Abstract

The present article critically and comprehensively reviews the most recent reports on smart sensors for determining glyphosate (GLP), an active agent of GLP-based herbicides (GBHs) traditionally used in agriculture over the past decades. Commercialized in 1974, GBHs have now reached 350 million hectares of crops in over 140 countries with an annual turnover of 11 billion USD worldwide. However, rolling exploitation of GLP and GBHs in the last decades has led to environmental pollution, animal intoxication, bacterial resistance, and sustained occupational exposure of the herbicide of farm and companies’ workers. Intoxication with these herbicides dysregulates the microbiome-gut-brain axis, cholinergic neurotransmission, and endocrine system, causing paralytic ileus, hyperkalemia, oliguria, pulmonary edema, and cardiogenic shock. Precision agriculture, i.e., an (information technology)-enhanced approach to crop management, including a site-specific determination of agrochemicals, derives from the benefits of smart materials (SMs), data science, and nanosensors. Those typically feature fluorescent molecularly imprinted polymers or immunochemical aptamer artificial receptors integrated with electrochemical transducers. Fabricated as portable or wearable lab-on-chips, smartphones, and soft robotics and connected with SM-based devices that provide machine learning algorithms and online databases, they integrate, process, analyze, and interpret massive amounts of spatiotemporal data in a user-friendly and decision-making manner. Exploited for the ultrasensitive determination of toxins, including GLP, they will become practical tools in farmlands and point-of-care testing. Expectedly, smart sensors can be used for personalized diagnostics, real-time water, food, soil, and air quality monitoring, site-specific herbicide management, and crop control.
Keywords: engineered nanomaterials, environmental pollution, glyphosate-based herbicides, lab-on-a-chip, precision agriculture, smart material-based sensors
1. Smart Materials-Based Sensors in Precision Agriculture
1.1. Ecological Contamination with Glyphosate
According to the United Nations Food and Agriculture Organization (FAO), the world’s population will attain ∼9.7 billion by 2050, corresponding to a 32% projected growth.1 A recent meta-analysis of projected global food demand revealed that the total global food demand should increase by 35% to 56% between 2010 and 2050.2 The food shortage threat belongs to global agriculture’s most harmful socio-economic and environmental challenges.3 Aside from the COVID-19 pandemic,4 increases in temperature and atmospheric CO2 concentration, the environmental pollution of agrochemicals arising from ill-considered farming and insufficient fertilizer delivery systems has become a civilization problem.5 2020-Forecasted global agrochemical annual use was 120 million tons for nitrogen-based fertilizers, 50 million tons for phosphate-based fertilizers, and over 2.6 million tons for pesticides.6,7 In recent decades, the nutrient-use efficiency (NUE) has dropped significantly, i.e., over 50% of the N, 85% of the P are not assimilated by crops,8,9 and less than 10% of the applied pesticides reach their targets.5 If not handled, these usages were estimated to increase by 50–90% by 2050.5,10
The first global initiative to solve agriculture’s challenges began with the Third Agricultural (Green) Revolution in the 1950s and 1960s.11 This technology transfer involved (i) high-throughput cultivation of high-yielding varieties of cereal seeds, (ii) improvement of the NUE by spatiotemporal biofertilization and microbial biodiversity, and (iii) reduction of the reactive nitrogen species use and nitrogen oxide emission. In the 1970s, it was followed by the Gene Revolution,12 based on the extensive use of genetically engineered (GE) herbicide-resistant crops, especially glyphosate (GLP)-resistant plants, which resulted in yield increases, tillage reduction, and enhanced the high technology-based weed management.13 Yet, despite several advantages of lowering greenhouse gas emission,14 the large-scale misuse of GLP-based herbicides (GBHs) and GLP-resistant crops and GLP-resistant weed-originated single mode-of-action herbicides has caused environmental pollution with GLP, herbicide resistance, superweeds, and pests generation, as well as consuming GE organisms and GBH-contaminated products, which have consequently re-empowered the expensive tillage.13,15 These outcomes have boosted the international debate on the policy controlling or forbidding GBH exploitation and, on the other hand, developing sensors for GLP contaminants that emerged in the environment over the last 50 years.16 Emerging malnourishment, inappropriate weed management, and critically imbalanced and anthropogenically altered P-cycle have been recognized by the U.S. National Science Foundation as some of the most crucial challenges of modern and future agriculture and ecology, which require advances both in proper fertilizing and devising portable and sensitive tools of chemical analysis in agriculture and ecology.3,17,18
1.2. Smart Materials in Precision Agriculture
The latest advancements in agriculture and ecology originated with precision agriculture (PA). According to the International Society of Precision Agriculture (ISPAg) (https://www.ispag.org),19 PA is “a management strategy that gathers, processes, and analyzes temporal, spatial, and individual data and combines them with other information to support management decisions according to estimated variability for improved resource use efficiency, productivity, quality, profitability and sustainability of agricultural production.” PA’s most crucial challenge is reducing herbicide resistance in weed management. In 2021, the herbicide market value accounted for ∼30 billion USD and was forecasted to reach ∼40 billion USD by 2027 (https://www.imarcgroup.com/herbicides-market). Expectedly, herbicide-resistant weed management (HRWM) shall comprise almost half of the modern agricultural activities, including tillage, crop and herbicide diversity, and growing GE herbicide-resistant plants.20 The increase in the NUE and crop productions will be achieved by applying artificial intelligence (AI)-excelled devices and smart materials (SMs),21 including engineered nanomaterials (ENMs) and biomaterials,22 and plant wearable (PW) sensors, actuators, and soft robotics.23 These PA HRWM nanobiotechnological tools enable direct, spatiotemporally targeted, and dose-dependent delivery as well as rapid, ultrasensitive, and selective determination of herbicides.
SMs are well-defined, self-sensing, self-healing, and stimuli-responsive materials that change their properties and act according to their surroundings or external stimuli, including microorganisms, chemical compounds, heat, pH, temperature, electromagnetic field, light, humidity, ultrasounds, pressure, and mechanical factors.21 The SMs are mostly based on electroactive, piezoelectric, shape memory, and biocompatible polymers. They are integrated with electronic devices implanted or installed in the site of interest.21,23 Moreover, these devices, e.g., lab-on-chips (LOCs), microrobots, or smartphone-assisted nanosensors, exploit incorporated computational algorithms that allow for acquiring, processing, and analyzing vast amounts of data in real-time, thus providing a model simulation of spatial and seasonal distribution as well as risk assessment of agrochemicals.3,17,18,24
Regarding PA-dedicated SM sensors, there is a need for advanced inexpensive, highly efficient, multifunctional, and flexible consumer- and operator-friendly tools that can determine analytes, including toxins, pests, herbicides, and microbes in nonlaboratory settings, hardly accessible locations, and diverse agroecosystems.25,26 Conventional multimodal chemosensors utilize immunochemical receptors, targeted to toxic fertilizers and pesticide residuals, and electrochemical and/or optical transducers. However, because of the environmental systems’ complexity, vulnerabilities, and uncertainties, these conventional sensors will hopefully be upgraded or replaced with smart sensors equipped with computational devices.27 In AI PA smart sensors, these conventional sensors are assembled or virtually connected with computational devices that convey the machine learning (ML), deep learning (DL), artificial neural networks, nanoinformatics, and translational bioinformatics algorithms to integrate, compute, process, analyze, and interpret the massive amounts of spatiotemporal data.17,3,18 Hence, PA SM sensors, based on smartphones, soft robotics, robotic-automated vehicles, and drones, provide rapid, mobile, and high-throughput analysis. Moreover, they afford high-quality, decision-making outcomes for herbicide misuse control and sustained and profitable agricultural production and weed management.3,17,18,28,29 For instance, DL-enhanced computing methods enable the analysis of high-resolution spectral images of herbicide-sprayed plants mapped by scanning transmission X-ray microscopy or X-ray fluorescence spectroscopy.30,31
Data science-excelled and PA-targeted SM sensors can generally analyze short- or long-term weather conditions, soil properties, plant diseases, pests, microbial communities, and industrial pollution.3,17,18 Smart sensors and sensor networks must sense and sustain optimal conditions for plant cultivation, including moisture, temperature, pH, nutrients, and agrochemicals.3 Environment-friendly AI PA targets the dynamic and complex nature of the local agroecosystem by synergistic application of theoretical prediction models and experimental stimuli-responsive delivery-detection tools. If attached or administrated to the soil, plant, or crops, the AI PA sensor can capture, digitize, and process images, as well as detect, monitor, respond, and regulate physicochemical stimuli and atmospheric conditions in an information-supported decision-making manner.18 Since pests cause 34% of crop loss globally, controlling and increasing crop yields is essential.32 By predicting the ecosystem components’ behavior, PA provides a real-time response to weather conditions, nutrient cycling, crop growth, plant phenotyping, disease diagnosis, weed infestation, insect damage, and food production, as well as emerging contamination with these agents. These data are then correlated to the site-targeted delivery, uptake, detection, retention, performance, interaction, and transformation of nanomaterial-based agrochemicals in plants.
1.3. Artificial Intelligence-Excelled SM Sensors
AI PA technologies have excelled in using self-powered wireless sensor networks (WSNs), weed patches, and PW sensors for remote and in situ monitoring.3,23 They involve nanosensors and nanomaterial-based delivery systems, PWs, and nanorobots that leverage online databases and algorithms of cheminformatics and translational bioinformatics.3,18 As a result, they enable spatiotemporal management of crops and livestock, low-cost in situ nutrient-sensing, precise agrochemical/fertilizer placement, and soil and plant tissue testing. By constantly measuring vegetative indices and evapotranspiration rates, AI PA helps control various and diverse plant growth conditions, nitrogen uptake, and secretion of nitrogen into the rhizosphere.
Before applying these sensors, one must overcome potential environmental pollution and mechanical breakdown risks. The plant-implantable or PW sensors should be constructed from biocompatible, mechanically resistant, nanotechnology-excelled materials, thus allowing well-defined interaction with microbiomes and metabolites exudated by plant leaves and roots.33−35 Although these sensors can be biotransformed or biodegraded in the phyllosphere, they are vulnerable to mechanical destruction, because of sudden weather changes. It is crucial to understand the (sensor material)-plant interactions, off-target performance, and long-term toxicity and persistence of the nanomaterial in the plant vascular structure and organelles, especially since the nanomaterials may penetrate the phloem and xylem, thus changing the fluid composition and flow rate.18 Finally, because of large-scale demands, energy storage costs must be well-considered. The efficient use of smart sensors requires efficient handling of enormous amounts of digitized output images and biophysical and physiological comprehension to solve real problems of scheduling sowing and controlling pesticide usage, as well as tracking and predicting crop growth and quality.36,37
Most advanced AI PA technologies propose intelligent self-powered WSNs and PW.3,23 Characterized by low-power demand, long-duration processing, and near-field communication (NFC), WSNs are coarsely distributed and interconnected sensors, typically exploited for environmental in situ and real-time monitoring. The WSNs, equipped with fully rechargeable batteries or battery-free solar energy harvesting systems for power supply, are easily controlled and power-supplied by smartphones, provided the distance from the source is less than several meters. However, they have limitations, including reduced light in the shaded, bushy, and foliaged places or prolonged signal transmission resulting from far distances from the aboveground microstrip antenna to the underground sensor.38,39 A similar technology underlies the modus operandi of PWs, i.e., the electronic devices implanted into the plant tissue for tracking and transmitting physiological parameters. Being an example of nanobionics, the PWs enable precise and continuous in-site or remote sensing of microclimate and tissue microenvironment changes, thus informing about the plant health and agrochemical performance.40
An outstanding recent review presents the state of the art of the nano biotechnologically excelled PW sensors.23 The present review focuses on developing analytical methods using conventional and SM-based sensors for GLP determination in water, food, crop, and soil samples. Expectedly, conventional sensors will soon be replaced by SM-based sensors. Examples of these sensors, including smartphone-integrated sensors, are briefly introduced and discussed herein.
2. Environmental and Human Toxicity of Glyphosate
2.1. Glyphosate Structure and Properties
Glyphosate, N-(phosphonomethyl) glycine (GLP), is a widely used broad-spectrum, nonselective, and postemergent herbicide for crop desiccation.41 Since the approval for agricultural use by U.S. Environmental Protection Agency (EPA) in 1974 and the authorization in the EU in 2002, GLP-based surfactants (GLP-SH) and GBHs have been commercialized as Roundup and RangerPro in over 140 countries, covering 350 million hectares of crops, with an annual turnover of 11 billion USD worldwide.42,43 According to the FAO, GBHs’ global market constitutes 18% of the total pesticide active ingredients (ActIs) and 92% of herbicide ActIs, with a global annual revenue accounting for 11 billion USD.43 As a powerful tool of modern crop management, prior-harvesting large-scale GBH interventions, called “green burndowns,” are expected to meet the growing demands for food and crops production, which are projected to increase to 100–110% by 2050.41,44
2.2. The Weed-Killing Activity of Glyphosate
GLP reversibly inhibits the 5-enolpyruvynyl-shikimate-3-phosphate synthase (EPSPS, EC 2.5.1.19) involved in the biosynthesis of aromatic amino acids in plants45 and some microorganisms.46−49 Roundup Ready crops are GLP-tolerant because they are genetically modified to carry the CP4 EPSPS gene derived from Agrobacterium sp. strain CP4, a naturally GLP-resistant rhizosphere bacteria.46 Moreover, the harmful efficacy of GLP against plant development strongly depends on the daily and circadian rhythms of the plant cells,42 and plants die within 4–20 days after crop spraying.50 This chronotherapeutic responsiveness of plants to GLP brings hope for optimized crop protection and safe food production security.
GBHs are commonly used in agriculture, industry, forestry, and weed management. From 1974 to 2014, the GBHs’ use increased 100-fold. However, the current regulations for the safety standards of GBH handling still rely on the studies performed in the late 1980s. Although the ∼90% growth of all GE seeds produced by Monsanto in 1996 is considered safe,51 and despite extensive research conducted in human biomonitoring, hazard assessments, epidemiological studies including occupationally exposed workers, pregnant women, and their offspring, and evaluation and standardization, the GBH safety to humans and the environment is still questioned.52
The major issues concern the environmental pollution that affects crops, soil, surface and groundwaters, sediment, and the spreading through wind and erosion, thus threatening wildlife and human occupational activities on farmlands and GBH factories.53−56 Various strategies for mitigating the global persistence and hazardous exposure to have been developed, as reviewed elsewhere.54
2.3. Human Toxicity of Glyphosate
The EPA-established daily chronic reference dose of GLP is 1.7 mg/kg of body weight, with a nonobservable adverse effect level of 50 mg/kg per day dose.43,57 Average urinary GLP levels for occupational exposure and nonoccupational exposure range from 0.26 to 73.5 μg/L and 0.13–7.6 μg/L, respectively.58 As an organophosphate (OP), GLP efficiently transmits orally, dermally, conjunctively, gastrointestinally, and via respiratory routes.56 According to the EFSA, GLP has low acute toxicity owing to the absence of the EPSP-metabolic pathway in vertebrates and the rapid degradation of GLP in mammals (half-life time of ∼5–10 h).57,58 However, occupational poisonings have become a global medical issue because of environmental pollution.59
Regarding genetic modification of GLP-tolerant crops, it is crucial to delineate the potential genotoxicity of the transgenic plants60−63 from the chemical toxicity of commercial GBH coformulants, including GLP isopropylamine (GLP-IPA) salt, polyoxyethyleneamine (POEA), and ppb traces of heavy metals, including chromium, cobalt, lead, or nickel64,65 and GLP metabolites, e.g., (aminomethyl) phosphonic acid (AMPA) and glufosinate.65 Because of the exquisitely disturbing impact of GBH surfactants on plant cell membranes,66 these are regarded as the most harmful GBH ingredients, demonstrated as more toxic than GLP alone in mammal cells.67−69
For example, a study on aquatic microorganisms (bacteria, microalgae, protozoa, and crustaceans) revealed the highest toxicity for POEA and GBH, whereas the only effect of GLP resulted from its acidity.70 Analogically, commercial GLP-SHs occurred more toxic than GLP-IPA in short-term acute toxicity trials (24 and 48 h).71 Moreover, in rats, 12-week exposure to GBH caused significant increases in kidney biomarkers, oxidative stress markers, and membrane-bound enzymes, indicating the accumulation of GLP residues in the kidneys, while GLP alone caused no nephrotoxicity.72
Concerning human toxicity, ingesting substantial GLP-SH volumes (100–500 mL) is associated with a human death rate up to 29.3%, depending on patients’ characteristics, such as age and intent of exposure.73,74 The uncoupling oxidative phosphorylation and POEA- or (heavy metals)-induced cardiovascular and cardiopulmonary harms are major lethal causes.74−76 According to a survey of medical reports of 107 patients, the ingestion of GLP-SH caused hypotension (47%), deterioration (38.6%), respiratory failure (30%), acute kidney injury (17.1%), and arrhythmia (10%). Interestingly, these complications depend on the volume of GLP-SH ingested and not the type of surfactant ingredient of the GBH.77 Emergency treatment of those intoxications comprises gastric lavage followed by hemodiafiltration and direct hemoperfusion, enabling the removal of GLP-IPA (228 Da) and surfactants (over 500 Da), respectively.78 Intensive care is required in severe GLP-SH intoxication, including dehydration, oliguria, paralytic ileus, hypovolemic shock, cardiogenic shock, pulmonary edema, hyperkalemia, and metabolic acidosis.79,78
3. Sensors and Separation Systems for GLP Determination
Nowadays, SM sensors are robustly applied to solve the most challenging environmental, socio-economic, and biomedical issues. Those involve rapid response to climate changes, real-time water, food, soil, and air quality monitoring, biotic and abiotic stress factors, nutrient recycling, sustained crop growth, biofuel production, point-of-care (POC) devices fabrication, and defense against bioterrorism.22,80 Receptor items of the modern SM sensors are emerging-field-deployable chemo- and biosensors and synthetic biology tools capable of detecting pathogenic pollution in various ecosystems and industrial environments. They involve molecularly imprinted polymers (MIPs), aptamers, viruses, prokaryotic or eukaryotic cells, and individual plants or insects that are able to sense single molecules or cells of emerging contaminants at pico- and femtomolar concentrations. Single-molecule/cell-based nanosensors allow determining agrochemical toxins as well as soil, plant, and insect-associated microbial communities. As such, they have become a powerful PA tool to track the impact of herbicides, pesticides, and insecticides on crop-associated microbiomes and ecosystems.81,82
Restricted concentrations of GLP in drinking water and food are 0.7 mg/L and 0.1–310 ppm, respectively.83 Various nanomaterial-based sensors for GLP have recently been prepared for detecting, adsorbing, and degrading GLP in real samples (Table 1). Conducting, semiconducting, and nonconducting polymers were prepared.84 For example, polyaniline-zeolite (PANI/ZSM-5 and PANI-FeZSM-5) composite-based adsorbents of efficient GLP adsorption capacity were prepared by oxidative polymerization.85,86 Polydopamine (PDA) was used to synthesize BiVO4/PDA-g-C3N4 photocatalyst sheets for exploiting dopamine self-polymerization, ultrasonic dispersion, and self-assembling. Under visible light irradiation, the BiVO4/PDAg-C3N4 photocatalysts degraded GLP more actively than the control composites prepared without PD.87 Fluorescent porous N-benzyl (carbazole derivative)-based polymer GLP detectors were synthesized in a one-step polymerization. The polymers of tunable pore sizes emitted bright cyan, blue, and green light upon ultraviolet (UV)-light excitation. GLP and other pesticides quenched the fluorescence of polymers according to the Stern–Volmer kinetics, thus demonstrating pesticide-specific recognition and determination.88 An intriguing report on a proteinoid polymer composite-based sensor for GLP was presented.89 The proteinoid polymer nanoparticles (NPs) were prepared by thermal step-growth polymerization of natural and unnatural amino acids in the presence of various agrochemicals. The agrochemicals interacted with the hollow NPs by encapsulation, integrating with the crude shell, or bound covalently/physically to the NP surface. Once hydrophobized and fluorescently labeled, the NPs were taken up by the plant and accumulated in the plant’s vascular system. This example shows the agrochemical-specific applications of these NPs to agriculture.89 Moreover, MIP-based GLP sensors of different properties and sensing parameters have been prepared.90−103 The current Review discusses several examples of these sensors (Table 2).
Table 1. Analytical Techniques for GLP Determination.
| analytical technique | LOD | LDCR | ref |
|---|---|---|---|
| FLD–HPLC | 0.04 mg/kg | 0.13–1000 mg/kg | (104) |
| ESI–MS–HPLC | 0.01 mg/kg | 0.04–1000 mg/kg | (104) |
| FLD–HPLC | 0.01 mg/kg | 0.005–0.5 mg/L | (109) |
| FLD–HPLC | 0.6 μg/L | 2–160 μg/L | (110) |
| LC–IRMS coupled with isotope labeling | <1 μM | n/a | (107) |
| HPLC with fluorescent labeling | 0.02 ng/mL | 1–3000 ng/mL | (111) |
| 0.002 mg/kg | n/a | ||
| HPLC–MS | 13 ng/mL | 13–500 ng/mL | (105) |
| HPLC–ICP–MS/MS | 8.2 μg/L | 27–218 μg/L | (108) |
| HPLC-DAD | 300 μg/L | 1–8 mg/L | (108) |
| UPLC–MS/MS | 0.05 μg/L | 0.1–200 μg/L | (112) |
| MIP-based adsorptive-extracting systems | 3.37 mg/mL | n/a | (90) |
| n/a | BF: 2.12–2.33 | (92) | |
| 0.05 μg/L | Recovery: 96% | (97) | |
| 0.043 μg/L | Recovery: 90.6–97.3% | (93) | |
| 700 μM | n/a | (101) | |
| SERS | <0.1 ppb | 0.01–0.2 ppm | (118) |
| SERS sensor based on GO/AgNPs/Ti NT arrays nanocomposite | 3 μg/L | 0.005–50 mg/L | (120) |
| EPSP-based interferometric sensing | 100 pM | 10–11–10–8 M | (117) |
| Colorimetry | 0.847 μM | 1–40 μM | (121) |
| 1.23 μM | 1–40 μM | ||
| MIP-based luminescent determination | 2 μg/mL | 1–40 μg/mL | (103) |
| Porous-carbazole fluorescent determination | 0.35 μM | n/a | (88) |
| DNA-labeled fluorescent magnetic core–shell NPs | 0.27 nM | 1–10000 nM | (116) |
| DNA-templated AuNC-based fluorescent determination | 5 μg/L | 15–100 μg/L | (122) |
| FRET fluorescent and colorimetric determination using SiNPs | 0.003 μg/mL | 0.15–1.5 μg/mL | (123) |
| Fluorescence of 4-butyl-3-thiosemicarbazide-labeled CDs | 0.27 μM | 0.4–30 μM | (124) |
| Fluorescence of 1,4-dihydroxyanthraquinone-labeled CDs | 0.8 ng/mL | 50–1300 ng/mL | (125) |
| Fluorescence of rhodamine B-embedded MOFs | 0.18 μM | 0.6–45 μM | (126) |
| Fluorescence of an UiO-67/Ce-MOF nanocomposite | 0.0062 μg/mL | 0.02–30 μg/mL | |
| Colorimetric/fluorescent/photothermal sensing by CDs-anchoring ferrocene MOF nanosheets | 0.0131 μg/mL | 0.039–3.19 μg/mL | (128) |
| 0.0015 μg/mL | 0.0088–3.98 μg/mL | ||
| Fluorescence-assisted immune-magnetic system | 88.8 ng/L | 0.0001–10 mg/L | (115) |
| FRET-assisted determination systems | 9.8 ng/kg | 0.02–2 μg/kg | (114) |
| 0.6 μM | 0.02–2 μM | (119) | |
| 0.79 μM | 0.5–20 μM | (113) | |
| Probe-free SPCE-mediated electrochemical sensing | 2 μM | 0.01–0.3 mM | (131) |
| Electrochemiluminescence | 0.1 nM | 0.1 nM–10 mM | (133) |
| HRP-based electrochemical sensor | 1.7 μg/L | 0.25–14 μg/L | (132) |
| Electrochemical sensing by enzymatic laser-induced graphene | 3.03 μM | 10–260 μM | (136) |
| (SWCNT/polyfluorene-bipyridine)-based water-gated transistor | 1 nM | 10–2–102 μM | (137) |
| Amperometric determination using copper NP-based sensor | 3.42 μM | 0–25 μM | (138) |
| EIS/immunoassay | 0.1 ng/mL | 0.1–72 ng/mL | (141) |
| EIS/immunoassay-based two-plex sensing platform | 1 ng/mL | 0.3–243 ng/mL | (142) |
| HRP-modified-pencil graphite electrode for CV and amperometry | 0.025 mg/L | 0.1–10 mg/L | (140) |
| Electrochemical sensing based on Ti3C2Tx/Cu nanocomposite | 24 fM | 0.1 pM – 1 μM | (139) |
| Machine learning-assisted electro-immunosensor | 0.01 ppm | 0.01–5 ppm | (130) |
| Urease-conjugated electrochemical sensing | 0.5 ppm | 0.5–50 ppm | (134) |
| Electroimmunochemical assay | 5 ng/L | 0–10000 ng/L | (129) |
| Electrochemiluminescent enzymatic immunoassay | 0.032 mM | 0.1–100 mM | (135) |
| MIP-based electrochemical systems | 0.8 pg/L | 1 pg/L −1 μg/L | (91) |
| 1 pM | 1 pM – 1 μM | (95) | |
| 0.27 ng/mL | 5–800 ng/mL | (102) | |
| 0.1 nM | 0.1–100 nM | (94) | |
| 0.35 ng/mL | 3.98–176.23 ng/mL | (96) | |
| n/a | n/a | (143) | |
| 4 nM | 0.025–500 μM | (144) | |
| 92 ng/L | 400–1200 ng/L | (100) | |
| AFM-assisted enzyme-based cantilever nanobiosensor | 0.028 mg/L | 0.01–10 mg/L | (145) |
| ELISA | 0.05 μg/L | 0.05–4 μg/L | (147) |
| 23 μg/kg | 50–4000 ng/L (50–4000 ng/g) | (150) | |
| GLP-ovalbumin conjugate-based ELISA | 2 ng/mL | 2–1000 ng/mL | (149) |
| HPLC–MS-assisted ELISA with online SPE | 3.2 ng/L | 50–500 ng/L | (148) |
| AuNP-oligonucleotide immuno-PCR sensing | 4.5 pg/g | 61.1 pg/g–31.3 ng/g | (146) |
| Fluorescent electrophoresis-coupled LOC | 0.17 μg/L | 0.17–845 μg/L | (152) |
| 3D-μPAD-QD-MIP colorimetric-catalytic sensor | 0.05–0.09 μg/L | 0.5–50 μg/L | (151) |
| MIP-based microfluidic-electrochemical systems | 0.002 μg/mL | 0.005–50 μg/mL | (98) |
| 247 nM | 0–1 mM | (99) | |
| Smartphone-integrated lab-in-a-syringe sensor | 2.81 nM | 0–10 μM | (154) |
| Smartphone-integrated enzyme-free fluorimetric paper sensor | 4.19 nM | 0–180 nM | (156) |
| Smartphone-assisted colorimetric and fluorescent sensor | 2.66 μM | 0–30 μM | (155) |
| Smartphone-assisted fluorescence/colorimetric/SERS assay | 0.738 nM | 0–0.12 μM | (158) |
| 2.26 nM | 0–220 nM | ||
| 0.186 nM | 0.5–12 nM | ||
| In-field robotics-assisted in-row weed control | n/a | n/a | (159) |
| Smartphone-assisted AChE-based colorimetric sensor | 0.15 μM | 1.5 nM – 15 μM | (157) |
Table 2. Analytical Parameters of MIP-Based Chemosensors for GLP Determination.
| functional monomer | cross-linking monomer | polymerization method | signal transduction technique | properties | ref |
|---|---|---|---|---|---|
| Acrylamide | EGDMA | T-FRP | Optical | LDCR: 5–40 μg/mL | (103) |
| LOD: 0.046 μg/mL | |||||
| N-Isopropylacrylamide | BAA | T-FRP | Optical | LDCR: 0.005–50 μg/mL | (98) |
| LOD: 0.002 μg/mL | |||||
| 3-(4-Sulfonylbutyl)-1-[3-(triethoxysilyl) propyl]-1H-imidazolium | TEOS | Sol–gel polycondensation | Optical | weLDCR: 0.1 nM - 800 μM | (94) |
| LOD: 0.1 nM | |||||
| N-Methacryl-l-cysteine | EGDMA | FRP, electropolymerization | Electrochemical | LDCR: 3.98–176.24 ng/mL | (96) |
| LOD: 0.35 ng/mL | |||||
| Pyrrole | Electropolymerization | Electrochemical | LDCR: 1 pM – 10 μM | (95) | |
| LOD: 1 pM | |||||
| p-Aminothiophenol | Electropolymerization | Electrochemical | LDCR: 1 pg/L–1 μg/L | (91) | |
| LOD 0.8 pg/L | |||||
| Pyrrole | Electropolymerization | Electrochemical | LDCR: 5–800 ng/L | (102) | |
| LOD: 0.27 ng/mL | |||||
| Pyrrole | Electropolymerization | Electrochemical | LDCR: 400–1200 ng/L | (100) | |
| LOD: 92 ng/mL | |||||
| Pyrrole | Electropolymerization | Electrochemical | LDCR: n/a | (143) | |
| LOD: n/a | |||||
| Acrylic acid and N-vinyl-2-pyrrolidone | DHEBA | Electropolymerization | Electrochemical | LDCR: 0.025–500 μM | (144) |
| LOD: 4 nM |
Various methods have been used to determine or extract traces of GLP and AMPA from liquids, solids, and plants (Table 1). Multimethod approaches, including chromatography,104−112 adsorption-extraction systems,90,92,93,97,101 optical,88,103,113−128 electrochemical,54,91,94−96,100,102,129−144 and scanning-probe techniques,145 immunochemistry,146−150 and microfluidic LOCs98,99,151−153 exploit modern GLP-sensitive materials to detect and analyze, as well as to extract and degrade, GLP traces. Hybrid nanomaterials used in these approaches include enzymes (AChE, ESPS), deoxyribonucleic acid (DNA)- or antibody-based aptamers, immune-magnetic conjugates, MIPs, and inorganic materials. Moreover, we herein discuss recent reports concerning the GLP determination by AI-excelled smartphone-integrated sensors,154−158 and an automated vegetable analyzer for targeted GLP delivery.159
3.1. Chromatography and Adsorptive-Extracting Systems for GLP
Chromatographic techniques are traditional tools for determining pesticides in liquids, foods, and clinical and environmental samples. They enable the pretreatment, separation, detection, or degradation of pesticides among other contaminants (micropollutants) of emerging concern, including endocrine-disrupting chemicals, plasticizers, artificial sweeteners, pharmaceuticals, personal care products, pyrethroid insecticides, and halogenated or organophosphorus retardants.160−162
For example, an intelligent postacquisition sample validation following mixed-mode solid-phase extraction (SPE) and ultraperformance liquid chromatography quadrupole-time-of-flight mass spectrometry (UPLC-Q-ToF-HRMS/MS) was employed for wide-scope target screening of 2316 emerging pollutants in wastewater samples collected from the Wastewater Treatment Plant of Athens. Upon validation of the method, it was employed to detect and quantify the influent and effluent wastewater connect of 398 selected contaminants of pesticides, opiates, and opioids, stimulants and sympathotomimetics, cannabinoids barbiturates, benzodiazepins, tranquilizers, analgesics, antibiotics, steroids, and industrial chemicals. This method allowed for determining the contents as low as 0.3 ng/L (perfluoroundecanoic acid), 0.4 ng/L (acetochlor, N-2,4-dimethylphenylformamide), and 0.5 ng/L (haloperidol, perfluoroheptanesulfonic acid).163 Moreover, HRMS-based suspect screening integrated with national monitoring data was recently applied in aquatic toxicology by investigating the presence of 16 not-well-explored pesticides and 242 pesticide transformation products in Swedish agricultural areas and streams. The study confirmed the occurrence of 11 transformation products and 12 tentatively identified ones.164
Furthermore, gas chromatography coupled to electron ionization mass spectrometry (GC-EIMS) was employed to determine levels of OP ethers in air and soil samples.165 Chromatographic analysis of hazardous agrochemicals usually involves two steps. First, the analyte is pretreated with optically active or polystyrene-coated magnetic NPs and then separated using GC or high-performance liquid chromatography (HPLC) coupled with a UV spectroscopic, fluorescent (FLD), or mass spectrometric (MS) detector. Most advanced adsorptive/separating chromatographic systems include nanotechnologically excellent adsorptive systems, engaging MIPs or silica NPs (SiNPs) of various porosity, developed surface area, and sorbing properties. Moreover, they are often modified with ionic liquids, silanes, amines, enzymes (AChE, carboxylesterases, laccases, or OP hydrolases), fluorescent, electrochemiluminescent, or surface-enhanced Raman spectroscopy (SERS) labels and magnetic beads. Finally, these systems are prepared as beads, wires, and sheets to improve their sensitivity, stability, amenability to modifications, and “on-site” applicability.166,167
Novel chromatographic techniques applied to GLP determination involve GC or HPLC coupled with UV spectroscopic,106 FLD, or electrospray ionization mass spectrometric (ESI-MS) detectors,104 often using fluorescent111 or carbon isotope (δ 13C) label probes.107 The HPLC-FLD and -(ESI-MS) determined GLP fluorenylmethyloxycarbonyl (FMOC) derivatives in maize in the 0.1–0.4 mg/kg range with the 79–86% efficacy of extraction recovery and the limit of quantification (LOQ) of 0.4 ng/mL.104 Similar FMOC-based HPLC methods determined GLP in soil109 and seawater110 with 0.01 mg/kg and 0.6 μg/mL LOD, respectively. With a different method, an HPLC precolumn was derivatized with 3,6-dimethoxy-9-phenyl-9H-carbazole-1-sulfonyl chloride (DPPC-Cl) to determine GLP in soybean with the LOD of 0.02 ng/mL and the extraction recovery exceeding 95%.111 The LC coupled to isotope-ratio MS (LC–IRMS) enabled the GLP determination in 21 commercial herbicide samples, revealing δ 13C values between −24 ‰ and −34 ‰ in the submicrogram concentration range.107 With porous (graphitized carbon absorbent)-based chromatography coupled to a three-quadrupole MS detector, the 6 ng/mL LOD of GLP in aqueous solutions was attained.105 In a similar study, with an HPLC coupled with an inductively coupled plasma (ICP-MS) detector or a diode array detector (DAD), GLP was determined with the LOD of 8.2 and 300 μg/L, respectively.108 Chromatographic analysis was employed for the GLP determination in real-life water samples from 10 agricultural provinces of China during various meteorological conditions.112 Specifically, UPLC-MS-MS was employed to provide the risk assessment and spatioseasonal distribution of GLP, AMPA, and glufosinate in China’s groundwater and surface water samples, from 2017 to 2018. GLP was determined with the LOD of 0.05 μg/L in the linear dynamic concentration range (LDCR) of 0.1–200 μg/L. Combined with the model simulations for potential leaching to water bodies, these quantitative results allowed us to establish that the spray drift deposition, runoff, and erosion are the main drivers of the aquatic GLP exposure from crop neighborhood, especially in summer and autumn seasons.
Inevitable progress will be made to address well-known limitations of GLP-targeted separating systems. Modern separation techniques, including capillary electrophoresis, GC, or LC, provide relatively low sensitivity (nanomolar) compared with optical, electrochemical, or immunochemical techniques that offer the detection of subnanomolar concentrations. Additionally, the blocky construction of modern systems disallows them for in-field use. Expectedly, the selectivity of future systems will be improved using tools of nanoinformatics, providing models and structures of analyte-receptor complexes of higher affinities. Finally, coupling these systems, based on aptamers, ionic liquids, NPs, and MIPs, with MS or FLD detectors, followed by their integration into the mobile microfluidic devices, shall enhance both sensitivity and applicability.168
3.1.1. MIP-Based Chromatography of GLP
Various chromatographic methods developed to remove or degrade GLP traces from environmental, biological, or grocery samples exploited the adsorptive-extracting properties of porous MIPs.169 The maximum adsorption capacity of GLP-selective MIPs, formulated via free radical polymerization (FRP) of acrylamide (AA) and ethylene glycol dimethacrylate (EGDMA), was evaluated as high as 3.37 mg/g90 (Figure 1G and 1H). A series of dual-templated methacrylic acid MAA-MIPs, imprinted with herbicides, including GLP, were fabricated by precipitation polymerization for water treatment. Efficiency, expressed by binding factors (BFs), KMIP/KNIP, where K is the partition coefficient, of chosen GLP-selective MAA-MIPs in tap water for GLP solution, ranged between 2.12 and 2.55.92 Moreover, based on 1-allyl-2-thiourea (ATU), GLP-detecting MIP cartridges were prepared to assess the (UPLC-MS-MS)-mediated GLP recovery from mineral and underground waters. The herbicides were totally retained from these real matrices, spiked with 0.5 μg/L GLP.97 The selective sorptive extraction of GLP from river water and soil samples, with mean recoveries ranging from 90.6 to 97.3%, was demonstrated for ATU- and 2-dimethyl aminoethyl methacrylate (DMAEM)-based MIPs, prepared by UV light-activated FRP93 (Figure 1A-1F). Eventually, in a recent study, positively charged (quaternary ammonium cation)-MIP quartz crystal microbalance (QCM) sensors were constructed for the (electrostatic interaction)-mediated binding of GLP from river waters.101
Figure 1.
MIP-based adsorption systems for GLP separation and determination. (A-D) SEM micrographs of the surface of stir-bars coated with a DMAEM-ATU-EGDMA (A,B) NIP and (C,D) MIP-GLP. Chromatograms recorded after the injection of a solution of 100 μg/L GLP and all analogs (E) before and (F) after performing the MIP (stir-bar)-based extraction. Adapted with permission from ref (93). Copyright 2016 Elsevier. (G) Time-dependent adsorption of GLP and AMPA on the AA-EGDMA MIPs at pH = 6.5 and 25 °C. (H) Isotherms for MIP-GLP and MIP-AMPA at 25 °C. Adapted with permission from ref (90). Copyright 2014 Elsevier.
The next-generation chromatographic sensors shall unite traditional multimodal separation and detection techniques, including thin-layer chromatography (TLC) or immunochromatography, with microfluidics and AI tools. Recent studies indicate the ultrasensitive on-site determination of toxins and pesticides in real samples using smartphone-coopted. Personal low-cost AI tools offer high-resolution photoimaging, portability, and instant online data availability that can be immediately shared. For example, an open-source smartphone-imaging app was developed to excel TLC screening and quantify pharmaceuticals.170 In another study, smartphone-based dual-channel immunochromatographic test strips labeled with polymer carbon QDs were fabricated for on-site simultaneous biomonitoring of cypermethrin, a pyrethroid pesticide, and its metabolite, 3-phenoxybenzoic acid, determined with LODs of 0.35 and 0.04 ng/mL, respectively.171 Expectedly, similar tools will soon be commercially available for GLP, as has already been demonstrated for other pesticides.
3.2. Optical Sensors for GLP
Recently, a series of optical strategies have been developed for pesticide sensing. Those include photonic, photoluminescent, photoelectric, electrochemiluminescent, and colorimetric methods. Over the past five years, carbon dot (CD)-based optical sensors equipped with aptamers, antibodies, enzymes, gold and silver nanoclusters (AuNCs and AgNCs), and nanoparticles (AuNPs and AgNPs), and MIPs as recognition units have been used as herbicide-derived signal indicators, catalysts, coreactants, and electrode surface modifiers.169,172 Moreover, novel carbon-based SERS biosensors with similarly excellent sensing properties were fabricated. They primarily include 0D carbon quantum dots (QDs), 1D carbon nanotubes (CNTs), 2D graphene, graphene oxide (GO), 3D carbon nanomaterials, and core–shell nanostructures. The SERS sensors were devised for selective and quantitative in situ analysis of agrochemicals by exploiting so-called localized “hotspots” produced during the application.173 QD-based chemosensors were used for the highly sensitive and selective detection of pesticide poisons in the clinical and forensic toxicological analysis of gaseous, anionic, phenolic, metallic, drug, and pesticide specimens. A breakthrough has been made by continuously applying whispering gallery modes (WGMs) to biosensing.174−176 Miniaturized size and excellent lasing properties of WGM-based microlasers, called resonators, were exploited in constructing label-free aptasensors177 and applied to detect single molecules, particles, cells, and molecular electrostatic changes at biointerfaces and barcode-type tagging and tracking.178−180 Furthermore, remarkable advances have been made in developing electrochemiluminescent (ECL) and photoelectrochemical sensors to analyze food quality. In most recent works, nanomaterial-based ECL luminophores have been synthesized and incorporated into immunoassay-, aptasensor-, and microfluidic systems for low-cost ultrasensitive determination of heavy metals, illegal additives, microbes, and pesticide contaminants in complex matrices.181 Food safety issues can be effectively solved by using brand-new photoelectrochemical biosensors. These devices, equipped with photoactive nanomaterial-based recognition units, quantitatively determine mycotoxins, antibiotics, and pesticides with high sensitivity at a significantly low signal-to-noise ratio.182
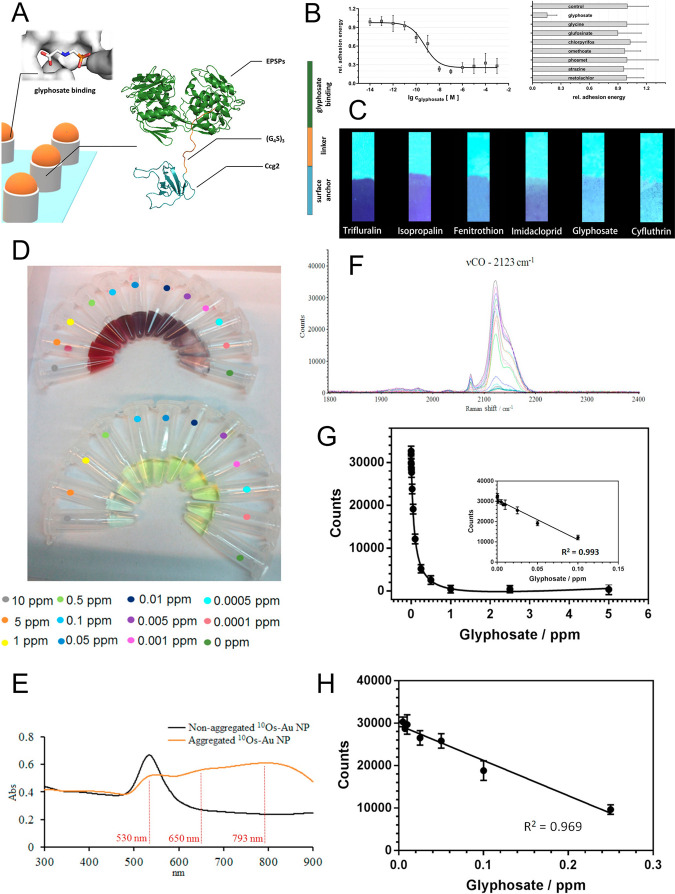
Modern optical methods of GLP sensing mainly exploit SERS, interferometry, chemiluminescence, colorimetry, fluorescence, and Förster resonance energy transfer (FRET). A SERS-based method, which exploits organometallic osmium carbonyl cluster-conjugated AuNPs, was used for AChE-mediated GLP determination with the LOD below 0.1 ppb118 (Figure 2D–2H).
Figure 2.
Optical sensors for GLP. (A) Scheme of a (glass slide)-adhered ESPS-based interferometric biochip (gray) specific for GLP (orange) deposited onto soft colloidal particles (SCPs), not shown. (B) Normalized relative adhesion energies of GLP-SCPs in the presence of GLP and other pesticides. (Left) GLP concentration dependence of adhesion energy. (Right) cross-reactivity of SCPs toward interferences. Adapted with permission from ref (117). Copyright 2020 Elsevier. (C) Strips of carbazole-based fluorescent porous polyaminals (PAN-C) test papers for sensing six pesticides in water at a concentration of 35 μM. Adapted with permission from ref (88). Copyright 2020 The American Chemical Society. A SERS sensor was used for GLP. (D) Dose-dependent colorimetric GLP determination by 10OsCO-AuNPs (top row), compared with a commercial standard (bottom row). (E) UV-NIR absorption spectra of nonaggregated and aggregated 10OsCO-AuNPs. (F) (GLP concentration)-dependent SERS spectra of 10OsCO-AuNPs (2123 cm–1). (G) GLP concentration dependence of the ν(CO) band intensity and (H) sensitivity assessment of the sensor in GLP-spiked beer samples. Adapted with permission from ref (118). Copyright 2020 Elsevier.
Regarding SERS sensors for GLP, an air-stable sensor was devised by using reduced GO (rGO)-wrapped dual-layer AgNPs on TiO2 NT arrays as a SERS substrate. This sensor’s adsorption capacity and SERS enhancement were excellent thanks to the tremendous electromagnetic field and chemical enhancement generated by localized SPR excitation of the dense dual-layer AgNPs uniformly deposited onto the TiO2 NTs and to facilitation of the charge transfer between the extensive π–π conjugations in the rGO. Because the GLP molecule does not have a specific chemical group, it is hardly detectable using a conventional SERS method. In the discussed study, GLP was determined by the enhanced adsorption area of the nanocomposite. That enabled GLP determination in environmental samples of waters and soils in the LDCR of 0.005–50 mg/L with the LOD of 3 μg/L, i.e., lower than the limit specified by the U.S. EPA and the European Union.120
Two UV–vis colorimetric sensors for GLP were devised by linking 3-chloro-4-methylpyridine with 4-(dimethylamino) benzaldehyde or 4-(dimethylamino) cinnamaldehyde in a one-step synthesis, resulting in 4-(2-(3-chloropyridin-4-yl) vinyl)-N,N-dimethylaniline (BP-Cl) or 4-(3-chloropyridin-4-yl) buta-1,3-dien-1-yl)-N,N-dimethylaniline (CP-Cl), respectively. In the GLP reaction with these sensing compounds, the N atom of GLP interacted with the Cl atom on the pyridine ring, resulting in highly sensitive and selective naked-eye detection of GLP, observed as color changes ranging from colorless to yellow (BP-Cl) and from yellow to orange (CP-Cl). In a naked-eye analysis, GLP was determined in tap water and potato samples with the LOD of 15 and 10 μM for BP-Cl and CP-Cl, respectively, whereas using UV–vis spectrophotometry, these LODs were of 0.847 and 1.23 μM, respectively, in the LDCR of 1–40 μM.121
GLP was determined by using interferometry and chemiluminescence. Picomolar traces of GLP were detected using an EPSP-decorated interferometric sensor with GLP-attached poly(ethylene glycol) (PEG)-based soft colloidal probes117 (Figure 2A,B). In a chemiluminescent-assisted method of GLP determination, the LOD of 46 ng/mL was reached using poly(vinyl chloride) (PVC)-MIP microbeads prepared by FRP of acrylamide and subsequent conjugation with PVC.103
Fluorescent sensors rely on the analyte-induced triggering or quenching of the receptor’s fluorescence, called the “ON/OFF strategy.” In this strategy, pesticides, including GLP, act as either direct or indirect quenchers or triggers of fluorescence. In the direct approach, GLP binding by the receptor modifies its electronic structure and fluorescent properties, thus enabling direct optical sensing and determination of the GLP content. In contrast, in the indirect approach, GLP interacts with the molecular trigger/quencher to subsequently turn on/off the receptor. Recent studies on GLP-based fluorescence modulation demonstrate examples of both approaches.
GLP-induced fluorescence was directly quenched using carbazole-based porous polyaminals88 (Figure 2C). Moreover, various hybrid fluorescent-magnetic immunosensors were fabricated to detect GLP in liquids. For example, water-in-oil microemulsion-based Co–V/SiO2 NPs, doped with rhodamine, enabled immunoassaying GLP-dsDNA double target/probe core–shell NPs with the LOD of 0.35 nM.116 Likewise, a magnetic-assisted oligonucleotide aptamer probe labeled with 6-carboxy-fluorescein was used for GLP sensing with the LOD of 88.8 ng/L.115 Finally, recently devised FRET-based sensors for GLP explored GLP-induced turn-on fluorescence following the aggregation of positively charged cysteamine-AuNPs and negatively charged CdTe QDs capped with thioglycolic acid. This FRET assay utilized GLP detection in apples with a 9.8 ng/kg LOD.114 In another study, the fluorescent carbon QD probe operating in the AND logic gate was successfully quenched by GLP, resulting in the GLP determination with the LOD of 0.6 μM.119 Finally, the GLP-induced FRET switching in a self-assembled nanosensing system, formulated from p-tert-butylcalix [4] arene-grafted ruthenium(II) bipyridine-doped SiNPs, was used for GLP determination with the LOD of 0.791 μM.113
The indirect-modulation fluorescence sensor mainly relies on the chelating properties of GLP, provided by its phosphonate and carboxyl groups, as well as the monoprotonated secondary amine nitrogen atom. A Cu2+-modulated DNA-templated AgNCs was used to determine GLP by GLP reaction with the Cu2+, a primary quencher. Upon chelation, the DNA AgNCs fluorescence was recovered, which enabled the stoichiometric determination of GLP in real samples in the LDCR of 15–100 μg/L and with the LOD of 5 μg/L.122 A similar approach was exploited in fluorescent and colorimetric sensing of Cu2+ and GLP by the (o-phenylenediamine)-SiNPs FRET interaction. The Cu2+ oxidation of o-phenyaldiamine disabled the fluorescence of SiNPs by FRET. Hence, by chelating Cu2+, GLP served as a secondary quencher by hindering the FRET donor’s oxidation and restoring the FRET acceptor’s emission. This approach allowed determining GLP in the LDCR of 0.15 to 1.5 μg/L with the LOD of 0.003 μg/mL.123 Consistently, the concept of the Cu2+-GLP system was used in a fluorescent 4-butyl-3-thiosemicarbazide-labeled CDs-based sensor. Cu2+-quenched fluorescence of the sensor was recovered by the GLP addition in a dose-dependent manner, which enabled GLP determination in real samples with the LOD of 0.27 μM.124 Likewise, a Cu2+-modulated 4-dihydroxyanthraquinone-CD nanosensor enabled for ultrasensitive sensing of GLP in vegetable samples with the LDCR of 50 to 1300 ng/mL and the LOD of 0.8 ng/mL.125 Moreover, GLP was rapidly determined in real samples of agri-food products (tea, soybean, wheat, cucumber) using (rhodamine B)-embedded amino-functionalized iron-based MOFs bonded with Cu2+ via Lewis interactions that resulted in fluorescence quenching. The addition of GLP resulted in Cu2+ chelation via hydrogen bonding, thus turning on the fluorescence of the nanosensor. That allowed for the GLP determination with the LOD of 0.18 μM in the 0.6–45 μM LDCR.126 Similarly, a hierarchical, highly porous fluorescent nanocomposite, UiO-67/Ce-PC, consisting of UiO-67 NPs grown on Ce-MOF-derived porous carbon, was devised to determine GLP in soybean, wheat, and corn. A large specific surface area and abundant metal active sites that alleviated the diffusion barrier and enhanced the GLP preenrichment ensured the determination. Additionally, the competitive coordination effect between GLP’s phosphonate groups and metalloorganic ligands (hydroxyl groups) attenuated the ligand-to-metal charge transfer between metallic nodes and organic struts, thus providing a dose-dependent fluorescence recovery upon GLP detection. The plot of the fluorescence enhancement response of UiO-67/Ce-PC toward GLP was linear in the range of 0.02–30 μg/mL with the LOD of 0.0062 μg/mL.127
An optical/temperature nanozyme platform, fabricated from nitrogen-doped CDs anchored onto Zr-based ferrocene MOF nanosheets, was devised for the sensitive and portable determination of GLP. The nanozyme mimicked peroxidase activity, i.e., oxidation of colorless 3,3′,5,5′-tetramethylbenzidine into a blue product in the presence of H2O2, which GLP readily suppressed. That enabled a trisignal response of fluorescence enhancement, absorbance, and temperature decrease. These features were exploited to construct a portable mini-photothermal device capable of colorimetric and fluorescent GLP determination in the 0.039–3.19 μg/mL LDCR with the LOD of 0.0131 μg/mL and the 0.0088–3.98 μg/mL LDCR with the LOD of 0.0015 μg/mL, respectively.128
Because of the analyte-triggered AChE inhibition, the optical sensors for GLP, based on the single molecule “turn on/off” on-site sensing mode, belong to the most sensitive and selective. Future optical technologies, compatible with smartphone-assisted kits and LOCs, include paper-, liquid-, and gel-based sensors. Double-signal fluorescence, phosphorescence, chemiluminescence, lateral flow immunoassay, or enzymatic fiber-optic biosensing are envisioned as the most promising operation strategies, enabling the in-field red-green-blue (RGB)-determination of GLP. Most recent advancements in optical sensors involve metasurface-based devices. Metasurfaces are 2D composite micro- or nanomaterials of the subwavelength thickness and desired geometry, allowing for adjusting the material’s refractive index of the material to positive, near-zero, or negative values (so-called negative refraction). Moreover, the nonlinear metasurface can transform the infrared signal into the visible signal, which the human eye or smartphone CCD can visualize.183 These exquisite features allow for devising a surface-enhanced infrared absorption-operating sensor for passive trapping and detecting glucose and proline with the LOD of ∼1 pg.184 In this context, the LODs of the devices discussed in the present review are comparable or one order of magnitude lower. Besides, devising optical sensors for agricultural use shall necessitate integrating ultrasensitive (of the order of ∼fM) devices with smartphones or portable meters, similar to commercial smart Raman spectroscopic alcohol meters.
3.3. Electrochemical Sensors for GLP
The contemporary electrochemical sensors provide a portable, simple, and sensitive determination of herbicides and pesticides in real water, food, and soil samples.185,186 Recently fabricated multimodal sensing devices contain aptamers, enzymes, graphene, CNTs, polymers, viruses, and cells as recognition units integrated with optical, piezoelectric, and microgravimetric transducers.187,188 The GLP concentration is mainly measured using amperometry, cyclic voltammetry (CV), differential pulse voltammetry (DPV), nonfaradic electrochemical impedance spectroscopy (EIS), and electrochemical surface plasmon resonance (SPR) spectroscopy.
Among other OP pesticides, including glufosinate and AMPA, GLP was determined amperometrically using a three-electrode sensor fabricated by (UV laser)-inscribing of OP-selective Cu NPs on a polyimide film. For GLP determination in natural water samples, the sensor exhibited the LOD of 3.42 μM, whereas for glufosinate and AMPA, the LOD was 7.28 and 17.78 μM, respectively. Moreover, the sensor prevailed in pesticide selectivity in the presence of ion and organic interferences in natural water.138 Recently, a very sensitive, highly conducting sensor for GLP was constructed based on Cu-benzene-1,3,5-tricarboxylate-loaded 2D Ti3C2Tx nanosheets deposited on a glassy carbon electrode (GCE). This nanocomposite was fabricated upon in situ copper component growth on chemically etching nanosheets that were subsequently dispersed and vacuum-dried on the GCE. Because of the Cu ions’ high affinity to GLP, the sensor sensitivity was excellent, resulting in the exquisitely low LOD of 26 fM and a broad LDCR of 100 fM to 1 μM.139
Carbon-based electrochemical sensors have recently become a powerful tool for tracing metabolism. The significant advantage of these materials is the enhancement of the electrochemical sensing performance by enlarging an active surface area. For example, a probe-free screen-printed carbon electrode (SPCE) was used for direct GLP sensing in tap water with micromolar sensitivity.131 An ECL horseradish peroxidase (HRP)-based sensor, formulated on a sulfonate polymer matrix, was used for the GLP determination with the LOD of 1.7 μg/L.132 In a recent study, a simple and accurate (pencil graphite electrode)-supported sensor containing an HRP enzyme, immobilized on a multiwalled CNTs (MWCNTs)-doped polysulfone membrane, was devised to detect GLP in the river and drinking water samples. This highly selective sensor was readily applied to the in-field determination of GLP, showing reproducible and repeatable CV and amperometric readouts in the LDCR of 0.1–10 mg/L and the LOD of 0.025 mg/L.140 Moreover, functionalized single-walled CNT (SWCNT)-based nanomaterials were used in electrochemical GLP sensing by providing transducing layers for water-gated transistor-based sensors. In particular, GLP was selectively determined with a sensor composed of networks of semiconducting, monochiral (6,5) SWCNTs featured with polyfluorene-bipyridine copolymer and a Cu2+-selective membrane. The functionality of this semiconducting sensor relied on the n-doping resulting from Cu2+ complexation by bipyridine. Adding GLP suppressed this complexation by competitive chelation of Cu2+, thus enabling the stoichiometric quantification of the herbicide analyte at nanomolar concentrations.137
Likewise, GLP was selectively determined using an amperometric sensor containing glycine oxidase, a flavoenzyme, immobilized on a platinum-decorated laser-induced graphene scaffold. Exquisite electronic and solid properties, including the LDCR of 10–260 μM and the LOD of 3.03 μM, allowed for selective determination of GLP with only minimal interference of common herbicides and insecticides, including atrazine, 2,4-dichlorophenoxyacetic acid, dicamba, parathion-methyl, paraoxon-methyl, malathion, chlorpyrifos, thiamethoxam, clothianidin, and imidacloprid. In the future, the sensor may enable food mapping and determining GLP in complex river waters and crop residue fluids.136
GLP was sensed by using electrochemical immunoassays. Recently, a label-free, portable, selective, and highly sensitive (LOD of 0.1 ng/mL in the LDCR of 0.1–72 ng/mL) sensor was devised to determine GLP in human urine.141 Its electrochemical platform consisted of a portable printed-circuit circular board with gold working and reference electrodes enabling nonfaradic EIS measurements. Its immunoassay-based platform included a monolayer of dithiobis(succinimidyl propionate), a thiol-based cross-linking monomer modified with a GLP antibody, and a coated gold electrode. The selectivity was assessed using typical herbicide interferences, including malathion, 3-phenoxybenzoic acid, and chlorpyrifos.141 Likewise, GLP and chlorpyrifos were determined in low- and high-fat food matrices using a two-plex, portable electrochemical-immunoassay nonfaradic ESI-based sensor. Both sides of this sensor were functionalized with the respective antibody. In low fat, the sensor determined GLP and chlorpyrifos with a 1-ng/mL LOD in the 0.3–243 ng/mL GLP/chlorpyrifos LDCR, whereas in high fat, the LOD was 1 ng/mL in the LDCR of 1–243 ng/mL.142
GLP was sensed with the LOD of 0.11 nM using sophisticated ECL sensors based on HRP-assisted in situ generations of ZnS QDs on ordered mesoporous carbon substrates.133 A bioconjugate of urease-AuNPs and an agarose-guar gum-entrapped biocomposite membrane was devised to detect the enzyme-inhibiting activity of GLP, enabling the determination of this herbicide in ppm traces.134 Similarly, GLP was determined with a sensitivity of 0.01 ppm (10 ng/mL) in fruits and vegetables using a field-deployable electrochemical immunosensor based on a polymer-metalized interdigitated two-electrode system, functionalized with a commercial GLP-antibody, and equipped with a portable reader and a machine-learning binary classifier130 (Figure 3). Likewise, an SPCE immunosensor, enabling the GLP determination with the 5 ng/L LOD, was fabricated using anti-GLP-IgG-modified magnetic beads and an HRP-conjugated-GLP tracer.129 Moreover, an electrodeposited monocrystalline silicon-PANI-HRP conjugate was constructed to evaluate an immunoassay for GLP sensing with the LOD of 5.44 μg/L.135 Continuously, a series of GLP-imprinted polymer-based electrochemical sensors was fabricated. Electropolymerized GLP-imprinted p-aminothiophenol metallorganic framework (MOF) films formed on AuNP surfaces determined GLP with a 0.8 pg/L LOQ.91 The LOD of 1 pM was determined using gravimetric-electrochemical GLP-selective polypyrrole (PPy) MIP films.95 Similar sensing efficacy (0.27 ng/mL) was demonstrated for an (Au-electrode)-deposited PPy-MIP sensor for GLP.102 Furthermore, in a recent study, a gold chip/electrode coated with a self-assembled monolayer of 11-(1H-pyrrol-1-yl) undecane-1-thiol, and PPy-MIPs, was exploited to determine GLP using CV, EIS, and electrochemical SPR analyses. Values of the dissociation constant, Kd, and free Gibbs energy change, ΔG0, accompanying the interaction of GLP with MIP-PPy were determined as Kd = 38.18 (±2) × 10–5 and ΔG0 = −19.51 (±0.2) kJ/mol.143 In a similar study, a graphite SPCE modified with a dual-MIP coated on an amino-mesoporous SiNPs-PtNPs core was used for DPV determination of paraquat and GLP. The herbicide-selective MIPs were fabricated by using a 3D-surface imprinting strategy that increased the conductivity and monodispersity of the sensor. A dual-MIP was fabricated by 3D-printing on the surface of mesoporous SiPtNPs, using acrylic acid and N-vinyl-2-pyrrolidone as functional monomers, and N,N′-(1,2-dihydroxyethylene) bis(acrylamide) (DHEBA) as the cross-linking monomer. The sensors allowed for simultaneous determination of both herbicides, paraquat, and GLP, in water samples in the LDCR of 0.025–500 μM and with the LOD of 3.1 nM and 4 nM, respectively.144
Figure 3.
Field deployable ElectrochemSENSE Platform for GLP sensing. (A) An ElectrochemSENSE sensor strip. (B) Solid–liquid interface contact angle generated by phosphate-buffered saline on a sensor substrate. (C) Scheme and (D) FT-IR spectra representing the sensor electrochemical immunoassay chemistry. (E) Immunochromatographic test results displaying a faint test line (GLP-free, green) and the absence of a test line for a 100 ppb GLP sample (red). (F) A scheme depicting the functionality of the ElectrochemSENSE Platform. (G) Custom electronic platform variant to connect to a personal computer for data acquisition and processing. (H) Custom electronic platform variant for determining the produced SAFE/UNSAFE response. Adapted with permission from ref (130). Copyright 2020 Elsevier.
A typical base-catalyzed sol–gel transition incorporation was performed to incorporate GLP and graphene QD labels into mesoporous organosilica MIPs. The GLP-induced quenching-mediated detection enabled GLP quantification at a subnanomolar level (0.017 ppb, 17 pg/mL).94 GLP was determined with the 0.35 ng/L LOD using a pencil graphite electrode dip-coated with AuNPs, then modified with the fabrication of glyphosate-glufosinate double-template MIP using atom transfer radical polymerization in the presence of templates and MWCNTs.96 Finally, GLP was ultrasensitively (the LOD of 92 ng/mL) determined by DPV using a composite of urchin-like AuNPs, Prussian Blue, and PPy-based GLP-MIPs, deposited by electropolymerization on the indium–tin oxide electrode.100
Electrochemical sensors for GLP are advantageous because of their excellent reproducibility and accuracy as well as high selectivity and sensitivity with a linear output among others. However, these features may deteriorate over time, as extended exposure to the target analyte usually shortens or limits the sensor lifetime, especially at variable temperatures. Once incorporated into the smart sensor, this device would require temperature compensation, which exploits the battery energy losses. Regarding sensitivity enhancement, biomimetic chemistry tools will measure the subfemtomolar GLP concentrations. Expectedly, the AChE-based nanozymes, containing MOFs and transient metal complexes, will be used to construct future sensors for GLP. In conclusion, although only a few examples of GLP-sensitive electrochemical smartphone-assisted sensors of subnanomolar LOD have so far been demonstrated, fabricating such tools is highly expected in the near future. For example, an (yttrium ferrite garnet)-embedded (graphitic carbon nitride)-based electrochemical POC sensor, integrated with a smartphone, was recently reported to determine pesticide mesotrione in food with the LOD of 0.95 nM.189
3.4. (Atomic Force Microscopy)-Based Sensors for GLP
Despite extreme usefulness, robustness, and sensitivity, atomic force microscopy (AFM) has hardly been used as a sensing tool. The main reasons included dimensions and the complicated curvature of the AFM tips. Recently, tip functionalization with various species has been optimized so that AFM has become an efficient technique for quantitatively determining chemicals, including herbicides and pesticides. Recently, atomic force spectroscopy, an AFM-derived technique, has been exploited to analyze imazaquin, metsulfuron-methyl, and atrazine samples using tips functionalized with the acetolactate synthase and antiatrazine antibody. This tip functionalization increased markedly (over 130, 140, and 175%, respectively) the adhesion force between the functionalized tips and the herbicides, distinguishing nonspecific and specific interactions between the tip-located biomolecules and the herbicides quantitatively.190
Regarding AFM-assisted GLP determination, a peroxidase-based AFM nanobiosensor was devised to evaluate GLP content in 0.01 to 10 mg/mL in zucchini extracts. This biosensor for GLP modus operandi relied on detecting GLP-induced changes in surface tension caused by GLP adsorption, followed by a conformational change in the peroxidase structure. The LOD was as low as 0.0238 mg/L.145
Low sensitivity, long scanning time, limited spatial resolution, and tip damage, i.e., significant drawbacks of AFM sensors, are expected to be addressed in the future. The construction of tuning-fork-balanced tips shall enable chiplike probing of biological and soft material samples. Applying tip-synchronized time-resolved electrostatic force microscopy will also allow for monitoring charge generation, transfer, and recombination, which is crucial for ultrasensitive enzymatic GLP determination in real samples.
3.5. Immunoassays and Immunosensors for GLP
Immunosensors are (affinity-ligand)-based sensing tools exploiting immunochemical antibody–antigen interactions. These interactions are quantified by transducers with immobilized antibodies. Over decades, the use of immunosensors, both labeled and nonlabeled, has become highly trending, as they comprise (single molecule)-operating sensing tools for food safety control, healthcare, and environmental monitoring.191 Usually, forming an immunocomplex with an analyte generates electrochemical, photochemical, and piezoelectric changes, contributing to multimodal and sensitive detection.192 Herbicides and pesticides belong to typical immunosensor analytes, as they are easily complexed by peptides and enzymes immobilized on the transducer surface. The affinity-based reactions resemble physiological and biochemical reactions because these analytes act as natural ligands.193 Besides, using MIPs, well-known as “plastic antibodies,” “semisynthetic enzymes,” and “artificial receptors,”194 enables low-cost pesticide residue determination in foods, feeds, medicines, and environmental samples.195 Various immunosensors have been constructed for GLP sensing. These include antibody- and aptamer-based immuno-assisted electrochemical and optical sensors.54 Most recent examples report conventional immunochemical methods for GLP determination using enzyme-linked immunosorbent assay (ELISA), which provides submicromolar LODs in liquids and animal feeds.147,150 The ELISA combination with an online SPE, followed by HPLC–MS analysis, enabled the GLP determination with the subnanomolar LOD.148 Advanced immunoassays utilize an immobilized GLP-ovalbumin conjugate and avian IgY antibodies for sensing ppb traces of GLP149 or an AuNP oligonucleotide-based biobarcode immuno-(polymerase chain reaction) (PCR) system with the LOD of 4.5 pg/g146 (Figure 4).
Figure 4.
Oligonucleotide-functionalized AuNP probe-based biobarcode immuno-PCR sensor for GLP. Characterization of the AuNP probe by (A) (sodium dodecyl sulfate)-(polyacrylamide gel) electrophoresis, SDS-PAGE, quantification (lanes 4–8: antibody at 0.49, 0.98, 1.95, and 7.81 μg, respectively) and (B) immunoassay using anti-GLP antibodies. (C) Amplification curves of real-time PCR and (D) the standard curve acquired for DNA samples in the 1 fM to 0.01 μM concentration range. (E) Amplification curves of real-time PCR and (F) the standard curve for the sensor using aqueous GLP solutions. (G) Melt curves of real-time PCR and (H–K) sensor stability over 30 days upon determining at 0, 0.5, 5, and 50 ng/mL GLP. Adapted with permission from ref (146). Copyright 2021 Elsevier.
Although contemporary immunosensors for GLP provide ultrasensitive determination, future trends promise the construction of sensors of higher standards. Because of the high production cost of contemporary biologically derived antibodies and enzymes, future immunosensors will be constructed from single atom/molecule or peptoid/enzyme mimetic catalysts containing single noble metal NCs or MOF nanozymes as recognition units. Since immunodetection of various environmentally emerging pesticide residuals can be executed with extremely low LODs (<pM),196 it is expected that the application of AI-excelled tools shall upgrade these values. As it was extensively summarized elsewhere,197 several smartphone-assisted immunosensors were so far devised for determining various biomedically or environmentally relevant analytes, including small molecules, macromolecules, viruses, and bacteria.
3.6. Microfluidic Lab-on-Chips for GLP
In recent decades, enormous progress has been made in devising microfluidic-assisted conventional and smart sensors. Technological progress enables microfabricating and miniaturizing microfluidic paper-based analytical devices (μPADs)198 and devising smartphone-coopted sensors operating in a microfluidic mode.199 In a traditional design, μPADs are constructed from conventional dipstick or lateral-flow setups. Once coupled with electrochemical immunosensors and LOCs, these eco-friendly devices have become onsite quantitative and semiqualitative equipment for POC medical diagnostics, food safety control, and environmental purity inspection. Particularly, microfluidic-assisted analytical devices enable direct, low-cost, sensitive, and real-time screening of chemical hazards or pathogens, including metal ions, nitrates and nitrites, phenols, pesticides and herbicides, and bacteria.200−203 Thanks to an impressive advance in biotechnological sciences, sophisticated living-cell-based microfluidic-handled biosensors have also been devised. They are mainly inspired by conventional in vitro bioassays, including the bacterial luminescence toxicity screen and the algal toxicity test by imaging pulse amplitude modulated fluorometry, which was demonstrated useful for pesticide determination in environmental samples.204 In the most advanced approach, algae-based biosensors sensitively, sustainably, and multiplexed analyzed agro-environmental samples. In these biosensors, whole algal cells and their photosynthetic complexes were used as miniaturized transducers to construct biomicrofluidic devices.205
Modern microfluidic-handled LOC sensors combine different methods, e.g., electrophoresis-coupled disposable microchips with laser-induced fluorescence detection for the rapid on-site interference-free determination of GLP residues in agricultural products, with the LOD of 0.34 μg/L and 84% recovery.152,153 (Direct injection)-UPLC with triple quadrupole MS was optimized to determine GLP traces in environmental waters with the LOD range of 0.05–0.09 μg/L and 76.3% recovery.151 Moreover, Mn-ZnS QD-embedded MIP, combined with a 3D-μPAD sensor, was fabricated for colorimetric-catalytic determination of GLP. Selective recognition of GLP by the poly(N-isopropylacrylamide) (PNIPAM)- and N,N′-methylenebis(acrylamide) (MBA)-based MIPs, formulated by thermally induced FRP, enabled selective GLP determination in whole grain samples with 0.002 μg/mL LOD98 (Figure 5A-C). Finally, an electrochemical LOC-assisted commercial MIP-based sensor for GLP was devised. It enabled rapid, online, and real-time determination of GLP in tap water with the LOD of 247 nM99 (Figure 5D-H).
Figure 5.
Multimodal microfluidic devices for GLP determination. (A) Colorimetric determination of GLP using Mn–ZnS QD-embedded MIPs combined with a 3D-μPAD, using a foldable sheet comprising three parts (top/center/bottom). (B) UV absorption spectra and (C) GLP calibration plot constructed based on the colorimetric determination of 0, 0.01, 0.05, 3, 5, 10, 20, 30 μg/mL GLP. Adapted with permission from ref (98). Copyright 2021 Elsevier. (D) The device and (E) cross-sectional view of the microfluidic MIP-based sensor with electrodeposited Au working electrodes and a poly(methyl methacrylate) flow cell with inlet and outlet channels. (F) Calibration curves and (G) continuous, reversible, and reproducible microfluidic chip-based determination of GLP. (H) A consistent (∼70%) electrochemical recovery of GLP from different MIP-based concentrators. Adapted with permission from ref (99). Copyright 2021 The American Chemical Society.
The next steps in devising future GLP-selective microfluidic-assisted sensors involve increasing sensitivity and integrating these sensors with AI tools including smartphones and mobile meters. These sensors will enable on-site ultrasensitive determination of GLP in foods, industrial waters, and biological fluids. Based on an ELISA assessment, such a tool has already been devised for on-site quantifying ppb (μg/kg) levels of aflatoxin B1 in moldy corn. The immunoassay sensor was 3D-printed on a plastic chip attachable to a smartphone.206 Similar sensitivity was achieved for various pesticides using a portable, smartphone-adaptable origami μPAD-based potentiostat. Relying on the pesticide-inhibited activity of the enzyme immobilized on the sensor’s transducing item allowed for chronoamperometric monitoring of paraoxon, 2,4-dichlorophenoxyacetic acid, and atrazine at a ppb level in river water samples.207
3.7. Smart Sensors and Plant Wearables for GLP
The necessity of developing the PW pest-, insect-, fungi-, and herbicide-specific sensing and delivery systems is crucial because of the off-target toxicity of these biocides. Among heavy metals, antibiotics and other drugs, food-derived growth factors, and industrial hydrocarbon wastes, these biocides are the most toxic environmental pollutants and hazards to human health.3 Because of various leakage from the target zone to the rhizosphere, waters, and air, relatively high half-time biocides destabilize the trophic chain’s matter cycle and endanger ecosystem safety.208−210 Thus, enormous progress has been made in AI-enhanced mobile or PW biocide-selective sensors.3,18 Miniaturized smart sensors, incorporated, e.g., in smartphones, PWs, and POCs or field-deployable devices, are equipped with nanophotonic antennas and dielectric metasurfaces that enable few-molecule sensitivity by confining incident light into intense hotspots of the electromagnetic fields, thus delivering strongly enhanced light-matter interactions.211 Regarding GLP sensing, an in-smartphone-incorporated mobile lab-in-a-syringe platform was designed for the rapid, visual, quantitative determination of organophosphorus pesticides via dual-mode colorimetry and fluorescence measurements. The platform was based on (cetyltrimethylammonium bromide)-coated NPs conjugated with a silica pad modified with red- and green-emission QDs. In the sensing reaction, thiocholine, the product of AChE-mediated hydrolysis of thioacetylcholine, induced the aggregation of NPs, thus giving rise to the color change. During the on-site GLP determination, the pesticide-caused enzymatic AChE inhibition changed the platform’s colorimetric and fluorescence properties, thus providing the signal output in the LDCR of 0 to 10 μM with the LOD of 2.81 nM154 (Figure 6). A similar study developed a smartphone-dedicated kit test for selective GLP determination based on the Cu(II)-pyrocatechol violet complex. The kit sensed 20 μM GLP in tap water by the “naked eye” test, quantifying GLP with the LOD of 2.66 μM.155 Moreover, a novel enzyme-free visual ratiometric fluorescence paper sensor for GLP was devised by assembling blue CDs and AuNCs and incorporating them into a portable smartphone platform. GLP sensing relied on the quick (2 s) GLP-induced quenching of CD fluorescence originating from CD-GLP complex formation. The sensor allowed instant GLP determination in real samples with the exquisite LOD of 4.19 nM in the broad LDCR of 0–190 nM.156 Furthermore, in a similar study, a diverse (thiocholine-AuNPs)-based colorimetric smartphone-assisted sensor was devised to detect eight pesticides, including GLP, thiram, imidacloprid, tribenuron methyl, nicosulfuron, thiofensulfuron methyl, dichlorprop, and fenoprop. The modus operandi of the sensors relied on the pesticide-induced inhibition of AChE-mediated hydrolysis of the Au–S covalent bond in the thiocholine-AuNPs complex. This bond cleavage resulted in the RGB-valued color change associated with the alteration of the SPR properties of AuNPs. All pesticides were determined in real samples of fruits, vegetables, and traditional Chinese herbs, with the LOD below 0.15 μM, thus satisfying the established specification of the U.S. EPA, noted as ∼3.91 μM, and demonstrating the sensor’s applicability.157 Finally, a smartphone-integrated triple-mode SERS/fluorimetric/colorimetric sensor for GLP was devised based on flowerlike Zn MOFs and the HRP activity-mimicking H(2) L ligand. Unique properties of this ligand, including the enzymatic catalysis and Cu2+-sensitive fluorescence, were used to detect GLP in a florescence-quenching manner based on the Cu2+ chelation by GLP. This inhibiting interaction decreased both the catalytic activity of the H(2) L ligand and the optical and plasmonic properties of the nanocomposite, thus allowing for quantitative fluorescent/colorimetric/SERS determination of GLP with the LOD of 0.738 (0–0.12 μM LDCR), 2.26 nM (0–220 nM LDCR), and 0.186 nM (0.5–12 nM LDCR), respectively. Moreover, the sensor was integrated with a portable (test strips)-smartphone sensing platform dedicated to POC testing GLP in food samples.158
Figure 6.
A smartphone-integrated lab-in-a-syringe device for the colorimetric and fluorescent on-site determination of GLP. (A) Colorimetric and (B) fluorescent imaging of rQDs-SiO2-gQDs+CTAB-AuNPs+AChE+ATch under daylight in 0 to 10 μM GLP. (C) RGB analysis of the fluorescence image using a smartphone color identifier application. (D) The ratio of green-to-red channel values of colorimetric and (E) fluorescent images in dependence on 0 to 10 μM GLP concentration. The respective insets depict plots of the ratios of green-to-red channel values versus 0–10 μM GLP concentration. Adapted with permission from ref (154). Copyright 2021 American Chemical Society.
The extremely high technological advancement of the presented pioneer examples of selective and sensitive SM-based sensors sets the direction of action in analytical chemistry. Expectedly, the multimodal smartphone-based sensors will equip the agronomic and POC laboratories, providing automated, computerized, and high-throughput toxin determination, despite the relatively high production and maintenance costs. In the near future, smartphone-augmented sensors will be commonly used as components of nanobionic PW sensors. For example, PWs based on thin nanofilm electronics, such as SWCNTs field-effect transistors, can now detect dimethyl methylphosphonate, a volatile nerve agent, at the ppm level.212 Moreover, the flexible, stretchable, and adhesive PW sensor, fabricated by direct writing of chitosan-based inks, was active toward plant mechanical injury-responsive healing and sensed the intoxication with methyl parathion and nitrites.213
3.8. Robotic Devices for GLP Delivery
The GLP-resistant weeds’ evolution in the farmlands forced scientists and farmers to develop novel GLP-free technologies for modern weed management.13,15 Among the development of transgenic crops displaying resistance to auxinic herbicides and herbicides displaying inhibitory activity against acetolactate synthase, acetyl-CoA carboxylase, hydroxyphenylpyruvate dioxygenase or bioherbicides, and sprayable herbicidal ribonucleic acid interference (RNAi) agents, the nonbiochemical solutions have been considered as well.13,214 Aside from SM AI-enhanced sensors, these innovations involve soft terrestrial robotics that will give rise to robotic weeding in the nearest future.13 In 2022, the global agricultural robots market, including unmanned aerial vehicles (UAV), drones, automated harvesting system drones, and driverless tractors, was estimated to be worth 5.9 million USD and is anticipated to reach a forecast value of 30.5 million USD by 2032 (https://www.futuremarketinsights.com/reports/agriculture-robotics-market, access on 21.05.2023).
Regarding GLP-oriented systems, a robot-based herbicide delivery system is a more advanced system for in-row weed control purposes. The robot was tailored to facilitate systematic and site-specific delivery of GLP and iodosulfuron droplets to four different weed species in a drop-on-demand manner without affecting carrot crops. The droplets were selectively sprayed on the leaves of the detected weeds within the plant row. Iodosulfuron, a sulfonylurea-based herbicidal inhibitor of acetolactate synthase, was used as an additive herbicide to control the species that GLP insufficiently controls. In indoor pot trials, amounts of 7.6 μg of GLP and 0.15 μg of iodosulfuron per droplet per plant were delivered, whereas in a field trial with the robot system involved, this amount was 5.3 μg of GLP per droplet. Moreover, the GLP amount was 10 times lower than GLP amount used in conventional crop spraying.159 In these in-field trials, the presented three-wheeled robot GLP delivery system displayed promising properties, including relatively low cost, maintainability, maneuverability, stability, and robustness, when compared to other commercial agricultural robots.215,216 (Figure 7). However, it would be required to perform quantitative environmental impact assessments using a comparative life cycle assessment of intra- and inter-row weeding management to claim its superior usefulness.217 Furthermore, worth mentioning the costs of devising and exploiting robotic GLP sensors/delivery systems, including costs of navigation hardware, machine vision technologies, and power consumption,216 are usually higher than those of conventional broadcast GLP analytical techniques, which must be considered when designing experiments or applying to industry. Not disregarding these cost limitations, in the long term, such robotic-based delivery systems may become a promising alternative for dosing a well-defined and strictly controlled amount of pesticides to the crops, thus reducing the risk of environmental contamination.
Figure 7.
Robots used for in-row weed control, in-field detection, and site-targeted delivery of herbicides. Adapted with permission from ref (159). Copyright 2018 Elsevier.
Robotics has become fundamental for constructing remote sensors (RSs) for GLP. They have been devised and implemented to monitor the agricultural and environmental quality of soil, crops, and water status. In particular, these tools have been applied to solve major issues of long-term herbicide-resistant weed management (HRWM).36,37 In the first step of modern HRWM, remote sensing is commonly implemented to detect and identify infested crops, followed by site-specific herbicide treatment of the weeds, mostly in their germination stage.218 Besides, RSs can be used for the man-free determination of herbicide-sprayed weeds and crops to eradicate the former or improve the latter’s growth using variable-rate technology (VRT).219−224
4. Future Prospective
Environment-protective farming (also called organic farming) is a goal of sustainable agriculture.225 Agriculture sustainability prevents soil degradation based on economic productivity, future crop yield maintenance, limited usage of agrochemicals, and eco-friendly integrated pest management and weed control. It ensures the health of the soil, air, water, livestock, animal, and humans.226 Concerning the latter, AI-excelled PA is expected to provide tools that enable rapid, mobile, and man-free delivery or detect harmful agrochemicals or biomaterials such as fertilizers and biocides. The AI technology aims to handle weed infestations because of their devastating role in crop cultivation by competing for water, nutrients, light, agricultural space, and air gases and by secreting potentially toxic exudates.227,228 Because weeds share the same zone and spread among crops, it is incredibly challenging to exterminate them without harming the crops. Besides, spectral images of herbicide-sprayed weeds and crops in early developmental stages are similar, which impedes proper visualization, recognition, and site-specific eradication.229−231 Nevertheless, AI-enhanced site-specific weed management, including weed patch mapping, will be possible.20,232 It shall allow for the application of nanoformulated herbicides in geospatially determined, stimuli-responsive, and dose-dependent manners, thus inhibiting weeds’ seed germination and decreasing weed biomass.218,233 Furthermore, tackling weed infestations can be addressed by UAV-handling of farmlands stricken with drought, air pollution, or heat caused by local climate changes that have been more frequent in the last ten years compared to previous decades.234
The limitation of the GBHs and agrochemical use was the founding myth of environment-protective farming, ecology, and weed science.225 Recent years’ technological advancement has displaced a previously in-force “many little hammers” approach based on the extensive labor and equipment-consuming field works.235 Nowadays, AI PA has been dominated by “digital farming,” providing the tool for intelligent and site-specific monitoring and improving crop protection. For instance, the controlled use of GBHs in recent years was associated with no-tillage agricultural systems, thus reducing their inevitable deficiencies, including soil degradation and high expenses. Thus, the current ban on GLP and GBH shall force farmers to return to conventional tillage systems as an effective weed management strategy for keeping crop-weed balance. So far, a series of no-tillage and no-GBH strategies have been developed to reduce the over-reliance on a few single modes of action herbicides, competitiveness in weeds, and dependency on herbicides, while enhancing the strategies of crop diversification, harvest weed seed control, and precision agriculture approaches, including RNAi technology, gene editing, robotics, and in-site or remote sensing.235
Expectedly, future devising of safe-by-design sensors for herbicides will mainly exploit AI tools of nanoinformatics,236−239 ML,240−243 and DL.18,244−247 Nanoinformatics-enhanced drug/sensor devising uses predictive risk assessment frameworks, including integrated approaches to testing and assessment for the in silico prediction, design, and optimization of the parameters of nanomaterials used as sensing components or delivery systems according to their agricultural destination.18,248 These parameters refer to structure and functionality as well as biocompatibility, stability, biodistribution, and transgenerational phytotoxicity of the material, thus providing prediction and translation of the long-term responses of the agroecosystem to current conditions, including efficient high or low doses of the agrochemical efficiently. This strategy is particularly promising because current experimental studies on nanomaterial toxicity require short-term exposures to relatively high doses of the agrochemical.18 Particularly, nanoinformatics models combine chemo- and bioinformatics tools with omics databases and in vitro, ex vivo, in vivo, and clinical data of the active agents and target or nontarget species, allowing for the de novo design of ActI or nanomaterials.237 The integrated web repositories and database were originally created using the data of eight model plant species, i.e., Arabidopsis thaliana, Oryza sativa, Solanum lycopersicum, Sorghum bicolor, Vitis vinifera, Solanum tuberosum, Medicago truncatula, and Glycine max. They enabled the founding of interspecies and intermolecular interaction networks such as Plant Omics Center, Plant Secretome, and Subcellular Proteome KnowledgeBase. Associated with analogic databases concerning microbiota, agrochemicals (PEST-CHEMGRIDS, Pesticide Properties Database, Pesticide Target Interaction Database, PubChem), hydroclimatic indicators, and socioeconomic and anthropogenic inputs, these nanoinformatics tools facilitate comprehensive and predictive determination of molecules, species, and plant diseases at every possible level.37,249−251 Regarding the nanoinformatics-based GLP sensing, the site-targeted performance of the future smart sensor shall be applied to the GLP-sprayed areas to determine GLP in the chemical environment of natural species.
The ML and DL methods have already been applied to crop sensing and phenotyping at all scales, including lands, fields, canopies, and leaves.240−243 Thus, presumably, these powerful computing tools, incorporated in the future smart sensors, will be easily harnessed to predict tripartite (e.g., “agrochemical-soil-plant,” “pathogen-treatment-plant”) interactions under variable climate conditions.18,244−247 The DL-enhanced algorithms will process the object and spectral data regarding the images’ numbers, types, sizes, and resolutions by grouping proximal pixels (of a few millimeters or centimeters) with homogeneous spectral value, combining them spectrally, topologically, and contextually.252 Upon spatiotemporal evaluation of the vegetation indices, including temperature, biomass, moisture, weed infestation, and nitrogen balance index (chlorophyll-to-polyphenol ratio) of these objects, the smart sensors shall identify the nature of the stress and apply proper fertilizers, agrochemicals, and irrigation waters in a site-specific manner using VRT-based equipment.253 In effect, the computational tool-excelled smart sensors will acquire and process massive amounts of data on “what,” “how,” “where,” and “when” the human intervention should be applied to address the most pressing needs, thus reducing the consumption of herbicides, such as GLP, to an absolute minimum.244−247
Acknowledgments
The National Centre for Research and Development of Poland financially supported the present research (Grant No. PhotonicSensing/1/2018 to W.K.).
Glossary
List of abbreviations
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- ActI
Active ingredient
- AFM
Atomic force microscopy
- AgNC
Silver nanocluster
- AgNP
Silver nanoparticle
- AHS
Agricultural Health Study
- AI
Artificial intelligence
- AMPA
(Aminomethyl) phosphonic acid
- ATU
1-Allyl-2-thiourea
- AuNC
Gold nanocluster
- AuNP
Gold nanoparticle
- BF
Binding factor
- CD
Carbon dot
- CNT
Carbon nanotube
- COVID-19
Coronavirus disease
- CTAB
Cetyltrimethylammonium bromide
- CV
Cyclic voltammetry
- DA
Dopamine
- DAD
Diode array detector
- DHEBA
N,N′-(1,2-Dihydroxyethylene) bis(acrylamide)
- DHP
Direct hemoperfusion
- DL
Deep learning
- DMAEM
2-Dimethyl aminoethyl methacrylate
- DNA
Deoxyribonucleic acid
- DPPC-Cl
3,6-Dimethoxy-9-phenyl-9H-carbazole-1-sulfonyl chloride
- ECETOC
European Centre for Ecotoxicology and Toxicology of Chemicals
- EChA
European Chemical Agency
- ECL
Electrochemiluminescence
- EFSA
European Food Safety Authority
- EGDMA
Ethylene glycol dimethacrylate
- EIS
Electrochemical impedance spectroscopy
- ELISA
Enzyme-linked immunosorbent assay
- ENM
Engineered nanomaterial
- EPA
Environmental Protection Agency
- EPSPS
5-Enolpyruvynyl-shikimate-3-phosphate synthase
- ESI-MS
Electrospray ionization mass spectrometry
- FAO
Food and Agriculture Organization
- FLD
Fluorescence detector
- FMOC
Fluorenylmethyloxycarbonyl (derivative)
- FRET
Förster resonance energy transfer
- FRP
Free radical polymerization
- GBH
Glyphosate-based herbicide
- GC
Gas chromatography
- GCE
Glassy carbon electrode
- GC-EIMS
Gas chromatography coupled to electron ionization mass spectrometry
- GE
Genetically engineered (organism)
- GLP
Glyphosate
- GLP-IPA
Glyphosate isopropylamine
- GLP-SH
Glyphosate surfactant
- GMO
Genetically modified organism
- GO
Graphene oxide
- HPLC
High-performance liquid chromatography
- HRMS
High-resolution mass spectrometry
- HRP
Horseradish peroxidase
- HRWM
Herbicide-resistant weed management
- ICP-MS
Inductively coupled plasma mass spectrometry
- Ig
Immunoglobulin
- IPA
Isopropylamine
- IRMS
Isotope-ratio mass spectrometry
- ISPAg
International Society of Precision Agriculture
- LC
Liquid chromatography
- LC–IRMS
Liquid chromatography coupled with isotope ratio mass spectrometry
- LDCR
Linear dynamic concentration range
- LOC
Lab-on-a-chip
- LOD
Limit of detection
- LOQ
Limit of quantification
- MAA
Methacrylic acid
- MBA
N,N′-Methylenebis(acrylamide)
- MIP
Molecularly imprinted polymer
- ML
Machine learning
- MOF
Metal–organic framework
- MS
Mass spectrometry
- MWCNT
Multiwalled carbon nanotube
- NC
Nanocluster
- NFC
Near-field communication
- NIR
Near-infrared (light)
- NMDA
N-Methyl-d-aspartate
- NT
Nanotube
- OP
Organophosphate
- OVA
Ovalbumin
- PA
Precision agriculture
- PAA
Polyacrylic acid
- μPAD
Microfluidic paper-based analytical device
- PANI
Polyaniline
- PCR
Polymerase chain reaction
- PDA
Polydopamine
- PEG
Poly(ethylene) glycol
- PNIPAM
Poly(N-isopropylacrylamide)
- POC
Point-of-care
- POEA
Polyoxyethyleneamine
- PPy
Polypyrrole
- PVC
Poly(vinyl chloride)
- PW
Plant wearable
- QCM
Quartz crystal microbalance
- QD
Quantum dot
- RGB
Red-green-blue (analysis)
- rGO
Reduced graphene oxide
- RNAi
Ribonucleic acid interference
- RS
Remote sensor
- SCP
Soft colloidal particle
- SDS-PAGE
(Sodium dodecyl sulfate)-(polyacrylamide gel electrophoresis)
- SERS
Surface-enhanced Raman spectroscopy
- SiNP
Silica nanoparticle
- SM
Smart material
- SPCE
Screen-printed carbon electrode
- SPE
Solid-phase extraction
- SPR
Surface plasmon resonance
- SWCNT
Single-walled carbon nanotube
- TLC
Thin-layer chromatography
- UAV
Unmanned aerial vehicle
- UPLC
Ultraperformance liquid chromatography
- UPLC-Q-ToF-HRMS/MS
Ultraperformance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry
- UV
Ultraviolet (light)
- VRT
Variable-rate technology
- WHO
World Health Organization
- WSN
Wireless sensor network
- ZSM
Zeolite Socony Mobil
Author Contributions
J.M.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - Original draft preparation, Visualization. K.K.: Formal Analysis, Visualization, Investigation. W.K.: Resources, Writing- Reviewing and Editing, Supervision, Project administration, Funding acquisition. P.S.S.: Resources, Writing - Reviewing and Editing, Supervision.
The authors declare no competing financial interest.
References
- The future of food and agriculture - Alternative pathways to 2050; Food and Agriculture Organization of the United Nations: Rome, 2018. [Google Scholar]
- van Dijk M.; Morley T.; Rau M. L.; Saghai Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. 10.1038/s43016-021-00322-9. [DOI] [PubMed] [Google Scholar]
- Yin H.; Cao Y.; Marelli B.; Zeng X.; Mason A. J.; Cao C. Soil Sensors and Plant Wearables for Smart and Precision Agriculture. Adv. Mater. 2021, 33, e2007764 10.1002/adma.202007764. [DOI] [PubMed] [Google Scholar]
- Sridhar A.; Balakrishnan A.; Jacob M. M.; Sillanpaa M.; Dayanandan N. Global impact of COVID-19 on agriculture: role of sustainable agriculture and digital farming. Environ. Sci. Pollut. Res. Int. 2023, 30, 42509. 10.1007/s11356-022-19358-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson L. M.; Pourzahedi L.; Laughton S.; Gao X.; Zimmerman J. B.; Theis T. L.; Westerhoff P.; Lowry G. V. Guiding the design space for nanotechnology to advance sustainable crop production. Nat. Nanotechnol. 2020, 15, 801–810. 10.1038/s41565-020-0706-5. [DOI] [PubMed] [Google Scholar]
- World fertilizer trends and outlook to 2020; Food and Agriculture Organization of the United States of America: Rome, 2017. [Google Scholar]
- Pesticides Industry Sales and Usage. Market Estimates; United States Environmental Protection Agency, 2017.
- Lassaletta L.; Billen G.; Grizzetti B.; Anglade J.; Garnier J. 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. 10.1088/1748-9326/9/10/105011. [DOI] [Google Scholar]
- Roberts T. L.; Johnston A. E. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. 10.1016/j.resconrec.2015.09.013. [DOI] [Google Scholar]
- Springmann M.; Clark M.; Mason-D’Croz D.; Wiebe K.; Bodirsky B. L.; Lassaletta L.; de Vries W.; Vermeulen S. J.; Herrero M.; Carlson K. M.; Jonell M.; Troell M.; DeClerck F.; Gordon L. J.; Zurayk R.; Scarborough P.; Rayner M.; Loken B.; Fanzo J.; Godfray H. C. J.; Tilman D.; Rockstrom J.; Willett W. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. 10.1038/s41586-018-0594-0. [DOI] [PubMed] [Google Scholar]
- Shahzad A. N.; Qureshi M. K.; Wakeel A.; Misselbrook T. Crop production in Pakistan and low nitrogen use efficiencies. Nat. Sustain. 2019, 2, 1106–1114. 10.1038/s41893-019-0429-5. [DOI] [Google Scholar]
- Bailey-Serres J.; Parker J. E.; Ainsworth E. A.; Oldroyd G. E. D.; Schroeder J. I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. 10.1038/s41586-019-1679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. O. Perspectives on transgenic, herbicide-resistant crops in the United States almost 20 years after introduction. Pest. Manag. Sci. 2015, 71, 652–657. 10.1002/ps.3863. [DOI] [PubMed] [Google Scholar]
- Brookes G.; Taheripour F.; Tyner W. E. The contribution of glyphosate to agriculture and potential impact of restrictions on use at the global level. GM Crops Food 2017, 8, 216–228. 10.1080/21645698.2017.1390637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Mahajan G.; Dahiya S.; Chauhan B. S. Challenges and Opportunities for Weed Management in No-Till Farming Systems. No-Till Farming Systems for Sustainable Agriculture 2020, 107–125. 10.1007/978-3-030-46409-7_7. [DOI] [Google Scholar]
- Husaini A. M. High-value pleiotropic genes for developing multiple stress-tolerant biofortified crops for 21st-century challenges. Heredity 2022, 128, 460–472. 10.1038/s41437-022-00500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew T. T. S.; Sarojam R.; Jang I. C.; Park B. S.; Naqvi N. I.; Wong M. H.; Singh G. P.; Ram R. J.; Shoseyov O.; Saito K.; Chua N. H.; Strano M. S. Species-independent analytical tools for next-generation agriculture. Nat. Plants 2020, 6, 1408–1417. 10.1038/s41477-020-00808-7. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Guo Z.; Ullah S.; Melagraki G.; Afantitis A.; Lynch I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat. Plants 2021, 7, 864–876. 10.1038/s41477-021-00946-6. [DOI] [PubMed] [Google Scholar]
- International Society of Precision Agriculture Precision Ag Definition. 2021. https://ispag.org/.
- Beckie H. J.; Harker K. N. Our top 10 herbicide-resistant weed management practices. Pest Manag. Sci. 2017, 73, 1045–1052. 10.1002/ps.4543. [DOI] [PubMed] [Google Scholar]
- Soto F.; Karshalev E.; Zhang F.; Esteban Fernandez de Avila B.; Nourhani A.; Wang J. Smart Materials for Microrobots. Chem. Rev. 2022, 122, 5365–5403. 10.1021/acs.chemrev.0c00999. [DOI] [PubMed] [Google Scholar]
- Lowry G. V.; Avellan A.; Gilbertson L. M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. 10.1038/s41565-019-0461-7. [DOI] [PubMed] [Google Scholar]
- Giraldo J. P.; Wu H.; Newkirk G. M.; Kruss S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 2019, 14, 541–553. 10.1038/s41565-019-0470-6. [DOI] [PubMed] [Google Scholar]
- Isozaki A.; Harmon J.; Zhou Y.; Li S.; Nakagawa Y.; Hayashi M.; Mikami H.; Lei C.; Goda K. AI on a chip. Lab Chip 2020, 20, 3074–3090. 10.1039/D0LC00521E. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Guo Z.; Ullah S.; Melagraki G.; Afantitis A.; Lynch I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat. Plants 2021, 7, 864–876. 10.1038/s41477-021-00946-6. [DOI] [PubMed] [Google Scholar]
- Vikesland P. J. Nanosensors for water quality monitoring. Nat. Nanotechnol. 2018, 13, 651–660. 10.1038/s41565-018-0209-9. [DOI] [PubMed] [Google Scholar]
- Gupta S.; Aga D.; Pruden A.; Zhang L.; Vikesland P. Data Analytics for Environmental Science and Engineering Research. Environ. Sci. Technol. 2021, 55, 10895–10907. 10.1021/acs.est.1c01026. [DOI] [PubMed] [Google Scholar]
- Ip R. H. L.; Ang L. M.; Seng K. P.; Broster J. C.; Pratley J. E. Big data and machine learning for crop protection. Comput. Electron. Agric. 2018, 151, 376–383. 10.1016/j.compag.2018.06.008. [DOI] [Google Scholar]
- Wittwehr C.; Blomstedt P.; Gosling J. P.; Peltola T.; Raffael B.; Richarz A. N.; Sienkiewicz M.; Whaley P.; Worth A.; Whelan M. Artificial Intelligence for chemical risk assessment. Comput. Toxicol. 2020, 13, 100114. 10.1016/j.comtox.2019.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendren C. O.; Lowry G. V.; Unrine J. M.; Wiesner M. R. A functional assay-based strategy for nanomaterial risk forecasting. Sci. Total Environ. 2015, 536, 1029–1037. 10.1016/j.scitotenv.2015.06.100. [DOI] [PubMed] [Google Scholar]
- Turner A. A.; Rogers N. M. K.; Geitner N. K.; Wiesner M. R. Nanoparticle affinity for natural soils: a functional assay for determining particle attachment efficiency in complex systems. Environ. Sci. Nano. 2020, 7, 1719–1729. 10.1039/D0EN00019A. [DOI] [Google Scholar]
- Oerke E. C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. 10.1017/S0021859605005708. [DOI] [Google Scholar]
- Motmainna M.; Juraimi A. S.; Uddin M. K.; Asib N. B.; Islam A. K. M. M.; Ahmad-Hamdani M. S.; Berahim Z.; Hasan M. Physiological and Biochemical Responses of Ageratum conyzoides, Oryza sativa f. spontanea (Weedy Rice) and Cyperus iria to Parthenium hysterophorus Methanol Extract. Plants 2021, 10, 1205. 10.3390/plants10061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay M. R.; Freitas D. N.; Mobed-Miremadi M.; Wheeler K. E. Machine learning provides predictive analysis into silver nanoparticle protein corona formation from physicochemical properties. Environ. Sci. Nano. 2018, 5, 64–71. 10.1039/C7EN00466D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motmainna M.; Juraimi A. S. B.; Uddin M. K.; Asib N. B.; Islam A. K. M. M.; Hasan M. Allelopathic potential of Malaysian invasive weed species on Weedy rice (Oryza sativa f. spontanea Roshev). Allelopathy J. 2021, 53, 53–67. 10.26651/allelo.j/2021-53-1-1327. [DOI] [Google Scholar]
- Roslim M. H. M.; Juraimi A. S.; Che’Ya N. N.; Sulaiman N.; Abd Manaf M. N. H.; Ramli Z.; Motmainna M. Using Remote Sensing and an Unmanned Aerial System for Weed Management in Agricultural Crops: A Review. Agronomy 2021, 11, 1809. 10.3390/agronomy11091809. [DOI] [Google Scholar]
- Wang D. S.; Cao W. J.; Zhang F.; Li Z. L.; Xu S.; Wu X. Y. A Review of Deep Learning in Multiscale Agricultural Sensing. Remote Sens-Basel 2022, 14, 559. 10.3390/rs14030559. [DOI] [Google Scholar]
- Zerger A.; Viscarra Rossel R.A.; Swain D.L.; Wark T.; Handcock R.N.; Doerr V.A.J.; Bishop-Hurley G.J.; Doerr E.D.; Gibbons P.G.; Lobsey C. Environmental sensor networks for vegetation, animal and soil sciences. IJAEO 2010, 12, 303–316. 10.1016/j.jag.2010.05.001. [DOI] [Google Scholar]
- Wong M. H.; Giraldo J. P.; Kwak S. Y.; Koman V. B.; Sinclair R.; Lew T. T. S.; Bisker G.; Liu P. W.; Strano M. S. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 2017, 16, 264–272. 10.1038/nmat4771. [DOI] [PubMed] [Google Scholar]
- Huang C. H.; Singh G. P.; Park S. H.; Chua N. H.; Ram R. J.; Park B. S. Early Diagnosis and Management of Nitrogen Deficiency in Plants Utilizing Raman Spectroscopy. Front. Plant Sci. 2020, 11, 663. 10.3389/fpls.2020.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. 10.1007/s13412-018-0517-2. [DOI] [Google Scholar]
- Belbin F. E.; Hall G. J.; Jackson A. B.; Schanschieff F. E.; Archibald G.; Formstone C.; Dodd A. N. Plant circadian rhythms regulate the effectiveness of a glyphosate-based herbicide. Nat. Commun. 2019, 10, 3704. 10.1038/s41467-019-11709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook C. M. Trends in glyphosate herbicide use in the United States and globally. Environmental sciences Europe 2016, 28, 3. 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D.; Balzer C.; Hill J.; Befort B. L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 20260–20264. 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbrunn E.; Eschenburg S.; Shuttleworth W. A.; Schloss J. V.; Amrhein N.; Evans J. N.; Kabsch W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 1376–1380. 10.1073/pnas.98.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke T.; Han H.; Healy-Fried M. L.; Fischer M.; Schonbrunn E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 13010–13015. 10.1073/pnas.0603638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke T.; Yang Y.; Han H.; Healy-Fried M.; Olesen S.; Becker A.; Schonbrunn E. Structural basis of glyphosate resistance resulting from the double mutation Thr97 -> Ile and Pro101 -> Ser in 5-enolpyruvylshikimate-3-phosphate synthase from Escherichia coli. J. Biol. Chem. 2009, 284, 9854–9860. 10.1074/jbc.M809771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander M.; Saloniemi I.; Omacini M.; Druille M.; Salminen J. P.; Saikkonen K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018, 642, 285–291. 10.1016/j.scitotenv.2018.05.377. [DOI] [PubMed] [Google Scholar]
- Smedbol E.; Lucotte M.; Labrecque M.; Lepage L.; Juneau P. Phytoplankton growth and PSII efficiency sensitivity to a glyphosate-based herbicide (Factor 540((R))). Aquat. Toxicol. 2017, 192, 265–273. 10.1016/j.aquatox.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Giesy J. P.; Dobson S.; Solomon K. R. Ecotoxicological risk assessment for Roundup (R) Herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. 10.1007/978-1-4612-1156-3_2. [DOI] [Google Scholar]
- Price B.; Cotter J. The GM Contamination Register: a review of recorded contamination incidents associated with genetically modified organisms (GMOs), 1997–2013. Int. J. Food Contam. 2014, 1, 5. 10.1186/s40550-014-0005-8. [DOI] [Google Scholar]
- Vandenberg L. N.; Blumberg B.; Antoniou M. N.; Benbrook C. M.; Carroll L.; Colborn T.; Everett L. G.; Hansen M.; Landrigan P. J.; Lanphear B. P.; Mesnage R.; vom Saal F. S.; Welshons W. V.; Myers J. P. Is it time to reassess current safety standards for glyphosate-based herbicides?. J. Epidemiol. Community Health 2017, 71, 613–618. 10.1136/jech-2016-208463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattani D.; Cesconetto P. A.; Tavares M. K.; Parisotto E. B.; De Oliveira P. A.; Rieg C. E. H.; Leite M. C.; Prediger R. D. S.; Wendt N. C.; Razzera G.; Filho D. W.; Zamoner A. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017, 387, 67–80. 10.1016/j.tox.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Reynoso E. C.; Torres E.; Bettazzi F.; Palchetti I. Trends and Perspectives in Immunosensors for Determination of Currently-Used Pesticides: The Case of Glyphosate, Organophosphates, and Neonicotinoids. Biosensors 2019, 9, 20. 10.3390/bios9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillezeau C.; van Gerwen M.; Shaffer R. M.; Rana I.; Zhang L.; Sheppard L.; Taioli E. The evidence of human exposure to glyphosate: a review. Environ. Health 2019, 18, 2. 10.1186/s12940-018-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen K. E.; Lifschitz A. L.; Lanusse C. E.; Virkel G. L. The herbicide glyphosate is a weak inhibitor of acetylcholinesterase in rats. Environ. Toxicol. Pharmacol. 2016, 45, 41–44. 10.1016/j.etap.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Kubsad D.; Nilsson E. E.; King S. E.; Sadler-Riggleman I.; Beck D.; Skinner M. K. Assessment of Glyphosate Induced Epigenetic Transgenerational Inheritance of Pathologies and Sperm Epimutations: Generational Toxicology. Sci. Rep. 2019, 9, 6372. 10.1038/s41598-019-42860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnage R.; Defarge N.; Spiroux de Vendomois J.; Seralini G. E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. 10.1016/j.fct.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Rana I.; Shaffer R. M.; Taioli E.; Sheppard L. Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: A meta-analysis and supporting evidence. Mutat. Res. 2019, 781, 186–206. 10.1016/j.mrrev.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk I.; Kovalchuk O.; Hohn B. Biomonitoring the genotoxicity of environmental factors with transgenic plants. Trends Plant Sci. 2001, 6, 306–310. 10.1016/S1360-1385(01)01985-9. [DOI] [PubMed] [Google Scholar]
- Conko G.; Kershen D. L.; Miller H.; Parrott W. A. A risk-based approach to the regulation of genetically engineered organisms. Nat. Biotechnol. 2016, 34, 493–503. 10.1038/nbt.3568. [DOI] [PubMed] [Google Scholar]
- Tsatsakis A. M.; Nawaz M. A.; Kouretas D.; Balias G.; Savolainen K.; Tutelyan V. A.; Golokhvast K. S.; Lee J. D.; Yang S. H.; Chung G. Environmental impacts of genetically modified plants: A review. Environ. Res. 2017, 156, 818–833. 10.1016/j.envres.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Delaney B.; Goodman R. E.; Ladics G. S. Food and Feed Safety of Genetically Engineered Food Crops. Toxicol. Sci. 2018, 162, 361–371. 10.1093/toxsci/kfx249. [DOI] [PubMed] [Google Scholar]
- Bradberry S. M.; Proudfoot A. T.; Vale J. A. Glyphosate poisoning. Toxicol. Rev. 2004, 23, 159–167. 10.2165/00139709-200423030-00003. [DOI] [PubMed] [Google Scholar]
- Nerozzi C.; Recuero S.; Galeati G.; Bucci D.; Spinaci M.; Yeste M. Effects of Roundup and its main component, glyphosate, upon mammalian sperm function and survival. Sci. Rep. 2020, 10, 11026. 10.1038/s41598-020-67538-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gress S.; Lemoine S.; Seralini G. E.; Puddu P. E. Glyphosate-based herbicides potently affect cardiovascular system in mammals: review of the literature. Cardiovasc. Toxicol. 2015, 15, 117–126. 10.1007/s12012-014-9282-y. [DOI] [PubMed] [Google Scholar]
- de Liz Oliveira Cavalli V. L.; Cattani D.; Heinz Rieg C. E.; Pierozan P.; Zanatta L.; Benedetti Parisotto E.; Wilhelm Filho D.; Mena Barreto Silva F. R.; Pessoa-Pureur R.; Zamoner A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013, 65, 335–346. 10.1016/j.freeradbiomed.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Mesnage R.; Bernay B.; Seralini G. E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Sribanditmongkol P.; Jutavijittum P.; Pongraveevongsa P.; Wunnapuk K.; Durongkadech P. Pathological and toxicological findings in glyphosate-surfactant herbicide fatality: a case report. Am. J. Forensic Med. Pathol. 2012, 33, 234–237. 10.1097/PAF.0b013e31824b936c. [DOI] [PubMed] [Google Scholar]
- Tsui M. T.; Chu L. M. Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. 10.1016/S0045-6535(03)00306-0. [DOI] [PubMed] [Google Scholar]
- Vincent K.; Davidson C. The toxicity of glyphosate alone and glyphosate-surfactant mixtures to western toad (Anaxyrus boreas) tadpoles. Environ. Toxicol. Chem. 2015, 34, 2791–2795. 10.1002/etc.3118. [DOI] [PubMed] [Google Scholar]
- Dedeke G. A.; Owagboriaye F. O.; Ademolu K. O.; Olujimi O. O.; Aladesida A. A. Comparative Assessment on Mechanism Underlying Renal Toxicity of Commercial Formulation of Roundup Herbicide and Glyphosate Alone in Male Albino Rat. Int. J. Toxicol. 2018, 37, 285–295. 10.1177/1091581818779553. [DOI] [PubMed] [Google Scholar]
- Lee H. L.; Chen K. W.; Chi C. H.; Huang J. J.; Tsai L. M. Clinical presentations and prognostic factors of a glyphosate-surfactant herbicide intoxication: a review of 131 cases. Acad. Emerg. Med. 2000, 7, 906–910. 10.1111/j.1553-2712.2000.tb02069.x. [DOI] [PubMed] [Google Scholar]
- Yang F. L.; Lu X. Acute obstructive fibrinous laryngotracheobronchitis induced by severe glyphosate surfactant intoxication: A case report. World J. Emerg. Med. 2020, 11, 125–126. 10.5847/wjem.j.1920-8642.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y.; Chang S. S.; Tseng C. P.; Deng J. F.; Lee C. C. Parenteral glyphosate-surfactant herbicide intoxication. Am. J. Emerg. Med. 2006, 24, 504–506. 10.1016/j.ajem.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Mutkule D. P.; Venkategowda P. M.; Mahendrakar K. Glyphosate surfactant herbicide poisoning and management. Indian J. Crit. Care Med. 2014, 18, 328–330. 10.4103/0972-5229.132508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S. J.; Park J. S.; Hong J. R.; Gil H. W.; Yang J. O.; Lee E. Y.; Song H. Y.; Hong S. Y. Surfactant volume is an essential element in human toxicity in acute glyphosate herbicide intoxication. Clin. Toxicol. (Phila.) 2011, 49, 892–899. 10.3109/15563650.2011.626422. [DOI] [PubMed] [Google Scholar]
- Ozaki T.; Sofue T.; Kuroda Y. Severe Glyphosate-Surfactant Intoxication Successfully Treated With Continuous Hemodiafiltration and Direct Hemoperfusion: Case Report. Ther. Apher. Dial. 2017, 21, 296–297. 10.1111/1744-9987.12565. [DOI] [PubMed] [Google Scholar]
- Roberts D. M.; Buckley N. A.; Mohamed F.; Eddleston M.; Goldstein D. A.; Mehrsheikh A.; Bleeke M. S.; Dawson A. H. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin. Toxicol. 2010, 48, 129–136. 10.3109/15563650903476491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. X.; Duncan T. V. Challenges and potential solutions for nanosensors intended for use with foods. Nat. Nanotechnol. 2021, 16, 251–265. 10.1038/s41565-021-00867-7. [DOI] [PubMed] [Google Scholar]
- French E.; Kaplan I.; Iyer-Pascuzzi A.; Nakatsu C. H.; Enders L. Emerging strategies for precision microbiome management in diverse agroecosystems. Nat. Plants 2021, 7, 256–267. 10.1038/s41477-020-00830-9. [DOI] [PubMed] [Google Scholar]
- Thavarajah W.; Verosloff M. S.; Jung J. K.; Alam K. K.; Miller J. D.; Jewett M. C.; Young S. L.; Lucks J. B. A primer on emerging field-deployable synthetic biology tools for global water quality monitoring. Npj Clean Water 2020, 3, 18. 10.1038/s41545-020-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency Glyphosate. https://www.epa.gov/ingredients-used-pesticide-products/glyphosate; 2015. [PubMed]
- Simoes F. R.; Mattoso L. H. C.; Vaz C. M. P. Conducting polymers as sensor materials for the electrochemical detection of pesticides. Sensor Lett. 2006, 4, 319–324. 10.1166/sl.2006.040. [DOI] [Google Scholar]
- Milojevic-Rakic M.; Janosevic A.; Krstic J.; Vasiljevic B. N.; Dondur V.; Ciric-Marjanovic G. Polyaniline and its composites with zeolite ZSM-5 for efficient removal of glyphosate from aqueous solution. Microporous Mesoporous Mater. 2013, 180, 141–155. 10.1016/j.micromeso.2013.06.025. [DOI] [Google Scholar]
- Milojevic-Rakic M.; Bajuk-Bogdanovic D.; Vasiljevic B. N.; Rakic A.; Skrivanj S.; Ignjatovic L.; Dondur V.; Mentus S.; Ciric-Marjanovic G. Polyaniline/FeZSM-5 composites - Synthesis, characterization and their high catalytic activity for the oxidative degradation of herbicide glyphosate. Microporous Mesoporous Mater. 2018, 267, 68–79. 10.1016/j.micromeso.2018.03.019. [DOI] [Google Scholar]
- Huo R.; Yang X. L.; Yang J. Y.; Yang S. Y.; Xu Y. H. Self-assembly synthesis of BiVO4/Polydopamine/g-C3N4 with enhanced visible light photocatalytic performance. Mater. Res. Bull. 2018, 98, 225–230. 10.1016/j.materresbull.2017.10.016. [DOI] [Google Scholar]
- Zhang B.; Li B.; Wang Z. G. Creation of Carbazole-Based Fluorescent Porous Polymers for Recognition and Detection of Various Pesticides in Water. ACS Sens. 2020, 5, 162–170. 10.1021/acssensors.9b01954. [DOI] [PubMed] [Google Scholar]
- Sasson E.; Pinhasi R. V.; Margel S.; Klipcan L. Engineering and use of proteinoid polymers and nanocapsules containing agrochemicals. Sci. Rep. 2020, 10, 9171. 10.1038/s41598-020-66172-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Mata K.; Corazza M. Z.; de Oliveira F. M.; de Toffoli A. L.; Tarley C. R. T.; Moreira A. B. Synthesis and characterization of cross-linked molecularly imprinted polyacrylamide for the extraction/preconcentration of glyphosate and aminomethylphosphonic acid from water samples. React. Funct. Polym. 2014, 83, 76–83. 10.1016/j.reactfunctpolym.2014.07.004. [DOI] [Google Scholar]
- Do M. H.; Florea A.; Farre C.; Bonhomme A.; Bessueille F.; Vocanson F.; Tran-Thi N. T.; Jaffrezic-Renault N. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int. J. Environ. Anal. Chem. 2015, 95, 1489–1501. 10.1080/03067319.2015.1114109. [DOI] [Google Scholar]
- Ghaeni F. A.; Karimi G.; Mohsenzadeh M. S.; Nazarzadeh M.; Motamedshariaty V. S.; Mohajeri S. A. Preparation of dual-template molecularly imprinted nanoparticles for organophosphate pesticides and their application as selective sorbents for water treatment. Sep. Sci. Technol. 2018, 53, 2517–2526. 10.1080/01496395.2018.1461112. [DOI] [Google Scholar]
- Gomez-Caballero A.; Diaz-Diaz G.; Bengoetxea O.; Quintela A.; Unceta N.; Goicolea M. A.; Barrio R. J. Water compatible stir-bar devices imprinted with underivatised glyphosate for selective sample clean-up. J. Chromatogr. A 2016, 1451, 23–32. 10.1016/j.chroma.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Lee J.; Shin I. S. Advanced method for fabrication of molecularly imprinted mesoporous organosilica with highly sensitive and selective recognition of glyphosate. Sci. Rep. 2019, 9, 10293. 10.1038/s41598-019-46881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazouz Z.; Rahali S.; Fourati N.; Zerrouki C.; Aloui N.; Seydou M.; Yaakoubi N.; Chehimi M. M.; Othmane A.; Kalfat R. Highly Selective Polypyrrole MIP-Based Gravimetric and Electrochemical Sensors for Picomolar Detection of Glyphosate. Sensors 2017, 17, 2586. 10.3390/s17112586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. B.; Jauhari D.; Tiwari M. P. Doubly imprinted polymer nanofilm-modified electrochemical sensor for ultra-trace simultaneous analysis of glyphosate and glufosinate. Biosens. Bioelectron. 2014, 59, 81–88. 10.1016/j.bios.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Puzio K.; Claude B.; Amalric L.; Berho C.; Grellet E.; Bayoudh S.; Nehme R.; Morin P. Molecularly imprinted polymer dedicated to the extraction of glyphosate in natural waters. J. Chromatogr. 2014, 1361, 1–8. 10.1016/j.chroma.2014.07.043. [DOI] [PubMed] [Google Scholar]
- Sawetwong P.; Chairam S.; Jarujamrus P.; Amatatongchai M. Enhanced selectivity and sensitivity for colorimetric determination of glyphosate using Mn-ZnS quantum dot embedded molecularly imprinted polymers combined with a 3D-microfluidic paper-based analytical device. Talanta 2021, 225, 122077. 10.1016/j.talanta.2020.122077. [DOI] [PubMed] [Google Scholar]
- Uka B.; Kieninger J.; Urban G. A.; Weltin A. Electrochemical Microsensor for Microfluidic Glyphosate Monitoring in Water Using MIP-Based Concentrators. ACS Sens. 2021, 6, 2738–2746. 10.1021/acssensors.1c00884. [DOI] [PubMed] [Google Scholar]
- Xu J. M.; Zhang Y.; Wu K. Q.; Zhang L. N.; Ge S. G.; Yu J. H. A molecularly imprinted polypyrrole for ultrasensitive voltammetric determination of glyphosate. Microchim. Acta 2017, 184, 1959–1967. 10.1007/s00604-017-2200-9. [DOI] [Google Scholar]
- Zarejousheghani M.; Jaafar A.; Wollmerstaedt H.; Rahimi P.; Borsdorf H.; Zimmermann S.; Joseph Y. Rational Design of Molecularly Imprinted Polymers Using Quaternary Ammonium Cations for Glyphosate Detection. Sensors 2021, 21, 296. 10.3390/s21010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; She Y. X.; Li T. F.; Zhao F. N.; Jin M. J.; Guo Y. R.; Zheng L. F.; Wang S. S.; Jin F.; Shao H.; Liu H. J.; Wang J. A highly selective electrochemical sensor based on molecularly imprinted polypyrrole-modified gold electrode for the determination of glyphosate in cucumber and tap water. Anal. Bioanal. Chem. 2017, 409, 7133–7144. 10.1007/s00216-017-0671-5. [DOI] [PubMed] [Google Scholar]
- Zhao P. N.; Yan M.; Zhang C. C.; Peng R. X.; Ma D. S.; Yu J. H. Determination of glyphosate in foodstuff by one novel chemiluminescence-molecular imprinting sensor. Spectrochim. Acta, Part A 2011, 78, 1482–1486. 10.1016/j.saa.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Bernal J.; Martin M. T.; Soto M. E.; Nozal M. J.; Marotti I.; Dinelli G.; Bernal J. L. Development and Application of a Liquid Chromatography-Mass Spectrometry Method To Evaluate the Glyphosate and Aminomethylphosphonic Acid Dissipation in Maize Plants after Foliar Treatment. J. Agric. Food Chem. 2012, 60, 4017–4025. 10.1021/jf3006504. [DOI] [PubMed] [Google Scholar]
- Goncharova E. N.; Statkus M. A.; Tsizin G. I.; Selimov R. N. HPLC Determination of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate Using a Hypercarb Porous Graphite Adsorbent. Mosc. Univ. Chem. Bull. 2018, 73, 265–271. 10.3103/S0027131418060056. [DOI] [Google Scholar]
- Koskinen W. C.; Marek L. J.; Hall K. E. Analysis of glyphosate and aminomethylphosphonic acid in water, plant materials and soil. Pest Manag. Sci. 2016, 72, 423–432. 10.1002/ps.4172. [DOI] [PubMed] [Google Scholar]
- Kujawinski D. M.; Wolbert J. B.; Zhang L. J.; Jochmann M. A.; Widory D.; Baran N.; Schmidt T. C. Carbon isotope ratio measurements of glyphosate and AMPA by liquid chromatography coupled to isotope ratio mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 2869–2878. 10.1007/s00216-012-6669-0. [DOI] [PubMed] [Google Scholar]
- Pimenta E. M.; da Silva F. F.; Barbosa E. S.; Cacique A. P.; Cassimiro D. L.; de Pinho G. P.; Silverio F. O. Quantification of Glyphosate and AMPA by HPLC-ICP-MS/MS and HPLC-DAD: A Comparative Study. J. Braz. Chem. Soc. 2020, 31, 298–304. 10.21577/0103-5053.20190175. [DOI] [Google Scholar]
- Sun L.; Kong D.; Gu W.; Guo X.; Tao W.; Shan Z.; Wang Y.; Wang N. Determination of glyphosate in soil/sludge by high performance liquid chromatography. J. Chromatogr. A 2017, 1502, 8–13. 10.1016/j.chroma.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Wang S.; Liu B.; Yuan D.; Ma J. A simple method for the determination of glyphosate and aminomethylphosphonic acid in seawater matrix with high performance liquid chromatography and fluorescence detection. Talanta 2016, 161, 700–706. 10.1016/j.talanta.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y.; Zhang Y.; Qu Q. S.; Wang G. X.; Wang C. Y. Determination of glyphosate and aminomethylphosphonic acid in soybean samples by high performance liquid chromatography using a novel fluorescent labeling reagent. Anal Methods-Uk 2013, 5, 6465–6472. 10.1039/c3ay41166d. [DOI] [Google Scholar]
- Geng Y.; Jiang L.; Zhang D.; Liu B.; Zhang J.; Cheng H.; Wang L.; Peng Y.; Wang Y.; Zhao Y.; Xu Y.; Liu X. Glyphosate, aminomethylphosphonic acid, and glufosinate ammonium in agricultural groundwater and surface water in China from 2017 to 2018: Occurrence, main drivers, and environmental risk assessment. Sci. Total Environ. 2021, 769, 144396. 10.1016/j.scitotenv.2020.144396. [DOI] [PubMed] [Google Scholar]
- Ashwin B. C. M. A.; Saravanan C.; Stalin T.; Mareeswaran P. M.; Rajagopal S. FRET-based Solid-state Luminescent Glyphosate Sensor Using Calixarene-grafted Ruthenium(II) bipyridine Doped Silica Nanoparticles. ChemPhysChem 2018, 19, 2768–2775. 10.1002/cphc.201800447. [DOI] [PubMed] [Google Scholar]
- Guo J. J.; Zhang Y.; Luo Y. L.; Shen F.; Sun C. Y. Efficient fluorescence resonance energy transfer between oppositely charged CdTe quantum dots and gold nanoparticles for turn-on fluorescence detection of glyphosate. Talanta 2014, 125, 385–392. 10.1016/j.talanta.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Jiang M.; Chen C.; He J.; Zhang H.; Xu Z. Fluorescence assay for three organophosphorus pesticides in agricultural products based on Magnetic-Assisted fluorescence labeling aptamer probe. Food Chem. 2020, 307, 125534. 10.1016/j.foodchem.2019.125534. [DOI] [PubMed] [Google Scholar]
- Lee H. U.; Jung D. U.; Lee J. H.; Song Y. S.; Park C.; Kim S. W. Detection of glyphosate by quantitative analysis of fluorescence and single DNA using DNA-labeled fluorescent magnetic core-shell nanoparticles. Sensor. Actuat. B-Chem. 2013, 177, 879–886. 10.1016/j.snb.2012.11.075. [DOI] [Google Scholar]
- Rettke D.; Doring J.; Martin S.; Venus T.; Estrela-Lopis I.; Schmidt S.; Ostermann K.; Pompe T. Picomolar glyphosate sensitivity of an optical particle-based sensor utilizing biomimetic interaction principles. Biosens. Bioelectron. 2020, 165, 112262. 10.1016/j.bios.2020.112262. [DOI] [PubMed] [Google Scholar]
- Tan M. J.; Hong Z. Y.; Chang M. H.; Liu C. C.; Cheng H. F.; Loh X. J.; Chen C. H.; Liao C. D.; Kong K. V. Metal carbonyl-gold nanoparticle conjugates for highly sensitive SERS detection of organophosphorus pesticides. Biosens. Bioelectron. 2017, 96, 167–172. 10.1016/j.bios.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Yuan Y. S.; Jiang J. Z.; Liu S. P.; Yang J. D.; Zhang H.; Yan J. J.; Hu X. L. Fluorescent carbon dots for glyphosate determination based on fluorescence resonance energy transfer and logic gate operation. Sensor. Actuat. B-Chem. 2017, 242, 545–553. 10.1016/j.snb.2016.11.050. [DOI] [Google Scholar]
- Butmee P.; Samphao A.; Tumcharern G. Reduced graphene oxide on silver nanoparticle layers-decorated titanium dioxide nanotube arrays as SERS-based sensor for glyphosate direct detection in environmental water and soil. J. Hazard. Mater. 2022, 437, 129344. 10.1016/j.jhazmat.2022.129344. [DOI] [PubMed] [Google Scholar]
- Aydin Z.; Keles M. A reaction-based system for the colorimetric detection of glyphosate in real samples. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 267, 120501. 10.1016/j.saa.2021.120501. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Ghalandari B.; Lin L.; Sang X.; Su W.; Divsalar A.; Ding X. A turn-on fluorescence sensor based on Cu(2+) modulated DNA-templated silver nanoclusters for glyphosate detection and mechanism investigation. Food Chem. 2022, 367, 130617. 10.1016/j.foodchem.2021.130617. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Li L.; Lin L.; Wang X.; Li J.; Liu H.; Liu X.; Huo D.; Hou C. A dual-signal sensing strategy based on ratiometric fluorescence and colorimetry for determination of Cu(2+) and glyphosate. Anal. Bioanal. Chem. 2022, 414, 2619–2628. 10.1007/s00216-022-03898-8. [DOI] [PubMed] [Google Scholar]
- Fu Z.; He J.; Li Y.; Ding H.; Gao X.; Cui F. A novel and ultrasensitive fluorescent probe derived from labeled carbon dots for recognitions of copper ions and glyphosate. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2023, 287, 122052. 10.1016/j.saa.2022.122052. [DOI] [PubMed] [Google Scholar]
- Wu J.; Chen X.; Zhang Z.; Zhang J. ″Off-on″ fluorescence probe based on green emissive carbon dots for the determination of Cu(2+) ions and glyphosate and development of a smart sensing film for vegetable packaging. Mikrochim. Acta 2022, 189, 131. 10.1007/s00604-022-05241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C. Q.; Pang Y. H.; Feng Y. W.; Shen X. F. A ratio fluorescence sensor based on rhodamine B embedded metal-organic framework for glyphosate detection in agri-food products. Food Chem. 2022, 394, 133446. 10.1016/j.foodchem.2022.133446. [DOI] [PubMed] [Google Scholar]
- Qiang Y.; Yang W.; Zhang X.; Luo X.; Tang W.; Yue T.; Li Z. UiO-67 decorated on porous carbon derived from Ce-MOF for the enrichment and fluorescence determination of glyphosate. Mikrochim. Acta 2022, 189, 130. 10.1007/s00604-022-05236-2. [DOI] [PubMed] [Google Scholar]
- Luo X.; Huang G.; Bai C.; Wang C.; Yu Y.; Tan Y.; Tang C.; Kong J.; Huang J.; Li Z. A versatile platform for colorimetric, fluorescence and photothermal multi-mode glyphosate sensing by carbon dots anchoring ferrocene metal-organic framework nanosheet. J. Hazard. Mater. 2023, 443, 130277. 10.1016/j.jhazmat.2022.130277. [DOI] [PubMed] [Google Scholar]
- Bettazzi F.; Romero Natale A.; Torres E.; Palchetti I. Glyphosate Determination by Coupling an Immuno-Magnetic Assay with Electrochemical Sensors. Sensors 2018, 18, 2965. 10.3390/s18092965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamu V. N.; Prasad S. ElectrochemSENSE: A platform towards field deployable direct on-produce glyphosate detection. Biosens. Bioelectron. 2020, 170, 112609. 10.1016/j.bios.2020.112609. [DOI] [PubMed] [Google Scholar]
- Noori J. S.; Dimaki M.; Mortensen J.; Svendsen W. E. Detection of Glyphosate in Drinking Water: A Fast and Direct Detection Method without Sample Pretreatment. Sensors 2018, 18, 2961. 10.3390/s18092961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songa E. A.; Arotiba O. A.; Owino J. H.; Jahed N.; Baker P. G.; Iwuoha E. I. Electrochemical detection of glyphosate herbicide using horseradish peroxidase immobilized on sulfonated polymer matrix. Bioelectrochemistry 2009, 75, 117–123. 10.1016/j.bioelechem.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Zhang Q. R.; Xu G. F.; Gong L. S.; Dai H.; Zhang S. P.; Li Y. L.; Lin Y. Y. An enzyme-assisted electrochemiluminescent biosensor developed on order mesoporous carbons substrate for ultrasensitive glyphosate sensing. Electrochim. Acta 2015, 186, 624–630. 10.1016/j.electacta.2015.10.081. [DOI] [Google Scholar]
- Vaghela C.; Kulkarni M.; Haram S.; Aiyer R.; Karve M. A novel inhibition based biosensor using urease nanoconjugate entrapped biocomposite membrane for potentiometric glyphosate detection. Int. J. Biol. Macromol. 2018, 108, 32–40. 10.1016/j.ijbiomac.2017.11.136. [DOI] [PubMed] [Google Scholar]
- Roman R. L.; Nagi L.; Silva L. L.; Fernandes S. C.; de Mello J. M. M.; Dal Magro J.; Fiori M. A. Monocrystalline silicon/polyaniline/horseradish peroxidase enzyme electrode obtained by the electrodeposition method for the electrochemical detection of glyphosate. J. Mater. Sci. Mater. El. 2020, 31, 9443–9456. 10.1007/s10854-020-03484-7. [DOI] [Google Scholar]
- Johnson Z. T.; Jared N.; Peterson J. K.; Li J.; Smith E. A.; Walper S. A.; Hooe S. L.; Breger J. C.; Medintz I. L.; Gomes C.; Claussen J. C. Enzymatic Laser-Induced Graphene Biosensor for Electrochemical Sensing of the Herbicide Glyphosate. Global challenges 2022, 6, 2200057. 10.1002/gch2.202200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci Leinen M.; Lindenthal S.; Heimfarth D.; Zaumseil J. Networks of as-dispersed, polymer-wrapped (6,5) single-walled carbon nanotubes for selective Cu(2+) and glyphosate sensing. Nanoscale 2022, 14, 13542–13550. 10.1039/D2NR02517E. [DOI] [PubMed] [Google Scholar]
- Bahamon-Pinzon D.; Moreira G.; Obare S.; Vanegas D. Development of a nanocopper-decorated laser-scribed sensor for organophosphorus pesticide monitoring in aqueous samples. Mikrochim. Acta 2022, 189, 254. 10.1007/s00604-022-05355-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Yao Y.; Zhao J.; Han X.; Chai C.; Dai P. A novel electrochemical sensor for glyphosate detection based on Ti(3) C(2) T (x) /Cu-BTC nanocomposite. RSC Adv. 2022, 12, 5164–5172. 10.1039/D1RA08064D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano-Intriago L. A.; Amorim C. G.; Araujo A. N.; Gritsok D.; Rodriguez-Diaz J. M.; Montenegro M. Development of an inexpensive and rapidly preparable enzymatic pencil graphite biosensor for monitoring of glyphosate in waters. Sci. Total Environ. 2023, 855, 158865. 10.1016/j.scitotenv.2022.158865. [DOI] [PubMed] [Google Scholar]
- Poudyal D. C.; Dhamu V. N.; Samson M.; Muthukumar S.; Prasad S. Portable Pesticide Electrochem-sensor: A Label-Free Detection of Glyphosate in Human Urine. Langmuir 2022, 38, 1781–1790. 10.1021/acs.langmuir.1c02877. [DOI] [PubMed] [Google Scholar]
- Poudyal D. C.; Dhamu V. N.; Samson M.; Muthukumar S.; Prasad S. Pesticide analytical screening system (PASS): A novel electrochemical system for multiplex screening of glyphosate and chlorpyrifos in high-fat and low-fat food matrices. Food Chem. 2023, 400, 134075. 10.1016/j.foodchem.2022.134075. [DOI] [PubMed] [Google Scholar]
- Balciunas D.; Plausinaitis D.; Ratautaite V.; Ramanaviciene A.; Ramanavicius A. Towards electrochemical surface plasmon resonance sensor based on the molecularly imprinted polypyrrole for glyphosate sensing. Talanta 2022, 241, 123252. 10.1016/j.talanta.2022.123252. [DOI] [PubMed] [Google Scholar]
- Thimoonnee S.; Somnet K.; Ngaosri P.; Chairam S.; Karuwan C.; Kamsong W.; Tuantranont A.; Amatatongchai M. Fast, sensitive and selective simultaneous determination of paraquat and glyphosate herbicides in water samples using a compact electrochemical sensor. Anal Methods-Uk 2022, 14, 820–833. 10.1039/D1AY02201F. [DOI] [PubMed] [Google Scholar]
- Muenchen D. K.; Martinazzo J.; Brezolin A. N.; de Cezaro A. M.; Rigo A. A.; Mezarroba M. N.; Manzoli A.; de Lima Leite F.; Steffens J.; Steffens C. Cantilever Functionalization Using Peroxidase Extract of Low Cost for Glyphosate Detection. Appl. Biochem. Biotechnol. 2018, 186, 1061–1073. 10.1007/s12010-018-2799-y. [DOI] [PubMed] [Google Scholar]
- Guan N.; Li Y.; Yang H.; Hu P.; Lu S.; Ren H.; Liu Z.; Soo Park K.; Zhou Y. Dual-functionalized gold nanoparticles probe based bio-barcode immuno-PCR for the detection of glyphosate. Food Chem. 2021, 338, 128133. 10.1016/j.foodchem.2020.128133. [DOI] [PubMed] [Google Scholar]
- Rendon-von Osten J.; Dzul-Caamal R. Glyphosate Residues in Groundwater, Drinking Water and Urine of Subsistence Farmers from Intensive Agriculture Localities: A Survey in Hopelchen, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. 10.3390/ijerph14060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis J.; Kantiani L.; Llorca M.; Rubio F.; Ginebreda A.; Fraile J.; Garrido T.; Farre M. Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 2335–2345. 10.1007/s00216-011-5541-y. [DOI] [PubMed] [Google Scholar]
- Selvi A. A.; Sreenivasa M. A.; Manonmani H. K. Enzyme-linked immunoassay for the detection of glyphosate in food samples using avian antibodies. Food Agric. Immunol. 2011, 22, 217–228. 10.1080/09540105.2011.553799. [DOI] [Google Scholar]
- Zhao J.; Pacenka S.; Wu J.; Richards B. K.; Steenhuis T.; Simpson K.; Hay A. G. Detection of glyphosate residues in companion animal feeds. Environ. Pollut. 2018, 243, 1113–1118. 10.1016/j.envpol.2018.08.100. [DOI] [PubMed] [Google Scholar]
- He S.; Cao X.; Wu H.; Li T.; Zhang M.; Liang Y.; Chen B. Rapid determination of glyphosate, aminomethyl phosphonic acid, glufosinate, and ethephon residues in environmental water by direct injection-ultra performance liquid chromatography-triple quadrupole mass spectrometry. Chin. J. Chromatogr. 2019, 37, 1179–1184. 10.3724/SP.J.1123.2019.05011. [DOI] [PubMed] [Google Scholar]
- Wei X.; Gao X.; Zhao L.; Peng X.; Zhou L.; Wang J.; Pu Q. Fast and interference-free determination of glyphosate and glufosinate residues through electrophoresis in disposable microfluidic chips. J. Chromatogr. A 2013, 1281, 148–154. 10.1016/j.chroma.2013.01.039. [DOI] [PubMed] [Google Scholar]
- Wei X.; Pu Q. Microchip electrophoresis for fast and interference-free determination of trace amounts of glyphosate and glufosinate residues in agricultural products. Methods Mol. Biol. 2015, 1274, 21–29. 10.1007/978-1-4939-2353-3_2. [DOI] [PubMed] [Google Scholar]
- Wei D. L.; Wang Y.; Zhu N. F.; Xiao J. X.; Li X. S.; Xu T.; Hu X. L.; Zhang Z.; Yin D. Q. A Lab-in-a-Syringe Device Integrated with a Smartphone Platform: Colorimetric and Fluorescent Dual-Mode Signals for On-Site Detection of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2021, 13, 48643–48652. 10.1021/acsami.1c13273. [DOI] [PubMed] [Google Scholar]
- Yadav P.; Zelder F. Detection of glyphosate with a copper(ii)-pyrocatechol violet based GlyPKit. Anal Methods-Uk 2021, 13, 4354–4360. 10.1039/D1AY01168E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Zhang Z.; Xu S.; Da L.; Lin D.; Jiang C. Enzyme-free and rapid visual quantitative detection for pesticide residues utilizing portable smartphone integrated paper sensor. J. Hazard. Mater. 2022, 436, 129320. 10.1016/j.jhazmat.2022.129320. [DOI] [PubMed] [Google Scholar]
- Zhao T.; Liang X.; Guo X.; Yang X.; Guo J.; Zhou X.; Huang X.; Zhang W.; Wang Y.; Liu Z.; Jiang Z.; Zhou H.; Zhou H. Smartphone-based colorimetric sensor array using gold nanoparticles for rapid distinguishment of multiple pesticides in real samples. Food Chem. 2023, 404, 134768. 10.1016/j.foodchem.2022.134768. [DOI] [PubMed] [Google Scholar]
- Guan J.; He Q.; Liu Q.; Chen X. Cu(2+) assisted carnation-like fluorescent metal-organic framework for triple-mode detection of glyphosate in food samples. Food Chem. 2023, 408, 135237. 10.1016/j.foodchem.2022.135237. [DOI] [PubMed] [Google Scholar]
- Utstumo T.; Urdal F.; Brevik A.; Dorum J.; Netland J.; Overskeid O.; Berge T. W.; Gravdahl J. T. Robotic in-row weed control in vegetables. Comput. Electron. Agric. 2018, 154, 36–45. 10.1016/j.compag.2018.08.043. [DOI] [Google Scholar]
- Alonso M. B.; Feo M. L.; Corcellas C.; Gago-Ferrero P.; Bertozzi C. P.; Marigo J.; Flach L.; Meirelles A. C.; Carvalho V. L.; Azevedo A. F.; Torres J. P.; Lailson-Brito J.; Malm O.; Diaz-Cruz M. S.; Eljarrat E.; Barcelo D. Toxic heritage: Maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ. Pollut. 2015, 207, 391–402. 10.1016/j.envpol.2015.09.039. [DOI] [PubMed] [Google Scholar]
- Rostvall A.; Zhang W.; Durig W.; Renman G.; Wiberg K.; Ahrens L.; Gago-Ferrero P. Removal of pharmaceuticals, perfluoroalkyl substances and other micropollutants from wastewater using lignite, Xylit, sand, granular activated carbon (GAC) and GAC+Polonite((R)) in column tests - Role of physicochemical properties. Water Res. 2018, 137, 97–106. 10.1016/j.watres.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Pico Y.; Belenguer V.; Corcellas C.; Diaz-Cruz M. S.; Eljarrat E.; Farre M.; Gago-Ferrero P.; Huerta B.; Navarro-Ortega A.; Petrovic M.; Rodriguez-Mozaz S.; Sabater L.; Santin G.; Barcelo D. Contaminants of emerging concern in freshwater fish from four Spanish Rivers. Sci. Total Environ. 2019, 659, 1186–1198. 10.1016/j.scitotenv.2018.12.366. [DOI] [PubMed] [Google Scholar]
- Gago-Ferrero P.; Bletsou A. A.; Damalas D. E.; Aalizadeh R.; Alygizakis N. A.; Singer H. P.; Hollender J.; Thomaidis N. S. Wide-scope target screening of > 2000 emerging contaminants in wastewater samples with UPLC-Q-ToF-HRMS/MS and smart evaluation of its performance through the validation of 195 selected representative analytes. J. Hazard. Mater. 2020, 387, 121712. 10.1016/j.jhazmat.2019.121712. [DOI] [PubMed] [Google Scholar]
- Menger F.; Bostrom G.; Jonsson O.; Ahrens L.; Wiberg K.; Kreuger J.; Gago-Ferrero P. Identification of Pesticide Transformation Products in Surface Water Using Suspect Screening Combined with National Monitoring Data. Environ. Sci. Technol. 2021, 55, 10343–10353. 10.1021/acs.est.1c00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Hao Y.; Li Y.; Yang R.; Wang P.; Zhang G.; Zhang Q.; Jiang G. Occurrence and distribution of organophosphate esters in the air and soils of Ny-Alesund and London Island, Svalbard, Arctic. Environ. Pollut. 2020, 263, 114495. 10.1016/j.envpol.2020.114495. [DOI] [PubMed] [Google Scholar]
- Bapat G.; Labade C.; Chaudhari A.; Zinjarde S. Silica nanoparticle based techniques for extraction, detection, and degradation of pesticides. Adv. Colloid Interface Sci. 2016, 237, 1–14. 10.1016/j.cis.2016.06.001. [DOI] [PubMed] [Google Scholar]
- He Q.; Liang J. J.; Chen L. X.; Chen S. L.; Zheng H. L.; Liu H. X.; Zhang H. J. Removal of the environmental pollutant carbamazepine using molecular imprinted adsorbents: Molecular simulation, adsorption properties, and mechanisms. Water Res. 2020, 168, 115164. 10.1016/j.watres.2019.115164. [DOI] [PubMed] [Google Scholar]
- Mazuryk J.; Klepacka K.; Piechowska J.; Kalecki J.; Derzsi L.; Piotrowski P.; Paszke P.; Pawlak D. A.; Berneschi S.; Kutner W.; Sharma P. S. In-Capillary Photodeposition of Glyphosate-Containing Polyacrylamide Nanometer-Thick Films. ACS applied polymer materials 2023, 5, 223–235. 10.1021/acsapm.2c01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y.; Soppera O.; Haupt K. Photopolymerization and photostructuring of molecularly imprinted polymers for sensor applications-a review. Anal. Chim. Acta 2012, 717, 7–20. 10.1016/j.aca.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Hauk C.; Boss M.; Gabel J.; Schafermann S.; Lensch H. P. A.; Heide L. An open-source smartphone app for the quantitative evaluation of thin-layer chromatographic analyses in medicine quality screening. Sci. Rep. 2022, 12, 13433. 10.1038/s41598-022-17527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Ruan X.; Song Y.; Smith J. N.; Vasylieva N.; Hammock B. D.; Lin Y.; Du D. Smartphone-Based Dual-Channel Immunochromatographic Test Strip with Polymer Quantum Dot Labels for Simultaneous Detection of Cypermethrin and 3-Phenoxybenzoic Acid. Anal. Chem. 2021, 93, 13658–13666. 10.1021/acs.analchem.1c03085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Liao X. F.; Hou Y. J.; Jia B. Y.; Fu L. Z.; Jia M. X.; Zhou L. D.; Lu J. H.; Kong W. J. Recent advances in synthesis and modification of carbon dots for optical sensing of pesticides. J. Hazard. Mater. 2022, 422, 126881. 10.1016/j.jhazmat.2021.126881. [DOI] [PubMed] [Google Scholar]
- Liang X.; Li N.; Zhang R. H.; Yin P. G.; Zhang C. M.; Yang N.; Liang K.; Kong B. Carbon-based SERS biosensor: from substrate design to sensing and bioapplication. Npg Asia Mater. 2021, 13, 8. 10.1038/s41427-020-00278-5. [DOI] [Google Scholar]
- Berneschi S.; Farnesi D.; Cosi F.; Conti G. N.; Pelli S.; Righini G. C.; Soria S. High Q silica microbubble resonators fabricated by arc discharge. Opt. Lett. 2011, 36, 3521–3523. 10.1364/OL.36.003521. [DOI] [PubMed] [Google Scholar]
- Berneschi S.; Baldini F.; Cosci A.; Farnesi D.; Conti G. N.; Tombelli S.; Trono C.; Pelli S.; Giannetti A. Fluorescence biosensing in selectively photo-activated microbubble resonators. Sensor. Actuat. B-Chem. 2017, 242, 1057–1064. 10.1016/j.snb.2016.09.146. [DOI] [Google Scholar]
- Cosci A.; Berneschi S.; Giannetti A.; Farnesi D.; Cosi F.; Baldini F.; Nunzi Conti G.; Soria S.; Barucci A.; Righini G.; Pelli S. Resonance Frequency of Optical Microbubble Resonators: Direct Measurements and Mitigation of Fluctuations. Sensors 2016, 16, 1405. 10.3390/s16091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunzi Conti G.; Berneschi S.; Soria S. Aptasensors Based on Whispering Gallery Mode Resonators. Biosensors 2016, 6, 28. 10.3390/bios6030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. D.; Vojta A. L.; Grant S. A.; Hunt H. K. Integrating Nanostructured Artificial Receptors with Whispering Gallery Mode Optical Microresonators via Inorganic Molecular Imprinting Techniques. Biosensors 2016, 6, 26. 10.3390/bios6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toropov N.; Cabello G.; Serrano M. P.; Gutha R. R.; Rafti M.; Vollmer F. Review of biosensing with whispering-gallery mode lasers. Light Sci. Appl. 2021, 10, 42. 10.1038/s41377-021-00471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer F.; Arnold S. Whispering-gallery-mode biosensing: label-free detection down to single molecules. Nat. Methods 2008, 5, 591–596. 10.1038/nmeth.1221. [DOI] [PubMed] [Google Scholar]
- Hao N.; Wang K. Recent development of electrochemiluminescence sensors for food analysis. Anal. Bioanal. Chem. 2016, 408, 7035–7048. 10.1007/s00216-016-9548-2. [DOI] [PubMed] [Google Scholar]
- Ge L.; Liu Q.; Hao N.; Kun W. Recent developments of photoelectrochemical biosensors for food analysis. J. Mater. Chem. B 2019, 7, 7283–7300. 10.1039/C9TB01644A. [DOI] [PubMed] [Google Scholar]
- Qin J.; Jiang S.; Wang Z.; Cheng X.; Li B.; Shi Y.; Tsai D. P.; Liu A. Q.; Huang W.; Zhu W. Metasurface Micro/Nano-Optical Sensors: Principles and Applications. ACS Nano 2022, 16, 11598–11618. 10.1021/acsnano.2c03310. [DOI] [PubMed] [Google Scholar]
- Miao X.; Yan L.; Wu Y.; Liu P. Q. High-sensitivity nanophotonic sensors with passive trapping of analyte molecules in hot spots. Light, science & applications 2021, 10, 5. 10.1038/s41377-020-00449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Vaid K.; Bansal S. A.; Kim K. H. Nanomaterial-based immunosensors for ultrasensitive detection of pesticides/herbicides: Current status and perspectives. Biosens. Bioelectron. 2020, 165, 112382. 10.1016/j.bios.2020.112382. [DOI] [PubMed] [Google Scholar]
- Li F. Q.; Yu Z. G.; Han X. D.; Lai R. Y. Electrochemical aptamer-based sensors for food and water analysis: A review. Anal. Chim. Acta 2019, 1051, 1–23. 10.1016/j.aca.2018.10.058. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Guo H.; Sun X. Recent progress on cell-based biosensors for analysis of food safety and quality control. Biosens. Bioelectron. 2019, 126, 389–404. 10.1016/j.bios.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Zambrano-Intriago L. A.; Amorim C. G.; Rodriguez-Diaz J. M.; Araujo A. N.; Montenegro M. Challenges in the design of electrochemical sensor for glyphosate-based on new materials and biological recognition. Sci. Total Environ. 2021, 793, 148496. 10.1016/j.scitotenv.2021.148496. [DOI] [PubMed] [Google Scholar]
- Rajaji U.; Chinnapaiyan S.; Chen S. M.; Govindasamy M.; Oliveira Filho J. I.; Khushaim W.; Mani V. Design and Fabrication of Yttrium Ferrite Garnet-Embedded Graphitic Carbon Nitride: A Sensitive Electrocatalyst for Smartphone-Enabled Point-of-Care Pesticide (Mesotrione) Analysis in Food Samples. ACS Appl. Mater. Interfaces 2021, 13, 24865–24876. 10.1021/acsami.1c04597. [DOI] [PubMed] [Google Scholar]
- Deda D. K.; Pereira B. B. S.; Bueno C. C.; da Silva A. N.; Ribeiro G. A.; Amarante A. M.; Franca E. F.; Leite F. L. The Use of Functionalized AFM tips as Molecular Sensors in the Detection of Pesticides. Mater. Res.-Ibero-Am. J. 2013, 16, 683–687. 10.1590/S1516-14392013005000043. [DOI] [Google Scholar]
- Fang L.; Liao X.; Jia B.; Shi L.; Kang L.; Zhou L.; Kong W. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 112255. 10.1016/j.bios.2020.112255. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Tian S.; Huang L.; Liu K.; Dong L.; Guo J. A smartphone-based biomedical sensory system. Analyst 2020, 145, 2873–2891. 10.1039/C9AN02294E. [DOI] [PubMed] [Google Scholar]
- Fang L.; Liao X. F.; Jia B. Y.; Shi L. C.; Kang L. Z.; Zhou L. D.; Kong W. J. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 164. 10.1016/j.bios.2020.112255. [DOI] [PubMed] [Google Scholar]
- Pan J.; Chen W.; Ma Y.; Pan G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. 10.1039/C7CS00854F. [DOI] [PubMed] [Google Scholar]
- Capoferri D.; Della Pelle F.; Del Carlo M.; Compagnone D. Affinity Sensing Strategies for the Detection of Pesticides in Food. Foods 2018, 7, 148. 10.3390/foods7090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Abd El-Aty A. M.; Eun J. B.; Shim J. H.; Zhao J.; Lei X.; Gao S.; She Y.; Jin F.; Wang J.; Jin M.; Hammock B. D. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. 10.1021/acs.jafc.2c05284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholafazad-Kordasht H.; Hasanzadeh M.; Seidi F. Smartphone based immunosensors as next generation of healthcare tools: Technical and analytical overview towards improvement of personalized medicine. Trends Anal. Chem. 2021, 145, 116455. 10.1016/j.trac.2021.116455. [DOI] [Google Scholar]
- Noviana E.; Ozer T.; Carrell C. S.; Link J. S.; McMahon C.; Jang I.; Henry C. S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. 10.1021/acs.chemrev.0c01335. [DOI] [PubMed] [Google Scholar]
- Sun B. R.; Zhou A. G.; Li X.; Yu H. Z. Development and Application of Mobile Apps for Molecular Sensing: A Review. ACS sensors 2021, 6, 1731–1744. 10.1021/acssensors.1c00512. [DOI] [PubMed] [Google Scholar]
- Alahmad W.; Varanusupakul P.; Varanusupakul P. Recent Developments and Applications of Microfluidic Paper-Based Analytical Devices for the Detection of Biological and Chemical Hazards in Foods: A Critical Review. Crit. Rev. Anal. Chem. 2023, 53, 233–20. 10.1080/10408347.2021.1949695. [DOI] [PubMed] [Google Scholar]
- Kudr J.; Zitka O.; Klimanek M.; Vrba R.; Adam V. Microfluidic electrochemical devices for pollution analysis-A review. Sensor. Actuat. B-Chem. 2017, 246, 578–590. 10.1016/j.snb.2017.02.052. [DOI] [Google Scholar]
- Kung C. T.; Hou C. Y.; Wang Y. N.; Fu L. M. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sensor. Actuat. B-Chem. 2019, 301, 126855. 10.1016/j.snb.2019.126855. [DOI] [Google Scholar]
- Zhang D. H.; Li C. C.; Ji D. L.; Wang Y. F. Paper-Based Microfluidic Sensors for Onsite Environmental Detection: A Critical Review. Crit. Rev. Anal. Chem. 2022, 52, 1432–1449. 10.1080/10408347.2021.1886900. [DOI] [PubMed] [Google Scholar]
- van de Merwe J. P.; Neale P. A.; Melvin S. D.; Leusch F. D. L. In vitro bioassays reveal that additives are significant contributors to the toxicity of commercial household pesticides. Aquat. Toxicol. 2018, 199, 263–268. 10.1016/j.aquatox.2018.03.033. [DOI] [PubMed] [Google Scholar]
- Antonacci A.; Scognamiglio V. Biotechnological Advances in the Design of Algae-Based Biosensors. Trends Biotechnol. 2020, 38, 334–347. 10.1016/j.tibtech.2019.10.005. [DOI] [PubMed] [Google Scholar]
- Li X.; Yang F.; Wong J. X. H.; Yu H. Z. Integrated Smartphone-App-Chip System for On-Site Parts-Per-Billion-Level Colorimetric Quantitation of Aflatoxins. Anal. Chem. 2017, 89, 8908–8916. 10.1021/acs.analchem.7b01379. [DOI] [PubMed] [Google Scholar]
- Arduini F.; Cinti S.; Caratelli V.; Amendola L.; Palleschi G.; Moscone D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. 10.1016/j.bios.2018.10.014. [DOI] [PubMed] [Google Scholar]
- McManus P.; Hortin J.; Anderson A. J.; Jacobson A. R.; Britt D. W.; Stewart J.; McLean J. E. Rhizosphere interactions between copper oxide nanoparticles and wheat root exudates in a sand matrix: Influences on copper bioavailability and uptake. Environ. Toxicol. Chem. 2018, 37, 2619–2632. 10.1002/etc.4226. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Ma Y. H.; Xie C. J.; Guo Z. L.; He X.; Valsami-Jones E.; Lynch I.; Luo W. H.; Zheng L. R.; Zhang Z. Y. Plant species-dependent transformation and translocation of ceria nanoparticles. Environ. Sci. Nano. 2019, 6, 60–67. 10.1039/C8EN01089G. [DOI] [Google Scholar]
- Zhang P.; Guo Z.; Zhang Z.; Fu H.; White J. C.; Lynch I. Nanomaterial Transformation in the Soil-Plant System: Implications for Food Safety and Application in Agriculture. Small 2020, 16, e2000705 10.1002/smll.202000705. [DOI] [PubMed] [Google Scholar]
- Tittl A.; John-Herpin A.; Leitis A.; Arvelo E. R.; Altug H. Metasurface-Based Molecular Biosensing Aided by Artificial Intelligence. Angew. Chem., Int. Ed. 2019, 58, 14810–14822. 10.1002/anie.201901443. [DOI] [PubMed] [Google Scholar]
- Lee K.; Park J.; Lee M. S.; Kim J.; Hyun B. G.; Kang D. J.; Na K.; Lee C. Y.; Bien F.; Park J. U. In-situ Synthesis of Carbon Nanotube-Graphite Electronic Devices and Their Integrations onto Surfaces of Live Plants and Insects. Nano Lett. 2014, 14, 2647–2654. 10.1021/nl500513n. [DOI] [PubMed] [Google Scholar]
- Tang W. Z.; Yan T. T.; Ping J. F.; Wu J.; Ying Y. B. Rapid Fabrication of Flexible and Stretchable Strain Sensor by Chitosan-Based Water Ink for Plants Growth Monitoring. Adv. Mater. Technol. 2017, 2, 1700021. 10.1002/admt.201700021. [DOI] [Google Scholar]
- Shaner D. L.; Beckie H. J. The future for weed control and technology. Pest Manag. Sci. 2014, 70, 1329–1339. 10.1002/ps.3706. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Miao Z.; Li N.; He C.; Sun T. Review of Current Robotic Approaches for Precision Weed Management. Current robotics reports 2022, 3, 139–151. 10.1007/s43154-022-00086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil G.; Casagrande D. E.; Cortes L.; Verschae R. Why the low adoption of robotics in the farms? Challenges for the establishment of commercial agricultural robots. Smart Agric. Techn. 2023, 3, 100069. 10.1016/j.atech.2022.100069. [DOI] [Google Scholar]
- Pradel M.; de Fays M.; Seguineau C. Comparative Life Cycle Assessment of intra-row and inter-row weeding practices using autonomous robot systems in French vineyards. Sci. Total Environ. 2022, 838, 156441. 10.1016/j.scitotenv.2022.156441. [DOI] [PubMed] [Google Scholar]
- Hagner M.; Lindqvist B.; Vepsalainen J.; Samori C.; Keskinen R.; Rasa K.; Hyvonen T. Potential of pyrolysis liquids to control the environmental weed Heracleum mantegazzianum. Environ. Technol. Inno. 2020, 20, 101154. 10.1016/j.eti.2020.101154. [DOI] [Google Scholar]
- Berni J. A. J.; Zarco-Tejada P. J.; Suarez L.; Fereres E. Thermal and Narrowband Multispectral Remote Sensing for Vegetation Monitoring From an Unmanned Aerial Vehicle. ITGRS 2009, 47, 722–738. 10.1109/TGRS.2008.2010457. [DOI] [Google Scholar]
- Deng L.; Mao Z. H.; Li X. J.; Hu Z. W.; Duan F. Z.; Yan Y. N. UAV-based multispectral remote sensing for precision agriculture: A comparison between different cameras. ISPRS J. Photogramm. 2018, 146, 124–136. 10.1016/j.isprsjprs.2018.09.008. [DOI] [Google Scholar]
- Lamb D. W.; Brown R. B. Remote-sensing and mapping of weeds in crops. JAER 2001, 78, 117–125. 10.1006/jaer.2000.0630. [DOI] [Google Scholar]
- Maimaitijiang M.; Ghulam A.; Sidike P.; Hartling S.; Maimaitiyiming M.; Peterson K.; Shavers E.; Fishman J.; Peterson J.; Kadam S.; Burken J.; Fritschi F. Unmanned Aerial System (UAS)-based phenotyping of soybean using multi-sensor data fusion and extreme learning machine. ISPRS J. Photogramm. 2017, 134, 43–58. 10.1016/j.isprsjprs.2017.10.011. [DOI] [Google Scholar]
- Moran M. S.; Inoue Y.; Barnes E. M. Opportunities and limitations for image-based remote sensing in precision crop management. Remote Sens. Environ. 1997, 61, 319–346. 10.1016/S0034-4257(97)00045-X. [DOI] [Google Scholar]
- Zwiggelaar R. A review of spectral properties of plants and their potential use for crop/weed discrimination in row-crops. Crop Protect. 1998, 17, 189–206. 10.1016/S0261-2194(98)00009-X. [DOI] [Google Scholar]
- Seufert V.; Ramankutty N.; Mayerhofer T. What is this thing called organic? - How organic farming is codified in regulations. Food Policy 2017, 68, 10–20. 10.1016/j.foodpol.2016.12.009. [DOI] [Google Scholar]
- Alreshidi E. Smart Sustainable Agriculture (SSA) Solution Underpinned by Internet of Things (IoT) and Artificial Intelligence (AI). Int. J. Adv. Comput. Sci. Appl. 2019, 10, 93–102. [Google Scholar]
- Shaw D. R. Translation of remote sensing data into weed management decisions. Weed Sci. 2005, 53, 264–273. 10.1614/WS-04-072R1. [DOI] [Google Scholar]
- Smith A. M.; Blackshaw R. E. Weed-crop discrimination using remote sensing: A detached leaf experiment. Weed Technol. 2003, 17, 811–820. 10.1614/WT02-179. [DOI] [Google Scholar]
- Chlingaryan A.; Sukkarieh S.; Whelan B. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: A review. Comput. Electron. Agric. 2018, 151, 61–69. 10.1016/j.compag.2018.05.012. [DOI] [Google Scholar]
- de Castro A. I.; Torres-Sanchez J.; Pena J. M.; Jimenez-Brenes F. M.; Csillik O.; Lopez-Granados F. An Automatic Random Forest-OBIA Algorithm for Early Weed Mapping between and within Crop Rows Using UAV Imagery. Remote Sens-Basel 2018, 10, 285. 10.3390/rs10020285. [DOI] [Google Scholar]
- Torres-Sanchez J.; Lopez-Granados F.; Pena J. M. An automatic object-based method for optimal thresholding in UAV images: Application for vegetation detection in herbaceous crops. Comput. Electron. Agric. 2015, 114, 43–52. 10.1016/j.compag.2015.03.019. [DOI] [Google Scholar]
- Lopez-Granados F. Weed detection for site-specific weed management: mapping and real-time approaches. Weed Res. 2011, 51, 1–11. 10.1111/j.1365-3180.2010.00829.x. [DOI] [Google Scholar]
- Oliveira M. C.; Osipitan O. A.; Begcy K.; Werle R. Cover crops, hormones and herbicides: Priming an integrated weed management strategy. Plant Sci. 2020, 301, 110550. 10.1016/j.plantsci.2020.110550. [DOI] [PubMed] [Google Scholar]
- Piveta L. B.; Roma-Burgos N.; Noldin J. A.; Viana V. E.; de Oliveira C.; Lamego F. P.; de Avila L. A. Molecular and Physiological Responses of Rice and Weedy Rice to Heat and Drought Stress. Agriculture 2021, 11, 9. 10.3390/agriculture11010009. [DOI] [Google Scholar]
- Korres N. E.; Burgos N. R.; Travlos I.; Vurro M.; Gitsopoulos T. K.; Varanasi V. K.; Duke S. O.; Kudsk P.; Brabham C.; Rouse C. E.; Salas-Perez R. New directions for integrated weed management: Modern technologies, tools and knowledge discovery. Adv. Agron. 2019, 155, 243–319. 10.1016/bs.agron.2019.01.006. [DOI] [Google Scholar]
- Karatzas P.; Melagraki G.; Ellis L. A.; Lynch I.; Varsou D. D.; Afantitis A.; Tsoumanis A.; Doganis P.; Sarimveis H. Development of Deep Learning Models for Predicting the Effects of Exposure to Engineered Nanomaterials on Daphnia magna. Small 2020, 16, e2001080 10.1002/smll.202001080. [DOI] [PubMed] [Google Scholar]
- Mouchlis V. D.; Afantitis A.; Serra A.; Fratello M.; Papadiamantis A. G.; Aidinis V.; Lynch I.; Greco D.; Melagraki G. Advances in de Novo Drug Design: From Conventional to Machine Learning Methods. Int. J. Mol. Sci. 2021, 22, 1676. 10.3390/ijms22041676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou C. A.; Brown N.; Pattichis C. S. Molecular optimization using computational multi-objective methods. Curr. Opin. Drug Discovery Devel. 2007, 10, 316–324. [PubMed] [Google Scholar]
- Winkler D. A. Role of Artificial Intelligence and Machine Learning in Nanosafety. Small 2020, 16, e2001883 10.1002/smll.202001883. [DOI] [PubMed] [Google Scholar]
- Rosell-Polo J. R.; Cheein F. A.; Gregorio E.; Andujar D.; Puigdomenech L.; Masip J.; Escola A. Advances in Structured Light Sensors Applications in Precision Agriculture and Livestock Farming. Adv. Agron. 2015, 133, 71–112. 10.1016/bs.agron.2015.05.002. [DOI] [Google Scholar]
- Singh A.; Ganapathysubramanian B.; Singh A. K.; Sarkar S. Machine Learning for High-Throughput Stress Phenotyping in Plants. Trends Plant Sci. 2016, 21, 110–124. 10.1016/j.tplants.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Singh A. K.; Ganapathysubramanian B.; Sarkar S.; Singh A. Deep Learning for Plant Stress Phenotyping: Trends and Future Perspectives. Trends Plant Sci. 2018, 23, 883–898. 10.1016/j.tplants.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Singh A.; Jones S.; Ganapathysubramanian B.; Sarkar S.; Mueller D.; Sandhu K.; Nagasubramanian K. Challenges and Opportunities in Machine-Augmented Plant Stress Phenotyping. Trends Plant Sci. 2021, 26, 53–69. 10.1016/j.tplants.2020.07.010. [DOI] [PubMed] [Google Scholar]
- Kamilaris A.; Prenafeta-Boldu F. X. Deep learning in agriculture: A survey. Comput. Electron. Agric. 2018, 147, 70–90. 10.1016/j.compag.2018.02.016. [DOI] [Google Scholar]
- LeCun Y.; Bengio Y.; Hinton G. Deep learning. Nature 2015, 521, 436–444. 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- Ma L.; Liu Y.; Zhang X. L.; Ye Y. X.; Yin G. F.; Johnson B. A. Deep learning in remote sensing applications: A meta-analysis and review. ISPRS J. Photogramm. 2019, 152, 166–177. 10.1016/j.isprsjprs.2019.04.015. [DOI] [Google Scholar]
- Zhong L. H.; Hu L. N.; Zhou H. Deep learning based multi-temporal crop classification. Remote Sens. Environ. 2019, 221, 430–443. 10.1016/j.rse.2018.11.032. [DOI] [Google Scholar]
- Afantitis A.; Melagraki G.; Isigonis P.; Tsoumanis A.; Varsou D. D.; Valsami-Jones E.; Papadiamantis A.; Ellis L. A.; Sarimveis H.; Doganis P.; Karatzas P.; Tsiros P.; Liampa I.; Lobaskin V.; Greco D.; Serra A.; Kinaret P. A. S.; Saarimaki L. A.; Grafstrom R.; Kohonen P.; Nymark P.; Willighagen E.; Puzyn T.; Rybinska-Fryca A.; Lyubartsev A.; Alstrup Jensen K.; Brandenburg J. G.; Lofts S.; Svendsen C.; Harrison S.; Maier D.; Tamm K.; Janes J.; Sikk L.; Dusinska M.; Longhin E.; Runden-Pran E.; Mariussen E.; El Yamani N.; Unger W.; Radnik J.; Tropsha A.; Cohen Y.; Leszczynski J.; Ogilvie Hendren C.; Wiesner M.; Winkler D.; Suzuki N.; Yoon T. H.; Choi J. S.; Sanabria N.; Gulumian M.; Lynch I. NanoSolveIT Project: Driving nanoinformatics research to develop innovative and integrated tools for in silico nanosafety assessment. Comput. Struct. Biotechnol. J. 2020, 18, 583–602. 10.1016/j.csbj.2020.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbedo J. G. A. Impact of dataset size and variety on the effectiveness of deep learning and transfer learning for plant disease classification. Comput. Electron. Agric. 2018, 153, 46–53. 10.1016/j.compag.2018.08.013. [DOI] [Google Scholar]
- Li Y.; Yang J. C. Meta-learning baselines and database for few-shot classification in agriculture. Comput. Electron. Agr. 2021, 182, 106055. 10.1016/j.compag.2021.106055. [DOI] [Google Scholar]
- Zhong F. M.; Chen Z. K.; Zhang Y. C.; Xia F. Zero- and few-shot learning for diseases recognition of Citrus aurantium L. using conditional adversarial autoencoders. Comput. Electron. Agr. 2020, 179, 105828. 10.1016/j.compag.2020.105828. [DOI] [Google Scholar]
- Blaschke T. Object based image analysis for remote sensing. ISPRS J. Photogramm. 2010, 65, 2–16. 10.1016/j.isprsjprs.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q.; Chang N. B.; Yang C. H.; Srilakshmi K. R. Combination of multispectral remote sensing, variable rate technology and environmental modeling for citrus pest management. J. Environ. Manage. 2008, 86, 14–26. 10.1016/j.jenvman.2006.11.019. [DOI] [PubMed] [Google Scholar]