live births | |
| Dent disease | CLCN5 (type 1) | 10,108 | 250 families worldwide | |
| OCRL (type 2) | 5,138 | |||
| Thick ascending limb and distal convoluted tubule diseases |
Bartter syndrome | SLC12A1 (type I) | 4,707 | 1 in 1,000,000 worldwide |
| KCNJ1 (type II) | 4,074 | |||
| CLCNKB (types III and IV) | 1,544 | |||
| BSND (type IV) | 3,472 | |||
| CLCNKA (type IV) | 2,581 | |||
| MAGED2 (type V) | 2,066 | |||
| Gitelman syndrome | C12A3 | 3,119 | 1 in 40,000 worldwide | |
| CLCNKB | 1,544 | |||
| Collecting duct diseases | Liddle syndrome | SCNN1A | 3,481 | 80 families worldwide |
| SCNN1B | 2,597 | |||
| SCNN1G | 3,507 | |||
| Distal renal tubular acidosis | ATP6V0A4 | 2,523 | <1 in 100,000 worldwide | |
| ATP6V1B1 | 1,542 | |||
| FOXI1 | 1,137 | |||
| SLC4A1 | 2,736 | |||
| WDR72 | 267 |
Select genetic kidney diseases with respective causative gene, cDNA size and prevalence. Some of the information is adapted from ref. 7. CKD, chronic kidney disease; PKD, polycystic kidney disease.
This Review discusses the potential for cell and gene therapies to treat kidney disease. We do not describe induced pluripotent stem cell-based regenerative medicine therapy or extracellular vesicles for kidney disease as those are covered elsewhere18-20. Herein, we focus on approved cell and gene therapies and those of similar technology being developed in the kidney field. We discuss the basics of cell and gene therapy including its technical aspects and history. This is followed by a discussion of the potential for cell and gene therapy of kidney disease based on successes within other organs and diseases, as well as genetic experiments in animal models that reveal the potential for gene therapy of kidney disease. Cell therapies for kidney disease are also reviewed. Finally, we discuss structural and vector considerations for targeting kidney disease and review recent advances and future directions.
A brief introduction to cell and gene therapy
Gene therapy is broadly defined as the use of genetic material to treat or prevent disease. This process includes but is not limited to the insertion or deletion of entire genes, resulting in gain or loss of gene function, or the editing of endogenous genes. Critically, gene therapies target somatic cells rather than germ cells to circumvent germline mutations and subsequent inherited genetic changes. Cell therapy, by contrast, involves the transfer of entire cells into a patient with goals including but not limited to replacing or repairing damaged tissue or cells or even for targeted destruction of pathological cells. Cell therapies cover a spectrum that ranges from simple transfusions of unmodified cells to the delivery of T cells genetically modified ex vivo to target specific cancers. In this Review, we restrict discussion to cell therapies that involve genetic modification.
Cell and gene therapy approaches
For cell and gene therapy to be successful, genetic cargo must be delivered to a target cell population. This target cell population is determined by knowledge about the pathological basis of the disease of interest. There is a wide range of approaches for designing gene therapies, allowing investigators to tailor the delivery, integration and method of editing the genome. Genetic material can be delivered through several vectors, including naked nucleic acids or those encapsulated in viruses or nanoparticles. Genetic cargo may then integrate into the genome or remain unintegrated and therefore episomal, or serve as a template to edit the genetic sequence.
The targeted cells can be modified in vivo or ex vivo. Both types of modification have been approved for use in patients. For instance, chimeric antigen receptor (CAR)-T cell-based therapies for cancer involve ex vivo modification of T cells, which are then adoptively transferred back into patients. Alternatively cells can be modified in vivo, instead of ex vivo. For example, adeno-associated virus (AAV) vectors have been used for gene delivery to both the retina and liver for inherited blindness and haemophilia, respectively. Therefore, cells can be genetically modified ex vivo and then transferred in vivo to modify a disease, or one can genetically modify cells directly in vivo.
Delivery to target cells is key for successful therapy. As such, viral vectors have gained prominence for use in gene therapy and are currently being used in the clinic for a wide range of diseases primarily owing to their intrinsic ability to more efficiently transduce cells compared with non-viral vectors. Adenovirus was used early in humans for gene therapy and soon after produced tragic results, involving immune response and death21. Because of this, adenovirus has not gained traction in clinical trials for treatment of genetic disease. It was hoped that helper-dependent adenovirus22, or gutless adenovirus devoid of immunogenic adenoviral genes, would overcome immunogenicity issues with adenovirus but this too has faced difficulties for treatment of inherited diseases, primarily owing to dose-dependent immune response to capsid proteins23. Lentivirus is a type of retrovirus that has been used for years for developing CAR-T cell immunotherapies. Aside from CAR-T cell development, lentivirus-mediated gene therapy has been approved for cerebral adrenoleukodystrophy and β-thalassaemia, wherein haematopoietic stem cells are modified with virus ex vivo and then infused.
AAV of the Parvoviridae family was discovered in the 1960s24, contains a DNA genome and has been shown to efficiently transduce various human cells, although it is not known to cause disease in humans. AAV-mediated gene therapy was first approved in the West as Glybera (alipogene tiparvovec) for lipoprotein lipase deficiency. This was followed by AAV gene therapy for retinal disease and haemophilia in the USA.
Cell and gene therapy approaches depend on understanding the genetic basis of the disease, having a vector capable of delivering genetic components to the target cells and the ability of the therapeutic agent to prevent or reverse pathology. For now, viruses are used overwhelmingly for approved gene therapy approaches. In time, novel technologies may overcome the use of viruses as delivery vehicles.
Pre-approval era
The history of cell and gene therapy includes both successes and failures. In 1990, the first gene therapy trial was conducted in which a 4-year-old child with severe combined immunodeficiency (SCID) received the adenosine deaminase gene through a viral vector25. Despite success of this trial and other gene therapy trials early on, progress in the field halted in 1999 with the death of an 18-year-old patient from a severe immune reaction to an adenovirus designed to treat a urea cycle disorder21. This tragic death prompted the field to strengthen safety measures surrounding the pre-existing clinical trials for gene therapies. The field slowly regained momentum in the early 2000s, with apparent curative therapy for X-linked SCID by retrovirally mediated delivery of the γc gene into CD34+ cells26. However, in subsequent years, 40% of treated patients developed leukaemia, and subsequent research into the underlying cause concluded that the vector led to oncogene activation27,28. More recently, a patient undergoing CRISPR gene editing for Duchenne muscular dystrophy died29. Although the cause of death is unclear, the FDA has placed a hold on this phase I study. These trials demonstrate the difficulties associated with gene therapies and paved the way for research into newer therapies that were eventually agency approved for treatment of patients. To date, 22 gene therapies, 21 RNA therapies and 59 non-genetically modified cell therapies have been approved globally for clinical use covering the gamut of ex vivo and in vivo therapies30. An additional ~4,000 cell and gene therapies are currently in development for clinical use30.
Approved cell and gene therapies
Approval of the first gene therapy outside of clinical trials occurred in Europe in 2012, with the authorization of Glybera31. This therapy, which involves the AAV-mediated delivery of the LPL gene into muscle, was approved for lipoprotein lipase deficiency31, but was later withdrawn in 2017 owing to commercial failure. Subsequently, in 2017, the FDA approved the first gene therapies in the USA, which included those targeting the eye and modification of T cells for cancer immunotherapy. CAR-T cell therapy involves genetic modification of T cells to redirect them against tumour antigens32. This form of therapy showed promise for many years in clinical trials and was finally approved by the FDA in 2017 under the name Kymriah(tisagenlecleucel), which used CD19-directed CAR-T cells to treat acute lymphoblastic leukaemia. The potential for genotoxicity exists with any integrating gene delivery vector. CAR-T cells were thought to be safe until recently two of ten patients developed CAR-T lymphoma in a clinical trial in Australia using a transposon system33. The cause of this adverse outcome remains to be definitively proved but may be related to the way in which the CAR-T cells were manufactured34. AAV-mediated gene therapies have expanded since Glybera, with Luxturna(voretigene neparvovec-rzyl) approved in 2017 for AAV-mediated gene therapy for RPE65 mutation-associated retinal dystrophy. This was later followed by approval for the use of AAV-delivered genes to treat spinal muscular atrophy type I and haemophilia B.
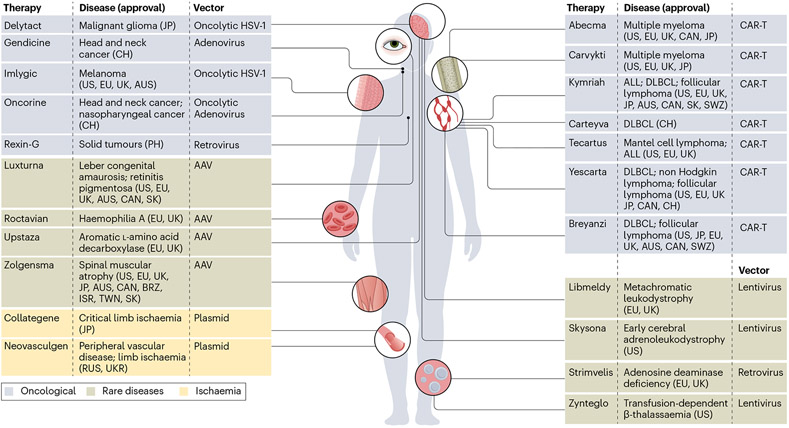
Several cell and gene therapies are now available, although they are mostly limited to certain tissues, including the retina, liver, muscle and the haematopoietic system (Fig. 1). This limited diversity in targetable tissue types is largely due to few available routes of delivery, risk of immunogenicity and poor specificity of cellular targeting. For example, retinal gene therapies have been developed owing to the advantageous immune privilege of the retina — a phenomena in which the eye limits inflammatory responses to preserve vision — as well as accessibility of delivery. Additionally, the liver is a relatively easily targetable organ owing to the predominance of only one cell type (hepatocytes) and high rates of transduction of viral vectors; for these reasons, therapies for several inherited hepatic diseases are currently in development. Finally, many blood-related disorders are prime targets for cell and gene therapy and have already seen success in clinical trials for leukaemia, lymphoma, myeloma, β-thalassaemia and haemophilia. In these diseases, a patient’s own cells can be modified ex vivo and then adoptively transferred back into the patient. The diversity in therapeutic approaches and diseases targeted for these more easily accessible tissues has provided hope for the gene therapy field of applying these insights to other tissues. A new range of treatments for kidney disease could be implemented if the field could adapt these strategies.
Fig. 1 ∣. Approved cell and gene therapies worldwide.
Approved gene therapies involve gene transfer in vivo (left) or modification of cells ex vivo that are transferred to patients (right). Various vectors are used with chimeric antigen receptor (CAR)-T cells generated using lentiviral or retroviral vectors. AAV, adeno-associated virus; ALL, acute lymphoblastic leukaemia; AUS, Australia; CAN, Canada; CH, China; DLBCL, diffuse large B cell lymphoma; EU, European Union; HSV-1, herpes simplex 1; ISR, Israel;JP,Japan; PH, Philippines; RUS, Russia; SK, South Korea; SWZ, Switzerland; TWN, Taiwan; UK, United Kingdom; UKR, Ukraine; USA, United States. Delytact (teserpaturev (Daiichi Sankyo and the University of Tokyo)); Gendicine (recombinant human p53 adenovirus (Shenzhen SiBiono GeneTech Co)); Imlygic (talimogene laherparepvec (BioVex Inc)); Oncorine (H101 (Shanghai Sunway Biotech)); Rexin-G (retroviral vector carrying mutant form of cyclin G1 gene)); Luxturna (voretigene neparvovec-rzyl (Spark Therapeutics, Inc.)); Roctavian (valoctocogene roxaparvovec (BioMarin)); Upstaza (eladocagene exuparvovec (PTC Therapeutics)); Zolgensma (onasemnogene abeparvovec (Novartis Gene Therapies, Inc.)); Collategene (HGF plasmid (AnGes, Inc.)); Neovasculgen (cambiogenplasmid (Human Stem Cell Institute)); Abecma (idecabtagene vicleucel (Bristol-Myers Squibb Pharma EEIG)); Carvykti (ciltacabtagene autoleucel (Janssen Biotech, Inc.)); Kymriah (tisagenlecleucel (Novartis Pharmaceuticals Corporation)); Carteyva (relmacabtagene autoleucel (JW Therapeutics)); Tecartus (brexucabtagene autoleucel (Kite Pharma, Inc)); Yescarta (axicabtagene ciloleucel (Kite Pharma Inc.)); Breyanzi (lisocabtagene maraleucel (Bristol-Myers Squibb Pharma EEIG)); Libmeldy (atidarsagene autotemcel (Orchard Therapeutics)); Skysona (elivaldogene autotemcel (Bluebird bio, Inc.)); Strimvelis (CD34+ cells transduced with retroviral vector that encodes for the ADA gene (GlaxoSmithKline)); Zynteglo (betibeglogene autotemcel (Bluebird bio, Inc.)).
The potential for gene therapy for kidney disease
The number of genetic targets for cell and gene therapy of kidney disease is not lacking (Table 1). Congenital nephrotic syndrome most commonly results from mutations in NPHS1 or NPHS2 and often leads to severe disease35. In 2010, researchers showed that inducibly expressing nephrin (encoded by NPHS1) in nephrin-deficient mice could prevent perinatal death36, which might have implications for gene therapy in patients with congenital nephrotic syndrome. However, incomplete phenotypic rescue was reported, as mostly normal kidney pathology reported at 1 week progressed to damage and proteinuria by week 6. Candidate reasons for this finding include the timing of transgene expression or use of rat nephrin in the mouse36. Nonetheless, congenital nephrotic syndromes remain potential targets for gene therapy35. Given the severity of this disease, such a novel therapeutic approach would be highly desirable, although perinatal or infant gene therapy will carry with it a high bar for safety.
Alport syndrome results from a defect in production of α3α4α5(IV) collagen in the glomerular basement membrane37, caused by a mutation in the COL4A3, COL4A4 or COL4A5 gene. Transfer of any of these genes to restore normal collagen expression in the glomerular basement membrane has not yet been achieved in humans. However, in a mouse model of Alport syndrome, an inducible transgenic system — which reactivated the COL4A3 gene — restored α3α4α5(IV) collagen expression from podocytes, thereby improving kidney function and extending lifespan38. These results demonstrate that restoration of collagen expression using gene therapy might therapeutically treat Alport syndrome in vivo. Slowing the progression of inherited renal diseases such as Alport syndrome is essential, as patients will progressively accumulate kidney damage until the point of end-stage kidney disease. Therefore, improvements in renal function even after glomerular basement membrane development may hold potential for patients with Alport syndrome.
In 2021, a similar study was conducted using a mouse model of autosomal dominant polycystic kidney disease (ADPKD), a disease that results when kidney tubule cells harbour mutations of either PKD1 or PKD2 (ref. 39). ADPKD is the most common monogenic kidney disorder, affecting more than 12 million patients worldwide40. Therefore, novel therapies that target PKD1 or PKD2 are a high priority for the kidney cell and gene therapy field. In that study, the authors conditionally inactivated Pkd1 or Pkd2 to induce ADPKD development, but subsequently induced re-expression of the inactivated gene. This model led to rapid reversal of key ADPKD features including proliferation, inflammation, extracellular matrix deposition and cell lining metaplasia. In animals that had developed cystic kidneys, the re-expression of PKD genes was able to reverse ADPKD in vivo, demonstrating that gene therapy could potentially reverse ADPKD even after cystic disease has developed. These findings hold promise for the use of gene therapy before patients develop key disease characteristics, but also for reversing disease pathogenesis.
Cell therapy for kidney disease
Although the use of mesenchymal stem cells and regenerative cell populations has been proposed for cell therapy of kidney disease20,41, herein we focus on gene-modified cell populations. Cell populations can be genetically modified and adoptively transferred to affect the kidney directly or to provide therapy for complications of kidney disease.
Haematopoietic stem and progenitor cell therapy
Haematopoietic stem and progenitor cells (HSPCs) are a promising avenue within renal cell therapy. One notable study investigated the use of HSPCs with a mouse model of cystinosis42, a lysosomal storage disorder characterized by the widespread accumulation of the amino acid cystine. Cystinosis affects several tissues and organs of the body, including the kidney, potentially leading to permanent kidney damage and failure. Through lentiviral transduction of HSPCs to express cystinosin — the pathogenically deficient cystine transporter in cystinosis — the authors demonstrated that transduced HSPCs differentiate and subsequently integrate into the kidney and other tissues. Importantly, such therapy led to a reduction in global cystine content and improved renal function. Thus, although not currently kidney specific, HSPC therapies hold therapeutic potential for diseases that affect multiple tissues, such as lysosomal storage disorders.
The use of HSPCs for kidney-targeted enhancements, such as to promote collagen deposition within the glomerulus, remains controversial. Researchers previously reported that bone marrow transplantation from wild-type mice could lead to collagen deposition in mouse models of Alport syndrome43,44. These results were challenged by a later publication suggesting that irradiation, which preceded transplantation, was responsible for improved renal histology and survival in these mice, rather than the effect of bone marrow transplantation45. The mechanisms that underlie this effect of irradiation are unknown. A subsequent study investigated the use of several cell therapies in a mouse model of Alport syndrome (Col4A3 knockout)46. Specifically, these researchers investigated the infusion of three cellular therapies, including wild-type bone marrow, wild-type unfractionated blood and human embryonic stem cells, and assessed their effects on kidney disease progression. All three cell therapies reportedly increased de novo α3(IV) collagen expression in the mice, as well as reduced proteinuria and histological damage. Importantly, the observed therapeutic effect occurred without irradiating or conditioning the mice before cell therapy infusions, indicating the promise of possible translation to the clinic. Overall, although the field remains controversial in this area, these studies raise the possibility of HSPC therapy as a treatment strategy for kidney disease.
Ex vivo modification and subsequent delivery of gene-modified autologous cells is another exciting avenue for cell therapies, as this strategy reduces the risk of immunogenicity and rejection. To assess the clinical effects of ex vivo modification of autologous cells, a recent study used lentivirus-mediated transduction in patients with Fabry disease47, which results from α-gal A deficiency and causes kidney disease over time. HSPCs were collected from five patients, genetically modified to express α-gal A and then injected back into the respective patients. Within 1 week after the therapy, all patients were noted to have reduced disease severity, including near-normal α-gal A activity and reductions in levels of both plasma and urine globotriaosylceramide (Gb3), which accumulates in Fabry disease. This early evidence of ex vivo cell therapy in the clinic for a lysosomal storage disorder that affects many organs, including the kidney, indicates that similar approaches may be effective for other renal diseases.
T lymphocyte-mediated cell therapy
Another large subclass of cell therapies centres around the modification of lymphocytes for cytotoxic or therapeutic effects. One application of this type of therapy is the delivery of specific proteins, as demonstrated by a 2017 study that used non-viral, transposon-mediated modification of T cells to act as a sustained peptide delivery platform48. In that study, mouse T cells were modified using the piggyBac transposon system to express erythropoietin and subsequently injected into mice. Sustained increases in haematocrit were observed for more than 20 weeks after injection, an effect that could be perpetuated by vaccination. This non-virally modified T cell approach has the potential to be applied to similar hormone or peptide-deficient diseases that affect the kidney, such as Fabry disease, or for therapy of complications of kidney disease.
Viral modification of T cells may similarly be an effective approach for delivering essential enzymes or peptides. A recent study generated cellular delivery vehicles — termed ‘micropharmacies’ — composed of CD4+ T cells modified ex vivo with a lentiviral vector49. These modified T cells were conditioned with rapamycin, an immunosuppressant that enhances survival of T cells and their memory capacity and reduces inflammation. The authors investigated the therapeutic potential of this ‘micropharmacy’ approach in several lysosomal storage disorders, including Fabry disease. Specifically, when investigating delivery of α-gal A in a mouse model of Fabry disease, they reported α-gal activity above wild-type levels and a systemic reduction in Gb3 upon delivery of modified micropharmacies from a patient with Fabry disease. Overall, ex vivo modification of T cells for therapeutic delivery appears to be a promising strategy for targeting systemic disorders such as lysosomal storage disorders. However, more research into the long-term effects of T cell therapy needs to be examined for use outside of the immunotherapy realm.
Both HSPCs and T cells offer vehicles for expression of therapeutic proteins to be delivered via the blood. Such approaches can act through bystander effects whereby therapeutic proteins can be expressed elsewhere and then taken up by target cells. Delivery and expression of structural proteins may not be amenable to such an approach.
Cell therapy for implantable devices
Implantable devices have the potential to replace organs or important organ functions. A notable example of an implantable device is an artificial kidney, although bioengineering functional kidneys is not without challenges50. An artificial kidney aims to recapitulate normal kidney function, requiring functional cells for reabsorption of salt and water. However, tubular cells — which serve to reabsorb salt and water — lose their ability to mimic in vivo function when removed from their in vivo niche and cultured, owing to loss of expression of key transporters51. To overcome this obstacle, researchers have used genetic engineering to enhance volumetric transport of renal epithelial cells52. Specifically, kidney cells have been engineered to overexpress sodium hydrogen exchanger 3 and aquaporin 1, resulting in increased volumetric transport, measured through a functional assay of water transport. Such modification of cells would be of great benefit in an implantable artificial kidney by improving reabsorption of salt and water much like the native kidney in vivo. Similarly, researchers have modified cultured proximal tubular cells to stably express organic ion transporters (OAT1 and OAT3), improving their ability for drug toxicity screening53. Therefore, cultured kidney tubular cells can be gene modified to better mimic their in vivo niche. Importantly, the ability to modulate renal transport is desirable not only for inherited renal diseases but also for acute and chronic kidney injuries in which transport is altered. Cell therapies that target renal transport could be applied to a broad range of diseases and patients, so further enquiry into transport modulation is essential for kidney cell therapy.
Technical considerations
Targeting
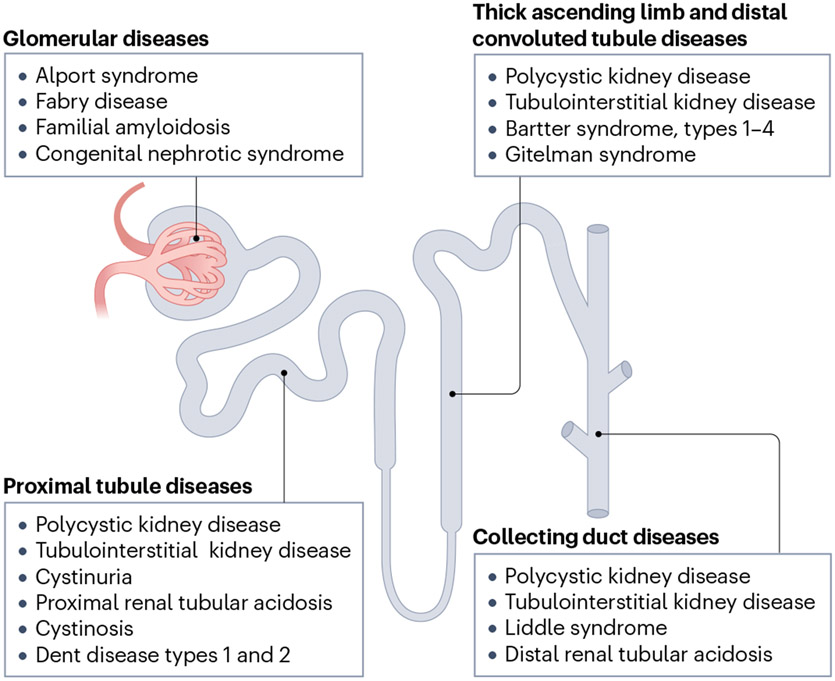
The nephron is a highly complicated structure that contains multiple specialized cell types, which in part explains why renal gene therapy has been difficult to achieve compared with other tissues. In contrast to the liver, where hepatocytes comprise more than 80% of the tissue, there are at least 26 unique cell types within the kidney54. Delivery of therapy products to a specific cell type within a specific region of the kidney has thus been the primary roadblock to kidney gene therapy. Notably, genetic kidney diseases arise from all parts of the nephron, meaning that any one of these cell types might need to be targeted to treat kidney disease (Fig. 2). Historically, viruses have not transduced the kidney efficiently, implying a lack of known viral receptor expression or accessibility, providing increased difficulty for viral targeting of key parts of the nephron. Engineering new viral capsids or particles to target specific kidney cell types could overcome this obstacle.
Fig. 2 ∣. Genetic kidney disease target location within the nephron, the basic functional unit of the kidney.
The major genetic kidney diseases for consideration of cell and gene therapy manifest disease in various cell types throughout the nephron structure.
Genetics
There are many considerations when deploying a gene therapy modality. One must consider promoter elements — deciding between ubiquitous and tissue-or cell-specific promoters or enhancers — whether the transgene cDNA will be codon optimized, whether additional elements will be added to enhance or stabilize expression such as the woodchuck hepatitis virus post-transcriptional regulatory element, and all of this must fit within the packaging capacity of the vector. Another important consideration is whether integration of genetic cargo is needed or preferred. Integration is needed for a dividing target cell population whereas episomal genetic cargo may suffice for quite some time for quiescent non-dividing target cells.
Accessibility
The size exclusion of the glomerulus remains another hurdle to overcome for renal therapeutic design. Particles travelling from the blood through the glomerulus into the urinary space have to pass through glomerular endothelium with 80–100-nm pores55, the glomerular basement membrane with a reported pore size of 3 nm (refs. 56,57) and interdigitating podocyte foot processes separated by 32 nm (ref. 58). Because of this, it is generally believed that particles above 10 nm and 50 kDa in size are actively excluded by the glomerular barrier59.
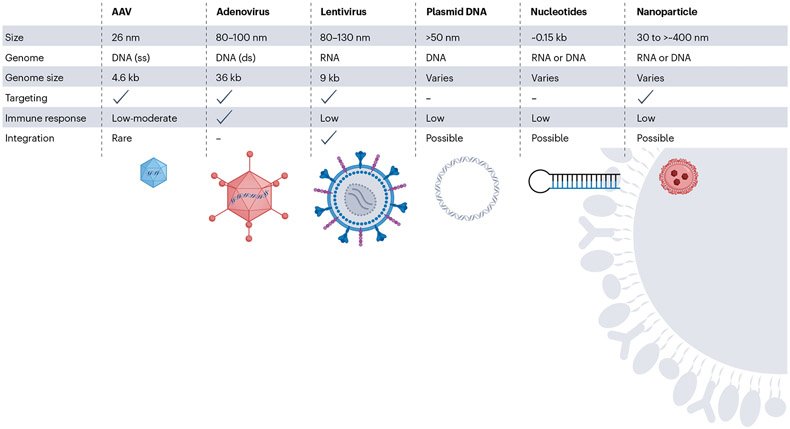
As described above, viral vectors are a frequently used delivery modality for many current gene therapies, but the size limitations of the three most common vectors must be considered (Fig. 3). The smallest of the viral vectors — adeno-associated virus (AAV) — is around 25 nm in size, whereas the largest viral vector — adenovirus — can reach a size of 100 nm. In addition to size exclusion, few serotypes of viral vectors have been shown to target the kidney, and as a result, higher viral doses are needed to achieve noticeable effects. Common viral receptors appear to be expressed in the kidney, such as AAVR/KIAA0319L for AAV60 and LDLR for the common VSV-G pseudotyped lentivirus61, implying that subcellular localization of the receptor (or receptors) or kidney architecture may contribute to a lack of kidney transduction. With these considerations in mind, nanoparticles are a promising route of delivery, given their variable size of between 1 nm and 400 nm, and because their modifiable shape and exterior could allow for enhanced targeting of a specific renal cell type.
Fig. 3 ∣. Vector considerations for cell and gene therapy of kidney disease.
Viral and non-viral vectors range in size and can package DNA or RNA with different capacity. Some can be engineered to target specific cells or tissues via capsid, envelope or particle modification. Immune response varies between vectors. Any time nucleic acids are delivered to cells, integration is a possibility, although the chance of integration can vary depending on vector type. Integration is part of lentivirus-mediated delivery but is possible, although rare, with other vector methodologies. AAV, adeno-associated virus; ds, double-stranded; ss, single-stranded.
Alternative routes for vectors to enter the kidney are important for overcoming the filtration limitations of the glomerulus. The nephron is widely accessible through several routes of injection, including anterograde delivery through the renal artery, retrograde delivery through the ureter, systemic administration and intraparenchymal administration. Each route of delivery has its own advantages, such that targeting of a specific cell type within the kidney might be enhanced by differential injection techniques. For instance, the tubular epithelium could be targeted on the apical side from the urinary space via retrograde ureteral or renal pelvis injection, but also via the basolateral side from particles traversing the blood endothelium. Additionally, the high rate of blood flow to the kidney may allow for increased exposure to vectors introduced through the circulation compared with tissues that receive relatively lower blood flow. Finally, although local injection to the kidney may improve specific targeting of renal gene and cell therapy, systemic administration is more accessible for translation to the clinic. Innovations in renal targeting of therapeutic vectors as well as optimizing the administration route of an individual therapy will eventually be required to maximize therapeutic benefit while minimizing toxicity and off-target effects. Nonetheless, many different delivery methods have been attempted over the years with none generating high enough transfection or transduction efficiency to gain traction for gene therapy of kidney disease59.
Vector type
One must also choose between viral and non-viral vectors, and if non-viral, whether delivery agents such as nanoparticles or liposomes will be used (Fig. 3). An additional consideration is whether pre-existing immunity to the gene transfer vector exists within the target patient population. If so, many patients may be excluded from receiving the therapy. All of these considerations have advantages and disadvantages, and by the time a gene therapy makes it to the clinic these various options have been extensively evaluated.
Vector choice involves consideration of packaging limits, differences in immunogenicity and whether cargo will remain episomal or integrate into the genome (Fig. 3). Adenovirus vectors have been in use for years in research for kidney gene transfer, with mixed results59; the clinical application of such a vector for kidney disease seems questionable. The use of adenovirus for kidney gene therapy has been reviewed elsewhere59. Here, we focus on vectors for kidney therapy that have reached clinical application in other tissues.
Lentivirus and retrovirus vectors are common tools for gene delivery. Although lentiviral transduction of the kidney has been poor62, recent work showed several months of symptomatic improvement in a mouse model of Dent disease — a kidney disorder caused by mutations in the CLCN5 gene — using retrograde ureteral injection of lentivirus carrying CLCN5 (ref. 63). Lentiviral and retroviral vectors transduce dividing cells, which may limit transduction of kidney tissue given that most renal cell types are postmitotic. Integration into the genome is part of the life cycle of these vectors, which, although being a useful attribute for sustained transgene expression, raises the possibility of genotoxicity. Nonetheless, integration could be favourable for certain kidney cell types that turn over over time, such as tubular cells. In particular, off-target toxicity could result from vector integration in cells outside of the kidney, unless kidney targeting specificity can be achieved. Pseudotyping with altered envelopes64,65, attaching monoclonal antibodies66 or other targeting moieties67 are strategies that have been used to retarget lentivirus to other cell types. Future research should aim to improve retargeting of lentivirus to the kidney for enhanced transduction.
AAV has emerged as a prominent viral vector for in vivo human gene therapy, even achieving approval for patient use. Although AAV serotypes have been identified that efficiently target tissues such as the liver, retina and muscle, few serotypes have shown kidney localization. Accordingly, AAV has not achieved widespread use in the kidney owing to variable transduction efficiency in multiple reports68-77. For instance, delivery of AAV9 in a mouse model of acrodysostosis demonstrated up to 70% transduction in kidney cortex tubular cells with subsequent restoration of disease symptoms78, but such success with AAV9 in the kidney has not been reported by others. In a separate AAV delivery study testing six AAV serotypes, researchers demonstrated transduction of kidney mesenchymal cells, including pericytes, fibroblasts and mesangial cells with a novel AAV serotype; however, they observed no kidney transduction with AAV9 (ref. 79). A recent report examined the biodistribution after injection of 124iodine-labelled AAV in non-human primates and found no uptake of AAV9 or AAVrh.10 in the kidney80. Re-engineering of AAV capsids may be necessary for efficient kidney transduction by AAV.
AAV is often preferred for its favourable viral safety profile, although the small packaging capacity compared with adenovirus and lentivirus is an important consideration for its use. The DNA within AAV can be made as single-stranded or self-complementary, resulting in a packaging capacity of 4.7 kb or 2.3 kb, respectively81. This limit is smaller than transgenes for the more common genetic kidney diseases such as PKD or Alport syndrome, which are too large for single AAV packaging (Table 1). To circumvent this issue, trans-splicing or the use of recombination-prone nucleic acid sequences can be used to stitch together larger transgenes from smaller pieces in cells. Researchers have successfully developed trans-splicing AAVs to reconstitute larger transgenes in cells82-85. However, success of trans-splicing AAVs in vivo in intact animals has been limited86,87. Integration of recombinant AAV is thought to be rare, although tumour development has been reported in mice after AAV-mediated gene delivery88-92. Although the native serotypes of AAV appear to be somewhat inefficient for kidney transduction, highly sensitive assays to detect transduction have suggested that AAV transduction of the kidney may be under-reported through using a highly sensitive assay to detect transduction93. Some researchers have reported transduction of tubular cells via retrograde injection of the ureter producing apparently higher transduction efficiency compared with intravenous injection of virus73. High dosage of AAV may be necessary for kidney transduction owing to current limitations on viral targeting. However, recent reports of patient deaths at high AAV dosage raise concerns94. AAV capsid engineering has enabled retargeting of AAV to various tissues and cell types95-99. Capsid engineering may offer new kidney-targeted AAVs, opening avenues towards a wide variety of gene therapy opportunities for kidney researchers. Overall, viral vectors are a leading choice for gene therapy approaches for several tissues and diseases. Identifying viral serotypes that better target the kidney and understanding the underlying mechanism behind those increased transduction rates are essential for pursuing viral vector-based therapies in the future.
Non-viral vectors, including nanoparticles, might be advantageous for kidney delivery owing to their modifiable size and reduced immunogenicity compared with viral vectors (Fig. 3). Their low manufacturing cost and flexibility in structural components are additional benefits. Several kidney-targeted non-viral gene delivery approaches have been attempted over the years, including liposomes100,101, ultrasound with microbubbles102-104, various vascular methods of naked DNA injection105-107 and hydrodynamic renal pelvis injection108. Promising nanoparticle approaches have been developed in recent years to overcome the hurdle of delivery to the kidney in vivo. For example, proximal tubule-targeted mesoscale nanoparticles have been developed109, with a reported renal uptake that was 26- to 94-fold higher than other tissues. Researchers have also developed proximal tubule-targeted nanocarriers that bind to megalin, a large receptor that mediates proximal tubule protein uptake110. Mesoscale nanoparticles that targeted proximal tubular nuclear factor-κB essential modulator were beneficial in a mouse model of acute kidney injury111. Diseases of the proximal tubule epithelium could benefit from this nanoparticle approach, as the renal specificity and lack of toxicity of this vector hold translational potential. In theory, nanoparticles can be used to deliver drugs, RNA, protein or DNA to target cells in vivo, although delivery of DNA has been less successful to date.
Emerging technologies and future advances
The future of effective kidney targeting and sustained phenotypic correction by cell and gene therapies remains hopeful. In the past year, several efforts have highlighted the field’s technological improvements and enhanced understanding of renal pathophysiology.
ADPKD is the most common monogenic renal disorder (Table 1). Recent insights into its disease pathology have offered hope for new avenues for treatment, including gene therapy. A 2022 paper investigated the inhibitory effect of the primarily mutated genes in ADPKD: PKD1 and PKD2 (ref. 112). Using a mouse model of ADPKD, it was found that a non-inactivated copy of Pkd1 produces mRNA that becomes repressed owing to cis binding in the 3′ untranslated region (UTR). Consequently, when that portion of the 3′ UTR motif is removed through CRISPR–Cas9 editing or is inhibited with an oligonucleotide, cyst size and growth are reduced. This attenuated disease pathogenesis was also seen with inhibition of the 3′ UTR in the non-inactivated copy of Pkd2. The robust mechanisms demonstrated here indicate multiple avenues for development of ADPKD gene therapy, as both gene editing and oligonucleotide delivery modified disease progression.
Both viral and non-viral approaches for kidney gene therapy have also gained traction recently. One study developed a novel nanoparticle coated with non-inhibitory plasminogen activator inhibitor 1R (PAI-1R) that selectively targets glomerular mesangial cells113. This nanoparticle was packaged with small interfering RNA to silence transforming growth factor-β1 (TGFβ1) in a rat model of human mesangial proliferative glomerulonephritis. Systemic TGFβ1 inhibitors are efficacious at slowing CKD progression, but harbour substantial risks of inflammation, as TGFβ1 is also a key anti-inflammatory factor114. Importantly for renal targeting, the PAI-1R-coated nanoparticles were able to target mesangial cells through glomerular vascular fenestrations, thereby bypassing the size restrictions of the glomerular filtration barrier. The authors observed that a single dose of the nanoparticle improved renal function, including significantly reducing urinary protein excretion and glomerular matrix accumulation 5 days after injection. Additionally, although TGFβ1 protein and mRNA levels were significantly inhibited in the glomerulus and the liver, no changes were observed in lung, spleen, arterial or renal medullary tissue. Although this study highlighted a model of glomerulonephritis, glomerulus-targeted nanoparticles could be modified for a wide range of glomerular diseases including both inherited and acquired pathologies.
Programmable nucleases can be used for various genomic manipulations that might have clinical implications. These include CRISPR–Cas9, transcription-activator-like effector nucleases and zinc-finger nucleases that induce targeted double-stranded breaks in the genome, leading to activation of the error-prone non-homologous end-joining pathway or template-dependent homologous directed repair115. Base editing can enable user-directed change of DNA or RNA bases to correct mutations or expression116. Prime editing uses a catalytically impaired Cas9 enzyme fused to reverse transcriptase wherein the guide RNA encodes targeting and the template for editing117. There are now several ongoing CRISPR clinical trials targeting various diseases118. Ultimately, the use of CRISPR–Cas for kidney gene delivery will depend upon effective delivery to the kidney.
Technologies have also become available for modulating gene transcription or protein translation without transgene delivery per se. For instance, anti-sense oligonucleotides have been used to enable exon skipping in an X-linked mouse model of Alport syndrome, resulting in improved collagen expression and animal survival119. AAV has been used to deliver suppressor tRNAs, enabling readthrough of nonsense mutations in vivo120. Time will tell whether such approaches are translatable for clinical application.
Notable advancements in viral vector design have improved feasibility for renal gene therapy. Viral pseudotyping — the process of producing viral vectors using viral envelope proteins from a different virus — allows investigators to alter the existing specificity of viral serotypes121,122. To improve renal targeting of lentivirus, a recent study designed lentivirus pseudotyped with the envelope from Zika virus, chosen for its affinity to renal tubular epithelial cells123. Interestingly, the pseudotyped virus, named ZIKV-E, demonstrated 100-fold higher transduction efficiency in renal tubular epithelial cells compared with the lentivirus-envelope control. ZIKV-E also demonstrated high transduction within the liver, brain, heart and spleen, indicating that further modification to the pseudotyped envelope would be necessary for selective renal targeting. In addition to pseudotyping of viral envelopes, capsid engineering of viruses also provides an avenue for enhanced renal specificity of cell and gene therapies124,125. High-throughput screening of diverse capsid libraries could allow for identification of kidney-targeted viruses. Additionally, identification of key peptides that facilitate renal transduction will be essential for understanding how transduction occurs within the kidney and therefore how the field could enhance renal cell and gene therapy efforts. Both viral and non-viral approaches for renal cell and gene therapy have drastically improved in the past few decades, but there are still major hurdles to overcome in targeting, efficiency and longevity before cell and gene therapies could be seen in the clinic.
Technological advances in cell and gene therapies may prove futile if costs are prohibitive. Costs of currently marketed products range from hundreds of thousands to millions of dollars per dose126. The hope would be that improved manufacturing and more available products would drive down costs. Much work needs to be done on many levels, from basic science and translational researchers through to drug companies and policy-makers, to make these therapies accessible and equitable for patient populations.
Conclusions
Cell and gene therapy for kidney disease is in its infancy. Although gene delivery to the kidney has been attempted for many years in various animal models, delivery of genetic cargo to the kidney remains the main obstacle for cell and gene therapy of kidney disease. The field will benefit from agency-approved cell and gene therapies for nonkidney diseases as well as the vectors used. However, breakthrough research needs to be carried out to target diseases of this complex but very important organ. Currently, almost all kidney diseases are treated with supportive care without the use of molecular therapies to target the underlying cause. This is unacceptable given the high burden, morbidity and mortality associated with kidney disease. Many patients are waiting for cell and gene therapy of kidney disease to improve and lengthen their lives. It is hoped that, in the near future, a toolbox of safe and effective cell and gene therapies for kidney disease will exist, revolutionizing the war against the various causes of CKD and its complications.
Key points.
Despite the use of cell and gene therapies in the clinic for other tissues, no such interventions are available that target the kidney.
Approximately 30% of chronic kidney diseases are inherited, and the genetic basis is well understood, meaning that they are suitable for targeting by cell or gene therapy before development of irreparable renal failure.
Genetic studies in mouse models have revealed the potential of gene therapy for kidney disease.
Delivery of therapeutic material to the kidney is the main hurdle to cell and gene therapy development.
Innovations in vector technology, delivery and an enhanced understanding of kidney disease pathogenesis provide hope for future kidney cell and gene therapies.
Acknowledgements
J.L.P. is supported by DK134046 and T32GM007347 from the NIH. M.H.W. is supported by BK004258 from the Department of Veterans Affairs and DK093660 and EB033676 from the NIH.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Evans M. et al. A narrative review of chronic kidney disease in clinical practice: current challenges and future perspectives. Adv. Ther 39, 33–43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill NR et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS ONE 11, e0158765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS & Coresh J Chronic kidney disease. Lancet 379, 165–180 (2012). [DOI] [PubMed] [Google Scholar]
- 4.United States Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2021). [Google Scholar]
- 5.Ginn SL, Amaya AK, Alexander IE, Edelstein M & Abedi MR Gene therapy clinical trials worldwide to 2017: an update. J. Gene Med 20, e3015 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Wheeler DC & Steiger J Evolution and etiology of cardiovascular diseases in renal transplant recipients. Transplantation 70, Ss41–Ss45 (2000). [PubMed] [Google Scholar]
- 7.Hildebrandt F. Genetic kidney diseases. Lancet 375, 1287–1295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrezenmeier E. et al. The underestimated burden of monogenic kidney disease in adults waitlisted for kidney transplantation. Genet. Med 23, 1219–1224 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai E, Takabatake Y, Mizui M & Isaka Y Gene therapy in renal diseases. Kidney Int. 65, 1551–1555 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Armstrong ME & Thomas CP Diagnosis of monogenic chronic kidney diseases. Curr. Opin. Nephrol. Hypertens 28, 183–194 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Connaughton DM et al. Monogenic causes of chronic kidney disease in adults. Kidney Int. 95, 914–928 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KDIGO Conference Participants. Genetics in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 101, 1126–1141 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayasinghe K. et al. Clinical impact of genomic testing in patients with suspected monogenic kidney disease. Genet. Med 23, 183–191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groopman EE et al. Diagnostic utility of exome sequencing for kidney disease. New Engl. J. Med 380, 142–151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore DF, Ries M, Forget EL & Schiffmann R Enzyme replacement therapy in orphan and ultra-orphan diseases: the limitations of standard economic metrics as exemplified by Fabry–Anderson disease. Pharmacoeconomics 25, 201–208 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Oder D, Nordbeck P & Wanner C Long term treatment with enzyme replacement therapy in patients with fabry disease. Nephron 134, 30–36 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Rohrbach M & Clarke JT Treatment of lysosomal storage disorders: progress with enzyme replacement therapy. Drugs 67, 2697–2716 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Grange C & Bussolati B Extracellular vesicles in kidney disease. Nat. Rev. Nephrol 18, 499–513 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biancone L & Camussi G Potential use of stem or progenitor cells for kidney regeneration. Nat. Rev. Nephrol 10, 67–68 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Bussolati B & Camussi G Therapeutic use of human renal progenitor cells for kidney regeneration. Nat. Rev. Nephrol 11, 695–706 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Couzin J & Kaiser J Gene therapy. As Gelsinger case ends, gene therapy suffers another blow. Science 307, 1028 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Brunetti-Pierri N & Ng P Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum. Mol. Genet 20, R7–R13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo P & Brunetti-Pierri N Challenges and prospects for helper-dependent adenoviral vector-mediated gene therapy. Biomedicines 2, 132–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atchison RW, Casto BC & Hammon WM Adenovirus-associated defective virus particles. Science 149, 754–756 (1965). [DOI] [PubMed] [Google Scholar]
- 25.Blaese RM et al. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science 270, 475–480 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Hacein-Bey-Abina S. et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. New Engl. J. Med 346, 1185–1193 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Hacein-Bey-Abina S. et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. New Engl. J. Med 348, 255–256 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Hacein-Bey-Abina S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Philippidis A. Brother of cure rare disease CEO dies in trial of Duchenne muscular dystrophy therapy. Hum. Gene Ther 33, 1224–1227 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Barrett D. et al. Gene, Cell, and RNA Therapy Landscape: Q3 2022 Quarterly Data Report, https://asgct.org/global/documents/asgct-citeline-q3-2022-report.aspx (2022). [Google Scholar]
- 31.Moran N. First gene therapy approved. Nat. Biotechnol 30, 1153 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Braendstrup P, Levine BL & Ruella M The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy 22, 57–69 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bishop DC et al. Development of CAR T-cell lymphoma in two of ten patients effectively treated with piggyBac modified CD19 CAR T-cells. Blood 138, 1504–1509 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Micklethwaite KP et al. Investigation of product derived lymphoma following infusion of piggyBac modified CD19 chimeric antigen receptor T-cells. Blood 138, 1391–1405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem MA Molecular stratification of idiopathic nephrotic syndrome. Nat. Rev. Nephrol 15, 750–765 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Juhila J. et al. Inducible nephrin transgene expression in podocytes rescues nephrin-deficient mice from perinatal death. Am. J. Pathol 176, 51–63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naylor RW, Morais M & Lennon R Complexities of the glomerular basement membrane. Nat. Rev. Nephrol 17, 112–127 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Lin X, Suh JH, Go G & Miner JH Feasibility of repairing glomerular basement membrane defects in Alport syndrome. J. Am. Soc. Nephrol 25, 687–692 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong K. et al. Renal plasticity revealed through reversal of polycystic kidney disease in mice. Nat. Genet 53, 1649–1663 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chebib FT & Torres VE Autosomal dominant polycystic kidney disease: core curriculum 2016. Am. J. Kidney Dis 67, 792–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tögel FE & Westenfelder C Mesenchymal stem cells: a new therapeutic tool for AKI. Nat. Rev. Nephrol 6, 179–183 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Harrison F. et al. Hematopoietic stem cell gene therapy for the multisystemic lysosomal storage disorder cystinosis. Mol. Ther 21, 433–444 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prodromidi EI et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cell 24, 2448–2455 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto H. et al. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc. Natl Acad. Sci. USA 103, 7321–7326 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katayama K. et al. Irradiation prolongs survival of Alport mice. J. Am. Soc. Nephrol 19, 1692–1700 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeBleu V. et al. Stem cell therapies benefit Alport syndrome. J. Am. Soc. Nephrol 20, 2359–2370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan A. et al. Lentivirus-mediated gene therapy for Fabry disease. Nat. Commun 12, 1178 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neil RT et al. Transposon-modified antigen-specific T lymphocytes for sustained therapeutic protein delivery in vivo. Nat. Commun 9, 1325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagree MS et al. Autologous, lentivirus-modified, T-rapa cell “micropharmacies” for lysosomal storage disorders. EMBO Mol. Med 14, e14297 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Fissell WH, Humes DH & Roy S Current strategies and challenges in engineering a bioartificial kidney. Front. Biosci 7, 215–228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khundmiri SJ, Chen L, Lederer ED, Yang CR & Knepper MA Transcriptomes of major proximal tubule cell culture models. J. Am. Soc. Nephrol 32, 86–97 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson MH et al. Genome engineering renal epithelial cells for enhanced volume transport function. Cell Mol. Bioeng 13, 17–26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieskens TT et al. A human renal proximal tubule cell line with stable organic anion transporter 1 and 3 expression predictive for antiviral-induced toxicity. AAPS J. 18, 465–475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Awqati Q & Oliver JA Stem cells in the kidney. Kidney Int. 61, 387–395 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Luft FC et al. Effects of moxalactam and cefotaxime on rabbit renal tissue. Antimicrob. Agents Chemother 21, 830–835 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanwar YS & Farquhar MG Presence of heparan sulfate in the glomerular basement membrane. Proc. Natl Acad. Sci. USA 76, 1303–1307 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa S. et al. High-resolution ultrastructural comparison of renal glomerular and tubular basement membranes. Am. J. Nephrol 19, 686–693 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Lahdenkari AT et al. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J. Am. Soc. Nephrol 15, 2611–2618 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Rubin JD & Barry MA Improving molecular therapy in the kidney. Mol. Diagn. Ther 24, 375–396 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pillay S. et al. An essential receptor for adeno-associated virus infection. Nature 530, 108–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finkelshtein D, Werman A, Novick D, Barak S & Rubinstein M LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl Acad. Sci. USA 110, 7306–7311 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis L & Park F Gene therapy research for kidney diseases. Physiol. Genomics 51, 449–461 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Yadav MK, Yoo KW, Atala A & Lu B Lentiviral vector mediated gene therapy for type I Dent disease ameliorates Dent disease-like phenotypes for three months in ClC-5 null mice. Mol. Ther. Methods Clin. Dev 27, 149–166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buchholz CJ, Mühlebach MD & Cichutek K Lentiviral vectors with measles virus glycoproteins - dream team for gene transfer? Trends Biotechnol. 27, 259–265 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Girard-Gagnepain A. et al. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs. Blood 124, 1221–1231 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Höfig I. et al. Systematic improvement of lentivirus transduction protocols by antibody fragments fused to VSV-G as envelope glycoprotein. Biomaterials 35, 4204–4212 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Buchholz CJ, Friedel T & Büning H Surface-engineered viral vectors for selective and cell type-specific gene delivery. Trends Biotechnol. 33, 777–790 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Chen S. et al. Gene delivery in renal tubular epithelial cells using recombinant adeno-associated viral vectors. J. Am. Soc. Nephrol 14, 947–958 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Chung DC et al. Adeno-associated virus-mediated gene transfer to renal tubule cells via a retrograde ureteral approach. Nephron Extra 1, 217–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grimm D. et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol 82, 5887–5911 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirsch ML, Green L, Porteus MH & Samulski RJ Self-complementary AAV mediates gene targeting and enhances endonuclease delivery for double-strand break repair. Gene Ther. 17, 1175–1180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kapturczak MH, Chen S & Agarwal A Adeno-associated virus vector-mediated gene delivery to the vasculature and kidney. Acta Biochim. Pol 52, 293–299 (2005). [PubMed] [Google Scholar]
- 73.Konkalmatt PR et al. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI insight 1, e85888 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rocca CJ, Ur SN, Harrison F & Cherqui S rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther. 21, 618–628 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zincarelli C, Soltys S, Rengo G & Rabinowitz JE Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther 16, 1073–1080 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Hillestad ML, Guenzel AJ, Nath KA & Barry MA A vector-host system to fingerprint virus tropism. Hum. Gene Ther 23, 1116–1126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubin JD, Nguyen TV, Allen KL, Ayasoufi K & Barry MA Comparison of gene delivery to the kidney by adenovirus, adeno-associated virus, and lentiviral vectors after intravenous and direct kidney injections. Hum. Gene Ther 30, 1559–1571 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozgur-Gunes Y. et al. Correction of a knock-in mouse model of acrodysostosis with gene therapy using a rAAV9-CAG-human PRKAR1A vector. Gene Ther. 29, 441–448 (2022). [DOI] [PubMed] [Google Scholar]
- 79.Ikeda Y, Sun Z, Ru X, Vandenberghe LH & Humphreys BD Efficient gene transfer to kidney mesenchymal cells using a synthetic adeno-associated viral vector. J. Am. Soc. Nephrol 29, 2287–2297 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ballon DJ et al. Quantitative whole-body imaging of I-124-labeled adeno-associated viral vector biodistribution in nonhuman primates. Hum. Gene Ther 31, 1237–1259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarty DM Self-complementary AAV vectors; advances and applications. Mol. Ther 16, 1648–1656 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Barbon E. et al. Development of a dual hybrid AAV vector for endothelial-targeted expression of von Willebrand factor. Gene Ther. 10.1038/s41434-020-00218-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghosh A, Yue Y & Duan D Efficient transgene reconstitution with hybrid dual AAV vectors carrying the minimized bridging sequences. Hum. Gene Ther 22, 77–83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carvalho LS et al. Evaluating efficiencies of dual AAV approaches for retinal targeting. Front. Neurosci 11, 503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reisinger E. Dual-AAV delivery of large gene sequences to the inner ear. Hear. Res 394, 107857 (2020). [DOI] [PubMed] [Google Scholar]
- 86.McClements ME & MacLaren RE Adeno-associated virus (AAV) dual vector strategies for gene therapy encoding large transgenes. Yale J. Biol. Med 90, 611–623 (2017). [PMC free article] [PubMed] [Google Scholar]
- 87.Colella P, Ronzitti G & Mingozzi F Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev 8, 87–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandler RJ et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Investig 125, 870–880 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nault JC et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet 47, 1187–1193 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Donsante A. et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 317, 477 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Russell DW & Grompe M Adeno-associated virus finds its disease. Nat. Genet 47, 1104–1105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berns KI et al. Adeno-associated virus type 2 and hepatocellular carcinoma. Hum. Gene Ther 26, 779–781 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lang JF, Toulmin SA, Brida KL, Eisenlohr LC & Davidson BL Standard screening methods underreport AAV-mediated transduction and gene editing. Nat. Commun 10, 3415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.No authors listed. High-dose AAV gene therapy deaths. Nat. Biotechnol 38, 910 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Dalkara D. et al. vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med 5, 189ra176 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Kay CN et al. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS ONE 8, e62097 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korbelin J. et al. Pulmonary targeting of adeno-associated viral vectors by next-generation sequencing-guided screening of random capsid displayed peptide libraries. Mol. Ther 24, 1050–1061 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lisowski L. et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506, 382–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tervo DG et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomita N. et al. Targeted gene therapy for rat glomerulonephritis using HVJ-immunoliposomes. J. Gene Med 4, 527–535 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Tomita N. et al. Direct in vivo gene introduction into rat kidney. Biochem. Biophys. Res. Commun 186, 129–134 (1992). [DOI] [PubMed] [Google Scholar]
- 102.Ka SM et al. Smad7 gene therapy ameliorates an autoimmune crescentic glomerulonephritis in mice. J. Am. Soc. Nephrol 18, 1777–1788 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Hou CC et al. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am. J. Pathol 166, 761–771 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koike H. et al. An efficient gene transfer method mediated by ultrasound and microbubbles into the kidney. J. Gene Med 7, 108–116 (2005). [DOI] [PubMed] [Google Scholar]
- 105.Maruyama H. et al. Kidney-targeted naked DNA transfer by retrograde renal vein injection in rats. Hum. Gene Ther 13, 455–468 (2002). [DOI] [PubMed] [Google Scholar]
- 106.Corridon PR et al. A method to facilitate and monitor expression of exogenous genes in the rat kidney using plasmid and viral vectors. Am. J. Physiol. Ren. Physiol 304, F1217–F1229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suda T, Suda K & Liu D Computer-assisted hydrodynamic gene delivery. Mol. Ther 16, 1098–1104 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Woodard LE et al. Kidney-specific transposon-mediated gene transfer in vivo. Sci. Rep 7, 44904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams RM et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 15, 2358–2364 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ordikhani F. et al. Selective trafficking of light chain-conjugated nanoparticles to the kidney and renal cell carcinoma. Nano Today 35, 100990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han SJ et al. Renal proximal tubular NEMO plays a critical role in ischemic acute kidney injury. JCI Insight 5, e139246 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lakhia R. et al. PKD1 and PKD2 mRNA cis-inhibition drives polycystic kidney disease progression. Nat. Commun 13, 4765 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu X, Zhang J, Tang A, Xu L & Huang Y A novel peptide ligand-coated nano-siRNA-lipoplex technology for kidney targeted gene therapy. Am. J. Transl. Res 14, 7362–7377 (2022). [PMC free article] [PubMed] [Google Scholar]
- 114.Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A & Rodrigues-Diez RR Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol 16, 269–288 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Maeder ML & Gersbach CA Genome-editing technologies for gene and cell therapy. Mol. Ther 24, 430–446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Porto EM, Komor AC, Slaymaker IM & Yeo GW Base editing: advances and therapeutic opportunities. Nat. Rev. Drug Discov 19, 839–859 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anzalone AV et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Henderson H. CRISPR Clinical Trials: A 2022 Update, https://innovativegenomics.org/news/crispr-clinical-trials-2022/ (2022). [Google Scholar]
- 119.Yamamura T. et al. Development of an exon skipping therapy for X-linked Alport syndrome with truncating variants in COL4A5. Nat. Commun 11, 2777 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang J. et al. AAV-delivered suppressor tRNA overcomes a nonsense mutation in mice. Nature 604, 343–348 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gutierrez-Guerrero A, Cosset FL & Verhoeyen E Lentiviral vector pseudotypes: precious tools to improve gene modification of hematopoietic cells for research and gene therapy. Viruses 12, 1016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Joglekar AV & Sandoval S Pseudotyped lentiviral vectors: one vector, many guises. Hum. Gene Ther. Methods 28, 291–301 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Liu J. et al. Efficient gene transfer to kidney using a lentiviral vector pseudotyped with zika virus envelope glycoprotein. Hum. Gene Ther 33, 1269–1278 (2022). [DOI] [PubMed] [Google Scholar]
- 124.VandenDriessche T. AAV capsid engineering: zooming in on the target. Hum. Gene Ther 28, 373–374 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Zolotukhin S & Vandenberghe LH AAV capsid design: a Goldilocks challenge. Trends Mol. Med 28, 183–193 (2022). [DOI] [PubMed] [Google Scholar]
- 126.Gene therapies should be for all. Nat. Med 27, 1311 (2021). [DOI] [PubMed] [Google Scholar]