SUMMARY
Research background
While it is clear that SARS CoV-2 coronavirus is the primary respiratory virus, there are no entirely clarified ways of transmission. Foodborne transmission has remained an unexplained path. Therefore, the goals of this paper are to examine and present an assessment of the most appropriate of the four selected kits for RNA extraction for the testing and detection of SARS-CoV-2 on food packaging surfaces, food surfaces, and in food. This will enable to indicate the possibility of infection through contact or direct food consumption.
Experimental approach
Finding the best technique is vital as RNA extraction is one of the essential elements in detecting SARS-CoV-2. This was achieved through an experiment with four commercial kits following the original manufacturers’ protocols, and with a modification of the original protocols that included the use of ethanol and isopropanol. The selected kit was used for RNA extraction from the swabs of packaging surfaces, food surface, and ready-to-eat food samples. The coronavirus was then identified using real-time reverse transcription-polymerase chain reaction (RT-PCR) assays to determine whether the SARS-CoV-2 virus or viral particles are present in the food chain with the overall purpose of demonstrating the possibility that food can contribute as a vehicle for the transmission of the virus.
Results and conclusions
The findings of this investigation made the most effective extraction kit and protocol stand out. The results of the applicability of the kit indicated a significant share of positive samples of viral SARS-CoV-2 virus particles on surfaces from the environment where infected persons with 'silent' COVID-19 infection, with mild symptoms or no symptoms, were present. However, according to the findings of the second part of the study, the virus was not detected on the examined samples of food packaging surfaces, food surfaces, and food.
Novelty and scientific contribution
The presented results distinguished one of the most suitable protocols for isolating RNA from environmental surface samples. The main contribution of the study is in the presentation of the results, that is, the examination of samples that are primarily related to the food chain, food packaging, food surfaces, and ready-to-eat food. The results of this study could also be helpful for further determination of the potential of food as a vector for the transmission of coronaviruses.
Keywords: SARS-CoV-2, food, RT-PCR, coronavirus, foodborne transmission, RNA extraction
INTRODUCTION
On 11 March 2020, the World Health Organization (WHO) declared the SARS-CoV-2 outbreak a global pandemic (1). The novel SARS-CoV-2 coronavirus led to the emergence of coronavirus disease (COVID-19), which caused over 6 million mortal cases, making it one of the deadliest pandemics in history. The WHO stated that it can spread from the mouth and nose in small liquid particles (2). However, during the SARS epidemic, direct contact with surfaces and faecal transmission was also reported (3). WHO points out that the SARS-CoV-2 virus can remain very stable at 4 °C, just like the SARS-CoV and Middle East respiratory syndrome (MERS) coronaviruses, that it is expected to behave similarly to its predecessors and that it could remain infectious at -20 °C for up to 2 years (4). In addition, many extensive studies confirm and clearly show the stability and variability of the persistence of SARS-CoV-2 and other coronaviruses in the environment, depending on different surfaces and the influence of climatic conditions on their stability (5–7). A couple of preliminary studies focus specifically on the importance of isolating and monitoring the occurrence of viruses in food and the stability of viruses on the surface of food, specifically salmon, shrimp, frozen chicken wings and pork (5, 8–12).
Although complex transmission modes have not been fully understood, there are indications that the contamination of the seafood market could be the source of the COVID-19 outbreak in Wuhan, PR China (12). It is important to highlight that there are no documents that report the transmission through foods or packaging materials, but the capability of the virus to remain infectious on those matrices warns caution (12). The stability of SARS-CoV-2 in a wide pH range (pH=3–10) allows its stability in most food products (12). The study of Huang et al. (13) indicates that the oral cavity is an important site for SARS-CoV-2 infection and points to saliva as a potential route of SARS-CoV-2 transmission. Available literature suggests that more studies are necessary to better understand transmission modes, especially the role of food that has the potential to act as a vehicle for the mentioned virus (12). Field investigation in food retailers concluded that if preventative measures together with sanitizing protocols are employed, the risk of exposure to SARS-CoV-2 is low (14).
Most of the research since the beginning of the pandemic was focused on the ’mainstream’ processing of clinical specimens to detect the virus that is the primary causative agent of the COVID-19 disease. Therefore, at the time of this experiment, there were not any standardized protocols or methodology for detecting SARS-CoV-2 in the environmental samples available. Corman et al. (15) pointed out that the testing of samples should ideally be carried out in two steps, i.e. proving the presence of at least two gene sequences, just like the recommendation of the WHO guide (16). DNA amplification and detection methods take advantage of the conservation of the nucleotide sequence of a viral genome, which enables viral identification with excellent specificity and high sensitivity (17).
Based on the aforementioned, this study aims to assess, through comparative tests, the most appropriate and efficient of the four selected kits for RNA extraction, as well as to provide the most efficient solution for the testing and detection of SARS-CoV-2 via swabs of packaging surfaces, food surfaces, and ready-to-eat food on a wide range of food products available on the Croatian market during the pandemic. Although the SARS-COV-2 virus was not isolated from the samples taken from the food chain, the results of the experimental part of the research, in which the applicability of the kit was tested, indicated a significant proportion of positive samples from the environment where infected persons with less pronounced symptoms or no symptoms were present. Considering that similar studies presented in this paper showed the possibility of infection through contact or direct consumption of food, it can be concluded that there is a potential for infection, but it is negligible.
MATERIALS AND METHODS
To test the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the surface of food, food packaging and in ready-to-eat food, it was necessary to evaluate the performance of commercially available kits for the isolation of viral ribonucleic acid (RNA).
Isolation kit efficiency assessment
In order to determine the best isolation kit for the target RNA, kits from four manufacturers were used, designated as kit 1, 2, 3, and 4 (Table 1). Kit manufacturers included in the test are Agilent Technologies (La Jolla, CA, USA), Bioron Diagnostics GmbH (Römerberg, Germany), EuroFins Technologies (Freiburg, Germany), and Qiagen (Venlo, Netherlands), manufacturers are listed alphabetically, kit numbers are random, and have no relation to the order of the kits in the displayed table.
Table 1. Overview of extraction kits compared in the study.
| Manufacturer | t(storage)/°C | Regulatory status | Made in |
|---|---|---|---|
| Agilent Technologies | room | RUO | La Jolla, CA, USA |
| Bioron Diagnostics GmbH | room/2-8 | CE-IVD | Romerberg, Germany |
| EuroFins Technologies | room | RUO | Freiburg, Germany |
| Qiagen | room | - | Venio, Netherlands |
CE-IVD=European conformity label for in-vitro diagnostics, RUO=research use only
RNA was isolated according to the manufacturer’s instructions for each selected kit. Verification of kit procedures comprises implementation of the mouse norovirus, because the commercial standard of SARS-CoV-2 virus was not available for controlled contamination (murine norovirus, median tissue culture infectious dose assay (TCID50/mL ≈108 copies/mL).
The standard is integral to VIR Seek Murine Norovirus (MNV), a quantitative real-time RT-PCR kit for food and environmental samples (EuroFins GeneScan Technologies, Freiburg, Germany). To determine the efficiency of PCR, equal volumes of MNV standard (10 µL) were added to the lysis buffer with varying virus dilutions (N(MNV)=undiluted, 10-1, 10-2 and 10-3). The assessment is based on the relative measure of the number of cycles at which the target analyte (after the RT-PCR reaction) curve intersects the threshold line, that is, the quantification cycle (Cq) values and other calculations shown in the statistical processing of the results.
Protocol modification based on ethanol/isopropanol use
In addition to the original protocol, testing was done with an alternative protocol in which ethanol (Scharlab, S.L., Sentmenat, Barcelona, Spain) or isopropanol (J.T. Baker, Deventer, the Netherlands) is used in the nucleic acid precipitation step. Modification within kits 1, 2 and 4, after sample lysis, was made during the RNA binding step; precisely equal volume of ethanol in the sample tube was replaced with equal volume of isopropanol, after which the procedure was the same according to the manufacturer’s protocol. Conversely, modification within kit 3 was also made after sample lysis, during the RNA binding step, but this time by adding equal volume of ethanol in the sample tube instead of equal volume of isopropanol.
Confirmation of applicability of the selected kit
The applicability of the kit and method was confirmed under the conditions that guaranteed the presence of the virus, firstly by participating in proficiency testing, after which a pilot test of surface samples (N=84) was conducted. Pilot test samples were taken in quarantine areas where people were asymptomatic or had weakly expressed symptoms of the COVID-19 disease. Such a condition of infected persons is the basic assumption for the contamination of items, for instance, food packaging in the retail chain, which was discussed in many studies (8, 18-21).
Selection and sampling
Different categories of food were selected and sampled, direct food surfaces and packaging surfaces of packaged food: food surface samples (N=60) from the retail chain (packaged, unpackaged, fresh (t=4 °C), frozen (t=-20 °C), imported and domestic origin) and samples of ready-to-eat (RTE) meals (N=40), precisely 18 cold and 22 hot ready-to-eat meals. Swab samples were taken in the refrigerator and freezer of a large shopping centre as shown in Table S1.
From all surfaces of food samples or surfaces of food packaging (except ready-to-eat meals), a swab sample was taken according to the instructions of the ISO 15216-2:2019 standard (22) and the WHO practical guide (16). Surface samples were taken using plastic swabs with a synthetic tip, pre-soaked in a sterile phosphate-buffered saline (PBS) solution. Wooden sticks and cotton wool should be avoided as they may result in false negatives. The recommended wiping area is 25-100 cm2, but whenever possible, an area of 100 cm2 was taken to increase the chance of virus detection.
After labelling the test tubes with swabs, the samples were immediately transported in refrigerators to the laboratory for analysis. Samples of ready-to-eat meals were taken from the hot-meal department in several retail chains. In addition, samples were taken of cold and hot ready meals in bulk, where we know from the experience that the possibility of microbiological contamination is greater. The sampling procedure was carried out according to the instructions of the Nordic Committee on Food Analysis (NMKL) (23), CEN ISO/TS 17728:2015 (24) and CAC/GL 50-2004 (25). Samples of swabs and food were transported and kept at (5±3) °C until testing.
RNA extraction from samples
The collected samples were processed using the methods that proved to be most effective, and RNA was isolated from the collected samples. Eluates obtained by extraction were used for a real-time reverse transcription-polymerase chain reaction (real-time RT-PCR).
Real-time reverse transcriptase-polymerase chain reaction
Real-time RT-PCR starts with the reverse transcription (RT) of viral RNA into cDNA, which is amplified until the amplicon appears. EuroFins Technologies detection kits were used for this study, validated, and designed for testing environmental samples and food surfaces, which can detect envelope protein (E-gene), RNA-dependent RNA polymerase (RdRp gene, inside the Orf1ab polyprotein gene), and nucleocapsid protein (N1/N2 gene). All tests were performed in duplicate.
In the initial screening step, the isolated RNA was tested for the E-gene present in the SARS- and MERS-related coronaviruses using the VIRSeek SARS-COV-2 screen kit using specific SARS-CoV-2 nucleotide sequence primers (EuroFins Technologies). The kit was developed as an initial screening test to be used with the VIRSeek SARS-CoV-2 Ident 2 and VIRSeek SARS-COV-2 Mplex kits to confirm a positive screening test. The confirmatory test is performed by detecting the RdRp gene with the VIRSeek SARS-COV-2 Ident 2 kit (EuroFins Technologies) or the N-gene sequence (N1 and N2) with the VIRSeek SARS-COV-2 Mplex kit (EuroFins Technologies). Assay quality control assurance is provided by an internal positive control (IPC) for each reaction and using a positive (PC) and negative control (NC). According to these instructions, all positive samples were confirmed for at least one more target gene in this study.
RdRp and N gene PCR kit primer combinations are highly specific for SARS-CoV-2 and do not cross-react with SARS-CoV, MERS-CoV, or seasonal human coronaviruses HKU1, OC43, NL63 or 229E. In addition, primers from these kits do not cross-react with cDNA from other common foodborne viruses, including norovirus genogroups I and II, hepatitis A and E, rotavirus, adenovirus, or astrovirus (17).
The PikoReal™ Real-Time PCR System (Thermo Fisher Scientific Oy, Vantaa, Finland) was used for testing. The total reaction mixture for real-time RT-PCR was prepared from 5 μL of BasicMix and 15 μL of OligoMix to which 5 μL of the sample were added. The thermal profile for reverse transcription is 10 min at 50 °C, followed by enzyme activation and reverse transcriptase inactivation for 3 min at 95 °C.
Thermal cycling was performed at 40 cycles, denaturation for 3 s at 95 °C, annealing, and extension for 30 s at 58 °C. The channel through which fluorescence was detected for the targeted E-gene is FAMTM and for the internal control Cy5TM. The results are displayed and evaluated through PikoReal™ software (26).
Statistical data interpretation
The efficiency of the extraction kit is determined by the slope (b) of the line of the linear regression, from which the required level of efficiency (E) >90 % and R2>0.95 are defined.
The calculation formula for the slope is as follows:
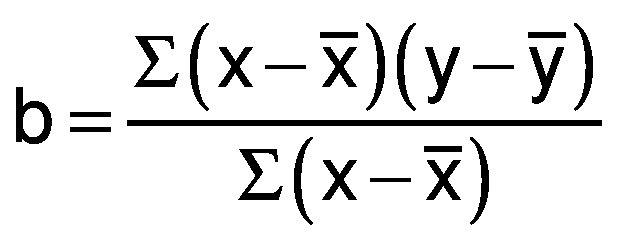 /1/ /1/
|
The formula for the efficiency percentage calculation from the slope is:
| E = -1 + 10(-1/b) /2/ |
The formula for the coefficient of determination calculation:
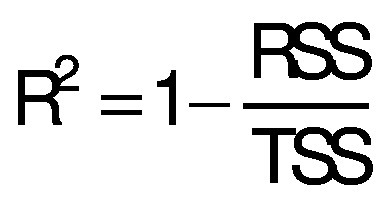 /3/ /3/
|
where R2 is the coefficient of determination, RSS is the sum of squares of residuals and TSS is the total sum of squares.
The software GraphPad Prism (27) and Microsoft Excel (28) were used for calculations.
RESULTS AND DISCUSSION
Evaluation of performance characteristics of kits
The focus of the first part of the test was the extraction kits, i.e. the evaluation of the performance characteristics of four commercially available kits for isolating viral RNA. However, considering that these are environmental samples in which a large amount of targeted viral RNA was not expected, potential improvement of RNA isolation with the implementation of the formal, original protocol (O) set by the manufacturer was additionally tested with an alternative modified protocol (M). Untoro et al. (29) conducted a similar study where they compared methanol, chloroform, and 2-isopropanol as alternative solvents to ethanol in the isolation of dengue virus type 2 RNA, and concluded that the used methanol and 2-isopropanol gave better results than ethanol. Generally speaking, the processing of environmental samples leads to a specific problem. Lever et al. (30) emphasize that all environmental samples have their peculiarities and may require a specific fine adjustment of the used components and their ratios.
In a modification of the protocol within the kits where the original protocol is based on ethanol, isopropanol was used (kits 1, 2 and 4). Within the kit that uses isopropanol in the original instructions, alternatively, ethanol was used (kit 3). Namely, it has been shown that isopropanol is a more suitable solution in certain analyses, especially when a low concentration of RNA/DNA is expected. On the other hand, DNA is less soluble in solutions containing isopropanol than in solutions containing ethanol. Precipitation with isopropanol was performed at room temperature to reduce the risk of solutes such as sucrose or sodium chloride co-precipitating with DNA/RNA (31). The results in Table 2 show that three extraction kits gave satisfactory results, while they were absent in the case of kit number 4, and they were excluded from further evaluation. The alternative protocol gave significantly better results in the case of kit 2. Kit 3 gave the second-best result according to the original protocol, while the alternative protocol did not provide better results. Overall, kit 1 showed the best results when following the original protocol.
Table 2. Results of the Cq values of the tested extraction kits show differences in target extraction efficiency of MNV RNA whether ethanol (EtOH) or isopropanol (IPA) was used during the extraction.
| N(MNV) | Cq | |||||||
|---|---|---|---|---|---|---|---|---|
| Kit 1 | Kit 2 | Kit 3 | Kit 4 | |||||
| EtOH (O) | IPA (M) | EtOH (O) | IPA (M) | EtOH (M) | IPA (O) | EtOH (O) | IPA (M) | |
| undiluted | 26.30 | 29.99 | 33.04 | 30.29 | 31.72 | 28.94 | 37.83 | 38.57 |
| 10-1 | 29.02 | 33.53 | 35.81 | 32.92 | 33.96 | 31.48 | 40.44 | 41.50 |
| 10-2 | 32.49 | 37.03 | 39.96 | 36.59 | 37.49 | 34.51 | NA | NA |
| 10-3 | 36.60 | 40.35 | 43.29 | 39.96 | 40.79 | 39.33 | NA | NA |
MNV=murine norovirus standard solution (various dilutions), O=original protocol, M=modified protocol, Cq=number of cycles at which the target analyte (after the RT-PCR reaction) curve intersects the threshold line
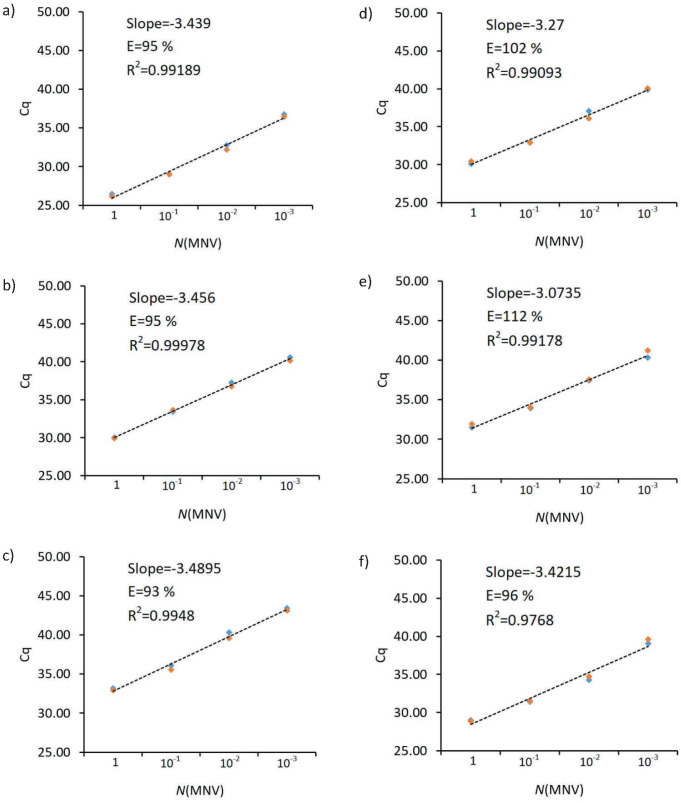
In addition, the efficiency (E) of the three extraction kits was tested by real-time RT-PCR detection of a duplicate series of a 10-fold dilution of the MNV standard. All tests showed satisfactory efficiency (E), while the R2 values of all kits except kit 3 (in which isopropanol was used) were >0.99, which meets the pre-defined required level (Fig. 1).
Fig. 1.
Presentation of the results of the efficiency test (E) of the three extraction kits tested by real-time RT-PCR detection of a double series of 10-fold dilution of the murine norovirus standard (MNV): a) kit 1 (ethanol), b) kit 1 (isopropanol), c) kit 2 (ethanol), d) kit 2 (isopropanol), e) kit 3 (ethanol) and f) kit 3 (isopropanol). Cq=number of cycles at which the target analyte (after the RT-PCR reaction) curve intersects the threshold line
A similar study was conducted by O'Brien et al. (32), but for wastewater surveillance of SARS-CoV-2. These kits are specific in that they may contain additional inhibitor removal steps, so they are not directly comparable. In their study, Ambrosi et al. (33) also focused on extraction efficiency and dealt with protocol modification. In that study, proper evaporation of ethanol (to reduce downstream interference of the RT-PCR reaction) and extended incubation time during elution and centrifugation are highlighted as critical steps in RNA extraction. The mentioned modifications proved effective and are recommended for application in the study's kits to improve RNA recovery for automatic and manual extraction. Finally, a study by Ransom et al. (34) shows a comparison of three kits (systems) for automatic RNA extraction from clinical samples where it is clear that all three kits are satisfactorily efficient, but one stands out with slightly better results in terms of higher Cq values after the RT-PCR test. The authors conclude that this is likely the result of the use of half the elution volume and thus a higher RNA concentration. Also, there are similar studies by van Kasteren et al. (35) and Shen et al. (36), but about the evaluation of diagnostic kits. Very few studies have carried out this type of evaluation, and it is impossible to compare the results of this study directly.
Confirmation of the suitability of the selected kits
The suitability of the selected extraction kit was double confirmed. The first confirmation of suitability was obtained by proficiency testing (LGC Standards Proficiency Testing, Bury, UK), during which three gene sequences: E, RdRp, and N, were successfully detected in the test sample (37). Furthermore, the method's suitability was confirmed through targeted pilot testing under conditions that guaranteed the presence of the virus (unpublished data). In particular, it was about testing surfaces in dedicated spaces (quarantine) where people who were asymptomatic or had weakly expressed symptoms of the COVID-19 disease were staying. At least two gene sequences were successfully confirmed in all samples.
The overall results of the targeted pilot testing are shown in Fig. 2. Logical conclusion is that the amount of positive samples is significant (32 %) and those results point to how important the implementation of all prescribed hygiene measures as well as those concerning social distancing are.
Fig. 2.
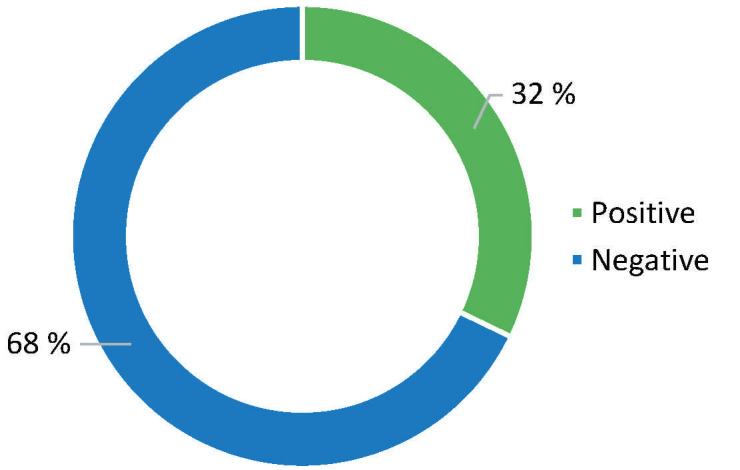
Ratio of total positive to negative samples from a targeted pilot testing of the presence of the SARS-CoV-2 virus or virus particles under conditions that guaranteed the presence of the virus
Namely, the basic assumption and risk arise when such persons work in the preparation, serving, or in food traffic, as pointed out by the German Federal Institute for Risk Assessment (BfR) (38), stating that the coronavirus can be found on cutlery and dishes by direct sneezing or coughing of an infected person and cause infection if it reaches the mucous membrane of the nose or eyes. However, it also points out that they have not recorded such infections so far. This assertion is supported by the fact highlighted in several studies that viral RNA detected on positive food samples most likely originates from infected persons within the processing and distribution chain before packaging. The reason could be that food processing facilities are often identified as hotspots for COVID-19 due to overcrowded workplaces, close contact with colleagues, shared transportation or housing (8, 39, 40). For example, one study found that physical contact and sharing food during a conference in Singapore resulted in a group of people suffering from COVID-19 (41).
Ong et al. (42) monitored the air and areas where symptomatic patients with mild and moderate clinical symptoms were present and showed that as many as 87 % of the samples were positive. For example, in another study, the results of environmental samples in intensive care units (ICU) and COVID units were significantly more pronounced, 54 out of 57, i.e. 94.7 % in the ICU, and 9 out of 9, i.e. 100 % in the COVID unit (43).
Examination of the presence of viruses on packaging surfaces, food surfaces and ready-to-eat food
After the applicability and suitability of the kit were confirmed by successful proficiency testing and targeted pilot testing under the conditions that guaranteed the presence of the virus, the kit was used in the testing of samples of food swabs and food packaging, which are considered to represent specific categories of food, taking into account diversity of the storage, storage temperature, and origin. As intended, RNA was extracted from all swabs and food samples (prepared meals), and the presence of the E-gene was detected using the VIRSeek SARS-CoV-2 Screen commercial kit. The results of all tested samples are summarized in Table 3.
Table 3. Presentation of the obtained results of testing surface swabs and ready-to-eat food.
| Target gene | Swab | Ready-to-eat meal | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| E | 0 (3 weeks positive) | 60 | 0 | 40 |
| RdRp | 0 (1 week positive) | 1 | NA | NA |
| N | 0 | 1 | NA | NA |
E=envelope protein, RdRp=RNA-dependent RNA polymerase, N=nucleocapsid protein, NA=not available
As for the screening kit, the settings of the automatic threshold limit and the criteria from the manufacturer's instructions were used (Cq≤38). The test results of the swabs of the samples showed one positive and two more samples with Cq>38. It should be noted that such high values are expected and usual in environmental samples. For example, in the study of Ong et al. (42), the average Cq value was 36.08. The samples that showed positive Cq values include all three samples from the category of fresh, refrigerated foods, i.e. fresh fruits and vegetables. All "positive" results have in common the absence of a characteristic sigmoid amplification curve, and as such, they cannot be considered fully valid results. Eventually, they can be characterized as weak positives. All "positive" samples, according to the recommendations, were additionally tested for the presence of the SARS-CoV-2 specific RdRP gene with the VIRSeek SARS-COV-2 Ident 2 kit. Only one sample had a positive result in terms of the obtained Cq value, but in this one too, the characteristic sigmoid amplification curve was absent. The same sample was also tested for the presence of the N gene with the VIRSeek SARS-COV-2 Mplex kit (EuroFins Technologies). No positive signal was recorded, i.e. no Cq value was expressed.
All 40 samples of the tested ready-to-eat meals were negative for E-gene presence. Generally speaking, the successful detection of viruses in food is a big challenge due to the physical and chemical properties of food, which include different matrices and the heterogeneous distribution of virus particles, low viral load, and very demanding isolation procedures (11, 44), which is why care should be taken of potentially false-negative results or underestimated amounts of viruses. There are a large number of diseases caused by food and associated with viral infections, the most common of which are hepatitis A virus (HAV), hepatitis E virus (HEV), and norovirus (NoV), but other viruses, including enterovirus (EV), human rotavirus (RV), hepatitis E virus (HEV), astrovirus, Aichi virus, sapovirus, coronavirus, parvovirus, and human adenovirus have the potential for food transmission (45). In contrast, no evidence has been found that the ingestion of ready-to-eat food is the route of transmission of the SARS-CoV-2 virus, as evidenced by numerous studies and risk assessments (8, 19, 44, 46-50). Most major food safety bodies, such as EFSA and FDA, have been obliged to clear any remaining doubts and closely monitor the situation. It is important to highlight that the early instances and outbreaks were linked to markets. Still, subsequent cases and outbreaks recurred in areas that were large warehouses of frozen food from many different parts of the world. The theory that the Huanan wholesale seafood market in Wuhan, PR China, was an early epicentre of the COVID-19 pandemic received its most considerable support yet in a comprehensive most recent study by Worobey et al. (51). However, it should not be forgotten that the sequencing of the genome of the SARS-CoV-2 virus isolated from the Xinfadi market in China confirmed that it is a European strain of the coronavirus (52), which turns the whole situation around a bit.
CONCLUSIONS
From this study, it can be concluded that food and the food chain may have a more significant role in the SARS-CoV-2 pandemic than was initially thought. Numerous studies partially tried to confirm and explain the significance of a food and food transport role, particularly emphasizing the cold chain and generally the contact transmission. Among others, one of the main goals of this study was to define the most effective extraction kit and the procedure among the four selected commercially available kits. The study results showed that in some kits, using isopropanol instead of ethanol in the precipitation step can give a higher yield of RNA. The presented results made it possible to highlight the most efficient extraction kit for this type of study, and the applicability of the featured kit was further confirmed in two additional steps. In the first step, through proficiency testing, and in the second step through a pilot test for the detection of the SARS-CoV-2 virus on surfaces that guaranteed the existence of the virus or virus particles, i.e. in quarantine areas where asymptomatic persons or persons with mild symptoms of the disease were staying. The pilot test results showed that a significant proportion of the tested samples were positive for viral particles of the SARS-CoV-2 virus. Such results confirmed the justification of suspicion that similar results could be achieved if such asymptomatic persons or persons with less pronounced symptoms worked in the food chain. The second part of the study presented a relatively wide range of samples primarily related to the food chain, i.e. food packaging, food surfaces, and the ready-to-eat food. The final results of testing the presence of SARS-CoV-2 virus or viral particles on food packaging surfaces, food surfaces, and ready-to-eat food showed that the virus is not significantly present, and according to the obtained results, it could be concluded that food does not represent a significant risk and probability of infection with the SARS-CoV-2 virus. However, this possibility should not be ruled out considering the weak positive results.
Systematic monitoring of the virus is recommended to gain a better insight into the dynamics and possibilities of virus transmission through food, which is corroborated by the numerous studies mentioned in this paper. Two focus points need to be pointed out, firstly, the oral cavity is a significant site for SARS-CoV-2 infection, and its direct role in virus transmission through the oral cavity requires further research, and secondly, in legislative frameworks and risk reduction techniques, frozen and chilled foods are largely overlooked as possible vectors.
SUPPLEMENTARY MATERIALS
Supplementary materials are available at: www.ftb.com.hr.
Table S1. Supplementary material list of analysed sample details.
| Sample no. | Sample name | Country of origin | Storage temperature | Packaging | Lot |
|---|---|---|---|---|---|
| 1 | White grapefruit | ZA | Chilled | Bulk | 43-03 |
| 2 | Williams pear (l=60 mm) |
Serbia | Chilled | Bulk | L:31002238201029 |
| 3 | Pomegranate variety Hicaz | Turkey | Chilled | Bulk | L:90450120TR |
| 4 | Cucumber variety Adrian | Spain | Chilled | Bulk | 44/01 |
| 5 | Tomato | Poland | Chilled | Bulk | 43-06 |
| 6 | Pepper mix variety CW | Spain | Chilled | Bulk | J26-THR |
| 7 | Zucchini variety Elite | Spain | Chilled | Bulk | 44/04 |
| 8 | Orange variety Valencia | ZA | Chilled | Bulk | 44/04 |
| 9 | Japanese apple persimmon | Spain | Chilled | Bulk | 4501 |
| 10 | Pineapple gold | Costa Rica | Chilled | Bulk | 44/02 |
| 11 | Apple variety Idared (l=70 mm) | Croatia | Chilled | Bulk | 4305 |
| 12 | Mandarin variety Satsuma | Croatia | Chilled | Bulk | 0359 |
| 13 | Apple variety Red delicious | Croatia | Chilled | Bulk | 4501 |
| 14 | Apple variety Jonagold | Croatia | Chilled | Bulk | L:99000099201028 |
| 15 | Champignons (m=500 g) |
Croatia | Chilled | Bulk | 4406 |
| 16 | Cabbage variety Coronet | Croatia | Chilled | Bulk | 43-01 |
| 17 | Paradise tomatoes | Croatia | Chilled | Packed | L031120 |
| 18 | Chard variety Verca | Croatia | Chilled | Bulk | / |
| 19 | Crystal Bovary salad | Croatia | Chilled | Bulk | 3010 |
| 20 | Apple variety Gala | Croatia | Chilled | Bulk | 4404 |
| 21 | Breaded fish sticks | Croatia | Frozen | Packed | L040820 20:26 |
| 22 | Quick-frozen chicken meat | Croatia | Frozen | Packed | 10.10.2020./10.01.2021. |
| 23 | Breaded chicken dinosaurs | Croatia | Frozen | Packed | 26.10.2020./26.10.2021. |
| 24 | Curry and beef roll | Croatia | Frozen | Packed | 110420 |
| 25 | Imperial vegetables (cauliflower, broccoli, carrots) |
Croatia | Frozen | Packed | 44124.42243 |
| 26 | Chicken fries | Croatia | Frozen | Packed | 27.10.2020/27.10.2021. |
| 27 | Pizza crispy pops | Croatia | Frozen | Packed | 18.08.2020./18.08.2021. |
| 28 | Creperols (ham, cheese, cucumbers) | Croatia | Frozen | Packed | 230620 |
| 29 | Summer mix (peas, carrots, red pepper, potato, sweet corn) |
Croatia | Frozen | Packed | 43801,41407 |
| 30 | Pie (chocolate/cherry) | Croatia | Frozen | Packed | / |
| 31 | Germknödel Toni Kaiser | Austria | Frozen | Packed | L 0284 11:52 |
| 32 | Broiler duckling | Hungary | Frozen | Packed | L20124051 |
| 33 | Mini natural baguette | Czech Republic | Frozen | Packed | L20125408 20125408 |
| 34 | French salad | Belgium | Frozen | Packed | 3:12 L20038-LN01 |
| 35 | Patagonian squid | Morocco | Frozen | Packed | 98/WOF/5219 |
| 36 | Vegetable trio (cauliflower, broccoli, carrots) | Belgium | Frozen | Packed | 08/04/22 18:57 L20099-LN01 |
| 37 | Bulgur mixture | Spain | Frozen | Packed | 16/10/2020 16 08:20 |
| 38 | French fries | Belgium | Frozen | Packed | L G02 20 217 14:45 |
| 39 | Breaded calamari rings | Spain | Frozen | Packed | 30/09/2020 01:09 |
| 40 | Seafood risotto | Belgium | Frozen | Packed | L1465350D |
| 41 | Long life milk V=1 L, w(mf)=2.8 % |
Croatia | Chilled | Packed | 23/01/21 23:27 000910K21 |
| 42 | Yogurt w(mf)=2.8 % |
Croatia | Chilled | Packed | 14-20-102274 |
| 43 | Chocolate hazelnut milk dessert | Croatia | Chilled | Packed | 13.12.20. 10:10 |
| 44 | Curd | Croatia | Chilled | Packed | 2011 20L598 |
| 45 | Solid yogurt w(mf)=3.2 % | Croatia | Chilled | Packed | 80-20-102601 |
| 46 | Long life milk V=1 L, w(mf)=3.5 % |
Slovenia | Chilled | Packed | 05.02.2021. 13:45:48 L6/U |
| 47 | Mozzarella | Czech Republic | Chilled | Packed | 20 11 20 10:50 20 298 Kt |
| 48 | Fresh cream | Serbia | Chilled | Packed | 28.11.2020. 01:00:50 |
| 49 | Skyr | Slovenia | Chilled | Packed | 29/11 00:27 29 |
| 50 | Mayonnaise | Serbia | Chilled | Packed | 05.2021 20:40 L02476839 |
| 51 | Broccoli variety Parthenon | Spain | Chilled | Bulk | 45/01 |
| 52 | Paprika variety Vedrana | North Macedonia | Chilled | Bulk | 44-03 |
| 53 | Seedless watermelon | Brazil | Chilled | Bulk | 44-02 |
| 54 | Red California pepper | Spain | Chilled | Bulk | 44/04 |
| 55 | Apple variety Granny Smith | Croatia | Chilled | Bulk | L:990000992010 |
| 56 | Pizza Margherita | Croatia | Frozen | Packed | L: 20247 |
| 57 | Pizza picante | Croatia | Frozen | Packed | L: 20245 |
| 58 | A cup of chestnut dessert premium | Croatia | Frozen | Packed | SN: 008294 Batch: 0003 |
| 59 | Peanut butter | USA | Frozen | Packed | L00233OL60 |
| 60 | Ice cream | France | Frozen | Packed | 12/2021 6 05:18 L005E3 DOE02 |
Footnotes
FUNDING
This work was carried out within the project Food Safety and Quality Center (KK.01.1.1.02.0004). The project is co-financed by the European Union from the European Regional Development Fund.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Hiscott J, Alexandridi M, Muscolini M, Tassone E, Palermo E, Soultsioti M, et al. The global impact of the coronavirus pandemic. Cytokine Growth Factor Rev. 2020;53:1–9. 10.1016/j.cytogfr.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation. Coronavirus [Internet]. [cited 2022 Nov 20]. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_1.
- 3.Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100–15. 10.1016/j.it.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 and food safety: Guidance for food businesses. Geneva, Switzerland: World Health Organization (WHO); 2020. Available from: https://www.who.int/publications/i/item/covid-19-and-food-safety-guidance-for-food-businesses.
- 5.Aboubakr HA, Sharafeldin TA, Goyal SM. Stability of SARS‐CoV‐2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound Emerg Dis. 2021;68(2):296–312. 10.1111/tbed.13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan KH, Sridhar S, Zhang RR, Chu H, Fung AYF, Chan G, et al. Factors affecting stability and infectivity of SARS-CoV-2. J Hosp Infect. 2020;106(2):226–31. 10.1016/j.jhin.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen HL, Chan MCW, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. 10.1016/S2666-5247(20)30003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han J, Zhang X, He S, Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ Chem Lett. 2021;19(1):5–16. 10.1007/s10311-020-01101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eslami H, Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19). AMB Express. 2020;10(1):92. 10.1186/s13568-020-01028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr AHW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol (Berl). 2005;194:1–6. 10.1007/s00430-004-0219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yépiz-Gómez MS, Gerba CP, Bright KR. Survival of respiratory viruses on fresh produce. Food Environ Virol. 2013;5(3):150–6. 10.1007/s12560-013-9114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yekta R, Vahid-Dastjerdi L, Norouzbeigi S, Mortazavian AM. Food products as potential carriers of SARS-CoV-2. Food Control. 2021;123:107754. 10.1016/j.foodcont.2020.107754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892–903. 10.1038/s41591-021-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh M, Sadat A, Abdi R, Colaruotolo LA, Francavilla A, Petker K, et al. Detection of SARS-CoV-2 on surfaces in food retailers in Ontario. Curr Res Food Sci. 2021;4:598–602. 10.1016/j.crfs.2021.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3): 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance, 19 March 2020. Geneva, Switzerland: World Health Organization (WHO); 2020. p. 7. Available from: https://www.who.int/publications/i/item/10665-331501.
- 17.Kirchheim F. VIRSeek SARS-CoV-2 Screen - Test kit for SARS-COV-2 screening (E-gene target) qualitative real-time RT-PCR from environmental and food surfaces. Freiburg, Germany; Eurofins Technologies GmbH. Available from: https://ingenasa.eurofins-technologies.com/media/7608/mnl_virseek_sars-cov-2_screen_5728200601_v1.pdf.
- 18.Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–51. 10.1016/j.coviro.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herstein JJ, Degarege A, Stover D, Austin C, Schwedhelm MM, Lawler JV, et al. Characteristics of SARS-CoV-2 transmission among meat processing workers in Nebraska, USA, and effectiveness of risk mitigation measures. Emerg Infect Dis. 2021;27(4):1032–8. 10.3201/eid2704.204800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceylan Z, Meral R, Cetinkaya T. Relevance of SARS-CoV-2 in food safety and food hygiene: Potential preventive measures, suggestions and nanotechnological approaches. VirusDis. 2020;31(2):154–60. 10.1007/s13337-020-00611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalava K. First respiratory transmitted food borne outbreak? Int J Hyg Environ Health. 2020;226:113490. 10.1016/j.ijheh.2020.113490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ISO 15216-2:2019. Microbiology of the food chain - Horizontal method for determination of hepatitis A virus and norovirus using real-time RT-PCR - Part 2: Method for detection. Geneva, Switzerland: International Organization for Standardization (ISO); 2019.
- 23.Procedure 12 Guide on sampling for analysis of foods. Oslo, Norway: Nordic Committee on Food Analysis (NMKL); 2014.
- 24.CEN ISO/TS 17728:2015. Microbiology of the food chain - Sampling techniques for microbiological analysis of food and feed samples. Geneva, Switzerland: International Organization for Standardization (ISO); 2015 (in Croatian).
- 25.CAC/GL 50-2004. General guidelines on sampling. Codex Alimentarius Commission (CAC): Codex Alimentarius; 2004. pp. 1–69. Available from: https://www.fao.org/uploads/media/Codex_2004_sampling_CAC_GL_50.pdf.
- 26.PikoReal Software, v. 2.2.248.601 for Windows, Thermo Fisher Scientific Oy, Vantaa, Finland; 2013.
- 27.GraphPad Prism, v. 9.4.0 673 for Windows, GraphPad Software, San Diego, CA, USA; 2022. Available from: www.graphpad.com.
- 28.Excel M. v. 2304/Build 16,0.16327.20248 for Windows, Microsoft Corporation, Redmond, Washington, USA; 2023. Available at: https://office.microsoft.com/excel.
- 29.Untoro YM, Sucipto TH, Setyawati H, Churrotin S, Amarullah IH, Wardhani P, et al. RNA isolation of dengue virus type 2 with different precipitation solvents. Jurnal Kimia Riset. 2018;3(1):52–7. 10.20473/jkr.v3i1.7455 [DOI] [Google Scholar]
- 30.Lever MA, Torti A, Eickenbusch P, Michaud AB, Šantl-Temkiv T, Jørgensen BB. A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types. Front Microbiol. 2015;6:476. 10.3389/fmicb.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green MR, Sambrook J. Precipitation of DNA with Isopropanol. Cold Spring Harb Protoc. 2017;2017(8):pdb.prot093385. 10.1101/pdb.prot093385 10.1101/pdb.prot093385 [DOI] [PubMed]
- 32.O’Brien M, Rundell ZC, Nemec MD, Langan LM, Back JA, Lugo JN. A comparison of four commercially available RNA extraction kits for wastewater surveillance of SARS-CoV-2 in a college population. Sci Total Environ. 2021;801:149595. 10.1016/j.scitotenv.2021.149595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambrosi C, Prezioso C, Checconi P, Scribano D, Sarshar M, Capannari M, et al. SARS-CoV-2: Comparative analysis of different RNA extraction methods. J Virol Methods. 2021;287:114008. 10.1016/j.jviromet.2020.114008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ransom EM, Potter RF, Wallace MA, Mitchell KF, Yarbrough ML, Burnham CAD, et al. Comparison of extraction methods and thermocyclers for SARS-CoV-2 molecular detection using clinical specimens. J Clin Microbiol. 2020;58(10): 10.1128/JCM.01622-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Kasteren PB, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A, et al. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol. 2020;128:104412. 10.1016/j.jcv.2020.104412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen L, Cui S, Zhang D, Lin C, Chen L, Wang Q. Comparison of four commercial RT-PCR diagnostic kits for COVID-19 in China. J Clin Lab Anal. 2021;35(1):e23605. 10.1002/jcla.23605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hygiene surface monitoring (HYGIENE) Individual Report - Round: HY295, Lab ID: HY0563. Bury, UK: LGC Standards; 2021. [Google Scholar]
- 38.Can SARS-CoV-2 be transmitted via food and objects? Berlin, Germany: German Federal Institute for Risk Assessment (BfR); 2022. Available from: https://www.bfr.bund.de/en/can_sars_cov_2_be_transmitted_via_food_and_objects_-244090.html.
- 39.Dyal JW, Grant MP, Broadwater K, Bjork A, Waltenburg MA, Gibbins JD, et al. COVID-19 among workers in meat and poultry processing facilities — 19 states, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):557–61. 10.15585/mmwr.mm6918e3 [DOI] [PubMed] [Google Scholar]
- 40.Waltenburg MA, Rose CE, Victoroff T, Butterfield M, Dillaha JA, Heinzerling A, et al. Coronavirus disease among workers in food processing, food manufacturing, and agriculture workplaces. Emerg Infect Dis. 2021;27(1):243–9. 10.3201/eid2701.203821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pung R, Chiew CJ, Young BE, Chin S, Chen MIC, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039–46. 10.1016/S0140-6736(20)30528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610. 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26(7):1583–91. 10.3201/eid2607.200885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch A, Gkogka E, le Guyader FS, Loisy-Hamon F, Lee A, van Lieshout L, et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int J Food Microbiol. 2018;285:110–28. 10.1016/j.ijfoodmicro.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrović T, D’Agostino M. Viral contamination of food. In: Barros-Velázquez J, editor. Antimicrobial food packaging. London, UK: Academic Press; 2016. pp. 65–79. [REMOVED HYPERLINKD8 FIELD] 10.1016/B978-0-12-800723-5.00005-X 10.1016/B978-0-12-800723-5.00005-X [DOI] [Google Scholar]
- 46.Coronavirus: No evidence that food is a source or transmission route. Parma, Italy: The European Food Safety Authority (EFSA); 2020. Available from: https://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route.
- 47.Food safety and the coronavirus disease 2019 (COVID-19). Silver Spring, MD, USA: US Food and Drug Administration (FDA); 2020. Available from: https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19.
- 48.Opinion Request No ANSES. 2020-SA-0037. Opinion on an urgent request to assess certain risks associated with COVID-19. Paris, France: French Agency for Food, Environmental and Occupational Health and Safety (ANSES); 2020. Available from: https://www.anses.fr/fr/system/files/SABA2020SA0037-1EN.pdf.
- 49.Qualitative risk assessment on the risk of food or food contact materials as a transmission route for SARS-CoV-2. London, UK: Food Standards Agency; 2020. Available from: https://www.food.gov.uk/print/pdf/node/4121.
- 50.Novel coronavirus and food safety. Canberra, Australia: Food Standards Australia & New Zealand; 2021. Available from: https://www.foodstandards.gov.au/consumer/safety/Pages/NOVEL-CORONAVIRUS-AND-FOOD-SAFETY.aspx.
- 51.Worobey M, Levy JI, Serrano LM, Crits-Christoph A, Pekar JE, Goldstein SA, et al. The Huanan seafood wholesale market in Wuhan was the early epicenter of the COVID-19 pandemic. Science. 2022;377(6609):951–9. 10.1126/science.abp8715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Pan Y, Zhao X, Shi W, Chen Z, Zhang S, et al. Genomic characterization of SARS-CoV-2 identified in a reemerging COVID-19 outbreak in Beijing’s Xinfadi market in 2020. Biosafety Health. 2020;2(4):202–5. 10.1016/j.bsheal.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]