Abstract
Background
Different first‐line drug classes for patients with hypertension are often assumed to have similar effectiveness with respect to reducing mortality and morbidity outcomes, and lowering blood pressure. First‐line low‐dose thiazide diuretics have been previously shown to have the best mortality and morbidity evidence when compared with placebo or no treatment. Head‐to‐head comparisons of thiazides with other blood pressure‐lowering drug classes would demonstrate whether there are important differences.
Objectives
To compare the effects of first‐line diuretic drugs with other individual first‐line classes of antihypertensive drugs on mortality, morbidity, and withdrawals due to adverse effects in patients with hypertension. Secondary objectives included assessments of the need for added drugs, drug switching, and blood pressure‐lowering.
Search methods
Cochrane Hypertension's Information Specialist searched the Cochrane Hypertension Specialized Register, CENTRAL, MEDLINE, Embase, and trials registers to March 2021. We also checked references and contacted study authors to identify additional studies. A top‐up search of the Specialized Register was carried out in June 2022.
Selection criteria
Randomized active comparator trials of at least one year's duration were included. Trials had a clearly defined intervention arm of a first‐line diuretic (thiazide, thiazide‐like, or loop diuretic) compared to another first‐line drug class: beta‐blockers, calcium channel blockers, alpha adrenergic blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, direct renin inhibitors, or other antihypertensive drug classes. Studies had to include clearly defined mortality and morbidity outcomes (serious adverse events, total cardiovascular events, stroke, coronary heart disease (CHD), congestive heart failure, and withdrawals due to adverse effects).
Data collection and analysis
We used standard Cochrane methodological procedures.
Main results
We included 20 trials with 26 comparator arms randomizing over 90,000 participants. The findings are relevant to first‐line use of drug classes in older male and female hypertensive patients (aged 50 to 75) with multiple co‐morbidities, including type 2 diabetes. First‐line thiazide and thiazide‐like diuretics were compared with beta‐blockers (six trials), calcium channel blockers (eight trials), ACE inhibitors (five trials), and alpha‐adrenergic blockers (three trials); other comparators included angiotensin II receptor blockers, aliskiren (a direct renin inhibitor), and clonidine (a centrally acting drug). Only three studies reported data for total serious adverse events: two studies compared diuretics with calcium channel blockers and one with a direct renin inhibitor.
Compared to first‐line beta‐blockers, first‐line thiazides probably result in little to no difference in total mortality (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.84 to 1.10; 5 trials, 18,241 participants; moderate‐certainty), probably reduce total cardiovascular events (5.4% versus 4.8%; RR 0.88, 95% CI 0.78 to 1.00; 4 trials, 18,135 participants; absolute risk reduction (ARR) 0.6%, moderate‐certainty), may result in little to no difference in stroke (RR 0.85, 95% CI 0.66 to 1.09; 4 trials, 18,135 participants; low‐certainty), CHD (RR 0.91, 95% CI 0.78 to 1.07; 4 trials, 18,135 participants; low‐certainty), or heart failure (RR 0.69, 95% CI 0.40 to 1.19; 1 trial, 6569 participants; low‐certainty), and probably reduce withdrawals due to adverse effects (10.1% versus 7.9%; RR 0.78, 95% CI 0.71 to 0.85; 5 trials, 18,501 participants; ARR 2.2%; moderate‐certainty).
Compared to first‐line calcium channel blockers, first‐line thiazides probably result in little to no difference in total mortality (RR 1.02, 95% CI 0.96 to 1.08; 7 trials, 35,417 participants; moderate‐certainty), may result in little to no difference in serious adverse events (RR 1.09, 95% CI 0.97 to 1.24; 2 trials, 7204 participants; low‐certainty), probably reduce total cardiovascular events (14.3% versus 13.3%; RR 0.93, 95% CI 0.89 to 0.98; 6 trials, 35,217 participants; ARR 1.0%; moderate‐certainty), probably result in little to no difference in stroke (RR 1.06, 95% CI 0.95 to 1.18; 6 trials, 35,217 participants; moderate‐certainty) or CHD (RR 1.00, 95% CI 0.93 to 1.08; 6 trials, 35,217 participants; moderate‐certainty), probably reduce heart failure (4.4% versus 3.2%; RR 0.74, 95% CI 0.66 to 0.82; 6 trials, 35,217 participants; ARR 1.2%; moderate‐certainty), and may reduce withdrawals due to adverse effects (7.6% versus 6.2%; RR 0.81, 95% CI 0.75 to 0.88; 7 trials, 33,908 participants; ARR 1.4%; low‐certainty).
Compared to first‐line ACE inhibitors, first‐line thiazides probably result in little to no difference in total mortality (RR 1.00, 95% CI 0.95 to 1.07; 3 trials, 30,961 participants; moderate‐certainty), may result in little to no difference in total cardiovascular events (RR 0.97, 95% CI 0.92 to 1.02; 3 trials, 30,900 participants; low‐certainty), probably reduce stroke slightly (4.7% versus 4.1%; RR 0.89, 95% CI 0.80 to 0.99; 3 trials, 30,900 participants; ARR 0.6%; moderate‐certainty), probably result in little to no difference in CHD (RR 1.03, 95% CI 0.96 to 1.12; 3 trials, 30,900 participants; moderate‐certainty) or heart failure (RR 0.94, 95% CI 0.84 to 1.04; 2 trials, 30,392 participants; moderate‐certainty), and probably reduce withdrawals due to adverse effects (3.9% versus 2.9%; RR 0.73, 95% CI 0.64 to 0.84; 3 trials, 25,254 participants; ARR 1.0%; moderate‐certainty).
Compared to first‐line alpha‐blockers, first‐line thiazides probably result in little to no difference in total mortality (RR 0.98, 95% CI 0.88 to 1.09; 1 trial, 24,316 participants; moderate‐certainty), probably reduce total cardiovascular events (12.1% versus 9.0%; RR 0.74, 95% CI 0.69 to 0.80; 2 trials, 24,396 participants; ARR 3.1%; moderate‐certainty) and stroke (2.7% versus 2.3%; RR 0.86, 95% CI 0.73 to 1.01; 2 trials, 24,396 participants; ARR 0.4%; moderate‐certainty), may result in little to no difference in CHD (RR 0.98, 95% CI 0.86 to 1.11; 2 trials, 24,396 participants; low‐certainty), probably reduce heart failure (5.4% versus 2.8%; RR 0.51, 95% CI 0.45 to 0.58; 1 trial, 24,316 participants; ARR 2.6%; moderate‐certainty), and may reduce withdrawals due to adverse effects (1.3% versus 0.9%; RR 0.70, 95% CI 0.54 to 0.89; 3 trials, 24,772 participants; ARR 0.4%; low‐certainty).
For the other drug classes, data were insufficient. No antihypertensive drug class demonstrated any clinically important advantages over first‐line thiazides.
Authors' conclusions
When used as first‐line agents for the treatment of hypertension, thiazides and thiazide‐like drugs likely do not change total mortality and likely decrease some morbidity outcomes such as cardiovascular events and withdrawals due to adverse effects, when compared to beta‐blockers, calcium channel blockers, ACE inhibitors, and alpha‐blockers.
Keywords: Aged; Female; Humans; Male; Middle Aged; Adrenergic beta-Antagonists; Adrenergic beta-Antagonists/adverse effects; Angiotensin Receptor Antagonists; Angiotensin Receptor Antagonists/adverse effects; Angiotensin-Converting Enzyme Inhibitors; Angiotensin-Converting Enzyme Inhibitors/adverse effects; Antihypertensive Agents; Antihypertensive Agents/adverse effects; Calcium Channel Blockers; Calcium Channel Blockers/adverse effects; Coronary Disease; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/drug therapy; Diuretics; Diuretics/adverse effects; Heart Failure; Heart Failure/drug therapy; Hypertension; Hypertension/chemically induced; Stroke; Stroke/drug therapy; Thiazides; Thiazides/adverse effects
Plain language summary
What are the benefits and harms of diuretics given as a first treatment compared to other drug classes for hypertension (high blood pressure)?
Key messages:
‐ Thiazides and thiazide‐like drugs (diuretics) probably decrease some adverse cardiovascular events compared to beta‐blockers, calcium channel blockers, ACE inhibitors, and alpha‐blockers when used as the first‐line drug for the treatment of hypertension.
‐ Total mortality is probably not different between diuretics and the other drug classes.
‐ First‐line diuretics likely reduce total cardiovascular events and heart failure compared to calcium channel blockers and alpha‐blockers.
‐ First‐line diuretics likely reduce withdrawals from the studies due to unwanted or harmful (adverse) effects compared to beta‐blockers, calcium channel blockers, ACE inhibitors, and alpha‐blockers.
What is hypertension (high blood pressure)?
Hypertension is defined using resting blood pressures: mild (140 to 159/90 to 99 mmHg), moderate (160 to 179/100 to 109 mmHg), and severe (180/110 mmHg or higher). Uncontrolled high blood pressure can lead to stroke, heart attack, heart failure, and kidney damage. Blood pressure‐lowering drugs have been proven to reduce these adverse events in people aged 60 years and older with moderate to severe elevations of blood pressure; they also reduce stroke in adults under 60 years old with hypertension.
How is hypertension treated?
This review focused on blood pressure‐lowering classes of drugs given as the initial drug treatment when lifestyle interventions are insufficient. The drug classes of interest include diuretics (e.g. hydrochlorothiazide, chlorthalidone); beta‐blockers (e.g. propranolol, atenolol); calcium channel blockers (e.g. amlodipine, nifedipine); angiotensin‐converting enzyme (ACE) inhibitors (e.g. lisinopril, enalapril); angiotensin receptor blockers (e.g. candesartan, losartan); renin inhibitors (e.g. aliskiren); alpha‐blockers (e.g. doxazosin); and centrally acting drugs (e.g. clonidine).
What did we want to find out?
We wanted to find out whether the benefits and harms of diuretics given first for hypertension differed from other drug classes.
What did we do?
We searched for studies that compared first‐line diuretics with other blood pressure‐lowering drug classes in people with hypertension. We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 20 studies that involved over 90,000 people with hypertension and lasted five years on average.
Main results
Mortality is probably not different between diuretics and the other drug classes when used in the first‐line setting. First‐line diuretics probably reduce cardiovascular events when compared to beta‐blockers. First‐line diuretics probably reduce cardiovascular events and heart failure when compared to calcium channel blockers. First‐line diuretics probably reduce stroke slightly when compared to ACE inhibitors. First‐line diuretics probably reduce total cardiovascular events, stroke, and heart failure when compared with alpha‐blockers. Diuretics likely reduce withdrawals due to adverse effects when compared to beta‐blockers, calcium channel blockers, ACE inhibitors, and alpha‐blockers. There were not enough data to compare against angiotensin receptor blockers and renin inhibitors.
What are the main limitations of the evidence?
More head‐to‐head trials are needed comparing low‐dose thiazides with angiotensin receptor blockers and renin inhibitors.
How up‐to‐date is the evidence?
The evidence is up‐to‐date to March 2021.
Summary of findings
Summary of findings 1. First‐line thiazides compared with first‐line beta‐blockers for hypertension in adults.
| First‐line thiazides versus first‐line beta‐blockers for hypertension in adults | ||||||
| Patient or population: adults with hypertension Setting: outpatients Intervention: first‐line thiazides Comparison: first‐line beta‐blockers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Risk ratio (95% CI) |
№ of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with beta‐blockers | Risk with thiazides | |||||
| Total mortality Duration: 1 to 5.8 years |
44 per 1000 | 42 per 1000 (37 to 48) | RR 0.96 (0.84 to 1.10) | 18,241 (5 studies) | ⨁⨁⨁◯ MODERATE1 | Probably little to no difference (I2 = 22%) |
| Total serious adverse events | — | — | — | — | — | None of the studies reported this outcome |
| Total cardiovascular events Duration: 1 to 5.8 years |
54 per 1000 | 48 per 1000 | RR 0.88 (0.78 to 1.00) |
18,135 (4 studies) |
⨁⨁⨁◯ MODERATE1 | First‐line diuretics probably lower cardiovascular events (I2 = 44%) (ARR = 0.6%) |
| Total stroke Duration: 1 to 5.8 years |
14 per 1000 | 12 per 1000 (9 to 15) | RR 0.85 (0.66 to 1.09) | 18,135 (4 studies) | ⨁⨁◯◯ LOW1,2 | May be little to no difference (I2 = 73%) |
| Total CHD Duration: 1 to 5.8 years |
35 per 1000 | 32 per 1000 | RR 0.91 (0.78 to 1.07) |
18,135 (4 studies) |
⨁⨁◯◯ LOW1,2 | May be little to no difference (I2 = 67%) |
| Total congestive heart failure Duration: 3.8 years |
10 per 1000 | 7 per 1000 (4 to 12) | RR 0.69 (0.40 to 1.19) | 6569 (1 study) | ⨁⨁◯◯ LOW1,3 | May be little to no difference |
| Withdrawals due to adverse effects Duration: 1 to 5.8 years |
101 per 1000 | 79 per 1000 (0.72 to 0.86) |
RR 0.78 (0.71 to 0.85) |
18,501 (5 studies) |
⨁⨁⨁◯ MODERATE2 |
First‐line diuretics probably lower withdrawals due to adverse effects (I2 = 91%) (ARR = 2.2%) |
| *The risk in the thiazide group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CHD: coronary heart disease; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level because studies had notable levels of unclear or high risk of bias.
2Downgraded one level because of notable inconsistency between the outcomes of studies.
3Downgraded one level due to imprecision.
Summary of findings 2. First‐line thiazides compared with first‐line calcium channel blockers for hypertension in adults.
| First‐line thiazides versus first‐line calcium channel blockers for hypertension in adults | ||||||
| Patient or population: adults with hypertension Setting: outpatients Intervention: first‐line thiazides Comparison: first‐line calcium channel blockers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with calcium channel blockers | Risk with thiazides | |||||
| Total mortality Duration: 1 to 5 years |
109 per 1000 | 111 per 1000 (105 to 118) | RR 1.02 (0.96 to 1.08) |
35,417 (7 studies) | ⨁⨁⨁◯ MODERATE1 | Probably little to no difference (I2 = 0%) |
| Total serious adverse events Duration: 1.75 to 3 years |
106 per 1000 | 116 per 1000 (103 to 131) |
RR 1.09 (0.97 to 1.24) |
7204 (2 studies) |
⨁⨁◯◯ LOW1,2 |
May be little to no difference (I2 = 80%) |
| Total cardiovascular events Duration: 1 to 5 years |
143 per 1000 | 133 per 1000 (127 to 140) |
RR 0.93 (0.89 to 0.98) |
35,217 (6 studies) |
⨁⨁⨁◯ MODERATE1 | Probably lower (I2 = 0%) (ARR=1.0%) |
| Total stroke Duration: 1 to 5 years |
34 per 1000 | 36 per 1000 (32 to 40) | RR 1.06 (0.95 to 1.18) |
35,217 (6 studies) | ⨁⨁⨁◯ MODERATE1 | Probably little to no difference (I2 = 0%) |
| Total CHD Duration: 1 to 5 years |
66 per 1000 | 66 per 1000 (61 to 71) |
RR 1.00 (0.93 to 1.08) |
35,217 (6 studies) |
⨁⨁⨁◯ MODERATE1 | Probably little to no difference (I2 = 0%) |
| Total congestive heart failure Duration: 1 to 5 years |
44 per 1000 | 32 per 1000 (29 to 36) | RR 0.74 (0.66 to 0.82) |
35,217 (6 studies) | ⨁⨁⨁◯ MODERATE1 | Probably lower (I2 = 10%) (ARR = 1.2%) |
| Withdrawals due to adverse effects Duration: 1 to 5 years |
76 per 1000 | 62 per 1000 (57 to 68) |
RR 0.81 (0.75 to 0.88) |
33,908 (7 studies) |
⨁⨁◯◯ LOW1,2 |
May be lower (I2 = 74%) (ARR = 1.4%) |
| *The risk in the thiazide group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARR: absolute risk reduction; CHD: coronary heart disease; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect | ||||||
1Downgraded one level because studies had notable levels of unclear or high risk of bias.
2Downgraded one level because of notable inconsistency between the outcomes of studies.
Summary of findings 3. First‐line thiazides compared with first‐line ACE inhibitors for hypertension in adults.
| First‐line thiazides versus first‐line ACE inhibitors for hypertension in adults | ||||||
| Patient or population: adults with hypertension Setting: outpatients Intervention: first‐line thiazides Comparison: first‐line ACE inhibitors | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with ACE inhibitors | Risk with thiazides | |||||
| Total mortality Duration: 1 to 5 years |
122 per 1000 | 122 per 1000 (116 to 130) | RR 1.00 (0.95 to 1.07) |
30,961 (3 studies) | ⨁⨁⨁◯ MODERATE1 | Probably little to no difference (I2 = 0%) |
| Total serious adverse events | — | — | — | — | — | None of the studies reported this outcome |
| Total cardiovascular events Duration: 2.6 to 5 years |
170 per 1000 | 165 per 1000 | RR 0.97 (0.92 to 1.02) |
30,900 (3 studies) |
⨁⨁◯◯ LOW1,2 | May be little to no difference (I2 = 55%) |
| Total stroke Duration: 2.6 to 5 years |
47 per 1000 | 41 per 1000 (37 to 46) | RR 0.89 (0.80 to 0.99) |
30,900 (3 studies) | ⨁⨁⨁◯ MODERATE1 | First‐line thiazides probably lower total stroke slightly (I2 = 0%) (ARR = 0.6%) |
| Total CHD Duration: 2.6 to 5 years |
79 per 1000 | 82 per 1000 | RR 1.03 (0.96 to 1.12) |
30,900 (3 studies) |
⨁⨁⨁◯ MODERATE1 | Probably little to no difference (I2 = 21%) |
| Total congestive heart failure Duration: 4 to 5 years |
45 per 1000 | 42 per 1000 (37 to 46) | RR 0.94 (0.84 to 1.04) |
30,392 (2 studies) | ⨁⨁⨁◯ MODERATE1 | Probably little to no difference (I2 = 36%) |
| Withdrawals due to adverse effects Duration: 1 to 5 years |
39 per 1000 | 29 per 1000 (25 to 33) |
RR 0.73 (0.64 to 0.84) |
25,254 (3 studies) |
⨁⨁⨁◯ MODERATE1 | First‐line thiazides probably lower withdrawals due to adverse effects (I2 = 14%) (ARR = 1.0%) |
| *The risk in the thiazide group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACE: angiotensin converting enzyme; ARR: absolute risk reduction; CHD: coronary heart disease; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect | ||||||
1Downgraded one level because studies had notable levels of unclear or high risk of bias.
2Downgraded one level because of notable inconsistency between the outcomes of studies.
Summary of findings 4. First‐line thiazides compared with first‐line alpha‐blockers for hypertension in adults.
| First‐line thiazides versus first‐line alpha‐blockers for hypertension in adults | ||||||
| Patient or population: adults with hypertension Setting: outpatients Intervention: first‐line thiazides Comparison: first‐line alpha‐blockers | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with alpha‐blockers | Risk with thiazides | |||||
| Total mortality Duration: 3.2 years |
57 per 1000 | 56 per 1000 (50 to 62) | RR 0.98 (0.88 to 1.09) |
24,316 (1 study) | ⨁⨁⨁◯ MODERATE1 | Probably little to no difference |
| Total serious adverse events | — | — | — | — | — | None of the studies reported this outcome |
| Total cardiovascular events Duration: 3 to 3.2 years |
121 per 1000 | 90 per 1000 | RR 0.74 (0.69 to 0.80) |
24,396 (2 studies) | ⨁⨁⨁◯ MODERATE1 | First‐line thiazides probably lower cardiovascular events (I2 = 0%) (ARR = 3.1%) |
| Total stroke Duration: 3 to 3.2 years |
27 per 1000 | 23 per 1000 (20 to 27) | RR 0.86 (0.73 to 1.01) |
24,396 (2 studies) | ⨁⨁⨁◯ MODERATE1 | First‐line thiazides probably lower stroke (I2 = 29%) (ARR = 0.4%) |
| Total CHD Duration: 3 to 3.2 years |
41 per 1000 | 40 per 1000 | RR 0.98 (0.86 to 1.11) |
24,396 (2 studies) |
⨁⨁◯◯ LOW1,2 | May be little to no difference (I2 = 52%) |
| Total congestive heart failure Duration: 3.2 years |
54 per 1000 | 28 per 1000 (24 to 31) | RR 0.51 (0.45 to 0.58) |
24,316 (1 study) | ⨁⨁⨁◯ MODERATE1 | First‐line thiazides probably lower heart failure (ARR = 2.6%) |
| Withdrawals due to adverse effects Duration: 1 to 3.2 years |
13 per 1000 | 9 per 1000 (7 to 12) |
RR 0.70 (0.54 to 0.89) |
24,772 (3 studies) |
⨁⨁◯◯ LOW1,2 |
First‐line thiazides may reduce withdrawals due to adverse effects (I2 = 82%) (ARR = 0.4%) |
| *The risk in the thiazide group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARR: absolute risk reduction; CHD: coronary heart disease; CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level because studies had notable levels of unclear or high risk of bias.
2Downgraded one level because of notable inconsistency between the outcomes of studies.
Background
Description of the condition
Elevated blood pressure (hypertension) is a chronic condition in which the blood pressure in the arteries is persistently elevated. It has been divided into three categories, based on resting blood pressures, measured in a standard way: mild hypertension (140 to 159/90 to 99 mmHg), moderate hypertension (160 to 179/100 to 109 mmHg), and severe hypertension (180/110 mmHg or higher) (James 2014). Most people with high blood pressure have no signs or symptoms and most have primary or essential hypertension, where there is no identifiable cause for the high blood pressure. Uncontrolled persistent resting high blood pressure increases the risk of stroke, heart attack, heart failure, and kidney damage (James 2014).
High blood pressure should initially be controlled by lifestyle changes, including eating a healthy diet with less salt, exercising regularly, quitting smoking, and maintaining a healthy weight. When these lifestyle changes are insufficient, treatment with antihypertensive drugs is recommended. Antihypertensive drugs have been proven to reduce mortality, stroke, myocardial infarction, and heart failure in adults 60 years of age and older with moderate to severe hypertension (Musini 2019), and to reduce stroke in adults under 60 (Musini 2017). Key guidelines do have an impact on how hypertension is managed globally (Whelton 2018; Williams 2018). However, they can be confusing for clinicians as they can be contradictory in their recommendations (Bakris 2019). We deliberately do not recommend any particular hypertension guideline, as all of the many available guidelines are conflicted to some degree due to funding and/or influence by the manufacturers of antihypertensive drugs (Ben‐Eltriki 2021). These conflicts tend to lead to non‐evidence‐based overdiagnosis and overtreatment.
Description of the intervention
One of the major decisions involved in the management of patients with elevated blood pressure is which class of drug to choose to start with (first‐line therapy). Presently, the available evidence is limited and lacks head‐to‐head comparisons of individual drug classes, which examine outcomes that are most important to patients with hypertension. There have been a number of systematic reviews assessing the effectiveness of antihypertensive therapy. However, most have used step care therapy and allowed the combination of different drug classes. Furthermore, they concentrated on overall effectiveness versus untreated controls (Collins 1990; Gueyffier 1996), or effectiveness in specific age groups (Insua 1994; MacMahon 1993; Musini 2017; Musini 2019; Thijs 1992). When different drug classes are combined in a systematic review, there is an underlying assumption that the lowering of blood pressure is independent of the drugs that are used and the mechanism by which decreased blood pressure is achieved. It is also possible that the pharmacological action by which a drug class lowers blood pressure will have additional effects in the body, which are independent of changes in blood pressure. These other actions, both known and unknown, could enhance or negate the benefits and harms of a drug and must be considered in the effect on different outcomes.
Thiazide diuretics are the most studied first‐line drug class and appear to have some advantages over the other drug classes (Wright 1999; Wright 2018). Thiazide and thiazide‐like diuretics are thus the most appropriate drug class to compare to other classes in head‐to‐head randomized controlled trials. The other classes include beta‐blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), calcium channel blockers, alpha‐adrenergic blockers, direct renin inhibitors, and centrally acting drugs.
How the intervention might work
Thiazide and thiazide‐like diuretics: the blood pressure‐lowering mechanism of action of thiazides is not fully understood. When administered acutely, thiazides lower blood pressure by causing diuresis, which reduces plasma volume and leads to a reduction in cardiac output. Chronic use of thiazides causes a reduction in blood pressure by lowering peripheral vascular resistance (vasodilation). Thiazides also may reduce blood pressure by inhibiting reabsorption of sodium (Na+) and chloride (Cl−) ions from the distal convoluted tubules in the kidneys by blocking the thiazide‐sensitive Na+‐Cl− symporter (Duarte 2010). They also increase calcium reabsorption at the distal tubule. By lowering the sodium concentration in the tubule epithelial cells, thiazides indirectly increase the activity of the basolateral Na+/Ca2+ antiporter, which facilitates the transport of Ca2+ from the epithelial cells into the renal interstitium. This movement of Ca2+ in turn decreases the intracellular Ca2+ concentration, which allows more Ca2+ to diffuse from the lumen of the tubules into epithelial cells via apical Ca2+‐selective channels (TRPV5). Thiazides are also thought to increase the reabsorption of Ca2+ by a mechanism involving the reabsorption of Na+ and Ca2+ in the proximal tubule in response to Na+ depletion. Some of this response may be due to augmentation of the action of parathyroid hormone (Longo 2010).
Beta‐blockers: beta‐blockers are competitive antagonists that block the receptor sites for the endogenous catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline) on adrenergic beta receptors of the sympathetic nervous system. Some block activation of all types of β‐adrenergic receptors and others are selective for one of the three known types of beta receptors, designated β1, β2 and β3 receptors. β1‐adrenergic receptors are located mainly in the heart and in the kidneys; β2‐adrenergic receptors are located mainly in the lungs, gastrointestinal tract, liver, uterus, vascular smooth muscle, and skeletal muscle; and β3‐adrenergic receptors are located in fat cells (Frishman 2005).
Angiotensin converting enzyme (ACE) inhibitors: ACE inhibitors block the conversion of angiotensin I (AI) to angiotensin II (AII). They thereby lower arteriolar resistance and increase venous capacity and lower resistance in blood vessels in the kidneys, and lead to increased excretion of sodium in the urine. Renin increases in concentration in the blood as a result of negative feedback of conversion of AI to AII. AI increases for the same reason; AII and aldosterone decrease. Bradykinin increases because of less inactivation by ACE (Dzau 1990).
Angiotensin II receptor blockers (ARBs): ARBs block the activation of AII AT1 receptors. Blockage of AT1 receptors directly causes vasodilation, reduces secretion of vasopressin, and reduces the production and secretion of aldosterone, among other actions. The combined effect reduces blood pressure (Rodgers 2001).
Calcium channel blockers: this class of antihypertensive drugs includes dihydropyridines and non‐dihydropyridines. They reduce blood pressure through various mechanisms, including: acting on vascular smooth muscle and causing an increase in arterial diameter (vasodilation); acting on cardiac muscles, where they reduce the force of contraction of the heart; slowing down the conduction of electrical activity within the heart and thus reducing the heart rate; and blocking the calcium signal on adrenal cortex cells thus directly reducing aldosterone production (Katz 1986).
Alpha‐adrenergic blockers: α1 adrenergic receptor blockers inhibit the binding of norepinephrine to the α1 receptors on the membrane of vascular smooth muscle cells. The primary effect of this inhibition is vasodilation, which decreases peripheral vascular resistance, leading to decreased blood pressure (Nash 1990).
Renin inhibitors: renin inhibitors bind the active site of the renin enzyme, thereby inhibiting its ability to cleave circulating angiotensinogen to AI and subsequently lowering circulating AI and AII concentrations (Shafiq 2008), leading to similar effects to the ACE inhibitors and ARBs.
Centrally acting drugs: these drugs act on the central nervous system to decrease sympathetic activity and reduce blood pressure. Examples include clonidine and alpha methyldopa.
Why it is important to do this review
A number of existing systematic reviews have compared first‐line drugs versus placebo or no treatment; these reviews concluded that thiazide diuretics are the first‐line therapy class associated with the best mortality and morbidity evidence (Psaty 1997; Wright 1999; Wright 2018). These findings would best be supported with a review of head‐to‐head randomized trials, where first‐line thiazide diuretics are compared with other drug classes. Previous attempts to do this include a review, which pooled data from first‐line drug treatment in antihypertensive trials (Collins 1990). These comparisons only included three trials that compared thiazides with beta‐blockers; one of these trials was not appropriate for this comparison as both treatment arms received thiazides (IPPSH 1985). Psaty 2003 performed a network meta‐analysis that combined direct and indirect comparisons of different first‐line drug classes and concluded that thiazide diuretics were as good as or better than other antihypertensive classes. Other Cochrane Reviews have compared first‐line beta‐blockers (Wiysonge 2017), calcium channel blockers (Zhu 2021), or inhibitors of the renin angiotensin system (Chen 2018), with other first‐line drug classes. Although some overlap exists between the comparisons in this Cochrane Review and other reviews (Chen 2018; Wiysonge 2017; Zhu 2021), this review is additive because it includes comparisons between diuretics and additional drug classes. Most importantly these reviews suggest that adverse cardiovascular outcomes are reduced more with first‐line diuretics as compared to the other classes of drugs. Therefore, using first‐line diuretics as the intervention is the most appropriate approach to this question.
This review builds on the previously published reviews Psaty 2003 and Wright 1999, with the objective of providing updated evidence about first‐line diuretics versus other classes of antihypertensive drugs to assist guideline developers and clinicians in choosing the most appropriate first‐line antihypertensive drug therapy based on the best available evidence of key effectiveness outcomes.
Objectives
Primary objective
To compare in head‐to‐head trials the effects of first‐line diuretic drugs versus other classes of antihypertensive drugs on morbidity, mortality, and withdrawals due to adverse drug effects in patients with hypertension.
Secondary objectives
To compare the percentage of patients requiring dose titration, addition of a second or third drug, and switching to other therapy.
To compare the blood pressure‐lowering efficacy at one year in the two groups.
Methods
Criteria for considering studies for this review
Types of studies
Trials were eligible if they:
were randomized controlled trials (RCTs); quasi‐randomized trials were not eligible for inclusion;
were of at least one year duration;
had study data that could be analyzed based on the intention‐to‐treat principle;
presented morbidity and mortality data that compared first‐line diuretics head‐to‐head with one or more other first‐line antihypertensive therapies.
Types of participants
Participants had to have a baseline resting blood pressure of at least 140 mmHg systolic or a diastolic blood pressure of at least 90 mmHg measured in a standard way on at least two occasions. Trials had to be limited to patients with elevated blood pressure or separately report outcome data on patients with elevated blood pressure as defined above.
Trials were not limited by any other factor or baseline risk. We assumed that age and co‐morbidities do not affect the relative risk reduction associated with drug treatment.
Types of interventions
Randomized controlled trials had to include treatment that was clearly defined as specific first‐line antihypertensive therapy: thiazide, thiazide‐like, or loop diuretics versus beta‐blockers, calcium channel blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, alpha‐adrenergic blockers, direct renin inhibitors, or centrally acting antihypertensive drugs. The majority (> 70%) of the patients in the treatment and control group should have been taking the first‐line drug class of interest after one year. Only initial combined therapy with potassium‐sparing diuretics (triamterene or amiloride) was allowed. These were included as there is evidence that they do not affect blood pressure (Heran 2012b). Supplemental drugs from other drug classes of interest were only allowed as stepped therapy in both groups, and only as long as they were not taken by over 50% of the patients. We assumed that these supplemental drugs may not systematically interact to affect the occurrence of the endpoints studied. We also assumed that there are no major differences in the effects of different drugs in the defined classes. All trials comparing a first‐line diuretic with one or more other first‐line antihypertensive drug classes were included irrespective of the dose used.
Types of outcome measures
Primary outcomes
Total mortality (death from all causes)
Total serious adverse events (patients with at least one serious adverse event)
Total number of people with at least one cardiovascular event including total stroke and total coronary heart disease (CHD) plus hospitalization or death from congestive heart failure and other significant vascular events such as ruptured aneurysms (does not include angina, transient ischemic attacks (TIAs), revascularization procedures or accelerated hypertension)
Total stroke including fatal and non‐fatal strokes
Total CHD including fatal and non‐fatal myocardial infarction and sudden or rapid cardiac death
Total congestive heart failure (death or hospitalization for heart failure)
Total withdrawals due to adverse effects
We analyzed all the primary outcomes as dichotomous outcomes, i.e. the number of people with at least one event. We excluded trials if they did not report any of the primary outcomes. When the trials did not report primary outcomes that exactly matched the above definitions, decisions by consensus among review authors were made based on maximizing the inclusion of the data and maintaining concordance with how the data were handled in previous systematic reviews (Chen 2018; Psaty 2003; Wiysonge 2017; Wright 1999). We assumed that the effects of antihypertensive treatment on outcomes would be independent of whether elevated blood pressure was defined in terms of systolic or diastolic pressure.
Secondary outcomes
Percentage of patients requiring dose titration and addition of a second or third drug
Percentage of patients switching to other antihypertensive therapies
Systolic and diastolic blood pressure at one year
Search methods for identification of studies
Electronic searches
We searched the following databases for randomized controlled trials (RCTs) without language or publication status restrictions:
the Cochrane Hypertension Specialized Register via the Cochrane Register of Studies (top‐up search 27 June 2022);
the Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 2) via the Cochrane Register of Studies (searched 25 March 2021);
MEDLINE Ovid (from 1998; searched 25 March 2021);
Embase Ovid (from 1998; searched 25 March 2021);
US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) (searched 26 March 2021);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched 26 March 2021).
Searches of MEDLINE and Embase were limited to 1998 onward (using the .dt. and.dc. commands, respectively) as it was assumed that pre‐1998 studies would have been identified by previous related systematic reviews (Psaty 2003; Wright 1999), and by searches of CENTRAL and the Cochrane Hypertension Specialized Register. In addition, we assessed the lists of references identified by the three Cochrane systematic reviews comparing first‐line therapy with beta‐blockers (Wiysonge 2017), calcium channel blockers (Zhu 2021), and drugs inhibiting the renin‐angiotension system (Chen 2018) with other classes of antihypertensive therapy to confirm that no trials comparing either of these classes with diuretics were missed. The Hypertension Specialized Register is updated weekly with new results from Ovid MEDLINE and Ovid Embase and updated monthly with searches of CENTRAL. Register searches for this review did not contain any exclusion commands.
The Information Specialist modeled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021)). We present the search strategies in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialized Register segment (which includes searches of MEDLINE and Epistemonikos for systematic reviews) to retrieve existing systematic reviews relevant to our topic, so that we could scan their reference lists for additional trials. The Specialized Register also includes searches of CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses, and Web of Knowledge.
We checked the bibliographies of included studies and relevant systematic reviews, including recent reviews comparing thiazide or thiazide‐like diuretics to other antihypertensive classes, to ensure identification of all relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
Data collection and analysis
Selection of studies
One review author (MR) screened the titles and abstracts resulting from the search strategies. We rejected articles on the initial screen only if it could be determined from the title or the abstract that the article was not a report of a randomized controlled trial (RCT) assessing diuretic monotherapy in a head‐to‐head comparison with another antihypertensive class in patients with hypertension. Two of three review authors (LP, MR, or JW) independently assessed the full‐text articles of studies that passed the initial screen according to the inclusion criteria listed in Criteria for considering studies for this review, with disagreements resolved through discussion or the involvement of a third review author (JW). We excluded trials that met the minimum inclusion criteria but only reported systolic and diastolic blood pressure outcomes.
Data extraction and management
Data extraction was completed by two review authors independently (MR, LP, or JW), cross‐checked and compared whenever possible to data from previously published meta‐analyses (Chen 2018; Psaty 2003; Wiysonge 2017; Wright 1999; Zhu 2021). The data extraction form included details of the study design, duration of treatment, baseline characteristics, number of patients lost to follow‐up, interventions, and outcomes.
Assessment of risk of bias in included studies
We assessed risk of bias in each trial using a modified version of Cochrane’s tool for assessing risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Six of the domains assessed were sequence generation, allocation concealment, blinding, incomplete outcome data, and within‐study selective outcome reporting. At least two of three review authors (LP, MR, or JW) independently assessed the risk of bias for each study based on these domains with ratings of 'low risk of bias', 'high risk of bias', and 'unclear risk'. We resolved discrepancies by discussion and consensus.
We also assessed trials for the use of supplemental drugs. We regarded high‐quality trials (low risk of bias) to be those designed such that the supplemental drugs for blood pressure not controlled by the first‐line drugs were the same for each arm of the trial. In this way, any difference in outcomes could be attributed to the first‐line drug. We judged trials designed to allow different supplemental drugs or in which the algorithms for treatment or stepped care with supplemental drug classes differed between comparative groups to be at high risk of bias.
Furthermore, we assessed trials for the presence of industry sponsorship (Lundh 2017). We considered studies that were clearly funded by a pharmaceutical company to have a high risk of bias. We judged studies with no clear industry sponsorship, but with authors who disclosed associations with pharmaceutical companies, to have an unclear risk of bias. We judged studies with no evidence of funding by a pharmaceutical company or author ties to pharmaceutical companies to have a low risk of bias.
Measures of treatment effect
Dichotomous data
We assessed dichotomous outcomes (total mortality, total serious adverse events, total cardiovascular events, total fatal and non‐fatal stroke, total coronary heart disease (CHD), total congestive heart failure, withdrawals due to adverse effects, dose titration and the addition of second or third drugs, and switching therapies) using the risk ratio (RR), along with the 95% confidence interval (CI).
Continuous data
We assessed continuous data (systolic and diastolic blood pressure) using the mean difference (MD) along with the 99% CI.
Unit of analysis issues
Studies with multiple treatment groups
We did not expect to find cluster‐RCTs for this clinical question as it would be very difficult to cluster by physician. Cross‐over RCTs are not possible because of the one‐year duration requirement. We assessed studies with multiple treatment groups (ALLHAT 2000/2002; Materson 1993) using the strategy of including each pair‐wise comparison separately according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not undertake a comparison of diuretics with all other antihypertensive drugs pooled to avoid double‐counting the first‐line diuretic group.
We attempted to include people with at least one event for each of the outcomes. However, in studies such as ALLHAT 2000/2002, where it was not entirely clear, we assumed that the data reported were people with at least one event and not total events.
Dealing with missing data
When published articles did not provide data for specific outcomes or provide sufficient detail to permit full assessment, we contacted the authors. Specifically, we did this and received additional data for the PREVER‐treatment 2016 trial.
When data were reported only as graph‐based images and not numerically, we estimated values following analysis with an imaging software (Rohatgi 2021).
Several studies described using an intention‐to‐treat (ITT) analysis (ALLHAT 2000/2002; ALPINE 2003; ANBP2 2003; DAPHNE 2002; HAPPHY 1987; INSIGHT 2000; MIDAS 1996; MRC 1985; MRC 1992; NESTOR 2004; PHYLLIS 2004; PREVER‐treatment 2016; SHELL 2003; VHAS 1997). In most cases, this was defined as the analysis of all randomized patients regardless of how long they remained in the trial (note that some studies further specified that one study visit or one treatment was required post‐randomization), and used last observed data with no declared strategy for imputing missing data. The PREVER‐treatment 2016 trial did state that it included imputed estimates from patients who were lost to follow‐up or who had minor protocol deviations; no further information on how estimates were imputed was provided.
Assessment of heterogeneity
We assessed heterogeneity of treatment effect between the trials using a standard Chi2 test for heterogeneity. We applied the fixed‐effect model to obtain summary statistics of pooled trials. We used the I2 statistic to estimate the percentage of variability due to heterogeneity rather than sampling error. If substantial heterogeneity was present (I2 value greater than 50%), then we explored reasons for heterogeneity using sensitivity analyses (Sensitivity analysis). These included the effect of small trials, the effect of supplementary drugs, the effect of high doses of thiazides, and the effect of thiazide or thiazide‐like drugs.
Assessment of reporting biases
We did not create funnel plots as there were fewer than 10 trials for each comparison. In future updates, if there are more than 10 trials in a comparison we will create funnel plots to identify evidence of small‐study effects by visual inspection of asymmetry and by Egger's test (Higgins 2021).
Data synthesis
We conducted data synthesis and analyses using the Cochrane Review Manager software, RevMan 5.4 (RevMan 2020). Quantitative analyses of outcomes were based on ITT results, where possible. We used a Mantel Haenszel fixed‐effect model for dichotomous outcomes, which we presented as a RR with 95% CI. We chose this model a priori because we anticipated that we would have large and small trials and we wanted the most weight to go to the larger trials. When substantial heterogeneity was present (I2 > 50%) we explored this using sensitivity analysis. We calculated absolute risk reduction (ARR) = risk difference x 100 and to number needed to treat for an additional beneficial outcome (NNTB) = 1/risk difference for outcomes that had moderate or higher certainty between diuretics and comparators. Continuous outcomes such as systolic and diastolic blood pressure are presented as a MD with 99% CI using an inverse variance fixed‐effect model. If the trial did not report the within‐study variance for decrease in blood pressure (ANBP2 2003; INSIGHT 2000; PHYLLIS 2004; SHELL 2003; VA 1982), we imputed the standard deviation (SD) from the average SD from the other trials. This imputation is acknowledged as a limitation, thus we reported the 99% CI instead of the standard 95% CI.
The data synthesis methods listed here differ from the original protocol (Reinhart 2011). These changes were approved following a review of updated analytical standards for meta‐analysis as well as discussion and consensus among the review authors.
Subgroup analysis and investigation of heterogeneity
The review protocol noted that results of trials restricted to patients with isolated systolic hypertension would be analyzed as a separate group; however, only one small study (Tresukosol 2005; N = 200) included only patients with isolated systolic hypertension and this subgroup analysis was therefore not possible.
Sensitivity analysis
To test the robustness of our results, we performed pre‐defined sensitivity analyses. We evaluated the effect of removing the largest trial (ALLHAT 2000/2002). We also investigated the effects of removing small trials (N < 1000 in each comparison). We tested the effect of supplemental drugs by first removing trials without supplemental drugs. We then assessed the effect further by removing trials where different supplemental drug classes or doses were allowed in each arm.
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro GDT software to present the summary of findings tables (GRADEpro GDT). As planned, we included all seven primary outcomes: total mortality, total serious adverse events, total cardiovascular events, total stroke, total coronary heart disease, total congestive heart failure, and withdrawals due to adverse events for four clinically important comparisons: first‐line thiazides versus first‐line beta‐blockers, first‐line thiazides versus first‐line calcium channel blockers, first‐line thiazides versus first‐line ACE inhibitors, and first‐line thiazides versus first‐line alpha‐blockers.
We considered five factors in grading the overall certainty of evidence: limitations in study design and implementation, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision in results, and high probability of publication bias. This approach specifies four levels of certainty: high, moderate, low, and very low certainty. The highest certainty rating is initially assigned to randomized trial evidence and may be downgraded by one level for each factor, up to a maximum of three levels for all factors. If there are severe problems for any one factor (when assessing limitations in study design and implementation, in concealment of allocation, loss of blinding, or attrition over 50% of participants during follow‐up), randomized trial evidence may fall by two levels due to that factor alone.
Results
Description of studies
This review includes all randomized head‐to‐head trials of at least one year duration comparing first‐line diuretics with other individual antihypertensive drug classes and reporting morbidity and mortality outcomes.
Results of the search
Electronic searches up to March 2021 retrieved 9646 unique, de‐duplicated records. Of these 9646 records, we considered 157 full‐text records potentially eligible after primary screening, and 20 studies (randomizing > 90,000 participants) met the inclusion criteria following the full‐text screen (Figure 1). These studies include the nine trials that met the inclusion criteria and had been published prior to 1998 from an earlier systematic review (Wright 1999). We also identified one ongoing study (NCT02217852) (see Characteristics of ongoing studies). A top‐up search of the Cochrane Hypertension Specialized Register to June 2022 retrieved 51 unique records, but did not yield additional included studies.
1.
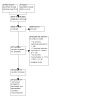
Included studies
We included a total of 20 parallel‐group randomized trials with 26 comparator arms in the review (see Characteristics of included studies). Study sample size ranged from fewer than 100 participants (DAPHNE 2002) to over 40,000 participants (ALLHAT 2000/2002); most studies had at least 500 participants and nine had over 1000 participants. Nearly all the studies took place in Western Europe and North America, except for three studies conducted in Japan (NICS‐EH 1999), Australia (ANBP2 2003), and Brazil (PREVER‐treatment 2016). All included studies enrolled participants with primary hypertension, and some assessed participants who had additional pre‐specified comorbidities or cardiovascular risk factors such as diabetes (ALLHAT 2000/2002; DAPHNE 2002; NESTOR 2004; PHYLLIS 2004). The average participant's age in most included studies was 50 to 60 years, although some trials specifically assessed participants who were older (55 years and older: ALLHAT 2000/2002; ANBP2 2003; INSIGHT 2000; NICS‐EH 1999; Tresukosol 2005; SHELL 2003). Five studies were restricted to males (Berglund 1981; DAPHNE 2002; HAPPHY 1987; Materson 1993; VA 1982); the remaining studies had both male and female participants.
Fifteen studies used first‐line thiazide diuretics: in 11 studies the drug was hydrochlorothiazide (ALPINE 2003; ANBP2 2003; DAPHNE 2002; INSIGHT 2000; Materson 1993; MIDAS 1996; MRC 1992; PHYLLIS 2004; Schmieder 2009; Tresukosol 2005; VA 1982); in two it was bendrofluazide (Berglund 1981; MRC 1985); in one it was trichlormethiazide (NICS‐EH 1999); and in one it was either hydrochlorothiazide or bendrofluazide (HAPPHY 1987). Five studies used first‐line thiazide‐like diuretics: chlorthalidone (ALLHAT 2000/2002; PREVER‐treatment 2016; SHELL 2003; VHAS 1997) and indapamide (NESTOR 2004). The largest trial, ALLHAT 2000/2002, used chlorthalidone, therefore the total number of participants treated with a thiazide‐like diuretic was similar to the number treated with a thiazide diuretic. First‐line thiazide and thiazide‐like diuretics were compared with the following first‐line antihypertensive drugs: calcium channel blockers (eight studies: ALLHAT 2000/2002; INSIGHT 2000; Materson 1993; MIDAS 1996; NICS‐EH 1999; SHELL 2003; Tresukosol 2005; VHAS 1997), beta‐blockers (six studies: Berglund 1981; HAPPHY 1987; Materson 1993; MRC 1985; MRC 1992; VA 1982), ACE inhibitors (five studies: ALLHAT 2000/2002; ANBP2 2003; Materson 1993; NESTOR 2004; PHYLLIS 2004), alpha‐adrenergic blockers (three studies: ALLHAT 2000/2002; DAPHNE 2002; Materson 1993), angiotensin II receptor blockers (two studies: ALPINE 2003; PREVER‐treatment 2016), direct renin inhibitor (Schmieder 2009), and a centrally acting drug, clonidine (Materson 1993).
The duration of follow‐up ranged from one year (six trials, ALPINE 2003; Berglund 1981; Materson 1993; NESTOR 2004; Schmieder 2009; VA 1982) to 5.8 years in the longest trial (MRC 1992). Five trials were five years or longer in duration (ALLHAT 2000/2002; MRC 1985; MRC 1992; NICS‐EH 1999; SHELL 2003). In all trials except one, the drugs were administered in standard doses once daily in the morning. In the one exception, the drugs were administered twice daily (MIDAS 1996). The thiazide or thiazide‐like doses were low‐dose except for three older trials where they were high‐dose (HAPPHY 1987; MRC 1985; VA 1982). High‐dose thiazides were standard therapy at the time these trials were conducted. The details of the drug doses are provided in the Characteristics of included studies table.
All included studies reported at least one primary outcome of interest and the most frequently reported outcomes included total mortality (16 studies), withdrawals due to adverse effects (16 studies), total CHD events (15 studies), total fatal and non‐fatal stroke events (14 studies), and total cardiovascular events (13 studies). Studies that reported changes in blood pressure but no other outcomes of interest were not included in this review.
Excluded studies
Fifty‐six excluded studies and the reasons for exclusion are described in the Characteristics of excluded studies table. The trial being less than 12 months in duration was a common reason for exclusion, occurring in 18 studies (Cho 2008; Cooper‐DeHoff 2010; Ebbs 2001; GENRES 2007; Iyalomhe 2014; Jordan 2012; Khan 2008; Klingbeil 2000; LIVE 1998; Mann 2002; Morgan 2004; Oshchepkova 2007; PEAR 2012; Pool 2009; Rasmussen 2006; SALT 2007; Schwartz 2013; Yasuda 2015). An equally common reason was that the study did not report any primary outcome. This was the reason in 18 studies (AVEC 2012; Caruso 2004; Galzerano 2004; Grassi 2006; Mahmud 2009; Posadzy‐Malaczynska 2014; Schram 2005; Shionoiri 2000; Sierra 2004; SPREAD 2006; Stritzke 2010; Tedesco 1998; Tedesco 1999; Trimarco 2011; Trimarco 2015; Veronesi 2007; Wilson 1963; Yogiantoro 2000). The third most common reason was that the study did not have or report data for a diuretic monotherapy arm. This was the reason in 11 studies (Appel 2010; Bakris 2010; Bebb 2007; CONVINCE 2003; COSMO‐CKD 2014; LIFE 2002; NORDIL 2000; PROGRESS 2001; STOP‐Hypertension‐2 1999; Syst‐Eur 1997; VADT 2011).
Neaton 1993 was identified as a study that met the inclusion criteria for this review. This study compared treatments from five different antihypertensive drug classes, including the thiazide‐like diuretic chlorthalidone, in male and female patients with hypertension for an average follow‐up of 4.4 years. The clinical event data, however, were not reported separately for the intervention arms, and when contacted the authors refused to provide the data separately per intervention arm. Should these data be received in the future, this study will be included in an update of this review.
Risk of bias in included studies
A summary of the assessment of risk of bias of the included studies is shown in Figure 2 and Figure 3. We judged many of the studies to have an unclear risk of bias. Several of the included studies were published prior to the introduction of standardized reporting methods for clinical trials, and lacked sufficient detail for an adequate bias assessment.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Treatment allocation by random sequence generation was adequately described and had a low risk of bias in five of the included studies (ALLHAT 2000/2002; NESTOR 2004; PHYLLIS 2004; PREVER‐treatment 2016; Schmieder 2009). Of these, only three studies had an adequate description of allocation concealment (ALLHAT 2000/2002; PREVER‐treatment 2016; Schmieder 2009). The remaining studies had an unclear risk of allocation bias as they did not have a detailed description of the randomization procedure or method of allocation concealment.
Blinding
Either study personnel alone (MRC 1985; MRC 1992), or both patients and study personnel (ANBP2 2003; HAPPHY 1987; SHELL 2003; VHAS 1997), were unblinded to treatment allocation in six trials, leading to a high risk of bias assessment for blinding. We judged blinding to be adequate in four studies (ALLHAT 2000/2002; Materson 1993; NICS‐EH 1999; VA 1982), with all other studies judged to have an unclear risk of bias, which in many cases was because of insufficient details describing the blinding protocol. Three studies used a double‐blind design for the original treatment assignment but any add‐on treatment was undertaken in an open‐label fashion without clear rationale; we judged these studies to have an unclear risk of bias (NESTOR 2004; PHYLLIS 2004; PREVER‐treatment 2016).
Outcome assessors were blind to treatment allocation in eight studies (ANBP2 2003; HAPPHY 1987; INSIGHT 2000; Materson 1993; MRC 1985; MRC 1992; NICS‐EH 1999; SHELL 2003). The remaining studies had insufficient information for outcome assessment blinding and we this judged the risk of bias to be unclear.
Incomplete outcome data
We judged the majority of included studies to have a low risk of bias for incomplete outcome data; intention‐to‐treat analysis was used, and dropout numbers were small and generally balanced between the treatment groups. We assessed a high risk of bias for the NICS‐EH 1999 and Tresukosol 2005 studies; the former had over 50% of patients discontinue and both used a per‐protocol analysis rather than intention‐to‐treat. We graded some studies as having an unclear risk of bias, as it was unclear whether or not the intention‐to‐treat analysis was carried out properly. Some studies failed to report data on discontinuation.
Selective reporting
A protocol was not available for the majority of studies and the risk of bias therefore remained unclear. For all studies that did have an accessible protocol (ALLHAT 2000/2002; INSIGHT 2000; NESTOR 2004; NICS‐EH 1999; VHAS 1997), we found no evidence of selective reporting. In the SHELL 2003 study, a protocol was not available but one of the secondary outcomes listed in the study methods was not reported in the results, thus we graded the study as having a high risk of bias for selective reporting.
Other potential sources of bias
We identified a high risk of bias resulting from inconsistent use of supplemental drugs in seven studies (ALPINE 2003; ANBP2 2003; HAPPHY 1987;INSIGHT 2000; MRC 1985; MRC 1992; SHELL 2003). We judged the remaining studies to have a low or unclear risk of bias because of either consistent add‐on treatment across all groups or no add‐on treatment permitted.
We also examined the role of industry sponsorship. Twelve studies were sponsored by a for‐profit company of the comparator drug and we considered them to have a high risk of bias (ALPINE 2003; ANBP2 2003; DAPHNE 2002; HAPPHY 1987; INSIGHT 2000; MIDAS 1996; NESTOR 2004; PHYLLIS 2004; Schmieder 2009; SHELL 2003; VA 1982; VHAS 1997). We considered six studies to have a low risk of bias (ALLHAT 2000/2002; Materson 1993; MRC 1985; MRC 1992; PREVER‐treatment 2016; Tresukosol 2005); two studies had insufficient information regarding sponsorship and we thus judged them to have an unclear risk of bias (Berglund 1981; NICS‐EH 1999).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
First‐line diuretics versus other classes of antihypertensive drugs
Total mortality
Total mortality was reported in 16 out of 20 studies (Analysis 1.1; Figure 4). The ALLHAT trial diuretic group was used for three separate comparisons, therefore only subtotals are shown. Mortality was similar when first‐line diuretics were compared with beta‐blockers (risk ratio (RR) 0.96, 95% confidence interval (CI) 0.84 to 1.10; Chi2 = 3.87 (P = 0.42); I2 = 0%; 5 studies, 18,241 participants; moderate‐certainty evidence) (Berglund 1981; HAPPHY 1987; MRC 1985; MRC 1992; VA 1982), calcium channel blockers (RR 1.02, 95% CI 0.96 to 1.08; Chi2 = 4.27 (P = 0.64); I2 = 0%; 7 studies, 35,417 participants; moderate‐certainty evidence) (ALLHAT 2000/2002; INSIGHT 2000; MIDAS 1996; NICS‐EH 1999; SHELL 2003; Tresukosol 2005; VHAS 1997), angiotensin converting enzyme (ACE) inhibitors (RR 1.00, 95% CI 0.95 to 1.07; Chi2 = 0.96 (P = 0.62); I2 = 0%; 3 studies, 30,961 participants; moderate‐certainty evidence) (ALLHAT 2000/2002; ANBP2 2003; NESTOR 2004), alpha‐adrenergic blockers (RR 0.98, 95% CI 0.88 to 1.09; 1 study, 24,316 participants; moderate‐certainty evidence) (ALLHAT 2000/2002); angiotensin II receptor blockers (RR 0.32, 95% CI 0.01 to 7.88; 1 study, 655 participants) (PREVER‐treatment 2016); and direct renin inhibitors (RR 0.34, 95% CI 0.01 to 8.31; 1 study, 1124 participants) (Schmieder 2009).
1.1. Analysis.

Comparison 1: First‐line diuretics versus active comparators: primary outcomes, Outcome 1: Total mortality
4.

Forest plot of comparison: 1 First‐line thiazides vs active comparators: primary outcomes, outcome: 1.1 Total mortality.
Sensitivity analyses
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, total mortality remained similar between first‐line diuretics and calcium channel blockers (RR 0.92, 95% CI 0.79 to 1.07; 6 studies, 11,114 participants), and between first‐line diuretics and ACE inhibitors (RR 1.08, 95% CI 0.90 to 1.31; 2 studies, 6652 participants). This sensitivity analysis was not possible for comparisons with beta‐blockers or alpha‐blockers. When small trials (< 1000 participants in each comparison) were excluded, leaving ALLHAT 2000/2002, ANBP2 2003, HAPPHY 1987, INSIGHT 2000, MRC 1985, MRC 1992, Schmieder 2009, SHELL 2003, and VHAS 1997, total mortality remained similar between first‐line diuretics and beta‐blockers (RR 0.96, 95% CI 0.84 to 1.11; 3 studies, 17,452 participants), first‐line diuretics and calcium channel blockers (RR 1.02, 95% CI 0.96 to 1.08; 4 studies, 33,920 participants), and between first‐line diuretics and ACE inhibitors (RR 1.00, 95% CI 0.95 to 1.07; 2 studies, 30,392 participants). This analysis was not possible for alpha‐blockers.
Supplemental drugs
In five trials, no supplemental drugs were allowed (Berglund 1981; DAPHNE 2002; Materson 1993; NICS‐EH 1999; VA 1982). When they were deselected, mortality was unaffected between first‐line diuretics and beta‐blockers (RR 0.96, 95% CI 0.84 to 1.11; 4 studies, 18,135 participants) and between first‐line diuretics and calcium channel blockers (RR 1.02, 95% CI 0.96 to 1.08; 6 studies, 35,003 participants). This analysis was not possible for ACE inhibitors and alpha‐blockers. When trials where different supplemental drug classes were allowed in each arm were removed (ALLHAT 2000/2002; ALPINE 2003; ANBP2 2003; HAPPHY 1987; INSIGHT 2000; MRC 1985; MRC 1992), total mortality remained similar between first‐line diuretics and beta‐blockers (RR 0.83, 95% CI 0.26 to 2.61; 2 studies, 789 participants), first‐line diuretics and calcium channel blockers (RR 0.86, 95% CI 0.69 to 1.06; 5 studies, 4795 participants), and between first‐line diuretics and ACE inhibitors (RR 2.02, 95% CI 0.18 to 22.17; 1 study, 569 participants). This analysis was not possible for alpha‐blockers.
Dosage of thiazides
In a sensitivity analysis exploring the cause of heterogeneity, we deselected the three trials where the thiazide dose was high (HAPPHY 1987; MRC 1985; VA 1982). This only affected the beta‐blocker comparison and total mortality became numerically reduced for the trials with low‐dose thiazides (RR 0.82, 95% CI 0.66 to 1.01; 2 studies, 2289 participants).
Total serious adverse events
In total, only three studies reported data for total serious adverse events (INSIGHT 2000; MIDAS 1996; Schmieder 2009), which was defined as participants who experienced one or more serious adverse events. Two of these studies compared diuretics with calcium channel blockers (RR 1.09, 95% CI 0.97 to 1.24; Chi2 = 5.04 (P = 0.02); I2 = 80%; 2 studies, 7204 participants; low‐certainty evidence) (INSIGHT 2000; MIDAS 1996), and one trial compared a diuretic to a direct renin inhibitor (RR 0.86, 95% CI 0.49 to 1.50; 1 study, 1124 participants) (Analysis 1.2; Figure 5) (Schmieder 2009).
1.2. Analysis.

Comparison 1: First‐line diuretics versus active comparators: primary outcomes, Outcome 2: Total serious adverse events
5.

Forest plot of comparison: 1 Thiazides vs active comparators: primary outcomes, outcome: 1.2 Total serious adverse events.
No sensitivity analyses were possible due to the limited number of trials.
Total cardiovascular events
A total of 13 studies reported data for the analysis of total cardiovascular events (Analysis 1.3; Figure 6). The ALLHAT trial diuretic group was used for three separate comparisons, therefore only subtotals are shown. Four studies compared diuretics to beta‐blockers (HAPPHY 1987; MRC 1985; MRC 1992; VA 1982), six compared diuretics to calcium channel blockers (ALLHAT 2000/2002; INSIGHT 2000; MIDAS 1996; NICS‐EH 1999; SHELL 2003; VHAS 1997), three compared diuretics to ACE inhibitors (ALLHAT 2000/2002; ANBP2 2003; PHYLLIS 2004), two compared diuretics to alpha‐adrenergic blockers (ALLHAT 2000/2002; DAPHNE 2002), and two compared diuretics to angiotensin II receptor blockers (ALPINE 2003; PREVER‐treatment 2016).
1.3. Analysis.

Comparison 1: First‐line diuretics versus active comparators: primary outcomes, Outcome 3: Total cardiovascular events
6.

Forest plot of comparison: 1 First‐line thiazides vs active comparators: primary outcomes, outcome: 1.3 Total cardiovascular events.
First‐line diuretics likely lower total cardiovascular events slightly compared to beta‐blockers (RR 0.88, 95% CI 0.78 to 1.00; Chi2 = 5.40 (P = 0.14); I2 = 44%; 4 studies, 18,135 participants; moderate‐certainty evidence). First‐line diuretics did not change total cardiovascular events as compared with ACE inhibitors (RR 0.97, 95% CI 0.92 to 1.02; Chi2 = 2.74 (P = 0.25); I2 = 27 %; 3 studies, 30,900 participants; low‐certainty evidence). Diuretics probably reduced total cardiovascular events compared with calcium channel blockers (RR 0.93, 95% CI 0.89 to 0.98; Chi2 = 1.73 (P = 0.89); I2 = 0%; 6 studies, 35,217 participants; moderate‐certainty evidence) and alpha‐adrenergic blockers (RR 0.74, 95% CI 0.69 to 0.80; Chi2 = 0.17 (P = 0.68); I2 = 0%; 2 studies, 24,396 participants; moderate‐certainty evidence). In two small trials, first‐line diuretics did not change total cardiovascular events compared to angiotensin receptor blockers (RR 1.47, 95% CI 0.25 to 8.79; Chi2 = 0.12 (P = 0.72); I2 = 0%; 2 studies, 1047 participants).
Sensitivity analyses
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, total cardiovascular events remained numerically less between first‐line diuretics and calcium channel blockers (RR 0.91, 95% CI 0.78 to 1.06; 5 studies, 10,914 participants). The lack of effect remained between diuretics and ACE inhibitors (RR 1.07, 95% CI 0.94 to 1.23; 2 studies, 6591 participants). The reductive effect between diuretics and alpha‐blockers remained (RR 0.53, 95% CI 0.10 to 2.71; 1 study, 80 participants). This sensitivity analysis was not possible for beta‐blockers. When small trials (< 1000 participants in each comparison) were excluded, total cardiovascular events continued to be reduced with diuretics compared to beta‐blockers (RR 0.88, 95% CI 0.77 to 1.00; 3 studies, 17,452 participants) and calcium channel blockers (RR 0.93, 95% CI 0.88 to 0.98; 2 studies, 30,624 participants). The lack of effect compared to ACE inhibitors remained (RR 0.97, 95% CI 0.92 to 1.02; 2 studies, 30,392 participants). The reduced effect with diuretics compared to alpha‐blockers also remained (RR 0.74, 95% CI 0.69 to 0.80; 1 study, 24,316 participants).
Supplemental drugs
When the trials with no supplemental drugs were deselected, the reduced cardiovascular events between first‐line diuretics and beta‐blockers were unaffected (RR 0.88, 95% CI 0.77 to 1.00; 3 studies, 17,452 participants), as was the comparison between diuretics and calcium channel blockers (RR 0.93, 95% CI 0.88 to 0.98; 5 studies, 34,803 participants), plus the comparison between diuretics and alpha‐blockers (RR 0.74, 95% CI 0.65 to 0.80; 1 study, 24,315 participants). This sensitivity analysis was not possible for ACE inhibitors. When trials where different supplemental drug classes were allowed in each arm were removed, the possible reduction in total cardiovascular events between diuretics and beta‐blockers was lost (RR 2.48, 95% CI 0.48 to 12.89; 1 study, 683 participants). The numerical reduction between diuretics and calcium channel blockers remained (RR 0.92, 95% CI 0.72 to 1.18; 4 studies, 4593 participants). The lack of effect between diuretics and ACE inhibitors remained (RR 1.01, 95% CI 0.21 to 4.95; 1 study, 508 participants). The numerical reduction between diuretics and alpha‐blockers remained (RR 0.53, 95% CI 0.10 to 2.71; 1 study, 80 participants). The lack of effect between diuretics and angiotensin receptor blockers remained (RR 1.93, 95% CI 0.18 to 21.22; 1 study, 655 participants).
Dosage of thiazides
In an exploratory sensitivity analysis where trials using high‐dose thiazides were deselected the reduction in total cardiovascular events with thiazides as compared to beta‐blockers became more certain (RR 0.72, 95% CI 0.57 to 0.91; 1 study, 2183 participants). There were no trials using high‐dose thiazides for the other drug classes.
Total fatal and non‐fatal stroke
Total stroke events were reported in 14 studies (Analysis 1.4; Figure 7). The ALLHAT trial diuretic group was used for three separate comparisons, therefore only subtotals are shown. Four studies compared diuretics to beta‐blockers (HAPPHY 1987; MRC 1985; MRC 1992; VA 1982), six compared diuretics to calcium channel blockers (ALLHAT 2000/2002; INSIGHT 2000; MIDAS 1996; NICS‐EH 1999; SHELL 2003; VHAS 1997), three compared diuretics with ACE inhibitors (ALLHAT 2000/2002; ANBP2 2003; PHYLLIS 2004), two compared diuretics with alpha‐adrenergic blockers (ALLHAT 2000/2002; DAPHNE 2002), and one compared diuretics with angiotensin II receptor blockers (PREVER‐treatment 2016).
1.4. Analysis.

Comparison 1: First‐line diuretics versus active comparators: primary outcomes, Outcome 4: Total stroke events
7.

Forest plot of comparison: 1 Thiazides vs active comparators: primary outcomes, outcome: 1.4 Total stroke events.
First‐line diuretics likely resulted in little to no difference in stroke as compared with beta‐blockers (RR 0.85, 95% CI 0.66 to 1.09; Chi2 = 11.08 (P = 0.01); I2 = 73%; 4 studies, 18,135 participants; low‐certainty evidence). First‐line diuretics probably did not change stroke events compared with calcium channel blockers (RR 1.06, 95% CI 0.95 to 1.18; Chi2 = 1.63 (P = 0.90); I2 = 0%; 6 studies, 35,217 participants; moderate‐certainty evidence). First‐line diuretics reduced total stroke events compared with ACE inhibitors (RR 0.89, 95% CI 0.80 to 0.99; Chi2 = 0.72 (P = 0.70); I2 = 0%; 3 studies, 30,900 participants; moderate‐certainty evidence), and probably reduced stroke compared to alpha‐blockers (RR 0.86, 95% CI 0.73 to 1.01; Chi2 = 1.48 (P = 0.70); I2 = 29%; 2 studies, 24,396 participants; moderate‐certainty evidence). First‐line diuretics did not change stroke in the one small trial compared to angiotensin receptor blockers (RR 2.90, 95% CI 0.12 to 70.96; 1 study, 655 participants).
Sensitivity analyses
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, total stroke remained similar between first‐line diuretics and calcium channel blockers (RR 1.04, 95% CI 0.81 to 1.34; 5 studies, 10,914 participants). The certainty of the evidence for a reduction was lost between diuretics and ACE inhibitors (RR 0.95, 95% CI 0.73 to 1.23; 2 studies, 6591 participants). The reduced effect between diuretics and alpha‐blockers was lost (RR 5.25, 95% CI 0.26 to 106.01; 1 study, 80 participants). When small trials (< 1000 participants in each comparison) were excluded, total stroke continued to be numerically reduced with diuretics compared to beta‐blockers (RR 0.82, 95% CI 0.64 to 1.00; 3 studies, 17,452 participants). The lack of effect on stroke compared to calcium channel blockers remained (RR 1.07, 95% CI 0.95 to 1.20; 2 studies, 30,624 participants). The reduction in stroke compared to ACE inhibitors remained (RR 0.89, 95% CI 0.80 to 0.99; 2 studies, 30,392 participants). The reduced effect with diuretics compared to alpha‐blockers also remained (RR 0.85, 95% CI 0.73 to 1.00; 1 study, 24,316 participants).
Supplemental drugs
When the trials with no supplemental drugs were deselected, the reduced stroke events between first‐line diuretics and beta‐blockers were unaffected (RR 0.82, 95% CI 0.64 to 1.00; 3 studies, 17,452 participants), as was the lack of effect between diuretics and calcium channel blockers (RR 1.06, 95% CI 0.95 to 1.18; 5 studies, 34,803 participants). There were no trials with no supplemental drugs in the ACE inhibitor comparison and the reduction in stroke in the comparison between diuretics and alpha‐blockers remained (RR 0.85, 95% CI 0.73 to 1.00; 1 study, 24,316 participants). When trials where different supplemental drug classes were allowed in each arm were removed, the lack of effect on total stroke events remained between diuretics and beta‐blockers (RR 6.94, 95% CI 0.36 to 133.83; 1 study, 683 participants), between diuretics and calcium channel blockers (RR 0.96, 95% CI 0.65 to 1.42; 4 studies, 4593 participants), between diuretics and ACE inhibitors (RR 0.34, 95% CI 0.01 to 8.21; 1 study, 508 participants), and between diuretics and alpha‐blockers (RR 5.25, 95% CI 0.26 to 106.01; 1 study, 80 participants). This sensitivity analysis was not possible for angiotensin receptor blockers.
Dosage of thiazides
In an exploratory sensitivity analysis where trials using high‐dose thiazides were deselected, the numerical reduction in total stroke events with thiazides as compared to beta‐blockers was unchanged (RR 0.82, 95% CI 0.56 to 1.20; 1 study, 2183 participants). There were no trials using high‐dose thiazides for the other drug classes.
Total coronary heart disease (CHD) events (fatal and non‐fatal myocardial infarction plus sudden death)
Fifteen studies were included in the analysis for total CHD events (Analysis 1.5; Figure 8). The ALLHAT trial diuretic group was used for three separate comparisons, therefore only subtotals have been shown. This outcome was not different when first‐line diuretics were compared to beta‐blockers (RR 0.91, 95% CI 0.78 to 1.07; Chi² = 8.98, df = 3 (P = 0.03); I² = 67%; 4 studies, 18,135 participants; moderate‐certainty evidence) (HAPPHY 1987; MRC 1985; MRC 1992; VA 1982), calcium channel blockers (RR 1.00, 95% CI 0.93 to 1.08; Chi² = 0.82, df = 5 (P = 0.98); I² = 0%; 6 studies, 35,217 participants; moderate‐certainty evidence) (ALLHAT 2000/2002; INSIGHT 2000; MIDAS 1996; NICS‐EH 1999; SHELL 2003; VHAS 1997), ACE inhibitors (RR 1.03, 95% CI 0.96 to 1.12; Chi² = 1.80, df = 2 (P = 0.41); I² = 0%; 3 studies, 30,900 participants; low‐certainty evidence) (ALLHAT 2000/2002; ANBP2 2003; PHYLLIS 2004), alpha‐adrenergic blockers (RR 0.98, 95% CI 0.86 to 1.11; Chi² = 2.10, df = 1 (P = 0.15); I² = 52%; 2 studies, 24,396 participants; moderate‐certainty evidence) (ALLHAT 2000/2002; DAPHNE 2002), and angiotensin II receptor blockers (RR 0.98, 95% CI 0.14 to 6.95; Chi² = 0.00, df = 1 (P = 0.99); I² = 0%; 2 studies, 1047 participants) (ALPINE 2003; PREVER‐treatment 2016).
1.5. Analysis.

Comparison 1: First‐line diuretics versus active comparators: primary outcomes, Outcome 5: Total coronary heart disease
8.

Forest plot of comparison: 1 Thiazides vs active comparators: primary outcomes, outcome: 1.5 Total coronary events.
Sensitivity analyses
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, total CHD events remained not different with diuretics compared to calcium channel blockers (RR 0.92, 95% CI 0.73 to 1.17; 5 studies, 10,914 participants); between diuretics and ACE inhibitors (RR 1.14, 95% CI 0.94 to 1.39; 2 studies, 6591 participants); and between diuretics and alpha‐blockers (RR 0.12, 95% CI 0.01 to 2.10; 1 study, 80 participants). When small trials (< 1000 participants in each comparison) were excluded, total CHD events continued to not be different with diuretics compared to beta‐blockers (RR 0.91, 95% CI 0.78 to 1.07; 3 studies, 17,452 participants); calcium channel blockers (RR 1.00, 95% CI 0.92 to 1.09; 2 studies, 30,624 participants); ACE inhibitors (RR 1.03, 95% CI 0.96 to 1.11; 2 studies, 30,392 participants); and alpha‐blockers (RR 0.99, 95% CI 0.87 to 1.12; 1 study, 24,316 participants).
Supplemental drugs
When the trials with no supplemental drugs were deselected, the lack of effect on CHD events remained between diuretics and beta‐blockers (RR 0.91, 95% CI 0.78 to 1.07; 3 studies, 17,452 participants), diuretics and calcium channel blockers (RR 1.00, 95% CI 0.93 to 1.08; 5 studies, 34,803 participants), and diuretics and alpha‐blockers (RR 0.99, 95% CI 0.87 to 1.12; 1 study, 24,316 participants). When trials where different supplemental drug classes were allowed in each arm were removed, the lack of effect on total CHD events remained between diuretics and beta‐blockers (RR 0.99, 95% CI 0.14 to 7.00; 1 study, 683 participants); diuretics and calcium channel blockers (RR 0.98, 95% CI 0.65 to 1.47; 4 studies, 4593 participants); diuretics and ACE inhibitors (RR 3.02, 95% CI 0.32 to 28.87; 1 study, 508 participants); diuretics and alpha‐blockers (RR 0.12, 95% CI 0.01 to 2.10; 1 study, 80 participants), and diuretics and angiotensin receptor blockers (RR 0.97, 95% CI 0.06 to 15.39; 1 study, 655 participants).
Dosage of thiazides
In an exploratory sensitivity analysis where trials using high‐dose thiazides were deselected the reduction in total coronary events with thiazides as compared to beta‐blockers became more certain (RR 0.61, 95% CI 0.43 to 0.87; 1 study, 2183 participants). There were no trials using high‐dose thiazides for the other drug classes.
Total congestive heart failure
In total, eight studies reported data for death or hospitalization due to heart failure (Analysis 1.6; Figure 9). The ALLHAT trial diuretic group was used for three separate comparisons, therefore only subtotals are shown. One study compared diuretics with beta‐blockers (HAPPHY 1987), six studies compared diuretics with calcium channel blockers (ALLHAT 2000/2002; INSIGHT 2000; MIDAS 1996; NICS‐EH 1999; SHELL 2003; VHAS 1997), two studies compared diuretics with ACE inhibitors (ALLHAT 2000/2002; ANBP2 2003), and one study compared diuretics with alpha‐adrenergic blockers (ALLHAT 2000/2002).
1.6. Analysis.

Comparison 1: First‐line diuretics versus active comparators: primary outcomes, Outcome 6: Total congestive heart failure
9.

Forest plot of comparison: 1 Thiazides vs active comparators: primary outcomes, outcome: 1.6 Total congestive heart failure.
First‐line diuretics may have resulted in little to no difference in heart failure compared with beta‐blockers (RR 0.69, 95% CI 0.40 to 1.19; 1 study, 6569 participants; low‐certainty evidence). Diuretics probably decreased heart failure compared to calcium channel blockers (RR 0.74, 95% CI 0.66 to 0.82; Chi2 = 5.98 (P = 0.35); I2 = 10%; 6 studies, 35,217 participants; moderate‐certainty evidence). Diuretics probably resulted in little to no difference in heart failure compared with ACE inhibitors (RR 0.94, 95% CI 0.84 to 1.04; Chi2 = 1.56 (P = 0.21); I2 = 36%; 2 studies, 30,392 participants; moderate‐certainty evidence). Diuretics decreased heart failure compared to alpha‐adrenergic blockers (RR 0.51, 95% CI 0.45 to 0.58; 1 study, 24,316 participants; moderate‐certainty evidence).
Sensitivity analyses
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, heart failure continued to be reduced by first‐line diuretics compared to calcium channel blockers (RR 0.65, 95% CI 0.43 to 0.99; 5 studies, 10,914 participants). The lack of effect between diuretics and ACE inhibitors remained (RR 1.13, 95% CI 0.82 to 1.56; 1 study, 6083 participants). This analysis was not possible for beta‐blockers and alpha‐blockers. When small trials (< 1000 participants in each comparison) were excluded, total heart failure continued to be reduced by diuretics compared to calcium channel blockers (RR 0.73, 95% CI 0.66 to 0.81; 2 studies, 30,624 participants). This analysis was not possible for beta‐blockers, ACE inhibitors, and alpha‐blockers.
Supplemental drugs
When the trials with no supplemental drugs were deselected, the reduced heart failure with diuretics compared to calcium channel blockers remained (RR 0.73, 95% CI 0.66 to 0.81; 5 studies, 34,803 participants). There were no trials with no supplemental drugs in the other comparisons. When trials where different supplemental drug classes were allowed in each arm were removed, the numerical reduction in total CHF events remained between diuretics and calcium channel blockers (RR 0.82, 95% CI 0.45 to 1.42; 4 studies, 4593 participants). This sensitivity analysis was not possible for beta‐blockers, ACE inhibitors, and alpha‐blockers.
A sensitivity analysis exploring the effect of high‐dose thiazides versus beta‐blockers was not possible as there was only one trial reporting this outcome.
Withdrawals due to adverse effects
Sixteen studies reported withdrawals due to adverse effects (Analysis 1.7; Figure 10). Five studies compared diuretics to beta‐blockers (HAPPHY 1987; Materson 1993; MRC 1985; MRC 1992; VA 1982), seven studies compared diuretics to calcium channel blockers (ALLHAT 2000/2002; INSIGHT 2000; Materson 1993; MIDAS 1996; NICS‐EH 1999; Tresukosol 2005; VHAS 1997), three studies compared diuretics to ACE inhibitors (ALLHAT 2000/2002; Materson 1993; NESTOR 2004), three studies compared diuretics to alpha‐adrenergic blockers (ALLHAT 2000/2002; DAPHNE 2002; Materson 1993), two studies compared diuretics to angiotensin II receptor blockers (ALPINE 2003; PREVER‐treatment 2016), one study compared a diuretic to a direct renin inhibitor (Schmieder 2009), and one study compared a diuretic to a centrally acting drug, clonidine (Materson 1993).
1.7. Analysis.

Comparison 1: First‐line diuretics versus active comparators: primary outcomes, Outcome 7: Withdrawals due to adverse effects
10.

Forest plot of comparison: 1 First‐line thiazides vs active comparators: primary outcomes, outcome: 1.7 Withdrawal due to adverse effects.
Withdrawals due to adverse effects may have been lowered for first‐line diuretics when compared with beta‐blockers (RR 0.78. 95% CI 0.71 to 0.85; Chi2 = 43.97 (P < 0.001); I2 = 91%; 5 studies, 18,501 participants; moderate‐certainty evidence), calcium channel blockers (RR 0.81, 95% CI 0.75 to 0.88; Chi2 = 22.67 (P < 0.001); I2 = 74%; 7 studies, 33,908 participants; low‐certainty evidence), ACE inhibitors (RR 0.73, 95% CI 0.64 to 0.84; Chi2 = 2.31 (P = 0.31); I2 = 14%; 3 studies, 25,254 participants; moderate‐certainty evidence), alpha‐blockers (RR 0.70, 95% CI 0.54 to 0.89; Chi2 = 10.95 (P = 0.004); I2 = 82%; 3 studies, 24,772 participants; low‐certainty evidence), and when compared with clonidine, a central nervous system (CNS)‐acting drug (RR 0.16, 95% CI 0.05 to 0.53; 1 study, 366 participants). There were no differences in withdrawals due to adverse effects when diuretics were compared with angiotensin II receptor blockers (RR 2.05, 95% CI 0.91 to 4.58; Chi2 = 1.74 (P = 0.17); I2 = 42%; 2 studies, 1047 participants) or with direct renin inhibitors (RR 1.39, 95% CI 0.88 to 2.20; 1 study, 1124 participants).
Sensitivity analysis
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, withdrawals due to adverse effects remained reduced by diuretics compared to calcium channel blockers (RR 0.72, 95% CI 0.66 to 0.80; 6 studies, 9605 participants), ACE inhibitors (RR 0.77, 95% CI 0.43 to 1.37; 2 studies, 945 participants), and alpha‐blockers (RR 0.33, 95% CI 0.17 to 0.63; 2 studies, 456 participants). This analysis was not possible for beta‐blockers. When small trials (< 1000 participants in each comparison) were deselected, withdrawals due to adverse effects continued to be reduced by diuretics compared to beta‐blockers (RR 0.78, 95% CI 0.71 to 0.86; 3 studies, 17,452 participants), calcium channel blockers (RR 0.81, 95% CI 0.74 to 0.88; 2 studies, 30,624 participants), ACE inhibitors (RR 0.73, 95% CI 0.63 to 0.84; 1 study, 24,309 participants), and alpha‐blockers (RR 0.81, 95% CI 0.62 to 1.07; 1 study, 24,316 participants).
Supplemental drugs
When the trials with no supplemental drugs were deselected, the reduction in withdrawals due to adverse effects remained between first‐line diuretics and beta‐blockers (RR 0.78, 95% CI 0.71 to 0.86; 3 studies, 17,452 participants), calcium channel blockers (RR 0.81, 95% CI 0.75 to 0.88; 5 studies, 33,121 participants), ACE inhibitors (RR 0.74, 95% CI 0.64 to 0.85; 2 studies, 24,878 participants), and alpha‐blockers (RR 0.81, 95% CI 0.62 to 1.07; 1 study, 24,316 participants). When trials where different supplemental drug classes were allowed in each arm were removed, the numerical reduction in withdrawals due to adverse effects remained between diuretics and beta‐blockers (RR 0.52, 95% CI 0.21 to 1.31; 2 studies, 1049 participants); calcium channel blockers (RR 0.51, 95% CI 0.60 to 1.09; 5 studies, 3284 participants); ACE inhibitors (RR 0.77, 95% CI 0.43 to 1.37; 2 studies, 945 participants), and alpha‐blockers (RR 0.33, 95% CI 0.17 to 0.83; 2 studies, 456 participants). The numerical increase between diuretics and angiotensin receptor blockers remained (RR 10.64, 95% CI 0.54 to 191.60; 1 study, 655 participants). This sensitivity analysis was not possible for renin inhibitors or CNS‐active drugs.
Dosage of thiazides
In an exploratory sensitivity analysis where trials using high‐dose thiazides were deselected, the reduction in withdrawals due to adverse effects with thiazides as compared to beta‐blockers became more prominent (RR 0.49, 95% CI 0.42 to 0.58; 2 studies, 2549 participants). There were no trials using high‐dose thiazides for the other drug classes.
Dose titration or add‐on therapy
Data for dose titration or add‐on therapy were available from 14 studies (Analysis 2.1; Figure 11). The ALLHAT trial diuretic group was used for three separate comparisons, therefore only subtotals are shown. Three studies compared diuretics with beta‐blockers (Berglund 1981; HAPPHY 1987; VA 1982), seven studies compared diuretics with calcium channel blockers (ALLHAT 2000/2002; INSIGHT 2000; MIDAS 1996; NICS‐EH 1999; SHELL 2003; Tresukosol 2005; VHAS 1997), two studies compared diuretics with ACE inhibitors (ALLHAT 2000/2002; NESTOR 2004), one study compared a diuretic with an alpha‐adrenergic blocker (ALLHAT 2000/2002), two studies compared diuretics with angiotensin II receptor blockers (ALPINE 2003; PREVER‐treatment 2016), and one study compared a diuretic with a direct renin inhibitor (Schmieder 2009).
2.1. Analysis.

Comparison 2: First‐line diuretics versus active comparators: secondary outcomes, Outcome 1: Dose titration and addition of second or third drug
11.

Forest plot of comparison: 2 Thiazides vs active comparators: secondary outcomes, outcome: 2.1 Dose titration and addition of second or third drug.
The need for dose titration or add‐on therapy was higher for first‐line diuretics when compared with beta‐blockers (RR 1.12, 95% CI 1.05 to 1.20; Chi2 = 34.56 (P < 0.00001); I2 = 94%; 3 studies, 7358 participants). The need for dose titration or add‐on therapy was lower for diuretics compared with calcium channel blockers (RR 0.97, 95% CI 0.94 to 0.99; Chi2 = 233.82 (P < 0.001); I2 = 97%; 7 studies, 35,417 participants), ACE inhibitors (RR 0.94, 95% CI 0.92 to 0.97; Chi2 = 0.55 (P = 0.46); I2 = 0%; 2 studies, 24,878 participants), and alpha‐adrenergic blockers (RR 0.85, 95% CI 0.83 to 0.88; 1 study, 24,316 participants). First‐line diuretics did not change the need for dose titration or add‐on therapy as compared to angiotensin receptor blockers (RR 0.94, 95% CI 0.85 to 1.03; Chi2 = 25.33 (P < 0.001); I2 = 96%; 2 studies, 1047 participants) and direct renin inhibitors (RR 1.10, 95% CI 0.98 to 1.24; 1 study, 1124 participants).
Sensitivity analyses
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, need for dose titration or add‐on therapy remained less with first‐line diuretics compared to calcium channel blockers (RR 0.80, 95% CI 0.75 to 0.85; 6 studies, 11,114 participants) and ACE inhibitors (RR 0.89, 95% CI 0.77 to 1.04; 1 study, 305 participants). This sensitivity analysis was not possible for beta‐blockers, alpha‐blockers, angiotensin receptor blockers, and renin inhibitors. When small trials (< 1000 participants in each comparison) were deselected, the need for dose titration or add‐on therapy continued to be greater with diuretics compared to beta‐blockers (RR 1.19, 95% CI 1.12 to 1.28; 1 study, 6569 participants). The need for dose titration or add‐on therapy continued to be lower for diuretics compared with calcium channel blockers (RR 0.94, 95% CI 0.91 to 0.96; 2 studies, 30,624 participants) and ACE inhibitors (RR 0.95, 95% CI 0.92 to 0.98; 1 study, 24,309 participants). This sensitivity analysis was not possible for alpha‐blockers, angiotensin receptor blockers, and renin inhibitors.
Supplemental drugs
When the trials with no supplemental drugs were deselected, the increased need for dose titration or add‐on therapy remained between first‐line diuretics and beta‐blockers (RR 1.19, 95% CI 1.12 to 1.28; 1 study, 6569 participants). This outcome remained reduced between diuretics and calcium channel blockers (RR 0.97, 95% CI 0.94 to 1.00; 6 studies, 35,003 participants). This sensitivity analysis was not possible for ACE inhibitors, alpha‐blockers, angiotensin receptor blockers, and renin inhibitors. When trials where different supplemental drug classes were allowed in each arm were removed, the increase in add‐on therapy for beta‐blockers reversed and became decreased (RR 0.60, 95% CI 0.48 to 0.75; 2 studies, 789 participants). The decrease between diuretics and calcium channel blockers also reversed to an increase (RR 1.34, 95% CI 1.21 to 1.49; 5 studies, 4793 participants). The decrease in add‐on therapy remained between diuretics and ACE inhibitors (RR 0.89, 95% CI 0.77 to 1.04; 1 study, 569 participants), and angiotensin receptor blockers (RR 0.76, 95% CI 0.65 to 0.88; 1 study, 655 participants). This sensitivity analysis was not possible for alpha‐blockers and renin inhibitors.
Dosage of thiazides
In an exploratory sensitivity analysis where trials using high‐dose thiazides were deselected, the increased need for dose titration or add‐on therapy with thiazides as compared to beta‐blockers was lost (RR 0.94, 95% CI 0.53 to 1.66; 1 study, 106 participants). There were no trials using high‐dose thiazides for the other comparisons.
Switching to other antihypertensive therapies
Five studies reported data for participants switching to other antihypertensive therapies (Analysis 2.2; Figure 12). These included one study comparing a diuretic with a beta‐blocker (Berglund 1981), two studies comparing diuretics with calcium channel blockers (MIDAS 1996; VHAS 1997), and two studies comparing diuretics with alpha‐adrenergic blockers (ALLHAT 2000/2002; DAPHNE 2002).
2.2. Analysis.

Comparison 2: First‐line diuretics versus active comparators: secondary outcomes, Outcome 2: Switching to other antihypertensive therapies
12.

Forest plot of comparison: 2 Thiazides vs active comparators: secondary outcomes, outcome: 2.2 Switching to other antihypertensive therapies.
First‐line diuretics did not affect switching when compared to beta‐blockers (RR 2.00, 95% CI 0.53 to 7.58; 1 study, 106 participants) or calcium channel blockers (RR 0.97, 95% CI 0.80 to 1.18; Chi2 = 0.58 (P = 0.45); I2 = 0%; 2 studies, 2297 participants). First‐line diuretics decreased the need to switch to other antihypertensives when compared with alpha‐adrenergic blockers (RR 0.10, 95% CI 0.09 to 0.12; Chi2 = 7.87 (P = 0.005); I2 = 87%; 2 studies, 24,396 participants).
Sensitivity analyses
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, the decrease in switching between diuretics and alpha‐blockers was lost (RR 0.79, 95% CI 0.19 to 3.30; 1 study, 80 participants). This sensitivity analysis was not possible for beta‐blockers, ACE inhibitors, and calcium channel blockers. When small trials (< 1000 participants in each comparison) were deselected, the lack of effect on switching between diuretics and alpha‐blockers remained (RR 0.10, 95% CI 0.08 to 0.12; 1 study, 24,316 participants). This sensitivity analysis was not possible for beta‐blockers, calcium channel blockers, and ACE inhibitors.
Supplemental drugs
When the trials with no supplemental drugs were deselected, the decreased switching remained between diuretics and alpha‐blockers (RR 0.10, 95% CI 0.08 to 0.12; 1 study, 24,316 participants). This sensitivity analysis was not possible for beta‐blockers, ACE inhibitors, and calcium channel blockers. When trials where different supplemental drug classes were allowed in each arm were removed, the decrease in switching between diuretics and alpha‐blockers was lost (RR 0.79, 95% CI 0.19 to 3.30; 1 study, 80 participants). This sensitivity analysis was not possible for beta‐blockers, ACE inhibitors, and calcium channel blockers.
A sensitivity analysis exploring the effect of high‐dose thiazides was not possible as there was only one trial reporting this outcome.
Systolic and diastolic blood pressure at one year
Blood pressure data were meta‐analyzed from trials that also reported at least one additional outcome of interest (mortality and morbidity); a total of 19 included studies reported 12‐month systolic and diastolic blood pressure data (Analysis 2.3; Analysis 2.4; Figure 13; Figure 14).
2.3. Analysis.

Comparison 2: First‐line diuretics versus active comparators: secondary outcomes, Outcome 3: Systolic blood pressure at 1 year
2.4. Analysis.

Comparison 2: First‐line diuretics versus active comparators: secondary outcomes, Outcome 4: Diastolic blood pressure at 1 year
13.

Forest plot of comparison: 2 Thiazides vs active comparators: secondary outcomes, outcome: 2.3 Systolic blood pressure.
14.

Forest plot of comparison: 2 Thiazides vs active comparators: secondary outcomes, outcome: 2.4 Diastolic blood pressure.
The studies analyzed in this review include five studies comparing diuretics with beta‐blockers (Berglund 1981; HAPPHY 1987; MRC 1985; MRC 1992; VA 1982), seven studies comparing diuretics with calcium channel blockers (ALLHAT 2000/2002; INSIGHT 2000; MIDAS 1996; NICS‐EH 1999; SHELL 2003; Tresukosol 2005; VHAS 1997), four studies comparing diuretics with ACE inhibitors (ALLHAT 2000/2002; ANBP2 2003; NESTOR 2004; PHYLLIS 2004), two studies comparing diuretics with alpha‐adrenergic blockers (ALLHAT 2000/2002; DAPHNE 2002), two studies comparing diuretics with angiotensin II receptor blockers (ALPINE 2003; PREVER‐treatment 2016), and one study comparing a diuretic with a direct renin inhibitor (Schmieder 2009).
The ALLHAT trial diuretic group was used for three separate comparisons, therefore only subtotals are shown. In seven of these trials, data were approximated from graphs using imaging software as numerical values were not reported (ANBP2 2003; Berglund 1981; HAPPHY 1987; INSIGHT 2000; PHYLLIS 2004; PREVER‐treatment 2016; SHELL 2003). In addition, Tresukosol 2005 reported blood pressure at 18 months only; this was included as we assumed that this would be approximately equivalent to 12‐month data. Six studies had their standard deviation imputed, therefore we have used 99% confidence intervals (ANBP2 2003; INSIGHT 2000; PHYLLIS 2004; PREVER‐treatment 2016; SHELL 2003; VA 1982).
First‐line diuretics reduced systolic blood pressure more than beta‐blockers (mean difference (MD) ‐2.94, 99% CI ‐3.58 to ‐2.29; Chi2 = 88.71 (P < 0.00001); I2 = 95%; 5 studies, 18,241 participants); calcium channel blockers (MD ‐1.36, 99% CI ‐1.80 to ‐0.92; Chi2 = 15.48 (P = 0.02); I2 = 61%; 7 studies, 31,585 participants); ACE inhibitors (MD ‐2.39, 99% CI ‐2.93 to ‐1.86; Chi2 = 25.67 (P < 0.0001); I2 = 88%; 4 studies, 27,289 participants), and alpha‐adrenergic blockers (MD ‐3.01, 99% CI ‐3.65 to ‐2.37; Chi2 = 0.68 (P = 0.41); I2 = 0%; 2 studies, 18,781 participants) (Analysis 2.3; Figure 13). First‐line diuretics numerically decreased systolic blood pressure more than angiotensin II receptor blockers (MD ‐1.93, 99% CI ‐4.32 to 0.47; Chi2 = 0.01 (P = 0.92); I2 = 0%; 2 studies, 1047 participants). In one trial, diuretics did not change systolic blood pressure compared to a direct renin inhibitor (MD 0.90, 99% CI ‐1.30 to 3.10; 1 study, 1124 participants).
First‐line diuretics did not change diastolic blood pressure compared to beta‐blockers (MD ‐0.29, 99% CI ‐0.65 to 0.07; Chi2 = 146.71 (P < 0.00001); I2 = 97%; 5 studies, 18,241 participants). Diuretics increased diastolic blood pressure as compared to calcium channel blockers (MD 0.47, 99% CI 0.20 to 0.73; Chi2 = 28.72 (P < 0.0001); I2 = 79%; 7 studies, 31,585 participants). Diuretics reduced diastolic blood pressure when compared to ACE inhibitors (MD ‐0.37, 99% CI ‐0.67 to ‐0.07; Chi2 = 8.40 (P = 0.04); I2 = 64%; 4 studies, 27,391 participants). Diuretics did not change diastolic blood pressure compared with alpha‐adrenergic blockers (MD 0.00, 99% CI ‐0.38 to 0.38; Chi2 = 0.00 (P = 1.00); I2 = 0%; 2 studies, 18,781 participants), angiotensin II receptor blockers (MD 0.04, 99% CI ‐1.21 to 1.29; Chi2 = 0.01 (P = 0.92); I2 = 0%; 2 studies, 1047 participants), and direct renin inhibitors (MD 1.00, 99% CI ‐0.44 to 2.44; 1 study, 1124 participants) (Analysis 2.4; Figure 14).
Sensitivity analysis
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, systolic blood pressure remained reduced by diuretics compared to calcium channel blockers (MD ‐1.00, 99% CI ‐1.70 to ‐0.30; 6 studies, 11,114 participants), ACE inhibitors (MD ‐1.01, 99% CI ‐1.93 to ‐0.09; 3 studies, 6906 participants), and alpha‐blockers (MD ‐7.00, 99% CI ‐19.43 to 5.43; 1 study, 80 participants). This sensitivity analysis was not possible for beta‐blockers, angiotensin receptor blockers, and renin inhibitors. When small trials (< 1000 participants in each comparison) were deselected, systolic blood pressure remained reduced by diuretics compared to beta‐blockers (MD ‐2.79, 99% CI ‐3.45 to ‐2.12; 3 studies, 17,452 participants), calcium channel blockers (MD ‐1.45, 99% CI ‐1.94 to ‐0.96; 2 studies, 26,792 participants), ACE inhibitors (MD ‐2.46, 99% CI ‐3.01 to ‐1.91; 2 studies, 26,466 participants), and alpha‐blockers (MD ‐3.00, 99% CI ‐3.64 to ‐2.36; 1 study, 18,702 participants). This sensitivity analysis was not possible for angiotensin receptor blockers and renin inhibitors.
Supplemental drugs
When the trials with no supplemental drugs were deselected, systolic blood pressure remained reduced by diuretics compared to beta‐blockers (MD ‐2.79, 99% CI ‐3.95 to ‐2.12; 3 studies, 17,454 participants), calcium channel blockers (MD ‐1.37, 99% CI ‐1.81 to ‐0.93; 6 studies, 31,171 participants), and alpha‐blockers (MD ‐3.00, 99% CI ‐3.64 to ‐2.36; 1 study, 18,701 participants). This sensitivity analysis was not possible for ACE inhibitors, angiotensin receptor blockers, and renin inhibitors. When trials where different supplemental drug classes were allowed in each arm were removed, systolic blood pressure remained reduced by diuretics compared to beta‐blockers (MD ‐5.08, 99% CI ‐7.59 to ‐2.57; 2 studies, 789 participants), calcium channel blockers (MD ‐1.00, 99% CI ‐2.00 to ‐0.00; 5 studies, 4793 participants), ACE inhibitors (MD ‐1.05, 99% CI ‐3.51 to 1.41; 2 studies, 823 participants), alpha‐blockers (MD ‐7.00, 99% CI ‐19.43 to 5.43; 1 study, 80 participants), and angiotensin receptor blockers (MD ‐2.00, 99% CI ‐5.02 to 1.02; 1 study, 655 participants). This sensitivity analysis was not possible for renin inhibitors.
Dosage of thiazides
In an exploratory sensitivity analysis where trials using high‐dose thiazides were deselected the reduction in systolic blood pressure with thiazides as compared to beta‐blockers remained (MD ‐4.02, 99% CI ‐5.69 to ‐2.36; 2 studies, 2289 participants). There were no trials using high‐dose thiazides for the other drug classes.
Small versus large trials
When the largest trial, ALLHAT 2000/2002, was deselected, diastolic blood pressure remained increased slightly by diuretics compared to calcium channel blockers (MD 0.43, 99% CI 0.04 to 0.81; 6 studies, 11,114 participants). The reduction as compared to ACE inhibitors was lost (MD 0.01, 99% CI ‐0.48 to 0.51; 3 studies, 6906 participants). The lack of effect compared to alpha‐blockers remained (MD 0.00, 99% CI ‐4.32 to 4.32; 1 study, 80 participants). This sensitivity analysis was not possible for beta‐blockers, angiotensin receptor blockers, and renin inhibitors. When small trials (< 1000 participants in each comparison) were deselected, diastolic blood pressure remained unaffected by diuretics compared to beta‐blockers (MD ‐0.05, 99% CI ‐0.42 to 0.33; 3 studies, 17,452 participants). The increase compared to calcium channel blockers remained (MD 0.34, 99% CI 0.04 to 0.63; 2 studies, 26,792 participants). The reduction as compared to ACE inhibitors persisted (MD ‐0.39, 99% CI ‐0.70 to ‐0.08; 2 studies, 26,568 participants). The lack of effect compared to alpha‐blockers remained (MD 0.00, 99% CI ‐0.38 to 0.38; 1 study, 18,701 participants). This sensitivity analysis was not possible for angiotensin receptor blockers and renin inhibitors.
Supplemental drugs
When the trials with no supplemental drugs were deselected, diastolic blood pressure remained unaffected by diuretics compared to beta‐blockers (MD ‐0.05, 99% CI ‐0.42 to 0.33; 3 studies, 17,452 participants). The increase compared to calcium channel blockers remained (MD 0.48, 99% CI 0.22 to 0.75; 6 studies, 31,171 participants). The lack of effect compared to alpha‐blockers remained (MD 0.00, 99% CI ‐0.38 to 0.38; 1 study, 18,701 participants). This sensitivity analysis was not possible for ACE inhibitors, angiotensin receptor blockers, and renin inhibitors. When trials where different supplemental drug classes were allowed in each arm were removed, diastolic blood pressure became reduced by diuretics compared to beta‐blockers (MD ‐3.73, 99% CI ‐5.15 to ‐2.31; 2 studies, 789 participants). The increase in diastolic blood pressure remained with diuretics compared to calcium channel blockers (MD 0.96, 99% CI 0.38 to 1.53; 5 studies, 4793 participants). The lack of effect remained for diuretics compared to ACE inhibitors (MD 0.12, 99% CI ‐1.31 to 1.56; 2 studies, 823 participants), alpha‐blockers (MD 0.00, 99% CI ‐4.32 to 4.32; 1 study, 80 participants), and angiotensin receptor blockers (MD 0.00, 99% CI ‐1.61 to 1.61; 1 study, 655 participants). This sensitivity analysis was not possible for renin inhibitors.
Dosage of thiazides
In an exploratory sensitivity analysis where trials using high‐dose thiazides were deselected, the lack of effect on diastolic blood pressure with thiazides as compared to beta‐blockers remained (MD 0.00, 99% CI ‐1.04 to 1.04; 2 studies, 2289 participants). There were no trials using high‐dose thiazides for the other drug classes.
Discussion
Summary of main results
The justification for the use of antihypertensive drugs in people with elevated blood pressure primarily comes from placebo/no treatment controlled trials in people aged 60 and over with moderate to severe elevations of blood pressure (> 160/100 mmHg) (Musini 2019). In that setting antihypertensive drugs reduce mortality and total cardiovascular events. In people aged 18 to 59 with mild to moderate elevations of blood pressure, evidence has only been found for a reduction in the incidence of stroke (low‐certainty) (Musini 2017). In people of all ages who are healthy except for mild elevations of blood pressure (140 to 159/90 to 99 mmHg) the evidence remains uncertain as to whether the benefits of drug therapy outweigh the harms (Diao 2012; Sheppard 2018; Sundström 2015). This uncertainty of evidence and conservative approach is supported by the recently updated review assessing blood pressure targets (Arguedas 2020). This target review demonstrates that the benefits of the intervention, trying to achieve a lower blood pressure target as compared to a standard target (≤ 140/90 mmHg), do not outweigh the harms associated with that intervention.
In clinical settings where antihypertensive therapy is indicated, it is important to know what drug class is the best to begin with. This review addresses that question and summary of findings tables are provided for individual drug class comparisons between thiazides and beta‐blockers (Table 1), calcium channel blockers (Table 2), ACE inhibitors (Table 3), and alpha‐blockers (Table 4). For these four comparisons, the data were sufficient to justify including them in the table. The amount of data for all comparisons with angiotensin receptor blockers, direct renin inhibitors, and centrally acting drugs was insufficient to justify including them in a summary of findings table.
When a clinician is deciding what drug to start for a patient with hypertension, it is most appropriate to look at the totality of data for each comparison. The comparison of diuretics to beta‐blockers is based on six RCTs, all studying a thiazide diuretic with fewer than 20,000 participants. As can be seen in Table 1, compared to beta‐blockers, diuretics likely reduce total cardiovascular events (absolute risk reduction (ARR) 0.6%; moderate‐certainty evidence) and withdrawals due to adverse effects (ARR 2.2%; moderate‐certainty evidence). For secondary outcomes, the proportion of participants requiring add‐on therapy was greater for diuretics than for beta‐blockers but it is worth noting that this finding was based on only one trial (HAPPHY 1987), which used a high‐dose thiazide and was judged to have a high risk of bias due to lack of blinding and industry involvement. In the two smaller trials where data were available, the opposite was true. Diuretics requiring more add‐on therapy does not fit with another major advantage of diuretics; at one‐year diuretics lowered systolic blood pressure by 2.6 mmHg more than beta‐blockers. The effect on diastolic blood pressure was similar for the two classes of drugs. In sensitivity analyses, these findings were insensitive to the size of trials and to supplemental drugs
In almost all the trials included in this review, the diuretic was a low‐dose thiazide. This is important because in the review comparing first‐line drug classes with placebo or no treatment (Wright 2018), low‐dose thiazides are defined and the evidence was consistent with the fact that high‐dose thiazides were not as good at reducing coronary heart disease events as low‐dose thiazides. The three trials where high‐dose thiazides were used were older trials comparing thiazides with beta‐blockers: HAPPHY 1987, MRC 1985, and VA 1982. In an exploratory sensitivity analysis where the high‐dose thiazide trials were deselected (see Effects of interventions), diuretics, as compared to beta‐blockers, reduced total mortality (RR 0.82, 95% CI 0.66 to 1.01), total cardiovascular events (RR 0.72, 95% CI 0.57 to 0.91), and total CHD (RR 0.61, 95% CI 0.43 to 0.87). In addition, the advantage of low‐dose thiazides in terms of withdrawals due to adverse effects became more prominent (RR 0.49, 95% CI 0.41 to 0.58). Thus, the totality of evidence comparing diuretics and beta‐blockers favors a low‐dose thiazide for hypertension, unless there is another indication for a beta‐blocker or a contraindication for a thiazide.
The comparison of first‐line diuretics with first‐line calcium channel blockers is based on eight RCTs with over 35,000 participants. Most of the data for this comparison used the thiazide‐like diuretic chlorthalidone compared to a dihydropyridine calcium channel blocker. As can be seen in Table 2, diuretics are likely not different from calcium channel blockers for total mortality, total stroke, and total coronary heart disease. However, diuretics as compared to calcium channel blockers likely decreased heart failure and total cardiovascular events (moderate‐certainty). Diuretics may reduce withdrawals due to adverse effects as compared to calcium channel blockers (low‐certainty). The decrease in total cardiovascular events is completely explained by the decrease in congestive heart failure. This effect was insensitive to the size of trials and the use of supplemental drugs. It was also insensitive to whether the diuretic was chlorthalidone or a thiazide. This means that choosing a diuretic over a calcium channel blocker would likely prevent 1.2% of patients from experiencing death or hospitalization for heart failure. In addition, it might prevent 1.4% of patients from withdrawing due to adverse effects. For secondary outcomes, diuretics had an advantage over calcium channel blockers in requiring less add‐on therapy but the data were heterogeneous. At one year, diuretics also lowered systolic blood pressure on average by 1.3 mmHg more than calcium channel blockers and calcium channel blockers lowered diastolic blood pressure on average by 0.5 mmHg more than diuretics. Thiazides likely prevent hospitalizations and death from heart failure compared to calcium channel blockers and are possibly better tolerated, therefore they are the preferred choice. The use of calcium channel blockers first‐line for hypertension undoubtedly leads to hospitalizations for heart failure. It is important for healthcare workers to recognize this as a potentially preventable cause of heart failure.
The comparison of first‐line diuretics with first‐line ACE inhibitors is based on five RCTs and over 30,000 participants. As can be seen in Table 3, diuretics are likely not different from ACE inhibitors for total mortality, total cardiovascular events, total coronary heart disease, and total congestive heart failure. However, diuretics likely decrease total stroke events (ARR 0.6%; moderate‐certainty) and withdrawals due to adverse effects (ARR 1.0%; moderate‐certainty). This means that choosing a diuretic over an ACE inhibitor likely would prevent 0.6% of patients from experiencing a stroke. It is important to appreciate that sensitivity analyses showed that this finding were dependent on the ALLHAT 2000/2002 trial. Prescribing a thiazide instead of an ACE inhibitor would likely prevent 1% of patients from withdrawing due to adverse effects (number needed to treat for an additional beneficial outcome (NNTB) 100). For secondary outcomes, diuretics had an advantage over ACE inhibitors in requiring less add‐on therapy. In keeping with this, at one year, diuretics lowered systolic blood pressure on average by 2.5 mmHg more than ACE inhibitors and lowered diastolic blood pressure on average by 0.4 mmHg more than ACE inhibitors. Thiazides are thus a preferred first‐line choice over ACE inhibitors. It is possible that thiazide‐related reduction of stroke as compared to ACE inhibitors may be due to the greater reduction in systolic and diastolic blood pressure.
The comparison of first‐line diuretics with first‐line alpha‐blockers is based on three RCTs with fewer than 25,000 participants and a mean follow‐up of 3.3 years. The shorter follow‐up duration was because the alpha‐blocker arm of the ALLHAT trial was stopped early when it became evident that doxazosin was inferior to the diuretic, chlorthalidone. As can be seen in Table 4, diuretics were likely not different from alpha‐blockers for total mortality and total coronary heart disease. However, diuretics likely decrease total cardiovascular events, total stroke, and total congestive heart failure compared to alpha‐blockers (moderate‐certainty). In addition, diuretics may reduce withdrawals due to adverse effects (low‐certainty). This means that choosing a thiazide over an alpha‐blocker would likely prevent 3.1% of patients from having an adverse cardiovascular event. This benefit was mostly due to a 2.6% reduction in total heart failure events. Withdrawals due to adverse effects may be 0.4% less for a diuretic. For secondary outcomes, thiazides had an advantage over alpha‐blockers in requiring less add‐on therapy, less switching, and by reducing systolic blood pressure at one year by 3 mmHg more than alpha‐blockers. The effect on diastolic blood pressure was not different. Thus despite having less head‐to‐head data, thiazides are a preferred first‐line choice over alpha‐blockers.
Only two small RCTs compared diuretics to angiotensin receptor blockers and only one RCT compared a diuretic to a direct renin inhibitor. In these trials, diuretics did not differ for mortality, total cardiovascular events, stroke, CHD, withdrawals due to adverse effects, and systolic and diastolic blood pressure compared to the comparators. In one RCT that compared a diuretic to a centrally acting drug the diuretic reduced withdrawals due to adverse effects by 8.5% at one year. None of the other outcomes were reported for this comparison.
Blood pressure data were available at one year for 17 of the 20 trials. These trials are useful for comparing the blood pressure‐lowering effect between diuretics and the other classes. These data should not be used as an estimate of the magnitude of blood pressure lowering for thiazides or the other classes of drugs because these studies allowed dose titration and addition of other drugs. The largest included trial showed that the first‐line thiazide‐like diuretic lowered systolic blood pressure at one year more than ACE inhibitors, calcium channel blockers, and alpha‐blockers (ALLHAT 2000/2002). This effect was confirmed when ALLHAT 2000/2002 was removed from the overall analysis. These findings are based on a large number of trials and were unaffected by sensitivity analyses testing the effect of small versus large trials or the use of supplementary drugs. Also, these findings are consistent with the Cochrane Review of the blood pressure‐lowering effect of thiazide diuretics, which as a class lower systolic blood pressure more than diastolic blood pressure and as a class have the greatest effect to lower pulse pressure (Musini 2014). This greater ability of thiazides to lower systolic blood pressure could have advantages in large population studies and could be an explanation for the fact that in this review diuretics reduced some morbidity outcomes more than other classes of drugs.
Overall completeness and applicability of evidence
The search strategy identified all relevant trials up until March 2021. A top‐up search of the Cochrane Hypertension Specialized Register to July 2022 retrieved 51 unique records, but no additional included studies. Overall, 16 of the 20 studies reported the primary outcome of total mortality. Unfortunately only three smaller studies reported total serious adverse events. The other most important outcome was total cardiovascular events and that was reported in 15 studies. These were in general lower with first‐line diuretics suggesting that they are the best choice for most patients with hypertension. The populations studied in these reviews were mostly older male and female patients (aged 50 to 75) with multiple co‐morbidities. In fact, in the largest trial with the greatest impact on the results, the ALLHAT 2000/2002 trial, over 40% of the patients had type 2 diabetes at baseline. Therefore, the results of this review are applicable to a wide spectrum of hypertensive patients including those with type 2 diabetes. This is important as patients with diabetes are often preferentially treated with drugs inhibiting the renin‐angiotensin system. The results are also primarily relevant to first‐line thiazides and thiazide‐like drugs starting with low doses. Only three trials started with high‐dose thiazides.
We did not find any trials studying first‐line loop diuretics and thus our review findings cannot be generalized to any diuretic. The fact that we did not find any trials studying loop diuretics is not surprising as loop diuretics are not currently considered first‐line drugs for the treatment of hypertension in major clinical practice guidelines (Whelton 2018; Williams 2018). In our published protocol, loop diuretics were included as we were under the assumption that they might lower blood pressure in a similar fashion to thiazides and thiazide‐like drugs (Musini 2014; Musini 2015). However, during the review development process, the field of hypertension management has evolved. For our next update, we will revise our approach and exclude loop diuretics from the first‐line diuretics to be studied.
Quality of the evidence
We have used a modified version of the Cochrane risk of bias tool by adding two domains under the 'Other' category. These two domains were the use of supplemental drugs and industry sponsorship. When comparing first‐line antihypertensive drugs the purest design would be to not allow any supplemental drugs. This was the case for five smaller studies in this review (Berglund 1981; DAPHNE 2002; Materson 1993; NICS‐EH 1999; VA 1982). However, common practice treatment of hypertension uses a stepped‐care approach. In this review, 15 trials allowed supplemental drugs. We have judged that trials where different supplemental drug classes or doses were allowed in the different arms were at high risk of bias. For future updates, we will explore the application of version 2 of the Cochrane tool for assessing risk of bias in randomized trials (RoB 2) for assessing risk of bias in included studies (Higgins 2021).
The largest study, with three comparator arms, was different from the other trials in allowing people on antihypertensive therapy at baseline to be enrolled (ALLHAT 2000/2002). This could create a legacy effect, although that is unlikely in that the trial lasted five years. Where possible we have deselected ALLHAT 2000/2002 in sensitivity analyses and the findings were insensitive to removing the trial. In other words the findings of ALLHAT were similar to the other trials.
In addition, the largest study with three comparator arms (ALLHAT 2000/2002) and two other large studies (MRC 1985; MRC 1992) were primarily funded by government sources and potentially less biased. Many of the other studies were designed and conducted with industry sponsorship, including some larger studies; in these studies, the industry sponsorship favored the comparator drug (ANBP2 2003; HAPPHY 1987; INSIGHT 2000; SHELL 2003) and thus were potentially biased against the diuretic arm. Only one small study favoring the thiazide arm was sponsored by a company (NESTOR 2004).
We considered five domains in grading the overall certainty of evidence: risk of bias or limitations in study design and implementation, unexplained heterogeneity or inconsistency of results, imprecision in results, indirectness of evidence, and high probability of publication bias. The majority of studies had a high risk of bias for funding, addition of supplemental drugs, performance bias due to lack of blinding, or attrition bias due to high losses to follow‐up. We have downgraded most of the outcomes due to these sources of bias and thus none of the outcomes are judged to be based on high‐certainty evidence. It should be noted that we have not considered the direction of bias in this judgment. Most of the funding bias was against the diuretic arm; thus it is possible that the true benefits of diuretics are larger and some of the findings should be judged as high‐certainty. We downgraded a few outcomes (mostly withdrawals due to adverse effects) due to inconsistency of the results. We downgraded one outcome due to imprecision: heart failure, for diuretics as compared to beta‐blockers. No outcomes were downgraded due to indirectness as we judged the populations studied to be a good reflection of the population being treated for hypertension in the real‐world setting. There was no evidence of publication bias and thus we did not downgrade the results for that domain. We assessed all comparisons for the main review outcomes as having moderate or low certainty (Table 1; Table 2; Table 3; Table 4).
As discussed above, the heterogeneity in the beta‐blocker data was eliminated by deselecting the trials where the thiazide doses were high. In addition to explaining the heterogeneity, it clarified that low‐dose thiazides reduced total cardiovascular events, total coronary heart disease events, and withdrawals due to adverse effects as compared to beta‐blockers. The heterogeneity in withdrawals due to adverse effects with calcium channel blockers was eliminated by deselecting ALLHAT 2000/2002 and Tresukosol 2005 where amlodipine was the calcium channel blocker. This would be worth exploring in more detail as withdrawals due to adverse effects were not a prespecified outcome in ALLHAT 2000/2002.
Potential biases in the review process
We made an assumption that all studies published prior to 1998 had been adequately screened and captured in Wright 1999, and literature searches were only conducted for the years including 1998 and onward. It remains possible that relevant studies published prior to these years may not have been identified in the previous systematic review. This potential was minimized by checking included studies against those of other similar systematic reviews (Chen 2018; Psaty 2003; Wiysonge 2017; Zhu 2021), and thus it is considered to be highly unlikely.
Studies were only included if they reported one of the primary outcomes of interest. Several studies that only reported changes in blood pressure were not assessed, therefore the results for changes in systolic and diastolic blood pressure do not include all studies that otherwise met the inclusion criteria.
We dealt with unit of analysis issues by not combining outcomes from the two multi‐arm trials (ALLHAT 2000/2002; Materson 1993). Another potential unit of analysis issue is that despite attempting to only include data from people with at least one event for each of the outcomes there may have been trials where events were reported. It is possible that for some outcomes we overcounted by including events rather than people with one event. This would be unlikely to cause bias as the increased numerator would have occurred in both groups.
The review identified three studies that only reported pooled data from patients treated with diuretics and other drug classes (CONVINCE 2003; Neaton 1993; STOP‐Hypertension‐2 1999). We sought unpublished data specific to the drug classes from these studies but data were not provided by the authors; if such data are obtained in the future, these studies will be included in the meta‐analysis.
Agreements and disagreements with other studies or reviews
Differences in mortality and cardiovascular outcomes between diuretics and other classes of antihypertensives have been assessed in other similar systematic reviews (Chen 2018; Psaty 2003; Thomopoulos 2015; Wiysonge 2017; Wright 1999; Zhu 2021). These other reviews have not taken the approach we have used here of looking at the totality of evidence for first‐line diuretics versus each of the other drug classes individually. Our findings favoring diuretics over beta‐blockers use the same trial data as Wiysonge 2017, though in that review they did not include withdrawals due to adverse effects as a primary outcome as we have. We have shown that diuretics and specifically thiazides tend to have lower adverse cardiovascular outcomes overall and reduce withdrawals due to adverse effects. Thus they are better tolerated as well as reducing systolic blood pressure more than beta‐blockers.
Our review showing that diuretics reduce congestive heart failure events compared with calcium channel blockers is concordant with a network meta‐analysis (Psaty 2003), as well as two more recent systematic reviews (Thomopoulos 2015; Zhu 2021). We have shown that diuretics also reduce withdrawals due to adverse effects and are thus better tolerated as well as reducing systolic blood pressure more than calcium channel blockers.
Our review identified a likely decreased incidence of stroke events for diuretics compared with ACE inhibitors, and these findings are supported by other systematic reviews (Chen 2018; Psaty 2003). This advantage is in addition to diuretics being better tolerated and reducing systolic blood pressure to a greater extent.
The comparison of diuretics and alpha‐blockers substantially favors thiazides and is supported by the network meta‐analysis (Psaty 2003). For other classes of drugs we have one trial showing that diuretics are better tolerated than the centrally acting drug, clonidine. For angiotensin receptor blockers and renin inhibitors we lack head‐to‐head randomized controlled trial evidence, but there is no reason to expect that they would have any significant advantages.
We believe that the approach used in this review is the approach that should be used by groups developing hypertension guidelines. The fact that reduced clinically significant morbidity is achieved with first‐line thiazides and thiazide‐like drugs as compared to the other first‐line drug classes should be reflected in all hypertension guidelines.
An important question arising from this review is why thiazides are better at reducing cardiovascular outcomes. It could be the fact that, as shown in this review, thiazides and thiazide‐like diuretics reduce systolic blood pressure and thus pulse pressure to a greater degree than other drug classes. The common belief that different antihypertensive drug classes lower blood pressure by the same amount warrants a revisit, in view of the current substantial evidence from this review and others that indicate otherwise (Heran 2008a; Heran 2008b; Heran 2012a; Musini 2014).
Authors' conclusions
Implications for practice.
The findings of this review have important implications for practice. These findings are relevant to the populations studied in these reviews, mostly older male and female hypertensive patients (aged 50 to 75) with multiple co‐morbidities, including type 2 diabetes. This does represent the majority of the population treated for hypertension. As such, the results of this review are applicable to a wide spectrum of hypertensive patients, including those with type 2 diabetes. The findings are limited to first‐line thiazide and thiazide‐like diuretic drugs compared to beta‐blockers, calcium channel blockers, ACE inhibitors, and alpha‐blockers.
When first‐line thiazides are compared to the other first‐line antihypertensive drug classes, thiazides may reduce a number of clinically important morbidity outcomes. As compared to first‐line beta‐blockers, thiazides are likely not different in their effect on mortality, likely reduce total cardiovascular events, may have no effect on stroke, coronary heart disease (CHD), and heart failure, likely reduce withdrawals due to adverse effects, likely reduce systolic blood pressure, and likely have no effect on diastolic blood pressure.
As compared to first‐line calcium channel blockers, first‐line thiazides are likely not different in their effect on mortality, may have no effect on serious adverse events, likely reduce total cardiovascular events, likely have no effect on stroke or CHD, likely reduce heart failure, may reduce withdrawals due to adverse effects, likely reduce systolic blood pressure, and likely increase diastolic blood pressure.
As compared to first‐line angiotensin converting enzyme (ACE) inhibitors, thiazides are likely not different in mortality, may not be different in total cardiovascular events, likely reduce stroke, likely have no effect on CHD or heart failure, likely reduce withdrawals due to adverse effects, likely reduce systolic blood pressure, and may reduce diastolic blood pressure.
As compared to first‐line alpha‐blockers, thiazides are likely not different in their effect on mortality, likely reduce total cardiovascular events and stroke, may have no effect on CHD, likely reduce heart failure, may reduce withdrawals due to adverse effects, likely reduce systolic blood pressure, and likely have no effect on diastolic blood pressure.
Data for comparison to other drug classes were insufficient, but no antihypertensive drug class had proven clinically important advantages over first‐line thiazides.
Implications for research.
It is important to note that there has been only one randomized trial designed and conducted to answer this question in the last 10 years (PREVER‐treatment 2016). We hope that this review will encourage further trials. Future head‐to‐head trials assessing mortality and morbidity should include an arm with a first‐line low‐dose thiazide as the standard of therapy. Independent, large, long‐duration head‐to‐head trials comparing first‐line, low‐dose thiazides with angiotensin receptor blockers and renin inhibitors are needed. Future research is needed to explore why thiazides are more effective at reducing some morbidity outcomes and better at lowering systolic blood pressure than other antihypertensive drug classes.
History
Protocol first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 9 May 2011 | Amended | New author added; minor edits to the search strategy. |
Acknowledgements
The authors would like to acknowledge the contributions of Dr. Vijaya Musini and Dr. Colin Dormuth to the protocol and earlier versions of this review. The authors acknowledge the assistance of Dr Kate Smolina for the translation and screening of Russian language studies, Ms Katherine Gale for assistance with screening and sensitivity analyses, and Stephen Adams for article retrieval.
Cochrane Hypertension supported the authors in the development of this review. L Puil, D Salzwedel and JM Wright are members of Cochrane Hypertension, but were not involved in the editorial process or decision‐making for this review. The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Michael Brown, Michigan State University College of Human Medicine, USA
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported the editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Heather Maxwell and Jenny Bellorini, c/o Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Costas Thomopoulos, Department of Cardiology, Helena Venizelou Hospital, Athens, Greece (clinical/content review); Eric Kam‐Pui Lee, The Chinese University of Hong Kong (clinical/content review); Yuda Turana, Department of Neurology, School of Medicine, Atma Jaya Catholic University of Indonesia (clinical/content review); Dimitris Konstantinidis, Hippokration Hospital, Athens, Greece (clinical/content review); Atsushi Mizuno, Department of Cardiovascular Medicine, St.Luke's International Hospital, Japan (clinical/content review); Pringgodigdo Nugroho, Faculty of Medicine, Universitas Indonesia Dr Cipto Mangunkusumo Hospital, Jakarta, Indonesia (clinical/content review); Alessia Gambaro, AOUI Verona, Verona, Italy (clinical/content review); Brian Duncan (consumer review); Rachel Richardson and Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review); Robin Featherstone, Cochrane Central Editorial Service and Candida Fenton, Cochrane Vascular (search review). Two additional peer reviewers provided clinical/content reviews, but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to March 24, 2021> Search Date: 25 March 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazides/ 2 exp sodium chloride symporter inhibitors/ 3 exp sodium potassium chloride symporter inhibitors/ 4 thiazide*.tw,kf. 5 ((sodium chloride adj2 cotransporter inhibit*) or (sodium chloride adj2 co‐transporter inhibit*) or (sodium chloride adj2 symporter inhibit*)).tw,kf. 6 ((ceiling adj2 diuretic*) or (loop adj2 diuretic*) or (potassium‐depleting adj2 diuretic*)).tw,kf. 7 (amiloride or amiclaran or amidal or amiduret trom or amikal or "amilo 5" or amiloberag or amilorid or amiloridehydrochlorhydrate or amiloridine or amipramidine or amyloride or arumil or berkamil or colectril or guanamprazine or kaluril or medamor or midamor or midoride or "mk 870" or modamide or nirulid or pandiuren).mp. 8 (azosemide or azosemid or luret or "ple 1053" or "sk 110").mp. 9 (bendroflumethiazide or aprinox or bendrofluazide or bendroflumethiazide or benzhydroflumethiazide or benzydroflumethiazide or benzyl hydroflumethiazide or benzylhydroflumethiazide or benzide or berkozide or bristuron or centonuron or centyl or esberizid or naturetin or naturine or neo naclex or neonaclex or naturetin or naturine or neonadex or pluryl or pluryle or repicin or salures or sinesalin or urizid).mp. 10 (bumetanide or budema or bumedyl or bumelex or bumet or bumetamide or bumethanide or bumetidine or bumex or burinax or burinex or busix or butinat or butinon or bymex or cambiex or drenural or farmadiuril or fontego or fordiuran or lixil or lunetoron or miccil or "pf 1593" or "pf‐1593" or pf1593 or primex or "ro 10 6338" or "ro 10‐6338" or "ro10 6338" or ro10‐6338).mp. 11 (butizide or buthiazide or eunephran or eunepran or isobutylhydrochlorothiazide or modenol or saltucin or thiabulazid or thiabutazide or thiobulazid or tiabutazide).mp. 12 (chlorothiazide or chlorosal or chlorothiazid or chlorothiazidum or chlorothiazine or chlorthiazide or chlotride or diachlor or diuril or diurilix or diuriwas wassermann milano or flumen or lyovac or saluric or warduzuide).mp. 13 (chlorthalidone or aquadon or chlorphthalidolone or chlortalidon or chlortalidone or clortalidone or chlorthalidine or chlorthalidon or chlorthialidone or clortalil or edemdal or hidronal or higroton or higrotona or hygroton or hylidone or hypertol or hythalton or igrolina or igroton or isoren or natriuran or oxodolin or oxodoline or phthalamidine or phthalamodine or phthalamudine or renon or servidone or thalitone or urandil or urofinil or zambesil).mp. 14 (cicletanine or "bn 1270" or "bn 50417" or "bn 50418" or bn1270 or bn50417 or bn50418 or cicletanide or cycletanide or justar or tenstaten or tenstatin).mp. 15 (clopamide or adurix or aquez or brinaldix or brinaldrix or brinedine or chlosudimeprimylum clopamid or clopamidum or clopamine).mp. 16 (clorexone or chlorexolone or flonatril or klorex or nefrolan or cyclothizaide or anhydron or doburil or fluidil or valmiran).mp. 17 (cyclopenthiazide or cyclomethiazide or cyclopenthiazine or cyclopentiazide or navidex or navidrex or navidrix or salimid or tsiklometiazid).mp. 18 (diapamide or thiamizide or tiamizide or fenquizone or idrolone).mp. 19 (eplerenone or "cgp 30 083" or "cgp 30083" or cgp30083 or elecor or eplerenon or epoxymexrenone or inspra or "sc 66110" or sc66110).mp. 20 (ethacrynic acid or edecril or edecrin or edecrina or endecril or etacrinic acid or etacrynate or etacrynic acid or ethacrinic acid or ethacrynate or ethacryonic acid or ethocrynic acid or ethycrynic acid or hydromedin or lyovac sodium edecrin or "mk 595" or "nsc 85791" or reomax or sodium ethacrynate or uregit or uregyt).mp. 21 (etozolin or elkapin or etazolin or etozoline or "go 687" or go687 or "goe 687" or goe687 or ozolinone ethyl ester or "w 2900" or w2900).mp. 22 (furosemide or aldic or aluzine or anfuramaide or aquarid or arasemide or cetasix or desal or diamazon or dirine or discoid or diumide or diural or diuresal or diurin or diurix or diurolasa or diusemide or diuspec or dryptal or durafurid or edenol or errolon or eutensin or eutensine or flurosemide or franyl or fretic or frumid or frusedan or frusehexal or frusema or frusemidor frusemide or frusid or fruzex or fumarenid or fumide or furanthril or furantral or furantril or furanturil or furasemide or furesin or furesis or furetic or furix or furmid or furo puren or furo‐basan or furo‐puren or furobasan or furomen or furomex or furomide or furomin or furopuren or furorese or furosamide or furoscan or furose or furosemid or furosemix or furosimide or furosix or furovite or fursemide or fusid or fusimex or hissuflux or hydro rapid or impugan or jufurix or kofuzon or kutrix or lasiletten or lasilix or lasix or laxis or laxur or "lb 502" or lb502 or luramide or marsemide or mirfat or odemase or odemex or oedemase or oedemex or pharmix or promedes or radisemide or rasitol or retep or salinex or seguril or selectofur or sigasalur or uremide or uresix or urex‐m or vesix or zafurida).mp. 23 (hydrochlorothiazide or apo‐hydro or aquarius or aquazide or bisalunil or bpzide or bremil or chlorosulthiadil or chlorsulfonamidodihydrobenzothiadiazine or cidrex or clothia or dehydratin or diaqua or dichlorosal or dichlothiazide or dichlotride or dichlozid or diclotride or didralin or dihydrochlorothiazide or dihydrodiuril or direma or disaluril or disothiazide or dithiazide or diu melusin or diumelusin or diurace or diurex or esidrex or esidrix or fluvin or hctz or hidrenox or hidril or hidroronol or hidrosaluretil or hudorex or hychlozide or hydrex‐semi or hydril or hydro aquil or hydrochlor or hydrochloro thiazide or hydrochlorothiamide or hydrochlorothiazid or hydrochlorothiazine or hydrochlorzide or hydrochlothiazide or hydro diuril or hydrodiuril or hydromal or hydrororonol or hydro saluric or hydrosaluric or hydrothide or hydro tonuron or hydrozide or hypothiazid or hypothiazide or ivaugan or maschitt or microzide or mictrin or nefrix or neoflumen or newtolide or niagar or oretic or pantemon or ridaq or sectrazide or tandiur or thiadril or thiaretic or thiuretic or urodiazin or urodiazine or urozide or vetidrex).mp. 24 (hydroflumethiazide or bristab or di ademil or diademil or dihydroflumethiazide or diraudixin or diucardin or hiserpin or hydrenox or leodrin or leodrine or metflorylthiadiazine or naclex or rontyl or saluron or sisuril or trifluoromethylhydrothiazide).mp. 25 (indapamide or agelan or apadex or arifon or damide or dapamax or diflerix or dixamid or extur or fludex or fluidema or frumeron or indahexal or indalix or indamol or indapam or indapress or indicontin or indoline or indopamide or inpamide or insig or ipamix or lorvas or loxide or lozol or metindamide or millibar or naplin or natrilix or natrix or noranat or pamid or pressural or pretanix or rinalix or sicco or tandix or tertensif or veroxil).mp. 26 (indacrinone or indacrinic acid or indacrynic acid or "mk 196").mp. 27 (mefruside or bay caron or bay1500 or baycaron or baycarone or mefrusid).mp. 28 (metolazone or barolyn or diulo or metalazone or metenix or metolazon or miclox or microx or mykrox or normelan or xuret or zaroxolyn).mp. 29 (methylclothiazide or aquatensen or enduron or enduron‐m or enduronum or methyclothiazide or methylchlorothiazide or thiazidil).mp. 30 (muzolimine or "bay g 2821" or "bay g2821" or "bayer g 2821" or "bayer g2821" or edrul or musolimino).mp. 31 (ozolinone or "go 3282" or go3282 or "goe 3282" or goe3282 or "goedecke 3282").mp. 32 phenoxybenzoic acid.mp. 33 (piretanide or arelix or arlix or eurelix or "hoe 118" or hoe118 or lafax or perbilen or "s 73 4118" or "s 734118" or s734118 or tauliz).mp. 34 (polythiazide or drenusil or nephril or polythiazide or renese).mp. 35 (quinethazone or aquamox or chinethazon or chinethazone or guinethazone or hydromox or kinetazone or quinethazon).mp. 36 (spironolactone or abbolactone or acelat or adultmin or alaton or alatone or aldace or aldactone or aldopur or aldospirone or almatol or aquareduct or berlactone or carospir or "crl 635" or crl635 or diram or duraspiron or "dyta urese" or dytaurese or espironolactona or flumach or frumikal or jenaspiron or hypazon or idrolattone or merabis or "novo spiroton" or "novo‐spiriton" or novospiroton or osiren or osyrol or pirolacton or pondactone or practon or prilactone or resacton or "sas 1060" or sas1060 or "sc 9420" or "sc‐9420" or sc9420 or spiractin or spiridon or spirix or spirobeta or "spiro ct" or spiroctan or spirogamma or spirohexal or spirolacton or spirolactone or spirolang or "spiro l.u.t." or spiron or spirone or spironex or spirono isis or spironol or spironolacton or spironolakton or spironone or spirospare or spirothiobarbiturate or spirotone or spiro von ct or supra puren or suprapuren or uractone or veroshpiron or verospiron or verospirone or xenalon or youlactone).mp. 37 (ticrynafen or "anp 3624" or "anp‐3624" or anp3624 or diflurex or selacryn or "skf 62698" or "skf‐62698" or skf62698 or selacryn or thienilic acid or thienylic acid or tienilic acid).mp. 38 tizolemide.mp. 39 (torsemide or "bm 02015" or "bm 2015" or bm02015 or bm2015 or demadex or diuremid or "jdl 464" or jdl464 or luprac or presaril or toradiur or torem or torrem or torasemide or unat or upcard).mp. 40 (triamterene or dyrenium or dytac or urocaudal or ademin or ademine or dyren or dyrenium or dytac or iatropur or jatropur or noridyl or "nsc 77625" or nsc77625 or pterofen or pterophene or "sk and f 8542" or "skf 8542" or skf8542 or teriam or triampterene or triamterence or triamterens or triamteril or triteren or uretren or urocaudal).mp. 41 (trichloromethiazide or aquazide or dichloromethylhydrochlorothiazide or diurese or esmarin or eurinol or fluitran or flutra or gangesol or hydrotrichlorothiazide or metahydrin or methahydrin or naqua or naquasone or salurin or triazide or trichlordiuride or trichlorex or trichlormethazide or trichlormethiazide or trichlormas or trichloromethylhydrochlorothiazide or triflumen or wadel).mp. 42 (tripamide or "adr 033" or adr033 or "e 614" or e614 or normonal).mp. 43 (xipamide or aquaforil or aquaphor or aquaphoril or aquavor or "bei 1293" or diurexan or lumitens or xipamid or xypamide or zipix).mp. 44 or/1‐43 45 exp angiotensin‐converting enzyme inhibitors/ 46 angiotensin converting enzyme inhibit*.tw,kf. 47 (ace adj2 inhibit*).tw,kf. 48 acei.tw,kf. 49 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw,kf. 50 or/45‐49 51 exp Angiotensin Receptor Antagonists/ 52 (angiotensin adj3 receptor antagon*).tw,kf. 53 (angiotensin adj3 receptor block*).tw,kf. 54 (arb or arbs).tw,kf. 55 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw,kf. 56 or/51‐55 57 exp calcium channel blockers/ 58 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw,kf. 59 (calcium adj2 (antagonist* or block* or inhibit*)).tw,kf. 60 or/57‐59 61 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 62 (reserpine or serpentina or rauwolfia or serpasil).mp. 63 (clonidine or adesipress or arkamin or caprysin or catapres* or catasan or chlofazolin or chlophazolin or clinidine or clofelin* or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin* or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 64 exp hydralazine/ 65 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).mp. 66 or/61‐65 67 exp adrenergic beta‐antagonists/ 68 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw,kf. 69 (beta adj2 (adrenergic* or antagonist* or block* or receptor*)).tw,kf. 70 or/67‐69 71 exp adrenergic alpha antagonists/ 72 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw,kf. 73 (adrenergic adj2 (alpha or antagonist*)).tw,kf. 74 ((adrenergic or alpha or receptor*) adj2 block*).tw,kf. 75 or/71‐74 76 50 or 56 or 60 or 66 or 70 or 75 77 hypertension/ 78 essential hypertension/ 79 (antihypertens* or hypertens*).tw,kf. 80 ((elevat* adj2 arterial pressur*) or (elevat* adj2 blood pressur*) or (elevat* adj2 diastolic pressur*) or (elevat* adj2 systolic pressur*)).tw,kf. 81 ((high adj2 arterial pressur*) or (high adj2 blood pressur*) or (high adj2 diastolic pressure) or (high adj2 systolic pressur*)).tw,kf. 82 ((rais* adj2 arterial pressur*) or (rais* adj2 blood pressur*) or (rais* adj2 diastolic pressure) or (rais* adj2 systolic pressur*)).tw,kf. 83 ((elevat* adj2 bp) or (elevat* adj2 dbp) or (elevat* adj2 sbp)).tw,kf. 84 ((high adj2 bp) or (high adj2 dbp) or (high adj2 sbp)).tw,kf. 85 ((rais* adj2 bp) or (rais* adj2 dbp) or (rais* adj2 sbp)).tw,kf. 86 or/77‐85 87 randomized controlled trial.pt. 88 controlled clinical trial.pt. 89 randomi*ed.ab. 90 placebo.ab. 91 dt.fs. 92 randomly.ab. 93 trial.ab. 94 groups.ab. 95 or/87‐94 96 animals/ not (humans/ and animals/) 97 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 98 (pregnancy‐induced or ocular hypertens* or preeclampsia or pre‐eclampsia).ti. 99 95 not (96 or 97 or 98) 100 44 and 76 and 86 and 99
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Hypertension Specialized Register via Cochrane Register of Studies
Search Date: 27 June 2022
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 thiazide* AND INREGISTER #2 (sodium chloride) NEAR2 (cotransporter inhibit* OR co‐transporter inhibit* OR symporter inhibit*) AND INREGISTER #3 (ceiling OR loop OR potassium‐depleting) NEAR2 (diuretic) AND INREGISTER #4 (amiloride OR amiclaran OR amidal OR amiduret trom OR amikal OR "amilo 5" OR amiloberag OR amilorid OR amiloridehydrochlorhydrate OR amiloridine OR amipramidine OR amyloride OR arumil OR berkamil OR colectril OR guanamprazine OR kaluril OR medamor OR midamor OR midoride OR "mk 870" OR modamide OR nirulid OR pandiuren) AND INREGISTER #5 (azosemide OR azosemid OR luret) AND INREGISTER #6 (bendroflumethiazide OR aprinox OR bendrofluazide OR bendroflumethiazide OR benzhydroflumethiazide OR benzydroflumethiazide OR benzyl hydroflumethiazide OR benzylhydroflumethiazide OR benzide OR berkozide OR bristuron OR centonuron OR centyl OR esberizid OR naturetin OR naturine OR neo naclex OR neonaclex OR naturetin OR naturine OR neonadex OR pluryl OR pluryle OR repicin OR salures OR sinesalin OR urizid) AND INREGISTER #7 (bumetanide OR budema OR bumedyl OR bumelex OR bumet OR bumetamide OR bumethanide OR bumetidine OR bumex OR burinax OR burinex OR busix OR butinat OR butinon OR bymex OR cambiex OR drenural OR farmadiuril OR fontego OR fordiuran OR lixil OR lunetoron OR miccil) AND INREGISTER #8 (butizide OR buthiazide OR eunephran OR eunepran OR isobutylhydrochlorothiazide OR modenol OR saltucin OR thiabulazid OR thiabutazide OR thiobulazid OR tiabutazide) AND INREGISTER #9 (chlorothiazide OR chlorosal OR chlorothiazid OR chlorothiazidum OR chlorothiazine OR chlorthiazide OR chlotride OR diachlor OR diuril OR diurilix OR diuriwas wassermann milano OR flumen OR lyovac OR saluric OR warduzuide) AND INREGISTER #10 (chlorthalidone OR aquadon OR chlorphthalidolone OR chlortalidon OR chlortalidone OR clortalidone OR chlorthalidine OR chlorthalidon OR chlorthialidone OR clortalil OR edemdal OR hidronal OR higroton OR higrotona OR hygroton OR hylidone OR hypertol OR hythalton OR igrolina OR igroton OR isoren OR natriuran OR oxodolin OR oxodoline OR phthalamidine OR phthalamodine OR phthalamudine OR renon OR servidone OR thalitone OR urandil OR urofinil OR zambesil) AND INREGISTER #11 (cicletanine OR cicletanide OR cycletanide OR justar OR tenstaten OR tenstatin) AND INREGISTER #12 (clopamide OR adurix OR aquez OR brinaldix OR brinaldrix OR brinedine OR chlosudimeprimylum clopamid OR clopamidum OR clopamine) AND INREGISTER #13 (clorexone OR chlorexolone OR flonatril OR klorex OR nefrolan OR cyclothizaide OR anhydron OR doburil OR fluidil OR valmiran) AND INREGISTER #14 (cyclopenthiazide OR cyclomethiazide OR cyclopenthiazine OR cyclopentiazide OR navidex OR navidrex OR navidrix OR salimid OR tsiklometiazid) AND INREGISTER #15 (diapamide OR thiamizide OR tiamizide OR fenquizone OR idrolone) AND INREGISTER #16 (eplerenone OR elecor OR eplerenon OR epoxymexrenone OR inspra) AND INREGISTER #17 (ethacrynic acid OR edecril OR edecrin OR edecrina OR endecril OR etacrinic acid OR etacrynate OR etacrynic acid OR ethacrinic acid OR ethacrynate OR ethacryonic acid OR ethocrynic acid OR ethycrynic acid OR hydromedin OR lyovac sodium edecrin OR "mk 595" OR "nsc 85791" OR reomax OR sodium ethacrynate OR uregit OR uregyt) AND INREGISTER #18 (etozolin OR elkapin OR etazolin OR etozoline OR ozolinone ethyl ester) AND INREGISTER #19 (furosemide OR aldic OR aluzine OR anfuramaide OR aquarid OR arasemide OR cetasix OR desal OR diamazon OR dirine OR discoid OR diumide OR diural OR diuresal OR diurin OR diurix OR diurolasa OR diusemide OR diuspec OR dryptal OR durafurid OR edenol OR errolon OR eutensin OR eutensine OR flurosemide OR franyl OR fretic OR frumid OR frusedan OR frusehexal OR frusema OR frusemidor frusemide OR frusid OR fruzex OR fumarenid OR fumide OR furanthril OR furantral OR furantril OR furanturil OR furasemide OR furesin OR furesis OR furetic OR furix OR furmid OR furo puren OR furo‐basan OR furo‐puren OR furobasan OR furomen OR furomex OR furomide OR furomin OR furopuren OR furorese OR furosamide OR furoscan OR furose OR furosemid OR furosemix OR furosimide OR furosix OR furovite OR fursemide OR fusid OR fusimex OR hissuflux OR hydro rapid OR impugan OR jufurix OR kofuzon OR kutrix OR lasiletten OR lasilix OR lasix OR laxis OR laxur OR luramide OR marsemide OR mirfat OR odemase OR odemex OR oedemase OR oedemex OR pharmix OR promedes OR radisemide OR rasitol OR retep OR salinex OR seguril OR selectofur OR sigasalur OR uremide OR uresix OR urex‐m OR vesix OR zafurida) AND INREGISTER #20 (hydrochlorothiazide OR apo‐hydro OR aquarius OR aquazide OR bisalunil OR bpzide OR bremil OR chlorosulthiadil OR chlorsulfonamidodihydrobenzothiadiazine OR cidrex OR clothia OR dehydratin OR diaqua OR dichlorosal OR dichlothiazide OR dichlotride OR dichlozid OR diclotride OR didralin OR dihydrochlORothiazide OR dihydrodiuril OR direma OR disaluril OR disothiazide OR dithiazide OR diu melusin OR diumelusin OR diurace OR diurex OR esidrex OR esidrix OR fluvin OR hctz OR hidrenox OR hidril OR hidroronol OR hidrosaluretil OR hudorex OR hychlozide OR hydrex‐semi OR hydril OR hydro aquil OR hydrochlor OR hydrochloro thiazide OR hydrochlorothiamide OR hydrochlorothiazid OR hydrochlorothiazine OR hydrochlorzide OR hydrochlothiazide OR hydro diuril OR hydrodiuril OR hydromal OR hydrororonol OR hydro saluric OR hydrosaluric OR hydrothide OR hydro tonuron OR hydrozide OR hypothiazid OR hypothiazide OR ivaugan OR maschitt OR microzide OR mictrin OR nefrix OR neoflumen OR newtolide OR niagar OR oretic OR pantemon OR ridaq OR sectrazide OR tandiur OR thiadril OR thiaretic OR thiuretic OR urodiazin OR urodiazine OR urozide OR vetidrex) AND INREGISTER #21 (hydroflumethiazide OR bristab OR di ademil OR diademil OR dihydroflumethiazide OR diraudixin OR diucardin OR hiserpin OR hydrenox OR leodrin OR leodrine OR metflorylthiadiazine OR naclex OR rontyl OR saluron OR sisuril OR trifluoromethylhydrothiazide) AND INREGISTER #22 (indapamide OR agelan OR apadex OR arifon OR damide OR dapamax OR diflerix OR dixamid OR extur OR fludex OR fluidema OR frumeron OR indahexal OR indalix OR indamol OR indapam OR indapress OR indicontin OR indoline OR indopamide OR inpamide OR insig OR ipamix OR lorvas OR loxide OR lozol OR metindamide OR millibar OR naplin OR natrilix OR natrix OR noranat OR pamid OR pressural OR pretanix OR rinalix OR sicco OR tandix OR tertensif OR veroxil) AND INREGISTER #23 (indacrinone OR indacrinic acid OR indacrynic acid) AND INREGISTER #24 (mefruside OR bay caron OR baycaron OR baycarone OR mefrusid) AND INREGISTER #25 (metolazone OR barolyn OR diulo OR metalazone OR metenix OR metolazon OR miclox OR microx OR mykrox OR normelan OR xuret OR zaroxolyn) AND INREGISTER #26 (methylclothiazide OR aquatensen OR enduron OR enduron‐m OR enduronum OR methyclothiazide OR methylchlorothiazide OR thiazidil) AND INREGISTER #27 (muzolimine OR edrul OR musolimino) AND INREGISTER #28 ozolinone AND INREGISTER #29 phenoxybenzoic acid AND INREGISTER #30 (piretanide OR arelix OR arlix OR eurelix OR lafax OR perbilen OR tauliz) AND INREGISTER #31 (polythiazide OR drenusil OR nephril OR polythiazide OR renese) AND INREGISTER #32 (quinethazone OR aquamox OR chinethazon OR chinethazone OR guinethazone OR hydromox OR kinetazone OR quinethazon) AND INREGISTER #33 (spironolactone OR abbolactone OR acelat OR adultmin OR alaton OR alatone OR aldace OR aldactone OR aldopur OR aldospirone OR almatol OR aquareduct OR berlactone OR carospir OR diram OR duraspiron OR "dyta urese" OR dytaurese OR espironolactona OR flumach OR frumikal OR jenaspiron OR hypazon OR idrolattone OR merabis OR "novo spiroton" OR "novo‐spiriton" OR novospiroton OR osiren OR osyrol OR pirolacton OR pondactone OR practon OR prilactone OR resacton OR spiractin OR spiridon OR spirix OR spirobeta OR "spiro ct" OR spiroctan OR spirogamma OR spirohexal OR spirolacton OR spirolactone OR spirolang OR "spiro l.u.t." OR spiron OR spirone OR spironex OR spirono isis OR spironol OR spironolacton OR spironolakton OR spironone OR spirospare OR spirothiobarbiturate OR spirotone OR spiro von ct OR supra puren OR suprapuren OR uractone OR veroshpiron OR verospiron OR verospirone OR xenalon OR youlactone) AND INREGISTER #34 (ticrynafen OR diflurex OR selacryn OR selacryn OR thienilic acid OR thienylic acid OR tienilic acid) AND INREGISTER #35 tizolemide AND INREGISTER #36 (torsemide OR "bm 02015" OR "bm 2015" OR bm02015 OR bm2015 OR demadex OR diuremid OR "jdl 464" OR jdl464 OR luprac OR presaril OR toradiur OR torem OR torrem OR torasemide OR unat OR upcard) AND INREGISTER #37 (triamterene OR dyrenium OR dytac OR urocaudal OR ademin OR ademine OR dyren OR dyrenium OR dytac OR iatropur OR jatropur OR noridyl OR pterofen OR pterophene OR teriam OR triampterene OR triamterence OR triamterens OR triamteril OR triteren OR uretren OR urocaudal) AND INREGISTER #38 (trichloromethiazide OR aquazide OR dichloromethylhydrochlorothiazide OR diurese OR esmarin OR eurinol OR fluitran OR flutra OR gangesol OR hydrotrichlorothiazide OR metahydrin OR methahydrin OR naqua OR naquasone OR salurin OR triazide OR trichlordiuride OR trichlorex OR trichlormethazide OR trichlormethiazide OR trichlormas OR trichloromethylhydrochlorothiazide OR triflumen OR wadel) AND INREGISTER #39 (tripamide OR normonal) AND INREGISTER #40 (xipamide OR aquaforil OR aquaphor OR aquaphoril OR aquavor OR diurexan OR lumitens OR xipamid OR xypamide OR zipix) AND INREGISTER #41 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40) #42 angiotensin converting enzyme inhibit* AND INREGISTER #43 ace NEAR2 inhibit* AND INREGISTER #44 acei OR aceis AND INREGISTER #45 (alacepril OR altiopril OR ancovenin OR benazepril OR captopril OR ceranapril OR ceronapril OR cilazapril OR deacetylalacepril OR delapril OR derapril OR enalapril OR epicaptopril OR fasidotril OR fosinopril OR foroxymithine OR gemopatrilat OR idapril OR imidapril OR indolapril OR libenzapril OR lisinopril OR moexipril OR moveltipril OR omapatrilat OR pentopril* OR perindopril* OR pivopril OR quinapril* OR ramipril* OR rentiapril OR saralasin OR s nitrosocaptopril OR spirapril* OR temocapril* OR teprotide OR trandolapril* OR utibapril* OR zabicipril* OR zofenopril*) AND INREGISTER #46 (#42 OR #43 OR #44 OR #45) #47 ((angiotensin receptor antagonist*) OR (angiotensin receptor block*)) AND INREGISTER #48 (arb OR arbs) AND INREGISTER #49 (abitesartan OR azilsartan OR candesartan OR elisartan OR embusartan OR eprosartan OR forasartan OR irbesartan OR losartan OR milfasartan OR olmesartan OR saprisartan OR tasosartan OR telmisartan OR valsartan OR zolasartan) AND INREGISTER #50 (#47 OR #48 OR #49) #51 ((calcium channel antagon*) OR (calcium channel block*) OR (calcium inhibit*)) AND INREGISTER #52 (amlodipine OR amrinone OR aranidipine OR barnidipine OR bencyclane OR benidipine OR bepridil OR cilnidipine OR cinnarizine OR clentiazem OR darodipine OR diltiazem OR efonidipine OR elgodipine OR etafenone OR fantofarone OR felodipine OR fendiline OR flunarizine OR gallopamil OR isradipine OR lacidipine OR lercanidipine OR lidoflazine OR lomerizine OR manidipine OR mibefradil OR nicardipine OR nifedipine OR niguldipine OR nilvadipine OR nimodipine OR nisoldipine OR nitrendipine OR perhexiline OR prenylamine OR semotiadil OR terodiline OR tiapamil OR verapamil) AND INREGISTER #53 (#51 OR #52) #54 (methyldopa OR alphamethyldopa OR amodopa OR dopamet OR dopegyt OR dopegit OR dopegite OR emdopa OR hyperpax OR hyperpaxa OR methylpropionic acid OR dopergit OR meldopa OR methyldopate OR medopa OR medomet OR sembrina OR aldomet OR aldometil OR aldomin OR hydopa OR methyldihydroxyphenylalanine OR methyl dopa OR mulfasin OR presinol OR presolisin OR sedometil OR sembrina OR taquinil OR dihydroxyphenylalanine OR methylphenylalanine OR methylalanine OR alpha methyl dopa) AND INREGISTER #55 (reserpine OR serpentina OR rauwolfia OR serpasil) AND INREGISTER #56 (clonidine OR adesipress OR arkamin OR caprysin OR catapres* OR catasan OR chlofazolin OR chlophazolin OR clinidine OR clofelin* OR clofenil OR clomidine OR clondine OR clonistada OR clonnirit OR clophelin* OR dichlorophenylaminoimidazoline OR dixarit OR duraclon OR gemiton OR haemiton OR hemiton OR imidazoline OR isoglaucon OR klofelin OR klofenil OR normopresan OR paracefan OR tesno timelets) AND INREGISTER #57 (hydralazin* OR hydrallazin* OR hydralizine OR hydrazinophtalazine OR hydrazinophthalazine OR hydrazinophtalizine OR dralzine OR hydralacin OR hydrolazine OR hypophthalin OR hypoftalin OR hydrazinophthalazine OR idralazina OR 1‐hydrazinophthalazine OR apressin OR nepresol OR apressoline OR apresoline OR apresolin OR alphapress OR alazine OR idralazina OR lopress OR plethorit OR praeparat) AND INREGISTER #58 (#54 OR #55 OR #56 OR #57) #59 ((adrenergic beta‐antagon*) OR (beta adrenergic*) OR (beta antagon*) OR (beta block*) OR (beta recept*)) AND INREGISTER #60 (acebutolol OR adimolol OR afurolol OR alprenolol OR amosulalol OR arotinolol OR atenolol OR befunolol OR betaxolol OR bevantolol OR bisoprolol OR bopindolol OR bornaprolol OR brefonalol OR bucindolol OR bucumolol OR bufetolol OR bufuralol OR bunitrolol OR bunolol OR bupranolol OR butofilolol OR butoxamine OR carazolol OR carteolol OR carvedilol OR celiprolol OR cetamolol OR chlORtalidone clORanolol OR cyanoiodopindolol OR cyanopindolol OR deacetylmetipranolol OR diacetolol OR dihydroalprenolol OR dilevalol OR epanolol OR esmolol OR exaprolol OR falintolol OR flestolol OR flusoxolol OR hydroxybenzylpinodolol OR hydroxycarteolol OR hydroxymetoprolol OR indenolol OR iodocyanopindolol OR iodopindolol OR iprocrolol OR isoxaprolol OR labetalol OR landiolol OR levobunolol OR levomoprolol OR medroxalol OR mepindolol OR methylthiopropranolol OR metipranolol OR metoprolol OR moprolol OR nadolol OR oxprenolol OR penbutolol OR pindolol OR nadolol OR nebivolol OR nifenalol OR nipradilol OR oxprenolol OR pafenolol OR pamatolol OR penbutolol OR pindolol OR practolol OR primidolol OR prizidilol OR procinolol OR pronetalol OR propranolol OR proxodolol OR ridazolol OR salcardolol OR soquinolol OR sotalol OR spirendolol OR talinolol OR tertatolol OR tienoxolol OR tilisolol OR timolol OR tolamolol OR toliprolol OR tribendilol OR xibenolol) AND INREGISTER #61 (#59 OR #60) #62 ((adrenergic alpha) OR (adrenergic antagon*) OR (adrenergic block*) OR (adrenergic receptor antagon*) OR (adrenergic receptor block*) OR (alpha block*)) AND INREGISTER #63 (alfuzosin OR bunazosin OR doxazosin OR metazosin OR neldazosin OR prazosin OR silodosin OR tamsulosin OR terazosin OR tiodazosin OR trimazosin) AND INREGISTER #64 (#62 OR #63) #65 (#46 OR #50 OR #53 OR #58 OR #61 OR #64) #66 RCT:DE AND INREGISTER #67 Review:ODE AND INREGISTER #68 (#66 OR #67) #69 (#41 AND #65 AND #68)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials (Issue 2, 2021) via Cochrane Register of Studies
Search Date: 25 March 2021
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 thiazide* AND CENTRAL:TARGET #2 sodium chloride NEAR2 (cotransporter inhibit* OR co‐transporter inhibit* OR symporter inhibit*) AND CENTRAL:TARGET #3 (ceiling OR loop OR potassium‐depleting) NEAR2 diuretic* AND CENTRAL:TARGET #4 (amiloride OR amiclaran OR amidal OR amiduret trom OR amikal OR "amilo 5" OR amiloberag OR amilorid OR amiloridehydrochlorhydrate OR amiloridine OR amipramidine OR amyloride OR arumil OR berkamil OR colectril OR guanamprazine OR kaluril OR medamor OR midamor OR midoride OR "mk 870" OR modamide OR nirulid OR pandiuren) AND CENTRAL:TARGET #5 (azosemide OR azosemid OR luret OR "ple 1053" OR "sk 110") AND CENTRAL:TARGET #6 (bendroflumethiazide OR aprinox OR bendrofluazide OR bendroflumethiazide OR benzhydroflumethiazide OR benzydroflumethiazide OR benzyl hydroflumethiazide OR benzylhydroflumethiazide OR benzide OR berkozide OR bristuron OR centonuron OR centyl OR esberizid OR naturetin OR naturine OR neo naclex OR neonaclex OR naturetin OR naturine OR neonadex OR pluryl OR pluryle OR repicin OR salures OR sinesalin OR urizid) AND CENTRAL:TARGET #7 (bumetanide OR budema OR bumedyl OR bumelex OR bumet OR bumetamide OR bumethanide OR bumetidine OR bumex OR burinax OR burinex OR busix OR butinat OR butinon OR bymex OR cambiex OR drenural OR farmadiuril OR fontego OR fordiuran OR lixil OR lunetoron OR miccil OR "pf 1593" OR "pf‐1593" OR pf1593 OR primex OR "ro 10 6338" OR "ro 10‐6338" OR "ro10 6338" OR ro10‐6338) AND CENTRAL:TARGET #8 (butizide OR buthiazide OR eunephran OR eunepran OR isobutylhydrochlorothiazide OR modenol OR saltucin OR thiabulazid OR thiabutazide OR thiobulazid OR tiabutazide) AND CENTRAL:TARGET #9 (chlorothiazide OR chlorosal OR chlorothiazid OR chlorothiazidum OR chlorothiazine OR chlorthiazide OR chlotride OR diachlor OR diuril OR diurilix OR diuriwas wassermann milano OR flumen OR lyovac OR saluric OR warduzuide) AND CENTRAL:TARGET #10 (chlorthalidone OR aquadon OR chlorphthalidolone OR chlortalidon OR chlortalidone OR clortalidone OR chlorthalidine OR chlorthalidon OR chlorthialidone OR clortalil OR edemdal OR hidronal OR higroton OR higrotona OR hygroton OR hylidone OR hypertol OR hythalton OR igrolina OR igroton OR isoren OR natriuran OR oxodolin OR oxodoline OR phthalamidine OR phthalamodine OR phthalamudine OR renon OR servidone OR thalitone OR urandil OR urofinil OR zambesil) AND CENTRAL:TARGET #11 (cicletanine OR "bn 1270" OR "bn 50417" OR "bn 50418" OR bn1270 OR bn50417 OR bn50418 OR cicletanide OR cycletanide OR justar OR tenstaten OR tenstatin) AND CENTRAL:TARGET #12 (clopamide OR adurix OR aquez OR brinaldix OR brinaldrix OR brinedine OR chlosudimeprimylum clopamid OR clopamidum OR clopamine) AND CENTRAL:TARGET #13 (clorexone OR chlorexolone OR flonatril OR klorex OR nefrolan OR cyclothizaide OR anhydron OR doburil OR fluidil OR valmiran) AND CENTRAL:TARGET #14 (cyclopenthiazide OR cyclomethiazide OR cyclopenthiazine OR cyclopentiazide OR navidex OR navidrex OR navidrix OR salimid OR tsiklometiazid) AND CENTRAL:TARGET #15 (diapamide OR thiamizide OR tiamizide OR fenquizone OR idrolone) AND CENTRAL:TARGET #16 (eplerenone OR "cgp 30 083" OR "cgp 30083" OR cgp30083 OR elecor OR eplerenon OR epoxymexrenone OR inspra OR "sc 66110" OR sc66110) AND CENTRAL:TARGET #17 (ethacrynic acid OR edecril OR edecrin OR edecrina OR endecril OR etacrinic acid OR etacrynate OR etacrynic acid OR ethacrinic acid OR ethacrynate OR ethacryonic acid OR ethocrynic acid OR ethycrynic acid OR hydromedin OR lyovac sodium edecrin OR "mk 595" OR "nsc 85791" OR reomax OR sodium ethacrynate OR uregit OR uregyt) AND CENTRAL:TARGET #18 (etozolin OR elkapin OR etazolin OR etozoline OR "go 687" OR go687 OR "goe 687" OR goe687 OR ozolinone ethyl ester OR "w 2900" OR w2900) AND CENTRAL:TARGET #19 (furosemide OR aldic OR aluzine OR anfuramaide OR aquarid OR arasemide OR cetasix OR desal OR diamazon OR dirine OR discoid OR diumide OR diural OR diuresal OR diurin OR diurix OR diurolasa OR diusemide OR diuspec OR dryptal OR durafurid OR edenol OR errolon OR eutensin OR eutensine OR flurosemide OR franyl OR fretic OR frumid OR frusedan OR frusehexal OR frusema OR frusemidor frusemide OR frusid OR fruzex OR fumarenid OR fumide OR furanthril OR furantral OR furantril OR furanturil OR furasemide OR furesin OR furesis OR furetic OR furix OR furmid OR furo puren OR furo‐basan OR furo‐puren OR furobasan OR furomen OR furomex OR furomide OR furomin OR furopuren OR furorese OR furosamide OR furoscan OR furose OR furosemid OR furosemix OR furosimide OR furosix OR furovite OR fursemide OR fusid OR fusimex OR hissuflux OR hydro rapid OR impugan OR jufurix OR kofuzon OR kutrix OR lasiletten OR lasilix OR lasix OR laxis OR laxur OR "lb 502" OR lb502 OR luramide OR marsemide OR mirfat OR odemase OR odemex OR oedemase OR oedemex OR pharmix OR promedes OR radisemide OR rasitol OR retep OR salinex OR seguril OR selectofur OR sigasalur OR uremide OR uresix OR urex‐m OR vesix OR zafurida) AND CENTRAL:TARGET #20 (hydrochlorothiazide OR apo‐hydro OR aquarius OR aquazide OR bisalunil OR bpzide OR bremil OR chlorosulthiadil OR chlorsulfonamidodihydrobenzothiadiazine OR cidrex OR clothia OR dehydratin OR diaqua OR dichlorosal OR dichlothiazide OR dichlotride OR dichlozid OR diclotride OR didralin OR dihydrochlORothiazide OR dihydrodiuril OR direma OR disaluril OR disothiazide OR dithiazide OR diu melusin OR diumelusin OR diurace OR diurex OR esidrex OR esidrix OR fluvin OR hctz OR hidrenox OR hidril OR hidroronol OR hidrosaluretil OR hudorex OR hychlozide OR hydrex‐semi OR hydril OR hydro aquil OR hydrochlor OR hydrochloro thiazide OR hydrochlorothiamide OR hydrochlorothiazid OR hydrochlorothiazine OR hydrochlorzide OR hydrochlothiazide OR hydro diuril OR hydrodiuril OR hydromal OR hydrororonol OR hydro saluric OR hydrosaluric OR hydrothide OR hydro tonuron OR hydrozide OR hypothiazid OR hypothiazide OR ivaugan OR maschitt OR microzide OR mictrin OR nefrix OR neoflumen OR newtolide OR niagar OR oretic OR pantemon OR ridaq OR sectrazide OR tandiur OR thiadril OR thiaretic OR thiuretic OR urodiazin OR urodiazine OR urozide OR vetidrex) AND CENTRAL:TARGET #21 (hydroflumethiazide OR bristab OR di ademil OR diademil OR dihydroflumethiazide OR diraudixin OR diucardin OR hiserpin OR hydrenox OR leodrin OR leodrine OR metflorylthiadiazine OR naclex OR rontyl OR saluron OR sisuril OR trifluoromethylhydrothiazide) AND CENTRAL:TARGET #22 (indapamide OR agelan OR apadex OR arifon OR damide OR dapamax OR diflerix OR dixamid OR extur OR fludex OR fluidema OR frumeron OR indahexal OR indalix OR indamol OR indapam OR indapress OR indicontin OR indoline OR indopamide OR inpamide OR insig OR ipamix OR lorvas OR loxide OR lozol OR metindamide OR millibar OR naplin OR natrilix OR natrix OR noranat OR pamid OR pressural OR pretanix OR rinalix OR sicco OR tandix OR tertensif OR veroxil) AND CENTRAL:TARGET #23 (indacrinone OR indacrinic acid OR indacrynic acid OR "mk 196") AND CENTRAL:TARGET #24 (mefruside OR bay caron OR bay1500 OR baycaron OR baycarone OR mefrusid) AND CENTRAL:TARGET #25 (metolazone OR barolyn OR diulo OR metalazone OR metenix OR metolazon OR miclox OR microx OR mykrox OR normelan OR xuret OR zaroxolyn) AND CENTRAL:TARGET #26 (methylclothiazide OR aquatensen OR enduron OR enduron‐m OR enduronum OR methyclothiazide OR methylchlorothiazide OR thiazidil) AND CENTRAL:TARGET #27 (muzolimine OR "bay g 2821" OR "bay g2821" OR "bayer g 2821" OR "bayer g2821" OR edrul OR musolimino) AND CENTRAL:TARGET #28 (ozolinone OR "go 3282" OR go3282 OR "goe 3282" OR goe3282 OR "goedecke 3282") AND CENTRAL:TARGET #29 phenoxybenzoic acid AND CENTRAL:TARGET #30 (piretanide OR arelix OR arlix OR eurelix OR "hoe 118" OR hoe118 OR lafax OR perbilen OR "s 73 4118" OR "s 734118" OR s734118 OR tauliz) AND CENTRAL:TARGET #31 (polythiazide OR drenusil OR nephril OR polythiazide OR renese) AND CENTRAL:TARGET #32 (quinethazone OR aquamox OR chinethazon OR chinethazone OR guinethazone OR hydromox OR kinetazone OR quinethazon) AND CENTRAL:TARGET #33 (spironolactone OR abbolactone OR acelat OR adultmin OR alaton OR alatone OR aldace OR aldactone OR aldopur OR aldospirone OR almatol OR aquareduct OR berlactone OR carospir OR "crl 635" OR crl635 OR diram OR duraspiron OR "dyta urese" OR dytaurese OR espironolactona OR flumach OR frumikal OR jenaspiron OR hypazon OR idrolattone OR merabis OR "novo spiroton" OR "novo‐spiriton" OR novospiroton OR osiren OR osyrol OR pirolacton OR pondactone OR practon OR prilactone OR resacton OR "sas 1060" OR sas1060 OR "sc 9420" OR "sc‐9420" OR sc9420 OR spiractin OR spiridon OR spirix OR spirobeta OR "spiro ct" OR spiroctan OR spirogamma OR spirohexal OR spirolacton OR spirolactone OR spirolang OR "spiro l.u.t." OR spiron OR spirone OR spironex OR spirono isis OR spironol OR spironolacton OR spironolakton OR spironone OR spirospare OR spirothiobarbiturate OR spirotone OR spiro von ct OR supra puren OR suprapuren OR uractone OR veroshpiron OR verospiron OR verospirone OR xenalon OR youlactone) AND CENTRAL:TARGET #34 (ticrynafen OR "anp 3624" OR "anp‐3624" OR anp3624 OR diflurex OR selacryn OR "skf 62698" OR "skf‐62698" OR skf62698 OR selacryn OR thienilic acid OR thienylic acid OR tienilic acid) AND CENTRAL:TARGET #35 tizolemide AND CENTRAL:TARGET #36 (torsemide OR "bm 02015" OR "bm 2015" OR bm02015 OR bm2015 OR demadex OR diuremid OR "jdl 464" OR jdl464 OR luprac OR presaril OR toradiur OR torem OR torrem OR torasemide OR unat OR upcard) AND CENTRAL:TARGET #37 (triamterene OR dyrenium OR dytac OR urocaudal OR ademin OR ademine OR dyren OR dyrenium OR dytac OR iatropur OR jatropur OR noridyl OR "nsc 77625" OR nsc77625 OR pterofen OR pterophene OR "sk and f 8542" OR "skf 8542" OR skf8542 OR teriam OR triampterene OR triamterence OR triamterens OR triamteril OR triteren OR uretren OR urocaudal) AND CENTRAL:TARGET #38 (trichloromethiazide OR aquazide OR dichloromethylhydrochlorothiazide OR diurese OR esmarin OR eurinol OR fluitran OR flutra OR gangesol OR hydrotrichlorothiazide OR metahydrin OR methahydrin OR naqua OR naquasone OR salurin OR triazide OR trichlordiuride OR trichlorex OR trichlormethazide OR trichlormethiazide OR trichlormas OR trichloromethylhydrochlorothiazide OR triflumen OR wadel) AND CENTRAL:TARGET #39 (tripamide OR "adr 033" OR adr033 OR "e 614" OR e614 OR normonal) AND CENTRAL:TARGET #40 (xipamide OR aquaforil OR aquaphor OR aquaphoril OR aquavor OR "bei 1293" OR diurexan OR lumitens OR xipamid OR xypamide OR zipix) AND CENTRAL:TARGET #41 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40) AND CENTRAL:TARGET #42 angiotensin converting enzyme inhibit* AND CENTRAL:TARGET #43 ace NEAR2 inhibit* AND CENTRAL:TARGET #44 acei OR aceis AND CENTRAL:TARGET #45 (alacepril OR altiopril OR ancovenin OR benazepril OR captopril OR ceranapril OR ceronapril OR cilazapril OR deacetylalacepril OR delapril OR derapril OR enalapril OR epicaptopril OR fasidotril OR fosinopril OR foroxymithine OR gemopatrilat OR idapril OR imidapril OR indolapril OR libenzapril OR lisinopril OR moexipril OR moveltipril OR omapatrilat OR pentopril* OR perindopril* OR pivopril OR quinapril* OR ramipril* OR rentiapril OR saralasin OR s nitrosocaptopril OR spirapril* OR temocapril* OR teprotide OR trandolapril* OR utibapril* OR zabicipril* OR zofenopril*) AND CENTRAL:TARGET #46 (#42 OR #43 OR #44 OR #45) AND CENTRAL:TARGET #47 ((angiotensin receptor antagonist*) OR (angiotensin receptor block*)) AND CENTRAL:TARGET #48 (arb OR arbs) AND CENTRAL:TARGET #49 (abitesartan OR azilsartan OR candesartan OR elisartan OR embusartan OR eprosartan OR forasartan OR irbesartan OR losartan OR milfasartan OR olmesartan OR saprisartan OR tasosartan OR telmisartan OR valsartan OR zolasartan) AND CENTRAL:TARGET #50 (#47 OR #48 OR #49) AND CENTRAL:TARGET #51 ((calcium channel antagon*) OR (calcium channel block*) OR (calcium inhibit*)) AND CENTRAL:TARGET #52 (amlodipine OR amrinone OR aranidipine OR barnidipine OR bencyclane OR benidipine OR bepridil OR cilnidipine OR cinnarizine OR clentiazem OR darodipine OR diltiazem OR efonidipine OR elgodipine OR etafenone OR fantofarone OR felodipine OR fendiline OR flunarizine OR gallopamil OR isradipine OR lacidipine OR lercanidipine OR lidoflazine OR lomerizine OR manidipine OR mibefradil OR nicardipine OR nifedipine OR niguldipine OR nilvadipine OR nimodipine OR nisoldipine OR nitrendipine OR perhexiline OR prenylamine OR semotiadil OR terodiline OR tiapamil OR verapamil) AND CENTRAL:TARGET #53 (#51 OR #52) AND CENTRAL:TARGET #54 (methyldopa OR alphamethyldopa OR amodopa OR dopamet OR dopegyt OR dopegit OR dopegite OR emdopa OR hyperpax OR hyperpaxa OR methylpropionic acid OR dopergit OR meldopa OR methyldopate OR medopa OR medomet OR sembrina OR aldomet OR aldometil OR aldomin OR hydopa OR methyldihydroxyphenylalanine OR methyl dopa OR mulfasin OR presinol OR presolisin OR sedometil OR sembrina OR taquinil OR dihydroxyphenylalanine OR methylphenylalanine OR methylalanine OR alpha methyl dopa) AND CENTRAL:TARGET #55 (reserpine OR serpentina OR rauwolfia OR serpasil) AND CENTRAL:TARGET #56 (clonidine OR adesipress OR arkamin OR caprysin OR catapres* OR catasan OR chlofazolin OR chlophazolin OR clinidine OR clofelin* OR clofenil OR clomidine OR clondine OR clonistada OR clonnirit OR clophelin* OR dichlorophenylaminoimidazoline OR dixarit OR duraclon OR gemiton OR haemiton OR hemiton OR imidazoline OR isoglaucon OR klofelin OR klofenil OR normopresan OR paracefan OR tesno timelets) AND CENTRAL:TARGET #57 (hydralazin* OR hydrallazin* OR hydralizine OR hydrazinophtalazine OR hydrazinophthalazine OR hydrazinophtalizine OR dralzine OR hydralacin OR hydrolazine OR hypophthalin OR hypoftalin OR hydrazinophthalazine OR idralazina OR 1‐hydrazinophthalazine OR apressin OR nepresol OR apressoline OR apresoline OR apresolin OR alphapress OR alazine OR idralazina OR lopress OR plethorit OR praeparat) AND CENTRAL:TARGET #58 (#54 OR #55 OR #56 OR #57) AND CENTRAL:TARGET #59 ((adrenergic beta‐antagon*) OR (beta adrenergic*) OR (beta antagon*) OR (beta block*) OR (beta recept*)) AND CENTRAL:TARGET #60 (acebutolol OR adimolol OR afurolol OR alprenolol OR amosulalol OR arotinolol OR atenolol OR befunolol OR betaxolol OR bevantolol OR bisoprolol OR bopindolol OR bornaprolol OR brefonalol OR bucindolol OR bucumolol OR bufetolol OR bufuralol OR bunitrolol OR bunolol OR bupranolol OR butofilolol OR butoxamine OR carazolol OR carteolol OR carvedilol OR celiprolol OR cetamolol OR chlORtalidone clORanolol OR cyanoiodopindolol OR cyanopindolol OR deacetylmetipranolol OR diacetolol OR dihydroalprenolol OR dilevalol OR epanolol OR esmolol OR exaprolol OR falintolol OR flestolol OR flusoxolol OR hydroxybenzylpinodolol OR hydroxycarteolol OR hydroxymetoprolol OR indenolol OR iodocyanopindolol OR iodopindolol OR iprocrolol OR isoxaprolol OR labetalol OR landiolol OR levobunolol OR levomoprolol OR medroxalol OR mepindolol OR methylthiopropranolol OR metipranolol OR metoprolol OR moprolol OR nadolol OR oxprenolol OR penbutolol OR pindolol OR nadolol OR nebivolol OR nifenalol OR nipradilol OR oxprenolol OR pafenolol OR pamatolol OR penbutolol OR pindolol OR practolol OR primidolol OR prizidilol OR procinolol OR pronetalol OR propranolol OR proxodolol OR ridazolol OR salcardolol OR soquinolol OR sotalol OR spirendolol OR talinolol OR tertatolol OR tienoxolol OR tilisolol OR timolol OR tolamolol OR toliprolol OR tribendilol OR xibenolol) AND CENTRAL:TARGET #61 (#59 OR #60) AND CENTRAL:TARGET #62 ((adrenergic alpha) OR (adrenergic antagon*) OR (adrenergic block*) OR (adrenergic receptor antagon*) OR (adrenergic receptor block*) OR (alpha block*)) AND CENTRAL:TARGET #63 (alfuzosin OR bunazosin OR doxazosin OR metazosin OR neldazosin OR prazosin OR silodosin OR tamsulosin OR terazosin OR tiodazosin OR trimazosin) AND CENTRAL:TARGET #64 (#62 OR #63) AND CENTRAL:TARGET #65 (#46 OR #50 OR #53 OR #58 OR #61 OR #64) AND CENTRAL:TARGET #66 MESH DESCRIPTOR Hypertension AND CENTRAL:TARGET #67 MESH DESCRIPTOR Essential Hypertension AND CENTRAL:TARGET #68 (antihypertens* OR hypertens*):TI,AB AND CENTRAL:TARGET #69 (elevat* OR high OR rais*) NEAR2 blood pressur* AND CENTRAL:TARGET #70 (#66 OR #67 OR #68 OR #69) AND CENTRAL:TARGET #71 #41 AND #65 AND #70 AND CENTRAL:TARGET
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Embase <1974 to 2021 March 24> Search Date: 25 March 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazide diuretic agent/ 2 exp loop diuretic agent/ 3 thiazide*.tw. 4 ((sodium chloride adj2 cotransporter inhibit*) or (sodium chloride adj2 co‐transporter inhibit*) or (sodium chloride adj2 symporter inhibit*)).tw. 5 ((ceiling adj2 diuretic*) or (loop adj2 diuretic*) or (potassium‐depleting adj2 diuretic*)).tw. 6 (amiloride or amiclaran or amidal or amiduret trom or amikal or "amilo 5" or amiloberag or amilorid or amiloridehydrochlorhydrate or amiloridine or amipramidine or amyloride or arumil or berkamil or colectril or guanamprazine or kaluril or medamor or midamor or midoride or "mk 870" or modamide or nirulid or pandiuren).mp. 7 (azosemide or azosemid or luret or "ple 1053" or "sk 110").mp. 8 (bendroflumethiazide or aprinox or bendrofluazide or bendroflumethiazide or benzhydroflumethiazide or benzydroflumethiazide or benzyl hydroflumethiazide or benzylhydroflumethiazide or benzide or berkozide or bristuron or centonuron or centyl or esberizid or naturetin or naturine or neo naclex or neonaclex or naturetin or naturine or neonadex or pluryl or pluryle or repicin or salures or sinesalin or urizid).mp. 9 (bumetanide or budema or bumedyl or bumelex or bumet or bumetamide or bumethanide or bumetidine or bumex or burinax or burinex or busix or butinat or butinon or bymex or cambiex or drenural or farmadiuril or fontego or fordiuran or lixil or lunetoron or miccil or "pf 1593" or "pf‐1593" or pf1593 or primex or "ro 10 6338" or "ro 10‐6338" or "ro10 6338" or ro10‐6338).mp. 10 (butizide or buthiazide or eunephran or eunepran or isobutylhydrochlorothiazide or modenol or saltucin or thiabulazid or thiabutazide or thiobulazid or tiabutazide).mp. 11 (chlorothiazide or chlorosal or chlorothiazid or chlorothiazidum or chlorothiazine or chlorthiazide or chlotride or diachlor or diuril or diurilix or diuriwas wassermann milano or flumen or lyovac or saluric or warduzuide).mp. 12 (chlorthalidone or aquadon or chlorphthalidolone or chlortalidon or chlortalidone or clortalidone or chlorthalidine or chlorthalidon or chlorthialidone or clortalil or edemdal or hidronal or higroton or higrotona or hygroton or hylidone or hypertol or hythalton or igrolina or igroton or isoren or natriuran or oxodolin or oxodoline or phthalamidine or phthalamodine or phthalamudine or renon or servidone or thalitone or urandil or urofinil or zambesil).mp. 13 (cicletanine or "bn 1270" or "bn 50417" or "bn 50418" or bn1270 or bn50417 or bn50418 or cicletanide or cycletanide or justar or tenstaten or tenstatin).mp. 14 (clopamide or adurix or aquez or brinaldix or brinaldrix or brinedine or chlosudimeprimylum clopamid or clopamidum or clopamine).mp. 15 (clorexone or chlorexolone or flonatril or klorex or nefrolan or cyclothizaide or anhydron or doburil or fluidil or valmiran).mp. 16 (cyclopenthiazide or cyclomethiazide or cyclopenthiazine or cyclopentiazide or navidex or navidrex or navidrix or salimid or tsiklometiazid).mp. 17 (diapamide or thiamizide or tiamizide or fenquizone or idrolone).mp. 18 (eplerenone or "cgp 30 083" or "cgp 30083" or cgp30083 or elecor or eplerenon or epoxymexrenone or inspra or "sc 66110" or sc66110).mp. 19 (ethacrynic acid or edecril or edecrin or edecrina or endecril or etacrinic acid or etacrynate or etacrynic acid or ethacrinic acid or ethacrynate or ethacryonic acid or ethocrynic acid or ethycrynic acid or hydromedin or lyovac sodium edecrin or "mk 595" or "nsc 85791" or reomax or sodium ethacrynate or uregit or uregyt).mp. 20 (etozolin or elkapin or etazolin or etozoline or "go 687" or go687 or "goe 687" or goe687 or ozolinone ethyl ester or "w 2900" or w2900).mp. 21 (furosemide or aldic or aluzine or anfuramaide or aquarid or arasemide or cetasix or desal or diamazon or dirine or discoid or diumide or diural or diuresal or diurin or diurix or diurolasa or diusemide or diuspec or dryptal or durafurid or edenol or errolon or eutensin or eutensine or flurosemide or franyl or fretic or frumid or frusedan or frusehexal or frusema or frusemidor frusemide or frusid or fruzex or fumarenid or fumide or furanthril or furantral or furantril or furanturil or furasemide or furesin or furesis or furetic or furix or furmid or furo puren or furo‐basan or furo‐puren or furobasan or furomen or furomex or furomide or furomin or furopuren or furorese or furosamide or furoscan or furose or furosemid or furosemix or furosimide or furosix or furovite or fursemide or fusid or fusimex or hissuflux or hydro rapid or impugan or jufurix or kofuzon or kutrix or lasiletten or lasilix or lasix or laxis or laxur or "lb 502" or lb502 or luramide or marsemide or mirfat or odemase or odemex or oedemase or oedemex or pharmix or promedes or radisemide or rasitol or retep or salinex or seguril or selectofur or sigasalur or uremide or uresix or urex‐m or vesix or zafurida).mp. 22 (hydrochlorothiazide or apo‐hydro or aquarius or aquazide or bisalunil or bpzide or bremil or chlorosulthiadil or chlorsulfonamidodihydrobenzothiadiazine or cidrex or clothia or dehydratin or diaqua or dichlorosal or dichlothiazide or dichlotride or dichlozid or diclotride or didralin or dihydrochlorothiazide or dihydrodiuril or direma or disaluril or disothiazide or dithiazide or diu melusin or diumelusin or diurace or diurex or esidrex or esidrix or fluvin or hctz or hidrenox or hidril or hidroronol or hidrosaluretil or hudorex or hychlozide or hydrex‐semi or hydril or hydro aquil or hydrochlor or hydrochloro thiazide or hydrochlorothiamide or hydrochlorothiazid or hydrochlorothiazine or hydrochlorzide or hydrochlothiazide or hydro diuril or hydrodiuril or hydromal or hydrororonol or hydro saluric or hydrosaluric or hydrothide or hydro tonuron or hydrozide or hypothiazid or hypothiazide or ivaugan or maschitt or microzide or mictrin or nefrix or neoflumen or newtolide or niagar or oretic or pantemon or ridaq or sectrazide or tandiur or thiadril or thiaretic or thiuretic or urodiazin or urodiazine or urozide or vetidrex).mp. 23 (hydroflumethiazide or bristab or di ademil or diademil or dihydroflumethiazide or diraudixin or diucardin or hiserpin or hydrenox or leodrin or leodrine or metflorylthiadiazine or naclex or rontyl or saluron or sisuril or trifluoromethylhydrothiazide).mp. 24 (indapamide or agelan or apadex or arifon or damide or dapamax or diflerix or dixamid or extur or fludex or fluidema or frumeron or indahexal or indalix or indamol or indapam or indapress or indicontin or indoline or indopamide or inpamide or insig or ipamix or lorvas or loxide or lozol or metindamide or millibar or naplin or natrilix or natrix or noranat or pamid or pressural or pretanix or rinalix or sicco or tandix or tertensif or veroxil).mp. 25 (indacrinone or indacrinic acid or indacrynic acid or "mk 196").mp. 26 (mefruside or bay caron or bay1500 or baycaron or baycarone or mefrusid).mp. 27 (metolazone or barolyn or diulo or metalazone or metenix or metolazon or miclox or microx or mykrox or normelan or xuret or zaroxolyn).mp. 28 (methylclothiazide or aquatensen or enduron or enduron‐m or enduronum or methyclothiazide or methylchlorothiazide or thiazidil).mp. 29 (muzolimine or "bay g 2821" or "bay g2821" or "bayer g 2821" or "bayer g2821" or edrul or musolimino).mp. 30 (ozolinone or "go 3282" or go3282 or "goe 3282" or goe3282 or "goedecke 3282").mp. 31 phenoxybenzoic acid.mp. 32 (piretanide or arelix or arlix or eurelix or "hoe 118" or hoe118 or lafax or perbilen or "s 73 4118" or "s 734118" or s734118 or tauliz).mp. 33 (polythiazide or drenusil or nephril or polythiazide or renese).mp. 34 (quinethazone or aquamox or chinethazon or chinethazone or guinethazone or hydromox or kinetazone or quinethazon).mp. 35 (spironolactone or abbolactone or acelat or adultmin or alaton or alatone or aldace or aldactone or aldopur or aldospirone or almatol or aquareduct or berlactone or carospir or "crl 635" or crl635 or diram or duraspiron or "dyta urese" or dytaurese or espironolactona or flumach or frumikal or jenaspiron or hypazon or idrolattone or merabis or "novo spiroton" or "novo‐spiriton" or novospiroton or osiren or osyrol or pirolacton or pondactone or practon or prilactone or resacton or "sas 1060" or sas1060 or "sc 9420" or "sc‐9420" or sc9420 or spiractin or spiridon or spirix or spirobeta or "spiro ct" or spiroctan or spirogamma or spirohexal or spirolacton or spirolactone or spirolang or "spiro l.u.t." or spiron or spirone or spironex or spirono isis or spironol or spironolacton or spironolakton or spironone or spirospare or spirothiobarbiturate or spirotone or spiro von ct or supra puren or suprapuren or uractone or veroshpiron or verospiron or verospirone or xenalon or youlactone).mp. 36 (ticrynafen or "anp 3624" or "anp‐3624" or anp3624 or diflurex or selacryn or "skf 62698" or "skf‐62698" or skf62698 or selacryn or thienilic acid or thienylic acid or tienilic acid).mp. 37 tizolemide.mp. 38 (torsemide or "bm 02015" or "bm 2015" or bm02015 or bm2015 or demadex or diuremid or "jdl 464" or jdl464 or luprac or presaril or toradiur or torem or torrem or torasemide or unat or upcard).mp. 39 (triamterene or dyrenium or dytac or urocaudal or ademin or ademine or dyren or dyrenium or dytac or iatropur or jatropur or noridyl or "nsc 77625" or nsc77625 or pterofen or pterophene or "sk and f 8542" or "skf 8542" or skf8542 or teriam or triampterene or triamterence or triamterens or triamteril or triteren or uretren or urocaudal).mp. 40 (trichloromethiazide or aquazide or dichloromethylhydrochlorothiazide or diurese or esmarin or eurinol or fluitran or flutra or gangesol or hydrotrichlorothiazide or metahydrin or methahydrin or naqua or naquasone or salurin or triazide or trichlordiuride or trichlorex or trichlormethazide or trichlormethiazide or trichlormas or trichloromethylhydrochlorothiazide or triflumen or wadel).mp. 41 (tripamide or "adr 033" or adr033 or "e 614" or e614 or normonal).mp. 42 (xipamide or aquaforil or aquaphor or aquaphoril or aquavor or "bei 1293" or diurexan or lumitens or xipamid or xypamide or zipix).mp. 43 or/1‐42 44 exp dipeptidyl carboxypeptidase inhibitor/ 45 angiotensin converting enzyme inhibit*.tw. 46 (ace adj2 inhibit*).tw. 47 acei.tw. 48 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or enalaprilat or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril* or pivopril or quinapril* or ramipril* or rentiapril or saralasin or s nitrosocaptopril or spirapril* or temocapril* or teprotide or trandolapril* or utibapril* or zabicipril* or zofenopril* or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. 49 or/44‐48 50 exp angiotensin receptor antagonist/ 51 (angiotensin adj3 receptor antagon*).tw. 52 (angiotensin adj3 receptor block*).tw. 53 (arb or arbs).tw. 54 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw. 55 or/50‐54 56 exp calcium channel blocking agent/ 57 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. 58 (calcium adj2 (antagonist* or block* or inhibit*)).tw. 59 or/56‐58 60 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 61 (reserpine or serpentina or rauwolfia or serpasil).mp. 62 (clonidine or adesipress or arkamin or caprysin or catapres* or catasan or chlofazolin or chlophazolin or clinidine or clofelin* or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin* or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 63 hydralazine/ 64 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).mp. 65 or/60‐64 66 exp beta adrenergic receptor blocking agent/ 67 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. 68 (beta adj2 (adrenergic* or antagonist* or block* or receptor*)).tw. 69 or/66‐68 70 exp alpha adrenergic receptor blocking agent/ 71 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. 72 (andrenergic adj2 (alpha or antagonist*)).tw. 73 ((andrenergic or alpha or receptor*) adj2 block*).tw. 74 or/70‐73 75 49 or 55 or 59 or 65 or 69 or 74 76 exp hypertension/ 77 (hypertens* or antihypertens*).tw. 78 ((elevat* adj2 arterial pressur*) or (elevat* adj2 blood pressur*) or (elevat* adj2 diastolic pressur*) or (elevat* adj2 systolic pressur*)).tw. 79 ((high adj2 arterial pressur*) or (high adj2 blood pressur*) or (high adj2 diastolic pressure) or (high adj2 systolic pressur*)).tw. 80 ((rais* adj2 arterial pressur*) or (rais* adj2 blood pressur*) or (rais* adj2 diastolic pressure) or (rais* adj2 systolic pressur*)).tw. 81 ((elevat* adj2 bp) or (elevat* adj2 dbp) or (elevat* adj2 sbp)).tw. 82 ((high adj2 bp) or (high adj2 dbp) or (high adj2 sbp)).tw. 83 ((rais* adj2 bp) or (rais* adj2 dbp) or (rais* adj2 sbp)).tw. 84 or/76‐83 85 randomized controlled trial/ 86 crossover procedure/ 87 double‐blind procedure/ 88 (randomi* or randomly).tw. 89 (crossover* or cross‐over*).tw. 90 placebo*.tw. 91 (doubl* adj blind*).tw. 92 assign*.tw. 93 allocat*.ab. 94 or/85‐93 95 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 96 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 97 (pregnancy‐induced or ocular hypertens* or preeclampsia or pre‐eclampsia).ti. 98 94 not (95 or 96 or 97) 99 43 and 75 and 84 and 98
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov
Search Date: 26 March 2021
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition or disease: Hypertension Other terms: (compared OR comparison OR versus OR vs) AND randomized Study type: Interventional Studies (Clinical Trials) Study Results: All Studies Intervention/treatment: (diuretic* OR sodium chloride symporter inhibitor* OR sodium potassium chloride symporter inhibitor* OR thiazide*)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: WHO International Clinical Trials Registry Platform (ICTRP)
Search Date: 26 March 2021
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Title: compared OR comparison OR other OR versus OR vs Condition: Hypertension Intervention: diuretics OR sodium chloride symporter inhibitors OR sodium potassium chloride symporter inhibitors OR thiazides Recruitment Status: ALL
Data and analyses
Comparison 1. First‐line diuretics versus active comparators: primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total mortality | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 vs beta‐blockers | 5 | 18241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.10] |
| 1.1.2 vs calcium channel blockers | 7 | 35417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.08] |
| 1.1.3 vs ACE inhibitors | 3 | 30961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.07] |
| 1.1.4 vs alpha adrenergic blockers | 1 | 24316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.09] |
| 1.1.5 vs angiotensin II receptor blockers | 1 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.88] |
| 1.1.6 vs direct renin inhibitors | 1 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.31] |
| 1.2 Total serious adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 vs calcium channel blockers | 2 | 7204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.24] |
| 1.2.2 vs direct renin inhibitors | 1 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.49, 1.50] |
| 1.3 Total cardiovascular events | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 vs beta‐blockers | 4 | 18135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 1.3.2 vs calcium channel blockers | 6 | 35217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.89, 0.98] |
| 1.3.3 vs ACE inhibitors | 3 | 30900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.02] |
| 1.3.4 vs alpha adrenergic blockers | 2 | 24396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.69, 0.80] |
| 1.3.5 vs angiotensin II receptor blockers | 2 | 1047 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.79] |
| 1.4 Total stroke events | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 vs beta‐blockers | 4 | 18135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.09] |
| 1.4.2 vs calcium channel blockers | 6 | 35217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.18] |
| 1.4.3 vs ACE inhibitors | 3 | 30900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 0.99] |
| 1.4.4 vs alpha adrenergic blockers | 2 | 24396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 1.4.5 vs angiotensin II receptor blockers | 1 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 70.96] |
| 1.5 Total coronary heart disease | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 vs beta‐blockers | 4 | 18135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.07] |
| 1.5.2 vs calcium channel blockers | 6 | 35217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.08] |
| 1.5.3 vs ACE inhibitors | 3 | 30900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.12] |
| 1.5.4 vs alpha adrenergic blockers | 2 | 24396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 1.5.5 vs angiotensin II receptor blockers | 2 | 1047 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.95] |
| 1.6 Total congestive heart failure | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 vs beta‐blockers | 1 | 6569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.19] |
| 1.6.2 vs calcium channel blockers | 6 | 35217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.66, 0.82] |
| 1.6.3 vs ACE inhibitors | 2 | 30392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.04] |
| 1.6.4 vs alpha adrenergic blockers | 1 | 24316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.45, 0.58] |
| 1.7 Withdrawals due to adverse effects | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 vs beta‐blockers | 5 | 18501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.71, 0.85] |
| 1.7.2 vs calcium channel blockers | 7 | 33908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.75, 0.88] |
| 1.7.3 vs ACE inhibitors | 3 | 25254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.84] |
| 1.7.4 vs alpha adrenergic blockers | 3 | 24772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.89] |
| 1.7.5 vs angiotensin II receptor blockers | 2 | 1047 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.91, 4.58] |
| 1.7.6 vs direct renin inhibitors | 1 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.88, 2.20] |
| 1.7.7 vs CNS‐acting drug | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.53] |
Comparison 2. First‐line diuretics versus active comparators: secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Dose titration and addition of second or third drug | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 vs beta‐blockers | 3 | 7358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.05, 1.20] |
| 2.1.2 vs calcium channel blockers | 7 | 35417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.94, 0.99] |
| 2.1.3 vs ACE inhibitors | 2 | 24878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.92, 0.97] |
| 2.1.4 vs alpha adrenergic blockers | 1 | 24316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.83, 0.88] |
| 2.1.5 vs angiotensin II receptor blockers | 2 | 1047 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.03] |
| 2.1.6 vs direct renin inhibitors | 1 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.24] |
| 2.2 Switching to other antihypertensive therapies | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 vs beta‐blockers | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.53, 7.58] |
| 2.2.2 vs calcium channel blockers | 2 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.18] |
| 2.2.3 vs alpha adrenergic blockers | 2 | 24396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.09, 0.12] |
| 2.3 Systolic blood pressure at 1 year | 19 | Mean Difference (IV, Fixed, 99% CI) | Subtotals only | |
| 2.3.1 vs beta‐blockers | 5 | 18241 | Mean Difference (IV, Fixed, 99% CI) | ‐2.94 [‐3.58, ‐2.29] |
| 2.3.2 vs calcium channel blockers | 7 | 31585 | Mean Difference (IV, Fixed, 99% CI) | ‐1.36 [‐1.80, ‐0.92] |
| 2.3.3 vs ACE inhibitors | 4 | 27289 | Mean Difference (IV, Fixed, 99% CI) | ‐2.39 [‐2.93, ‐1.86] |
| 2.3.4 vs alpha adrenergic blockers | 2 | 18781 | Mean Difference (IV, Fixed, 99% CI) | ‐3.01 [‐3.65, ‐2.37] |
| 2.3.5 vs angiotensin II receptor blockers | 2 | 1047 | Mean Difference (IV, Fixed, 99% CI) | ‐1.93 [‐4.32, 0.47] |
| 2.3.6 vs direct renin inhibitors | 1 | 1124 | Mean Difference (IV, Fixed, 99% CI) | 0.90 [‐1.30, 3.10] |
| 2.4 Diastolic blood pressure at 1 year | 19 | Mean Difference (IV, Fixed, 99% CI) | Subtotals only | |
| 2.4.1 vs beta‐blockers | 5 | 18241 | Mean Difference (IV, Fixed, 99% CI) | ‐0.29 [‐0.65, 0.07] |
| 2.4.2 vs calcium channel blockers | 7 | 31585 | Mean Difference (IV, Fixed, 99% CI) | 0.47 [0.20, 0.73] |
| 2.4.3 vs ACE inhibitors | 4 | 27391 | Mean Difference (IV, Fixed, 99% CI) | ‐0.37 [‐0.67, ‐0.07] |
| 2.4.4 vs alpha adrenergic blockers | 2 | 18781 | Mean Difference (IV, Fixed, 99% CI) | 0.00 [‐0.38, 0.38] |
| 2.4.5 vs angiotensin II receptor blockers | 2 | 1047 | Mean Difference (IV, Fixed, 99% CI) | 0.04 [‐1.21, 1.29] |
| 2.4.6 vs direct renin inhibitors | 1 | 1124 | Mean Difference (IV, Fixed, 99% CI) | 1.00 [‐0.44, 2.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ALLHAT 2000/2002.
| Study characteristics | ||
| Methods | Randomized, double‐blind, active‐controlled clinical trial | |
| Participants | North American patients aged ≥ 55 with stage 1 or 2 hypertension and at least 1 other CHD risk factor 2000 analysis (chlorthalidone vs doxazosin): 24,335 patients; mean age, 67 years; 11,383 F:12,952 M 2002 analysis (chlorthalidone vs amlodipine vs lisinopril): 33,357 patients; mean age, 66.9 years; 15,638 F:17,719 M |
|
| Interventions | Chlorthalidone 12.5 mg to 25 mg daily Amlodipine 2.5 mg to 10 mg daily Lisinopril 10 mg to 40 mg daily Doxazosin 2 mg to 8 mg daily |
|
| Outcomes | Combined fatal CHD or non‐fatal MI All‐cause mortality Stroke (fatal and non‐fatal) Combined CHD (the primary outcome, coronary revascularization, hospitalized angina) Combined CVD (combined CHD, stroke, other treated angina, HF (fatal, hospitalized, or treated non hospitalized), and peripheral arterial disease) BP at 1 year Duration: mean follow‐up 3.2 years for doxazosin and 5 years for the other comparisons |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (ALLHAT 2002): "By telephone, participants were randomly assigned to chlorthalidone, amlodipine, or lisinopril in a ratio of 1.7:1:1. The concealed randomisation scheme was generated by computer, implemented at the clinical trials centre, stratified by centre and blocked in random block sizes of 5 or 9 to maintain balance." Quote (ALLHAT 2000): "... assigned by a computer‐generated randomisation schedule to 1 of 4 treatments", 1:1:1:1. "Randomization was stratified by centre and blocked over time to maintain the ratio." |
| Allocation concealment (selection bias) | Low risk | Quote (ALLHAT 2000): "The randomisation code was held only by the ALLHAT Clinical Trials Center (CTC)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (ALLHAT 2002): "Step 1 drugs were encapsulated and identical in appearance so that the identity of each agent was double‐masked at each dosage level." Supplemental therapy (step 2 and 3 drugs), as well as any other administered drugs (including low doses of open‐label step 1 drug class), were administered open‐label if patients failed to meet goal BP. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (ALLHAT 2002): "Study outcomes were assessed at follow‐up visits and reported to the clinical trials centre... In addition, searches for outcomes were accomplished through the Center for Medicare and Medicaid Services, the Department of Veterans Affairs, the National Death Index, and the Social Security Administration databases. A death was ascertained by clinic report or by match with the aforementioned databases plus a confirmatory death certificate... Medical reviewers from the clinical trials centre verified the physician‐assigned diagnoses of outcomes using death certificates and hospital discharge summaries. More detailed information was collected on a random (10%) subset of CHD and stroke events to validate the procedure of using physician diagnoses." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (ALLHAT 2002): "Data were analysed according to participants’ randomised treatment assignments regardless of their subsequent medications (intent‐to‐treat analysis)." ALLHAT 2002 paper withdrawals at 5 years: figure 1. Total withdrawals: chlorthalidone 16%; amlodipine 15%; lisinopril 20%; WDAE chlorthalidone 2%; amlodipine 5%; lisinopril 3%; "Other nonmedical reasons" 1% each. Reasons and percentages seem comparable; no differential dropout. Cross‐overs itemized and comparable. At trial closeout similar numbers had unknown vital status. |
| Selective reporting (reporting bias) | Low risk | All outcomes for this review were reported. |
| Use of supplemental drugs | High risk | Supplemental drugs and doses could be chosen from a predefined list at the discretion of study investigator, thus were not identical for all patients. Quote (ALLHAT 2002): "For patients in any of the four treatment arms who are unable to attain satisfactory blood pressure control on the maximum tolerable dosage of their first‐line drug, a choice of second‐ and third‐line drugs are provided in open‐label form for use in addition to (not substitution for) the first‐line drug unless the first‐line drug is not tolerated. The choice of second‐line drug(s) is at the discretion of the treating study investigator". Step 2: atenolol 25 to 100 mg/day, reserpine 0.05 to 0.2 mg/day or clonidine 0.1 to 0.3 mg twice per day. Step 3: hydralazine, 25 to 100 mg twice per day. |
| Industry sponsorship | Low risk | Study was supported by contract with the National Heart, Lung, and Blood Institute (NHLBI). The ALLHAT investigators acknowledged contributions of study medications supplied by Pfizer Inc. (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol‐Myers Squibb (pravastatin), and financial support provided by Pfizer to the NHLBI. The companies were not involved in the conduct, analysis or publication of the results of the trial. |
ALPINE 2003.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled, parallel‐group trial | |
| Participants | 392 patients from Sweden with hypertension (SBP 140 to 179 mmHg and/or DBP 90 to 104 mmHg), no severe concomitant disease including diabetes Mean age 55 years 207 F:185 M |
|
| Interventions | Candesartan 16 mg daily Hydrochlorothiazide 25 mg daily |
|
| Outcomes | BP at 1 year Patient well‐being (subjective symptom assessment) Plasma glucose, serum insulin, OGTT Total plasma cholesterol, LDL‐C, HDL‐C, triglycerides AEs leading to withdrawal or change in therapy Duration: 12 months |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that participants were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was described as double‐blind (including add‐on treatment), but no additional details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of outcome assessment blinding was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat approach was used. Quote: "The discontinuation rates were low, 8.2 and 7.1% [for candesartan and hydrochlorothiazide], respectively." One patient was excluded from ITT due to lack of outcome data. PP analysis also reported. No patients were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although there was no evidence of selective reporting and all outcomes in methods were reported, it is not possible to fully assess without a protocol that confirms the list of prespecified outcomes. |
| Use of supplemental drugs | High risk | Supplemental drugs differed between groups. Quote: "If sitting systolic or diastolic blood pressure was above the target pressure at any visit during the treatment period, double‐blind treatment with 2.5–5.0 mg felodipine extended‐release was added to the candesartan group and 50–100 mg atenolol was added to the hydrochlorothiazide group. No further antihypertensive treatment was allowed." |
| Industry sponsorship | High risk | Quote: "The study was financed by the Department of Public Health and Clinical Medicine, Umea University, Sweden together with AstraZeneca R&D, Molndal, Sweden and Hassle Lakemedel AB, Sweden" |
ANBP2 2003.
| Study characteristics | ||
| Methods | Randomized, open‐label trial | |
| Participants | 6083 patients in Australia aged 65 to 84 years with hypertension (SBP ≥ 160 mmHg or DBP ≥ 90 mmHg) Mean age 72 years 3102 F:2981 M |
|
| Interventions | Enalapril Hydrochlorothiazide (recommended agents, although other ACE inhibitors and diuretics were permitted; doses not provided) |
|
| Outcomes | Combined endpoint of all cardiovascular events (coronary and cerebrovascular events, both fatal and nonfatal) or death from any cause Individually reported events: all‐cause mortality, coronary event, MI, HF, cerebrovascular event, stroke BP at 1 year Duration: median follow‐up 4 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that patients were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study used an open‐label design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An end‐point committee whose members were unaware of the treatment group assignments adjudicated all potential end points." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analysis was used. Quote: "All subjects who underwent randomisation were included in the final analysis. For subjects who were lost to follow‐up monitoring, we used the last available data; vital status was ascertained for all but two subjects." A total of 2.2% of patients in the ACE inhibitor group and 3.3% of patients in the diuretic group were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Without a protocol, cannot fully determine if all prespecified outcomes have been reported. |
| Use of supplemental drugs | High risk | Add‐on therapy was permitted at physician's discretion with no clear algorithm. Since this was an open‐label trial this could lead to bias. Quote: "To achieve the blood‐pressure goals, the addition of beta‐blockers, calcium‐channel blockers, and alpha‐blockers was recommended in both groups" |
| Industry sponsorship | High risk | Quote: "Supported by the Australian Commonwealth Department of Health and Aging; the National Health and Medical Research Council of Australia; and Merck Sharp & Dohme, Australia." |
Berglund 1981.
| Study characteristics | ||
| Methods | Randomized | |
| Participants | 106 male patients in Sweden with hypertension (SBP > 170 mmHg or DBP > 105 mmHg) Aged 47 to 54 (mean age not reported) |
|
| Interventions | Bendroflumethiazide 2.5 mg to 5 mg daily Propranolol 80 mg to 160 mg twice daily |
|
| Outcomes | OGTT, serum insulin Triglycerides, serum cholesterol Serum potassium, total body potassium, serum urate AEs Mortality BP at 1 year Duration: 12‐month follow‐up |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that patients were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "All 106 patients were maintained on the medication they were initially randomized to for the first year of follow‐up. During the second to sixth year 4 patients in the bendroflumethiazide group (1 death) and 3 in the propranolol group (all deaths) were lost to follow‐up." Analyzed those with 6 years of treatment (38 bendroflumethiazide and 37 propranolol) and according to original group regardless of treatment. About 30% no longer taking treatment of randomization. After 10 years of follow‐up, 7 patients in the bendroflumethiazide group and 9 patients in the propranolol group died or were otherwise lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No information about prespecified outcomes; unable to assess. |
| Use of supplemental drugs | Low risk | Dose increase was permitted but no supplemental drugs. Quote: "The dose was doubled to 5 mg bendroflumethiazide daily and 160 mg propranolol twice daily if after 2 months' treatment the BP was above 160 systolic or 95 mmHg diastolic. If the BP was not reduced below these limits by this dose increment, no further increment was made". |
| Industry sponsorship | Unclear risk | Quote: "This study was supported by a grant from the Swedish Association against Heart and Chest Diseases". Unclear if this organization receives sponsorship from for‐profit companies. |
DAPHNE 2002.
| Study characteristics | ||
| Methods | Randomized, double‐blind trial | |
| Participants | 80 male patients from the Netherlands, aged 45 to 70 years with essential hypertension (DPB 95 to 115 mmHg), peripheral atherosclerosis and hypercholesterolemia Mean age 59 years |
|
| Interventions | Doxazosin 1 mg to 16 mg daily Hydrochlorothiazide 12.5 mg to 100 mg daily |
|
| Outcomes | AEs Cholesterol, triglycerides, LDL‐C, HDL‐C, IDL‐C Carotid intimal‐medial thickness, femoral intimal‐medial thickness BP at 1 year Duration: 3 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that patients were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was described as double‐blind, but no additional details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of outcome assessment blinding was provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was used. Quote: "A total of 29 patients [70.7%] in the doxazosin group and 27 [69.2%] in the HCTZ group completed the study." This represents a relatively large loss of patients with some difference between the 2 groups. |
| Selective reporting (reporting bias) | Unclear risk | Without a protocol, cannot fully determine if all prespecified outcomes have been reported. |
| Use of supplemental drugs | Low risk | Dose increase was permitted but no supplemental drugs. Quote: "Dose adjustment was allowed during the rest of the study when DBP was consistently above 90 mmHg. For doxazosin the regimen was 1 mg, 2 mg, 4 mg, 8 mg and 16 mg once a day; for HCTZ the dosing was 12.5 mg, 25 mg, 50 mg and 100 mg once a day." |
| Industry sponsorship | High risk | Quote: "The study was made possible by an unrestricted grant from Pfizer Netherlands BV." |
HAPPHY 1987.
| Study characteristics | ||
| Methods | Randomized, open‐label trial | |
| Participants | 6569 male patients from 15 countries in Europe and North America aged 40 to 64 with hypertension (DBP 100 to 130 mmHg) Mean age 52 years |
|
| Interventions | Bendroflumethiazide 5 mg daily or hydrochlorothiazide 50 mg daily Atenolol 100 mg daily or metoprolol 200 mg daily |
|
| Outcomes | Serum potassium, creatinine, cholesterol, urate Mortality (cause‐specific) Non‐fatal MI Non‐fatal stroke AEs BP at 1 year Duration: mean follow‐up 3.8 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to open treatment with a diuretic or a beta‐blocker, after stratification into nine groups according to predicted CHD risk based upon age, serum cholesterol, smoking habits and SBP... Individual centres could choose to use either atenolol or metoprolol and bendrofluazide or hyrochlorothiazide. The fact that there was no randomisation between centres choosing different alternatives, militated against a valid comparison of the two beta‐blockers or of the two diuretics used in the trial." No further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were randomised to open treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent end‐point committee reviewed the diagnoses of the end‐points without knowing to which treatment patients had been randomised." Criteria for endpoints were well defined in the methods. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the crude withdrawal rate, calculated as the number of withdrawn patients divided by the total number of patients, was 8.9 and 7.9% in the diuretic and beta‐blocker groups, respectively (NS), corresponding to an annual withdrawal rate of 2.4% per year for the diuretic treated group and 2.1 % for the beta‐blocker treated group." Reasons for patient withdrawal are itemized and appear similar. Quote: "The analyses were made on an 'intention‐to‐treat' basis." |
| Selective reporting (reporting bias) | Unclear risk | Methods are well documented, but without a protocol, cannot fully determine if all prespecified outcomes have been reported. |
| Use of supplemental drugs | High risk | Additional treatment was consistent across first four steps, but was then free of choice in this non‐blinded trial.
Step 1: hydralazine (75 mg)
Step 2: hydralazine (150 mg)
Step 3: step 2 + spironolactone (75 mg)
Step 4: step 2 + spironolactone (150 mg)
Step 5: step 4 + optional drug Quote: "If the goal BP was not attained with the drugs and doses shown in the schedule, other drugs, free of choice, were added." |
| Industry sponsorship | High risk | Quote: "The trial was supported economically by AB Hassle, Mcilndal, a subsidiary of AB ASTRA, Sweden and ICI, Macclesfield, UK." |
INSIGHT 2000.
| Study characteristics | ||
| Methods | Randomized, double‐blind trial | |
| Participants | 6321 patients from 8 countries (western Europe and Israel) aged 55 to 80 years with BP ≥ 150/95 mmHg or ≥ 160 mmHg SBP Mean age 65 years 3392 F:2929 M |
|
| Interventions | Nifedipine 30 mg daily Co‐amilozide (hydrochlorothiazide 25 mg plus amiloride 2.5 mg daily) |
|
| Outcomes | Composite endpoint of death from any cardiovascular or cerebrovascular cause, together with non‐fatal stroke, MI, and HF Total mortality Death from vascular cause Non‐fatal vascular events (including TIA, angina, renal failure) BP at 1 year AEs Duration: 1.75 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study used dynamic randomization (minimization). Quote: "As well as the risk factors in table 1, randomisation also took into account patients’ sex, age, and whether or not they were receiving aspirin." No further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was double‐blind. Quote: "All patients received one active and one placebo tablet taken at the same time of day." No further information provided on blinding of personnel and no details provided on the blinding or dose increases and add‐on therapy for patients or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent critical events committee assessed all endpoints according to prespecified criteria. The members of this committee were unaware of the treatment group and blood pressure of each patient." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | An intention‐to‐treat analysis was used. Total withdrawals were large and different between the 2 groups: 39.9% in the nifedipine group and 33.5% in the thiazide group. This high and different attrition in the 2 groups could lead to bias. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified criteria no longer available on website; unable to assess. |
| Use of supplemental drugs | High risk | Additional treatment was consistent across first three steps, but was then based on clinician's choice. Quote: "There were four optional, dose‐titration steps... These extra dose steps were: dose doubling of the randomised drug; addition of atenolol 25 mg daily (or enalapril 5 mg daily if atenolol contraindicated); dose‐doubling of the additional drug; and addition of any other antihypertensive drug (other than calcium‐channel blockers or diuretics). These titration steps could be done in that order at any visit". |
| Industry sponsorship | High risk | Quote: "The study was funded conducted and reported by Bayer AG". |
Materson 1993.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled study for a period of 1 year | |
| Participants | 1292 male veterans with resting diastolic blood pressure of 95 mmHg to 109 mmHg | |
| Interventions | Placebo or 1 of the 6 drugs: hydrochlorothiazide 12.5 mg to 50 mg/day; atenolol 25 mg to 100 mg/day; captopril 25 mg to 100 mg/day; clonidine 0.2 mg to 0.6 mg/day; a sustained preparation of diltiazem 120 mg to 360 mg/day or prazosin 4 mg to 20 mg/day | |
| Outcomes | Withdrawals due to adverse effects Duration 1 year |
|
| Notes | Morbidity and mortality not reported. Blood pressure not reported at 1 year. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of achieving allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding maintained. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of withdrawals due to adverse effects outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rate of withdrawals in each arm. |
| Selective reporting (reporting bias) | Low risk | Withdrawals due to adverse effects reported in each group. |
| Use of supplemental drugs | Low risk | No supplemental drugs allowed. |
| Industry sponsorship | Low risk | Veterans administration trial. No industry involvement. |
MIDAS 1996.
| Study characteristics | ||
| Methods | Multicenter, randomized, double‐bind, controlled clinical trial | |
| Participants | 883 patients in the USA aged ≥ 40 years with DBP 90 to 115 mmHg Mean age 58 years 194 F:689 M |
|
| Interventions | Hydrochlorothiazide 12.5 mg to 25 mg twice daily Isradipine 2.5 mg to 5.0 mg twice daily |
|
| Outcomes | IMT Any major vascular event (stroke, MI, CHF, angina, sudden death and other cardiovascular disease‐related death) Any major vascular procedure (endarterectomy, CABG and angioplasty) Any non‐major vascular events/procedures (TIA, AF, PVC, femoral/popliteal bypass graft, aortic valve replacement and palpitation) BP at 1 year Duration: 3 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients... were randomised into 2 treatment groups... The randomisation process was stratified and blocked by clinic to provide equal probability of assignment to either treatment group throughout the study." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided, although add‐on enalapril, if used, was administered open‐label. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was described as double‐blind, but no additional details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All reported clinical events were reviewed, adjudicated, and classified by the MIDAS Investigators' Morbidity and Mortality Committee, consisting of 6 clinicians, each from a different clinical centre; all were blinded to the randomisation assignments... Members of this committee were required to reach a unanimous decision, based on clinical judgment, on how each reported event should be classified." Quote: "After completion of the trial, when investigators were unblinded to the results on clinical events obtained by the Morbidity and Mortality Committee, concern was expressed as to whether objective criteria had been consistently applied in adjudication of clinical events, especially those classified as 'hospitalized angina pectoris'. Accordingly an external ad hoc panel of 3 recognized authorities in the fields of cardiology and epidemiology was appointed. Using standard clinical definitions and the hierarchy described herein, this ad hoc committee independently reviewed and adjudicated selected clinical events while blinded to the randomisation assignments of the participants. The final analysis of clinical events reported in this article is based on the classification of events reported by this ad hoc committee." Because unblinding occurred (and was the reason for the ad hoc committee), risk is assessed as unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat approach was used. Quote: "[At the study end] Twenty percent of those on isradipine treatment and 18% of those on hydrochlorothiazide treatment had withdrawn from their respective study medications." Relatively high attrition over 3 years. |
| Selective reporting (reporting bias) | Low risk | All cardiovascular and BP outcomes in the protocol reported. |
| Use of supplemental drugs | Low risk | Only enalapril permitted for add‐on therapy. Quote: "Those who do not have responses (whose diastolic blood pressure is not controlled) to the first dose of the study drugs will have their doses doubled. The small proportion of participants who then still do not demonstrate adequate blood pressure control will receive open‐label enalapril in doses from 2.5 to 10 mg twice daily." |
| Industry sponsorship | High risk | Quote: "This study was supported in part by Sandoz Research Institute (SRI), Sandoz Pharmaceuticals, East Hanover, NJ". |
MRC 1985.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | 17,354 patients in the UK aged 35 to 64 with hypertension (DBP 90 to 109 mmHg; SBP < 200 mmHg) Mean age 52 years 8306 F:9048 M |
|
| Interventions | Bendrofluazide 10 mg daily Propranolol up to 240 mg daily Placebo |
|
| Outcomes | Stroke
Coronary events
All CV events
All‐cause mortality BP at 1 year Duration: 5.5 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated at entry... Randomisation was in stratified blocks of eight within each sex, 10 year age group, and clinic." No information provided for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "four treatments: the thiazide diuretic bendrofluazide; placebo tablets that looked like bendrofluazide; the beta blocker propranolol; and placebo tablets that looked like propranolol. The two placebo groups were treated as one in all analyses." Quote: "When the protocol was written, it was judged unreasonable to ask general practitioners to undertake such adjustments in a double blind study, and the trial was therefore single blind only." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evidence on which the diagnosis of each terminating event was based was assessed by an arbitrator ignorant of the treatment regimen... The arbitrator used WHO criteria for classification." Adjudication was independent and blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "All analyses presented here are based on randomised treatment ("intention to treat") categories. Thus data for all participants are presented as if the individual was still in the treatment group to which he was originally randomised, although substantial percentages of patients (see below) were in fact withdrawn from their randomly allocated regimen during follow up." Quote: "The total five and a half year cumulative percentages of men who stopped taking their randomised treatment, including both those withdrawn from their randomly allocated regimen but continuing on follow up and those lapsing from the trial, were 43% of the bendrofluazide group, 42% of the propranolol group, and 47% of the placebo group. For women the figures were 33%, 40%, and 40% respectively. The cumulative percentages of people not taking either primary active drug by five and a half years were smaller: 33% of men originally randomised to bendrofluazide and 34% of men randomised to propranolol and 28% and 31% respectively of women." |
| Selective reporting (reporting bias) | Unclear risk | No information about prespecified outcomes is available on which to make this assessment. |
| Use of supplemental drugs | High risk | Supplemental drugs differed between groups for a portion of the study. Quote: "Supplementary treatment was added if blood pressure did not respond satisfactorily to the primary drug. Methyldopa was originally used as a supplement to bendrofluazide and guanethidine as a supplement to propranolol, but later methyldopa was used whatever the primary drug." The primary paper does not report proportion who were initially treated with different supplementary drugs. |
| Industry sponsorship | High risk | Quote: "The working party thanks... Flockhart and Co Ltd for tablets of bendrofluazide and placebo; Imperial Chemical Industries Ltd for financial support and for tablets of propranolol and placebo; CIBA Laboratories for supplies of guanethidine; and Merck Sharp and Dohme Ltd for a mobile screening unit, funds for its staffing, and supplies of methyldopa." |
MRC 1992.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled, single‐blind trial | |
| Participants | 4396 patients in the UK aged 65 to 74 with hypertension (SBP 160 to 209 mmHg; DBP < 115 mmHg) Mean age 70 years 2560 F:1836 M |
|
| Interventions | Amiloride 2.5 to 5 mg daily and hydrochlorothiazide 25 to 50 mg daily Atenolol 50 mg Placebo |
|
| Outcomes | Stroke (fatal or non‐fatal) Coronary events (sudden death due to coronary cause, fatal and non‐fatal MI) Other cardiovascular events All‐cause mortality BP at 1 year Duration: mean follow‐up 5.8 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All trial entrants were randomly allocated in equal proportions to one of four treatment categories... Randomisation was in stratified blocks of eight within each sex and clinic." No information provided for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The trial was single blind: patients did not know which treatment group they were in, but the doctors and nurses did." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The diagnostic evidence for each terminating event was assessed by an arbitrator, blind to the treatment regimen. World Health Organisation criteria for classification of strokes and coronary events were used. All available documentation was reviewed, including copies of general practitioners' notes, hospital inpatient or outpatient notes..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The primary results are based on a comparison of groups according to their randomised treatment ‐ that is, on an intention to treat basis." Quote: "Over the five and a half years about 25% of people were lost to follow up. The cumulative percentages of people who stopped taking their randomised treatment, including both those withdrawn but continuing on follow up and those lost to follow up, were 48% of the diuretic group, 63% of the beta‐blocker group, and 53% of the placebo group." Insufficient detail to determine if ITT was carried out correctly. Differential dropouts in terms of reasons (beta‐blocker group had more withdrawals, for both suspected major side effects (333 WDAE, 12 inadequate control); diuretic 160 WDAE and 1 inadequate control; placebo 82 WDAE and 175 inadequate control). |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not identified as prespecified (other than the outcome on which a sample size calculation was reported); unable to assess. |
| Use of supplemental drugs | High risk | Quote: "Drug regimens for those on active treatment were modified if blood pressure had not responded after 12 weeks or if target pressure had not been achieved after six months. The most common change necessary was an increase in atenolol to 100 mg daily (225 patients). When further control was necessary the other trial drug was used to supplement the drug allocated by randomisation. After this, the calcium channel blocker nifedipine was used in doses of up to 20 mg daily. Any other supplementary drugs were also allowed at this stage (further details on request)." There is potential confounding by the fact that the other drug was used in treatment arms; 11% to 16% of patients received the drug opposite to the one they were assigned. |
| Industry sponsorship | Low risk | MRC funded trial. Only the drugs were supplied by the different companies. |
NESTOR 2004.
| Study characteristics | ||
| Methods | Randomized, multinational, double‐blind, double‐dummy, parallel‐group trial | |
| Participants | 570 patients from 18 countries with type 2 diabetes, essential hypertension (SBP 140 to 180 mmHg, DBP < 110 mmHg) and persistent microalbuminuria (20 to 200 g/min) Mean age 60 years 71 F:129 M |
|
| Interventions | Indapamide SR 1.5 mg daily Enalapril 10 mg daily |
|
| Outcomes | AEs UACR, AER, creatinine clearance, fractional albumin clearance BP at 1 year Duration: 1 year |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients who fulfil all of the inclusion criteria will be randomly allocated‐ to one of the two study treatments by a computerized randomisation procedure." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was double‐blind and double‐dummy. Quote: "Treatment wiII be administered daily in the form of one tablet (indapamide SR or placebo) plus one capsule (enalapril or placebo)." Supplemental drugs were administered in an open‐label fashion. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All ABPM recordings were edited by the investigators and sent to the Central Committee for validation by an expert.... Assessment of safety was based mainly on analysis of adverse events, ECG parameters, body mass index and biochemical parameters." Unclear if assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analysis was used. Quote: "Two hundred and forty‐seven (87%) and 255 (89%) patients completed the study at week 52 in the indapamide SR and in the enalapril groups, respectively." |
| Selective reporting (reporting bias) | Low risk | All cardiovascular and blood pressure outcomes listed in the protocol were reported. |
| Use of supplemental drugs | Low risk | Stepped treatment algorithm was the same for all participants. Quote: "From week 6 of the double‐blind period, the addition of open label treatment will be possible, with amlodipine 5 to 10 mg once daily as a first step and atenolol 50 to 100 mg once daily as a second step." |
| Industry sponsorship | High risk | Quote: "This study was supported by an unrestricted grant from Institut de Recherches Internationales Servier" |
NICS‐EH 1999.
| Study characteristics | ||
| Methods | Phase IV, multicenter, randomized, double‐blind, controlled, comparative clinical trial | |
| Participants | 414 patients in Japan aged 60 and older with hypertension (SBP 160 to 220 mmHg and DBP < 115 mmHg) with no history of cardiovascular complications Mean age 70 years 277 F:137 M |
|
| Interventions | Trichlormethiazide 2 mg daily Nicardipine 20 mg twice daily |
|
| Outcomes | Cardiovascular complications Erythrocyte count, total leucocyte count, hemoglobin concentration, hematocrit, total protein, albumin, total bilirubin, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, alkaline phosphatase, lactate dehydrogenase, sodium, potassium, chlorine, calcium, phosphorus, blood urea nitrogen, creatinine, uric acid, blood glucose, triglycerides, total cholesterol, high‐density lipoprotein‐cholesterol, urinary protein, urinary glucose and urinary sediments AEs BP at 1 year Duration: 5 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that patients were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy method was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "For patients who had any end point, the attending physician's judgment was assessed blindly by the Steering Committee and the diagnosis was confirmed." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Per protocol analysis was used to analyze the results of this trial" At week 140, 101 patients (50.2%) remained in the trichlormethiazide group and 84 patients (41.2%) remained in the nicardipine group. High and differential dropouts. |
| Selective reporting (reporting bias) | Low risk | Protocol published with interim analyses; does not appear to be evidence of selective outcome reporting. |
| Use of supplemental drugs | Low risk | Dose increase was permitted but no supplemental drugs. Quote: "The dosage was increased by up to 2‐fold when the antihypertensive effect achieved was not sufficient. The only antihypertensive agents allowed were the trial drugs, although a potassium supplement was administered when necessary". |
| Industry sponsorship | Unclear risk | No specific indication of any funding or sponsorship. |
PHYLLIS 2004.
| Study characteristics | ||
| Methods | Randomized, double‐blind trial | |
| Participants | 508 patients from Italy with hypertension (SBP 150 to 210 mmHg, DBP 95 to 115 mmHg), hypercholesterolemia and asymptomatic carotid atherosclerosis Mean age 58 years 304 F:204 M |
|
| Interventions | Hydrochlorothiazide 25 mg daily plus placebo Fosinopril 20 mg daily plus placebo Hydrochlorothiazide 25 mg daily plus pravastatin 40 mg daily Fosinopril 20 mg daily plus pravastatin 40 mg daily |
|
| Outcomes | IMT Total cholesterol, LDL‐C, HDL‐C, triglycerides Glucose, creatinine, urate, potassium MI, stroke and CVD events BP at 1 year Duration: 2.6 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer generated with a block size of 4." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients and study personnel were blinded to treatment assignment." Placebos were used to maintain blinding (triple‐dummy system). Add‐on therapy was permitted in an open‐label fashion. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of outcome assessment blinding was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An intention‐to‐treat analysis was used. No information on patient discontinuations was provided. |
| Selective reporting (reporting bias) | Unclear risk | No information about prespecified outcomes is available on which to make this assessment. |
| Use of supplemental drugs | Low risk | Only nifedipine was permitted as add‐on therapy. Quote: "If DBP was not >90 mm Hg or >95 mm Hg with a fall of ≥10 mm Hg, open‐label nifedipine gastrointestinal therapeutic system (GITS), 30 mg QD, was added after 3 months to be eventually increased to 60 mg after 6 months." |
| Industry sponsorship | High risk | Quote: "PHYLLIS was an investigator‐generated trial sponsored by Bristol‐Myers Squibb Italy, Rome, and Menarini, Florence. All authors have received research grants or lecture honoraria from the sponsors." |
PREVER‐treatment 2016.
| Study characteristics | ||
| Methods | Randomized, double‐blind trial | |
| Participants | 655 patients aged 30 to 70 years from Brazil with hypertension (SBP 140 to 159 mmHg or DBP 90 to 99 mmHg) after a 3‐month lifestyle intervention phase Mean age 54 years 321 F:334 M |
|
| Interventions | Chlorthalidone/amiloride 12.5 mg to 25 mg/2.5 mg to 5 mg daily Losartan 50 mg to 100 mg daily Optional add‐on: amlodipine 5 mg to 10 mg daily (month 6 and 9) followed by propranolol 40 mg to 80 mg twice a day (month 12 and 15) |
|
| Outcomes | Proportion of patients with controlled hypertension Use of non‐study BP‐lowering medications Development or worsening of microalbuminuria and left ventricular mass Fatal and nonfatal major cardiovascular events Safety BP at 1 year Duration: 18 months |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... participants were randomly assigned in a 1:1 ratio to a chlorthalidone along with amiloride combination pill or to losartan. Randomization was based on a computer‐generated list, using validated software, with variable block sizes of 4, 6, 8, or 10 and was stratified by center." |
| Allocation concealment (selection bias) | Low risk | Quote: "To guarantee concealment of the allocation list, randomization was implemented through a 24‐h web‐based automated system." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Participants, members of the steering committee, healthcare staff... were blinded as to whether patients received chlorthalidone/amiloride or losartan...The two study drugs were identical in size, shape, color, taste, and texture." However, the add‐on drugs amlodipine and propanolol were open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "... outcome assessors but not members from the data safety monitoring committee were blinded as to whether patients received chlorthalidone/amiloride or losartan." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Methods state that "Trial results were analyzed using the intention‐to‐treat approach"; however, patients with incomplete follow‐up do not appear to have been included in the evaluation of outcomes. Dropouts: chlorthalidone/amlodipine: 6.9%; losartan: 7.1%; reasons for withdrawals generally similar. Evaluation at 18 months: chlorthalidone/amlodipine 310/333 (93.1%); losartan 299/322 (92.9%). Follow‐up included 27 participants off trial drugs in diuretic arm and 31 in other arm. Handling of dropouts: analysis stated as "intention‐to‐treat". Use of a random‐effects linear model with adjustment for within‐participant correlation among the longitudinal data; model included an indicator variable for time, an interaction term for treatment by time, and the variable treatment. Quote: "Results or imputed estimates were included from participants who were lost to follow‐up, who had minor protocol deviations, such as missing one or more visits or measurement of only one BP value at a study visit, and whose study visits occurred on days other than scheduled." |
| Selective reporting (reporting bias) | Low risk | Published protocol identified the following outcomes: BP variation; proportion of use of add‐on drugs; adverse events; development of worsening of microalbuminuria; left ventricular hypertrophy (ECG); fatal or major cardiovascular events. All were reported. Ambiguous information was clarified with authors. |
| Use of supplemental drugs | Low risk | Stepped treatment algorithm was the same for all patients. Quote: "At the third month study visit, the dose was doubled if BP remained uncontrolled. If BP was uncontrolled at the 6‐month visit, amlodipine 5mg once a day was added, in an open fashion, and increased to 10mg if necessary at the 9‐month visit. At the 12‐month visit, propranolol 40 twice a day was prescribed for patients with uncontrolled BP, and doubled at the fifteenth month visit if necessary." |
| Industry sponsorship | Low risk | Quote: "Sources of funding: this study was funded by grants from the Department of Science and Technology (DECIT), Health Ministry; National Council of Research (CNPq) and Agency for Funding of Studies and Projects (FINEP), Science and Technology Ministry; National Institute of Health Technology Assessment (IATS); and Funding of Incentive to Research (FIPE), Hospital de Clinicas de Porto Alegre, all in Brazil. The sponsors had no participation in the design and conduct of the study, preparation and approval of the manuscript." |
Schmieder 2009.
| Study characteristics | ||
| Methods | Randomized, double‐blind, active‐controlled, dose‐titration trial followed by an extension phase | |
| Participants | 1124 patients from 6 European countries with hypertension (SBP 140 to 179 mmHg and/or DBP 90 to 104 mmHg), no severe concomitant disease and no diabetes Mean age 56 years 505 F:618 M |
|
| Interventions | Aliskiren 150 mg to 300 mg daily Hydrochlorothiazide 12.5 mg to 25 mg daily Optional add‐on treatment of amlodipine 5 mg to 10 mg daily |
|
| Outcomes | AEs Potassium, creatinine, BUN BP at 1 year Duration: 1 year |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization by centre was performed by the interactive voice response system provider with the use of a validated system that automates the random assignment of patients to randomisation numbers." Unclear if patients who were initially randomized to placebo treatment were then assigned to aliskiren or hydrochlorothiazide at 6 weeks in a randomized manner. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization data were kept strictly confidential until the time of unblinding." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was described as double‐blind, but no additional details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of outcome assessment blinding was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An intention‐to‐treat analysis was used. Quote: "[During the 26‐week double‐blind period] the overall number of discontinuations was significantly higher with the hydrochlorothiazide regimen than with the aliskiren regimen (15.8% versus 10.2%, respectively)." Quote: "[During the 26‐week extension] the proportion of patients discontinuing in this phase of the study was higher with the hydrochlorothiazide regimen than with the aliskiren regimen (6.7% versus 3.2%, respectively)." |
| Selective reporting (reporting bias) | Unclear risk | Unable to fully assess (no available protocol listing prespecified outcomes). |
| Use of supplemental drugs | Low risk | Only amlodipine permitted as add‐on therapy. Quote: "For patients not achieving the target BP of less than 140/90 mmHg, addition of amlodipine 5 mg was permitted from week 12, with titration to 10 mg from week 18." |
| Industry sponsorship | High risk | Quote: "This study was supported by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA." |
SHELL 2003.
| Study characteristics | ||
| Methods | Randomized, double‐blind trial | |
| Participants | 1882 patients from Italy aged ≥ 60 with SBP ≥ 160 mmHg and DBP ≤ 95 mmHg Mean age 72 years 1154 F:728 M |
|
| Interventions | Chlorthalidone 12.5 mg to 25 mg daily Lacidipine 4 mg to 6 mg daily |
|
| Outcomes | Composite of fatal and non‐fatal stroke, sudden death, fatal and non‐fatal myocardial infarction, fatal and nonfatal congestive heart failure, myocardial revascularization and carotid endarterectomy All‐cause mortality TIA Non‐Q myocardial infarction AEs BP at 1 year Duration: up to 5 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was made by BETA Trial Center, Genoa (Italy), using a sequentially based criterion." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was conducted in an open fashion. However, in 12 additional centers, patients were followed in double‐blind fashion for the first year of treatment to evaluate objectively the efficacy and tolerability of the drugs employed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Events were assessed according to predefined criteria by an independent committee unaware of the treatment group to which patients belonged." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "During follow‐up, patients who remained on randomised treatment were 79.5% in the lacidipine group and 75.5% in the chlorthalidone group." Quote: "Data were analysed on an intention‐to‐treat basis by BETA Trial Center." |
| Selective reporting (reporting bias) | High risk | All primary outcomes are listed in results; "non‐Q myocardial infarction" was listed as a secondary outcome but it is not mentioned in the results. Protocol listing prespecified outcomes not available. |
| Use of supplemental drugs | Low risk | Any ACE inhibitor permitted. Quote: "If the systolic blood pressure response was not satisfactory (reduction ≤20 mmHg and absolute value >160 mmHg) at the end of the first 4 weeks, treatment was titrated upward first by increasing the dose of the initial monotherapy (chlorthalidone 25 mg and lacidipine 6 mg) and by bringing back the monotherapy dose to the initial step and adding fosinopril 10 mg o.d. or any other ACE inhibitor at an equivalent dose after another 4 weeks of treatment." |
| Industry sponsorship | High risk | Quote: "The trial was sponsored by Laboratori Guidotti s.p.a., Pisa, Italy." |
Tresukosol 2005.
| Study characteristics | ||
| Methods | Randomized | |
| Participants | 200 patients aged 60 to 80 years with well‐established history of mild to moderate isolated systolic hypertension (SBP > 160 mmHg, DBP < 90 mmHg) Mean age 69 years 68 F:32 M |
|
| Interventions | Amlodipine 5 mg to 10 mg daily Hydrochlorothiazide 25 mg to 50 mg daily |
|
| Outcomes | LVMI Death MI, stroke AEs Cost of treatment BP at 1 year Duration: 1.5 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that participants were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of blinding was provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of outcome assessment blinding was provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per‐protocol analysis; no indication of an intention‐to‐treat analysis. A total of 27.5% of participants discontinued treatment from the amlodipine group, whereas 12.2% of participants discontinued from the hydrochlorothiazide group (differential dropout rate). |
| Selective reporting (reporting bias) | Unclear risk | Unable to fully assess (no available protocol listing prespecified outcomes) |
| Use of supplemental drugs | Low risk | Only prazosin permitted as add‐on therapy. Quote: "After the 6‐month ECHO measurement, only Prazosin 1‐20 mg per day could be added for those who had sitting systolic blood pressure above 160 mmHg in order to achieve optimal sitting systolic blood pressure below 140 mmHg." |
| Industry sponsorship | Low risk | Supported by grants of the National Research Council of Thailand. |
VA 1982.
| Study characteristics | ||
| Methods | Multicenter, randomized, double‐blind trial | |
| Participants | 683 male patients aged 21 to 65 years with hypertension (DBP 95 to 114 mmHg) Mean age 50 years |
|
| Interventions | Propranolol 80 mg to 640 mg daily Hydrochlorothiazide 50 mg to 200 mg daily |
|
| Outcomes | Reasons for discontinuation AEs Blood chemistry BP at 1 year Duration: 12 months |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that patients were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was double‐blind. Quote: "The code name for the identical appearing tablets containing either propranolol or hydrochlorothiazide was 'propazide.' The six strengths of both preparations were referred to as propazide B, C, D, E, F, and G." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of outcome assessment blinding was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Among the 394 patients who entered the long‐term treatment phase, 302 completed the 12 month follow‐up, while 92 were terminated... Administrative reasons for termination included 14 patients receiving propranolol and 18 receiving hydrochlorothiazide... More terminations owing to medical causes occurred in the propranolol group as compared with the patients receiving hydrochlorothiazide. There were 46 medical terminations, of which 35 were associated with propranolol and 11 with hydrochlorothiazide (P<.001)," Unclear how missing data were accounted for in patients who dropped out. Differential dropout in terms of reason for withdrawal. |
| Selective reporting (reporting bias) | Unclear risk | Unable to fully assess (no available protocol listing prespecified outcomes). |
| Use of supplemental drugs | Low risk | Dose increase was permitted but no supplemental drugs. |
| Industry sponsorship | High risk | Quote: "This study was supported by a grant from Ayerst Laboratories, Inc." |
VHAS 1997.
| Study characteristics | ||
| Methods | Multicenter, randomized, parallel‐group trial | |
| Participants | 1414 patients in Italy aged 40 to 65 years with hypertension (msSBP ≥ 160 and msDBP ≥ 95 mmHg) Mean age 54 years 722 F:690 M |
|
| Interventions | Chlorthalidone 25 mg daily Verapamil 240 mg daily |
|
| Outcomes | ECG Serum glucose, creatinine total and HDL‐C, triglycerides, urate, BUN, AST, ALT, sodium and potassium AEs Cardiovascular events (stroke, MI, TIA, angina, HF, revascularization procedures) BP at 1 year Duration: 2 years |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study indicates that patients were randomly allocated to treatment groups, but no further information provided regarding the method of randomization. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "After the first 6 months of double‐blind treatment, the patients returned to being administered their previous treatment according to an open design for an additional 18 months (open treatment)." No details provided on measures used to achieve double‐blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The details of cardiovascular events were verified, according to predetermined criteria, by experts blind to the randomised treatment assigned". Unclear if physicians conducting the clinical examinations throughout the study were blind to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "1464 entered the run‐in period and 1414 were allocated randomly to double‐blind treatment. All of them made at least one visit during the treatment period and could be included in intention‐to‐treat analyses... In total 1099 patients completed the 2‐year treatment period; 315 dropped out (21.6% of the verapamil group and 22.9% of the chlorthalidone group)." Unclear how missing data were accounted for in participants who dropped out. Per‐protocol analysis also reported. |
| Selective reporting (reporting bias) | Low risk | Assessment is based on predetermined criteria for cardiovascular events. |
| Use of supplemental drugs | Low risk | Only captopril permitted as add‐on treatment. Quote: "After 1 month, we added 25 mg captopril once a day to the double‐blind treatment for patients whose blood pressures had not been lowered to the goal values (a DBP while sitting < 90 mmHg or < 95 mmHg with a reduction of at least 10% from baseline values). After the second month we increased the captopril dose to 25 mg twice a day for patients whose blood pressures had not yet responded to the combined treatment." |
| Industry sponsorship | High risk | No specific indication of any funding or sponsorship in primary manuscript but in 1998 paper substudy in the Journal of Hypertension (16(11):1667‐76) quote: "This study was supported by a scientific grant from Knoll Farmaceutici Spa and Ravizza Farmaceutici Spa". |
ABPM: ambulatory blood pressure monitoring; ACE: angiotensin converting enzyme; AER: albumin excretion rate; AEs: adverse events; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BP: blood pressure; BUN: blood urea nitrogen; CABG: coronary artery bypass graft; CHD: coronary heart disease; CHF: chronic heart failure; CVD: cardiovascular disease; DBP: diastolic blood pressure; ECG: electrocardiogram; F: female; HDL: high‐density lipoprotein; HF: heart failure; IMT: intimal‐medial thickness; ITT: intention‐to‐treat; LDL: low‐density lipoprotein; LVMI: left ventricular mass index; M: male; MI: myocardial infarction; MRC: medical research council; ms: mean sitting; OGTT: oral glucose tolerance test; PP: per protocol; PVC: premature ventricular complex; SBP: systolic blood pressure; TIA: transient ischemic attack; UACR: urine albumin‐creatinine ratio; WDAE: withdrawal due to adverse event
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACCOMPLISH 2008 | Control group was combination therapy not a single first‐line drug class |
| Appel 2010 | No diuretic monotherapy treatment group |
| AVEC 2012 | No primary outcomes reported |
| Bakris 2010 | No diuretic monotherapy treatment group |
| Bebb 2007 | No diuretic monotherapy treatment group |
| Caruso 2004 | No primary outcomes reported |
| Cho 2008 | Treatment duration < 12 months |
| COLM investigators 2014 | Combination therapy and not first‐line single drug class |
| CONVINCE 2003 | Data for patients treated with diuretic monotherapy pooled with other treatment groups; no diuretic monotherapy data available from publication |
| Cooper‐DeHoff 2010 | Treatment duration < 12 months |
| COPE 2011 | Assesses diuretics in combination with calcium channel blockers after initial failure of calcium channel blockers alone |
| COSMO‐CKD 2014 | No diuretic monotherapy; patients on background RAS inhibitor |
| Ebbs 2001 | Treatment duration < 12 months |
| Galzerano 2004 | No primary outcomes reported |
| GENRES 2007 | Treatment duration < 12 months |
| Grassi 2006 | No primary outcomes reported; BP data only |
| Iyalomhe 2014 | Treatment duration < 12 months |
| Jordan 2012 | Treatment duration < 12 months |
| Khan 2008 | Treatment duration < 12 months |
| Klingbeil 2000 | Treatment duration < 12 months |
| LIFE 2002 | No diuretic monotherapy treatment group |
| LIVE 1998 | Treatment duration < 12 months; median follow‐up of 11 months |
| Mahmud 2009 | Congress abstract only; no primary outcomes reported and duration of treatment unclear |
| Mallion 2004 | No active comparator assessed |
| Mann 2002 | Treatment duration < 12 months |
| Morgan 2004 | Treatment duration < 12 months |
| Neaton 1993 | Outcomes were not reported separately for different comparators; study authors were contacted but refused to provide data separately for different groups |
| NORDIL 2000 | Data for patients treated with diuretic monotherapy pooled with other treatment groups; no diuretic monotherapy data available from publication |
| Oshchepkova 2007 | Treatment duration < 12 months |
| PEAR 2012 | Treatment duration < 12 months |
| Peng 2015 | Inappropriate patient group (high‐normal at baseline; not hypertensive) |
| Pool 2009 | Treatment duration < 12 months |
| Posadzy‐Malaczynska 2014 | No primary outcomes reported |
| PROGRESS 2001 | No diuretic monotherapy treatment group |
| Rasmussen 2006 | Treatment duration < 12 months |
| SALT 2007 | Treatment duration < 12 months |
| Schram 2005 | No primary outcomes reported; BP data only |
| Schwartz 2013 | Treatment duration < 12 months |
| SHEP 1991 | Systolic Hypertension in the Elderly Program (SHEP) trial; no head‐to‐head comparison with another active comparator |
| Shionoiri 2000 | No primary outcomes reported; BP data only |
| Sierra 2004 | Congress abstract only; no primary outcomes reported. |
| Solorzano 2011 | Congress abstract; retrospective analysis of trial data but unclear if RCT |
| SPREAD 2006 | Outcomes not relevant; BP data only |
| STOP‐Hypertension‐2 1999 | Data for patients treated with diuretic monotherapy pooled with other treatment groups; no diuretic monotherapy data available from publication |
| Stritzke 2010 | Congress abstract only; no primary outcomes reported |
| Syst‐Eur 1997 | No diuretic monotherapy treatment group |
| Tedesco 1998 | No primary outcomes reported |
| Tedesco 1999 | No primary outcomes reported |
| Trimarco 2011 | Congress abstract only; insufficient data regarding primary outcomes |
| Trimarco 2015 | No primary outcome data reported; abstract only |
| VADT 2011 | No diuretic monotherapy treatment group |
| Veronesi 2007 | No primary outcomes reported |
| Wilson 1963 | No primary outcomes reported |
| Yasuda 2015 | Treatment duration < 12 months |
| Yogiantoro 2000 | Congress abstract only; no primary outcomes reported |
| Yurenev 1992 | Patients with left ventricular hypertrophy were included, not specifically a hypertension population. Study was included in Thomopoulos 2015 systematic review. |
BP: blood pressure; RAS: renin angiotensin inhibitors; RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02217852.
| Study name | NCT02217852 |
| Methods | Randomized, open‐label, controlled clinical trial |
| Participants | Adult Tibetan patients with diagnosed hypertension grade 1 to 3, aged 18 to 80 years old |
| Interventions | Nitrendipine 10 to 20 mg orally twice daily Hydrochlorothiazide 12.5 mg to 25 mg orally 4 times daily Captopril plus hydrochlorothiazide, 25 mg to 50 mg orally 3 times for captopril and 12.5 to 25 mg orally 4 times a day for hydrochlorothiazide Beijing hypotensive No.0, 1 pile orally 4 times a day or less |
| Outcomes | Change in blood pressure (12 months), change in target organ damage |
| Starting date | August 2014 |
| Contact information | Xiaoping Chen, MD, West China Hospital, Chengdu, Sichuan, China, 610041 xiaopingchen11@126.com |
| Notes | — |
Differences between protocol and review
Our review methods were updated to reflect the latest Cochrane methodology. The section Data collection and analysis was reported under the following subheadings: 'Selection of studies', 'Data extraction and management', 'Assessment of risk of bias in included studies', 'Measures of treatment effect', 'Unit of analysis issues', 'Dealing with missing data', 'Assessment of heterogeneity', 'Assessment of reporting biases', 'Data synthesis', 'Subgroup analysis', 'Sensitivity analysis' and 'Summary of findings and assessment of the certainty of evidence'.
In the original protocol spironolactone was included as a potassium‐sparing diuretic that would be allowed in combination with a thiazide. We now realize that this was incorrect as spironolactone has proven blood pressure‐lowering effects (Batterink 2010). It is no longer included under Types of interventions.
Total congestive heart failure events was added as a separate primary outcome of interest in the review. This was added after a significant difference in this outcome was noted for the comparison of calcium channel blockers with diuretics in a separate Cochrane Review (Chen 2018). We also included 'direct renin inhibitors' as a comparator drug class.
A separate sensitivity analysis was conducted excluding the ALLHAT 2000/2002 trial, as this was the largest trial and it might have had substantial influence over several meta‐analyses. The inclusion of ALLHAT 2000/2002 also prevented an overall comparison (i.e. diuretics versus all other classes) as the diuretic arm would be included in the meta‐analysis multiple times. Overall comparisons were therefore undertaken after the exclusion of ALLHAT 2000/2002 in sensitivity analyses.
Contributions of authors
Marcia Reinhart: led this review, conducted screening, extracted data, drafted review in RevMan 5, incorporated comments from fellow authors into the draft.
Lorri Puil: participated in screening, data extraction, the writing of the discussion and conclusions, and finalization of the draft.
Douglas Salzwedel: designed and executed the search strategies, participated in screening, and assisted in editing the final draft.
James M Wright: formulated the idea for the protocol, extracted data, participated in risk of bias judgments, and the writing and interpretation of the results, discussion, summary of findings tables, and conclusions.
Sources of support
Internal sources
-
Faculty of Medicine, University of British Columbia, Canada
Salary and infrastructure support
External sources
-
British Columbia Ministry of Health, Canada
Infrastructure grant to our parent organization, the Therapeutics Initiative
Declarations of interest
Marcia Reinhart: Thermo Fisher Scientific (employment, since October 2020).
Lorri Puil: no relevant interests; Editor of Cochrane Hypertension but was not involved in any part of the editorial process of this review.
Douglas Salzwedel: no relevant interests; Information Specialist of Cochrane Hypertension but was not involved in any part of the editorial process of this review.
James Wright: no relevant interests; Co‐ordinating Editor of Cochrane Hypertension but was not involved in any part of the editorial process of this review.
New
References
References to studies included in this review
ALLHAT 2000/2002 {published data only}
- ALLHAT Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003;42:239-46. [DOI] [PubMed] [Google Scholar]
- Bang CN, Soliman E, Simpson LM, Davis B, Devereux R, Okin P. Electrocardiographic left ventricular hypertrophy predicts cardiovascular morbidity and mortality in hypertensive patients: the ALLHAT STUDY. Journal of the American College of Cardiology 2015;1:A1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Davis BR, Bettencourt J, Margolis KL, Goff DC Jr, Black H, et al. Cardiovascular outcomes using doxazosin vs. chlorthalidone for the treatment of hypertension in older adults with and without glucose disorders: a report from the ALLHAT study. Journal of Clinical Hypertension 2004;6:116-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2006;166:2191-201. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Jones CL, Davis BR, Basile JN, Goff DC Jr, Ciocon JO, et al. Baseline characteristics of the diabetic participants in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Diabetes Care 2001;24:654-8. [DOI] [PubMed] [Google Scholar]
- Black HR, Davis B, Barzilay J, Nwachuku C, Baimbridge C, Marginean H, et al. Metabolic and clinical outcomes in nondiabetic individuals with the metabolic syndrome assigned to chlorthalidone, amlodipine, or lisinopril as initial treatment for hypertension: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Diabetes Care 2008;31:353-60. [DOI] [PubMed] [Google Scholar]
- Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Journal of Clinical Hypertension 2002;4:393-404. [DOI] [PubMed] [Google Scholar]
- Cushman WC, Ford CE, Einhorn PT, Wright JT Jr, Preston RA, Davis BR, et al. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Journal of Clinical Hypertension 2008;10:751-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BR, Cutler JA, Furberg CD, Wright JT, Farber MA, Felicetta JV, et al. Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: further analyses from the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial. [Summary for patients in Ann Intern Med. 2002 Sep 3;137(5 Part 1):I38; PMID: 12204046]. Annals of Internal Medicine 2002;137:313-20. [DOI] [PubMed] [Google Scholar]
- Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JTJr, Cushman WC, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. American Journal of Hypertension 1996;9:342-60. [DOI] [PubMed] [Google Scholar]
- Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, et al. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation 2008;118:2259-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, et al. Role of diuretics in the prevention of heart failure: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation 2006;113:2201-10. [DOI] [PubMed] [Google Scholar]
- Dewland TA, Soliman EZ, Davis BR, Magnani JW, Yamal JM, Piller LB, et al. Predictors of incident conduction system disease in the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Circulation 2014;130:A16491. [DOI] [PubMed] [Google Scholar]
- Grimm RH, Davis BR, Piller LB, Cutler JA, Margolis KL, Barzilay J, et al. Heart failure in ALLHAT: did blood pressure medication at study entry influence outcome? Journal of Clinical Hypertension 2009;11:466-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm RHJr, Margolis KL, Papademetriou VV, Cushman WC, Ford CE, Bettencourt J, et al. Baseline Characteristics of participants in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2001;37:19-27. [DOI] [PubMed] [Google Scholar]
- Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, et al. Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial). Journal of the American College of Cardiology 2009;54:2023-31. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Davis BR, Cutler JA, Furberg CD, Lairson DR, Shlipak MG, et al. Cost-effectiveness of chlorthalidone, amlodipine, and lisinopril as first-step treatment for patients with hypertension: an analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Journal of General Internal Medicine 2008;23:509-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, Levitan EB, Lynch AI, Simpson LM, Whittle J, Davis BR, et al. Effect of chlorthalidone, amlodipine, and lisinopril on visit-to-visit variability of blood pressure: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Journal of Clinical Hypertension 2014;16:323-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller LB, Davis BR, Cutler JA, Cushman WC, Wright JT, Williamson J, et al. Validation of heart failure events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants assigned to doxazosin and chlorthalidone. Trials 2002;3(10):10. [DOI: 10.1186/1468-6708-3-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller LB, Simpson LM, Baraniuk S, Habib GB, Rahman M, Basile JN, et al. Characteristics and long-term follow-up of participants with peripheral arterial disease during ALLHAT. Journal of General Internal Medicine 2014;1129:1475-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschan M, Ford CE, Cutler JA, Graumlich JF, Pavlik V, Cushman C, et al. How much effect of different antihypertensive medications on cardiovascular outcomes is attributable to their effects on blood pressure? Statistics in Medicine 2013;32:884-97. [DOI] [PubMed] [Google Scholar]
- Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT Jr, Whelton PK, et al. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2005;165:936-46. [DOI] [PubMed] [Google Scholar]
- Reisin E, Graves JW, Yamal J-M, Barzilay JI, Pressel SL, Einhorn PT, et al. Blood pressure control and cardiovascular outcomes in normal-weight, overweight, and obese hypertensive patients treated with three different antihypertensives in ALLHAT. Journal of Hypertension 2014;32:1503-13; discussion 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams T, Auchus AP, Oparil S, Wright C, Wright J, Furlan AJ, et al. Baseline quality of life and risk of stroke in the antihypertensive and lipid lowering to prevent heart attack (ALLHAT) trial. Stroke 2015;46:3078-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ALLHAT Officers. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA 2000;283:1967-75. [PubMed] [Google Scholar]
- The ALLHAT Officers. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981-97. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Barzilay J, Cushman WC, Davis BR, Iiamathi E, Kostis JB, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2005;165:1401-9. [DOI] [PubMed] [Google Scholar]
- Wright JT Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005;293:1595-608. [DOI] [PubMed] [Google Scholar]
- Wright JT Jr, Harris-Haywood S, Pressel S, Barzilay J, Baimbridge C, Bareis CJ, et al. Clinical outcomes by race in hypertensive patients with and without the metabolic syndrome: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 2008;168:207-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamal JM, Oparil S, Davis BR, Alderman MH, Calhoun DA, Cushman WC, et al. Stroke outcomes among participants randomized to chlorthalidone, amlodipine or lisinopril in ALLHAT. Journal of the American Society of Hypertension 2014;8:808-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
ALPINE 2003 {published data only}
- Lindholm LH, Kartman B, Carlberg B, Persson M, Svensson A, Samuelsson O. Cost implications of development of diabetes in the ALPINE study. Journal of Hypertension - Supplement 2006;24:S65-72. [DOI] [PubMed] [Google Scholar]
- Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study). Journal of Hypertension 2003;21(8):1563-74. [DOI] [PubMed] [Google Scholar]
ANBP2 2003 {published data only}
- Chowdhury EK, Owen A, Ademi Z, Krum H, Johnston CI, Wing Lindon MH, et al. Short- and long-term survival in treated elderly hypertensive patients with or without diabetes: findings from the Second Australian National Blood Pressure study. American Journal of Hypertension 2014;27:199-206. [DOI] [PubMed] [Google Scholar]
- Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, et al. A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly. New England Journal of Medicine 2003;348(7):583-92. [DOI] [PubMed] [Google Scholar]
Berglund 1981 {published data only}
- Berglund G, Andersson O, Widgren B. Low-dose antihypertensive treatment with a thiazide diuretic is not diabetogenic. A 10-year controlled trial with bendroflumethiazide. Acta Medica Scandinavica 1986;220(5):419-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Berglund G, Andersson O. beta-blockers or diuretics in hypertension? A six year follow-up of blood pressure and metabolic side effects. Lancet 1981;1(8223):744-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
DAPHNE 2002 {published data only}
- Hoogerbrugge N, Groot E, Heide LHM, Ridder MAJ, Birkenhageri JC, Stijnen T, et al. Doxazosin and hydrochlorothiazide equally affect arterial wall thickness in hypertensive males with hypercholesterolaemia (the DAPHNE study). Doxazosin Atherosclerosis Progression Study in Hypertensives in the Netherlands. Netherlands Journal of Medicine 2002;60:354-61. [PubMed] [Google Scholar]
HAPPHY 1987 {published data only}
- Wilhelmsen L, Berglund G, Elmfeldt D, Fitzsimons T, Holzgreve H, Hosie J, et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. Journal of Hypertension 1987;5(5):561-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
INSIGHT 2000 {published data only}
- Brown MJ, Palmer CR, Castaigne A, De Leeuw PW, Mancia G, Rosenthal T, et al. Principal results from the International Nifedipine GITS Study: Intervention as a Goal in Hypertension Treatment (INSIGHT). European Heart Journal Supplements 2001;3:B20-6. [Google Scholar]
- Brown MJ, Palmer CR, Castaigne A, Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 2000;356(9227):366-72. [DOI] [PubMed] [Google Scholar]
- Gariepy J, Simon A, Chironi G, Moyse D, Levenson J. Large artery wall thickening and its determinants under antihypertensive treatment: the IMT-INSIGHT study. Journal of Hypertension 2004;22:137-43. [DOI] [PubMed] [Google Scholar]
- Heagerty A, Deverly A, Palmer C, Kaplinsky E, Salvetti A, Wahlgren NG, et al. The role of the critical event committee in a major cardiovascular outcome study. Blood Pressure 2002;11(6):339-44. [DOI] [PubMed] [Google Scholar]
- Mancia G, Brown M, Castaigne A, Leeuw P, Palmer CR, Rosenthal T, et al. Outcomes with nifedipine GITS or Co-amilozide in hypertensive diabetics and nondiabetics in Intervention as a Goal in Hypertension (INSIGHT). Hypertension 2003;41:431-6. [DOI] [PubMed] [Google Scholar]
- Mancia G, Omboni S, Parati G, Investigators of the Insight Abpm substudy. Twenty-four hour ambulatory blood pressure in the International Nifedipine GITS Study Intervention as a Goal in Hypertension Treatment (INSIGHT). Journal of Hypertension 2002;20:545-53. [DOI] [PubMed] [Google Scholar]
- Mancia G, Ruilope L, Palmer C, Brown M, Castaigne A, De Leeuw P, et al. Effects of nifedipine GITS and diuretics in isolated systolic hypertension--a subanalysis of the INSIGHT study. Blood Pressure 2004;13:310-5. [DOI] [PubMed] [Google Scholar]
- Mancia G, Ruilope LM, Brown MJ, Palmer CR, Rosenthal T, Castaigne A, et al. The effect of nifedipine GITS on outcomes in patients with previous myocardial infarction: a subgroup analysis of the INSIGHT study. British Journal of Cardiology 2002;9(7):401-5. [Google Scholar]
- Simon A, Gariepy J, Moyse D, Levenson J. Differential effects of nifedipine and co-amilozide on the progression of early carotid wall changes. Circulation 2001;103:2949-54. [DOI] [PubMed] [Google Scholar]
- Simon A, Gariepy J, Provost JC, IMT-INSIGHT Group. Nifedipine, hypertension, and arterial wall thickness in extracoronary arteries: design features of a clinical trial using non invasive ultrasound end points. Journal of Cardiovascular Pharmacology 1996;28:527-32. [Google Scholar]
Materson 1993 {published data only}
- Gottdiener JS, Reda DJ, Williams DW, Materson BJ, Cushman W, Anderson RJ. Effect of single-drug therapy on reduction of left atrial size in mild to moderate hypertension: comparison of six antihypertensive agents. Circulation 1998;98(2):140-8. [DOI] [PubMed] [Google Scholar]
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single‐drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. New England Journal of Medicine 1993;328:914-21. [DOI: 10.1056/NEJM199304013281303] [DOI] [PubMed] [Google Scholar]
MIDAS 1996 {published data only}
- Borhani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa-Terris M, Carr AA, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). A randomized controlled trial. JAMA 1996;276(10):785-91. [PubMed] [Google Scholar]
- Byington RP, Furberg CD, Craven TE, Pahor M, Sowers JR. Isradipine in prediabetic hypertensive subjects. Diabetes Care 1998;21(12):2103-10. [DOI] [PubMed] [Google Scholar]
MRC 1985 {published data only}
- Medical Research Council Working Party on Mild Hypertension. MRC trial of treatment of mild hypertension: principal results. Medical Research Council Working Party. British Medical Journal 1985;291(6488):97-104. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart S. Results of MRC (UK) trial of drug therapy for mild hypertension. Clinical and Investigative Medicine 1987;10(6):616-20. [PubMed] [Google Scholar]
MRC 1992 {published data only}
- Carr MJ, Bao Y, Pan J, Cruickshank K, McNamee R. The predictive ability of blood pressure in elderly trial patients. Journal of Hypertension 2012;30(9):1725-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ (Clinical Research Ed.) 1992;304:405-12. [DOI: 10.1136/bmj.304.6824.405] [DOI] [PMC free article] [PubMed] [Google Scholar]
NESTOR 2004 {published data only}
- Marre M, Puig JG, Kokot F, Fernandez M, Jermendy G, Opie L, et al. Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes. Journal of Hypertension 2004;22(8):1613-22. [DOI: 10.1097/01.hjh.0000133733.32125.09] [DOI] [PubMed] [Google Scholar]
- Puig JG, Marre M, Kokot F, Fernandez M, Jermendy G, Opie L, et al. Efficacy of indapamide SR compared with enalapril in elderly hypertensive patients with type 2 diabetes. American Journal of Hypertension 2007;20:90-7. [DOI] [PubMed] [Google Scholar]
NICS‐EH 1999 {published data only}
- Kuwajima I, Kuramoto K, Ogihara T, Iimura O, Abe K, Saruta T, et al. Tolerability and safety of a calcium channel blocker in comparison with a diuretic in the treatment of elderly patients with hypertension: secondary analysis of the NICS-EH. Hypertension Research - Clinical & Experimental 2001;24:475-80. [DOI] [PubMed] [Google Scholar]
- Kuwajima I, Kuramoto K. Randomized double-blind comparison of a calcium channel blocker and a diuretic in elderly hypertensives: a final result of the National Interventional Cooperative Study in Elderly Hypertension (NICS-EH). Journal of Stroke and Cerebrovascular Diseases 2000;9(2):29-30. [Google Scholar]
- National Intervention Cooperative Study in Elderly Hypertensives (NICS-EH) Study Group. Randomized double-blind comparison of a calcium antagonist and a diuretic in elderly hypertensives. Hypertension 1999;34(5):1129-33. [PMID: ] [PubMed] [Google Scholar]
PHYLLIS 2004 {published data only}
- Zanchetti A, Crepaldi G, Bond MG, Gallus G, Veglia F, Mancia G, et al. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis: principal results of PHYLLIS—a randomized double-blind trial. Stroke 2004;35(12):2807-12. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Crepaldi G, Bond MG, Gallus GV, Veglia F, Ventura A, et al. Systolic and pulse blood pressures (but not diastolic blood pressure and serum cholesterol) are associated with alterations in carotid intima-media thickness in the moderately hypercholesterolaemic hypertensive patients of the Plaque Hypertension Lipid Lowering Italian Study. Journal of Hypertension 2001;19(1):79-88. [DOI] [PubMed] [Google Scholar]
PREVER‐treatment 2016 {published data only}
- Bertoluci C, Foppa M, Santos ABS, Fuchs SC, Fuchs FD. Diuretics are similar to losartan on echocardiographic target-organ damage in stage I hypertension. PREVER-Treatment Study. Arquivos Brasileiros de Cardiologia 2019;112(1):87-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs FD, Fuchs SC, Moreira LB, Gus M, Nobrega AC, Poli-de-Figueiredo CE, et al. A comparison between diuretics and angiotensin-receptor blocker agents in patients with stage I hypertension (PREVER-treatment trial): study protocol for a randomized double-blind controlled trial. Trials [Electronic Resource] 2011;12:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs FD, Scala LC, Vilela-Martin JF, Mello RB, Mosele F, Whelton PK, et al. Effectiveness of chlorthalidone/amiloride versus losartan in patients with stage I hypertension: results from the PREVER-treatment randomized trial. Journal of Hypertension 2016;34:798-806. [DOI] [PubMed] [Google Scholar]
Schmieder 2009 {published data only}
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Bush C, Keefe DL. Aliskiren-based therapy lowers blood pressure more effectively than hydrochlorothiazide-based therapy in obese patients with hypertension: sub-analysis of a 52-week, randomized, double-blind trial. Journal of Hypertension 2009;27:1493-501. [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, Weissbach N, et al. Long-Term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12-month randomized, double-blind comparator trial with hydrochlorothiazide. Circulation 2009;119:417-25. [DOI] [PubMed] [Google Scholar]
SHELL 2003 {published data only}
- Malacco E, Mancia G, Rappelli A, Menotti A, Zuccaro M S, Coppini A. Treatment of isolated systolic hypertension: the SHELL study results. Blood Pressure 2003;12:160-7. [DOI] [PubMed] [Google Scholar]
Tresukosol 2005 {published data only}
- Tresukosol D, Sriyuthasak O, Thongtang V. Amlodipine and hydrochlorothiazide for isolated systolic hypertension in the Thai elderly. Siriraj Medical Journal 2005;57(9):374-9. [Google Scholar]
VA 1982 {published data only}
- Freis E. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II. Results of long-term therapy. JAMA 1982;248(16):2004-11. [PMID: ] [PubMed] [Google Scholar]
- Freis ED. Veterans Administration Cooperative Study Group on Hypertensive Agents: effects of age on treatment results. American Journal of Medicine 1991;90(3A):20S-3S. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ramirez EA, Talmers FN. Propranolol or hydrochlorothiazide alone for the initial treatment of hypertension. IV: effect on plasma glucose and glucose tolerance. Hypertension 1985;7(6):1008-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
VHAS 1997 {published data only}
- Rosei EA, Dal Palu C, Leonetti G, Magnani B, Pessina A, Zanchetti A. Clinical results of the Verapamil inHypertension and Atherosclerosis Study. VHAS Investigators. Journal of Hypertension 1997;15(11):1337-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Rosei EA, Dal Palu C, Leonetti G, Magnani B, Pessina A. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness. Journal of Hypertension 1998;16:1667-76. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACCOMPLISH 2008 {published data only}
- Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. New England Journal of Medicine 2008;359:2417-28. [DOI] [PubMed] [Google Scholar]
Appel 2010 {published data only}
- Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. New England Journal of Medicine 2010;363(10):918-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
AVEC 2012 {published data only}
- Hajjar I, Hart M, Chen Y-L, Mack W, Milberg W, Chui H, et al. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double-blind randomized clinical trial. Archives of Internal Medicine 2012;172:442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Hart M, Chen YL, Mack W, Novak V, Chui HC, et al. Antihypertensive therapy and cerebral hemodynamics in executive mild cognitive impairment: results of a pilot randomized clinical trial. Journal of the American Geriatrics Society 2013;61:194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Hart M, Milberg W, Novak V, Lipsitz L. The rationale and design of the antihypertensives and vascular, endothelial, and cognitive function (AVEC) trial in elderly hypertensives with early cognitive impairment: role of the renin angiotensin system inhibition. BMC Geriatrics 2009;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00605072. The antihypertensives and vascular, endothelial and cognitive function trial [The antihypertensives and vascular, endothelial and cognitive function trial (AVEC trial)]. clinicaltrials.gov/show/NCT00605072 (first received 30 January 2008).
Bakris 2010 {published data only}
- Bakris G, White WB, Weber MA, Sica D, Perez A, Cao C, et al. Results of a double-blind randomized study comparing chlorthalidone and hydrochlorothiazide combined with the new angiotensin receptor blocker azilsartan medoxomil in primary hypertension. Journal of Clinical Hypertension 2010;12:530. [Google Scholar]
Bebb 2007 {published data only}
- Bebb C, Kendrick D, Coupland C, Madeley R, Stewart J, Brown K, et al. A cluster randomised controlled trial of the effect of a treatment algorithm for hypertension in patients with type 2 diabetes. British Journal of General Practice 2007;57(535):136-43. [PMC free article] [PubMed] [Google Scholar]
Caruso 2004 {published data only}
- Caruso D, D'Avino M, Acampora C, Romano L, Bevilacqua N, Caruso G, et al. Effects of losartan and chlorthalidone on blood pressure and renal vascular resistance index in non-diabetic patients with essential hypertension and normal renal function. Journal of Cardiovascular Pharmacology 2004;44:520-4. [DOI] [PubMed] [Google Scholar]
Cho 2008 {published data only}
- Cho YS, Lee HY, Youn TJ, Chung WY, Chae IH, Choi DJ, et al. A randomized prospective study to compare the effects of three anti-hypertensives on plasma adipokines and arterial stiffness. Journal of Hypertension 2008;26:S44. [Google Scholar]
COLM investigators 2014 {published data only}
- Ogihara T, Saruta T, Rakugi H, Saito I, Shimamoto K, Matsuoka H, et al: COLM Investigators. Combinations of olmesartan and a calcium channel blocker or a diuretic in elderly hypertensive patients: a randomized, controlled trial. Journal of Hypertension 2014;32:2054-63; discussion 2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONVINCE 2003 {published data only}
- Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, Neaton JD, et al. Results of the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) trial by geographical region. Journal of Hypertension 2005;23:1099-106. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA 2003;289:2073-82. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RHJ, et al. Baseline characteristics and early blood pressure control in the CONVINCE trial. Hypertension 2001;37:12-8. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RHJ, et al. Rationale and design for the Controlled ONset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) Trial. Controlled Clinical Trials 1998;19:370-90. [DOI] [PubMed] [Google Scholar]
Cooper‐DeHoff 2010 {published data only}
- Cooper-DeHoff RM, Wen S, Beitelshees AL, Zineh I, Gums JG, Turner ST, et al. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension 2010;55:61-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
COPE 2011 {published data only}
- Matsuzaki M, Ogihara T, Umemoto S, Rakugi H, Matsuoka H, Shimada K, et al. Prevention of cardiovascular events with calcium channel blocker-based combination therapies in patients with hypertension: a randomized controlled trial. Journal of Hypertension 2011;29:1649-59. [DOI] [PubMed] [Google Scholar]
COSMO‐CKD 2014 {published data only}
- Ando K, Nitta K, Rakugi H, Nishizawa Y, Yokoyama H, Nakanishi T, et al. Comparison of the antialbuminuric effects of benidipine and hydrochlorothiazide in renin-angiotensin system (RAS) inhibitor-treated hypertensive patients with albuminuria: the COSMO-CKD (Combination strategy on renal function of benidipine or diuretics treatment with RAS inhibitors in a chronic kidney disease hypertensive population) Study. International Journal of Medical Sciences 2014;11(9):897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbs 2001 {published data only}
- Ebbs D. A comparison of selected antihypertensives and the use of conventional vs ambulatory blood pressure in the detection and treatment of hypertension. Cardiology 2001;96(Suppl 1):3-9. [DOI] [PubMed] [Google Scholar]
Galzerano 2004 {published data only}
- Galzerano D, Tammaro P, Cerciello A, Breglio R, Mallardo M, Lama D, et al. Freehand three-dimensional echocardiographic evaluation of the effect of telmisartan compared with hydrochlorothiazide on left ventricular mass in hypertensive patients with mild-to-moderate hypertension: a multicentre study. Journal of Human Hypertension 2004;18:53-9. [DOI] [PubMed] [Google Scholar]
GENRES 2007 {published data only}
- Donner KM, Hiltunen TP, Suonsyrja T, Hannila-Handelberg T, Tikkanen I, Antikainen M, et al. CYP2C9 genotype modifies activity of the renin-angiotensin-aldosterone system in hypertensive men. Journal of Hypertension 2009;27(10):2001-9. [DOI] [PubMed] [Google Scholar]
- Hiltunen TP, Suonsyrja T, Hannila-Handelberg T, Paavonen KJ, Miettinen HE, Strandberg T, et al. Predictors of antihypertensive drug responses: initial data from a placebo-controlled, randomized, cross-over study with four antihypertensive drugs (The GENRES Study). American Journal of Hypertension 2007;20:311-8. [DOI] [PubMed] [Google Scholar]
- Suonsyrja T, Donner K, Hannila-Handelberg T, Fodstad H, Kontula K, Hiltunen TP. Common genetic variation of beta1-and beta2-adrenergic receptor and response to four classes of antihypertensive treatment. Pharmacogenetics and Genomics 2010;20(5):342-5. [DOI] [PubMed] [Google Scholar]
Grassi 2006 {published data only}
- Grassi G, Quarti-Trevano F, Scopelliti F, Seravalle G, Cuspidi C, Mancia G. Effects of long-term lercanidipine or hydrochlorothiazide administration on hypertension-related vascular structural changes. Blood Pressure 2006;15(5):268-74. [DOI] [PubMed] [Google Scholar]
Iyalomhe 2014 {published data only}
- Iyalomhe G, Omogbai E, Iyalomhe O. Electrolyte effects during initiation of antihypertensive therapy with amlodipine or hydrochlorothiazide in diabetic Nigerians. Basic and Clinical Pharmacology and Toxicology 2014;115:54. [Google Scholar]
Jordan 2012 {published data only}
- Jordan J, Boye SW, Breton SL, Keefe DL, Engeli S, Prescott MF. Antihypertensive treatment in patients with class 3 obesity. Therapeutic Advances in Endocrinology & Metabolism 2012;3(3):93-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2008 {published data only}
- Khan M, Khan RA, Islam F, Laghari J, Jamali SN. To study the efficacy of losartan on urinary uric acid excretion in thiazide induced hyperuricemic and hypertensive patients. Pakistan Journal of Pharmaceutical Sciences 2011;24(4):583-7. [PubMed] [Google Scholar]
- Khan M, Mashori GR, Memon KM. Safety of losartan in hypertensive patients with thiazide induced hyperuricemia. Journal of the Liaquat University of Medical and Health Sciences 2008;7(3):163-7. [Google Scholar]
Klingbeil 2000 {published data only}
- Klingbeil AU, John S, Schneider MP, Delles C, Weidinger GP, Schmieder RE. Valsartan but not a thiazide improves aortic augmentation pressure index in essential hypertension: a randomized, placebo controlled study. Circulation 2000;102(18):416-7. [Google Scholar]
LIFE 2002 {published data only}
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, De Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359(9311):995-1003. [DOI] [PubMed] [Google Scholar]
- De Simone G, Lyle PA. Body mass index and cardiovascular events in patients with hypertension and left ventricular hypertrophy. Cardiology Review 2006;23(6):22-6. [Google Scholar]
LIVE 1998 {published data only}
- Ambrosioni DE. Management of cardiovascular risk in patients with left ventricular hypertrophy. [French]. Presse Medicale 2002;31(Spec Iss 2):2HS13-6. [PubMed] [Google Scholar]
- Gosse P, Dubourg O, Gueret P. Regression of left ventricular hypertrophy with echocardiography: some lessons from the live study. Journal of Hypertension 2003;21(1):217-21. [DOI] [PubMed] [Google Scholar]
- Gosse P, Guez D, Gueret P, Dubourg O, Beauchet A, Cordoue A, et al. Centralized echocardiogram quality control in a multicenter study of regression of left ventricular hypertrophy in hypertension. Journal of Hypertension 1998;16:531-5. [DOI] [PubMed] [Google Scholar]
- Gosse P, Sheridan DJ, Zannad F, Dubourg O, Gueret P, Karpov Y, et al. Regression of left ventricular hypertrophy in hypertensive patients treated with indapamide SR 1.5 mg versus enalapril 20 mg: the LIVE study. Journal of Hypertension 2000;18:1465-75. [DOI] [PubMed] [Google Scholar]
Mahmud 2009 {published data only}
- Mahmud A, Feely J. Central aortic pressure is a better determinant of anti-hypertensive response than brachial pressure in young hypertensive patients. British Journal of Clinical Pharmacology 2009;68(2):274-5. [Google Scholar]
Mallion 2004 {published data only}
- Mallion J-M, Chamontin B, Asmar R, De Leeuw PW, O'Brien E, Duprez D, et al. Twenty-four-hour ambulatory blood pressure monitoring efficacy of perindopril/indapamide first-line combination in hypertensive patients: the REASON study. American Journal of Hypertension 2004;17:245-51. [DOI] [PubMed] [Google Scholar]
Mann 2002 {published data only}
- Mann SJ, Gerber LM. Psychological characteristics and responses to antihypertensive drug therapy. Journal of Clinical Hypertension 2002;4:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2004 {published data only}
- Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. American Journal of Hypertension 2004;17(2):118-23. [DOI] [PubMed] [Google Scholar]
Neaton 1993 {published data only (unpublished sought but not used)}
- Neaton JD, Grimm RH, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, et al. Treatment of mild hypertension study: final results. JAMA 1993;270(6):713-24. [PubMed] [Google Scholar]
NORDIL 2000 {published data only}
- Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359-65. [DOI] [PubMed] [Google Scholar]
- Svensson-Farbom P, Wahlstrand B, Almgren P, Dahlberg J, Fava C, Kjeldsen S, et al. A functional variant of the NEDD4L gene is associated with beneficial treatment response with β-blockers and diuretics in hypertensive patients. Journal of Hypertension 2011;29:388-95. [DOI] [PubMed] [Google Scholar]
Oshchepkova 2007 {published data only}
- Oshchepkova EV, Dmitriev VA, Titiov VN, Rogoza AN, Masenko VP. The activity of nonspecific inflammation in hypertensive patients. Terapevticheskii Arkhiv 2007;79:18-25. [PubMed] [Google Scholar]
PEAR 2012 {published data only}
- Moore M, Yan G, Hou W, Chapman AB, Langaee T, Schwartz GL, et al. Clinical predictors of dysglycemic effects associated with use of beta blockers and thiazide diuretics. Clinical Pharmacology and Therapeutics 2012;91:S24-5. [Google Scholar]
- Moore MJ, Gong Y, Hou W, Hall K, Schmidt SO, Curry RW, et al. Predictors for glucose change in hypertensive participants following short-term treatment with atenolol or hydrochlorothiazide. Pharmacotherapy 2014;34:1132-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner ST, Schwartz GL, Chapman AB, Beitelshees AL, Gums JG, Cooper-Dehoff RM, et al. Power to identify a genetic predictor of antihypertensive drug response using different methods to measure blood pressure response. Journal of Translational Medicine 2012;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandell AG, Lobmeyer MT, Gawronski BE, Langaee TY, Gong Y, Gums JG, et al. G protein receptor kinase 4 polymorphisms: beta-blocker pharmacogenetics and treatment-related outcomes in hypertension. Hypertension 2012;60(4):957-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peng 2015 {published data only}
- Peng J, Zhao Y, Zhang H, Liu Z, Wang Z, Tang M, et al. Prevention of metabolic disorders with telmisartan and indapamide in a Chinese population with high-normal blood pressure. Hypertension Research 2015;38(2):123-31. [DOI] [PubMed] [Google Scholar]
Pool 2009 {published data only}
- Pool JL, Glazer R, Crikelair N, Levy D. The role of baseline blood pressure in guiding treatment choice: a secondary analysis of the use of valsartan/hydrochlorothiazide as initial therapy in hypertensive adults in a randomized, double-blind, placebo-controlled trial. Clinical Drug Investigation 2009;29(12):791-802. [DOI] [PubMed] [Google Scholar]
Posadzy‐Malaczynska 2014 {published data only}
- Posadzy-Malaczynska A, Rajpold K, Woznicka-Leskiewicz L, Marcinkowska J. Hemodynamic and metabolic effects of estrogen plus progestin therapy in hypertensive postmenopausal women treated with an ACE-inhibitor or a diuretic. Clinical Research in Cardiology 2014;104:38-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
PROGRESS 2001 {published data only}
- He FJ, MacGregor GA. Blood pressure and stroke; the PROGRESS trial. Journal of the Renin-Angiotensin-Aldosterone System 2001;2:153-5. [DOI] [PubMed] [Google Scholar]
Rasmussen 2006 {published data only}
- Rasmussen S, Borrild N, Vang Andersen J. Efficacy and safety of 24 weeks of therapy with bendroflumethiazide 1.25 mg/day or 2.5 mg/day and potassium chloride compared with enalapril 10 mg/day and amlodipine 5 mg/day in patients with mild to moderate primary hypertension: a multicentre, randomised, open study. Clinical Drug Investigation 2006;26:91-101. [DOI] [PubMed] [Google Scholar]
SALT 2007 {published data only}
- Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation 2007;116(3):268-75. [DOI] [PubMed] [Google Scholar]
Schram 2005 {published data only}
- Schram MT, Ittersum FJ, Spoelstra-de Man A, Dijk RA, Schalkwijk CG, Ijzerman RG, et al. Aggressive antihypertensive therapy based on hydrochlorothiazide, candesartan or lisinopril as initial choice in hypertensive type II diabetic individuals: effects on albumin excretion, endothelial function and inflammation in a double-blind, randomized clinical trial. Journal of Human Hypertension 2005;19(6):429-37. [DOI] [PubMed] [Google Scholar]
- Spoelstra-de Man AME, Ittersum FJ, Schram MT, Kamp O, Dijk RAJM, Ijzerman RG, et al. Aggressive antihypertensive strategies based on hydrochlorothiazide, candesartan or lisinopril decrease left ventricular mass and improve arterial compliance in patients with type II diabetes mellitus and hypertension. Journal of Human Hypertension 2006;20:599-611. [DOI] [PubMed] [Google Scholar]
Schwartz 2013 {published data only}
- Schwartz GL, Bailey K, Chapman AB, Boerwinkle E, Turner ST. The role of plasma renin activity, age, and race in selecting effective initial drug therapy for hypertension. American Journal of Hypertension 2013;26:957-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
SHEP 1991 {published data only}
- Kostis JB, Sedjro JE, Cabrera J, Cosgrove NM, Pantazopoulos JS, Kostis WJ, et al. Visit-to-visit blood pressure variability and cardiovascular death in the Systolic Hypertension in the Elderly Program. Journal of Clinical Hypertension 2014;16:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostis JB. Effect of chlorthalidone treatment on mortality: 20-year follow-up of the systolic hypertension in the elderly program (SHEP). European Heart Journal 2011;32:971-2. [Google Scholar]
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program. JAMA 1991;265:3255-64. [PMID: ] [PubMed] [Google Scholar]
Shionoiri 2000 {published data only}
- Shionoiri H, Kosaka T, Kita E, Takizawa T, Takasaki I. Comparison of long-term therapeutic effect of an ACE inhibitor, temocapril, with that of a diuretic on microalbuminuria in non-diabetic essential hypertension. Hypertension Research - Clinical & Experimental 2000;23:593-600. [DOI] [PubMed] [Google Scholar]
Sierra 2004 {published data only}
- Sierra C, Sobrino J, Antonio MT, Larrousse M, Domenech M, Coca A, et al. Effect of antihypertensive therapy with amlodipine or diuretic on silent cerebrovascular damage: a randomized, double blind, comparative study. Journal of Hypertension 2004;22:S85. [Google Scholar]
Solorzano 2011 {published data only}
- Solorzano G, Chen F, Mychaleckyj JC, Chen WM, Hsu FC, Sale M, et al. Antihypertensive class and stroke recurrence: an analysis of the VISP trial. Stroke 2011;42(3):e348. [Google Scholar]
SPREAD 2006 {published data only}
- Fan X, Wang P, Huan T, Yang X, Chen J, Zheng Y, et al. Low-dose hydrochlorothiazide monotherapy is the most effective and tolerable antihypertensive drug compared with long-acting nifedipine, atenolol, or captopril monotherapy in Chinese patients with hypertension: The SPREAD study. Circulation 2006;113:E314. [Google Scholar]
STOP‐Hypertension‐2 1999 {published data only}
- Ekbom T, Linjer E, Hedner T, Lanke J, De FU, Wester PO, et al. Cardiovascular events in elderly patients with isolated systolic hypertension. A subgroup analysis of treatment strategies in STOP-Hypertension-2. Blood Pressure 2004;13:137-41. [DOI] [PubMed] [Google Scholar]
- Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Schersten B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999;354:1751-6. [DOI] [PubMed] [Google Scholar]
- Hansson L. Results of the STOP-Hypertension-2 trial. Blood Pressure. Supplement 2000;9(2):17-20. [PubMed] [Google Scholar]
- Lindholm LH, Anderson H, Ekbom T, Hansson L, Lanke J, Dahlof B, et al. Relation between drug treatment and cancer in hypertensives in the Swedish Trial in Old Patients with Hypertension 2: a 5-year, prospective, randomised, controlled trial. Lancet 2001;358(9281):539-44. [DOI] [PubMed] [Google Scholar]
Stritzke 2010 {published data only}
- Stritzke J, Mortensen K, Markus MR, Lieb W, Luchner A, Doring A, et al. Impact of medical treatment on central aortic systolic blood pressure - The MONICA/KORA study. Journal of Hypertension 2010;28:e133. [Google Scholar]
Syst‐Eur 1997 {published data only}
- Fagard RH, Staessen JA, Thijs L, Celis H, Birkenhager WH, Bulpitt CJ, et al. Prognostic significance of electrocardiographic voltages and their serial changes in elderly with systolic hypertension. Hypertension 2004;44(4):459-64. [DOI] [PubMed] [Google Scholar]
- Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, De Leeuw PW, et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. Journal of Hypertension 2003;21(12):2251-7. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tedesco 1998 {published data only}
- Tedesco MA, Ratti G, Aquino D, Limongelli G, di Salvo G, Mennella S, et al. Effects of losartan on hypertension and left ventricular mass: a long-term study. Journal of Human Hypertension 1998;12:505-10. [DOI] [PubMed] [Google Scholar]
Tedesco 1999 {published data only}
- Tedesco MA, Ratti G, Mennella S, Manzo G, Grieco M, Rainone AC, et al. Comparison of losartan and hydrochlorothiazide on cognitive function and quality of life in hypertensive patients. American Journal of Hypertension 1999;12:1130-4. [DOI] [PubMed] [Google Scholar]
Trimarco 2011 {published data only}
- Trimarco V. Persistence and compliance to treatment with diuretics as first choice in antihypertensive therapy. High Blood Pressure and Cardiovascular Prevention 2011;18:152. [Google Scholar]
Trimarco 2015 {published data only}
- Trimarco V, Izzo R, Migliore T, Rozza F, Marino M, Manzi MV, et al. Should thiazide diuretics be given as first line antihypertensive therapy or in addition to other medications? High Blood Pressure and Cardiovascular Prevention 2015;22:55-9. [DOI] [PubMed] [Google Scholar]
VADT 2011 {published data only}
- Anderson RJ, Bahn GD, Moritz TE, Kaufman D, Abraira C, Duckworth W. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care 2011;34(1):34-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veronesi 2007 {published data only}
- Veronesi M, Cicero AF, Prandin MG, Dormi A, Cosentino E, Strocchi E, et al. A prospective evaluation of persistence on antihypertensive treatment with different antihypertensive drugs in clinical practice. Vascular Health andRrisk Management 2007;3(6):999-1005. [PMC free article] [PubMed] [Google Scholar]
Wilson 1963 {published data only}
- Wilson WR, Okun R, Tetreault L. Methyldopa and hydrochlorothiazide in primary hypertension: controlled clinical trial of drugs singly and in combination. JAMA 1963;185:819-25. [DOI] [PubMed] [Google Scholar]
Yasuda 2015 {published data only}
- Yasuda G, Saka S, Ando D, Hirawa N. Effects of doxazosin as the third agent on morning hypertension and position-related blood pressure changes in diabetic patients with chronic kidney disease. Clinical and Experimental Hypertension 2015;37:75-81. [DOI] [PubMed] [Google Scholar]
Yogiantoro 2000 {published data only}
- Yogiantoro M, Bakri S, Kabo P. Efficacy and tolerability of indapamide sr 1.5 mg in mild to moderate hypertension: a double-blind study compared to amlodipine 5 mg. In: 13th Asian Colloquium in Nephrology ~ Kartika Plaza Beach Hotel, Bali. 2000:180-90.
Yurenev 1992 {published data only}
- Yurenev AP, Dyakonova HG, Novikov ID, Vitols A, Pahl L, Haynemann G, et al. Management of essential hypertension in patients with different degrees of left ventricular hypertrophy. Multicenter trial. American Journal of Hypertension 1992;5:182S-9S. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02217852 {published data only}
- NCT02217852. Treatment of hypertension in Tibetan adult population [A randomized, open-label, positive drug controlled clinical trials to compare the efficacy of nitrendipine and hydrochlorothiazide, captopril plus hydrochlorothiazide and Beijing hypotensive No.0 in Tibetan hypertension]. clinicaltrials.gov/show/NCT02217852 (first received 15 August 2014).
Additional references
Arguedas 2020
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets in adults with hypertension. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD004349. [DOI: 10.1002/14651858.CD004349.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakris 2019
- Bakris G, Ali W, Parati G. ACC/AHA Versus ESC/ESH on Hypertension Guidelines. Journal of the American College of Cardiology 2019;73(23):3018-26. [DOI: 10.1016/j.jacc.2019.03.507] [DOI] [PubMed] [Google Scholar]
Batterink 2010
- Batterink J, Stabler SN, Tejani AM, Fowkes CT. Spironolactone for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. Art. No: CD008169. [DOI: 10.1002/14651858.CD008169.pub2] [DOI] [PubMed] [Google Scholar]
Ben‐Eltriki 2021
- Ben-Eltriki M, Cassels A, Erviti J, Wright JM. Why we need a single independent international hypertension clinical practice guideline. Hypertension Research 2021;44(8):1037-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2018
- Chen YJ, Li LJ, Tang WL, Song JY, Qiu R, Li Q, et al. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD008170. [DOI: 10.1002/14651858.CD008170.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1990
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke and coronary heart disease - part 2, short term reduction in blood pressure: overview of randomised drug trials in epidemiological context. Lancet 1990;335:827-38. [DOI] [PubMed] [Google Scholar]
Diao 2012
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD006742. [DOI: 10.1002/14651858.CD006742.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duarte 2010
- Duarte JD, Cooper-DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Review of Cardiovascular Therapy 2010;8(6):793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dzau 1990
- Dzau VJ. Mechanism of action of angiotensin-converting enzyme (ACE) inhibitors in hypertension and heart failure. Role of plasma versus tissue ACE. Drugs 1990;39(Suppl 2):11-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Frishman 2005
- Frishman WH, Cheng-Lai A, Nawarskas J. Current Cardiovascular Drugs. Fourth edition. Current Science Group, 2005. [Google Scholar]
Gueyffier 1996
- Gueyffier F, Froment A, Gouton M. New meta-analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. Journal of Human Hypertension 1996;10:1-8. [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003823. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003822. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2012a
- Heran BS, Galm BP, Wright JM. Blood pressure lowering efficacy of alpha blockers for primary hypertension. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD004643. [DOI: 10.1002/14651858.CD004643.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2012b
- Heran BS, Chen JMH, Wang JJ, Wright JM. Blood pressure lowering efficacy of potassium‐sparing diuretics (that block the epithelial sodium channel) for primary hypertension. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD008167. [DOI: 10.1002/14651858.CD008167.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Insua 1994
- Insua JT, Sacks HS, Lau TS, Lau J, Reitman D, Pagano D, et al. Drug treatment of hypertension in the elderly: a meta-analysis. Annals of Internal Medicine 1994;121:355-62. [DOI] [PubMed] [Google Scholar]
IPPSH 1985
- IPPSH Collaboration Group. Cardiovascular risk and risk factors in a randomized trial of beta blocker, oxeprenolol: the International Prospective Primary Prevention study in hypertension (IPPSH). Hypertension 1985;3:379-92. [DOI] [PubMed] [Google Scholar]
James 2014
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507-20. [DOI: 10.1001/jama.2013.284427] [DOI] [PubMed] [Google Scholar]
Katz 1986
- Katz AM. Pharmacology and mechanisms of action of calcium-channel blockers. Journal of Clinical Hypertension 1986;2(3 Suppl):28s-37s. [PubMed] [Google Scholar]
Longo 2010
- Longo, DL. Harrison's Principals of Internal Medicine. Vol. 2. New York: McGraw-Hill, 2012. [ISBN 978-0-07-174887-2] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017;(2). [DOI: 10.1002/14651858.MR000033.pub3] [MR000033] [DOI] [PMC free article] [PubMed]
MacMahon 1993
- MacMahon S, Rogers A. The effects of blood pressure reduction in older patients: an overview of five randomized controlled trials in the elderly hypertensives. Clinical & Experimental Hypertension (New York) 1993;15(6):967-78. [DOI] [PubMed] [Google Scholar]
Musini 2014
- Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD003824. [DOI: 10.1002/14651858.CD003824.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Musini 2015
- Musini VM, Rezapour P, Wright JM, Bassett K, Jauca CD. Blood pressure‐lowering efficacy of loop diuretics for primary hypertension. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD003825. [DOI: 10.1002/14651858.CD003825.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Musini 2017
- Musini VM, Gueyffier F, Puil L, Salzwedel DM, Wright JM. Pharmacotherapy for hypertension in adults aged 18 to 59 years. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD008276. [DOI: 10.1002/14651858.CD008276.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Musini 2019
- Musini VM, Tejani AM, Bassett K, Puil L, Wright JM. Pharmacotherapy for hypertension in adults 60 years or older [Pharmacotherapy for hypertension in the elderly]. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD000028. [DOI: 10.1002/14651858.CD000028.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nash 1990
- Nash DT. Alpha-adrenergic blockers: mechanism of action, blood pressure control, and effects of lipoprotein metabolism. Clinical Cardiology 1990;13(11):764-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Psaty 1997
- Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Hechbert SR, et al. Health outcomes associated with antihypertensive therapies used as first line agents. A systematic review and meta-analysis. JAMA 1997;277:739-45. [PubMed] [Google Scholar]
Psaty 2003
- Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003;289:2534-44. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen, Denmark: The Cochrane Collaboration, 2020.
Rodgers 2001
- Rodgers JE, Patterson JH. Angiotensin II-receptor blockers: clinical relevance and therapeutic role. American Journal of Health-system Pharmacy: AJHP 2001;58(8):671-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rohatgi 2021 [Computer program]
- WebPlotDigitizer. Rohatgi A, Version 4.5. Pacifica, CA: Ankit Rohatgi, August 2021. https://automeris.io/WebPlotDigitizer.
Shafiq 2008
- Shafiq MM, Menon DV, Victor RG. Oral direct renin inhibition: premise, promise, and potential limitations of a new antihypertensive drug. American Journal of Medicine 2008;121(4):265-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheppard 2018
- Sheppard JP, Stevens S, Stevens R, Martin U, Mant J, Hobbs FD, et al. Benefits and harms of antihypertensive treatment in low-risk patients with mild hypertension. JAMA Internal Medicine 2018;178(12):1626-34. [DOI: 10.1001/jamainternmed.2018.4684] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sundström 2015
- Sundström J, Arima H, Jackson R, Turbull F, Rahimi K, Chalmers J, et al. Effects of blood pressure reduction in mild hypertension. A systematic review and meta-analysis. Annals of Internal Medicine 2015;162:184-91. [DOI] [PubMed] [Google Scholar]
Thijs 1992
- Thijs L, Fagard R, Lijnen P, Staessen J, VanHoof R, Amery A. A meta-analysis of outcome trials in elderly hypertensives. Journal of Hypertension 1992;10:1103-9. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2015
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: head-to-head comparisons of various classes of antihypertensive drugs--overview and meta-analyses. Journal of Hypertension 2015;33(7):1321-41. [DOI] [PubMed] [Google Scholar]
Whelton 2018
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Journal of the American College of Cardiology May 2018;71(19):e127-e248. [DOI: 10.1016/j.jacc.2017.11.006] [DOI] [PubMed] [Google Scholar]
Williams 2018
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. Journal of Hypertension 2018;36(10):1953-2041. [DOI] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta-blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD002003. [DOI: 10.1002/14651858.CD002003.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2018
- Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD001841. [DOI: 10.1002/14651858.CD001841.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2021
- Zhu J, Chen N, Zhou M, Guo J, Zhu C, Zhou J, et al. Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD003654. [DOI: 10.1002/14651858.CD003654.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Reinhart 2011
- Reinhart M, Musini VM, Salzwedel DM, Dormuth C, Wright JM. First-line diuretics versus other classes of antihypertensive drugs for hypertension. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD008161. [DOI: 10.1002/14651858.CD008161.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1999
- Wright JM, Lee CH, Chambers GK. Systematic review of anti-hypertensive therapies: does the evidence assist in choosing a first-line drug? Canadian Medical Association Journal 1999;161(1):25-32. [PMC free article] [PubMed] [Google Scholar]
Wright 2009
- Wright JM, Musini VM, Salzwedel DM, Dormuth C. First-line diuretics versus other classes of antihypertensive drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD008161. [DOI: 10.1002/14651858.CD008161] [DOI] [PMC free article] [PubMed] [Google Scholar]


